Abstract
Study Design:
Cross-sectional study.
Objectives:
To define multilevel lumbar developmental spinal stenosis (DSS) using a composite score model and to determine its prevalence.
Methods:
This was a cohort study of 2385 openly recruited subjects with lumbosacral (L1-S1) MRIs. All subjects with previous spinal surgery or spinal deformities were excluded. The anteroposterior (AP) vertebral canal diameter was measured by two independent observers. Any associations between level-specific vertebral canal diameter and subject body habitus were analysed with non-parametric tests. Three or more stenotic levels, equivalent to a composite score of 3 or more, were considered as multilevel DSS. The median values of these subjects’ AP canal diameters were used to construct the multilevel DSS values. Receiver operating characteristic analysis was utilized to determine the ability of these cut-off values to screen for DSS by presenting their area under curve, sensitivity and specificity.
Results:
Subject body habitus was poorly correlated with AP vertebral canal diameter. Multilevel DSS was identified as L1<19 mm, L2<19 mm, L3<18 mm, L4<18 mm, L5<18 mm, S1<16 mm with 81%–96% sensitivity and 72%–91% specificity. The prevalence of multilevel DSS in this cohort was 7.3%.
Conclusions:
Utilizing a large homogeneous cohort, the prevalence of multilevel DSS is determined. Our cut-offs provide high diagnostic accuracy. Patients with multiple levels that fulfil these criteria may be at-risk of spinal canal compressions at multiple sites.
Level of Evidence:
III
Keywords: developmental spinal stenosis, lumbar spine, magnetic resonance imaging, multilevel, vertebral canal, prevalence
Introduction
Developmental lumbar spinal stenosis (DSS), as illustrated by Verbiest, 1 refers to a pre-existing narrowing of the lumbar bony canal. There is a lower threshold whereby patients are more susceptible to neural compression with mild degeneration, such as milder degrees of ligamentum flavum hypertrophy and disc herniation. It is important to differentiate DSS from degenerative lumbar spinal stenosis as they have different pathogenesis and treatments. 2 It was reported that spinal stenosis was associated with genetic mutations such as COL9A2, Trp2, and Trp3,3,4 while LRP5 mutation is associated with DSS. 5 Hence, this is a result of spinal canal maldevelopment rather than a result of degenerative changes.
As DSS is considered to be a developmental event, 6 multilevel stenosis is expected and these patients are prone to developing neural compression at multiple levels.7-9 This contributes to a high risk of reoperation up to 22% if levels with DSS are not decompressed in the initial surgery.10-12 Therefore, it is crucial to identify any stenotic levels prior to the index surgery as they may also be indicated for prophylactic decompression. This can only be achieved by using a precise and accurate, standardized diagnostic tool. Over the years, numerous radiological cut-offs have been proposed to define DSS7,13-16 without consensus. Some proposed cut-offs were of low sensitivity, indicating that many cases were underdiagnosed. 14 The imaging modalities were also inconsistent, ranging from axial or sagittal magnetic resonance imaging (MRI), to computed tomography (CT), myelography with contrast, and plain radiographs.7-9,13-15,17-19 Furthermore, the orientation of patients during MRI or plain radiography also varied between studies, including supine and lateral standing.7,9,13-16 In addition, the sample size in each individual study was very limited which is not representative of the overall population. Most importantly, the definition of DSS were based on single level measurements without consideration of multilevel involvement. With the above discrepancies, there is a need for a better definition of DSS.
Despite the inconsistency, it is well-recognized that the anteroposterior (AP) spinal canal diameter is significantly shorter in patients with DSS than the general “normal” population.7,9,13,14 Therefore, due to a lack of consensus and weak significance in defining DSS as stated above, a cross-sectional study is conducted by utilizing population-based cohort to redefine the diagnostic criteria of DSS with a multilevel component and to determine the population-based prevalence of multilevel DSS. These new values provide better representation of a developmental origin, and is based on a large sample size with high sensitivity and specificity.
Materials and Methods
Study Design and Population
This was a cohort study assessing 2385 subjects14,20-23 who were openly recruited from the general population via newspaper advertisement, posters and emails regardless of social or economic status between 2010-2015. Patients with previous lumbar surgery or history of scoliosis as a child were excluded. The selection was not based on the presence or absence of clinical symptoms. All subjects were of Chinese ethnicity, and underwent axial and sagittal MRI of the lumbosacral spine (L1-S1). Ethics review was conducted by a local institutional review board (UW 13-570). Written consent was obtained for every subject. As subjects were openly recruited from the population for MRIs without specifically targeting spinal stenosis, they were considered to be representative of the general population. Information regarding any low back pain or radicular leg pain in the past month and year from the date of MRI was obtained. There was no missing data.
MRI Protocol
1.5 or 3 T HD MRI machines were used for imaging in all subjects in supine position. For axial scans, the field of view was 21 cm × 21 cm; for sagittal scans, the field of view was 28 cm × 28 cm. Slice thickness was 4 mm and 5 mm, and slice spacing was 0.4 mm and 1 mm for axial and sagittal scans respectively. The imaging matrix was 218 × 256 for axial scans and 448 × 336 for sagittal scans. For T1 and T2, the repetition time were 500-800 ms and 3320 ms respectively, and the echo time were 9.5 ms and 85 ms for T1 and T2 respectively. There were 11 slices per vertebral level and parallel slices were made according to the disc and pedicle levels.
Measurements
Two investigators were blinded to all clinical information and performed the measurements independently. Methods of measurements were standardized before data collection began. A subset of 40 subjects were randomly selected for intraobserver and interobserver reliability assessments prior to measuring the main cohort. The first and second round of measurements were performed at least 3 weeks apart. All images were measured using Philips DICOM Viewer 3.0 (Philips, Andover, MA, USA).
AP vertebral canal diameters and AP vertebral body diameters were obtained from T1-weighted axial MR images (Figure 1). The images were the cuts with the thickest pedicle diameter that also included the vertebral body, pedicle and lamina. The pedicle level was chosen to specifically measure the bony spinal canal diameter. This had been shown to be a useful and reproducible measurement for DSS. 14
Figure 1.
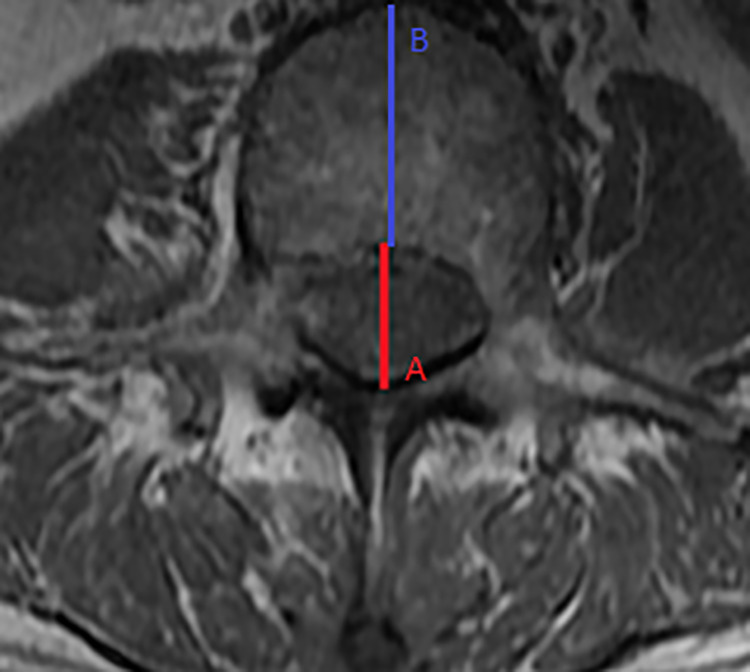
Axial magnetic resonance imaging: (A) Midline anteroposterior (AP) vertebral canal diameter; (B) Midline AP vertebral body diameter.
Redefining DSS
To specifically address the issue of defining DSS with a multilevel component, we utilized previously established cut-off values of the midline AP canal diameter 14 : L1 < 20 mm, L2 < 19 mm, L3 < 19 mm, L4 < 17 mm, L5 < 16 mm, S1 < 16 mm. These values were defined by comparing axial MRIs of surgical cases with controls and thus were more clinically relevant. However, they were based on single level diagnoses and could only indicate a level that was sporadically narrower than others or subject to measurement variations. Using our 2385 subjects, we dichotomized the canal measurements of each vertebral level as having DSS (score of 1) versus non-DSS (score of 0). This scoring system indicated whether a spinal level was developmentally stenotic and we defined a DSS “composite score” which represented the sum of the 6 individual vertebral level scores. Such scoring system was developed for better illustration and easier communication in this study. Those with a composite score of 3 or above, representing 3 or more stenotic levels, were considered as multilevel DSS. This was decided as the definition as two operative levels of lumbar spinal stenosis would coincide with three vertebral levels (i.e. L3-5 decompression).
Statistical Analysis
Descriptive statistics were conducted and presented as mean and range. Intraobserver and interobserver reliability assessments were based on Cronbach α analysis. Excellent reliability was noted with α values of 0.90 to 1.00 and good reliability was noted with 0.80 to 0.89. 24 To investigate any relationship between the canal diameter at each vertebral level and parameters relevant to patient body habitus including age, sex, body mass index (BMI) and the AP vertebral body diameter, nonparametric correlation tests using Spearman’s correlation and Kendall’s tau-b were used for continuous and binary variables respectively. The results of any relationship found between AP canal diameter and various patient factors were taken into account when redefining the cut-off values for multilevel DSS. A correlation coefficient between −0.3 and 0.3 was noted to be poor, while a value of 0.3 to 0.5 or −0.3 to −0.5 was noted to have low correlation. 25
The level-specific median AP spinal canal diameter measurements of subjects with a composite score of 3 or more were used as a reference to indicate any subjects with multilevel DSS. Any canal diameter that was smaller than the proposed values 14 was considered a DSS level. Receiver operating characteristic (ROC) curve and the area under curve (AUC) were used to assess the diagnostic property of these proposed values, as compared to previous values. The best cut-off value with optimal sensitivity and specificity was applied. An AUC of 0.8 to 0.9 was considered excellent, while AUC of more than 0.9 was considered outstanding. 26 A decrease in AUC suggested worse diagnostic accuracy. Lastly, these new values were used to screen for multilevel DSS in this cohort and to identify its prevalence. SPSS Statistics 26 (IBM SPSS Inc., Chicago, IL, USA) was used for analysis. A P-value of less than 0.05 was considered statistically significant.
Results
There were 913 males (38.3%) and 1472 females (61.7%) with mean age of 51.1 (range: 16.7-77.9) and 51.2 (range: 17.4-86.3) respectively. The mean body weight was 69.6 kg for males and 57.2 kg for females, mean body height was 1.69 m and 1.57 m respectively, and mean BMI was 24.2 kg/m2 and 23.2 kg/m2 respectively. The mean and range of AP vertebral canal diameters and AP vertebral body diameters are presented in Table 1. Correlations of canal diameter and age, sex, BMI, and AP vertebral body diameter were poor or statistically insignificant, with correlation coefficients within −0.3 and 0.3 (Table 2). Excellent interobserver (α = 0.90-0.96) and intraobserver reliability (α = 0.92-0.99 and α = 0.92-0.99) between the two independent observers were noted.
Table 1.
Vertebral Canal and Vertebral Body Measurements.
| Measurement | Mean (mm) | Range (mm) |
|---|---|---|
| Axial anteroposterior vertebral canal diameter | ||
| L1 | 21.2 | 16.7-29.6 |
| L2 | 20.8 | 15.3-30.2 |
| L3 | 20.3 | 14.7-29.7 |
| L4 | 20.1 | 14.1-28.9 |
| L5 | 20.1 | 12.7-32.3 |
| S1 | 18.6 | 9.4-30.3 |
| Axial anteroposterior vertebral body diameter | ||
| L1 | 25.8 | 18.5-39.0 |
| L2 | 27.1 | 19.5-37.7 |
| L3 | 28.6 | 20.2-39.5 |
| L4 | 28.8 | 16.4-40.0 |
| L5 | 29.2 | 20.2-42.3 |
| S1 | 27.7 | 12.6-38.8 |
mm: millimetres.
Table 2.
Correlation Between the Vertebral Canal Diameter and Various Parameters.
| Parameters | L1 | L2 | L3 | L4 | L5 | S1 |
|---|---|---|---|---|---|---|
| Age | -0.094** | -0.031 | -0.045** | -0.006 | -0.036 | -0.006 |
| Sex | 0.090** | 0.015 | -0.014 | 0.061** | 0.101** | 0.233** |
| BMI | -0.034 | -0.041 | -0.071** | -0.033 | -0.007 | 0.052 |
| AP Vertebral Body Diameter | -0.009 | -0.007 | -0.003 | 0.014 | 0.035 | 0.018 |
** Statistically significant at the 0.05 level.
BMI: Body mass index, AP: anteroposterior.
By using previously defined DSS values, subjects with DSS were identified and composite scores were calculated (Table 3). The median AP vertebral canal diameter for subjects with a composite score of 3 or more (n=155) was also calculated (Table 4).
Table 3.
Distribution of Composite Scores.
| Composite Score | Number of Subjects | Most Prevalent Level(s) |
|---|---|---|
| 0 | 1473 | N/A |
| 1 | 542 | L3 (n=193) |
| 2 | 215 | L1+L3 (n=57) |
| 3 | 107 | L1+L2+L3 (n=55) |
| 4 | 35 | L1+L2+L3+S1 & L1+L2+L3+L4 (n=14 each) |
| 5 | 11 | L1+L2+L3+L4+S1 (n=5) |
| 6 | 2 | L1+L2+L3+L4+L5+S1 |
Table 4.
Median Anteroposterior Vertebral Canal Diameter in Patients With Composite Score of 3 or More.
| Vertebral Level | Mean (range) |
|---|---|
| L1 | 18.9 mm (16.7-19.9) |
| L2 | 18.3 mm (15.3-19.6) |
| L3 | 18.0 mm (14.7-19.0) |
| L4 | 16.6 mm (14.9-17.0) |
| L5 | 15.4 mm (14.4-15.9) |
| S1 | 14.6 mm (11.8-16.6) |
mm: millimeters
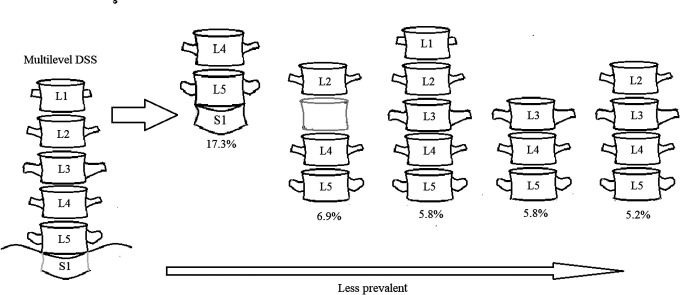
By comparing the AUCs of previously defined DSS values and the new multilevel DSS values, all levels had larger AUCs for multilevel DSS values with a range of 0.812-0.977 (p<0.001), suggesting excellent to outstanding diagnostic accuracy (Table 5). Therefore, we implemented the new multilevel DSS values for L1-S1. Based on the ROC analysis, multilevel DSS values were refined with best sensitivity and specificity for each vertebral level (Table 5). By using the new values on the same cohort, 173 subjects had multilevel DSS, which was equivalent to a prevalence of 7.3%. Multilevel stenosis involving L4+L5+S1 (17.3%) was most common, followed by L2+L4+L5 (6.9%) (Figure 2). Comparing between the 173 subjects with multilevel DSS and 2212 subjects without multilevel DSS, there was a greater likelihood for radicular leg pain (Table 6).
Table 5.
Comparison of Receiver Operating Characteristic Analysis for DSS Values.
| Vertebral Level | Multilevel DSS Cut-off Values | Sensitivity, % | Specificity, % | Area Under Curve of Diagnosing Multilevel DSS Using Prior( 12 ) DSS Values (P-value) | Area Under Curve of Diagnosing Multilevel DSS Using New Multilevel DSS Values (P-value) |
|---|---|---|---|---|---|
| L1 | 19 mm | 95.7 | 90.9 | 0.906 (< 0.001) | 0.977 (< 0.001) |
| L2 | 19 mm | 92.8 | 87.9 | 0.934 (< 0.001) | 0.965 (< 0.001) |
| L3 | 18 mm | 94.5 | 90.9 | 0.933 (< 0.001) | 0.951 (< 0.001) |
| L4 | 18 mm | 80.9 | 78.8 | 0.875 (< 0.001) | 0.892 (< 0.001) |
| L5 | 18 mm | 84.7 | 71.9 | 0.814 (< 0.001) | 0.882 (< 0.001) |
| S1 | 16 mm | 80.7 | 73.1 | 0.772 (< 0.001) | 0.812 (< 0.001) |
DSS = Developmental lumbar spinal stenosis, mm: millimetres, %: percentage.
Figure 2.
Patterns of Multilevel Lumbar Developmental Spinal Stenosis. L4+L5+S1 multilevel stenosis was most prevalent, followed by L2+L4+L5 multilevel stenosis.
Table 6.
Prevalence of Pain.
| DSS | Non-DSS | P-value | |
|---|---|---|---|
| Number of Subjects | n=173 | n=2212 | |
| LBP in the past month | 92 (53.2%) | 1157 (52.3%) | 0.351 |
| LBP in the past year | 120 (69.4%) | 1468 (66.4%) | 0.069 |
| Radicular leg pain in the past month | 61 (35.3%) | 610 (27.6%) | 0.008 |
| Radicular leg pain in the past year | 83 (48.0%) | 820 (37.1%) | 0.001 |
DSS: developmental spinal stenosis; LBP: low back pain.
Discussion
Multilevel involvement is one of the most distinctive features in patients with DSS.7-9 This is an inborn defect rather than a degenerative change because it is considered as a maldevelopment during fetal and post-natal period. The current study showed that different demographics are poorly correlated with the canal size, and such correlations were negligible,25,27 which adds evidence to a developmental origin. In addition, patients with DSS are reported to have high risk of reoperation especially at the adjacent levels.10-12,28,29 This may be contributed by the presence of multilevel stenosis because pre-existing narrowed canals that are asymptomatic tend not to be investigated and operated. Furthermore, it is well-recognized that the lamina is significantly shorter in patients with DSS which contributes to a shorter AP canal diameter.7,9,13,14,30 The narrowed vertebral spinal canal predisposes patients to neural compression even with only mild degeneration. As current available evidence7-9,13-15,17-19 fails to reach consensus on defining DSS, this study redefined the cut-offs for DSS with a large-scale population-based cohort, and found the prevalence of multilevel DSS in southern Chinese. Our proposed values are different from previous proposed critical values, below which patients required surgery.1,14 However, our results reflect the involvement of multilevel stenosis in the general population, and provides a guideline to which patients should be screened to better identify those at-risk of multilevel compression and risk of reoperation. Nevertheless, DSS is an important consideration during pre-operative assessment with nearly four times greater odds of adjacent level reoperation after the index decompression surgery. 10 Surgeons should consider whether pre-emptive decompression is warranted especially in those with pre-existing degeneration. Further studies are required to determine whether this practice is justifiable.
Many studies assessed the prevalence of lumbar spinal stenosis and degenerative lumbar spinal stenosis, but failed to address DSS specifically.21,31,32 Our study reported a prevalence of 7.3%, suggesting that multilevel DSS is not an uncommon pathology in the general population. We redefined new cut-off values for DSS based on the presence of multilevel stenosis. This is an appropriate definition as multilevel stenosis is a significant radiological feature in DSS, and is a major factor for reoperation risk. By using ROC analysis, we were able to refine the cut-offs with the best sensitivity and specificity. These values allowed us to identify more underdiagnosed cases accurately. Any subject with a composite score of 3 or more should be closely followed up because they are prone to develop compressive symptoms when they age with degeneration. These cut-off figures are representative of multilevel involvement.
Based on our results, L4-S1 was the most common multilevel pattern. This was of great importance as many symptoms arise from stenotic L4-S1 such as low back pain and sciatica,11,12,33 and many of the surgical cases are targeting these three levels. 10 Furthermore, 4 out of 5 patterns (Figure 2) and more than 50% of subjects with multilevel DSS in our cohort had narrowed lumbosacral levels in a consecutive manner, suggesting a possible consecutive involvement for this pathology. All newly proposed values had better AUCs than the previous established DSS values. 14 Similar to our study, the authors also used subjects from the general population and without surgery to develop DSS cut-offs for L1-L3. However, our larger sample size and better AUCs indicated our proposed values were more appropriate for population screening. Conversely, the previous study only included values for L4-S1 in a surgical cohort. This is reflected by improved AUCs of L4-S1 in our study, suggesting better diagnostic accuracy. These refined criteria provide superior diagnosis of multilevel stenosis in the underlying population, which serves the purpose of this study.
It is important to obtain accurate and precise measurements to define DSS. The AP canal diameter was used in this study as it is proven to be one of the most reliable spinal canal parameters to reflect canal development.7,9,13,14 Many authors have used both axial and midsagittal MRI to evaluate the canal size, but midsagittal images are prone to inaccuracies. The canal diameter can be affected by the posterior curvature of the vertebrae and endplate disease.14,34 Moreover, poor positioning and spinal deformities such as scoliosis greatly affect the possibility of obtaining a good midline cut for measurements. Therefore, greater variability is expected for midsagittal measurements, while axial measurements are preferable and more reliable.
Although our study provides some insights upon the definition and prevalence of multilevel DSS in the general population, there are several limitations in this study. Our results are based on southern Chinese and thus may not be generalizable to other populations. Results should be validated in different populations. Having said that, focusing on one ethnicity allowed us to minimize confounders such as genetic variations. In addition, as our subjects were openly recruited from the general population, the amount of male and female participants in this analysis could not be controlled as with the age of subjects. We also only excluded patients with known history of scoliosis, any deformities that are occult or unbeknownst to the subjects may have been present. This was also a cross-sectional study design. Prospective follow-up of subjects to determine how many patients develop spinal stenosis symptoms requiring surgery should be performed. This is necessary to determine the risk of life-time symptomatology. Future study should also examine the relationship of multilevel DSS with other potential developmental phenotypes such as Schmorl’s nodes and degenerative disc disease, and its development through skeletal growth. Although the diagnostic criteria is based on bony parameters and should be static, our measurements were performed on supine MRIs. Whether changes alter with dynamic MRI should be studied.
This study provides a more comprehensive understanding of the scope of DSS in a population. The large-scale population-based study provides the population-based prevalence of multilevel DSS and a better justified imaging definition. Multilevel DSS is not an uncommon pathology with specific patterns of level involvement. By emphasizing the presence of multilevel stenosis, our proposed DSS values serve to identify patients with multilevel DSS for better characterization of patients at-risk of multilevel stenosis and provides a guideline for determining which spinal levels may require consideration for preemptive decompression.
Footnotes
Author Contribution: Marcus Kin Long Lai and Prudence Wing Hang Cheung are equal contributors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by the Master of Research in Medicine (MRes) programme at the University of Hong Kong, AOSpine Asia Pacific Regional Grant and the Hong Kong Theme-Based Research Scheme (T12-708/12 N).
ORCID iD: Prudence Wing Hang Cheung, BDSc(Hons)  https://orcid.org/0000-0002-3213-7373
https://orcid.org/0000-0002-3213-7373
Dino Samartzis, DSc  https://orcid.org/0000-0002-7473-1311
https://orcid.org/0000-0002-7473-1311
Jason Pui Yin Cheung, MBBS, MMedSc, MS, PDipMDPath, MD, FHKCOS, FHKAM, FRCSEd  https://orcid.org/0000-0002-7052-0875
https://orcid.org/0000-0002-7052-0875
References
- 1.Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br. 1954;36-B(2):230–237. [DOI] [PubMed] [Google Scholar]
- 2.Lai MKL, Cheung PWH, Cheung JPY. A systematic review of developmental lumbar spinal stenosis. Eur Spine J. 2020;29(9):2173–2187. [DOI] [PubMed] [Google Scholar]
- 3.Hyun SJ, Park BG, Rhim SC, et al. A haplotype at the COL9A2 gene locus contributes to the genetic risk for lumbar spinal stenosis in the Korean population. Spine (Phila Pa 1976). 2011;36(16):1273–1278. [DOI] [PubMed] [Google Scholar]
- 4.Noponen-Hietala N, Kyllonen E, Mannikko M, et al. Sequence variations in the collagen IX and XI genes are associated with degenerative lumbar spinal stenosis. Ann Rheum Dis. 2003;62(12):1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung JPY, Kao PYP, Sham P, et al. Etiology of developmental spinal stenosis: a genome-wide association study. J Orthop Res. 2018;36(4):1262–1268. [DOI] [PubMed] [Google Scholar]
- 6.Lai MKL, Cheung PWH, Song YQ, Samartzis D, Cheung JPY. Pedigree analysis of lumbar developmental spinal stenosis: determination of potential inheritance patterns. J Orthop Res. 2020. [Epub] [DOI] [PubMed] [Google Scholar]
- 7.Kitab SA, Alsulaiman AM, Benzel EC. Anatomic radiological variations in developmental lumbar spinal stenosis: a prospective, control-matched comparative analysis. Spine J. 2014;14(5):808–815. [DOI] [PubMed] [Google Scholar]
- 8.Postacchini F, Pezzeri G. CT scanning versus myelography in the diagnosis of lumbar stenosis. A preliminary report. Int Orthop. 1981;5(3):209–215. [DOI] [PubMed] [Google Scholar]
- 9.Postacchini F, Pezzeri G, Montanaro A, Natali G. Computerised tomography in lumbar stenosis. A preliminary report. J Bone Joint Surg Br. 1980;62-B(1):78–82. [DOI] [PubMed] [Google Scholar]
- 10.Cheung PWH, Fong HK, Wong CS, Cheung JPY. The influence of developmental spinal stenosis on the risk of re-operation on an adjacent segment after decompression-only surgery for lumbar spinal stenosis. Bone Joint J. 2019;101-B(2):154–161. [DOI] [PubMed] [Google Scholar]
- 11.Reale F, Delfini R, Gambacorta D, Cantore GP. Congenital stenosis of lumbar spinal canal: comparison of results of surgical treatment for this and other causes of lumbar syndrome. Acta Neurochir (Wien). 1978;42(3-4):199–207. [DOI] [PubMed] [Google Scholar]
- 12.Verbiest H.Results of surgical treatment of idiopathic developmental stenosis of the lumbar vertebral canal. A review of twenty-seven years’ experience. J Bone Joint Surg Br. 1977;59(2):181–188. [DOI] [PubMed] [Google Scholar]
- 13.Chatha DS, Schweitzer ME. MRI criteria of developmental lumbar spinal stenosis revisited. Bull NYU Hosp Jt Dis. 2011;69(4):303–307. [PubMed] [Google Scholar]
- 14.Cheung JP, Samartzis D, Shigematsu H, Cheung KM. Defining clinically relevant values for developmental spinal stenosis: a large-scale magnetic resonance imaging study. Spine (Phila Pa 1976). 2014;39(13):1067–1076. [DOI] [PubMed] [Google Scholar]
- 15.Cheung JPY, Ng KKM, Cheung PWH, Samartzis D, Cheung KMC. Radiographic indices for lumbar developmental spinal stenosis. Scoliosis Spinal Disord. 2017;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verbiest H. Fallacies of the present definition, nomenclature, and classification of the stenoses of the lumbar vertebral Cana. Spine (Phila Pa 1976). 1976;1(4):217–225. [Google Scholar]
- 17.Kitab S, Habboub G, Abdulkareem SB, Alimidhatti MB, Benzel E. Redefining lumbar spinal stenosis as a developmental syndrome: does age matter? J Neurosurg Spine. 2019;31(3):357–365. [DOI] [PubMed] [Google Scholar]
- 18.Kitab S, Lee BS, Benzel EC. Redefining lumbar spinal stenosis as a developmental syndrome: an MRI-based multivariate analysis of findings in 709 patients throughout the 16- to 82-year age spectrum. J Neurosurg Spine. 2018;29(6):654–660. [DOI] [PubMed] [Google Scholar]
- 19.Mrowka R, Pieniazek J. Developmental narrowing of the spinal canal in the lumbar region. Zentralbl Neurochir. 1986;47(2):144–148. [PubMed] [Google Scholar]
- 20.Cheung KM, Samartzis D, Karppinen J, Luk KD. Are “patterns” of lumbar disc degeneration associated with low back pain? New insights based on skipped level disc pathology. Spine (Phila Pa 1976). 2012;37(7):E430–438. [DOI] [PubMed] [Google Scholar]
- 21.Mok FP, Samartzis D, Karppinen J, Luk KD, Fong DY, Cheung KM. ISSLS prize winner: prevalence, determinants, and association of Schmorl nodes of the lumbar spine with disc degeneration: a population-based study of 2449 individuals. Spine (Phila Pa 1976). 2010;35(21):1944–1952. [DOI] [PubMed] [Google Scholar]
- 22.Samartzis D, Karppinen J, Chan D, Luk KD, Cheung KM. The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: a population-based study. Arthritis Rheum. 2012;64(5):1488–1496. [DOI] [PubMed] [Google Scholar]
- 23.Samartzis D, Karppinen J, Mok F, Fong DY, Luk KD, Cheung KM. A population-based study of juvenile disc degeneration and its association with overweight and obesity, low back pain, and diminished functional status. J Bone Joint Surg Am. 2011;93(7):662–670. [DOI] [PubMed] [Google Scholar]
- 24.Vangeneugden T, Laenen A, Geys H, Renard D, Molenberghs G. Applying concepts of generalizability theory on clinical trial data to investigate sources of variation and their impact on reliability. Biometrics. 2005;61(1):295–304. [DOI] [PubMed] [Google Scholar]
- 25.Mukaka MM.Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69–71. [PMC free article] [PubMed] [Google Scholar]
- 26.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315–1316. [DOI] [PubMed] [Google Scholar]
- 27.Akoglu H. User’s guide to correlation coefficients. Turk J Emerg Med. 2018;18(3):91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radcliff K, Curry P, Hilibrand A, et al. Risk for adjacent segment and same segment reoperation after surgery for lumbar stenosis: a subgroup analysis of the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976). 2013;38(7):531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javalkar V, Cardenas R, Tawfik TA, et al. Reoperations after surgery for lumbar spinal stenosis. World Neurosurg. 2011;75(5-6):737–742. [DOI] [PubMed] [Google Scholar]
- 30.Clark GA, Panjabi MM, Wetzel FT. Can infant malnutrition cause adult vertebral stenosis? Spine (Phila Pa 1976). 1985;10(2):165–170. [DOI] [PubMed] [Google Scholar]
- 31.Kalichman L, Cole R, Kim DH, et al. Spinal stenosis prevalence and association with symptoms: the Framingham Study. Spine J. 2009;9(7):545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yabuki S, Fukumori N, Takegami M, et al. Prevalence of lumbar spinal stenosis, using the diagnostic support tool, and correlated factors in Japan: a population-based study. J Orthop Sci. 2013;18(6):893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CK, Hansen HT, Weiss AB. Developmental lumbar spinal stenosis. Pathology and surgical treatment. Spine (Phila Pa 1976). 1978;3(3):246–255. [DOI] [PubMed] [Google Scholar]
- 34.Roussouly P, Nnadi C. Sagittal plane deformity: an overview of interpretation and management. Eur Spine J. 2010;19(11):1824–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]