What is clinically important difference?
Simply stated, clinically important difference (CID) is the difference in outcome scores between 2 groups that can be considered clinically relevant. 1 The notion of CID began as a way to assist in the interpretation of quality-of-life instruments and other patient-reported outcome measures (PROM). 2 It was introduced initially as the minimal clinically important difference (MCID) and later as the minimal important difference (MID), which omits the “clinical” to allow for use with laboratory or functional tests. Clinically important difference focuses on the perceptions of patients or clinicians and reflects a change in outcomes that is not necessarily minimal. The table below summarizes the variations in terminology the reader may encounter (Table 1).
Table 1.
Common Clinically Important Difference Terminology.
| Terminology | Definitions and/or Common Applications |
|---|---|
| Minimal clinically important difference (MCID) | The smallest difference that patients perceive as beneficial. In the absence of troublesome side effects or excessive cost, this difference would mandate a change in the patient’s management. |
| Minimal important difference (MID) | Currently the dominant terminology in literature (although MCID is still used). MID omits the “clinical” of MCID. Therefore, MID could also be based on a change in a laboratory marker or a functional test. |
| Clinically important difference (CID) | CID reflects clinically important change that is not necessarily minimal. The term is also used in contrast to the clinically important responder (CIR). |
| Clinically important responder (CIR) | Amount of change an individual needs to report to consider they have experienced a meaningful treatment benefit. Individuals who achieved the threshold would be identified as a responder. |
How is CID Determined?
Generally, CID is estimated using 2 methodologies: the distribution- and anchor-based methods. Distribution-based methods rely on statistical properties of the distribution of PROM scores expressing a change score difference relative to some measure of variability such as the standard deviation. However, this method fails to include patients’ perspectives and therefore limits the usefulness of these estimates. On the other hand, anchor-based methods compare the association between the difference in the PROM scores to a closely related concept measured by an independent or external criterion (anchor). The anchor should be easily understandable and relevant to the patient for decision-making. For example, the change in the severity of pain on a 0-100 scale (PROM) can be compared to a global rating of change (anchor) that asks a patient whether the ability to function at home since spine surgery has gotten worse, has stayed the same, is a bit better, is significantly better or is completely better.
Why is CID Important?
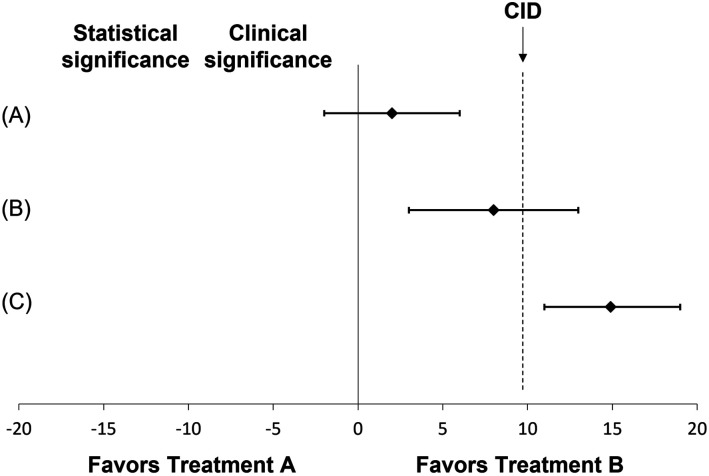
Clinically important difference can be used to evaluate the clinical significance of the study results. Often, studies report the difference between groups only in terms of its statistical significance based on the P-values. However, believing that a statistically significant result indicates a clinically important finding can lead to a flawed application of study results. 3 Consider Figure 1 comparing the mean difference between 2 treatments and assuming the CID for a hypothetical outcome is equal to 10. In example (A), the results are not significant statistically nor clinically, whereas example (B) is statistically significant but not clinically important. Only example (C) demonstrates a result that is both statistically and clinically important.
Figure 1.
Examples of results demonstrating the relationship between statistical and clinical differences.
How is CID Interpreted?
Clinically important difference can be assessed at the group or the individual level. At the group-level, the patient response to treatment is being measured by the PROM comparing the average change from baseline across all patients in the 2 different treatment groups according to the CID. At the individual level, a comparison is made of the proportion of patients in each group who meet the prespecified criterion for CID and is considered a “responder.” The proportion of responders is then compared between groups to assess whether a treatment is considered to provide a clinically important benefit at the individual level. Whether investigators assess the group- or individual-level CID, results demonstrating the effects of an intervention should be presented in relation to the CID.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Joseph R. Dettori https://orcid.org/0000-0002-0216-8363
References
- 1.Coon CD, Cappelleri JC. Interpreting change in scores on patient-reported outcome instruments. Ther Innov Regul Sci. 2016;50(1):22-29. [DOI] [PubMed] [Google Scholar]
- 2.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407-415. [DOI] [PubMed] [Google Scholar]
- 3.Dettori JR, Norvell DC, Chapman JR. P-value worship: Is the idol significant? Global Spine J. 2019;9(3):357-359. [DOI] [PMC free article] [PubMed] [Google Scholar]