Abstract
Study Design:
A retrospective study.
Objectives:
To investigate the incidence, management and outcome of delayed deep surgical site infection (SSI) after the spinal deformity surgery.
Methods:
This study reviewed 5044 consecutive patients who underwent spinal deformity corrective surgery and had been followed over 2 years. Delayed deep SSI were defined as infection involving fascia and muscle and occurring >3 months after the initial procedure. An attempt to retain the implant were initially made for all patients. If the infection failed to be eradicated, the implant removal should be put off until solid fusion was confirmed, usually more than 2 years after the initial surgery. Radiographic data at latest follow-up were compared versus that before implant removal.
Results:
With an average follow-up of 5.3 years, 56 (1.1%) patients were diagnosed as delayed deep SSI. Seven (12.5%) patients successfully retained instrumentation and there were no signs of recurrence during follow-up (average 3.4 years). The remaining patients, because of persistent or recurrent infection, underwent implant removal 2 years or beyond after the primary surgery, and solid fusion was detected in any case. However, at a minimum 1-year follow-up (average 3.9 years), an average loss of 9° in the thoracic curve and 8° in the thoracolumbar/lumbar curves was still observed.
Conclusions:
Delayed deep SSI was rare after spinal deformity surgery. To eradicate infection, complete removal of implant may be required in the majority of delayed SSI. Surgeons must be aware of high likelihood of deformity progression after implant removal, despite radiographic solid fusion.
Keywords: delayed deep surgical site infection, spinal deformity, incidence, management, implant removal, progression deformity
Introduction
Delayed deep surgical site infection (SSI) after spinal deformity surgery is an uncommon but catastrophic complication, which usually results in prolonged hospitalization, additional costs and significant increases in morbidity.1,2 The incidence of delayed infection varied depending on multiple factors. Specially, diagnosis was well recognized as a significant risk factor and neuromuscular scoliosis tend to present a higher rate of infection than other diagnoses. 3 Previous literatures have reported that the rate of delayed infection ranged from 1.4% to 3.0%.1,3-9 However, it remains controversial for the definition of “delayed,” as the criterion of more than 1 month, 3 months, 6 months, and 1 year after the initial surgery were adopted by several studies.1,5,6,10
The subsequent management and outcome differ for delayed versus acute deep SSIs. For acute infection, debridement with irrigation, closed suction/irrigation system and antibiotic administration is preferable for its powerful ability to eliminating infection.2,10,11 However, when it comes to delayed SSI, recurrent infection always occurred after extensive debridement if implant was retained.12,13 In the patients with delayed infection, because of an indolent course, the bacteria have enough time to adhere to implant and form a glycocalyx layer, resisting the penetration of antibiotics and host immune factors. 12 Thus, most studies recommended removing instrumentation to completely eradicate delayed infection.10,12 Nevertheless, radiological results in patients with early implant removal frequently become disappointing, with progression of either scoliosis or kyphosis in the fusion area, finally leading to necessity of revision surgery.14-16 It was then suggested that solid fusion should be obtained when implant removal was considered for resolving refractory infection.17,18 Despite the radiographic evidence of fusion, significant loss of correction was still observed in those patients after the removal.15,16
To date, delayed deep SSI following spinal deformity surgery are still yet to be fully defined in a relatively large series of patients. Meanwhile, there remains a lack of consensus on the standardized treatment of delayed SSI, especially for the indications and timing for implant removal. Therefore, this study was performed with the purpose in 3 folds: 1) to investigate the incidence of delayed deep SSI after the spinal deformity surgery in a single center with a large sample; 2) to evaluate the effectiveness of the standard management for delayed SSI; 3) and to further elucidate the influence of implant removal on the progression of deformity.
Materials and Methods
We retrospectively reviewed a consecutive series of patients who had undergone spinal deformity surgery at our institution between January 1998 and August 2018. The inclusion criteria were as follows: (1) primarily diagnosed as scoliosis (with or without kyphosis); (2) received spinal fusion and instrumentation; (3) had a minimum follow-up of 2 years after the index procedure; (4) had a complete set of outcome measures and radiological examinations. Patients treated with growing rods or VEPTR procedure were excluded, as were any patient who was identified with acute infection or specific infection, such as tuberculosis, tetanus and brucellosis. This study was approved by the Institutional Review Board of Affiliated Drum Tower Hospital of Nanjing University Medical School, and informed consent have been exempt from requirement in the study.
A total of 8427 consecutive spinal deformity patients underwent fusion surgery during the study period. There were 3383 patients who were excluded because of a short follow-up (<2 years), only anterior fusion or a lack of radiological examinations. The remaining 5044 patients with an average follow-up period of 5.3 years (range, 2 to 20 years) were finally enrolled. Regarding the diagnoses, 2874 (57.0%) were with idiopathic scoliosis, 1533 (30.4%) with congenital scoliosis, 245 (4.9%) with neuromuscular scoliosis, 302 (6.0%) with scoliosis related to a syndrome, and 90 (1.8%) with scoliosis related to other diagnoses; 510 underwent combined anterior and posterior spinal fusion and 4534 received posterior-only spinal fusion, respectively. The patients were divided into 2 groups according to the date of the initial surgery: 1998 to 2007 and 2008 to 2018.
Diagnosis of Deep SSI
A deep SSI was defined following the Center for Disease Control and Prevention criteria as an infection involving the fascial and muscle layers. 19 The deep SSI was diagnosed when patients had at least one of the following.19-22 (1) purulent drainage from the deep incision; (2) a deep incision that spontaneously dehisces, or is deliberately opened or aspirated by a surgeon AND organism(s) identified from the deep soft tissues of the incision by a culture or non-culture based microbiologic testing method which is performed for purposes of clinical diagnosis or treatment AND patient has at least one of the following clinical symptoms: fever (>38°C); local pain or tenderness; (3) gross anatomical or histopathologic exam, or imaging test showed that an abscess or other evidence of infection involving deep wound. In this study, delayed SSI was specified as an infection occurred beyond 3 months after the initial procedure.17,19
Treatment Protocol
As soon as deep SSI was diagnosed, an immediate debridement with irrigation was performed. The infected surgical site was approached through the same incision used in the initial procedure. First, the purulent secretion or pus was completely removed. Second, necrotic tissue and cyst wall around the abscess were eliminated thoroughly, as were the biofilm that adhered to the implant. Then, the surgical site was irrigated in the following order: normal saline (1000 ml), 0.2% (v/v) diluted povidone-iodine solution (400 ml), normal saline (1000 ml), hydrogen peroxide solution (100 ml) and normal saline (5000 ml). The extensive irrigation with hydrogen peroxide and dilute povidone-iodine was used in both decades. After these procedures, a closed suction/irrigation system was placed. Muscle and fascia were closed tightly, followed by subcutaneous and skin layer. The purulent drainage and necrotic tissue before, during and after debridement were repeatedly taken for bacterial culture and drug sensitivity test. The present study incubated cultures for up to 10-14 days to prevent from missing organisms such as coagulase-negative staphylococcus and Propionibacterium acnes.
The sterile normal saline was used for continuous irrigation after surgery. The inflow was set at 500 ml/4 h. Usually the irrigation was performed at least 2 weeks. When the drainage became clear and bacterial culture of drainage was repeatedly negative, the drain tubes was suggested to be removed. Based on the infected organisms and drug sensitivity test results, intravenous broad-spectrum antibiotics that were initially administrated were replaced by sensitive antibiotics and subsequently oral antibiotics under the guidance of infectious specialists.
Any effort should be initially made to retain the implants. If the infection would not be eradicated after debridement with irrigation, subsequent treatment strategies were decided on basis of the infection timing. For patients who developed infection within 2 years after initial surgery, regular dressing changes and oral antibiotics were employed in suppressing the infection and the implant removal was postponed. The implant could not be removed until solid fusion was radiographically confirmed by careful evaluation of the fusion mass, usually more than 2 years after the initial surgery. For patients that developed infection beyond 2 years after initial surgery, implant removal could be directly performed when there was strong evidence of solid fusion. According to the age at revision surgery, the patients who had undergone instrumentation removal were divided into 2 groups: younger than 18 years (<18 years) and older than 18 years (≥18 years). Then, based on the timing of removal, these patients were further classified as implant removal within or beyond 3 years after the initial surgery.
All patients were followed up at 3, 6, and 12 months after successful eradication of infection and then at a 1-year interval. At each visit, the wound condition was carefully inspected, and laboratory examinations and plain radiographs were taken for determining whether infection recurred.
Radiographic Evaluation
Full-length X-rays of the total spine in a standardized standing position were taken before the initial procedure, before the revision surgery (implant retention or removal), after the revision surgery and at the latest visit. The following coronal parameters were collected: 1) major coronal Cobb angle (thoracic curve, and thoracolumbar/lumbar (TL/L) curve); 2) apical vertebral translation (AVT), the distance from the central sacral vertical line to the midpoint of the apical vertebra; 3) coronal balance (CB), the horizontal distance between the C7 plumb line and the center of the sacrum.
The following sagittal parameters were collected: 1) thoracic kyphosis (TK): the angle between the superior endplate of T5 and the inferior endplate of T12; 2) thoracolumbar junctional angle: the angle between the superior endplate of T10 and the inferior endplate of L2; 3) lumbar lordosis (LL), the angle subtended by the superior end plate line of L1 and the superior end plate line of S1; 4) sagittal vertical axis (SVA), the perpendicular distance between the C7 plumb line and posterior-superior endplate of the S1. Kyphotic angles were recorded as positive and lordotic angles as negative.
Statistical Analyses
Statistical analysis was performed using SPSS version 22.0 (IBM Corp., Armonk, New York, USA). The difference in the prevalence of delayed infection between first and second decade was analyzed by Chi-square test or Fisher exact test. Due to limited patients in the groups of other diagnoses, the analyses were not carried out. The radiographic parameters before the initial procedure, before the revision surgery, after the revision surgery and at the latest visit were compared by paired t test. The unpaired t test was used to determine differences in the radiographic parameters between implant removal within and beyond 3 years after initial surgery, as well as that between age <18 years and age ≥18 years. A P value of less than 0.05 was considered statistically significant.
Results
Fifty-six patients (56/5044, 1.1%) were diagnosed as delayed deep SSI. There were 25 males and 31 females with an average age of 20.1 years (range, 8-45 years) at the primary surgery. Of them, 44 patients (44/4534, 1.0%) received posterior-only spinal fusion and 12 patients (12/510, 2.4%) underwent combined anterior and posterior spinal fusion. Fusion length averagely spanned 11.4 levels (2-18), which was significantly higher than that (9.2, range, 1-18) in the patients without delayed infection (P < 0.01). Four patients had a spinal fusion to sacrum, and 6 patients to pelvis, respectively. The mean and median time from initial surgery to infection onset was 42 months (range 4-204 months) and 32 months, respectively (Figure 1). The presenting signs of infection included: 47 patients with wound sinus and purulent drainage, 10 with wound dehisce, 15 with local pain, 6 with fever >38.0°C, and 6 with abscess. A total of 46 patients (82.1%, 46/56) were culture-positive; of them, 11 patients had polymicrobial infections. Most cultured pathogens were gram-positive (62.1%, 36/58), but a remarkable rate (37.9%, 22/58) of gram-negative organisms was also noted (Table 1).
Figure 1.
Time distribution of deep SSI after the initial procedure.
Table 1.
Microbiology of the Infections.
| Organism | Gram stain | Number |
|---|---|---|
| Staphylococcus aureus | Gram-positive | 7 |
| Methicillin sensitive | 5 | |
| Methicillin resistant | 2 | |
| coagulase-negative staphylococcus | Gram-positive | 12 |
| Staphylococcus epidermidis | 10 | |
| Staphylococcus haemolyticus | 1 | |
| Staphylococcus simulans | 1 | |
| Propionibacterium acnes | Gram-positive | 12 |
| Escherichia coli | Gram-negative | 6 |
| Pseudomonas aeruginosa | Gram-negative | 8 |
| hemolytic streptococcus | Gram-positive | 3 |
| Enterobacter cloacae | Gram-negative | 3 |
| Klebsiella pneumoniae | Gram-negative | 2 |
| Enteroccoccus faecalis | Gram-positive | 2 |
| Proteus mirabilis | Gram-negative | 1 |
| Bacteriodes fragilis | Gram-negative | 1 |
| Serratia marcescens | Gram-negative | 1 |
Infection Rate and Trend Analysis
The rate of infection varied widely among different diagnoses. Idiopathic scoliosis had the lowest incidence of deep SSI as 0.7% (21/2874). The highest rate of deep SSI was observed in neuromuscular scoliosis (4.9%, 12/245), including 2 (2/26, 7.7%) with polio, 7 (7/101, 6.9%) with spina bifida, 2 (2/25, 8.0%) with cerebral palsy, and 1 (1/53, 1.9%) with myopathy, respectively. No infected cases were found in the other subtypes comprising syringomyelia, spinal cord injury and Friedreich ataxia (Table 2).
Table 2.
Infection Rates by Diagnosis.
| Diagnosis | Case | Rate |
|---|---|---|
| Idiopathic | 21/2874 | 0.7% |
| Congenital | 16/1533 | 1.0% |
| Syndromic | 5/302 | 1.7% |
| Neuromuscular | 12/245 | 4.9% |
| Polio | 2/26 | 7.7% |
| Spina bifida | 7/101 | 6.9% |
| Cerebral palsy | 2/25 | 8.0% |
| Myopathy | 1/53 | 1.9% |
| Other | 0/40 | 0% |
| Other | 2/90 | 2.2% |
The cases in the second decade was twice as many as that in the first decade, but the distribution of diagnoses did not change significantly. The overall infection rate was slightly but significantly lower in the second decade than that in the first decade (0.7% vs. 2.0%, P < 0.05). In terms of diagnoses, patients with idiopathic, congenital, neuromuscular and syndromic scoliosis also showed a clear downward trend (Table 3).
Table 3.
Infection Rates Based on Time Period.
| 1998-2007 | 2008-2018 | |||||
|---|---|---|---|---|---|---|
| Diagnosis | Number | Infection | Rate | Number | Infection | Rate |
| Idiopathic | 927 (57.8%) | 14 | 1.5% | 1947 (56.6%) | 7 | 0.4% |
| Congenital | 465 (30.0%) | 6 | 1.3% | 1068 (31.1%) | 10 | 0.9% |
| Neuromuscular | 94 (5.9%) | 9 | 9.6% | 151 (4.4%) | 3 | 2.0% |
| Syndromic | 89 (5.5%) | 3 | 3.4% | 213 (6.2%) | 2 | 0.9% |
| Other | 30 (1.9%) | 0 | 0% | 60 (1.7%) | 2 | 3.3% |
| Total | 1605 | 32 | 2.0% | 3439 | 24 | 0.7% |
Management of Delayed Deep SSI
The implant was retained in 7 patients, in which average time from primary arthrodesis to infection onset was 9 months (range 4 to 18 months). This interval was significantly shorter than average timing (42 months) of delayed infection (P < 0.05). The average number of reoperations was 1.7, with 1 reoperation in 3 cases (2 Staphylococcus aureus and 1 Escherichia coli), 2 in 3 cases (1 Escherichia coli, 1 Enteroccoccus faecalis and 1 Enterobacter cloacae) and 3 in 1 case (methicillin-resistant S. aureus), respectively. With a mean follow-up of 3.4 years (range 1 to 7 years) after eradication of infection, all wounds healed successfully and there were no signs of recurrent infection.
Complete implant removal was performed in 49 patients. Of them, 12 developed infection within 2 years after the initial surgery (average 16 months, range 12-21 months). Attempts to retain the instrumentation were made for them, via debridement with irrigation and continuous irrigation/suction drainage. In 4 cases of them, a partial implant removal was also done for screw loosening or prominent instrumentation. The implant had to be removed at 2 years after initial surgery for refractory infection. Before removal, all received dressing change to keep wound clean. In the remaining 37 patients who developed infection beyond 2 years after initial surgery, the implant removal was also performed because of failure in eliminating the infection. A solid fusion mass without pseudarthrosis were detected in any case during the removal surgery. Then, closed suction/irrigation systems were used for an average of 8 days and all wounds healed uneventfully. With an average followed up of 2.8 years (range 3 months to 8 years), none was identified with recurrent infection during the period.
Progression of Deformity
Thirty-three patients who were followed up for >1 year (average 3.9 years, range 1-8 years), including 7 with implant retention and 26 with implant removal, were brought into analysis. In the patients with implant removal, the median and average time from initial surgery to implant removal were 2.8 and 3.8 years (range 2 to 8 years), respectively.
Curve correction in the patients with implant retention, as expected, was effectively sustained (Figure 2). In the patients with implant removal, however, an immediate loss of 3° in thoracic curves and 5° in TL/L curves were observed after implant removal. Further increase of curve magnitude occurred during the follow-up. At the latest visit, Cobb angles of both thoracic curves and TL/L curves were significantly higher than those before the implant removal (P < 0.05). Total loss averaged 9° for thoracic curves and 8° for TL/L curves, respectively (Figure 3). Also, these patients had a trend, although not significant, toward higher AVT and high TK (P = 0.07). Whereas, other radiological parameters, such as CB, LL, SVA and T10/L2 junction angle, did not change significantly (Table 4). Moreover, compared to group of age ≥18 years, we observed slight tendency for greater progression of deformity in group of age <18 years (P = 0.06). However, no significant differences were detected in deformity progression between the patients who underwent implant removal <3 years after initial surgery and those >3 years (Table 5).
Figure 2.
Time course of scoliosis evolution of the Cobb angle (A, B) and AVT (C).
Figure 3.
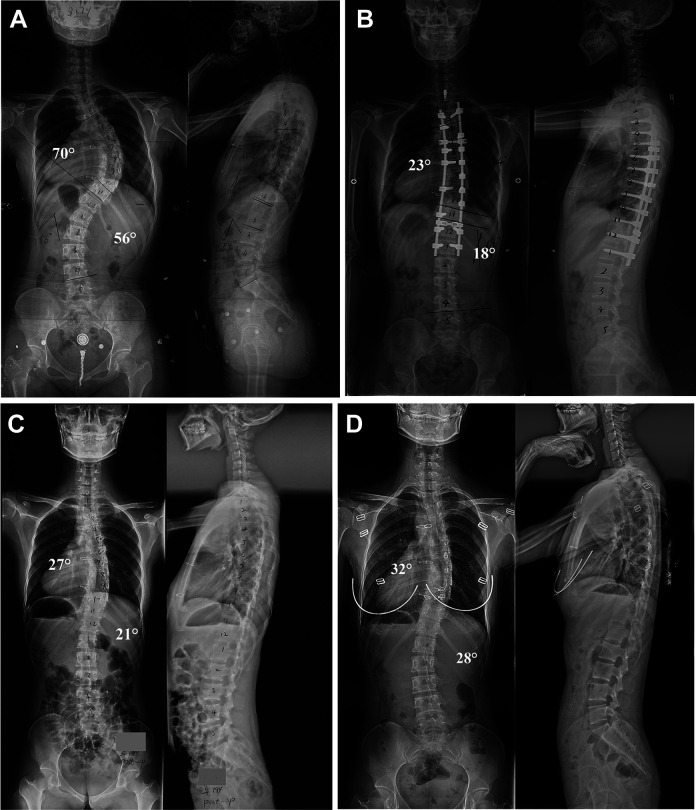
Anteroposterior and lateral preoperative radiographs showing a 17-year-old girl with adolescent idiopathic scoliosis (A). Radiographs made at 2 years and 6 months after posterior spinal fusion and immediately before implant removal secondary to deep SSI showed that the major curve notably improved and the correction was sustained (B). After complete implant removal, an immediate loss of correction was detected (C). Radiographs 2 years after implant removal demonstrated further increase of magnitude in the thoracic and lumbar curves (D).
Table 4.
Coronal and Sagittal Parameters as Measured Before the Initial Surgery, Before the Implant Removal, After the Implant Removal, and at the Latest Visit.
| Before the initial surgery | Before the removal | After the removal | Latest visit | Loss of correction* | P values | |
|---|---|---|---|---|---|---|
| Main curve Cobb angles | ||||||
| Thoracic curve (°) | 62 (32 to 114) | 30 (0 to 76) | 33 (0 to 76) | 39 (8 to 78) | 9 (1 to 24) | 0.000 |
| TL/L curve (°) | 55 (27 to 105) | 24 (4 to 48) | 29 (7 to 53) | 32 (9 to 55) | 8 (2 to 26) | 0.007 |
| CB (mm) | −3 (−50 to 26) | −6 (−43 to 20) | −5 (−27 to 28) | −1 (−33 to 30) | 7 (−26 to 24) | 0.148 |
| AVT (mm) | 54 (23 to 97) | 31 (8 to 78) | 34 (10 to 82) | 39 (11 to 88) | 8 (2 to 33) | 0.075 |
| Sagittal alignment | ||||||
| TK (°) | 22 (3 to 95) | 23 (6 to 47) | 24 (5 to 51) | 29 (4 to 58) | 6 (−15 to 27) | 0.074 |
| T10/L2 junction (°) | −1 (−20 to 18) | 0 (−20 to 20) | −2 (−12 to 10) | 5 (−13 to 37) | 5 (−5 to 40) | 0.115 |
| LL (°) | −50 (−87 to −31) | −43 (−65 to −35) | −44 (−76 to −25) | −45 (−75 to −26) | −2 (−36 to 16) | 0.780 |
| SVA (°) | −25 (−61 to 67) | −23 (−55 to −8) | −17 (−51 to 27) | −11 (−62 to 48) | 12 (−22 to 57) | 0.192 |
The latest visit data were compared versus data before implant removal via the paired t test.
* indicates measurement at latest visit— measurement before the implant removal.
TL/L, thoracolumbar/lumbar; CB, coronal balance; AVT, apical vertebral translation; TK, thoracic kyphosis; LL, lumbar lordosis; SVA, sagittal vertical axis.
Table 5.
Comparison of Main Curve Cobb Angles Between Age <18 Years and Age ≥18 Years and Comparison of Main Curve Cobb Angles Between Implant Removal Within and Beyond 3 Years After Initial Surgery.
| Age <18 years (n = 10*) | Age ≥18 years (n = 15) |
P values |
|
|---|---|---|---|
| Thoracic curve (°) | |||
| Before the initial procedure | 50 (40-55) | 69 (32-114) | 0.108 |
| Before the removal | 22 (7-34) | 34 (0-76) | 0.204 |
| After the removal | 26 (9-51) | 37 (0-76) | 0.325 |
| The latest visit | 35 (16-58) | 42 (8-78) | 0.481 |
| △ Thoracic curve (°) | 13 (8-24) | 7 (1-13) | 0.065 |
| TL/L curve (°) | |||
| Before the initial procedure | 57 (27-105) | 54 (29-88) | 0.809 |
| Before the removal | 22 (4-43) | 27 (11-48) | 0.530 |
| After the removal | 28 (7-47) | 32 (16-53) | 0.632 |
| The latest visit | 33 (9-51) | 32 (16-55) | 0.973 |
| △ TL/L curve (°) | 11 (2-26) | 5 (3-8) | 0.263 |
| 2-3 Years after initial procedure (n = 12) |
≥ 3 Years after initial procedure (n = 13*) |
||
| Thoracic curve (°) | |||
| Before the initial procedure | 63 (42-102) | 60 (32-114) | 0.824 |
| Before the removal | 31 (12-55) | 30 (0-76) | 0.908 |
| After the removal | 34 (14-59) | 32 (0-76) | 0.850 |
| The latest visit | 41 (24-68) | 38 (8-78) | 0.680 |
| △ Thoracic curve (°) | 10 (1-24) | 8 (2-12) | 0.277 |
| TL/L curve (°) | |||
| Before the initial procedure | 52 (30-88) | 58 (27-105) | 0.690 |
| Before the removal | 26 (15-48) | 24 (4-45) | 0.796 |
| After the removal | 31 (20-53) | 29 (7-47) | 0.853 |
| The latest visit | 35 (20-55) | 31 (9-50) | 0.645 |
| △ TL/L curve (°) | 9 (5-26) | 7 (2-20) | 0.685 |
△ indicates measurement at latest visit-measurement before the implant removal.
* indicates one patient who received reinstrumenation 1 month after implant removal was not carried into analysis.
TL/L, thoracolumbar/lumbar.
Till the latest follow-up, 2 patients underwent reinstrumentation and fusion. One developed infection 7 years after initial surgery and received the implant removal. Then, because of deformity progression from a hemivertebra below the original fusion, this patient underwent reinstrumentation 1 month after implant removal. Another patient developed infection 2.5 years after initial surgery and then received the implant removal. She underwent reinstrumentation at 3 years after removal due to significant loss of correction.
Discussion
To our knowledge, this is a retrospective study of delayed deep SSI with the largest sample volume of patients who had undergone spinal deformity surgery at a single institution. The current study investigated the timing, incidence, trend change, and microbiology of delayed infection. Furthermore, we proposed our own treatment algorithm for delayed infection, and then analyzed the follow-up outcomes.
Infection Rate
In a prior study, Cahill et al. 17 reported a delayed infection rate of 1.9% in cohort of 1543 patients with average follow-up of 4.3 years (at least 2 years) after spinal deformity surgery, with similarly designed studies showing delayed deep SSI rates varying from 1.4% to 3.0%.1,3-9 More recently, the data in a single institution showed an infection rate of 1.6% for 1070 cases of pediatric scoliosis. 1 However, these cohorts contained a large percentage of neuromuscular cases, which were at high risk of infection. In our series, we found the highest rate of deep SSI in patients with neuromuscular scoliosis, consistent with previous literature. However, as majority of patients were idiopathic and congenital scoliosis, the infection rate of 1.1% following primary arthrodesis was a little lower than previously reported incidence. The change of diagnosis constitution could substantially influence the overall incidence of infection. Moreover, our results showed that the fusion length in the SSI group was significantly higher than that in non-SSI group. Additional levels fused may be proxies for increased surgical time and blood loss. These factors have been reported as significant risk factors for increased delayed SSI rates. 23
Based on trend analysis, we found that noteworthy declines in the incidence of infection parallels an increase in the number of patients treated. This phenomenon may be secondary to improved understanding of risk factors related to infection. 17 Also, differences in the incidence of infection could be argued to be a result of varied surgical techniques and prophylactic measures, including implant choice, hospital facilities and antibiotic administration. 24 There were no significant changes in pre-, intra- and postoperative prophylaxis between the early and the later cohort. The first or second generation cephalosporin, such as cefazolin and cefuroxime, were the main antibiotics used in our center. Previously, many studies addressed the use of intrawound antibiotics for the prevention of infection in spinal surgery. 25 Some surgeons frequently used powdered vancomycin before closure in order to thoroughly decontaminate the wound.25,26 However, it is still debated whether these approaches are effective and which method is to be preferred. Thus, no intrawound antibiotics were ever used in our center.
The major advance in surgical techniques was that surgeons have shifted from anterior-posterior to posterior-only approaches. Majority of combined anterior and posterior spinal fusion were performed in the first decade, while posterior-only spinal fusion predominated in the second decade. Compared with anterior-posterior approach, posterior-only approach was characterized with less invasive and shorter surgical time. Moreover, as increasing number of surgeries, accumulation of surgical experiences and improvement of surgical proficiency significantly shorten the surgical time and reduce the blood loss, which may help reduce risk of infection. When it comes to implants, most surgeries in the first decade were performed with TRSH and CD-Horizon instrumentations, while CD-Horizon legacy instrumentation predominated in the second decade. However, there were no differences in materials among them and all these implants were made of titanium alloy.
Management of Delayed Deep SSI
The management of delayed deep SSIs after spinal deformity surgery has long been an important concern among spine surgeons. Many authors concluded that eradication of delayed infection was not possible without complete implant removal.10,12,18 Two possible explanations could account for this result. First, due to a long quiescent period, the organisms had enough time to proliferate and form protective glycocalyx. 21 Second, debridement without implant removal results in areas underneath rods and screws that cannot be thoroughly debrided, leaving retained areas of infected tissue. 10 In our cohort, only 7 patients who had a relatively short quiescent period successfully retained the implant.
In terms of microbiology, most common organism in the retention group was gram-negative organisms and S. aureus, which had relatively high virulence and short time to infection onset. 27 Whereas, low-virulent bacteria such as S. epidermidis and p. acnes were recognized as main organisms in the patients with implant removal. For low-virulent bacteria, it remained undetected in the early intervals causing widespread biofilm formation, later leading to a resilient onset of SSI. 28 As reported by previous studies, high incidence of failure in management of SSI were often seen with infections of low-virulent bacteria.2,10,24 Finally, only by removing the instrumentation can the delayed infection be completely eradicated.
However, early implant removal can result in the significant progression of deformity. Cahill et al. 17 reported that average loss of 30.3° in the patients who underwent implant removal within 1 year after the initial surgery. The subsequent studies proposed stable fusion mass and absence of pseudarthrosis as the indications for implant removal.16,18,29 According to the experience in our center, the existence of infection seems not to disturb the process of spinal fusion, and with the support of instrumentation, good quality of fusion can normally be achieved beyond 2 years after the initial surgery. For patients who developed refractory infection within 2 years after surgery, debridement without implant removal followed by dress change and antibiotic administration were employed until solid fusion was evidenced.
Progression of Deformity After Implant Removal
Most studies agreed that the implant removal after spinal deformity surgery was a risk factor for curve progression. In the present study, the scoliotic curves in the patients who retained implant remained stable. Following the implant removal, however, we noticed significant increases in coronal curves with an average follow-up of more than 3 years, despite solid fusion. Several hypotheses have been proposed to explain such a finding. Kotani et al. 29 speculated that pseudarthrosis could be covered by a weak sheet of bone similar to a mature fusion bone. Both current techniques and surgical explorations were inadequate for reliably excluding the presence of partial cleft or occult pseudarthrosis in the fusion mass.14,15,29 On the contrary, Potter et al. believed that diffuse regional settling of the curves, instead of focal decompensation, demonstrated that solid fusion was obtained. 15 The bending of the immature fusion mass may contribute to the progression of deformity. 16 Other factors such as amount of fusion and partial facetectomy, as well as altered mechanical stress were also mentioned.15,29 In addition to the above factors, our results showed that patients younger than 18 years at the removal were more likely to suffer from high loss of correction. This phenomenon may be attributed to the skeletal immaturity in pediatric or adolescent patients. Without the constraint of instrumentation, the remaining growth in these patients can lead to rapid progression of deformity. In light of a high likelihood of deformity progression after implant removal, patients and their families should be notified with potentially significant loss of correction and even possibility of revision surgery with instrumented correction.
Furthermore, we found that no significant difference in deformity progression between groups of instrumentation removal less and more than 3 years after initial surgery. This result strengthened that deformity progression may be unavoidable after implant removal, but high degree of fusion mass maturation and relatively stable curves can be achieved beyond 2 years after surgery. In our series, an average loss of less than 10° was tolerated clinically and not up to the standard of radiographic progression advocated by prior literature.14,17 Thus, if implant had to be removed to completely clear the infection, implant removal should not be performed until adequate fusion was obtained, usually beyond 2 years after the primary surgery.
Limitations
This study has several limitations. First, this study has the limitation inherent to its retrospective nature. Our data showed that the mean time from initial surgery to infection onset was 42 months and a large percentage of patients developed infection beyond 2 years after the initial surgery. When only looking at 2-year follow-up, some patients without deep SSI, especially for those in the second decade, are still at risk for further infection. The short follow-up duration may lead to possible missed infections. Thus, the incidence of delayed deep SSI could be underestimated and a long-term follow-up is needed to assess precise infection rate. Second, primary surgery-related data were incomplete or not captured in our data collection system, precluding a comprehensive analysis of risk factors for deep SSI. Last, our cohort had a relatively short follow-up period after successful management of infection that might lead to underestmation for adverse events, such as recurrent infection, severe progression of deformity and revision surgeries.
Conclusion
In conclusion, our results showed that delayed deep SSI was a rare complication after spinal deformity surgery. By analysis of etiology, neuromuscular scoliosis presented the highest rates of infection. Eradication of infection with implant retention can hardly be expected in the majority of delayed deep SSI. When implant removal cannot be avoided, the surgery should be put off until no less than 2 years after the index procedure. Meanwhile, surgeons must be aware of high likelihood of deformity progression after implant removal, particularly for young patients.
Footnotes
Authors’ Note: This manuscript has not been previously published. The manuscript submitted does not contain information about medical device(s)/drug(s). No relevant financial activities outside the submitted work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Grant No. 81772422) and the Natural Science Foundation of Jiangsu Province (BE2017606).
ORCID iD: Bo Yang, MD  https://orcid.org/0000-0003-3397-3370
https://orcid.org/0000-0003-3397-3370
Zezhang Zhu, MD  https://orcid.org/0000-0001-8569-3595
https://orcid.org/0000-0001-8569-3595
Xu Sun, MD  https://orcid.org/0000-0002-7181-877X
https://orcid.org/0000-0002-7181-877X
References
- 1.Warner SJ, Uppstrom TJ, Miller AO, et al. Epidemiology of deep surgical site infections after pediatric spinal fusion surgery. Spine (Phila Pa 1976). 2017;42(3):E163–E168. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Glotzbecker M, Hedequist D. Surgical site infection after pediatric spinal deformity surgery. Curr Rev Musculoskelet Med. 2012;5(2):111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramo BA, Roberts DW, Tuason D, et al. Surgical site infections after posterior spinal fusion for neuromuscular scoliosis: a thirty-year experience at a single institution. J Bone Joint Surg Am. 2014;96(24):2038–2048. [DOI] [PubMed] [Google Scholar]
- 4.Rihn JA, Lee JY, Ward WT. Infection after the surgical treatment of adolescent idiopathic scoliosis: evaluation of the diagnosis, treatment, and impact on clinical outcomes. Spine (Phila Pa 1976). 2008;33(3):289–294. [DOI] [PubMed] [Google Scholar]
- 5.Garg S, LaGreca J, Hotchkiss M, Erickson M. Management of late (>1 y) deep infection after spinal fusion: a retrospective cohort study. J Pediatr Orthop. 2015;35(3):266–270. [DOI] [PubMed] [Google Scholar]
- 6.Rohmiller MT, Akbarnia BA, Raiszadeh K, Raiszadeh K, Canale S. Closed suction irrigation for the treatment of postoperative wound infections following posterior spinal fusion and instrumentation. Spine (Phila Pa 1976). 2010;35(6):642–646. [DOI] [PubMed] [Google Scholar]
- 7.Mistovich RJ, Jacobs LJ, Campbell RM, Spiegel DA, Flynn JM, Baldwin KD. Infection control in pediatric spinal deformity surgery: a systematic and critical analysis review. JBJS Rev. 2017;5(5):e3. [DOI] [PubMed] [Google Scholar]
- 8.Peng XQ, Sun CG, Fei ZG, Zhou QJ. Risk factors for surgical site infection after spinal surgery: a systematic review and meta-analysis based on twenty-seven studies. World Neurosurg. 2019;123: e318–e329. [DOI] [PubMed] [Google Scholar]
- 9.Pesenti S, Pannu T, Andres-Bergos J, et al. What are the risk factors for surgical site infection after spinal fusion? A meta-analysis. Eur Spine J. 2018;27(10):2469–2480. [DOI] [PubMed] [Google Scholar]
- 10.Hedequist D, Haugen A, Hresko T, Emans J. Failure of attempted implant retention in spinal deformity delayed surgical site infections. Spine (Phila Pa 1976). 2009;34(1):60–64. [DOI] [PubMed] [Google Scholar]
- 11.van Rhee MA, de Klerk LW, Verhaar JA. Vacuum-assisted wound closure of deep infections after instrumented spinal fusion in six children with neuromuscular scoliosis. Spine J. 2007;7(5):596–600. [DOI] [PubMed] [Google Scholar]
- 12.Di Silvestre M, Bakaloudis G, Lolli F, Giacomini S. Late-developing infection following posterior fusion for adolescent idiopathic scoliosis. Eur Spine J. 2011;20(suppl 1):S121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen SH, Lee CH, Huang KC, Hsieh PH, Tsai SY. Postoperative wound infection after posterior spinal instrumentation: analysis of long-term treatment outcomes. Eur Spine J. 2015;24(3):561–570. [DOI] [PubMed] [Google Scholar]
- 14.Rathjen K, Wood M, McClung A, Vest Z. Clinical and radiographic results after implant removal in idiopathic scoliosis. Spine (Phila Pa 1976). 2007;32(20):2184–2188. [DOI] [PubMed] [Google Scholar]
- 15.Potter BK, Kirk KL, Shah SA, Kuklo TR. Loss of coronal correction following instrumentation removal in adolescent idiopathic scoliosis. Spine (Phila Pa 1976). 2006;31(1):67–72. [DOI] [PubMed] [Google Scholar]
- 16.Farshad M, Sdzuy C, Min K. Late implant removal after posterior correction of AIS with pedicle screw instrumentation—a matched case control study with 10-year follow-up. Spine Deform. 2013;1(1):68–71. [DOI] [PubMed] [Google Scholar]
- 17.Cahill PJ, Warnick DE, Lee MJ, et al. Infection after spinal fusion for pediatric spinal deformity: thirty years of experience at a single institution. Spine (Phila Pa 1976). 2010;35(12):1211–1217. [DOI] [PubMed] [Google Scholar]
- 18.Nunez-Pereira S, Pellise F, Rodriguez-Pardo D, et al. Implant survival after deep infection of an instrumented spinal fusion. Bone Joint J. 2013;95-B(8):1121–1126. [DOI] [PubMed] [Google Scholar]
- 19.Du JY, Poe-Kochert C, Thompson GH, Son-Hing JP, Hardesty CK, Mistovich RJ. Risk factors for early infection in pediatric spinal deformity surgery: a multivariate analysis. Spine Deform. 2019;7(3):410–416. [DOI] [PubMed] [Google Scholar]
- 20.Liu JM, Deng HL, Chen XY, et al. Risk factors for surgical site infection after posterior lumbar spinal surgery. Spine (Phila Pa 1976). 2018;43(10):732–737. [DOI] [PubMed] [Google Scholar]
- 21.Yin D, Liu B, Chang Y, Gu H, Zheng X. Management of late-onset deep surgical site infection after instrumented spinal surgery. BMC Surg. 2018;18(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan W, Liu X, Zhou X, Pei L, Zhu Y. Management of early deep wound infection after thoracolumbar instrumentation: continuous irrigation suction system versus vacuum-assisted closure system. Spine (Phila Pa 1976). 2018;43(18):E1089–E1095. [DOI] [PubMed] [Google Scholar]
- 23.Shen J, Liang J, Yu H, Qiu G, Xue X, Li Z.Risk factors for delayed infections after spinal fusion and instrumentation in patients with scoliosis. Clinical article. J Neurosurg Spine. 2014;21(4):648–652. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal A, Kelkar A, Agarwal AG, et al. Implant retention or removal for management of surgical site infection after spinal surgery. Global Spine J. 2020;10(5):640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemans JVC, Oner FC, Wijdicks SPJ, Ekkelenkamp MB, Vogely HC, Kruyt MC. The efficacy of intrawound vancomycin powder and povidone-iodine irrigation to prevent surgical site infections in complex instrumented spine surgery. Spine J. 2019;19(10):1648–1656. [DOI] [PubMed] [Google Scholar]
- 26.Kim HS, Lee SG, Kim WK, Park CW, Son S. Prophylactic intrawound application of vancomycin powder in instrumented spinal fusion surgery. Korean J Spine. 2013;10(3):121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdul-Jabbar A, Berven SH, Hu SS, et al. Surgical site infections in spine surgery: identification of microbiologic and surgical characteristics in 239 cases. Spine (Phila Pa 1976). 2013;38(22):E1425–1431. [DOI] [PubMed] [Google Scholar]
- 28.Haidar R, Najjar M, Der Boghossian A, Tabbarah Z. Propionibacterium acnes causing delayed postoperative spine infection: review. Scand J Infect Dis. 2010;42(6-7):405–411. [DOI] [PubMed] [Google Scholar]
- 29.Kotani T, Akazawa T, Lumawig JM, Sakuma T, Minami S. Reinstrumentation for rapid curve progression after implant removal following posterior instrumented fusion in adolescent idiopathic scoliosis: a case report. Scoliosis. 2013;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]