Abstract
Study Design:
Retrospective cohort.
Objective:
The purpose was to investigate the incidence of and risk factors for complications associated with vertebroplasty (VP) or kyphoplasty (KP) for osteoporotic vertebral compression fracture (OVCF) using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database.
Methods:
A cohort of patients undergoing VP/KP was constructed from the 2011-2013 ACS-NSQIP dataset using Current Procedural Terminology (CPT) codes. The incidences of minor complications (i.e. urinary tract infection, pneumonia, renal insufficiency, superficial infection, wound dehiscence), major complications (i.e. reoperation, deep vein thrombosis, pulmonary embolism, sepsis, dialysis, cardiac arrest, deep infection, stroke), and mortality within 30 days post-surgery were investigated, and their risk factors were assessed using logistic regression modeling.
Results:
Of 1932 patients undergoing VP/KP, 166 (8.6%) experienced a complication, including minor complications in 53 (2.7%), major complications in 95 (4.9%), and death in 40 (2.1%). Multivariate logistic regression analysis indicated that the adjusted odds ratios (95% confidence interval [CI]) of mortality was significantly associated with ASA 4: 16.604 (1.956-140.959) and increased creatinine (≥ 1.3 mg/dL): 3.494 (1.128-10.823). History of chronic obstructive pulmonary disease was associated with minor complications. Increased WBC count and hypoalbuminemia (<3.0 g/dL) were also associated with major complications.
Conclusions:
The major complication and mortality rates associated with VP/KP were 4.9% and 2.1% respectively, higher than previous reports. Increased creatinine and ASA 4 were independently associated with mortality after VP/KP. Therefore, cautious monitoring and counseling is needed for elderly, patients with preexisting kidney disease or ASA 4 undergoing VP/KP.
Keywords: vertebroplasty, kyphoplasty, American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP), short-term complication, mortality
Introduction
Osteoporotic vertebral compression fractures (OVCF) are a growing public health burden causing chronic back pain, physical inactivity, and disability, particularly in the elderly population.1-4 While OVCF can be managed conservatively with bed rest, pain medication, and bracing,5,6 vertebral augmentation procedures, such as vertebroplasty (VP) and kyphoplasty (KP), are other treatment options that can be employed upon failure of conservative treatments.7-9
Both VP/KP have been widely performed to alleviate pain and promote ambulation in patients with OVCF.2,10-12 Though some debate persists,13,14 both procedures are considered effective treatments for OVCFs, showing decreased pain and improved function.11,15,16 Over the past decade, rates of vertebral augmentation procedures have increased, mostly in an older population. In a study of over 61 000 patients, VP/KP was performed over 40x more often in older patients (>80 years) compared to younger patients (40-59 years). 2
However, previous studies regarding VP/KP have focused on the efficacy or cost-effectiveness of these procedures rather than their complications.11,13,14,16 Furthermore, most studies about complications related to VP/KP have dealt with cement leakage after the procedure, rather than broader medical complications.17,18 Few meta-analyses and/or systematic reviews are available regarding general complications after vertebral augmentation procedures.10,11,15 Therefore, the purpose of this study was to investigate the incidence of and risk factors for complications and mortality associated with VP/KP for OVCF by using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database.
Materials and Methods
NSQIP Database
The de-identified ACS-NSQIP database was used for the current retrospective cohort study. ACS-NSQIP prospectively collects more than 200 variables pertaining to patient characteristics, comorbid conditions, operative details, 30-day postoperative outcomes, and mortality for many surgical procedures. In the NSQIP database, preoperative, intraoperative, and 30-day postoperative variables are prospectively collected from operative reports, medical records, and patient interviews to assess the 30-day adjusted surgical outcomes.19,20 Clinical data are collected throughout the entire 30-day postoperative period. Over 600 hospitals participate in the NSQIP registry, and data are collected prospectively by trained and audited data collection specialists in accordance with rigorous definitions. Due to the lack of protected health information in the publicly available database, the institutional review board (IRB) did not require review.
Study Population and Data Collection
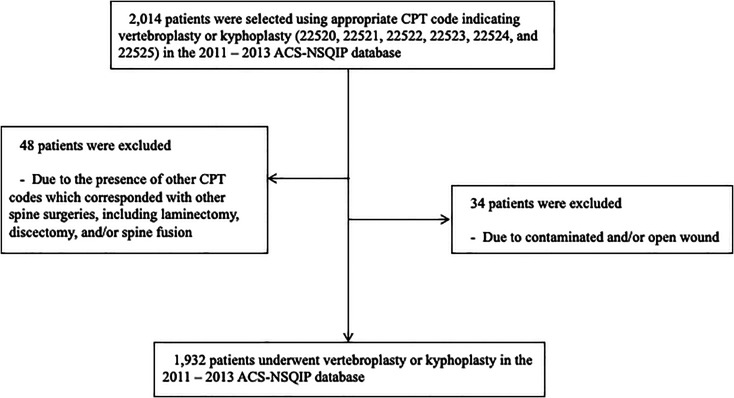
The 2011 to 2013 ACS-NSQIP database was used in this study. VP/KP cases were identified using primary Current Procedural Terminology (CPT) codes: 22 520, 22 521, 22 522, 22 523, 22 524, and 22 525. Among these, the patients who underwent other spine surgeries, including laminectomy, discectomy, and/or spine fusion with VP/KP, were excluded using other CPT codes. Furthermore, patients with contaminated and/or open wound were excluded. Thus, 1932 patients who underwent VP/KP were included in this study ( Figure 1 ).
Figure 1.
Flow chart about cohort construction for vertebroplasty or kyphoplasty using current procedural terminology.
Variables
Several independent variables were collected including demographic data, medical comorbidities, preoperative conditions (e.g. American Society of Anesthesiologist classification of physical health [ASA], and smoking), medical history, 10% loss of body weight in the 6 months prior to surgery, body mass index (BMl) (kg/m2), preoperative platelet count (× 1000 cells/mcL), preoperative white blood cell (WBC) count (cells/mL), preoperative blood urea nitrogen (BUN) (mg/dL), preoperative creatinine (Cr) (mg/dL), preoperative albumin (g/dL), preoperative hematocrit (%), and surgical characteristics, including methods (VP/KP), and operative time (≤ 30 minutes or > 30 minutes), were obtained.
The primary outcomes were the incidence of minor complications, major complications, and mortality within 30-days post-surgery. In addition, risk factors for each outcome after VP/KP were identified. The complications were categorized into minor and major based on previous studies.21,22 The list of minor and major complications is presented in Table 1.
Table 1.
Incidence of Death, Major, and Minor Complications.
| Complication | Value (no. [%]) |
|---|---|
| Minor complications | 53 (2.7) |
| Urinary tract infection | 30 (1.6) |
| Pneumonia | 18 (0.9) |
| Progressive renal insufficiency | 4 (0.2) |
| Superficial surgical site infection | 1 (0.1) |
| Wound dehiscence | 0 (0) |
| Major complications | 95 (4.9) |
| Return to operation room | 61 (3.2) |
| Deep vein thrombosis | 14 (0.7) |
| Pulmonary embolism | 13 (0.7) |
| Sepsis | 9 (0.5) |
| Septic shock | 4 (0.2) |
| Renal failure requiring dialysis | 4 (0.2) |
| Cardiac arrest | 4 (0.2) |
| Myocardial infarction | 1 (0.1) |
| Cerebrovascular accident with neurologic deficit | 1 (0.1) |
| Deep wound infection | 0 (0) |
| Organ space infection | 0 (0) |
| Death | 40 (2.1) |
| Minor complications + Major complications + Death | 166 (8.6) |
Statistical Analysis
The influence of potential risk factors for minor complications, major complications, and death was initially evaluated using univariate logistic regression analysis and/or the chi-square test. The variables that were significantly associated with complications and/or mortality at P < 0.20 in the univariate analysis were entered into the multivariate logistic regression model, along with age, sex, and potentially important variables as significant predictors for complications and/or mortality. The α level was set at 0.05 for significance. All statistical analyses were performed using the SPSS, version 20.0.0 statistical software package (SPSS, Inc., Chicago, IL, USA).
Results
Demographics and Comorbidities
In total, 1932 patients who underwent VP/KP were registered in the NSQIP database between 2011 and 2013 ( Figure 1 ). Table 2 describes the demographic data, medical morbidities, and surgical characteristics of this cohort. The mean age ± standard deviation was 74.9 ± 11.9. Most patients were white (87.9%) and female (71.0%). Hypertension was the most prevalent co-existing condition (64.8%). For surgical characteristics, VP and KP were performed in 197 (9.9%) and 1769 (90.1%) patients, respectively. Operation time of less than 30 minutes were observed in 1164 patients (59.1%).
Table 2.
Patients’ Demographic and Characteristics.
| No. with available data | Value | |
|---|---|---|
| Demographic data | ||
| Age (year) | 1932 | 74.9 ± 11.9 |
| < 64 | - | 344 (17.8) |
| 65-74 | - | 458 (23.7) |
| 75-84 | - | 688 (35.6) |
| ≥ 85 | - | 442 (22.9) |
| Female sex | 1932 | 1372 (71.0) |
| Race (no. [%]) | 1932 | |
| White | - | 1699 (87.9) |
| Black | - | 39 (2.0) |
| Hispanic | - | 107 (5.6) |
| Asian | - | 84 (4.3) |
| Unknown | - | 3 (0.2) |
| BMI (kg/m2) | 1904 | 26.6 ± 6.0 |
| Under weight (< 17.9) | - | 60 (3.1) |
| Normal (18.0-24.9) | - | 809 (42.5) |
| Overweight (25.0-29.9) | - | 579 (30.4) |
| Moderate obese (30-35) | - | 293 (15.4) |
| Severe obese (≥ 35) | - | 163 (8.6) |
| Preoperative laboratory data | ||
| White blood cell count (cells/mL) | 1805 | 7.6 ± 3.0 |
| Blood urea nitrogen (mg/dL) | 1783 | 18.3 ± 9.1 |
| Increased blood urea nitrogen (≥ 20.0 mg/dL) | - | 614 (34.4) |
| Creatinine (mg/dL) | 1794 | 0.9 ± 0.6 |
| Increased creatinine (≥ 1.3 mg/dL) | - | 211 (11.8) |
| Hematocrit (%) | 1805 | 37.4 ± 5.1 |
| Platelet count (× 1000 cells/mcL) | 1802 | 243.2 ± 83.7 |
| Albumin (g/dL) | 1052 | 3.6 ± 0.6 |
| Hypoalbuminemia (< 3.0 g/dL) | - | 198 (18.8) |
| Preoperative condition | ||
| ASA classification | 1932 | |
| 1 and 2 | - | 501 (26.0) |
| 3 | - | 1232 (63.9) |
| 4 | - | 195 (10.1) |
| Smoking* | 1932 | 250 (12.9) |
| Medical comorbidities | ||
| Diabetes | 1932 | 332 (16.7) |
| Dyspnea | 1932 | 253 (13.1) |
| Functional status prior to surgery | 1932 | |
| Independent | - | 1640 (84.9) |
| Partially dependent | - | 200 (10.4) |
| Totally dependent | - | 26 (1.3) |
| Unknown | 66 (3.4) | |
| History of Chronic Obstructive Pulmonary Disease | 1932 | 263 (13.9) |
| History of Congestive Heart Failure in 30 days before surgery | 1932 | 29 (1.5) |
| Hypertension requiring medication | 1932 | 1251 (64.8) |
| Current dialysis | 1932 | 17 (0.9) |
| Steroid use§ | 1932 | 231 (12.0) |
| Bleeding disorder | 1932 | 139 (7.2) |
| Transfusion within 72 hours before surgery | 1932 | 16 (0.8) |
| > 10% loss of body weight‡ | 1932 | 26 (1.3) |
| Surgical Characteristics | ||
| Method of Anesthesia General anesthesia |
1932 | 1712 (88.6) |
| MAC or local anesthesia | 220 (11.4) | |
| Method | 1932 | |
| Vertebroplasty | - | 191 (9.9) |
| Kyphoplasty | - | 1741 (90.1) |
| Operation time (minutes) | 1932 | 33.0 ± 23.0 |
| ≤ 30 minutes | - | 1142 (59.1) |
| > 30 minutes | - | 790 (40.9) |
The values are given as the mean ± the standard deviation or numbers (%). BMI, Body Mass Index; ASA, American Society of Anesthesiologist; no, numbers, * means smoking within the past year. § means oral or parenteral use of steroid in 30 days before surgery for a chronic medical condition. ‡ means 10% loss of body weight in the 6 months prior to surgery.
Incidence of Minor Complications, Major Complications, and Death
Within 30 days after surgery, the overall complication rate was 8.6% (166 patients) with a minor complication rate of 2.7% (53 patients), a major complication rate of 4.9% (95 patients), and a mortality rate of 2.1% (40 patients) (Table 1). In the case of minor complications, the prevalence of urinary tract infection (UTI) and pneumonia were the highest at 1.6% (30 patients) and 0.9% (18 patients), respectively. Among the major complications, the prevalence of reoperation was 3.2% (61 patients), while that of pulmonary embolism was 0.7% (13 patients). Among 40 patients who died, 5 patients had a precedent pneumonia, 1 patient had a precedent deep vein thrombosis, and 4 patients had cardiac arrest. The death-related events of the other patients were not known.
Odds Ratio of Risk Factors
Univariate and multivariate logistic regression analysis identified multiple risk factors for 30-day 1 or more minor complications, 1 or more major complications, and mortality (Tables 3-5). For minor complications, history of chronic obstructive pulmonary disease (P = 0.037; OR, 2.599; CI, 1.061-6.367) were significant risk factors (Table 3). Lower level of albumin (< 3.0 g/dL) (P = 0.005; OR, 2.392; CI, 1.305-4.383) and increased WBC count (P = 0.004; OR, 1.117; CI, 1.037-1.204) were significant risk factors for major complications (Table 4). For mortality, ASA 4 (P = 0.010; OR, 16.604; 95% confidence interval [CI], 1.956-140.959), increased level of creatinine (≥ 1.3 mg/dL) (P = 0.030; OR, 3.494; CI, 1.128-10.823), and MAC or local anesthesia (P = 0.019; OR, 3.049; CI, 1.197-7.762) were significant risk factors (Table 5).
Table 3.
Risk Factors for Minor Complications by Univairate Logistic Regression Analysis and Multivariate Logistic Regression Analysis After Vertebroplasty or Kyphoplasty.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Risk factors for minor complications | P value | Odds Ratio (95% CI) |
P value | Odds Ratio (95% CI) |
| Age (< 64 vs. 65-74) (< 64 vs. 75-84) (< 64 vs. ≥ 85) |
0.423 0.198 0.160 0.096 |
1.981 (0.699-5.610) 2.030 (0.755-5.456) 2.382 (0.857-6.619) |
0.732
0.764 0.455 0.319 |
1.249 (0.293-5.327) 1.690 (0.427-6.694) 2.090 (0.490-8.912) |
| Male gender | 0.184 |
1.576 (0.805-3.084) |
0.295 | 0.601 (0.231-1.558) |
| BMI Normal vs. Underweight Normal vs. Overweight Normal vs. Moderate obese Normal vs. Severe obese |
0.581 0.533 0.127 0.218 0.778 |
1.606 (0.362-7.123) 1.667 (0.865-3.211) 1.646 (0.745-3.637) 1.172 (0.389-3.530) |
0.238
0.604 0.022 0.137 0.594 |
1.567 (0.202-15.151) 2.972 (1.168-7.563) 2.368 (0.760-7.383) 1.576 (0.296-8.380) |
| ASA classification (1 and 2 vs. 3) (1 and 2 vs. 4) |
0.184 0.086 0.113 |
1.961 (0.908-4.234) 2.295 (0.821-6.416) |
0.967
0.858 0.978 |
1.101 (0.381-3.184) 0.980 (0.237-4.046) |
| Albumin (< 3.0 g/dL) | 0.211 |
1.647 (0.753-3.600) |
0.181 |
1.777 (0.765-4.131) |
| Blood urea nitrogen (≥ 20.0 mg/dL) | 0.028 |
1.866
(1.068 - 3.260) |
0.813 |
1.100 (0.498-2.429) |
| Creatinine (≥ 1.3 mg/dL) | 0.412 |
1.378 (0.640-2.969) |
||
| White blood cell count | 0.002 |
1.103
(1.038 - 1.172) |
0.076 |
1.093 (0.991-1.206) |
| Smoking* | 0.636 |
1.203 (0.560-2.582) |
||
| Diabetes | 0.239 |
1.481 (0.770-2.851) |
0.704 |
1.210 (0.466-3.094) |
| Dyspnea | 0.040 |
1.989
(1.031 - 3.839) |
0.783 |
1.157 (0.411-3.255) |
| Functional status prior to surgery (Independent vs. partially and totally dependent) |
0.033
|
2.100
(1.060 - 4.163) |
0.838 |
0.893 (0.304-2.628) |
| History of Chronic Obstructive Pulmonary Disease | 0.026 |
2.070
(1.092 - 3.923) |
0.037 |
2.599
(1.061 - 6.367) |
| Hypertension requiring medication | 0.014 |
2.388
(1.193 - 4.784) |
0.863 |
1.087 (0.422-2.802) |
| History of Congestive Heart Failure in 30 days before surgery | 0.815 |
1.271 (0.170-9.522) |
||
| Current dialysis | 0.999 | |||
| Steroid use | 0.049 |
1.975
(1.002 - 3.893) |
0.829 |
1.117 (0.410-3.045) |
| Bleeding disorder | 0.920 |
1.055 (0.375-2.966) |
|
|
| Transfusion within 72 hours before surgery | 0.403 |
2.390 (0.310-18.432) |
||
| > 10% loss of body weight‡ | 0.998 | |||
| MAC or local anesthesia | 0.988 |
0.993 (0.420-2.351) |
||
| Procedure methods (Kyphoplasty vs. Vertebroplasty) |
0.202 |
0.607 (0.282-1.307) |
0.192 |
0.506 (0.182-1.407) |
| Operation time (≤ 30 minutes vs. > 30 minutes) |
0.111 |
0.617 (0.341-1.118) |
0.673 |
0.841 (0.337-1.721) |
CI, confidence interval; BMI, Body Mass Index; ASA, American Society of Anesthesiologist; * means current smoking for recent 1 year. Hypoalbuminemia was defined as a serum albumin of less than 3.0 g/dL. ‡ means 10% loss of body weight in the 6 months prior to surgery.
Table 4.
Risk Factors for Major Complications by Univairate Logistic Regression Analysis and Multivariate Logistic Regression Analysis After Vertebroplasty or Kyphoplasty.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Risk factors for major complications | P value | Odds Ratio (95% CI) |
P value | Odds Ratio (95% CI) |
| Age (< 64 vs. 65-74) (< 64 vs. 75-84) (< 64 vs. ≥ 85) |
0.577
0.730 0.918 0.374 |
0.887 (0.449-1.752) 1.033 (0.560-1.904) 1.334 (0.707-2.517) |
0.724
0.404 0.605 0.927 |
0.671 (0.264-1.710) 0.798 (0.340-1.874) 1.043 (0.423-2.576) |
| Male gender | 0.568 |
1.138 (0.730-1.775) |
0.610 |
0.847 (0.448-1.601) |
| BMI Normal vs. Underweight Normal vs. Overweight Normal vs. Moderate obese Normal vs. Severe obese |
0.377
0.777 0.054 0.612 0.410 |
0.811 (0.190-3.464) 1.605 (0.991-2.599) 1.180 (0.622-2.238) 1.374 (0.645-2.930) |
0.305
0.760 0.139 0.660 0.058 |
0.772 (0.090-5.816) 1.650 (0.850-3.202) 1.229 (0.491-3.076) 2.465 (0.970-6.267) |
| ASA classification (1 and 2 vs. 3) (1 and 2 vs. 4) |
0.081
0.641 0.037 |
1.129 (0.678-1.881) 2.043 (1.043-4.003) |
0.518
0.929 0.363 |
1.036 (0.473-2.271) 1.571 (0.593-4.158) |
| Albumin (< 3.0 g/dL) | 0.001 |
2.620
(1.497 - 4.585) |
0.005 |
2.392
(1.305 - 4.383) |
| Blood urea nitrogen (≥ 20.0 mg/dL) | 0.292 |
1.262 (0.819-1.943) |
||
| Creatinine (≥ 1.3 mg/dL) | 0.088 |
1.627 (0.930-2.847) |
0.467 |
1.312 (0.631-2.727) |
| White blood cell count | 0.001 |
1.095
(1.040 - 1.153) |
0.004 |
1.117
(1.037 - 1.204) |
| Smoking* | 0.825 |
1.070 (0.587-1.952) |
||
| Diabetes | 0.962 |
1.013 (0.584-1.758) |
||
| Dyspnea | 0.157 |
1.479 (0.860-2.543) |
0.508 |
1.285 (0.611-2.701) |
| Functional status prior to surgery (Independent vs. partially and totally dependent) |
0.125
|
1.547 (0.886-2.701) |
0.309 |
1.474 (0.698-3.112) |
| History of Chronic Obstructive Pulmonary Disease | 0.580 |
1.174 (0.666-2.070) |
||
| Hypertension requiring medication | 0.443 |
1.189 (0.764-1.852) |
||
| History of Congestive Heart Failure in 30 days before surgery | 0.621 |
1.442 (0.338-6.154) |
||
| Current dialysis | 0.999 | |||
| Steroid use | 0.070 |
1.653 (0.960-2.847) |
0.946 |
1.026 (0.493-2.132) |
| Bleeding disorder | 0.636 |
1.198 (0.568-2.524) |
|
|
| Transfusion within 72 hours before surgery | 0.177 |
2.800 (0.627-12.502) |
0.490 |
1.844 (0.325-10.474) |
| > 10% loss of body weight‡ | 0.514 |
1.625 (0.378-6.978) |
||
| MAC or local anesthesia | 0.696 |
1.132 (0.608-2.110) |
||
| Procedure methods (Kyphoplasty vs. Vertebroplasty) |
0.237 |
1.661 (0.717-3.850) |
0.282 |
1.932 (0.583-6.407) |
| Operation time (≤ 30 minutes vs. > 30 minutes) |
0.037 |
0.621
(0.398 - 0.971) |
0.470 |
0.806 (0.448-1.448) |
CI, confidence interval; BMI, Body Mass Index; ASA, American Society of Anesthesiologist; * means current smoking for recent 1 year. Hypoalbuminemia was defined as a serum albumin of less than 3.0 g/dL. ‡ means 10% loss of body weight in the 6 months prior to surgery.
Table 5.
Risk Factors for Mortality by Univairate Logistic Regression Analysis and Multivariate Logistic Regression Analysis After Vertebroplasty or Kyphoplasty.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Risk factors for mortality | P value | Odds Ratio (95% CI) |
P value | Odds Ratio (95% CI) |
| Age (< 64 vs. 65-74) (< 64 vs. 75-84) (< 64 vs. ≥ 85) |
0.093
0.413 0.144 0.030 |
1.764 (0.453-6.872) 2.533 (0.728-8.811) 3.993 (1.147-13.905) |
0.784
0.979 0.584 0.489 |
0.976 (0.157-6.070) 1.608 (0.294-8.810) 1.862 (0.320-10.838) |
| Male gender | 0.621 |
1.184 (0.606-2.311) |
0.307 |
0.595 (0.220-1.611) |
| BMI Normal vs. Underweight Normal vs. Overweight Normal vs. Moderate obese Normal vs. Severe obese |
0.066
0.067 0.059 0.682 0.630 |
2.818 (0.931-8.527) 0.413 (0.165-1.035) 0.825 (0.328-2.074) 0.740 (0.217-2.519) |
0.326
0.086 0.402 0.810 0.571 |
3.772 (0.830-17.135) 0.611 (0.193-1.932) 1.171 (0.323-4.248) 1.632 (0.300-8.869) |
| ASA classification (1 and 2 vs. 3) (1 and 2 vs. 4) |
< 0.001
0.105 < 0.001 |
2.739 (0.810-9.260) 14.838 (4.273 - 51.526 ) |
< 0.001
0.275 0.010 |
3.212 (0.395-26.147) 16.604 (1.956 - 140.959) |
| Albumin (< 3.0 g/dL) | < 0.001 |
4.036
(1.979 - 8.229) |
0.087 |
2.174 (0.895-5.284) |
| Blood urea nitrogen (≥ 20.0 mg/dL) | 0.017 |
2.143
(1.143 - 4.018) |
0.448 |
0.678 (0.249-1.849) |
| Creatinine (≥ 1.3 mg/dL) | 0.001 |
3.349
(1.676 - 6.691) |
0.030 |
3.494
(1.128 - 10.823) |
| White blood cell count | 0.002 |
1.108
(1.038 - 1.182) |
0.062 |
1.107 (0.995-1.232) |
| Smoking* | 0.577 |
0.743 (0.262-2.107) |
||
| Diabetes | 0.320 |
1.465 (0.690-3.107) |
||
| Dyspnea | 0.196 |
1.681 (0.766-3.689) |
0.252 |
0.471 (0.130-1.710) |
| Functional status prior to surgery (Independent vs. partially and totally dependent) |
0.032
|
2.304
(1.076 - 4.932) |
0.209 |
1.901 (0.698-5.175) |
| History of Chronic Obstructive Pulmonary Disease | 0.015 |
2.413
(1.191 - 4.891) |
0.487 |
1.424 (0.525-3.864) |
| Hypertension requiring medication | 0.093 |
1.897 (0.898-4.009) |
0.610 |
0.758 (0.261-2.202) |
| History of Congestive Heart Failure in 30 days before surgery | 0.604 |
1.707 (0.227-12.864) |
||
| Current dialysis | < 0.001 |
10.876
(2.998 - 39.460) |
0.977 |
1.030 (0.139-7.619) |
| Steroid use | 0.550 |
1.307 (0.543-3.149) |
||
| Bleeding disorder | 0.490 |
1.446 (0.507-4.123) |
|
|
| Transfusion within 72 hours before surgery | 0.012 |
7.060
(1.550 - 32.152) |
0.270 |
3.435 (0.383-30.825) |
| > 10% loss of body weight‡ | 0.003 |
6.589
(1.895 - 22.913) |
0.087 |
4.676 (0.801-27.291) |
| MAC or local anesthesia | < 0.001 |
3.470
(1.738 - 6.928) |
0.019 |
3.049
(1.197 - 7.762) |
| Procedure methods (Kyphoplasty vs. Vertebroplasty) |
0.278 |
0.614 (0.254-1.482) |
0.918 |
0.937 (0.271-3.241) |
| Operation time (≤ 30 minutes vs. > 30 minutes) |
0.908 |
0.963 (0.508-1.825) |
||
CI, confidence interval; BMI, Body Mass Index; ASA, American Society of Anesthesiologist; * means current smoking for recent 1 year. Hypoalbuminemia was defined as a serum albumin of less than 3.0 g/dL. ‡ means 10% loss of body weight in the 6 months prior to surgery.
Discussion
The present study intended to identify the incidences of and risk factors for complications and mortality after VP/KP. Overall complication rates were 8.6%: the rate of minor complications, major complications, and mortality were 2.7%, 4.9%, and 2.1%, respectively. Mortality was independently associated with an ASA 4 or above and increased creatinine. Major complications were independently associated with lower albumin and increased WBC count.
The current complication and mortality rates in patients undergoing VP/KP were higher than those in previous systematic reviews and meta-analyses,11,17,23-25 which have shown a perioperative complication rate of 0.9% and a mortality rate of 0.01%. These differences can be explained by 2 reasons. First, it should be acknowledged that previous systematic reviews and meta-analyses used case series or comparative studies with a small numbers of patients—most of them had less than 100 patients.17,23 Therefore, complication and/or mortality rates might be underestimated due to insufficient sample sizes. Second, meta-analyses and systematic reviews may be subject to publication bias, which means a “positive” result (usually in favor of a new treatment) are more likely to be published. 26 Therefore, general complications after vertebral augmentation procedures may neglected in previous studies. Interestingly, a recent study using a large, national database reported a 1.5% mortality rates after vertebral augmentation, confirming that larger samples may have the power to detect higher complication and mortality rates. 27
Increased level of creatinine (≥ 1.3 mg/dL), ASA 4, and MAC or local anesthesia were independently associated with 30 day mortality after VP/KP. Renal insufficiency is a known risk factor for complications after all spinal procedures, and it is important to note that these risk factors remain significant even in a minor, percutaneous cement augmentation procedure. 27 In addition, ASA classification has also been considered as an independent risk factor for postoperative mortality in previous studies.22,28,29 Interestingly, the use of local anesthesia was associated with mortality, but we recommend interpreting these results with caution. MAC or local anesthesia is a less invasive form of anesthesia, and in theory should lead to less complications; however, it is possible that general anesthesia was avoided in patients with complex medical histories and many comorbidities. Thus, MAC or local anesthesia may have been used in sicker patients, still at risk for postoperative complications despite the less invasive form of sedation. Regardless, we recommend that the decision to place elderly patients under general anesthesia, as opposed to conscious sedation, should be jointly discussed with the anesthesia and surgical team to make the optimal choice for each patient.
Increased WBC count was also associated with an increased odds of for major complications. Previous studies agree with these results, which have shown that leukocytosis was a significant risk factor for postoperative complication and/or prolonged length of stay after hip fracture surgery and above-knee amputation.30,31 Furthermore, increased WBC count might be related to an asymptomatic or non-diagnosed infection prior to surgery. When performing VP/KP, complications are rarely related to the surgical technique, which often lasts under an hour, and more related to the prone positioning and co-morbidities of an elderly population. Thus, the initial decision to undergo the procedure may be the most important decision in avoiding complications after VP/KP. It is our hope that surgeons can use this information preoperatively to optimally select patients for surgery by threshold cut-off values for creatinine, WBC, and ASA class and defer VP/KP in patients meeting these criteria. At-risk patients should see a general practitioner to correct these metabolic disturbances, and only then should VP/KP be considered.
For proper interpretation of the current results, several methodological aspects should be considered. First, the current cohort included very elderly patients: the mean age was 74.9 years. This should be attributable to higher rates of mortality and complication rates compared to previous studies.10,11,17,23 However, this also reflects that many elderly patients (even more than 75 years old) undergo VP/KP in real clinical practice. The prevalence of OVCF has increased to 40% in patients older than 80 years. 32 Therefore, with the increasing age of the general population, vertebral augmentation procedures will be performed in more elderly patients. In addition, though many of the complications might appear to be unrelated to the cement injection procedure itself, such as UTI, pneumonia, or renal insufficiency, they may be associated with prone position and co-morbidities which may be too much for certain elderly patients to undergo. ACS-NSQIP data did not include any complication which was not associated with targeted surgical procedure and/or related anesthesia.19,33,34 The ACS NSQIP tracks patients for 30 days after their operation, providing a more complete picture of their care. Ideally, we would have been able to assess surgery-specific complications, such as neurologic complications from cement leakage or venous embolism, but unfortunately, this was not possible with the current data collection methods. Second, coding errors in the NSQIP dataset would influence the current results.35,36 The wide variation in hospital settings, hospital quality, surgical strategy, and surgeons’ expertise in a nationwide database can also confound the present results. Third, the current data set draws from 2011-2013, which represents an older time period that may reflect different practice patterns than more recent data. It is our hope the current analyses can be used as a baseline comparison for future studies to assess potential changes in complications after VP/KP procedures for more recent years. Fourth, the current data was collected retrospectively from a national database. Despite the strength of a large, heterogeneous sample, the retrospective methodology reflected wide variation in hospital setting, hospital quality, surgical strategy, and surgeons’ expertise, which may lend itself to a biased and/or less accurate sample. However, despite these limitations, this study provides the largest sample size in a single study for reporting short-term postoperative mortality and complications following vertebral augmentation procedures such as VP/KP across the nation.
Conclusion
In conclusion, the major complication and mortality rates after VP/KP were 4.9% and 2.1%, respectively, in the current study, which were higher than in previous systematic reviews and/or meta-analyses.4,10,11,17,23 Mortality was independently associated with an ASA 4 or above and increased creatinine. Though we cannot determine if the morbidity was associated with the specific spinal procedure or associated perioperative care, in any case, cautious monitoring and counseling are recommended for elderly patients with preexisting kidney disease and/or ASA 4.
Acknowledgments
The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Scott L. Zuckerman, MD, MPH  https://orcid.org/0000-0003-2951-2942
https://orcid.org/0000-0003-2951-2942
References
- 1.Melton LJ, 3rd. Epidemiology of spinal osteoporosis. Spine (Phila Pa 1976). 1997;22(24 Suppl):2S–11S. [DOI] [PubMed] [Google Scholar]
- 2.Goz V, Koehler SM, Egorova NN, et al. Kyphoplasty and vertebroplasty: trends in use in ambulatory and inpatient settings. Spine J. 2011;11(8):737–744. [DOI] [PubMed] [Google Scholar]
- 3.Lips P, van Schoor NM. Quality of life in patients with osteoporosis. Osteoporos Int. 2005;16(5):447–455. [DOI] [PubMed] [Google Scholar]
- 4.Kim DH, Vaccaro AR. Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J. 2006;6(5):479–487. [DOI] [PubMed] [Google Scholar]
- 5.Longo UG, Loppini M, Denaro L, Maffulli N, Denaro V. Osteoporotic vertebral fractures: current concepts of conservative care. B Med Bull. 2012;102:171–189. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Yi JM, Cho HG, et al. Comparative study of the treatment outcomes of osteoporotic compression fractures without neurologic injury using a rigid brace, a soft brace, and no brace: a prospective randomized controlled non-inferiority trial. J Bone Joint Surg Am. 2014;96(23):1959–1966. [DOI] [PubMed] [Google Scholar]
- 7.Wardlaw D, Van Meirhaeghe J. Another chapter for vertebral compression fractures. Lancet. 2010;376(9746):1031–1033. [DOI] [PubMed] [Google Scholar]
- 8.Silva AMG, Tan SSH, Makaranda MC, Chen JLT. Compression fractures in the setting of diffuse idiopathic skeletal hyperostosis. Asian Spine J. 2015;9(4):629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnakumar R, Lenke LG. “Sternum-into-abdomen” deformity with abdominal compression following osteoporotic vertebral compression fractures managed by 2-level vertebral column resection and reconstruction. Spine (Phila Pa 1976). 2015;40(18):E1035–1039. [DOI] [PubMed] [Google Scholar]
- 10.Hulme PA, Krebs J, Ferguson SJ, Berlemann U. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine (Phila Pa 1976). 2006;31(17):1983–2001. [DOI] [PubMed] [Google Scholar]
- 11.McGirt MJ, Parker SL, Wolinsky JP, Witham TF, Bydon A, Gokaslan ZL. Vertebroplasty and kyphoplasty for the treatment of vertebral compression fractures: an evidenced-based review of the literature. Spine J. 2009;9(6):501–508. [DOI] [PubMed] [Google Scholar]
- 12.Hegazy R, El-Mowafi H, Hadhood M, Hannout Y, Allam Y, Silbermann J. The outcome of radiofrequency kyphoplasty in the treatment of vertebral compression fractures in osteoporotic patients. Asian spine J. 2019;13(3):459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361(6):557–568. [DOI] [PubMed] [Google Scholar]
- 14.Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361(6):569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eck JC, Nachtigall D, Humphreys SC, Hodges SD. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta-analysis of the literature. Spine J. 2008;8(3):488–497. [DOI] [PubMed] [Google Scholar]
- 16.Klazen CA, Lohle PN, de Vries J, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet. 2010;376(9746):1085–1092. [DOI] [PubMed] [Google Scholar]
- 17.Hochmuth K, Proschek D, Schwarz W, Mack M, Kurth AA, Vogl TJ. Percutaneous vertebroplasty in the therapy of osteoporotic vertebral compression fractures: a critical review. Eur Radiol. 2006;16(5):998–1004. [DOI] [PubMed] [Google Scholar]
- 18.Zhang JD, Poffyn B, Sys G, Uyttendaele D. Comparison of vertebroplasty and kyphoplasty for complications. Orthop Surg. 2011;3(3):158–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khuri SF, Daley J, Henderson W, et al. The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA surgical quality improvement program. Ann Surg. 1998;228(4):491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basques BA, Fu MC, Buerba RA, Bohl DD, Golinvaux NS, Grauer JN. Using the ACS-NSQIP to identify factors affecting hospital length of stay after elective posterior lumbar fusion. Spine (Phila Pa 1976). 2014;39(6):497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalanithi PS, Patil CG, Boakye M. National complication rates and disposition after posterior lumbar fusion for acquired spondylolisthesis. Spine (Phila Pa 1976). 2009;34(18):1963–1969. [DOI] [PubMed] [Google Scholar]
- 22.Schoenfeld AJ, Ochoa LM, Bader JO, Belmont PJ, Jr. Risk factors for immediate postoperative complications and mortality following spine surgery: a study of 3475 patients from the National Surgical Quality Improvement Program. J Bone Join Surg Am. 2011;93(17):1577–1582. [DOI] [PubMed] [Google Scholar]
- 23.Taylor RS, Fritzell P, Taylor RJ. Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J. 2007;16(8):1085–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi SH, Kim DY, Koo JW, Lee SG, Jeong SY, Kang CN. Incidence and management trends of osteoporotic vertebral compression fractures in South Korea: a nationwide population-based study. Asian Spine J. 2020;14(2):220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winkelmann M, Mavropoulos T, Decker S, Omar M, Krettek C, Müller CW. Radiological and clinical outcomes of balloon kyphoplasty versus radiofrequency kyphoplasty in the treatment of vertebral compression fractures. Asian Spine J. 2018;12(5):862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker E, Hernandez AV, Kattan MW. Meta-analysis: its strengths and limitations. Clev Clin J Med. 2008;75(6):431–439. [DOI] [PubMed] [Google Scholar]
- 27.Toy JO, Basques BA, Grauer JN. Morbidity, mortality, and readmission after vertebral augmentation: analysis of 850 patients from the American College of Surgeons national surgical quality improvement program database. Spine (Phila Pa 1976). 2014;39(23):1943–1949. [DOI] [PubMed] [Google Scholar]
- 28.Pateder DB, Gonzales RA, Kebaish KM, Cohen DB, Chang JY, Kostuik JP. Short-term mortality and its association with independent risk factors in adult spinal deformity surgery. Spine (Phila Pa 1976). 2008;33(11):1224–1228. [DOI] [PubMed] [Google Scholar]
- 29.Hackett NJ, De Oliveira GS, Jain UK, Kim JY. ASA class is a reliable independent predictor of medical complications and mortality following surgery. Int J Surg (London, England). 2015;18:184–190. [DOI] [PubMed] [Google Scholar]
- 30.Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma. 2014;28(2):63–69. [DOI] [PubMed] [Google Scholar]
- 31.Wise ES, McMaster WG, Jr, Williamson K, Wergin JE, Hocking KM, Brophy CM. Preoperative predictors of 30-day mortality and prolonged length of stay after above-knee amputation. Ann Vas Surg. 2015;31:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melton LJ, 3rd, Kan SH, Frye MA, Wahner HW, O’Fallon WM, Riggs BL. Epidemiology of vertebral fractures in women. Am J Epidemiol. 1989;129(5):1000–1011. [DOI] [PubMed] [Google Scholar]
- 33.Davenport DL, Holsapple CW, Conigliaro J. Assessing surgical quality using administrative and clinical data sets: a direct comparison of the University Healthsystem Consortium Clinical Database and the National Surgical Quality Improvement Program data set. Am J Med Qual. 2009;24(5):395–402. [DOI] [PubMed] [Google Scholar]
- 34.Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons national surgical quality improvement program approach. Adv Surg. 2010;44:251–267. [DOI] [PubMed] [Google Scholar]
- 35.Mull HJ, Borzecki AM, Loveland S, et al. Detecting adverse events in surgery: comparing events detected by the Veterans Health Administration Surgical Quality Improvement Program and the patient safety indicators. Am J Surg. 2014;207(4):584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorence DP, Ibrahim IA. Benchmarking variation in coding accuracy across the united states. J Health Care Finance. 2003;29(4):29–42. [PubMed] [Google Scholar]