Abstract
BACKGROUND
Arteriovenous malformations (AVMs) of the brain are vessel conglomerates of feeding arteries and draining veins that carry a risk of spontaneous and intraoperative rupture. Augmented reality (AR)-assisted neuronavigation permits continuous, real-time, updated visualization of navigation information through a heads-up display, thereby potentially improving the safety of surgical resection of AVMs.
OBSERVATIONS
The authors report a case of a 37-year-old female presenting with a 2-year history of recurrent falls due to intermittent right-sided weakness and increasing clumsiness in the right upper extremity. Magnetic resonance imaging, magnetic resonance angiography, and cerebral angiography of the brain revealed a left parietal Spetzler-Martin grade III AVM. After endovascular embolization of the AVM, microsurgical resection using an AR-assisted neuronavigation system was performed. Postoperative angiography confirmed complete obliteration of arteriovenous shunting. The postsurgical course was unremarkable, and the patient remains in excellent health.
LESSONS
Our case describes the operative setup and intraoperative employment of AR-assisted neuronavigation for AVM resection. Application of this technology may improve workflow and enhance patient safety.
Keywords: AVM, arteriovenous malformation, augmented reality, virtual reality, image-guided surgery, neuronavigation
ABBREVIATIONS : 3D = three-dimensional, AR = augmented reality, AVM = arteriovenous malformation, CTA = computed tomography angiography, HUD = heads-up display, MCA = middle cerebral artery, MRA = magnetic resonance angiography, MRI = magnetic resonance imaging, VR = virtual reality
Arteriovenous malformations (AVMs) of the brain are conglomerates of dysplastic vessels with direct shunting of blood from feeding arteries to draining veins without an interposed capillary bed.1,2 Altered hemodynamic forces in this low-resistance, high-pressure vascular network lead to an estimated 1% annual rate of spontaneous ruptures with intracranial hemorrhage and a fivefold increased risk of rehemorrhage yearly.1,2 Microsurgical resection is recognized as an outstanding treatment option that offers excellent cure rates and an acceptable risk profile.3,4 A single cohort of AVM resections has reported an estimated intraoperative rupture rate of approximately 5%.5
Augmented reality (AR) technology is a novel approach for intraoperative image guidance using preoperative imaging studies to create a real-time, updated, three-dimensional (3D) virtual model of anatomical structures superimposed on the real-world surgical field visible on a microscope’s heads-up display (HUD).6,7 This differs from virtual reality (VR)-assisted neuronavigation, which requires the surgeon to mentally construct a 3D model of the surgical field from 2D imaging data and can lead to workflow disruption.8 Given the heterogeneous angioarchitecture of AVMs and their close relationship to surrounding structures, AR-based neuronavigation could be particularly helpful in establishing and maintaining an understanding of AVM topology pre- and intraoperatively.9 Although the employment of AR technology has been described in a variety of neurosurgical pathologies in the past few years, literature characterizing its use in conjunction with AVM resection remains scarce. In this case report, we present our standard AVM resection procedure assisted by AR neuronavigation to improve surgical outcomes and increase patient safety.
Illustrative Case
A 37-year-old female presented with a 2-year history of intermittent right upper and lower motor weakness causing frequent nonsyncopal falls. She also experienced 4 months of increasing clumsiness and episodes of sensory disturbance in her right upper extremity. Her medical history was significant for migraines with aura, sciatic leg pain, and a prior L3–5 lumbar fusion. Her neurological examination was unremarkable with full strength in all extremities and no sensory deficits.
Diagnostic workup with computed tomography angiography (CTA), magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), and 3D rotational angiography revealed a Spetzler-Martin grade III AVM (size 3–6 cm, superficial drainage, eloquent cortex) centered within the left parietal lobe without evidence of rupture. The AVM nidus measured 2.5 × 2.4 × 3.4 cm. Arterial supply was composed of a parietal branch of the middle cerebral artery (MCA) and an occipital branch of the posterior cerebral artery. Venous drainage was superficial and directed superiorly and laterally toward the superior sagittal sinus and transverse sinus, respectively (Fig. 1A).
FIG. 1.
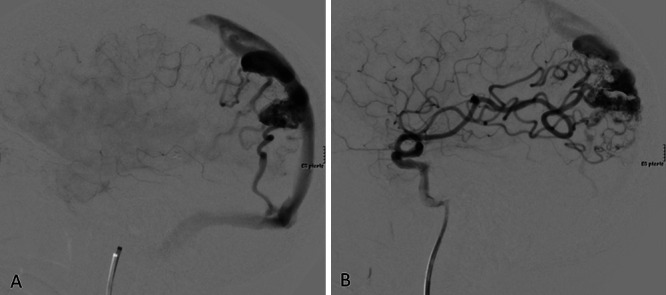
Cerebral angiography of left parietal AVM pre- and postembolization. A: Preembolization angiogram of the left internal carotid artery shows a lateral view of the malformation draining into the superior sagittal sinus and left transverse sinus. B: Postembolization angiogram of the left internal carotid artery demonstrates obliteration of the feeding MCA branch.
Endovascular embolization with Onyx achieved excellent obliteration of the deep MCA feeder (Fig. 1B), without compromise of venous outflow. Arteriovenous shunting persisted with arterial supply from an anterior division of the posterior parietal artery of the MCA. The nidus appeared to be reduced by 20%–30%. Postembolization MRA confirmed the presence of venous drainage into the superior sagittal sinus via a large-caliber tortuous vein.
The patient was prepared for stereotactic computer-assisted volumetric resection. A brain contrast MRI scan was obtained after endovascular embolization and was then loaded into the frameless stereotactic neuronavigation system (BrainLab Curve). Using the BrainLab SmartBrush software (BrainLab version 3.0), we then segmented the draining veins, feeder arteries, and eloquent structures on the 3D model by assigning different colors. The color-coded 3D model was then injected into the HUD. The patient was positioned supine on the operating table with the head turned and secured with the Doro skull clamp (pro med instruments GmbH).
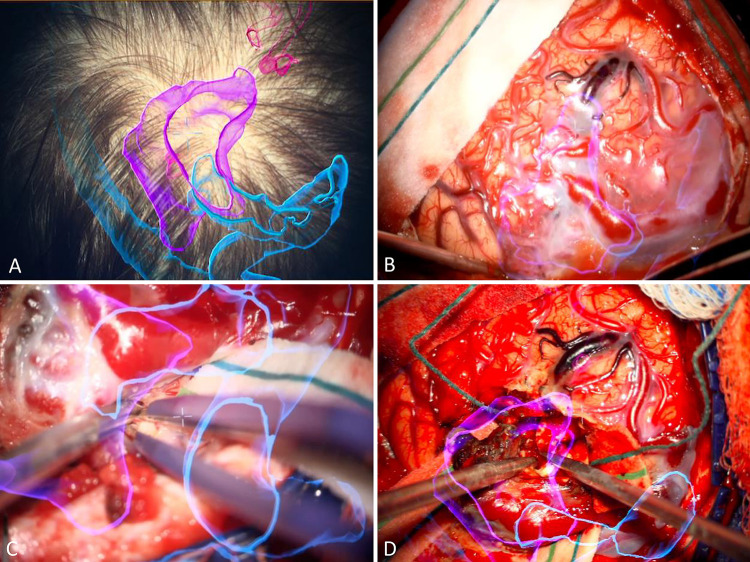
The frameless reference cluster was attached to the head frame, and stereotactic registration was performed. Once registration accuracy was confirmed, a second stereotactic frameless reference frame was attached to the microscope (Zeiss KINEVO 900). The microscope was brought into the surgical field before the sterile prep and draping. The HUD was turned on and used to assess patient positioning, design the skin incision, and plan the craniotomy (Fig. 2A).
FIG. 2.
AR neuronavigation system for computer-assisted volumetric resection of AVM pre- and intraoperatively. A: HUD visualization of AVM nidus (purple), superior sagittal sinus, left transverse sinus, major draining veins (blue), and MCA feeders (pink) for stereotactic planning of craniotomy. B: Intraoperative HUD visualization with real-time tracking of AVM nidus (purple) and adjacent structures. C and D: Intraoperative photographs of HUD-guided intranidal dissection of AVM feeders and draining veins.
After the left parietal craniotomy, the microscope was brought in before dural opening. Upon opening the dura, the main draining vein was visualized on the cortical surface. Correlating the angiographic anatomy with the intraoperative findings and HUD, the major feeding arteries were traced directly into the nidus under high visual magnification and were circumferentially coagulated and divided (Fig. 2B, Video 1). Complete discontinuation of arterial blood flow was confirmed using indocyanine green videoangiography. At this point, the only remaining attachment was the single draining vein, which was then coagulated and divided.
VIDEO 1. Clip showing AR-assisted microsurgical resection of a brain AVM. Click here to view.
The patient’s postoperative course was unremarkable. Immediately after surgery, she was extubated and admitted to the neurosurgical intensive care unit for monitoring and blood pressure control. Her neurological examination results remained stable from her preoperative exam. Postsurgical angiography confirmed complete resection of the AVM without evidence of arteriovenous shunting or early filling veins (Fig. 3). The patient was discharged on the third postoperative day. At 3-month follow-up, she remains in excellent condition with complete resolution of presenting symptoms.
FIG. 3.
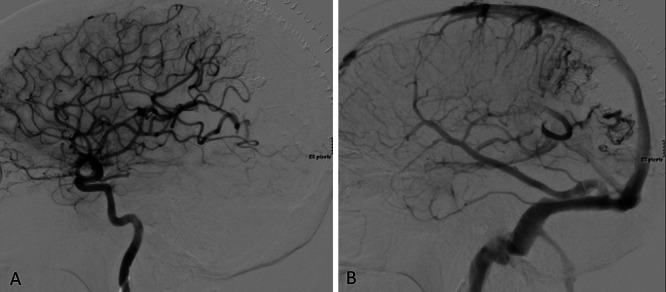
Postsurgical angiography with complete separation of arterial and venous phase. Postsurgical angiogram of the left internal carotid artery in lateral view confirming complete obliteration of the AVM with restored arterial circulation (A) and venous drainage (B).
Discussion
Observations
Neurosurgery as a specialty relies greatly on radiological imaging and has pioneered the use of AR-based neuronavigation for clinical and training purposes.8 AR technology has been used for the resection of various neoplastic lesions and a small number of neurovascular lesions.7 Review of the literature revealed only eight studies investigating the application of AR-projecting HUD for AVM resection, and of those, even fewer were performed on high-grade AVMs.6,10–16
Our setup matches the approach described by Cabrilo et al.,6 where virtual segmentations from preoperative imaging studies (CTA, MRA, 3D angiogram) are integrated into the frameless stereotactic system and projected onto the microscope’s HUD as a 3D overlay. However, we advance this approach by integrating the patient’s brain contrast MRI that was directly obtained after endovascular treatment. This approach permits visualization of postembolization updated AVM architecture information on the HUD. The frameless stereotactic navigation system integrates the microscope’s spatial, focus, and zoom parameters, thus permitting automated updating real-time tracking of the target lesion.6 We believe this is a major advance to VR-assisted neuronavigation where the surgeon’s attention is divided between the operative field and the navigation screens in order to mentally align the intraoperative findings with the patient’s MRI data.6,8,10
In addition, preoperative color segmentation allows intraoperative distinction and tracking of draining veins, feeder arteries, and eloquent structures through the HUD and can guide the surgeon in the critical process of arteriovenous dissection.8 Early benefits of the HUD include planning of patient positioning and the surgical approach, and further advantages in AVM resection include intraoperative guidance of superficial and deep dissection of arterial feeders and draining veins.10 In this case, the AR outline accurately corresponded to the true alignment found during surgery and smoothly adjusted to the zoom and focus functions of the microscope.
Lessons
Augmented reality provides a significant advance to VR-assisted neuronavigation by enabling neurosurgeons to optimize their surgical approach as well as to identify and track the AVM in real-time mode and with direct visualization on the HUD. MRI data that are obtained directly after endovascular treatment permit visualization of updated navigation information, potentially minimizing alignment error, improving workflow, and enhancing patient safety and care.
The use of AR technology has some limitations that deserve discussion, though none of them were significant during this case. Previous studies noted that a delay in AR projection on HUD may provide the surgeon with inaccurate data for a short latency of time.8 The use of high-speed wireless routers permitting rapid data transfer can provide a solution.8 Deng et al.17 found that registration, tracking, and calibration of different devices may result in alignment inaccuracies; therefore, compatibility of devices is of highest importance.8 Last, the system does not account for intraoperative brain tissue shift due to positioning changes or cerebrospinal fluid leakage, resulting in an increased AR alignment error.8,18,19 Kantelhardt et al.20 addressed this limitation by manually repositioning the shifted VR segments according to imaging studies, and the intraoperative reregistration allowed excellent, easy-to-perform restoration of AR overlay accuracy.
There exist specific limitations of AR technology in AVM resection. For example, previous studies reported an issue of insufficient depth perception.6 The human eye incorporates depth cues such as texture and shading during accommodation, and this is not constituted by the planes of the virtual image on the HUD.21 Although the integration of color-segmented 3D models alleviates the accommodation discrepancy, surgeons may encounter depth perception mismatches when navigating along the z-axis.6,21 Furthermore, the limited ability to distinguish between types of arterial feeders and the complex angioarchitecture of AVMs may limit the usefulness of AR.6 In contrast to the report by Cabrilo et al.,6 where the navigation information of draining veins was incongruent to the 3D model constructed from preembolization imaging studies, we received updated navigation information because we had injected postembolization imaging studies into the HUD.
The small number of reported AR-assisted AVM resections in the current literature does not permit a final conclusion of its usefulness for widespread clinical application at this point. Although more prospective studies are needed to evaluate the benefits and barriers of this technology, recent preclinical efforts have been made to incorporate the concept of time into the AR environment to better assess hemodynamics in vascular pathologies.22 This is achieved by injecting 3D rotational angiography, 3D time-of-flight MRI, and 4D phase-contrast MRI data into an AR-capable device.22 Although not integrated into the intraoperative HUD yet, this technology may allow visualization of animated blood flow during neurovascular surgery.22 By enabling the surgeon to navigate in space and time through an advanced 4D AR HUD, this technology may help identify the different types of arterial feeders and draining veins, further increasing workflow and patient safety.
Acknowledgments
We thank Laura Salgado-Lopez, MD, for assistance with manuscript editing. We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité – Universitätsmedizin Berlin.
Disclosures
Dr. Bederson reported travel reimbursement for a teaching engagement from Brainlab and travel reimbursement for a teaching engagement from Zeiss outside the submitted work. No other disclosures were reported.
Author Contributions
Conception and design: Scherschinski, Schlachter, Yaeger, Bederson. Acquisition of data: Scherschinski, Schlachter, Oemke, Yaeger. Analysis and interpretation of data: Scherschinski, McNeill, Schlachter, Yaeger. Drafting the article: Scherschinski, McNeill, Shuman. Critically revising the article: Scherschinski, McNeill, Shuman, Oemke, Yaeger. Reviewed submitted version of manuscript: Scherschinski, McNeill, Schlachter, Shuman, Oemke, Yaeger. Approved the final version of the manuscript on behalf of all authors: Scherschinski. Administrative/technical/material support: McNeill, Oemke. Study supervision: Schlachter.
Supplemental Information
Video
Video 1. https://vimeo.com/689695641.
References
- 1. Lawton MT, Rutledge WC, Kim H, et al. Brain arteriovenous malformations. Nat Rev Dis Primers. 2015;1:15008. doi: 10.1038/nrdp.2015.8. [DOI] [PubMed] [Google Scholar]
- 2. Friedlander RM. Clinical practice. Arteriovenous malformations of the brain. N Engl J Med. 2007;356(26):2704–2712. doi: 10.1056/NEJMcp067192. [DOI] [PubMed] [Google Scholar]
- 3. Lawton MT, Lang MJ. The future of open vascular neurosurgery: perspectives on cavernous malformations, AVMs, and bypasses for complex aneurysms. J Neurosurg. 2019;130(5):1409–1425. doi: 10.3171/2019.1.JNS182156. [DOI] [PubMed] [Google Scholar]
- 4. Potts MB, Lau D, Abla AA, Kim H, Young WL, Lawton MT. Current surgical results with low-grade brain arteriovenous malformations. J Neurosurg. 2015;122(4):912–920. doi: 10.3171/2014.12.JNS14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Torné R, Rodríguez-Hernández A, Lawton MT. Intraoperative arteriovenous malformation rupture: causes, management techniques, outcomes, and the effect of neurosurgeon experience. Neurosurg Focus. 2014;37(3):E12. doi: 10.3171/2014.6.FOCUS14218. [DOI] [PubMed] [Google Scholar]
- 6. Cabrilo I, Bijlenga P, Schaller K. Augmented reality in the surgery of cerebral arteriovenous malformations: technique assessment and considerations. Acta Neurochir (Wien) 2014;156(9):1769–1774. doi: 10.1007/s00701-014-2183-9. [DOI] [PubMed] [Google Scholar]
- 7. Meola A, Cutolo F, Carbone M, Cagnazzo F, Ferrari M, Ferrari V. Augmented reality in neurosurgery: a systematic review. Neurosurg Rev. 2017;40(4):537–548. doi: 10.1007/s10143-016-0732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tagaytayan R, Kelemen A, Sik-Lanyi C. Augmented reality in neurosurgery. Arch Med Sci. 2018;14(3):572–578. doi: 10.5114/aoms.2016.58690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kersten-Oertel M, Chen SS, Drouin S, Sinclair DS, Collins DL. Augmented reality visualization for guidance in neurovascular surgery. Stud Health Technol Inform. 2012;173:225–229. [PubMed] [Google Scholar]
- 10. Mascitelli JR, Schlachter L, Chartrain AG, et al. Navigation-linked heads-up display in intracranial surgery: early experience. Oper Neurosurg (Hagerstown) 2018;15(2):184–193. doi: 10.1093/ons/opx205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cabrilo I, Bijlenga P, Schaller K. Augmented reality in the surgery of cerebral aneurysms: a technical report. Neurosurgery. 2014;10(suppl 2):252–261. doi: 10.1227/NEU.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 12. Vassallo R, Kasuya H, Lo BWY, Peters T, Xiao Y. Augmented reality guidance in cerebrovascular surgery using microscopic video enhancement. Healthc Technol Lett. 2018;5(5):158–161. doi: 10.1049/htl.2018.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kersten-Oertel M, Gerard I, Drouin S, et al. Augmented reality in neurovascular surgery: feasibility and first uses in the operating room. Int J CARS. 2015;10(11):1823–1836. doi: 10.1007/s11548-015-1163-8. [DOI] [PubMed] [Google Scholar]
- 14. Kockro RA, Tsai YT, Ng I, et al. Dex-ray: augmented reality neurosurgical navigation with a handheld video probe. Neurosurgery. 2009;65(4):795–808. doi: 10.1227/01.NEU.0000349918.36700.1C. [DOI] [PubMed] [Google Scholar]
- 15. King AP, Edwards PJ, Maurer CR, Jr, et al. A system for microscope-assisted guided interventions. Stereotact Funct Neurosurg. 1999;72(2-4):107–111. doi: 10.1159/000029708. [DOI] [PubMed] [Google Scholar]
- 16.Edwards PJ, Johnson LG, Hawkes DJ, Fenlon MR, Strong AJ, Gleeson MJ. Clinical experience and perception in stereo augmented reality surgical navigation. In: Yang GZ, Jiang TZ, editors. Medical Imaging and Augmented Reality. Lecture Notes in Computer Science. Vol. 3150. Springer; 2004. pp. 369–376. [Google Scholar]
- 17. Deng W, Li F, Wang M, Song Z. Easy-to-use augmented reality neuronavigation using wireless tablet PC. Stereotact Funct Neurosurg. 2014;92:17–24. doi: 10.1159/000354816. [DOI] [PubMed] [Google Scholar]
- 18. Shuhaiber JH. Augmented reality in surgery. Arch Surg. 2004;139(2):170–174. doi: 10.1001/archsurg.139.2.170. [DOI] [PubMed] [Google Scholar]
- 19. Mikhail M, Mithani K, Ibrahim GM. Presurgical and intraoperative augmented reality in neuro-oncologic surgery: clinical experiences and limitations. World Neurosurg. 2019;128:268–276. doi: 10.1016/j.wneu.2019.04.256. [DOI] [PubMed] [Google Scholar]
- 20. Kantelhardt SR, Gutenberg A, Neulen A, Keric N, Renovanz M, Giese A. Video-assisted navigation for adjustment of image-guidance accuracy to slight brain shift. Oper Neurosurg (Hagerstown) 2015;11(4):504–511. doi: 10.1227/NEU.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 21. Guha D, Alotaibi NM, Nguyen N, Gupta S, McFaul C, Yang VXD. Augmented reality in neurosurgery: a review of current concepts and emerging applications. Can J Neurol Sci. 2017;44(3):235–245. doi: 10.1017/cjn.2016.443. [DOI] [PubMed] [Google Scholar]
- 22. Karmonik C, Elias SN, Zhang JY, et al. Augmented reality with virtual cerebral aneurysms: a feasibility study. World Neurosurg. 2018;119:e617–e622. doi: 10.1016/j.wneu.2018.07.222. [DOI] [PubMed] [Google Scholar]