Abstract
Monoterpenes with an unsaturated hydrocarbon structure are mineralized anaerobically by the denitrifying β-proteobacterium Alcaligenes defragrans. Organic acids occurring in cells of A. defragrans and culture medium were characterized to identify potential products of the monoterpene activation reaction. Geranic acid (E,E-3,7-dimethyl-2,6-octadienoic acid) accumulated to 0.5 mM in cells grown on α-phellandrene under nitrate limitation. Cell suspensions of A. defragrans 65Phen synthesized geranic acid in the presence of β-myrcene, α-phellandrene, limonene, or α-pinene. Myrcene yielded the highest transformation rates. The alicyclic acid was consumed by cell suspensions during carbon limitation. Heat-labile substances present in cytosolic extracts catalyzed the formation of geranic acid from myrcene. These results indicated that a novel monoterpene degradation pathway must be present in A. defragrans.
The mineralization of organic matter by microorganisms is often severely hampered by the chemical structure of the substrate. In the presence of oxygen, mono- and dioxygenases catalyze the oxidative functionalization of recalcitrant compounds, i.e., hydrocarbons. Microbial mineralization of such substances also occurs in nature in the absence of molecular oxygen, and in the last decade anaerobic bacteria have been isolated on substances such as alkylbenzenes (6, 18, 22), alkanes (1, 7, 25, 26), and alkenes (15). Thus far, the biochemistry of the initial activation reactions has been revealed only for toluene. Catalysis via radicals seems to be involved in the addition of toluene to fumarate by the enzyme benzylsuccinate synthase, a putative glycine radical enzyme (3, 21). This novel enzyme raises the question of how the activation of alkanes and alkenes commences.
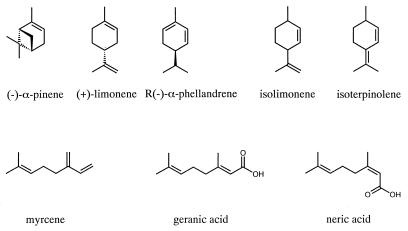
Monoterpenes are ubiquitous alkenes in nature (Fig. 1). The physiological trait of anaerobic mineralization of monoterpenes to carbon dioxide has been demonstrated by the enrichment and isolation of denitrifying strains of Alcaligenes defragrans and Thauera terpenica (11, 12, 16). During growth of bicyclic monoterpenes, e.g., α-pinene, traces of monocyclic monoterpenes are detected, suggesting menthadienes as intermediates (19). A. defragrans cometabolically catalyzes the transformation of isolimonene to isoterpinolene. This 3,1-hydrogen-Δ1-Δ3-mutase reaction confirms the microbial activation of alkene bonds that are not polarized by adjacent functional groups. It also indicates the necessity for a sp2 hybridization of the C-1 atom in order for microbial monoterpene oxidation to occur (19).
FIG. 1.
Monoterpene structures.
It seems likely that the unsaturated monoterpenes may be transformed initially into an organic acid, because intermediates in catabolic pathways are usually organic acids, e.g., in the citrate cycle. If this acid cannot diffuse through membranes, the carbon source is fixed in the cell. To explore this hypothesis, we characterized the organic acids in cells and culture medium and studied the formation of geranic acid (3,7-dimethyl-2,6-trans,trans-octadienoic acid) in cells of A. defragrans 65Phen grown on different monoterpenes. Experiments with cell suspensions and cell-free extracts demonstrated a transformation of myrcene to geranic acid by cytosolic fractions.
MATERIALS AND METHODS
Materials.
A. defragrans strains 51Men, 54PinT, 62Car, and 65Phen were maintained in our laboratory under selective conditions (11). Monoterpenes of highest purity (≥99%) were obtained from Fluka (Deisenhofen, Germany); limonene (98% purity) and a monoterpene mixture (technical-grade phellandrene, 50% purity) were used for 10-liter fermentations. Geranic acid (85% purity) was obtained from Aldrich (Steinheim, Germany) and contained two isomers, E,E-3,7-dimethyl-2,6-octadienoic acid (geranic acid) and Z,E-3,7-dimethyl-2,6-octadienoic acid (neric acid). Monoterpenes have a low solubility of 50 to 200 μM in water (9); presented concentrations are solely calculated values that relate to the aqueous phase in the experiment.
Culture conditions and cell harvest.
Microorganisms were cultured without oxygen as described (11, 29). The Hungate technique was applied in all experiments to obtain anoxic conditions (23). Cultivation on acetate (60 mM) and nitrate (40 mM) occurred in 5-liter bottles. Large-scale cultures on monoterpenes were established in a 10-liter fermentor (Biostat A; Braun Biotech, Melsungen, Germany) equipped with pH and temperature controls maintaining pH 7.0 (with sulfuric acid) and 30°C, respectively. The freshwater medium (10 liters) contained 100 mM nitrate and was inoculated with 1 liter of a culture recently grown on a monoterpene. A four-blade impeller was run at 150 rpm to optimize mass transfer from the monoterpene phase (10 or 22 mM monoterpene, representing a monoterpene or nitrate limitation, respectively) to the aqueous phase. Cell harvest started with the addition of an additional reductant establishing a final concentration of 50 μM FeIICl2 and 2 mM dithiothreitol. Then cells were transferred by gas pressure to centrifuge tubes. After centrifugation (11,300 × g for 20 min at 4°C), the pellet weights were determined, and the cells were suspended in equal weights of anoxic, nitrate-free medium, were transferred to serum flasks, were frozen in liquid nitrogen, and were stored at −80°C. Monoterpenes were detected by smell in cells of nitrate-limited cultures, but not in cells of monoterpene-limited cultures.
Anaerobic cell suspension experiments.
Aliquots (20 ml) of frozen cell suspensions were rapidly thawed and diluted in 80 ml of anoxic, nitrate-free medium to an optical density at 660 nm of 20 to 30. The suspension was stirred for 20 min at room temperature with a magnetic stir bar and then dispensed into 15-ml vials or 30-ml serum bottles. The experiments were started by the addition of carbon sources and/or nitrate from anoxic stock solutions. The nitrate stock solution contained 5 M sodium nitrate. Manageable stock solutions of monoterpenes (100 mM) were obtained by dilution into 2,2,4,4,6,8,8-heptamethylnonane. Incubation took place at 28°C in the dark with efficient phase mixing using an internal magnetic bar. Subsamples for nitrate and geranic acid analyses were withdrawn anaerobically with nitrogen-flushed syringes. Reactions were stopped by the addition of 0.4 ml of 100 mM sodium hydroxide per ml of sample. For time-dependent analysis of monoterpene turnover, experiments were started in replicates and finished separately after defined variable incubation times by extraction with 0.4 ml of hexane per ml of suspension.
Preparation of anaerobic cell-free extracts and in vitro experiments.
Cells were suspended in 1 volume of anoxic buffer, 100 mM HEPES, pH 7.0, inside an anaerobic chamber and were passed three times through a French pressure cell at 7.6 MPa. Membrane and cytosolic fractions were obtained by centrifugation at 150,000 × g for 45 min. Assays were prepared anaerobically with 1 ml of extract in 2-ml vials by using monoterpene and nitrate stock solutions as described above for cell suspension experiments. The same incubation conditions and termination reactions were applied as in cell suspension experiments.
Metabolite preparation.
For the preparation of free fatty acids, 20 g of wet cells were disintegrated with a French pressure cell and then dialyzed against 0.75 liters of distilled water for 24 h at 4°C. The water containing the metabolites as well as the culture broth of the fermentation (10 liters) were acidified to pH 2.0 with sulfuric acid (60% wt/vol) and were extracted three times with 0.1 liters of diethyl ether per liter of sample. The combined ether phases were clarified by centrifugation (3,800 × g for 10 min at 4°C) and were extracted three times with 300 ml of 50 mM sodium hydroxide per liter of ether phase. The aqueous phases were neutralized with 2 N hydrochloric acid and were concentrated by freeze-drying. For gas chromatography (GC) and GC-mass spectrometry (GC-MS) analysis, 10 to 20 mg of sample was derivatized with 2 ml of boron trifluoride in methanol (10% wt/vol) at 60°C for 1 h. The methyl esters were extracted with 1.25 ml of hexane. Alternatively, 10 mg of sample was resuspended in 2.5 ml of tertiar-butyl-methyl ether and was dissolved by the addition of 100 μl of 2 N hydrochloric acid. A mixture of 100 μl of the ether phase and 50 μl of 0.2 M trimethylsulfonium hydroxide in methanol was sampled for GC injection at 250°C. The high injection temperature is required to pyrolyze the reagent to methanol and dimethylsulfide (2).
Monoterpenes were recovered by the addition of 0.4 ml of hexane per ml of cell suspension, intensive mixing for 10 min, and phase separation by centrifugation (3,800 × g for 10 min at 4°C). Camphene in hexane was added as an internal standard prior to GC analysis.
For high-pressure liquid chromatography (HPLC) analysis of acids, 1-ml samples were stopped with 0.4 ml of 100 mM sodium hydroxide solution and were incubated at 80°C for 20 min. After centrifugation (11,300 × g for 20 min), the supernatants were acidified with 150 μl of phosphoric acid (1.5 M) and were centrifuged again. Supernatants obtained were filtered (pore size, 0.45 μm) prior to HPLC analysis.
Quantification of fatty acids was calibrated with freshly prepared standard solutions. Calculation of cellular concentrations of fatty acids was based on the amount of acids found and the wet weight of the cell pellet assuming a cell density of 1 g liter−1.
Chemical analyses.
Whole-cell protein was determined after lysis by the method of Bradford (17). Nitrite and nitrate analysis by HPLC and monoterpene analysis by GC were performed as described (10, 16). Fatty acid methyl esters were analyzed on a capillary column coated with 5% diphenyl–95% dimethyl-polysiloxan (0.32 mm by 50 m; film thickness, 0.5 μm) (SE-54; Machery-Nagel, Düren, Germany) with hydrogen as carrier gas at 40 cm s−1 with the following temperature program: injection port temperature, 250°C; column temperature, 60°C for 2 min, increasing to 200°C at a rate of 4°C min−1, 200°C for 0.1 min, increasing to 220°C at a rate of 10°C min−1, 220°C for 5 min; detection temperature, 280°C. For GC-MS, methyl esters were separated on a DB-5 column (3.32 mm by 30 m; film thickness, 0.25 μm) (J&W Scientific, Folsom, Calif.) by using helium as carrier gas and a temperature gradient (60 to 250°C at a rate of 4°C min−1), and mass spectra were obtained by using a Finnigan MAT 8200 system (Finnigan, Bremen, Germany) in the EI mode (70 eV) with a scan speed of 1 s decade−1 and an ion source temperature of 200°C. Organic acids were separated by reversed-phase HPLC on a Spherisorb ODS2 column (5 by 250 mm) (ODS2 made by PhaseSep, Deeside, United Kingdom; column obtained from Grom, Herrenberg, Germany) with 1 ml min−1 0.75 mM phosphoric acid in water-acetonitrile (45:55 [vol:vol]) as eluent at 25°C. The separation of the isocratic system was optimized by variation of the acetonitrile content. A band capacity factor of 5 was achieved, thus geranic acid was retained on the column at five times the dead time of the HPLC system. UV detection was performed as a scan and at 215 and 235 nm. The detection limit was 1 μM geranic acid in the injected 50-μl sample.
RESULTS
Monoterpene and nitrate consumption rates in vivo.
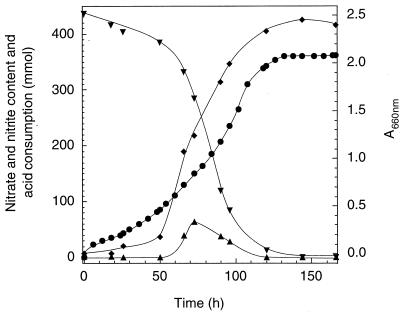
We determined the detrimental effect of high concentrations of nitrate and of monoterpenes on A. defragrans to optimize single-fed batch fermentations. Accumulation of nitrite (of up to 16 mM) and other metabolites of the denitrification process did not hamper growth on 100 mM nitrate. Balanced growth on this amount of nitrate consumes 15 mM monoterpene (12). A. defragrans 65Phen tolerated a pure α-phellandrene phase of 3.5‰ vol/vol, corresponding to 22 mM. The hardy traits of A. defragrans allowed growth in a pH-controlled fermentor as single-fed batch culture on 22 mM monoterpene and 100 mM nitrate in the absence of an organic carrier phase (Fig. 2). Alcalinization of the fermentation medium is attributed to the catabolic reaction: one proton is consumed during the reduction of one nitrate molecule. Maximum denitrification rates were 218 nmol of nitrate (mg of protein)−1 min−1. This corresponds to a dissimilatory monoterpene oxidation rate of 19.5 nmol of monoterpene (mg of protein)−1 min−1 and according to earlier quantitative determination (12) to a total monoterpene consumption rate of 31 nmol of monoterpene (mg of protein)−1 min−1.
FIG. 2.
Graph of growth of A. defragrans 65Phen on 22 mM α-phellandrene and 100 mM nitrate in a pH-controlled 4.5-liter single-fed batch fermentation. Nitrate (▾) content decreased with a small transient nitrite (▴) accumulation. Microbial growth (⧫) correlated with the consumption of acid (●) due to metabolic activity.
Free fatty acids in cells and culture fluid.
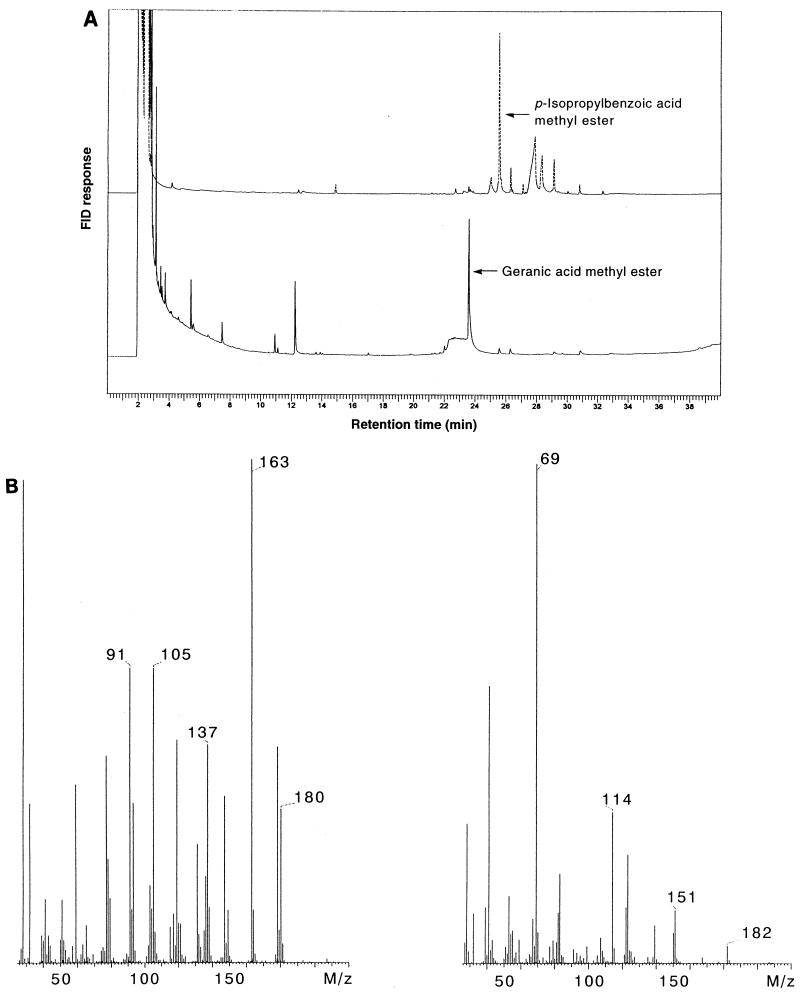
Cells and the medium of a nitrate-limited single-fed batch culture of A. defragrans 65Phen on 22 mM α-phellandrene and 100 mM nitrate were selected for the analyses of metabolites. Water-soluble cellular compounds with masses of less than 10 kDa, the exclusion size of the dialysis tubing, and culture medium were surveyed via acidification, ether extraction, alkaline extraction, and derivatization for GC and GC-MS analyses for the presence of acidic oxidation products of the anaerobic monoterpene mineralization pathway. Initially, we used boron trifluoride as the derivatization reagent. However, GC-MS analyses showed methanol adducts, e.g., 3,7-dimethyl-3-methoxy-6-octenoic acid methyl ester. We found in control experiments that boron trifluoride in methanol was not an appropriate derivatization reagent for monoterpenes; geraniol yielded over 40 distinct compounds according to GC analysis (data not shown). In contrast to the catalysis by the Lewis acid boron trifluoride, the derivatization with trimethylsulfonium hydroxide is considered to proceed via methyl transfer and to avoid the formation of cationic intermediates that may isomerize to other monoterpenes or add methanol. GC analyses of methylated acids prepared with trimethylsulfonium hydroxide exhibited a lower number of substances present in the extracts (Fig. 3). Identification of these metabolites was initially based on GC-MS analyses and was confirmed by retention time analyses and coinjection with an authentic standard (data not shown). Cumic acid (p-isopropyl-benzoic acid) was present in cells as well as in culture medium at concentrations of 25 and 20 μM, respectively. Geranic acid had accumulated to a concentration of 470 μM in cells, but the culture liquid contained only 2 μM geranic acid.
FIG. 3.
(A) Results of GC analyses of fatty acids present in the culture medium (top trace) and in cells (bottom trace) of a 10-liter fermentation of A. defragrans 65Phen. Cells were grown on 22 mM α-phellandrene (technical grade) and 100 mM nitrate. (B) Mass spectra of p-isopropyl-benzoate methyl ester (left) and of geranic acid methyl ester (right) obtained by GC-MS analyses.
Retention time analyses as well as the mass spectrum indicated the presence of the trans,trans configuration (E,E); the trans,cis-isomer neric acid was not found. We developed an isocratic HPLC method with a band capacity factor of 5 for geranic acid to utilize the sensitive UV detectibility of the α,β-unsaturated fatty acid and confirmed the identification of the major metabolite as geranic acid (data not shown). Analyses of the monoterpenes utilized as growth substrate for the fermentations revealed the absence of geranic acid, as judged by GC and by HPLC. Cells from several fermentations on α-phellandrene or limonene and nitrate were analyzed by the HPLC method; geranic acid was found in the biomass of nitrate-limited, but not in that of monoterpene-limited, cultures. In addition, anaerobic cells grown on acetate with a limiting amount of nitrate did not contain geranic acid.
Monoterpene, geranic acid, and nitrate metabolism in anaerobic cell suspensions.
Actively metabolizing cell suspensions of A. defragrans 65Phen were established to study the geranic acid formation. Cells were grown with a limited amount of limonene (10 mM) and 100 mM nitrate to avoid denitrification with monoterpenes and with intracellular storage compounds (polyhydroxyalkanoates) as carbon sources in these cell suspension experiments. The biomass was harvested in the late exponential growth phase and was suspended under strictly anoxic conditions. Cell suspensions reduced nitrate with a specific rate of 1.83 nmol of nitrate (mg of protein)−1 min−1. Consumption of α-phellandrene proceeded with a specific rate of 123 pmol (mg of protein)−1 min−1. Heat-inactivation (20 min at 95°C) stopped all catabolic activities of the cells. In further experiments, the degradation of limonene or α-pinene and the cometabolic transformation of isolimonene to isoterpinolene during limonene utilization confirmed that the established cell suspensions were metabolically active.
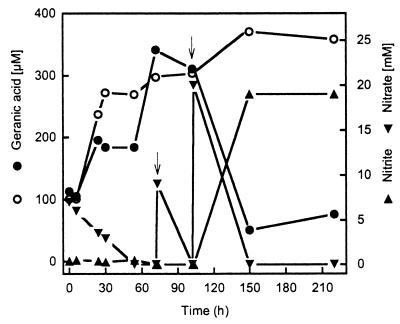
The biomass of nitrate-limited fermentations contained geranic acid and a residual amount of growth-supporting monoterpene probably associated with lipid phases. The fate of geranic acid during a cell suspension experiment was studied in cells of that kind grown on α-phellandrene (Fig. 4). In the absence of nitrate, the concentration of geranic acid increased from 101 to 370 μM in the assay. In denitrifying cell suspensions, the initial increase of geranic acid stopped at 195 μM. Nitrate depletion correlated with a further increase to 341 μM geranic acid. This formation of geranic acid may originate from the microbial metabolism of residual α-phellandrene. The denitrifying cell suspensions started to consume geranic acid after the reduction of over 16 mM nitrate. Three-quarters of the geranic acid concentration disappeared. At this stage, nitrate reduction within the cell suspension was limited to nitrite formation, likely a sign of electron donor limitation.
FIG. 4.
Metabolism of geranic acid in the presence (●) and in the absence (○) of nitrate by dense cell suspensions of A. defragrans 65Phen. Cells were grown on 22 mM α-phellandrene (technical grade) and 100 mM nitrate. Arrows indicate the addition of nitrate (9 and 20 mM) after nitrate consumption.
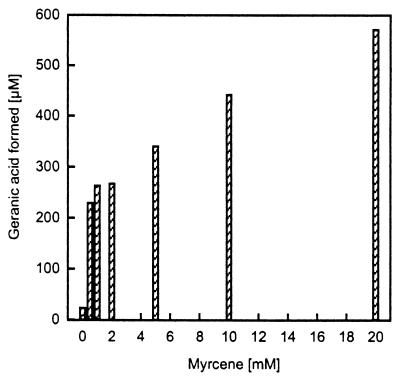
The amounts of geranic acid formed in the different experiments were small with respect to the monoterpene supplied. Hence, we tested various monoterpenes as precursors for geranic acid in nitrate-limited cell suspensions with 5 mM nitrate and 4 mM monoterpene. The denitrifying cells produced, within 2 days, 50, 54, and 68 μM geranic acid from α-pinene, limonene, and α-phellandrene, respectively. The acyclic β-myrcene supported the synthesis of 508 μM geranic acid, presenting a transformation rate of approximately 13% of the myrcene supplied. This result from HPLC analysis was confirmed by GC analysis. Cell suspension experiments with different amounts of myrcene showed an increased formation of geranic acid in correlation with an increased supply of myrcene (Fig. 5). Myrcene was not present, according to GC analysis, in the monoterpenes α-pinene, limonene, and α-phellandrene that did support geranic acid formation in the cell suspension experiments. But GC analyses of the 98%-pure limonene and the phellandrene (technical grade)—both were utilized in large fermentations—showed the presence of myrcene in an amount sufficient to theoretically balance the amount of geranic acid formed.
FIG. 5.
Geranic acid formation by cell suspensions of A. defragrans 65Phen in relation to the amount of myrcene provided in the experiment. Cells were grown on 15 mM α-phellandrene (technical grade) and 100 mM nitrate.
Geranic acid formation in cell-free extracts.
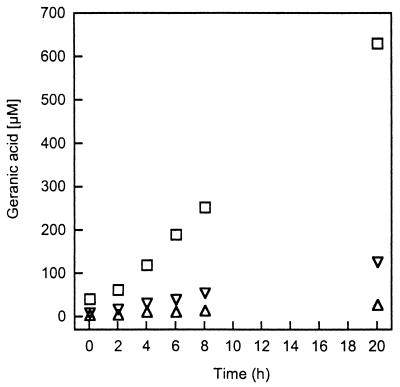
Cells of strain 65Phen were fractionated to locate the site of geranic acid formation. The catalytic activity was preserved after cell disintegration by passage through a French pressure cell under anoxic conditions. Over 90% of the activity was recovered in the soluble fraction after ultracentrifugation, which indicates a cytosolic location. After a small lag phase, the reaction proceeded linearly to a total turnover of 0.6 mM (6%) myrcene within 1 day of incubation (Fig. 6). During this time, the geranic acid synthesis rate was 52 pmol (mg of protein)−1 min−1 at a protein concentration of 10 mg ml−1. The reaction required myrcene but did not require nitrate. Incubation of the extract for 10 min at 95°C completely inactivated the capacity of the extract to form geranic acid (Table 1).
FIG. 6.
Time-dependence of geranic acid formation from myrcene by cell-free cytosolic extracts. Each value was obtained by analysis of an entire assay. Extracts were obtained from cells grown on 15 mM α-phellandrene (technical grade) and 100 mM nitrate. ▵, ▿, and □ represent extracts containing 1.15, 1.85, and 10.1 mg of protein ml−1, respectively.
TABLE 1.
Geranic acid formation by cell-free cytosolic extractsa
| Extract type | Protein content (mg ml−1) | Myrcene provided (mM) | Geranic acid formed (mM) |
|---|---|---|---|
| Complete | 10.1 | 10 | 0.590 |
| No myrcene | 10.1 | 0 | 0.000 |
| With heat treatment (95°C for 10 min) | 10.1 | 10 | 0.000 |
| With nitrate (20 mM) | 10.1 | 10 | 0.581 |
| Diluted (1:5) | 1.85 | 10 | 0.127 |
| Diluted (1:10) | 1.15 | 10 | 0.029 |
Assays were incubated for 20 h.
Growth experiments with A. defragrans.
Myrcene supported denitrifying growth of A. defragrans strains 51Men, 62Car, and 65Phen, but not growth of the type strain 54PinT (12). In an extended survey, ocimene was identified as the first acyclic monoterpene that supports growth of all four strains. The degradation of myrcene and of α-phellandrene (positive control) was studied with A. defragrans 65Phen in monoterpene-limited experiments containing 1 mM monoterpene and 20 mM nitrate, to verify the complete mineralization of myrcene. After 15 days of incubation, the cultures were in the late stationary growth phase. The consumption of 0.90 mM myrcene and 0.97 mM α-phellandrene proceeded with the denitrification of 7.6 and 8.1 mM nitrate and a biomass formation of 18.4 and 21.6 mg of protein liter−1, respectively. Geranic acid was not detectable in these cultures.
Other substrates tested as sole organic carbon sources included monoterpenoic acids and methylcyclohexenes. Cumic acid (0.5 mM) and the available isomer mixture of geranic and neric acid (0.5 mM) did not support denitrification and growth of all A. defragrans strains. Molecules lacking the isopropyl group, 1-methyl-cyclohexene and 1-methyl-cyclohexa-1,4-diene, were not utilized, in contrast to the corresponding monoterpenes, menth-1-ene and γ-terpinene.
DISCUSSION
The mineralization of hydrocarbons by aerobic microorganisms requires molecular oxygen (for reviews of monoterpenes, see references 20, 27, and 28). In the absence of this cosubstrate, anaerobic bacteria have to use a different biochemistry, which still contains many unknown features (13). Being interested in alkenes, we chose natural monoterpenes as substrates for the isolation of anaerobic, nitrate-respiring bacteria (16). In this study, we describe the first identification of an ionic product obtained from the initial anaerobic hydrocarbon activation reaction. Geranic acid (E,E-3,7-dimethyl-2,6-octadienoic acid) was found as the major metabolite in nitrate-limited cells that were grown anaerobically on monoterpenes. The identification of geranic acid involved GC and HPLC analyses with mass spectra, flame ionization, and UV scan detection.
In experiments with cell suspensions, we showed that cyclic monoterpenes (α-pinene, limonene, and α-phellandrene) and myrcene served as metabolic precursors of geranic acid. Transformation of myrcene occurred at the highest rate. This may be attributed to the fact that the other compounds have to undergo a ring opening reaction in order to become acyclic. The transformation of myrcene to geranic acid was also observed with cytosolic extracts as catalyst. The heat sensitivity and the correlation between transformation rate and protein content of the assay suggest an enzymatic reaction. The rates obtained in vitro are currently lower than the in vivo rates. However, similar observations were reported for the degradation of toluene by denitrifying Thauera aromatica (21).
Geranic acid has not thus far been observed as a product of myrcene metabolism in microorganisms. Degradation of myrcene by aerobic bacteria is initiated by an oxidation of one of the terminal methyl groups yielding myrcene-8-ol (E-2-methyl-6-methylen-2,7-octadien-1-ol) (20, 24). Fungi cometabolically form several diols, e.g., 2-methyl-6-methylen-7-octene-2,3-diol, 6-methyl-2-ethenyl-5-heptene-1,2-diol, and 7-methyl-3-methylen-6-octene-1,2-diol (30). Further oxidation of these compounds does not involve the formation of geranic acid. Hence, A. defragrans seems to contain an uncharacterized pathway for myrcene oxidation.
Potential intermediates between myrcene and geranic acid are the hydration products geraniol and linalool. Neither compound supports the growth of A. defragrans (12), and we did not detect either compound during our survey of neutral metabolites of the monoterpene metabolism (19). Geranic acid itself was consumed slowly in cell suspensions. This raises the likelihood that the formation of geranic acid may be an evasion reaction in response to the deficiency of electron acceptor. Still, our development of the first in vitro assay for anaerobic alkene transformation has provided the means to study a novel enzyme reaction. The formation of geranic acid from α-pinene, limonene, and α-phellandrene requires a ring opening reaction. Thermal rearrangement of α- and β-pinenes is industrially used to obtain ocimene and myrcene, respectively. The mechanism involves biradicals and comprises a decyclization reaction (8). A transformation of cyclic monoterpenes into acyclic monoterpenes via ionic intermediates has, to our knowledge, never been reported (5, 8). The opposite is well known: all natural cyclic monoterpenes are synthesized via cationic intermediates (4, 14). Hence, radical enzymology may be involved in the oxidation of bicyclic and monocyclic monoterpenes and of myrcene to geranic acid.
ACKNOWLEDGMENTS
We thank Peter Schulze, Universität Bremen, for GC-MS analyses.
This study was supported by the Max-Planck-Society and the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Aeckersberg F, Bak F, Widdel F. Anaerobic oxidation of saturated hydrocarbons to CO2 by a new type of sulfate-reducing bacterium. Arch Microbiol. 1991;156:4–14. [Google Scholar]
- 2.Butte W. Rapid method for the determination of fatty-acid profiles from fats and oils using trimethylsulfonium hydroxide for transesterification. J Chromatogr. 1983;261:142–145. [Google Scholar]
- 3.Coschigano P W, Wehrman T S, Young L Y. Identification and analysis of genes involved in anaerobic toluene metabolism by strain T1: putative role of a glycine free radical. Appl Environ Microbiol. 1998;64:1650–1656. doi: 10.1128/aem.64.5.1650-1656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croteau R. Biosynthesis and catabolism of monoterpenoids. Chem Rev. 1987;87:929–954. [Google Scholar]
- 5.Cruz Costa M C, Johnstone R A W, Whittaker J D. Catalysis of gas and liquid phase ionic and radical rearrangements of α- and β-pinene by metal(IV)phosphate polymers. J Mol Catal A Chem. 1996;104:251–259. [Google Scholar]
- 6.Dolfing J, Zeyer J, Binder-Eicher P, Schwarzbach R P. Isolation and characterization of a bacterium that mineralizes toluene in the absence of molecular oxygen. Arch Microbiol. 1990;154:336–341. doi: 10.1007/BF00276528. [DOI] [PubMed] [Google Scholar]
- 7.Ehrenreich P, Behrends A, Harder J, Widdel F. Anaerobic oxidation of alkanes by newly isolated denitrifying bacteria. Arch Microbiol. 2000;173:58–64. doi: 10.1007/s002030050008. [DOI] [PubMed] [Google Scholar]
- 8.Erman W F. Chemistry of the monoterpenes: an encyclopedic handbook, part A. New York, N.Y: Marcel Dekker, Inc.; 1985. [Google Scholar]
- 9.Fichan I, Larroche C, Gros J B. Water solubility, vapor pressure, and activity coefficients of terpenes and terpenoids. J Chem Eng Data. 1999;44:56–62. [Google Scholar]
- 10.Foß S, Harder J. Microbial transformation of a tertiary allylalchol: regioselective isomerisation of linalool to geraniol without nerol formation. FEMS Microbiol Lett. 1997;149:71–75. [Google Scholar]
- 11.Foß S, Harder J. Thauera linaloolentis sp. nov. and Thauera terpenica sp. nov., isolated on oxygen-containing monoterpenes (linalool, menthol and eucalyptol) and nitrate. Syst Appl Microbiol. 1998;21:365–373. doi: 10.1016/s0723-2020(98)80046-5. [DOI] [PubMed] [Google Scholar]
- 12.Foß S, Heyen U, Harder J. Alcaligenes defragrans sp. nov., description of four strains isolated on alkenoic monoterpenes ((+)-menthene, α-pinene, 2-carene and α-phellandrene) and nitrate. Syst Appl Microbiol. 1998;21:237–244. doi: 10.1016/s0723-2020(98)80028-3. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs G. Novel reactions and catalytic mechanisms in anaerobic microorganisms. FEMS Microbiol Rev. 1999;22:339–340. [Google Scholar]
- 14.Gibbs R A. Prenyl transfer and the enzymes of terpenoid and steroid biosynthesis. In: Sinnott M, editor. Comprehensive biological catalysis: a mechanistic reference. Vol. 1. London, United Kingdom: Academic Press; 1998. pp. 31–118. [Google Scholar]
- 15.Gilewicz M, Monpert G, Acquaviva M, Mille G, Bertrand J C. Anaerobic oxidation of 1-n-heptadecene by a marine denitrifying bacterium. Appl Microbiol Biotechnol. 1991;36:252–256. [Google Scholar]
- 16.Harder J, Probian C. Microbial degradation of monoterpenes in the absence of molecular oxygen. Appl Environ Microbiol. 1995;61:3804–3808. doi: 10.1128/aem.61.11.3804-3808.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harder J, Probian C. Anaerobic mineralisation of cholesterol by a novel type of dentrifying bacterium. Arch Microbiol. 1997;167:269–274. doi: 10.1007/s002030050442. [DOI] [PubMed] [Google Scholar]
- 18.Heider J, Spormann A M, Beller H R, Widdel F. Anaerobic bacterial metabolism of hydrocarbons. FEMS Microbiol Rev. 1999;22:459–473. [Google Scholar]
- 19.Heyen U, Harder J. Cometabolic isoterpinolene formation from isolimonene by denitrifying Alcaligenes defragrans. FEMS Microbiol Lett. 1998;169:67–71. [Google Scholar]
- 20.Iurescia S, Marconi A M, Tofani D, Gambacorta A, Paternò A, Devirgiliis C, van der Werf M J. Identification and sequencing of β-myrcene catabolism genes from Pseudomonas sp. strain M1. Appl Environ Microbiol. 1999;65:2871–2876. doi: 10.1128/aem.65.7.2871-2876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leuthner B, Leutwein C, Schulz H, Hörth P, Haehnel W, Schlitz E, Schägger H, Heider J. Biochemical and genetic characterization of benzylsuccinate synthase from Thauera aromatica: a new glycyl radical enzyme catalysing the first step in anaerobic toluene metabolism. Mol Microbiol. 1998;28:615–628. doi: 10.1046/j.1365-2958.1998.00826.x. [DOI] [PubMed] [Google Scholar]
- 22.Lovley D R, Baedecker M J, Lonergan D J, Cozzarelli I M, Phillips E J P, Siegel D I. Oxidation of aromatic contaminants coupled to microbial iron reduction. Nature. 1989;339:297–300. [Google Scholar]
- 23.Macy J M, Snellen J E, Hungate R E. Use of syringe methods for anaerobiosis. Am J Clin Nutr. 1972;25:1318–1323. doi: 10.1093/ajcn/25.12.1318. [DOI] [PubMed] [Google Scholar]
- 24.Narushima H, Omori T, Minoda Y. Microbial oxidation of beta-myrcene. In: Verina C, Singh K, editors. Advances in biotechnology. Vol. 3. Oxford, England: Pergamon Press; 1982. pp. 525–531. [Google Scholar]
- 25.Rueter P, Rabus R, Wilkes H, Aeckersberg F, Rainey F A, Jannasch H W, Widdel F. Anaerobic oxidation of hydrocarbons in crude oil by new types of sulphate-reducing bacteria. Nature. 1994;372:455–458. doi: 10.1038/372455a0. [DOI] [PubMed] [Google Scholar]
- 26.So C M, Young L Y. Isolation and characterization of a sulfate-reducing bacterium that anaerobically degrades alkanes. Appl Environ Microbiol. 1999;65:2969–2976. doi: 10.1128/aem.65.7.2969-2976.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trudgill P W. Microbial metabolism and transformation of selected monoterpenes. In: Ratledge C, editor. Biochemistry of microbial degradation. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 33–61. [Google Scholar]
- 28.van der Werf M J, Swarts H J, de Bont J A M. Rhodococcus erythropolis DCL14 contains a novel degradation pathway for limonene. Appl Environ Microbiol. 1999;65:2092–2102. doi: 10.1128/aem.65.5.2092-2102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Widdel F, Bak F. Gram-negative mesophilic sulfate-reducing bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. 2nd ed. Berlin, Germany: Springer; 1992. pp. 3352–3378. [Google Scholar]
- 30.Yamazaki Y, Hayashi Y, Hori N, Mikami Y. Microbial conversion of β-myrcene. Agric Biol Chem. 1988;52:2921–2922. [Google Scholar]