Abstract
Ichthyoses are hereditary skin disorders characterized by the formation of scales and defects in the outermost layer of the epidermis. In dogs, at least six different breed-specific ichthyoses including a relatively common PNPLA1-related autosomal recessive ichthyosis in Golden Retrievers are known. In this study, we investigated 14 Golden Retrievers with scales that were not homozygous for the mutant PNPLA1 allele suggesting a genetically distinct new form of ichthyosis. Histopathological examinations showed lamellar, orthokeratotic hyperkeratosis, and mildly hyperplastic epidermis that led to the diagnosis of a nonepidermolytic ichthyosis. Combined linkage and homozygosity mapping in 14 cases and 30 nonaffected family members delimited a critical interval of ∼12.7 Mb on chromosome 23. Whole-genome sequencing of an affected dog revealed a single protein-changing variant within this region that was not present in 795 control genomes. The identified variant is a 14 bp deletion in the ABHD5 gene (c.1006_1019del), leading to a frameshift and altering the last 14 codons p.(Asp336Serfs*6). The genotypes at this variant showed perfect cosegregation with the ichthyosis phenotype in a large family comprising 14 cases and 72 controls. ABHD5 encodes an acyltransferase required for lipid metabolism. In humans, variants in ABHD5 cause Chanarin-Dorfman syndrome, a neutral lipid storage disease with ichthyosis. Our data in dogs together with the knowledge on the effects of ABHD5 variants in humans strongly suggest ABHD5:c.1006_1019del as candidate causative genetic variant for a new canine form of ichthyosis, which we propose to designate as Golden Retriever ichthyosis type 2 (ICH2).
Keywords: Canis lupus familiaris, dog, dermatology, genodermatosis, metabolism, lipid storage disorder, animal model, precision medicine, veterinary medicine
Introduction
Ichthyoses comprise a heterogeneous group of cornification disorders characterized by generalized dry and scaly skin. Various genetic defects have been described, all disrupting the skin barrier and leading to hyperkeratosis and scaling of the skin (Oji et al. 2010; Schmuth et al. 2013). The classification of ichthyoses distinguishes between syndromic and nonsyndromic forms. Nonsyndromic ichthyoses refer to those with the phenotypic manifestation of the disease limited to the skin whereas syndromic ichthyoses additionally involve other organs. Ichthyoses can be further subdivided into epidermolytic and nonepidermolytic ichthyoses. This differentiation is light microscopy based. Epidermolytic ichthyoses are accompanied by epidermolytic hyperkeratosis at the ultrastructural level. Typical findings are intracellular vacuolization and formation of small intraepidermal blisters (Oji et al. 2010).
In humans, at least 67 different genes have been described to be related with different forms of ichthyosis (Uitto et al. 2020). In dogs, several breed-specific ichthyoses have been described. However, in only six of the canine ichthyoses, the underlying genetic defect has been identified (Mauldin 2013; Leeb et al. 2017). A heterozygous missense variant in ASPRV1 caused an autosomal dominant form of nonepidermolytic ichthyosis in a German Shepherd (Bauer et al. 2017; OMIA 002099-9615). A mild epidermolytic ichthyosis in Norfolk terriers is caused by a splice-site variant in KRT10 (Credille et al. 2005; OMIA 001415-9615). In American Bulldogs, a frameshift deletion in NIPAL4 was found to cause autosomal recessive congenital ichthyosis (Casal et al. 2017; OMIA 001980-9615). A splice site variant in SLC27A4 was described in Great Danes with a clinically severe, autosomal recessive syndromic ichthyosis (Metzger et al. 2015; OMIA 001973-9615). An autosomal recessive nonepidermolytic ichthyosis in Jack Russell Terriers is caused by a LINE-1 insertion into the TGM1 gene (Credille et al. 2009; OMIA 000546-9615). Probably the most common canine ichthyosis is an autosomal recessive ichthyosis in Golden Retrievers (OMIA 001588-9615). The first case report is from 2004 (Hall and Yager 2004) and the disease phenotype has been well characterized in the following years (Guaguère et al. 2007, 2009; Cadiergues et al. 2008; Mauldin et al. 2008). The underlying genetic defect is a homozygous insertion-deletion (indel) variant in PNPLA1 encoding patatin like phosphatase domain containing 1. The protein plays a key role in lipid organization and metabolism of the epidermal barrier and the defective protein in the affected Golden Retrievers causes malformation of the intercellular stratum corneum lipid layer and abnormal desquamation (Grall et al. 2012).
In this study, we investigated 14 Golden Retrievers with clinical and histopathological signs of nonepidermolytic ichthyosis. Despite the phenotypic similarity to the PNPLA1-related ichthyosis, none of these dogs carried the mutant PNPLA1 allele in a homozygous state. In the present study, we therefore aimed to characterize this presumably new form of inherited ichthyosis and to unravel the causative genetic variant. We propose to term this specific phenotype Golden Retriever ichthyosis type 2 (ICH2, OMIA 002368-9615).
Materials and methods
Ethics statement
The Golden Retrievers in this study were privately owned and skin biopsies and blood samples for diagnostic purposes were collected with the consent of their owners. The collection of blood samples was approved by the Cantonal Committee for Animal Experiments (Canton of Bern; permit BE71/19). All animal experiments were done in accordance with local laws and regulations.
Clinical and histopathological examinations
A physical examination was performed by the attending veterinarians. Two to four 4–6 mm skin punch biopsies per dog were taken and routinely processed for histopathology. Hematoxylin and eosin (H&E) stained slides were reviewed by board certified veterinary pathologists (DJW and EAM).
Animal selection for genetic analyses
This study included 482 Golden Retrievers. They comprised 86 closely related dogs including 14 ICH2 affected and 72 unaffected relatives originating from North America. The remaining 396 dogs were Golden Retrievers of European origin from the Vetsuisse Biobank.
DNA extraction
Genomic DNA was extracted from EDTA blood using the Maxwell® RSC Whole Blood DNA with the Maxwell® RSC instrument (Promega, Dübendorf, Switzerland).
Linkage analysis and homozygosity mapping
Genotype data for 44 Golden Retrievers comprising dogs from 7 litters and their parents were obtained with 220 k Illumina CanineHD BeadChips by Geneseek/Neogen (Supplementary File S1, 11 unaffected parents, 14 affected, and 19 unaffected offspring). For all dogs, the call rate was >95%. Using PLINK v1.9 (Purcell et al. 2007), markers on the sex chromosomes or with unknown positions were removed. We further removed markers that had missing genotypes in any of the 44 dogs, Mendel errors, or a minor allele frequency <0.01. The final pruned dataset contained 110,720 markers and was organized into four separate subfamilies comprising between 5 and 23 dogs. To analyze the data for parametric linkage, an autosomal recessive inheritance model with full penetrance, a disease allele frequency of 0.55 and the Merlin software (Abecasis et al. 2002) were applied.
For homozygosity mapping, the genotype data for the 14 ICH2 affected dogs were used. Markers with call rates <100% and markers on the sex chromosomes were excluded. The --homozyg-group option in PLINK was used on a final dataset of 198,921 markers to search for extended regions of homozygosity. The output intervals were matched against the intervals from linkage analysis in Excel spreadsheets to find overlapping regions (Supplementary Table S1). A tped-file containing the markers on chromosome 23 was visually inspected in an Excel spreadsheet to precisely delimit the homozygous shared haplotype in the cases (Supplementary Table S1). All positions correspond to the CanFam3.1 reference genome assembly.
Whole-genome sequencing
An Illumina TruSeq PCR-free library with an insert size of ∼330 bp was prepared from one ICH2 affected dog and sequenced at 26x coverage on an Illumina NovaSeq 6000 instrument. The reads were mapped to the dog CanFam3.1 reference genome assembly as previously described (Jagannathan et al. 2019). The sequence data were deposited under study accession PRJEB16012 and sample accession SAMEA8797074 at the European Nucleotide Archive.
Variant calling and variant filtering
Variant calling was performed using GATK HaplotypeCaller (McKenna et al. 2010) in gVCF mode as described (Jagannathan et al. 2019). To predict the functional effects of the called variants, the SnpEff software (Cingolani et al. 2012), together with NCBI annotation release 105 for the CanFam 3.1 genome reference assembly was used. For filtering of private variants, we used 795 control genomes (Supplementary Table S2). Numbering within the canine ABHD5 gene corresponds to the NCBI RefSeq accession numbers XM_542689.5 (mRNA) and XP_542689.2 (protein).
Targeted genotyping
We used Sanger sequencing to confirm the candidate variant ABHD5:c.1006_1019del. A 389 bp (or 375 bp in case of the mutant allele) PCR product was amplified from genomic DNA using AmpliTaqGold360Mastermix (Thermo Fisher Scientific, Waltham, MA, USA) and primers 5′-CTG CTG GCC CTG TCA TTA GT-3′ (Primer F) and 5′-CAG GCT CTC TCT CCC ACA TT-3′ (Primer R). After treatment with exonuclease I and alkaline phosphatase, we sequenced the amplicons in both directions on an ABI 3730 DNA Analyzer (Thermo Fisher Scientific Corporation, Waltham, MA, USA). Sanger sequences were analyzed with the Sequencher 5.1 software (Gene Codes Corporation, Ann Arbor, MI, USA). Subsequently, targeted genotyping of dogs was performed by fragment length analysis of PCR products on a 5200 Fragment Analyzer (Agilent, Basel, Switzerland).
The PNPLA1:c.1445_1447delinsTACTACTA variant causing another form of ichthyosis in Golden Retrievers (Grall et al. 2012) was genotyped by Sanger sequencing of PCR amplicons as described above. A 300 bp PCR product (or 305 bp in case of the mutant allele) was amplified with the primers 5’-GGC CCT GAT AGT GAA GGA CA-3’ (Primer F) and 5’-TCC TAA CAC CTG CTC CTG CT-3’ (Primer R). The reverse primer was used for sequencing.
Results
Clinical description
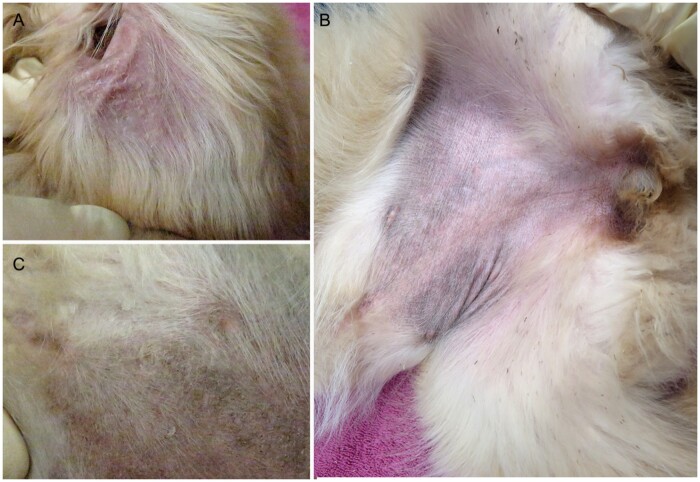
ICH2 affected dogs had large white to gray and powdery to adherent scale throughout the hair coat. The abdominal skin was mildly hyperpigmented. Thick white scale was adherent to the concave surface of the pinnae (Figure 1).
Figure 1.
Clinical images from an 11-week-old Golden Retriever with ICH2. (A) Adherent scales on the inner pinna. (B) Thick scales on the ventral thorax and (C) abdominal hyperpigmentation.
Histopathological examination
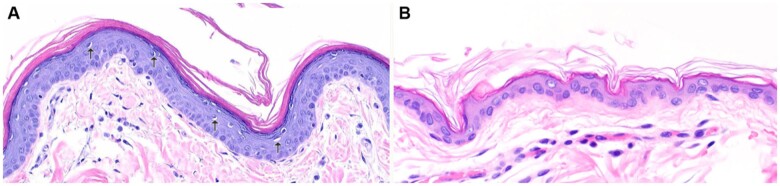
The skin biopsies of affected dogs showed moderate epidermal hyperplasia without dermal inflammation. The main change was expansion of the stratum corneum by laminar orthokeratotic hyperkeratosis. Numerous keratinocytes in the granular layer contained perinuclear clear spaces (Figure 2). The clinical signs together with the histopathological findings led to the diagnosis of a nonepidermolytic ichthyosis.
Figure 2.
Histopathology of a Golden Retriever with ICH2. (A) Skin biopsy from an affected dog reveals marked thickening of the epidermis with expansion of the stratum corneum by laminar orthokeratotic hyperkeratosis. Numerous keratinocytes have perinuclear clear spaces (arrows). (B) Normal skin from an unaffected Golden Retriever. H&E 200X.
Genetic analysis
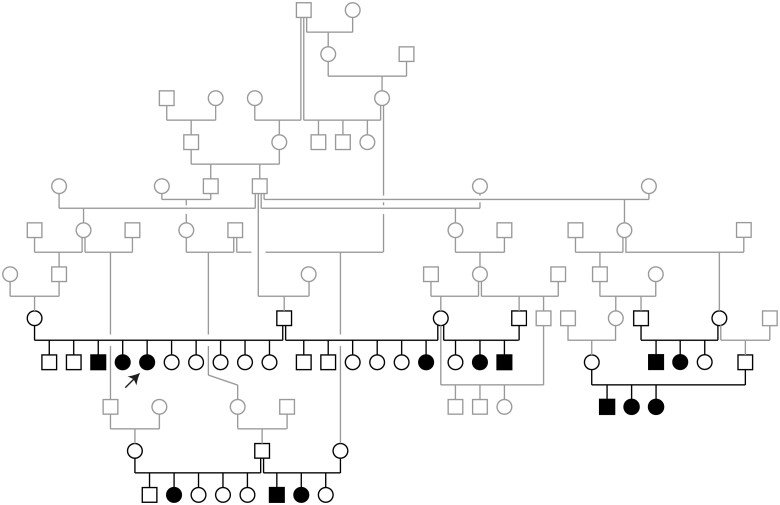
The 14 available cases belonged to seven different litters, each with unaffected parents. Both male and female dogs were affected. The pedigree showed multiple inbreeding loops and was strongly suggestive for a monogenic autosomal recessive inheritance (Figure 3).
Figure 3.
Pedigree of the investigated Golden Retriever family. Squares represent males and circles represent females. The affected dogs are indicated by filled symbols. Note the multiple inbreeding loops within this pedigree. All affected dogs have shared common ancestors in their maternal and paternal lineages. The 44 dogs that were genotyped on microarrays and used for the linkage analysis are indicated in black and constitute four separate subfamilies. The other dogs are shown in gray. The arrow indicates the dog that was used for whole-genome sequencing.
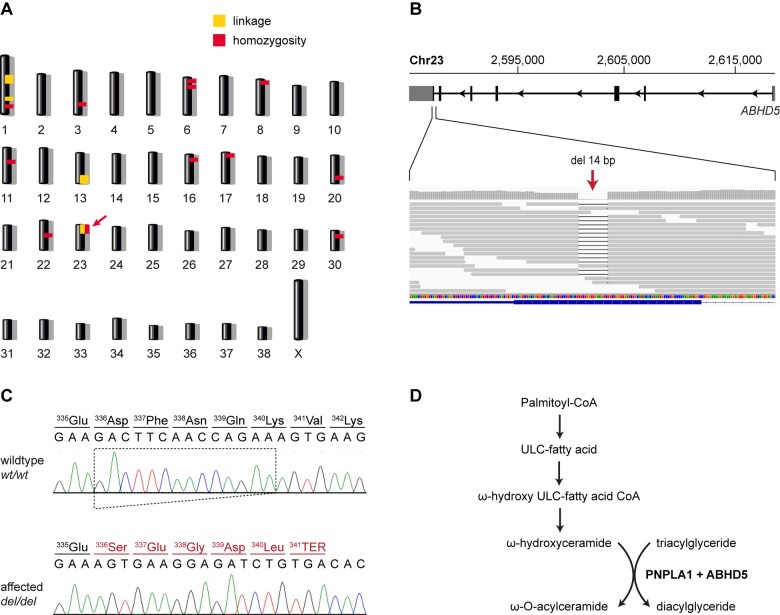
By combining linkage analysis and homozygosity mapping, we determined the critical interval for the disease-causing genetic variant. A single ∼12.7 Mb segment on chromosome 23 showed both linkage in the families and shared homozygous genotypes in all 14 available cases (Figure 4A). The LOD score of the linked interval on chromosome 23 was 2.75 (Table 1, Supplementary Figure S1). The exact coordinates of the critical interval were Chr23:1–12,734,912 (Supplementary Table S1).
Figure 4.
Mapping of the ICH2 locus and details of the ABHD5:c.1006_1019del variant. (A) Combined linkage and homozygosity mapping revealed a single overlapping region on chromosome 23 indicated by an arrow. (B) Integrative Genomics Viewer (IGV) screenshot showing the position of the deletion in the short-read alignments of the ICH2 affected dog. (C) Sanger sequencing confirmed the detected 14 bp frameshift deletion. The altered reading frame and the premature stop codon are indicated in red. (D) Metabolic pathway for the synthesis of ω-O-acylceramide, an essential lipid required to maintain skin barrier function (Haydar Eskiocak et al. 2019). In the last step of this pathway, ABHD5 acts as a coactivator of PNPLA1 and may help to provide the required triacylglycerides within the endoplasmic reticulum (Ohno et al. 2018). A lack of either ABHD5 or PNPLA1 leads to ichthyosis in humans (Lefevre et al. 2001; Grall et al. 2012).
Table 1.
Results of linkage analysis in 44 dogs from 7 litters and their parents
| Chrom | Start (Mb) | Stop (Mb) | Max LOD score | αa |
|---|---|---|---|---|
| 1 | 41.498 | 48.203 | 0.43 | 0.65 |
| 1 | 69.318 | 69.332 | 3.46 | 1 |
| 1 | 105.516 | 106.520 | 0.23 | 0.63 |
| 13 | 57.599 | 63.242 | 1.07 | 0.73 |
| 23 | 0.000 | 12.822 | 2.75 | 1 |
α indicates an estimated proportion of the four subfamilies that show linkage. The dogs were organized in four subfamilies (see Materials and Methods and Figure 3).
The genome of one affected dog was sequenced at 26x coverage. Assuming that the disease allele is rare in the dog population, we filtered for private homozygous variants that were absent from 795 control genomes (Supplementary Table S2). This analysis identified 32 private homozygous variants in the critical interval, of which only one was predicted to be protein changing (Table 2, Supplementary Table S3).
Table 2.
Results of variant filtering in the ICH2 affected Golden Retriever against 795 control genomes
| Filtering step | Variants |
|---|---|
| All variants in the affected Golden Retriever | 3,040,308 |
| Private variants | 1,110 |
| Private variants in critical interval | 32 |
| Private protein-changing variants in critical interval | 1 |
Only homozygous variants are reported.
The identified candidate variant was a 14 bp deletion in the last exon of ABHD5, XM_542689.5:c.1006_1019del (Figure 4, B and C). The formal genomic designation of the variant is Chr23:2,587,000–2,587,013del (CanFam3.1). It causes a frameshift, resulting in a premature stop codon and altering the last 14 codons of the open reading frame, XP_542689.2:p.(Asp336Serfs*6).
We confirmed the presence of this variant in the index case by Sanger sequencing and genotyped all 85 relatives. The genotypes at the variant showed perfect cosegregation with the ICH2 phenotype. All 14 available cases were homozygous for the deletion. The 11 parents of the 14 cases who represent obligate carriers were all heterozygous. The remaining 61 unaffected relatives were either heterozygous or homozygous for the wildtype allele. The mutant allele was absent from 396 Golden Retrievers of European origin (Table 3, Supplementary Table S4).
Table 3.
Genotypes at the ABHD5: c.1006_1019del variant in Golden Retrievers of North American and European origin
| Dogs | wt/wt | wt/del | del/del |
|---|---|---|---|
| ICH2 affected Golden Retrievers (n = 14) | — | — | 14 |
| Obligate carriers for ICH2 (n = 11) | — | 11 | — |
| Unaffected other relatives (n = 61) | 35 | 26 | — |
| Golden Retrievers of European origin (n = 396) | 396 | — | — |
Discussion
In this study, we investigated 14 Golden Retrievers with a new form of ichthyosis termed ICH2 that so far appears to be limited to dogs from North America. Histopathology classified ICH2 as nonepidermolytic ichthyosis. The 14 available cases came from seven different litters, all of which were related. The clinical and histological presentation of ICH2-affected dogs strongly resembled that of the well-known PNPLA1-related ichthyosis in Golden Retrievers (Grall et al. 2012). Experienced breeders reported more severe and adherent scaling in ICH2-affected Golden Retrievers. Histologically, the epidermis in ICH2-affected dogs is thicker than in dogs with the PNPLA1-related ichthyosis dogs and this may correspond with a more severe barrier defect. ICH2 cases tend to have more keratinocytes with perinuclear clear spaces than dogs with the PNPLA1-related ichthyosis.
Using a hypothesis-free positional approach we delimited a ∼12.7 Mb interval or roughly 0.5% of the 2.4 Gb dog genome for the ICH2 locus. Contrasting the genome sequence of an affected dog against 795 control genomes identified a single private homozygous coding variant within the critical interval, a 14 bp deletion in the last exon of the ABHD5 gene. ABHD5 encodes α-β hydrolase domain containing 5 also known under the alias name CGI-58. This protein is involved in the biosynthesis of major components of the skin barrier formation, especially ω-O-acylceramide (Ghosh et al. 2008; Ohno et al. 2018). Studies in Abhd5-/- knockout mice showed drastically reduced triglyceride (TG) hydrolase activity in the epidermis. The defective epidermal TG catabolism led to an impaired synthesis of acylceramides and defective formation of the corneocyte lipid envelope resulting in a dysfunctional permeability barrier of the skin (Radner et al. 2010). ABHD5 also activates adipose triglyceride lipase (ATGL), which catalyzes the initial step of lipolysis converting TG to diglycerides (DG) (Zimmermann et al. 2004; Lass et al. 2006).
ABHD5 loss-of-function variants in humans were reported to cause Chanarin-Dorfman syndrome (CDS) (OMIM # 275630, Dorfman et al. 1974; Chanarin et al. 1975; Lefevre et al. 2001). CDS is a rare autosomal recessive inherited syndromic form of ichthyosis. In CDS, a nonepidermolytic ichthyosis is often accompanied by hepatic steatosis with hepatomegaly, myopathy, and neurological disorders due to lipid droplet accumulation in various tissues (Dorfman et al. 1974; Chanarin et al. 1975; Bruno et al. 2008).
More than ten pathogenic human ABHD5 variants have been described, many of which are affecting the α-β hydrolase domain, lipid binding region, pseudocatalytic domain, or acyltransferase domain (Lefevre et al. 2001; Schweiger et al. 2009). The ABHD5:c.1006_1019del frameshift deletion identified in the affected Golden Retrievers of this study alters the last 14 codons of the open reading frame. They include the last 10 codons of the α-β hydrolase domain (Schweiger et al. 2009). A comparable ABHD5 nonsense variant truncating the last 14 codons of the homologous human sequence was reported in four CDS patients from a consanguineous family (Aggarwal et al. 2012). This strongly suggests an essential function of the C-terminal tail of ABHD5 and supports the hypothesis that the observed ABHD5:c.1006_1019del frameshift deletion is indeed causative for the ichthyosis in the investigated Golden Retrievers.
In summary, we discovered a new autosomal recessive ichthyosis in Golden Retrievers, which we propose to designate as Golden Retriever ichthyosis type 2 (ICH2). The identified candidate causative variant enables genetic testing to prevent the unintentional breeding of affected puppies. Further studies are warranted to clarify whether the phenotypic changes in the affected dogs are limited to the skin or whether ICH2 also affects other organs like CDS in humans.
Data availability
The genome sequence data were submitted to the European Nucleotide Archive (ENA). All accession numbers are listed in Supplementary Table S2.
Supplementary material is available at G3 online.
Supplementary Material
Acknowledgments
The authors are grateful to all dog owners who donated samples and shared health and pedigree information of their dogs. The authors thank Nathalie Besuchet Schmutz, Catia Coito, Marion Ernst, and Daniela Steiner for expert technical assistance, the Next Generation Sequencing Platform of the University of Bern for performing the high-throughput sequencing experiments, and the Interfaculty Bioinformatics Unit of the University of Bern for providing high performance computing infrastructure. The authors thank the Dog Biomedical Variant Database Consortium (Gus Aguirre, Catherine André, Danika Bannasch, Doreen Becker, Brian Davis, Cord Drögemüller, Kari Ekenstedt, Kiterie Faller, Oliver Forman, Steve Friedenberg, Eva Furrow, Urs Giger, Christophe Hitte, Marjo Hytönen, Vidhya Jagannathan, Tosso Leeb, Frode Lingaas, Hannes Lohi, Cathryn Mellersh, Jim Mickelson, Leonardo Murgiano, Anita Oberbauer, Sheila Schmutz, Jeffrey Schoenebeck, Kim Summers, Frank van Steenbeek, and Claire Wade) for sharing whole-genome sequencing data from control dogs. They also acknowledge all researchers who deposited dog or wolf whole-genome sequencing data into public databases.
Conceptualization, M.L.C. and T.L.; investigation, S.K., D.J.W., K.H., A.B.D., E.A.M., and M.L.C.; data curation, V.J.; writing—original draft, S.K., D.J.W., K.H., A.B.D., E.A.M., M.L.C., and T.L.; writing—review and editing, S.K., D.J.M., K.H., A.B.D., V.J., E.A.M., M.L.C., and T.L.; supervision, T.L. All authors have read and agreed to the published version of the manuscript.
Contributor Information
Sarah Kiener, Institute of Genetics, Vetsuisse Faculty, University of Bern, 3001 Bern, Switzerland; Dermfocus, University of Bern, Bern 3001, Switzerland.
Dominique J Wiener, Department of Veterinary Pathobiology, Texas A&M University, College of Veterinary Medicine and Biomedical Sciences, College Station, TX 77843-4467, USA.
Kaitlin Hopke, Department of Small Animal Clinical Sciences, Texas A&M University, College of Veterinary Medicine and Biomedical Sciences, College Station, TX 77843-4474, USA.
Alison B Diesel, Department of Small Animal Clinical Sciences, Texas A&M University, College of Veterinary Medicine and Biomedical Sciences, College Station, TX 77843-4474, USA.
Vidhya Jagannathan, Institute of Genetics, Vetsuisse Faculty, University of Bern, 3001 Bern, Switzerland.
Elizabeth A Mauldin, Department of Clinical Sciences and Advanced Medicine, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA.
Margret L Casal, Department of Clinical Sciences and Advanced Medicine, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA.
Tosso Leeb, Institute of Genetics, Vetsuisse Faculty, University of Bern, 3001 Bern, Switzerland; Dermfocus, University of Bern, Bern 3001, Switzerland.
Funding
This research was funded by the Swiss National Science Foundation, grant number 310030_200354.
Conflicts of interest
M.L.C. is affiliated with the PennGen Laboratory, which offers genetic testing for dogs. The other authors declare no conflict of interest.
Literature cited
- Abecasis GR, Cherny SS, Cookson WO, Cardon LR.. 2002. Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 30:97–101. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Maras JS, Alam S, Khanna R, Gupta SK, et al. 2012. Novel nonsense mutation of ABHD5 in Dorfman-Chanarin syndrome with unusual findings: a challenge for genotype-phenotype correlation. Eur J Med Genet. 55:173–177. [DOI] [PubMed] [Google Scholar]
- Bauer A, Waluk DP, Galichet A, Timm K, Jagannathan V, et al. 2017. A de novo variant in the ASPRV1 gene in a dog with ichthyosis. PLoS Genet. 13:e1006651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno C, Bertini E, Di Rocco M, Cassandrini D, Ruffa G, et al. 2008. Clinical and genetic characterization of Chanarin–Dorfman syndrome. Biochem Biophys Res Commun. 369:1125–1128. [DOI] [PubMed] [Google Scholar]
- Cadiergues MC, Patel A, Shearer DH, Fermor R, Miah S, et al. 2008. Cornification defect in the golden retriever: clinical, histopathological, ultrastructural and genetic characterisation. Vet Dermatol. 19:120–129. [DOI] [PubMed] [Google Scholar]
- Casal ML, Wang P, Mauldin EA, Lin G, Henthorn PS.. 2017. A defect in NIPAL4 is associated with autosomal recessive congenital ichthyosis in American Bulldogs. PLoS One. 12:e0170708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanarin I, Patel A, Slavin G, Wills EJ, Andrews TM, et al. 1975. Neutral-lipid storage disease: a new disorder of lipid metabolism. Br Med J. 1:553–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, et al. 2012. A program for annotating and predicting the effects of single nucleotide polymorphisms. Fly (Austin). 6:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Credille KM, Barnhart KF, Minor JS, Dunstan RW.. 2005. Mild recessive epidermolytic hyperkeratosis associated with a novel keratin 10 donor splice-site mutation in a family of Norfolk terrier dogs. Br J Dermatol. 153:51–58. [DOI] [PubMed] [Google Scholar]
- Credille KM, Minor JS, Barnhart KF, Lee E, Cox ML, et al. 2009. Transglutaminase 1-deficient recessive lamellar ichthyosis associated with a LINE-1 insertion in Jack Russell terrier dogs. Br J Dermatol. 161:265–272. [DOI] [PubMed] [Google Scholar]
- Dorfman ML, Hershko C, Eisenberg S, Sagher F.. 1974. Ichthyosiform dermatosis with systemic lipidosis. Arch Dermatol. 110:261–266. [PubMed] [Google Scholar]
- Ghosh AK, Ramakrishnan G, Chandramohan C, Rajasekharan R.. 2008. CGI-58, the causative gene for Chanarin-Dorfman syndrome, mediates acylation of lysophosphatidic acid. J Biol Chem. 283:24525–24533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grall A, Guaguère E, Planchais S, Grond S, Bourrat E, et al. 2012. PNPLA1 mutations cause autosomal recessive congenital ichthyosis in Golden Retriever dogs and humans. Nat Genet. 44:140–147. [DOI] [PubMed] [Google Scholar]
- Guaguère E, Bensignor E, Muller A, Degorce-Rubiales F, Andre C.. 2007. Epidemiological, clinical, histopathological and ultrastructural aspects of ichthyosis in Golden Retrievers: a report of 50 cases. Vet Dermatol. 18:382–383. [Google Scholar]
- Guaguère E, Bensignor E, Küry S, Degorce-Rubiales F, Muller A, et al. 2009. Clinical, histopathological and genetic data of ichthyosis in the Golden Retriever: a prospective study. J Small Anim Pract. 50:227–235. [DOI] [PubMed] [Google Scholar]
- Hall JA, Yager J.. 2004. Diagnostic dermatology. Can Vet J. 45:872–873. [PMC free article] [PubMed] [Google Scholar]
- Haydar Eskiocak A, Missaglia S, Moro L, Durdu M, Tavian D.. 2019. A novel mutation of ABHD5 gene in a Chanarin Dorfman patient with unusual dermatological findings. Lipids Health Dis. 18:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannathan V, Drögemüller C, Leeb T, Aguirre G, André C, et al. ; Dog Biomedical Variant Database Consortium (DBVDC). 2019. A comprehensive biomedical variant catalogue based on whole genome sequences of 582 dogs and eight wolves. Anim Genet. 50:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, et al. 2006. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 3:309–319. [DOI] [PubMed] [Google Scholar]
- Leeb T, Müller EJ, Roosje P, Welle M.. 2017. Genetic testing in veterinary dermatology. Vet Dermatol. 28:4–e1. [DOI] [PubMed] [Google Scholar]
- Lefevre C, Jobard F, Caux F, Bouadjar B, Karaduman A, et al. 2001. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin-Dorfman syndrome. Am J Hum Genet. 69:1002–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauldin EA. 2013. Canine ichthyosis and related disorders of cornification. Vet Clin North Am Small Anim Pract. 43:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauldin EA, Credille KM, Dunstan RW, Casal ML.. 2008. The clinical and morphologic features of nonepidermolytic ichthyosis in the Golden Retriever. Vet Pathol. 45:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, et al. 2010. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J, Wöhlke A, Mischke R, Hoffmann A, Hewicker-Trautwein M, et al. 2015. A novel SLC27A4 splice acceptor site mutation in Great Danes with ichthyosis. PLoS One. 10:e0141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y, Nara A, Nakamichi S, Kihara A.. 2018. Molecular mechanism of the ichthyosis pathology of Chanarin–Dorfman syndrome: stimulation of PNPLA1-catalyzed ω-O-acylceramide production by ABHD5. J Dermatol Sci. 92:245–253. [DOI] [PubMed] [Google Scholar]
- Oji V, Tadini G, Akiyama M, Blanchet Bardon C, Bodemer C, et al. 2010. Revised nomenclature and classification of inherited ichthyoses: results of the First Ichthyosis Consensus Conference in Soreze 2009. J Am Acad Dermatol. 63:607–641. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, et al. 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radner FP, Streith IE, Schoiswohl G, Schweiger M, Kumari M, et al. 2010. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58). J Biol Chem. 285:7300–7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuth M, Martinz V, Janecke AR, Fauth C, Schossig A, et al. 2013. Inherited ichthyoses/generalized Mendelian disorders of cornification. Eur J Hum Genet. 21:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R.. 2009. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am J Physiol Endocrinol Metab. 297:E289–E296. [DOI] [PubMed] [Google Scholar]
- Uitto J, Youssefian L, Saeidian AH, Vahidnezhad H.. 2020. Molecular genetics of keratinization disorders - what's new about ichthyosis. Acta Derm Venereol. 100:adv00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, et al. 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 306:1383–1386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genome sequence data were submitted to the European Nucleotide Archive (ENA). All accession numbers are listed in Supplementary Table S2.
Supplementary material is available at G3 online.