Abstract
There are currently no standardized therapies for Parkinson disease (PD). Curcumin shows anti-amyloidogenic properties in vitro and may be a promising treatment for PD. We evaluated the effects of curcumin supplementation on clinical scales and misfolded, phosphorylated α-synuclein (p-syn) accumulation in skin biopsies in 19 PD patients who received curcumin supplementation for 12 months and 14 PD patients to treated with curcumin. The patients underwent autonomic (COMPASS-31), motor (MDS-UPDRS and H&Y) and nonmotor (NMSS) questionnaires and skin biopsies to evaluate clinical involvement and p-syn load in skin nerves at the beginning and the end of study. Curcumin and curcuminoid levels were assayed in plasma and CSF. Supplemented patients showed detectable CSF curcuminoid levels that were lower than those in plasma. They showed a decrease of COMPASS-31 and NMSS scores, and a slight p-syn load decrease versus untreated patients who displayed a worsening of these parameters despite increased levodopa doses. Multiple regression models showed a significant effect of curcumin supplementation in decreasing the worsening of the clinical parameters and p-syn load at after curcumin treatment. These data suggest that curcumin can cross the blood-brain barrier, that it is effective in ameliorating clinical parameters and that it shows a tendency to decrease skin p-syn accumulation in PD patients.
Keywords: α-Synuclein, Clinical scales, Curcumin, Dietary supplement, Parkinson disease, Protein misfolding, Skin biopsy
INTRODUCTION
Parkinson disease (PD) is the second-most common neurodegenerative disease estimated to occur in approximately 1% of individuals >60 years of age (1). This is expected to more than double by 2030 because of the increasing global aging population (1). Conventional treatments for PD, such as levodopa (L-dopa) and dopamine agonists, are not completely effective due to long-term side effects and their lack of effect on disease progression. A critical problem in testing disease-modifying therapies is the lack of available biomarkers that could allow biomarker-aided clinical trials of novel therapeutics (2). Skin biopsies may disclose pathological (i.e. phosphorylated) α-synuclein (p-syn) deposition in skin nerves in vivo (3–10), This is similar to the potential of RT-QuIC assays for disclosing abnormal α-syn seeding properties in vivo (11, 12). Additionally, the reliability of skin biopsy as a diagnostic tool has been supported by previous studies with excellent reproducibility (13).
Emerging treatment approaches in PD include natural substances such as curcumin, the main component of turmeric spice found in the root of the Curcuma longa plant (14, 15); it shows antioxidant, anti-inflammatory, and anti-amyloidogenic properties (16–20). Despite its interesting properties, the effect of curcumin on PD patients has yet to be assessed thoroughly.
The aim of this study was to ascertain the effect of a chronic supplementation with curcumin in patients with PD. Specific endpoints assessed include a clinical evaluation by means of motor and nonmotor scales, and the load of p-syn in skin nerves.
MATERIALS AND METHODS
Study Design and Patient Population
Forty consecutive patients fulfilling clinical and instrumental diagnostic criteria for idiopathic PD (21) and p-syn inclusions in skin nerves were screened for this study at the IRCCS Institute of Neurological Sciences of Bologna from December 2016 to July 2020. Inclusion criteria for this study were a late-onset disorder (>45 years old), absence of acquired or genetic diseases explaining PD, preserved cognitive function (Mini-Mental State Examination corrected ≤24), and the presence of p-syn deposits in skin nerves. In addition, all patients (except 2 patients in each curcumin-supplemented and untreated groups) were treated with L-dopa alone or in combination with dopamine agonists with good control of motor symptoms. The clinical diagnosis of PD was supported by specific diagnostic tests. Nigrostriatal dopamine transporter imaging by means of [123I]ioflupane single photon emission-computed tomography (SPECT) showed abnormal findings in all recruited patients and brain magnetic resonance imaging excluded an atypical parkinsonism.
All recruited patients were re-evaluated after 1 year. Twenty-one patients agreed to start the curcumin supplementation (supplemented group): 2 g per day of curcumin as curcumin phospholipids as Meriva (1 g b.i.d. of Maxicur) for 1 year, maintaining stability of the dosage of L-dopa and/or dopamine agonists. At the time of re-evaluation, 2 patients were lost because they chose not to undergo the lumbar puncture required by the study protocol.
Nineteen PD patients did not agree to undergo to the study protocol as they felt more protected by increasing the traditional (i.e. L-dopa) therapy (untreated group as controls). Clinical involvement was quantified using specific questionnaires to objectively define autonomic (i.e. COMPASS-31) (22), motor (MDS-UPDRS-III in off state and Hoehn and Yahr scale [H&Y] scores) and nonmotor (Non-Motor Symptoms Scale [NMSS]) (23) involvement. Each clinical evaluation was associated with an anonymized code and was done blindly by clinicians who did not know which treatment group the patient would join. The second evaluation was also made blinded to the previous evaluation as it was made by a different clinician who did not know the result of the first evaluation.
Curcumin Treatment
Meriva is standardized to contain 18%–22% of curcuminoids including curcumin, demethoxycurcumin (DMC), and bisdemethoxycurcumin (BDMC) dispersed in a phospholipid matrix. The remaining amount of Meriva (78%–82%) contains the delivery system (phytosome) that is needed to increase the bioavailability of curcuminoids (24). When orally administered, Meriva is metabolized in the following major metabolites: tetrahydrocurcumin (THC) and hexahydrocurcumin (HHC) (25).
Ethical Statements
Clinical safety (evaluation of vital signs and any adverse effects) was performed according to current food supplement legislation and Good Clinical Practice procedures. All human subject research was performed in accordance with the guidelines described in the Declaration of Helsinki regarding international clinical research involving human beings. Informed written consent was obtained from all patients who participated in this study. The study was approved by the local Human Ethics Committee (Comitato Etico Interaziendale Bologna-Imola AUSL Bologna, Code CE 16103).
Skin Biopsy
Three-millimeter punch biopsies were taken from proximal (C7 paravertebral) and distal (thigh and leg) hairy skin sites (5, 9). At each skin site a second biopsy 3–4 cm away from the first sample was taken to increase the rate of p-syn positivity (5, 9). Following previously published procedures, skin samples were immediately fixed in cold Zamboni fixative and kept at 4°C overnight (26, 27).
About 10-μm-thick sections were obtained using a freezing sliding microtome (HM550, Thermo Scientific, Waltham, MA). They were double-immunostained overnight with a panel of primary antibodies including rabbit monoclonal p-syn at Ser 129 (p-syn; 1:500, Abcam, Cambridge, UK, cat. no. ab-51253) and mouse pan-neuronal marker protein gene product 9.5 (1:750; Abcam, cat. no. ab72911). Sections were then washed, and secondary antibodies were added for an incubation of 1 hour. As secondary antibodies, an antimouse Alexa Fluor(R) 488 (1:400; Jackson ImmunoResearch, West Grove, PA, cat. num. 715-545-150) or rabbit cyanine dye fluorophores 3.18 (1:200, Jackson ImmunoResearch, cat. num. 711-165-152) were used. The microscopic analysis and criteria followed to define a p-syn positivity were previously described (5, 9, 26, 27). The analysis was made in a blinded fashion to the treatment group and the follow-up phase, by attributing to the skin sample the same anonymized code used for clinical evaluation. P-syn staining was rated as positive when a single skin nerve fiber showed a positive staining at high magnification (×40). Misfolded α-syn in skin nerves was quantified as previously described (3, 5, 26): skin samples positive for p-syn (samples rate: number of positive skin samples/number of skin samples analyzed for each patient in percentage) and skin adnexa (i.e. sweat glands, arterioles, and muscle arrector pilorum) showing in their own autonomic innervation p-syn deposits (adnexa rate [%]: number of skin adnexa innervated by autonomic nerves containing p-syn deposits/all skin adnexa contained in the skin sample).
Blood and CSF Measurements of Curcumin and Its Metabolites
Plasma and CSF Determination of CUR, DMC, and BDMC
A method has been established for the determination of curcumin and curcuminoids (demethoxycurcumin [DMC] and bisdemethoxycurcumin [BDMC]) in human plasma and cerebrospinal fluid (Kymos Pharma Services, S.L., Barcelona, Spain). The determination of free and total curcuminoids concentrations in human plasma and CSF (0.2 mL) was carried out by HPLC-MS/MS after liquid-liquid extraction with ethyl acetate. The compound Curcumin-d6 was used as an internal standard of curcumin, the compound Demethoxycurcumin-d7 was used as internal standard of demethoxycurcumin and the compound Bisdemethoxycurcumin-d8 was used as the internal standard of bisdemethoxycurcumin. The calibration range of the method is defined from 2 to 1000 ng/mL for curcuminoids. In order to evaluate the total curcuminoids concentration (free and conjugated) a previous enzymatic hydrolysis with β-glucuronidase from bovine liver and sulfatase from Helix pomatia was done with the plasma samples, whereas for the determination of free curcuminoids, in which the deconjugation process should be avoided, the same procedure was applied but the enzymatic solution was replaced by solvent and the incubation period was omitted. The method was proved to be selective, linear, precise, and accurate for the determination of free and total curcuminoids (28).
Plasma and CSF Determination of THC and HHC
The determination of free and total THC keto, THC enol, and HHC concentrations in human plasma and cerebrospinal fluid (200 µL) was carried out by HPLC-MS/MS after liquid-liquid extraction with ethyl acetate (qualified method by Kymos Pharma Services, S.L., Barcelona, Spain). The compounds THC keto-d6, THC enol-d6, and HHC-d6 were used as internal standards of THC keto, THC enol, and HHC, respectively. The calibration range of the method in human plasma was defined to be from 10 to 1000 ng/mL, and in cerebrospinal fluid, was defined to be from 2 to 1000 ng/mL.
In order to determine the total THC keto, THC enol, and HHC concentration (free and conjugated) a previous enzymatic hydrolysis with β-glucuronidase from bovine liver and sulfatase from Helix pomatia was done in plasma and in CSF samples, whereas for the determination of free metabolites, in which the deconjugation process should be avoided, the same procedure was applied but the enzymatic solution was replaced by solvent and the incubation period was omitted.
The method proved to be selective, linear, precise, and accurate for the determination of free and total THC keto, THC enol, and HHC in human plasma and in CSF (28, 29). Two patients affected by multiple sclerosis and chronic inflammatory demyelinating polyradiculoneuropathy who had not taken curcumin in the last year and underwent lumbar puncture for diagnostic purposes in relationship to their neurological disorder were used to verify the reliability of the method used to quantify curcumin and its metabolites in the plasma and CSF.
Statistical Analysis
The statistical evaluation of data was done by B-W-Subject ANOVA, a Mixed Analysis of Variance Design for the difference in mean, the only probabilistic model correctly applicable to the type of experimental design to be evaluated: between groups (controls and untreated, curcumin); within observation periods (basal, 12 months). In the presence of significant interaction, treatment x time was applied. Tukey-Kramer Multiple Comparison Test for nonconfounded means to identify, among the observed mean, any differences within the planned observation periods and between the 2 groups to be compared. The variables analyzed with this procedure were MDS-UPDRS-III, COMPASS-31 score, H&Y score, NMSS score, skin sample rates, and adnexa rate. Multiple Regression, Prediction Profiler, and Spearman ρ were applied to study whether the clinical scales were influenced by confounders such as L-dopa dosage, age, sex, and disease duration. In particular, Spearman ρ for the correlative study between curcuminoid plasma analysis and clinical/demographic variables between basal and 12 months. All comparisons were conducted at p < 0.05 level. Statistical procedures were performed using the JMP14 Pro software from SAS Institute, Inc.
RESULTS
Curcumin-supplemented and untreated patients did not show significant differences regarding age, sex, and disease duration (Table 1). Baseline clinical scales showed slightly higher motor disability (i.e. MDS-UPDRS and H&Y) in the curcumin group, together with a corresponding higher L-dopa dosage (Table 1). By contrast, remaining scales and p-syn load did not differ between the 2 groups of patients (Table 1). Interestingly, during the 12 months of curcumin administration, patients did not exhibit any serious adverse events and the dietary supplement was well-tolerated, thus confirming the good safety profile of the curcumin formulation used in the study.
TABLE 1.
Baseline Clinical and Demographic Data of Parkinson Disease Patients
| Number | Age (years) | Sex Male:Female | Disease Duration (years) | UPDRS Off Score | H&Y Score | C-31 Score | NMSS Score | Samples Rate (%) | Adnexa Rate (%) | L-Dopa (mg/day) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Supplemented | 19 | 72 ± 2 | 9:10 | 5 ± 1 | 26 ± 3 | 2 ± 0.2 | 17 ± 3 | 11 ± 1 | 54 ± 8 | 13 ± 3 | 270 ± 20 |
| Untreated | 14 | 72 ± 2 | 9:5 | 6 ± 1 | 16 ± 2 | 1 ± 0.2 | 8 ± 2 | 10 ± 1 | 40 ± 7 | 10 ± 4 | 180 ± 30 |
| p-Value | 0.98 | 0.2 | 0.62 | 0.11 | 0.01* | 0.15 | 0.96 | 0.55 | 0.99 | 0.01* |
Data are reported as mean±SE.
C-31, COMPASS-31; H&Y, Hoehn and Yahr scale; NMSS, Non-Motor Symptoms Scale; UPDRS, Unified Parkinson Disease Rating Scale.
Significant differences.
Determination of Curcumin Metabolites in Plasma and Cerebrospinal Fluids
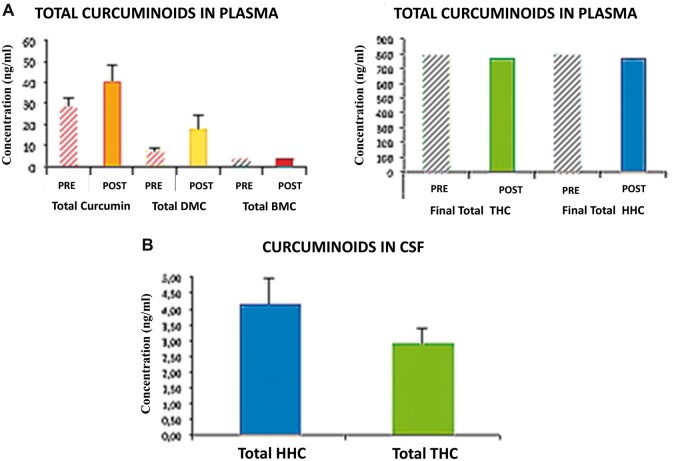
Patients who did not take curcumin did not show detectable traces of curcumin, curcuminoids, and metabolites, either in the plasma or in the CSF, supporting the reliability of the method used. In the group supplemented with curcumin, at the end of the supplementation period (12 months), the plasma levels were measured before (pre) and after (post) the last morning tablet intake. Patients showed a clear increase of total curcumin, DMC (p < 0.05), and BDMC (p < 0.05), while no significant changes were found for THC or HHC (Fig. 1). Plasma THC and HHC reached steady-state concentrations. Supplemented patients showed detectable HHC and THC in CSF, although lower than plasma levels, whereas no detectable traces of total curcumin, DMC, and BDMC were found in the same sample (Fig. 1).
FIGURE 1.
Curcumin and metabolites determination in CSF and plasma. (A) Plasma quantification of curcumin, curcuminoids, and metabolites by a standardized method. At the end of the supplementation period (12 months), after the last morning tablet intake, patients showed a clear increase of total curcumin, demethoxycurcumin (DMC), and bisdemethoxycurcumin (BDMC) compared to the values measured before the last tablet intake. No significant changes were found for the major derived metabolites such as tetrahydrocurcumin (THC) and total hexahydrocurcumin (HHC). Controls did not show detectable traces of curcumin, curcuminoids, or metabolites in plasma. (B) Similarly supplemented patients showed detectable THC and HHC in CSF although the levels were lower than those in the plasma. No detectable traces of total curcumin, DMC, and BDMC were found in the same sample. Controls did not show detectable curcumin or metabolites in CSF.
The Effect of Curcumin on Clinical Outcome
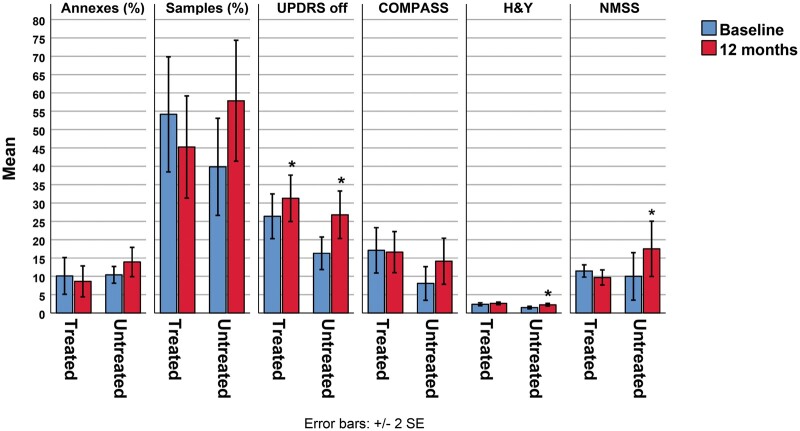
The untreated group showed worsening of all clinical scales despite the increase of L-dopa dosage (Table 2). The curcumin-supplemented group, which was not allowed to increase L-dopa dosage, showed an increase only of MDS-UPDRS and H&Y scores, although the increases were less than those in the untreated group. It should be emphasized that COMPASS-31 and NMSS decreased over the follow-up with an opposite trend compared to the untreated group (Table 2 and Fig. 2). PD patients with shorter disease duration (<6 years, 9 patients) had a better outcome than patients with longer disease duration, based on mean changes in used clinical scales: MDS-UPDRS (4 vs 7, respectively), H&Y (0.2 vs 0.4), COMPASS-31 (−2 vs 1), and NMSS (−3 vs −1).
TABLE 2.
Follow-Up Changes of Clinical Scales and Phosphorylated α-Synuclein Load in Curcumin-Supplemented and Untreated Parkinson Disease Patients
| Supplemented | p-Value | Untreated | p-Value | |
|---|---|---|---|---|
| UPDRS off (score) | 5 ± 5 | 0.0016* | 11 ± 5 | 0.0001* |
| H&Y (score) | 0.2 ± 0.4 | 0.0655 | 0.7 ± 0.4 | 0.0001* |
| COMPASS-31 (score) | −0.5 ± 3 | 0.99 | 6 ± 4 | 0.11 |
| NMSS (score) | −2 ± 0.6 | 0.49 | 8 ± 7 | 0.0001* |
| Samples rate (%) | −9 ± 8 | 0.52 | 18 ± 15 | 0.1 |
| Adnexa rate (%) | −5 ± 3 | 0.6 | 4 ± 8 | 0.93 |
| L-dopa (mg/day) | 0 | – | 150 ± 120 | 0.59 |
p-Values refer to comparisons from baseline (Table 1) and follow-up measurements. Data are reported as mean±SE.
Significant differences.
FIGURE 2.
The effect of curcumin on clinical outcome and pathological form of α-synuclein in skin nerves. P-syn samples and adnexa rates increased in untreated patients differently from supplemented patients showing a decrease of p-syn deposits although the difference from baseline is not significant. Untreated patients show a worsening of all clinical scales despite the increase of L-dopa dosage. Patients supplemented with curcumin showed an increase only of MDS-UPDRS and H&Y scores. Although less than the untreated group, COMPASS-31 and NMSS decreased over the follow-up with an opposite trend compared to the untreated group. *Asterisk indicates significant changes from baseline.
The Curcumin Effect on Pathological Form of α-Syn in Skin Nerves
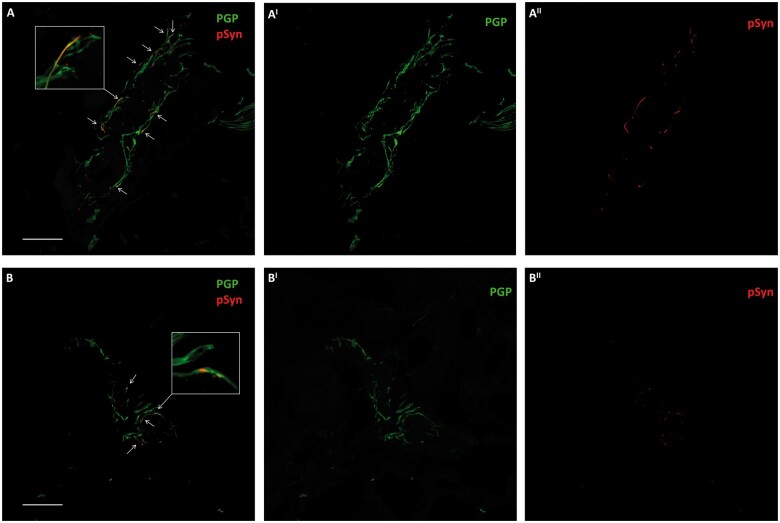
P-syn samples and adnexa rates increased in the untreated group differently from the supplemented group, showing a slight decrease of p-syn deposits. This was clearer in patients with shorter disease duration, although the difference from baseline was not significant (Table 2; Figs. 2 and 3).
FIGURE 3.
Phosphorylated α-synuclein (p-syn) deposits in skin nerves in a supplemented PD patient during the follow-up period. Confocal microscope (×400) study of p-syn in skin nerves of a patient with PD at baseline (A) and after the follow-up period (B). Abundant nerve fibers are found around a skin arteriole identified by PGP 9.5. Some of these fibers presented abnormal neuritic inclusions of misfolded α-synuclein depicted by staining phosphorylation at Ser 129 (p-syn, arrows). The number of fibers expressing p-syn was higher at baseline (A) than after the follow-up (B). The number of deposits (arrows) around a skin arteriole was decreased after 1 year of supplementation of curcumin (B). Higher magnification images of nerve fibers showing misfolded α-synuclein aggregates are in boxes. Scale bar: 50 µm.
Linear Correlation among Investigated Variables
In CSF total HHC was correlated with total curcumin post (r = 0.6; p < 0.03), DMC post (r = 0.6; p < 0.04), THC enol form pre and post (r = 0.6; p < 0.02), total THC keto form pre (r = 0.7; p < 0.02) and post (r = 0.6; p < 0.03), total THC pre and post (r = 0.7; p < 0.01), total HHC pre and post (r = 0.8; p < 0.01), and an inverse correlation with the THC enol/keto ratio (r = 0.8; p < 0.01). Furthermore, the THC enol/keto pre-ratio was correlated with MDS-UPDRS (r = 0.5; p < 0.04) and COMPASS-31 (r = 0.5; p < 0.03) at the end of follow-up. Finally, NMSS score was correlated with p-syn load both considering skin samples (r = 0.5; p < 0.05) and skin adnexa (r = 0.5; p < 0.05) at the follow-up.
Multiple Regression Model Controlling for L-Dopa, Age, Sex, and Disease Duration
The regression model showed a significant effect of curcumin supplementation in decreasing the worsening of MDS-UPDRS, H&Y, and NMSS compared to untreated patients (p < 0.001). Similarly, the model showed a significant decrease of the p-syn load, considering skin samples in the supplemented group compared to untreated PD (p < 0.03).
DISCUSSION
The main results of our study indicate that curcumin: (1) is able to cross the blood-brain barrier in the form of its active metabolites when formulated in phospholipids; (2) is effective in ameliorating/stabilizing motor and nonmotor symptoms in PD; and (3) shows a tendency to decrease misfolded α-syn deposits in skin nerves as supported by the multiple regression model.
Curcumin is the main component of turmeric spice, a natural polyphenol compound found in the root of the Curcuma longa plant. Several studies have demonstrated that polyphenols show antioxidant, anti-inflammatory, and anti-amyloidogenic properties. These properties are particularly relevant for treatment of neurodegenerative diseases, such as PD (14, 16, 18).
In this study, our primary aim was to test for the first time the possibility that curcumin may improve clinical impairments and p-syn load in skin nerves of idiopathic PD patients. Our data indicate a beneficial effect of curcumin on the clinical outcome. In fact, PD patients supplemented with curcumin, despite a stable L-dopa dosage, showed an improvement of nonmotor scales while the worsening of the motor scale was less than expected in the control patients who, by contrast, increased their dosage of L-dopa. The significant effect of curcumin supplementation on MDS-UPDRS, H&Y, and NMSS scores was supported by a multiple regression model considering age, sex, and disease duration. In particular, the disease duration seems important for the curcumin supplementation, as PD patients with shorter disease duration presented a better clinical outcome, likely because of a restricted neuronal damage in the early disease course of PD (30). Accordingly, a compound with a potential neuroprotective effect may induce a better outcome if taken in this disease phase. Our data indicate a greater effect of the used dosage of curcumin on autonomic symptoms (Table 2), mainly arising from peripheral nerves in PD (26) and a less-effective impact on motor dysfunctions, arising from central nervous system damage. This is consistent with the higher level of curcumin in the plasma versus the CSF. However, an important point to consider for future studies is the possibility of curcumin entering neurons to act on abnormal deposits of misfolded α-synuclein, potentially affecting its anti-aggregation properties.
In agreement with the main effect of curcumin on autonomic symptoms, we found that patients treated with curcumin tend to have a reduction in p-syn load in skin nerves at the time of follow-up, compared to untreated patients showing increased skin aggregates. Although these data need to be supported by additional studies showing a direct effect of curcumin on skin p-syn aggregates; the current experimental model supports a possible effect of curcumin on misfolded α-synuclein aggregates. In fact, curcumin directly binds to α-synuclein in vitro, and can both inhibit and reverse the formation of toxic α-synuclein aggregate species (17, 19, 20), thus promoting and stabilizing nonaggregate forms of α-syn (15). Curcumin may also improve the motor behavior of mice and binds Lewy bodies in human brain tissue (15, 16). With respect to tolerability, no adverse events were observed in the supplemented (treated) group during the long-term administration (12 months) confirming that the profile of the curcumin phospholipids formulation and dosage used in the study are indeed safe.
The current study presents the following limitations: (1) We consider this to be a preliminary investigation to test the possible effect of curcumin on clinical scales and accumulation of misfolded p-syn in PD patients. However, we underline that disease duration and baseline clinical scales (except H&Y) and p-syn load did not differ between supplemented and untreated PD patients at the outset (Table 1), supporting a similar degree of clinical involvement. The greater intake of L-dopa by the supplemented patients at baseline (Table 1) suggests that this group of patients may have had a tendency to develop a more aggressive form of the disease. Furthermore, baseline clinical scale evaluations and skin biopsies were evaluated blindly supporting the reliability of the data presented, though we were aware a larger, double-blinded trial is needed to confirm the preliminary indications of the current study. (2) No dietary and lifestyle factors such as protein intake or BMI that could influence levodopa-responsiveness were monitored during the study, although all recruited patients reported no changes in dietary and lifestyle habits during at the time of follow-up.
ACKNOWLEDGMENTS
We would like to express our gratitude to Kymos Pharma Services, S.L., Barcelona (Spain) for the excellent work on the analytical part of the project. We also thank Tami Dalberg for the English revision of the manuscript and Dr Priya Rangan, PhD, for their in-depth English review of the manuscript.
Contributor Information
Vincenzo Donadio, IRCCS Istituto delle Scienze Neurologiche di Bologna, UOC Clinica Neurologica, Bologna, Italy.
Alex Incensi, IRCCS Istituto delle Scienze Neurologiche di Bologna, UOC Clinica Neurologica, Bologna, Italy.
Giovanni Rizzo, IRCCS Istituto delle Scienze Neurologiche di Bologna, UOC Clinica Neurologica, Bologna, Italy.
Enrico Fileccia, IRCCS Istituto delle Scienze Neurologiche di Bologna, UOC Clinica Neurologica, Bologna, Italy.
Francesco Ventruto, IRCCS Istituto delle Scienze Neurologiche di Bologna, UOC Clinica Neurologica, Bologna, Italy.
Antonella Riva, R&D Department, Indena S.p.A., Milan, Italy.
Domenico Tiso, Medical Department, Agave Group, Bologna, Italy.
Martino Recchia, R&D Department, Indena S.p.A., Milan, Italy.
Veria Vacchiano, IRCCS Istituto delle Scienze Neurologiche di Bologna, UOC Clinica Neurologica, Bologna, Italy.
Rossella Infante, IRCCS Istituto delle Scienze Neurologiche di Bologna, UOC Clinica Neurologica, Bologna, Italy.
Giovanna Petrangolini, R&D Department, Indena S.p.A., Milan, Italy.
Pietro Allegrini, R&D Department, Indena S.p.A., Milan, Italy.
Silvia Avino, Medical Department, Agave Group, Bologna, Italy.
Roberta Pantieri, IRCCS Istituto delle Scienze Neurologiche di Bologna, UOC Neurologia, Bologna, Italy.
Barbara Mostacci, IRCCS Istituto delle Scienze Neurologiche di Bologna, UOC Clinica Neurologica, Bologna, Italy.
Patrizia Avoni, IRCCS Istituto delle Scienze Neurologiche di Bologna, UOC Clinica Neurologica, Bologna, Italy; Dipartimento di Scienze Biomediche e Neuromotorie, Università di Bologna, Italy.
Rocco Liguori, Dipartimento di Scienze Biomediche e Neuromotorie, Università di Bologna, Italy.
Vincenzo Donadio, Alex Incensi, Patrizia Avoni and Rocco Liguori contributed equally to this work.
AR, GP, and PAl are employees of Indena. DT and SA are employees of Agave Group. The other authors have no duality or conflicts of interest to declare.
REFERENCES
- 1. Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of 408. people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007;68:384–6 [DOI] [PubMed] [Google Scholar]
- 2. Dehay B, Bourdenx M, Gorry P, et al. Targeting α-synuclein for treatment of Parkinson’s disease: Mechanistic and therapeutic considerations. Lancet Neurol 2015;14:855–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Donadio V, Incensi A, Cortelli P, et al. Skin sympathetic fiber α-synuclein deposits: A potential biomarker for pure autonomic failure. Neurology 2013;80:725–32 [DOI] [PubMed] [Google Scholar]
- 4. Doppler K, Ebert S, Uçeyler N, et al. Cutaneous neuropathy in Parkinson’s disease: A window into brain pathology. Acta Neuropathol 2014;128:99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donadio V, Incensi A, Rizzo G, et al. A new potential biomarker for dementia with Lewy bodies: Skin nerve α-synuclein deposits. Neurology 2017;89:318–26 [DOI] [PubMed] [Google Scholar]
- 6. Doppler K, Jentschke HM, Schulmeyer L, et al. Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson’s disease. Acta Neuropathol 2017;133:535–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antelmi E, Donadio V, Incensi A, et al. Skin nerve phosphosylated α-synuclein deposits in idiopathic REM sleep behavior disorder. Neurology 2017;88:2128–31 [DOI] [PubMed] [Google Scholar]
- 8. Melli G, Vacchi E, Biemmi V, et al. Cervical skin denervation associates with alpha-synuclein aggregates in Parkinson disease. Ann Clin Transl Neurol 2018;5:1394–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donadio V. Skin nerve α-synuclein deposits in Parkinson’s disease and other synucleinopathies: A review. Clin Auton Res 2019;29:577–85 [DOI] [PubMed] [Google Scholar]
- 10. Tsukita K, Sakamaki-Tsukita H, Tanaka K, et al. Value of in vivo α-synuclein deposits in Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 2019;34:1452–63 [DOI] [PubMed] [Google Scholar]
- 11. Donadio V, Wang Z, Incensi A, et al. In vivo diagnosis of synucleinopathies: A comparative study of skin biopsy and RT-QuIC. Neurology 2021;96:e2513–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Z, Becker K, Donadio V, et al. Skin α-synuclein aggregation seeding activity as a novel biomarker for Parkinson disease. JAMA Neurol 2020;78:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Donadio V, Doppler K, Incensi A, et al. Abnormal α-synuclein deposits in skin nerves: Intra- and inter-laboratory reproducibility. Eur J Neurol 2019;26:1245–51 [DOI] [PubMed] [Google Scholar]
- 14. Fan X, Zhang C, Liu D-B, et al. The clinical applications of curcumin: Current state and the future. Curr Pharm Des 2013;19:2011–31 [PubMed] [Google Scholar]
- 15. Spinelli KJ, Osterberg VR, Meshul CK, et al. Curcumin treatment improves motor behavior in α-synuclein transgenic mice. PLoS One 2015;10:e0128510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pan J, Li H, Ma J-F, et al. Curcumin inhibition of JNKs prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease through suppressing mitochondria dysfunction. Transl Neurodegener 2012;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pandey N, Strider J, Nolan WC, et al. Curcumin inhibits aggregation of α-synuclein. Acta Neuropathol 2008;115:479–89 [DOI] [PubMed] [Google Scholar]
- 18. Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Adv Exp Med Biol 2007;595:197–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahmad B, Lapidus LJ. Curcumin prevents aggregation in α-synuclein by increasing reconfiguration rate. J Biol Chem 2012;287:9193–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Radbakhsh S, Barreto GE, Bland AR, et al. Curcumin: A small molecule with big functionality against amyloid aggregation in neurodegenerative diseases and type 2 diabetes. Biofactors 2021;47:570–86 [DOI] [PubMed] [Google Scholar]
- 21. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015;30:1591–601 [DOI] [PubMed] [Google Scholar]
- 22. Pierangeli G, Turrini A, Giannini G, et al. Translation and linguistic validation of the Composite Autonomic Symptom Score COMPASS 31. Neurol Sci 2015;36:1897–902 [DOI] [PubMed] [Google Scholar]
- 23. Cova I, Di Battista ME, Vanacore N, et al. Validation of the Italian version of the Non Motor Symptoms Scale for Parkinson’s disease. Parkinsonism Relat Disord 2017;34:38–42 [DOI] [PubMed] [Google Scholar]
- 24. Cuomo J, Appendino G, Dern AS, et al. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J Nat Prod 2011;74:664–9 [DOI] [PubMed] [Google Scholar]
- 25. Asher GN, Xie Y, Moaddel R, et al. Randomized pharmacokinetic crossover study comparing 2 curcumin preparations in plasma and rectal tissue of healthy human volunteers. J Clin Pharmacol 2017;57:185–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Donadio V, Incensi A, Del Sorbo F, et al. Skin nerve phosphorylated α-synuclein deposits in Parkinson’s disease with orthostatic hypotension. J Neuropathol Exp Neurol 2018;77:942–9 [DOI] [PubMed] [Google Scholar]
- 27. Donadio V, Incensi A, Rizzo G, et al. Spine topographical distribution of skin α-synuclein deposits in idiopathic Parkinson disease. J Neuropathol Exp Neurol 2017;76:384–9 [DOI] [PubMed] [Google Scholar]
- 28. Ringman JM, Frautschy SA, Teng E, et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res Ther 2012;4:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Begum AN, Jones MR, Lim GP, et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J Pharmacol Exp Ther 2008;326:196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Braak H, Rub U, Gai WP, et al. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna) 2003;110:517–36 [DOI] [PubMed] [Google Scholar]