Abstract
Introduction
The US Food and Drug Administration most recently announced its intention to ban menthol cigarettes and cigars nationwide in April 2021. Implementation of the ban will require evidence that it would improve public health. This paper simulates the potential public health impact of a ban on menthol in cigarettes and cigars through its impacts on smoking initiation, smoking cessation and switching to nicotine vaping products (NVPs).
Methods
After calibrating an established US simulation model to reflect recent use trends in cigarette and NVP use, we extended the model to incorporate menthol and non-menthol cigarette use under a status quo scenario. Applying estimates from a recent expert elicitation on the behavioural impacts of a menthol ban, we developed a menthol ban scenario with the ban starting in 2021. We estimated the public health impact as the difference between smoking and vaping-attributable deaths and life-years lost in the status quo scenario and the menthol ban scenario from 2021 to 2060.
Results
As a result of the ban, overall smoking was estimated to decline by 15% as early as 2026 due to menthol smokers quitting both NVP and combustible use or switching to NVPs. These transitions are projected to reduce cumulative smoking and vaping-attributable deaths from 2021 to 2060 by 5% (650 000 in total) and reduce life-years lost by 8.8% (11.3 million). Sensitivity analyses showed appreciable public health benefits across different parameter specifications.
Conclusions and relevance
Our findings strongly support the implementation of a ban on menthol in cigarettes and cigars.
Keywords: advocacy, electronic nicotine delivery devices, public policy, smoking caused disease
Introduction
While US cigarette smoking prevalence has declined substantially in the past decade, the prevalence of menthol smoking has remained constant.1–5 Menthol cigarettes now represent 35% of cigarette sales6 and are disproportionately used by youth, young adults, women and African-Americans.3 7 Menthol cigarette use has been associated with increased smoking initiation and reduced smoking cessation.8–11 In response, the European Union, Canada, Brazil, Ethiopia and Turkey have banned menthol in cigarettes.12 In the USA, more than 20 localities and the state of Massachusetts have banned menthol cigarettes.13 Recently, the Food and Drug Administration announced its intention to implement a nationwide ban on menthol in cigarettes and cigars.14 A stronger evidence base is urgently needed about whether such a ban would improve public health.15 16
A small body of research has examined the potential impact of banning menthol in cigarettes. A simulation model17 projected that a menthol ban would have major impacts on smoking prevalence and smoking-attributable deaths. However, that model simulated a ban starting in 2010 and did not consider the impact of switching to nicotine vaping products (NVPs, also known as e-cigarettes). Additionally, recent evidence finds that a menthol ban would likely increase smoking cessation, with more limited evidence of reducing smoking initiation and switching from smoking to other products.18 To better gauge the potential impact of a menthol cigarette and cigar ban in the vaping era, we conducted an expert elicitation to explicitly consider the impact of the ban on smoking initiation and cessation and on NVP use.19
This paper applies the results of our expert elicitation to evaluate a US menthol ban on all combustibles, including cigarettes and cigars. We use the previously developed smoking and vaping model (SAVM)20 to simulate the impact of the ban on cigarette and NVP use. We extend that model to distinguish menthol and non-menthol cigarette use and to estimate the public health impact of a menthol ban on combustible tobacco products.
Methods
The SAVM is a compartmental model that simulates the public health impact of cigarette and NVP use over time for a specific set of birth cohorts in a given population.20 The model is publicly available as a Microsoft Excel file with a user manual.21 We extend SAVM to project menthol and non-menthol cigarette use in the absence of a ban (status quo scenario) and in the presence of a ban (menthol ban scenario). We estimate the public health impact as the difference in smoking and vaping-attributable deaths (SVADs) and life-years lost (LYLs) between scenarios. Further description of the model and model equations are found in online supplemental file 1.
tobaccocontrol-2021-056604supp001.pdf (823.6KB, pdf)
Status quo scenario
The SAVM20 first projects never, current and former smoking prevalence using age and sex-specific initiation and cessation rates for each cohort of males and females by individual age (0–85) beginning in 2013. The model parameters were estimated by applying an age-period-cohort statistical smoking model to National Health Interview Survey (NHIS) data through 2013,22–25 thereby incorporating trends before NVP use became more prevalent in 2013.26 Current smoking is defined as having smoked ≥100 cigarettes during one’s lifetime and currently smoking at least some days. Current smokers become former smokers after having quit for 2 years to reflect cessation net of relapse. Future smoking prevalence is based on the estimated initiation and cessation rates.
Overlaying the smoking model, SAVM incorporates switching from smoking to regular NVP use, NVP initiation and cessation, and smoking initiation and cessation.20 To simplify the analysis and because dual use is often unstable,27–31 dual users of cigarettes and NVPs are included in SAVM as current smokers.30 Those who vape de novo or who switch from smoking to vaping before age 35 are treated as exclusive vapers, reflecting the minimal smoking-related mortality risks of smokers who quit by age 35.32 33 Those who switch from smoking to vaping after age 35 become former smokers who vape.
An earlier version of the SAVM generally validated well,20 but underestimated the decline in smoking. Given the importance of smoking initiation to future smoking rates, we recalibrated model parameters using 2013–2018 NHIS data, as described in online supplemental file 1.
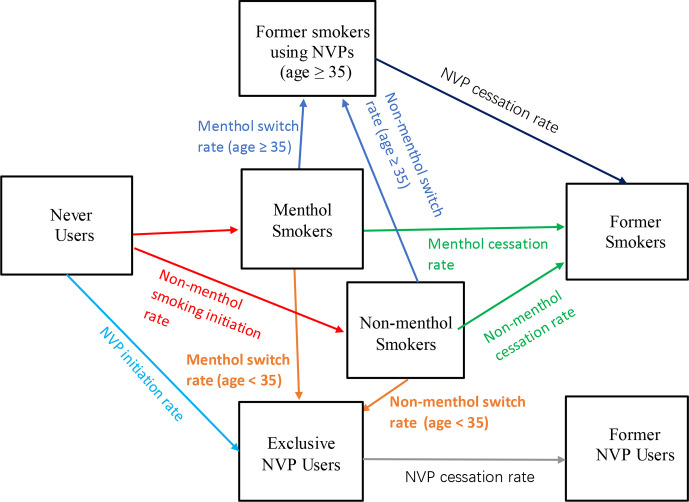
To incorporate menthol use, we differentiate menthol and non-menthol smokers in the model by age and gender. Using data from the 2013/2014 to 2016/2017 Population Assessment of Tobacco and Health (PATH) study, menthol smokers are defined as those whose regular brand is flavoured to taste like menthol.34 Transitions in the status quo scenario are illustrated in figure 1.
Figure 1.
Transitions between smoking and nicotine vaping product (NVP) use states in the status quo scenario.
Smoking initiation rates for menthol and non-menthol smokers are determined assuming a constant proportion of menthol smokers among all smokers at age 30 (MP30), an age when most initiation and smoking patterns have become established.35 36 Based on our analysis of PATH data (see online supplemental file 2), less than 3% of smokers switch between menthol and non-menthol cigarettes or initiate smoking after age 30. Using PATH data for ages 25–35, MP30 is estimated as the average proportion of menthol smokers and is applied to smoker initiation rates at each age a in year t as:
tobaccocontrol-2021-056604supp002.pdf (749.8KB, pdf)
While this method does not explicitly model differences in the trajectories of menthol and non-menthol use prior to age 30, it implicitly allows for initiation as well as switching between menthol and non-menthol use through age 30.
Age and year-specific cessation rates of menthol and non-menthol smokers are based on transforming overall cessation using the menthol proportion at each age (MPa) and the ratio of the menthol to non-menthol cessation rates (RMNCr), yielding:
Based on recent studies11 37–41 and PATH data, we set RMNCr=0.8 for all ages and both genders.
To allow for different switching rates from menthol and non-menthol smoking to NVP use, we apply a similar method using the ratio of menthol to non-menthol switching (RMNSw). We assume that switching rate declines annually by 10% beginning in 2018 (to reflect that those most amenable to vaping have already switched).
Based on recent PATH data, we set RMNSw at 0.9 for all ages and both genders.
Given limited evidence of differential mortality,42 43 we assume no difference in the mortality rates of menthol and non-menthol smokers.
Online supplemental file 1 shows projected trends. The proportion of menthol smokers among all smokers shows an upward trend, consistent with trends reported in recent studies.1–6
Menthol ban scenario
We model a federal menthol ban beginning in 2021. While the model focuses on cigarette use, the ban is assumed to apply to both cigarettes and cigars. We focused on the effect of a ban on both, since little cigars have been found to be a close substitute for cigarettes.44–46 Were cigars (especially little cigars) exempted, many preban menthol cigarette smokers would likely switch to menthol cigars.
We rely on the aforementioned expert elicitation.19 Finalised in September 2020, the elicitation was specifically developed to assess the impact of a menthol ban on smoking initiation and cessation and on NVP use.19 The panel of experts was selected using a three-pronged approach: (1) selection of lead and senior authors of studies identified in a scoping review on the impact of menthol and flavour bans18; (2) a search in Scopus to identify individuals who are the most published authors on the topic of menthol tobacco and with an H-Index of ≥20; and (3) the advice of an external advisory panel. After selecting 12 of the 82 experts with the highest rated criteria 1 and 2 above and with no reported conflicts of interest, our final sample comprised 11 experts after one invitation was declined.
Experts were asked to estimate transitions regarding current tobacco and NVP use patterns under a menthol cigarette and cigar ban, including continued (illicit) menthol cigarette or cigar smoker47; switching to non-menthol cigarettes or cigars, smokeless tobacco or novel nicotine delivery products (NNDPs, including NVPs and heated tobacco products); or ceasing all nicotine product use. Because mortality risks for cigars are similar to or less than those for cigarettes,48 49 estimated panel transitions into cigar use are modelled as non-menthol cigarette use. For convenience, the small percentage of estimated transitions to smokeless tobacco use (2% for ages 18–24 and <1% for ages 35–54) is also transferred to non-menthol cigarette use. Although the elicitation included heated tobacco products in NNDPs, we treat all such transitions as NVP use based on relatively similar risks.50–52 The elicitation methodology and results are described further in online supplemental file 2.
The experts first estimated the impact of a menthol ban on smoking initiation for those aged 12–24 who, absent a ban, would have initiated menthol smoking by age 24. Based on experts’ mean estimates, 38.3% of otherwise menthol smokers would instead become non-menthol smokers, 2.4% illicit menthol smokers, 17.3% NVP users and 42.0% would not use cigarettes or NVPs. These adjustments are applied to the initiation rates of otherwise menthol smokers in 2021 and as ongoing transitions in future years.
For those already menthol smokers, experts considered transitions over a 2-year period under the status quo and under a menthol ban. We model the experts’ estimates of mean net transitions (the difference in 2-year transitions under the status quo and a menthol ban). Among current menthol smokers aged 18–24, 10.1% switch to illicit menthol combustibles, 48.0% switch to non-menthol combustibles, 24.2% switch to NVPs and 17.7% quit all product use. These transitions are applied to menthol smokers through age 30. Among current menthol smokers aged 35–54, 8.8% switch to illicit menthol cigarettes and cigars, 59.1% switch to non-menthol tobacco use, 17.3% switch to NVPs and 14.7% quit all product use. These transitions are applied to menthol smokers above age 30. Current non-menthol smokers were assumed to be unaffected by the ban.
Public health outcomes
Smoking-attributable deaths are estimated as the excess mortality risk at each age for current and former smokers multiplied by their respective populations. Vaping-attributable deaths are measured in the same way, except vaping excess mortality risk is initially set at 15% of excess smoking risk, higher than previously published estimates.53 54 Total LYLs are estimated at each age by the number of SVADs multiplied by the expected years of life remaining of a never smoker.
We estimate the public health impact of a menthol ban as the differences in SVADs and LYLs in the status quo and menthol ban scenarios over a 40-year period, 2021–2060. To address uncertainties about the values of variables applied to both scenarios, we conduct sensitivity analyses of the public health impacts with excess mortality risks of NVPs at 5% and 25% that of excess smoking risks, with smoking and NVP initiation and cessation transitions and rates of switching from cigarettes to NVPs varied by −10% and +10% of their baseline levels and with the ratio of menthol to non-menthol cessation and menthol to non-menthol switching equal to 1.
Results
Public health impact under the base case status quo and menthol ban scenarios
Table 1 presents the 2021–2060 menthol and non-menthol smoking and NVP prevalence, SVADs and LYLs from the model for US adults (aged >18), males and females combined (weighted by population). Results from 2026 and 2060 are presented to display illustrative short-term and long-term status. Online supplemental file 3 provides breakdowns by gender and with the time period extended from 2060 to 2080.
Table 1.
Smoking and NVP prevalence, smoking and vaping-attributable deaths, life-years lost and public health impact for both genders combined, age 18 and above, 2021–2060
| Status quo scenario | |||||
| Category | Category/year | 2021 | 2026 | 2060 | Cumulative impact* |
| Prevalence | Menthol smoker | 5.4% | 4.5% | 2.4% | −55.7% |
| Non-menthol smoker | 7.1% | 5.7% | 2.7% | −62.6% | |
| Total smokers† | 12.6% | 10.2% | 5.1% | −59.6% | |
| Former smoker | 19.4% | 18.4% | 9.2% | −52.7% | |
| Exclusive NVP user‡ | 3.5% | 4.7% | 5.8% | 64.4% | |
| Former NVP user | 0.2% | 0.6% | 4.6% | 1972.5% | |
| Smoking and vaping-attributable deaths§ | Menthol smoker | 77 455 | 74 136 | 39 418 | 2 402 279 |
| Non-menthol smoker | 122 242 | 106 124 | 37 923 | 2 909 245 | |
| Former smoker | 175 798 | 189 490 | 192 368 | 8 500 851 | |
| Exclusive NVP user‡ | 5031 | 7296 | 11 032 | 392 107 | |
| Former NVP user | 0 | 0 | 1717 | 12 811 | |
| Total | 380 525 | 377 046 | 282 457 | 14 217 294 | |
| Life-years lost | Menthol smoker | 1 335 250 | 1 242 012 | 556 131 | 37 846 630 |
| Non-menthol smoker | 1 949 502 | 1 655 744 | 581 810 | 45 122 020 | |
| Former smoker | 1 323 247 | 1 404 460 | 1 050 414 | 53 496 563 | |
| Exclusive NVP user | 86 635 | 122 874 | 181 241 | 6 494 346 | |
| Former smoker-NVP user | 85 815 | 117 704 | 50 734 | 4 246 249 | |
| Former NVP user | 0 | 2 | 32 110 | 278 716 | |
| Total | 4 694 635 | 4 425 092 | 2 401 706 | 143 238 275 | |
| Menthol ban scenario | |||||
| Category | Category/year | 2021 | 2026 | 2060 | Cumulative impact* |
| Prevalence | Menthol smoker | 5.4% | 0.3% | 0.1% | −98.5% |
| Non-menthol smoker | 7.1% | 8.4% | 4.2% | −40.9% | |
| Total smokers† | 12.6% | 8.7% | 4.3% | −65.7% | |
| Former smoker | 19.4% | 19.1% | 9.2% | −52.4% | |
| Exclusive NVP user‡ | 3.5% | 5.7% | 7.4% | 108.0% | |
| Former NVP user | 0.2% | 0.6% | 5.6% | 2418.0% | |
| Smoking and vaping-attributable deaths§ | Menthol smoker | 77 455 | 6792 | 2557 | 271 469 |
| Non-menthol smoker | 122 242 | 151 299 | 55 379 | 4 157 520 | |
| Former smoker | 175 798 | 191 098 | 195 744 | 8 620 599 | |
| Exclusive NVP user | 5031 | 10 768 | 12 859 | 499 475 | |
| Former smoker-NVP user | 5011 | 10 640 | 6815 | 413 819 | |
| Former NVP users | 0 | 0 | 1895 | 14 010 | |
| Total | 380 525 | 359 958 | 268 435 | 13 563 073 | |
| Life-years lost | Menthol smoker | 1 335 250 | 111 678 | 30 555 | 4 174 157 |
| Non-menthol smoker | 1 949 502 | 2 403 756 | 841 520 | 64 926 659 | |
| Former smoker | 1 323 247 | 1 424 993 | 1 065 194 | 54 531 402 | |
| Exclusive NVP user‡ | 86 635 | 122 874 | 181 241 | 6 494 346 | |
| Former smoker-NVP user | 85 815 | 168 033 | 56 050 | 5 418 265 | |
| Former NVP user | 0 | 2 | 35 817 | 306 840 | |
| Total | 4 694 635 | 4 113 651 | 2 182 890 | 131 927 198 | |
| Difference between menthol status quo and menthol ban scenario¶ | |||||
| Relative reduction in prevalence | Menthol smoker | – | −92.5% | −96.5% | – |
| Non-menthol smoker | – | 47.4% | 58.0% | – | |
| Total smokers† | – | −14.7% | −15.1% | – | |
| Total NVP users‡ | – | 22.6% | 26.5% | – | |
| Gain | Averted deaths | – | 17 088 | 14 022 | 654 221 |
| Averted life-years lost | – | 311 441 | 218 817 | 11 311 077 | |
*The cumulative impact is measured in terms of the relative change from 2021 to 2060 for prevalence rates (ie, (2060–2021)/2021) and the sum of the smoking and vaping-attributable deaths or life-years lost over the years 2021 through 2060.
†Total smokers include menthol and non-menthol smokers.
‡Exclusive NVP users include de novo exclusive NVP users and former smokers now using NVPs.
§The number of smoking and vaping-attributable deaths and life-years lost is rounded to the nearest integer.
¶The difference between the Status quo and Menthol ban scenarios includes the comparisons for prevalence in relative terms and for health gains in absolute terms. Relative reduction in prevalence is measured as the relative difference between the status quo scenario and the menthol ban scenario (ie, (postban–preban)/preban) in years 2026 and 2060; the health gain is measured as the change in averted deaths and life-years lost from the Status quo scenario and the Menthol ban scenario.
NVP, nicotine vaping product.
tobaccocontrol-2021-056604supp003.pdf (236.3KB, pdf)
Under the status quo scenario, adult (age >18) menthol smoking prevalence declines from 5.4% in 2021 to 4.5% in 2026 and 2.4% in 2060, while non-menthol smoking prevalence declines from 7.1% in 2021 to 5.7% in 2026 and 2.7% in 2060. Cumulative SVADs from 2021 to 2060 of 14.2 million translate to 143.2 million LYLs.
Under the menthol ban scenario, adult menthol smoking prevalence declines to 0.3% in 2026 and 0.1% in 2060, while non-menthol smoking prevalence increases to 8.4% in 2026 but declines to 4.2% in 2060. Cumulative SVADs of 13.6 million translate to 131.9 million LYLs.
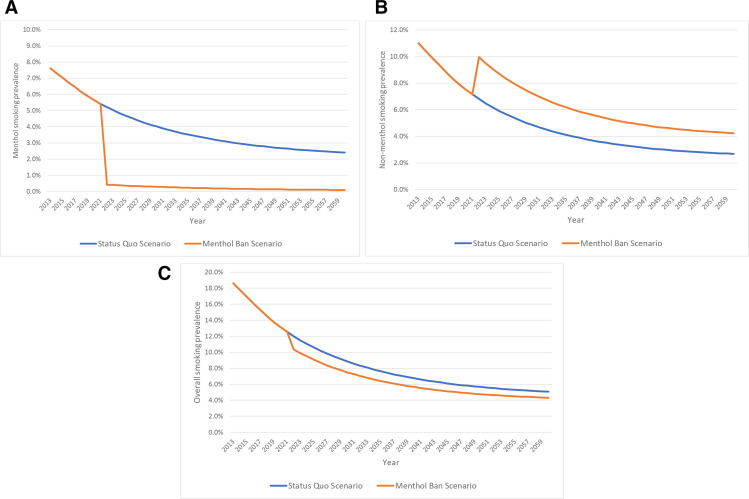
Figure 2A–C shows menthol, non-menthol and overall smoking prevalence from 2013 to 2060 under the status quo scenario and menthol ban scenario. By 2060, combined menthol and non-menthol smoking prevalence falls from 5.1% under the status quo to 4.3% with a menthol ban, a 15.1% relative reduction. Exclusive NVP prevalence increases by 25% under the menthol ban compared with status quo scenario (7.4% vs 5.8%). Cumulative SVADs by 2060 are reduced by 650 000 (4.6% relative reduction), and LYLs are reduced by 11.3 million (7.9% relative reduction).
Figure 2.
(A) Current menthol smoking prevalence (age 18 and above), menthol SAVM, status quo and menthol ban scenarios, 2013–2060. (B) Current non-menthol smoking prevalence (age 18 and above), menthol SAVM, status quo and menthol ban scenarios, 2013–2060. (C) Current overall smoking prevalence (age 18 and above), menthol SAVM, status quo and menthol ban scenarios, 2013–2060. SAVM, smoking and vaping model.
Sensitivity to NVP relative risks and NVP transition parameters
Table 2 provides sensitivity analyses to variations in model parameters relative to the baseline levels (case 1). With NVP risk at 5% of excess smoking mortality risks and baseline levels of other parameters (case 2), both total averted SVADs and LYLs increase by 5%. With NVP risks at 25% (case 3), averted SVADs and LYLs both decline by 5%.
Table 2.
Sensitivity analysis of averted smoking and vaping-attributable deaths and life-years lost to NVP relative risks and individual transition parameters, both genders combined, all ages, 2021–2060
| Case | Description | Smoking and vaping-attributable deaths averted | % change* | Smoking and vaping-attributable life-years lost averted | % change* |
| 1 | Base case with NVP at 15% of cigarette excess mortality risk | 654 221 | – | 11 311 077 | – |
| 2 | NVP risk at 5% of cigarette-attributable excess mortality risk | 687 209 | 5.0 | 11 924 114 | 5.4 |
| 3 | NVP risk at 25% of cigarette-attributable excess mortality risk | 622 425 | −4.9 | 10 707 764 | −5.3 |
| 4 | Reduce overall smoking initiation rates by 10% | 647 128 | −1.1 | 11 083 049 | −2.0 |
| 5 | Increase overall smoking initiation rates by 10% | 661 201 | 1.1 | 11 535 131 | 2.0 |
| 6 | Reduce overall smoking cessation rates by 10% | 702 353 | 7.4 | 11 979 548 | 5.9 |
| 7 | Increase overall smoking cessation rates by 10% | 609 459 | −6.8 | 10 679 917 | −5.6 |
| 8 | Menthol cessation rate the same as non-menthol rate | 461 006 | −29.5 | 8 577 213 | −24.2 |
| 9 | Reduce overall switching rate by 10% | 670 082 | 2.4 | 11 612 042 | 2.7 |
| 10 | Increase overall switching rate by 10% | 638 805 | −2.4 | 11 019 404 | −2.6 |
| 11 | Menthol cessation rate the same as non-menthol rate | 461 006 | −29.5 | 8 577 213 | −24.2 |
| 12 | Reduce the annual decline in switching rate from 10% to 0% | 520 179 | −20.5 | 8 830 696 | −21.9 |
| 13 | Reduce NVP initiation rates by 10% | 654 443 | 0.03 | 11 318 431 | 0.1 |
| 14 | Increase NVP initiation rates by 10% | 654 001 | −0.03 | 11 303 795 | −0.1 |
| 15 | Reduce NVP cessation rates by 10% | 650 266 | −0.6 | 11 253 710 | −0.5 |
| 16 | Increase NVP cessation rates by 10% | 657 768 | 0.5 | 11 363 818 | 0.5 |
*% change is in terms of the relative difference from the base case (eg, (687 209–654 221)/654 221 for case 2 relative to case 1).
NVP, nicotine vaping product.
With a 10% change in the smoking initiation rate (case 4, case 5), averted LYLs vary by 2% in the opposite direction and vary by 6% in the same direction with a 10% change in the overall smoking cessation rate (case 6, case 7). Equating the menthol to non-menthol cessation rate (case 8) reduces the averted LYLs by 24%. With a 10% change in the overall switching rate to NVP use (case 9, case 10), averted LYLs vary by about 3% in the opposite direction. Assuming the same switching rate from menthol smokers as non-menthol smokers (case 11) reduces averted LYLs by 24%. Maintaining the switching rate at the 2018 level rather than assuming a 10% annual decline (case 12) reduces averted LYLs by 22%.
The results were relatively insensitive to NVP initiation (0.1% change) and cessation rates (0.6% change) (cases 13–16).
Discussion
In the absence of a ban on menthol in cigarettes and cigars, the proportion of smokers who smoke menthol cigarettes is likely to continue to increase over time even as overall smoking prevalence declines. With a ban implemented in 2021, we estimated that combined menthol and non-menthol cigarette smoking would decline by 14.7% by 2026 and by 15.1% by 2060 relative to combined smoking in the absence of a ban. With these reductions, SVADs were estimated to fall by about 5% and LYLs by 8.8%, translating to 650 000 deaths averted (16 250 per year) and 11.3 million life-years gained (almost 300 000 per year) over a 40-year period. These impacts are large relative to other tobacco control policies,55 and the public health gains are observed over a wide range of parameter values in the model. Further, while we focus on health gains over a 40-year period, much of the impact is on initiation and related health effects that occur after 40 years. When the analysis is extended to consider a 60-year period, life-years gained increase from 11.3 to 14.7 million (see online supplemental file 3).
Our analysis expands on previous research by incorporating NVP use. A relatively large percentage of menthol smokers, particularly young menthol smokers, switch to NVP use. While increased NVP use presents its own risks, sensitivity analyses indicated that assuming NVP excess mortality risks are 25% those of smokers still yields 620 000 deaths averted and 10.7 million life-years gained under a ban. Increasing the NVP initiation rate and reducing the NVP cessation rate also had minimal effects, despite our assumption that cessation from NVPs is no more likely than from cigarettes. Nevertheless, these risks and the potential for NVPs to be a gateway to smoking, while uncertain, could influence the public health impact of a menthol ban in combustibles. If these prove to be a significant problem, stronger policies may be needed to reduce NVP use among youth. However, while the public health implications were relatively insensitive to changes in the rate of NVP initiation and cessation, they were sensitive to rates of switching from cigarette to NVP use, suggesting that policies to reduce NVP use among youth could also reduce their use by adults, thereby reducing adult smoking cessation.
Our results are conservative in some respects. First, we considered impacts through 2060. Beyond 2060, deaths averted would increase both in absolute and relative terms as the effects on younger generations of reduced smoking are fully realised. We also limited the direct effects of a menthol ban on current menthol smokers to 2 years. Further increases might be expected over time (eg, via additional cessation from illicit menthol or from non-menthol smoking by previous menthol smokers). Our analysis does not consider the effects of a menthol ban on non-menthol smokers. Peer effects of reduced menthol smoking by family, friends, parents or coworkers may motivate more non-menthol smokers to quit.18 While the expert elicitation expected relatively small impacts on non-menthol smokers,19 a 5% reduction in non-menthol use (as suggested by one expert) spread equally between NVPs and no tobacco use would further avert 69 000 deaths (a 9% increase compared with our baseline findings) and 1.1 million LYLs (10% increase) by 2060. Finally, we do not explicitly include current cigar use in this application of the model. Public health benefits are also likely to accrue for current cigar smokers, who may quit all use or switch to NVPs in reaction to a ban.
We did not perform analyses of subpopulations within the USA. Our expert elicitation19 suggested larger impacts on African-Americans. Under a menthol ban, experts estimated 48% of African-Americans who would otherwise initiate menthol smoking would not initiate smoking or vaping compared with 39% for the overall population, and African-American menthol smokers aged 35–54 would be more likely to quit all tobacco use (27% vs 22%). With African-Americans having disproportionately high rates of menthol smoking,3 7 56 a menthol ban would reduce downstream health disparities in smoking-related morbidity and mortality.57 58
Limitations
The results depend on parameters and assumptions underlying the model. While the model was calibrated to incorporate the increase in NVP use through 2018, youth NVP rates increased further in 201959 60 and then fell substantially in 2020,59 indicating that NVP use is difficult to predict. Although some evidence suggests that NVP use may increase smoking initiation,61 62 recent increases in youth vaping coincide with rapid declines in smoking by youth and young adults.63 64 If these reductions in youth and young adult smoking are not maintained, the increased smoking rates among youth and young adults would lead to a larger impact from a menthol ban.
Another limitation is that SAVM does not distinguish dual use of NVPs and cigarettes from exclusive cigarette use. While some studies indicate stable levels of dual use,65 66 other studies indicate dual use is an unstable use state, with high rates of transition to exclusive NVP use or cigarette smoking.27–31 Moreover, some studies suggest similar health risks for dual users as for exclusive smokers,67–69 although others have suggested higher levels.70–72 Further study is warranted on health impacts and patterns of dual use. The model also does not distinguish the health impact experienced by exclusive menthol cigarette smokers who switch to cigar use as a result of a menthol ban. While a recent study found similar levels of biomarker-based risk exposure of exclusive cigar and exclusive cigarette users48 73 and smoking patterns of little cigar users have been found to be similar to those of cigarette users,74 further exploration is warranted on the health impacts of cigar use, especially different types of cigars, for example, little cigars, cigarillos or large cigars. Those switching to smokeless tobacco were also not distinguished. While our expert panel indicated minimal switching to smokeless tobacco, current marketing of oral products, such as ON!,75 may increase the likelihood of switching to these products.
The results are also subject to uncertainties regarding the impact of a menthol ban. The menthol ban transitions were based on results of an expert elicitation.19 While we adopted a well-defined selection process that screened for menthol-related research expertise, the results are dependent on the selected reviewers.19 In addition, because expert elicitations rely on opinions, they are subject to heuristics and biases that are difficult to correct.76–79 The opinions of individual experts differed considerably, especially regarding the extent of switching to exclusive NVP and no use. However, the use of median rather than mean estimates of net transitions (not shown) had little effect on the results. The elicitation results are also consistent with our recent review of menthol ban studies,18 while the magnitude of our findings is broadly consistent with those of a previous menthol ban model17 and a recent study of menthol bans.80
We modelled a ban on menthol applied to both cigarettes and cigars to restrict substitution from cigarettes to little cigars.81–84 We did, however, ask the experts about the impact of a menthol ban on just cigarettes, which the experts indicated would have substantially less impact. We also asked experts about the impact of a menthol ban that is extended to all nicotine delivery products, including NVPs, and they indicated that menthol smokers were less likely to switch out of menthol cigarette use (ie, into NVPs or no regular use) in that scenario compared with a ban limited to cigarettes and cigars. This outcome is consistent with expectations that menthol smokers would be especially likely to switch to menthol NVPs.85 The effects of a menthol ban will also depend on other tobacco control policies. In particular, higher cigarette taxes would reduce smoking initiation and increase cessation,86–90 and increased enforcement of age 21 purchase laws would likely reduce smoking initiation.91 92 While these policies would reinforce the effects of a ban, they may reduce its relative impact, as suggested by our sensitivity analyses regarding reduced smoking initiation and increased smoking cessation.
Finally, the results depend on the modelling approach. Further research might consider expanded categories of nicotine delivery product types (eg, inclusion of smokeless tobacco, distinguishing NVP device type) and multiproduct use, feedback loops via system dynamics models (eg, due to reactions by government or industry to policy changes) and heterogeneity of the population via microsimulation (eg, differential effects by race or socioeconomic status).24 25
Conclusion
Our findings strongly support the implementation of a ban on menthol in cigarettes and cigars on public health grounds. These gains reflect reduced smoking initiation and increased smoking cessation. Support for a menthol ban is strengthened by sensitivity analyses showing that large public health benefits accrue under a broad range of model parameters. Additional public health benefits may be expected through reductions in menthol cigar use.
What this paper adds.
The US Food and Drug Administration (FDA) recently announced its intention to ban menthol in combustible products. Previous research has focused on the relationship between menthol cigarette use and initiation and cessation and on the impact of menthol use on overall smoking, but has not considered the potential impact on future cigarette and nicotine vaping product use if a ban of menthol in cigarettes and cigars were to be implemented. Additional evidence is needed by the FDA on its public health impact.
Our model estimates that such a menthol ban on cigarettes and cigars could prevent 650 000 premature tobacco-related deaths and reduce life-years lost by 11 million over a 40-year period. These gains accrue under a broad range of assumptions.
Our findings strongly support implementation of a ban on menthol in cigarettes and cigars on public health grounds.
Acknowledgments
We would like to thank Stephanie Land for her helpful comments.
Footnotes
Twitter: @AlexCLiber, @cdoug
Contributors: DTL and RM supervised the project and were the principal writers of the original manuscript and the revised manuscript. ZY conducted the original data analysis and wrote the original methods and results sections. YL conducted the original data analysis and helped write the original methods and results sections. CC led the original expert elicitation and helped write the methods section and revisions. LMS-R and NT helped lead the original expert elicitation and helped write the methods section and revisions. MK conducted the data analysis on the expert elicitation and helped write the methods section and revisions. RMi, JLH, NFL, SS, AFB, ACL and CD helped write the original paper and revisions. JJ helped conduct the analysis, and write the original paper and revisions. KEW was a major contributor to the original paper and revisions.
Funding: This project was funded through the National Cancer Institute (NCI) and the Food and Drug Administration (FDA) grant U54CA229974.
Disclaimer: The opinions expressed in this article are the authors’ own and do not reflect the views of the National Institutes of Health, the Department of Health and Human Services, or the US government.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The model and data will be provided upon request. The model and data will be provide upon request.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Kuiper NM, Gammon D, Loomis B, et al. Trends in sales of flavored and menthol tobacco products in the United States during 2011-2015. Nicotine Tob Res 2018;20:698–706. 10.1093/ntr/ntx123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cigarette report for 2018, 2020. Available: www.ftc.gov/reports/federal-trade-commission-cigarette-report-2019-smokeless-tobacco-report-2019 [Accessed 07 Oct 2020].
- 3. Giovino GA, Villanti AC, Mowery PD, et al. Differential trends in cigarette smoking in the USA: is menthol slowing progress? Tob Control 2015;24:28–37. 10.1136/tobaccocontrol-2013-051159 [DOI] [PubMed] [Google Scholar]
- 4. Mattingly DT, Hirschtick JL, Meza R, et al. Trends in prevalence and sociodemographic and geographic patterns of current menthol cigarette use among U.S. adults, 2005-2015. Prev Med Rep 2020;20:101227. 10.1016/j.pmedr.2020.101227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Villanti AC, Mowery PD, Delnevo CD, et al. Changes in the prevalence and correlates of menthol cigarette use in the USA, 2004-2014. Tob Control 2016;25:ii14–20. 10.1136/tobaccocontrol-2016-053329 [DOI] [PubMed] [Google Scholar]
- 6. Delnevo CD, Giovenco DP, Villanti AC. Assessment of menthol and Nonmenthol cigarette consumption in the US, 2000 to 2018. JAMA Netw Open 2020;3:e2013601. 10.1001/jamanetworkopen.2020.13601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. CDC . Menthol and cigarettes, 2020. Available: https://www.cdc.gov/tobacco/basic_information/tobacco_industry/menthol-cigarettes/index.html [Accessed 07 Nov 2020].
- 8. Malone RE. It's the 21st century: isn't it past time to ban menthol cigarette sales? Tob Control 2017;26:359–69. 10.1136/tobaccocontrol-2017-053862 [DOI] [PubMed] [Google Scholar]
- 9. Wagener TL, Meier E, Hale JJ, et al. Pilot investigation of changes in readiness and confidence to quit smoking after e-cigarette experimentation and 1 week of use. Nicotine Tob Res 2014;16:108–14. 10.1093/ntr/ntt138 [DOI] [PubMed] [Google Scholar]
- 10. Delnevo CD, Gundersen DA, Hrywna M, et al. Smoking-Cessation prevalence among U.S. smokers of menthol versus non-menthol cigarettes. Am J Prev Med 2011;41:357–65. 10.1016/j.amepre.2011.06.039 [DOI] [PubMed] [Google Scholar]
- 11. Villanti AC, Collins LK, Niaura RS, et al. Menthol cigarettes and the public health standard: a systematic review. BMC Public Health 2017;17:983. 10.1186/s12889-017-4987-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Staden SR, Groenewald M, Engelbrecht R, et al. Carboxyhaemoglobin levels, health and lifestyle perceptions in smokers converting from tobacco cigarettes to electronic cigarettes. S Afr Med J 2013;103:865–8. 10.7196/samj.6887 [DOI] [PubMed] [Google Scholar]
- 13. States & Localities That Have Restricted the Sale Of Flavored Tobacco Products, 2020. Available: https://www.tobaccofreekids.org/assets/factsheets/0398.pdf [Accessed 28 Sep 2020].
- 14. FDA commits to evidence-based actions aimed at saving lives and preventing future generations of smokers, 2021. Available: https://www.fda.gov/news-events/press-announcements/fda-commits-evidence-based-actions-aimed-saving-lives-and-preventing-future-generations-smokers [Accessed 06 May 2021].
- 15. Schroth KRJ, Villanti AC, Kurti M, et al. Why an FDA ban on menthol is likely to survive a tobacco industry lawsuit. Public Health Rep 2019;134:300–6. 10.1177/0033354919841011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Extension of certain tobacco product compliance deadlines related to the final Deeming rule: guidance for industry, 2017. Available: https://www.fda.gov/downloads/TobaccoProducts/Labeling/RulesRegulationsGuidance/UCM557716.pdf [Accessed 02 Nov 2017].
- 17. Levy DT, Pearson JL, Villanti AC, et al. Modeling the future effects of a menthol ban on smoking prevalence and smoking-attributable deaths in the United States. Am J Public Health 2011;101:1236–40. 10.2105/AJPH.2011.300179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cadham CJ, Sanchez-Romero LM, Fleischer NL, et al. The actual and anticipated effects of a menthol cigarette ban: a scoping review. BMC Public Health 2020;20:1055. 10.1186/s12889-020-09055-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levy DT, Cadham CJ, Sanchez-Romero LM. An expert elicitation on the effects of a ban on menthol cigarettes and Cigars in the United States. Washington, D.C.: Georgetown University. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levy DT, Tam J, Sanchez-Romero LM, et al. Public health implications of vaping in the USA: the smoking and vaping simulation model. Popul Health Metr 2021;19:19. 10.1186/s12963-021-00250-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. University of Michigan Tobacco Centers for Tobacco Control , 2021. Available: https://tcors.umich.edu/Resources_Download.php?FileType=SAV_Model [Accessed 01 Jul 2021].
- 22. Holford TR, Levy DT, Meza R. Comparison of smoking history patterns among African American and white cohorts in the United States born 1890 to 1990. Nicotine Tob Res 2016;18 Suppl 1:S16–29. 10.1093/ntr/ntv274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holford TR, Levy DT, McKay LA, et al. Patterns of birth cohort-specific smoking histories, 1965-2009. Am J Prev Med 2014;46:e31–7. 10.1016/j.amepre.2013.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jeon J, Holford TR, Levy DT, et al. Smoking and lung cancer mortality in the United States from 2015 to 2065: a comparative modeling approach. Ann Intern Med 2018;169:684–93. 10.7326/M18-1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tam J, Levy DT, Jeon J, et al. Projecting the effects of tobacco control policies in the USA through microsimulation: a study protocol. BMJ Open 2018;8:e019169. 10.1136/bmjopen-2017-019169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levy DT, Yuan Z, Li Y, et al. An examination of the variation in estimates of e-cigarette prevalence among U.S. adults. Int J Environ Res Public Health 2019;16:3164. 10.3390/ijerph16173164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coleman B, Rostron B, Johnson SE, et al. Transitions in electronic cigarette use among adults in the population assessment of tobacco and health (path) study, waves 1 and 2 (2013-2015). Tob Control 2019;28:50–9. 10.1136/tobaccocontrol-2017-054174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Azagba S, Shan L, Latham K. Adolescent dual use classification and its association with nicotine dependence and quit intentions. J Adolesc Health 2019;65:195–201. 10.1016/j.jadohealth.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 29. Robertson L, Hoek J, Blank M-L, et al. Dual use of electronic nicotine delivery systems (ends) and smoked tobacco: a qualitative analysis. Tob Control 2019;28:13–19. 10.1136/tobaccocontrol-2017-054070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Borland R, Murray K, Gravely S, et al. A new classification system for describing concurrent use of nicotine vaping products alongside cigarettes (so-called 'dual use'): findings from the ITC-4 Country Smoking and Vaping wave 1 Survey. Addiction 2019;114 Suppl 1:24–34. 10.1111/add.14570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brouwer AF, Jeon J, Hirschtick JL, et al. Transitions between cigarette, ends and dual use in adults in the path study (waves 1-4): multistate transition modelling accounting for complex survey design. Tob Control 2022;31:424–31. 10.1136/tobaccocontrol-2020-055967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality--beyond established causes. N Engl J Med 2015;372:631–40. 10.1056/NEJMsa1407211 [DOI] [PubMed] [Google Scholar]
- 33. Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med 2013;368:351–64. 10.1056/NEJMsa1211127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Population Assessment of Tobacco and Health (PATH) Study. Inter-university Consortium for Political and Social Research [distributor], 2018. Available: https://www.icpsr.umich.edu/icpsrweb/NAHDAP/studies/36498/datadocumentation
- 35. U.S. Department of Health and Human Services . The health consequences of Smoking—50 years of progress: a report of the surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- 36. USDHHS . Preventing tobacco use among youth and young adults: a report of the surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2012. [Google Scholar]
- 37. Fu SS, Okuyemi KS, Partin MR, et al. Menthol cigarettes and smoking cessation during an aided quit attempt. Nicotine Tob Res 2008;10:457–62. 10.1080/14622200801901914 [DOI] [PubMed] [Google Scholar]
- 38. Hoffman AC, Simmons D. Menthol cigarette smoking and nicotine dependence. Tob Induc Dis 2011;9 Suppl 1:S5. 10.1186/1617-9625-9-S1-S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Preliminary scientific evaluation of the possible public health effects of menthol versus Nonmenthol cigarettes, 2013. Available: https://wayback.archive-it.org/7993/20170404193211/https://www.fda.gov/downloads/ScienceResearch/SpecialTopics/PeerReviewofScientificInformationandAssessments/UCM361598.pdf [Accessed 10 Dec 2019].
- 40. Mills SD, Hao Y, Ribisl KM, et al. The relationship between menthol cigarette use, smoking cessation, and relapse: findings from waves 1 to 4 of the population assessment of tobacco and health study. Nicotine Tob Res 2021;23:966–75. 10.1093/ntr/ntaa212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schneller LM, Bansal-Travers M, Mahoney MC, et al. Menthol cigarettes and smoking cessation among adult smokers in the US. Am J Health Behav 2020;44:252–6. 10.5993/AJHB.44.2.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoffman AC. The health effects of menthol cigarettes as compared to non-menthol cigarettes. Tob Induc Dis 2011;9 Suppl 1:S7. 10.1186/1617-9625-9-S1-S7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jones MR, Tellez-Plaza M, Navas-Acien A. Smoking, menthol cigarettes and all-cause, cancer and cardiovascular mortality: evidence from the National health and nutrition examination survey (NHANES) and a meta-analysis. PLoS One 2013;8:e77941. 10.1371/journal.pone.0077941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Delnevo CD, Giovenco DP, Miller Lo EJ. Changes in the Mass-merchandise cigar market since the tobacco control act. Tob Regul Sci 2017;3:S8–16. 10.18001/trs.3.2(suppl1).2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cohn A, Cobb CO, Niaura RS, et al. The other Combustible products: prevalence and correlates of little Cigar/Cigarillo use among cigarette smokers. Nicotine Tob Res 2015;17:1473–81. 10.1093/ntr/ntv022 [DOI] [PubMed] [Google Scholar]
- 46. Richardson A, Rath J, Ganz O, et al. Primary and dual users of little cigars/cigarillos and large cigars: demographic and tobacco use profiles. Nicotine Tob Res 2013;15:1729–36. 10.1093/ntr/ntt053 [DOI] [PubMed] [Google Scholar]
- 47. Stoklosa M. No surge in illicit cigarettes after implementation of menthol ban in nova Scotia. Tob Control 2019;28:702–4. 10.1136/tobaccocontrol-2018-054552 [DOI] [PubMed] [Google Scholar]
- 48. Chang CM, Corey CG, Rostron BL, et al. Systematic review of cigar smoking and all cause and smoking related mortality. BMC Public Health 2015;15:390. 10.1186/s12889-015-1617-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Christensen CH, Rostron B, Cosgrove C, et al. Association of cigarette, cigar, and pipe use with mortality risk in the US population. JAMA Intern Med 2018;178:469–76. 10.1001/jamainternmed.2017.8625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mallock N, Pieper E, Hutzler C, et al. Heated tobacco products: a review of current knowledge and initial assessments. Front Public Health 2019;7:287. 10.3389/fpubh.2019.00287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Simonavicius E, McNeill A, Shahab L, et al. Heat-not-burn tobacco products: a systematic literature review. Tob Control 2019;28:582–94. 10.1136/tobaccocontrol-2018-054419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rodrigo G, Jaccard G, Tafin Djoko D, et al. Cancer potencies and margin of exposure used for comparative risk assessment of heated tobacco products and electronic cigarettes aerosols with cigarette smoke. Arch Toxicol 2021;95:283–98. 10.1007/s00204-020-02924-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McNeill A, Brose L, Calder R, et al. Evidence review of ecigarettes and heated tobacco products 2018. A report commissioned by public health England. London: Public Health England, 2018. [Google Scholar]
- 54. RCo P. Nicotine without smoke. tobacco harm reduction. London: Royal College of Physicians, 2016. [Google Scholar]
- 55. Levy DT, Tam J, Kuo C, et al. The impact of implementing tobacco control policies: the 2017 tobacco control policy Scorecard. J Public Health Manag Pract 2018;24:448–57. 10.1097/PHH.0000000000000780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cornelius ME, Wang TW, Jamal A, et al. Tobacco Product Use Among Adults - United States, 2019. MMWR Morb Mortal Wkly Rep 2020;69:1736–42. 10.15585/mmwr.mm6946a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alexander LA, Trinidad DR, Sakuma K-LK, et al. Why we must continue to investigate menthol's role in the African American smoking paradox. Nicotine Tob Res 2016;18 Suppl 1:S91–101. 10.1093/ntr/ntv209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moolchan ET, Fagan P, Fernander AF, et al. Addressing tobacco-related health disparities. Addiction 2007;102 Suppl 2:30–42. 10.1111/j.1360-0443.2007.01953.x [DOI] [PubMed] [Google Scholar]
- 59. Gentzke AS, Wang TW, Jamal A, et al. Tobacco Product Use Among Middle and High School Students - United States, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1881–8. 10.15585/mmwr.mm6950a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang TW, Gentzke AS, Creamer MR, et al. Tobacco Product Use and Associated Factors Among Middle and High School Students - United States, 2019. MMWR Surveill Summ 2019;68:1–22. 10.15585/mmwr.ss6812a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Levy DT, Borland R, Villanti AC, et al. The application of a Decision-Theoretic model to estimate the public health impact of Vaporized nicotine product initiation in the United States. Nicotine Tob Res 2017;19:149–59. 10.1093/ntr/ntw158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Khouja JN, Suddell SF, Peters SE, et al. Is e-cigarette use in non-smoking young adults associated with later smoking? A systematic review and meta-analysis. Tob Control 2021;30:8–15. 10.1136/tobaccocontrol-2019-055433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Levy DT, Warner KE, Cummings KM, et al. Examining the relationship of vaping to smoking initiation among US youth and young adults: a reality check. Tob Control 2019;28:629–35. 10.1136/tobaccocontrol-2018-054446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Meza R, Jimenez-Mendoza E, Levy DT. Trends in tobacco use among adolescents by grade, sex, and race, 1991-2019. JAMA Netw Open 2020;3:e2027465. 10.1001/jamanetworkopen.2020.27465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bandi P, Cahn Z, Goding Sauer A, et al. Trends in e-cigarette use by age group and Combustible cigarette smoking histories, U.S. adults, 2014-2018. Am J Prev Med 2021;60:151–8. 10.1016/j.amepre.2020.07.026 [DOI] [PubMed] [Google Scholar]
- 66. Owusu D, Huang J, Weaver SR, et al. Patterns and trends of dual use of e-cigarettes and cigarettes among U.S. adults, 2015-2018. Prev Med Rep 2019;16:101009. 10.1016/j.pmedr.2019.101009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Goniewicz ML, Smith DM, Edwards KC, et al. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and Combustible cigarettes. JAMA Netw Open 2018;1:e185937. 10.1001/jamanetworkopen.2018.5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shahab L, Goniewicz ML, Blount BC, et al. Nicotine, carcinogen, and toxin exposure in long-term e-cigarette and nicotine replacement therapy users: a cross-sectional study. Ann Intern Med 2017;166:390–400. 10.7326/M16-1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shahab L, Goniewicz ML, Blount BC, et al. E-Cigarettes and toxin exposure. Ann Intern Med 2017;167:525–6. 10.7326/L17-0315 [DOI] [PubMed] [Google Scholar]
- 70. King JL, Reboussin BA, Wiseman KD, et al. Adverse symptoms users attribute to e-cigarettes: results from a national survey of US adults. Drug Alcohol Depend 2019;196:9–13. 10.1016/j.drugalcdep.2018.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li D, Sundar IK, McIntosh S, et al. Association of smoking and electronic cigarette use with wheezing and related respiratory symptoms in adults: cross-sectional results from the population assessment of tobacco and health (path) study, wave 2. Tob Control 2020;29:140–7. 10.1136/tobaccocontrol-2018-054694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Osei AD, Mirbolouk M, Orimoloye OA, et al. Association between e-cigarette use and cardiovascular disease among never and current Combustible-Cigarette smokers. Am J Med 2019;132:949–54. 10.1016/j.amjmed.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 73. Chang CM, Rostron BL, Chang JT, et al. Biomarkers of exposure among U.S. adult cigar smokers: population assessment of tobacco and health (path) study wave 1 (2013-2014). Cancer Epidemiol Biomarkers Prev 2019;28:943–53. 10.1158/1055-9965.EPI-18-0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Corey CG, Holder-Hayes E, Nguyen AB, et al. Us adult cigar smoking patterns, purchasing behaviors, and reasons for use according to cigar type: findings from the population assessment of tobacco and health (path) study, 2013-2014. Nicotine Tob Res 2018;20:1457–66. 10.1093/ntr/ntx209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Altria enters growing oral nicotine products category with on! pouch product. Available: https://www.bloomberg.com/press-releases/2019-06-03/altria-enters-growing-oral-nicotine-products-category-with-on-pouch-product [Accessed 04 Feb 2021].
- 76. Iglesias CP, Thompson A, Rogowski WH, et al. Reporting guidelines for the use of expert judgement in model-based economic evaluations. Pharmacoeconomics 2016;34:1161–72. 10.1007/s40273-016-0425-9 [DOI] [PubMed] [Google Scholar]
- 77. Morgan MG. Use (and abuse) of expert elicitation in support of decision making for public policy. Proc Natl Acad Sci U S A 2014;111:7176–84. 10.1073/pnas.1319946111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Knol AB, Slottje P, van der Sluijs JP, et al. The use of expert elicitation in environmental health impact assessment: a seven step procedure. Environ Health 2010;9:19. 10.1186/1476-069X-9-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. O’Hagan A. Expert knowledge elicitation: subjective but scientific. Am Stat 2019;73:69–81. 10.1080/00031305.2018.1518265 [DOI] [Google Scholar]
- 80. Chung-Hall J, Fong GT, Meng G, et al. Evaluating the impact of menthol cigarette bans on cessation and smoking behaviours in Canada: longitudinal findings from the Canadian arm of the 2016-2018 ITC four country smoking and Vaping surveys. Tob Control 2022;31:556–63. 10.1136/tobaccocontrol-2020-056259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Delnevo CD, Hrywna M, Giovenco DP, et al. Close, but no cigar: certain cigars are pseudo-cigarettes designed to evade regulation. Tob Control 2017;26:349–54. 10.1136/tobaccocontrol-2016-052935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gammon DG, Loomis BR, Dench DL, et al. Effect of price changes in little cigars and cigarettes on little cigar sales: USA, Q4 2011-Q4 2013. Tob Control 2016;25:538–44. 10.1136/tobaccocontrol-2015-052343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jawad M, Lee JT, Glantz S, et al. Price elasticity of demand of non-cigarette tobacco products: a systematic review and meta-analysis. Tob Control 2018;27:689–95. 10.1136/tobaccocontrol-2017-054056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Messer K, White MM, Strong DR, et al. Trends in use of little cigars or cigarillos and cigarettes among U.S. smokers, 2002-2011. Nicotine Tob Res 2015;17:515–23. 10.1093/ntr/ntu179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Buckell J, Marti J, Sindelar JL. Should flavours be banned in cigarettes and e-cigarettes? evidence on adult smokers and recent quitters from a discrete choice experiment. Tob Control 2019;28:168–75. 10.1136/tobaccocontrol-2017-054165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Huang J, Gwarnicki C, Xu X, et al. A comprehensive examination of own- and cross-price elasticities of tobacco and nicotine replacement products in the U.S. Prev Med 2018;117:107–14. 10.1016/j.ypmed.2018.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pesko MF, Huang J, Johnston LD, et al. E-Cigarette price sensitivity among middle- and high-school students: evidence from monitoring the future. Addiction 2018;113:896–906. 10.1111/add.14119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stoklosa M, Drope J, Chaloupka FJ. Prices and e-cigarette demand: evidence from the European Union. Nicotine Tob Res 2016;18:1973–80. 10.1093/ntr/ntw109 [DOI] [PubMed] [Google Scholar]
- 89. Zheng Y, Zhen C, Dench D, et al. U.S. demand for tobacco products in a system framework. Health Econ 2017;26:1067–86. 10.1002/hec.3384 [DOI] [PubMed] [Google Scholar]
- 90. Chaloupka FJ, Sweanor D, Warner KE. Differential Taxes for Differential Risks--Toward Reduced Harm from Nicotine-Yielding Products. N Engl J Med 2015;373:594–7. 10.1056/NEJMp1505710 [DOI] [PubMed] [Google Scholar]
- 91. Friedman AS, Buckell J, Sindelar JL. Tobacco-21 laws and young adult smoking: quasi-experimental evidence. Addiction 2019;114:1816–23. 10.1111/add.14653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Friedman AS, Wu RJ. Do local Tobacco-21 laws reduce smoking among 18 to 20 Year-Olds? Nicotine Tob Res 2020;22:1195–201. 10.1093/ntr/ntz123 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
tobaccocontrol-2021-056604supp001.pdf (823.6KB, pdf)
tobaccocontrol-2021-056604supp002.pdf (749.8KB, pdf)
tobaccocontrol-2021-056604supp003.pdf (236.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The model and data will be provided upon request. The model and data will be provide upon request.