Abstract
Objectives:
Evaluate the relationship between calorie intake and post-discharge outcomes in hospitalized patients with heart failure (HF).
Background:
Malnutrition increases adverse outcomes in HF, and dietary sodium restriction may inadvertently worsen nutritional intake.
Methods:
In a dietary intervention trial, baseline nutritional intake in HF inpatients was estimated via Block Food Frequency Questionnaire (FFQ) and a Nutritional Risk Index (NRI) was calculated. Insufficient calorie intake was defined at <90% of metabolic needs, and a 15-point micronutrient deficiency score was created. Adjusted linear, logistic, and negative binomial regression were used to evaluate associations between insufficient calorie intake and quality of life (Kansas City Cardiomyopathy Questionnaire Clinical Summary [KCCQ-CS]), readmission risk, and days rehospitalized over 12 weeks.
Results:
Among 57 participants (age 70±8 years, 31% female, body mass index 32±8 kg/m2), median sodium and calorie intake were 2,987 (IQR 2,160–3,540) mg/day and 1,602 (IQR 1,2012,142) kcal/day; 11% of patients screened as malnourished by NRI. All patients consuming <2,000 mg/day sodium had insufficient calorie intake; this group also more frequently had dietary micronutrient and protein deficiencies. At 12 weeks, patients with insufficient calorie intake had less improvement in KCCQ-CS (β=−14.6, 95% CI −27.3_−1.9), higher readmission odds (OR 14.5, 95% CI 2.2–94.4), and more days rehospitalized (IRR 31.3, 95% CI 4.3–229.3).
Conclusions:
Despite high prevalence of obesity and rare overt malnutrition, insufficient calorie intake was associated with poorer post-discharge quality of life and increased readmission burden in patients with HF. Inpatient dietary assessment could improve readmission risk stratification and identify patients for nutritional intervention.
Keywords: nutrition, diet, sodium, hospitalization
INTRODUCTION
Dietary sodium excess can precipitate HF hospitalization by causing fluid retention (1), and a low sodium diet is the most widely-advised self-care behavior in HF (2). However, in several randomized trials this strategy increased readmission and death in recently discharged patients (3,4). Dietary sodium restriction can inadvertently worsen nutritional status (5), which could explain these counter-intuitive results. Malnutrition is highly prevalent in heart failure (HF) inpatients, strongly predicts functional decline, readmission, and death (6–10), and may be central to the “post-hospital syndrome,” a depletion of physiological reserve that increases readmission risk for up to six weeks post-discharge (11). Interventions to improve nutritional status in patients discharged from HF hospitalization have the potential to increase post-discharge quality of life, improve exercise capacity, and reduce readmission burden (12,13). Despite these observations, no previous studies have formally described dietary deficiencies or their consequences in U.S. patients hospitalized for decompensated HF. The Geriatric OUt-of-hospital Randomized MEal Trial in Heart Failure (GOURMET-HF; NCT02148679) study evaluated the effects of home-delivered, nutritionally robust low sodium meals on health-related quality of life following discharge from HF hospitalization (13). As part of baseline testing, GOURMET-HF participants completed a Block Food Frequency Questionnaire (FFQ; see Supplementary Data), which was used to estimate nutrient and energy (calorie) intake. Our primary hypotheses were that patients with insufficient calorie intake to meet metabolic needs would have less post-discharge improvement in functional/symptom-related quality of life and greater readmission burden at 12 weeks. We also hypothesized that patients with low estimated sodium intake would more frequently have insufficient calorie intake, as well as greater prevalence of dietary protein and micronutrient deficiencies.
METHODS
The GOURMET-HF study was approved by University of Michigan’s IRBMED, the VA Ann Arbor’s IRB, and Columbia University’s IRB and registered at clinicaltrials.gov (NCT02148679).The primary results of the GOURMET-HF study have been previously published (13). In brief, 66 patients aged ≥ 55 years who had been hospitalized for decompensated HF were randomized at hospital discharge to receive prepackaged, home-delivered, low sodium, nutritionally robust meals for 4 weeks vs. usual care. The primary outcome of the three-site study was the inter-group change between hospital discharge and 4 weeks post-discharge in the Overall Summary score from the Kansas City Cardiomyopathy Questionnaire (KCCQ). Randomized participants were followed for 12 weeks, at which point the KCCQ was repeated and dates of hospitalizations and deaths were obtained.
Nutrient and energy intake estimation
During the enrollment hospitalization, most GOURMET-HF participants completed a Block FFQ. This 110-item questionnaire records the habitual intake of commonly consumed foods in the United States and can be used to estimate overall nutrient and energy intake. The instrument was developed based on the National Health and Nutrition Examination Survey, and contains adjustment questions to increase accuracy in assessing macronutrient and overall energy intake. We estimated baseline intake of calories, protein, sodium, and other micronutrients in all GOURMET-HF participants that completed a Block FFQ. Analysis of dietary nutrient content is performed with standardized software (NutritionQuest, Berkley, CA), and results have previously been validated against multiple-day dietary records in older adults (14).
Estimated Energy Needs
Caloric intake needs depend on the resting energy expenditure (REE) and the amount of physical activity performed, which combined define the total energy expenditure (TEE). The gold standard for measuring REE is indirect calorimetry. When indirect calorimetry is not available (as in this study), predictive equations are used to estimate REE and TEE. The Academy of Nutrition and Dietetics guidelines for caloric intake in HF (15) are based primarily on indirect calorimetry performed by Aquilani et al. in 57 young (age 52±3 years), non-obese, non-diabetic, stable participants with HF and reduced ejection fraction (16).
The GOURMET-HF study enrolled hospitalized older adults (age 71±8 years) who were often obese (body mass index 33±7 kg/m2) and had multiple comorbid conditions (e.g. >50 % with diabetes mellitus); approximately one-third had HF with preserved ejection fraction. Due to the substantial differences between this group and the Aquilani et al. cohort, we looked to previous work in older adults and in hospitalized patients, and estimated TEE using discharge body weight and the revised Harris-Benedict equation with a multiplier of 1.1 (17,18) for the analysis:
For the primary analysis, we conservatively defined insufficient calorie intake at below 90% of the estimated TEE by these equations. We performed secondary analyses using Academy of Nutrition and Dietetics caloric intake guidance for advanced HF, defining insufficient threshold at below 18 kcal/kg/day * 1.1 (multiplier for sedentary activity level).
Micronutrient and protein deficiencies
In similar manner to recent work by Lennie et al. in outpatients with HF (6), we assigned one point for each of 15 micronutrients with insufficient estimated intake: magnesium, folate, calcium, iron, selenium, zinc, niacin, thiamine, riboflavin, and vitamins A, B6, B12, C, D, and E. We did not include vitamin K or potassium, as medication use (e.g. warfarin or spironolactone) may affect dietary guidance for individual patients. We then summed the deficiencies to create a micronutrient deficiency score 1–15. Thresholds for adequate intake were defined according to Dietary Reference Intake recommendations from the Institute of Medicine’s Food and Nutrition Board (19). Using the Academy of Nutrition and Dietetics recommendations to prevent catabolism in HF (15), we defined adequate protein intake as 1.1 g/kg based on discharge body weight.
Relationship between dietary components
We compared the dietary micronutrient deficiency score and adequacy of protein intake above and below the median sodium intake using 2-sample t-testing after confirming normality using the Shapiro-Wilk test. We graphically assessed the relationship between sodium intake and the adequacy of calorie intake.
Malnutrition screening
The Nutritional Risk Index (NRI) uses serum albumin and the ratio of body weight to ideal body weight to assess the risk of nutrition-related complications. Our group and others have shown that the NRI predicts death and readmission in patients hospitalized for HF (10,20).
The NRI is calculated as follows:
NRI = (1.519 × serum albumin, g/dL) + [41.7 × discharge weight (kg)/ideal body weight(kg)] Scores of >100, 97.5–100, 83.5–97.5, and <83.5 indicate no, mild, moderate, and severe risk of nutrition-related complications. We used the Devine and Robinson formulas to calculate ideal body weight for men and women, respectively (10). Due to the high prevalence of obesity in this sample, as in our prior work (10) we replaced the ratio of discharge to ideal body weight = 1 if discharge weight was greater than estimated ideal body weight and used this modified NRI in outcome analysis. We also calculated the Prognostic Nutritional Index (PNI), a non-weight based measure that predicts adverse outcomes in outpatients with HF (9):
PNI = (10 x serum albumin, g/dL) + (0.005 x total lymphocyte count, mm3) Scores of >38, 35–38, and <35, respectively, indicate no, moderate, or severe malnutrition.
Relationship between insufficient calorie intake and outcomes
We evaluated the association of insufficient caloric intake with two outcomes, obtained at 12 weeks post-discharge: 1) change in the KCCQ Clinical Summary score [KCCQ-CS, the average of HF symptom and physical limitations domains (21)], and 2) all-cause hospital readmission. We adjusted for other known predictors of each outcome. For change in KCCQ-CS, we used linear regression adjusted for age, sex, discharge KCCQ-CS, randomization status (home-delivered meals vs. usual care), micronutrient deficiency score, and sodium intake (dichotomized at the median).
No patients died over the 12 weeks post-discharge. We used logistic regression to evaluate the association between insufficient calorie intake and all-cause rehospitalization over 12 weeks of follow-up, adjusted for NRI category (normally nourished, mild, or moderate nutritional risk) and the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) risk model. This multivariable score stratifies patients with HF for mortality risk within 60 days of discharge, and includes age, weight, comorbidities, and admission blood pressure, creatinine, and serum sodium (22). We assessed discrimination for all-cause readmission with and without NRI and caloric intake. For days rehospitalized, which represent over-dispersed count data, we used negative binomial regression, adjusted for NRI category, OPTIMIZE-HF score, randomization to meals vs. usual care, and the number of cardiovascular hospitalizations within the prior year (categorized as zero, one, two or more)(23).
RESULTS
Dietary characteristics
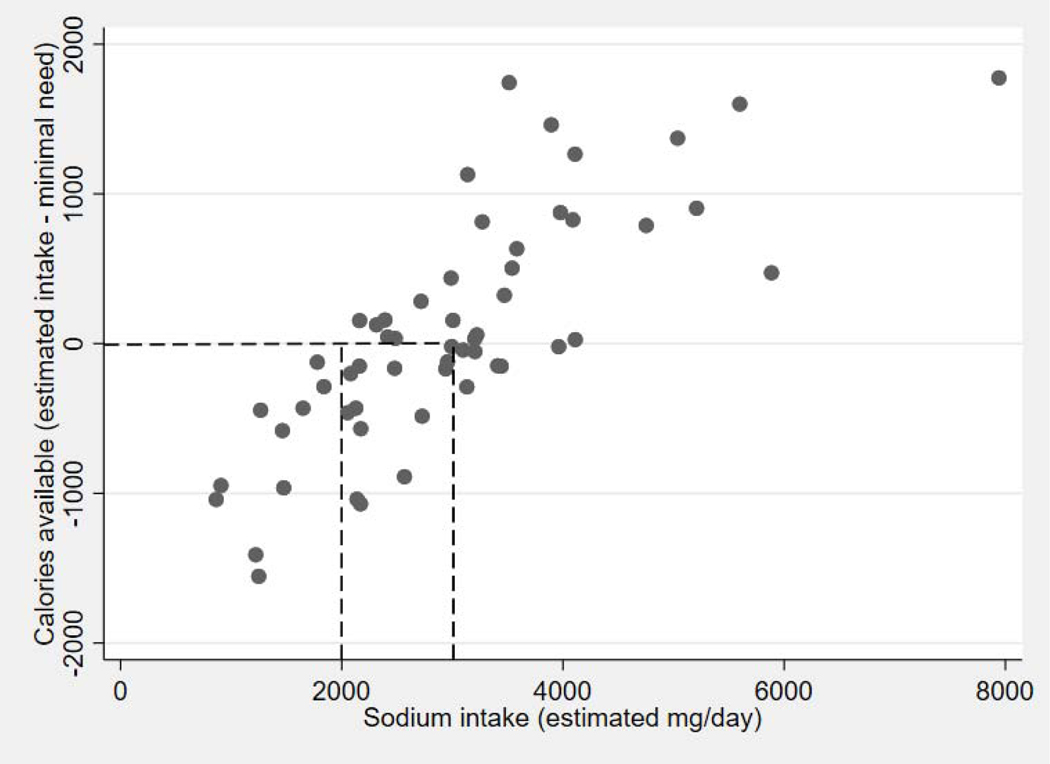
In the 57 randomized GOURMET-HF participants with completed FFQ, the median sodium intake was 2,987 mg/day (IQR 2,160–3,540 mg/day), and the median calorie intake was 1,602 kcal/day (IQR 1,201–2,142 kcal/day); 53% (29/57) of participants had insufficient caloric intake (as defined above, <90% of estimated TEE). Body mass index at discharge was significantly higher and age was younger in patients with insufficient calorie intake. Several comorbidities were numerically, but not statistically, more common (Table 1). Most patients below the median sodium intake and all with estimated sodium intake <2000 mg/day (the most commonly recommended sodium intake for patients with HF) had insufficient calorie intake (Figure 1).
Table 1.
Demographic and clinical characteristics
| Overall cohort | Adequate calorie intake | Insufficient calorie intake | |
|---|---|---|---|
| Age (years) | 70±8 | 73±8 | 67±8* |
| Sex (% female) | 31% | 31% | 31% |
| Body mass index (kg/m2) | 32±8 | 30±7 | 34±8* |
| Ejection fraction (%) | 38±19 | 38±19 | 40±19 |
| Coronary artery disease | 43% | 38% | 47% |
| Atrial fibrillation | 51% | 38% | 61% |
| Stroke/vascular disease | 27% | 32% | 24% |
| Diabetes mellitus | 58% | 54% | 62% |
| Chronic kidney disease (eGFR <60) | 65% | 54% | 76% |
Abbreviations: eGFR, estimated glomerular filtration rate
p<0.05 for comparision between adequate and insufficient calorie intake
Figure 1. Relationship between sodium intake and energy available above minimal needs.
Scatter-plot relationship between sodium intake and the energy available for physical activity. Values below zero on the y-axis represent insufficient calorie intake.
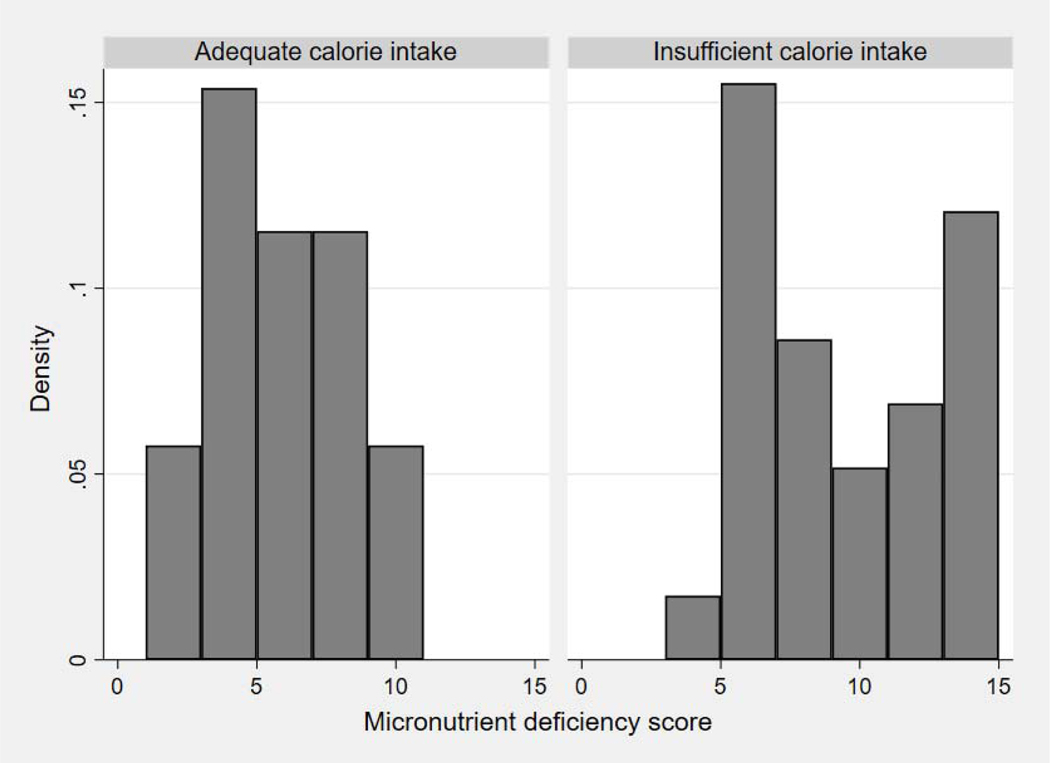
Similarly, all patients with sodium intake <2000 mg/day had insufficient protein intake. When expressed as a ratio of adequate intake, protein consumption was significantly lower in patients with insufficient calorie intake (ratio 0.5±0.2 vs. 1.0 ±0.3, p <0.001) and in those consuming less than the median sodium intake (ratio 0.5±0.2 vs. 0.9 ±0.3, p <0.001). In addition, the number of dietary micronutrient deficiencies was greater among patients with insufficient calorie intake (9±4 vs. 5±3 points; Figure 2) as well as in those consuming less than the median sodium intake (10±3 vs. 5±2 points, p < 0.001).
Figure 2. Relationship between calorie intake and dietary micronutrient deficiencies.
Histogram of dietary micronutrient deficiency scores, with groups defined by insufficient or adequate calorie intake. Higher scores represent a larger number of micronutrient deficiencies.
Malnutrition screening
In the GOURMET-HF cohort, using the standard NRI equation 11% (6/53) patients screened as at risk of nutrition-related complications. With the weight ratio modified as above, the prevalence of normal nutrition and mild, moderate, or severe risk of nutrition-related complications was 41%, 17%, 41%, and 0%, respectively. Using the PNI, 6% (4/62) of participants with complete data screened as having moderate malnutrition, and none had severe malnutrition.
Clinical outcomes
The KCCQ-CS was available at discharge and 12 weeks in 50/57 participants with complete FFQ data. The KCCQ-CS scores were similar at hospital discharge in patients with adequate and insufficient caloric intake [median 48 (IQR 34–60) vs. median 46 (IQR 28–57), p=0.60]. The KCCQ-CS increased from discharge to week 12 [median 46 (IQR 31–59) to median 63 (IQR 45–80), p<0.001]. After adjustment for randomization assignment, discharge KCCQ-CS, age, and sex, insufficient calorie intake was associated with less improvement in KCCQ-CS at week 12 (Table 2; ≥7 points is a clinically significant difference). Results were slightly attenuated but similar using the Academy of Nutrition and Dietetics threshold for calorie intake in advanced HF (β= −12.7 points, p=0.058).
Table 2.
Unadjusted and adjusted analyses for clinical outcomes
| Change in KCCQ-CS [β (95% CI)] | P | All-cause readmission [OR (95% CI)] | P | Days rehospitalized [IRR (95% CI)] | P | |
|---|---|---|---|---|---|---|
| Unadjusted analysis | ||||||
| Insufficient calorie intake | −11.7 (−25.2 – 1.84) | 0.09 | 4.5 (1.3–15.2) | 0.02 | 4.6 (1.4–15.9) | 0.02 |
| Adjusted analyses | ||||||
| Insufficient calorie intake | −14.6 (−27.3 -- −1.9) | 0.03 | 14.5 (2.2–94.4) | 0.005 | 31.3 (4.3–229.3) | 0.001 |
| Age (years) | −0.6 (−1.5 – 0.2) | 0.17 | ||||
| Sex (female) | −8.2 (−21.4 – 5.1) | 0.22 | ||||
| Discharge KCCQ-CS (per point) | −0.5 (−0.8 -- −0.2) | 0.001 | 0.96 (0.9–1.0) | 0.09 | ||
| Randomization assignment | 4.3 (−8.2 – 16.7) | 0.50 | 3.2 (0.6–16.8) | 0.17 | ||
| OPTIMIZE-HF risk score (per point) | 1.2 (1.0–1.4) | 0.02 | 1.1 (0.99–1.3) | 0.08 | ||
| Cardiovascular admissions in 12 mos. | reference | |||||
| (0, reference) | ||||||
| 1 | 2.6 (0.1–53.8) | 0.53 | ||||
| 2 or more | 1.7 (0.1–24.1) | 0.70 | ||||
| Nutritional Risk Index (not at risk, reference) | reference | reference | ||||
| Mild risk | 25.2 (2.5–253.6) | 0.006 | 6.7 (0.9–47.4) | 0.06 | ||
| Moderate risk | 7.7 (1.3–45.9) | 0.02 | 2.5 (0.6–11.4) | 0.23 | ||
Abbreviations: KCCQ-CS, Kansas City Cardiomyopathy Questionnaire Clinical Summary; OPTIMIZE-HF, Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure
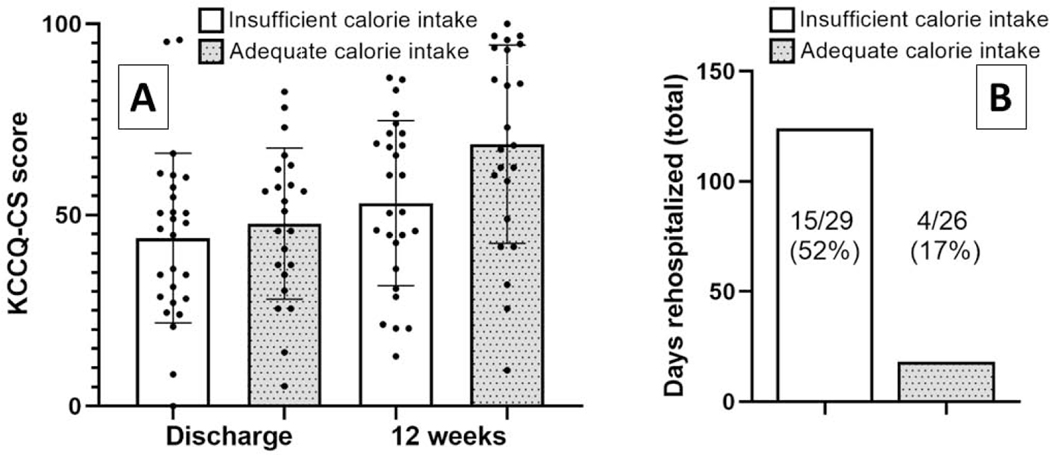
Complete data for risk stratification and estimated caloric needs were available for 53/57 patients with FFQ data. After adjustment for OPTIMIZE-HF risk score and NRI category as above, insufficient calorie intake was strongly associated with a greater risk of readmission (Table 2). Adding the NRI category to the OPTIMIZE-HF risk score increased the C-statistic for all-cause readmission from 0.61 to 0.78, and adding caloric intake further increased the C-statistic to 0.85. After adjustment for randomization assignment, discharge KCCQ-CS, previous hospitalizations, OPTIMIZE-HF risk score, and NRI category as above, insufficient calorie intake was also independently associated with a greater number of days rehospitalized (Table 2). Among patients with insufficient caloric intake, 52% (15/29) were rehospitalized for a total of 124 days, while 17% (4/24) patients with above-threshold calorie intake were readmitted for a total of 18 days. This finding did not relate to standard prognostic factors, as the number of previous cardiovascular hospitalizations and OPTIMIZE-HF risk score did not differ by calorie intake. Results were similar using the Academy of Nutrition and Dietetics guidance for advanced HF to define insufficient calorie intake. Sodium intake was not associated with any of the outcomes studied (all p >0.05).
DISCUSSION
In older patients hospitalized with HF, reported low sodium intake was associated with dietary protein and micronutrient deficiencies and insufficient caloric intake to support baseline metabolic needs. In turn, insufficient baseline caloric intake predicted poorer post-discharge quality of life and increased burden of readmission over 12 weeks post-discharge. These observations were independent of standard HF prognostic factors and a commonly used nutritional risk screener.
Excess dietary sodium can contribute to volume overload, and may precipitate a substantial proportion of HF hospitalizations (1). Accordingly, dietary guidance in HF has historically focused on restriction of sodium intake (2). Recently hospitalized patients with HF would seem to be the ideal population for interventions to reduce dietary sodium, but published trials raise concerns about this approach. The largest study randomized 1,927 Italian patients 1:1 to low (1,840 mg/day) vs. moderate (2,760 mg/day) sodium diet during hospitalization for HF, with the assigned diet continuing post-discharge. While these data are challenging to interpret due to differential inpatient use of hypertonic saline, participants assigned to the low-sodium diet had a much greater risk of death and readmission over long-term follow-up (3). The U.S. Heart Failure Adherence and Retention Trial study randomized outpatients with HF to a self-management intervention vs. usual education. After propensity-matching, participants who reported sodium intake <2,500 mg/day by questionnaire had a higher long-term risk of hospitalization than those with higher sodium intake (4).
The potential adverse effects of sodium restriction in HF are often attributed to volume contraction and neurohormonal activation. While this may occur in some cases, little attention has focused on the role of non-sodium dietary components, which were not analyzed in the trials cited above. In a small intervention study in outpatients with HF, successful dietary sodium restriction was associated with decreased consumption of several key micronutrients (5). Large food diary studies in U.S. and South Korean outpatients with HF link dietary micronutrient deficiencies to increased risk of all-cause and cardiovascular hospitalization (6,7). We also observed greater dietary micronutrient deficiency in inpatients who reported guideline-recommended sodium restriction.
In addition to micronutrient deficits, it is possible that patients with HF who restrict sodium may not consume enough calories to maintain muscle mass and physical function, particularly in the vulnerable post-hospital setting (11). Sodium and calorie intake are closely correlated in the U.S. population. All GOURMET-HF participants who reported adherence with the commonly used <2000 mg/day threshold for sodium intake had calorie intake below their estimated metabolic needs. Protein intake was also more often deficient in such patients. When calorie intake is low, adequate protein consumption helps prevent loss of lean muscle mass (24). Given the high prevalence of frailty and sarcopenia in HF (25), protein intake may be especially important in this population.
A recent meta-analysis noted that more than half of inpatients with decompensated HF screen positive for undernutrition (comprising overt malnutrition or at risk of nutrition-related complications). Depending on the screening instrument used, malnutrition was associated with a two- to four-fold increase in mortality, and also predicted more frequent hospital readmissions (8).Overt cardiac cachexia is a worrisome sign in HF, and most nutrition screening instruments incorporate body mass index and/or recent weight loss as key variables. However, these instruments were evaluated in HF cohorts with relatively low prevalence of obesity (8,9). Only 11% of GOURMET-HF participants screened as at-risk by the standard NRI equation, 6% by PNI, and none were found to have severe malnutrition. Moreover, while higher body mass index is generally considered a favorable prognostic factor in HF, we found that insufficient calorie intake was more common in obese patients. These data outline a missed opportunity to evaluate and intervene on malnutrition in obese older inpatients with HF.
The potential value of post-discharge dietary intervention was clearly illustrated by the Nutritional Intervention in Malnourished Hospitalized Patients with Heart Failure study. In this trial, 120 Spanish HF inpatients at risk of malnutrition were randomized 1:1 to a six-month intensive nutritional intervention. Over the next year, intervention group participants had a markedly lower combined risk of HF readmission or death (27 vs. 61%) and all-cause mortality (20 vs 48%) (12). This intervention comprised monthly outpatient visits with a physician-led dietitian team, and could be difficult to replicate on a large scale. The primary results of the GOURMET-HF pilot suggest that home-delivered meals have scalable potential to improve post-discharge outcomes (13). The optimal nutritional characteristics for dietary intervention have not been fully defined. Epidemiological evidence supports the Dietary Approaches to Stop Hypertension (DASH) and Mediterranean diet patterns (26). Pilot intervention studies also suggest potential for the DASH diet (27,28) in HF as well as for increased intake of unsaturated fatty acids in HFpEF (29). In cachectic patients with HF, protein supplementation can increase body weight and has the potential to improve quality of life (30).
Our findings demonstrate the prognostic value of dietary assessment in hospitalized patients with HF, and also provide another possible method to select appropriate patients for dietary intervention. It is important to note that calorie restriction has been shown to improve exercise capacity in obese patients with stable HFpEF (31). However, the sustainability of related fat mass loss and physiological benefits is uncertain (32), as are the effects of calorie restriction in more ill HF populations. More research is urgently needed to define which patients with HF should consume more and which fewer calories to improve short- and long-term outcomes.
Limitations
We analyzed a small cohort of patients who participated in a dietary intervention trial. While GOURMET-HF was both geographically and ethnically diverse, our results need to be validated in larger community-based samples. The FFQ used in this study has been criticized for the potential to produce biased estimates of nutrient intakes (33). However, the estimated daily sodium intake in GOURMET-HF was comparable to other studies in patients with HF that used questionnaires or repeated food records (4,6). While repeated food records are not suitable for inpatient use, dietary assessment could also be done via calorie count or dietitian interview (15).
We evaluated two malnutrition screening instruments (the NRI and PNI) that are prognostic in patients with HF across varied clinical settings (8–10). However, very few GOURMET-HF patients were identified as malnourished by these instruments, possibly because of the high prevalence of obesity. A recent consensus statement on nutrition in HF advocated including questionnaire-based and nutrition-focused physical examination data for effective nutritional risk stratification in this population (34). Regarding caloric needs, the REE and TEE in patients with HF are not well-established in contemporary cohorts. We used two different, conservatively designed thresholds for adequate calorie intake and found similar results with both. Although we adjusted for other known predictors of adverse outcomes, we cannot assign causality to insufficient calorie intake. We also cannot definitively ascribe any potential harm from low sodium intake to associated dietary deficiencies. However, we believe our results highlight the need for more comprehensive dietary counseling in HF beyond sodium restriction.
CONCLUSIONS
In older patients hospitalized for HF, insufficient calorie intake is independently associated with poorer quality of life and greater readmission burden at 12 weeks post-discharge.
Supplementary Material
Central Illustration: Calorie intake and (A) change in KCCQ-CS, (B) readmission burden over 12 weeks.
Panel A of the Central Illustration represents a column scatter plot of KCCQ-CS scores at baseline and Week 12. Panel B represents a bar graph of total days rehospitalized over 12 weeks, overlaid with the number of patients who were rehospitalized. White bars denote insufficient and grey bars adequate calorie intake.
Abbreviation: KCCQ-CS, Kansas City Cardiomyopathy Questionnaire
CLINICAL PERSPECTIVES
Dietary sodium restriction, guideline-recommended for patients with symptomatic heart failure (HF), may inadvertently contribute to malnutrition, a major risk factor for poor outcomes. In HF inpatients, low dietary sodium intake was frequently accompanied by inadequate intake of protein, micronutrients, and calories. In turn, insufficient calorie intake was associated with poorer post-discharge quality of life and increased readmission burden. Inpatient dietary assessment could improve readmission risk stratification and has the potential to identify patients with HF who could benefit from nutritional intervention.
TRANSLATIONAL OUTLOOK
Insufficient calorie intake was associated with poor post-discharge outcomes in this cohort of patients hospitalized for HF. However, other lines of evidence in animals and humans indicate that caloric restriction can produce cardiovascular and anti-aging benefits. More work is urgently needed to define energy needs and clarify appropriate calorie intake in acute and chronic HF. Similarly, better methods to assess dietary quality and promote high-quality nutritional intake in patients with HF are needed.
Acknowledgments
Funding: the GOURMET-HF trial was jointly funded by NIH/NIA (R21-AG047939) and PurFoods, LLC (Ankeny, IA)
Relationship with industry: unrestricted research funding from PurFoods, LLC
ABBREVIATIONS:
- DASH
Dietary Approaches to Stop Hypertension
- FFQ
Food Frequency Questionnaire
- HF
heart failure
- KCCQ-CS
Kansas City Cardiomyopathy Questionnaire Clinical Summary
- NRI
Nutritional Risk Index
- OPTIMIZE-HF
Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure
- PNI
Prognostic Nutrition Index
- REE
resting energy expenditure
- TEE
total energy expenditure
Footnotes
CLINICAL TRIAL REGISTRATION: https://clinicaltrials.gov/ct2/show/NCT02148679
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Michalsen A, Konig G, Thimme W. Preventable causative factors leading to hospital admission with decompensated heart failure. [See comments.]. Heart 1998;80:437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta D, Georgiopoulou VV, Kalogeropoulos AP et al. Dietary Sodium Intake in Heart Failure. Circulation 2012;126:479–485. [DOI] [PubMed] [Google Scholar]
- 3.Paterna S, Fasullo S, Parrinello G et al. Short-term effects of hypertonic saline solution in acute heart failure and long-term effects of a moderate sodium restriction in patients with compensated heart failure with New York Heart Association class III (Class C) (SMAC-HF Study). Am J Med Sci 2011;342:27–37. [DOI] [PubMed] [Google Scholar]
- 4.Doukky R, Avery E, Mangla A et al. Impact of Dietary Sodium Restriction on Heart Failure Outcomes. JACC: Heart Failure 2016;4:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jefferson K, Ahmed M, Choleva M et al. Effect of a Sodium-Restricted Diet on Intake of Other Nutrients in Heart Failure: Implications for Research and Clinical Practice. J Card Fail 2015;21:959–962. [DOI] [PubMed] [Google Scholar]
- 6.Lennie TA, Andreae C, Rayens MK et al. Micronutrient Deficiency Independently Predicts Time to Event in Patients with Heart Failure. Journal of the American Heart Association 2018:(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song EK, Moser DK, Kang SM, Lennie TA. Association of Depressive Symptoms and Micronutrient Deficiency With Cardiac Event-Free Survival in Patients With Heart Failure. J Card Fail 2015;21:945–51. [DOI] [PubMed] [Google Scholar]
- 8.Lin H, Zhang H, Lin Z, Li X, Kong X, Sun G. Review of nutritional screening and assessment tools and clinical outcomes in heart failure. Heart Fail Rev 2016;21:549–565. [DOI] [PubMed] [Google Scholar]
- 9.Sze S, Pellicori P, Kazmi S et al. Prevalence and Prognostic Significance of Malnutrition Using 3 Scoring Systems Among Outpatients With Heart Failure. A Comparison With Body Mass Index 2018;6:476–486. [DOI] [PubMed] [Google Scholar]
- 10.Adejumo OL, Koelling TM, Hummel SL. Nutritional Risk Index predicts mortality in hospitalized advanced heart failure patients. J Heart Lung Transplant 2015;34:1385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dharmarajan K, Hsieh AF, Kulkarni VT et al. Trajectories of risk after hospitalization for heart failure, acute myocardial infarction, or pneumonia: retrospective cohort study. BMJ : British Medical Journal 2015;350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonilla-Palomas JL, Gámez-López AL, Castillo-Domínguez JC et al. Nutritional Intervention in Malnourished Hospitalized Patients with Heart Failure. Arch Med Res 2016;47:535–540. [DOI] [PubMed] [Google Scholar]
- 13.Hummel SL, Karmally W, Gilespie BG et al. Home-Delivered Meals Postdischarge From Heart Failure Hospitalization: the GOURMET-HF Pilot Study. Circulation: heart failure 2018;11:e004886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mares-Perlman JA, Klein BE, Klein R, Ritter LL, Fisher MR, Freudenheim JL. A diet history questionnaire ranks nutrient intakes in middle-aged and older men and women similarly to multiple food records. J Nutr 1993;123:489–501. [DOI] [PubMed] [Google Scholar]
- 15.Kuehneman T, Gregory M, de Waal D et al. Academy of Nutrition and Dietetics Evidence-Based Practice Guideline for the Management of Heart Failure in Adults. J Acad Nutr Diet 2018;118:2331–2345. [DOI] [PubMed] [Google Scholar]
- 16.Aquilani R, Opasich C, Verri M et al. Is nutritional intake adequate in chronic heart failure patients? J Am Coll Cardiol 2003;42:1218–1223. [DOI] [PubMed] [Google Scholar]
- 17.Boullata J, Williams J, Cottrell F, Hudson L, Compher C. Accurate Determination of Energy Needs in Hospitalized Patients. J Am Diet Assoc 2007;107:393–401. [DOI] [PubMed] [Google Scholar]
- 18.Siervo M, Bertoli S, Battezzati A et al. Accuracy of predictive equations for the measurement of resting energy expenditure in older subjects. Clin Nutr 2014;33:613–9. [DOI] [PubMed] [Google Scholar]
- 19.Medicine Io. Nutrient Recommendations: Dietary Reference Intakes (DRI). 2010. [Google Scholar]
- 20.Aziz EF, Javed F, Pratap B et al. Malnutrition as assessed by nutritional risk index is associated with worse outcome in patients admitted with acute decompensated heart failure: an ACAP-HF data analysis. Heart international 2011;6:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMurray JJV, Packer M, Desai AS et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor CM, Abraham WT, Albert NM et al. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J 2008;156:662–73. [DOI] [PubMed] [Google Scholar]
- 23.Hummel SL, Katrapati P, Gillespie BW, Defranco AC, Koelling TM. Impact of prior admissions on 30-day readmissions in medicare heart failure inpatients. Mayo Clin Proc 2014;89:623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koliaki C, Spinos T, Spinou M, Brinia Mu E, Mitsopoulou D, Katsilambros N. Defining the Optimal Dietary Approach for Safe, Effective and Sustainable Weight Loss in Overweight and Obese Adults. Healthcare (Basel, Switzerland) 2018;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorodeski EZ, Goyal P, Hummel SL et al. Domain Management Approach to Heart Failure in the Geriatric Patient. Present and Future 2018;71:1921–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levitan EB, Lewis CE, Tinker LF et al. Mediterranean and DASH diet scores and mortality in women with heart failure: The Women’s Health Initiative. Circ Heart Fail 2013;6:1116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hummel SL, Seymour EM, Brook RD et al. Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail 2013;6:1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rifai L, Pisano C, Hayden J, Sulo S, Silver MA. Impact of the DASH diet on endothelial function, exercise capacity, and quality of life in patients with heart failure. Proceedings (Baylor University Medical Center) 2015;28:151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carbone S, Billingsley HE, Canada JM et al. Unsaturated Fatty Acids to Improve Cardiorespiratory Fitness in Patients With Obesity and HFpEF. The UFA-Preserved Pilot Study 2019;4:563–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rozentryt P, von Haehling S, Lainscak M et al. The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: a randomized, double-blind pilot study. Journal of cachexia, sarcopenia and muscle 2010;1:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: A randomized clinical trial. JAMA 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houston DK, Miller ME, Kitzman DW et al. Long-Term Effects of Randomization to a Weight Loss Intervention in Older Adults: A Pilot Study. Journal of Nutrition in Gerontology and Geriatrics 2019;38:83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cobb LK, Anderson CAM, Elliott P et al. Methodological Issues in Cohort Studies That Relate Sodium Intake to Cardiovascular Disease Outcomes: A Science Advisory From the American Heart Association. Circulation 2014. [DOI] [PubMed] [Google Scholar]
- 34.Vest AR, Chan M, Deswal A et al. Nutrition, Obesity, and Cachexia in Patients With Heart Failure: A Consensus Statement from the Heart Failure Society of America Scientific Statements Committee. J Card Fail 2019;25:380–400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.