Abstract
Objectives
Gamma-glutamyl transferase (GGT) enzyme is a routinely used diagnostic marker to detect various systemic diseases, elevation of which indicates destructive activity. Elevated GGT levels in GCF of destructive periodontal diseases hence can be expected. Hence, the aim of this study was to investigate if gamma glutamyl transferase would be a good indicator of on-going disease activity and to also assess the effect of non-surgical therapy on Gamma glutamyl transferase in gingival crevicular fluid of clinically healthy and chronic periodontitis patients.
Methods
GCF samples from 20 chronic periodontitis patients and 20 clinically healthy individuals of age group 35–45 years were collected. Clinical parameters were recorded and GGT levels in GCF assessed using semi-autoanalyser before and after appropriate non-surgical periodontal therapy in both the groups.
Results
GGT levels were higher in chronic periodontitis group compared to healthy group at baseline. There was a significant reduction in the GGT levels and clinical parameters at 30th and 90th day post treatment.
Conclusion
The significant reduction in the GGT levels after Non Surgical Periodontal Therapy at every recall interval may indicate that GGT can be used as a potential diagnostic marker of periodontitis.
Keywords: Gamma glutamyl transferase, Periodontitis, Biomarkers, Oxidative stress
Clinical relevance
GCF collected from chronic periodontitis patients showed higher levels of GGT in chronic periodontitis patients compared to healthy group. The GGT levels were reduced post treatment in periodontitis patients. Hence GGT can be used as a potential diagnostic marker of periodontal disease.
1. Introduction
Periodontitis an inflammation of the supporting tissues of the teeth has multifactorial etiology. Virulent pathogens, susceptible host and absence of beneficial species are a few of the many causative and predisposing factors. Periodontal diseases are a source of Reactive Oxygen Species (ROS) produced by different cells, their traditional role being that of killing bacteria.1,2 Due to the incident collateral damage by ROS, host has a few protective anti-oxidant mechanisms, to either eradicate ROS or reverse the damage caused by them.3 In addition to the anti- and pro-oxidant activity, host also produces a number of enzyme families released from connective tissue, epithelial or inflammatory cells. One such enzyme is Gamma-glutamyl transpeptidase (GGT) which is routinely used as a diagnostic marker enzyme to detect liver and bile ducts diseases.4 It is an intracellular enzyme which infiltrates saliva, serum and GCF and plays an important role in antioxidant defence mechanisms.5
Elevated GGT levels lead to pro-oxidant activity which in turn leads to generation of injurious reactive oxygen species or nitric oxide.6 An imbalance in the pro-oxidant–antioxidant levels leads to oxidative stress (OS) causing potential damage. OS influences the pathogenesis of many chronic inflammatory conditions associated with ageing.7
Glutathione is an anti-oxidant which prevents the damage by ROS. GGT plays a well-established pivotal role in glutathione regulation and degradation.8 Normal range of GGT is 0–51IU/L. The destruction of cells of the liver and bile duct releases GGT in to the serum, which enters the circulation, hence referred to as the ‘leaking enzyme’. Elevated serum GGT activity is thus one of the good indicators of on-going destructive activity.9
Active periodontal destruction, involves degradation of connective tissue as well as resorption of bone, which results in leaking of GGT enzyme to serum, saliva and Gingival Crevicular Fluid (GCF).5 GCF is regarded as the most suitable medium to investigate pathobiological reactions within the periodontal tissues. Not many studies have been conducted on the levels of GGT in GCF before and after appropriate non-surgical therapy. Hence this study was conducted to investigate whether GCF levels of GGT could be used as a reliable marker for detecting ongoing destructive activity in patients with chronic periodontitis.
2. Methodology
After Institutional Ethical Clearance was obtained for the study twenty patients diagnosed with chronic generalised periodontitis (Experimental Group) and twenty diagnosed as clinically healthy individuals (Control Group) were selected from the Outpatient Department of Periodontology for the study. Patients of both genders in the age range of 35–45 years were included. The enrolees were systemically healthy with no history of antibiotic or oral antiseptic therapy or periodontal therapy in the previous 3–6 months. Chronic generalised periodontitis patients exhibiting more than 5 mm probing depth in 30% of the sites as per AAP 1999 criteria were included in the study.10
Alcoholics, smokers, pregnant/lactating mothers, medically compromised patients, immunocompromised individuals and non-compliant patients were excluded from the study. Written informed consent was obtained from each patient.
2.1. Clinical parameters
Plaque index, (Silness and Loe, 1964), Gingival index. (Loe and Silness, 1963), Gingival Bleeding Index (Ainamo and Bay, 1975), Probing pocket depth was recorded using a pressure sensitive probe (Bluedent®). Clinical parameters were recorded at baseline, 1 month and 3 months.
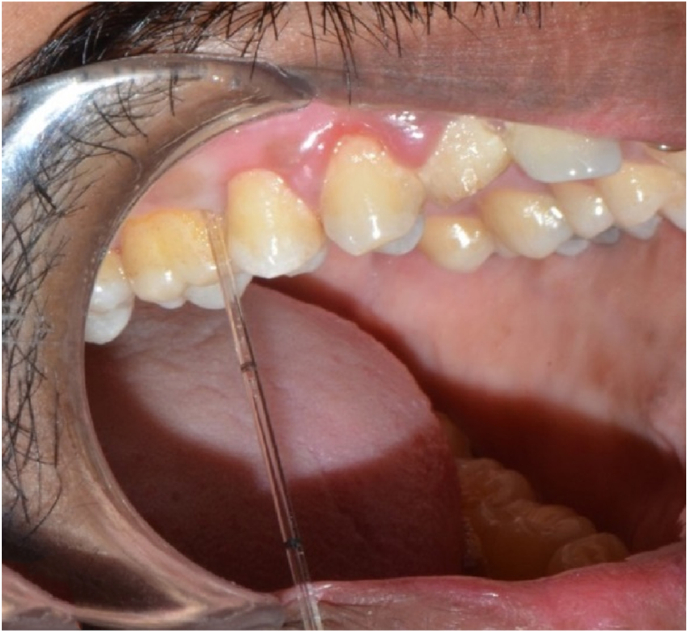
GCF samples were collected a day after the assessment of clinical parameters to prevent the contamination of the samples with blood from freshly probed sites. The patients were instructed to gargle vigorously with sterile water to dislodge all debris. Sites marked for collection were cleared of supragingival plaque, dried thoroughly and isolated using cotton rolls. Samples were obtained from sites with the deepest probing depths (Fig. 1).
Fig. 1.
Pipette placed in the gingival crevice to collect GCF.
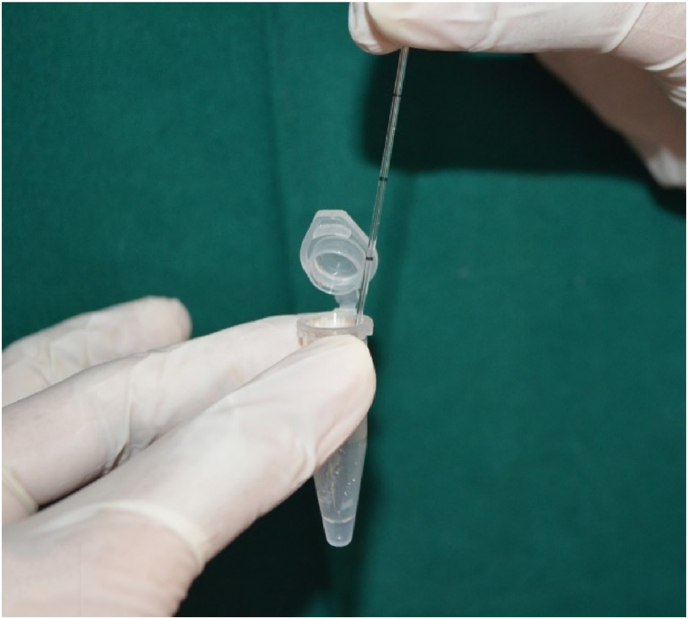
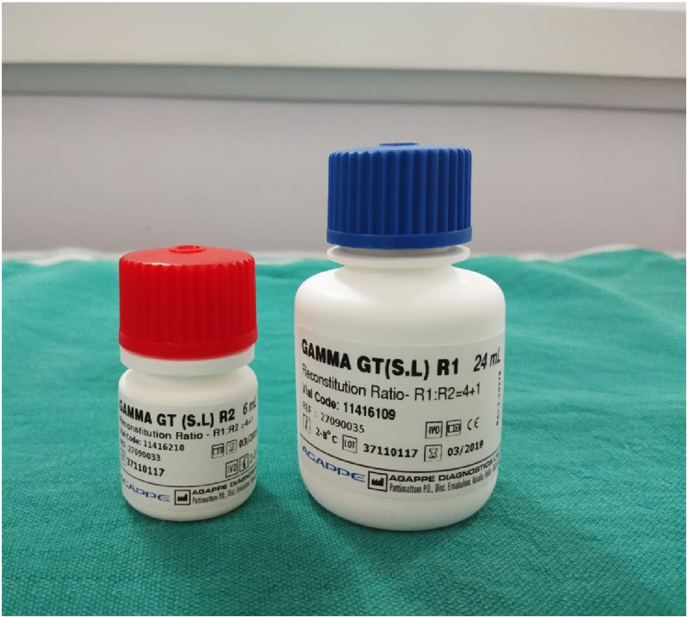
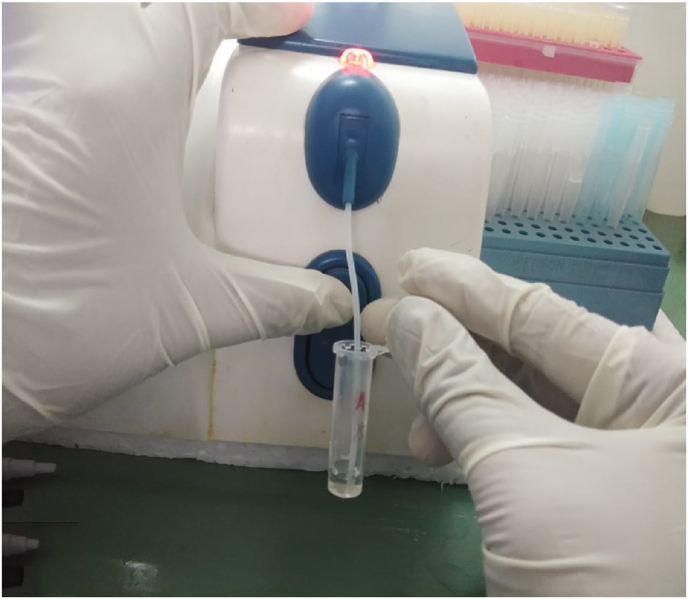
GCF collection was done by intracrevicular method by placing the caliberated volumetric micropipettes at the specific site before and after the therapy at regular time intervals (Fig. 1) and a standardized volume of 1 μL of GCF was collected (Fig. 2). The collected GCF was immediately transferred to a test tube containing 0.1 ml of phosphate buffer saline (pH 7, 0.05 M) (Fig. 2). The test tubes were immediately sent to the biochemical lab for analysis. The levels of GGT were assessed using Agappe reagent kit (Fig. 3) and Robonik Semi Autoanalyzer (Fig. 4).
Fig. 2.
GCF sample transferred to phosphate buffered saline.
Fig. 3.
Reagents added to the GCF sample.
Fig. 4.
Aspiration of sample by Robonik Auto-analyzer*
*ROBONIK INDIA PVT LTD, Plot No. 3 & 4, MIDC Industrial Area, Morivali, Near Ladi Naka, Ambernath (W) - 421 501, Thane, INDIA.
Statistical Analysis: The data was analyzed using Statistical Package for Social Sciences, version 18.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics including mean and standard deviation were computed. The normality of data was analyzed by the Shapiro-Wilk test. The parametric tests were used to check differences in mean scores between and within groups. Repeated Analysis of Variance (ANOVA) with post hoc analysis was employed to check change in mean of study variables at different study intervals. Unpaired t-test was used to compare mean change in study variables between study groups. Pearson's correlation was used to check correlation of GGT levels with Plaque index, Gingival index, Bleeding on probing and Probing pocket depth at various study intervals. A p value of <0.05 was considered as significant for all statistical inferences
3. Results
The mean BOP scores are represented in Table 1. Chronic periodontitis group had a higher level of BOP at baseline when compared to the control sites which had lower scores. The mean BOP in the experimental group after SRP reduced at 1 month and 3 months post therapy which were statistically significant (p < 0.001). On comparison with the healthy controls, the difference in the mean BOP between both the groups was statistically significant at all time intervals (p < 0.001).
Table 1.
Intergroup comparison of Bleeding on probing scores at baseline, 1 month and 3 months.
| BLEEDING ON PROBING | Group | Mean | Std. Deviation | Std. Error | t | p |
|---|---|---|---|---|---|---|
| Baseline | Control | 0.17 | 0.1 | 0.01 | −17.87 | <0.001 |
| Experiment | 0.86 | 0.1 | 0.03 | |||
| After 1month | Control | 0.09 | 0.1 | 0.01 | −11.79 | <0.001 |
| Experiment | 0.43 | 0.1 | 0.02 | |||
| After 3months | Control | 0.04 | 0.1 | 0.01 | −6.01 | 0.237 |
| Experiment | 0.13 | 0.1 | 0.01 |
The mean probing pocket depth, and changes in the PPD, are represented in Table 3 & Table 4. The mean probing depths of healthy sites at baseline were lower than that of experimental sites. Post nonsurgical periodontal therapy, the mean PPD reduced significantly in both the groups at all time intervals. However, the periodontitis group showed greatest reduction in PPD at the end of 3 months which was statistically highly significant (p < 0.001).
Table 3.
Comparison of all the parameters recorded for Group B between different time intervals using Post Hoc tests.
| Dependent Variable | (I) | (J) | Mean Difference (I-J) | Sig. | 95% Confidence Interval |
|
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| GGT levels UL | Baseline | 1 Month | 30.47 | <0.001 | 26.13 | 34.80 |
| 3 Month | 38.22 | <0.001 | 33.88 | 42.55 | ||
| 1 Month | 3 Month | 7.75 | <0.001 | 3.41 | 12.08 | |
| Plaque index | Baseline | 1 Month | .57 | <0.001 | .38 | .76 |
| 3 Month | 1.17 | <0.001 | .98 | 1.35 | ||
| 1 Month | 3 Month | .59 | <0.001 | .40 | .78 | |
| Gingiva lindex | Baseline | 1 Month | .34 | <0.001 | .22 | .46 |
| 3 Month | .91 | <0.001 | .79 | 1.04 | ||
| 1 Month | 3 Month | .57 | <0.001 | .44 | .69 | |
| Bleeding on probing | Baseline | 1 Month | .42 | <0.001 | .32 | .52 |
| 3 Month | .72 | <0.001 | .62 | .81 | ||
| 1 Month | 3 Month | .29 | <0.001 | .20 | .39 | |
| Probing pocket depth | Baseline | 1 Month | 2.04 | <0.001 | 1.58 | 2.49 |
| 3 Month | 3.87 | <0.001 | 3.41 | 4.33 | ||
| 1 Month | 3 Month | 1.83 | <0.001 | 1.37 | 2.29 | |
a The mean difference is significant at the 0.05 level.
Table 4.
Intergroup comparison of Probing depth scores at baseline, 1 month and 3 months.
| PROBING DEPTH | Group | Mean | Std. Deviation | Std. Error | t | P |
|---|---|---|---|---|---|---|
| Baseline | Control | 3.05 | 0.3 | 0.08 | −18.80 | 0.004 |
| Experiment | 6.13 | 0.6 | 0.13 | |||
| After 1month | Control | 2.32 | 0.1 | 0.04 | −10.61 | 0.006 |
| Experiment | 4.09 | 0.7 | 0.16 | |||
| After 3months | Control | 1.84 | 0.1 | 0.03 | −4.18 | 0.002 |
| Experiment | 2.26 | 0.4 | 0.09 |
The mean GGT levels of both the groups at different time intervals are represented in Table 5. Elevated levels of GGT (mean 43.06) were seen in periodontitis group when compared to healthy controls (10.11 mean) at baseline. Post SRP, the levels of GGT reduced in both the groups at the end of 1 month and 3 months recall period, which were statistically significant (p < 0.001). However, the experimental group showed the highest reduction (mean 4.84) having a p value less than 0.001.
Table 5.
Intergroup comparison of GGT levels at baseline, 1 month and 3 months.
| GGT Level | Group | Mean | Std. Deviation | Std. Error | t | P |
|---|---|---|---|---|---|---|
| Baseline | Control | 10.11 | 6.0 | 1.34 | −13.54 | <0.001 |
| Experiment | 43.06 | 9.0 | 2.02 | |||
| After 1month | Control | 6.36 | 4.4 | .98 | −5.12 | <0.001 |
| Experiment | 12.59 | 3.1 | .71 | |||
| After 3months | Control | 3.77 | 3.2 | .73 | −1.2 | 0.237 |
| Experiment | 4.84 | 2.2 | .50 |
Plaque and Gingival Index scores for either group progressively improved from baseline to 1 and 3 month recalls. Improvements in the form of reduction in scores and mean differences, on an average were statistically significant.
4. Discussion
In the present study, GCF from chronic periodontitis demonstrated higher levels of GGT as compared to healthy subjects. GGT levels have been consistently higher when sampled from Chronic Periodontitis patients, however most of the studies on GGT have used either serum or saliva as the source of GGT. In conformation to higher levels of GGT in previous studies, our study showed a positive correlation between periodontal destruction and GGT levels.11,12
GGT is a marker for underlying oxidative stress,8 which plays a key role in chronic inflammatory diseases such as periodontal disease, diabetes mellitus.13 It is even known to increase risk of hospitalization.8 GGT is detrimental to periodontal health as it independently induces expression of “receptor activator of nuclear factor kappa beta ligand” [RANKL] in the bone marrow stromal cells, which is a bone resorbing factor.3 In addition, in the presence of iron it is an important compound in the manufacture of free radicals. It is also a pro-atherogenic factor14 and also linked to metabolic syndrome.15 Despite there being no dearth of evidence that GGT levels are a marker for oxidative stress and oxidative stress directly related to quite a few systemic diseases, it still remains an under-rated marker for both cardiovascular disease and metabolic syndrome.15
In diseases/conditions like Tuberculosis, Menopause, Alzheimer's disease, hypertension, GGT has been used as a marker for oxidative stress. Similarly, elevated levels of GGT in Chronic Periodontitis are a potential marker for underlying oxidative stress and thereby an indicator of resulting cellular damage in the periodontium. Moderate levels of ROS/RNS (reactive oxygen species and reactive nitrogen species) act as signals to promote cell proliferation and survival; however severe increase of ROS/RNS can induce cell death. This balance ensures that the cells respond properly to endogenous and exogenous stimuli. Change in this balance can lead to oxidative stress and cell death.16 A higher GGT level perhaps adds to this already high oxidative stress and brings about cell death.
At baseline mean GGT levels were significantly higher in the experimental group than in controls with clinically healthy gingiva. Four patients in the experimental group had GGT levels highest being 53. Three of these patients however were former smokers with no history of smoking for the past one year. Former smokers who are also ageing, continue to show signs of oxidative stress despite having quit smoking for quite a while. In addition to increased levels of GGT, there is the added inflammatory burden from chronic periodontitis. We have excluded smokers as a group, however former smokers were included. Ageing itself is associated with increases oxidative stress owing to gradual loss of organ and tissue function apparently.17 This could have been one of the reasons why the above mentioned three patients might have exhibited high GGT levels. Association between smoking, ageing and GGT levels18 perhaps needs to be explored further.
Increased reduction in the GGT levels in the experimental group at 1 month post-phase I, and at 3 month recall could be attributed to the fact that non-surgical therapy results in a reduction of bacterial load, inflammatory burden and a further reduction in probing depth due to formation of long junctional epithelium. Reduced pocket depths may result in recolonization with beneficial bacteria which are protective against inflammation.19
GGT levels for the healthy group also reduced from baseline; but the reductions were not significant or dramatic as there was no inflammation or periodontal destruction at baseline to begin with. Difference between baseline GGT for control and experimental group was statistically highly significant. However, 3 month GGT levels between the two groups were compared and the difference was not significant. This clearly indicates that at the end of 3 months experimental group(chronic periodontitis) experienced a significant reduction in oxidative stress and reduced inflammatory burden owing to vgourous phase I therapy, which reflected in the reduced GGT levels.
The 3 month GGT levels of the experimental group was lower than baseline GGT level of control group, indicating that scaling and root planing has excellent initial results in bringing the oxidative stress down to GGT levels that resemble that of a clinically healthy tissue (Table 5).
Bleeding on probing, which is an important clinical indicator of gingival inflammation also reduced considerably in both groups. The baseline difference between both the groups were statistically highly significant, while at 3 months the difference was not significant indicating that the gingival inflammation in the experimental group resolved to nearly healthy levels and at 3 months it had dropped below the baseline BOP of control group
Mean GI scores, plaque scores and probing pocket depth scores reduced considerably from baseline to 3 months since both groups received SRP and oral hygiene instructions and periodic re-enforcements (Table 2 and Table 3).
Table 2.
Comparison of all the parameters recorded for Group A between different time interval.
| Dependent Variable | (I) group | (J) group | Mean Difference (I-J) | Sig. | 95% Confidence Interval |
|
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| GGT levels UL | Baseline | 1 Month | 3.74 | 0.038 | 0.16 | 7.33 |
| 3 Month | 6.33 | <0.001 | 2.7 | 9.92 | ||
| 1 Month | 3 Month | 2.59 | 0.199 | −0.99 | 6.17 | |
| Plaque index | Baseline | 1 Month | 0.19 | <0.001 | 0.14 | 0.24 |
| 3 Month | 0.29 | <0.001 | 0.24 | 0.33 | ||
| 1 Month | 3 Month | 0.09 | <0.001 | 0.04 | 0.14 | |
| Gingival index | Baseline | 1 Month | 0.17 | <0.001 | 0.11 | 0.23 |
| 3 Month | 0.27 | <0.001 | 0.21 | 0.33 | ||
| 1 Month | 3 Month | 0.10 | <0.001 | 0.04 | 0.15a | |
| Bleeding on probing | Baseline | 1 Month | 0.08 | <0.001 | 0.06 | 0.11 |
| 3 Month | 0.13 | <0.001 | 0.11 | 0.16 | ||
| 1 Month | 3 Month | 0.04 | <0.001 | 0.02 | 0.07 | |
| Probing pocket depth | Baseline | 1 Month | 0.73 | <0.001 | 0.52 | 0.93 |
| 3 Month | 1.20 | <0.001 | 1.00 | 1.40 | ||
| 1 Month | 3 Month | 0.47 | <0.001 | 0.26 | 0.67 | |
The mean difference is significant at the 0.05 level.
Correlation between healthy group's clinical parameters and their GGT levels exhibits some discrepancy. While both groups received the same level of rigorous non-surgical periodontal therapy at baseline, the clinical parameters in control(clinically healthy) do not exhibit a significant change because the BOP or PPD or PI scores were not high, hence the effect of scaling was not as significant as it was with the experimental group. Hence the reduction in GGT in the control group is not as much as in the experimental group. The correlation co-efficients reflect this phenomenon where the GGT levels do not correlate with the small changes in the clinical parameters (Table 6).
Table 6.
Correlation between clinical parameters and Gamma Glutamyl Transferase levels.
| Recall Intervals | Variables | Experimental |
Healthy |
|||
|---|---|---|---|---|---|---|
| r value | p value | r value | p value | |||
| Baseline | GGT | Plaque index | −0.042 | 0.86 | −0.179 | 0.45 |
| Gingival index | −0.390 | 0.09 | 0.186 | 0.43 | ||
| Bleeding on probing | −0.240 | 0.31 | −0.034 | 0.89 | ||
| Probing pocket depth | 0.510 | 0.02 | −0.611 | 0.004 | ||
| 1 Month | GGT | Plaque index | −0.030 | 0.91 | −0.054 | 0.82 |
| Gingival index | .0163 | 0.49 | 0.174 | 0.46 | ||
| Bleeding on probing | 0.263 | 0.26 | −0.097 | 0.68 | ||
| Probing pocket depth | 0.093 | 0.69 | −0.372 | 0.11 | ||
| 3 Months | GGT | Plaque index | 0.122 | 0.61 | −0.275 | 0.24 |
| Gingival index | −0.217 | 0.36 | −0.340 | 0.14 | ||
| Bleeding on probing | −0.035 | 0.88 | −0.304 | 0.19 | ||
| Probing pocket depth | 0.084 | 0.73 | −0.070 | 0.77 | ||
Probing depths and GGT levels had a correlation co-efficient value (r-value) closer to −1 at baseline as compared to other parameters. Activity of pathogens is highest in the deepest portion of the periodontal pocket hence deeper the pocket, higher the GGT levels. The r-values at 1 month exhibited a positive correlation between GI, PPD and BOP. Adequate plaque control led to the reduction in inflammatory infiltrate in the tissues resulting in reduced GGT levels and a positive correlation. However, PI exhibited a slight negative correlation. This can be attributed to isolated areas of plaque accumulation whose duration of accumulation was probably not long enough to evoke a clinical gingival inflammation, hence perhaps the discrepancy (as reflected by GI and BOP scores which pointed more towards a clinically healthy gingiva) (Table 6).
In the present study, chronic periodontitis patients showed higher levels of GGT as compared to healthy controls. Other studies showing similar results includes those by Sreeram M et al.,10 Koregol AC et al.,5 Todorovic T et al.20 indicating the positive correlation between the amount of destruction and the value of GGT obtained from the present study.
GGT may not be a sole indicator of destructive activity of the underlying disease, but it is definitely an indicator of oxidative stress. Our study also demonstrated that meticulous, conscientious non-surgical therapy led to a fall in GGT levels. While elective periodontal therapy rectifies anatomic risk factors, the vital importance of non-surgical therapy cannot be emphasized enough. Scaling, root planing, chemical and mechanical plaque control and maintenance visits not only keep the local irritants under control but keep a check on oxidative stress as well. In the future, perhaps periodontal therapy needs to include unconventional adjuncts for successfully and feasibly dealing with oxidative stress as well, since oxidative stress happens to be the effect and eventually the perpetuating factor for periodontal disease. Hence, a comprehensive type of treatment plan that also aims to boost glutathione levels involving unorthodox measures like diet modification etc in conjunction with vigorous non-surgical periodontal therapy, would give promising results. Limitations of the study are the smaller sample size and GCF collection would have been better quantified with periotron.
5. Conclusion
In our study, GGT levels were higher in chronic periodontitis group compared to healthy group at baseline. After Non Surgical Periodontal Therapy there was a significant reduction in the GGT levels at every recall interval. This indicates that GGT can be used as a potential diagnostic marker of periodontitis. However, meticulous exploration of its potential as a biomarker needs to be done so that concepts of ageing, inflammation and oxidative stress can be routinely used in correlation with periodontal disease and include these concepts in treatment planning as well.
References
- 1.Socransky S.S., Haffajee A.D. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63:322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 2.Chapple I.L. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997;24:287–296. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 3.Dahiya P., Kamal R., Gupta R., Bharadwaj R., Chaudhary K., Kaur S. Reactive oxygen species in periodontitis. J Indian Soc Periodontol. 2013;17:411–416. doi: 10.4103/0972-124X.118306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mason J.E., Starke R.D., Van Kirk J.E. Gamma-glutamyl transferase: a novel cardiovascular risk biomarker. Prev Cardiol. 2010;13:36–41. doi: 10.1111/j.1751-7141.2009.00054.x. [DOI] [PubMed] [Google Scholar]
- 5.Koregol A.C., Kalburgi N.B., Kamat Wagh A.U., Warad S. Gamma glutamyl transpeptidase, smokeless tobacco, chronic periodontitis: exploring the Link. J Clin Diagn Res. 2017;11:ZC17–ZC20. doi: 10.7860/JCDR/2017/23598.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita T., Yamazaki Y., Fujiharu C., et al. Serum Gamma-glutamyl transferase level is associated with periodontal disease independent of drinking habits in Japanese adults. Int Med J Exp Clin Res. 2014;20:2109–2116. doi: 10.12659/MSM.891204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khansari N., Shakiba Y., Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 8.Hong S., et al. Gamma-glutamyl transferase variability and the risk of hospitalisation for heart failure. Heart Jul. 2020;106(14):1080–1086. doi: 10.1136/heartjnl-2019-316271. [DOI] [PubMed] [Google Scholar]
- 9.Dabra S., China K., Kaushik A. Salivary enzyme as a diagnostic marker for detection of gingival/periodontal disease and their correlation with the severity of the disease. J Indian Soc Periodontol. 2012;16:358–364. doi: 10.4103/0972-124X.100911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.1999 International workshop for a classification of periodontal diseases and conditions. Papers. Ann Periodontol. 1999;4:1–112. doi: 10.1902/annals.1999.4.1.i. [DOI] [PubMed] [Google Scholar]
- 11.Sreeram M., Suryakar A.N., Dani N.H. Is gamma glutamyl transpeptidase a biomarker for oxidative stress in periodontitis? J Indian Soc Periodontol. 2015;19:150–154. doi: 10.4103/0972-124X.149032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haffajee A.D., Socransky S.S., Goodson J.M. Clinical parameters as predictors of destructive periodontal disease activity. J Clin Periodontol. 1983;10:257–265. doi: 10.1111/j.1600-051x.1983.tb01274.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Andrukhov O., Rausch-Fan X. Oxidative stress and antioxidant system in periodontitis. Front Physiol. 2017;8:910. doi: 10.3389/fphys.2017.00910. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emdin M., Passino C., Pompella A., Paolicchi A. Gamma-glutamyltransferase as a cardiovascular risk factor. Eur Heart J. 2006;27:2145–2146. doi: 10.1093/eurheartj/ehl151. Epub 2006 Jul 11. [DOI] [PubMed] [Google Scholar]
- 15.Neuman M.G., Malnick S., Chertin L. Gamma glutamyl transferase - an underestimated marker for cardiovascular disease and the metabolic syndrome. J Pharm Pharmaceut Sci. 2020;23(1):65–74. doi: 10.18433/jpps30923. [DOI] [PubMed] [Google Scholar]
- 16.Trachootham D., Lu W., Ogasawara, Valle N.R., Huang P. Redox regulation of cell survival. Antioxidants Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lihuori I., et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donohue J.F. Ageing, smoking and oxidative stress. Thorax. 2006;61:461–462. doi: 10.1136/thx.2005.053058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenstein G. Periodontal response to mechanical non-surgical therapy: a review. J Periodontol. 1992;63:118–130. doi: 10.1902/jop.1992.63.2.118. [DOI] [PubMed] [Google Scholar]
- 20.Todorovic T., Dozic I., Vicente-Barrero M., et al. Salivary enzymes and periodontal disease. Med Oral Patol Oral Cir Bucal. 2006;11:E115–E119. [PubMed] [Google Scholar]