Abstract
Background
Cognitive dysfunction, presenting as learning and memory impairment, is a common manifestation in many chronic diseases of the nervous system. Some of these diseases include depression, epilepsy, and Alzheimer’s disease. To date, few drugs or medicinal products have shown ability to improve learning and memory deficits. Neuroprotection is one of the mechanisms by which memory could be improved. The extract of Xylopia aethiopica and its kaurene derivative, xylopic acid, have previously demonstrated neuroprotective effects in animal models. The aim of the present study was to investigate the effect of an extract of Xylopia aethiopica fruit and xylopic acid, on learning and memory using murine models.
Materials and methods
Unripe Xylopia aethiopica fruits were collected, dried, and extracted using 70% v/v ethanol. Xylopic acid was isolated from the fruits using petroleum ether, concentrated with ethyl acetate and then recrystallized with petroleum ether before purifying with ethanol (96%v/v). Institute of Cancer Research (ICR) mice received oral doses of the extract of Xylopia aethiopica (XAE; 30, 100 and 300 mg/kg), xylopic acid (XA; 30, 100 and mg/kg), citicoline (300 mg/kg), piracetam (300 mg/kg) or ketamine (30 mg/kg) and saline (vehicle). The animals were then taken through the Morris water maze test (MWM), spontaneous alternation Y-maze test (Y-maze), and novel object recognition test (NOR), to assess learning and memory.
Results
In the NOR test, XAE (30, 100 and 300 mg/kg) and XA (30, 100 and 300 mg/kg) increased the percentage exploration and recognition index (p = 0.0005 and p < 0.0001, respectively) when compared to both vehicle and ketamine groups. Similarly, doses of XAE and XA as used in the NOR test increased the percentage alternation in the Y-maze test. Although XAE and XA treatments decreased the latencies to find hidden platform in the MWM test, it was not significantly different from the vehicle group. However, this decrease in latency differed significantly when compared to the ketamine group. Interestingly, both XAE and XA treatments increased the percentage frequency to the target quadrant in the probe trial of the MWM. It is noteworthy that in all the three models used, both the extract and xylopic acid performed better than piracetam and citicoline, the reference drugs.
Conclusion
The ethanolic extract of Xylopia aethiopica fruit and xylopic acid improved exploratory learning and recognition memory, spatial working, recognition, and reference memories in the behavioral tests.
Abbreviations: AUC, Area under the curve; CTC, Citicoline; HPLC, High performance liquid chromatography; ICR, Institute of Cancer Research; KET, Ketamine; MWM, Morris water maze; NOR, Novelty object recognition; PCT, Piracetam; TLC, Thin layer chromatography; VEH, Vehicle; XA, Xylopic acid; XAE, Extract of Xylopia aethiopica; Y-maze, Spontaneous alternation Y-maze
Keywords: Xylopia aethiopica, Xylopic acid, Nootropic, Novelty object recognition, Spontaneous alternation Y-maze, Morris water maze
Highlights
-
•
Xylopia aethiopica extract and xylopic acid improve exploratory learning and recognition memory.
-
•
Xylopia aethiopica extract and xylopic acid ameliorate spatial working and reference memories.
-
•
Xylopia aethiopica extract and xylopic acid are better than piracetam and citicoline in improving learning and memory.
1. Introduction
Learning and memory are cognitive functions that aid fundamental processes in humans (Vanderveren et al., 2017). This explains anxiety generated when there is impairment in learning and memory. There may be instances when there will be a decline in higher cognitive functions such as attention, learning, and memory, often termed dementia (Pahaye et al., 2017). Dementia affects quality of life especially in the elderly. Dementia is also common in people suffering from other central nervous system disorders such as depression, schizophrenia, Alzheimer’s disease, anxiety, among others (Anjula et al., 2015, Dhingra et al., 2005). Worldwide, 47 million people live with dementia of which about 63% occur in low- and middle-income countries (WHO., 2017). Incidence of dementia is expected to increase to 75 million worldwide by 2030 (Prince et al., 2016, WHO, 2017).
Current drugs used to improve learning and memory deficits (nootropics), such as citicoline and piracetam, have minimal efficacy, undesirable side effects and are relatively expensive (Malykh and Sadaie, 2010, Talih and Ajaltouni, 2015). There is, therefore, the need to identify and/or develop other agents with high efficacy and few adverse effects.
Reports suggest that a majority of people in low- and middle-income countries depend on herbal or traditional medicines due to the fact that these agents are cost-effective, and show some degree of efficacy in the management of conditions such as depression, epilepsy, and cancer (Chugh et al., 2018). The learning and memory-enhancing effect of medicinal plants like maca, St. John’s wort, ginseng, and Gingko biloba have been reported (El Tabaa et al., 2017, Heo et al., 2008, Khalifa, 2001, Rubio et al., 2011). The aforementioned studies suggest that plant sources may hold promise in the management of dementia and related memory problems.
Xylopia aethiopica is a plant of the family Annonaceae with a straight crown and buttressed stem (Irvine, 1961). It is commonly known as ‘Ethiopian pepper’ or ‘African pepper’. It grows in Ghana, Angola, Ethiopia, Burkina Faso, Gabon, Nigeria, and other parts of tropical Africa. The plant is known to possess a number of pharmacological properties. Some of which include antidepressant-like (Biney et al., 2016), antimicrobial (Fleischer et al., 2008), analgesic (Woode et al., 2012), anti-inflammatory (Obiri and Osafo, 2013), and anti-helminthic (Ekeanyanwu and Etienajirhevwe, 2012, Suleiman et al., 2005) effects. Many studies have been conducted on the kaurenes, a class of diterpenes, isolated from X. aethiopica (Biney et al., 2014). Xylopic acid (XA), one of these kaurenes has anti-inflammatory (Osafo et al., 2018), neuroprotective (Biney et al., 2015), antiplasmodial (Boampong et al., 2013) and diuretic (Somova et al., 2001) effects. Other known properties of the fruit exact of Xylopia aethiopica (XAE) and XA on the central nervous system include analgesic (Woode et al., 2012), anti-allodynic (Ameyaw et al., 2014) and antidepressant-like (Biney et al., 2016) effects. Despite the aforementioned neuroactive effects, there is paucity of data on the effect of XAE and XA on learning and memory. Thus, the present study evaluated the effect of XAE and XA on learning and memory using murine models.
2. Materials and methods
2.1. Drugs and chemicals
Piracetam (Cebrotonin) was purchased from Walter Ritter GmbH+ Co. KG (Hamburg- Germany), ketamine was from Psychotropics India Ltd. (Uttarakhand, India), and citicoline (Somazina) was from Ferrer International, S.A (Barcelona, Spain). Ethanol and ethyl acetate were obtained from Merck KGaA (Darmstadt, Germany) and petroleum ether was from Science Company (Lakewood, USA).
2.2. Plant collection and extraction
Fresh fruits of X. aethiopica were collected from the Botanical Garden of Kwame Nkrumah University of Science and Technology (KNUST) (06° 41'6.39N; 01° 33' 45.35W) between the periods of March and June 2017. The fruits of X. aethiopica were authenticated at the Center for Plant Medicine Research, Akuapem-Mampong, Ghana, with voucher specimen number: CPMR 4888-21-06-2017. The fruits were shade-dried for about four weeks till they were easy to break. It was then pulverized to powder with a hammer mill. Two (2) kg of the powder was used for extraction using the cold maceration method with 70% v/v ethanol for two-consecutive 72-h periods. The rotary evaporator (at 60 °C) was used to produce a semi-solid mass. The semi-solid mass was dried on a water bath with a temperature of 78–79 °C and afterwards kept in a desiccator. The percent yield was 46.9% w/w.
2.3. Qualitative Phytochemical Screening of XAE
The method by Trease and Evans (1989) was used to screen the XAE for the presence of alkaloids, saponins, tannins, glycosides, flavonoids, sterols, and terpenoids using the following chemicals and reagents: alkaloids (Dragendoff’s reagent, HCl), saponins (distilled water), tannins (1% FeCl3), glycosides (H2SO4, 20% NaOH, Fehling’s A and B solutions), flavonoids (98% ethanol, HCl, Zn metal), sterols (chloroform, acetic anhydride, H2SO4) and terpenoids (chloroform, H2SO4).
2.4. Isolation and purification of xylopic acid
Xylopic acid was isolated using the methods described by Woode et al. (2012) and Biney et al. (2014). About 0.30 kg of the powdered fruits were placed in a cylindrical jar and soaked with 2.5 L of petroleum ether and allowed to stand for 72 h. The extract was collected and concentrated with a rotatory evaporator at 60 °C. A volume of 5 ml ethyl acetate was added to the concentrate for it to facilitate the crystallization of xylopic acid and allowed to stand for 5 days. The crystals formed were washed with petroleum ether. The xylopic acid was purified by recrystallization in 96% v/v ethanol. The concentrated solution obtained was filtered while hot and the crystals of the xylopic acid deposited at the bottom of the cylindrical jar.
The purity of the xylopic acid was determined using high-performance liquid chromatography (HPLC), thin-layer chromatography (TLC), and melting point of isolate (Ameyaw et al., 2014). The chromatograph consisted of LC-10AT Shimadzu pump with a programmable absorbance detector (783 A Applied Biosystems) and Shimadzu CR501 Chromatopac. Phenomenex Hypersil 20-micron C18 200 × 3.20 mm column was used. The mobile phase consisted of methanol and water (9:1) eluted isocratically at 0.5 mlmin-1. Portions of 20 μl of a suitable concentration of pure xylopic acid were loaded and injected into the column after dissolving in the mobile phase at 60°C. The eluent was monitored at 206 nm. Portions of the extract and xylopic acid were loaded and injected. Peaks were noted as components of the extract and xylopic acid.
2.5. Animals
Male and female Institute of Cancer Research (ICR) mice (20–25 g) were obtained from the Centre for Plant Medicine Research, Akuapem-Mampong, Ghana. The mice were kept at the Animal Experimentation Unit of the Department of Medical Microbiology, School of Biomedical and Allied Health Sciences, University of Ghana. The mice were housed in cages (34 × 47 × 18 cm3) (n = 8 per cage) with wood shavings as bedding, fed with a commercial pellet diet obtained from (GAFCO, Tema, Ghana) and water. All behavioral studies were carried out at the Neuropsychopharmacology Research Laboratory, Department of Medical Pharmacology, University of Ghana Medical School, Korle-Bu. Behavioral studies were conducted in the light cycle with experimentally naive mice. The mice were handled according to the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised 1996. Ethical clearance for this research was obtained from the College of Health Sciences Ethical and Protocol Review Committee, University of Ghana (CHS-Et/M.2- P1.8/2017–2018). Different cohorts of mice were used for the different behavioral tests.
2.6. Experimental protocol
The study was an acute study which employed different behavioral tests to achieve the results. The doses were selected based on previous work by Biney et al. (2014). (Table 1).
Table 1.
A table showing the experimental protocol of the different behavioral tests.
| Behavioral Model | Animal Grouping | Duration |
|---|---|---|
| Novelty Object Recognition (NOR) | XAE (30, 100, 300 mg/kg p.o.) | 8 experimental days i.e. 3 days for habituation phase, 3 days for familiarization phase and 2 days for testing phase |
| XA (30, 100, 300 mg/kg p.o.) | ||
| Piracetam (300 mg/kg p.o.) | ||
| Ketamine (30 mg/kg i.p.) | ||
| Saline (10 ml/kg p.o) | ||
| Spontaneous Alternation Y-maze (Y-maze) | XAE (30, 100, 300 mg/kg p.o.) | 8 experimental days i.e. 5 days for training and 3 days for testing |
| XA (30, 100, 300 mg/kg p.o.) | ||
| Citicoline (300 mg/kg p.o.) | ||
| Ketamine (30 mg/kg i.p.) | ||
| Saline (10 ml/kg p.o) | ||
| Morris water maze (MWM) | XAE (30, 100, 300 mg/kg p.o.) | 6 experimental days i.e. 1 day for training, 4 days for testing and 1 day for probe trial |
| XA (30, 100, 300 mg/kg p.o.) | ||
| Citicoline (300 mg/kg p.o.) | ||
| Ketamine (30 mg/kg i.p.) | ||
| Saline (10 ml/kg p.o) |
2.7. Novel object recognition (NOR) test
The test was carried out according to methods described by Ennanceur and Delacour (1988) and Moscardo et al. (2012), with minor modifications. NOR test measured exploratory learning and recognition memory. Mice were randomly assigned into nine groups (n = 8) i.e. 3 groups for XAE, 3 groups XA, 1 group each for ketamine, piracetam and vehicle (saline). The test involved three (3) phases namely the habituation, familiarization, and testing. In the habituation phase, the mice were kept in an open field (33 × 33 × 20 cm3) for 5 min twice daily with a 6 h interval for 3 consecutive days. Twenty-four hours after the last day of habituation, the familiarization phase was carried out with 2 identical (shape, color, and size) objects placed in the open field, 20 cm apart. Mice were placed at the centre of the field for 10 min and expected to explore the objects freely. This phase lasted for three days. Behavioral assessment of each mouse was done for 10 min with a public domain software JWatcher, version 1.0 (University of California, Los Angeles, USA, and Macquarie University, Sydney, Australia). Exactly 24 h after the last familiarization phase, the testing phase was performed which lasted for 2 days. One hour before each test day, the mice were pre-treated with XAE (30, 100 and 300 mg/kg p.o.), XA (30, 100 and 300 mg/kg p.o.), piracetam (300 mg/kg p.o.), ketamine (30 mg/kg i.p) and vehicle (saline, 10 ml/kg p.o.). The drugs were administered for 2 days during the test days. The test was carried out for 5 min with one of the identical objects replaced with a new object. The duration mice spent interacting with the objects was recorded. The recognition index was then calculated to demonstrate the level of discrimination against the familiar object. To make it easier for the animals to explore with the novel object and be able to discriminate against the familiar one environmental cues were hung in the study environment (Bevins et al., 2002).
The formula for the calculation according to d’Isa et al. (2014) is .
2.8. Spontaneous Alternation Y-maze (Y-maze)
The spontaneous alternation Y-maze test was performed according to methods described by Choi and Choi (2016) and Fu et al. (2008) to evaluate spatial, working and recognition memory. The Y-maze setup consisted of 3 arms labeled A, B and C interconnected at an angle of 120°. The mice were randomly divided into 9 groups (n = 8) i.e. 3 for XAE, 3 for XA and 1 each for ketamine, citicoline and vehicle (saline). The experiment consisted of training and testing phases. In the training phase, mice had free access to explore all 3 arms of the Y-maze for 5 min. Before commencement of the trial, each mouse was placed in the start arm facing the centre of the maze and its behavior tracked with JWatcher, version 1.0. The start arms were selected randomly during the training period for 5 days. The spontaneous alternation behavior was calculated as the number of times a mouse consecutively enters all 3 different arms without entering the previous arm divided by the total number of arm entries. Entry was defined the whole body of the mouse getting into half the distance of the arm. The testing phase was carried out 24 h after the last training day. One hour before each test day, the mice were pre-treated with XAE (30, 100 and 300 mg/kg p.o.), XA (30, 100 and 300 mg/kg p.o.), citicoline (300 mg/kg p.o.), ketamine (30 mg/kg i.p) and vehicle (saline, 10 ml/kg p.o.). The drugs were administered for 3 consecutive days during the test days. The test phase was performed for 3 consecutive days and consisted of 2 trials. The first trial measured working memory in the mice by scoring the number of alternations the mouse made in the Y-maze when one arm of the maze was blocked. This was done for 5 min. Approximately 10–15 min after the first trial, the second trial was performed with the partition that was used in blocking the arm in the first trial removed and the mouse allowed entry to all 3 arms for 2 min. Spatial recognition memory was measured during this trial. To eliminate olfactory traces of previous maze users which had the tendency to affect the percentage alternation in the Y-maze (Bats et al., 2001, Hughes, 2004), 70% ethanol was used to clean the maze in between trials.
2.9. Morris water maze (MWM)
The Morris water maze test was carried out according to methods described by Morris (1981), Morris et al. (1986), Nunez (2008), Sun and Alkon (2004), and Barnhart et al. (2015), to assess hippocampal-dependent spatial/place learning and working memory. The test involved 3 phases i.e. the training, testing, and probe trials. The experiment was done in an 85 cm diameter pool of water at a temperature of 25°C. The maze was divided into 4 quadrants ((South (S), North (N), West (W) and East (E)). The mice were trained on the first day to locate a visible platform placed l1 cm above the water surface. The mice were placed on the platform for 20 s for orientation. After the 20 s, they were lowered gently into the water to swim and locate the platform within 60 s after which those that did not locate the platform after the 60 s were guided to the platform and allowed to re-orient for an additional 20 s. They were then removed and dried. This was repeated twice after a 30 min inter-trial interval. On day 2, the testing phase started one hour after pre-treating the mice with XAE (30, 100 and 300 mg/kg p.o.), XA (30, 100 and 300 mg/kg p.o.), citicoline (300 mg/kg p.o.), ketamine (30 mg/kg i.p) or vehicle (saline, 10 ml/kg p.o.). The drugs were administered from days 2–5 (during the testing phase and probe trial). For four days, prior to each test day, animals received various treatments as described above. During the testing phase, a non-toxic dye was used to make the platform invisible. The platform was placed 2 cm below the water surface. The mice were kept in the water facing the wall. The mice were allowed 60 s to locate the platform. The trial ended when a mouse located the platform within the 60 s period. Those that did not locate the platform within the 60 s were guided. The testing was repeated after 30 min. The probe trial was done 24 h after the last training day, which was the 6th day. The starting locations are indicated in Table 2 below. To make it easier for the mice to navigate towards the hidden platform, distal cues were placed around the pool. This facilitated spatial learning and memory.
Table 2.
Starting locations for Morris water maze.
| Day | Trial 1 | Trial 2 |
|---|---|---|
| 1 | SW | NW |
| 2 | NE | SE |
| 3 | SW | SE |
| 4 | NW | NE |
| 5 | SE | NW |
| 6 (probe trial) | NE |
2.9.1. Duration of study
The study lasted for 9 months from July 2017 to April 2018.
2.10. Statistical analysis
GraphPad Prism for windows version 8.0 (GraphPad Software, San Diego, CA, USA) was used for data and statistical analysis, and p-value < 0.05 was considered statistically significant. The time-course curves were subjected to two-way (treatment × time) repeated measures analysis of variance (ANOVA) with Bonferroni’s post hoc test. Percentage exploration with objects, percentage alternation, and change in time taken to find a hidden platform for each treatment was calculated in the arbitrary unit as the area under the curve (AUC). Differences in AUCs were analyzed by one-way ANOVA followed by Tukey’s post hoc test.
3. Results
3.1. Phytochemical test
Phytochemical screening of the ethanolic (70%) extract of Xylopia aethiopica revealed the presence of alkaloids, saponins, flavonoids, and glycosides (Table 3).
Table 3.
Preliminary phytochemical screening of the ethanolic fruit extract of Xylopia aethiopica.
| Constituent (s) | Inference |
|---|---|
| Alkaloids | Present |
| Saponins | Present |
| Tannins | Absent |
| Flavonoids | Present |
| Glycosides | Absent |
3.2. High-performance liquid chromatography (HPLC) and determination of some properties of xylopic acid
Previously, we used HPLC to characterize the extract and to determine the purity of the isolated xylopic acid. The number of peaks as well as the percentage purity from that work has been previously reported in the work done by (Ameyaw et al., 2018).
3.3. Novelty object recognition
3.3.1. Effect of extract of Xylopia aethiopica (XAE) and xylopic acid (XA) treatment on percentage time spent with a novel object
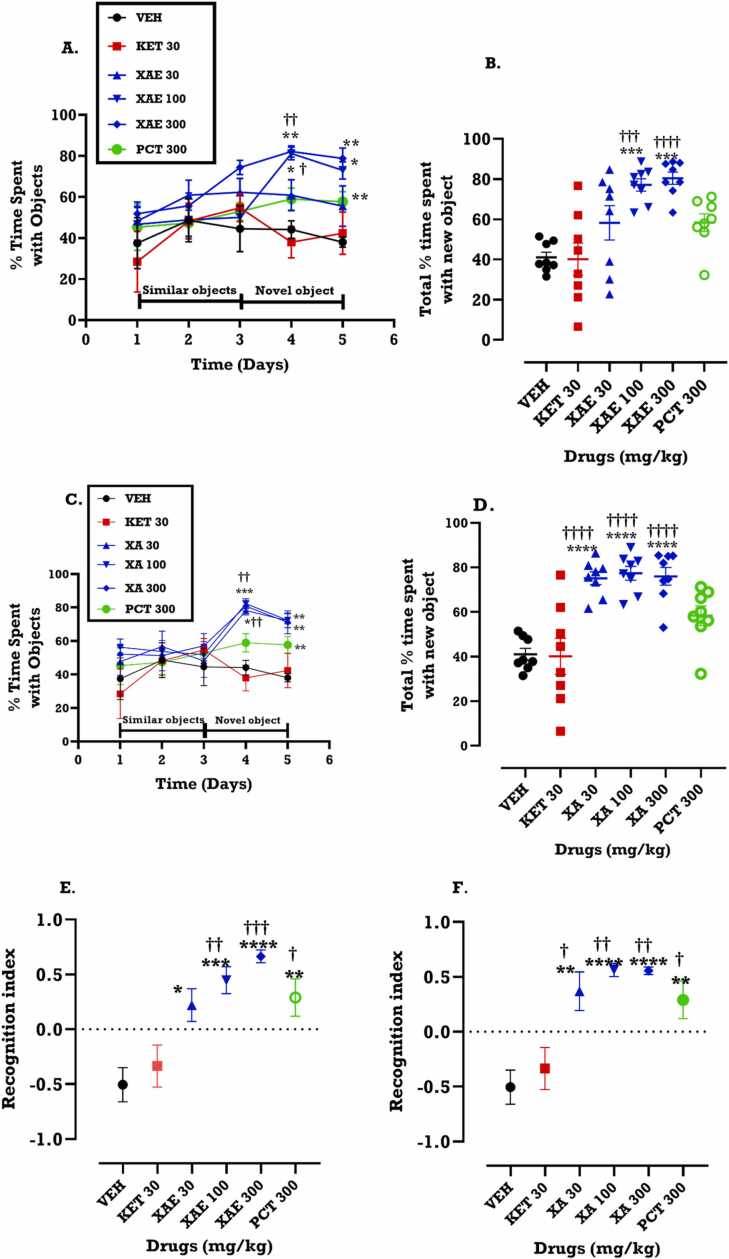
From the time-course curve, XAE (100 and 300 mg/kg) significantly increased the percentage time spent with the novel object (p = 0.0044). Unlike ketamine, XAE (100 and 300 mg/kg) increased the time spent with the novel object, on the first day of test an effect which was still present on the second day of the test (Fig. 1A). The results reveal that XAE (F5,42 = 9.662; p < 0.0001) increased the total time spent (calculated as AUC) exploring the new object when compared to the vehicle (VEH) or ketamine (Fig. 1B). The results for the recognition index to assess the level of discrimination against the familiar object during the test demonstrated that XAE significantly increased the discrimination against the familiar object (F5,42 = 9.527; p < 0.0001) (Fig. 1E). Similarly, XA (100 and 300 mg/kg) significantly increased the percentage of time spent with the novel object as seen from the time-course curve (p = 0.0088; Fig. 1C) and the total time spent with the new object F5,42 = 14.78; P < 0.0001 (Fig. 1D). The results showed an increase in the recognition index for XA (F5,42 = 10.16; p < 0.0001) (Fig. 1F).
Fig. 1.
Effects of XAE (30, 100 and 300 mg/kg) and XA (30, 100 and 300 mg/kg) treatment on the percentage time spent with the novel object in the NOR test. Data are presented as mean ± SEM (n = 8) for the time-course graphs (A, C) and analyzed by Two-way ANOVA followed by Bonferroni’s test. The total times spent with the new object are presented as the areas under the curve (AUCs) (B, D) (One-way ANOVA followed by Tukey’s multiple comparison test). Significantly different from the saline (VEH): *p < 0.05 **p < 0.01, ****p < 0.0001; significantly different from ketamine: ††p < 0.01, †††p < 0.001 and ††††p <0.0001. The recognition index is presented (E, F) (One-way ANOVA followed by Tukey’s multiple comparison test). Significantly different from the saline (VEH): *p < 0.05 **p < 0.01, ****p < 0.0001; significantly different from ketamine: †p < 0.05, ††p < 0.01.
3.4. Spontaneous alternation Y-maze
3.4.1. Effect of extract of Xylopia aethiopica (XAE) and xylopic acid (XA) treatment on working memory in the Y-maze test
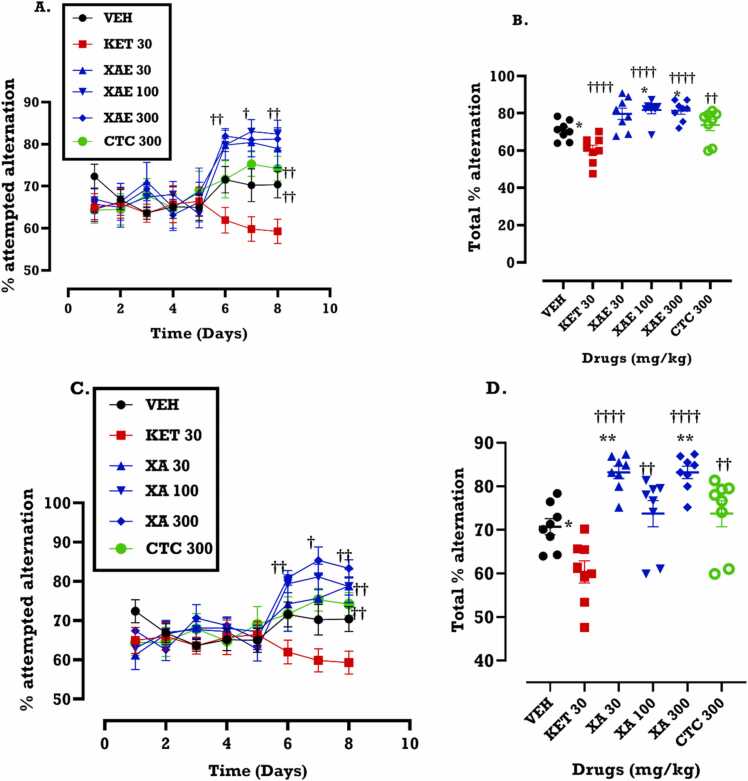
From the time-course graph, XAE (30, 100 and 300 mg/kg) increased the percentage attempted alternation (the number of times a mouse makes an attempt to enter the blocked arm of the Y-maze divided by the total number of alternations the mouse makes in the unblocked arms of the Y-maze multiplied by 100) in the Y-maze (p < 0.0001) when compared to the ketamine group. The effect of XAE was sustained for the first two days after extract treatment ( i.e. experimental day 6 and 7) (Fig. 2A) but declined by the third day (experimental day 8). Compared to the placebo (vehicle) group, the increase in percentage alternation induced by citicoline, the reference drug, was marginal on all treatment days (Fig. 2B, D). However, we observed a significant increase in total percentage alternation in comparison with the placebo when the effect of XAE for the three days of drug treatment was summed up (Fig. 2B). Similar effects were observed with XA treatment (30, 100 and 300 mg/kg), as it significantly increased percentage of attempted alternation in the blocked arm of the Y-maze (p < 0.0001). The effect of XA began on the first day of treatment (Fig. 2C) and further increased on the second day of treatment. This effect declined by the third day of treatment. Mice that received XAE (F4,10 = 207.8, p < 0.0001) and XA (F4,10 =81.97, p < 0.0001) demonstrated significant increase in the percentage attempted alternation in the blocked arm of the Y-maze, indicating a significant improvement in spatial working memory. This is seen in the area under the curve (AUC) in Fig. 2B and Fig. 2D for XAE and XA respectively.
Fig. 2.
Effects of XAE (30, 100 and 300 mg/kg) and XA (30, 100 and 300 mg/kg) treatment on the percentage attempted alternation in the blocked arm of the Y-maze in the spontaneous alternation Y-maze test measuring spatial working memory. Data are presented as mean ± SEM (n = 8) for the time-course graphs (A, C) and analyzed by Two-way ANOVA followed by Bonferroni’s test and their areas under the curve (AUCs) (B, D) (One-way ANOVA followed by Tukey’s multiple comparison test).Significantly different from the saline (VEH): *p < 0.05***p < 0.001, ****p < 0.0001; Significantly different from ketamine: ††p < 0.01, †††p < 0.001 and ††††p <0.0001.
3.4.2. Effect of extract of Xylopia aethiopica (XAE) and xylopic acid (XA) treatment on spatial recognition memory in the Y-maze test
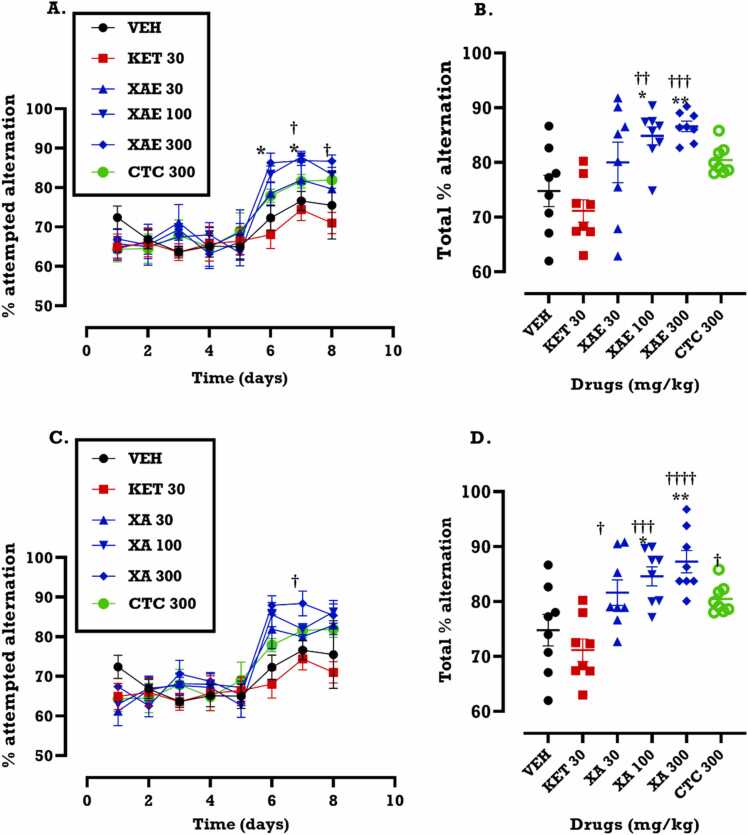
From the time-course curve (Fig. 3), ketamine decreased while XAE, XA and CTC increased the percentage alternation in the Y-maze. The effect of XAE increased slightly on day 6 (first day of treatment) (Fig. 3A) until the last day of treatment. Similarly, XA increased percentage alternation in arms of the Y-maze when the blocked arm was opened (p = 0.0243). The effect of XA increased on days 6 and 7 (Fig. 3C) before declining on day 8. XAE (p < 0.0001) and XA (p < 0.0001) significantly increased the percentage alternation performed by mice in the opened arm of the Y-maze, indicating a significant improvement in spatial recognition memory. This is seen in the area under the curve (AUC) in Fig. 3B and D for XAE and XA respectively. Compared to the vehicle (VEH), the reference drug group, CTC, showed insignificant effect unlike the extract and xyopic acid (Fig. 3 B, D).
Fig. 3.
Effects of XAE (30, 100 and 300 mg/kg) and XA (30, 100 and 300 mg/kg) treatment on the percentage alternation in the previously blocked arm (i.e. opened) of the Y-maze in the spontaneous alternation Y-maze test measuring spatial recognition memory. Data are presented as mean ± SEM (n = 8) for the time-course graphs (A, C) and analysis of differences done by Two-way ANOVA followed by Bonferroni’s test. The areas under the curve (AUCs) (B, D) were analyzed by One-way ANOVA followed by Tukey’s multiple comparison test. Significantly different from the saline (VEH): *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001,; significantly different from ketamine: ††p < 0.01, †††p < 0.001 and ††††p < 0.0001.
3.5. Morris water maze
3.5.1. Effect of extract of Xylopia aethiopica (XAE) and xylopic acid (XA) treatment on spatial memory the Morris water maze test
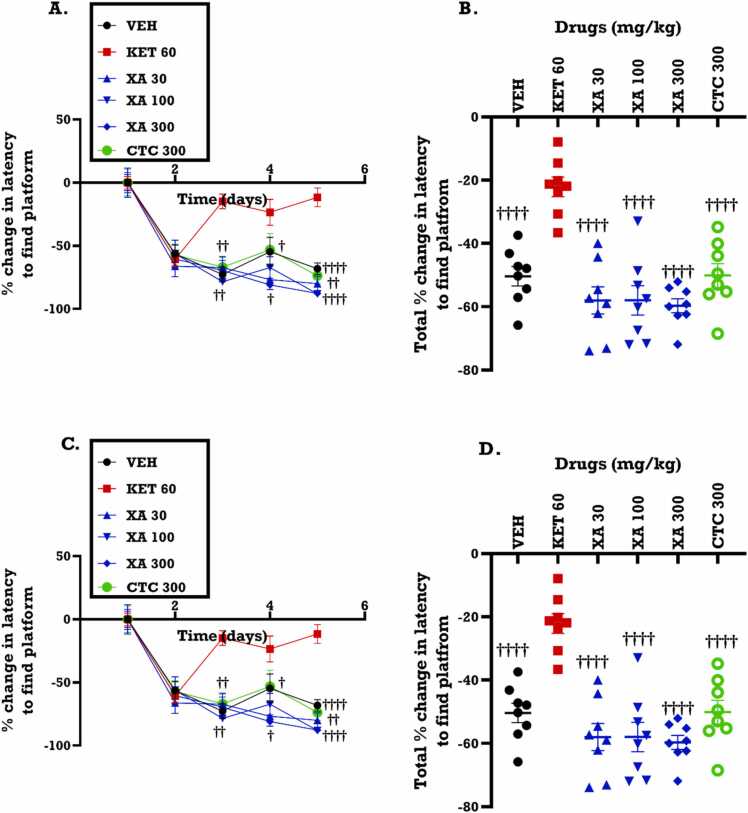
Treatment with ketamine, a drug that impairs cognitive function, caused difficulties in the mice ability to locate hidden platform. Over time, the latency to locate the platform did not decrease in the ketamine group as would be expected if learning had taken place and by trial day 5, the ketamine group performed significantly worse than the control group (Fig. 4A–D). In both time-course graphs (Fig. 4A, C) and areas under the curve (Fig. 4B, D), the effects of XAE and XA was not significantly different from the vehicle treatment, though slight decreases in the latencies were observed in the treated groups.
Fig. 4.
Effects of XAE (30, 100 and 300 mg/kg) and XA (30, 100 and 300 mg/kg) treatment on the percentage latency to locate hidden platform in the Morris water maze test. Data are presented as mean ± SEM (n = 8) for the time-course graphs (A, C) and analyzed by Two-way ANOVA followed by Bonferroni’s test and their areas under the curve (AUCs) (B, D) (One-way ANOVA followed by Tukey’s multiple comparison test). Significantly different from the saline (VEH): **p < 0.01; significantly different from ketamine: ††p < 0.01 and ††††p < 0.0001.
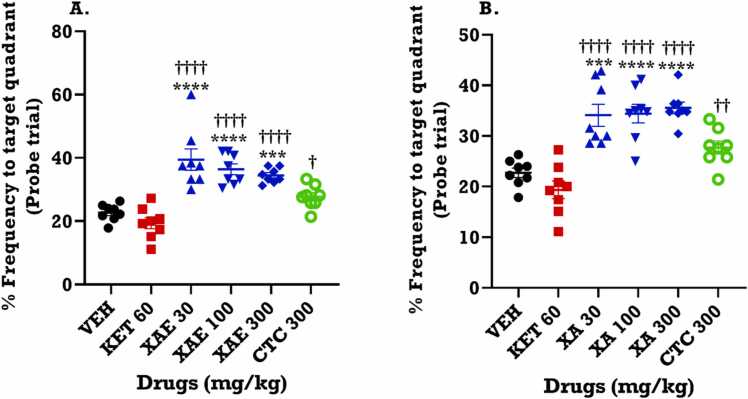
3.5.2. Probe trial to evaluate memory retention in the Morris water maze (MWM) test
In order to test for memory retention, the probe trial was carried out. With the escape platform removed, the number of times mice enter the quadrant that previously hid the platform was used as a measure of memory retention. In this study, XAE (F5,42 = 18.80; p < 0.0001) and XA (F5,42 =18.42; p < 0.0001) significantly increased the percentage frequency of entry to the quadrant that previously hid the escape platform, in comparison to vehicle (VEH) and ketamine (Fig. 5A, B). This indicates that the reference memory was improved in the probe trial of the MWM test by the test drugs. Ketamine, however, did not show any significant effect on memory retention when compared to VEH.
Fig. 5.
Effects of XAE (30, 100 and 300 mg/kg) and XA (30, 100 and 300 mg/kg) percentage frequency of probe trial in the Morris water maze test measuring reference memory. Data are presented as mean ± SEM (n = 8) for (A) XAE and (B) XA groups. Significantly different from the saline (VEH): ***p < 0.001 and ****p < 0.0001. Significantly different from Ketamine: ††p < 0.05, ††p < 0.01 and ††††p < 0.0001 (Analysis by differences by One-way ANOVA followed by Tukey’s multiple comparison test).
4. Discussion
The findings from this study suggests that Xylopia aethiopica (XAE) and its major kaurene diterpenoid, xylopic acid (XA), have broad spectrum nootropic effects in murine models. It is worth noting that the findings on phytochemical constituents of XAE and XA corroborate earlier studies (Biney et al., 2014, John-Dewole et al., 2012). It is our view that the effect of the extract may be due to the xylopic acid component alone or the xylopic acid together with other phytochemicals. This assertion however requires further investigations.
Exploratory learning deals with establishing a relationship between existing knowledge and new content or concept, while working memory is temporarily holding information and using it in cognitive tasks. Reference memory is a form of long-term memory that utilizes two aspects of episodic memory which are content and place dimensions of activity (Miyake and Shah, 1999, Nadel and Hardt, 2011). Recognition memory comprises recollection and familiarity (Squire et al., 2007). The behavioral tests employed in this investigation aided assessment of exploratory, working, recognition and reference memories. XAE and XA enhanced all parameters except for spatial learning as measured by the Morris water maze test. The extracts also enhanced memory retention as assessed with the probe trial of the Morris water maze (Baldi et al., 2005, Brose et al., 2019, Vorhees and Williams, 2006, Vorhees and Williams, 2014). Additionally, XAE and XA improved learning and memory in the novel object recognition (NOR) and Y-maze. Although the extract didn’t enhance initial learning during the Morris water maze (MWM) tests, it enhanced memory retention of the platform location.
In the NOR test, the XAE and XA produced significant increase in percentage time spent with the new object when one of the familiar objects was replaced. A high measure in percentage time spent with the new object is indicative of improvement in exploratory learning and recognition memory (Bevins et al., 2002; Ennaceur, 2010). Although rodents may differ somewhat from humans, the conditions of the NOR test mimics studies that involve human cognition (Lueptow, 2017). Studies indicate that the integrity of the hippocampus and cerebral cortex are critical for the type of recognition memory assessed in the NOR test (Aggleton et al., 2010, Buckmaster et al., 2004). It has been observed that the perirhinal cortex is needed for object recognition after a short retention time while the hippocampus is required for long-term object recognition (Reger et al., 2009). Therefore it will be prudent in the future to investigate the in vitro effects of XAE and XA on hippocampal plasticity as this is believed to be critical to memory formation. Co-treatment of animals with ketamine, which impairs memory formation via known pathways could also contribute towards identifying the brain regions and pathways via which XAE and XA exert their nootropic effects.
Whiles the NOR assesses recognition memory, the Y-maze can be used to assess spatial working memory, which is hippocampal-dependent (Boon and Simpson, 2012, Kraeuter et al., 2019). Mice with normal working memory remember arms previously visited and demonstrate preference for less frequented arms (Kraeuter et al., 2019, Lalonde, 2002). In the Y-maze test, XAE and XA caused significant increase in the percentage number of attempted alternations when one of the arms was blocked and percentage alternations when the blockade was removed. This implies that XAE and XA enhanced the ability of mice to discriminate against the two open arms they were familiar with, making attempts to enter the blocked arm and eventually frequenting that arm once the blockade was removed. This means that there may be enhancement in recognition memory as was previously shown by the NOR tests, with additional benefit that includes spatial working memory (Kraeuter et al., 2019). Taken together, we postulate that XAE and XA improve these two types of memory.
The MWM test is a classical test that assesses the ability of mice to use distal cues to navigate towards a submerged platform and remember its location. Although XAE and XA did not significantly reduce the latency to locate the hidden platform in comparison to controls, neither did citicoline, the reference drug used in this test. Citicoline has previously been shown to be restorative in nature, improving spatial learning and memory following an insult or in diseased states where learning and memory is compromised (Zhao et al., 2006). Therefore it is expected that, any initial benefits of either XAE or XA will be evident if we had chosen to use a pathological group of animals rather than healthy controls. Nonetheless, XAE and XA significantly increased the percentage frequency of entry to the quadrant that previously hid the platform on the 6th day in the probe trial. This effect was significantly higher than citicoline, implying that XAE and XA enhanced reference memory of mice (Baldi et al., 2005, Brose et al., 2019, Vorhees and Williams, 2006, Vorhees and Williams, 2014). It has been shown that the hippocampus, prefrontal cortex, cingulate cortex, neostriatum, entorhinal cortex, perirhinal cortex and N-methyl-D-aspartate (NMDA) receptor function influence mice behavior in this model (D’Hooge and De Deyn, 2001, Vorhees and Williams, 2006). It is thus, possible that the effects of XAE and XA may involve any of the above mentioned structures or systems. A combination of focal ischaemias, in vitro tests and administration of NMDA receptor antagonists will help narrow possible targets of XAE and XA in the nervous system.
Using three animal models (NOR, Y-maze and MWM), we have been able to demonstrate the nootropic effects of XAE and XA. Several factors such as stress, sensory stimuli and environmental cues could affect the performance of mice in the behavioral tests used (Bevins et al., 2002, Crawley, 2000, Nelissen et al., 2018, Weitzner et al., 2015). However, considering the fact that the findings are consistent across all three models, we can conclude that steps taken to mitigate the influence of confounders were adequate.
Altogether, our findings show that XAE and XA produce nootropic effects comparable to reference drugs used (piracetam and citicoline). Whereas evidence supporting the use of piracetam is unclear, citicoline is prescribed for age-related memory loss and diseased states marked by cognitive deficits. These deficits typically affect quality of life, reduce creativity, slow academic performance especially in children, and may increase the risk of developing attention-deficit hyperactivity disorder (ADHD), dyslexia, and Alzheimer’s disease (Fine et al., 2008, Holmes et al., 2010). Since XAE and XA improved exploratory learning, working, recognition, and reference memory in mice, further studies exploring their mechanisms of action and safety is required.
5. Conclusion
The current study showed that ethanolic (70%) extract of the fruit of Xylopia aethiopica and xylopic acid enhance exploratory, working, recognition, and reference memories in mice.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
We declare that we have no conflict of interest.
Acknowledgment
The authors want to thank the staff of the Animal Experimentation Unit of the Department of Medical Microbiology, School of Biomedical and Allied Health Sciences, University of Ghana, and the technical staff of the Department of Pharmacology and Toxixology, School of Pharmacy, University of Ghana.
Contributor Information
Awo Efua Koomson, Email: aekoomson002@st.ug.edu.gh.
Kennedy Kwami Edem Kukuia, Email: kkekukuia@ug.edu.gh, edemkennedy@yahoo.com.
Patrick Amoateng, Email: pamoateng@ug.edu.gh.
Robert Peter Biney, Email: robert.biney@ucc.edu.gh.
Thomas Amatey Tagoe, Email: tatagoe@ug.edu.gh.
Jeffrey Amoako Mensah, Email: jeffreyamoako.mensah@utah.edu.
Elvis Ofori Ameyaw, Email: eameyaw@ucc.edu.gh.
Joseph Torbi, Email: jtorbi@st.ug.edu.gh.
Seth Kwabena Amponsah, Email: skamponsah@ug.edu.gh.
References
- Aggleton J.P., Albasser M.M., Aggleton D.J., Poirier G.L., Pearce J.M. Lesions of the rat perirhinal cortex spare the acquisition of a complex configural visual discrimination yet impair object recognition. Behav. Neurosci. 2010;124:55–68. doi: 10.1037/a0018320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameyaw E.O., Woode E., Boakye-Gyasi E., Abotsi W.K.M., Kyekyeku J.O., Adosraku R.K. Anti-allodynic and Anti-hyperalgesic effects of an ethanolic extract and xylopic acid from the fruits of Xylopia aethiopica in murine models of neuropathic pain. Pharma. Res. 2014;6(2):172–179. doi: 10.4103/0974-8490.129041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameyaw E.O., Asmah K.B., Biney R.P., Henneh I.T., Owusu‑Agyei P., Prah J., Forkuo A.D. Isobolographic analysis of co-administration of two plant-derived antiplasmodial drug candidates, cryptolepine and xylopic acid, in Plasmodium berghei. Malaria J. 2018;17(153):1–11. doi: 10.1186/s12936-018-2283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjula S., Sarvesh S., Hemant S., Pratap S., Dheeraj K., Amod K.S., Rajendra N. a, Rakesh K.D. An experimental study to evaluate the effect of mucuna pruriens on learning and memory in mice. Int. J. Innov. Sci. Res. 2015;4(4):144–148. [Google Scholar]
- Baldi E., Efoudebe M., Lorenzini C.A., Bucherelli C. Spatial navigation in the Morris water maze: working and long lasting reference memories. Neurosci. Lett. 2005;378:176–180. doi: 10.1016/j.neulet.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Barnhart C.D., Yang D., Lein P.J. Using the Morris water maze to assess spatial learning and memory in weanling mice. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0124521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bats S., Thoumas J.L., Lordi B., Tonon M.C., Lalonde R., Caston J. The effects of a mild stressor on spontaneous alternation in mice. Behav. Brain Res. 2001;118:11–15. doi: 10.1016/s0166-4328(00)00285-0. [DOI] [PubMed] [Google Scholar]
- Bevins R.A., Besheer J., Palmatier M.I., Jensen H.C., Pickett K.S., Eurek S. Novel-object place conditioning: behavioral and dopaminergic processes in expression of novelty reward. Behav. Brain Res. 2002;129(1–2):41–50. doi: 10.1016/s0166-4328(01)00326-6. [DOI] [PubMed] [Google Scholar]
- Biney R.P., Boakye-Gyasi E., Benneh C.K., Woode E. Neuroprotective effects of xylopic acid on lipopolysaccharide-induced neuroinflammation. Planta Med. 2015;81(16) [Google Scholar]
- Biney R.P., Mante P.K., Boakye-Gyasi E., Kukuia K.E., Woode E. Neuropharmacological effects of an ethanolic fruit extract of Xylopia aethiopica and xylopic acid, a kaurene diterpene isolate, in mice. West Afr. J. Pharmacy. 2014;25(1):106–117. [Google Scholar]
- Biney R.P., Benneh C.K., Ameyaw E.O., Boakye-Gyasi E., Woode E. Xylopia aethiopica fruit extract exhibits antide-pressant-like effect via interaction with serotonergic neurotransmission in mice. J. Ethnopharmacol. 2016;184:49–57. doi: 10.1016/j.jep.2016.02.023. [DOI] [PubMed] [Google Scholar]
- Boampong J.N., Ameyaw E.O., Aboagye B., Asare K., Kyei S., Donfack J.H., Woode E. The curative and prophylactic effects of xylopic acid on plasmodium berghei infection in mice. J. Parasitol. Res. 2013;2013:1–7. doi: 10.1155/2013/356107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon W.C., Simpson E.R. Handbook of Neuroendocrinology. Academic Press; 2012. Neuroendocrine inherited or induced aromatase enzyme deficits. [Google Scholar]
- Brose R.D., Savonenko A., Devenney B., Smith K.D., Reeves R.H. Hydroxyurea improves spatial memory and cognitive plasticity in mice and has a mild effect on these parameters in a down syndrome mouse model. Front. Aging Neurosci. 2019;11(96):1–14. doi: 10.3389/fnagi.2019.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster C.A., Eichenbaum H., Amaral D.G., Suzuki W.A., Rapp P.R. Entorhinal cortex lesions disrupt the relational organization of memory in monkeys. J. Neurosci. 2004;24:9811–9825. doi: 10.1523/JNEUROSCI.1532-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.J., Choi Y.S. Effects of electromagnetic radiation from smartphones onlearning ability and hippocampal progenitor cell proliferation in mice. OsongPublic Health Res. Perspect. 2016;7:12–17. doi: 10.1016/j.phrp.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh N.A., Bali S., Koul A. Integration of botanicals in contemporary medicine: road blocks, checkpoints and go-ahead signals. Integr. Med. Res. 2018;7(2):109–125. doi: 10.1016/j.imr.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J.N. 1st edition. Wiley-Liss; 2000. What’s Wrong with My Mouse Behavioral Phenotyping of Transgenic and Knockout Mice. [Google Scholar]
- D’Hooge R., De Deyn P.P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- d’Isa R., Brambilla R., Fasano S. Vol. 1120. Springer Science+ Business Media LLC; 2014. Behavioral methods for the study of the Ras–ERK pathway in memory formation and consolidation: passive avoidance and novel object recognition tests; pp. 131–157. (Ras Signaling: Methods and Protocols, Methods in Molecular Biology). [DOI] [PubMed] [Google Scholar]
- Dhingra D., Parle M., Kulkarni S.K. Genetic basis of Alzheimer’s disease. Indian J. Pharm. Sci. 2005;67(4):409–413. [Google Scholar]
- Ekeanyanwu R.C., Etienajirhevwe O.F. In vitro anthelmintic potentials of Xylopia aethiopica and Monodora myristica from Nigeria. Afr. J. Biochem. Res. 2012;6(9):115–120. [Google Scholar]
- El Tabaa M.M., Sokkar S.S., Ramadan E.S., El Salam I.Z.A., Zaid A. Neuroprotective role of Ginkgo biloba against cognitive deficits associated with Bisphenol A exposure: an animal model study. Neurochem. Int. 2017;108:199–212. doi: 10.1016/j.neuint.2017.03.019. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues behavioral Brain Research. behavioral Brain Research. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Ennanceur A., Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav. Brain Res. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fine E.M., Delis D.C., Wetter S.P., Jacobson M.W., Hamilton J.M., Peavy G., Goldstein J., McDonald C., Corey-Bloom J., Bondi M.W., Salmon D.P. Identifying the “source” of recognition memory deficits in patients with Huntington’s disease or Alzheimer’s disease: Evidence from the CVLT-II. J. Clin. Exp. Neuropsychol. 2008;30(4):463–470. doi: 10.1080/13803390701531912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer T.C., Mensah M.L.K., Mensah A.Y., Komlaga G., Gbedema S.Y., Skaltsa H. Antimicrobial Activity of Essential Oils of Xylopia aethiopica. African Journal of Traditional. Complem. Alt. Med. 2008;5(4):391–393. doi: 10.4314/ajtcam.v5i4.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Wang C., Wang J., Lei Y., Ma Y. Long-term exposure to extremely low-frequency magnetic fields impairs spatial recognition memory in mice. Clin. Exp. Pharmacol. Physiol. 2008;35:797–800. doi: 10.1111/j.1440-1681.2008.04922.x. [DOI] [PubMed] [Google Scholar]
- Heo J.H., Lee S.T., Chu K., Oh M.J., Park H.J., Shim J.Y., Kim M. An open-label trial of Korean red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzheimer’s disease. Eur. J. Neurol. 2008;15(8):865–868. doi: 10.1111/j.1468-1331.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- Holmes J., Gathercole S., Dunning D. In: Holmes J., editor. Vol. 39. Academic Press; Burlington: 2010. Poor working memory: impact and interventions; pp. 1–43. (Advances in Child Development and Behavior: Developmental Disorders and Interventions). [DOI] [PubMed] [Google Scholar]
- Hughes R.N. Review: the value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. 2004;28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Irvine F.R. Oxford University Press,; 1961. Woody Plants of Ghana: With Special Reference to Their Uses.〈https://books.google.com.gh/books?id=_ftUAAAAMAAJ〉 [Google Scholar]
- John-Dewole O.O., Agunbiade S.O., Alao O.O., O.A A. Phytochemical and antimicrobial studies of extract of the fruit of Xylopia aethiopica for medicinal importance. J. Biotechnol. Pharm. Res. 2012;3(6):118–122. [Google Scholar]
- Khalifa A.E. Hypericum perforatum as a nootropic drug: enhancement of retrieval memory of a passive avoidance conditioning paradigm in mice. J. Ethnopharmacol. 2001;76(1):49–57. doi: 10.1016/s0378-8741(01)00210-0. [DOI] [PubMed] [Google Scholar]
- Kraeuter A.-K., Guest P.C., Sarnyai Z. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol. Biol. 2019;1916:105–111. doi: 10.1007/978-1-4939-8994-2_10. [DOI] [PubMed] [Google Scholar]
- Lalonde R. Review: The neurobiological basis of spontaneous alternation. Neurosci. Biobehav. Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Lueptow L.M. Novel object recognition test for the investigation of learning and memory in mice. J. Visualized Exp. 2017;126(55718) doi: 10.3791/55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykh A.G., Sadaie M.R. Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders. Drugs. 2010;70:287–312. doi: 10.2165/11319230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Miyake A., Shah P. Models of working memory. Cambridge University Press. 1999 [Google Scholar]
- Morris R.G., Anderson E., Lynch G.S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319(774–776) doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Morris R.G.M. Spatial localization does not require the presence of local cues. Learn. Motiv. 1981;12:239–260. [Google Scholar]
- Moscardo E., Salvetti B., Becchi S., Bertini G., Fabene P.F. The novel object recognition test in rodents: which are the essential methodological aspects? Meas. Behav. 2012:476. [Google Scholar]
- Nadel L., Hardt O. Update on Memory Systems and Processes. Neuropsychopharmacology. 2011;36:251–273. doi: 10.1038/npp.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen E., Prickaerts J., Blokland A. Acute stress negatively affects object recognition early memory consolidation and memory retrieval unrelated to state-dependency. Behav. Brain Res. 2018;345:9–12. doi: 10.1016/j.bbr.2018.02.027. [DOI] [PubMed] [Google Scholar]
- Nunez J. Morris water maze experiment. J. Visualized Exp. 2008:19. doi: 10.3791/897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obiri D.D., Osafo N. Aqueous ethanol extract of the fruit of Xylopia aethiopica (Annonaceae) exhibits anti-anaphylactic and anti-inflammatory actions in mice. J. Ethnopharmacol. 2013;148(3):940–945. doi: 10.1016/j.jep.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Osafo N., Obiri D.D., Antwi A.O., Yeboah O.K. The acute anti-inflammatory action of xylopic acid isolated from Xylopia aethiopica. J. Basic Clin. Physiol. Pharmacol. 2018;29(6):659–669. doi: 10.1515/jbcpp-2018-0019. [DOI] [PubMed] [Google Scholar]
- Pahaye D.B., Bum E.N., Taïwé S.G., Ngoupaye G.T., Sidiki N., Moto F.C., Kouemou N., Njapdounke S.J., Nkantchoua G., Kandeda A., Omam J.P., Mairaira V., Ojong J.L. Neuroprotective and Antiamnesic Effects of Mitragyna inermis Willd (Rubiaceae) on Scopolamine-Induced Memory Impairment in Mice. Hindawi. Behav. Neurol. 2017;2017:1. doi: 10.1155/2017/5952897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince, M., Comas-Herrera, A., Knapp, M., Guerchet, M., Karagiannidou, M. , 2016. Improving healthcare for people living with dementia (World Alzheimer Report 2016, Issue. A. s. D. International.
- Reger M.L., Hovda D.A., Giza C.C. Ontogeny of rat recognition memory measured by the novel object recognition task. Dev. Psychobiol. 2009;51(8):672–678. doi: 10.1002/dev.20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio J., Yucra S., Gasco M., Gonzales G.F. Dose-response effect of black maca (Lepidium meyenii) in mice with memory impairment induced by ethanol. Toxicol. Mech. Methods. 2011;21(8):628–634. doi: 10.3109/15376516.2011.583294. [DOI] [PubMed] [Google Scholar]
- Somova L.J., Shode F.O., Moodley K., Govender Y. Cardiovascular and diuretic activity of Kaurene derivatives of Xylopia aethiopica and Alepidea amatymbica. J. Ethnopharmacol. 2001;77(2–3):165–174. doi: 10.1016/s0378-8741(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Squire L.R., Wixted J.T., Clark R.E. Recognition memory and the medial temporal lobe: a new perspective. Nature Reviews Neuroscience. 2007;8(11):872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleiman M.M., Mamman M., YO A., JO A. Anthelmintic activity of the crude methanol extract of Xylopia aethiopica against Nippostrongylus brasiliensis in rats. Veterinarski Arhiv. 2005;75(6):487–495. [Google Scholar]
- Sun M.K., Alkon D.L. Induced depressive behaviour impairs learning and memory in rats. Neuroscience. 2004;129:129–139. doi: 10.1016/j.neuroscience.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Talih F., Ajaltouni J. Probable nootropic induced psychiatric adverse effects: a series of four cases. Innov. Clin. Neurosci. 2015;12(11–12):21–25. [PMC free article] [PubMed] [Google Scholar]
- Trease G.E., Evans W.C. Pharmacognosy. 13 ed. ELBS/Bailliere Tindall; 1989. [Google Scholar]
- Vanderveren E., Bijttebier P., Hermans D. The Importance of memory specificity and memory coherence for the self: linking two characteristics of autobiographical memory. Front. Psychol. 2017;8:2250. doi: 10.3389/fpsyg.2017.02250. ISSN=1664-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees C.V., Williams M.T. Assessing spatial learning and memory in rodents. ILAR J. 2014;55(2):310–332. doi: 10.1093/ilar/ilu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzner D.S., Engler-Chiurazzi E.B., Kotilinek L.A., Ashe K.H., Reed M.N. Morris water maze test: optimization for mouse strain and testing environment. J. Vis. Exp. 2015;100:1–11. doi: 10.3791/52706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2017. 10 facts on dementia.
- Woode E., Ameyaw E.O., Boakye-Gyasi E., Abotsi W.K.M. Analgesic effects of an ethanol extract of the fruits of Xylopia aethiopica (Dunal) A. Rich (Annonaceae) and the major constituent, xylopic acid in murine models. J. Pharmacy Bioallied Sci. 2012;4:291–301. doi: 10.4103/0975-7406.103251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.-J., Liu Y., Chen X.-L., Liu J.-X., Tian Y.-F., Zhang P.-B., Kang Q.-Y., Qiu F. Effect of citicoline on spatial learning and memory of rats after focal cerebral ischemia. J. South. Med. Univ. 2006;26(2):174–176. [PubMed] [Google Scholar]