Abstract
Background
Postpartum depression is a mood disorder that affects about 9–20% of women after child birth. Reports suggest that gestational iron deficiency can cause a deficit in behavioral, cognitive and affective functions and can precipitate depressive symptoms in mothers during the postpartum period. The present study examined the effect of iron supplementation on depressive behavior during postpartum period in a rat model.
Method
Female Sprague-Dawley rats were crossed. Pregnant rats received iron, fluoxetine, desferrioxamine or vehicle throughout the period of gestation. During the postpartum period, mothers from all groups were taken through the open field test (OFT), forced swim test (FST), novelty-induced hypophagia (NIH) and sacrificed for histological examination of the brains.
Results
Results showed that rats treated with iron-chelating agent, desferrioxamine, and vehicle during gestation exhibited increased immobility scores in the FST, increased latency to feed and reduced feeding in the NIH with corresponding decreased number of neurons and dendritic branches in the cortex of the brain. These depression-related effects were attenuated by perinatal iron supplementation which showed decreased immobility scores in the FST comparable to rats treated with fluoxetine, a clinically effective antidepressant. Iron treatment also decreased latency to feeding while increasing feeding behavior in the NIH. Iron-treated dams had a higher number of neurons with dendritic connections in the frontal cortex compared to vehicle- and desferrioxamine-treated groups.
Conclusion
The results suggest that, iron supplementation during gestation exerts an antidepressant-like effect in postpartum Sprague-Dawley rats, attenuates neuronal loss associated with depression and increases dendritic spine density.
Abbreviations: AUC, area under the curve; DFx, desferrioxamine; Fe, iron; Flx, fluoxetine; FST, Forced swim test; ID, iron deficiency; HPA, hypothalamic-pituitary-adrenal; NIH, novelty-induced hypophagia; OFT, open field test; PPD, Post-partum depression; VEH, vehicle
Keywords: Post-partum depression, Iron, Desferrioxamine, Fluoxetine, Golgi staining
Highlights
-
•
Iron supplementation during gestation exerts an antidepressant-like effect in postpartum Sprague-Dawley rats.
-
•
Iron supplementation during gestation attenuates neuronal loss associated with depression.
-
•
Iron-treated dams had a higher number of neurons with dendritic connections in the frontal cortex.
1. Introduction
Postpartum depression (PPD) is a non-psychotic depressive disorder that begins 6–12 weeks post-delivery, but can persist even up to 1 year following child birth (Sheikh et al., 2015, Etebary et al., 2010). PPD affects between 9% and 20% of women following child birth (McCloskey and Reno, 2019), and with many cases underreported and/or undiagnosed. PPD can affect both mother and child. In the mother, maternal duties and marital relationship may be affected. In the child, social, emotional, psychomotor and cognitive development may be affected (Letourneau et al., 2012, McCloskey and Reno, 2019, Roomruangwong et al., 2016), and this can persist to adolescent age (Sheikh et al., 2017, Sinclair and Murray, 1998, Tahirkheli et al., 2014). The clinical symptoms of PPD are not very different from that of major depressive disorder and manifests as lack of interest in previously pleasurable activities, insomnia or hypersomnia, crying spells, negative maternal attitudes, significant weight loss or weight gain, paranoia, among others (Beck, 1998, Andrews-Fike, 1999, Etebary et al., 2010, Sheikh et al., 2015). These symptoms cause marked impairment in everyday activities of mothers.
The causes of PPD remain unknown even though several intersecting risk factors including biological, psychological, socio-economic, familial and cultural have been implicated (Yim et al., 2015, Nestler et al., 2002, Craddock and Forty, 2006). Some of the biological and psychological risk factors include rapid decline in the levels of hormones following child birth, alterations in neurotransmitter levels such as serotonin and noradrenaline, dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, nutritional and/or metabolic imbalance (Etebary et al., 2010, Bina, 2008, McCloskey and Reno, 2019). Emerging studies still try to understand association between the aforementioned risk factors and PPD.
Iron is an essential element in living organisms (Beard, Hendricks, and Perez, 2005). Iron is a component of various biomolecules such as haemoglobin, myoglobin, oxidative enzymes, and respiratory chain proteins (Etebary et al. 2010). In nerve cells, iron plays a significant role in oxide reductase reactions, neurotransmission and myelin formation (Beard, 2001, Beard et al., 2002). It also serves as a co-factor for the synthesis of neurotransmitters such as serotonin, dopamine, noradrenaline and gamma-aminobutyric acid (GABA). Furthermore, iron deficiency is the single most common nutrient deficiency in women of reproductive age (Taha et al. 2014). Many studies have documented the link between iron deficiency, iron deficiency anaemia and/or low ferritin with PPD (Albacar et al., 2011, Beard et al., 2005, Etebary et al., 2010, Sheikh et al., 2015, Alharbi and Abdulghani, 2014, Milman, 2011). A number of studies have also reported iron supplementation and dietary intervention as potential remedy for PPD (Bodnar and Wisner, 2005, Aubuchon-Endsley et al., 2012). So far there is not enough data on the effect of perinatal iron administration on PPD to support such assertions. Therefore, the current study sought to investigate the role of perinatal iron administration on postpartum depressive Sprague-Dawley rats.
2. Materials and methods
2.1. Chemicals and reagents
Fluoxetine hydrochloride (Prozac) was purchased from Bristol laboratories Ltd. Berkhamsted, Hertfordshire, HP4 1 EG, UK. The iron supplement (crystalline ferrous sulphate) was obtained from Sigma-Aldrich Inc., St. Louis, MO, USA. Desferrioxamine methane sulphonate was purchased from Novartis Pharma Stein AG, Stein, Switzerland. Potassium dichromate (KₐCrₐO₇), Mercuric chloride (HgClₐ) and Potassium chromate (KₐCrO₄) used for the Golgi-cox solution were purchased from Merck KGaA, Darmstadt, Germany.
2.2. Experimental animals and housing
Eighty female (180 – 250 g) and twenty male (200 – 250 g) Sprague-Dawley rats at 6 weeks old were obtained from the Centre for Plant Medicine Research (CPMR), Mampong, Eastern Region, Ghana, and kept at the Animal House of the Department of Medical Microbiology, School of Biomedical and Allied Health Science, University of Ghana. The rats were housed in standard cages (800 cm2 × 14 cm) with a minimum floor area of 200 cm2 per animal and soft wood shavings as bedding material. The animals were maintained at room temperature (27 ± 1), relative humidity of 45–65% with a 12 h light/dark cycle and fed with normal commercial pellet diet (AGRIMAT, Kumasi, Ghana), with water provided ad libitum. The rats were made to acclimatize to this environment for 1 week prior to experimental procedures. Ethical clearance was received from the Ethical and Protocol Review Committee of the College of Health Sciences, University of Ghana, (CHS-Et/M.4-P2.8/2017–2018). All animal studies were carried out in accordance with the National Institute of Health guide for the care and use of laboratory animals (NIH Publication No. 8023, revised 1996).
2.3. Experimental design
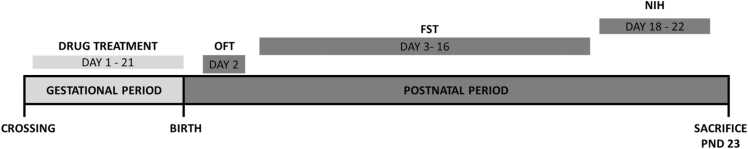
Eighty (80) young adult (6 weeks old) female rats were crossed. Five rats (1 male: 4 females) were initially housed in clear polyurethane cages under standard laboratory conditions for crossing. The day of vaginal plug (male sperm deposit in female) was captured as gestational day (GD) 1. Pregnant rats were randomly allocated into 9 groups, n = 10. Group I (VEH group): pregnant rats received saline (10 ml/kg) orally as the negative control. Groups II, III, IV (Flx group): pregnant rats were treated with fluoxetine (3; 10; 30 mgkg−1, p.o; n = 10) respectively as the positive control group. Group V (DFx group): pregnant rats were treated with desferrioxamine (50 mgkg−1, s.c) as the iron-deficient group. Group VI, VII, VIII (Fe group): pregnant rats were treated with iron supplement (0.005, 0.8, 8 mgkg−1, p.o). Doses of iron were extrapolated from human doses and were selected to reflect borderline low iron group (0.005 mgkg−1), iron sufficient (0.8 mgkg−1) and borderline iron overload (8.0 mgkg−1) respectively. Drug administration started on GD 1 and continued throughout the period of gestation (GD 21). After giving birth (postpartum), the female rats were taken through the open field test (OFT) on day 2, followed by the forced swim test (FST) from days 3–16 and later the novelty-induced hypophagia (NIH) from days 18–22, as shown in Fig. 1. The rats were sacrificed on day 23 to harvest the brain for histological examination.
Fig. 1.
Experimental design.
2.3.1. Open field test (OFT)
The test was based on that described previously by Kasture et al. (2002). The open field apparatus was constructed with plywood and the walls painted white. It measured 72 cm × 72 cm × 36 cm (l × b × h). Black lines were drawn on the floor with a marker and were visible on the white floor. The lines divided the floor into sixteen 18 × 18 cm squares. A central square of (18 cm × 18 cm) was drawn in the middle of the open field. The set up was illuminated by a 100 W bulb placed about 150 cm directly above the centre of the apparatus floor. On the test day animals were transferred from the animal house to the behavioral test room at least 1 h prior to the test for habituation. The test period was initiated by starting the video recorder and placing a single rat in the centre of the central square of the apparatus. The animal was allowed to move freely in the arena for 5 min. Each session was recorded by a video camera suspended approximately 100 cm above the arena. In order to evaluate the locomotor activity of the animal, the latency to leave the centre square and total line crossings (as all four paws crossing over the line) were recorded as previously described (Salari, Bakhtiari, and Homberg, 2015).
2.3.2. Forced swimming test (FST)
The test was carried out as previously described with modifications (Slattery and Cryan, 2012, Arbabi et al., 2014, Porsolt et al., 1977). The set up used for the FST was a transparent polypropylene cylindrical tank (height 40 cm, diameter 25 cm) and filled with clean water to a height 30 cm at 27 ± 1. Animals were transported from the animal house to the behavioral test room at least an hour prior to the FST for habituation. Postpartum rats from each treatment group were taken through FST by placing them individually in the water on postpartum day 2 to induce a state of helplessness. On postpartum day 3 to postpartum day 16 rats were again placed in the swim tanks and allowed to swim for 5 min. Behavioral assessment was measured using a public domain software JWatcher, version 1.0 (University of California, Los Angeles, USA, and Macquarie University, Sydney, Australia) during the 5-min test period. The predominant behavior for each animal was assessed in 5-second blocks throughout the 5-min test and used as the mean behavior. The behavioral components scored included immobility (floating upright in the water without active movement except for twitches, shivers, or corrective wall bouncing), swimming (movement of the hind limbs or tail resulting in active horizontal motions more than necessary to solely maintain their head above water) and climbing (active movements in and out of the water with forepaws, usually directed against the walls of the container) (Amoateng et al., 2018).
2.3.3. Weight changes
PPD can cause a decline in weight. Hence the effect of perinatal of iron, desferrioxamine, and fluoxetine treatment on weight variation during the 14-day forced swimming test (FST) period was investigated. Weights of animals were taken on day 0, 3, 6, 9, 12, 14 of the FST.
2.3.4. Novelty-induced hypophagia (NIH)
The procedure for the NIH test was based on that described by Dulawa (2009). Postpartum rats were housed singly for 24 h and then trained to drink sweetened condensed milk (dilution factor = 3, stored at 4 ) drawn into 10 ml serological pipettes for three consecutive days in the home cage. For home-cage testing, rats were removed from their cages briefly to position the pipettes containing milk in wire lids and initial readings were taken and then quickly returned to their cages and a timer was started. The latency to drink and the volume consumed were recorded every 5 min for a 30 min period. Latency to drink is defined as the time taken for the rat to first lick the sipper. To be counted as a lick, the tongue should make contact with the sipper; merely sniffing the sipper was not counted. The sipper was positioned such that a rat resting on the floor of the cage could drink comfortably. Home cage testing was conducted under relatively dim lighting. Rats that never drank during the 30 min of home cage testing were eliminated from the experiment. For novel-cage (which is a standard home cage without bedding) testing, pipettes containing the milk solution were positioned and any drops of milk solution on the floor of the novel cage during pipette positioning was cleaned thoroughly. Rats were then quickly placed into the novel cage and a timer started. The latency to drink and the volume consumed were recorded every 5 min for 30 min. The novel-cage testing was performed under bright lighting with white floor under the cages to increase aversiveness of the cage.
2.4. Histological evaluation of the volume of neurons in the brain
2.4.1. Golgi-Cox solution
The Golgi-Cox stain was used as previously described with modifications (Das et al., 2013, Zaqout and Kaindl, 2016). The Golgi-Cox solution was prepared by mixing 5% w/v solutions of potassium dichromate (), mercuric chloride () and potassium chromate () in a ratio of 5:5:4 respectively.
2.4.2. Tissue collection and preservation
Postpartum rats were sacrificed after FST by anaesthetising them using diethyl ether. Brain tissues from the postpartum rats were then extracted immediately after the animals were perfused transcardially with 0.9% saline. Brain tissues were divided into two transverse halves and post-fixed in a 40 ml bottle containing Golgi-Cox solution for twenty-four (24) h. After 24 h, each brain sample was removed and the solution discarded. The sample bottle was washed and filled with new Golgi-Cox solution. The brain samples were placed back into the solution and stored in dark for fourteen (14) days. After which they were removed from the Golgi-Cox solution, slightly blotted with tissue paper and transferred into a 30% w/v sucrose solution and stored in the refrigerator until sectioning was done at 50 using a microtome (Das, Reuhl, and Zhou, 2013).
2.4.3. Tissue processing
Upon removal from the sucrose solution, each brain sample was divided into three coronal sections, placed in histological cassettes (Rotilabor embedding cassettes; K114.1, Carl Roth GmbH, Germany) and passed through an ethanol series of 70% ethanol for 1 h, 95% ethanol for 1.5 h and 100% ethanol twice for 2 h each. The processed tissues were placed in molten paraffin wax for a total time of 3 h after which they were embedded in molten paraffin wax and placed in the refrigerator at 4 until sectioning.
2.4.4. Sectioning
The microtome used for sectioning was a Leica RM 2235 manual microtome. The refrigerated tissue blocks of the vehicle-, fluoxetine-, desferrioxamine- and iron-treated groups were mounted on the microtome, sectioned at 50 and placed on water. The floating sections were picked with a brush and mounted on the gelatine coated slides. The sections were blotted with tissue paper and direct, downward moderate pressure was applied with the heel of the palm (Gibb and Kolb, 1998) so that the sections were firmly glued to the gelatine slides. The slides with sections were transferred to racks and kept for drying in the dark for 3 days.
2.4.5. Colour development
The racks with the slides were dewaxed by passing them through xylene twice for 2 min each. The racks were then passed through 100% ethanol twice for 2 min each and placed in a jar filled 50% ethanol for 5 min before being placed in a 3:1 ammonia solution for 8 min in the dark at room temperature. The sections were washed with double distilled water twice for 5 min each. Next, the racks with the slides were immersed in 1% sodium thiosulfate solution to fix the stain for 5 min at room temperature in the dark. The racks with the slides were washed in distilled water twice for 1 min each. The sections were then incubated in 5% Mallory stain C as a counter stain for 1 min. The sections were subsequently passed through an ethanol series of 70%, 95% and 100% (twice) for 5 min each for complete dehydration. Afterwards, the racks with the slides were placed in fresh xylene for 2 min in the dark and then the slides were taken out carefully and mounted with DPX (mixture of distyrene, a plasticizer, and xylene) and allowed to dry under the fume hood for 3 days before examining under the microscope.
2.5. Neuronal count
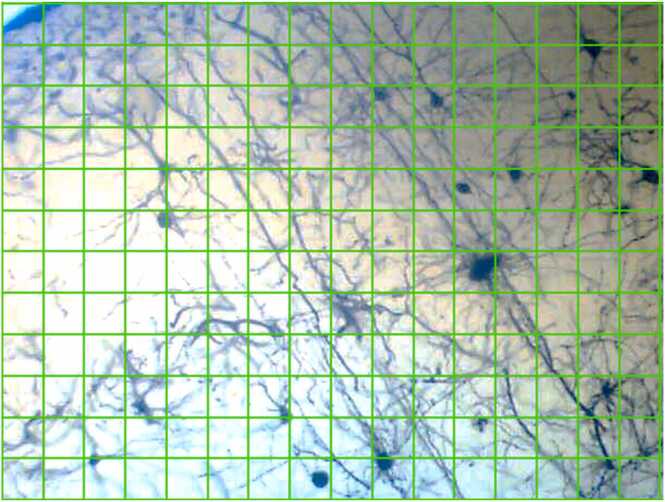
The slides were observed under a light microscope (Leica Galen III-1154XV) at low (x100) and high (x400) magnifications. Images were captured using a coupled device (CD) eye piece (Lenovo Q350 USB PC Camera). All images were enhanced using Adobe Photoshop CS6 version 13.0 × 64. Using ImageJ Software, a stereological grid consisting of uniformly spaced points, 1 cm × 1 cm was superimposed over each micrograph of the brain tissue to count the number of test points which intersected with the cell bodies (Fig. 2).
Fig. 2.
A picture showing the stereological grid (1 cm × 1 cm) superimposed on a brain section of the cortex.
2.5.1. Evaluation of dendritic spine characteristics
Evaluation of dendritic length and spine characteristics was done as described previously by Zhu et al. (2018) and Risher et al. (2014). Dendritic spines were analysed and counted with a Fiji version of Image J software (version 1.53c, NIH) and the freely available Reconstruct software (http://synapses.clm.utexas.ed). Further analysis of the spine morphological characteristics was done as described by Risher et al. (2014).
Briefly, three independent coronal sections per rat were used for analysis. A Z-stack images of the Golgi spines were processed with the Image J software and imported as series images in the Reconstruct software. The series images were calibrated and processed for the analysis of dendritic spine density and classification. The dendritic segment was identified for all section to be 10 µm in length uninterrupted. The length and width of the spines were measured using the reconstruct software.
The data for the spine length and width were imported and computed in a spreadsheet. The morphological characteristics of the spines were classified based on the length, width and length-to-width ration of the spines as described by Risher et al. (2014). The results were then exported to Graph pad prism for the statistical analysis.
2.6. Statistics
GraphPad Prism Version 8.01 for Windows (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses. Data was expressed as mean ± SEM (Standard Error of Mean). P < 0.05 was considered statistically significant when data of test groups were compared with that of vehicle-control in a one-way ANOVA followed by Tukey’s test or a two-way ANOVA followed by a Bonferroni’s post hoc test where applicable.
The ED50 (concentration responsible for 50% of the maximal effect) of iron/fluoxetine was determined using a repetitive computer least squares method in Prism for Windows version 8.01 (GraphPad Software, San Diego, CA, USA) with the following nonlinear regression (four-parameter logistic equation).
Where, X is the logarithm of concentration. Y is the response, starting at ‘a′ and ending at point ‘b′ with a sigmoid shape. The fitted midpoints (ED50s) of the curves were compared statistically using the F test.
3. Results
3.1. Changes in animal weight
There was a general increase in the weights of all the animals weighed during the 14-day FST test. The effect of drug treatments on the weight variation was however not statistically significant. Refer to Table 1, Table 2.
Table 1.
The effects of supplemental Fe (0.005, 0.8 and 08.0 mgkg−1) on the weights (g) of rats during a 14-day forced swimming test.
| Body weight (g) |
||||
|---|---|---|---|---|
| Day | Vehicle | Fe 0.005 | Fe 0.08 | Fe 8 |
| 0 | 211.840 ± 6.479 | 216.930 ± 6.567 | 207.042 ± 3.769 | 222.050 ± 7.405 |
| 3 | 208.527 ± 732 | 220.260 ± 8.022 | 208.404 ± 3.409 | 221.868 ± 8.230 |
| 6 | 212.481 ± 7.049 | 226.717 ± 8.152 | 210.759 ± 2.960 | 222.396 ± 8.834 |
| 9 | 215.557 ± 6.968 | 226.717 ± 7.526 | 208.902 ± 3.248 | 223.253 ± 8.336 |
| 12 | 216.429 ± 6.902 | 226.096 ± 7.879 | 209.387 ± 3.096 | 224.725 ± 8.033 |
| 14 | 215.363 ± 6.633 | 225.435 ± 8.007 | 211.556 ± 3.274 | 225.075 ± 7.619 |
Table 2.
The effects of fluoxetine (3, 10 and 30 mgkg−1) and desferrioxamine (50 mgkg−1) on the weights (g) of rats in a 14-day during the forced swimming test.
| Body weight (g) |
|||||
|---|---|---|---|---|---|
| Day | Vehicle | Flx 3 | Flx 10 | Flx 30 | DFx 50 |
| 0 | 211.840 ± 6.479 | 220.281 ± 8.551 | 220.155 ± 5.852 | 207.691 ± 6.924 | 179.533 ± 9.469 |
| 3 | 208.527 ± 732 | 218.774 ± 9.283 | 223.630 ± 6.147 | 207.604 ± 7.832 | 199.319 ± 9.464 |
| 6 | 212.481 ± 7.049 | 221.241 ± 8.935 | 225.923 ± 6.460 | 213.018 ± 8.042 | 199.417 ± 8.491 |
| 9 | 215.557 ± 6.968 | 223.089 ± 8.512 | 229.182 ± 5.860 | 212.325 ± 7.752 | 201.930 ± 8.968 |
| 12 | 216.429 ± 6.902 | 222.688 ± 8.859 | 230.447 ± 6.481 | 211.042 ± 8.024 | 203.054 ± 9.102 |
| 14 | 215.363 ± 6.633 | 222.896 ± 8.274 | 230.344 ± 6.495 | 215.804 ± 8.150 | 203.847 ± 9.023 |
3.2. Open field test
3.2.1. Effect of perinatal iron, fluoxetine and desferrioxamine treatment on locomotor activity for dam in the OFT
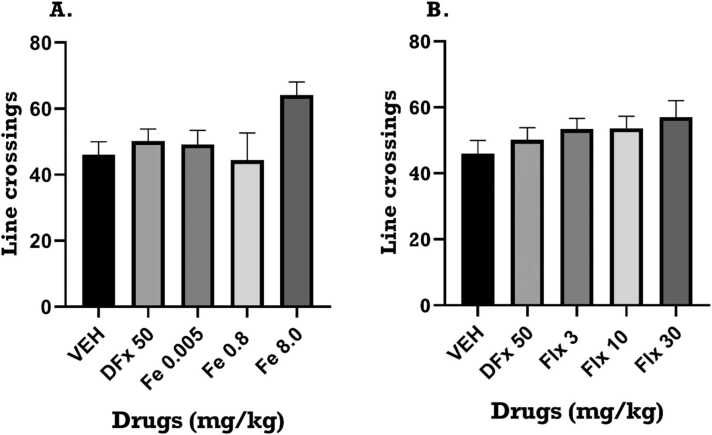
The open field test was used to assess locomotor activity. A one-way ANOVA revealed that perinatal iron treatment caused no significant change in the number of line crossings (F4, 10 = 0.8026; p = 0.5332) in OFT. Similarly, perinatal fluoxetine and desferrioxamine treatment caused no significant changes in the number of line crossings. Refer to Fig. 3A, B.
Fig. 3.
Effect of (a) iron, Fe (0.005–8 mg kg−1) and (b) fluoxetine, Flx (3 – 30.0 mg kg−1) treatment on number of line crossings respectively in the open field test. Data is presented as mean ± SEM of their number of line crossings. Significantly different from control: p < 0.05 One-way ANOVA followed by Tukey post hoc test.
3.3. Forced swimming test
3.3.1. Effect of perinatal iron, fluoxetine and desferrioxamine treatment on mean immobility
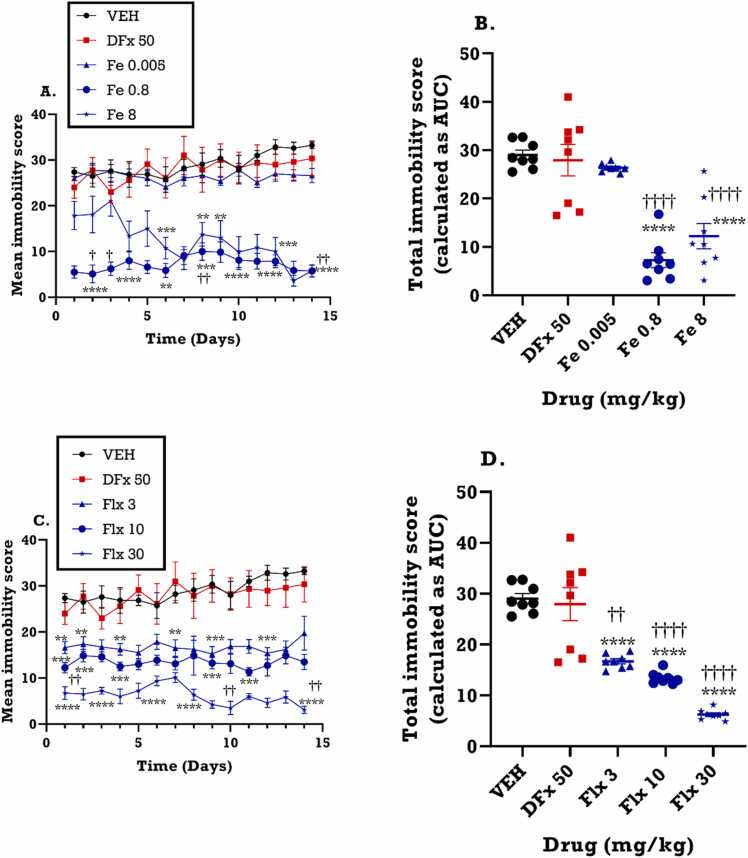
From the time course curve, both vehicle and desferrioxamine groups exhibited an increase in immobility behavior throughout the 14-day period. In contrast, the medium dose of iron (0.8 mgkg−1), representing the equivalent iron dose given to humans during pregnancy, caused a significant decrease in immobility from day 3 of the FST (F4, 52 = 210.3; p<0.0001) (Fig. 4A) and the effect was sustained throughout the 14 days of test. Similarly, fluoxetine decreased immobility scores just as the iron treatment (F4,52 = 222.8; p<0.0001) (Fig. 4C). To investigate the overall impact of the treatments on dams, the total immobility score presented as area under the curve (AUCs) was calculated. From the AUCs, both iron (F4,65 = 175.9; p<0.0001) and fluoxetine (F4,65 = 396.0; p<0.0001) treatment during gestation significantly decreased the total immobility scores of the dams as shown in Fig. 4B and Fig. 4D. Conversely, perinatal desferrioxamine (DFx) treatment had no significant effect on the total immobility score (AUC) of the dams (Fig. 4B, D) when compared to the vehicle group. However, both iron and fluoxetine treatment produced a significantly lower immobility score when compared to DFx.
Fig. 4.
Effects of Fe (0.005–8 mgkg−1) and fluoxetine (3 – 30 mgkg−1) treatment on the duration of immobility in the forced swim test. Data are presented as both (A, C): time course curves and the (B, D): mean ± SEM of the total immobility score (area under the curves; AUCs). Significantly different from vehicle-treated group: **p< 0.01, * ** p < 0.001, ****p < 0.0001 using two-way ANOVA followed by Bonferroni’s test (A, C) and one-way ANOVA followed by Tukey’s test (B, D). Comparison with DFx-treated group: ††p < 0.01, ††††p < 0.0001; two-way ANOVA followed by Bonferroni’s (A, C) test and one-way ANOVA followed by Tukey’s test (B, D).
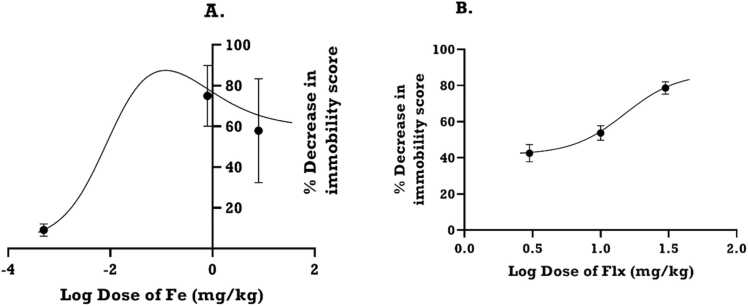
3.3.2. Log dose-response curves of iron and fluoxetine for dams
The log-dose response curve for iron (Fe), obtained from mean immobility scores (Fig. 4), showed that increasing iron dose increased the diminution of the immobility score until it dipped, giving a bell shaped curve (Fig. 5A). In contrast, fluoxetine (Flx) gave a sigmoidal curve (Fig. 5B). Thus, for Fe, there were two ED50s estimated (0.13 and 0.68 mgkg−1) whereas the ED50 for Flx was 15.09 mgkg−1. The antidepressant efficacy (Emax) calculated from the dose-response curve for Fe peaked at 86% but decreased with increasing drug dose to plateau below 60% (Fig. 5A). In contrast, the Emax of Flx did not plateau even after 86% (Fig. 5B).
Fig. 5.
Dose–response curves of (A): iron (Fe) and (B): fluoxetine (Flx) showing % decrease in immobility in the forced swimming test in rats. Each point is the mean ± S.E.M. of 8 animals.
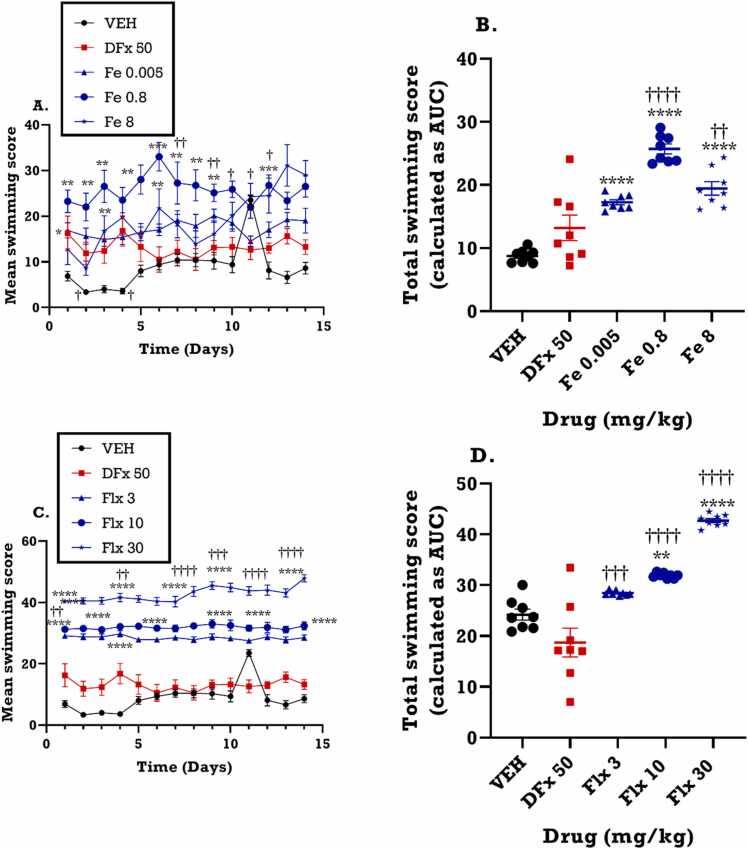
3.3.3. Effect of perinatal iron, fluoxetine and desferrioxamine treatment on mean swimming score for dams
Iron increased the swimming score of the dams in the FST (F4, 52 = 91.36; p<0.0001). This effect produced by iron was sustained throughout the duration of the FST (Fig. 6A). In a similar fashion, the time-course graph of fluoxetine (F4,52 = 1158.0, p<0.0001) (Fig. 6C), showed an increase and sustained effect on the swimming score from day 1 to day 14 of the FST. Both iron (F4, 65 =35.54; p< 0.0001) and fluoxetine (F4,65 =388.6, p<0.0001) significantly increased the total swimming score of the dams as depicted in the area under the curve (AUC) (Fig. 6B, D). As previously noted from the dose response curve, the medium dose of iron (0.8 mgkg−1) produced the greatest increase in swimming score (F4, 65 = 15.99; p< 0.05) than the highest dose (8.0 mgkg−1) (F4,65 = 10.09; p<0.05), giving a bell-shaped effect (Fig. 6B). DFx did not exert any significant effect on swimming score of the dams when compared to the vehicle control group. Compared to DFx however, iron and fluoxetine significantly increased the total swimming score (Fig. 6B, D).
Fig. 6.
Effects of iron (Fe, 0.005–8 mgkg−1) and fluoxetine (Flx, 3 – 30 mgkg−1) treatment on the swimming score in the forced swim test. Data are presented as both (A, C): time course curves and the (B, D): Mean ± SEM of their total swimming score presented as areas under the curve (AUCs). Significantly different from control: *p < 0.05, * * p < 0.01, * ** p < 0.001; two-way ANOVA followed by Bonferroni’s test (A, C) and one-way ANOVA followed by Tukey’s test (B, D). Comparison with DFx-treated group: †p < 0.05, ††p < 0.01, †††p < 0.001, ††††p < 0.0001; two-way ANOVA followed by Bonferroni’s (A, C) test and one-way ANOVA followed by Tukey’s test (B, D).
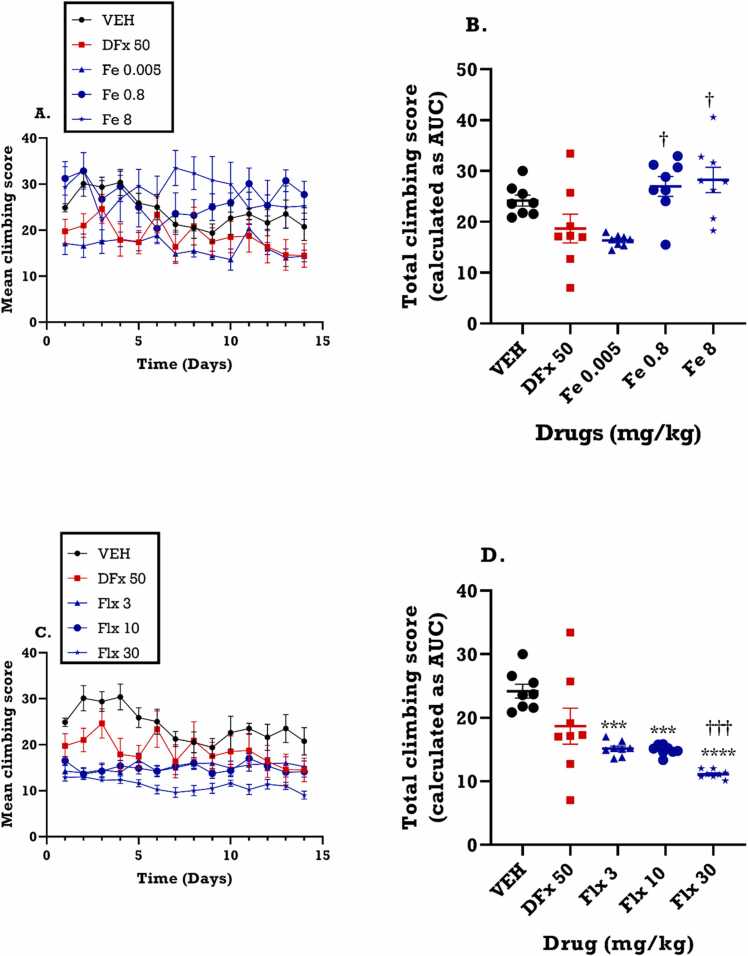
3.3.4. Effect of perinatal iron, fluoxetine and desferrioxamine treatment on mean climbing score for dams
From the time course curve (Fig. 7A), iron did not exert a significant effect on the climbing score in the FST. Similarly, fluoxetine (Fig. 7C), did not significantly alter the climbing scores in the FST (F3, 55 = 79.86; p<0.0001). The total climbing score for the 14-day period however indicated that perinatal iron treatment increased the climbing score when compared to control and desferrioxamine whereas fluoxetine decreased the total climbing score (Fig. 7B, D).
Fig. 7.
Effects of Fe (0.005–8 mgkg−1) and fluoxetine (3 – 30 mgkg−1) treatment on the climbing score in the forced swim test. Data are presented as both (A, C): time course curve and (B, D): Mean ± SEM of the total climbing score presented as areas under the curves (AUCs). Significantly different from control: * **p < 0.001; two-way ANOVA followed by Bonferroni’s test (A, C) and one-way ANOVA followed by Tukey’s test (B, D). Comparison with DFx-treated group: †p < 0.05, †††p < 0.001; two-way ANOVA followed by Bonferroni’s (A, C) test and one-way ANOVA followed by Tukey’s test (B, D).
3.4. Novelty-induced hypophagia (NIH)
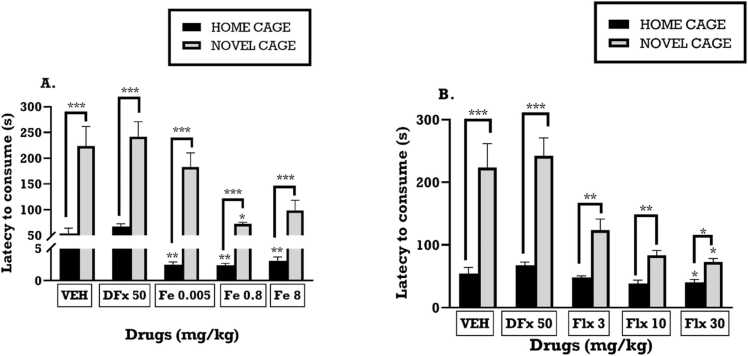
3.4.1. Effect of perinatal iron, fluoxetine and desferrioxamine treatment on the latency to consume a palatable meal in the home cage and novel cage
From the NIH, iron treatment significantly decreased the latency to drink diluted condensed milk in the novel cage (F4,30 = 10.14; p<0.0001) (Fig. 8A) as well as the home cage (F4, 30 = 6.988; p=0.0004) (Fig. 8A) when compared with vehicle control group. Similarly, fluoxetine had a significant effect on the latency in the home cage (F4,35 = 3.696; p=0.0130) (Fig. 8B) and the novel cage in a dose dependent manner (F4, 35 = 18.02; p<0.0001) (Fig. 8B). Even though the latency to consume the milk was generally higher in the novel cage than the home cage, both iron and fluoxetine significantly reduced the latencies in the novel cage when compared to vehicle group (Fig. 8A, B). Though perinatal DFx treatment did not cause a significant change in the latency of the dams to drink the milk in both the home and novel cages, the latency in the novel cage was higher than the home cage (Fig. 8A, B).
Fig. 8.
The effects of perinatal (A): iron (0.005–8 mgkg−1), (B): fluoxetine (3 – 30 mgkg−1) treatment on the latency to consume a palatable meal in the home cage and novel cage. Values are presented as means SEM. * **p < 0.0001;* *p < 0.001 when compared to control group with one-way ANOVA followed by Tukey’s test (A, B).
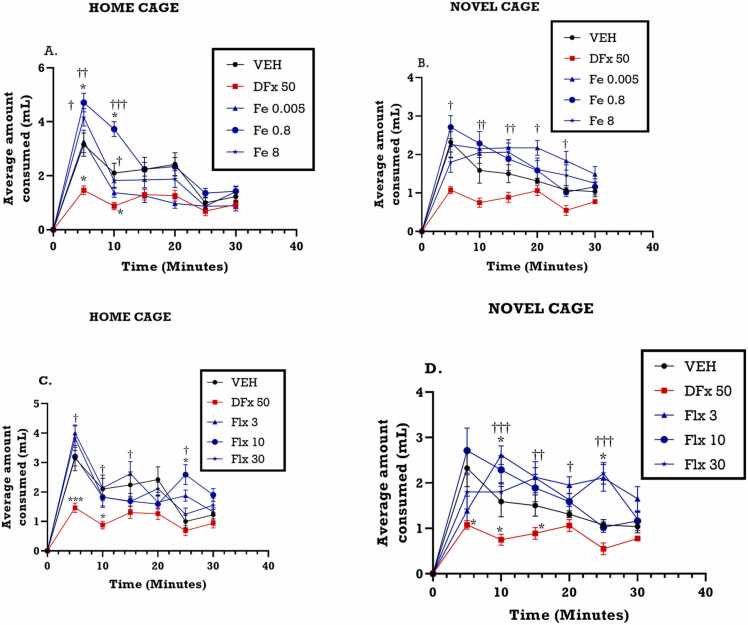
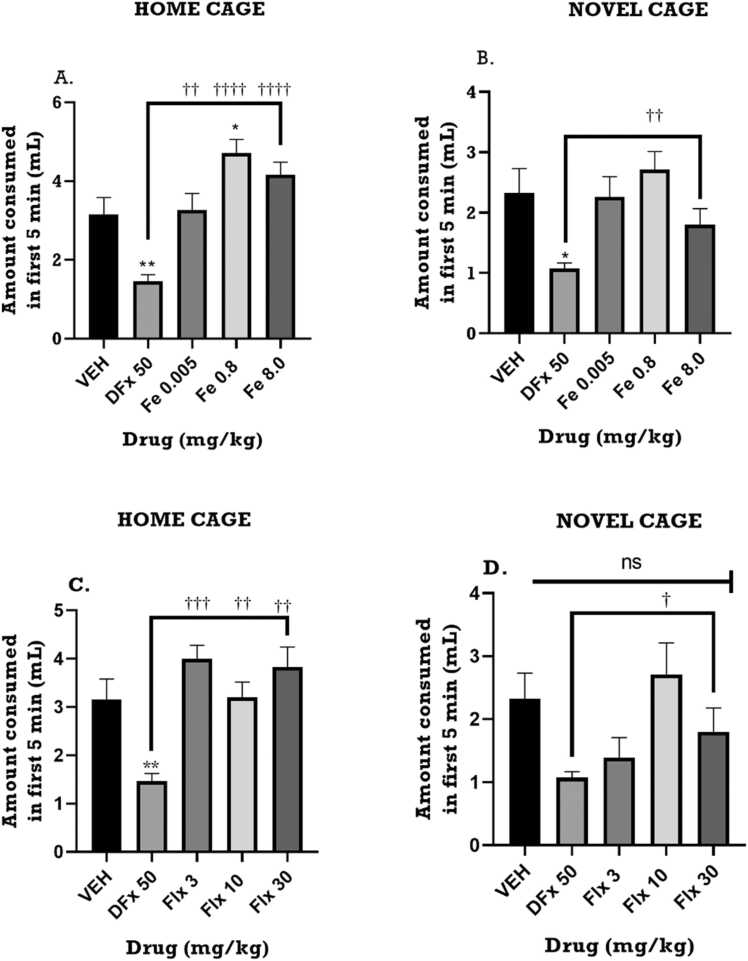
3.4.2. Effect of perinatal iron, fluoxetine and desferrioxamine on the consumption of a palatable meal for the first 5 min in the home cage and novel cage
From the time course curve, average milk consumption measured over 5 min interval for the 30 min period, was generally increased by iron in the home cage but not the novel cage when compared with the vehicle group (Fig. 9A, B). In contrast, desferrioxamine treatment significantly reduced milk consumption during the same period (Fig. 9A, B). Fluoxetine treatment demonstrated similar pattern as the iron group. Analysis of milk consumption during the first 5 min, which measures anxiety-related behavior, showed a general increase in consumption in the home cage but a relative decrease in the novel cage. During the first 5 min, perinatal iron treatment significantly increased the milk consumption of the dams in the home cage (F4,30 = 3.908; p< 0.0114) (Fig. 9A) but not in novel cage (F4,34 = 0.5477, p=0.7021) (Fig. 9B). Perinatal fluoxetine treatment revealed a similar pattern just as the iron group did in the home cage (F4, 34 = 3.792; p=0.0121) (Fig. 9C) as well as the novel cage (F4,34 = 3.732; p<0.0140) (Fig. 9D). Desferrioxamine significantly reduced the milk consumption in the first 5 min Fig. 10.
Fig. 9.
Effects of perinatal (A, B) iron (0.005–8 mgkg−1) and (C, D) fluoxetine (3 – 30 mgkg−1) treatment on the amount of milk consumed in first five-minute interval for 30 min of the novelty-induced hypophagia test. Data are presented as Mean ± SEM of their consumption within the first five minutes. Significantly different from control: p < 0.001; two-way ANOVA followed by Bonferroni’s test and one-way ANOVA followed by Tukey’s test. Comparison with DFx-treated group: †p < 0.05, †††p < 0.001; two-way ANOVA followed by Bonferroni’s test and one-way ANOVA followed by Tukey’s test.
Fig. 10.
Effects of perinatal (A, B) iron (0.005–8 mgkg−1) and (C, D) fluoxetine (3 – 30 mgkg−1) treatment on the amount of milk consumed in the first five minutes of the novelty-induced hypophagia test. Data are presented as Mean ± SEM of their consumption within the first five minutes. Significantly different from control: p < 0.001; two-way ANOVA followed by Bonferroni’s test and one-way ANOVA followed by Tukey’s test. Comparison with DFx-treated group: †p < 0.05, †††p < 0.001; two-way ANOVA followed by Bonferroni’s test and one-way ANOVA followed by Tukey’s test.
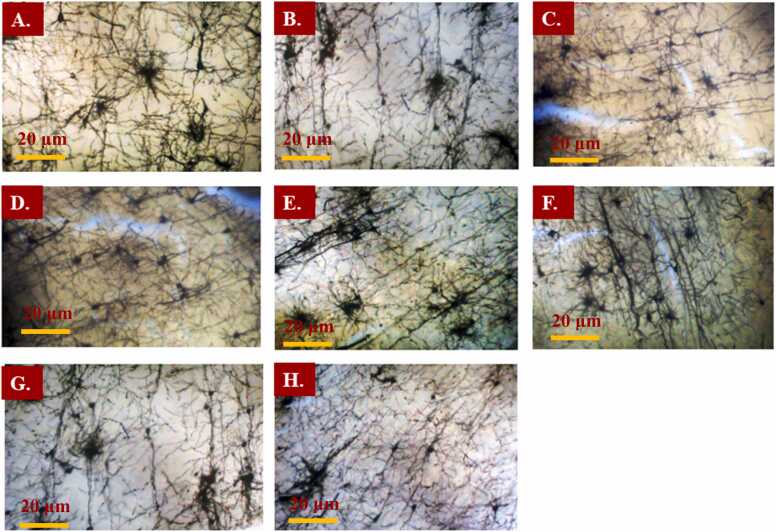
3.5. Histological examination of the brain tissue
The histological examination of the brain revealed visible cell bodies with prominent axons and dendrites. The slides were examined at x100 magnification using a light microscope. Results show that DFx group had reduced number of neurons with fewer dendritic branches when compared to the iron and fluoxetine group. Refer to Fig. 11.
Fig. 11.
Photomicrographs of Golgi-Cox stained brain cortex sections of the dams of A: Vehicle, B: Desferrioxamine, C, D, E: Iron (Fe 0.005, 0.8, 8 mgkg−1) and F: Fluoxetine treatment groups (3, 10, 30 mgkg−1). Magnification: × 100. Scale bar: 20 µm.
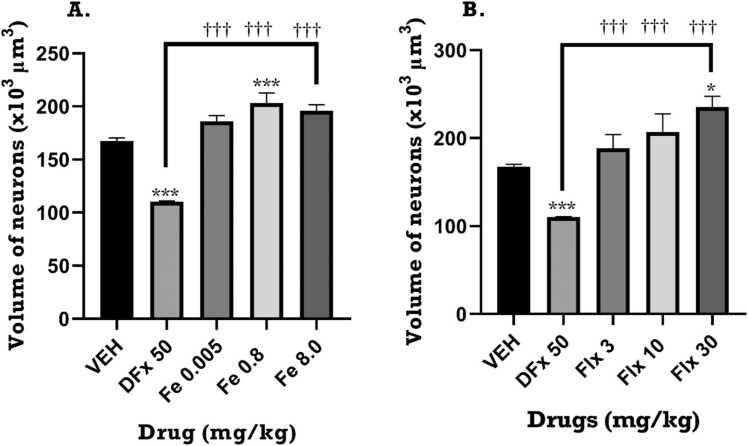
3.6. Volume of neurons for dams
One-way ANOVA of the effect of treatment on volume of neurons showed that there was a significant increase in the absolute volume of neurons in the frontal cortex of the rat brain for both iron (F4, 14 = 11.02, p = 0.0011) and fluoxetine (F4, 14 = 12.82, p = 0.0006) treatment groups compared to the vehicle group. Also, the volume of neurons in the iron and fluoxetine-treated groups was significantly higher compared to DFx-treated group. Fig. 12.
Fig. 12.
Effects of perinatal iron (0.005–8 mgkg−1), fluoxetine (3 – 30 mgkg−1) and desferrioxamine (50 mgkg−1) treatment on the volume of neurons in the prefrontal cortex of postpartum dams. Data are presented as Mean ± SEM of the number of neurons. Significantly different from control: p < 0.001; two-way ANOVA followed by Bonferroni’s test and one-way ANOVA followed by Tukey’s test. Comparison with DFx-treated group: †p < 0.05, †††p < 0.001; two-way ANOVA followed by Bonferroni’s test and one-way ANOVA followed by Tukey’s test.
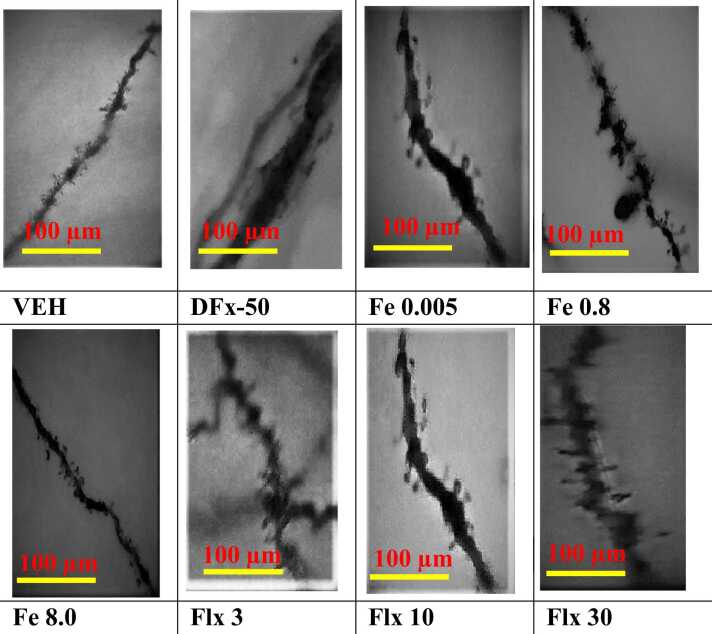
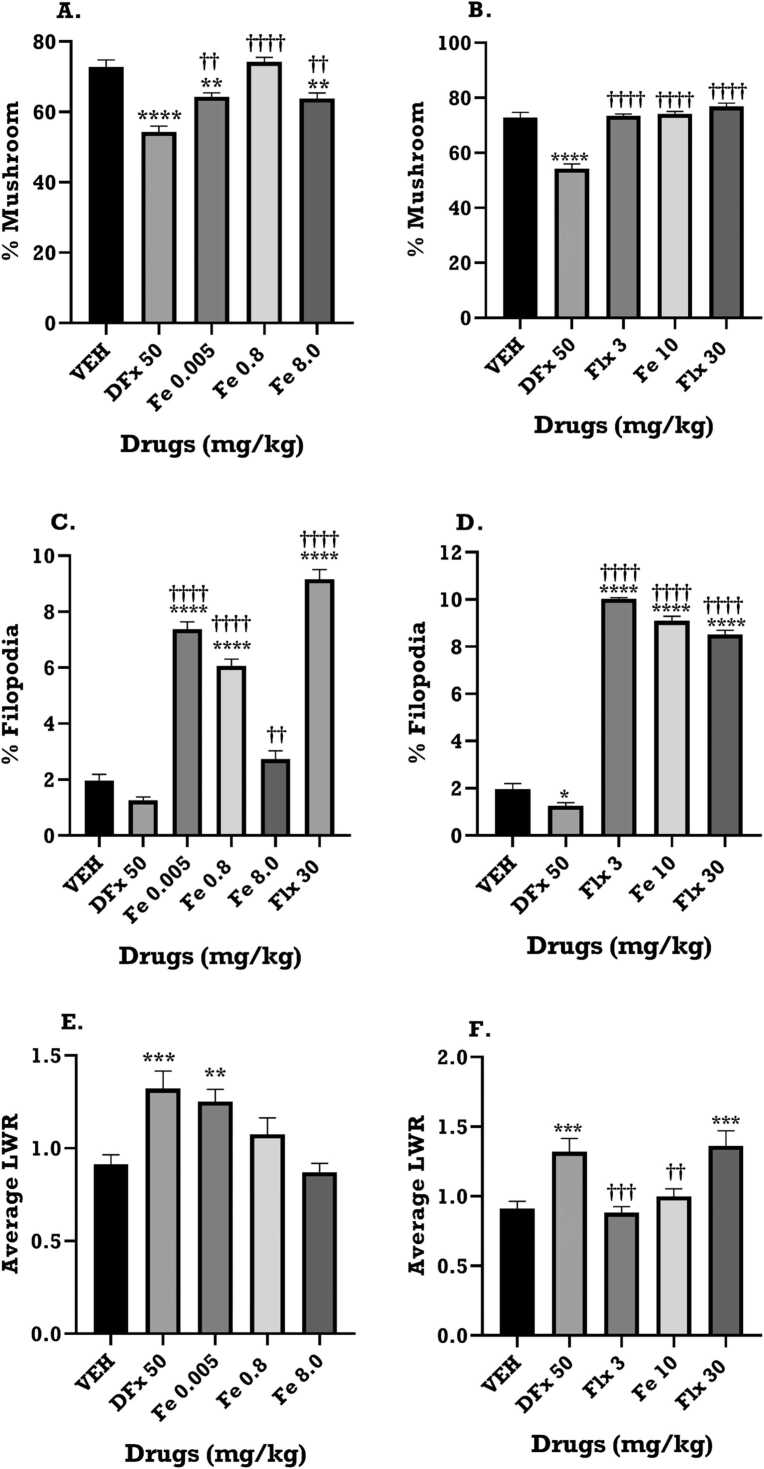
3.7. Effect of iron treatment on dendritic spine characteristics
The Z-stack images of the Golgi-spine in the frontal cortex revealed the dendritic branches and spine morphologies that were analysed in the Reconstruct software for morphological classification. The results show that the DFx group has reduced dendritic branches and spine density when compared to the iron and fluoxetine groups (Fig. 13). A difference in dams’ spine morphology and density was observed between experimental groups, (Fig. 14 and Table 3, Table 4). The iron and fluoxetine treatment showed a significant increase in the percentage of the mature mushroom-type spines when compared to the DFx group (p < 0.05, Fig. 14A, B). It is worth noting that DFx sharply decreased the percentage of the mature mushroom spines when compared to the vehicle group (Fig. 14A, B). The iron and fluoxetine treatment significantly increased the proliferation of the immature filopodia-type spines when compared to the vehicle and DFx groups (p < 0.05, Fig. 14C, D). There was a significant increase in the average length-to-width ratio for the DFx, iron and fluoxetine groups when compared to the vehicle group, reflecting longer, narrow spines identified in the frontal cortex of the dams (p < 0.05, Fig. 14 E, F).
Fig. 13.
Representative images of Golgi-Cox stained dendritic spines of the dams of VEH: Vehicle, DFx: Desferrioxamine: Iron (Fe 0.005, 0.8, 8 mgkg−1) and Fluoxetine (Flx 3, 10, 30 mgkg−1) treatment group. Scale bar: 100 µm.
Fig. 14.
The Golgi-spine analysis of the frontal cortex of the dams of VEH: Vehicle, DFx: Desferrioxamine: Iron (Fe 0.005, 0.8, 8 mgkg-1) and Flx 30: Fluoxetine treatment group report spine proliferation and maturation. (A, B) Bar graph showing the percentage of mature mushroom spines in the various experimental groups. (C, D) Bar graph showing the percentage of immature filopodia-type spines in the various experimental groups. (E, F) Bar graph showing the average length-to-width-ratio (LWR) in the various experimental groups. Data are presented as Mean ± SEM. Significantly different from control: * **p < 0.001, * *p < 0.01; two-way ANOVA followed by Bonferroni’s test and one-way ANOVA followed by Tukey’s test. Comparison with DFx-treated group: ††p < 0.01, †††p < 0.001; two-way ANOVA followed by Bonferroni’s test and one-way ANOVA followed by Tukey’s test.
Table 3.
Characteristics of dendritic spines in the frontal cortex regions of the dams of VEH: Vehicle, DFx: Desferrioxamine, and Iron (Fe 0.005, 0.8, 8 mgkg−1) treatment group.
| Treatment mg/kg (n = sample size) | |||||
|---|---|---|---|---|---|
| Spine Characteristics | VEH (n = 400) | DFx-50 (n = 320) | Fe 0.0005 (n = 400) | Fe 0.8 (n = 360) | Fe 8.0 (n = 470) |
| Mushroom (width > 0.6 µm) | 300 (75%) | 190 (59.38%) | 250 (62.5%) | 260 (72.22%) | 280 (59.57%) |
| Filopodia (length > 2 µm) | 10 (2.5%) | 5 (1.56) | 30 (7.5%) | 20 (5.56%) | 10 (2.13%) |
| Stubby (LWR < 1 µm) | 5 (1.25%) | 0 (0%) | 10 (2.5%) | 10 (2.78%) | 30 (6.39%) |
| Long thin (length < 1 µm) | 10 (2.5%) | 10 (3.12%) | 40 (10%) | 10 (2.78%) | 90 (19.17%) |
| Thin (length < 2 µm) | 50 (12.5%) | 60 (18.72%) | 70 (17.5%) | 20 (5.56%) | 150 (31.95%) |
| Branches (2 or more) | 5 (1.25%) | 55 (17.16%) | 40 (10%) | 40 (11.12%) | 10 (2.13%) |
Table 4.
Characteristics of dendritic spines in the frontal cortex regions of the dams of VEH: Vehicle, DFx: Desferrioxamine, and Fluoxetine (Flx 3, 10, 30 mgkg−1) treatment group.
| Treatment mg/kg (n = sample size) | |||||
|---|---|---|---|---|---|
| Spine Characteristics | VEH (n = 400) | DFx-50 (n = 320) | Flx 3 (n = 300) | Flx 10 (n = 330) | Flx 30 (n = 410) |
| Mushroom (width > 0.6 µm) | 300 (75%) | 190 (59.38%) | 220 (73.33%) | 245 (74.24%) | 315 (76.83%) |
| Filopodia (length > 2 µm) | 10 (2.5%) | 5 (1.56) | 30 (10.0%) | 30 (9.09%) | 35 (8.54%) |
| Stubby (LWR < 1 µm) | 5 (1.25%) | 0 (0%) | 4 (1.33%) | 5 (1.52%) | 5 (1.22%) |
| Long thin (length < 1 µm) | 10 (2.5%) | 10 (3.12%) | 5 (1.67%) | 5 (1.52%) | 5 (1.22%) |
| Thin (length < 2 µm) | 50 (12.5%) | 60 (18.72%) | 5 (1.67%) | 5 (1.52%) | 5 (1.22%) |
| Branches (2 or more) | 5 (1.25%) | 55 (17.16%) | 36 (12.0%) | 40 (12.12%) | 45 (10.98%) |
4. Discussion
The present study was conducted to assess the effects of perinatal iron treatment on depressive-like behavior and prefrontal cortex neurons in postpartum Sprague-Dawley rats. We report that iron exerts antidepressant-like effects in post-partum depressed rats and that these behavioral effects are linked to increased neuronal volume and dendritic branching.
Attenuation of immobility behavior in the forced swimming test (FST) is considered the primary index for antidepressant activity in this model (Petit-Demouliere et al., 2005). The FST results of this study found that perinatal iron treatment elicited a significant reduction in immobility behavior with an associated increase in swimming and climbing behavior during FST in the postpartum period. This suggests antidepressant-like effect of iron in post-partum rats. Numerous studies have established that antidepressants in clinical use are able to reduce immobility behavior in rodent FST while other drugs devoid of antidepressant potential fail to give the same response (Petit-Demouliere et al., 2005). It has also been demonstrated that specific components of active behavior distinguish between neurochemically unique antidepressants (Cryan et al., 2002, Lucki, 1997). For instance, drugs that decrease immobility by causing an upsurge in the swimming behavior without significantly altering the climbing behavior are purported to mediate their antidepressant effect via the serotoninergic pathway. Conversely, drugs which act via increasing catecholaminergic neurotransmission selectively increase climbing behavior (Cryan et al., 2005, Detke and Lucki, 1996, Slattery and Cryan, 2012, Page et al., 1999, Rénéric et al., 2001). Since iron induced a significant reduction in immobility score while increasing both swimming and climbing scores in the dams, it is plausible that it’s antidepressant-like effects may be mediated by serotoninergic and noradrenergic pathways. In this study, we realized that on day 11 of the FST, the vehicle control group showed a steep increase in swimming behavior while the low-dose iron group caused a sharp increase in the climbing score. It is plausible that on the day 11, there was a sudden spike in serotoninergic or noradrenergic neurotransmission respectively which might have contributed to the respective observations. These assertions require further investigation to fully explain.
Postnatal administration of iron has been proven in both experimental and observational research to lessen the burden of major depression and depression in post-partum women (Grantham-McGregor and Ani, 2001, Georgieff, 2011). Iron is involved in oxygenation of brain parenchyma and thus allows normal function of the brain (Mlyniec et al., 2014). Apart from this, iron is essential for brain energy production, normal neuronal cell function, neurotransmitter synthesis as well as myelination (Dusek et al., 2012). The involvement of brain iron in these important neuronal functions draw strong correlation between iron levels and behavior (Kim and Wessling-Resnick, 2014). It has been demonstrated that deficiency in iron results in attenuation of monoamine metabolism as well as myelination (Kim and Wessling-Resnick, 2014). Metabolism of neurotransmitters such as glutamate and GABA is altered by fluctuations in brain iron level. These neurotransmitters are involved in the regulation of mood, neuronal activity, and anxiety (Kim and Wessling-Resnick, 2014). Thus, it is not surprising that changes in the level and function of these neurotransmitters have been shown to precipitate emotional and psychological problems (Wang et al., 2020, Noori et al., 2018). Iron also acts as a cofactor for the synthesis of tyrosine hydroxylase and tryptophan hydroxylase enzymes (Kim and Wessling-Resnick, 2014). These enzymes are responsible for the synthesis of catecholamines and serotonin respectively which are also implicated in the pathophysiology of depression. Studies in humans have shown that iron supplementation in patients with depression has beneficial effects and improves symptoms (Kim and Wessling-Resnick, 2014, Mlyniec et al., 2014). It is possible that behavioral effect of iron may be due to its aforementioned roles in the brain. Further studies may be required to ascertain the specific mechanisms involved.
For the dams treated with desferrioxamine, there were depression-related behavior. Desferrioxamine is a blood brain barrier (BBB) permeable iron chelator designed for its potential use in the treatment of neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) (Salvador, 2010). It was administered subcutaneously during gestation to induce gestational iron deficiency. Being an iron chelator, it is possible that it induced perinatal iron deficiency in the dams, which negatively affected behavior.
Iron deficiency (ID) is the most common single nutrient deficiency that affects most women in the reproductive age (Etebary et al., 2010, Sheikh et al., 2015). Maternal ID has been linked with disruptive effects on mental health of women, including deficits in cognitive function, short term memory, verbal learning, attention span/concentration, intelligence and mood (Beard et al., 2005, Georgieff, 2008, Georgieff, 2017). According to Etebary et al. (2010), ID can lead to depression and negatively affect the oxidative capacity of tissues. Severe ID results in reduced haemoglobin concentration and eventually disrupts the oxygen carrying capacity of blood (Haas and Brownlie Iv, 2001). Several studies have shown a positive association between deficiency in iron and depressive disorders (Corwin et al., 2003, Beard and Connor, 2003, Georgieff, 2008, Radlowski and Johnson, 2013). Other studies have shown that cognitive impairment and postpartum depression in women are closely linked with iron deficiency anaemia and that depressive disorders responds to iron therapy (Beard et al., 2005, Etebary et al., 2010). Furthermore, iron deficiency has been postulated to affect the neuronal surface protein Thy1 in a rat model and as a result alters the release of neurotransmitters with compromised synaptic efficacy. This could contribute to a variety of abnormal communications between neurons. (Lozoff et al., 2006, Wang et al., 2004, Erikson et al., 2000). Hence, the effect of iron deficiency-induced alterations in neurotransmission could cause depressive-like traits in the postpartum period as well as other neurobehavioral disorders implicated by impaired neurotransmission. It comes as no surprise therefore that, desferrioxamine which was expected to cause iron deficiency, produced depressive-like symptoms in our study.
It is worth mentioning that neither the iron treatment not fluoxetine or desferrioxamine had any significant impact on weight variation in the post-partum rats. Usually, weight changes occur in depression or post-partum depression (Carter et al., 2000, Herring et al., 2008, Konttinen et al., 2019). However, no such effects were seen in the treatment groups studied.
The open field test (OFT) was used to assess the effect of perinatal iron supplementation on anxiety related behavior as well as motor activity in postpartum Sprague-Dawley rats. Usually, agents that cause increased locomotor activity in OFT may also decrease immobility scores in the FST and this can result in false positive outcomes. Compounds such as stimulants, convulsants and anticonvulsants can all cause increased locomotor activity with decreased immobility scores in FST meanwhile these agents are devoid of antidepressant activity (Arbabi et al., 2014, Butterweck, 2003, Slattery and Cryan, 2012). The open field test (OFT) was therefore employed in this study to rule out any likely effect of perinatal iron treatment on locomotor activity that can contribute to bias in the FST results. The OFT results for the postpartum dams showed that, there was no significant differences between the iron treatment groups and the vehicle group. These findings indicate that iron supplementation during gestation, which was observed to reduce immobility score in FST was not a result any significant effect on locomotor activity.
A third model, the novelty induced hypophagia (NIH) test, was utilized to further clarify the possible effects of iron. NIH is a conflict test in which animals are confronted with a choice to approach and consume a palatable meal in a new environment while avoiding the new environment (Dulawa and Hen, 2005). Unlike some hyponeophagia models that incorporate overnight fasting of the animals, in this test the use of a familiar and highly palatable meal (condensed milk) made food deprivation unnecessary (Merali et al., 2004). The same parameters were assessed in the home and novel cages to control for the effects of drug treatment on appetite. The dependent variables used to assess anxiety-related behavior in this study were the latency to drink the milk and consumption of the palatable milk within the first five minutes of the test. Our results indicate that the novel cage was anxiety provoking since the latency to drink was generally increased while consumption of milk within the first 5 min of the test was decreased in the novel cage relative to the home cage. Even though the latency to consume was generally higher in the novel cage compared to the home cage, there was a significant decrease in latency in the iron-treated dams compared to the control in the novel cage. This suggest that perinatal iron treatment ameliorated anxiety effects in postpartum Sprague-Dawley rats. This finding is consistent with other studies which have demonstrated anxiolytic properties of iron both in rats (Beard et al., 2002, Eseh and Zimmerberg, 2005) as well as humans. The observation that perinatal iron treatment may reduce anxiety is welcoming because anxiety is one of the major and debilitating features in post-partum depression. Further studies are however needed to further explain the observation.
Alterations such as decrease in the volume of the subgenual prefrontal cortex and a decrease in the number of neurons and glia (Drevets, 2000, Rajkowska, 2000, Duman, 2002) in the cerebral cortex of patients with major depressive disorder or bipolar disorder has been observed. Since the symptoms of depressive episodes occurring at any time is not different from those of PPD, it is hypothesized that similar changes are likely to be observed in the brains of PPD patients. Neuronal atrophy and neuronal loss in the hippocampus, as well as cerebral cortex, could result from a number of factors including glutamatergic excitoxicity, hyperactivation of the HPA axis, excitoxins, viral or bacterial infections, hypoxia-ischemia, or vulnerability to stress or other insults of genetic background (Duman et al., 1997, Duman et al., 2000) as well as nutritional deficiencies such as ID (Salvador, 2010, Yien and Paw, 2016). Although acute exposure to ID and any of the above factors alone may not be enough to cause structural and behavioral alterations, the cumulative effects over time could have devastating consequences on neuronal architecture, morphology and number. ID has been associated with several structural and functional changes in the brain including diminished BDNF expression affecting synaptic remodelling, reduction in dendritic length in pyramidal neurons (Tran et al. 2015), decrease in branching complexity of cortical neurons (Greminger et al. 2014) and eventual loss of neurons (Yien and Paw, 2016, Salvador, 2010). These changes are similar to those observed in the brain during depressive states (Miguel-Hidalgo and Rajkowska, 2002). In this study, perinatal iron treatment in Sprague-Dawley rats was able to attenuate neuronal loss in postpartum Sprague-Dawley rats as well as prevent gross alterations in the neuronal architecture of the prefrontal cortex as well as prevent gross alterations in the neuronal architecture of the prefrontal cortex. Our study showed that iron supplementation could influence dendritic spine characteristics. Alterations in dendritic spines can occur after rodents are exposed to various stimuli, including drugs, hypoxia, psychiatric diseases like depression as well as neurodevelopmental and neurodegenerative diseases (Nishiyama, 2019, Barrientos et al., 2018, Herms and Dorostkar, 2016, Saraceno et al., 2012, Penzes et al., 2011). There are various types of dendritic spines namely filopodia, mushroom, stubby and thin. Each of these have their specific function in the brain. For instance, filopodia spines (the long, thin dendritic membrane projections without a clear head), commonly observed in developing neurons are considered dendritic spine precursors. They may also be found in mature neurons undergoing neuroplasticity or during brain injury (Yoshihara et al., 2009). The increase in percentage filopodia in post-partum dams in our study seem to suggest that iron may be inducing neuroplastic changes that contributed to the reduction in depressive behavior. The highest dose of iron produced a reduction in filopodia percentage although this percentage was significantly higher than those produce by desferrioxamine. This may suggest that despite the usefulness of iron to the brain, high levels of it may be detrimental. Desferrioxamine, which induced depressive behaviour in the rats, reduced the number of filopodia spines, suggesting a reduction in neuroplasticity which often underlies depressive behaviour. Desferrioxamine is a known hypoxia-mimetic agent and it is possible that the reduction in the dendritic spines may be due to hypoxia-induced apoptosis (Guo et al., 2006). Mushroom spines are long-lived dendritic spines that constitute the main postsynaptic type of functional mature spines whereas thin and stubby spines are immature and plastic spines (Bourne and Harris, 2007, Petrak et al., 2005, Kasai et al., 2003). Just like the percentage filopodia, iron treatment increased the percentage mushroom and thin and stubby spines. These iron-induced changes may contribute to the beneficial behavioural effects in the rats. Some studies proffer that it is the neuroplastic changes in dendritic spines that form the basis of behavioral homeostasis established by antidepressants (Bessa et al., 2009). Our study lends credence to this assertion since both fluoxetine and iron which demonstrated anti-depressant effect also produced characteristic neuroplastic changes in the dendritic spines morphology. Moreover, although we did not investigate specific biochemical and molecular pathways to support our work, we hypothesize that the BDNF-CREB pathway may contribute to the effects seen. Considering the role of the brain-derived neurotrophic factor (BDNF)-CREB pathway in synaptic plasticity and neurogenesis, we are currently investigating the possible contribution of this molecular pathways(s) on different aspects of initial data obtained in the current study.
Taken together, iron demonstrated a significant antidepressant-like effect in the forced swim test and exhibited significant increases in neuron volume and dendritic spines in the medial prefrontal cortex of the postpartum rat brain.
5. Conclusion
The present study demonstrates that iron supplementation during gestation ameliorates depression in postpartum rats as observed in the behavioural tests carried out. Iron supplementation also prevents neuronal loss in the rat brain as well as increase dendritic spine density. The study shows that perinatal iron supplementation may be helpful in reducing susceptibility to post-partum depression while iron deficiency does the opposite.
Ethics approval and consent to participate
Ethical clearance was received from the Ethical and Protocol Review Committee of the College of Health Sciences, University of Ghana, (CHS-Et/M.4-P2.8/2017-2018). All animal experiments were carried out in accordance with the National Institute of Health guide for the care and use of laboratory animals (NIH Publication No. 8023, revised 1996).
Funding
This study was supported with funding from the Carnegie Corporation of New York under the "Building a New Generation of Academics in Africa" (BANGA-Africa) Project, awarded to Dr. Kennedy Kwami Edem Kukuia (UG-BA/PD-026/2016-2017).
Acknowledgements
The authors wish to thank Mr. Samuel Mensah of the Department of Anatomy, University of Ghana Medical School, University of Ghana and the technicians from the Department of Medical Pharmacology, University of Ghana, for their technical support.
Competing interest
We declare that we have no competing interest.
Contributor Information
Kennedy Kwami Edem Kukuia, Email: kkekukuia@ug.edu.gh.
Joseph Torbi, Email: jtorbi@st.ug.edu.gh.
Patrick Amoateng, Email: pamoateng@ug.edu.gh.
Kevin Kofi Adutwum-Ofosu, Email: kadutwum-ofosu@ug.edu.gh.
Awo Efua Koomson, Email: aekoomson@ug.edu.gh.
Frimpong Appiah, Email: fappiah023@st.ug.edu.gh.
Thomas Amatey Tagoe, Email: tatagoe@ug.edu.gh.
Jeffrey Amoako Mensah, Email: jamensah@st.ug.edu.gh.
Elvis Ofori Ameyaw, Email: eameyaw@ucc.edu.gh.
Ofosua Adi-Dako, Email: oadi-dako@ug.edu.gh.
Seth Kwabena Amponsah, Email: skamponsah@ug.edu.gh.
References
- Albacar G., Sans T., Martin-Santos R. An association between plasma ferritin concentrations measured 48h after delivery and postpartum depression. J. Affect. Disord. 2011;131:136–142. doi: 10.1016/j.jad.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Alharbi A.A., Abdulghani H.M. Risk factors associated with postpartum depression in the Saudi population. Neuropsychiatr. Dis. Treat. 2014;10:311–316. doi: 10.2147/NDT.S57556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoateng P., Kukuia K.K.E., Mensah J.A., Osei-Safo D., Adjei S., Eklemet A.A., Vinyo E.A., Karikari T.K. An extract of Synedrella nodiflora (L) Gaertn exhibits antidepressant properties through monoaminergic mechanisms. Metab. Brain Dis. 2018;33:1359–1368. doi: 10.1007/s11011-018-0244-0. [DOI] [PubMed] [Google Scholar]
- Andrews-Fike C. A review of postpartum depression. Prim care companion. J. Clin. Psychiatry. 1999;1:9–14. doi: 10.4088/pcc.v01n0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbabi L., Buharuldin M.T.H., Moklas M.A.M., Fakurazi S., Muhammad S.I. Antidepressant-like effects of omega-3 fatty acids in postpartum model of depression in rats. Behav. Brain Res. 2014;271:65–71. doi: 10.1016/j.bbr.2014.05.036. [DOI] [PubMed] [Google Scholar]
- Aubuchon-Endsley N.L., Thomas D.G., Kennedy T.S., Grant S.L., Valtr T. Interactive relations among maternal depressive symptomatology, nutrition, and parenting. Women Health. 2012;52:197–213. doi: 10.1080/03630242.2012.662933. [DOI] [PubMed] [Google Scholar]
- Barrientos C., Knowland D., Wu M.M.J., Lilascharoen V., Huang K.W., Malenka R.C., et al. Cocaine-induced structural plasticity in input regions to distinct cell types in nucleus accumbens. Biol. Psychiatry. 2018;84:893–904. doi: 10.1016/j.biopsych.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard J. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr. 2001;Volume 131(2):568S–580S. doi: 10.1093/jn/131.2.568S. February 2001. [DOI] [PubMed] [Google Scholar]
- Beard J., Erikson K.M., Jones B.C. Neurobehavioral analysis of developmental iron deficiency in rats. Behav. Brain Res. 2002;134:517–524. doi: 10.1016/s0166-4328(02)00092-x. [DOI] [PubMed] [Google Scholar]
- Beard J.L., Hendricks M.K., Perez E.M. Maternal iron deficiency anemia affects postpartum emotions and cognition. J. Nutr. 2005;135:267–272. doi: 10.1093/jn/135.2.267. [DOI] [PubMed] [Google Scholar]
- Beard J.L., Connor J.R. Iron status and neural functioning. Annu. Rev. Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- Beck C.T. The effects of postpartum depression on child development: a meta-analysis. Arch. Psychiatric Nurs. 1998;12:12–20. doi: 10.1016/s0883-9417(98)80004-6. [DOI] [PubMed] [Google Scholar]
- Bessa J.M., Ferreira D., Melo I., Marques F., Cerqueira J.J., Palha J.A., Almeida O.F., Sousa N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol. Psychiatry. 2009;14(8):764–773. doi: 10.1038/mp.2008.119. 739. [DOI] [PubMed] [Google Scholar]
- Bina R. The impact of cultural factors upon postpartum depression: a literature review. Health Care Women Int. 2008;29:568–592. doi: 10.1080/07399330802089149. [DOI] [PubMed] [Google Scholar]
- Bodnar L.M., Wisner K.L. Nutrition and depression: implications for improving mental health among childbearing-aged women. Biol. Psychiatry. 2005;58:679–685. doi: 10.1016/j.biopsych.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne J., Harris K.M. Do thin spines learn to be mushroom spines that remember? Curr. Opin. Neurobiol. 2007;17(3):381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Butterweck V. Step by step removal of hyperforin and hypericin: activity profile of different Hypericum preparations in behavioural models. Life Sci. 2003;73:627–639. doi: 10.1016/s0024-3205(03)00314-x. [DOI] [PubMed] [Google Scholar]
- Carter A.S., Baker C.W., Brownell K.D. Body mass index, eating attitudes, and symptoms of depression and anxiety in pregnancy and the postpartum period. Psychosom. Med. 2000;62:264–270. doi: 10.1097/00006842-200003000-00019. [DOI] [PubMed] [Google Scholar]
- Corwin E.J., Murray-Kolb L.E., Beard J.L. Low hemoglobin level is a risk factor for postpartum depression. J. Nutr. 2003;133:4139–4142. doi: 10.1093/jn/133.12.4139. [DOI] [PubMed] [Google Scholar]
- Craddock N., Forty L. Genetics of affective (mood) disorders. Eur. J. Hum. Genet. 2006;14:660–668. doi: 10.1038/sj.ejhg.5201549. [DOI] [PubMed] [Google Scholar]
- Cryan J.F., Markou A., Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol. Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cryan J.F., Valentino R.J., Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Das G., Reuhl K., Zhou R. The Golgi-Cox method. Neural Dev. 2013;1018:313–321. doi: 10.1007/978-1-62703-444-9_29. [DOI] [PubMed] [Google Scholar]
- Detke M.J., Lucki I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav. Brain Res. 1996;73:43–46. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- Drevets W.C. Neuroimaging studies of mood disorders. Biol. Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Dulawa S.C. Novelty-Induced Hypophagia. Neuromethods. 2009;42:247–259. [Google Scholar]
- Dulawa S.C., Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci. Biobehav. Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Duman R.S. Pathophysiology of depression: the concept of synaptic plasticity. Eur. Psychiatry. 2002;17:306–310. doi: 10.1016/s0924-9338(02)00654-5. [DOI] [PubMed] [Google Scholar]
- Duman R.S., Heninger G.R., Nestler E.J. A molecular and cellular theory of depression. Arch. Gen. Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duman R.S., Malberg J., Nakagawa S., D’Sa C. Neuronal plasticity and survival in mood disorders. BiolPsychiatry. 2000;48:732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- Dusek P., Jankovic J., Le W. Iron dysregulation in movement disorders. Neurobiol. Dis. 2012;46:1–18. doi: 10.1016/j.nbd.2011.12.054. [DOI] [PubMed] [Google Scholar]
- Erikson K., Jones B., Beard J.L. Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr. 2000;130:2831–2837. doi: 10.1093/jn/130.11.2831. [DOI] [PubMed] [Google Scholar]
- Eseh R., Zimmerberg B. Age-dependent effects of gestational and lactational iron deficiency on anxiety behavior in rats. Behav. Brain Res. 2005;164:214–221. doi: 10.1016/j.bbr.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Etebary S., Nikseresht S., Sadeghipour H.R., Zarrindast M.R. Postpartum depression and role of serum trace elements. Iran. J. Psychiatry. 2010;5:40–46. [PMC free article] [PubMed] [Google Scholar]
- Georgieff M.K. The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochem. Soc. Trans. 2008;36:1267–1271. doi: 10.1042/BST0361267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieff M.K. Long-term brain and behavioral consequences of early iron deficiency. Nutr. Rev. 2011;69:S43–S48. doi: 10.1111/j.1753-4887.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieff M.K. Iron assessment to protect the developing brain. Am. J. Clin. Nutr. 2017;106(Suppl):1588S–1593SS. doi: 10.3945/ajcn.117.155846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb R., Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J. Neurosci. Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Grantham-McGregor S., Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J. Nutr. 2001;131:649S–668SS. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- Greminger R.A., Lee D.L., Shrager P., Mayer-Proschel M. Gestational iron deficiency differentially alters the structure and function of white and gray matter brain regions of developing rats. J. Nutr. 2014;144:1058–1066. doi: 10.3945/jn.113.187732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Song L.P., Jiang Y., Liu W., Yu Y., Chen C.G. Hypoxia-mimetic agents desferrioxamine and cobalt chloride induce leukemic cell apoptosis through different hypoxia-inducible factor-1α independent mechanisms. Apoptosis. 2006;11:67–77. doi: 10.1007/s10495-005-3085-3. [DOI] [PubMed] [Google Scholar]
- Haas J.D., Brownlie Iv T. Iron-deficiency anemia: reexamining the nature and magnitude of the public health problem. Summary: implications for research and programs. J. Nutr. 2001;131:697S–701S. doi: 10.1093/jn/131.2.697S. [DOI] [PubMed] [Google Scholar]
- Herms J., Dorostkar M.M. Dendritic spine pathology in neurodegenerative diseases. Annu. Rev. Pathol. 2016;11:221–250. doi: 10.1146/annurev-pathol-012615-044216. [DOI] [PubMed] [Google Scholar]
- Herring S.J., Rich-Edwards J.W., Oken E., Rifas-Shiman S.L., Kleinman K.P., Gillman M.W. Association of postpartum depression with weight retention 1 year after childbirth. Obesity. 2008;16:1296–1301. doi: 10.1038/oby.2008.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Matsuzaki M., Noguchi J., Yasumatsu N., Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26(7):360–368. doi: 10.1016/S0166-2236(03)00162-0. (https://doi.org/) [DOI] [PubMed] [Google Scholar]
- Kasture V., Deshmukh V.K., Chopde C. Anxiolytic and anticonvulsive activity ofSesbania grandiflora leaves in experimental animals. Phytother. Res. 2002;16(5):455–460. doi: 10.1002/ptr.971. 16: 455-60. [DOI] [PubMed] [Google Scholar]
- Kim J., Wessling-Resnick M. Iron and mechanisms of emotional behavior. J. Nutr. Biochem. 2014;25:1101–1107. doi: 10.1016/j.jnutbio.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konttinen H., van Strien T., Männistö S., Jousilahti P., Haukkala A. Depression, emotional eating and long-term weight changes: a population-based prospective study. Int. J. Behav. Nutr. Phys. Act. 2019;16:1–11. doi: 10.1186/s12966-019-0791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau N.L., Dennis C.L., Benzies K., Duffett-Leger L., Stewart M., Tryphonopoulos P.D., Watson W. Postpartum depression is a family affair: Addressing the impact on mothers, fathers, and children. Issues Ment. Health Nurs. 2012;33:445–457. doi: 10.3109/01612840.2012.673054. [DOI] [PubMed] [Google Scholar]
- Lozoff B., Beard J., Connor J., Felt B., Georgieff M.K., Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 2006;64(5):S34–S91. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav. Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- McCloskey R.J., Reno R. Complementary health approaches for postpartum depression: a systematic review. Soc. Work Ment. Health. 2019;17:106–128. [Google Scholar]
- Merali Z., Khan S., Michaud D.S., Shippy S.A., Anisman H. Does amygdaloid corticotropin-releasing hormone (CRH) mediate anxiety-like behaviors? Dissociation of anxiogenic effects and CRH release. Eur. J. Neurosci. 2004;20:229–239. doi: 10.1111/j.1460-9568.2004.03468.x. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo J.J., Rajkowska G. Morphological brain changes in depression: can antidepressants reverse them? CNS Drugs. 2002;16:361–372. doi: 10.2165/00023210-200216060-00001. [DOI] [PubMed] [Google Scholar]
- Milman N. Postpartum anaemia I: definition, prevalence, causes, and consequences. Ann. Hematol. 2011;90:1247–1253. doi: 10.1007/s00277-011-1279-z. [DOI] [PubMed] [Google Scholar]
- Mlyniec K., Davies C.L., Gomez De Aguero I.S., Pytka K., BudziszewskA B., Nowak G. Essential elements in depression and anxiety. Part I. Pharmacol. Rep. 2014;66:534–544. doi: 10.1016/j.pharep.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Nestler E.J., Barrot M., DiLeone R.J., Eisch A.J., Gold S.J., Monteggia L.M. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nishiyama J. Plasticity of dendritic spines: molecular function and dysfunction in neurodevelopmental disorders. Psychiatry Clin. Neurosci. 2019;73:541–550. doi: 10.1111/pcn.12899. [DOI] [PubMed] [Google Scholar]
- Noori H.R., Mervin L.H., Bokharaie V., Durmus Ö., Egenrieder L., Fritze S., Gruhlke B., et al. Systemic neurotransmitter responses to clinically approved and experimental neuropsychiatric drugs. Nat. Commun. 2018;9:1–14. doi: 10.1038/s41467-018-07239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M.E., Detke M.J., Dalvi A., Kirby L.G., Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology. 1999;147:162–167. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- Penzes P., Cahill M.E., Jones K.A., VanLeeuwen J.E., Woolfrey K.M. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit-Demouliere B., Chenu F., Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology. 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Petrak L.J., Harris K.M., Kirov S.A. Synaptogenesis on mature hippocampal dendrites occurs via filopodia and immature spines during blocked synaptic transmission. J. Comp. Neurol. 2005;484(2):183–190. doi: 10.1002/cne.20468. [DOI] [PubMed] [Google Scholar]
- Porsolt R.D., Pichon M.Le, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Radlowski E.C., Johnson R.W. Perinatal iron deficiency and neurocognitive development. Front. Hum. Neurosci. 2013;7:1–11. doi: 10.3389/fnhum.2013.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol. Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rénéric J.P., Bouvard M., Stinus L. Idazoxan and 8–OH‑DPAT modify the behavioral effects induced by either NA, or 5–HT, or dual NA/5–HT reuptake inhibition in the rat forced swimming test. Neuropsychopharmacol. 2001;24:379–390. doi: 10.1016/S0893-133X(00)00214-1. [DOI] [PubMed] [Google Scholar]
- Risher, W.Christopher, Tuna Ustunkaya, Jonnathan Singh Alvarado, and Cagla %J PloS one Eroglu. 2014. 'Rapid Golgi analysis method for efficient and unbiased classification of dendritic spines', 9: e107591. [DOI] [PMC free article] [PubMed]
- Roomruangwong C., Kanchanatawan B., Sirivichayakul S., Maes M. Antenatal depression and hematocrit levels as predictors of postpartum depression and anxiety symptoms. Psychiatry Res. 2016;238:211–217. doi: 10.1016/j.psychres.2016.02.039. [DOI] [PubMed] [Google Scholar]
- Salari A.-A., Bakhtiari A., Homberg J.R. Activation of GABA-A receptors during postnatal brain development increases anxiety- and depression-related behaviors in a time- and dose-dependent manner in adult mice. Eur. Neuropsychopharmacol. 2015;25:1260–1274. doi: 10.1016/j.euroneuro.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Salvador G.A. Iron in neuronal function and dysfunction. BioFactors. 2010;36:103–110. doi: 10.1002/biof.80. [DOI] [PubMed] [Google Scholar]
- Saraceno G.E., Castilla R., Barreto G.E., Gonzalez J., Kölliker-Frers R.A., Capani F. Hippocampal dendritic spines modifications induced by perinatal asphyxia. Neural Plast. 2012;2012 doi: 10.1155/2012/873532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh M., Hantoushzadeh S., Shariat M., Farahani Z., Ebrahiminasab O. The efficacy of early iron supplementation on postpartum depression, a randomized double‑blind placebo‑controlled trial. Eur. J. Nutr. 2015:1–8. doi: 10.1007/s00394-015-1140-6. [DOI] [PubMed] [Google Scholar]
- Sheikh M., Hantoushzadeh S., Shariat M., Farahani Z., Ebrahiminasab O. The efficacy of early iron supplementation on postpartum depression, a randomized double-blind placebo-controlled trial. Eur. J. Nutr. 2017;56:901–908. doi: 10.1007/s00394-015-1140-6. [DOI] [PubMed] [Google Scholar]
- Sinclair D., Murray L. Effects of postnatal depression on children’s adjustment to school. Teacher’s reports. Br. J. Psychiatry J. Ment. Sci. 1998;172:58–63. doi: 10.1192/bjp.172.1.58. [DOI] [PubMed] [Google Scholar]
- Slattery D.A., Cryan J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protocols. 2012;7:1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- Taha A., Azhar S., Lone T., Murtaza G., Khan S.A., Mumtaz A., Asad M.H., et al. Iron deficiency anaemia in reproductive age women attending obstetrics and gynecology outpatient of university health centre in Al-Ahsa, Saudi Arabia. Afr. J. Tradit. Complement. Altern. Med. 2014;11:339–342. doi: 10.4314/ajtcam.v11i2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahirkheli N.N., Cherry A.S., Tackett A.P., McCaffree M.A., Gillaspy S.R. Postpartum depression on the neonatal intensive care unit: current perspectives. Int. J. Women’s Health. 2014;6:975–987. doi: 10.2147/IJWH.S54666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran P.V., Kennedy B.C., Lien Y.C., Simmons R.A., Georgieff M.K. Fetal iron deficiency induces chromatin remodeling at the Bdnf locus in adult rat hippocampus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308:R276–R282. doi: 10.1152/ajpregu.00429.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Yang J., Pan F., Ho R.C., Huang J.H. Editorial: neurotransmitters and emotions. Front. Psychol. 2020;11 doi: 10.3389/fpsyg.2020.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wiesinger J., Beard J., Felt B., Menzies S., Earley C., Allen R., Connor J. Thy1 expression in the brain is affected by iron and is decreased in Restless Legs Syndrome. J. Neurol. Sci. 2004;220:59–66. doi: 10.1016/j.jns.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Yien Y.Y., Paw B.H. A role for iron deficiency in dopaminergic neurodegeneration. PNAS. 2016;113:3417–3418. doi: 10.1073/pnas.1601976113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim I.S., Stapleton L.R.T., Guardino C.M./, Hahn-Holbrook J., Schetter C.D. Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu. Rev. Clin. Psychol. 2015;11:99–137. doi: 10.1146/annurev-clinpsy-101414-020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y., De Roo M., Muller D. Dendritic spine formation and stabilization. Curr. Opin. Neurobiol. 2009;19:146–153. doi: 10.1016/j.conb.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Zaqout S., Kaindl A.M. Golgi-Cox staining step by step. Front. Neuroanat. 2016;10 doi: 10.3389/fnana.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Xiu-Ling, Jing-Jing Chen, Fei Han, Chuan Pan, Ting-Ting Zhuang, Ya-Fei Cai, and Ya-Ping J Psychopharmacology Lu. 2018. 'Novel antidepressant effects of Paeonol alleviate neuronal injury with concomitant alterations in BDNF, Rac1 and RhoA levels in chronic unpredictable mild stress rats', 235: 2177–91. [DOI] [PubMed]