Abstract
This paper describes the light-directed functionalization of anisotropic gold nanoparticles with different thiolated-DNA oligomer (oligo) sequences. The starting nanoconstruct are gold nanostars (AuNS) uniformly grafted with one oligo sequence that are then exposed to fs-laser pulses at the plasmon resonance of the branches. The excitation selectively cleaves Au-S bonds at the tips of the branches to create vacant areas for functionalization with a different thiolated oligo sequence. Nanoconstructs synthesized by this approach present one oligo sequence on the AuNS body and branches and a different sequence at the tips. Furthermore, this architecture enables the formation of nanoparticle superstructures consisting of AuNS cores and small Au satellite nanoparticles at controlled locations after DNA hybridization. Our approach enables selective engineering of oligo presentation at the single-particle level and opens prospects for sophisticated design of nanoscale assemblies that are important in a wide range of biological applications.
Keywords: Gold nanoparticles, multiple ligands, DNA, ligand exchange, core-satellite structures, nanoassemblies
Graphical abstract

Core-satellite metal nanoparticle assemblies are emerging as nanoconstructs for sensing,1, 2 imaging,3, 4 and therapeutics.5, 6 Gold nanoparticles (AuNPs) are advantageous as building blocks for assembled structures because they can be synthesized in a wide variety of shapes and sizes7, 8 and functionalized with surface ligands containing a thiol group.9, 10 Thiolated oligonucleotides (oligos) are effective linkers for bio-programmable nanoparticle constructs because the sequences are tailorable, and hybridization between complementary strands is reversible.6, 11–13 Although spheres are the most common shape of oligo-functionalized AuNPs, control over the placement of different oligos at specific locations is challenging because of the uniform surface curvature and reactivity; also, the chemical and physical properties of different sequences of DNA are similar.14, 15 Methods have been developed to create AuNPs with regio-selective surface ligands by phase segregation, where oligos and polymers each cover different regions of the AuNP; however, this process introduces hydrophobic ligands that can cause NP aggregation.16–19 Other approaches use blocking agents to prevent oligo functionalization in specific areas20 or pre-assembled DNA templates to orient oligos with respect to each other,21 but they cannot control oligo location on a single NP or facilitate the attachment of different oligo sequences.
AuNPs with anisotropic shapes offer a complementary approach to construct building blocks for assemblies by functionalizing specific structural features with surface ligands.13, 22–24 Nanorods and nanoprisms terminate in different crystal facets with different binding energies for thiolated oligos that enable selective functionalization.12, 25–29 However, the synthesis of seed-mediated anisotropic AuNPs usually requires capping agents such as cetyl trimethyl ammonium bromide (CTAB), which have two key drawbacks for biological applications: (1) the surfactant is cytotoxic; and (2) the molecular bilayer formed binds tightly to the Au surface and inhibits functionalization of AuNPs with thiolated oligos.30, 31 Furthermore, the placement of oligos on the desired crystal facets depends sensitively on ligand concentration and incubation time, which could lead to oligos binding non-specifically.13, 29, 32–34
Highly anisotropic AuNPs support multiple localized surface plasmon (LSP) resonances that can be individually excited to produce localized heat and hot electrons at specific wavelengths.35–38 Excitation of LSPs has been exploited in the design of AuNPs for the delivery and release of therapeutic oligos.39 Light irradiation at the LSP resonance can thermally dehybridize doublestranded DNA grafted to particle surfaces,38, 40 release single-stranded DNA by reshaping gold nanorods into spheres,33, 41 and excite hot electrons that can cleave Au-S bonds.36, 39 Gold nanostars (AuNS) are structures with multiple branches and sharp tips that show strong, concentrated near-field enhancement at their LSP wavelengths.39, 42–44 Previously, we demonstrated that therapeutic oligos (DNA aptamers) can be released from AuNS inside cancer cells near the cell nucleus under fs-pulses; notably, the AuNS maintained its shape both in vitro and after light irradiation.39
Here we show a light-mediated approach to direct the placement of different DNA strands onto specific regions of AuNPs (Scheme 1). AuNS densely functionalized with single-stranded DNA are the starting nanoconstructs and subjected to fs-pulsed light at the LSP resonance of a branch to release DNA selectively from the tips. The extent of DNA cleavage is controlled by laser power and irradiation time. The ligand-free areas at the tips of the AuNS can then be functionalized with a second thiolated DNA sequence to generate nanoconstructs with two different oligo sequences at the body and tips of the AuNS. This site-selective functionalization of AuNS with different oligo sequences can facilitate the assembly of AuNPs into complex superstructures.
Scheme 1. DNA replacement on tips of AuNS branches.

DNA1 (grey) releases from the surface upon irradiation, leaving space at the tips for DNA2 (red) to conjugate.
AuNS were synthesized in a one-pot reaction with the biocompatible Good’s Buffer, HEPES, which acts as both the capping and reducing agent.45 The heterogeneous mixture of AuNS shapes resulted in an average hydrodynamic diameter of ~40 nm and a peak LSP resonance near 800 nm (Figure 1a). Transmission electron microscopy (TEM) analysis on this AuNS synthesis procedure revealed that particles with three or more branches make up ~70% of the AuNS population.46 Figures 1b–c show typical seven and six-branched particles, respectively. Finite-difference time-domain (FDTD) calculations of AuNS shapes under 820-nm light showed high electric field intensities at the tips of the branches aligned with the polarization of light (Figure 1d–e; Figure S1). Minimal enhancement occurred at the long branches not aligned with the light polarization or short branches that did not have LSP resonances at 820 nm.
Figure 1. Simulations of near-field enhancement of AuNS from 820-nm light irradiation.
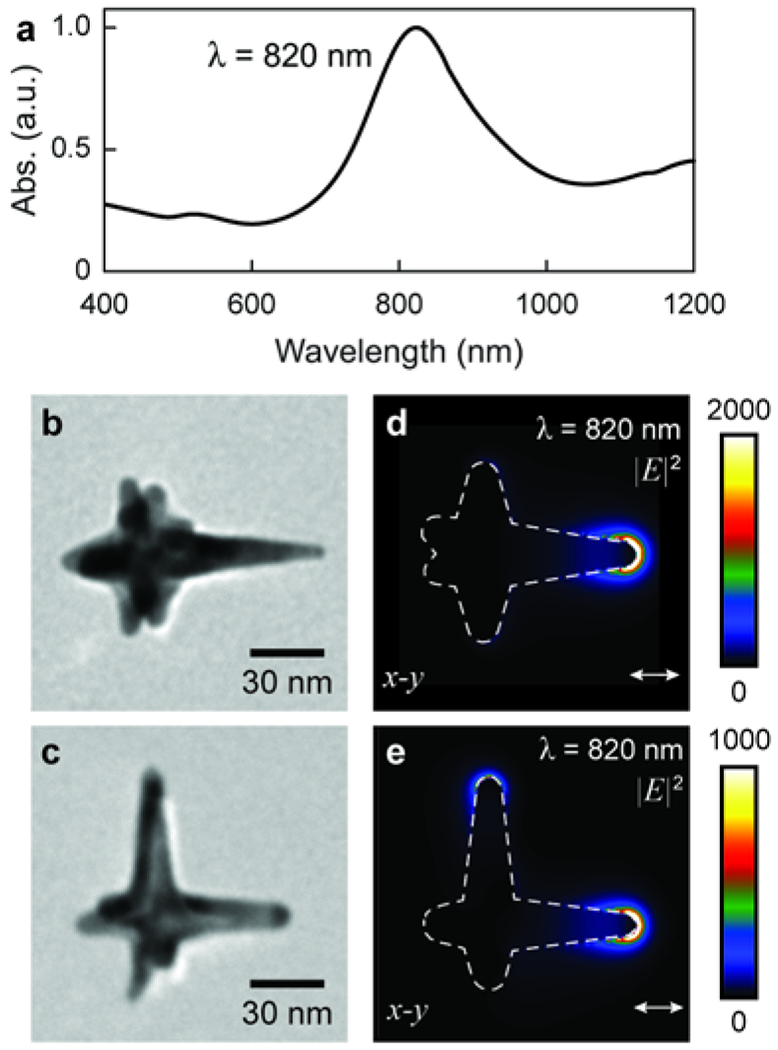
(a) UV-Vis absorbance spectra of bulk AuNS solution (normalized to highest peak). (b-c) TEM images of AuNS with (b) one and (c) two long branches. (d-e) FDTD simulations show the near-field enhancement of plasmonic gold nanostars is unevenly distributed among branches when irradiated at the LSP of the bulk solution, which leads to selective tip DNA replacement.
AuNS were first fully functionalized with sequence 1 (DNA1; 5’ SH-R-TTT TTT TTT TTT TTT TTT TTT TTT 3’; Supporting Information, Methods). DNA1@AuNS (1900 ± 100 DNA1 strands/AuNS) in water were irradiated with a 1 kHz Ti:sapphire laser at 800 nm for either 4 or 10 s. Since AuNPs reshape in aqueous solution near 1.5 W/cm2,41–47 which we also confirmed (Figure S2), we used lower powers (0.3, 0.5, 0.7, and 0.9 W/cm2) to break the Au-S bond and release the oligos from the AuNS tips.
Figure 2 shows how the amount of DNA1 released from AuNS is controlled by both irradiation time and laser power. At 0.3 W/cm2 for 4 s, 200 ± 60 DNA1 strands were cleaved from the AuNS (10% of starting amount), and at 0.9 W/cm2 for the same irradiation time, 1300 ± 90 DNA1 strands were cleaved (68%). At low laser powers and shorter irradiation times (≤0.9 W/cm2, 4 s), the AuNS released DNA1 with unobserved or only minor reshaping of the NP cores (Figures S2-S3; Table S1). At higher laser powers and longer irradiation times (≥0.5 W/cm2, 10 s), AuNS reshaped into spherical particles (Figures S2-S3; Table S1); therefore, DNA released only from the tips cannot be differentiated from the total amount of cleaved DNA at these higher irradiation powers because of contributions from reshaping (Figure S4).
Figure 2. Higher laser power and longer irradiation times increase DNA release from AuNS.

As laser power (0.0, 0.3, 0.5, 0.7, or 0.9 W/cm2) and exposure time (4 or 10 s) increases, more DNA releases from the AuNS. P-values were calculated using a T-test. All samples had p-values <0.05 in comparison to non-irradiated DNA1@AuNS (0% release).
Following irradiation of AuNS to release DNA1, AuNS were functionalized with a second thiolated DNA oligo sequence (DNA2; 5’ TCCATGACGTTCCTGACGTT-R-SH 3’) (Supporting Information, Methods). To quantify oligo attachment during this second round of conjugation, we added a Cy3 fluorophore to the 5’ end of DNA2 (Cy3-DNA2). We found that to avoid any additive adsorption of DNA2 oligos between DNA1 strands on the AuNS body and to ensure that DNA2 only adsorbed on the newly exposed bare regions, AuNS must be densely functionalization with DNA1 (>1900 strands/AuNS) prior to irradiation (Figure S5).
Figure 3 shows that the amount of DNA1 released during irradiation is similar to the amount of Cy3-DNA2 after 2 h of incubation in a salt solution (Supporting Information, Methods). The slightly higher than one-to-one replacement of Cy3-DNA2 with increased power and conjugation time (average 10% increase) may be attributed to a combination of the tips having a small, positive radius of curvature48, 49 and the oligo(ethylene glycol) spacer between the thiol and nucleotide bases in DNA2 having less electrostatic repulsion than the charged DNA1 backbone.50 A 24-h incubation period increased the amount of DNA2 on the surface by an additional average of 30% for each power and time condition (Figure S6), which suggests that longer incubation times allow for increased density of DNA2 at the AuNS tips. In contrast, without high concentrations of salt (0.6 M Na+) during incubation, little DNA2 functionalization was observed (Figures S7-8). Moreover, Cy3-DNA2 was not detected on non-irradiated, AuNS fully functionalized with DNA1 (Figure 3); hence, there was no exchange of DNA1 for DNA2 during the second round of functionalization.
Figure 3. Irradiated DNA1@AuNS add DNA2 strands to the surface through a second round of functionalization.

DNA1@AuNS were irradiated at 0, 0.3, 0.5, 0.7 or 0.9 W/cm2 to release DNA1 from the surface of AuNS. The particles then went through a second round of functionalization in a 0.6 M [Na+] solution for 2 h to add DNA2 to the vacant spaces. p-values were calculated using a t-test. All samples had p-values < 0.01 compared to non-irradiated DNA1@AuNS (0.0 W/cm2).
Because the NP sizes are below the diffraction limit at visible wavelengths, the Cy3-labeled DNA2 used for optical quantification cannot be used to characterize the location of DNA2 on the AuNS. Therefore, we attached 5-nm spherical AuNPs by DNA hybridization (DNA4@spheres) to DNA2 and then used TEM to visualize the assemblies.6, 51-54 To overcome NP-to-NP electrostatic repulsion from the negatively charged DNA shells and to increase hybridization within the assemblies,53 we used a linker DNA (DNA3) complementary to both DNA2 on the AuNS and DNA4 on the 5-nm AuNPs (Figure 4a; Scheme S2).
Figure 4. DNA4@AuNP5-nm hybridize to DNA2 on AuNS.

(a) Scheme of DNA2, DNA3, and DNA4 hybridization. (b-e) TEM images of DNA4@spheres hybridized to (b) DNA1@AuNS (DNA1@AuNS), (c) irradiated DNA1@AuNS (0.5 W/cm2 for 4 s) followed by a second round of functionalization to place DNA2 at the tips and keep DNA1 at the body (DNA1Body−DNA2Tips@AuNS), (d) irradiated DNA2@AuNS (0.5 W/cm2 for 4 s) followed by a second round of functionalization to place DNA1 at the tips and keep DNA2 at the body (DNA2Body−DNA1Tips@AuNS), and (e) DNA2@AuNS.
Figures 4b-d indicate the locations of DNA2 strands via hybridization with DNA4@spheres on three different AuNS nanoconstructs with different presentations of DNA1 and DNA2. Figure 4b shows a control experiment for the hybridization process; the association of DNA4@spheres occurs for all regions of AuNS functionalized with DNA2 (DNA2@AuNS) (Figures S9 and S10; Supporting Information, Methods). Figures 4c-d each show irradiated AuNS (0.5 W/cm2 for 4 s) with selective placement of the second DNA oligo. In Figure 4c, DNA1@AuNS were first irradiated and then functionalized with DNA2 (DNA1Body-DNA2Tips@AuNS) similar to that in Figure 3 but without Cy3. These assemblies show spheres around the tips of the AuNS branches (Figure S11), locations that are consistent with the calculated near-field enhancement (Figure 1, Figures S1 and S12). In contrast, Figure 4d shows assemblies with the opposite configuration, where DNA2 surrounds the body (indicated by 5-nm spheres), and DNA1 is at the tips (DNA2Body-DNA1Tips@AuNS). These nanoconstructs demonstrate the specificity of the DNA2 placement and selective tip functionalization.
In summary, we developed an optical approach to direct the placement of two different DNA sequences on a single AuNP. Pulsed laser excitation at the LSP wavelength of the plasmonic AuNS can release DNA from the branch tips that can then be functionalized with a second thiolated DNA sequence. This biocompatible approach allows for regio-selective placement of multiple oligo sequences while preserving the properties of an oligo-only ligand shell that can enable unique assemblies of NP superstructures via DNA hybridization. We expect that the ability to position NPs relative to each with molecular precision will facilitate the design and synthesis of complex nanoconstruct architectures for sub-cellular sensing and imaging.
Supplementary Material
Acknowledgements.
This work was supported by the National Cancer Institute of the National Institutes of Health under Award U54CA199091 (E.E.C., A.L., T.W.O.) and NSF RAISE NSF CMMI-1848613 (J. H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work made use of the BioCryo facility of Northwestern University’s NUANCE Center, which has received support from the SHyNE Resource (NSF ECCS-2025633), the IIN, and Northwestern’s MRSEC program (NSF DMR-1720139). Metal analysis was performed at the Northwestern University Quantitative Bio-element Imaging Center generously supported by NASA Ames Research Center NNA06CB93G. We thank the Mirkin Lab (Northwestern University, Evanston, Illinois) for the DNA synthesis, and the Northwestern University High Throughput Analysis Laboratory (HTA) for equipment use.
Footnotes
Supporting Information Available: The Supporting Information is available free of charge at XXX.
Chemicals and materials, experimental procedures with details on the synthesis, functionalization, and hybridization of AuNS and AuNP-spheres, additional TEM images of AuNP superstructures.
The authors declare no competing financial interest.
REFERENCES
- 1.De Silva Indrasekara AS; Norton SJ; Geitner NK; Crawford BM; Wiesner MR; Vo-Dinh T, Tailoring the Core–Satellite Nanoassembly Architectures by Tuning Internanoparticle Electrostatic Interactions. Langmuir 2018, 34 (48), 14617–14623. [DOI] [PubMed] [Google Scholar]
- 2.Ha M; Kim J-H; You M; Li Q; Fan C; Nam J-M, Multicomponent Plasmonic Nanoparticles: From Heterostructured Nanoparticles to Colloidal Composite Nanostructures. Chemical reviews 2019, 119 (24), 12208–12278. [DOI] [PubMed] [Google Scholar]
- 3.Mazur F; Liu L; Li H; Huang J; Chandrawati R, Core-satellite gold nanoparticle biosensors for monitoring cobalt ions in biological samples. Sensors and Actuators B: Chemical 2018, 268, 182–187. [Google Scholar]
- 4.Huschka R; Neumann O; Barhoumi A; Halas NJ, Visualizing Light-Triggered Release of Molecules Inside Living Cells. Nano Letters 2010, 10 (10), 4117–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilhelm S; Tavares AJ; Dai Q; Ohta S; Audet J; Dvorak HF; Chan WCW, Analysis of nanoparticle delivery to tumours. Nature Reviews Materials 2016, 1 (5), 16014. [Google Scholar]
- 6.Ohta S; Glancy D; Chan WCW, DNA-controlled dynamic colloidal nanoparticle systems for mediating cellular interaction. Science 2016, 351 (6275), 841. [DOI] [PubMed] [Google Scholar]
- 7.De Sio L; Placido T; Comparelli R; Lucia Curri M; Striccoli M; Tabiryan N; Bunning TJ, Next-generation thermo-plasmonic technologies and plasmonic nanoparticles in optoelectronics. Progress in Quantum Electronics 2015, 41, 23–70. [Google Scholar]
- 8.Chen H; Kou X; Yang Z; Ni W; Wang J, Shape- and Size-Dependent Refractive Index Sensitivity of Gold Nanoparticles. Langmuir 2008, 24 (10), 5233–5237. [DOI] [PubMed] [Google Scholar]
- 9.Bürgi T, Properties of the gold–sulphur interface: from self-assembled monolayers to clusters. Nanoscale 2015, 7 (38), 15553–15567. [DOI] [PubMed] [Google Scholar]
- 10.Bain CD; Troughton EB; Tao YT; Evall J; Whitesides GM; Nuzzo RG, Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. Journal of the American Chemical Society 1989, 111 (1), 321–335. [Google Scholar]
- 11.Jin R; Wu G; Li Z; Mirkin CA; Schatz GC, What Controls the Melting Properties of DNA-Linked Gold Nanoparticle Assemblies? Journal of the American Chemical Society 2003, 125 (6), 1643–1654. [DOI] [PubMed] [Google Scholar]
- 12.Xu L; Kuang H; Xu C; Ma W; Wang L; Kotov NA, Regiospecific Plasmonic Assemblies for in Situ Raman Spectroscopy in Live Cells. Journal of the American Chemical Society 2012, 134 (3), 1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang G; Zhang Y; Liang X; Takarada T; Maeda M, Regioselective DNA Modification and Directed Self-Assembly of Triangular Gold Nanoplates. Nanomaterials (Basel) 2019, 9 (4), 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xing H; Wang Z; Xu Z; Wong NY; Xiang Y; Liu GL; Lu Y, DNA-Directed Assembly of Asymmetric Nanoclusters Using Janus Nanoparticles. ACS Nano 2012, 6 (1), 802–809. [DOI] [PubMed] [Google Scholar]
- 15.Hayes OG; McMillan JR; Lee B; Mirkin CA, DNA-Encoded Protein Janus Nanoparticles. Journal of the American Chemical Society 2018, 140 (29), 9269–9274. [DOI] [PubMed] [Google Scholar]
- 16.Chen G; Gibson KJ; Liu D; Rees HC; Lee J-H; Xia W; Lin R; Xin HL; Gang O; Weizmann Y, Regioselective surface encoding of nanoparticles for programmable self-assembly. Nature Materials 2019, 18 (2), 169–174. [DOI] [PubMed] [Google Scholar]
- 17.Chen T; Yang M; Wang X; Tan LH; Chen H, Controlled Assembly of Eccentrically Encapsulated Gold Nanoparticles. Journal of the American Chemical Society 2008, 130 (36), 11858–11859. [DOI] [PubMed] [Google Scholar]
- 18.Tan LH; Xing H; Chen H; Lu Y, Facile and Efficient Preparation of Anisotropic DNA-Functionalized Gold Nanoparticles and Their Regioselective Assembly. Journal of the American Chemical Society 2013, 135 (47), 17675–17678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iida R; Kawamura H; Niikura K; Kimura T; Sekiguchi S; Joti Y; Bessho Y; Mitomo H; Nishino Y; Ijiro K, Synthesis of Janus-Like Gold Nanoparticles with Hydrophilic/Hydrophobic Faces by Surface Ligand Exchange and Their Self-Assemblies in Water. Langmuir 2015, 31 (14), 4054–4062. [DOI] [PubMed] [Google Scholar]
- 20.Feng L; Dreyfus R; Sha R; Seeman NC; Chaikin PM, DNA patchy particles. Advanced materials (Deerfield Beach, Fla.) 2013, 25 (20), 2779–83. [DOI] [PubMed] [Google Scholar]
- 21.Edwardson TGW; Lau KL; Bousmail D; Serpell CJ; Sleiman HF, Transfer of molecular recognition information from DNA nanostructures to gold nanoparticles. Nature Chemistry 2016, 8, 162. [DOI] [PubMed] [Google Scholar]
- 22.Rotz MW; Culver KSB; Parigi G; MacRenaris KW; Luchinat C; Odom TW; Meade TJ, High Relaxivity Gd(III)–DNA Gold Nanostars: Investigation of Shape Effects on Proton Relaxation. ACS Nano 2015, 9 (3), 3385–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zagorovsky K; Chou LYT; Chan WCW, Controlling DNA–nanoparticle serum interactions. Proceedings of the National Academy of Sciences 2016, 113 (48), 13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yue J; Pallares RM; Cole LE; Coughlin EE; Mirkin CA; Lee A; Odom TW, Smaller CpG-Conjugated Gold Nanoconstructs Achieve Higher Targeting Specificity of Immune Activation. ACS applied materials & interfaces 2018, 10 (26), 21920–21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson CJ; Dujardin E; Davis SA; Murphy CJ; Mann S, Growth and form of gold nanorods prepared by seed-mediated, surfactant-directed synthesis. Journal of Materials Chemistry 2002, 12 (6), 1765–1770. [Google Scholar]
- 26.Nikoobakht B; El-Sayed MA, Evidence for Bilayer Assembly of Cationic Surfactants on the Surface of Gold Nanorods. Langmuir 2001, 17 (20), 6368–6374. [Google Scholar]
- 27.Nikoobakht B; El-Sayed MA, Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chemistry of Materials 2003, 15 (10), 1957–1962. [Google Scholar]
- 28.Wang Y; Zeiri O; Meshi L; Stellacci F; Weinstock IA, Regioselective placement of alkanethiolate domains on tetrahedral and octahedral gold nanocrystals. Chemical Communications 2012, 48 (78), 9765–9767. [DOI] [PubMed] [Google Scholar]
- 29.Caswell KK; Wilson JN; Bunz UHF; Murphy CJ, Preferential End-to-End Assembly of Gold Nanorods by Biotin–Streptavidin Connectors. Journal of the American Chemical Society 2003, 125 (46), 13914–13915. [DOI] [PubMed] [Google Scholar]
- 30.Wang S; Lu W; Tovmachenko O; Rai US; Yu H; Ray PC, Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes. Chemical Physics Letters 2008, 463 (1), 145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi H; Niidome Y; Niidome T; Kaneko K; Kawasaki H; Yamada S, Modification of Gold Nanorods Using Phosphatidylcholine to Reduce Cytotoxicity. Langmuir 2006, 22 (1), 2–5. [DOI] [PubMed] [Google Scholar]
- 32.Nie Z; Fava D; Rubinstein M; Kumacheva E, “Supramolecular” Assembly of Gold Nanorods End-Terminated with Polymer “Pom-Poms”: Effect of Pom-Pom Structure on the Association Modes. Journal of the American Chemical Society 2008, 130 (11), 3683–3689. [DOI] [PubMed] [Google Scholar]
- 33.Link S; Burda C; Nikoobakht B; El-Sayed MA, Laser-Induced Shape Changes of Colloidal Gold Nanorods Using Femtosecond and Nanosecond Laser Pulses. The Journal of Physical Chemistry B 2000, 104 (26), 6152–6163. [Google Scholar]
- 34.Wang ZL; Mohamed MB; Link S; El-Sayed MA, Crystallographic facets and shapes of gold nanorods of different aspect ratios. Surface Science 1999, 440 (1), L809–L814. [Google Scholar]
- 35.Jain PK, Taking the Heat Off of Plasmonic Chemistry. The Journal of Physical Chemistry C 2019, 123 (40), 24347–24351. [Google Scholar]
- 36.Jain PK; Qian W; El-Sayed MA, Ultrafast Cooling of Photoexcited Electrons in Gold Nanoparticle–Thiolated DNA Conjugates Involves the Dissociation of the Gold–Thiol Bond. Journal of the American Chemical Society 2006, 128 (7), 2426–2433. [DOI] [PubMed] [Google Scholar]
- 37.Poon L; Zandberg W; Hsiao D; Erno Z; Sen D; Gates BD; Branda NR, Photothermal Release of Single-Stranded DNA from the Surface of Gold Nanoparticles Through Controlled Denaturating and Au–S Bond Breaking. ACS Nano 2010, 4 (11), 6395–6403. [DOI] [PubMed] [Google Scholar]
- 38.Jones MR; Millstone JE; Giljohann DA; Seferos DS; Young KL; Mirkin CA, Plasmonically Controlled Nucleic Acid Dehybridization with Gold Nanoprisms. ChemPhysChem 2009, 10 (9 – 10), 1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dam DHM; Lee JH; Sisco PN; Co DT; Zhang M; Wasielewski MR; Odom TW, Direct Observation of Nanoparticle–Cancer Cell Nucleus Interactions. ACS Nano 2012, 6 (4), 3318–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamashita S; Fukushima H; Akiyama Y; Niidome Y; Mori T; Katayama Y; Niidome T, Controlled-release system of single-stranded DNA triggered by the photothermal effect of gold nanorods and its in vivo application. Bioorganic & Medicinal Chemistry 2011, 19 (7), 2130–2135. [DOI] [PubMed] [Google Scholar]
- 41.Wijaya A; Schaffer SB; Pallares IG; Hamad-Schifferli K, Selective Release of Multiple DNA Oligonucleotides from Gold Nanorods. ACS Nano 2009, 3 (1), 80–86. [DOI] [PubMed] [Google Scholar]
- 42.Hua Y; Chandra K; Dam DHM; Wiederrecht GP; Odom TW, Shape-Dependent Nonlinear Optical Properties of Anisotropic Gold Nanoparticles. The Journal of Physical Chemistry Letters 2015, 6 (24), 4904–4908. [DOI] [PubMed] [Google Scholar]
- 43.Dam DHM; Culver KSB; Odom TW, Grafting Aptamers onto Gold Nanostars Increases in Vitro Efficacy in a Wide Range of Cancer Cell Types. Molecular Pharmaceutics 2014, 11 (2), 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knudson MP; Li R; Wang D; Wang W; Schaller RD; Odom TW, Polarization-Dependent Lasing Behavior from Low-Symmetry Nanocavity Arrays. ACS Nano 2019, 13 (7), 7435–7441. [DOI] [PubMed] [Google Scholar]
- 45.Xie J; Lee JY; Wang DIC, Seedless, Surfactantless, High-Yield Synthesis of Branched Gold Nanocrystals in HEPES Buffer Solution. Chemistry of Materials 2007, 19 (11), 2823–2830. [Google Scholar]
- 46.Culver KSB; Shin YJ; Rotz MW; Meade TJ; Hersam MC; Odom TW, Shape-Dependent Relaxivity of Nanoparticle-Based T1 Magnetic Resonance Imaging Contrast Agents. The Journal of Physical Chemistry C 2016, 120 (38), 22103–22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skrabalak SE; Chen J; Au L; Lu X; Li X; Xia Y, Gold Nanocages for Biomedical Applications. Advanced materials (Deerfield Beach, Fla.) 2007, 19 (20), 3177–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hill HD; Millstone JE; Banholzer MJ; Mirkin CA, The role radius of curvature plays in thiolated oligonucleotide loading on gold nanoparticles. ACS nano 2009, 3 (2), 418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee O-S; Prytkova TR; Schatz GC, Using DNA to Link Gold Nanoparticles, Polymers and Molecules: a Theoretical Perspective. The journal of physical chemistry letters 2010, 1 (12), 1781–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hurst SJ; Lytton-Jean AKR; Mirkin CA, Maximizing DNA loading on a range of gold nanoparticle sizes. Analytical chemistry 2006, 78 (24), 8313–8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Storhoff JJ; Elghanian R; Mucic RC; Mirkin CA; Letsinger RL, One-Pot Colorimetric Differentiation of Polynucleotides with Single Base Imperfections Using Gold Nanoparticle Probes. Journal of the American Chemical Society 1998, 120 (9), 1959–1964. [Google Scholar]
- 52.Claridge SA; Goh SL; Fréchet JMJ; Williams SC; Micheel CM; Alivisatos AP, Directed Assembly of Discrete Gold Nanoparticle Groupings Using Branched DNA Scaffolds. Chemistry of Materials 2005, 17 (7), 1628–1635. [Google Scholar]
- 53.Huo F; Lytton-Jean AKR; Mirkin CA, Asymmetric Functionalization of Nanoparticles Based on Thermally Addressable DNA Interconnects. Advanced Materials 2006, 18 (17), 2304–2306. [Google Scholar]
- 54.Chou LYT; Song F; Chan WCW, Engineering the Structure and Properties of DNA-Nanoparticle Superstructures Using Polyvalent Counterions. Journal of the American Chemical Society 2016, 138 (13), 4565–4572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


