Abstract
Natural killer (NK) cells are innate lymphoid cells that eliminate cancer cells, produce cytokines, and are being investigated as a nascent cellular immunotherapy. Impaired NK cell function, expansion, and persistence remain key challenges for optimal clinical translation. One promising strategy to overcome these challenges is cytokine-induced memory-like (ML) differentiation, whereby NK cells acquire enhanced antitumor function after stimulation with interleukin-12 (IL-12), IL-15, and IL-18. Here, reduced-intensity conditioning (RIC) for HLA-haploidentical hematopoietic cell transplantation (HCT) was augmented with same-donor ML NK cells on day +7 and 3 weeks of N-803 (IL-15 superagonist) to treat patients with relapsed/refractory acute myeloid leukemia (AML) in a clinical trial (NCT02782546). In 15 patients, donor ML NK cells were well tolerated, and 87% of patients achieved a composite complete response at day +28, which corresponded with clearing high-risk mutations, including TP53 variants. NK cells were the major blood lymphocytes for 2 months after HCT with 1104-fold expansion (over 1 to 2 weeks). Phenotypic and transcriptional analyses identified donor ML NK cells as distinct from conventional NK cells and showed that ML NK cells persisted for over 2 months. ML NK cells expressed CD16, CD57, and high granzyme B and perforin, along with a unique transcription factor profile. ML NK cells differentiated in patients had enhanced ex vivo function compared to conventional NK cells from both patients and healthy donors. Overall, same-donor ML NK cell therapy with 3 weeks of N-803 support safely augmented RIC haplo-HCT for AML.
INTRODUCTION
Acute myeloid leukemia (AML) is a common hematologic malignancy with many patients suffering poor outcomes (1). Allogeneic hematopoietic cell transplantation (allo-HCT) is a potentially curative therapy for patients with AML that relies on donor immune system recognition of patient leukemia [graft versus leukemia (GvL)], primarily by T and natural killer (NK) cells. Allo-HCT outcomes are best when implemented as consolidation for patients in complete remission (CR) (2). The efficacy of allo-HCT is limited by the presence of active leukemia and treatment-related toxicities proportionate to transplant conditioning intensity (3). In addition, human leukocyte antigen (HLA)–matched siblings or unrelated donors are not always available, which has driven the expansion of the donor pool to include allogeneic HLA-haploidentical relatives (haplo-HCT) (4). Unfortunately, patients with relapsed/refractory (rel/ref) AML treated with reduced-intensity conditioning (RIC) and haplo-HCT experience dismal outcomes, with small cohorts observing less than 10% 1-year leukemia-free survival (LFS) and overall survival (OS), with AML relapse being a primary determinant (5). Thus, developing new transplantation strategies that incorporate safe and effective immunotherapies for treating high-risk AML, especially in older and less-fit patients, remains a clinical priority.
NK cells are innate lymphoid cells that play key roles in defense against pathogens and antitumor immune responses (6). Prior studies have explored adoptively transferring HLA-haploidentical NK cells to treat patients with AML given their potential for GvL effects without the risk of graft-versus-host disease (GvHD) (7). Although promising, many efforts resulted in only modest NK cell expansion and antileukemia activity (8). To enhance donor NK cell antileukemia activity, our group advanced a memory-like (ML) NK cell therapy that differentiates after combined interleukin-12 (IL-12), IL-15, and IL-18 cytokine activation (9). This donor ML NK cell adoptive therapy strategy was investigated in a first-in-human phase 1 clinical trial for patients with rel/ref AML, where promising remission rates and minimal toxicities were observed (10, 11). Mass cytometry identified expanding and persisting donor ML NK cells for 3 weeks (11), followed by the expected recipient-mediated donor NK cell rejection after the recipient’s immune system recovers from lymphodepleting chemotherapy. We hypothesized that providing ML NK cells in an immune-compatible setting (with ML NK cells matched to T cells) would overcome expansion and persistence barriers, thereby allowing the full potential of ML NK cells to be assessed in vivo.
To test this, a clinical trial was developed for high-risk patients with AML not in remission, who were treated with an RIC [fludarabine/cyclophosphamide/total body irradiation (Flu/Cy/TBI)] haplo-HCT on day 0, posttransplant cyclophosphamide on days +3 and + 4, and then same-donor ML NK cells administered on day +7 supported by N-803 (an IL-15 superagonist) administered over 3 weeks (12). This strategy capitalizes on the early lymphodepletion after HCT conditioning (13) and the matching of donor T cells from the HCT graft to the administered same-donor ML NK cells. We reasoned that short-term anti-AML responses in a high-risk population would be mediated by ML NK cells as observed previously (11), with long-term responses likely dependent on developing donor alloreactive T cells later in the HCT time course. AML responses were assessed by standard clinical criteria and highly sensitive next-generation sequencing (NGS) to track specific AML mutations. To identify donor ML NK cells in recipient samples, single-cell RNA sequencing coupled with oligonucleotide-tagged antibodies for selected NK markers [cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq)] and mass cytometry were used, in addition to NK cell function assays. This study reveals a remarkable expansion of ML NK cells in vivo, followed by prolonged functional persistence.
RESULTS
Participants
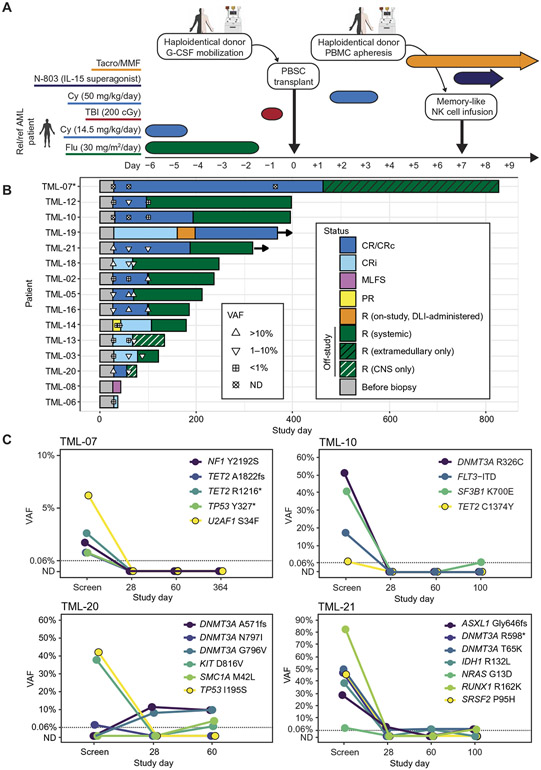
From April 2017 to March 2020, 15 patients with rel/ref AML were enrolled (Table 1 and Fig. 1A). Patients had undergone a median of 3 lines of therapy (range, 1 to 8) for AML before receiving the study treatment, with 14 (94%) patients having received cytarabine-containing intensive chemotherapy. At the time of enrollment, patients had active disease: eight with AML blasts >5% and the remainder with measurable residual disease (MRD) positivity by flow cytometry or fluorescence in situ hybridization (FISH). All 14 patients with complete chromosomal and NGS genotyping data met European LeukemiaNet 2017 criteria for adverse risk disease (14). Eight (54%) patients had complex karyotype disease, with seven (47%) having mutated TP53. All 15 patients received Flu/Cy/TBI RIC and granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood stem cell (PBSC) grafts. All 15 HLA-haploidentical donors underwent successful ML NK cell production on day +6 followed by infusion into patients on day +7 (fig. S1), following the same procedure as the phase 1 study (11). To determine whether the infusion products were affected by the 1-week prior G-CSF donor mobilization (15), IL-12/IL-15/IL-18–activated products from nonmobilized donors were compared to the 1-week prior G-CSF–mobilized donors by mass cytometry (fig. S1, D to F). Apart from increased CD25 and CD137 on G-CSF–exposed donor NK cells after cytokine activation, subset composition and functional molecules granzyme B and perforin were similar to untreated products. All patients received the target dose of 0.5 × 106 to 10 × 106 NK cells/kg, resulting in an absolute dose of 2.3 × 108 to 1 × 109 ML NK cells (fig. S1, B and C). The NK cell products were highly purified, viable, and activated (fig. S1). Thirteen of 15 patients received all four planned doses of N-803; 2 other patients received two doses of N-803 because of early relapse or mortality (tables S1 and S2). Median follow-up was 7.0 months (range, 1.2 to 27.5 months) at interim data cutoff.
Table 1. Patient characteristics.
MRD, measurable residual disease; FISH, fluorescence in situ hybridization; ELN, European LeukemiaNet.
| Characteristic | Measure |
|---|---|
| Median age, years (range) | 67 (19 to 73) |
| Male/female, no. of patients | 11/4 |
| Median/mean bone marrow blasts, % (range) | 6/21 (0 to 89) |
| Disease history, no. | |
| De novo AML | 10 |
| Secondary or treatment-related AML | 5 |
| Median number of prior therapies, no. (range) | 3 (1 to 8) |
| Prior therapy regimens, no. of patients | |
| Intensive chemotherapy with cytarabine | 14 |
| Hypomethylating agent only | 1 |
| Disease status, no. of patients | |
| Active disease | 10 |
| MRD positive by flow cytometry | 3 |
| MRD positive by FISH | 2 |
| Cytogenetics/FISH, no. of patients | |
| Complex | 8 |
| Isolated trisomy 13 | 1 |
| Isolated trisomy 8 | 1 |
| Isolated del 5q | 1 |
| t(1;17) + del 19 | 1 |
| Normal | 3 |
| Recurrently mutated genes, no. of patients | |
| TP53 | 7 |
| DNMT3A | 6 |
| TET2 | 4 |
| SF3B1 | 3 |
| ASXL1 | 3 |
| CBL | 3 |
| SRSF2 | 2 |
| FLT3 | 2 |
| NRAS | 2 |
| RUNX1 | 2 |
| SUZ12 | 2 |
| 2017 ELN Genetic Risk Stratification, no. of patients | |
| Adverse | 14 |
| Intermediate | 1* |
| Favorable | 0 |
NGS genotype not available, classified as intermediate based on normal karyotype.
Fig. 1. RIC haplo-HCT was augmented by ML NK cells and N-803.
(A) Study schema. Patients with rel/ref AML underwent RIC with fludarabine (Flu), cyclophosphamide (Cy), and total body irradiation (TBI) and then received a donor-derived G-CSF–mobilized peripheral blood stem cell (PBSC) graft on day 0, posttransplant cyclophosphamide on day +3/+4, and donor-derived memory-like (ML) NK cells (produced using nonmobilized PBMCs) on day +7 with N-803 (IL-15 superagonist) support. GvHD prophylaxis consisted of tacrolimus (Tacro) and mycophenolate mofetil (MMF). (B) Swimmer’s plot of patient outcomes. Disease status is indicated by bar color: complete response (CR), cytogenetic complete response (CRc; marked by *), morphologic leukemia-free state (MLFS), partial response (PR), and relapse (R). DLI indicates donor lymphocyte infusion. CNS indicates central nervous system. Patients alive at the last follow-up are indicated by right arrows. Symbols indicate the highest variant allele fraction (VAF) at a given time point of mutations initially identified at the time of screening. ND indicates not detected. (C) Mutation VAF trends over time for patients TML-07, TML-10, TML-20, and TML-21. Dotted lines at VAF = 0.06% represent the limit of detection upper bound across all variants. Variants detected at VAF 0.06% or lower are plotted on the dotted line; variants not detected are plotted below on the y axis at ND.
ML NK cells were safe and well tolerated
Adverse events (AEs) attributed to ML NK cells or N-803 were minimal: One patient developed grade 1 cytokine release syndrome (CRS), and six patients experienced grade 1 or 2 injection site reactions, none of which led to treatment modifications. All grade ≥3 AEs, regardless of attribution, occurring in two or more patients are listed in table S3. Ten (67%) patients developed acute GvHD (grade 1: 4, grade 2: 6). No patient developed steroid-refractory acute GvHD. Two of 10 (20%) evaluable patients developed chronic GvHD, 1 of which was mild and only involved the skin, whereas another patient had moderate skin and gastrointestinal involvement. These rates of GvHD were comparable to expected rates with haplo-HCT (16), with no exacerbations attributed to ML NK cells or N-803. Two patients experienced nonrelapse mortality (NRM) in the setting of grade 5 AEs. One patient suffered primary graft failure and died from related complications on day +43; another died on day +37 from complications of sepsis. Fourteen of 15 patients achieved neutrophil engraftment (median, day +21; range, 16 to 35), and 13 of 15 patients achieved platelet engraftment (median, day +30; range, 14 to 49).
Haplo-HCT augmented by ML NK cells and N-803 induced remission in most patients, including TP53 mutation clearance
All 15 patients were evaluable by day +28 bone marrow (BM) biopsy, at which point composite CR [any of CR, CR with incomplete recovery of PB counts (CRi), or cytogenetic CR (CRc); further defined in Supplementary Materials and Methods] was achieved by 13 (87%) patients (Fig. 1B). Of the other two patients, one suffered primary graft failure having achieved a morphologic leukemia-free state at day +28, and the other achieved a partial response at day +28 and converted to CRi at day +44 only receiving therapy per protocol. Twelve (80%) patients were alive by day +100, of which four remained in composite CR at that time. Median OS was 7.0 months (range, 1.2 to 27.5), with 29% 1-year OS. Of the four patients beyond 365 days after HCT at interim data cutoff, one was free from relapse, and one other had relapsed but achieved a second CR with only donor lymphocyte infusions and no other systemic antileukemia therapy. Median event-free survival among all 15 patients was 3.2 months (range, 1.2 to 15.4). Median LFS among 14 of 15 patients achieving composite CR at any time after HCT was 2.2 months (range, 0.3 to 14.4). Reasons for treatment discontinuation were disease progression (n = 13) and NRM (n = 2). Of 13 patients suffering disease relapse, 3 were isolated extramedullary relapses: 2 in the central nervous system and 1 myeloid sarcoma.
We performed deep [median 2538× coverage; variant allele fraction (VAF) limit of detection 0.06% or more stringent per mutation] error-corrected NGS of 40 genes recurrently mutated in AML (17) using baseline study screening PB samples to evaluate somatic mutations present pretreatment (fig. S2, A and B). TP53 mutations, which confer a poor prognosis in AML both at diagnosis and before HCT (18, 19), were the most commonly identified variants in our cohort (seven patients having 13 unique variants). To relate clinical outcomes and clearance of mutations after HCT, we also sequenced patients’ day +28 and selected follow-up PB samples (Fig. 1, B and C, fig. S3, and data files S1 and S2). Nine of 13 patients with detectable pretreatment mutations had all mutations reduced to VAF <1% at day +28. For two patients (transplant memory-like, TML-07 and TML-10), all mutations from their screening samples were not detectable (ND) at day +28 (Fig. 1C). Complete mutation clearance at day +28 was associated with longer remissions, as these two patients had the two longest LFS durations (433 and 163 days, respectively) and two of the three longest OS durations (826 and 396 days, respectively) in our cohort. Reemergence of mutations previously cleared to ND did not always indicate imminent relapse: Patient TML-10 went 95 days between the first post-HCT detectable mutation and formal clinical relapse. Serial profiling also suggested sustained GvL effects, with some patients having rapid but incomplete mutation clearance by day +28, followed by persistent suppression of disease-associated variants at low VAFs, or a progressive decline in VAFs over time after HCT. Of seven patients with mutated TP53, two (TML-07 and TML-20) cleared their TP53 mutations to ND (Fig. 1C). Eight of 13 total mutations in TP53 were cleared to ND at any point after HCT, with 3 mutations being cleared after previously remaining detectable at day +28. These findings suggest that haplo-HCT augmented by ML NK cells and N-803 has antileukemic activity against TP53-mutated AML.
Donor NK cells expand robustly after adoptive transfer
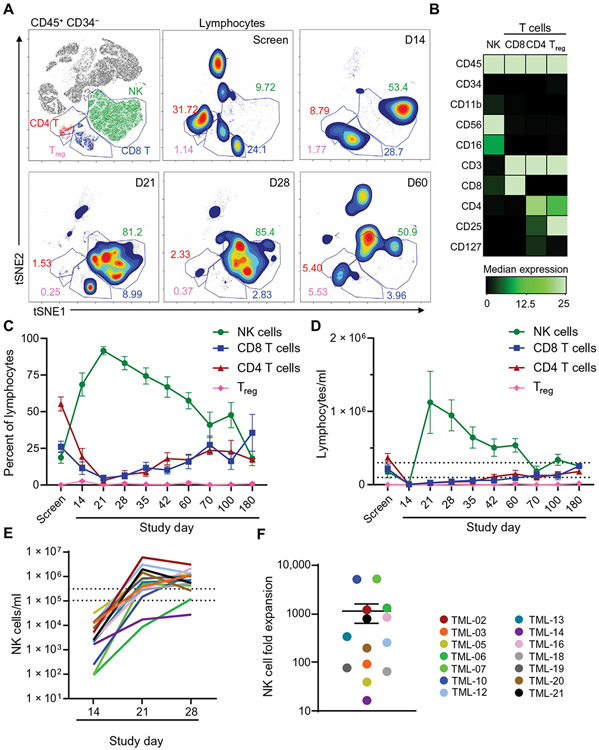
To understand NK cell persistence and expansion in an immune-compatible environment, mass cytometry was used on donor and patient samples over time (Fig. 2). We hypothesized that, by administering donor NK cells early post-HCT where NK cells are matched to graft-derived lymphocytes, the transferred ML NK cells would expand and persist in patients in the absence of allo-rejection by T cells (11). Using an unbiased clustering approach, NK cells represented the main lymphocyte subset for 2 months after infusion [P = 0.0001; two-way analysis of variance (ANOVA) with Dunnett’s multiple comparison; Fig. 2]. NK cell expansion peaked at day +21, 2 weeks after NK cell infusion in most patients (Fig. 2C). NK cell frequencies were elevated for months, only returning to screening amounts by day+180. PB CD4+ T cell and regulatory T cell frequencies remained low during this period compared to patient screening samples, which is consistent with previous reports on immune reconstitution after haplo-HCT (Fig. 2C) (20). Absolute NK cell numbers remained elevated above the normal range for 2 months (Fig. 2D). NK cell expansion kinetics varied by individual and occurred over 2 to 3 weeks (Fig. 2E). Maximum expansion also varied (16- to 5170-fold) from day +14 to peak expansion (Fig. 2F), corresponding with declining serum IL-15 concentrations (fig. S4). This expansion occurred despite ongoing provision of tacrolimus and mycophenolate mofetil immunosuppression as standard GvHD prophylaxis.
Fig. 2. NK cells are the most abundant lymphocyte after NK cell administration.
Patient PB was obtained and assessed by mass cytometry at the indicated time points. (A) FlowSOM identified the major lymphocyte populations. Representative overlay and density plots are shown for samples isolated from TML-05, with each lymphocyte population indicated. Gray indicates nonlymphocyte/myeloid populations. Inset numbers depict frequency of cells within the indicated gate on lymphocytes (T, B, and NK cells). (B) Markers used for FlowSOM clustering for each population shown in (A). Treg, regulatory T cell. (C) Summary data from (A) are shown for 14 patients. (D) Total lymphocyte concentrations per milliliter of PB are shown for 14 patients. (E) Total NK cells in PB are shown for each patient. (F) Summary data are shown depicting maximum fold expansion from days +7 to +21 or +28. Each dot represents a patient. The dashed lines in (D) and (E) indicate average NK cell counts for healthy donors. Data are presented as means ± SEM.
ML NK cells identified by mass cytometry expand and persist after adoptive transfer
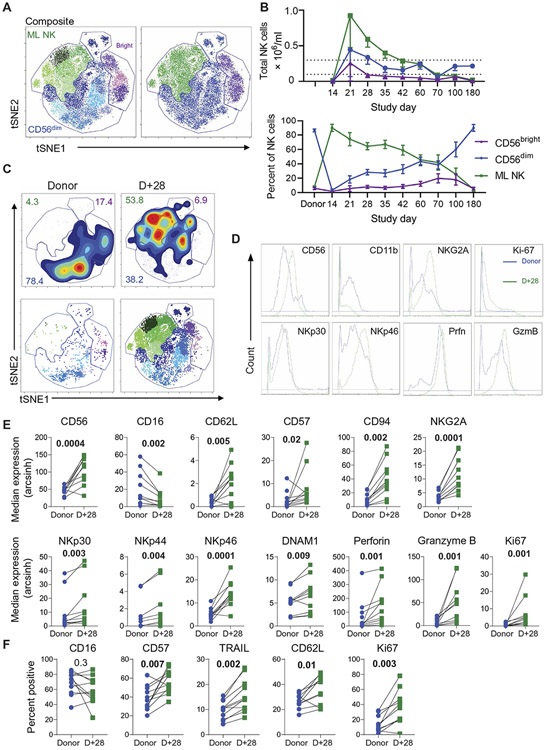
Previous studies have reported that CD56bright NK cells are the main NK cell subset reconstituting early after haplo-HCT and that ML NK cells are distinct from conventional CD56bright and CD56dim NK cells (11, 13, 20). In addition, ML NK cells differentiating 1 week in vivo, confirmed by donor- or recipient-specific HLA expression, were similar in phenotype to in vitro differentiated ML NK cells (10). Here, HLA-based NK cell tracking was not possible because the immune cells reconstituting after haplo-HCT are HLA-matched to the infused same-donor NK cells. We therefore used an unbiased approach [t-distributed stochastic neighbor embedding (tSNE) and FlowSOM, a visualization technique to analyze mass cytometry data using a self-organizing map] to identify NK cell subsets using our previously reported multidimensional analysis strategy (Fig. 3, A and B, and figs. S5 and S6) (10, 11). Consistent with our prior report, purified NK cells, activated product, and early in vivo differentiated ML NK cells were distinct from each other and were detected in the BM (fig. S5) (11). To understand long-term ML NK cell persistence, CD56dim, CD56bright, and ML NK cells were annotated and binned, and frequencies and absolute numbers were assessed over time (Fig. 3, A to C, and fig. S6) from samples that were thawed, stained, and assessed in batch. As expected, conventional CD56dim and CD56bright subsets were identified in donor blood and purified NK cells. ML NK cells were detectable at significantly increased frequencies over baseline until ≥day +70 (P < 0.01 to 0.0001; Tukey’s multiple comparison test; Fig. 3, B and C). CD56bright NK cells were stable and unexpectedly comprised a minority NK cell population in our patients’ PB (Fig. 3B) (20). At day +28, ML NK cells were most abundant. These cells were distinct from conventional subsets and expressed a variety of markers consistent with ML NK cell phenotype (Fig. 3, C to F, and fig. S6). CD56, CD62L, NKG2A, and activating receptors (NKp30, NKp44, NKp46, and DNAM-1) were all increased (Fig. 3, D to F), consistent with the in vivo ML NK cell phenotype (11). Also consistent with the ML NK cell phenotype are increased effector molecules perforin, granzyme B, tumor necrosis factor (TNF)–related apoptosis-inducing ligand, and proliferation marker Ki-67 (Fig. 3, D to F, and fig. S6). CD57 and killer immunoglobulin (Ig)–like receptors (KIRs) were increased in ML NK cells differentiated for 3 weeks in patients in the current study, whereas changes in these proteins were not observed in prior studies, which followed ML NK cells over a shorter time period (Fig. 3, E and F, and fig. S7). Overall changes in KIR expression corresponded to increased KIR diversity, suggesting ongoing maturation, rather than specific KIR subsets preferentially expanding in vivo. These data support that ML NK cells are distinct from conventional NK (cNK) subsets and persist for months in the immune-compatible setting. A limited set of AML samples was analyzed by mass cytometry for alterations in NK cell receptor ligands upon relapse, which revealed reductions in activating NK cell receptor ligands (fig. S8).
Fig. 3. ML NK cells persist in patient PB after transfer in the RIC-HCT setting.
The multidimensional phenotype of FlowSOM-gated ML NK cells was assessed using viSNE. (A) Representative viSNE plots for patient TML-05 are shown, demonstrating NK cell subset (CD56bright, CD56dim, and ML NK) binning. (B) Summary percent (bottom) and total numbers (top) of NK cell subsets are shown for 14 patients. (C) Representative density plots of donor and patient NK cells at days +28 and +60 are shown, and inset numbers represent frequency of cells within the gate (top). A second FlowSOM was used to identify NK cell subsets (see figs. S5 and S6), and distinct subsets within viSNE were identified (bottom). (D) Overlay histograms are shown of donor (blue) and day +28 (green) PB bulk NK cells for the expression of the indicated marker. (E and F) Summary data from (D) depicting median expression (E) or percent positive (F) are shown for 12 donor-patient pairs. Data are presented in arcsinh transformed scale and are depicted as means ± SEM and were compared using paired t or Wilcoxon test, as appropriate. P values are indicated above each plot. TRAIL, TNF-related apoptosis-inducing ligand.
ML NK cells are distinct from cNK cells as measured by single-cell transcriptomics
To provide orthogonal evidence of ML NK cell persistence, we used CITE-seq, which defined the NK cell receptor repertoire using a custom NK cell–specific panel of oligonucleotide-tagged antibodies (table S4), while simultaneously performing single-cell RNA sequencing (21). First, we generated an unbiased classifier for ML NK cells using our 1-week in vitro ML NK cell differentiation method (22) to generate control cNK [low-dose (LD) IL-15] and ML NK cells. This method reliably resulted in established blood subsets of cNK cells (CD56bright and CD56dim; fig. S9A) and ML NK cells while recapitulating exposure of in vivo differentiating NK cells to IL-15. CITE-seq analyses revealed distinct changes in cNK and ML NK cell transcriptional states, evident by changes in the uniform manifold approximation and projection (UMAP) space and differentially expressed genes (fig. S9, A to C). ML NK cells were primarily comprised of three clusters (ML1 to ML3), with minimal transcriptional overlap with LD cNK cells (fig. S9, B and C). To generate the ML NK cell classifier, the most highly variable genes across both control and ML NK cells were selected, and a weighted nearest neighbor analysis was used to jointly represent mRNA and protein expression, allowing us to incorporate NK cell receptors poorly enriched by RNA expression alone (such as CD57, CD56, and NKp46) (23). Because the ML NK cells included in the classifier had differentiated for 1 week, this strategy is biased toward early ML NK cell differentiation and provides a conservative starting point for identifying in vivo differentiated ML NK cells at day +28.
Donor NK cells with a distinct ML NK cell program expand and persist in recipients
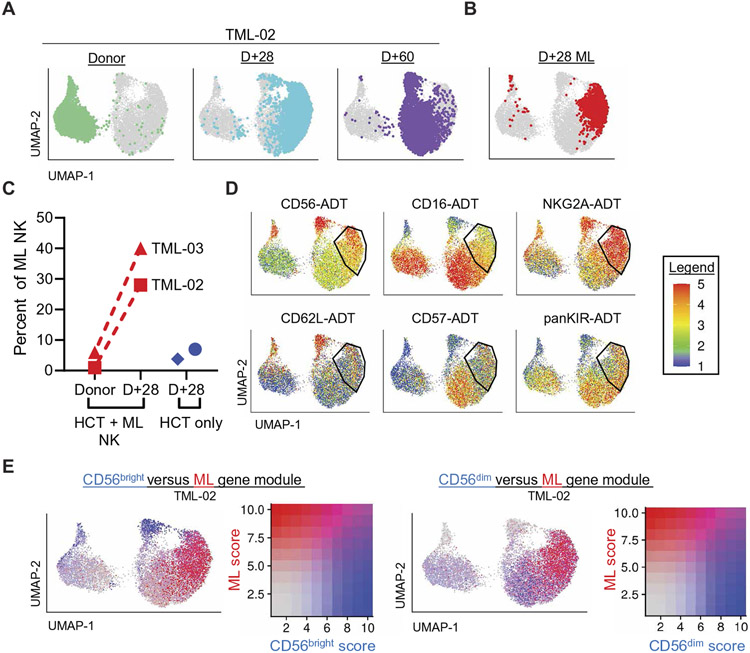
We next performed transfer learning between the in vitro dataset and two patients’ baseline donor and day +28 NK cells to identify ML NK cells. The classifier identified ML NK cells as 28% and 40% of total NK cells at day +28 in TML-02 and in TML-03, respectively. As expected, ML NK cells were very rare at baseline (1 to 6%; Fig. 4, A to C). To confirm that this ML NK cell population was specific to patients receiving ML NK cellular therapy and not an aberrant NK cell population developing after HCT, transfer learning was performed on NK cells from patients who underwent haplo-HCT without NK cell infusion (HCT only; Fig. 4C). Minimal ML NK cells were identified in HCT-only patients at day +28, despite sufficient IL-15 concentrations (fig. S4), indicating that ML NK cells identified in TML patients reflect ML NK cell adoptive therapy (Fig. 4, A to C, and fig. S9, D to F). Furthermore, the classified ML NK cells had an NK cell receptor profile analogous to our mass cytometry findings (Fig. 4D and fig. S9G).
Fig. 4. CITE-seq identifies ML NK cells persisting for 2 months in patients.
(A) UMAP visualization of TML-02 with cells highlighted by color at each time point: Donor (sea green), +D28 (turquoise), +D60 (purple). (B) ML NK cells identified by classifier are highlighted in red on the UMAP visualization of TML-02; see also fig. S9. (C) Proportions of ML NK in total NK cells at baseline/donor, day +28, and in patients receiving haplo-HCT transplant without ML NK cells are shown. (D) Expression of key ML antibody-derived tags (ADTs) on TML-02 are shown; min.cutoff = “q02,” max.cutoff = “q98.” (E) CD56bright and CD56dim as annotated (blue), and ML (red) NK cell gene module scoring was overlaid on TML-02 UMAP visualization. Cells are ordered on the UMAP by binned expression.
We corroborated this analysis with two additional bioinformatic approaches. First, each cell was scored by the expression of genes enriched in CD56bright, CD56dim, and ML NK cells as defined by our in vitro dataset. We found enrichment of gene signatures for cNK cell subsets in the expected clusters. High expression of the ML NK cell gene module was observed in most non-CD56bright NK cells at day +28, and joint expression of CD56dim and ML NK cell gene programs was present in many cells at day +60, consistent with ongoing changes in ML NK cells (Fig. 4E and fig. S9H). This was further supported by using matchSCore2, which defines a probability score of a cell belonging to a given reference cell type. Cells with a high probability of ML NK cell designation were identified at day +28 in additional NK cell clusters that persisted to day +60 (fig. S9, I and J).
In vivo differentiated ML NK cells have transcriptomic evidence of enhanced NK cell function
We next compared the day +28 classified ML NK cells to other NK cell populations to delineate their transcriptional program. Analyzing samples from two patients (TML-02 and TML-03), we found several shared differentially expressed genes between ML NK cells compared to all other NK cells. These included high expression of major histocompatibility complex class II–associated genes (HLA-DRA and CD74) and cytotoxicity-associated genes (GZMA, GZMK, and GNLY), as well as reduced expression of granzyme M (GZMM; Fig. 5A and fig. S10, A to C). ML NK cells had a unique NK cell repertoire consisting of increased expression of KLRC1 (NKG2A), KIR2DL4, and CD300A and decreased expression of the inhibitory receptor KLRB1 (CD161). In addition, ML NK cells had differential expression of intracellular signaling mediators including up-regulation of CD3E (Fig. 5A and fig. S10A). Furthermore, ML NK cells had differentially expressed costimulatory and adhesion receptors (Fig. 5B and fig. S10D). ML NK cells also had distinct expression of genes encoding cytokines and chemokines including Lymphotoxin beta (LTB), chemokine-like factor (CKLF), and fibroblast growth factor binding protein 2 (FGFBP2) (fig. S11, A and B). Last, ML NK cells had a distinct transcription factor profile including increased HOPX and ID2 expression, which have been reported to play pivotal roles in NK cell sensitivity to IL-15 (24). ML NK cells had decreased expression of NR4A2 and ZEB2, which has been implicated in murine NK cell maturation (Fig. 5C and fig. S11C) (25, 26). Thus, in vivo differentiated ML NK cells had several transcriptomic features consistent with enhanced NK cell function.
Fig. 5. ML NK cells are transcriptionally distinct from cNK cells.
(A to C) Select differentially expressed genes conserved across both patients in ML NK cells (indicated within the gate) compared to all other clusters were visualized on TML-02 UMAP. Expression of activation, effector function, and NK receptors (A); adhesion and co-receptors (B); and transcription factors (C) are shown. Data were analyzed using a Wilcoxon rank sum test. n = 2 independent experiments. Cells are ordered on UMAPs according to normalized binned expression; see figs. S10 and S11. Adjusted P value or q value of <0.05 for all.
Previously, it has been reported that cytomegalovirus (CMV)–adaptive NK cells up-regulate the gene encoding Killer Cell Lectin Like Receptor 2 (NKG2C), KLRC2, in CD56dim NK cells, and down-regulate the genes encoding Promyelocytic Leukemia Zinc Finger [PLZF; gene: Zinc Finger and BTB Domain Containing 16 (ZBTB16)], Fc Epsilon Receptor Ig (FcεR1γ;FCER1G), EWS/FLI1 activated transcript 2 [EAT-2; gene: SH2 Domain Containing 1B (SH2D1B)], and Spleen Tyrosine Kinase (SYK) (27, 28). In contrast, ML NK cells consistently expressed SH2D1B and FCER1G, with low KLRC2 and variable ZBTB16 (PLZF) expression (fig. S12, A and B). A subset of patients was further analyzed at days +28 to +35 for intracellular FcεR1γ, PLZF, SYK, and EAT-2 (fig. S12C). Despite expected variability of these markers across multiple patients, in vivo differentiated ML NK cells were distinct from CMV-adaptive NK cells from healthy donors (fig. S12, D to F). We further found that in vivo differentiated ML NK cells do not express CD45RO, a marker associated with T cell memory (fig. S12G), suggesting that ML NK cells have a distinct molecular program.
ML NK cells exhibit a unique pseudotime differentiation trajectory
We performed trajectory analysis to determine how the phenotype of ML NK cells changes in vivo in TML-02 from days +28 to +60 using Monocle2 (29). Trajectory analysis revealed three distinct NK cell states, with the conventional CD56bright subset localized in state 1, conventional CD56dim subset predominant in state 2 and present in state 1, and ML NK cells localized to state 3 (Fig. 6, A to C, and fig. S13). At day +28, ML NK cells in state 3 predominated. At day +60, ML NK cells within state 3 persisted, with increased proportions of cNK cell states 1 and 2 present, consistent with partial reconstitution of the cNK cell compartment from the graft. Moreover, about 50% of NK cells remained in state 3 with increased pseudotime progression of ML NK cells following the time course of our sample set. The phenotype of ML NK cells within state 3 changed as the ML NK cells progressed from days +28 to +60 with significant changes in the transcription factors GATA3 (q = 2.3 × 10−72), KLF2 (q = 0), and ZEB2 (q = 5.4 × 10−51), along with ID2 (q = 5.8 × 10−105) and HOPX (q = 1.4 × 10−112), although of smaller magnitude. CD3E, GZMK, and CD44 decreased in expression, which were enriched in the day +28 classified ML NK cells (Fig. 6D). We also observed decreased NCAM1 and increased SPON2 expression over time. Thus, pseudotime analysis reveals ongoing transcriptional changes in vivo by ML NK cells, consistent with continued differentiation from days +28 to +60.
Fig. 6. ML NK cells differentiate in patients.
(A) A monocle trajectory plot is shown for samples from TML-02, depicting three cell states and their proportions over time. (B) ADT expression of CD56, CD16, and CD57 was overlaid on the TML-02 trajectory. (C) ML NK cells are abundant in cell state 3 (red). (D) Select significantly changed genes across time in state 3 are shown. Points are colored by time, and the line depicts expression as a function of pseudotime. Differential gene test in Monocle2 using a regression model was used to determine genes changing as a function of time. Adjusted P value or q value of <0.05 for all.
In vivo differentiated ML NK cells exhibit enhanced functionality ex vivo
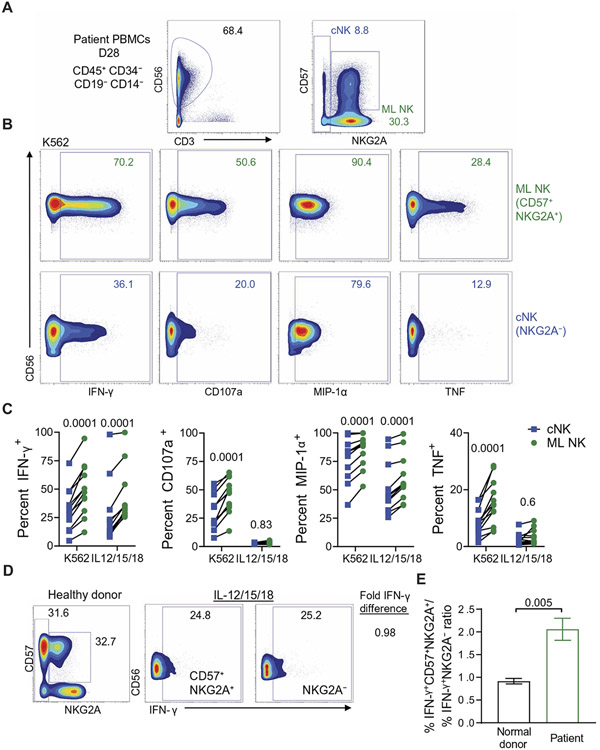
ML NK cells were originally described by their enhanced functionality in response to multiple stimuli, including CD16 ligation (30), cytokines (31), and hematologic or solid tumors (10, 11, 32). We hypothesized that in vivo differentiated ML NK cells would also exhibit enhanced responses compared to cNK cells within the same patient environment and that this would be specific to patients treated with ML NK cells. To evaluate whether in vivo differentiated ML NK cells exhibit enhanced functionality compared to cNK cells, we used mass cytometry to assess cytokine production [interferon-γ (IFN-γ), TNF, macrophage inflammatory protein 1α (MIP-1α)] and degranulation (CD107a) in response to stimulation. Patient PB mononuclear cells (PBMCs) were isolated at day +28 and incubated with K562 cells or IL-12/IL-15/IL-18 for 6 hours, followed by cytokine and surface CD107a analysis by mass cytometry. NK cells (CD45+CD34−CD19−CD14−CD3−CD56+) were identified using Boolean gating, and conventional mature NK cells (NKG2A−) and in vivo differentiated ML NK cells (CD57+NKG2A+) were characterized (Fig. 7A). Using this approach, donor cNK cells within the patient demonstrated significantly reduced cytokine and degranulation responses to K562 (P = 0.0001) and reduced MIP-1α and IFN-γ responses to IL-12/IL-15/IL-18 stimulation compared to in vivo differentiated ML NK cells (P = 0.0001; Fig. 7, B and C). Next, we compared the relative IFN-γ production between patient cNK and ML NK cells to normal donor cNK cells that were CD57+NKG2A+ and NKG2A−, observing that these conventional populations in healthy donors respond similarly to cytokines (Fig. 7D). However, when comparing IFN-γ expression fold changes between ML NK and cNK cells within patients, ML NK cells demonstrated significantly increased IFN-γ production relative to cNK cells (P = 0.005; Fig. 7E). In this analysis, CD57−NKG2A+ NK cells were excluded because they may confound the clear delineation between ML NK cells and cNK cells by containing newly developing NK cells from the graft (NKG2A+CD56bright or immature CD56dim), thereby hindering definitive ML NK cell identification. CD57 and NKG2A were also chosen because they are present on ML NK cells at day +28 and remained consistent between unstimulated and stimulated conditions, unlike the activating NK cell receptors normally used to identify ML NK cells via mass cytometry. These data support that ML NK cells differentiated in vivo maintain their enhanced functionality compared to cNK cells, even after expansion and cell division.
Fig. 7. In vivo differentiated ML NK cells demonstrate enhanced functionality ex vivo.
Freshly isolated patient PBMCs (day +28) were stimulated with K562 or IL-12/IL-15/IL-18 for 6 hours and characterized by mass cytometry. (A) A representative gating strategy shows identification of in vivo differentiated ML NK cells (NKG2A+CD57+) and cNK cells (NKG2A−). (B) Representative plots show ML NK (green) and cNK (blue) responses to K562. Inset numbers represent the frequency of cells within the gate. (C) Summary data from (B) are shown, with each patient depicted. (D)Patient and healthy donor PBMCs were stimulated with IL-12/IL-15/IL-18, and IFN-γ responses were compared. Representative flow plots comparing relative IFN-γ responses in NKG2A+CD57+ and NKG2A− from healthy donors are shown. The ratio of IFN-γ+ cells in each subset was quantified (right) as % IFN-γ+NKG2A+CD57+/% IFN-γ+NKG2A−. (E) Summary data from (D) are shown. Data in (E) are from 12 healthy donors and 12 patients. Each dot represents an individual patient. Data were compared using a (C) two-way ANOVA or (E) Mann-Whitney test.
DISCUSSION
Here, we report interim clinical and correlative analyses of a single-arm, phase 2 clinical trial using RIC haplo-HCT augmented by donor-derived ML NK cell adoptive transfer supported by the IL-15 superagonist N-803 to treat rel/ref AML. Despite the extremely poor prognosis of this subgroup of patients, 87% of patients achieved a composite CR at day +28, and 4 of 14 evaluable patients were alive after 1 year, with some patients achieving deep and sustained control of TP53-mutated disease. Clinically relevant CRS was not observed, and GvHD was not exacerbated by same-donor ML NK cell therapy and N-803. Orthogonal immune correlative data using both mass cytometry and CITE-seq demonstrate that ML NK cells expand an average of 1104-fold and persist for at least 60 days after a single dose in this immune-compatible setting. Likewise, a distinct transcriptional and proteomic profile of ML NK cells was identified, including increased expression of genes related to effector function. Furthermore, in vivo differentiated ML NK cells demonstrate persistently enhanced effector functions after extensive cell division. Additional studies are warranted to better understand the unique biology of ML NK cells and their potential as a therapy after HCT.
Developing new therapies for patients with rel/ref AML remains an area of unmet clinical need. Recently approved targeted therapies have been shown to induce responses in rel/ref AML; however, these are typically short in duration and not curative (33). Unfortunately, many patients with rel/ref AML are not candidates for myeloablative conditioning (MAC) as part of an HCT strategy because of poor fitness and comorbidities. Additional barriers to conventional HCT approaches include the presence of active disease, older age, lacking suitable fully matched sibling donors, and unfavorable disease kinetics that make timing an unrelated donor difficult (4, 34).In turn, RIC haplo-HCT strategies are attractive from tolerability and logistical perspectives, but the post-HCT relapse risk remains high using standard approaches (3, 35, 36). Augmenting RIC haplo-HCT with ML NK cell adoptive transfer and N-803 injections was feasible and well tolerated in our study. All enrolled patients underwent the planned HCT, ML NK cell infusion, and at least two of four planned N-803 injections.There were no new safety signals, with only limited CRS and transient injection site reactions. The incidences of GvHD and graft failure were also comparable to expected rates with RIC haplo-HCT.
NK cells are a nascent cellular therapy for hematologic malignancies and solid cancers, with multiple types of NK cell products in clinical development (8). Using mass cytometry and CITE-seq, improved ML NK cell expansion and persistence were clear in this immune-matched setting, compared to prior work in the nonimmune-matched setting (10, 11). The first reports of in vivo NK cell expansion were in the setting of haploidentical NK cell adoptive therapy with IL-2 or IL-15 cytokine support, where expansion was observed to day +14 in a subset of patients (37, 38). Recently, K562mbIL-21 feeder cell–expanded NK cells with haplo-HCT were used for myeloid malignancies; however, NK cells analyzed after transfer were not distinct when compared to patients who did not receive NK cells (39). Similarly, a recent clinical trial using cord blood–derived expanded chimeric antigen receptor (CAR) NK cells found donor-derived NK cells to constitute 1% or less of total NK cells 7 to 14 days after transfer, despite membrane-bound IL-15 expression by the CAR NK cells, suggesting a limited persistence in this immune-incompatible setting (40). In an unmatched environment, the window of opportunity for ML NK cells to expand and function is constrained due to allo-rejection by recipient T cells. Here, NK cells and T cells are immune-compatible, allowing the potential for ML NK cell expansion and persistence to be evaluated. When contrasted to historical controls without NK cell therapy (13), there was a marked increase in total blood NK cells (from about 100/μl to about 1000/μl) in patients receiving same-donor ML NK cells. Thus, these data suggest that strategies to avoid T cell–mediated allo-rejection may improve on immune-incompatible allogeneic ML NK cell therapy. This study suggests that rapid ML NK expansion likely delays CD56bright NK cell reconstitution from the graft, and better understanding the underlying mechanism is important. In addition to NK cells, memory stem T cells are present early after HCT, are responsive to IL-15, and may promote GvL. Additional studies examining alternative effector populations that can contribute to tumor clearance are warranted (41). Overall, these data demonstrate enhanced adoptive NK cell therapy persistence and expansion, which can be harnessed in future clinical trials.
This study reveals insights into ML and cNK cell subsets using in-depth CITE-seq transcriptional and proteomic analyses. Using a previously unreported CITE-seq classifier informed by in vitro differentiated ML and cNK cells, we identify an ML NK cell profile that is consistent with our mass cytometry data (CD56hi, CD16+/−, KIR+/−, and CD62Lhi/NKG2Ahi) and with our previous clinical trial using donor and recipient HLA tracking by HLA-specific monoclonal antibodies (11). Haplo-HCT patients who did not receive ML NK cells had negligible ML NK cells, indicating that it is unlikely that the ML NK cell population identified here represents an aberrant post-HCT NK cell subset. Moreover, this classifier provides a robust, reproducible method for identifying ML NK cells and thus can be used as a model for future work on extending the classifier to automatically identifying ML NK cells at post-HCT time points beyond day +28 and in other clinical contexts. Intriguingly, ML NK cells had a cell surface receptor profile with similarities to central memory T cells (CD44hi and CD62Lhi) and the recently described virtual memory T cells, including high NKG2A and KIR expression, along with increased IFN-γ production after cytokine stimulation (25, 42-44). Further studies are warranted to determine whether there are similarities in the ML NK cell molecular program and memory T cell subsets. Results from this study demonstrate that the ML NK cell molecular program induces long-lived (days +28 to +60) effector cells with enhanced functionality passed on after cell division in vivo, which are distinct from CMV-adaptive NK cells. Prior studies demonstrated that NKG2A is an important ML NK cell checkpoint, whereas KIR interactions were ignored by in vitro differentiated ML NK cells. Future studies examining the contributions of these inhibitory signals on in vivo ML NK cell responses will be critical in determining whether durability can be improved by checkpoint blockade. Using this information and approach, we now have the tools and understanding to explore whether ML NK cells represent a physiologic program, existing in normal donors. Extremely low, but detectable, ML NK cell frequencies were observed in normal donor samples. These findings will lead to new avenues of research, including interrogating the presence of these cells in solid tumors and in response to various infections, and will yield insights into the possible mechanisms into how they may develop naturally.
Comparing the clinical outcomes of our cohort of patients to other studies is challenging. To the best of our knowledge, there is no prospective study comprised exclusively of patients with active, adverse risk AML undergoing RIC-HCT. One retrospective analysis reported a median OS of 3.5 months in patients with primary refractory or unsuccessfully treated relapsed AML undergoing MAC allo-HCT (2), although fewer than half of these patients had adverse risk genetics compared to 14 of 15 patients in our cohort. Furthermore, although not optimized for MRD and NGS testing, 14 of 15 patients in our study were “very high” risk and 1 was “high” risk by Disease Risk Index (45). McCurdy et al. (46) cited a 3-year Progression-free survival (PFS) of 22% among 60 high- or very high–risk patients undergoing RIC haplo-HCT with posttransplant cyclophosphamide for a range of hematologic malignancies. However, this cohort included only one patient with active, adverse risk AML, two features nearly universal in our cohort. Nonetheless, the preliminary efficacy and safety signals of our strategy using RIC haplo-HCT augmented by ML NK cell infusion and N-803 injections are encouraging. Posttransplant correlative genomics revealed several insights into the activity of this therapeutic approach, with tracking of AML-associated mutations, including TP53 variants, over time revealing successful clearance in a large portion of patients. Reemergence of these mutations did not precede imminent relapse, because mutations could persist at low VAFs for several months before clinical relapse, suggesting ongoing GvL effects.
There are limitations to the current study. The clinical results are from a relatively small sample size (N = 15) and represent the interim analysis of a single-arm clinical trial. Benchmarking these clinical results to the standard of care for this complex patient cohort was challenging on the basis of the available published clinical trials. It was not possible to evaluate relapsed AML for NK cell–related immune evasion, because we were unable to comprehensively obtain adequate AML samples at the time of relapse. Future studies will prioritize strategies to overcome these limitations of our correlative sample set. Last, the unfortunately high relapse rate in our patients indicates that further efforts to improve remission durability will be crucial.
In summary, we report clinical and correlative results of ML NK cell therapy supported by N-803 after haplo-HCT for heavily pretreated, high-risk patients with AML. We observed an excellent safety profile and a high response rate, albeit for short durations. Somatic mutation analyses revealed that ML NK cells hold potential for clearing somatic mutations associated with poor outcomes, including TP53 variants. Multidimensional correlative analyses showed that ML NK cells persisted for at least 60 days, corroborated by robust expansion in patients and a unique phenotypic and transcriptional profile. Future correlative studies and clinical work are warranted to better understand ML NK cell biology and to improve the durability of disease control in this setting.
MATERIALS AND METHODS
Study design
This single-center, single-arm, open-label phase 2 study (registered at ClinicalTrials.gov as #NCT02782546) enrolled patients with AML and HLA-haploidentical donors. From April 2017 to March 2020, 39 individuals were consented and screened for eligibility, and 30 individuals were enrolled (15 patients and 15 donors). Eligible patients were of age 18 years or older with refractory AML defined by (i) failure to achieve CR after two or more cycles of induction therapy (primary induction failure), or (ii) AML relapsed after obtaining a CR and then failing to achieve a second CR after one or more cycles of reinduction therapy. Patients had a Karnofsky performance status >60% at enrollment. Cytoreductive therapies including leukapheresis and hydroxyurea were allowed. Patients underwent RIC consisting of Flu (30 mg/m2 per day on days −6 through −2), cyclophosphamide (14.5 mg/kg per day on days −6 and −5), and TBI at 200 centigrays on day −1. Donors underwent G-CSF mobilization (days −4 through 0) and PBSC collection by apheresis on day 0 (goal of >4 × 106 CD34+ cells/kg). Patients received the PBSC graft infusion on day 0 and then posttransplant cyclophosphamide at 50 mg/kg on days +3 and +4. GvHD prophylaxis consisted of tacrolimus and mycophenolate mofetil starting on day +5 (continued until days +180 and +35, respectively, in the absence of GvHD). Donors underwent ML NK cell production on day +6, consisting of nonmobilized PBMC collection by large-volume (20 liters) apheresis followed by preactivation with recombinant human (rh) IL-12, rhIL-15, and rhIL-18 under good manufacturing practice conditions. Patients received ML NK cell infusion on day +7 (acceptable ML NK cell dose range was 0.5 × 106 to 10 × 106 cells/kg) (11). Patients received subcutaneous N-803 (previously referred to as ALT-803) at 10 μg/kg starting on day +7. Patients received G-CSF injections daily from day +14 until neutrophil engraftment. Additional details are described in Supplementary Materials and Methods.
End points and assessments
Patients were followed for 48 months and analyzed on an intention-to-treat basis, described further in Supplementary Materials and Methods. Patients were censored at the time of last follow-up or at interim data cutoff on 22 January 2021. Responses were assessed by the Revised International Working Group Criteria for AML (47). All patients who received ML NK cell infusion and one dose of N-803 and then underwent day +28 BM biopsy for response assessment were evaluable for disease response. AEs were defined by the National Cancer Institute Common Terminology Criteria for Adverse Events v4. Engraftment and GvHD were assessed by the Center for International Blood and Marrow Transplant Research criteria. This is an interim analysis with scientific correlative data and no preplanned statistical testing of clinical outcomes.
Chromosomal and mutation analysis
All patients underwent metaphase karyotyping and FISH for cytogenetic assessments plus targeted genotyping per institutional standard of care at the time of enrollment. Retrospective mutational assessment was performed using HaloPlex high-sensitivity (HS) amplicon sequencing (Agilent) targeting 40 genes mutated in AML (17). Genomic DNA (gDNA) was isolated from PBMCs using the DNeasy Blood & Tissue Kit (Qiagen, #69506) per the manufacturer’s protocols. HaloPlex HS libraries were generated using 50 ng of input gDNA per sample per the manufacturer’s protocols. Individual libraries were assessed using the High Sensitivity DNA Kit (Agilent, #5067-4626) on a 2100 Bioanalyzer instrument (Agilent, #G2939BA). Libraries were normalized by molarity for pooling and then sequenced on the NovaSeq 6000 S4 platform (Illumina) to obtain about 40 million reads per sample. Variant calling is described in Supplementary Materials and Methods.
Patient samples
PB and BM aspirates were obtained from patients who provided written informed consent before participating and were treated on a Washington University Institutional Review Board (IRB)-approved clinical trial (Human Research Protection Office #201610088). PBMCs were isolated as previously described (11). Briefly, mononuclear cells were isolated by Ficoll-Paque PLUS (GE Health) centrifugation. Samples were viably frozen or assessed immediately.
Mass cytometry
Lymphocytes were stained fresh or from cryopreserved samples, and subsets were identified using FlowSOM as described in Supplementary Materials and Methods (table S5) (11). Metaclusters were manually annotated, and NK cell metacluster was used for subsequent phenotypic analysis. FlowSOM-gated NK cells for each patient-donor pair were analyzed individually using viSNE, a visualization tool for high-dimensional single-cell data based on the tSNE algorithm (proportional sampling, 1000 iterations, perplexity = 30, theta = 0.5; table S5) (10, 11). The viSNE NK cell subsets were then identified using FlowSOM (tSNE1 and tSNE2; all events, hierarchical consensus, 20 metaclusters, 196 clusters, 15 iterations, random seed, scales not normalized). Data were analyzed using Prism v9.0 and Cyto-bank (48). All donor products were assessed, and all but TML-08 patient samples were assessed for phenotype by mass cytometry. TML-08 had insufficient material to assess. Functional experiments were performed as previously described from freshly isolated PBMC (11).
CITE-seq sample and data processing
Samples were thawed in batch and sorted for live NK cells by negative selection (see Supplementary Materials and Methods). Sorted cells were loaded onto a 10x Chromium Instrument (5’v1 chemistry), and libraries were sequenced on an Illumina NovaSeq6000. The resulting FASTQ files were aligned to a genome consisting of the GRCh38 and mm10 reference using CellRanger (default settings, v.3.0.1).
CITE-seq initial data processing details
The CellRanger filtered barcode matrix for each sample was imported into Seurat (v.3.9.9; R) (21) for normalization, clustering, visualization, and differential expression by patient as previously described (23). Differentially expressed genes in ML NK cells were identified by re-assigning cells identified as ML NK cells according to transfer learning to an ML NK cell cluster. A Wilcoxon rank sum test was conducted between ML NK and all other clusters.
In vitro control and ML CITE-seq dataset
The in vitro dataset was generated by isolating PB NK cells from leukoreduction chambers, and control and ML NK cells were generated as described (22). At the end of differentiation, the NK cells were stained with the addition of Hashtag antibodies (table S4), allowing for pooling the control and ML NK cells into the same 10X Chromium run. The resulting matrices were demultiplexed (quantile = 0.90) after normalizing hashtag staining by centered log ratio transformation per feature in Seurat.
Statistical analysis
Before statistical analyses, data were tested for normal distribution (Shapiro-Wilk). If data were not normally distributed, then appropriate nonparametric tests were used (GraphPad Prism v9), with all statistical comparisons indicated in the figure legends. Uncertainty is represented in figures as the SEM, except where otherwise indicated. All comparisons used a two-sided α = 0.05 for significance testing. CITE-seq statistical analyses were conducted in R (3.5, 3.6, or 4.0) as described in Materials and Methods and figure legends.
Supplementary Material
Acknowledgments:
We would like to acknowledge the Siteman Flow Cytometry (B. Eades), Immune Monitoring Laboratory (S. Oh), McDonnell Genome Institute (C. Fronick, B. Fulton, K. Haub, and J. Ponce), and Biological Therapy Core Facility. We thank the Genome Technology Access Center (GTAC) at the McDonnell Genome Institute (MGI) at Washington University School of Medicine for help with genomics services.
Funding:
The research reported in this publication was supported by grants from the NIH National Cancer Institute (P50CA171963 to T.A.F., A.F.C., and M.M.B.-E.; U01CA248235 to M. Griffith; R01CA205239 to T.A.F.; P30CA91842 to T.A.F. and GTAC/MGI; and K12CA1667540 to M.M.B.-E.); NIH National Heart, Lung, and Blood Institute (T32HL007088 to J.A.F., D.A.R.-G., and P. Wong); NIH National Institute of General Medical Sciences (T32GM139799 to J.A.F.); NIH National Institute of Allergy and Infectious Diseases (F30AI161318 to C.C.C.); NIH National Center for Research Resources (UL1TR002345 to GTAC/MGI); the American Association of Immunologists: Intersect Fellowship Program for Computational Scientists and Immunologists to J.A.F., A.A.P., and T.A.F.; the American Society of Clinical Oncology Young Investigator Award via Conquer Cancer Foundation to M.T.J.; the Washington University MGI Pilot Grant to T.A.F.; and the Siteman Cancer Center Investment Program to T.A.F. The clinical costs of the trial were supported in part by ImmunityBio Inc. (P.S.-S.).
Footnotes
Competing interests: M.M.B.-E. and T.A.F. are inventors on patent/patent applications (15/983,275,62/963,971, and PCT/US2019/060005) licensed to Wugen Inc. and held/submitted by Washington University that cover aspects of ML NK cell biology. This results in potential royalties to M.M.B.-E., T.A.F., and Washington University from Wugen Inc. P.S.-S. and ImmunityBio hold a patent (#11,173,191) that covers combination therapies using an IL-15–based superagonist complex and an antibody to effectively treat subjects with cancer and infectious diseases. Unrelated to this work, J.A.F. is an inventor on patent/patent application (WO 2019/152387, US 63/018,108) licensed to Kiadis Inc. and held/submitted by Nationwide Children’s Hospital on TGF-β resistant, expanded NK cells. M.M.B.-E. has equity, consulting, and royalty interest in Wugen Inc. Unrelated to this work, J.A.F. has a monoclonal antibody unrelated to the present work licensed to EMD Millipore. Unrelated to this work, C.C.C. reports equity in Pionyr Immunotherapeutics. Unrelated to this work, D.A.R.-G. receives consulting fees from Cartography Inc. Unrelated to this work, M.A.S. reports receiving honorary and consulting from Amgen, Astellas, CareDx, Dova Pharmacteticals, Equillium Inc., FlatIron Inc., GSK, Gilead Sciences Inc., Incyte, Janssen, Novo Nordisk, Partners Therapeutics, Pfizer, and Sanofi Genzyme. In addition, M.A.S. reports previously serving on speakers’ bureaus and receiving honorary outside of the submitted work from Abbvie, Merck, and Takeda. Unrelated to this work, R.R. reports research funding from Crispr Therapeutics and Glycostem (SAB). Unrelated to this work, G.L.U. reports consulting for Novartis, Abbvie, Agios GSK, and Jazz. Unrelated to this work, P. Westervelt reports paid consultancy with Pfizer. W.-R.L. is an employee of MilliporeSigma. D.H.S. reports consulting for Wugen Inc. J.F.D. has equity interest in Wugen Inc. Unrelated to this work, A.G. reports consulting or advisory role for Kite (a Gilead Company), Amgen, Atara, Wugen, and Celgene; research funding from Kite and Amgen; and honoraria from Kite. P.S.-S. is the executive chairman, director, employee, and a majority holder of ImmunityBio Inc. T.A.F. reports researching funding from HCW Biologics Inc., ImmunityBio, Wugen, and the NIH during the conduct of the study. T.A.F. has equity, research funding, consulting, and royalty interest in Wugen Inc. Unrelated to this work, T.A.F. also reports consulting for Compass Therapeutics and Affimed; consulting from Kiadis, Nkarta, and Nektar; and advises (equity interest) Indapta and OrcaBio. All other authors declare that they have no competing interests.
Data and materials availability: All data associated with this study are present in the paper or the Supplementary Materials. Raw sequencing files will be available to qualified investigators at the time of publication at GSE185989 and phs002681. Clinical data will be deposited at www.clinicaltrials.gov (#NCT02782546) in accordance with guidelines. For questions about data requests, contact the corresponding author.
SUPPLEMENTARY MATERIALS
REFERENCES AND NOTES
- 1.Estey EH, Acute myeloid leukemia: 2019 update on risk-stratification and management. Am. J. Hematol 93, 1267–1291 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y, Mielcarek M, Estey EH, Appelbaum FR, Walter RB, Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: Time to move toward a minimal residual disease-based definition of complete remission? J. Clin. Oncol 34, 329–336 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, Maziarz RT, Warlick ED, Fernandez HF, Alyea EP, Hamadani M, Bashey A, Giralt S, Geller NL, Leifer E, Le-Rademacher J, Mendizabal AM, Horowitz MM, Deeg HJ, Horwitz ME, Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J. Clin. Oncol 35, 1154–1161 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCurdy SR, Luznik L, How we perform haploidentical stem cell transplantation with posttransplant cyclophosphamide. Blood 2019, 513–521 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaiswal SR, Zaman S, Chakrabarti A, Sen S, Mukherjee S, Bhargava S, Ray K, O’Donnell PV, Chakrabarti S, Donnell PVO, Chakrabarti S, O’Donnell PV, Chakrabarti S, Improved outcome of refractory/relapsed acute myeloid leukemia after post-transplantation cyclophosphamide-based haploidentical transplantation with myeloablative conditioning and early prophylactic granulocyte colony-stimulating factor–mobilized donor lymphocyte infusions. Biol. Blood Marrow Transplant 22, 1867–1873 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S, Functions of natural killer cells. Nat. Immunol 9, 503–510 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Berrien-Elliott MM, Romee R, Fehniger TA, Improving natural killer cell cancer immunotherapy. Curr. Opin. Organ Transplant 20, 671–680 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimasaki N, Jain A, Campana D, NK cells for cancer immunotherapy. Nat. Rev. Drug Discov 19, 200–218 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Gang M, Wong P, Berrien-Elliott MM, Fehniger TA, Memory-like natural killer cells for cancer immunotherapy. Semin. Hematol 57, 185–193 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, Leong JW, Abdel-Latif S, Schneider SE, Willey S, Neal CCC, Yu L, Oh ST, Lee Y-S, Mulder A, Claas F, Cooper MA, Fehniger TA, Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med 8, 357ra123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berrien-Elliott MM, Cashen AF, Cubitt CC, Neal CC, Wong P, Wagner JA, Foster M, Schappe T, Desai S, McClain E, Becker-Hapak M, Foltz JA, Cooper ML, Jaeger N, Srivatsan SN, Gao F, Romee R, Abboud CN, Uy GL, Westervelt P, Jacoby MA, Pusic I, Stockerl-Goldstein KE, Schroeder MA, DiPersio J, Fehniger TA, Multidimensional analyses of donor memory-like NK Cells reveal new associations with response after adoptive immunotherapy for leukemia. Cancer Discov. 10, 1854–1871 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han KP, Zhu X, Liu B, Jeng E, Kong L, Yovandich JL, Vyas VV, Marcus WD, Chavaillaz P-AA, Romero CA, Rhode PR, Wong HC, Han VK, Zhua X, Liua B, Jenga E, Konga L, Yovandichb JL, Vyasc PRV, Marcusa WD, Chavaillaza P-A, Romeroa CA, Rhodea HCW, IL-15:IL-15 receptor alpha superagonist complex: High-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine 56, 804–810 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo A, Oliveira G, Berglund S, Greco R, Gambacorta V, Cieri N, Toffalori C, Zito L, Lorentino F, Piemontese S, Morelli M, Giglio F, Assanelli A, Stanghellini MTL, Bonini C, Peccatori J, Ciceri F, Luznik L, Vago L, NK cell recovery after haploidentical HSCT with posttransplant cyclophosphamide: Dynamics and clinical implications. Blood 131, 247–262 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien H-F, Wei AH, Löwenberg B, Bloomfield CD, Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129, 424–447 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller JS, Prosper F, McCullar V, Natural killer (NK) cells are functionally abnormal and NK cell progenitors are diminished in granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cell collections. Blood 90, 3098–3105 (1997). [PubMed] [Google Scholar]
- 16.Ciurea SO, Zhang M-J, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, Armand P, Antin JH, Chen J, Devine SM, Fowler DH, Luznik L, Nakamura R, O’Donnell PV, Perales M-A, Pingali SR, Porter DL, Riches MR, Ringdén OTH, Rocha V, Vij R, Weisdorf DJ, Champlin RE, Horowitz MM, Fuchs EJ, Eapen M, Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood 126, 1033–1040 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnell EK, Newcomer KF, Skidmore ZL, Krysiak K, Anderson SR, Lukas, Wartman D, Oh ST, Welch JS, Stockerl-Goldstein Keith, E., Vij R, Cashen AF, Pusic I, Westervelt P, Abboud CN, Ghobadi A, Uy GL, Schroeder MA, Dipersio JF, Politi MC, David, Spencer H, Duncavage EJ, Ley TJ, Griffith M, Jacoby MA, Griffith OL, Krysiak K, Anderson SR, Wartman LD, Oh ST, Welch JS, Stockerl-Goldstein KE, Vij R, Cashen AF, Pusic I, Westervelt P, Abboud CN, Ghobadi A, Uy GL, Schroeder MA, Dipersio JF, Politi MC, Spencer DH, Duncavage EJ, Ley TJ, Griffith M, Jacoby MA, Griffith OL, Impact of a 40-gene targeted panel test on physician decision making for patients with acute myeloid leukemia. JCO Precis. Oncol, 5, 191–203 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welch JS, Petti AA, Miller CA, Fronick CC, O’Laughlin M, Fulton RS, Wilson RK, Baty JD, Duncavage EJ, Tandon B, Lee Y-S, Wartman LD, Uy GL, Ghobadi A, Tomasson MH, Pusic I, Romee R, Fehniger TA, Stockerl-Goldstein KE, Vij R, Oh ST, Abboud CN, Cashen AF, Schroeder MA, Jacoby MA, Heath SE, Luber K, Janke MR, Hantel A, Khan N, Sukhanova MJ, Knoebel RW, Stock W, Graubert TA, Walter MJ, Westervelt P, Link DC, DiPersio JF, Ley TJ, TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N. Engl. J. Med 375, 2023–2036 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan O, Hunter A, Talati C, Sallman DA, Asghari H, Song J, Hussaini M, Bejanyan N, Elmariah H, Kuykendall AT, Padron E, Komrokji RS, List AF, Lancet JE, Sweet KL, Mishra A, Impact of TP53 gene mutation clearance and conditioning intensity on outcome in MDS or AML patients prior to allogeneic stem cell transplantation. Blood 134, 149 (2019). [Google Scholar]
- 20.Rambaldi B, Kim HT, Reynolds C, Chamling Rai S, Arihara Y, Kubo T, Buon L, Gooptu M, Koreth J, Cutler C, Nikiforow S, Ho VT, Alyea EP, Antin JH, Wu CJ, Soiffer RJ, Ritz J, Romee R, Impaired T- and NK-cell reconstitution after haploidentical HCT with posttransplant cyclophosphamide. Blood Adv. 5, 352–364 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoeckius M, Hafemeister C, Stephenson W, Houck-Loomis B, Chattopadhyay PK, Swerdlow H, Satija R, Smibert P, Simultaneous epitope and transcriptome measurement in single cells. Nat. Methods 14, 865–868 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gang M, Marin ND, Wong P, Neal CC, Marsala L, Foster M, Schappe T, Meng W, Tran J, Schaettler M, Davila M, Gao F, Cashen AF, Bartlett NL, Mehta-Shah N, Kahl BS, Kim MY, Cooper ML, DiPersio JF, Berrien-Elliott MM, Fehniger TA, CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood 136, 2308–2318 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foltz JA, Hess BT, Bachanova V, Bartlett NL, Berrien-Elliott MM, McClain E, Becker-Hapak M, Foster M, Schappe T, Kahl B, Mehta-Shah N, Cashen AF, Marin ND, McDaniels K, Moreno C, Mosior M, Gao F, Griffith OL, Griffith M, Wagner JA, Epperla N, Rock AD, Lee J, Petti AA, Soon-Shiong P, Fehniger TA, Phase I trial of N-803, an IL15 receptor agonist, with rituximab in patients with indolent non-hodgkin lymphoma. Clin. Cancer Res 27, 3339–3350 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delconte RB, Shi W, Sathe P, Ushiki T, Seillet C, Minnich M, Kolesnik TB, Rankin LC, Mielke LA, Zhang JG, Busslinger M, Smyth MJ, Hutchinson DS, Nutt SL, Nicholson SE, Alexander WS, Corcoran LM, Vivier E, Belz GT, Carotta S, Huntington ND, The helix-loop-helix protein ID2 governs NK cell fate by tuning their sensitivity to interleukin-15. Immunity 44, 103–115 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Chen J, López-Moyado IF, Seo H, Lio C-WJ, Hempleman LJ, Sekiya T, Yoshimura A, Scott-Browne JP, Rao A, NR4A transcription factors limit CAR T cell function in solid tumours. Nature 567, 530–534 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Helden MJ, Goossens S, Daussy C, Mathieu A-L, Faure F, Marçais A, Vandamme N, Farla N, Mayol K, Viel S, Degouve S, Debien E, Seuntjens E, Conidi A, Chaix J, Mangeot P, de Bernard S, Buffat L, Haigh JJ, Huylebroeck D, Lambrecht BN, Berx G, Walzer T, Terminal NK cell maturation is controlled by concerted actions of T-bet and Zeb2 and is essential for melanoma rejection. J. Exp. Med 212, 2015–2025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TDD, Han H, Chiang SCCCC, Foley B, Mattsson K, Larsson S, Schaffer M, Malmberg K-JJ, Ljunggren H-GG, Miller JSS, Bryceson YTT, Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 42, 443–456 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith SL, Kennedy PR, Stacey KB, Worboys JD, Yarwood A, Seo S, Solloa EH, Mistretta B, Chatterjee SS, Gunaratne P, Allette K, Wang Y-C, Smith ML, Sebra R, Mace EM, Horowitz A, Thomson W, Martin P, Eyre S, Davis DM, Diversity of peripheral blood human NK cells identified by single-cell RNA sequencing. Blood Adv. 4, 1388–1406 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, Trapnell C, Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner JA, Berrien-Elliott MM, Rosario M, Leong JW, Jewell BA, Schappe T, Abdel-Latif S, Fehniger TA, Cytokine-induced memory-like differentiation enhances unlicensed NK cell anti-leukemia and FcγRIIIa-triggered responses. Biol. Blood Marrow Transplant 23, 398–404 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA, Cytokine activation induces human memory-like NK cells. Blood 120, 4751–4760 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marin ND, Krasnick BA, Becker-Hapak M, Conant L, Goedegebuure SP, Berrien-Elliott MM, Robbins KJ, Foltz JA, Foster M, Wong P, Cubitt CC, Tran J, Wetzel CB, Jacobs M, Zhou AY, Russler-Germain D, Marsala L, Schappe T, Fields RC, Fehniger TA, Memory-like differentiation enhances NK cell responses to melanoma. Clin. Cancer Res 27, 4859–4869 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daver N, Wei AH, Pollyea DA, Fathi AT, Vyas P, DiNardo CD, New directions for emerging therapies in acute myeloid leukemia: The next chapter. Blood Cancer J. 10, 107 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kekre N, Antin JH, Hematopoietic stem cell transplantation donor sources in the 21st century: Choosing the ideal donor when a perfect match does not exist. Blood 124, 334–343 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Santoro N, Labopin M, Ciceri F, Van Lint MT, Nasso D, Blaise D, Arcese W, Tischer J, Bruno B, Ehninger G, Koc Y, Santarone S, Huang XJ, Savani BN, Mohty M, Ruggeri A, Nagler A, Impact of conditioning intensity on outcomes of haploidentical stem cell transplantation for patients with acute myeloid leukemia 45 years of age and over. Cancer 125, 1499–1506 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Bejanyan N, Zhang M, Bo-Subait K, Brunstein C, Wang H, Warlick ED, Giralt S, Nishihori T, Martino R, Passweg J, Dias A, Copelan E, Hale G, Gale RP, Solh M, Kharfan-Dabaja MA, Diaz MA, Ganguly S, Gore S, Verdonck LF, Hossain NM, Kekre N, Savani B, Byrne M, Kanakry C, Cairo MS, Ciurea S, Schouten HC, Bredeson C, Munker R, Lazarus H, Cahn J-Y, van Der Poel M, Rizzieri D, Yared JA, Freytes C, Cerny J, Aljurf M, Palmisiano ND, Pawarode A, Bacher VU, Grunwald MR, Nathan S, Wirk B, Hildebrandt GC, Seo S, Olsson RF, George B, de Lima M, Hourigan CS, Sandmaier BM, Litzow M, Kebriaei P, Saber W, Weisdorf D, Myeloablative conditioning for allogeneic transplantation results in superior disease-free survival for acute myelogenous leukemia and myelodysplastic syndromes with low/ intermediate but not high disease risk index: A center for international blood and marrow transplant research study. Transplant. Cell. Ther 27, 68.e1–68.e9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lewis D, Hippen K, McGlave P, Weisdorf DJ, Blazar BR, Miller JS, Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood 123, 3855–3863 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooley S, He F, Bachanova V, Vercellotti GM, DeFor TE, Curtsinger JM, Robertson P, Grzywacz B, Conlon KC, Waldmann TA, McKenna DH, Blazar BR, Weisdorf DJ, Miller JS, First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 3, 1970–1980 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciurea S, Schafer J, Bassett R, Denman C, Cao K, Willis D, Rondon G, Chen J, Soebbing D, Kaur I, Gulbis A, Ahmed S, Rezvani K, Shpall E, Lee D, Champlin R, Phase 1 clinical trial using mbIL21 ex vivo–expanded donor-derived NK cells after haploidentical transplantation. Blood 130, 1857–1868 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, Kerbauy LN, Overman B, Thall P, Kaplan M, Nandivada V, Kaur I, Cortes AN, Cao K, Daher M, Hosing C, Cohen EN, Kebriaei P, Mehta R, Neelapu S, Nieto Y, Wang M, Wierda W, Keating M, Champlin R, Shpall EJ, Rezvani K, Kerbauy LN, Overman B, Thall P, Kaplan M, Nandivada V, Kaur I, Cortes AN, Cao K, Daher M, Hosing C, Cohen EN, Kebriaei P, Mehta R, Neelapu S, Nieto Y, Wang M, Wierda W, Keating M, Champlin R, Shpall EJ, Rezvani K, Kerbauy LN, Overman B, Thall P, Kaplan M, Nandivada V, Kaur I, Cortes AN, Cao K, Daher M, Hosing C, Cohen EN, Kebriaei P, Mehta R, Neelapu S, Nieto Y, Wang M, Wierda W, Keating M, Champlin R, Shpall EJ, Rezvani K, Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med 382, 545–553 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, Bondanza A, Bordignon C, Peccatori J, Ciceri F, Lupo-Stanghellini MT, Mavilio F, Mondino A, Bicciato S, Recchia A, Bonini C, IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 121, 573–584 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Turner SJ, Bennett TJ, La Gruta NL, CD8 + T-cell memory: The why, the when, and the how. Cold Spring Harb. Perspect. Biol 13, a038661 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bezman NA, Kim CC, Sun JC, Min-Oo G, Hendricks DW, Kamimura Y, Best JA, Goldrath AW, Lanier LL; The Immunological Genome Project Consortium, Molecular definition of the identity and activation of natural killer cells. Nat. Immunol 13, 1000–1009 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Albrecht I, Niesner U, Janke M, Menning A, Loddenkemper C, Kühl AA, Lepenies I, Lexberg MH, Westendorf K, Hradilkova K, Grün J, Hamann A, Epstein JA, Chang H-D, Tokoyoda K, Radbruch A, Persistence of effector memory Th1 cells is regulated by Hopx. Eur. J. Immunol 40, 2993–3006 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Armand P, Gibson CJ, Cutler C, Ho VT, Koreth J, Alyea EP, Ritz J, Sorror ML, Lee SJ, Deeg HJ, Storer BE, Appelbaum FR, Antin JH, Soiffer RJ, Kim HT, A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood 120, 905–913 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mcurdy SR, Kanakry JA, Showel MM, Tsai HL, Bolaños-Meade J, Rosner GL, Kanakry CG, Perica K, Symons HJ, Brodsky RA, Gladstone DE, Huff CA, Pratz KW, Prince GT, Dezern AE, Gojo I, Matsui WH, Borrello I, McDevitt MA, Swinnen LJ, Smith BD, Levis MJ, Ambinder RF, Luznik L, Jones RJ, Fuchs EJ, Kasamon YL, Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood 125, 3024–3031 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD; International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia, Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J. Clin. Oncol 21, 4642–4649 (2003). [DOI] [PubMed] [Google Scholar]
- 48.Kotecha N, Krutzik PO, Irish JM, Web-based analysis and publication of flow cytometry experiments. Curr. Protoc. Cytom Chapter 10, Unit10.17 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H, Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM (2013); arXiv:1303.3997v2. [Google Scholar]
- 50.Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, Miller CA, Mardis ER, Ding L, Wilson RK, VarScan 2: Somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 22, 568–576 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rimmer A, Phan H, Mathieson I, Iqbal Z, Twigg SRF, Wilkie AOM, Mcvean G, Lunter G, Integrating mapping-, assembly- and haplotype-based approaches for calling variants in clinical sequencing applications. Nat. Genet 46, 912–918 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye K, Schulz MH, Long Q, Apweiler R, Ning Z, Pindel: A pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics 25, 2865–2871 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zunder ER, Finck R, Behbehani GK, Amir E-AD, Krishnaswamy S, Gonzalez VD, Lorang CG, Bjornson Z, Spitzer MH, Bodenmiller B, Fantl WJ, Pe’er D, Nolan GP, Palladium-based mass tag cell barcoding with a doublet-filtering scheme and single-cell deconvolution algorithm. Nat. Protoc 10, 316–333 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Gassen S, Callebaut B, Van Helden MJ, Lambrecht BN, Demeester P, Dhaene T, Saeys Y, FlowSOM: Using self-organizing maps for visualization and interpretation of cytometry data. Cytometry A 87, 636–645 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Diggins KE, Ferrell PB Jr., Irish JM, Methods for discovery and characterization of cell subsets in high dimensional mass cytometry data. Methods 82, 55–63 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM, Hao Y, Stoeckius M, Smibert P, Satija R, Comprehensive integration of single-cell data. Cell 177, 1888–1902.e21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, Lee MJ, Wilk AJ, Darby C, Zager M, Hoffman P, Stoeckius M, Papalexi E, Mimitou EP, Jain J, Srivastava A, Stuart T, Fleming LM, Yeung B, Rogers AJ, McElrath JM, Blish CA, Gottardo R, Smibert P, Satija R, Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoeckius M, Zheng S, Houck-Loomis B, Hao S, Yeung BZ, Mauck WM, Smibert P, Satija R, Satija R, Cell hashing with barcoded antibodies enables multiplexing and doublet detection for single cell genomics. Genome Biol. 19, 224 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tirosh I, Izar B, Prakadan SM, Wadsworth MH, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, Fallahi-Sichani M, Dutton-Regester K, Lin J-R, Cohen O, Shah P, Lu D, Genshaft AS, Hughes TK, Ziegler CGK, Kazer SW, Gaillard A, Kolb KE, Villani A-C, Johannessen CM, Andreev AY, Van Allen EM, Bertagnolli M, Sorger PK, Sullivan RJ, Flaherty KT, Frederick DT, Jané-Valbuena J, Yoon CH, Rozenblatt-Rosen O, Shalek AK, Regev A, Garraway LA, Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mereu E, Lafzi A, Moutinho C, Ziegenhain C, McCarthy DJ, Álvarez-Varela A, Batlle E, Sagar DG, Lau JK, Boutet SC, Sanada C, Ooi A, Jones RC, Kaihara K, Brampton C, Talaga Y, Sasagawa Y, Tanaka K, Hayashi T, Braeuning C, Fischer C, Sauer S, Trefzer T, Conrad C, Adiconis X, Nguyen LT, Regev A, Levin JZ, Parekh S, Janjic A, Wange LE, Bagnoli JW, Enard W, Gut M, Sandberg R, Nikaido I, Gut I, Stegle O, Heyn H, Benchmarking single-cell RNA-sequencing protocols for cell atlas projects. Nat. Biotechnol 38, 747–755 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.