Abstract
Background & objectives:
Rabbit model is commonly used to demonstrate the proof of concept in cartilage tissue engineering. However, limited studies have attempted to find an ideal source of rabbit mesenchymal stem cells (MSCs) for cartilage repair. This study aimed to compare the in vitro chondrogenic potential of rabbit MSCs isolated from three sources namely infrapatellar fat pad (IFP), periosteum (P) and bone marrow (BM).
Methods:
Rabbit MSCs from three sources were isolated and characterized using flow cytometry and multi-lineage differentiation assay. Cell proliferation was assessed using trypan blue dye exclusion test; in vitro chondrogenic potential was evaluated by histology and gene expression and the outcomes were compared amongst the three MSC sources.
Results:
MSCs from three sources shared similar morphology and expressed >99 per cent positive for CD44 and CD81 and <3 per cent positive for negative markers CD34, CD90 and human leukocyte antigen – DR isotype (HLA-DR). The BM-MSCs and IFP-MSCs showed significantly higher cell proliferation (P<0.001) than the P-MSCs from passage 4. Histologically, BM-MSCs formed a thicker cartilage pellet (P<0.01) with abundant matrix deposition than IFP and P-MSCs during chondrogenic differentiation. The collagen type 2 staining was significantly (P<0.05) higher in BM-MSCs than the other two sources. These outcomes were further confirmed by gene expression, where the BM-MSCs demonstrated significantly higher expression (P<0.01) of cartilage-specific markers (COL2A1, SOX9 and ACAN) with less hypertrophy.
Interpretation & conclusions:
This study demonstrated that BM-MSCs had superior chondrogenic potential and generated better cartilage than IFP and P-MSCs in rabbits. Thus, BM-MSCs remain a promising candidate for rabbit articular cartilage regeneration.
Keywords: Bone marrow, chondrogenic differentiation, collagen type 2, infrapatellar fat pad, mesenchymal stem cells, MSC markers, periosteum, rabbit
Articular cartilage has low regeneration potential due to its avascular nature and dense extracellular matrix content, which impedes the recruitment of sparsely populated chondrocyte to the site of defect1. Peterson’s group seminally demonstrated the articular cartilage regeneration using culture-expanded autologous chondrocyte transplantation2. In the last two decades, this tissue engineering approach for cartilage defects has demonstrated a significant improvement in joint function and alleviated pain2. However, this procedure requires harvest of chondrocytes from an autologous source. The availability of the donor articular cartilage is limited in adult humans and causes local morbidity. Another limitation is the in vitro expansion of chondrocytes leading to fibroblastic de-differentiation. Upon transplantation, the dedifferentiated cells form mechanically inferior fibrocartilage3. These shortcomings necessitate finding an alternative source for the chondrocyte.
Mesenchymal stem cells (MSCs) have the ability to multipotent differentiation, self-renewal and easy expandability, storage and off-shelf accessibility making them an ideal source for cell therapy4. The property of immune evasion, immunosuppression and their ability to secrete various therapeutic factors add to their potential utility in articular cartilage tissue regeneration5.
MSCs can be sourced from various tissues such as bone marrow (BM), fat, periosteum, synovium and peripheral blood6. All MSCs were previously considered same due to their similar morphological appearance and the expression of common surface markers. However, their multipotent ability is different for different sources, for instance, MSCs isolated from the adipose tissue are more committed towards adipogenic lineage and displayed poor chondrogenic differentiation7. Therefore, it is essential to find an ideal source of MSCs which delivers high chondrogenic potential and use it for cartilage tissue regeneration.
BM-MSCs serve as a gold standard for cartilage tissue engineering due to its chondrogenic and proliferative potential, and nearly 50 per cent of the reported clinical studies are using BM-MSCs for the cartilage repair8. However, due to its age-related decline in quantity, alternative sources are investigated. Studies have shown that infrapatellar fat pad (IFP) tissue is a rich source of MSC with high chondrogenic potential9. During embryonic development, IFP, periosteum, BM-MSCs and articular cartilage are derived from the common progenitor cells and therefore it is pertinent to consider for cartilage repair. The periosteal MSCs are the main source of chondrocyte during fracture healing. The cambium layer of periosteum contains abundant stem cells, and it exhibits age-independent proliferation and high chondrogenic differentiation potential10.
Although the rabbit model is being used extensively for studying articular cartilage regeneration, only limited studies have been performed comparing the chondrogenic potential of its MSC sources11. The aim of this study was to evaluate the chondrogenic potential of rabbit MSCs derived from easily harvestable sources around the knee such as IFP and periosteum and compare the outcome with the standard of care source, i.e. BM-MSCs.
Material & Methods
Sample collection: The study was approved by the Institutional Review Board, and the Institutional Animal Ethics Committee approved the use of animals for the study. This study was carried out between November 2013 and October 2016 (three years) at Centre for Stem Cell Research, Christian Medical College, Vellore, Tamil Nadu, India. Animal procedures were performed in accordance with the guidelines of the Committee for the purpose of control and supervision of experiments on animals, Ministry of Environment and Forest, Government of India. Three New Zealand white male rabbits (aged eight months and 3-3.5 kg in weight) were euthanized using an overdose of anaesthesia. Animals were prepared for the harvest by cleaning the operation sites using a 10 per cent (W/V) povidone-iodine solution (3M India Limited, Pune). Three to five millilitres of BM was aspirated from the iliac crest and collected in a tube containing 1 ml of heparin (Gland Pharma Limited, Telangana). The IFP was harvested from the knees and the periosteum was harvested from the femur and tibia. Samples were collected in a 50 ml centrifuge tube containing alpha - minimum essential medium (α-MEM). All three tissues were transferred to the laboratory and processed within two hours from the time of collection. Reagents used in this study were obtained from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise specified.
Isolation and culture of mesenchymal stem cells from harvested tissues: Collected BM was re-suspended in phosphate-buffered saline (PBS) at a ratio of 1:2 and carefully layered upon Ficoll-Paque (GE Healthcare Life Sciences, NJ, USA) in a 15 ml centrifuge tube. The tube was centrifuged at 400 g for 30 min and the buffy coat containing mononuclear cells was collected and transferred to a 50 ml centrifuge tube. The buffy coat was diluted in 20 ml PBS and centrifuged at 700 g for 10 min. After two washes, supernatant was discarded; final cell pellet was re-suspended in MSC culture medium [α-MEM supplemented with 10 per cent foetal bovine serum (FBS), 5 ng/ml fibroblast growth factor-(FGF)-2 (PeproTech, Rocky Hill, NJ, USA), 50 μg/ml gentamicin and 2 μg/ml amphotericin B] and plated in a 25 cm2 tissue culture flask.
The periosteum and the IFP samples were washed twice with PBS containing gentamicin (100 μg/ml) and amphotericin B (4 μg/ml) and then minced into fine pieces. The periosteum was incubated with 2 mg/ml of collagenase (Worthington Biochemical Corporation, Freehold, NJ, USA) for three hours and the IFP was incubated with 1 mg/ml collagenase for eight hours at 37°C for digestion. After incubation, undigested debris was removed by filter through a 100 μm cell strainer (Corning, Durham, NC, USA) and the filtrate was centrifuged at 2000 rpm for ten minutes. After discarding the supernatant, cell pellets were re-suspended and cultured in 25 cm2 tissue culture flasks containing MSC culture medium.
Cultures were maintained at a 37°C incubator with five per cent CO2 atmosphere. Three days after initiation of culture, the non-adhered cells were removed by PBS wash and the fresh medium was added, and after that, the medium was replenished every 3-4 days.
Characterization: Cells isolated from three sources were characterized using rabbit-specific MSC markers to confirm their phenotype. Third passage cells (n=3) were used for the characterization experiment. Rabbit MSC-positive markers CD44 and CD81 and negative markers such as CD34, CD90 and HLA-DR were used for analysis. Antibodies were selected to have reactivity/cross-reaction with rabbit species. Except for CD44 (AbD Serotec, Oxford, UK), all other antibodies were obtained from Becton–Dickinson (BD) Biosciences (BD Biosciences, San Jose, CA, USA). Non-specific mouse immunoglobulin G antibody was used as an isotype control. The number of cells stained positive and their fluorescent intensity was measured using BD FACS Calibur™ (BD Biosciences, San Jose, CA, USA). The results were analyzed using BD CellQuest™ software version 6.0 (BD Biosciences, San Jose, CA, USA).
Cell proliferation assay: MSCs from all three sources were seeded in six well plates at a density of 3000 cells/cm2 and cultured with MSC culture medium. When the cultures reached 90 per cent confluence, cells were trypsinized using 0.05 per cent Trypsin-EDTA and then passaged at a seeding density of 3000 cells/cm2. Cells were taken to passage six and the cell yield was quantitated at the end of each passage using a Neubauer chamber. This experiment was performed in triplicates.
Multi-lineage differentiation assay: The multipotent ability of MSC isolated from three sources was confirmed by differentiating them into chondrogenic, osteogenic and adipogenic lineage. In all three sources, the differentiation assay was initiated using third passaged MSCs with an identical number of cells. For osteogenic and adipogenic lineage, 1.5×104 cells/well seeded in 24 well plate and chondrogenic lineage; 1.5×105 cells/well in 96 well plate was used. After 24 h culture, the MSC culture medium was replaced with chondrogenic differentiation medium (Dulbecco’s modified Eagle’s medium-high glucose +1X insulin–transferrin–selenium+50 μg/ml ascorbic acid+100 nM dexamethasone+40 μg/ml L-proline), osteogenic (α-MEM+10% FBS+10 nM Dexamethasone+10 mM β-glycerophosphate+ 50 μg/ml ascorbic acid) and adipogenic induction medium (α-MEM+10% FBS+10 μM isobutyl-methylxanthine+100 μM indomethacin+1 μM dexamethasone+10 μg/ml insulin), respectively. Differentiation mediums were replenished every three days once for four weeks. After differentiation, cells were fixed with four per cent paraformaldehyde for 15 min and then 500 μl of Alizarin Red and Oil O Red stains were added on osteogenic and adipogenic differentiated cells, respectively, and incubated for 10 min at room temperature12. Subsequently, cells were washed twice with distilled water to remove the excess stains and observed under the Leica DM1000 light microscope (Leica Microsystems, Wetzlar, Germany).
The percentage of area stained with Oil O Red dye was quantitated using ImageJ software version 1.52a (National Institute of Health, USA). The quantity of Alizarin Red bound with mineralized matrix was estimated by the cetylpyridinium chloride extraction method at 550 nm absorbance using a microplate reader.
After four weeks of chondrogenic differentiation, cartilage pellet derived from three sources were photographed and then the size of the pellet was measured using ImageJ software. Later, the pellet was fixed with 4 per cent paraformaldehyde for 24 h at room temperature. After routine histological processing of pellet, 5 μM tissue sections were prepared from each sample using Leica Biosystems RM2245 Semi-Automated Rotary Microtome (Leica Microsystems, Wetzlar, Germany) and stained with Safranin O dye to visualize the deposition of glycosaminoglycan (GAG) in the pellet11,13. A Leica DM1000 light microscope was used to observe the staining, and the results were documented using Leica MC170 HD camera (Leica Microsystems, Wetzlar, Germany).
Quantitation of cartilage-specific markers: After chondrogenic differentiation of all three MSC sources, the total RNA was isolated as per the protocol mentioned in the TRI reagent datasheet. The total RNA was reverse transcribed into cDNA using PrimeScript™ RT Reagent Kit (Takara, Otsu, Japan). Expression of cartilage-specific genes was quantitated by QuantStudio™ 12K Flex Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA) using PrimeScript RT-PCR Kit (Takara, Otsu, Japan). Rabbit primers used in this assay were designed using Primer-BLAST software and the details are mentioned in Supplementary Table I. The housekeeping gene glyceraldehyde-phosphate-dehydrogenase was used to normalize the expression of the target gene. The relative gene expression was calculated using the ΔΔCt method and the outcome was represented in fold change14.
Supplementary Table SI.
Rabbit primers used to study the expression of cartilage-specific genes
| Gene | Sequence (5’->3’) | Amplicon size | |
|---|---|---|---|
|
| |||
| Forward primer | Reverse primer | ||
| COL2A1 | ACCGCAAGGATTTCAAGGCA | AGGTTTTCCAGCTTCGCCAT | 124 |
| SOX9 | GAAGCTCTGGAGACTGCTGAA | CCCATTCTTCACCGACTTCCT | 136 |
| ACAN | GTTACCGTCACTTCCCCGAC | AGTCCTGAGCGTTGTTGTTGA | 129 |
| COL10A1 | CTTTCCGGGATGCCTCTTGT | TACCGCTGGGTAAGCTTTGG | 96 |
| GAPDH | ATTTGAAGGGCGGAGCCAAA | AGGATGCGTTGCTGACAATCT | 123 |
Immunohistochemistry: The deposition of collagen type 2 (a cartilage-specific marker) during chondrogenic differentiation was confirmed by immunohistochemistry (IHC). Briefly, antigen was retrieved by incubating the sections with hyaluronidase (3 mg/ml) for one hour at 37°C. After blocking the endogenous peroxidase (3% H2O2 for 20 min) and non-specific antigen binding (10% FBS for one hour), sections were incubated with primary antibody (DSHB, Iowa, USA, clone: II-II6B3, 1:5 dilution) for one hour at 37°C. Subsequently, sections were incubated with horseradish peroxidase-conjugated secondary antibody for one hour at 37°C. The EnVision™ FLEX DAB+Chromogen and Substrate Buffer (Dako, Glostrup, Denmark) were used to visualize the antigen. Finally, sections were counterstained with haematoxylin and observed under a Leica DM1000 light microscope (Leica Microsystems, Wetzlar, Germany). The percentage of staining on cartilage pellets was quantitated using ImageJ software with the IHC profiler plugin.
Statistical analysis: The data were analyzed using GraphPad Prism software version 6 (GraphPad Prism, La Jolla, CA, USA), and the outcomes were expressed in mean ± standard deviation. Two-way analysis of variance (ANOVA) with post hoc analysis using Tukey’s multiple comparison test (because the data met the assumption of normal distribution evaluated using the Kolmogorov–Smirnov test) was applied for the cell proliferation data. One-way ANOVA with the post hoc analysis using Tukey’s multiple comparison test was used to assess the statistical significance for the intensity of staining and gene expression assay. The calculated P value below 0.05 was considered significant.
Results
Isolation of mesenchymal stem cell (MSC) from three sources: The average time taken by the cells to reach confluence was 14.66±2.08 days for BM-MSCs, 5.66±1.54 days for P-MSCs and 6.33±1.52 days for IFP-MSCs (Table I). The mean cell yield from the primary culture was 1.32×106 (BM-MSCs), 1.43×106 (IFP-MSCs) and 2.35×106 (P-MSCs) (Table II). As shown in Fig. 1, MSCs from all three sources were plastic adherent, with fibroblast-like morphology.
Table I.
Time taken for mesenchymal stromal cells from three sources to achieve confluence
| Tissue source | Time taken for primary cells to reach confluence (days) | |||
|---|---|---|---|---|
|
| ||||
| Sample 1 | Sample 2 | Sample 3 | Mean±SD | |
| BM | 14 | 13 | 17 | 14.66±2.08 |
| IFP | 5 | 5 | 7 | 5.66±1.15 |
| P | 6 | 8 | 5 | 6.33±1.52 |
SD, standard deviation; BM, bone marrow, IFP, infrapatellar fat pad; P, periosteum
Table II.
Number of cells isolated from three tissue sources (at the end of primary culture)
| Tissue source | Cell yield from the primary culture (×106 cells) | |||
|---|---|---|---|---|
|
| ||||
| Sample 1 | Sample 2 | Sample 3 | Mean±SD | |
| BM | 1.66 | 1.36 | 0.95 | 1.32±0.35 |
| IFP | 1.89 | 1.68 | 0.743 | 1.43±0.61 |
| P | 2.535 | 2.46 | 2.06 | 2.35±0.25 |
Fig. 1.
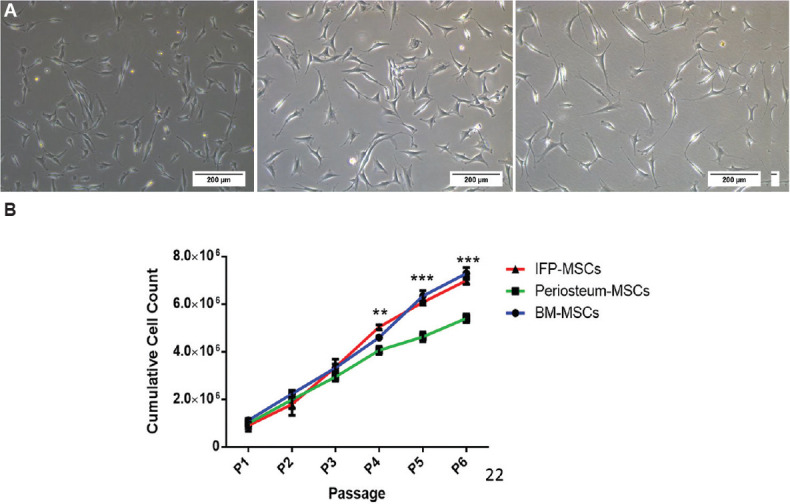
Morphology and proliferation potential of mesenchymal stem cells (MSCs) from three tissues. (A) Phase-contrast microscopic images (×10 magnification) of bone marrow (BM)- MSCs, infrapatellar fat pad (IFP)- MSCs and periosteum (P) - MSCs at day one after the first passage showing similar multipolar elongated fibroblastic morphology (scale bar=200 μM). (B) Comparison of cell proliferation potential of MSCs indicates the IFP and BM-MSCs had a significantly higher proliferation rate. P **<0.01; ***<0.001.
Cell proliferation: The MSCs from three sources were cultured at a density of 3000 cells/cm2 from the first passage to the sixth passage. At the end of each passage, cell yield was quantitated and a similar growth trend was found until the third passage. Compared to P-MSCs (4.05×106), the cell proliferation of BM-MSCs (4.59×106, P=ns) and IFP-MSCs (5.03×106, P<0.001) was higher at the fourth passage (Fig. 1). At the end of the sixth passage, the cell proliferation of BM-MSCs and IFP-MSCs was 7.29×106 and 6.98×106, respectively, were significantly (P<0.001) higher compared to P-MSCs (5.4×106) (Supplementary Table II).
Supplementary Table SII.
Proliferation potential of BM- MSCs, IFP- MSCs and periosteum (P)- MSCs
| Cell yield at the end of each passage (×106 cells) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Passage | BM (B) | IFP (I) | ||||||
|
|
|
|||||||
| Sample 1 | Sample 2 | Sample 3 | Mean±SD | Sample 1 | Sample 2 | Sample 3 | Mean±SD | |
| Passage 1 | 1.042 | 1.188 | 1.118 | 1.116±0.073 | 0.635 | 1.076 | 0.975 | 0.895±0.231 |
| Passage 2 | 2.083 | 2.377 | 2.240 | 2.233±0.147 | 1.270 | 2.153 | 1.950 | 1.791±0.462 |
| Passage 3 | 3.137 | 3.427 | 3.404 | 3.322±0.161 | 2.965 | 3.634 | 3.443 | 3.347±0.344 |
| Passage 4 | 4.607 | 4.645 | 4.544 | 4.599±0.051 | 4.938 | 5.130 | 5.051 | 5.040±0.097 |
| Passage 5 | 6.426 | 6.505 | 6.070 | 6.334±0.232 | 6.070 | 6.146 | 6.004 | 6.073±0.071 |
| Passage 6 | 7.517 | 7.330 | 7.030 | 7.292±0.246 | 7.120 | 7.013 | 6.829 | 6.987±0.147 |
|
| ||||||||
| Passage | P | Statistics (P) | ||||||
|
| ||||||||
| Sample 1 | Sample 2 | Sample 3 | Mean±SD | |||||
|
| ||||||||
| Passage 1 | 0.772 | 1.110 | 1.095 | 0.992±0.190 | P (NS) | |||
| Passage 2 | 1.545 | 2.220 | 2.190 | 1.985±0.381 | P (NS) | |||
| Passage 3 | 2.816 | 3.157 | 2.861 | 2.945±0.185 | P (NS) | |||
| Passage 4 | 4.050 | 4.230 | 3.885 | 4.055±0.172 | B versus P (P<0.05) | |||
| B versus I (P=NS) | ||||||||
| I versus P (P<0.001) | ||||||||
| Passage 5 | 4.728 | 4.755 | 4.387 | 4.623±0.205 | B versus P (P<0.001) | |||
| B versus I (P=NS) | ||||||||
| I versus P (P<0.001) | ||||||||
| Passage 6 | 5.508 | 5.512 | 5.197 | 5.406±0.180 | B versus P (P<0.001) | |||
| B versus I (P=NS) | ||||||||
| I versus P (P<0.001) | ||||||||
SD, standard deviation; BM, bone marrow, IFP, infrapatellar fat pad; P, periosteum
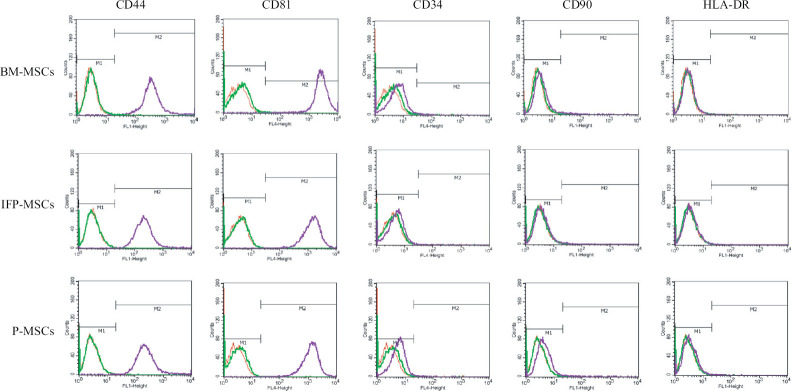
Rabbit mesenchymal stem (MSC) surface marker expression analysis: The cells isolated from all three sources were >99 per cent positive for CD44 and CD81 markers and stained negative (<3%) for CD34, CD90 and HLA-DR (Fig. 2).
Fig. 2.
The histograms representing the expression of rabbit - MSCs surface markers in the cells from three sources shows >99 per cent positive for CD44 and CD81 and <3 per cent positive for negative markers (CD34, CD90 and HLA-DR). Note that CD90, a human - MSCs marker, was not expressed in rabbit - MSCs. In the histogram, red colour peak indicates unstained cells, green indicates isotype controls and purple indicates the surface marker.
Multi-lineage differentiation assay:
Adipogenic differentiation: After 28 days of adipogenic differentiation, all three MSC samples produced lipid droplets and stained with Oil Red O staining. The percentage of the stained area calculated using ImageJ software showed BM-MSCs (15.88%) and IFP-MSCs (15.18%) produced significantly (P<0.01) more lipids compared to P-MSCs (4.3%) (Fig. 3).
Fig. 3.
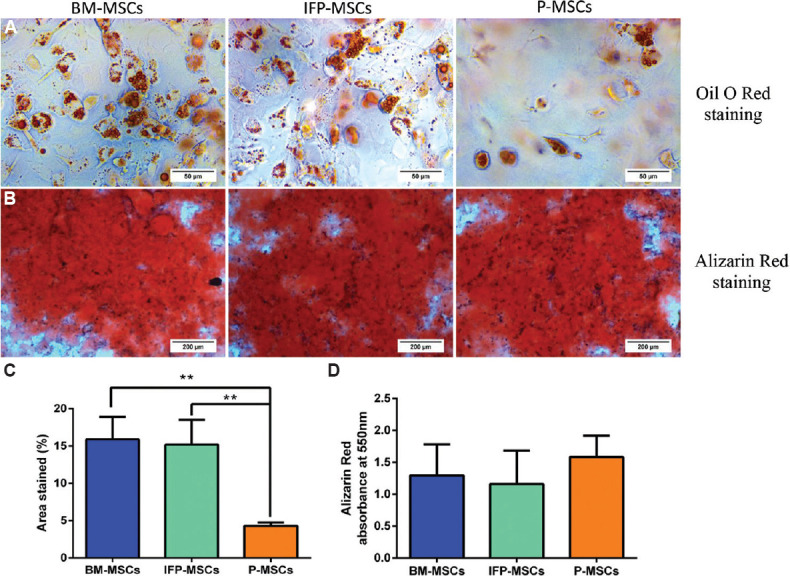
Adipogenic and osteogenic differentiation potential of MSCs from three sources. (A) Oil O Red staining showed lipid droplet formation in BM- MSCs, infrapatellar fat pad- MSCs and P- MSCs. (B) Light microscopic images showed the calcium deposits stained with Alizarin Red staining. (C) Percentage of area stained with Oil Red O indicates BM- MSCs and infrapatellar fat pad- MSCs formed significantly higher lipid droplets than P- MSCs. P **<0.01. (D) Quantification of Alizarin Red staining indicates MSCs from all three tissues deposited similar amount of calcium with no significant difference. MSC, mesenchymal stem cells.
Osteogenic differentiation: After osteogenic differentiation, Alizarin Red staining was performed to stain the calcium deposits. The MSCs from three sources showed a similar intensity of Alizarin Red staining with no significant difference between sources (Fig. 3).
Chondrogenic differentiation: After four weeks of differentiation, the cross-sectional measurement showed cartilage pellets produced from the BM-MSCs (1.159 mm) were bigger than IFP-MSCs (0.86 mm) and significantly (P<0.01) larger compared to P-MSCs (0.68 mm) (Fig. 4). During differentiation, the size of cartilage pellet increases due to the deposition of the extracellular matrix, therefore, the histological staining for GAG and collagen type 2 immunostaining were performed to confirm the outcome. Safranin O staining demonstrated the profound deposition of GAG in the BM-MSCs derived cartilage compared to IFP-MSCs and P-MSCs (Fig. 4).
Fig. 4.
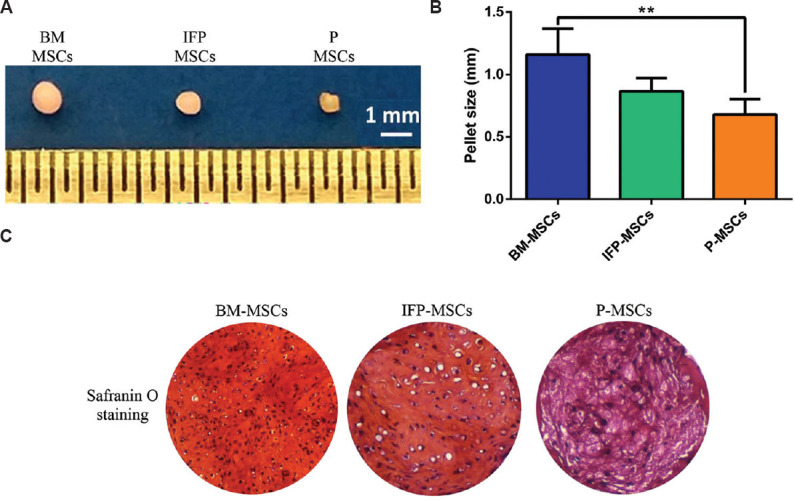
Chondrogenic differentiation of MSCs from three sources. (A) The photograph represents the gross appearance of cartilage pellets derived from three MSC sources with a scale (1-cm markings) showing the BM- MSCs generated the largest size pellet. (B) Cross-sectional measurement confirmed that BM- MSCs had a significantly thicker cartilage than P- MSCs. (C) Light microscopic images (×10) illustrate Safranin O staining of cartilage pellets showed profuse matrix deposition in BM- MSCs compared to IFP-P-MSCs. P **<0.01. IFP-P-MSCs, infrapatellar fat pad and periosteum- MSCs.
Collagen type 2 immunostaining illustrated that the percentage of positive staining was significantly higher in the cartilage pellet derived from BM-MSCs than the other two sources. Whereas, IFP-MSCs also showed a significantly high positive staining than the periosteum MSCs (Fig. 5). These outcomes indicate that MSCs from BM have higher chondrogenic potential compared to IFP-MSCs and P-MSCs.
Fig. 5.
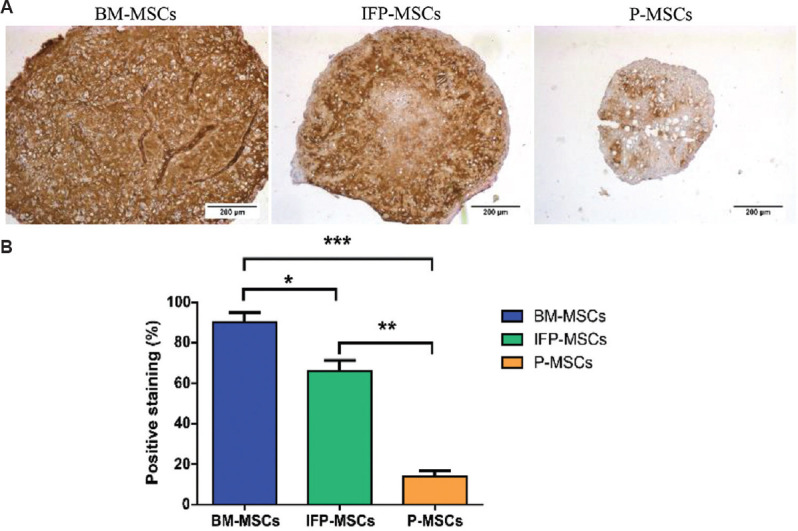
Collagen type 2 immunostaining on the cartilage pellets. (A) Light microscopic images indicate cartilage pellet derived from bone marrow (BM)- MSCs deposited more collagen type 2 as seen with high intensity of collagen type 2 staining in BM- MSCs compared to the other two tissue sources. (B) Quantification of collagen type 2 staining using the immunohistochemistry profiler confirmed that the percentage of staining was higher in BM- MSCs than IFP- MSCs and P- MSCs. P *<0.05, **<0.01, ***<0.001.
Real-time polymerase chain reaction (PCR): Gene expression for COL2A1 and ACAN was significantly higher (P<0.001) in BM-MSCs compared to IFP-MSCs and P-MSCs (Fig. 6). The expression of SOX9 (the cartilage master transcription factor) was found significantly higher by 2.33 folds in BM-MSCs and 2.30 folds in IFP-MSCs than the P-MSCs. The hypertrophic cartilage marker, COL10A1, was significantly upregulated in P-MSCs, 6.27 folds higher than BM-MSCs and four folds higher than IFP-MSCs. The quantitative data confirmed that the BM-MSCs have significantly higher chondrogenic potential and less hypertrophic marker expression compared to MSCs from IFP and periosteum sources.
Fig. 6.
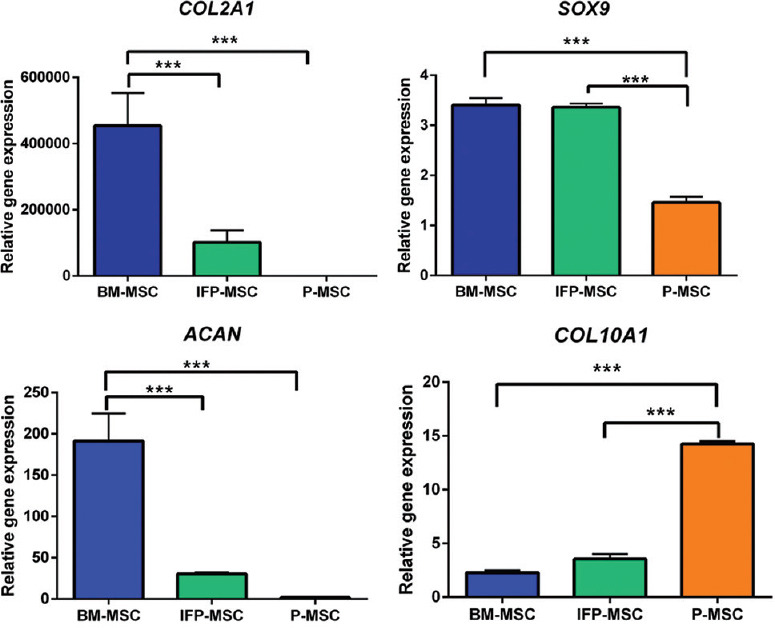
Quantitative analysis of cartilage-specific genes (COL2A1, SOX9 and ACAN) and hypertrophic chondrocyte gene (COL10A1) expressions in the chondrocyte pellet derived from three MSC sources. The BM- MSCs showed significantly higher expressions of COL2A1, SOX9 and ACAN expression with less hypertrophic differentiation (COL10A1), collectively the results in Figs 4 and 5 indicate that BM- MSCs have the best chondrogenic potential. P ***<0.001.
Discussion
MSCs are widely considered for cartilage tissue engineering as these are plentiful, easy to isolate and have a multipotent differentiation potential5. These are especially relevant after 40 yr of age, where the autologous chondrocytes are less available and exhibit poor regenerative capacity13. Wakitani et al15 first demonstrated the regeneration of a full-thickness articular cartilage defect in 12 patients using autologous BM-MSC, and the histological score was improved in the treatment group. Around 46 clinical studies have been reported using MSCs as cell sources for the cartilage repair and the outcomes showed varied success mainly because of using different sources of MSCs8.
In this study, we compared three tissue sources for the MSCs harvest: BM from the iliac crest, periosteum from the tibial surface and IFP from the knee. These are easily accessible to the orthopaedic surgeon and can be harvested with minimum morbidity during the time of arthroscopy. A direct comparison of these three selected sources is not reported previously. Two studies (human and rat) comparing the five different musculoskeletal sources of MSCs (adipose, BM, muscle, periosteum and synovium) showed the synovium, BM and periosteum to have the best chondrogenic potential in that order12,16. Although synovial MSCs are reported to have high chondrogenic potential, this was not used as a comparison in this study because of its poor chondrogenic potential in degenerative conditions17 and involvement in inflammation and hypertrophy18. Furthermore, Baboolal et al19 reported that the synovial fluid from OA joint reduces the binding capacity of synovium MSCs. This may negatively affect the regeneration potential of synovium MSCs since the early attachment of transplanted cells is vital for its survival, growth and differentiation20. The embryonic development similarity to cartilage (mesoderm lineage) dictated the choice of periosteum and the IFP, the two sources, for comparison. IFP being extra-synovial would be less affected by the degenerative process in osteoarthritis and its morpho-functional similarity to synovium which has otherwise shown good chondrogenesis21.
While comparing the proliferation potential between the three selected sources, we found the BM- and IFP-MSCs to have significantly higher proliferation potential compared to periosteal MSCs. In literature, a direct comparison of these three sources has not been performed and the studies compared periosteal MSCs with BM-MSCs have shown periosteal MSCs had high proliferative potential than BM-MSCs12,16,22. A similar study comparing IFP-MSCs with BM also showed BM-MSCs had a poor proliferation potential22. This may be directly linked to the culture condition that influences cell proliferation. In this study, MSCs from all three sources were cultured in α-MEM media supplemented with FGF-2 (5 ng/ml), to improve the self-renewal potential of MSCs23. Bianchi et al23 have shown that the addition of FGF-2 to the culture increases the proliferation (21 folds) of BM-MSCs compared to the control. Whereas, BM-MSCs cultured without FGF-2 undergoes cellular senescence as early as the second passage24. The same is not true for IFP-MSCs and P-MSCs. Previous studies have attempted to identify an optimal MSC source without the addition of FGF-2 and this could be one of the reasons behind their lower proliferation rate12,16,22. In this study, we reported an optimized protocol for culturing rabbit BM-MSC using FGF-2 with a good outcome. It is noteworthy that FGF2 is being used in the clinical trials for the expansion of stem cells.
In this study, BM-MSCs were isolated using Ficoll-Paque, a widely adopted density gradient centrifugation method25. Other techniques of MSC isolation include direct plating of whole BM or RBC lysis method. During our initial standardization, we found Ficoll-Paque provided more colonies than the RBC lysis method (data not shown), hence this was used for the rest of the study. Although we did not test the direct seeding of BM culture method, literature suggests that the direct plating of rabbit whole BM delivered more cell yield than other techniques25. Similarly, MSCs from solid tissues can be isolated either by enzymatic or explant culture methods. In this study, enzymatic method for the isolation of P-MSCs and IFP-MSCs was employed from their sources similar to previous studies26.
The classical way to characterize the MSCs is the evaluation of surface marker expression that reflects the proliferative and differentiation ability of the MSCs; it is a useful guide to the suitability of the cells for transplantation. Nevertheless, there is no single surface marker known to be specifically expressed in MSCs alone. In 2006, Dominici et al27 delineated the criteria to define MSCs, the human MSCs should express >95 per cent positive for MSC markers (CD105, CD73 and CD90) and <2 per cent positive for haematopoietic markers (CD45, CD34, CD14 or CD19 and HLA-DR) identified by flow cytometry. In rabbits, the presence of cell surface markers and its percentage is different from humans28. In this study, we found the CD81 and CD44 markers expressed in all three rabbit MSCs but were negative for CD34 and HLA-DR markers. Except CD90, the expression of all other surface markers has been reported earlier. Martinez-Lorenzo et al29 showed rabbit BM-MSCs expressed 98 per cent positive for CD90 marker. However, a review from Lee et al28 indicated that the expression of CD90 markers in rabbit MSCs is inconsistent and it is an inconclusive marker for rabbit MSCs.
In the present study, the histological analysis of chondrogenic, osteogenic and adipogenic lineage differentiation showed that adipogenic lineage was better in the BM and infrapatellar fat. Periosteum MSCs showed poor adipogenic differentiation potential, which is in agreement with the previous study12. Osteogenic lineage differentiation was equal in all three sources. The adipogenic and osteogenic differentiation was mainly performed to demonstrate the multipotent ability of MSCs, and therefore, it was not quantitated using quantitative PCR.
This study shows that histologically BM-MSCs had the best outcome for chondrogenic differentiation regarding the size of the pellet, quality of extracellular matrix and collagen type 2 deposition. A significant increase in collagen and aggrecan gene expression supported this finding. Previous publications in contrast, have reported a high chondrogenic potential of IFP-MSCs compared to BM-MSC, but their results showed a high expression of cartilage-specific markers and high GAG content (3/6 samples) in BM-MSCs compared to IFP-MSCs9, 22.
Kanawa et al30 showed that the BM-MSCs exhibit an age-dependent decline in the chondrogenic potential when comparing MSCs isolated from the elderly patients with the young adults. In the present study, eight month old rabbits which correspond to 18 yr of human life were used31. Also since articular cartilage defects are usually reported in young adults due to sports injury or osteochondritis dissecans32, hence, the outcome of this study is relevant and applicable for the treatment of a young individual’s cartilage defect. Furthermore, since the allogeneic MSCs are also being used for articular cartilage regeneration, the findings of our study suggests that the BM-MSCs harvested from a younger donor can be considered as these show the best chondrogenic potential.
The cambium layer of periosteum contains multipotent stem cells. Upon skeletal maturity, the cambium layer becomes thinner and loses its chondrogenic potential33. In the rabbit, thinning of the cambium layer was observed as early as in a six month old rabbit33. In this study, the periosteum was harvested from adult rabbits (8 months old) and could be one of the reasons for its poor chondrogenic differentiation. The expression of hypertrophic markers was significantly higher in the P-MSCs than the other two sources. The periosteum is reported to have an intermediate endochondral pathway in fracture repair, and therefore, an expression of hypertrophic markers is expected8. The gene expression outcome, histology and gross measurements confirmed that MSCs derived from BM source are superior in chondrocyte differentiation than other sources, and it is more suitable to use it for cartilage tissue engineering.
The study’s main limitation was that it was carried out in a rabbit model and the findings will have to be validated again in humans for translation. The rabbit was chosen for this study as it is the commonest animal model used for generating proof of concept. Three animals may be considered as too few for this study, however, this sample size was based on the numbers allocated by the Animal Ethics Committee, as in literature, 2-3 animals have been used for similar studies.
In conclusion, we have demonstrated an optimized culture condition for rabbit BM-MSCs and illustrated its high cartilage forming ability over the other two MSCs sources. Considering, the rabbit animal model is predominantly used for cartilage tissue engineering, the study outcome will aid to achieve a better treatment option for the management of cartilage defects using BM-derived MSCs.
Footnotes
Financial support & sponsorship: This work was supported by the Science and Engineering Research Board (SR/SO/HS-190/2012), Department of Science and Technology, Government of India.
Conflicts of Interest: None.
References
- 1.Mobasheri A, Kalamegam G, Musumeci G, Batt ME. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 2014;78:188–98. doi: 10.1016/j.maturitas.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Peterson L, Brittberg M, Kiviranta I, Åkerlund EL, Lindahl A. Autologous chondrocyte transplantation:Biomechanics and long-term durability. Am J Sports Med. 2002;30:2–12. doi: 10.1177/03635465020300011601. [DOI] [PubMed] [Google Scholar]
- 3.Lin L, Zhou C, Wei X, Hou Y, Zhao L, Fu X, et al. Articular cartilage repair using dedifferentiated articular chondrocytes and bone morphogenetic protein 4 in a rabbit model of articular cartilage defects. Arthritis Rheum. 2008;58:1067–75. doi: 10.1002/art.23380. [DOI] [PubMed] [Google Scholar]
- 4.Kot M, Baj-Krzyworzeka M, Szatanek R, Musiał-Wysocka A, Suda-Szczurek M, Majka M. The importance of HLA assessment in “off-the-shelf”allogeneic mesenchymal stem cells based-therapies. Int J Mol Sci. 2019;20:5680. doi: 10.3390/ijms20225680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hached F, Vinatier C, Le Visage C, Gondé H, Guicheux J, Grimandi G, et al. Biomaterial-assisted cell therapy in osteoarthritis:From mesenchymal stem cells to cell encapsulation. Best Pract Res Clin Rheumatol. 2017;31:730–45. doi: 10.1016/j.berh.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Via AG, Frizziero A, Oliva F. Biological properties of mesenchymal stem cells from different sources. Muscles Ligaments Tendons J. 2012;2:154. [PMC free article] [PubMed] [Google Scholar]
- 7.Trujillo NA, Popat KC. Increased adipogenic and decreased chondrogenic differentiation of adipose derived stem cells on nanowire surfaces. Materials (Basel) 2014;7:2605–30. doi: 10.3390/ma7042605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park YB, Ha CW, Rhim JH, Lee HJ. Stem cell therapy for articular cartilage repair:Review of the entity of cell populations used and the result of the clinical application of each entity. Am J Sports Med. 2018;46:2540–52. doi: 10.1177/0363546517729152. [DOI] [PubMed] [Google Scholar]
- 9.Garcia J, Mennan C, McCarthy HS, Roberts S, Richardson JB, Wright KT. Chondrogenic potency analyses of donor-matched chondrocytes and mesenchymal stem cells derived from bone marrow infrapatellar fat pad, and subcutaneous fat. Stem Cells Int. 2016;2016:6969726. doi: 10.1155/2016/6969726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferretti C, Mattioli-Belmonte M. Periosteum derived stem cells for regenerative medicine proposals:Boosting current knowledge. World J Stem Cells. 2014;6:266–77. doi: 10.4252/wjsc.v6.i3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koga H, Muneta T, Nagase T, Nimura A, Ju YJ, Mochizuki T, et al. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis:Suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008;333:207–15. doi: 10.1007/s00441-008-0633-5. [DOI] [PubMed] [Google Scholar]
- 12.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues:Superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–9. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 13.Barbero A, Grogan S, Schäfer D, Heberer M, Mainil-Varlet P, Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage. 2004;12:476–84. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–62. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 17.Nagase T, Muneta T, Ju YJ, Hara K, Morito T, Koga H, et al. Analysis of the chondrogenic potential of human synovial stem cells according to harvest site and culture parameters in knees with medial compartment osteoarthritis. Arthritis Rheum. 2008;58:1389–98. doi: 10.1002/art.23418. [DOI] [PubMed] [Google Scholar]
- 18.Attur M, Samuels J, Krasnokutsky S, Abramson SB. Targeting the synovial tissue for treating osteoarthritis (OA):Where is the evidence? Best Pract Res Clin Rheumatol. 2010;24:71–9. doi: 10.1016/j.berh.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Baboolal TG, Mastbergen SC, Jones E, Calder SJ, Lafeber FP, McGonagle D. Synovial fluid hyaluronan mediates MSC attachment to cartilage, a potential novel mechanism contributing to cartilage repair in osteoarthritis using knee joint distraction. Ann Rheum Dis. 2016;75:908–15. doi: 10.1136/annrheumdis-2014-206847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S, Choi E, Cha MJ, Hwang KC. Cell adhesion and long-term survival of transplanted mesenchymal stem cells:A prerequisite for cell therapy. Oxid Med Cell Longev. 2015;2015:632902. doi: 10.1155/2015/632902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.do Amaral RJ, Almeida HV, Kelly DJ, O'Brien FJ, Kearney CJ. Infrapatellar fat pad stem cells:From developmental biology to cell therapy. Stem Cells Int. 2017;2017:6843727. doi: 10.1155/2017/6843727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sasaki A, Mizuno M, Ozeki N, Katano H, Otabe K, Tsuji K, et al. Canine mesenchymal stem cells from synovium have a higher chondrogenic potential than those from infrapatellar fat pad, adipose tissue, and bone marrow. PLoS One. 2018;13:e0202922. doi: 10.1371/journal.pone.0202922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bianchi G, Banfi A, Mastrogiacomo M, Notaro R, Luzzatto L, Cancedda R, et al. Ex vivo enrichment of mesenchymal cell progenitors by fibroblast growth factor 2. Exp Cell Res. 2003;287:98–105. doi: 10.1016/s0014-4827(03)00138-1. [DOI] [PubMed] [Google Scholar]
- 24.Coutu DL, François M, Galipeau J. Inhibition of cellular senescence by developmentally regulated FGF receptors in mesenchymal stem cells. Blood. 2011;117:6801–12. doi: 10.1182/blood-2010-12-321539. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Zhang F, Shi H, Tan R, Han S, Ye G, et al. Comparisons of rabbit bone marrow mesenchymal stem cell isolation and culture methods in vitro. PLoS One. 2014;9:e88794. doi: 10.1371/journal.pone.0088794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDuffee LA. Comparison of isolation and expansion techniques for equine osteogenic progenitor cells from periosteal tissue. Can J Vet Res. 2012;76:91–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 28.Lee TC, Lee TH, Huang YH, Chang NK, Lin YJ, Chien PW, et al. Comparison of surface markers between human and rabbit mesenchymal stem cells. PLoS One. 2014;9:e111390. doi: 10.1371/journal.pone.0111390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martínez-Lorenzo MJ, Royo-Cañas M, Alegre-Aguarón E, Desportes P, Castiella T, García-Alvarez F, et al. Phenotype and chondrogenic differentiation of mesenchymal cells from adipose tissue of different species. J Orthop Res. 2009;27:1499–507. doi: 10.1002/jor.20898. [DOI] [PubMed] [Google Scholar]
- 30.Kanawa M, Igarashi A, Ronald VS, Higashi Y, Kurihara H, Sugiyama M, et al. Age-dependent decrease in the chondrogenic potential of human bone marrow mesenchymal stromal cells expanded with fibroblast growth factor-2. Cytotherapy. 2013;15:1062–72. doi: 10.1016/j.jcyt.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Dutta S, Sengupta P. Rabbits and men:Relating their ages. J Basic Clin Physiol Pharmacol. 2018;29:427–35. doi: 10.1515/jbcpp-2018-0002. [DOI] [PubMed] [Google Scholar]
- 32.Mithoefer K, Hambly K, Logerstedt D, Ricci M, Silvers H, Villa SD. Current concepts for rehabilitation and return to sport after knee articular cartilage repair in the athlete. J Orthop Sports Phys Ther. 2012;42:254–73. doi: 10.2519/jospt.2012.3665. [DOI] [PubMed] [Google Scholar]
- 33.O'Driscoll SW, Saris DB, Ito Y, Fitzimmons JS. The chondrogenic potential of periosteum decreases with age. J Orthop Res. 2001;19:95–103. doi: 10.1016/S0736-0266(00)00014-0. [DOI] [PubMed] [Google Scholar]