Abstract
Background & objectives:
Evaluation of bone marrow infiltration in lymphoma is usually done by bone marrow biopsy (BMB). This study analyzed the utility of 18F-fluorodeoxyglucose positron emission tomography/computerized tomography (18F-FDG PET/CT) to detect bone marrow involvement (BMI) compared to BMB.
Methods:
Treatment-naïve lymphoma patients underwent both 18F-FDG PET/CT scan and BMB before treatment initiation. BMI detected on PET/CT was compared with BMB.
Results:
The study population consisted of 80 patients and comprised 37 Hodgkin’s lymphoma (HL) patients, 30 aggressive non-HL (NHL) and 13 indolent NHL patients. The majority of the aggressive NHLs were diffuse large B-cell lymphoma (20/30) and major indolent lymphoma was follicular lymphoma (5/13). When compared to BMB, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of focal (±diffuse) marrow FDG uptake on 18F-FDG PET/CT were 100, 61.3, 33.3 and 100 per cent, respectively, for HL; 100, 65.4, 30.8 and 100 per cent, respectively, for aggressive NHL and 75, 80, 85.7 and 66.7 per cent, respectively, for indolent NHL. When comparing marrow involvement on 18F-FDG PET/CT to baseline BMB and/or resolution of bone marrow FDG uptake at interim/end-of-treatment 18F-FDG PET/CT, the sensitivity, specificity, PPV and NPV were 100 per cent each for HL and aggressive NHL and 77.3, 100, 100 and 66.7 per cent, respectively, for indolent NHL.
Interpretation & conclusions:
18F-FDG PET/CT has a good sensitivity and NPV for detecting BMI in HL and aggressive lymphoma. The low specificity and PPV improved if marrow uptake pattern on interim or end-of-treatment 18F-FDG PET/CT scan was analyzed. In patients with HL who are staged with18F-FDG PET/CT at baseline and followed up with an interim/end-of-treatment PET/CT, baseline BMB may be avoided. For all other lymphoma subtypes, BMB may be essential if there is no marrow FDG uptake on PET/CT scan performed at baseline.
Keywords: Bone marrow involvement, 18F-FDG PET/CT, lymphoma, trephine biopsy
Lymphoma is diagnosed by cell morphology and immunophenotyping of the primarily involved tissue. Before starting treatment, the patient is staged with the Ann Arbor staging system for prognostication and treatment planning1. Pre-treatment staging is done by contrast-enhanced computerized tomography (CECT) of the neck, thorax, abdomen, pelvis or combined positron emission tomography/CT (PET/CT) of the whole body. 18F-fluorodeoxyglucose (18F-FDG) PET/CT is the preferred staging modality for FDG-avid lymphoma2. Assessment of bone marrow involvement (BMI) is an essential component in staging which is done with a bone marrow biopsy (BMB) and this is invariably performed, even when the likelihood of involvement is low. BMB is an invasive, painful and time-consuming procedure with a relatively long turnaround time. Considering the high sensitivity of 18F-FDG PET/CT in the detection of marrow involvement, the absolute indication of BMB in some of the histological subtypes has been questioned3.
In Hodgkin lymphoma (HL), given the high sensitivity of 18F-FDG PET/CT in detecting marrow infiltration, BMB is avoided for patients who are staged with 18F-FDG PET/CT4. In diffuse large B-cell lymphoma (DLBCL), PET/CT is more sensitive than BMB, but it may miss low-volume (10 to 20% of the marrow) or diffuse involvement of the marrow and involvement of the marrow by a discordant low-grade lymphoma if present. Thus, marrow involvement on 18F-FDG PET/CT scan is usually sufficient to designate advanced-stage disease, but if the scan is negative, a BMB is indicated3,5,6. Data are inconsistent concerning other aggressive non-HLs (NHLs) and indolent NHL where the utility of 18F-FDG PET/CT in detecting BM involvement is not clear. This study was planned to assess the utility of 18F-FDG PET/CT in detecting bone marrow involvement in all lymphoma subtypes.
Material & Methods
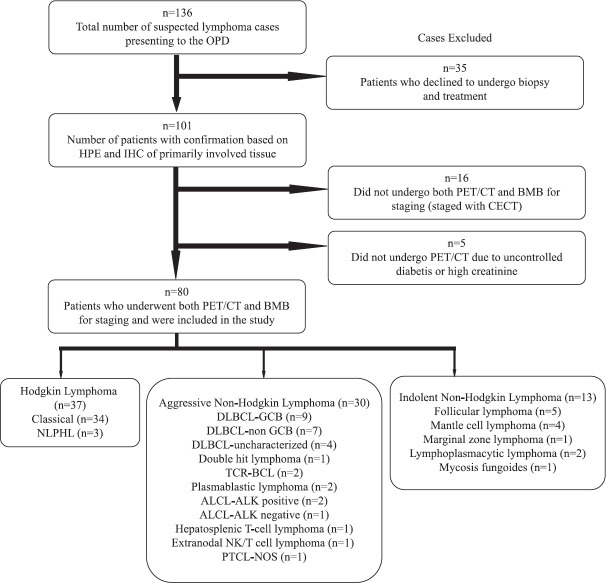
A prospective study was conducted in the department of Hematology, Nil Ratan Sircar Medical College and Hospital, Kolkata, from March 2017 to September 2018. Institutional ethical clearance was obtained before the initiation of the study. Written informed consent was obtained from each patient and/or their legal guardians at the time of enrolment into the study. Newly diagnosed, treatment-naïve patients of all ages with a histological diagnosis of lymphoma were included. The patients underwent staging with 18F-FDG PET/CT and unilateral iliac crest BMB. 18F-FDG PET/CT was performed before BMB, and the maximum time gap between the two procedures was four days. Patients who had received any form of chemotherapy or steroid before the staging, who required immediate cytoreduction due to aggressive presentation before staging was complete, patients in whom 18F-FDG PET/CT was contraindicated and those staged with CECT were excluded. A total of 136 patients were clinically suspected as lymphoma during the study period, of whom 80 patients met the inclusion criteria and were enrolled in the study. The Figure shows the flow diagram of the study showing reasons for exclusion.
Figure.
Consort diagram of the study. ALCL: anaplastic large cell lymphoma; BMB: bone marrow biopsy; DLBCL: diffuse large B-cell lymphoma; GCB: germinal centre B-cell; NLPHL: nodular lymphocyte-predominant Hodgkin lymphoma; PET/CT: positron emission technology/computerized tomography; PTCL-NOS: peripheral T-cell lymphoma not otherwise specific; TCR-BCL: T-cell-rich B-cell lymphoma; CECT, contrast-enhanced computerized tomography; ALK, anaplastic lymphoma kinase.
18F-FDG PET/CT acquisition: All 18F-FDG PET/CT scans were performed using the Siemens Biograph mCT (Erlangen, Germany) PET/CT scanner. Intravenous 18F-FDG was administered at a dose of 10 mCi for adults and 0.21 mCi/kg for children (dose range was 2.73 mCi to 10 mCi or 101 MBq to 370MBq). The radiotracer uptake period was 45-60 min. All patients fasted for six hours before the scan procedure and the fasting blood glucose before the procedure was less than 150 mg/dl. A low-dose whole-body CT scan was performed followed by a PET scan from the base of the skull to mid-thigh, with special acquisition of the brain in all cases. PET images were reconstructed using ordered subset expectation maximization reconstruction algorithms.
18F-FDG PET/CT interpretation (qualitative): FDG uptake more than that of the liver, localized in the marrow, was considered positive. Focal pattern (at least one) with or without diffuse involvement of marrow was considered as positive. Isolated diffuse involvement of the marrow was not considered positive for lymphoma infiltration. 18F-FDG PET/CT marrow involvement was considered as true positive if there was marrow involvement on histology and/or the FDG uptake in the marrow resolved post-therapy as evaluated by interim or end-of-treatment 18F-FDG PET/CT scan. Patients where 18F-FDG PET/CT either showed no marrow involvement or showed diffuse marrow involvement along with a negative BMB were considered as true negative. No marrow uptake on 18F-FDG PET/CT and a positive BMB was a false negative. A negative BMB with a positive focal (±diffuse) lesion on 18F-FDG PET/CT which failed to show resolution on follow up interim or end-of-treatment PET/CT scan (in the event of complete metabolic response of all other nodal or extranodal sites involved by the lymphoma at baseline evaluation) was considered false positive.
18F-FDG PET/CT interpretation (semi-quantitative): The standardized uptake value (SUVmax) normalized for body weight was calculated in patients with positive FDG uptake in marrow from the most intense area. When there was no abnormal FDG uptake in the marrow, reference SUVmax was measured from the posterior superior iliac spine on both sides and the mean of these values was taken for analysis.
Bone marrow biopsy (BMB): The bone marrow procedure was performed after the PET/CT in all patients. Unilateral iliac crest bone marrow aspirate (BMA) and BMB (at least 2 cm length) were obtained under local anaesthesia. The BMB sections were processed and stained with haematoxylin and eosin stain. The pattern of infiltration and cell morphology was assessed in comparison with the morphology of the tissue of primary diagnosis. Immunohistochemistry with an appropriate and adequate panel was used whenever necessary to confirm the marrow involvement.
Statistical analysis: Measures of diagnostic performance of 18F-FDG PET/CT were estimated by sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) along with the corresponding confidence intervals (CI). Differences between groups for the continuous variable were compared using the Mann–Whitney U test and for categorical variables using the Chi-square test and Fisher’s exact test. Statistical analysis was done using Prism version 7.05 (GraphPad Software, San Diego, USA).
Results
The demographic profile of the patients included in the study is shown in Table I. Of the 80 patients included, there were 37 HL cases, of whom majority 34 (91.9%) were classical HL and rest 3 (8.1%) were nodular lymphocytic predominant HL. Among the 43 NHL patients, 30 were aggressive NHL and 13 were indolent NHL. The majority of the aggressive NHLs were of B-cell origin (n=24), and DLBCL (n=20) was the most common histological subtype. Among the indolent NHL, 12 were B-cell lymphoma cases and the common subtypes were follicular lymphoma (5/13) and mantle cell lymphoma (4/13).
Table I.
Demographic details of the patients included in the study (n=80)
| Details of patients | HL, n (%) | Aggressive NHL, n (%) | Indolent NHL, n (%) |
|---|---|---|---|
| Total number of cases | 37 | 30 | 13 |
| Median age (yr) | 23 | 47 | 55 |
| Range (yr) | 3-62 | 7-85 | 40-79 |
| Sex | |||
| Male | 26 (70.3) | 22 (73.3) | 9 (69.2) |
| Female | 11 (29.7) | 8 (26.7) | 4 (30.8) |
| B-symptoms | |||
| Fever | 21 (56.8) | 17 (56.7) | 5 (38.5) |
| Weight loss | 16 (43.2) | 17 (56.7) | 5 (38.5) |
| Night sweats | 18 (48.6) | 13 (43.3) | 5 (38.5) |
| Clinical organ involvement | |||
| Lymphadenopathy | 37 (100) | 23 (76.7) | 11 (84.6) |
| Hepatomegaly | 15 (35.1) | 6 (20) | 3 (23.1) |
| Splenomegaly | 11 (29.7) | 7 (23.3) | 3 (23.1) |
| Site of involvement | |||
| Nodal | 28 (75.7) | 15 (50) | 7 (53.8) |
| Extranodal | 0 | 6 (20) | 1 (7.7) |
| Nodal/extranodal | 9/37 (24.3) | 9/30 (30) | 5/13 (38.5) |
| Ann Arbor stage | |||
| I | 2 (5.4) | 6 (20) | 1 (7.7) |
| II | 9 (24.3) | 1 (3.3) | 1 (7.7) |
| III | 6 (16.2) | 5 (16.7) | 1 (7.7) |
| IV | 20 (54.1) | 18 (60) | 10 (76.9) |
| Cell type | |||
| B-cell | 37 (100) | 24 (80) | 12 (92.3) |
| T-cell | 0 | 5 (16.7) | 1 (7.7) |
| NK/T-cell | 0 | 1 (3.3) | 0 |
HL, Hodgkin lymphoma; NHL, non-HL
18F-FDG PET/CT showed marrow infiltration in 18 patients of HL and 13 and seven of aggressive and indolent NHLs, respectively. This was in contrast with marrow infiltration on BM histology where HL showed infiltration in six and aggressive NHL in four patients. Eight patients with indolent lymphoma showed marrow involvement on histology which was higher as opposed to 18F-FDG PET/CT.
When marrow infiltration on 18F-FDG PET/CT was compared with the BMB, there was 67 per cent concordance between the two modalities in HL compared to 72 per cent in NHL. Indolent NHL showed a higher concordance than aggressive NHL (77 vs. 70%). 18F-FDG PET/CT showed excellent sensitivity and NPV in both HL (LR+ 2.58; 95% CI: 1.66-4.02) and NHL (LR+ 2.58; 95% CI: 1.46-4.57). The specificity and PPV of 18F-FDG PET/CT were low in both the subtypes. Among the NHL subtypes, the sensitivity and NPV (LR+ 2.89; 95% CI: 1.6-4.9) were higher in the aggressive subtype compared to the indolent subtype while the specificity and PPV (LR+ 3.75; 95% CI: 0.62-22.64) were higher in the indolent subtype compared to the aggressive subtype. When 18F-FDG PET/CT was compared with the gold standard BMB, the sensitivity, specificity, PPV and NPV were 100, 61.3, 33.3 and 100 per cent, respectively, for HL; 100, 65.4, 30.8 and 100 per cent, respectively, for aggressive NHL and 75, 80, 85.7 and 66.7 per cent, respectively, for indolent NHL (Table II). In all cases, a follow up 18F-FDG PET/CT scan at the interim or end of treatment was done. In these interim or end-of-treatment scans, if there was a resolution of all nodal or extranodal FDG-avid lesions present at baseline, the marrow FDG uptake was analyzed. This analysis showed that there was a resolution of the FDG-avid marrow lesion seen at baseline in all cases (n=38), suggesting that the FDG avidity was probably due to lymphoma involvement. There were 22 cases where initial BMB showed no lymphoma infiltrate, but PET/CT showed the presence of focal (±diffuse) FDG-avid lesion at baseline which showed complete resolution after treatment of lymphoma. When the presence of marrow involvement on histology at baseline and/or disappearance of FDG uptake on follow up PET/CT scan were taken as the reference standard, the specificity and PPV of PET/CT improved to 100 per cent for all subtypes of lymphoma. However, for indolent lymphoma, the sensitivity (77.3%; 95% CI: 39.9-97.2%) and NPV (66.7%; 95% CI: 37.1-87.2%) remained low (Table III).
Table II.
Analysis of PET/CT in detecting bone marrow infiltration in cases showing marrow involvement in bone marrow biopsy at diagnosis
| Type of lymphoma | BM histology showing lymphoma infiltration | BM status on PET | Percentage (95% CI) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| PET+ | PET− | Sensitivity | Specificity | PPV | NPV | ||
| HL | BMB positive | 6 | 0 | 100 (54.1-100) | 61.3 (42.2-78.2) | 33.3 (24.3-43.8) | 100 |
| BMB negative | 12 | 19 | |||||
| NHL | BMB positive | 10 | 2 | 83.3 (51.6-97.9) | 67.7 (48.6-83.3) | 50 (36.2-63.9) | 91.3 (73.7-97.5) |
| BMB negative | 10 | 21 | |||||
| Aggressive NHL | BMB positive | 4 | 0 | 100 (39.8-100) | 65.4 (44.3-82.8) | 30.8 (20.8-42.9) | 100 |
| BMB negative | 9 | 17 | |||||
| Indolent NHL | BMB positive | 6 | 2 | 75 (34.9-96.8) | 80 (28.4-99.5) | 85.7 (49.8-97.3) | 66.7 (35.8-87.8) |
| BMB negative | 1 | 4 | |||||
PET+ and PET−, PET positive and negative for marrow infiltration by lymphoma. PET-CT, positron emission tomography–computed tomography; PPV, positive predictive value; NPV, negative predictive value; HL, Hodgkin lymphoma; NHL, non-HL; BM, bone marrow; BMB, BM biopsy; CI, confidence interval
Table III.
Analysis of PET/CT in detecting bone marrow infiltration in cases with bone marrow biopsy involvement at diagnosis and/or disappearance of marrow fluorodeoxyglucose uptake post-treatment
| Type of lymphoma | BM infiltration at diagnosis (any modality) | BM status on PET | Percentage (95% CI) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| PET+ | PET- | Sensitivity | Specificity | PPV | NPV | ||
| HL | Positive | 18 | 0 | 100 (81.5-100) | 100 (82.4-100) | 100 | 100 |
| Negative | 0 | 19 | |||||
| NHL | Positive | 20 | 2 | 90.9 (70.9-98.9) | 100 (83.9-100) | 100 | 91.3 (73.7-97.5) |
| Negative | 0 | 21 | |||||
| Aggressive NHL | Positive | 13 | 0 | 100 (75.3-100) | 100 (80.5-100) | 100 | 100 |
| Negative | 0 | 17 | |||||
| Indolent NHL | Positive | 7 | 2 | 77.3 (39.9-97.2) | 100 (39.8-100) | 100 | 66.7 (37.1-87.2) |
| Negative | 0 | 4 | |||||
BMB+ and BMB−, BMB positive and negative for marrow infiltration by lymphoma; PET+ and PET−, PET positive and negative for marrow infiltration by lymphoma. BM, bone marrow; BMB, BM biopsy; PET-CT, positron emission tomography–computed tomography; PPV: positive predictive value; NPV: negative predictive value; HL, Hodgkin lymphoma; NHL: non-HL; CI, confidence interval
There was a significant difference in the mean SUVmax on PET/CT between those who showed marrow infiltration on BMB compared to those who did not 7.49 vs. 3.13; P<0.001).
Discussion
It has been a routine practice to perform BMB for staging in all cases of lymphoma. Most of the studies analyzing the utility of 18F-FDG PET/CT in the evaluation of marrow infiltration are of retrospective design7,8,9. This study has a prospective design, similar to a few other published studies10,11.
In this study, patients were staged simultaneously with both 18F-FDG PET/CT and BMB. The marrow uptake results on 18F-FDG PET/CT were analyzed with respect to the results of marrow histology on BMB. The presence of BMI on BMB alone (approach A) was considered for evaluating sensitivity, specificity, PPV and NPV. Alternatively, several studies10,12,13 have taken data of positive BMB at diagnosis together with the resolution of FDG PET uptake on treatment as determined by follow up PET scan (interim or end of treatment) (approach B) as the standard for calculations of the utility of 18F-FDG PET/CT in detecting marrow infiltration in lymphoma staging.
In HL using approach (A), the sensitivity, specificity, PPV and NPV were 100, 61.3, 33.3 and 100 per cent, respectively, which improved to 100 per cent for each of the four parameters by approach (B). Retrospective analysis with approach (A), showed that 18F-FDG PET/CT had a sensitivity, specificity, PPV and NPV of 93.6 to 99, 56, 53 and 99.4 to 99 per cent, respectively7,11. A meta-analysis showed that 18F-FDG PET/CT had an excellent pooled sensitivity of 94.5 per cent in comparison to a poor pooled sensitivity of 39.4 per cent for BMB in detecting marrow infiltration14. A prospective study, reported a sensitivity and NPV of 100 per cent each. However, the specificity and PPV were 86 and 71 per cent, respectively10. From the data in the present study, a positive 18F-FDG PET/CT marrow involvement in HL may be sufficient for staging if a follow up 18F-FDG PET/CT is planned for response assessment.
There were 30 patients with aggressive NHL in this study. The sensitivity, specificity, PPV and NPV of PET/CT were 100, 65.4, 30.8 and 100 per cent, respectively, by approach (A), which improved to 100 per cent for all four parameters on approach (B). A study of high-grade B-cell NHL reported a sensitivity of 52.7 per cent and NPV of 81.7 per cent7. The inclusion of diffuse marrow FDG uptake as positive reduced the sensitivity and NPV in this study. A meta-analysis6 showed that the sensitivity and specificity of 18F-FDG PET/CT ranged from 70.8 to 95.8 and 99.0 to 100 per cent, respectively, with pooled estimates of 88.7 and 99.8 per cent, respectively. Using approach (A) in DLBCL, the sensitivity and NPV of PET/CT in detecting BMI were 86 to 100 and 98 to 100 per cent, respectively15,16. Chen et al12 analyzed 93 patients with DLBCL, Burkitt’s lymphoma, lymphoblastic lymphoma and ALCL by approach (B). There was a concordance of 76 per cent between PET/CT and BMB. The sensitivity, specificity, PPV and NPV of PET/CT were 95, 98, 97 and 96 per cent, respectively. In an evaluation of aggressive NHL cases, the sensitivity, specificity, PPV and NPV of PET/CT were 100, 94, 93 and 100 per cent, respectively10. In aggressive NHL like DLBCL, the marrow may show discordant involvement with low-grade histology or may have a low-volume marrow involvement (10-20% tumour load in the marrow) and 18F-FDG PET/CT may be falsely negative in such cases7,17. This may result in false downstaging of the disease. Our study did not have any such case of aggressive NHL which led to better specificity and PPV. Based on these observations, patients of aggressive NHL who undergo baseline staging and treatment follow up by 18F-FDG PET/CT scan, BMB may be avoided at baseline for staging. However, baseline BMB is justified in cases where marrow is not involved in 18F-FDG PET/CT3.
There were 13 patients with indolent NHL in the present study, of whom eight cases had marrow involvement on histology. The sensitivity, specificity, PPV and NPV were 75, 80, 85.7 and 66.7 per cent, respectively, with approach (A) and 88.9, 100, 100 and 80 per cent, respectively, with approach (B). Sengar et al18 showed a sensitivity (43.7%) and NPV (50%) of the 18F-FDG PET-using approach (A). The specificity (81.2%) and PPV (77.8%) were relatively higher for follicular lymphoma. In the same study, approach (B) showed that the specificity and PPV of 18F-FDG PET/CT improved to 100 per cent each, but the sensitivity and NPV remained 43 and 50 per cent, respectively. In follicular lymphoma using approach (A), the PPV and NPV were 100 and 48.5 per cent8 while the sensitivity and specificity were 67 and 85 per cent9. Mittal et al10 prospectively analyzed 15 follicular lymphomas and two small lymphocytic lymphomas using approach (B). They also found that specificity and PPV of PET/CT were 100 per cent each, while the sensitivity and NPV were comparatively lower at 50 and 70 per cent, respectively. The excellent specificity and PPV signify that in indolent lymphoma in general and follicular and mantle cell lymphoma in particular, BMB for staging may be avoided if there is positive FDG-marrow uptake on PET/CT. However, in the case of no marrow uptake on PET/CT, BMB should be done to exclude marrow involvement.
The pattern of FDG uptake in the marrow needs special consideration. In the present study, patients who were considered positive for marrow involvement on 18F-FDG PET/CT had at least one focal uptake pattern, with or without diffuse marrow uptake. Isolated diffuse uptake was not considered as positive for marrow involvement. Focal involvement on PET/CT in HL and both diffuse/focal involvement in high grade B-cell NHL were predictive of bone marrow involvement on histology7. High concordance between focal pattern on 18F-FDG PET/CT and marrow infiltration on BMB (86%) was seen in follicular lymphoma13. The isolated diffuse pattern on 18F-FDG PET/CT may be due to benign and reactive conditions, more so in HL19,20. In the present study, patients with focal involvement on 18F-FDG PET/CT that were missed by BMB showed involvement of marrow at sites other than the iliac crest, resulting in sampling error. Detection of such cases requires a PET/CT-guided targeted biopsy. These focal lesions, however, disappeared on follow up scan post-treatment signifying their true positive nature.
With regard to the optimal SUVmax cut-off, previous studies10,21 showed that marrow involvement on histology was significantly associated with a higher SUVmax in all lymphoma subtypes. Considering histological subtypes, marrow SUVmax in patients with follicular lymphoma (5.4 vs. 1.8; P<0.001)9 and HL (3.0 vs. 1.2; P<0.001)12 were significantly higher in patients with marrow involvement on histology than in those in whom BM was not involved. In the present study, there were three cases of DLBCL, in whom PET/CT showed a SUVmax of more than 10, but BMB failed to identify marrow involvement. This may be because these cases either showed involvement at a site other than the iliac crest or showed focal lesions in the iliac bone which were not sampled in the blinded BMB procedure.
The strength of the study was its prospective nature. Each patient was followed up with a repeat 18F-FDG PET/CT scan during/after treatment, and the disappearance of the marrow uptake helped in delineating the true positive nature of disease involvement. The limitation was the sample size, especially concerning indolent lymphoma which was under-represented in the study. Furthermore, unilateral BMB was performed in all cases. The performance of bilateral BMB would have possibly increased the diagnostic yield, but the same is seldom practically performed in clinical practice. A targeted 18F-FDG PET/CT-directed BMB at baseline would have improved the sensitivity of the procedure and would have made our approach (B) of data analysis more evidence based and concrete. This could be a potential hypothesis for future studies22. A larger, preferably multicentric study would validate the results and add to the much-required data pool for better decision-making in clinical practice.
The choice of 18F-FDG PET/CT or BMB for detecting BMI in lymphoma staging should be made primarily based on the histological subtype of lymphoma determined by lymph node/extranodal site biopsy. 18F-FDG PET/CT should be the method of the first choice for staging HL and BMB may be avoided if a follow up 18F-FDG PET/CT is planned. In aggressive (DLBCL) and indolent NHL (follicular lymphoma), if baseline and follow up 18F-FDG PET/CT is performed, and if there is the presence of marrow FDG uptake, the need of a BMB may be precluded. However, if marrow FDG uptake on PET/CT is absent, then a BMB should be done. However, due to meagre data in lymphoma subtypes other than HL, DLBCL and follicular lymphoma, a BMB is indicated especially if there is a negative bone marrow FDG uptake on PET/CT until large-scale data are available to answer this question.
Acknowledgment
Authors acknowledge the contribution of Dr Amburanjan Santra, Department of Nuclear Medicine, Nilratan Sircar Medical College and Hospital for his contribution in interpretation of the PET/CT scan.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease:Cotswolds meeting. J Clin Oncol. 1989;7:1630–6. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 2.Weiler-Sagie M, Bushelev O, Epelbaum R, Dann EJ, Haim N, Avivi I, et al. (18)F-FDG avidity in lymphoma readdressed:A study of 766 patients. J Nucl Med. 2010;51:25–30. doi: 10.2967/jnumed.109.067892. [DOI] [PubMed] [Google Scholar]
- 3.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma:The Lugano classification. J Clin Oncol. 2014;32:3059–68. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichenauer DA, Aleman BMP, André M, Federico M, Hutchings M, Illidge T, et al. Hodgkin lymphoma:ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv19–29. doi: 10.1093/annonc/mdy080. [DOI] [PubMed] [Google Scholar]
- 5.Adams HJA, Kwee TC. Do not abandon the bone marrow biopsy yet in diffuse large B-cell lymphoma. J Clin Oncol. 2015;33:1217. doi: 10.1200/JCO.2014.58.7360. [DOI] [PubMed] [Google Scholar]
- 6.Adams HJA, de Klerk JMH, Fijnheer R, Dubois SV, Nievelstein RAJ, Kwee TC. False-negative FDG-PET in histologically proven extensive large cell bone marrow involvement in diffuse large B-cell lymphoma. Am J Hematol. 2015;90:681. doi: 10.1002/ajh.23986. [DOI] [PubMed] [Google Scholar]
- 7.Chen-Liang TH, Martin-Santos T, Jerez A, Senent L, Orero MT, Remigia MJ, et al. The role of bone marrow biopsy and FDG-PET/CT in identifying bone marrow infiltration in the initial diagnosis of high grade non-Hodgkin B-cell lymphoma and Hodgkin lymphoma. Accuracy in a multicenter series of 372 patients. Am J Hematol. 2015;90:686–90. doi: 10.1002/ajh.24044. [DOI] [PubMed] [Google Scholar]
- 8.Perry C, Lerman H, Joffe E, Sarid N, Amit O, Avivi I, et al. The value of PET/CT in detecting bone marrow involvement in patients with follicular lymphoma. Medicine (Baltimore) 2016;95:2910. doi: 10.1097/MD.0000000000002910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ujjani CS, Hill EM, Wang H, Nassif S, Esposito G, Ozdemirli M, et al. (18) F-FDG PET-CT and trephine biopsy assessment of bone marrow involvement in lymphoma. Br J Haematol. 2016;174:410–6. doi: 10.1111/bjh.14071. [DOI] [PubMed] [Google Scholar]
- 10.Mittal BR, Manohar K, Malhotra P, Das R, Kashyap R, Bhattacharya A, et al. Can fluorodeoxyglucose positron emission tomography/computed tomography avoid negative iliac crest biopsies in evaluation of marrow involvement by lymphoma at time of initial staging? Leuk Lymphoma. 2011;52:2111–6. doi: 10.3109/10428194.2011.593273. [DOI] [PubMed] [Google Scholar]
- 11.Hassan A, Siddique M, Bashir H, Riaz S, Wali R, Mahreen A, et al. 18F-FDG PET-CT imaging versus bone marrow biopsy in pediatric Hodgkin's lymphoma:A quantitative assessment of marrow uptake and novel insights into clinical implications of marrow involvement. Eur J Nucl Med Mol Imaging. 2017;44:1198–206. doi: 10.1007/s00259-017-3647-y. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Wang S, He K, Ma C, Fu H, Wang H. PET/CT predicts bone marrow involvement in paediatric non-Hodgkin lymphoma and may preclude the need for bone marrow biopsy in selected patients. Eur Radiol. 2018;28:2942–50. doi: 10.1007/s00330-018-5306-5. [DOI] [PubMed] [Google Scholar]
- 13.Lee EYP, Gill H, Wang Y, Kwong YL, Khong PL. Bone marrow uptake of indolent non-Hodgkin lymphoma on PET/CT with histopathological correlation. Nucl Med Commun. 2015;36:1035–41. doi: 10.1097/MNM.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 14.Cheng G, Alavi A. Value of 18F-FDG PET versus iliac biopsy in the initial evaluation of bone marrow infiltration in the case of Hodgkin's disease:A meta-analysis. Nucl Med Commun. 2013;34:25–31. doi: 10.1097/MNM.0b013e32835afc19. [DOI] [PubMed] [Google Scholar]
- 15.Vishnu P, Wingerson A, Lee M, Mandelson MT, Aboulafia DM. Utility of bone marrow biopsy and aspirate for staging of diffuse large B cell lymphoma in the era of positron emission tomography with 2-Deoxy-2-[Fluorine-18] fluoro-deoxyglucose integrated with computed tomography. Clin Lymphoma Myeloma Leuk. 2017;17:631–6. doi: 10.1016/j.clml.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Teagle AR, Barton H, Charles-Edwards E, Dizdarevic S, Chevassut T. Use of FDG PET/CT in identification of bone marrow involvement in diffuse large B cell lymphoma and follicular lymphoma:Comparison with iliac crest bone marrow biopsy. Acta Radiol. 2017;58:1476–84. doi: 10.1177/0284185117701305. [DOI] [PubMed] [Google Scholar]
- 17.Tilly H, Gomes da Silva M, Vitolo U, Jack A, Meignan M, Lopez-Guillermo A, et al. Diffuse large B-cell lymphoma (DLBCL):ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26((Suppl 5)):v116–25. doi: 10.1093/annonc/mdv304. [DOI] [PubMed] [Google Scholar]
- 18.Sengar M, Jain H, Rangarajan V, Agrawal A, Menon H, Epari S, et al. Correlation of FDG-PET/CT with bone marrow aspiration and biopsy at the initial staging in follicular lymphoma:Can we omit bone marrow biopsy in some? Blood. 2014;124:4436. [Google Scholar]
- 19.El-Galaly TC, d'Amore F, Mylam KJ, de Nully Brown P, Bøgsted M, Bukh A, et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol. 2012;30:4508–14. doi: 10.1200/JCO.2012.42.4036. [DOI] [PubMed] [Google Scholar]
- 20.Adams HJA, Kwee TC, Fijnheer R, Dubois SV, Nievelstein RAJ, de Klerk JMH. Diffusely increased bone marrow FDG uptake in recently untreated lymphoma:Incidence and relevance. Eur J Haematol. 2015;95:83–9. doi: 10.1111/ejh.12483. [DOI] [PubMed] [Google Scholar]
- 21.Xiao-Xue W, Xinyue H, Lijun Z. Whole body FDG-PET/CT for the assessment of bone marrow infiltration in patients with newly diagnosed lymphoma. Med Clin (Barc) 2020;154:61–5. doi: 10.1016/j.medcli.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 22.Elamir Y, Elazab M, Owis AS, Elsayed HF. PET/CT and bone marrow biopsy (BMB) in evaluating bone marrow in lymphoma. Egypt J Radiol Nucl Med. 2020;51:201. [Google Scholar]