Abstract
Objective
To describe a family with primary familial brain calcifications (PFBCs) and leukoencephalopathy associated with a novel variant in PDGFB.
Methods
We present 3 generations of a family with PFBC associated with a previously unreported variant in PDGFB.
Results
A 24-year-old woman with migraine, bipolar disorder, and functional neurologic disorder was found to have bilateral calcifications of the basal ganglia and frontally predominant periventricular white matter disease. Her father had mild cognitive impairment and action tremor of the hands with basal ganglia and cerebellar calcifications found incidentally on head CT. Her paternal grandmother had severe parkinsonism and dementia with calcifications of the basal ganglia and cerebellum and diffuse, confluent periventricular white matter disease. Genetic testing in both the proband and her father revealed a PDGFB variant (NM_002608.3:c.298C>T:p.Arg100Cys) not reported in publicly available databases. Multiple in silico analysis tools support pathogenicity.
Discussion
Our report identifies a novel PDGFB variant associated with PFBC and highlights the rare association of leukoencephalopathy with PDGFB-associated PFBC.
Primary familial brain calcification (PFBC) is a rare, progressive disease characterized by bilateral calcifications of the basal ganglia and other brain structures.1 The phenotype is variable and can include neuropsychiatric symptoms, movement disorders, a cerebellar syndrome, seizures, and headaches. Pathogenic mutations in 6 genes have been identified in patients with PFBC (PDGFB, PDGFRB, SLC20A2, XPR1, MYORG, and JAM2).1 There are over 20 previously reported probable disease-causing variants in PDGFB.1 Leukoencephalopathy has been rarely reported in PFBC associated with variants in PDGFB.2-4 Here, we report a novel missense PDGFB variant in a family with PFBC and leukoencephalopathy.
Case Report
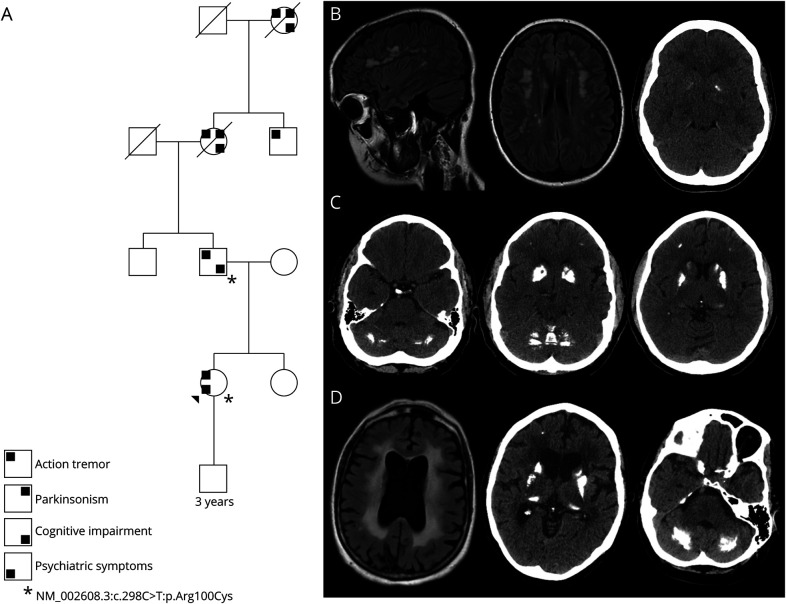
A 24-year-old woman presented to our academic movement disorders center with neuropsychiatric symptoms, migraines, and episodes of transient neurologic symptoms. Since the age of 15 years, she had a history of depression with self-harming behaviors, panic attacks, mood instability, and attention deficit disorder, which required intermittent stimulant use. She reported nonstereotyped episodes of variable symptoms that involved dysesthesia in multiple body parts, subjective bilateral leg “heaviness,” bilateral leg and hand shaking with retained awareness, and monocular visual dark spots that alternated between eyes. These symptoms were not associated with headaches. Prolonged EEGs were normal. Brain imaging revealed bilateral basal ganglia calcifications and frontal subcortical white matter T2-weighted hyperintensities (Figure, B). CSF studies, including oligoclonal bands, were normal. Clinical examination revealed an intermittent, high-frequency bilateral hand tremor that was distractible and entrained to contralateral hand tasks. Frontal release signs were present. She scored 28 of 30 on cognitive screening.
Figure. Pedigree and Patient Images.
(A) Family tree with symptomatology and genetic testing were available. Neurologic symptoms were present in the proband's father, paternal grandmother, paternal great-uncle, and paternal great-grandmother. (B) Proband MRI and CT with bilenticular calcification, TCS = 5. (C) CT scan of the proband's father, TCS = 45. (D) MRI and CT scan of the proband's paternal grandmother, TCS = 53. TCS = total calcification score (range 0–80).
The patient's father developed an action-induced hand tremor at the age of 18 years and subjective memory complaints in his late 20s. The paternal grandmother was diagnosed with parkinsonism in her late 40s with poor levodopa response, falls, severe tremor, and dementia. There was also a paternal great-uncle with tremor, and the paternal great-grandmother had severe parkinsonism, tremor, and dementia as well. The proband's father and paternal grandmother were found to have calcifications of the bilateral frontal lobes, basal ganglia, and cerebellum on head CT (Figure, C and D). The paternal grandmother's brain MRI showed global volume loss and confluent periventricular white matter changes. Brain MRIs were unavailable for the father, paternal great-uncle, and paternal great-grandmother.
In the proband and father, targeted sequencing covering the coding regions (plus 10 bases of flanking noncoding DNA at 20x next-generation sequencing reads) of SLC20A2, PDGFB, PDGFRB, and XPR1 was performed through a CLIA/CAP-certified commercial laboratory (PreventionGenetics, test code 10171). Both the proband and father were found to have a heterozygous missense variant in the PDGFB gene (NM_002608.3:c.298C>T), predicted to result in the amino acid substitution p.Arg100Cys.
Discussion
We report a novel variant of interest in PDGFB (c.298C>T:p.Arg100Cys) not previously reported in public databases in a family with brain calcifications and leukoencephalopathy spanning 3 generations.5 In this phenotypically heterogenous family, clinical manifestations included migraines, neuropsychiatric symptoms, severe parkinsonism, tremor, cognitive impairment, and functional neurologic disorder. Leukoencephalopathy in PDGFB-associated PFBC has been described in a family with a c.3G>C variant (p.Met1Ile) and in a patient with an intragenic deletion of exons 2 to 5.2,3 Pontine and periventricular white matter changes have been reported in a patient with a variant in exon 4 (c.C365>T:p.Pro122Leu) of PDGFB.4 Aicardi-Goutières syndrome was also considered as a possible cause of intracranial calcifications and leukoencephalopathy inherited in an autosomal dominant pattern. However, other signs and symptoms of this syndrome were not reported in any family members, including profound early disability, infantile regression, dystonia, excessive startle, oculomotor abnormalities, and epilepsy.6 In addition, the presence of causal variants in other genes associated with PFBC cannot be excluded because only the 4 genes associated with autosomal dominant PFBC were sequenced.
Although reported as a variant of uncertain significance by the performing laboratory, multiple in silico protein analysis tools (MutationTaster, PolyPhen-2, SIFT, and Provean) predicted the variant to be damaging.7 Furthermore, the Arg residue at position 100 is highly conserved with a phastCons score of 1.0. Prior variants of interest have been reported in exon 4.2-4 This along with segregation of the variant in multiple generations with apparent clinical phenotypes support pathogenicity.
The observations in this family expand the list of variants in PDGFB and add to genetic causes of leukoencephalopathies. We further highlight the range of possible clinical and radiographic phenotypes within a single family harboring a monogenic disease. The presence of leukoencephalopathy and brain calcifications in patients with a wide range of neurologic symptoms, including functional neurologic disorder, inherited in an autosomal dominant pattern, should prompt clinicians to consider testing for PDGFB mutations. Go to Neurology.org/NG for full disclosures. Funding information is provided at the end of the article.
Appendix. Authors
Contributor Information
Jack Shen, Email: shenjk@ucmail.uc.edu.
Amelle Shillington, Email: amelle.shillington@cchmc.org.
Alberto J. Espay, Email: espayaj@ucmail.uc.edu.
Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures. Go to Neurology.org/NG for full disclosures.
References
- 1.Balck A, Schaake S, Kuhnke NS, et al. Genotype–phenotype relations in primary familial brain calcification: systematic MDSGene review. Mov Disord. 2021;36(11):2468-2480. doi: 10.1002/mds.28753. [DOI] [PubMed] [Google Scholar]
- 2.Biancheri R, Severino M, Robbiano A, et al. White matter involvement in a family with a novel PDGFB mutation. Neurol Genet. 2016;2(3):e77. doi: 10.1212/NXG.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicolas G, Rovelet-Lecrux A, Pottier C, et al. PDGFB partial deletion: a new, rare mechanism causing brain calcification with leukoencephalopathy. J Mol Neurosci. 2014;53:171-175. doi: 10.1007/s12031-014-0265-z. [DOI] [PubMed] [Google Scholar]
- 4.Keogh MJ, Pyle A, Daud D, et al. Clinical heterogeneity of primary familial brain calcification due to a novel mutation in PDGFB. Neurology. 2015;84(17):1818-1820. doi: 10.1212/WNL.0000000000001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karczewski KJ, Francioli LC, Tiao G, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434-443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Livingston JH, Crow YJ. Neurologic phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR1, and IFIH1: Aicardi-Goutières syndrome and beyond. Neuropediatrics. 2016;47(6):355-360. doi: 10.1055/s-0036-1592307. [DOI] [PubMed] [Google Scholar]
- 7.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]