Abstract
Background and Objectives
Genetic variants affect both Parkinson disease (PD) risk and manifestations. Although genetic information is of potential interest to patients and clinicians, genetic testing is rarely performed during routine PD clinical care. The goal of this study was to examine interest in comprehensive genetic testing among patients with PD and document reactions to possible findings from genome sequencing in 2 academic movement disorder clinics.
Methods
In 203 subjects with PD (age = 63 years, 67% male), genome sequencing was performed and filtered using a custom panel, including 49 genes associated with PD, parkinsonism, or related disorders, as well as a 90-variant PD genetic risk score. Based on the results, 231 patients (age = 67 years, 63% male) were surveyed on interest in genetic testing and responses to vignettes covering (1) familial risk of PD (LRRK2); (2) risk of PD dementia (GBA); (3) PD genetic risk score; and (4) secondary, medically actionable variants (BRCA1).
Results
Genome sequencing revealed a LRRK2 variant in 3% and a GBA risk variant in 10% of our clinical sample. The genetic risk score was normally distributed, identifying 41 subjects with a high risk of PD. Medically actionable findings were discovered in 2 subjects (1%). In our survey, the majority (82%) responded that they would share a LRRK2 variant with relatives. Most registered unchanged or increased interest in testing when confronted with a potential risk for dementia or medically actionable findings, and most (75%) expressed interest in learning their PD genetic risk score.
Discussion
Our results highlight broad interest in comprehensive genetic testing among patients with PD and may facilitate integration of genome sequencing in clinical practice.
Advances in genetic knowledge have implicated more than 100 genes in Parkinson disease (PD) risk, including both rare and common variants.1,2 Along with potential implications for counseling families, identification of these genetic risk factors may also affect clinical decision making. Several clinical trials for targeted therapies in PD are now recruiting patients with GBA-PD or LRRK2-PD, motivating large-scale efforts to identify eligible subjects.3 In addition, numerous studies highlight links between specific genetic variants and heterogeneous PD symptoms. For example, GBA-PD is characterized by more rapid disease progression and an increased risk of dementia.4-7 APOE genotype, which has an established role in clinical risk stratification for Alzheimer disease,8 is also associated with Lewy body dementia.9,10 In LRRK2-PD, several studies have suggested a more benign disease course, whereas others reveal a higher likelihood of the postural instability gait difficulty motor phenotype.11,12 Finally, although most common PD risk variants (i.e., population allele frequency >1%) have only modest effect sizes in isolation, a genetic risk score incorporating dozens of single nucleotide polymorphisms predicts accelerated PD progression13,14 and may also facilitate early diagnosis.15 Because it is derived from common variant genotypes, the PD genetic risk score may be applicable to many patients with PD. Similar algorithms have been clinically validated for risk assessment in other common and complex genetic disorders, such as cardiovascular disease.16,17
Most current commercial gene panels for PD are largely restricted to testing for monogenic causes of mendelian PD. The available tests also vary widely in the number of genes and variants tested, frequently include loci with uncertain evidence, and do not include common genetic variants or assess a genetic risk score.18 Compared with targeted panel assays, genome sequencing comprehensively examines both rare and common variants in most genes. In addition, results can be readily filtered and reported based on all available evidence, making possible iterative analytic updates following new discoveries and without requiring an additional blood draw or new data generation. The American College of Medical Genetics and Genomics (ACMG) recommends offering testing for genes conferring a risk of a serious medical condition for which there is an effective intervention or need for surveillance.19 Such medically actionable genetic findings unrelated to PD can be readily screened using genome sequencing.
Despite the growing potential to affect clinical decision making, genetic testing is rarely used in the routine clinical assessment of PD,20 yet most patients with PD have a strong desire to know their genetic information.21-23 Besides potential barriers for reimbursement, many neurologists feel poorly prepared to provide counseling to patients regarding results.24 In addition, other issues that may arise from comprehensive testing using genome sequencing have not been fully explored, such as disclosure of genetic risk scores or potential discovery of medically actionable findings unrelated to PD diagnosis. To address these gaps, it is essential to document not only the spectrum of findings from genome sequencing that would be expected in clinical practice but also the possible reactions of patients to learning of these results.
Methods
Subject Recruitment
Subjects were recruited from 2 academic movement disorder centers (Baylor College of Medicine and University of Maryland School of Medicine). During routine clinic visits between 2013 and 2019, patients with PD who were diagnosed by a movement disorder specialist were offered participation in a DNA bank repository. Demographics and selected clinical details were collected through chart review and confirmed based on an unstructured interview at the time of sample collection in the clinic, including a family history of PD (defined as any affected blood relative), age at symptom onset, and results from cognitive screening. The DNA bank repository protocol permits genome sequencing for research but does not allow for disclosure of results. Therefore, in a separate study conducted between 2018 and 2019, patients with PD from both clinics were recruited for an approximately 15-minute survey in which responses were recorded to several clinical vignettes including genetic testing results (below). Individuals with dementia and those who had previously undergone clinical genetic testing for PD were excluded from the survey study. There were 203 subjects who contributed DNA samples for genome sequencing and 231 who completed the survey. Twenty-eight subjects participated in both parts of the study.
Standard Protocol Approvals, Registrations, and Patient Consents
All subjects provided informed consent. The institutional review boards at Baylor College of Medicine and University of Maryland approved both the biospecimen collection/sequencing and survey study included in this report.
Custom PD Gene Panel
We developed a custom PD gene panel for filtering sequencing results and creation of personalized PD genome risk profile reports (eTable 1, links.lww.com/NXG/A530). We included 5 categories of genes/variants, including both (1) 5 highly penetrant PD risk genes (SNCA, LRRK2, VPS35, PARK2/PRKN, and PINK1) and (2) 3 moderately penetrant risk/modifier genes (GBA, SMPD1, and APOE). APOE has an established association with cognitive impairment in PD.9,25 Because idiopathic PD clinical manifestations sometimes overlap with other genetic syndromes that cause parkinsonism (e.g., dopamine-responsive dystonias26), we also included an additional category (3) including 41 such genes (eTable 1). All genes selected for the panel were based on careful literature review with the following inclusion criteria: at least 1 pathogenic variant reported in 3 separate families by 2 or more independent groups or at least 1 large sequencing study revealing a significant association with PD or parkinsonism. For the PD genetic risk score (category 4), we considered 90 risk variants identified by PD genome-wide association study (GWAS) meta-analysis, weighted by their individual odds ratios for PD.16,27 We divided the genetic risk scores into low, medium, and high risk based on quintiles (first quintile defined as low risk, second–fourth quintile as medium risk, and fifth quintile as high risk).28 Subjects with a high vs low score were compared in terms of age at onset, family history, and the Montreal Cognitive Assessment score using the Student t test. Finally (category 5), our panel included 59 genes unrelated to PD, but which were recommended for secondary reporting by the ACMG.29
Genome Sequencing
DNA was extracted from peripheral blood lymphocytes or saliva samples using standard protocols. Whole-genome sequencing data were generated for 203 samples at BCM-HGSC using established library preparation and sequencing methods. Libraries were prepared using KAPA Hyper PCR-free library reagents (KK8505; Kapa Biosystems Inc., Wilmington, MA) on Beckman robotic workstations (Biomek FX and FXp models). In brief, DNA (750 ng) was sheared into fragments of approximately 200–600 bp using the Covaris E220 system (96 well format; Covaris, Inc., Woburn, MA) followed by purification of the fragmented DNA using AMPure XP beads. A double size selection step was used, with different ratios of AMPure XP beads, to select a narrow size band of sheared DNA molecules for library preparation. DNA end-repair and 3′-adenylation were then performed in the same reaction followed by ligation of Illumina unique dual barcode adapters (Cat# 20022370) to create PCR-Free libraries, and the library was run on the Fragment Analyzer (Advanced Analytical Technologies, Inc., Ames, IA) to assess the library size and the presence of remaining adapter dimers. This was followed by a qPCR assay using the KAPA Library Quantification Kit (KK4835) using their SYBR FAST qPCR Master Mix for size estimation and quantification. WGS libraries were sequenced on the Illumina NovaSeq instrument using the S4 flow cells with 24 libraries in each lane. Sequencing reagents used included the NovaSeq 6000 S4 Reagent Kit (Cat# 20012866) and NovaSeq Xp 4-Lane Kit (Cat# 20021665). Libraries were loaded at an average concentration of 365 pM to generate 150-bp paired-end reads. Sequence data were processed using HgV (Mercury V, version 17.5), the HGSC workflow management system, and mapped to human reference build hg19.30,31 Unique aligned sequences in these samples varied between 69.6 and 285.7 Gb, with an average of 129.89 Gb per sample. The average median insert size was 420 bp, and the average mode insert size was 399 bp. For the analysis of copy number variation at the PRKN locus, we applied the Dragen calling algorithm with Circular Binary Segmentation caller using the self-normalization method (Illumina, San Diego, CA, version 3.8.4), which integrates both depth-based calling and split read methods to identify potential copy number variation within 500 kb of the gene.
Variant Filtering/Analysis
All variants, including both exonic and intronic variants, were initially annotated using the ClinVar32 and Human Gene Mutation Database (HGMD) in April 2019, supplemented by literature review and discussion by our multidisciplinary team, which included movement disorder neurologists, a clinical geneticist, a genetic counselor, and basic scientists.33 With the exception of GBA (see below), all variants considered pathogenic in this study were annotated in ClinVar as either pathogenic/likely pathogenic or in HGMD as DM, indicating disease causing. In the case of GBA, database annotation is largely based on the risk for Gaucher disease; however, we additionally considered 2 GBA variants, p.E365K and p.T408M, with strong literature support for an increased risk for PD, but which are nonpathogenic for Gaucher.34,35 For all variants, we additionally performed literature review to document consistent reports from multiple independent families and/or large cohort sequencing studies or support from experimental validation in model organisms. This supportive evidence is documented in eTable 2 (links.lww.com/NXG/A530). For category 1 genes, following the exclusion of ClinVar benign or likely benign variants, all other identified findings with MAF <5% were considered variants of uncertain significance (VUS) (eTable 3).
Survey Study
The survey comprised 4 clinical vignettes developed based on empiric results from our genome sequencing study (see eMethods for complete survey, links.lww.com/NXG/A530). The vignettes highlighted the following scenarios: family risk of PD due to a LRRK2 variant (vignette 1); personal dementia risk in a carrier of a GBA variant (vignette 2); negative findings for highly penetrant variants and reporting of the genetic risk score (vignette 3); and discovery of a medically actionable variant in BRCA1 (vignette 4). These vignettes were designed both as an educational tool and to assess how patient interest in genetic testing may be affected by different results. Vignettes and follow-up questions were read aloud to subjects. The interest level for comprehensive genetic testing was assessed based on a Likert scale (0, “much less interested,” to 5, “much more interested”). Qualitatively, we also recorded comments from subjects on factors influencing their decision making. Comments responding to at least 1 vignette were recorded from 133 of 231 subjects. We also requested feedback on how doctors might better prepare patients for genetic testing in PD.
Data Availability
Complete results from the survey are available in eTable 4 (links.lww.com/NXG/A530). For all subjects who consented for public data sharing, sequencing data are in process for release in relevant genomic databases. The complete sequencing data are also available on request by contacting the corresponding author, Dr. Shulman (joshua.shulman@bcm.edu).
Results
Genome Sequencing
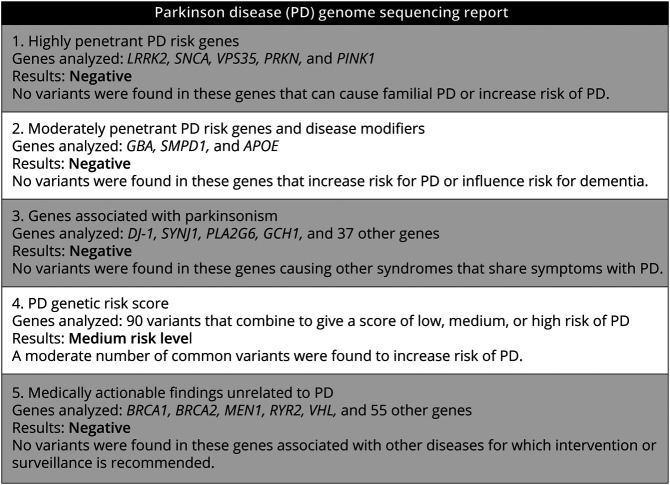
We performed genome sequencing in 203 unselected, unrelated subjects with PD. Subject demographics and clinical characteristics are summarized in Table 1. For filtering and annotation of personal genomes, we developed a custom PD gene and variant panel including 5 categories of reportable genetic risk (Methods). The gene panel was designed to facilitate generation of individual PD Genome Sequencing Reports (Figure 1). Because genome sequencing was conducted under a protocol that did not allow for disclosure of results, the individual findings instead informed our development of several clinical vignettes that we subsequently presented to a largely nonoverlapping sample of subjects with PD.
Table 1.
Subject Characteristics
Figure 1. Sample PD Genome Sequencing Report.
This report represents the most common results based on application of our customized PD gene panel (see also eTable 1, links.lww.com/NXG/A530, for all genes included in categories 1–3).
Among highly penetrant gene variants (category 1; see also vignette 1, below), our sample included 7 subjects with a LRRK2 pathogenic variant (3%) and 1 heterozygous carrier of a pathogenic PRKN single nucleotide variant. In this individual, sequence data were further interrogated to exclude the possibility of a copy number variant. An additional 28 subjects (14%) were discovered to have a VUS in at least 1 category 1 gene (eTable 3, links.lww.com/NXG/A530). We next examined variants in genes associated with a moderate risk of PD and disease modifiers (category 2; vignette 2). We discovered 21 carriers of GBA pathogenic variants (10%). Notably, 1 subject was a carrier of both a GBAE356K and a LRRK2M1646T variant. For APOE, 45 subjects were heterozygous for the e4 allele. Two individuals were homozygous for APOE e4, but based on available clinical documentation, both individuals were not known to have cognitive impairment. Notably, 2 subjects were carriers of SMPD1 variants previously reported to be pathogenic for Niemann-Pick Type A (p.E517V, c.1264-1G>T); however, these alleles have not been definitively associated with PD. Table 2 shows all reportable pathogenic variants in genes from categories 1 and 2. No individuals in our sample were discovered to have variants linked to other genetic causes of parkinsonism (category 3).
Table 2.
Sequencing Results
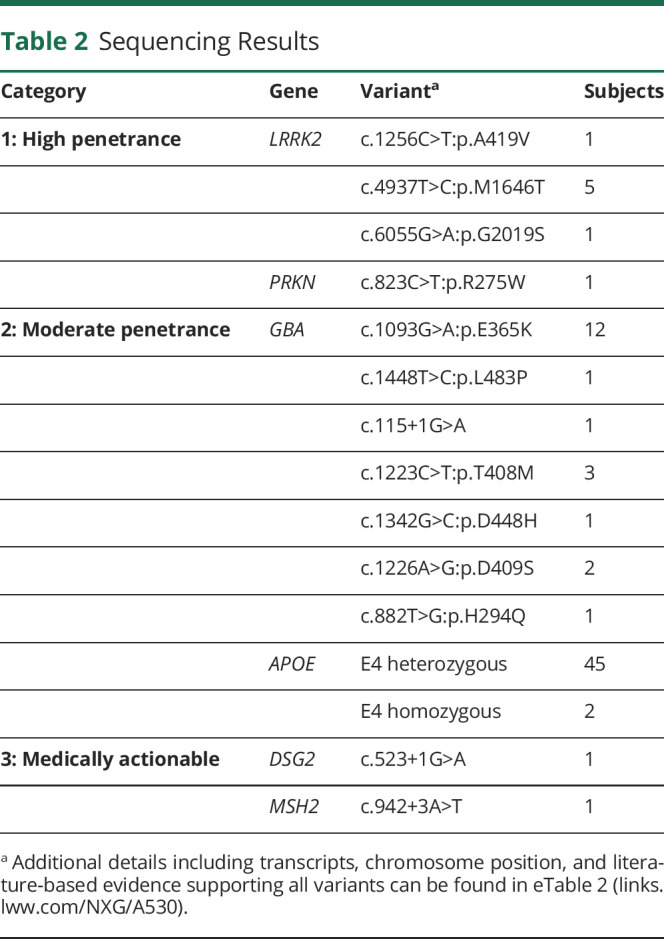
We next examined the 90-variant PD genetic risk score (category 4), which was normally distributed in our sample (Figure 2). After dividing into quintiles,28 there were 41 subjects each with a high or low genetic risk score. Of note in comparisons of the low- vs high-risk groups, we found no significant difference between positive family history (p = 0.3), age at symptom onset (p = 0.6), or level of cognitive function (p = 0.2), despite reports of such associations in much larger sample sizes.36,37 As expected, most subjects (n = 122) had a score consistent with a moderate risk, and for most of these (n = 104), no other genes were flagged from categories 1 to 3 (representative report, Figure 1; vignette 3). Finally, we examined 59 ACMG medically actionable findings, unrelated to PD risk (category 5; vignette 4). We discovered 2 individuals harboring such alleles, including a DSG2 variant associated with arrhythmogenic right ventricular cardiomyopathy and a variant in MSH2, which causes Lynch syndrome, conferring an increased risk for colon and other cancers (Table 2).29
Figure 2. Genetic Risk Score Distribution.
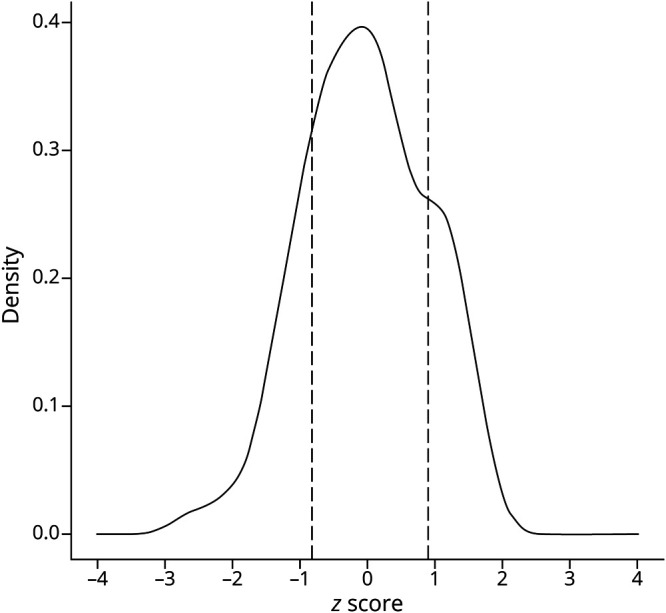
Density plot for the PD genetic risk score. Z scores reflect standard deviations from the mean. The low- and high-risk groups are indicated by dashed lines, highlighting cutoffs for the first and fifth quintiles, respectively. PD = Parkinson disease.
Survey Study
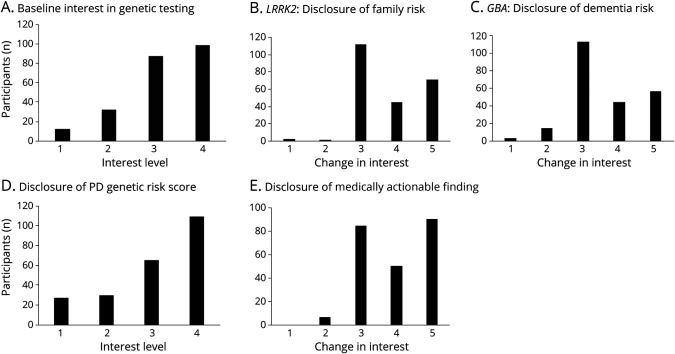
Because our institutional review board protocol did not permit disclosure of genome sequencing results to participants, we enrolled a largely nonoverlapping sample of 231 subjects with PD from our 2 centers for a survey study, in which representative results were presented as part of clinical vignettes. Subject demographics are summarized in Table 1. A minority of subjects (n = 28) completing the survey also contributed samples for genome sequencing. Before hearing the vignettes, most participants (81%) responded that they were interested in genetic testing for PD (Figure 3). There was no significant difference in interest among respondents with a family history of PD (p = 0.18) or those contributing samples for genome sequencing (p = 0.34). Most respondents (82%) indicated that they would inform at-risk family members about the potential finding of a high-penetrance PD risk variant, such as LRRK2G2019S (vignette 1). Only 2 subjects (1%) reported reduced interest in comprehensive genetic testing based on the potential implications for familial risk. Feedback often reflected concern for children or grandchildren. One subject commented, “I just want to know what I can do to keep my kids from getting this,” and another offered, “I don't want to do anything that would hurt our grandchildren.” Three subjects expressed concern about potential insurance denial or job discrimination, either for themselves or their relatives.
Figure 3. Survey Results.
Results from survey questions gauging subject interest in comprehensive genetic testing at baseline (A) or following several clinical vignettes, including disclosure of an LRRK2 variant (B, vignette 1), GBA variant (C, vignette 2), genetic risk score (D, vignette 3), or secondary, medically actionable finding unrelated to PD (E, vignette 4). The survey responses for “interest level” (A and C), included 1, “not interested”; 2, “a little interested”; 3, “moderately interested”; and 4, “very interested.” For “change in interest” (B, C, and E), responses included 1, “much less interested”; 2, “a little less interested”; 3, “no change”; 4, “a little more interested”; and 5, “much more interested.” Most subjects reported no change or increased interest in genetic testing following disclosures. For complete survey and all results, see eMethods and eTable 4 (links.lww.com/NXG/A530). PD = Parkinson disease.
Clinical vignette 2 focused on the potential for an increased dementia risk following discovery of a GBA variant. Only 16 subjects (7%) reported reduced interest in genetic testing, whereas 101 (44%) were, in fact, more interested in genetic testing after learning that genetic variants might indicate a heightened risk for PD dementia. When informed that genetic testing results, such as discovery of a GBA variant, would not currently change treatment decisions for the majority of patients, 75% of subjects responded that this did not change their interest in genetic testing.
For most patients, genetic testing is not expected to reveal a highly penetrant PD risk variant. Instead, vignette 3 highlights that a genetic risk score can be computed based on a large number of genetic polymorphisms. The majority of subjects (75%) expressed an interest in learning their PD genetic risk score. Among the subjects who were not interested in their PD genetic risk score, 4 expressed concerns that PD must be predominantly influenced by environmental factors, making genetic testing less relevant. Finally, most participants (88%) expressed a desire to receive information on medically actionable variants unrelated to PD following vignette 4, which included discussion of a pathogenic BRCA1 variant. In fact, 61% reported that they were even more interested in genetic testing considering the possibility of learning such information.
Other general comments from subjects praised the potential utility of genetic testing for long-term care planning, improved understanding of PD, and promoting new therapies. Six subjects noted minimal interest in genetic testing due to the lack of availability of disease-modifying treatments. One volunteered, “I don't think it should be discussed unless there is a cure.” Four subjects raised concerns about the cost of genetic testing. Six reported being unaware that PD is influenced by genetics or that testing was available. Several subjects were surprised to learn that genetic risk in PD is more complicated than in other disorders. One subject expressed that before the survey, he was concerned that genetic testing was clinically useful but was being withheld from patients because of cost. The most frequent comment at the end of the survey was a request for more information. Fifteen subjects desired more education about genetic testing during doctor visits. Seventeen requested that written material on genetics in PD be made available. Three suggested a didactic class or seminar, and 5 asked for references to websites or online videos. Many expressed an increased interest in genetic testing that was specifically related to new information learned from completing the survey.
Discussion
A substantial majority of patients with PD seen at 2 academic medical centers were interested in information about genetic risk, including the opportunity to inform at-risk family members (72%), discover their possible risk of dementia (75%), obtain their genetic risk score (75%), and learn about potential medically actionable variants (88%). This is largely consistent with other published surveys.21-23 Despite rapidly expanding genetic knowledge about PD, including many established variants that affect disease risk and clinical manifestations, testing is rarely pursued in routine PD clinical care. Most commercial genetic testing panels are largely limited to monogenic causes of familial PD.18 Moreover, such panels often include genes with limited evidence or omit more recently discovered loci. By contrast, genome sequencing offers more comprehensive genetic analysis. We obtained genome sequencing from 203 patients with PD and developed a custom gene and variant panel for filtering and analysis. In our sample, 29 subjects had reportable, diagnostic findings of either high- or moderate-penetrance PD risk or modifying alleles among 49 genes examined. These results are overall consistent with other published work.38-40 We also computed a 90-variant PD genetic risk score for all subjects and examined secondary findings for 59 medically actionable genes. It is important to note that compared with a targeted gene panel assay, genome sequencing coupled with focused filtering for genes relevant to PD permits refiltering and updated reporting based on newly published findings.41 For example, since completing our analyses, the ACMG has already updated its recommendations on medically actionable findings to include 73 genes.19
Because sequencing was performed under a research protocol that did not allow for disclosure of genetic results to subjects, we designed a survey to assess reactions to clinical vignettes including representative findings. For the majority of subjects, interest in genetic testing remained strong or increased despite potential revelations of familial PD risk, personal risk of PD dementia, or lack of immediate effect on treatment decisions. Interest in clinical genetic testing for PD will also likely rise with increased awareness of clinical trials for disease-modifying treatments in carriers of LRRK2 and GBA variants.3,42 If such therapies prove successful, results of this and other larger multicenter studies (e.g., PD GENEration43) will be important to inform roll-out of more routine genetic testing for PD, including guidelines for effective communication with patients and families.
Based on recent studies,24 many neurologists feel unprepared to communicate authoritatively on PD genetics, but some of the issues are commonly encountered in genetic counseling for other neurogenetic syndromes. For example, the discovery of high-penetrance LRRK2 variants may trigger interest in cascade testing among family members. However, in the case of moderately penetrant variants, such as GBA or APOE, the implications for relatives may be more difficult to convey and for patients to conceptualize. Despite the potential for clinically meaningful findings, most patients are unlikely to discover responsible high- or moderate-penetrance PD risk variants, at least based on our current genetic knowledge. In our study, many subjects were surprised to learn that such negative results are the most likely outcome (86% of our sequencing sample). Inclusion of the PD genetic risk score expands the potential applicability of genetic testing, and we documented strong interest in such results. Higher genetic risk scores have been shown to be significantly associated with PD progression,44,45 cognitive impairment,13 and levodopa-induced dyskinesia.46 Nevertheless, most neurologists may not be familiar with genetic risk scores. In addition, available risk scores are derived from GWAS conducted almost exclusively in European-ancestry populations, making them poorly generalizable for other groups.
Strengths of this study include genetic sampling from 2 high-volume academic movement disorders clinic populations and inclusion of more than 200 subjects for both the genome sequencing and survey arms of the study. We also developed a custom panel for filtering and annotation of genome sequencing results based on systematic criteria and literature review. The clinical vignettes developed for our survey may be a valuable tool for engaging and educating patients as part of pretest genetic counseling. We also acknowledge some important limitations. Adoption of genome sequencing as a first-line clinical genetic test for PD may be limited by higher cost for data generation and analysis compared with single gene panels. Moreover, compared with single nucleotide variants, the detection of copy number variants may be less reliable, and our analysis did not systematically consider such alleles, which can be important contributors to PD risk.46 In addition, expert consensus annotation of PD pathogenic variants is currently lacking to guide reporting of results for many genes. Notably, in the case of GBA and SMPD1, current database annotations are focused on lysosomal storage disorders rather than PD. Finally, attitudes about genetic testing from our academic movement disorders clinics may not be generalizable, including for more culturally and ethnically diverse populations. Overall, our findings highlight broad interest in comprehensive genetic testing among patients with PD and can help inform successful integration of genome sequencing in clinical practice.
Acknowledgment
The authors thank all participants in this study. Kim Bambarger, Veronica Fallon, Christina Griffin, and Phillip Heiser provided coordination and support for sample collection and processing.
Glossary
- ACMG
American College of Medical Genetics and Genomics
- HGMD
Human Gene Mutation Database
- PD
Parkinson disease
- VUS
variants of uncertain significance
Appendix. Authors
Contributor Information
Emily J. Hill, Email: emily.j.hill8@gmail.com.
Laurie A. Robak, Email: robak@bcm.edu.
Rami Al-Ouran, Email: rami.alouran@gmail.com.
Jennifer Deger, Email: jennifer.deger@bcm.edu.
Jamie C. Fong, Email: jamie.fong@bcm.edu.
Paul Jerrod Vandeventer, Email: paul.vandeventer@bcm.edu.
Emily Schulman, Email: emily.schulman@som.umaryland.edu.
Sindhu Rao, Email: sindhu.rao@bcm.edu.
Hiba Saade, Email: hiba.saade@bcm.edu.
Joseph M. Savitt, Email: jsavitt@som.umaryland.edu.
Rainer von Coelln, Email: rvoncoelln@som.umaryland.edu.
Neeja Desai, Email: desai.neeja14@gmail.com.
Harshavardhan Doddapaneni, Email: doddapan@bcm.edu.
Sejal Salvi, Email: sejal.salvi@bcm.edu.
Shannon Dugan-Perez, Email: sdugan@bcm.edu.
Donna M. Muzny, Email: donnam@bcm.edu.
Amy L. McGuire, Email: amcguire@bcm.edu.
Zhandong Liu, Email: zhandonl@bcm.edu.
Richard A. Gibbs, Email: agibbs@bcm.edu.
Chad Shaw, Email: cashaw@bcm.edu.
Joseph Jankovic, Email: josephj@bcm.edu.
Lisa M. Shulman, Email: lshulman@som.umaryland.edu.
Study Funding
This work was supported by the Huffington Foundation, McGee Foundation, and the Jan and Dan Duncan Neurological Research Institute at Texas Children's Hospital.
Disclosure
The authors report no disclosures relevant to the manuscript. L.M. Shulman receives research funding from the NIH (R01AG059651), The Rosalyn Newman Foundation, and the Eugenia and Michael Brin Family. She receives royalties from Oxford University Press and Johns Hopkins University Press. J. Jankovic has received research or training grants from AbbVie Inc., Acadia Pharmaceuticals, Cerevel Therapeutics, CHDI Foundation, Dystonia Coalition, Emalex Biosciences, Inc., F. Hoffmann-La Roche Ltd., Huntington Study Group, Medtronic Neuromodulation, Merz Pharmaceuticals, Michael J. Fox Foundation for Parkinson Research, NIH, Neuraly, Inc., Neurocrine Biosciences, Parkinson's Foundation, Parkinson Study Group, Prilenia Therapeutics, Revance Therapeutics, Inc., and Teva Pharmaceutical Industries Ltd. J. Jankovic has served as a consultant for Aeon BioPharma, Allergan, Inc., Merck & Co, Inc., Revance Therapeutics, and Teva Pharmaceutical Industries Ltd. J. Jankovic has received royalties from Cambridge; Elsevier, MedLink: Neurology, Lippincott Williams and Wilkins, UpToDate, and Wiley-Blackwell. L. Robak receives funding from NIH (K08NS112467). J.M. Shulman consults for the Adrienne Helis Malvin & Diana Helis Henry Medical Research Foundations. Go to Neurology.org/NG for full disclosures.
Publication History
Previously published at MedRxiv doi: 10.1101/2021.11.23.21266755. Received by Neurology: Genetics November 27, 2021. Accepted in final form April 22, 2022. Submitted and externally peer reviewed. The handling editor was Alexandra Durr, MD, PhD.
References
- 1.Bandres-Ciga S, Diez-Fairen M, Kim JJ, Singleton AB. Genetics of Parkinson's disease: an introspection of its journey towards precision medicine. Neurobiol Dis. 2020;137:104782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan MMX, Malek N, Lawton MA, et al. Genetic analysis of Mendelian mutations in a large UK population-based Parkinson's disease study. Brain. 2019;142(9):2828-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider SA, Alcalay RN. Precision medicine in Parkinson's disease: emerging treatments for genetic Parkinson's disease. J Neurol. 2020;267(3):860-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brockmann K, Srulijes K, Pflederer S, et al. GBA-associated Parkinson's disease: reduced survival and more rapid progression in a prospective longitudinal study. Mov Disord. 2015;30(3):407-411. [DOI] [PubMed] [Google Scholar]
- 5.Liu G, Boot B, Locascio JJ, et al. Specifically neuropathic Gaucher's mutations accelerate cognitive decline in Parkinson's. Ann Neurol. 2016;80(5):674-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009;361(17):1651-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark LN, Kartsaklis LA, Gilbert RW, et al. Association of glucocerebrosidase mutations with dementia with Lewy bodies. Arch Neurol. 2009;66(5):578-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groot C, Sudre CH, Barkhof F, et al. Clinical phenotype, atrophy, and small vessel disease in APOE2 carriers with Alzheimer disease. Neurology. 2018;91(20):E1851-E1859. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Luo J, Liu L, Fu H, Tang L. The genetic association between apolipoprotein E gene polymorphism and Parkinson disease: a meta-analysis of 47 studies. Medicine (Baltimore). 2018;97(43):e12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirza SS, Saeed U, Knight J, et al. APOE4, white matter hyperintensities, and cognition in Alzheimer and Lewy body dementia. Neurology. 2019;93(19):e1807-e1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Healy DG, Falchi M, O’sullivan SS, et al. Articles Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol. 2008;7(7):583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alcalay RN, Mirelman A, Saunders-Pullman R, et al. Parkinson disease phenotype in Ashkenazi jews with and without LRRK2 G2019S mutations. Mov Disord. 2013;28(14):1966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul KC, Schulz J, Bronstein JM, Lill CM, Ritz BR. Association of polygenic risk score with cognitive decline and motor progression in Parkinson disease. JAMA Neurol. 2018;75(3):360-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibanez L, Dube U, Saef B, et al. Parkinson disease polygenic risk score is associated with Parkinson disease status and age at onset but not with alpha-synuclein cerebrospinal fluid levels. BMC Neurol. 2017;17(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nalls MA, McLean CY, Rick J, et al. Diagnosis of Parkinson's disease on the basis of clinical and genetic classification: a population-based modelling study. Lancet Neurol. 2015;14(10):1002-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paquette M, Chong M, Thériault S, Dufour R, Paré G, Baass A. Polygenic risk score predicts prevalence of cardiovascular disease in patients with familial hypercholesterolemia. J Clin Lipidol. 2017;11(3):725-732.e5. [DOI] [PubMed] [Google Scholar]
- 17.Murdock DR, Venner E, Muzny DM, et al. Genetic testing in ambulatory cardiology clinics reveals high rate of findings with clinical management implications. Genet Med. 2021;23(12):2404-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook L, Schulze J, Verbrugge J, et al. The commercial genetic testing landscape for Parkinson's disease. Parkinsonism Relat Disord. 2021;92:107-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller DT, Lee K, Chung WK, et al. ACMG SF v3.0 list for reporting of secondary findings in clinical exome and genome sequencing: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23(8):1381-1390. [DOI] [PubMed] [Google Scholar]
- 20.Gatto EM, Walker RH, Gonzalez C, et al. Worldwide barriers to genetic testing for movement disorders. Eur J Neurol. 2021;28(6):1901-1909. [DOI] [PubMed] [Google Scholar]
- 21.Maloney KA, Alaeddin DS, von Coelln R, et al. Parkinson's disease: patients' knowledge, attitudes, and interest in genetic counseling. J Genet Couns. 2018;27(5):1200-1209. [DOI] [PubMed] [Google Scholar]
- 22.Mulhern M, Bier L, Alcalay RN, Balwani M. Patients' opinions on genetic counseling on the increased risk of Parkinson disease among Gaucher disease carriers. J Genet Couns. 2018;27(3):675-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakanaka K, Waters CH, Levy OA, et al. Knowledge of and interest in genetic results among Parkinson disease patients and caregivers. J Genet Couns. 2014;23(1):114-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alcalay RN, Kehoe C, Shorr E, et al. Genetic testing for Parkinson disease: current practice, knowledge, and attitudes among US and Canadian movement disorders specialists. Genet Med. 2020;22(3):574-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul KC, Rausch R, Creek MM, et al. APOE, MAPT, and COMT and Parkinson's disease susceptibility and cognitive symptom progression. J Parkinsons Dis. 2016;6(2):349-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weissbach A, Wittke C, Kasten M, Klein C. ‘Atypical’ Parkinson's disease—genetic. Int Rev Neurobiol. 2019;149:207-235. [DOI] [PubMed] [Google Scholar]
- 27.Nalls MA, Blauwendraat C, Bandres-Ciga S, et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 2019;18(12):1091-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambert SA, Abraham G, Inouye M. Towards clinical utility of polygenic risk scores. Hum Mol Genet. 2019;28(R2):R133-R142. [DOI] [PubMed] [Google Scholar]
- 29.Kalia SS, Adelman K, Bale SJ, et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet Med. 2017;19(2):249-255. [DOI] [PubMed] [Google Scholar]
- 30.Farek J, Hughes D, Mansfield A, et al. xAtlas: scalable small variant calling across heterogeneous next-generation sequencing experiments. bioRxiv 295071; doi: 10.1101/295071 [DOI] [PMC free article] [PubMed]
- 31.Reid JG, Carroll A, Veeraraghavan N, et al. Launching genomics into the cloud: deployment of Mercury, a next generation sequence analysis pipeline. BMC Bioinformatics. 2014;15:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Accessed on April 1, 2019. ncbi.nlm.nih.gov/clinvar/.
- 33.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallett V, Ross JP, Alcalay RN, et al. GBA P.T369M substitution in Parkinson disease: polymorphism or association? A meta-analysis. Neurol Genet. 2016;2(5):e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y, Deng L, Zhong Y, Yi M. The association between E326K of GBA and the risk of Parkinson's disease. Parkinsons Dis. 2018;2018:1048084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu G, Peng J, Liao Z, et al. Genome-wide survival study identifies a novel synaptic locus and polygenic score for cognitive progression in Parkinson's disease. Nat Genet. 2021;53(6):787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pihlstrøm L, Fan CC, Frei O, et al. Genetic stratification of age‐dependent Parkinson's disease risk by polygenic hazard score. Mov Disord. 2022;37(1):62-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blauwendraat C, Reed X, Kia DA, et al. Frequency of loss of function variants in LRRK2 in Parkinson disease. JAMA Neurol. 2018;75(11):1416-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rana HQ, Balwani M, Bier L, Alcalay RN. Age-specific Parkinson disease risk in GBA mutation carriers: information for genetic counseling. Genet Med. 2013;15(2):146-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alcalay RN, Caccappolo E, Mejia-Santana H, et al. Frequency of known mutations in early-onset Parkinson disease: implication for genetic counseling: the consortium on risk for early onset Parkinson disease study. Arch Neurol. 2010;67(9):1116-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El Mecky J, Johansson L, Plantinga M, et al. Reinterpretation, reclassification, and its downstream effects: challenges for clinical laboratory geneticists. BMC Med Genomics. 2019;12(1):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senkevich K, Rudakou U, Gan-Or Z. New therapeutic approaches to Parkinson's disease targeting GBA, LRRK2 and Parkin. Neuropharmacology. 2022;202:108822. [DOI] [PubMed] [Google Scholar]
- 43.Alcalay RN, Hall A, Marder K, et al. PD GENEration: mapping the future of Parkinson's disease [online]. 2021. Accessed October 23, 2021. parkinson.org/PDGENEration.
- 44.Lee MJ, Pak K, Kim JH, et al. Effect of polygenic load on striatal dopaminergic deterioration in Parkinson disease. Neurology. 2019;93(7):e665-e674. [DOI] [PubMed] [Google Scholar]
- 45.Pihlstrøm L, Morset KR, Grimstad E, Vitelli V, Toft M. A cumulative genetic risk score predicts progression in Parkinson's disease. Mov Disord. 2016;31(4):487-490. [DOI] [PubMed] [Google Scholar]
- 46.Eusebi P, Romoli M, Paoletti FP, Tambasco N, Calabresi P, Parnetti L. Risk factors of levodopa-induced dyskinesia in Parkinson's disease: results from the PPMI cohort. NPJ Parkinsons Dis. 2018;4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Complete results from the survey are available in eTable 4 (links.lww.com/NXG/A530). For all subjects who consented for public data sharing, sequencing data are in process for release in relevant genomic databases. The complete sequencing data are also available on request by contacting the corresponding author, Dr. Shulman (joshua.shulman@bcm.edu).