Abstract
Background
Airway clearance techniques are supposed to be a necessary adjunct for the enhancement of impaired peripheral clearance in cystic fibrosis (CF). The objective was to assess the effect of one physiotherapy session (autogenic drainage: AD) on mucus clearance (sputum wet weight) and impulse oscillometry system (IOS) indices, including those obtained from extended Resistance-Inertance-Compliance (eRIC) modelling, considering the degree of bronchial congestion.
Methods
Thirty children with CF (median age: 12.7 years) in a stable condition prospectively underwent IOS measurements at baseline and after AD. They were divided in two groups: with (visual analog scale of bronchial congestion by the physiotherapist ≥ 5/10) and without (scale < 5/10) bronchial congestion. Paired-comparison of the effects of AD on airway resistance measurements was done with Wilcoxon test.
Results
The congestion scale correlated with the wet weight of sputum production during the session (Pearson test: p < 0.0001, R = 0.66). Ten children had bronchial congestion and 20 were without congestion. In the whole group, R5–20 Hz significantly decreased after AD (P = 0.049), which was related to a decrease in the children with congestion (P = 0.025), whereas it was not significantly modified in the children without congestion (P = 0.327). The eRIC model allowed the calculation of the peripheral resistance of the respiratory system, which also decreased in the children with congestion (P = 0.037), however, not modified in the children without congestion (P = 0.390).
Conclusion
One session of autogenic drainage has the ability to decrease peripheral resistance obtained from IOS measurements, more specifically in children with CF with moderate to severe bronchial congestion.
Trial registration
ClinicalTrials.gov Identifier: NCT04094441.
Keywords: Chest physiotherapy, Impulse oscillometry, Lung model, Cystic fibrosis, Childhood
Background
In 1985, Kirilloff and colleagues posed the question, “Does chest physical therapy work?”, and in their review, they concluded that chest physical therapy has frequently been shown to be of benefit in cystic fibrosis (CF) [1]. Generally, patients with other chronic lung diseases have shown improvement following chest physical therapy if they produce large volumes of sputum [1]. Moreover, Bateman and colleagues reported that radioaerosol clearance increased fivefold in the central and intermediate lung regions and fourfold in peripheral lung regions when compared with control days [2], suggesting the ability of airway clearance techniques to mobilise distal airway secretions. These findings confirmed that cough only partially compensated for impaired mucociliary clearance and airway clearance techniques were a necessary adjunct for the enhancement of impaired peripheral lung clearance [2].
Airway clearance techniques are usually commenced as soon as the diagnosis of CF is made. In a systematic review, Warnock and Gates evaluated the different modalities of airway clearance techniques in CF [3]. The results of this review showed that airway clearance techniques had short-term effects in the terms of increasing mucus transport [3]. Overall, these techniques seem to provide similar effects in terms of airway clearance and recent guidelines state that, in most cases, there is little evidence to support the use of one technique over another [4, 5]. In a long-term comparative study in adolescents with CF, autogenic drainage (AD) was as effective as postural drainage, and participants showed strong preference for AD [6]. The effects of airway clearance techniques on pulmonary function indices remain debated, since either no effect, detrimental or beneficial effects have been shown, mostly using spirometry [3].
Based on this background, we hypothesised that AD in children with CF could improve peripheral airway indices, depending on the volume of sputum cleared during the session. Sakarya and colleagues showed that the indices obtained from the impulse oscillometry system (IOS) were improved after treatment of an acute exacerbation in children with CF [7]. IOS is a variant of the forced oscillation technique, which allows passive measurement of total respiratory system impedance. In this method, indices describing resistance to airflow (respiratory system resistance, RRS) and reactive indices that mostly relate to the efficient storage and return of energy by the lung (respiratory system reactance, XRS) are obtained [8]. Since IOS measurements involve frequencies and impedances, it is possible to correlate the measurements to respiratory system models consisting of analogous electrical components [8], to provide parameter estimates that are physiologically more realistic and relevant. The extended Resistance-Inertance-Compliance (eRIC) model gives access to two peripheral markers of small airway disease, peripheral compliance and resistance of the respiratory system [8]. The objective of this prospective study in children with CF was to assess the effect of a single AD session on mucus clearance (sputum wet weight) and IOS indices, including those obtained from eRIC modelling, taking into account the degree of bronchial congestion.
Methods
Design (cross-sectional study)
This interventional study was registered (ClinicalTrials.gov Identifier: NCT04094441) on September 19, 2019.
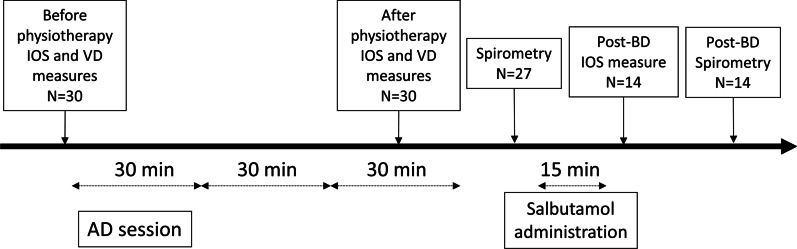
The subjects did not perform physical therapy on the morning of the tests and received no bronchodilator or mucolytic treatment in the 12 h preceding the tests. Since forced expiration has been associated with cough, spirometry was not performed at baseline in order not to induce a bias related to expectoration of mucus. The timeline of study procedures is provided in Fig. 1.
Fig. 1.
Timeline of study procedures. IOS denotes impulse oscillometry system; VD denotes anatomical dead space; AD denotes autogenic drainage. 27/30 children performed spirometry after the AD session and among them 14 had a bronchodilator test (salbutamol 400 µg) due to airflow limitation
Patients
Children 4–18 years with CF followed at the Cystic Fibrosis Centre (CRCM) at Robert Debré hospital were enrolled during their annual check-ups. All subjects were clinically stable (no antibiotics for an exacerbation in the preceding 4 weeks). This study conformed to the standards set by the latest revision of the Declaration of Helsinki and was approved by an ethics committee (CPP SUD-OUEST et OUTRE MER II, n° IDRCB: 2017-AO2426-47). The parents of the children gave written informed consent; assent was also obtained from children.
Chest physiotherapy
Assessment of AD session: before the session, the degree of bronchial congestion was evaluated by both the child (absence versus presence) and physiotherapist (based on auscultation, visual analogic scale, from 0 [absence] to 10 [maximum]). The children with CF were then divided in two groups according to the visual analogue scale: < 5/10 (absence to mild bronchial congestion denoted no congestion) and ≥ 5/10 (moderate to severe bronchial congestion denoted congestion). All sputum samples produced during the session were collected and weighed (within 15 min after the end of the session). The sputum weight was determined in the pulmonary function test unit by an independent investigator, who was blinded to the congestion rating.
AD was performed by four experienced chest physiotherapists devoted only to children with CF. AD is an airway clearance technique developed by Chevallier that is based on non-forced expiration during controlled breathing at different levels of vital capacity [9, 10]. Briefly, while sitting, the patient first performs diaphragmatic breathing at low lung volume following a cycle of slow inspiration through the nose to total pulmonary capacity, followed by a pause of three seconds, then non-forced exhalation through the nose or mouth to residual volume. These steps are repeated until mucus movement can be felt. The entire breathing cycle is repeated at progressively increasing lung volumes, so that mucus is moved from the small to the mid-sized to the large airways. Finally, mucus is evacuated from the trachea by huffing. In this study, the cycles were repeated for a total AD session of approximately 30 min.
Children practiced chest physiotherapy on a usual basis (three times a week with a physiotherapist).
Pulmonary function tests
Impedance of the respiratory system was measured using an IOS (Master Scope Body, Carefusion Technologies, Yorba Linda, California, USA), as previously described [8, 11]. The quality criterion for IOS measurements was a coherence value ≥ 0.6 at 5 Hz [11]. Five trials of IOS were repeated and we kept only the three recordings yielding the lowest coefficient of variation of impedance at 5 Hz for analysis, as previously described [11] and accordingly to recent guidelines [12]. We used the following IOS variables: impedance at 5, 10, 15, 20, 25, 30 and 35 Hz, resistance and reactance at 5 Hz and 20 Hz, fall in resistance between R5 and R20 (R5–20 Hz), area under the reactance curve (AX) and resonance frequency (Fres). The % predicted and z-scores of the IOS variables were calculated according to Gochicoa-Rangel et al. [13]. The assessment of IOS was between 30 and 60 min after AD session based on the results of Hortal and Hjelte [14].
We used one mechanistic model capable of accounting for significant frequency dependence of the respiratory impedance, which has previously been described [8]. The RIC model is constituted by a Resistance, an Inertance (IRS) and a Compliance of the respiratory system. In the eRIC model, resistance is partitioned in the central resistance (RcentralRS) and peripheral resistance (RperipheralRS) of the respiratory system, while the peripheral compliance of the respiratory system (CpRS) includes the parenchymal and chest wall compliances. The model was fitted to the impedance data (5–35 Hz) and the minimization of a performance index allowed the calculation of model indices [8]. To determine the relative appropriateness of the various inverse model topologies, we used the corrected Akaike information criterion (AICc) [8].
Anatomical dead space (VD) measurement was obtained using the apparatus for multiple breath washout measurement (Exhalyzer D, Eco Medics AG, Duernten, Switzerland with Spiroware 3.1.6 software) based on the analysis of expired CO2 pressure. The mean VD obtained from five respiratory cycles was recorded. VD (cm3) was further normalised by height (cm), due to the close relationship between these two factors. One may hypothesise that mucus clearance due to AD could increase “free” anatomical dead space.
Spirometry (Master Scope Body, Carefusion Technologies, Yorba Linda, California, USA) was obtained according to international guidelines [15] in older (aged ≥ 6 years) children only. The z-scores of the spirometry indices were calculated based on Global Lung Initiative-2012 reference values [16]. A bronchodilator test (salbutamol 400 µg using an inhaler device) was further performed in children with airflow limitation (z-score of FEV1/FVC < − 1.645). A secondary objective was to evaluate the peripheral effects of salbutamol.
Statistical analyses
The power calculation for this study was based on data published for CF patients in a study that evaluated the changes in respiratory system resistance and reactance in adult CF subjects after a single AD session [17]. As a result, we planned to include a similar number of patients (n = 30). Our primary objective was to show that peripheral resistance (R5–20 Hz) decreased after AD. Using 30 participants, it allowed to show a decrease in R5–20 Hz of 0.05 kPa/L/s after one AD session with a Standard Deviation of 0.095 for its change (α, two-tailed, of 0.05 and β of 0.20) using the T statistic (with a non-centrality parameter) [18].
The results are expressed as median [25th–75th percentile] for continuous data and as frequency (percentage) for categorical data, except when stated. Comparisons between baseline and after AD or between after AD and after salbutamol were performed using the Wilcoxon rank paired test. Comparisons of the continuous variables between the two groups of children were performed using the Mann–Whitney U test [19]. Categorical variables were compared using a chi-square test with Fisher correction when needed. The other tests are described in the text. A P value < 0.05 was deemed significant. No correction for multiple testing was done due to the pathophysiological design of the study [20]. Statistical analyses were performed with StatView 5.0 (SAS institute, Cary, North Carolina, USA) software.
Results
IOS and eRIC model qualities
The mean (± standard deviation) of the coefficients of variation of Fres, R20Hz, R5Hz, R5–20 Hz, X5Hz and AX were 6 ± 3, 5 ± 4, 6 ± 3, 7 ± 4, 10 ± 10, 12 ± 11; respectively.
The intraclass correlation coefficient within-test of Fres, R20Hz, R5Hz, R5–20 Hz, X5Hz and AX were excellent: 0.95 [0.92; 0.98], 0.98 [0.96; 0.99], 0.98 [0.95; 0.99], 0.98 [0.95; 0.99], 0.94 [0.91; 0.97], 0.93 [0.91; 0.97]; respectively.
Performance index at baseline, after physiotherapy and after salbutamol were 0.0095 [0.0048; 0.0198], 0.0068 [0.0046; 0.0093] and 0.0073 [0.0045; 0.0138] kPa2 s2 L−2, respectively. The corrected Akaike information criterion (appropriateness of the various inverse model topologies) at baseline, after AD and after salbutamol were − 86 [− 96; − 76], − 91 [− 96; − 86] and − 90 [− 97; − 81], respectively. Overall, the model adequately fitted the experimental data (Fig. 2).
Fig. 2.
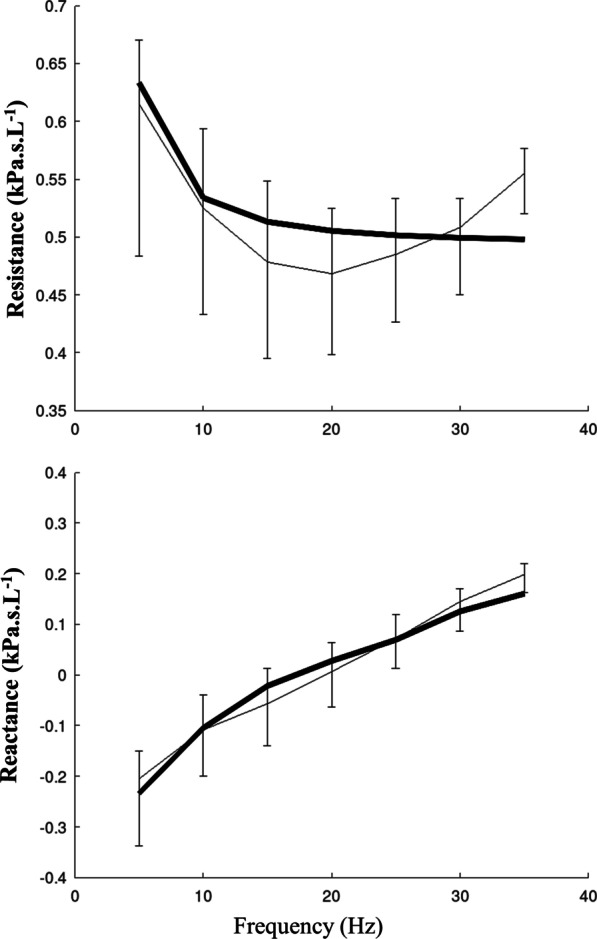
The impedance spectra obtained by fitting the model. Respiratory system resistance (upper panel) and reactance (lower panel) obtained by fitting the model (thick solid lines) to the baseline data of the experimental measurements represented by the median values and the 25th and 75th percentiles for each frequency (thin lines). Overall, the model adequately fitted experimental data
Between-group comparisons
The clinical and functional characteristics of the 30 enrolled children with CF are described in Tables 1 and 2, respectively. The range of age was 5 to 18 years, 4 children were younger than 7 years and 6 children were older than 16 years. The congestion score given by the physiotherapist before the session correlated with the wet weight of sputum production during the session (Pearson test, P < 0.0001, R = 0.66). There was moderate agreement between the child and the physiotherapist (% of agreement: 73%, Cohen’s kappa: 0.478) (Table 1). Results of the pulmonary function tests were not significantly different between the two groups of children (Table 2).
Table 1.
Clinical characteristics of the 30 CF children
| Characteristics | With bronchial congestion N = 10 |
Without bronchial congestion N = 20 |
Between 2 groups (with and without) at baseline |
|---|---|---|---|
| Ethnicity C/B/A/M | 9/0/1/0 | 16/2/0/2 | 0.253 |
| Age, years | 14.6 [9.7; 16.3] | 11.8 [7.9; 14.5] | 0.333 |
| Sex, female/male | 7/3 | 5/15 | 0.045 |
| Weight, kg | 40.5 [33.8; 48.0] | 40.0 [23.0; 50.5] | 0.552 |
| Weight z-score | − 0.60 [− 0.84; − 0.03] | − 0.66 [− 1.13; 0.04] | 0.391 |
| Height, cm | 150 [139; 160] | 146 [122; 160] | 0.843 |
| Height z-score | − 0.44 [− 1.03; 0.16] | − 0.75 [− 1.04; 0.00] | 0.644 |
| BMI z-score | − 0.48 [− 0.58; − 0.03] | − 0.34 [− 0.89; 0.71] | 0.509 |
| Pancreatic insufficiency, n | 10 | 17 | 0.519 |
| Previous pseudomonas aeruginosa colonisation, n | 10 | 13 | 0.093 |
| Dornase alfa/azithromycin, n | 9/3 | 17/5 | ND |
| Inhaled β-agonist/corticosteroid, n | 1/1 | 3/3 | ND |
| CFTR modulator therapy, n | 4 | 5 | ND |
| Mutation | |||
| Homozygous /heterozygous/other* | 4/4/2 | 7/6/7 | 0.689 |
| Score (VAS) of congestion | 6.5 [5.0; 7.0] | 2.0 [1.0; 3.0] | ND |
| Sputum weight, g | 7.9 [6.1; 12.1] | 1.7 [0.9; 4.5] | < 0.001 |
| Congestion absent/present# | 1/9 | 13/7 | 0.007 |
Ethnicity Caucasian/Black/Asian/Mixed; BMI denotes Body Mass Index; VAS denotes Visual Analogical Score
* homozygous and heterozygous ΔF508 mutations
#based on child’s opinion, absent = absent (or almost absent), present = frank presence
VAS denotes visual analog scale and ND denotes not done. Bold P values are those < 0.05
Table 2.
Functional characteristics of the 30 CF children
| Characteristics | With bronchial congestion | Without bronchial congestion | After versus before AD, all patients | After salbutamol versus before all patients | Between 2 groups (with and without) at baseline | ||||
|---|---|---|---|---|---|---|---|---|---|
| N = 10 | N = 20 | ||||||||
| Lung function | Before AD | After AD | After salbutamol | Before AD | After AD | After salbutamol | |||
| VD, cm3 | 80 [71; 112] | 90 [75; 104] | ND | 76 [44; 121] | 85 [55; 120] | ND | 0.097 | 0.509 | |
| VD/height, cm2 | 0.53 [0.46; 0.70] | 0.61 [0.55; 0.66] | ND | 0.53 [0.37; 0.70] | 0.60 [0.41; 0.75] | ND | 0.098 | 0.660 | |
| Spirometry, patients, n | 10 | 7 | 17 | 7 | |||||
| FEV1, L | ND | 1.66 [1.14; 2.32] | 1.87 [1.38; 2.42] | ND | 2.01 [1.56; 2.59] | 1.81 [0.97; 2.49] | 0.007 | ||
| FEV1, z-score | ND | − 2.49 [− 3.51; − 2.09] | − 2.30 [− 3.47; − 1.80] | ND | − 0.72 [− 2.41; 0.14]$ | − 2.38 [− 3.89; − 2.25] | |||
| FVC, L | ND | 2.33 [1.55; 3.31] | 2.38 [1.89; 3.35] | ND | 2.73 [1.97; 3.54] | 2.51 [1.77; 3.47] | 0.195 | ||
| FVC, z-score | ND | − 1.53 [− 2.66; − 0.35] | − 1.18 [− 2.34; − 1.11] | ND | − 0.16 [− 1.52; 0.48] | − 1.54 [− 2.03; − 0.35] | |||
| FEV1/FVC | ND | 0.75 [0.70; 0.77] | 0.78 [0.72; 0.79] | ND | 0.81 [0.70; 0.83] | 0.69 [0.59; 0.76] | 0.064 | ||
| FEV1/FVC, z-score | ND | − 2.08 [− 2.53; − 1.44] | − 1.84 [− 2.31; − 1.68] | ND | − 1.04 [− 2.31; − 0.84] | − 2.39 [− 3.48; − 1.49] | |||
| IOS, patients, n | 10 | 10 | 10 | 20 | 20 | 20 | |||
| Fres, Hz | 20.97 [14.34; 23.93] | 18.82 [13.88; 26.12] | 11.9 [11.2; 25.3] | 19.21 [13.76; 25.13] | 18.99 [14.74; 22.95] | 18.3 [14.5; 25.5] | 0.943 | 0.016 | |
| Fres, z-score | 0.85 [− 0.70; 1.02] | 0.54 [− 0.87; 2.61] | − 0.90 [− 3.79; 2.35] | 0.46 [− 1.01; 1.89] | 0.18 [− 0.87; 0.96] | 0.17 [− 0.84; 1.12] | 0.481 | ||
| R20Hz, kPa/L/s | 0.47 [0.31; 0.58] | 0.43 [0.28; 0.54] | 0.43 [0.29; 0.52] | 0.47 [0.39; 0.61] | 0.49 [0.39; 0.61] | 0.43 [0.33; 0.65] | 0.607 | 0.075 | |
| R20Hz, z-score | 1.53 [0.84; 2.25] | 1.60 [0.70; 2.18] | 1.59 [0.32; 2.00] | 1.64 [1.16; 2.68] | 1.70 [1.04; 2.42] | 1.62 [1.31; 2.37] | 0.660 | ||
| R5Hz, kPa/L/s | 0.64 [0.38; 0.80] | 0.60 [0.37; 0.75] | 0.41 [0.35; 0.76] | 0.59 [0.45; 0.88] | 0.61 [0.46; 0.85] | 0.51 [0.42; 1.16] | 0.217 | 0.034 | |
| R5Hz, z-score | 1.31 [0.51; 2.35] | 1.10 [0.20; 2.56] | 0.84 [0.15; 1.69] | 1.32 [0.54; 2.46] | 1.17 [0.54; 1.85] | 1.17 [0.83; 3.77] | 0.895 | ||
| R5–20 Hz, kPa/L/s | 0.17 [0.06; 0.22] | 0.12 [0.05; 0.22] | 0.07 [0.02; 0.23] | 0.15 [0.07; 0.32] | 0.14 [0.06; 0.19] | 0.11 [0.07; 0.34] | 0.049 | 0.278 | |
| R5–20 Hz, z-score | 0.47 [0.04; 1.70] | 0.33 [− 0.32; 1.42] | 0.25 [− 0.36; 1.12] | 0.55 [− 0.42; 1.57] | 0.49 [− 0.49; 0.87] | 0.62 [0.30; 2.00] | 0.509 | ||
| X5Hz, kPa/L/s | − 0.24 [− 0.34; − 0.20] | − 0.23 [− 0.33; − 0.19] | − 0.16 [− 0.33; − 0.14] | − 0.19 [− 0.31; − 0.15] | − 0.20 [− 0.27; − 0.15] | − 0.16 [− 0.31; − 0.12] | 0.604 | 0.041 | |
| X5Hz, z-score | 1.59 [0.58; 2.27] | 1.48 [0.87; 1.67] | 0.83 [0.67; 1.52] | 0.88 [0.06; 1.72] | 0.57 [0.16; 1.21] | 0.76 [0.45; 2.11] | 0.065 | ||
| AX, kPa/L | 1.34 [0.73; 2.76] | 1.21 [0.68; 2.87] | 0.39 [0.35; 2.97] | 1.18 [0.50; 2.98] | 1.16 [0.58; 2.06] | 0.86 [0.41; 3.97] | 0.328 | 0.016 | |
| AX, % z-score | 0.92 [0.70; 1.91] | 1.25 [0.45; 1.73] | 0.84 [0.31; 2.02] | 0.82 [0.21; 2.75] | 0.76 [0.00; 1.28] | 0.90 [0.51; 2.63] | 0.253 | ||
| eRIC model | |||||||||
| RcentralRS, kPa/L/s | 0.49 [0.32; 0.56] | 0.44 [0.29; 0.52] | 0.42 [0.29; 0.48] | 0.49 [0.40; 0.60] | 0.50 [0.40; 0.62] | 0.41 [0.33; 0.63] | 0.600 | 0.116 | 0.403 |
| IRS × 100, kPa/L/s2 | 1.02 [0.90; 1.16] | 0.91 [0.88; 1.01] | 0.97 [0.90; 1.09] | 0.97 [0.76; 1.15] | 0.98 [0.75; 1.10] | 0.95 [0.80; 1.13] | 0.159 | 0.023 | 0.567 |
| CpRS × 100, L/kPa | 7.83 [5.57; 11.63] | 7.15 [5.90; 12.48] | 16.14 [5.51; 18.86] | 8.80 [4.00; 15.28] | 9.44 [7.00; 15.37] | 11.05 [4.00; 16.34] | 0.416 | 0.011 | 0.930 |
| RperipheralRS, kPa/L/s | 0.89 [0.68; 1.04] | 0.71 [0.50; 0.94] | 0.77 [0.62; 5.00] | 1.00 [0.77; 1.67] | 1.01 [0.66; 1.05] | 0.67 [0.51; 1.14] | 0.082 | 0.311 | 0.403 |
VD is the anatomical volume of airways; AD denotes Autogenic Drainage
$ The 7 children with airflow limitation who had a bronchodilator test had z-scores of FEV1 of − 2.72 [− 3.79; − 2.31] and FVC of − 1.55 [− 1.90; − 1.00] that have to be compared to the “After salbutamol” results
ND denotes not done. Bold P values are those < 0.05
The correlations between VD and height were highly significant (before physiotherapy: R = 0.91; after physiotherapy: R = 0.86, p < 0.0001 for both) justifying the normalisation
Effect of chest physiotherapy (30 children)
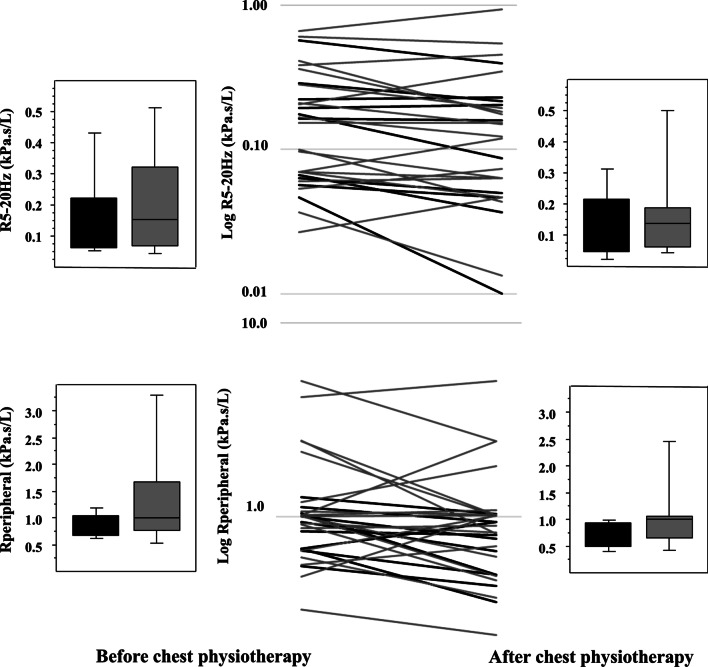
The effects of AD on two peripheral markers of resistance (R5–20 Hz and RperipheralRS) are described in Fig. 3, taken into account the presence or absence of bronchial congestion.
Fig. 3.
Indices of peripheral resistance assessed before and after AD. The upper panels describe R5-R20Hz resistance of the respiratory system before (left panel) and after (right panel) a single AD session. Box and whisker plots show median, 25th and 75th percentiles, and 10th and 90th percentiles in children with CF with (dark grey) and without (light grey) bronchial congestion. The middle panel describes individual data of the 30 children: black lines for the 10 children with bronchial congestion and light grey lines for the 20 children without bronchial congestion. To better highlight individual changes of R5–20 Hz, the Y-axis is a logarithmic base 10 scale. A significant decrease in the raw value of R5–20 Hz was observed in the children with bronchial congestion (P = 0.025) whereas it was not significant in those without congestion (P = 0.327). The lower panels describe RperipheralRS computed using eRIC model before (left panel) and after (right panel) a single AD session. Box and whisker plots show median, 25th and 75th percentiles, and 10th and 90th percentiles in children with CF with (dark grey) and without (light grey) bronchial congestion. The middle panel describes individual data of the 30 children: black lines for the 10 children with bronchial congestion and light grey lines for the 20 children without bronchial congestion. To better highlight individual changes of RperipheralRS, the Y-axis is a logarithmic base 10 scale. A significant decrease in RperipheralRS (P = 0.037) is evidenced after physiotherapy in the children with bronchial congestion, which was not significant in those without congestion (P = 0.390)
In the whole group (n = 30), R5–20 Hz significantly decreased after AD (P = 0.049, Table 1), which was related to a decrease in the 10 children with congestion (Fig. 3), whereas it was not significantly modified in the 20 children without congestion. The change in z-scores of R5–20 Hz (pre minus post AD value) in children with congestion was + 0.13 [− 0.03; + 0.79] and 0.00 [− 0.32; + 0.25] in those without congestion. RperipheralRS was not significantly modified after AD in the whole group. Nevertheless, it significantly decreased in the children with congestion (Fig. 3), whereas it was not modified in the no congestion group.
The other indices obtained from IOS or eRIC model were not significantly modified after AD (data not shown).
When the effects of AD were evaluated based on the opinion of the child (absence, n = 14 or presence of congestion, n = 16), similar results (a significant effect of AD in children with presence of congestion) were evidenced for both R5–20 Hz (P = 0.010) and RperipheralRS (P = 0.026).
AD did not significantly modify VD, neither in the whole population (P = 0.097) nor in children with (P = 0.138) or without (P = 0.489) bronchial congestion.
Effect of salbutamol (14 children)
Bronchodilator administration was associated with a significant increase in FEV1 in the 14 children who performed postbronchodilator spirometry due to due to airflow limitation persisting after AD (Table 2), which was due to its effect in children having had bronchial congestion: P = 0.018 versus children without bronchial congestion (children without bronchial congestion, P = 0.172). Among the IOS indices, Fres (P = 0.016), AX (P = 0.016), R5Hz (P = 0.034) and X5Hz (P = 0.041) were significantly modified by the bronchodilator. The eRIC model showed significant modifications of IRS and CpRS (Table 2).
Discussion
Main results
The main result of our prospective study is to demonstrate that one session of AD performed by a physiotherapist has the ability to decrease two indices of peripheral resistance obtained from IOS measurement, specifically in children with CF with moderate to severe bronchial congestion. Additionally, bronchodilator administration after AD improved other indices of lung function, suggesting complementary benefits.
Effect of physiotherapy on mucus clearance
Sputum weight was in agreement with the weight expected based on the literature on CF patients [21, 22]. The main effect of airway clearance techniques occurs in the presence of large volumes of sputum production in other bronchial diseases [1]. Our results in CF are similar, suggesting that the improvement in peripheral resistance of the respiratory system was related to mucus withdrawal from the distal airways in the children depicting moderate to severe bronchial congestion.
In a similar study, Wallaert et al. performed forced oscillation tests before and after a single AD session [17], but did not consider the degree of bronchial congestion. Their results showed a moderate decrease in inspiratory resistance in the global (Rrs5) and proximal (Rrs11 and Rrs19) airways, but not in the distal compartment (Rrs5–Rrs19) in 30 adult CF subjects [17]. The authors hypothesised that AD did not significantly increase the luminal calibre of the distal airways. They argued that the sputum coming from the proximal part of the lung is significantly cleared immediately after the AD session, in contrast to the more peripheral secretions. It must be emphasised that their adult population exhibited moderate to severe obstructive ventilatory disorders that may have prevented distal mucus clearance. Their results are in contradiction with those showing that cough only partially compensated for impaired mucociliary clearance and that airway clearance techniques were a necessary adjunct for the enhancement of impaired peripheral lung clearance [2].
Effect of AD on resistance
The eRIC model adequately fitted the experimental data of children with CF, the modeling error was inferior to the error observed in asthmatic children [8]. It emphasizes that small airway indices (peripheral resistance and compliance of the respiratory system) are important contributors of respiratory system impedance in CF disease. We show that R5–20 Hz and peripheral resistance decreased by 30% and 20% (% baseline) respectively after AD in children with bronchial congestion, which is a small effect as expected. For instance, in the study of Pfleger and colleagues, the FEV1 significantly increased by 2.24% predicted or 80 mL after chest physiotherapy [23]. One may wonder whether this level of improvement is higher than the repeatability of IOS measurement and whether it is clinically significant. We show an excellent repeatability that was even better than that obtained by others [24]. Nevertheless, the change in z-score of R5–20 Hz is lower than its within-visit variability in children with bronchial congestion, which emphasizes that a significant statistical effect cannot be guaranteed in all participants. But, the level of R5–20 Hz decrease is within the range of values defining significant bronchodilator response (~ 15 to 40%) [24, 25]. This level of improvement is also in accordance with that observed following treatment of CF exacerbation [26–28].
Similarly, in a recent study, significant changes were observed in the values of R5–R20Hz and AX, after an oscillatory positive expiratory pressure session and thoracic compression, in the comparison with rest in adult patients with non-CF bronchiectasis, also suggesting peripheral effects of airway clearance techniques [29]. The beneficial effect of oscillatory positive expiratory pressure, combined with DNase, has also been recently shown in children/adolescents with CF who showed an immediate decrease in airway resistance and reactance [30].
Effect of AD on anatomical dead space
AD, despite mucus clearance, was unable to increase the anatomical dead space in our study. This result could be related to the specific distal effect of AD that was evidenced using IOS measurements. Moreover, using functional respiratory imaging, Leemans and colleagues showed that the volume of the airways decreased following high-frequency chest wall oscillation in CF patients, whereas resistance increased [22]. They explained this result based on a mucus shift from the periphery towards the central lung regions [22], a proposition confirmed by fluid dynamics simulation works [31]. In this latter study, the authors showed that the main effect of chest physiotherapy is to reduce the hydrodynamic resistance of the airway tree by secretion redistribution in the tree [31].
Effect of salbutamol
The long-term effects of bronchodilator administration in CF remain debated [32]. In the evaluation of bronchodilator response in 33 CF patients, Öztürk and colleagues found significant changes in IOS (decrease in AX, Fres and R5-R20Hz) and spirometry (increase in FEV1) after bronchodilator use [33]. We observed quite similar results. It has been shown that sympathomimetics relieve bronchospasm in many CF subjects, but since they enhance airway compressibility, they thereby decrease peripheral expiratory airflow in some subjects [34]. The increase in respiratory system compliance that is demonstrated using the eRIC model is in agreement with the enhancement of airway compressibility. Overall, the effects of bronchodilator administration seem complementary to those of AD.
Study limitations
Our study has limitations that are related to its design. Only short-term effects of AD were evaluated in a restricted sample of children with CF. The two-group subdivision may seem artifactual since sputum production during the AD session was a continuum, but similar results were obtained based on the child’s opinion. Moreover, in the whole group, a decrease in R5–R20Hz was demonstrated. Our results were obtained in children with mildly affected lung function and may not be generalizable to all subjects with CF. The predicted values of IOS that have been used were developed in Mexican children [13], which may constitute a limitation; but their adequacy for Caucasian children has further been demonstrated [35].
Sputum elimination was also determined in the present study. For this purpose, the wet sputum weight was measured immediately after the interventions. It is well known that mucus quantity, as volume or weight, cannot represent mucus depuration without considering the possible losses by swallowing or errors in its collection [36]. Furthermore, saliva contamination can represent a confounding factor for this measurement. Even if the assessment of bronchial congestion using auscultation and sputum weight has inherent limitations, their correlation is reassuring; the strong correlation (r value 0.6 to 0.8) evidenced also suggests that interobserver variability of bronchial congestion assessment was restricted, may be due to the involvement of experienced and specialized physiotherapists, which is a limitation for the generazibility of our results since different physiotherapists could obtain different results, even using a similar technique. Computer Aided Lung Sound Analysis is proposed as an objective, non-invasive, bedside clinical measure with the potential to monitor and assess the effects of airway clearance therapy [37], which remains to be demonstrated. Whether other kinds of airway clearance techniques can afford similar benefits remains to be evaluated and it remains to be highlighted that AD in our study improved lung function in children with bronchial congestion. Finally, since our study was not a randomized clinical trial, we show that AD is associated with a change in R5–20 Hz but a causal relationship cannot be formally inferred. The changes evidenced were very small and probably not associated with any short-term clinical effect. This was beyond the subject of our study, which focused on the distal effects of AD, which deserved to be demonstrated.
In conclusion, one session of AD performed by a physiotherapist has the ability to decrease peripheral resistance obtained from IOS measurements, more specifically in children with CF with moderate to severe bronchial congestion.
Acknowledgements
The authors thank the nurses of the pulmonary function testing unit who performed the spirometry and IOS measurements.
Author contributions
Conceptualization: PB, BM and CD2; Formal analysis: PB, CD2; Investigation: PB, MG, GB, EDCN, RJ, AVF, LLC, VH; Methodology: GB, EDCN, RJ, AVF, PB, BM, VH; Project administration: CD2, MG, LLC, VH; Supervision: CD2, AVF, VH; Validation: PB, BM, CD2; Roles/Writing—original draft: PB, CD2; Writing—review & editing: all authors. All authors read and approved the final manuscript.
Funding
VirtualChest project, supported by a grant from the National Agency for Research (N° ANR-16-CE19-0014-05). Sponsor: Assistance Publique – Hôpitaux de Paris.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study conformed to the standards set by the latest revision of the Declaration of Helsinki and was approved by an ethics committee (CPP SUD-OUEST et OUTRE MER II, n° IDRCB: 2017-AO2426-47). The parents of the children gave written informed consent; assent was also obtained from children.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kirilloff LH, Owens GR, Rogers RM, Mazzocco MC. Does chest physical therapy work? Chest. 1985;88:436–444. doi: 10.1378/chest.88.3.436. [DOI] [PubMed] [Google Scholar]
- 2.Bateman JR, Newman SP, Daunt KM, Sheahan NF, Pavia D, Clarke SW. Is cough as effective as chest physiotherapy in the removal of excessive tracheobronchial secretions? Thorax. 1981;36:683–687. doi: 10.1136/thx.36.9.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warnock L, Gates A. Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis. Cochrane Database Syst Rev. 2015;2015:CD001401. doi: 10.1002/14651858.CD001401.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellani C, Duff AJA, Bell SC, Heijerman HGM, Munck A, Ratjen F, et al. ECFS best practice guidelines: the 2018 revision. J Cyst Fibros. 2018;17:153–178. doi: 10.1016/j.jcf.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Button BM, Wilson C, Dentice R, Cox NS, Middleton A, Tannenbaum E, et al. Physiotherapy for cystic fibrosis in Australia and New Zealand: a clinical practice guideline. Respirology. 2016;21:656–667. doi: 10.1111/resp.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McIlwaine M, Wong LT, Chilvers M, Davidson GF. Long-term comparative trial of two different physiotherapy techniques; postural drainage with percussion and autogenic drainage, in the treatment of cystic fibrosis. Pediatr Pulmonol. 2010;45:1064–1069. doi: 10.1002/ppul.21247. [DOI] [PubMed] [Google Scholar]
- 7.Sakarya A, Uyan ZS, Baydemir C, Anık Y, Erdem E, Gokdemir Y, et al. Evaluation of children with cystic fibrosis by impulse oscillometry when stable and at exacerbation. Pediatr Pulmonol. 2016;51:1151–1158. doi: 10.1002/ppul.23449. [DOI] [PubMed] [Google Scholar]
- 8.Bokov P, Bafunyembaka G, Medjahdi N, Bernard A, Essalhi M, Houdouin V, et al. Cross-sectional phenotyping of small airway dysfunction in preschool asthma using the impulse oscillometry system. J Asthma. 2021;58:573–585. doi: 10.1080/02770903.2020.1719133. [DOI] [PubMed] [Google Scholar]
- 9.McCormack P, Burnham P, Southern KW. Autogenic drainage for airway clearance in cystic fibrosis. Cochrane Database Syst Rev. 2017;10:CD009595. doi: 10.1002/14651858.CD009595.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnham P, Stanford G, Stewart R. Autogenic drainage for airway clearance in cystic fibrosis. Cochrane Database Syst Rev. 2021;12:CD009595. doi: 10.1002/14651858.CD009595.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bokov P, Gerardin M, Le Clainche L, Houdouin V, Delclaux C. Impulse oscillometry indices to detect an abnormal lung clearance index in childhood cystic fibrosis. Pediatr Pulmonol. 2021 doi: 10.1002/ppul.25649. [DOI] [PubMed] [Google Scholar]
- 12.King GG, Bates J, Berger KI, Calverley P, de Melo PL, Dellacà RL, et al. Technical standards for respiratory oscillometry. Eur Respir J. 2020;55:1900753. doi: 10.1183/13993003.00753-2019. [DOI] [PubMed] [Google Scholar]
- 13.Gochicoa-Rangel L, Torre-Bouscoulet L, Martínez-Briseño D, Rodríguez-Moreno L, Cantú-González G, Vargas MH. Values of impulse oscillometry in healthy Mexican children and adolescents. Respir Care. 2015;60:119–127. doi: 10.4187/respcare.03374. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez Hortal MC, Hjelte L. Time point to perform lung function tests evaluating the effects of an airway clearance therapy session in cystic fibrosis. Respir Care. 2014;59:1537–1541. doi: 10.4187/respcare.02823. [DOI] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallaert E, Perez T, Prevotat A, Reychler G, Wallaert B, Le Rouzic O. The immediate effects of a single autogenic drainage session on ventilatory mechanics in adult subjects with cystic fibrosis. PLoS ONE. 2018;13:e0195154. doi: 10.1371/journal.pone.0195154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow S-C, Shao J, Wang H. A note on sample size calculation for mean comparisons based on noncentral t-statistics. J Biopharm Stat. 2002;12:441–456. doi: 10.1081/BIP-120016229. [DOI] [PubMed] [Google Scholar]
- 19.Lee SW. Methods for testing statistical differences between groups in medical research: statistical standard and guideline of Life Cycle Committee. Life Cycle. 2022;2:e1. doi: 10.54724/lc.2022.e1. [DOI] [Google Scholar]
- 20.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. doi: 10.1097/00001648-199001000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Phillips GE, Pike SE, Jaffé A, Bush A. Comparison of active cycle of breathing and high-frequency oscillation jacket in children with cystic fibrosis. Pediatr Pulmonol. 2004;37:71–75. doi: 10.1002/ppul.10358. [DOI] [PubMed] [Google Scholar]
- 22.Leemans G, Belmans D, Van Holsbeke C, Becker B, Vissers D, Ides K, et al. The effectiveness of a mobile high-frequency chest wall oscillation (HFCWO) device for airway clearance. Pediatr Pulmonol. 2020;55:1984–1992. doi: 10.1002/ppul.24784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfleger A, Steinbacher M, Schwantzer G, Weinhandl E, Wagner M, Eber E. Short-term effects of physiotherapy on ventilation inhomogeneity in cystic fibrosis patients with a wide range of lung disease severity. J Cyst Fibros. 2015;14:627–631. doi: 10.1016/j.jcf.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Knihtilä H, Kotaniemi-Syrjänen A, Pelkonen AS, Kalliola S, Mäkelä MJ, Malmberg LP. Small airway oscillometry indices: repeatability and bronchodilator responsiveness in young children. Pediatr Pulmonol. 2017;52:1260–1267. doi: 10.1002/ppul.23794. [DOI] [PubMed] [Google Scholar]
- 25.Park J-H, Lee JH, Kim H-J, Jeong N, Jang H-J, Kim H-K, et al. Usefulness of impulse oscillometry for the assessment of bronchodilator response in elderly patients with chronic obstructive airway disease. J Thorac Dis. 2019;11:1485–1494. doi: 10.21037/jtd.2019.03.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren CL, Brucker JL, Rovitelli AK, Bordeaux KA. Changes in lung function measured by spirometry and the forced oscillation technique in cystic fibrosis patients undergoing treatment for respiratory tract exacerbation. Pediatr Pulmonol. 2006;41:345–349. doi: 10.1002/ppul.20390. [DOI] [PubMed] [Google Scholar]
- 27.Buchs C, Coutier L, Vrielynck S, Jubin V, Mainguy C, Reix P. An impulse oscillometry system is less efficient than spirometry in tracking lung function improvements after intravenous antibiotic therapy in pediatric patients with cystic fibrosis. Pediatr Pulmonol. 2015;50:1073–1081. doi: 10.1002/ppul.23301. [DOI] [PubMed] [Google Scholar]
- 28.Loukou I, Moustaki M, Deligianni A, Sardeli O, Douros K. Forced oscillation technique for monitoring the respiratory status of children with cystic fibrosis: a systematic review. Children (Basel) 2021;8:857. doi: 10.3390/children8100857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Souza Simoni LH, Dos Santos DO, de Souza HCD, Baddini-Martinez JA, Santos MK, Gastaldi AC. Acute effects of oscillatory PEP and thoracic compression on secretion removal and impedance of the respiratory system in non-cystic fibrosis bronchiectasis. Respir Care. 2019;64:818–827. doi: 10.4187/respcare.06025. [DOI] [PubMed] [Google Scholar]
- 30.Gonçalves Wamosy RM, Castilho T, Almeida ACDS, de Assumpção MS, Ludwig Neto N, Schivinski CIS. Immediate effect of inhalation therapy combined with oscillatory positive expiratory pressure on the respiratory system of children with cystic fibrosis. Int J Clin Pract. 2021;75:e14659. doi: 10.1111/ijcp.14659. [DOI] [PubMed] [Google Scholar]
- 31.Mauroy B, Flaud P, Pelca D, Fausser C, Merckx J, Mitchell BR. Toward the modeling of mucus draining from human lung: role of airways deformation on air-mucus interaction. Front Physiol. 2015;6:214. doi: 10.3389/fphys.2015.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barry PJ, Flume PA. Bronchodilators in cystic fibrosis: a critical analysis. Expert Rev Respir Med. 2017;11:13–20. doi: 10.1080/17476348.2017.1246358. [DOI] [PubMed] [Google Scholar]
- 33.Kartal Öztürk G, Eşki A, Gülen F, Demir E. Is Impulse oscillometry system a useful method for the evaluation and follow-up of patients with cystic fibrosis? Pediatr Allergy Immunol Pulmonol. 2021;34:15–22. doi: 10.1089/ped.2020.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eber E, Oberwaldner B, Zach MS. Airway obstruction and airway wall instability in cystic fibrosis: the isolated and combined effect of theophylline and sympathomimetics. Pediatr Pulmonol. 1988;4:205–212. doi: 10.1002/ppul.1950040404. [DOI] [PubMed] [Google Scholar]
- 35.Gochicoa-Rangel L, Del Río-Hidalgo R, Hernández-Ruiz J, Rodríguez-Moreno L, Martínez-Briseño D, Mora-Romero U, et al. Validating reference equations for impulse oscillometry in healthy Mexican children. Respir Care. 2017;62:1156–1165. doi: 10.4187/respcare.05247. [DOI] [PubMed] [Google Scholar]
- 36.Arens R, Gozal D, Omlin KJ, Vega J, Boyd KP, Keens TG, et al. Comparison of high frequency chest compression and conventional chest physiotherapy in hospitalized patients with cystic fibrosis. Am J Respir Crit Care Med. 1994;150:1154–1157. doi: 10.1164/ajrccm.150.4.7921452. [DOI] [PubMed] [Google Scholar]
- 37.Marques A, Bruton A, Barney A. Clinically useful outcome measures for physiotherapy airway clearance techniques: a review. Phys Therapy Rev. 2006;11:299–307. doi: 10.1179/108331906X163441. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.