Abstract
Introduction
Fluid challenges are widely adopted in critically ill patients to reverse haemodynamic instability. We reviewed the literature to appraise fluid challenge characteristics in intensive care unit (ICU) patients receiving haemodynamic monitoring and considered two decades: 2000–2010 and 2011–2021.
Methods
We assessed research studies and collected data regarding study setting, patient population, fluid challenge characteristics, and monitoring. MEDLINE, Embase, and Cochrane search engines were used. A fluid challenge was defined as an infusion of a definite quantity of fluid (expressed as a volume in mL or ml/kg) in a fixed time (expressed in minutes), whose outcome was defined as a change in predefined haemodynamic variables above a predetermined threshold.
Results
We included 124 studies, 32 (25.8%) published in 2000–2010 and 92 (74.2%) in 2011–2021, overall enrolling 6,086 patients, who presented sepsis/septic shock in 50.6% of cases. The fluid challenge usually consisted of 500 mL (76.6%) of crystalloids (56.6%) infused with a rate of 25 mL/min. Fluid responsiveness was usually defined by a cardiac output/index (CO/CI) increase ≥ 15% (70.9%). The infusion time was quicker (15 min vs 30 min), and crystalloids were more frequent in the 2011–2021 compared to the 2000–2010 period.
Conclusions
In the literature, fluid challenges are usually performed by infusing 500 mL of crystalloids bolus in less than 20 min. A positive fluid challenge response, reported in 52% of ICU patients, is generally defined by a CO/CI increase ≥ 15%. Compared to the 2000–2010 decade, in 2011–2021 the infusion time of the fluid challenge was shorter, and crystalloids were more frequently used.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-04056-3.
Keywords: Fluids, Fluid challenge, Fluid bolus, Fluid responsiveness, Critically ill patients
Introduction
Fluid administration in the intensive care unit (ICU) is one of the most common and disputed interventions triggered at the bedside by several clinical variables [1, 2].
Fluid therapy aims to increase stroke volume (SV) and cardiac output (CO) to optimise systemic blood flow and tissue perfusion. As with any therapeutic intervention, the final clinical effect elicited may vary because of a complex interplay between the patient's intrinsic conditions and the therapy itself.
Fluid responsiveness can occur only if both ventricles work on the ascending, steep part of the Frank–Starling curve, i.e. in cases where CO is preload dependent [3, 4]. Preload dependency is assessed using a diagnostic test performed by infusing a fixed aliquot of fluid, the fluid challenge [5–7]. From a clinical perspective, this approach also allows titration of fluid administration (when the patient becomes no longer responsive to the fluid challenge) and reduces the risk of fluid overload, which worsens the outcome of ICU patients [8, 9].
Several variables defining the characteristics of the fluid challenge have been further investigated in studies adopting continuous haemodynamic monitoring, showing that the amount of fluids given, the rate of administration, and the threshold adopted to define fluid responsiveness impact the outcome of a fluid challenge [10–12]. Moreover, despite conflicting results on shock reversal efficacy between crystalloids and colloids, crystalloids are now recommended as the first-line fluid type in patients with septic shock, being inexpensive and widely available. Also, the administration of colloids compared to crystalloids has not demonstrated any clear benefit in the literature [13, 14].
However, neither the nature, mode of administration, and method to assess the effectiveness of the fluid challenge are standardised in current clinical practice, and the definition of fluid challenge responsiveness is also variable among studies [15–18].
Whether or not these findings have modified the modalities of fluid challenge and the definition of fluid responsiveness in published studies is uncertain. To address this issue, we systematically reviewed existing literature from the year 2000. We appraised the characteristics of fluid challenges in critically ill patients (i.e., amount and kind of fluid administration, time of infusion, hemodynamic variables, and thresholds for fluid responsiveness) enrolled in research studies receiving continuous haemodynamic monitoring and assessed the relationship between the reported fluid responsiveness and predefined independent variables. Secondarily, we compared data from studies published in 2011–2021 versus those published in 2000–2010.
Material and methods
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis-Protocols (PRISMA-P) guidelines (Additional file 1: Table S1). The study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) in November 2021 (CRD42021284761).
Search strategy
A systematic literature search was performed, including the following databases: PUBMED®, EMBASE®, and the Cochrane Controlled Clinical trials register. The following keywords and their related MeSh terms were used: “fluid challenge”, “fluid responsiveness”, “stroke volume variation”, “pulse pressure variation”, “dynamic indices OR indexes”, “passive leg raising”, OR “passive leg raising test”, “functional haemodynamic test OR tests”. Included papers were also examined to identify other studies of interest missed during the primary search.
Study selection and inclusion criteria
Articles enrolling at least 20 adult critically ill patients, written in English and published from 1st January 2000 to 31st December 2021 in indexed scientific journals, were considered. Editorials, commentaries, letters to the editor, opinion articles, reviews, and meeting abstracts were excluded. Studies enrolling paediatric or obstetric populations were excluded. References of selected papers, review articles, commentaries, and editorials on this topic were also reviewed to identify other studies of interest missed during the primary search. When multiple publications of the same research group/centre described potentially overlapping cohorts, the most recent publications were selected.
A fluid challenge was defined as an infusion of a definite quantity of fluid (expressed as a volume in mL or ml/kg) in a fixed time (expressed in minutes), whose outcome was defined as a change in one of the following haemodynamic variables above a predetermined threshold: CO, cardiac index (CI), SV, SV index (SVI), or surrogate of SV, i.e., velocity–time integral (VTI) in the left ventricular outflow tract and aortic blood flow (ABF), as assessed by transthoracic, transoesophageal echocardiography or oesophageal Doppler. We included studies adopting both a specific (i.e., Ringer lactate, saline, etc.) and a broad definition (i.e., crystalloids, colloids, etc.) of the fluid used for the fluid challenge. Studies adopting changes in systemic arterial pressure to define fluid responsiveness were excluded. Finally, we considered the predefined clinical reasons and triggers to start fluid challenge infusion.
Data extraction
Three couples of examiners independently evaluated titles and abstracts. The articles were then subdivided into three subgroups: “included” and “excluded” (if the two examiners agreed with the selection) or “uncertain” (in case of disagreement). In the case of “uncertain” classification, discrepancies were resolved by further examination performed by one of the three expert authors (A.M., X.M., or M.C.). We used a standardised electronic spreadsheet (Microsoft Excel, V 14.4.1; Microsoft, Redmond, WA) to extract data from all included studies, recording: the study setting (type of study, geographical area and time, where and when the study was carried out, and sample size), patient characteristics (gender, age, reason for admission, underlying diseases, ICU scores of severity, mode of ventilation, and inotropic/vasopressor support), criteria for haemodynamic instability, fluid challenge characteristics, pre- and post-fluid challenge haemodynamic variables. When necessary, the corresponding authors of the included studies were contacted to obtain missing data related to trial demographics, methods, and outcomes (Additional file 1: Table S2).
Statistical analysis
Statistical analysis was conducted on the summary statistics described in the selected articles (e.g., means, medians, proportions) and, therefore, the statistical unit of observation for all the selected variables was the single study and not the patient. Due to the discrepancy between the overall patients enrolled in the trials over the two considered decaders, the comparisons were not weighted for study size.
Fluid challenge was the exposure variable, and clinical and haemodynamic characteristics were considered outcome variables. Descriptive statistics of individual studies used different statistical indicators for central tendency and variability, such as means and standard deviations (i.e., age, tidal volume, fluid responders, severity scores), whereas absolute and relative frequencies were adopted for qualitative variables. To show one indicator for the quantitative variables, we collected means with standard deviations (SD) or medians and inter-quartile ranges (IQR).
Student's t test or Mann–Whitney test in case of parametric or nonparametric distributions, respectively, were used to assess the difference in mean values between responders and non-responders.
Statistical analyses were conducted using GraphPad PRISM® 8 (GraphPad Software Inc., San Diego, CA, USA) and STATA®15 (StataCorp, College Station, TX, USA). For all comparisons, we considered p values < 0.05 significant.
Results
The electronic search identified 3,963 potentially relevant studies. Figure 1 and Additional file 1: Table S3 provide a detailed description of the selection process flow. After evaluating 160 full-text manuscripts, the inclusion criteria were met by 124 studies, 32 (25.8%) published in the period 2000–2010 and 92 (74.2%) in the period 2011–2021. Ten studies (8.1%) required revision by senior examiners because of disagreement regarding inclusion criteria between the initial examiners. We did not find any further relevant publications by reviewing the bibliography of the selected studies.
Fig. 1.
Flow of the studies; *Reasons for studies' exclusion are reported in the Supplementary materials
The general characteristics of the patients are reported in Table 1. We included 6,086 patients, with a median (IQR) of 38 (30–59) patients enrolled in each study. Six studies (4.8%) [20–25] were retrospective, while the others were prospective. The median (IQR) period of enrolment [reported in 66 (52.8%) studies] was 12 (6–18) months. At baseline, 2,985 (49.0%) patients received norepinephrine, 179 (2.9%) dopamine, 416 (6.8%) dobutamine, and 177 (2.8%) epinephrine.
Table 1.
Patient characteristics at enrolment
| General characteristics | Overall (n = 6086) |
% DR | 2011–2021 (n = 1243) |
2000–2010 (n = 4843) |
p value** |
|---|---|---|---|---|---|
| Age (year) | 63 (59–65) | (95.9) | 63 (59–65) | 63 (58–67) | 0.52 |
| Male (n, %) | 3552 (58.3) | (87.1) | 2837 (58.6) | 715 (57.5) | 0.52 |
| SAPS II (points) | 53 (45–59) | (39.5) | 55 (45–60) | 53 (40–57) | 0.34 |
| SOFA (points) | 10 (7–11) | (22.5) | 10 (7–11) | 11 (9–14) | 0.10 |
| APACHE (points) | 20 (19–26) | (22.5) | 24 (19–27) | 19 (12–21) | 0.12 |
| Data regarding respiratory support | (70.9) | ||||
| Totally controlled ventilatory support (n; %) | 2658 (56.6) | 1955 (56.2) | 703 (57.9) | 0.29 | |
| PCV (n; %) | 150 (3.2) | 128 (3.6) | 22 (1.8) | ||
| VCV (n; %) | 2428 (51.7) | 1747 (50.2) | 681 (56.1) | ||
| APRV (n; %) | 80 (1.7) | 80 (2.3) | 0 (0–0) | ||
| Partially controlled ventilatory support (n; %) | 1503 (32.0) | 1161 (33.4) | 342 (28.1) | 0.05 | |
| ACV (n; %) | 1256 (26.7) | 990 (28.4) | 266 (21.9) | ||
| PSV (n; %) | 247 (5.3) | 171 (4.9) | 76 (6.2) | ||
| Spontaneously breathing (n; %) | 530 (10.1) | 362 (10.4) | 168 (13.8) | 0.001 | |
| VT (mL/Kg ideal body weight) | 7 (7–8) | (70.9) | 7 (6–8) | 8 (7–9) | 0.03 |
| Type of patients* | (91.9) | ||||
| Sepsis/septic shock (%) | 3546 (50.6) | 2823 (50.5) | 723 (51.2) | 0.63 | |
| Haemorrhagic/hypovolemic shock (%) | 344 (4.9) | 252 (13.9) | 92 (6.5) | < 0.0001 | |
| Trauma (%) | 120 (1.7) | 101 (1.8) | 19 (1.3) | 0.25 | |
| ARDS/pneumonia (%) | 1741 (24.8) | 1428 (25.5) | 313 (22.2) | 0.009 | |
| Postoperative optimization (%) | 1005 (14.3) | 780 (13.9) | 225 (15.9) | 0.06 | |
| Cardiogenic (%) | 193 (2.7) | 160 (2.8) | 33 (2.3) | 0.31 | |
| Other (%) | 53 (0.7) | 47 (2.8) | 6 (0.5) | 0.12 |
Data presented as median (25th–75th IQR), as appropriate; % DR, percentage of studies reporting the data indicated, SAPS, simplified acute physiology score; SOFA, sequential organ failure assessment; APACHE, Acute Physiologic Assessment and Chronic Health Evaluation; PCV, pressure-controlled ventilation; VCV, volume-controlled ventilation; APRV, Airway pressure release ventilation; ACV, assisted-controlled ventilation; PSV, pressure support ventilation; ARDS, acute respiratory distress syndrome; VT, tidal volume. *The overall number of patients stratified by typology is more significant as compared to the overall number of patients included in the studies because of partial overlapping classification (i.e., sepsis/septic shock and ARDS/pneumonia); **p value refers to the comparison between 2000–2010 versus 2011–2021 subgroups
The reliability of a functional haemodynamic test in predicting fluid responsiveness was assessed in 46 (37.1%) studies. Comparing the two considered decades, no difference was found in the rate of FC administration [17 min (17–33) vs. 33 min (17–50); p = 0.39), in the percentage of fluid responders [52% (43–67) vs. 53% (45–60); p = 0.91], in the percentage of studies adopting crystalloids over colloids [63.6% vs. 67.9%; p = 1.00), or in the threshold of increase in CO or surrogates adopted to define fluid responsiveness (10% over 15%) [18.2% vs. 24.1%; p = 1.00).
Forty-four studies (35.4%) investigated the reliability of a dynamic index in predicting fluid responsiveness. Comparing the two considered decades, no differences were found in the rate of FC administration [17 min (17–25) vs. 29 min (13–33) p = 0.42), or in the rate of fluid responders [53% (41–62) vs. 50% (44–56) p = 0.81), or in the threshold of increase in CO or surrogates adopted to define fluid responsiveness (10% over 15%) (78.5% vs. 66.67 p = 0.42), as compared to studies in the decade 2000–2010. On the contrary, in the decade 2010-2021 we adopted more frequently crystalloids (21.4% vs. 60.0% p = 0.024).
Fluid challenge characteristics and haemodynamic monitoring
Overall, the included studies infused 6,333 fluid challenges. The median (IQR) proportion of fluid responders was 52 (44–62)% (Table 2).
Table 2.
Fluid challenge characteristics and haemodynamic monitoring in the included studies
| References | Year | Vol (ml) | Vol (ml/kg) | Time (min) | Rate (ml/min) | Responsiveness cut-off | Type of fluid | Monitoring device | % R |
|---|---|---|---|---|---|---|---|---|---|
| Mahjoub et al. [35] | 2010 | 500 | – | 30 | 17 | SV ≥ 15% | CRYS—Saline | CARDIOQ | 76 |
| Feissel et al. [37] | 2004 | 500 | – | 20 | 25 | CO ≥ 15% | COLL—HES 6% | ECO—TT/TE | 41 |
| Marik et al. [39] | 2013 | 500 | – | 10 | 50 | SVI ≥ 10% | CRYS—Saline | NICOM | 53 |
| Wyffels et al. [41] | 2007 | 500 | – | 20 | 25 | CI ≥ 15% | COLL—HES 6% | PAC | 62 |
| Jozwiak et al. [43] | 2017 | 500 | – | 10 | 50 | CI ≥ 15% | CRYS—Saline | CAL—PiCCO2 | 50 |
| Monnet et al. [45] | 2009 | 500 | – | 10 | 50 | CI ≥ 15% | CRYS—Saline | CAL—PiCCO2 | 70 |
| Monnet et al. [47] | 2012 | 500 | – | 20 | 25 | CI ≥ 15% | CRYS—Saline | CAL—PiCCO2 | 55 |
| Vaquer et al. [20] | 2020 | 500 | – | 30 | 17 | SVI ≥ 15% | CRYS—Saline | CAL—PiCCO2 | 34 |
| Chen et al. [49] | 2021 | 500 | – | 40 | 13 | CI ≥ 15% | COLL—HES 6% | CAL—PiCCO2 | 60 |
| Abdullah et al. [49] | 2021 | 500 | – | 10 | 50 | SVI ≥ 15% | CRYS—Saline | UNCAL—FLOWTRAC/VIGILEO | 46 |
| Messina et al. [52] | 2021 | 500 | – | 10 | 50 | SVI ≥ 10% | CRYS—Ringer A/L | UNCAL—MOSTCARE | 48 |
| Taccheri et al. [54] | 2021 | 500 | – | 10 | 50 | CI ≥ 10% | CRYS—Saline | CAL—PiCCO2 | 50 |
| Kaur et al. [56] | 2021 | 500 | – | 20 | 25 | CO ≥ 15% | CRYS—Ringer A/L | UNCAL—FLOWTRAC/VIGILEO | 67 |
| Biasucci et al. [58] | 2019 | 500 | – | 30 | 17 | CI ≥ 15% | COLL—HES 6% | PAC | 60 |
| Gavaud et al. [60] | 2019 | 500 | – | 15 | 33 | CO ≥ 10% | CRYS—Saline | ECO—TT/TE | 90 |
| Dépret et al. [62] | 2019 | 500 | – | 10 | 50 | CI ≥ 15% | CRYS—Saline | CAL—PiCCO2 | 50 |
| Messina et al. [64] | 2019 | 500 | – | 10 | 50 | CI ≥ 15% | CRYS—Saline | UNCAL—MOSTCARE | 66 |
| Vistisen et al. [66] | 2018 | 500 | – | 30 | 17 | SV ≥ 10% | CRYS—Saline | NICOM | 23 |
| Xu et al. [68] | 2017 | 500 | – | 15 | 33 | CI ≥ 15% | COLL—Gelatine | PAC | 45 |
| Preau et al. [69] | 2017 | – | 6 | 30 | – | SVI ≥ 10% | COLL—Gelatine | CAL—PiCCO2 | 55 |
| Machare-Delgado et al. [71] | 2011 | – | 6 | 10 | – | SVI ≥ 10% | CRYS—Saline | ECO—TT/TE | 32 |
| Monnet et al. [73] | 2013 | – | 7 | 30 | – | CI ≥ 15% | CRYS—Saline | CAL—PiCCO2 | 49 |
| Monnet et al. [74] | 2007 | 500 | – | 8 | 67 | ABF ≥ 15% | CRYS—Saline | ECO—TT/TE | 54 |
| Ishihara et al. [76] | 2013 | 250 | – | 20 | 13 | CI ≥ 15% | COLL—Dextran 10% | CAL—PiCCO | 54 |
| Monge Garcia et al. [78] | 2012 | 500 | – | 30 | 17 | CO ≥ 15% | COLL—HES 6% | CARDIOQ | 57 |
| Luzi et al. [80] | 2013 | 500 | – | 30 | 17 | SV ≥ 15% | CRYS—Saline | ECO—TT/TE | 50 |
| Dong et al. [82] | 2012 | 500 | – | 30 | 17 | SVI ≥ 15% | COLL—HES 6% | CAL—PiCCO | 69 |
| Jabot et al. [84] | 2008 | – | 20 | 10 | – | CI ≥ 15% | CRYS—Saline | CAL—PiCCO | 100 |
| Préau et al. [86] | 2010 | 500 | – | 30 | 17 | SV ≥ 15% | COLL—HES 6% | ECO—TT/TE | 41 |
| Monnet et al. [88] | 2006 | 500 | – | 10 | 50 | ABF ≥ 15% | CRYS—Saline | ECO—TT/TE | 52 |
| Monnet et al. [47] | 2012 | 500 | – | 20 | 25 | CI ≥ 15% | CRYS—Saline | CAL—PiCCO | 56 |
| Monnet et al. [91] | 2013 | 500 | – | 30 | 17 | CI ≥ 15% | CRYS—Saline | CAL—PiCCO | 43 |
| Loupec et al. [93] | 2011 | 500 | – | 10 | 50 | CO ≥ 15% | COLL—HES 6% | ECO—TT/TE | 53 |
| Monnet et al. [95] | 2012 | – | 8 | 30 | – | CI ≥ 15% | CRYS—Saline | CAL—PiCCO | 44 |
| Huang et al. [97] | 2008 | – | 7 | 40 | – | CI ≥ 15% | COLL—HES 6% | CAL—PiCCO | 46 |
| Khwannimit et al. [98] | 2012 | 500 | – | 30 | 17 | SVI ≥ 15% | COLL—HES 6% | UNCAL—FLOWTRAC/VIGILEO | 57 |
| Fischer et al. [100] | 2013 | – | 7 | 15 | – | CI ≥ 15% | COLL—HES 6% | CAL—PiCCO | 71 |
| Kramer et al. [102] | 2004 | – | 7 | 15 | – | CO ≥ 15% | Blood | PAC | 29 |
| Yazigi et al. [104] | 2012 | – | 10 | 20 | – | SVI ≥ 15% | COLL—HES 6% | PAC | 68 |
| Wyler von Ballmoos et al. [106] | 2010 | 200 | 7 | 10 | 20 | SV ≥ 10% | COLL—HES 6% | PAC | 28 |
| Michard et al. [108] | 2000 | 500 | 6 | 30 | 17 | CI ≥ 15% | COLL—HES 6% | PAC | 40 |
| Lakhal et al. [110] | 2011 | 500 | – | 30 | 17 | CO ≥ 10% | COLL—Gelatine | PAC | 40 |
| Muller et al. [112] | 2012 | 500 | – | 15 | 33 | VTI ≥ 15% | COLL—HES 6% | ECO—TT/TE | 50 |
| Giraud et al. [114] | 2011 | 500 | – | 10 | 50 | CI ≥ 15% | CRYS—Saline | PAC | 47 |
| Suehiro et al. [116] | 2012 | 500 | – | 30 | 17 | CI ≥ 15% | CRYS—Ringer A/L | UNCAL—FLOWTRAC/VIGILEO | 48 |
| Perner et al. [117] | 2006 | – | 4 | 30 | – | CI ≥ 10% | COLL—Dextran 6% | CAL—PiCCO | 47 |
| Smorenberg et al. [118] | 2013 | 250 | – | 15 | 17 | SVI ≥ 10% | COLL—Gelatine | PAC | 44 |
| Monnet et al. [119] | 2012 | – | 10 | 30 | – | CI ≥ 15% | CRYS—Saline | CAL—PiCCO | 42 |
| Yonis et al. [121] | 2017 | 500 | – | 15 | 33 | CI ≥ 10% | CRYS—Saline | CAL—PiCCO | 33 |
| Xiao-ting et al. [122] | 2015 | 500 | – | 15 | 33 | CI ≥ 10% | CRYS—Saline | CAL—PiCCO | 70 |
| Biais et al. [124] | 2009 | 500 | – | 15 | 33 | SV ≥ 15% | CRYS—Saline | UNCAL—FLOWTRAC/VIGILEO | 67 |
| Mallat et al. [126] | 2015 | 500 | – | 15 | 33 | CI ≥ 15% | COLL—HES 6% | CAL—PiCCO | 45 |
| Maizel et al. [127] | 2007 | 500 | – | 15 | 33 | CO ≥ 15% | CRYS—Saline | ECO—TT/TE | 50 |
| Lamia et al. [129] | 2007 | 500 | – | 15 | 33 | SV ≥ 15% | CRYS—Saline | ECO—TT/TE | 59 |
| Silva et al. [131] | 2004 | 500 | – | 30 | 17 | CI ≥ 10% | COLL—HES 6% | PAC | 63 |
| Cecconi et al. [133] | 2012 | 250 | – | 5 | 50 | SV ≥ 15% | COLL—HES 6% /Dextran 10% | CAL—LiDCO | 39 |
| Georges et al. [135] | 2018 | 500 | – | 15 | 33 | CO ≥ 15% | CRYS—Saline | ECO—TT/TE | 56 |
| Monnet et al. [137] | 2013 | 500 | – | 30 | 17 | CI ≥ 15% | CRYS—Saline | CAL—PiCCO | 52 |
| Monnet et al. [139] | 2005 | 500 | – | 10 | 50 | VTI ≥ 15% | CRYS—Saline | ECO—TT/TE | 53 |
| Biais et al. [141] | 2012 | 500 | – | 15 | 33 | SV ≥ 15% | CRYS—Saline | UNCAL—MOSTCARE | 54 |
| Lakhal et al. [115] | 2013 | 500 | – | 30 | 17 | CO ≥ 10% | COLL—Gelatine | PAC | 37 |
| Michard et al. [144] | 2003 | 500 | – | 30 | 17 | SVI ≥ 15% | COLL—HES 6% | CAL—PiCCO | 49 |
| References | Year | Vol (ml) | Vol (ml/kg) | Time (min) | Rate (ml/min) | Responsiveness cut-off | Type of fluid | Monitoring device | % R |
|---|---|---|---|---|---|---|---|---|---|
| Préau et al. [36] | 2012 | – | 6 | 30 | – | SV ≥ 15% | COLL—HES 6% | ECO—TT/TE | 44 |
| Caille et al. [38] | 2008 | 500 | – | 15 | 33 | CI ≥ 10% | COLL—HES 6% | ECO—TT/TE | 43 |
| Mahjoub et al. [40] | 2012 | 500 | – | 20 | 25 | SV ≥ 15% | CRYS—Saline | ECO—TT/TE | 71 |
| Wu et al. [42] | 2014 | 500 | – | 15 | 33 | CO ≥ 15% | CRYS—Saline | ECO—TT/TE | 54 |
| Fellahi et al. [44] | 2012 | – | 7 | 15 | – | CI ≥ 15% | COLL—HES 6% | CAL—PiCCO | 84 |
| Smorenberg et al. [46] | 2017 | 500 | – | 30 | 17 | CO ≥ 10% | COLL—HES 6% | CAL—LiDCO | 62 |
| Muller et al. [48] | 2011 | 500 | – | 15 | 33 | VTI ≥ 15% | COLL—HES 6% | ECO—TT/TE | 54 |
| Monnet et al. [28] | 2011 | 500 | – | 20 | 25 | CI ≥ 15% | CRYS—Saline | CAL—PiCCO | 62 |
| Monge Garcia et al. [50] | 2008 | 500 | – | 30 | 17 | SVI ≥ 15% | CRYS—Saline | UNCAL—FLOWTRAC/VIGILEO | 37 |
| Natalini et al. [51] | 2006 | 500 | – | 30 | 17 | CI ≥ 15% | CRYS—Saline | PAC | 59 |
| Mahjoub et al. [53] | 2009 | 500 | – | 30 | 17 | SV ≥ 15% | COLL—Gelatine | ECO—TT/TE | 66 |
| Fischer et al. [55] | 2013 | – | 7 | 15 | – | CI ≥ 15% | COLL—HES 6% | CAL—PiCCO | 73 |
| Vistisen et al. [57] | 2009 | 500 | – | 90 | 6 | CI ≥ 15% | COLL—HES 6% | PAC | 74 |
| Kupersztych-Hagege et al. [59] | 2013 | 500 | – | 10 | 50 | CI ≥ 15% | CRYS—Saline | CAL—PiCCO | 40 |
| Monge Garcìa et al. [61] | 2009 | 500 | – | 30 | 17 | SVI ≥ 15% | COLL—HES 6% | UNCAL—FLOWTRAC/VIGILEO | 50 |
| Lakhal et al. [63] | 2012 | 500 | – | 30 | 17 | CO ≥ 10% | COLL—Gelatine | PAC | 39 |
| Soubrier et al. [65] | 2007 | 500 | – | 20 | 25 | CI ≥ 15% | COLL—HES 6% | ECO—TT/TE | 59 |
| Fellahi et al. [67] | 2012 | 500 | – | 15 | 33 | CI ≥ 15% | COLL—HES 6% | CAL—PiCCO | 56 |
| Osman et al. [22] | 2007 | 500 | – | 20 | 25 | CI ≥ 15% | COLL—HES 6% | PAC | 43 |
| Lakhal et al. [70] | 2010 | 500 | – | 30 | 17 | CO ≥ 10% | COLL—Gelatine | CAL—PiCCO | 42 |
| De Oliveira-Costa et al. [72] | 2012 | 1000 | – | 30 | 33 | CI ≥ 15% | CRYS—Saline | PAC | 46 |
| Velissaris et al. [23] | 2011 | 500 | – | 30 | 17 | CI ≥ 10% | COLL | PAC | 52 |
| Monnet et al. [75] | 2011 | 500 | – | 10 | 50 | CI ≥ 10% | CRYS—Saline | CAL—PiCCO | 84 |
| Muller et al. [77] | 2010 | 500 | – | 30 | 17 | SVI ≥ 10% | COLL—HES 6% | CAL—PiCCO | 72 |
| Muller et al. [79] | 2008 | 500 | – | 30 | 17 | SVI ≥ 15% | COLL—HES 6% | CAL—PiCCO | 51 |
| Heenen et al. [81] | 2006 | 500 | – | 30 | 17 | CO ≥ 15% | COLL—HES 6% | PAC | 43 |
| De Backer et al. [83] | 2005 | 500 | – | 30 | 17 | CI ≥ 15% | COLL—HES 6% | PAC | 55 |
| Le Dorze et al. [85] | 2018 | 250 | – | 5 | 50 | SV ≥ 10% | CRYS—Saline | CARDIOQ | 35 |
| Wu et al. [87] | 2018 | 250 | – | 15 | 17 | SVI ≥ 15% | CRYS—Saline | ECO—TT/TE | 45 |
| Si et al. [89] | 2018 | 250 | – | 30 | 8 | SVI ≥ 15% | COLL—Albumine | CAL—PiCCO | 63 |
| Pouska et al. [90] | 2018 | – | 5 | 20 | – | SVI ≥ 15% | CRYS—Saline | CAL—PiCCO2 | 49 |
| Xu et al. [92] | 2017 | 250 | – | 10 | 25 | SV ≥ 10% | CRYS—Saline | NICOM | 41 |
| Soussi et al. [94] | 2017 | 500 | – | 15 | 33 | CI ≥ 15% | CRYS—Ringer A/L | CAL—PiCCO2 | 31 |
| Mallat et al. [96] | 2016 | 500 | – | 15 | 33 | CI ≥ 15% | CRYS—Saline | CAL—PiCCO | 52 |
| Aya et al. [32] | 2016 | 250 | – | 5 | 50 | CO ≥ 10% | CRYS—Ringer A/L | CAL—LiDCO | 50 |
| Hamimy et al. [99] | 2016 | 500 | – | 15 | 33 | SV ≥ 10% | CRYS—Saline | CARDIOQ | 89 |
| Liu et al. [101] | 2016 | 500 | – | 20 | 25 | CO ≥ 15% | CRYS—Saline | CAL—PiCCO | 54 |
| Guérin et al. [103] | 2015 | 500 | – | 10 | 50 | CI ≥ 15% | CRYS—Saline | CAL—PiCCO2 | 50 |
| Airapetian et al. [105] | 2015 | 500 | – | 15 | 33 | CO ≥ 10% | CRYS—Saline | ECO—TT/TE | 49 |
| Messina et al. [107] | 2015 | 500 | – | 10 | 50 | CI ≥ 15% | CRYS—Saline | UNCAL—MOSTCARE | – |
| Cecconi et al. [109] | 2015 | 250 | – | 8 | 33 | CO ≥ 10% | COLL—Gelatine | CAL—PiCCO | 50 |
| Soliman et al. [111] | 2015 | 500 | – | 10 | 50 | CI ≥ 15% | COLL—HES 6% | ECO—TT/TE | 56 |
| Nunes et al. [113] | 2014 | 500 | – | 30 | 17 | CI ≥ 15% | CRYS—Ringer A/L | PAC | 65 |
| Lakhal et al. [115] | 2013 | 500 | – | 30 | 17 | CO ≥ 10% | COLL—Gelatine | PAC | 37 |
| Monge García et al. [21] | 2015 | 500 | – | 30 | 17 | CO ≥ 10% | 0,COLL—Gelatine | CARDIOQ | 67 |
| Cecconi et al. [26] | 2013 | 250 | – | 5 | 50 | CO ≥ 10% | – | CAL—LiDCO | 43 |
| Hu et al. [24] | 2013 | 300 | – | 20 | 15 | CI ≥ 10% | COLL—HES 6% | CAL—PiCCO | 52 |
| Schnell et al. [120] | 2013 | 500 | – | 23 | 22 | ABF ≥ 10% | CRYS—Saline | ECO—TT/TE | 49 |
| Pranskunas et al. [27] | 2013 | 500 | – | 30 | 17 | SV ≥ 10% | – | CAL—VIGILANCE | 68 |
| Elsayed et al. [123] | 2021 | – | 4 | 15 | – | CO ≥ 15% | CRYS—Ringer A/L | ECO—TT/TE | 35 |
| Bataille et al. [125] | 2021 | 500 | – | 15 | 33 | SV ≥ 15% | CRYS—Ringer A/L | ECO—TT/TE | 50 |
| De Santis et al. [25] | 2021 | 500 | – | 30 | 17 | CI ≥ 10% | – | CAL—PiCCO | 58 |
| Kumar et al. [128] | 2021 | – | 10 | 30 | – | CI ≥ 10% | CRYS—Saline | UNCAL—FLOWTRAC/VIGILEO | 64 |
| Braun et al. [130] | 2020 | 500 | – | 15 | 33 | SV ≥ 15% | CRYS—Ringer A/L | CAL—PiCCO | 43 |
| Huette et al. [132] | 2020 | 500 | – | 10 | 50 | SV ≥ 15% | CRYS—Ringer A/L | ECO—TT/TE | 77 |
| Abdelfattah et al. [134] | 2020 | 500 | – | 15 | 33 | CO ≥ 15% | CRYS—Saline | ECO—TT/TE | 55 |
| Jacquet-Lagrèze et al. [136] | 2019 | 500 | – | 20 | 25 | CI ≥ 15% | CRYS—Ringer A/L | CAL—PiCCO | 38 |
| Beurton et al. [138] | 2019 | 500 | – | 10 | 50 | CI ≥ 15% | CRYS—Saline | CAL—PiCCO2 | 60 |
| Roger et al. [140] | 2019 | 500 | – | 10 | 50 | SV ≥ 15% | CRYS—Ringer A/L | ECO—TT/TE | 53 |
| Mukhtar et al. [142] | 2019 | 500 | – | 16 | 31 | SV ≥ 15% | COLL—Albumine | ECO—TT/TE | 68 |
| Trifi et al. [143] | 2019 | 500 | – | 15 | 33 | SV ≥ 15% | CRYS—Saline | ECO—TT/TE | 70 |
| Giraud et al. [145] | 2018 | 500 | – | 10 | 50 | CO ≥ 15% | CRYS—Saline | CAL—PiCCO | 45 |
COLL, colloids; CRYS, crystalloids; HES 6%, hydroxyethyl starch 6%; Ringer A\L, ringer acetate\lactate; CAL, thermodilution/chemodilution calibrated device; UNCAL, pulse wave analysis uncalibrated device; ABF, aortic blood flow; CO, cardiac output; CI, cardiac index; SV, stroke volume; SVI, stroke volume index; VTI, velocity-time integral; CardioQ, Deltex Medical Ltd, Chichester, UK; ECO-TT\TE, transthoracic\transoesophageal echocardiography; FLOWTRAC/VIGILEO, Edwards Lifescience Corporation, Irvine, Ca, USA; LIDCO, LIDCO group plc, London, UK; MOSTCARE, Pressure Recording Analytical Method, PRAM, Vytech Health®, Padova, Italy; NICOM, Non-Invasive Continuous Cardiac Output, Imedex, France; PAC, pulmonary artery catheter; PiCCO/ProAQT/PICCO2, PULSION Medical Systems; R, responders; Vol, volume
In 19 studies (15.3%), the volume of the fluid challenge was reported in mL/kg, with a median (IQR) of 7 (6–8) mL/kg (Table 2). A fixed volume of 500 mL was administered in 95 (76.6)% of the included studies. The median (IQR) of the dispensed volume of fluid was 500 (500–500) mL, infused in a median (IQR) of 18 (11–30) min. Then, the median (IQR) infusion rate was 25 (17–33) mL/min.
CO/CI was used as target variables in 78 (62.9%) studies, while SV/SVI was used in 40 (32.2%) studies. The other six studies (4.8%) adopted SV surrogates (ABF in 4 studies and VTI in two studies). In 88 (70.9%) studies, the threshold adopted to define the fluid responsiveness was an increase of the considered variable ≥ 15% from baseline (Table 2).
Three studies (2.4%) [25–27] did not report the type of fluid used for the fluid challenge. Among the others, crystalloids were used in 68 (56.6)% studies, colloids in 52 (43.3) %, and blood in one (0.8)% (Table 2).
The majority of the studies [49 (39.5%)] used transpulmonary thermodilution/dye dilution calibrated haemodynamic monitoring; 22 (17.7%) studies adopted the pulmonary artery catheter monitoring. Echocardiography (either transthoracic or transoesophageal) was used in 31 (25.0)% of studies, and 5 (4.0%) used oesophageal doppler monitoring. Uncalibrated pulse wave analysis monitoring was used in the other 14 (11.2)% studies (Table 2). Finally, bioreactance was adopted in three studies (2.4%). Haemodynamic pre–post-fluid challenge variables in responders and non-responders populations are reported in Table 3.
Table 3.
Haemodynamic parameters before and after fluid challenge administration in responders and non-responders
| Haemodynamic variable | % DR | Pre FC | Post FC | % change pre versus post FC |
p value–pre FC R versus NR |
p value pre FC versus post FC |
|---|---|---|---|---|---|---|
| CI (L/min/m2) | 44.3 | |||||
| R | 2.8 (2.5–3.2) | 3.6 (3.0–4.1) | 29 (23–33) | 0.0003 | < 0.0001 | |
| NR | 3.3 (2.7–3.6) | 3.4 (2.9–3.7) | 5 (0–6) | 0.09 | ||
| SVI (ml/m2) | 22.6 | |||||
| R | 29 (26–33) | 39 (36–42) | 29 (25–38) | 0.0001 | < 0.0001 | |
| NR | 36 (31–41) | 37 (31–42) | 3 (− 1; 7) | 0.05 | ||
| MAP (mmHg) | 73.4 | |||||
| R | 70 (68–74) | 82 (77–85) | 14 (10–18) | 0.005 | < 0.0001 | |
| NR | 74 (70–80) | 78 (75–85) | 6 (4–8) | < 0.0001 | ||
| SAP (mmHg) | 33.9 | |||||
| R | 104 (99–108) | 123 (113–129) | 17 (12–22) | 0.002 | < 0.0001 | |
| NR | 109 (105–118) | 116 (109–123) | 5 (4–8) | < 0.0001 | ||
| PAOP (mmHg) | 14.5 | |||||
| R | 11 (10–12) | 15 (12–16) | 28 (18–45) | 0.05 | < 0.0001 | |
| NR | 13 (10–14) | 16 (13–18) | 28 (15–37) | 0.005 | ||
| CVP (mmHg) | 42.7 | |||||
| R | 9 (7–11) | 11 (10–13) | 30 (19–41) | 0.03 | < 0.0001 | |
| NR | 10 (8–12) | 13 (10–15) | 26 (15–38) | < 0.0001 | ||
| HR (beats/min) | 75.8 | |||||
| R | 98 (88–105) | 94 (86–101) | − 3 (− 4; − 1) | 0.03 | < 0.0001 | |
| NR | 94 (86–101) | 91 (84–98) | − 2 (− 3; − 1) | < 0.0001 | ||
| PPV (%) | 30.6 | |||||
| R | 15 (12–18) | 9 (5–11) | − 42 (− 53; − 29) | < 0.0001 | < 0.0001 | |
| NR | 8 (6–10) | 7 (5–9) | − 15 (− 28; 0) | 0.0002 | ||
| SVV (%) | 14.5 | |||||
| R | 14 (12–17) | 10 (6–12) | − 36 (− 45; − 30) | < 0.0001 | 0.0002 | |
| NR | 11 (8–13) | 9 (6–11) | − 22 (− 33; − 9) | 0.002 |
Data are presented as median (25th–75th interquartile) in responders (R) and non-responders (NR), n = 21; FC, fluid challenge; CI, cardiac index; SVI, stroke volume index; MAP, mean arterial pressure; SAP, systolic pressure variation; HR, heart rate; PPV, pulse pressure variation; SVV, stroke volume variation, CVP, central venous pressure; PAOP, pulmonary artery occluded pressure; %DR, percentage of data reported in the studies
Trigger of fluid challenge administration.
Hypotension (i.e., systolic or mean arterial pressure below a fixed value or reduced by a fixed percentage from baseline) was used in 68 (62.4)% of studies. Oliguria (i.e. a drop in urine output below 0.5 mL/h for 2 or 3 consecutive hours) was used in 54 (49.5)% studies, skin mottling or peripheral hypoperfusion in 47 (43.1)% studies, tachycardia (i.e. an increase in heart rate above 100–110 beats/min) in 43 (39.4)%, the need for initiating the infusion or reducing the dose of vasoactive drugs in 41 (37.6)% studies, an increase in blood lactate in 34 (31.2)% studies, a diagnosis of sepsis/septic shock in 12 (11.0)% studies, and renal or hepatic dysfunction in seven (6.4)% studies. Fifteen studies (12.1%) did not report any trigger to start fluid challenge administration.
Comparison of publication periods 2011–2021 versus 2000–2010
The comparison between the 2000–2010 and 2011–2021 decades is reported in Table 4. The percentage of fluid responders (52% for both the decades) and the volume infused (500 mL) were comparable. On the contrary, the infusion time was lower in the last decade (a median of 15 (10–30) min vs 30 (15–30) min, p = 0.03). Crystalloids were used in 61.9% of studies published between 2011–2021 and 34.3% in the 2000–2010 decade (p = 0.007) (Figs. 2 and S1 in the Additional file 1).
Table 4.
Comparison between 2011–2021 and 2000–2010 decades regarding the modality of fluid challenge administration
| General characteristics | 2011–2021 | 2000–2010 | p value |
|---|---|---|---|
| Fluid responders (%) | 52 (45–60) | 52 (43–62) | 0.32 |
| Crystalloids versus colloids (n. of studies) | 57 versus 32 | 11 versus 20 | 0.007 |
| Volume (ml) | 500 (500–500) | 500 (500–500) | 0.32 |
| Time of infusion (min) | 15 (10–30) | 30 (15–30) | 0.03 |
| Threshold 10% versus 15% (n. of studies) | 30 versus 62 | 6 versus 26 | 0.17 |
| CO/CI versus SV/SVI (n. of studies) | 61 versus 30 | 18 versus 12 | 0.51 |
CO, cardiac output; CI, cardiac index; SV, stroke volume; SVI, stroke volume index
Fig. 2.
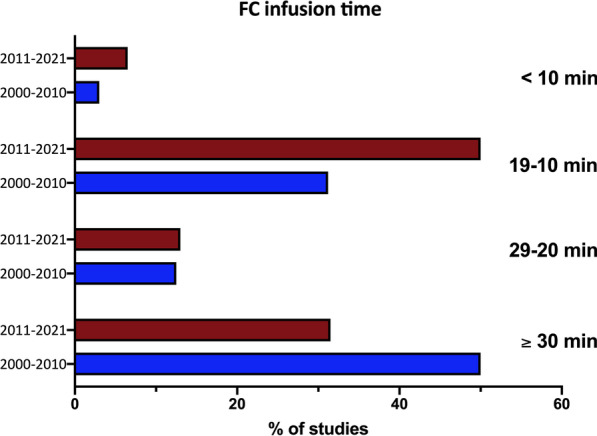
Percentage of studies in the two decades adopting different infusion timings. Fluid challenge, fluid challenge
CO/CI was used in 67% of the studies published in 2011–2021 and in 60% of those published in 2000–2010 (p = 0.51). The threshold adopted was an increase in CO or surrogates ≥ 15% in 67.4% of the studies of the 2011–2021 decade and in 81.2% of the studies published in 2000–2010 (p = 0.17) (Additional file 1: Figure S1).
Discussion
The results of this review, including research studies investigating the fluid challenge effect in critically ill adult patients receiving haemodynamic monitoring, may be summarised as follows: 1) fluid challenge is usually performed infusing a bolus of 500 mL of fluid, most often a crystalloid, in less than 20 min; 2) the response to fluid challenge is usually defined as a CI or CO increase ≥ 15% as compared to baseline; 3) positive response to fluid challenge is reported in about 50% of ICU patients; 4) the most common trigger for fluid challenge administration is usually the occurrence of hypotension, followed by oliguria and clinical signs of hypoperfusion; 5) the comparison between the 2000–2010 and 2011–2021 decades of publication showed no difference in the percentage of fluid responders (52% on average for both the decades), the volume infused (500 ml), and the criteria defining fluid responsiveness. On the contrary, compared to the 2000–2010 decade, in the period 2011–2021, the fluid challenge infusion time was lower, and crystalloids were more frequently used.
Fluid challenge characteristics
Among the included studies, the fluid challenge usually consisted of a median volume of 500 mL administered over a 20-min period and defined as a positive response by an increase ≥ 15% of CO or surrogate. These characteristics and responsiveness definition are to be considered good practice, for the response of CO to a fluid bolus is poorly followed by the simultaneous changes in arterial pressure [28, 29] or heart rate [30]. However, this is not the case in clinical practice, where the fluid challenge effect is often assessed by a rise in arterial blood pressure [16].
Interestingly, 500 mL was also the median volume fluid challenge used in the FENICE study (an observational study including 311 centres across 46 countries) [16], whereas a fluid challenge of 250 mL is usually adopted in high-risk surgical patients undergoing goal-directed therapy optimisation [31]. The use of large volumes for fluid challenge optimisation should be balanced to the detrimental risk of fluid overload [9], primarily if safety limits (i.e., increase in CVP) dynamically indicate fluid non-responsiveness are rarely used [19]. Since fluid challenge volume should be at least 4 mL/kg [32], smaller fluid challenge volumes may be considered for repetitive tests.
Moreover, the FENICE study reported a median of 24 min of infusion time and a rate of 17 mL/min [16]. Hence, the volume and rate of administration seem comparable between clinical and research settings. On the contrary, the infusion time was lower in the last decade (a median of 15 min vs 30 min, p = 0.03), indicating a trend towards the increase in the infusion rate in more recent studies. This global inception cohort study evaluated the clinical use of the fluid challenge in daily practice, whereas our review considered only research papers adopting the fluid challenge as a part of a protocol, limiting the comparison with the results of the FENICE. Moreover, in contrast with a previous metanalysis, including ICU studies up to 2014 [19], crystalloids are used in most studies. Crystalloids have been used in two-thirds of the studies from 2011 to 2021, compared to one-third from 2000 to 2010. These data indicate an alignment between research studies, recent guidelines, and metanalyses [13, 14].
Limitations
Limitations of our review have to be considered when extrapolating the results to clinical practice. First, the present study does not report any outcome endpoints. A recent large randomised-controlled trial showed no difference in mortality rate among ICU patients receiving different fluid bolus infusion rates [33]. However, the faster rate adopted in this study (5.5 mL/min) is below the median rate found in the studies included in the present review (25 mL/min) [33]. The administration of aliquots of fluids at a slow rate should not probably be indicated as a fluid challenge. Moreover, all the included studies are research papers whose aim was to evaluate the haemodynamic changes after the fluid challenge infusion or assess the reliability of indexes or functional haemodynamic tests in predicting the response to a fluid challenge. We did not include studies on the fluid challenge clinical use in ICU patients.
Another potential source of bias is related to the different haemodynamic monitorings used to assess fluid challenge responsiveness. When considering the median cut-off value identifying responders from non-responders, the accuracy of measurement of the changes in CO, or its surrogates, is undoubtedly relevant. Additionally, the reliability of different monitorings in tracking the dynamic trends of CO may not be consistent and may be below the boundaries of accuracy and precision of the Critchley–Critchley criteria [34]. Hence, the reproducibility of CO measurements obtained by the different monitoring systems may be limited. Moreover, cut-off values and measurement techniques potentially induce heterogeneity in response to the fluid challenge administration. As confirmed, responders ranged from 23 to 100% across the included studies (Table 2). The use of echocardiography is associated with high proportions of fluid responders compared to other haemodynamic monitoring devices. The operator-dependent bias may affect the evaluation of SV changes after fluid challenge.
We excluded studies in which the fluid challenge response has been assessed without haemodynamic monitoring and, hence assessing changes in systemic arterial pressures, potentially limiting the whole comparability of the technique in the two considered decades. Finally, the overall number of patients enrolled in the trials of the two considered decades was considerably different. This could bias the comparisons between the two groups if weighted for study size.
Conclusions
This systematic review, including research studies on fluid challenge use in critically ill adult patients receiving haemodynamic monitoring, showed a positive response in 52% of the patients. This test was usually performed infusing a bolus of 500 mL fluid, more often a crystalloid, in less than 20 min, and fluid responsiveness was generally indicated as a CI or CO increase ≥ 15% compared to baseline. Fluid challenge administration is usually triggered by hypotension. In the 2011–2021, the infusion time was shorter, and crystalloids were more frequently used than in the 2000–2010 decade.
Supplementary Information
Additional file 1. Table S1. PRISMA-DTA checklist. Table S2. Extracted data in each study assessed for eligibility. Table S3. Full-text articles excluded, not fitting eligibility criteria. Table S4. Studies on functional haemodynamic tests or dynamic indexes of fluid responsiveness. Figure S1. Characteristics of fluid challenge administration and monitoring along the considered years.
Acknowledgements
We are thankful to Dr. Katerina Negri for the linguistic revision of this manuscript.
Abbreviations
- CI
Cardiac index
- SVI
Stroke volume index
- CO
Cardiac output
- SV
Stroke volume
- ICU
Intensive care unit
- CVP
Central venous pressure
- VTI
Velocity–time integral in the left ventricular outflow tract
- ABF
Aortic blood flow
Author contributions
AM designed the study, performed data analysis, and drafted the manuscript; EM: helped in data analysis and manuscript preparation; LC, LP, AL, AS, and DR substantially contributed to data collection and interpretation; MC, XM, and GE substantially contributed to data interpretation and manuscript draft. All the authors approved the final version of the paper and agreed to be accountable for all aspects of the work, thereby ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work has not been funded by an external source.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Humans ethics statement, adult consent to participate written and human accordance statement
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Messina received travel expenses and registration for meetings, congresses, and courses and lecture fees from Vygon, Edwards, Philips, and Getinge. Xavier Monnet is a member of the medical advisory board of Pulsion Medical Systems (Getinge), and has given lectures for Baxter. Prof. Cecconi is a consultant of Edwards Lifesciences (Directed Systems Consultancy).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369:1243–1251. doi: 10.1056/NEJMra1208627. [DOI] [PubMed] [Google Scholar]
- 2.Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the european society of intensive care medicine. Intensive Care Med. 2014;40:1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369:1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 4.Magder S. Bench-to-bedside review: an approach to hemodynamic monitoring–guyton at the bedside. Crit Care. 2012;16:236. doi: 10.1186/cc11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cecconi M, Parsons AK, Rhodes A. What is a fluid challenge? Curr Opin Crit Care. 2011;17:290–295. doi: 10.1097/MCC.0b013e32834699cd. [DOI] [PubMed] [Google Scholar]
- 6.Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. 2011;1:1. doi: 10.1186/2110-5820-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monnet X, Marik PE, Teboul JL. Prediction of fluid responsiveness: an update. Ann Intensive Care. 2016;6:111. doi: 10.1186/s13613-016-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hjortrup PB, Haase N, Bundgaard H, Thomsen SL, Winding R, Pettila V, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the classic randomised, parallel-group, multicentre feasibility trial. Intensive Care Med. 2016;42:1695–1705. doi: 10.1007/s00134-016-4500-7. [DOI] [PubMed] [Google Scholar]
- 9.Marik PE, Linde-Zwirble WT, Bittner EA, Sahatjian J, Hansell D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: An analysis of a large national database. Intensive Care Med. 2017;43:625–632. doi: 10.1007/s00134-016-4675-y. [DOI] [PubMed] [Google Scholar]
- 10.Messina A, Palandri C, De Rosa S, Danzi V, Bonaldi E, Montagnini C, et al.: Pharmacodynamic analysis of a fluid challenge with 4 mL kg(-1) over 10 or 20 min: A multicenter cross-over randomized clinical trial. J Clin Monit Comput 2021 [DOI] [PMC free article] [PubMed]
- 11.Aya HD, Rhodes A, Chis Ster I, Fletcher N, Grounds RM, Cecconi M. Hemodynamic effect of different doses of fluids for a fluid challenge: a quasi-randomized controlled study. Crit Care Med. 2017;45:e161–e168. doi: 10.1097/CCM.0000000000002067. [DOI] [PubMed] [Google Scholar]
- 12.Messina A, Sotgiu G, Saderi L, Cammarota G, Capuano L, Colombo D, et al.: Does the definition of fluid responsiveness affect passive leg raising reliability? A methodological ancillary analysis from a multicentric study. Minerva Anestesiol 2021 [DOI] [PubMed]
- 13.Lewis SR, Pritchard MW, Evans DJ, Butler AR, Alderson P, Smith AF, et al. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst Rev. 2018;8:CD000567. doi: 10.1002/14651858.CD000567.pub7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finfer S, Liu B, Taylor C, Bellomo R, Billot L, Cook D, et al. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care. 2010;14:R185. doi: 10.1186/cc9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, et al. Fluid challenges in intensive care: The fenice study: a global inception cohort study. Intensive Care Med. 2015;41:1529–1537. doi: 10.1007/s00134-015-3850-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messina A, Longhini F, Coppo C, Pagni A, Lungu R, Ronco C, et al.: Use of the fluid challenge in critically ill adult patients: a systematic review. Anesth Analg 2017 [DOI] [PubMed]
- 18.Messina A, Dell'Anna A, Baggiani M, Torrini F, Maresca GM, Bennett V, et al. Functional hemodynamic tests: A systematic review and a metanalysis on the reliability of the end-expiratory occlusion test and of the mini-fluid challenge in predicting fluid responsiveness. Crit Care. 2019;23:264. doi: 10.1186/s13054-019-2545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messina A, Longhini F, Coppo C, Pagni A, Lungu R, Ronco C, et al. Use of the fluid challenge in critically ill adult patients: a systematic review. Anesth Analg. 2017;125:1532–1543. doi: 10.1213/ANE.0000000000002103. [DOI] [PubMed] [Google Scholar]
- 20.Vaquer S, Chemla D, Teboul JL, Ahmad U, Cipriani F, Oliva JC, et al. Volume infusion markedly increases femoral dp/dtmax in fluid-responsive patients only. Crit Care Med. 2020;48:1487–1493. doi: 10.1097/CCM.0000000000004515. [DOI] [PubMed] [Google Scholar]
- 21.Monge Garcia MI, Guijo Gonzalez P, Gracia Romero M, Gil Cano A, Oscier C, Rhodes A, et al. Effects of fluid administration on arterial load in septic shock patients. Intensive Care Med. 2015;41:1247–1255. doi: 10.1007/s00134-015-3898-7. [DOI] [PubMed] [Google Scholar]
- 22.Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007;35:64–68. doi: 10.1097/01.CCM.0000249851.94101.4F. [DOI] [PubMed] [Google Scholar]
- 23.Velissaris D, Pierrakos C, Scolletta S, De Backer D, Vincent JL. High mixed venous oxygen saturation levels do not exclude fluid responsiveness in critically ill septic patients. Crit Care. 2011;15:R177. doi: 10.1186/10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu B, Xiang H, Liang H, Yu L, Xu T, Yang JH, et al. Assessment effect of central venous pressure in fluid resuscitation in the patients with shock: a multi-center retrospective research. Chin Med J (Engl) 2013;126:1844–1849. [PubMed] [Google Scholar]
- 25.De Santis P, De Fazio C, Franchi F, Bond O, Vincent JL, Creteur J, et al.: Incoherence between systemic hemodynamic and microcirculatory response to fluid challenge in critically ill patients. J Clin Med 2021;10 [DOI] [PMC free article] [PubMed]
- 26.Cecconi M, Aya HD, Geisen M, Ebm C, Fletcher N, Grounds RM, et al. Changes in the mean systemic filling pressure during a fluid challenge in postsurgical intensive care patients. Intensive Care Med. 2013;39:1299–1305. doi: 10.1007/s00134-013-2928-6. [DOI] [PubMed] [Google Scholar]
- 27.Pranskunas A, Koopmans M, Koetsier PM, Pilvinis V, Boerma EC. Microcirculatory blood flow as a monitoring to select icu patients eligible for fluid therapy. Intensive Care Med. 2013;39:612–619. doi: 10.1007/s00134-012-2793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monnet X, Letierce A, Hamzaoui O, Chemla D, Anguel N, Osman D, et al. Arterial pressure allows monitoring the changes in cardiac output induced by volume expansion but not by norepinephrine. Crit Care Med. 2011;39:1394–1399. doi: 10.1097/CCM.0b013e31820edcf0. [DOI] [PubMed] [Google Scholar]
- 29.Pierrakos C, Velissaris D, Scolletta S, Heenen S, De Backer D, Vincent JL. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Med. 2012;38:422–428. doi: 10.1007/s00134-011-2457-0. [DOI] [PubMed] [Google Scholar]
- 30.Ait-Hamou Z, Teboul JL, Anguel N, Monnet X. How to detect a positive response to a fluid bolus when cardiac output is not measured? Ann Intensive Care. 2019;9:138. doi: 10.1186/s13613-019-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messina A, Pelaia C, Bruni A, Garofalo E, Bonicolini E, Longhini F, et al.: Fluid challenge during anesthesia: A systematic review and meta-analysis. Anesth Analg 2018 [DOI] [PubMed]
- 32.Aya HD, Ster IC, Fletcher N, Grounds RM, Rhodes A, Cecconi M. Pharmacodynamic analysis of a fluid challenge. Crit Care Med. 2016;44:880–891. doi: 10.1097/CCM.0000000000001517. [DOI] [PubMed] [Google Scholar]
- 33.Zampieri FG, Machado FR, Biondi RS, Freitas FGR, Veiga VC, Figueiredo RC, et al. Effect of slower vs faster intravenous fluid bolus rates on mortality in critically ill patients: the basics randomized clinical trial. JAMA. 2021;326:830–838. doi: 10.1001/jama.2021.11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. 1999;15:85–91. doi: 10.1023/A:1009982611386. [DOI] [PubMed] [Google Scholar]
- 35.Mahjoub Y, Touzeau J, Airapetian N, Lorne E, Hijazi M, Zogheib E, et al. The passive leg-raising maneuver cannot accurately predict fluid responsiveness in patients with intra-abdominal hypertension. Crit Care Med. 2010;38:1824–1829. doi: 10.1097/CCM.0b013e3181eb3c21. [DOI] [PubMed] [Google Scholar]
- 36.Preau S, Dewavrin F, Soland V, Bortolotti P, Colling D, Chagnon JL, et al. Hemodynamic changes during a deep inspiration maneuver predict fluid responsiveness in spontaneously breathing patients. Cardiol Res Pract. 2012;2012:191807. doi: 10.1155/2012/191807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive Care Med. 2004;30:1834–1837. doi: 10.1007/s00134-004-2233-5. [DOI] [PubMed] [Google Scholar]
- 38.Caille V, Jabot J, Belliard G, Charron C, Jardin F, Vieillard-Baron A. Hemodynamic effects of passive leg raising: an echocardiographic study in patients with shock. Intensive Care Med. 2008;34:1239–1245. doi: 10.1007/s00134-008-1067-y. [DOI] [PubMed] [Google Scholar]
- 39.Marik PE, Levitov A, Young A, Andrews L. The use of bioreactance and carotid doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest. 2013;143:364–370. doi: 10.1378/chest.12-1274. [DOI] [PubMed] [Google Scholar]
- 40.Mahjoub Y, Benoit-Fallet H, Airapetian N, Lorne E, Levrard M, Seydi AA, et al. Improvement of left ventricular relaxation as assessed by tissue doppler imaging in fluid-responsive critically ill septic patients. Intensive Care Med. 2012;38:1461–1470. doi: 10.1007/s00134-012-2618-9. [DOI] [PubMed] [Google Scholar]
- 41.Wyffels PA, Durnez PJ, Helderweirt J, Stockman WM, De Kegel D. Ventilation-induced plethysmographic variations predict fluid responsiveness in ventilated postoperative cardiac surgery patients. Anesth Analg. 2007;105:448–452. doi: 10.1213/01.ane.0000267520.16003.17. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y, Zhou S, Zhou Z, Liu B. A 10-second fluid challenge guided by transthoracic echocardiography can predict fluid responsiveness. Crit Care. 2014;18:R108. doi: 10.1186/cc13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jozwiak M, Depret F, Teboul JL, Alphonsine JE, Lai C, Richard C, et al. Predicting fluid responsiveness in critically ill patients by using combined end-expiratory and end-inspiratory occlusions with echocardiography. Crit Care Med. 2017;45:e1131–e1138. doi: 10.1097/CCM.0000000000002704. [DOI] [PubMed] [Google Scholar]
- 44.Fellahi JL, Fischer MO, Rebet O, Massetti M, Gerard JL, Hanouz JL. A comparison of endotracheal bioimpedance cardiography and transpulmonary thermodilution in cardiac surgery patients. J Cardiothorac Vasc Anesth. 2012;26:217–222. doi: 10.1053/j.jvca.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 45.Monnet X, Osman D, Ridel C, Lamia B, Richard C, Teboul JL. Predicting volume responsiveness by using the end-expiratory occlusion in mechanically ventilated intensive care unit patients. Crit Care Med. 2009;37:951–956. doi: 10.1097/CCM.0b013e3181968fe1. [DOI] [PubMed] [Google Scholar]
- 46.Smorenberg A, Cherpanath TGV, Geerts BF, de Wilde RBP, Jansen JRC, Maas JJ, et al. A mini-fluid challenge of 150ml predicts fluid responsiveness using modelflow(r) pulse contour cardiac output directly after cardiac surgery. J Clin Anesth. 2018;46:17–22. doi: 10.1016/j.jclinane.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 47.Monnet X, Bleibtreu A, Ferre A, Dres M, Gharbi R, Richard C, et al. Passive leg-raising and end-expiratory occlusion tests perform better than pulse pressure variation in patients with low respiratory system compliance. Crit Care Med. 2012;40:152–157. doi: 10.1097/CCM.0b013e31822f08d7. [DOI] [PubMed] [Google Scholar]
- 48.Muller L, Toumi M, Bousquet PJ, Riu-Poulenc B, Louart G, Candela D, et al. An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid responsiveness: The mini-fluid challenge study. Anesthesiology. 2011;115:541–547. doi: 10.1097/ALN.0b013e318229a500. [DOI] [PubMed] [Google Scholar]
- 49.Chen YH, Lai YJ, Huang CY, Lin HL, Huang CC. Effects of positive end-expiratory pressure on the predictability of fluid responsiveness in acute respiratory distress syndrome patients. Sci Rep. 2021;11:10186. doi: 10.1038/s41598-021-89463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monge Garcia MI, Gil Cano A, Diaz Monrove JC. Arterial pressure changes during the valsalva maneuver to predict fluid responsiveness in spontaneously breathing patients. Intensive Care Med. 2009;35:77–84. doi: 10.1007/s00134-008-1295-1. [DOI] [PubMed] [Google Scholar]
- 51.Natalini G, Rosano A, Taranto M, Faggian B, Vittorielli E, Bernardini A. Arterial versus plethysmographic dynamic indices to test responsiveness for testing fluid administration in hypotensive patients: a clinical trial. Anesth Analg. 2006;103:1478–1484. doi: 10.1213/01.ane.0000246811.88524.75. [DOI] [PubMed] [Google Scholar]
- 52.Messina A, Romano SM, Ozdemirkan A, Persona P, Tarquini R, Cammarota G, et al.: Multivariable haemodynamic approach to predict the fluid challenge response: a multicentre cohort study. Eur J Anaesthesiol 2020 [DOI] [PubMed]
- 53.Mahjoub Y, Pila C, Friggeri A, Zogheib E, Lobjoie E, Tinturier F, et al. Assessing fluid responsiveness in critically ill patients: False-positive pulse pressure variation is detected by doppler echocardiographic evaluation of the right ventricle. Crit Care Med. 2009;37:2570–2575. doi: 10.1097/CCM.0b013e3181a380a3. [DOI] [PubMed] [Google Scholar]
- 54.Taccheri T, Gavelli F, Teboul JL, Shi R, Monnet X. Do changes in pulse pressure variation and inferior vena cava distensibility during passive leg raising and tidal volume challenge detect preload responsiveness in case of low tidal volume ventilation? Crit Care. 2021;25:110. doi: 10.1186/s13054-021-03515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fischer MO, Coucoravas J, Truong J, Zhu L, Gerard JL, Hanouz JL, et al. Assessment of changes in cardiac index and fluid responsiveness: a comparison of nexfin and transpulmonary thermodilution. Acta Anaesthesiol Scand. 2013;57:704–712. doi: 10.1111/aas.12108. [DOI] [PubMed] [Google Scholar]
- 56.Kaur KB, Nakra M, Mangal V, Singh S, Taank P, Marwah V. Comparative evaluation of stroke volume variation and inferior vena cava distensibility index for prediction of fluid responsiveness in mechanically ventilated patients. Ann Card Anaesth. 2021;24:327–332. doi: 10.4103/aca.ACA_113_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vistisen ST, Struijk JJ, Larsson A. Automated pre-ejection period variation indexed to tidal volume predicts fluid responsiveness after cardiac surgery. Acta Anaesthesiol Scand. 2009;53:534–542. doi: 10.1111/j.1399-6576.2008.01893.x. [DOI] [PubMed] [Google Scholar]
- 58.Biasucci DG, Cina A, Calabrese M, Antoniucci ME, Cavaliere C, Bevilacqua F, et al. Size and shape of the inferior vena cava before and after a fluid challenge: a pilot study. Minerva Anestesiol. 2019;85:514–521. doi: 10.23736/S0375-9393.18.13041-0. [DOI] [PubMed] [Google Scholar]
- 59.Kupersztych-Hagege E, Teboul JL, Artigas A, Talbot A, Sabatier C, Richard C, et al. Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth. 2013;111:961–966. doi: 10.1093/bja/aet282. [DOI] [PubMed] [Google Scholar]
- 60.Gavaud A, Nguyen LS, Caubel A, Grillet G, Donal E, Belliard G. Respiratory variability of pulmonary velocity-time integral as a new gauge of fluid responsiveness for mechanically ventilated patients in the icu. Crit Care Med. 2019;47:e310–e316. doi: 10.1097/CCM.0000000000003642. [DOI] [PubMed] [Google Scholar]
- 61.Monge Garcia MI, Gil Cano A, Diaz Monrove JC. Brachial artery peak velocity variation to predict fluid responsiveness in mechanically ventilated patients. Crit Care. 2009;13:R142. doi: 10.1186/cc8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Depret F, Jozwiak M, Teboul JL, Alphonsine JE, Richard C, Monnet X. Esophageal doppler can predict fluid responsiveness through end-expiratory and end-inspiratory occlusion tests. Crit Care Med. 2019;47:e96–e102. doi: 10.1097/CCM.0000000000003522. [DOI] [PubMed] [Google Scholar]
- 63.Lakhal K, Ehrmann S, Benzekri-Lefevre D, Runge I, Legras A, Dequin PF, et al. Brachial cuff measurements of blood pressure during passive leg raising for fluid responsiveness prediction. Ann Fr Anesth Reanim. 2012;31:e67–72. doi: 10.1016/j.annfar.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 64.Messina A, Colombo D, Barra FL, Cammarota G, De Mattei G, Longhini F, et al. Sigh maneuver to enhance assessment of fluid responsiveness during pressure support ventilation. Crit Care. 2019;23:31. doi: 10.1186/s13054-018-2294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soubrier S, Saulnier F, Hubert H, Delour P, Lenci H, Onimus T, et al. Can dynamic indicators help the prediction of fluid responsiveness in spontaneously breathing critically ill patients? Intensive Care Med. 2007;33:1117–1124. doi: 10.1007/s00134-007-0644-9. [DOI] [PubMed] [Google Scholar]
- 66.Vistisen ST, Krog MB, Elkmann T, Vallentin MF, Scheeren TWL, Solling C. Extrasystoles for fluid responsiveness prediction in critically ill patients. J Intensive Care. 2018;6:52. doi: 10.1186/s40560-018-0324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fellahi JL, Fischer MO, Dalbera A, Massetti M, Gerard JL, Hanouz JL. Can endotracheal bioimpedance cardiography assess hemodynamic response to passive leg raising following cardiac surgery? Ann Intensive Care. 2012;2:26. doi: 10.1186/2110-5820-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu B, Yang X, Wang C, Jiang W, Weng L, Hu X, et al. Changes of central venous oxygen saturation define fluid responsiveness in patients with septic shock: a prospective observational study. J Crit Care. 2017;38:13–19. doi: 10.1016/j.jcrc.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 69.Preau S, Bortolotti P, Colling D, Dewavrin F, Colas V, Voisin B, et al. Diagnostic accuracy of the inferior vena cava collapsibility to predict fluid responsiveness in spontaneously breathing patients with sepsis and acute circulatory failure. Crit Care Med. 2017;45:e290–e297. doi: 10.1097/CCM.0000000000002090. [DOI] [PubMed] [Google Scholar]
- 70.Lakhal K, Ehrmann S, Runge I, Benzekri-Lefevre D, Legras A, Dequin PF, et al. Central venous pressure measurements improve the accuracy of leg raising-induced change in pulse pressure to predict fluid responsiveness. Intensive Care Med. 2010;36:940–948. doi: 10.1007/s00134-010-1755-2. [DOI] [PubMed] [Google Scholar]
- 71.Machare-Delgado E, Decaro M, Marik PE. Inferior vena cava variation compared to pulse contour analysis as predictors of fluid responsiveness: a prospective cohort study. J Intensive Care Med. 2011;26:116–124. doi: 10.1177/0885066610384192. [DOI] [PubMed] [Google Scholar]
- 72.Oliveira-Costa CD, Friedman G, Vieira SR, Fialkow L. Pulse pressure variation and prediction of fluid responsiveness in patients ventilated with low tidal volumes. Clinics (Sao Paulo) 2012;67:773–778. doi: 10.6061/clinics/2012(07)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Monnet X, Julien F, Ait-Hamou N, Lequoy M, Gosset C, Jozwiak M, et al. Lactate and venoarterial carbon dioxide difference/arterial-venous oxygen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit Care Med. 2013;41:1412–1420. doi: 10.1097/CCM.0b013e318275cece. [DOI] [PubMed] [Google Scholar]
- 74.Monnet X, Chemla D, Osman D, Anguel N, Richard C, Pinsky MR, et al. Measuring aortic diameter improves accuracy of esophageal doppler in assessing fluid responsiveness. Crit Care Med. 2007;35:477–482. doi: 10.1097/01.CCM.0000254725.35802.17. [DOI] [PubMed] [Google Scholar]
- 75.Monnet X, Jabot J, Maizel J, Richard C, Teboul JL. Norepinephrine increases cardiac preload and reduces preload dependency assessed by passive leg raising in septic shock patients. Crit Care Med. 2011;39:689–694. doi: 10.1097/CCM.0b013e318206d2a3. [DOI] [PubMed] [Google Scholar]
- 76.Ishihara H, Hashiba E, Okawa H, Saito J, Kasai T, Tsubo T. Neither dynamic, static, nor volumetric variables can accurately predict fluid responsiveness early after abdominothoracic esophagectomy. Perioper Med (Lond) 2013;2:3. doi: 10.1186/2047-0525-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muller L, Louart G, Bousquet PJ, Candela D, Zoric L, de La Coussaye JE, et al. The influence of the airway driving pressure on pulsed pressure variation as a predictor of fluid responsiveness. Intensive Care Med. 2010;36:496–503. doi: 10.1007/s00134-009-1686-y. [DOI] [PubMed] [Google Scholar]
- 78.Monge Garcia MI, Gil Cano A, Gracia Romero M, Monterroso Pintado R, Perez Madueno V, Diaz Monrove JC. Non-invasive assessment of fluid responsiveness by changes in partial end-tidal co2 pressure during a passive leg-raising maneuver. Ann Intensive Care. 2012;2:9. doi: 10.1186/2110-5820-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muller L, Louart G, Bengler C, Fabbro-Peray P, Carr J, Ripart J, et al. The intrathoracic blood volume index as an indicator of fluid responsiveness in critically ill patients with acute circulatory failure: a comparison with central venous pressure. Anesth Analg. 2008;107:607–613. doi: 10.1213/ane.0b013e31817e6618. [DOI] [PubMed] [Google Scholar]
- 80.Luzi A, Marty P, Mari A, Conil JM, Geeraerts T, Lepage B, et al. Noninvasive assessment of hemodynamic response to a fluid challenge using femoral doppler in critically ill ventilated patients. J Crit Care. 2013;28:902–907. doi: 10.1016/j.jcrc.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 81.Heenen S, De Backer D, Vincent JL. How can the response to volume expansion in patients with spontaneous respiratory movements be predicted? Crit Care. 2006;10:R102. doi: 10.1186/cc4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dong ZZ, Fang Q, Zheng X, Shi H. Passive leg raising as an indicator of fluid responsiveness in patients with severe sepsis. World J Emerg Med. 2012;3:191–196. doi: 10.5847/wjem.j.issn.1920-8642.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31:517–523. doi: 10.1007/s00134-005-2586-4. [DOI] [PubMed] [Google Scholar]
- 84.Jabot J, Teboul JL, Richard C, Monnet X. Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med. 2009;35:85–90. doi: 10.1007/s00134-008-1293-3. [DOI] [PubMed] [Google Scholar]
- 85.Le Dorze M, Huche F, Coelembier C, Rabuel C, Payen D. Impact of fluid challenge increase in cardiac output on the relationship between systemic and cerebral hemodynamics in severe sepsis compared to brain injury and controls. Ann Intensive Care. 2018;8:74. doi: 10.1186/s13613-018-0419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Préau S, Saulnier F, Dewavrin F, Durocher A, Chagnon JL. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med. 2010;38:819–825. doi: 10.1097/CCM.0b013e3181c8fe7a. [DOI] [PubMed] [Google Scholar]
- 87.Wu J, Wang Z, Wang T, Yu T, Yuan J, Zhang Q, et al. Evaluation of the fluid responsiveness in patients with septic shock by ultrasound plus the passive leg raising test. J Surg Res. 2018;224:207–214. doi: 10.1016/j.jss.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 88.Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34:1402–1407. doi: 10.1097/01.CCM.0000215453.11735.06. [DOI] [PubMed] [Google Scholar]
- 89.Si X, Cao DY, Chen J, Wu JF, Liu ZM, Xu HL, et al. Effect of systolic cardiac function on passive leg raising for predicting fluid responsiveness: a prospective observational study. Chin Med J (Engl) 2018;131:253–261. doi: 10.4103/0366-6999.223841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pouska J, Tegl V, Astapenko D, Cerny V, Lehmann C, Benes J. Impact of intravenous fluid challenge infusion time on macrocirculation and endothelial glycocalyx in surgical and critically ill patients. Biomed Res Int. 2018;2018:8925345. doi: 10.1155/2018/8925345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Monnet X, Guerin L, Jozwiak M, Bataille A, Julien F, Richard C, et al. Pleth variability index is a weak predictor of fluid responsiveness in patients receiving norepinephrine. Br J Anaesth. 2013;110:207–213. doi: 10.1093/bja/aes373. [DOI] [PubMed] [Google Scholar]
- 92.Xu J, Peng X, Pan C, Cai S, Zhang X, Xue M, et al. Fluid responsiveness predicted by transcutaneous partial pressure of oxygen in patients with circulatory failure: a prospective study. Ann Intensive Care. 2017;7:56. doi: 10.1186/s13613-017-0279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Loupec T, Nanadoumgar H, Frasca D, Petitpas F, Laksiri L, Baudouin D, et al. Pleth variability index predicts fluid responsiveness in critically ill patients. Crit Care Med. 2011;39:294–299. doi: 10.1097/CCM.0b013e3181ffde1c. [DOI] [PubMed] [Google Scholar]
- 94.Soussi S, Vallee F, Roquet F, Bevilacqua V, Benyamina M, Ferry A, et al. Measurement of oxygen consumption variations in critically ill burns patients: are the fick method and indirect calorimetry interchangeable? Shock. 2017;48:532–538. doi: 10.1097/SHK.0000000000000885. [DOI] [PubMed] [Google Scholar]
- 95.Monnet X, Dres M, Ferre A, Le Teuff G, Jozwiak M, Bleibtreu A, et al. Prediction of fluid responsiveness by a continuous non-invasive assessment of arterial pressure in critically ill patients: comparison with four other dynamic indices. Br J Anaesth. 2012;109:330–338. doi: 10.1093/bja/aes182. [DOI] [PubMed] [Google Scholar]
- 96.Mallat J, Lemyze M, Meddour M, Pepy F, Gasan G, Barrailler S, et al. Ratios of central venous-to-arterial carbon dioxide content or tension to arteriovenous oxygen content are better markers of global anaerobic metabolism than lactate in septic shock patients. Ann Intensive Care. 2016;6:10. doi: 10.1186/s13613-016-0110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang CC, Fu JY, Hu HC, Kao KC, Chen NH, Hsieh MJ, et al. Prediction of fluid responsiveness in acute respiratory distress syndrome patients ventilated with low tidal volume and high positive end-expiratory pressure. Crit Care Med. 2008;36:2810–2816. doi: 10.1097/CCM.0b013e318186b74e. [DOI] [PubMed] [Google Scholar]
- 98.Khwannimit B, Bhurayanontachai R. Prediction of fluid responsiveness in septic shock patients: comparing stroke volume variation by flotrac/vigileo and automated pulse pressure variation. Eur J Anaesthesiol. 2012;29:64–69. doi: 10.1097/EJA.0b013e32834b7d82. [DOI] [PubMed] [Google Scholar]
- 99.Hamimy W, Mukhtar A, Zaghloul A, Salem M. Comparing transesophageal doppler corrected systolic flow time versus central venous pressure as a guide for fluid resuscitation in septic shock. Egypt J Anaesthesia. 2019;32:181–187. doi: 10.1016/j.egja.2015.12.004. [DOI] [Google Scholar]
- 100.Fischer MO, Pelissier A, Bohadana D, Gerard JL, Hanouz JL, Fellahi JL. Prediction of responsiveness to an intravenous fluid challenge in patients after cardiac surgery with cardiopulmonary bypass: a comparison between arterial pulse pressure variation and digital plethysmographic variability index. J Cardiothorac Vasc Anesth. 2013;27:1087–1093. doi: 10.1053/j.jvca.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 101.Liu Y, Wei LQ, Li GQ, Yu X, Li GF, Li YM. Pulse pressure variation adjusted by respiratory changes in pleural pressure, rather than by tidal volume, reliably predicts fluid responsiveness in patients with acute respiratory distress syndrome. Crit Care Med. 2016;44:342–351. doi: 10.1097/CCM.0000000000001371. [DOI] [PubMed] [Google Scholar]
- 102.Kramer A, Zygun D, Hawes H, Easton P, Ferland A. Pulse pressure variation predicts fluid responsiveness following coronary artery bypass surgery. Chest. 2004;126:1563–1568. doi: 10.1378/chest.126.5.1563. [DOI] [PubMed] [Google Scholar]
- 103.Guerin L, Teboul JL, Persichini R, Dres M, Richard C, Monnet X. Effects of passive leg raising and volume expansion on mean systemic pressure and venous return in shock in humans. Crit Care. 2015;19:411. doi: 10.1186/s13054-015-1115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yazigi A, Khoury E, Hlais S, Madi-Jebara S, Haddad F, Hayek G, et al. Pulse pressure variation predicts fluid responsiveness in elderly patients after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2012;26:387–390. doi: 10.1053/j.jvca.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 105.Airapetian N, Maizel J, Alyamani O, Mahjoub Y, Lorne E, Levrard M, et al. Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients? Crit Care. 2015;19:400. doi: 10.1186/s13054-015-1100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wyler von Ballmoos M, Takala J, Roeck M, Porta F, Tueller D, Ganter CC, et al. Pulse-pressure variation and hemodynamic response in patients with elevated pulmonary artery pressure: a clinical study. Crit Care. 2010;14:R111. doi: 10.1186/cc9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Messina A, Colombo D, Cammarota G, De Lucia M, Cecconi M, Antonelli M, et al. Patient-ventilator asynchrony affects pulse pressure variation prediction of fluid responsiveness. J Crit Care. 2015;30:1067–1071. doi: 10.1016/j.jcrc.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 108.Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, et al. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162:134–138. doi: 10.1164/ajrccm.162.1.9903035. [DOI] [PubMed] [Google Scholar]
- 109.Cecconi M, Monge Garcia MI, Gracia Romero M, Mellinghoff J, Caliandro F, Grounds RM, et al. The use of pulse pressure variation and stroke volume variation in spontaneously breathing patients to assess dynamic arterial elastance and to predict arterial pressure response to fluid administration. Anesth Analg. 2015;120:76–84. doi: 10.1213/ANE.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 110.Lakhal K, Ehrmann S, Benzekri-Lefevre D, Runge I, Legras A, Dequin PF, et al. Respiratory pulse pressure variation fails to predict fluid responsiveness in acute respiratory distress syndrome. Crit Care. 2011;15:R85. doi: 10.1186/cc10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Soliman RA, Samir S, el Naggar A, El Dehely K. Stroke volume variation compared with pulse pressure variation and cardiac index changes for prediction of fluid responsiveness in mechanically ventilated patients. The Egypt J Crit Care Med. 2015;3:9–16. doi: 10.1016/j.ejccm.2015.02.002. [DOI] [Google Scholar]
- 112.Muller L, Bobbia X, Toumi M, Louart G, Molinari N, Ragonnet B, et al. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: Need for a cautious use. Crit Care. 2012;16:R188. doi: 10.1186/cc11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nunes TS, Ladeira RT, Bafi AT, de Azevedo LC, Machado FR, Freitas FG. Duration of hemodynamic effects of crystalloids in patients with circulatory shock after initial resuscitation. Ann Intensive Care. 2014;4:25. doi: 10.1186/s13613-014-0025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Giraud R, Siegenthaler N, Gayet-Ageron A, Combescure C, Romand JA, Bendjelid K. Scvo(2) as a marker to define fluid responsiveness. J Trauma. 2011;70:802–807. doi: 10.1097/TA.0b013e3181e7d649. [DOI] [PubMed] [Google Scholar]
- 115.Lakhal K, Ehrmann S, Perrotin D, Wolff M, Boulain T. Fluid challenge: Tracking changes in cardiac output with blood pressure monitoring (invasive or non-invasive) Intensive Care Med. 2013;39:1953–1962. doi: 10.1007/s00134-013-3086-6. [DOI] [PubMed] [Google Scholar]
- 116.Suehiro K, Rinka H, Ishikawa J, Fuke A, Arimoto H, Miyaichi T. Stroke volume variation as a predictor of fluid responsiveness in patients undergoing airway pressure release ventilation. Anaesth Intensive Care. 2012;40:767–772. doi: 10.1177/0310057X1204000503. [DOI] [PubMed] [Google Scholar]
- 117.Perner A, Faber T. Stroke volume variation does not predict fluid responsiveness in patients with septic shock on pressure support ventilation. Acta Anaesthesiol Scand. 2006;50:1068–1073. doi: 10.1111/j.1399-6576.2006.01120.x. [DOI] [PubMed] [Google Scholar]
- 118.Smorenberg A, Lust EJ, Beishuizen A, Meijer JH, Verdaasdonk RM, Groeneveld AB. Systolic time intervals vs invasive predictors of fluid responsiveness after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2013;44:891–897. doi: 10.1093/ejcts/ezt108. [DOI] [PubMed] [Google Scholar]
- 119.Monnet X, Picard F, Lidzborski E, Mesnil M, Duranteau J, Richard C, et al. The estimation of cardiac output by the nexfin device is of poor reliability for tracking the effects of a fluid challenge. Crit Care. 2012;16:R212. doi: 10.1186/cc11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schnell D, Camous L, Guyomarc'h S, Duranteau J, Canet E, Gery P, et al. Renal perfusion assessment by renal doppler during fluid challenge in sepsis. Crit Care Med. 2013;41:1214–1220. doi: 10.1097/CCM.0b013e31827c0a36. [DOI] [PubMed] [Google Scholar]
- 121.Yonis H, Bitker L, Aublanc M, Perinel Ragey S, Riad Z, Lissonde F, et al. Change in cardiac output during trendelenburg maneuver is a reliable predictor of fluid responsiveness in patients with acute respiratory distress syndrome in the prone position under protective ventilation. Crit Care. 2017;21:295. doi: 10.1186/s13054-017-1881-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xiao-ting W, Hua Z, Da-wei L, Hong-min Z, Huai-wu H, Yun L, et al. Changes in end-tidal co2 could predict fluid responsiveness in the passive leg raising test but not in the mini-fluid challenge test: a prospective and observational study. J Crit Care. 2015;30:1061–1066. doi: 10.1016/j.jcrc.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 123.Elsayed AI, Selim KA, Zaghla HE, Mowafy HE, Fakher MA. Comparison of changes in ppv using a tidal volume challenge with a passive leg raising test to predict fluid responsiveness in patients ventilated using low tidal volume. Indian J Crit Care Med. 2021;25:685–690. doi: 10.5005/jp-journals-10071-23875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Biais M, Vidil L, Sarrabay P, Cottenceau V, Revel P, Sztark F. Changes in stroke volume induced by passive leg raising in spontaneously breathing patients: Comparison between echocardiography and vigileo/flotrac device. Crit Care. 2009;13:R195. doi: 10.1186/cc8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bataille B, de Selle J, Moussot PE, Marty P, Silva S, Cocquet P. Machine learning methods to improve bedside fluid responsiveness prediction in severe sepsis or septic shock: an observational study. Br J Anaesth. 2021;126:826–834. doi: 10.1016/j.bja.2020.11.039. [DOI] [PubMed] [Google Scholar]
- 126.Mallat J, Meddour M, Durville E, Lemyze M, Pepy F, Temime J, et al. Decrease in pulse pressure and stroke volume variations after mini-fluid challenge accurately predicts fluid responsiveness. Br J Anaesth. 2015;115:449–456. doi: 10.1093/bja/aev222. [DOI] [PubMed] [Google Scholar]
- 127.Maizel J, Airapetian N, Lorne E, Tribouilloy C, Massy Z, Slama M. Diagnosis of central hypovolemia by using passive leg raising. Intensive Care Med. 2007;33:1133–1138. doi: 10.1007/s00134-007-0642-y. [DOI] [PubMed] [Google Scholar]
- 128.Kumar N, Malviya D, Nath SS, Rastogi S, Upadhyay V. Comparison of the efficacy of different arterial waveform-derived variables (pulse pressure variation, stroke volume variation, systolic pressure variation) for fluid responsiveness in hemodynamically unstable mechanically ventilated critically ill patients. Indian J Crit Care Med. 2021;25:48–53. doi: 10.5005/jp-journals-10071-23440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33:1125–1132. doi: 10.1007/s00134-007-0646-7. [DOI] [PubMed] [Google Scholar]
- 130.Braun F, Proenca M, Wendler A, Sola J, Lemay M, Thiran JP, et al. Noninvasive measurement of stroke volume changes in critically ill patients by means of electrical impedance tomography. J Clin Monit Comput. 2020;34:903–911. doi: 10.1007/s10877-019-00402-z. [DOI] [PubMed] [Google Scholar]
- 131.Silva E, De Backer D, Creteur J, Vincent JL. Effects of fluid challenge on gastric mucosal pco2 in septic patients. Intensive Care Med. 2004;30:423–429. doi: 10.1007/s00134-003-2115-2. [DOI] [PubMed] [Google Scholar]
- 132.Huette P, Abou-Arab O, Longrois D, Guinot PG. Fluid expansion improve ventriculo-arterial coupling in preload-dependent patients: a prospective observational study. BMC Anesthesiol. 2020;20:171. doi: 10.1186/s12871-020-01087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cecconi M, Monti G, Hamilton MA, Puntis M, Dawson D, Tuccillo ML, et al. Efficacy of functional hemodynamic parameters in predicting fluid responsiveness with pulse power analysis in surgical patients. Minerva Anestesiol. 2012;78:527–533. [PubMed] [Google Scholar]
- 134.Mohammad Abdelfattah WoM, Saad-eldeen Elgammal S, Mohammad Elsayed K, Said Mowafy SM, Mohammad Abdalla R. Distensibility index of inferior vena cava and pulse pressure variation as predictors of fluid responsiveness in mechanically ventilated shocked patients. J Emerg Med Trauma Acute Care 2020;2020
- 135.Georges D, de Courson H, Lanchon R, Sesay M, Nouette-Gaulain K, Biais M. End-expiratory occlusion maneuver to predict fluid responsiveness in the intensive care unit: an echocardiographic study. Crit Care. 2018;22:32. doi: 10.1186/s13054-017-1938-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jacquet-Lagreze M, Bouhamri N, Portran P, Schweizer R, Baudin F, Lilot M, et al. Capillary refill time variation induced by passive leg raising predicts capillary refill time response to volume expansion. Crit Care. 2019;23:281. doi: 10.1186/s13054-019-2560-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Monnet X, Bataille A, Magalhaes E, Barrois J, Le Corre M, Gosset C, et al. End-tidal carbon dioxide is better than arterial pressure for predicting volume responsiveness by the passive leg raising test. Intensive Care Med. 2013;39:93–100. doi: 10.1007/s00134-012-2693-y. [DOI] [PubMed] [Google Scholar]
- 138.Beurton A, Teboul JL, Girotto V, Galarza L, Anguel N, Richard C, et al. Intra-abdominal hypertension is responsible for false negatives to the passive leg raising test. Crit Care Med. 2019;47:e639–e647. doi: 10.1097/CCM.0000000000003808. [DOI] [PubMed] [Google Scholar]
- 139.Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, et al. Esophageal doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive Care Med. 2005;31:1195–1201. doi: 10.1007/s00134-005-2731-0. [DOI] [PubMed] [Google Scholar]
- 140.Roger C, Zieleskiewicz L, Demattei C, Lakhal K, Piton G, Louart B, et al. Time course of fluid responsiveness in sepsis: the fluid challenge revisiting (fcrev) study. Crit Care. 2019;23:179. doi: 10.1186/s13054-019-2448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Biais M, Cottenceau V, Stecken L, Jean M, Ottolenghi L, Roullet S, et al. Evaluation of stroke volume variations obtained with the pressure recording analytic method. Crit Care Med. 2012;40:1186–1191. doi: 10.1097/CCM.0b013e31823bc632. [DOI] [PubMed] [Google Scholar]
- 142.Mukhtar A, Awad M, Elayashy M, Hussein A, Obayah G, El Adawy A, et al. Validity of mini-fluid challenge for predicting fluid responsiveness following liver transplantation. BMC Anesthesiol. 2019;19:56. doi: 10.1186/s12871-019-0728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Trifi A, Abdellatif S, Daly F, Nasri R, Touil Y, Ben Lakhal S. Ultrasound stroke volume variation induced by passive leg raising and fluid responsiveness: An observational cohort study. Med Intensiva (Engl Ed) 2019;43:10–17. doi: 10.1016/j.medin.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 144.Michard F, Alaya S, Zarka V, Bahloul M, Richard C, Teboul JL. Global end-diastolic volume as an indicator of cardiac preload in patients with septic shock. Chest. 2003;124:1900–1908. doi: 10.1378/chest.124.5.1900. [DOI] [PubMed] [Google Scholar]
- 145.Giraud R, Abraham PS, Brindel P, Siegenthaler N, Bendjelid K. Respiratory changes in subclavian vein diameters predicts fluid responsiveness in intensive care patients: a pilot study. J Clin Monit Comput. 2018;32:1049–1055. doi: 10.1007/s10877-018-0103-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. PRISMA-DTA checklist. Table S2. Extracted data in each study assessed for eligibility. Table S3. Full-text articles excluded, not fitting eligibility criteria. Table S4. Studies on functional haemodynamic tests or dynamic indexes of fluid responsiveness. Figure S1. Characteristics of fluid challenge administration and monitoring along the considered years.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.