Abstract
Background
Despite the standardization and optimization of disinfection protocols, duodenoscope-related infections (DRIs) remain an emerging threat for patients undergoing endoscopic retrograde cholangiopancreatography (ERCP). Single-use duodenoscopes could represent a potential alternative avenue to circumvent the problem of reprocessing and thus risk of exogenous patient-to-patient transmission. The aim of our study was to test the feasibility and technical success rate of a recently made available single-use duodenoscope.
Methods
We carried out a thorough and standardized evaluation of the usability, performance and safety of a recently developed single-use duodenoscope in 52 consecutive patients scheduled for ERCP in a single center. Outcomes included performance ratings of the single-use duodenoscopes, adverse events (assessed at 3 days and 1 week), and crossover rate to a reusable duodenoscope.
Results
The ERCP completion rate with a single-use duodenoscope was 90.4%, rising to 94.2% after crossover to reusable duodenoscope. The mean American Society for Gastrointestinal Endoscopy (ASGE) grade was 2.7, with 27 procedures (51.9%) considered as advanced level complexity (ASGE grade 3 & 4). Performance rating found that 94% of the therapeutic treatments were comparable to those using a traditional reusable duodenoscope. Overall satisfaction amounted to 80%. No major adverse events were attributable to the single-use endoscope.
Conclusions
Single-use duodenoscopes can provide an alternative to avoid the intensive and often inconsistent results of cleaning and disinfection procedures. We confirmed the feasibility, adequate performance characteristics and safety of a recently developed first-generation single-use duodenoscope over a broad range of ERCP procedures, in terms of both indication and complexity.
Keywords: Single-use duodenoscope, ERCP, duodenoscope-related infection, performance
Introduction
Multidrug resistant organisms (MDRO) represent an emerging global public health threat. In the context of endoscopy, duodenoscopes used for endoscopic retrograde cholangiopancreatography (ERCP) are most prevalent and notorious for scope contamination and thus for exogenous patient-to-patient duodenoscope-related infections (DRIs). These latter include potentially devastating infections with antibiotic-resistant bacteria such as carbapenem-resistant Enterobacteriaceae (CRE) and Pseudomonas aeruginosa and vancomycin-resistant enterococci [1].
The first article reporting a DRI with CRE was published only recently, in 2014 [2]. In response to this troubling report and other single-center reported outbreaks, the Food and Drug Administration (FDA) conducted a post-market surveillance study, which showed a higher-than-expected contamination rate of 9%: 3.6% low-to-moderate-concern organisms and 5.4% high-concern organisms (i.e., more often associated with clinical disease) [3]. A recent meta-analysis further set the stage by reporting a duodenoscope contamination rate of 15% [4]. As a logic consequence, all disinfection protocols were subsequently subjected to critical review. Optimizing to double high-level disinfection (HLD) (i.e., complete manual cleaning followed by automated reprocessing, with the entire process repeated) led to a reduction to 9.4% of scopes still showing positive cultures. By changing the pre-cleaning solution to a non-enzymatic solution (detergent), the contamination rate was further reduced to 4.8%, with known pathogens still being isolated in 0.2-0.8% of cases [5]. In contrast, other authors have reported that double HLD did not reduce culture positivity rates compared with single HLD in facilities with an already low positive culture rate [6]. So, at best, optimizing disinfection protocols for duodenoscopes can reduce, but not eliminate, the rate of positive cultures for known pathogens and for organisms of low pathogenic potential. The implications of this are massive, given the constant and persistent need for overall unadulterated vigilance and awareness, safeguarding sufficiently trained staff and personnel in terms of handling and reprocessing scopes, implementation of standardized microbiological surveillance strategies, logistics to meticulously manage outbreaks (track & trace, increasing scope fleet, quarantine facilities for contaminated scopes) while balancing and securing the daily operationality of an ERCP program.
Apart from the above, other risk mitigation (ideally elimination) strategies are urgently needed. One of the major essentials in DRIs is the intrinsic complex design (elevator function) of the reusable duodenoscope, the main hurdle to thorough and efficient cleaning [7]
Going “disposable” or single-use could therefore represent a potential alternative avenue to circumvent the problem of reprocessing and thus the risk of exogenous patient-to-patient transmission. The DRI issue could not be more contemporary, if one considers the highly contagious COVID-19 pandemic and its inherent risk of aerosolization during disinfection [8]. Recently, Boston-Scientific introduced the first approved single-use, sterile duodenoscope, Exalt™ Model D. Until now, 4 reports have been published on this device in 381 patients overall [1,9-11] (Fig. 1). We aimed to report our single center cohort experience and evaluation of feasibility, safety and performance characteristics with this novel device in 52 consecutive patients.
Figure 1.
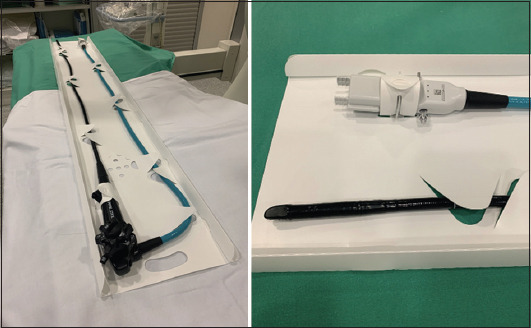
Exalt single-use duodenoscope before use (left), with a detailed view of the plug-in socket and tip of the endoscope (right)
Patients and methods
Study design
This project involved a prospective single-center cohort study that evaluated the first and currently only CE marked and FDA cleared single-use duodenoscope (EXALT Model D, Boston Scientific Corporation, Marlborough, MA) in 52 consecutive patients undergoing ERCP from October until November 2020. Because of compelling circumstances (i.e., DRI with MDRO) whilst in a 3rd wave of escalating COVID-19 infections, our biliopancreatic endoscopy unit was obliged to make an almost exclusive crossover to single-use duodenoscopes to contain a potential MDRO outbreak. All procedures were performed by 3 expert endoscopists and an endoscopy fellow at a tertiary referral center in Belgium. No modifications were made to the devices prior to their use nor were any practice training or lead-in period given to the endoscopists.
The primary endpoint was to test the feasibility and performance of the single-use duodenoscope in 52 consecutive patients. Secondary endpoints included safety (adverse events assessed at 3 days until 4 weeks thereafter) and incidence of crossover to a (backup) reusable duodenoscope. The study was approved by the Institutional Review Board of the University Hospitals Leuven.
Patients
All consecutive patients between October 6th and November 5th 2020 with a formal clinical indication for ERCP and who consented were included in the analysis. No patients were excluded, nor were any specifically selected a priori for this period, nor for the analysis.
Intervention
All ERCP procedures were performed under general anesthesia and according to the institution’s conventional standard of care. For each procedure a single-use sterile disposable Exalt Model D duodenoscope (Boston Scientific Corporation, Marlborough, MA) was used. Procedures were equally divided between endoscopists.
Relevant patient demographic data and relevant clinical history were collected from the medical records at baseline and during hospitalization, including sex, age, ERCP history (including prior sphincterotomy), current indication for ERCP, American Society of Anesthesiologists classification, history of preprocedural MDRO, utilization of antibiotic prophylaxis, empirical antibiotic usage, and nonsteroidal anti-inflammatory drugs given for prevention of post-ERCP pancreatitis.
The following procedural parameters were recorded: total procedure time (i.e., time from induction to extubation), ERCP completion rate, type of therapeutic treatments completed or attempted, type of advanced diagnostic/therapeutic procedure, reason for failed procedure or crossover to reusable duodenoscope, and American Society for Gastrointestinal Endoscopy (ASGE) grade for the complexity of the ERCP procedure.
Handling and operating characteristics of the scope were assessed by means of evaluation of roll-in and ERCP-specific maneuvers and a set of predefined ERCP performance characteristics, as previously defined [8]. For consistency, the same assessment methodology was applied.
More specifically, roll-in maneuvers consisted of navigation to the duodenal papilla, adequate visualization of the papilla, followed by the optional insertion of an ancillary device through the working channel, left/right and up/down flexing of the tip, and withdrawal of the single-use duodenoscope. This maneuver specifically evaluates the ability to insert and advance the scope to the ampullary region and take a stable position in front of it, and rate the expected confidence in the ability to perform deep cannulation. All aspects of this maneuver needed to be met before a given endoscopist could rate it as confident (successful) or not.
ERCP-specific maneuvers related to diagnostic or therapeutic related interventions using additional ancillary devices applied through the scope once the roll-in maneuver was completed. These included sphincterotomy, cannulation, duct clearance, stent insertion/removal, performing cholangioscopy/brushing or balloon dilatation, etc. Overall, subjective performance rating scores were given to 14 ERCP maneuvers (options include not preferred/neutral/preferred relative to the reusable duodenoscope normally used). In addition, 23 device performance characteristics were scored, ranging from 1 [not preferred] to 5 [comparable to prior historical experience with reusable duodenoscope] on a Likert scale, and median overall satisfaction with the single use duodenoscope during the procedure (Likert scale of 1 [unsatisfied] to 10 [very satisfied]). Finally, serious adverse events were assessed at 72 h up to 4 weeks after the ERCP procedure.
Statistical analysis
Descriptive statistics included mean, standard error of mean, and range or %.
Results
Patient characteristics (Table 1)
Table 1.
Patient demographics and relevant clinical history
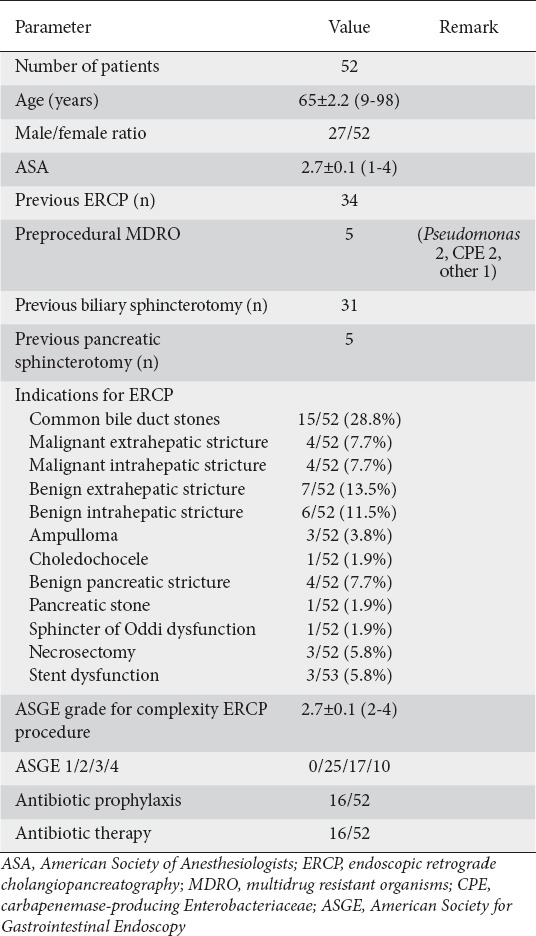
During a one-month period (6 October until 5 November 2020, amid the second COVID-19 peak in Belgium), we recruited 52 patients for the study. The patients’ demographics are shown in table 1. Mean age was 65 years. Sex distribution was balanced (male/female 27/25).
Thirty-four patients (65.4%) had undergone one or more previous ERCPs. The most frequent indications were clearance of common bile duct stones (15/52, 28.8%) and benign extrahepatic strictures (7/52, 13.5%). Five patients (9.6%) had a preexisting infection with MDRO, 16 patients (30.8%) received antibiotic prophylaxis and 16 patients (30.8%) were already receiving treatment dosing of antibiotics at the time of the ERCP.
Feasibility
The mean ASGE grade for procedure complexity was 2.7. More than half of the procedures were more advanced, classed as ASGE grades 3 and 4 (27 procedures, 51.9%). The grade 4 procedures included 4 biliary interventions combined with single-operator cholangioscopy (spyglass-guided electrohydraulic lithotripsy and assisted biopsies), papillectomy, removal of pancreatic stones and endoscopic mucosectomy.
The mean duration of an ERCP was 55.5 min from induction until extubation. The ERCP completion rate with a single-use duodenoscope was 90.4% overall. After crossover to a reusable duodenoscope the completion rate reached 94.2% (Table 2). More specifically, in one patient with a malignant stricture, we obtained biliary drainage via endoscopic ultrasound-guided hepaticogastrostomy. In the remaining 4 patients (7.7%) we switched to a reusable duodenoscope. The first of these latter 4 patients was a 90-year-old male with obstructive jaundice caused by pancreatic cancer. The reason for the crossover was repeated inadvertent slipping of the endoscope out of the duodenum. After switching to a reusable duodenoscope (Olympus TJF-Q180V, Tokyo, Japan) the operator was able to successfully cannulate the bile duct and insert an uncovered self-expandable metallic stent. The second patient was a 69-year-old female with intrahepatic cholangiocarcinoma. The single-use duodenoscope could not traverse a stenosis at the bulbo-duodenal junction. After crossover to a reusable duodenoscope we managed to drain liver segments 6 and 7 transpapillary by placement of an uncovered metallic stent. The third patient was a 54-year-old male with symptomatic chronic pancreatitis and ductal stones. The single-use duodenoscope could not safely be negotiated over a large hiatal hernia with intrathoracic stomach. The fourth patient was a 71-year-old female with liver metastases related to breast cancer and signs of stent dysfunction. The rigidity of the single-use duodenoscope prevented it traversing a stenosis of the bulbo-duodenal junction.
Table 2.
Procedural characteristics
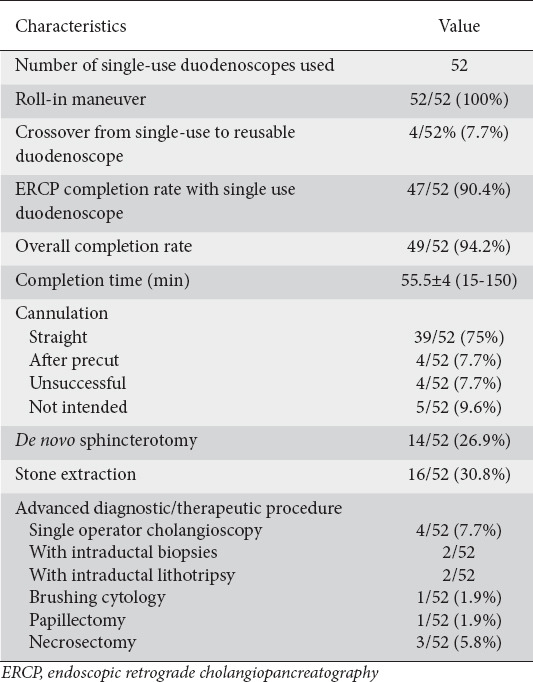
Performance
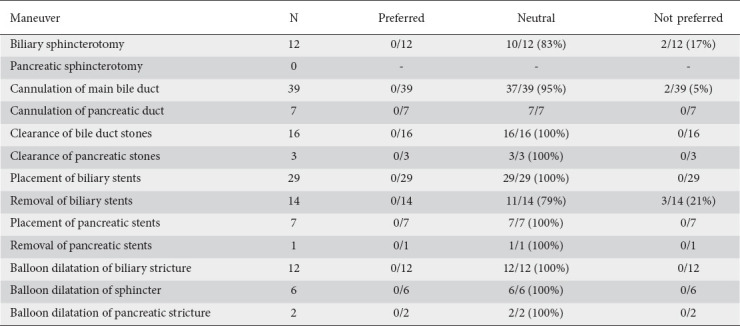
We scored 14 specific options of ERCP maneuvers (Table 3). Most of the ratings were neutral (141/148, 95.3%) when comparing the single-use duodenoscope with the reusable duodenoscope. Only 7 (4.7%) maneuvers were labeled as not preferred. The most common reasons for a “not preferred” rating were problems with cannulation (n=2) due to either inadvertent slipping or inability to maintain a straight position in front of the papilla, sphincterotomy (n=2) due to the elevator’s insufficient upward bending capacity, and removal of biliary stents (n=3) through the scope.
Table 3.
Ratings of endoscopic retrograde cholangiopancreatography maneuvers (n=52)
We also documented performance characteristics for 23 ERCP maneuvers. Seventeen maneuvers (73.9%) had a median score of 5 (comparable to prior historical experience with the reusable duodenoscope) (Fig. 2). Navigation/pushability/torquability of the scope, range of motion, selective cannulation and tip deflection were appreciated slightly less than with a reusable duodenoscope. Elevator function was scored as the least favorable performance characteristic. Overall satisfaction regarding use of the single-use duodenoscope was rated as 8.
Figure 2.
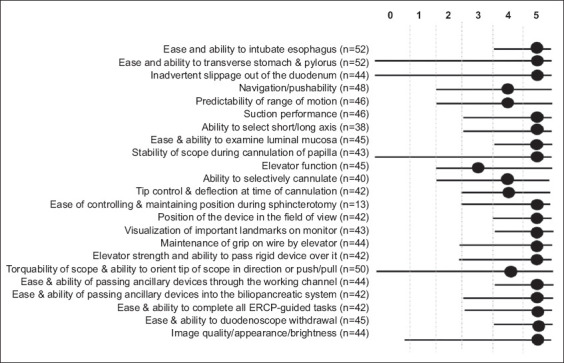
Averaged ratings on 23 endoscopic retrograde cholangiopancreatography (ERCP) performance characteristics
Safety
The complication rate was low (1.9%) and not primarily related to duodenoscope maneuvering/handling; one patient had a post-sphincterotomy bleeding treated endoscopically.
Discussion
Emerging MDROs, and DRI in particular, compel endoscopists to up their game, given the potentially devastating outcome of a DRI in a given patient on the one hand, and the inseparable risk of setting off local aggressive outbreaks on the other [3,4]. Rubin et al reported 32 DRI outbreaks from 2000-2017. These documented outbreaks comprise a population of 400 patients and over 20 patient deaths [12].
Despite substantial improvement in available materials, detergents, quality, and standardized handling of scope disinfection, even the most meticulously executed protocol cannot attain a zero risk of DRI [4-5,7-8]. The complex design of a duodenoscope makes it difficult to clean all the intricate surfaces (narrow lumens). Moreover, case-reports have even documented biofilm on internal areas (such as behind the lens and the O-ring) of a reusable duodenoscope that was involved in an outbreak of Pseudomonas aeruginosa [13]. In addition to disinfection, one also needs rigid microbiological surveillance to maintain oversight and permanent quality control of the disinfection protocol. A recent study performed in The Netherlands sampled 155 duodenoscopes (intended to be ready for use in patients) and demonstrated a contamination rate of 22% for any microorganism and 15% for microorganisms with a gastrointestinal or oral origin [14]. “Going disposable” by using a single-use duodenoscope might therefore represent an alternative approach that circumvents the whole chain of consecutive disinfection steps, with its indisputable inherent flaws.
In this study, we prospectively evaluated the first and currently only CE-marked and FDA-cleared single-use duodenoscope (EXALT Model D, Boston Scientific Corporation, Marlborough, MA) in terms of its applicability and performance in 52 consecutive patients undergoing ERCP from October until November 2020, amid a second COVID-19 wave in Belgium. The assessment was performed by 4 different operators (3 experts, 1 fellow) in procedures for diverse indications covering the whole range of complexity, and with at least half of the procedures being ASGE 3-4 (advanced procedures), in patients ranging from 9-98 years old. Apart from the reduced number of procedures as a result of COVID-19, our study cohort mirrors real-life daily endoscopic practice in our tertiary center, thus allowing the generalization of our findings to large-volume ERCP units. Typical ERCP maneuvers, performance characteristics of the scope and overall satisfaction were semi-qualitatively scored using previously accepted scores [1].
In our hands we observed a high ERCP completion rate with single-use duodenoscopes and an applicability, maneuverability and technical performance comparable with our vast historical experience with reusable duodenoscopes, leading to an overall acceptable level of satisfaction with the disposable duodenoscope. The crossover rate to reusable duodenoscopes in our cohort amounted to 7.7%. However, if one analyzes the supposed 5 failures (see Results, Feasibility section), one of them did not relate to the type of duodenoscope but rather reflected the impossibility of achieving a retrograde approach to the malignant hilar stricture, which was then drained via endoscopic ultrasound-assisted hepaticogastrostomy. In the other 4 cases, the rigid nature of the single-use duodenoscope, seemingly manifested as reduced pushability, predictability of range of motion, and torquability of the scope, gives the operator less confidence and feedback to traverse difficult obstacles, such as a bulbo-duodenal stenosis or a large hiatal hernia, as was the case in our patients. On the other hand, the extra stiffness of the single-use duodenoscope was felt to expedite single-operator cholangioscopy, as the angle of the duodenoscope was found to be steeper in all cholangioscopy procedures compared to that of a reusable duodenoscope, although this remains to be validated in a larger series (Fig. 3). An apparent working point is the elevator function, which did not always allow maximal bending up of materials upon cannulation (Fig. 4). No direct complications were observed related to duodenoscope-related maneuvering/handling, although its rigidity calls for a gentler introduction.
Figure 3.
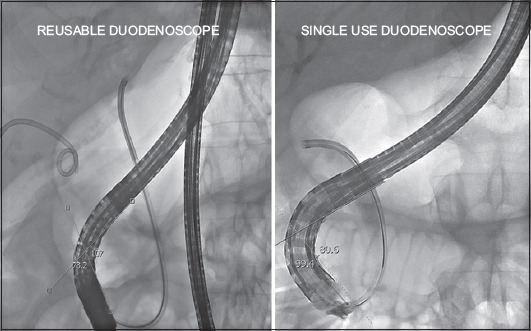
Stiffness of the single-use duodenoscope potentially expediting single-operator cholangioscopy by steepening the duodenoscope angulation directed towards the ampullary region (80° for single-use duodenoscope vs. 107° for reusable duodenoscope)
Figure 4.
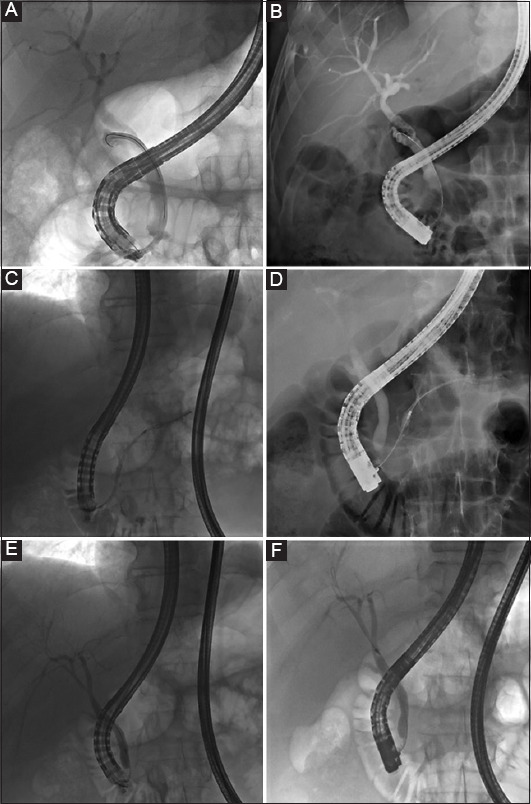
A comparison between single-use (A,C,E) and reusable duodenoscope (B,D,F) in terms of the degree of lifting of the elevator with an inserted device. (A,B) represents the procedural cholangiograms of a male patient with a cystic duct syndrome with the SpyScope introduced in the cystic duct in A, and a diagnostic catheter in B. (C-F) represent the procedural cholangiograms of a male patient with chronic pancreatitis and benign distal biliary duct stenosis. C-D reflect the introduction of diagnostic catheter in the main pancreatic duct, while E-F show the insertion of the catheter in the common bile duct
If we compare our observations to reported series using the same single-use duodenoscope [1,9-11] (Table 4), results are quite comparable in over 433 patients in terms of crossover rate (overall average 6.3%), procedural completion rate (overall average 94.8%), performance characteristics (overall average 4.5/5), overall satisfaction (overall average 8.5/10), and safety. From the perspective of clinical use, these data are encouraging and set the stage for further development and optimization of a single-use duodenoscope platform, as this is the only clinical pathway at present that carries a zero risk of patient-to-patient transferred DRIs, the ultimate safety prerequisite for any given patient.
Table 4.
Review of currently available literature on the single-use duodenoscope

The major remaining challenges at this point will be cost-effectiveness, affordability and ecological impact. Although this seems a “no-brainer” for the former 2 issues, the truth is not. Bang et al [15] analyzed the adaptability of “going disposable” by adopting a cost-analysis model in which the per-procedural cost of reusable duodenoscopes was weighed to the break-even costs for transitioning to single-use duodenoscopes. The authors considered the acquisition cost of a reusable duodenoscope at $35,000 with a (conservative) life-expectancy of 3 years and annual use in 200 patients. In the scenario where this disposable duodenoscope would be substituted for a single-use (at a cost of $3000 per device), it would lead to a cost of $367,200 ($612/procedure), which is 10 times more than the present cost, to treat the same number of patients. The authors meticulously highlighted the complex interaction between variable infection rate and ERCP volume and documented a lower cost ($800 vs. ≥$1300) for high-volume centers (≥150 ERCPs/year). This difference in cost is explained by the fact that investment costs (such as the fixed costs of purchasing a reusable duodenoscope, video tower, maintenance, annual and non-foreseen repairs, deliveries, personnel, microbiological surveillance) can be allotted to a higher volume. As the field will undoubtedly evolve rapidly in the near future (several manufacturers, design optimization, cost-reduction), more micro-costing approaches are urgently needed, taking the abovementioned and different scenarios into consideration. For the time being, it would seem medically appropriate and responsible to reserve single-use duodenoscopes for ERCP in high-risk/immunocompromised patients [16], or to smother an evolving DRI-outbreak. Solutions in terms of recycling used single-use duodenoscopes will need to be addressed appropriately.
In conclusion, despite HLD protocols, there is still a substantial contamination rate of processed duodenoscopes, resulting in a possible risk for patient-to-patient DRIs with antibiotic-resistant bacteria. Single-use duodenoscopes can provide an alternative to eliminate patient-to-patient exogenous contamination, with good performance characteristics, also in high complexity procedures. Ongoing innovation in single-use duodenoscope, as well as micro-costing approaches are to be pursued, so that the device may truly take its place in the daily safe and resilient care of patients undergoing ERCP for biliopancreatic disorders.
Summary Box.
What is already known:
Duodenoscope-related infections remain an emerging threat for patients undergoing endoscopic retrograde cholangiopancreatography (ERCP), despite all efforts at optimizing disinfection protocols for duodenoscopes, which can reduce, but not eliminate, the problem
What the new findings are:
Single-use duodenoscopes can provide an alternative to avoid the intensive and often inconsistent results of cleaning and disinfection procedures
We confirm the feasibility, adequate performance characteristics and safety of a recently introduced first-generation single-use duodenoscope over a broad range of ERCP procedures, in terms of both indication and complexity
Biography
University Hospitals Leuven, Belgium; AZ Damiaan, Ostend, Belgium
Footnotes
Conflict of Interest: WL and SVDM hold the BSC chair on interventional echo endoscopy for hepatobiliary disease and received consultancy fees from BSC. HvM has a consultancy agreement with BSC. DP, EV, AS, MD, WM, KB declare no conflicts of interest
References
- 1.Muthusamy VR, Bruno MJ, Kozarek RA, et al. Clinical evaluation of a single-use duodenoscope for endoscopic retrograde cholangiopancreatography. Clin Gastroenterol Hepatol. 2020;18:2108–2117. doi: 10.1016/j.cgh.2019.10.052. [DOI] [PubMed] [Google Scholar]
- 2.Epstein L, Hunter JC, Arwady MA, et al. New Delhi metallo-b-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA. 2014;312:1447–1455. doi: 10.1001/jama.2014.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration:Medical Devices;Medical Device Safety;Safety Communications. The FDA continues to remind facilities of the importance of following duodenoscope reprocessing instructions:FDA Safety Communication (April 12, 2019) [Accessed 20 May 2022]. Available from: https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm635828.htm .
- 4.Larsen S, Russell RV, Ockert LK, et al. Rate and impact of duodenoscope contamination:a systematic review and meta-analysis. EClinicalMedicine. 2020;25:100451. doi: 10.1016/j.eclinm.2020.100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rex DK, Sieber M, Lehman GA, et al. A double-reprocessing high-level disinfection protocol does not eliminate positive cultures from the elevators of duodenoscopes. Endoscopy. 2018;50:588–596. doi: 10.1055/s-0043-122378. [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration. Reprocessing of reusable medical devices. Infections associated with reprocessed duodenoscopes. [Accessed 20 May 2022]. Available from: https://www.fda.gov/medical-devices/reprocessing-reusable-medical-devices/infections-associated-reprocessed-duodenoscopes .
- 7.Bartles RL, Leggett JE, Hove S, et al. A randomized trial of single versus double high-level disinfection of duodenoscopes and linear echoendoscopes using standard automated reprocessing. Gastrointest Endosc. 2018;88:306–313. doi: 10.1016/j.gie.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Bilal M, Berzin TM, Cohen J, Sawhney MS, Pleskow DK. ERCP in patients with COVID-19 infection—is a single-use duodenoscope the safer option? Endoscopy. 2020;52:932. doi: 10.1055/a-1180-8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bang JY, Hawes R, Varadarajulu S. Equivalent performance of single-use and reusable duodenoscopes in a randomised trial. Gut. 2021;70:838–844. doi: 10.1136/gutjnl-2020-321836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Napoléon B, Gonzalez JM, Grandval P, et al. Evaluation of the performances of a single-use duodenoscope:prospective multi-center national study. Dig Endosc. 2022;34:215–221. doi: 10.1111/den.13965. [DOI] [PubMed] [Google Scholar]
- 11.Slivka A, Ross AS, Sejpal DV, et al. EXALT Single-use Duodenoscope Study Group. Single-use duodenoscope for ERCP performed by endoscopists with a range of experience in procedures of variable complexity. Gastrointest Endosc. 2021;94:1046–1055. doi: 10.1016/j.gie.2021.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Rubin ZA, Kim S, Thaker AM, Muthusamy VR. Safely reprocessing duodenoscopes:current evidence and future directions. Lancet Gastroenterol Hepatol. 2018;3:499–508. doi: 10.1016/S2468-1253(18)30122-5. [DOI] [PubMed] [Google Scholar]
- 13.Verfaillie CJ, Bruno MJ, Voor in 't Holt AF, et al. Withdrawal of a novel-design duodenoscope ends outbreak of a VIM-2-producing Pseudomonas aeruginosa. Endoscopy. 2015;47:493–502. doi: 10.1055/s-0034-1391886. [DOI] [PubMed] [Google Scholar]
- 14.Rauwers AW, Voor In 't Holt AF, Buijs JG, et al. High prevalence rate of digestive tract bacteria in duodenoscopes:a nationwide study. Gut. 2018;67:1637–1645. doi: 10.1136/gutjnl-2017-315082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bang JY, Sutton B, Hawes R, Varadarajulu S. Concept of disposable duodenoscope:at what cost? Gut. 2019;68:1915–1917. doi: 10.1136/gutjnl-2019-318227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S, Russell D, Mohamadnejad M, et al. Risk factors associated with the transmission of carbapenem-resistant Enterobacteriaceae via contaminated duodenoscopes. Gastrointest Endosc. 2016;83:1121–1129. doi: 10.1016/j.gie.2016.03.790. [DOI] [PubMed] [Google Scholar]


