Abstract
Background
The Barrx™ radiofrequency ablation (RFA) catheter system comes in several different variations and sizes and is widely used for the eradication of Barrett’s esophagus (BE). The Barrx™ 360 and 360 Express system is used to perform circumferential RFA, while the Barrx™ focal catheter system is used for secondary focal RFA or primary treatment of short-segment BE. We aimed to investigate the number and type of complications and device failures associated with the Barrx™ RFA catheter system.
Method
We analyzed post-marketing surveillance data from the Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database from August 2011 to August 2021.
Results
During the study period, 148 unique reports detailing 78 device issues and 87 patient-related adverse events were identified. The most reported adverse events for patients were stenosis secondary to treatment (n=15, 17.24%), mucosal laceration of the esophagus (n=13, 14.94%), chest pain (n=10, 11.49%), and odynophagia/dysphagia (n=7, 8.05%). The most common device complication was failure of the device to deploy when activated (n=10, 13.82%). Other device malfunctions included material deformation/distortion (n=7, 8.97%), catheter breakage (n=6, 7.69%), connection problems between the generator cable and RFA device (n=6, 7.69%), and failure of the balloon to properly inflate (n=5, 6.41%).
Conclusion
Findings from the MAUDE database highlight patient and device complications that endoscopists should be aware of prior to RFA of BE with the Barrx™ RFA catheter system.
Keywords: Barrx, radiofrequency ablation, Barrett’s esophagus, MAUDE, adverse events
Introduction
Radiofrequency ablation (RFA) is a widely accepted method for eradicating Barrett’s esophagus (BE). RFA has shown efficacy in achieving reversal to normal appearing mucosa in patients with low-grade dysplasia BE and reducing progression from high-grade dysplasia to esophageal cancer in BE patients. RFA has been incorporated into both American and European guidelines for the management of BE [1-3].
The commercially available system to endoscopically ablate BE via RFA is marketed under the trade name Barrx™ (Medtronic, Minneapolis, Minnesota). The Barrx™ system consists of different catheters that deliver radiofrequency energy to BE tissue. The Barrx™ 360 and 360 Express systems are used to perform circumferential RFA. In contrast, the Barrx™ focal catheter system is used for secondary focal RFA or primary treatment for short-segment BE. Patients usually begin their treatment with a circumferential ablation procedure. After a minimum of 8 weeks patients are rescheduled to undergo a second ablation procedure to evaluate for and ablate any residual BE. Follow-up ablations can involve the use of the circumferential ablation device or a more localized focal ablation. Ablation can be repeated every 2-3 months until all BE has been eradicated. Absence of residual BE is verified by endoscopic inspection 2-3 months after the last treatment [4].
Although there have been multiple large-scale studies evaluating the clinical efficacy of the Barrx™ system for RFA, there is a paucity of data available regarding the technical failures and clinical adverse events encountered [5-7]. In a multicenter randomized trial comparing RFA with endoscopic surveillance in patients with BE and a confirmed diagnosis of low-grade dysplasia, RFA reduced the risk of progression to high-grade dysplasia or adenocarcinoma by 25.0% (95% confidence interval 14.1-35.9%). Complete eradication of BE occurred in 92.6% of patients with dysplasia and 88.2% of patients with intestinal metaplasia. Treatment-related adverse events occurred in 19.1% of patients undergoing ablation [7]. The aim of this study was to evaluate the adverse events and device malfunctions associated with the use of all commercially available catheters compatible with the Barrx™ system using data from the Food and Drug Administration’s (FDA) Manufacturer and User Facility Device Experience (MAUDE) database from 2011-2021.
Materials and methods
We analyzed post-marketing surveillance data for each different Barrx™ RFA catheters using the FDA’s MAUDE database. The MAUDE database classifies reports into 3 different categories: device-associated deaths, serious injuries, and malfunctions. The MAUDE database receives several hundred thousand medical device reports (MDRs) yearly. Reporting can be mandatory, submitted by manufacturers, importers, and device user facilities, or voluntary, submitted by individuals such as healthcare professionals, patients, and consumers. The website is freely accessible to the public here: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.com. The MAUDE database is updated monthly and reflects the data of the most recent update. MDRs include information regarding the specific device, event date, event type, a manufacturer narrative and an event description. The FDA uses MDRs to monitor device performance and detect safety-related issues. The FDA can also use MDRs for risk/benefit assessment of products.
All commercially available catheters compatible with the Barrx™ system were evaluated using the MAUDE database. Barrx™ currently has several RFA systems on the market that are used to treat BE, including the 360 Express RFA Balloon Catheter, the RFA Focal Catheters, the 360 RFA Catheter, the 360 Soft Sizing Balloon, and the Channel RFA Endoscopic Catheter. The 360 Express RFA Balloon Catheter is a newer model of the 360 RFA Catheter, still available on the market. The earlier model 360 RFA catheter does not have autosizing capabilities, so a separate soft sizing balloon must be used prior to ablation. The RFA Focal Catheters come in 3 different sizes: 90, 60, and ultra-long. The electrode dimension for the Barrx™ 90 is 20 mm (l) × 13 mm (w) (ablation area 2.6 cm2); for the Barrx™ 60 it is 15 mm (l) × 10 mm (w) (ablation area 1.6 cm2); and for the Barrx™ Ultra Long it is 40 mm (l) × 13 mm (w) (ablation area 5.2 cm2). The Channel RFA Endoscopic Catheter fits through the working channel of a flexible endoscope and delivers energy to smaller areas of tissue [8].
We queried the MAUDE database from August 2011 to August 2021. Each individual device report was investigated for date of event, model number, event type, device problem, and patient problem. Duplicate reports and reports related to retrospective studies or literary reviews that did not provide details on the number of patients or events reported were excluded. Complications were categorized into 1 of 2 categories: patient-related adverse events or device-related issues. If reports were found related to the same patient where different catheters were used, these reports were included in this study and sorted by the device used so as to avoid double counting. Data was organized into Microsoft Excel (Microsoft, Redmond, WA). The Excel functions of sort and filter were used to narrow down reports by model number, patient-related adverse events, and device problems associated with each model.
Results
One hundred forty-eight unique reports detailing 78 device issues and 87 patient-related adverse events were identified. Reports that included literature reviews, where the number of patients involved could not be determined, were not included in the analysis.
Patient-related adverse events
Endoscopic impression
The most frequently reported patient-related adverse event was esophageal stenosis secondary to treatment (n=15, 17.2%). Ten cases of post-treatment esophageal stenosis were related to the use of the 360 Express RFA Balloon Catheter, 3 to the 360 RFA Balloon Catheter, and 2 to the 360 Soft Sizing Balloon. All 10 of the patients who developed an esophageal stenosis following treatment via the 360 Express RFA Balloon Catheter required subsequent dilation post-procedure. In these patients, stenoses were diagnosed at follow-up visits that ranged from 2 weeks to 4 months post-procedure. For the 360 RFA Balloon Catheter, 2 patients required dilation post-procedure for symptomatic dysphagia, while the third required no intervention despite the presence of a stenosis. Neither of the 2 patients who developed stenosis following treatment via the 360 Soft Sizing Balloon required intervention.
Mucosal laceration of the esophagus (n=13, 14.94%) was another commonly reported adverse event, with 10 cases being associated with the 360 Express Balloon Catheter. Four of the mucosal laceration cases required endoscopic clip placement to close the laceration, 1 required cautery of the laceration to stop bleeding, 1 progressed to a perforation, and 4 cases required no intervention. The patient who had a perforation of the esophagus after laceration was seen in the emergency department 4 h post-procedure, presenting with pain and respiratory distress with free air in the neck and mediastinum. Esophagogastroduodenoscopy with stent placement was performed, and the report ends stating the patient was stable. Mucosal laceration of the esophagus in 2 cases related to the 360 RFA Balloon Catheter and 1 case related to the 360 Soft Sizing Balloon required no intervention.
Clinical symptoms
Chest pain (n=10, 11.49%), dysphagia/odynophagia (n=7, 8.05%), and gastrointestinal hemorrhage secondary to treatment (n=8, 9.20%) were other commonly reported patient-related adverse events. Chest pain was reported most frequently for the 360 Express Balloon Catheter, with 6 cases. Odynophagia/dysphagia was reported twice for the 360 Express Balloon Catheter. The 360 RFA Balloon Catheter had 2 reported cases of odynophagia/dysphagia. For the RFA Focal Catheter, gastrointestinal hemorrhage with 3 cases and chest pain with 3 cases were the most commonly reported patient-related adverse events. Other adverse events are reported in Table 1.
Table 1.
Summary of patient-related adverse events
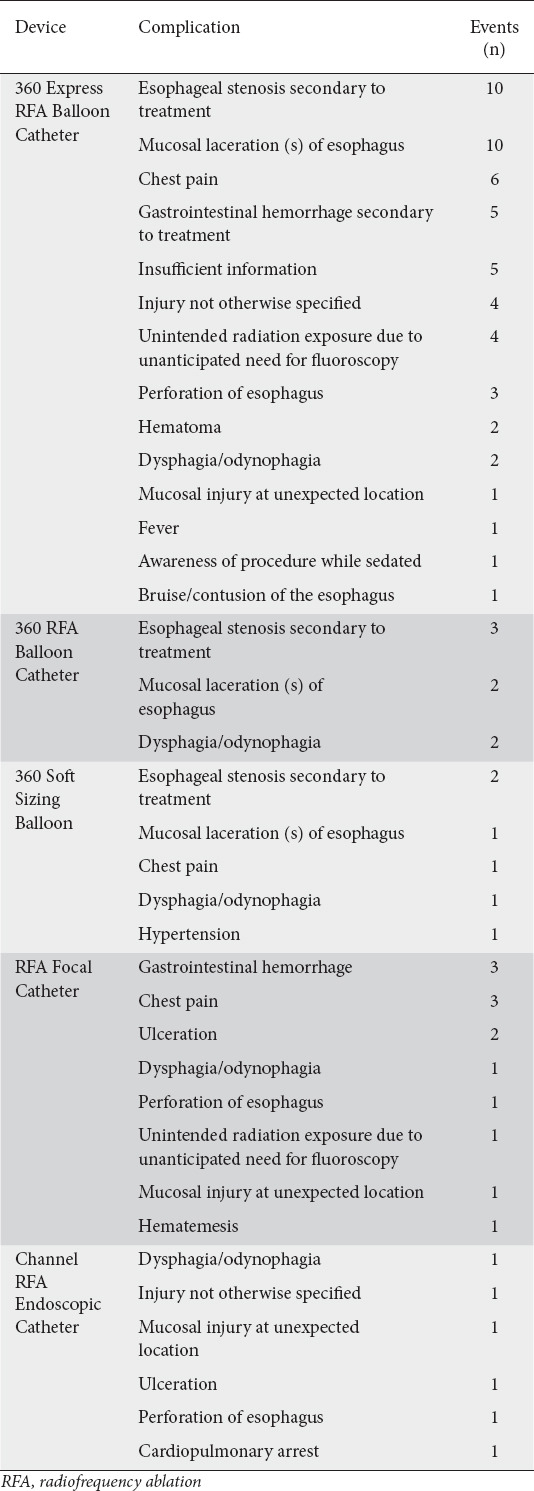
The 360 Express RFA Balloon Catheter had the most patient-related adverse events reported (n=53, 60.92%), followed by the RFA Focal Catheter (n=13, 14.94%). There was one reported death secondary to gastrointestinal hemorrhage. This occurred in a patient who had undergone RFA with the Barrx™ Ultra Long device 10 days previously. The patient in this case developed hematemesis following reinstitution of anticoagulation therapy and died despite interventions to achieve hemostasis.
Device-related issues
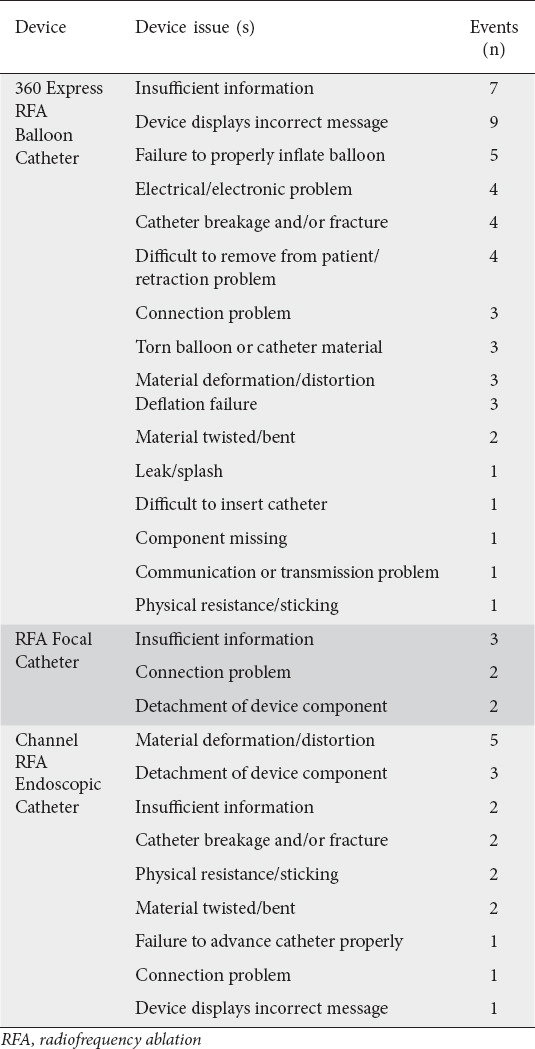
The 360 Express RFA Catheter had the most device problems reported (n=52, 66.67%), followed by the Channel RFA Endoscopic Catheter (n=19, 24.36%). One of the most common device malfunctions for all instruments was an error code being displayed on the RFA generator during attempts at catheter usage (n=10, 12.82%). The most common error message reported was e73, “catheter electronics fault”, which prevented the device from delivering energy. Another common device malfunction was material deformation/distortion (n=7, 8.97%), which affected both the 360 Express RFA Catheter and the Channel RFA catheter, preventing repeat use of the catheter. For the 360 Express RFA catheter material deformation/distortion refers to the end of the catheter being frayed, unraveled or detached from the electrode after removal from the patient. For the Channel RFA catheter this referred to the catheter electrode splitting from the silicone patty or improperly folding in on itself when passed through the working channel of the endoscope.
Catheter breakage was another common problem (n=6, 7.69%). This refers to the ablative surface of the catheter becoming dislodged when the catheter was retracted. All broken parts were removed from patients under direct observation and no harm to patients was reported in any of the reports. Connection problems between the generator cable and RFA device were another common device malfunction (n=6, 7.69%); 3 of these reports were related to one event where the user opened 4 packages before finding one that would connect to the generator cable. No information was provided by the device manufacturer for these reports, so it is unclear if this was due to operator error. Instruments involved in these 3 reports included a Channel RFA catheter, the 90 RFA focal catheter, and the 60 RFA focal catheter.
Failure of the balloon to properly inflate was reported for the 360 Express RFA catheter (n=5, 6.41%); for all events, failure of the balloon to inflate occurred during the procedure when the catheter was in the esophagus. For 3 of these events, there was no patient harm, and a new balloon was used to finish the procedure. The other 2 events resulted in patient harm. Esophageal mucosal damage with bleeding was reported for one event immediately post-procedure; this did not result in a perforation. For the second event, esophageal bleeding was reported 2 weeks post-ablation, this patient was on an anticoagulant and was admitted to the hospital. No further information was provided on this patient’s outcome.
Other less frequent device issues are reported in Table 2. Insufficient information accounted for a total of 12 device malfunctions; these reports were unclear regarding the specifics of the device malfunction.
Table 2.
Summary of device issues
Discussion
BE is diagnosed when there is an extension of salmon-colored mucosa into the tubular esophagus, extending ≥1 cm proximal to the gastroesophageal junction, seen during upper endoscopy, with biopsy confirmation of the presence of intestinal metaplasia. BE is well known to increase the risk of progression to esophageal adenocarcinoma [5].
A 2016 systematic review and meta-analysis showed that the overall risk of adverse events after using RFA in addition to endoscopic mucosal resection was about 9%. As in the present study, esophageal stenosis secondary to treatment was the most commonly reported adverse event, occurring in 6% of all patients [9]. Similarly, a 2013 systemic review and meta-analysis showed that esophageal stricture was the most common adverse event, reported in 5% of patients, followed by pain (3%) and bleeding (1%) [10]. A multicenter, comparative cohort study comparing the manual 360 with the self-sizing 360 Express showed that both systems had similar safety and efficacy. The average number of treatments to achieve complete eradication of intestinal metaplasia was 3 [1,9].
Our analysis of the MAUDE database revealed 15 patients who developed esophageal stenosis secondary to treatment. All of the events were related to the circumferential ablation devices (360 RFA catheter and 360 Express RFA catheter). There were more instances of esophageal stenosis with the 360 Express RFA catheter, perhaps because the 360 Express has been more widely used since its introduction [10].
Chest pain, dysphagia, and general post-procedure discomfort were other adverse events commonly reported by patients. Generally, pain and dysphagia occurred 3-4 days post-procedure [11-13]. In our study, chest pain and dysphagia were reported in 17 cases. Patients reported chest pain at timepoints ranging from immediately following endoscopy to a week post-procedure. Chest pain and dysphagia were reported for all devices studied.
Our study found 5 cases of perforation of the esophagus: 3 for the 360 Express RFA catheter, one for the RFA Focal Catheter, and one for the Channel RFA Endoscopic Catheter. In a 2016 meta-analysis, the authors also found a low rate of esophageal perforation. Five cases were reported, producing a rate of 0.6% [9]. From the 5 cases in our study, 2 instruments were returned to the manufacturer for investigation, in both cases the 360 Express RFA catheter. One device was found to be without defect, while the other was found to have a damaged electrode that had separated from the balloon; this may have caused difficulty in removing the device after ablation. In 3 of the 5 cases it was reported that patients were in stable condition after having perforation of the esophagus treated. It should be noted that limited information has been provided for these cases, with not all reports providing detailed patient follow up or manufacturer reports and no root cause for the perforations that have been identified.
Our study found only one reported death. This death was associated with use of the Ultra Long Catheter and was secondary to a gastrointestinal hemorrhage that occurred 10 days after treatment. The patient had cardiac comorbidities and in this case a blood thinner was withheld 7 days prior to treatment but had to be resumed 7 days after treatment. The patient presented to the Emergency Department with hematemesis 10 days after treatment and died soon after. It should be noted that, at this time, there is limited information regarding this death and no supplemental information was provided to the manufacturer for the report. The device in question was not returned to the manufacturer and no supplemental manufacturer report was issued; no root cause for this death was identified.
To date, only a few studies have assessed the rate of device malfunction events associated with RFA devices. Our study found that the most common device malfunction was an error code being displayed on the RFA generator. When an error code is displayed, the provider is unable to perform the ablation until the error code is resolved, if possible. This increases procedure time, causing increased sedation time for the patient, frustration to the provider, and the disposal of catheters and other resources used for the ablation procedure. These error codes vary in meaning and are often difficult for the end-user to decipher. Medtronic provides a manual for the Barrx™ HaloFlex Generator, but this manual does not provide clear instructions on how to resolve many error codes.
The most commonly reported error code was e73, “catheter electronic fault”. Reports including this error code did not specify how this issue was resolved, and the catheters and generators used were not returned to the manufacturer. Additional error codes reported included e95. The Medtronics Barrx™ HaloFlex Generator manual shows that the e95 error code may be seen in the presence of an air leak when using a balloon-based catheter system and provides steps for verifying the connection between the instruments used. Several of the reports that indicated that an error code was displayed did not specify which error code they received while attempting RFA. The Medtronics Barrx™ HaloFlex Generator manual states that, in the event of an error code, endoscopic evaluation must be used to verify complete deflation of the balloon before removal.
The second most common device malfunction was material deformation/distortion. This occurred with both the 360 Express RFA Catheter and the Channel RFA Catheter. In all cases where devices were returned, after inspection by the device manufacturer it was concluded that the malfunctions were related to user error. A common trend among the manufacturer report narratives was misuse of the Channel RFA Catheter, where users inserted it into the introducer incorrectly and forcibly pushed the catheter through the scope. A field safety notice was issued in February of 2018 by Medtronic for the use of the Barrx™ catheter system that included the directions to not advance or retract the catheter if excessive resistance is met and encouraged users to observe the other instructions and warnings mentioned in the instructions for use. Data collected in this study did not show a difference in number of device malfunction reports submitted after Medtronic issued the field safety notice in 2018.
Many of the limitations of this study are inherent to the data formatting contained in the MAUDE database itself. Events can be submitted that are incomplete, inaccurate, untimely, unverified, or biased. Endoscopists and office staff are not instructed to input device malfunctions or patient-related adverse events into the MAUDE database, and many may not be aware of its existence. Therefore, there may be underreporting of adverse events or device malfunctions. Additionally, the incidence or prevalence of an event cannot be determined by the MAUDE database, because events may be under-reported and there may be a lack of information regarding the frequency of device use.
Additional limitations could include a lack of operator experience and low procedural volume, which could lead to user error. The operator’s individual experience with the RFA system cannot be deduced from the MAUDE database. For the 148 reports in this study only 15 reports included a manufacturer report, where the instrument in question was sent back to the manufacturer. In these 15 reports, the manufacturer determined that 10 of the cases were due to user error. One of these reports noted that the physician performing the ablation had only just learned RFA. For 5 of the reports the manufacturer could not determine the root cause after inspection of the damaged instrument. The MAUDE database does not report who was the operator of the instrument or how much experience they had had with using the instrument in question. Therefore, there may be more cases of user error due to operator experience than were reported.
Actual complication rates cannot be calculated from the MAUDE database, since the total number of Barrx™ RFA catheters used without complications during this time period is not available. This is a limitation of all of the many published studies that utilize MAUDE database information. Lastly, no patient information, such as age, sex, comorbidities or operative reports, is available in the MAUDE database.
The MAUDE database is only one of the FDA’s many post-market surveillance data resources. Not all device malfunctions contained corresponding manufacturer reports, and end-users were not required to return failed or broken devices to the manufacturer for analysis.
RFA is widely used for the treatment of BE. Our analysis of the FDA MAUDE database of currently commercially available endoscopic RFA devices revealed patterns and trends among common device malfunctions and patient-related adverse events. Endoscopists should be aware that stenosis of the esophagus, mucosal laceration and pain are among the most encountered potential clinical adverse events seen in patients undergoing RFA. Esophageal perforation is a rare but potentially major adverse event with RFA treatment. Common device malfunctions include the generator displaying an error code preventing use of the catheter as well as material deformation of the catheters themselves. Healthcare providers performing endoscopic RFA in patients with BE should have an understanding and awareness of these clinical adverse events and device malfunctions.
Summary Box.
What is already known:
Radiofrequency ablation (RFA) is a widely accepted method for eradicating Barrett’s esophagus (BE)
RFA has been incorporated into both American and European guidelines for the management of BE
Although there have been multiple large-scale studies evaluating the clinical efficacy of the Barrx™ system for RFA, there is a paucity of data available regarding the technical failures and clinical adverse events encountered with the Barrx™ system
What the new findings are:
Our analysis of the MAUDE database for the Barrx™ system showed similar findings to previous meta-analyses as regards patient-related adverse events
Endoscopists should be aware that stenosis of the esophagus, mucosal laceration and pain are among the most encountered potential clinical adverse events seen in patients undergoing RFA
Common device malfunctions include the generator displaying an error code preventing use of the catheter, as well as material deformation of the catheters themselves
Biography
Rocky Vista University College of Osteopathic Medicine, Ivins, Utah; University of Utah School of Medicine, Salt Lake City, Utah; Porter Adventist Hospital, Centura Health, Denver, Colorado, USA
Footnotes
Conflict of Interest: None
References
- 1.Kahn A, Priyan H, Dierkhising RA, et al. Outcomes of radiofrequency ablation by manual versus self-sizing circumferential balloon catheters for the treatment of dysplastic Barrett's esophagus:a multicenter comparative cohort study. Gastrointest Endosc. 2021;93:880–887. doi: 10.1016/j.gie.2020.07.056. [DOI] [PubMed] [Google Scholar]
- 2.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140:1084–1091. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Weusten B, Bisschops R, Coron E, et al. Endoscopic management of Barrett's esophagus:European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2017;49:191–198. doi: 10.1055/s-0042-122140. [DOI] [PubMed] [Google Scholar]
- 4.Pouw RE, Sharma VK, Bergman JJ, Fleischer DE. Radiofrequency ablation for total Barrett's eradication:a description of the endoscopic technique, its clinical results and future prospects. Endoscopy. 2008;40:1033–1040. doi: 10.1055/s-0028-1103421. [DOI] [PubMed] [Google Scholar]
- 5.Shaheen NJ, Falk GW, Iyer PG, Gerson LB. American College of Gastroenterology, ACG clinical guideline:diagnosis and management of Barrett's esophagus. Am J Gastroenterol. 2016;111:30–50. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pouw RE, Klaver E, Phoa KN, et al. Radiofrequency ablation for low-grade dysplasia in Barrett's esophagus:long-term outcome of a randomized trial. Gastrointest Endosc. 2020;92:569–574. doi: 10.1016/j.gie.2020.03.3756. [DOI] [PubMed] [Google Scholar]
- 7.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia:a randomized clinical trial. JAMA. 2014;311:1209–1217. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 8.Navaneethan U, Thosani N, Goodman A, et al. ASGE Technology Committee. Radiofrequency ablation devices. VideoGIE. 2017;2:252–259. doi: 10.1016/j.vgie.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qumseya BJ, Wani S, Desai M, et al. Adverse events after radiofrequency ablation in patients with Barrett's esophagus:a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1086–1095. doi: 10.1016/j.cgh.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett's esophagus:systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1245–1255. doi: 10.1016/j.cgh.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belghazi K, Pouw RE, Sondermeijer C, et al. A single-step sizing and radiofrequency ablation catheter for circumferential ablation of Barrett's esophagus:Results of a pilot study. United European Gastroenterol J. 2018;6:990–999. doi: 10.1177/2050640618768919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan R, Mulki R, Peter S. The role of ablation in the treatment of dysplastic Barrett's esophagus. Ther Adv Gastrointest Endosc. 2021;14:26317745211049967. doi: 10.1177/26317745211049967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Condon A, Muthusamy VR. The evolution of endoscopic therapy for Barrett's esophagus. Ther Adv Gastrointest Endosc. 2021;14:26317745211051834. doi: 10.1177/26317745211051834. [DOI] [PMC free article] [PubMed] [Google Scholar]


