Abstract
Background
Primary gastric squamous cell carcinoma (PGSCC) is an uncommon type of gastric malignancy estimated to comprise around 0.04-0.5% of all gastric malignancies. PGSCC’s long-term survival has been quoted to range from 17-50% depending on stage, with surgery arguably representing the most useful modality for prolonging oncologic survival. Nevertheless, reliable data on its effectiveness are still lacking in the literature.
Method
A systematic literature search of the Medline, Cochrane library and Scopus databases was undertaken, to identify cases of surgically managed PGSCC reporting patient-related outcomes.
Results
In total, 23 case reports and 1 case series incorporating 38 patients were identified. Mean patient age was 61.2 years and the male/female ratio was 18:1. Most tumors were high-stage at the time of diagnosis, with the T4 stage predominating in the patient pool (n=15, 50%) along with a high percentage of lymphatic spread (N positive tumors, n=15, 47%). All patients underwent curative-intent surgical resection and were subsequently followed for an average of 30.7±14 months. Extrapolated survival data revealed a projected 3- and 5-year overall survival of 62.2% and 51.9%, respectively, while the 3-year probability for being disease-free was calculated to be 30.8%. T4 stage and lymphatic spread were found to be predictors of poor survival in univariate but not in multivariate analysis.
Conclusion
Notwithstanding the methodological limitations inherent to the present review, the obtained results, when superimposed on existing cross-sectional survival data, suggest significantly enhanced patient survival following surgery, solidifying its role in the management of patients with PGSCC.
Keywords: Primary gastric squamous cell carcinoma, gastric cancer, systematic review
Introduction
Primary gastric squamous cell carcinoma (PGSCC) is an uncommon type of gastric malignancy. Although the true prevalence of this disease cannot be accurately measured because of its rarity, it is roughly estimated to be around 0.04-0.5% of all gastric neoplasms [1-3]. Pure PGSCC closely resembles squamous cell carcinomas (SCCs) encountered in other organs and can be distinctively identified by the criteria established by Boswell and Helwig [4], i.e., the formation of keratinizing cell masses with pearl formation, the mosaic pattern of cell arrangement, the presence of intercellular bridges and the identification of keratin within neoplastic cells. In 2011, the Japanese Gastric Cancer Association published their updated diagnostic criteria, which postulate that: (i) PGSCC should originate entirely from the gastric mucosa; and (ii) all tumor cells must be squamous neoplastic cells, without a glandular component.
The exact pathogenetic mechanism leading to the development of SCC in the stomach is yet to be determined. Boswell and Helwig [4] hypothesized that gastric SCC arises from metaplastic foci thought to exist as a response to injurious insults, such as corrosive agent ingestion or peptic ulcer disease, or conceivably from chronic exposure to tobacco and alcohol [5,6]. Although this explanation is the most plausible one, it has also been suggested that ectopic squamous cell nests encountered in the stomach may also arise de novo from gastric pluripotent stem cells, which undergo malignant transformation and thus give rise to PGSCCs [7]. Attempts at identifying a causal link between human papilloma virus infection and squamous cell gastric cancer have been unsuccessful to this date [8,9].
Taking into account the histologic resemblance of PGSCC to other types of SCC, a similarly aggressive tumor behavior is to be expected. Reports suggest that the prognosis of this particular subtype of gastric cancer is poor [10,11], with expected survival rates of 17-50%, depending on stage and surgical resectability [3,11]. Although surgery combined with chemotherapy and/or radiation therapy is pursued in the treatment of patients with PGSCC, it should be emphasized that reliable long-term data on its effectiveness are still lacking. The aim of the present systematic review was to identify potential prognostic factors related to the oncologic survival of patients with resectable PGSCC and to gauge their long-term survival rates.
Materials and methods
Search strategy
A systematic literature search for articles published until December 2021 was performed using Medline, Cochrane library and Scopus databases. The MESH terms, used in combination with the Boolean operators AND/OR, were: “gastric malignancies”, “primary”, “gastric squamous cell carcinoma”, “gastrectomy”. Two authors (AS, DP) independently screened the obtained abstract list for potentially eligible articles and after full-text evaluation they were evaluated for inclusion in the systematic review. The reference lists of all potentially eligible studies were manually reviewed for any further publications of relevance. Any discrepancies were resolved by a third author (DS). The present systematic review was conducted according to PRISMA guidelines [12].
Inclusion and exclusion criteria
Studies were considered eligible if the following criteria were met: 1) studies reporting cases of PGSCC; and 2) involved neoplasms were surgically excised with curative intent. Exclusion criteria were: 1) SCCs not originating from the stomach; 2) metastatic SCCs; 3) primary gastric SCCs not surgically excised or excised with palliative intent; 4) not curative-intent surgery; 5) studies not in the English language; 6) non-human studies; and 7) letters/reviews/editorials without explicit data on patient therapy and survival.
Data extraction and data of interest
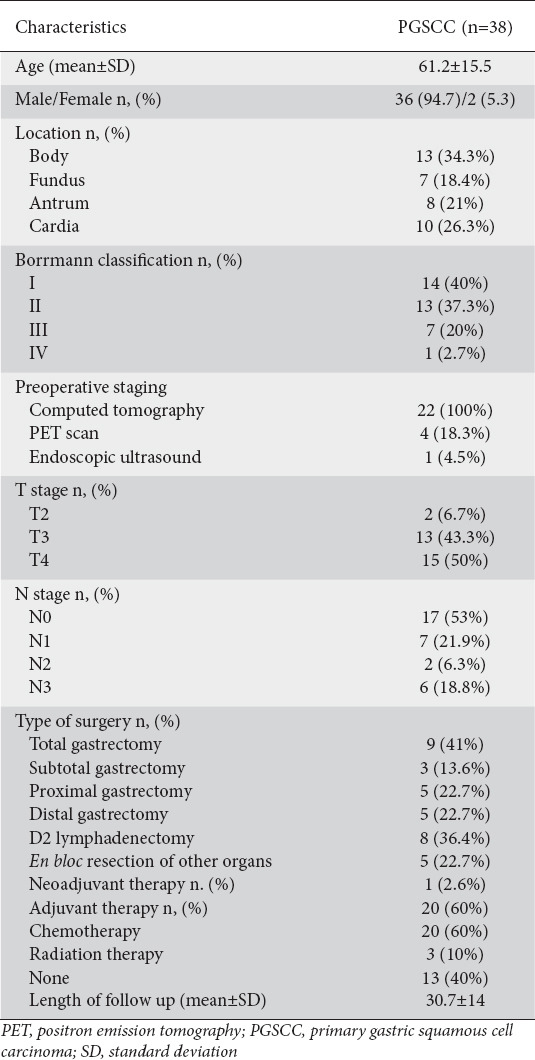
Data extracted from eligible studies were entered into standardized Excel spreadsheets (Microsoft, Redmond, Washington, USA) independently by 2 authors (AS, DP) and were subsequently crosschecked for accuracy and completeness by a third author (DS). All ensuing disagreements were resolved by consensus. Data of interest were the name of the first author, the year of publication, the type of study, the number and demographics of patients involved, the characteristics of the tumor involved (location, size, Borrmann classification), as well as oncologic (TNM classification, type of adjuvant therapy employed) and survival outcomes.
Statistical analysis
Survival time was measured as the period from the date of surgery until death or recurrence or until the most recent follow up. Overall and disease-free survivals were extrapolated using the Kaplan-Meier method. Prognostic factors associated with survival evaluated in this study included age, sex, Borrmann classification, T-stage, N-stage, and use of adjuvant therapy. Univariate and multivariate analyses were performed using the Cox proportional hazards method and all computations were performed using the SPSS statistical package version 26 (SPSS Inc, Chicago, IL, USA). All P-values equal to or less than 0.05 were considered statistically significant.
Results
The initial database search yielded 991 results in total, of which 24 met the inclusion/exclusion criteria described above. The PRISMA flowchart of study selection is depicted in Fig. 1. The entire study pool was comprised of 23 case reports and 1 retrospective cohort study, totaling 38 patients with PSGCC [1,2,19-28,9,29-32,11,13-18] (the details of the included studies are available as Supplementary Table 1 (188.3KB, pdf) ). Included studies were published between 2004 and 2019.
Figure 1.
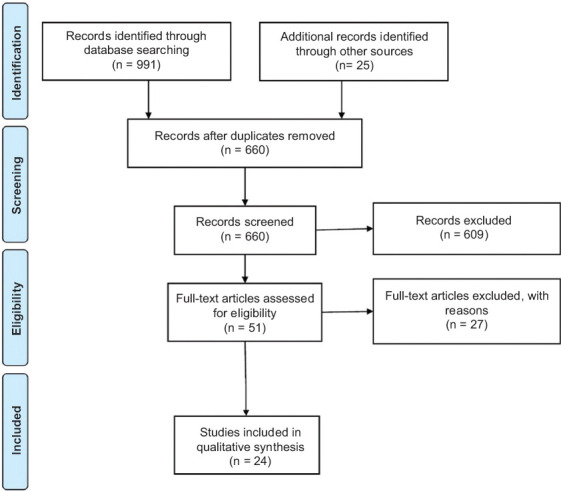
PRISMA flowchart of study selection
Overall, 38 patients (36 male, 2 female), with mean age 61.2 years, were identified. The majority of evaluated tumors were located in the gastric body (n=13, 34.3%), followed by the cardia (n=10, 26.3%), the antrum (n=8, 21%), and the fundus (7, 18.4%). With regard to the endoscopic appearance of the PSGCC, most lesions were either polypoid (Borrmann type I, n=14, 40%) or fungating (Borrmann type II, n=13, 37.3%), with a minority being ulcerated (Borrmann type III, n=7, 20%) or diffuse (Borrmann type IV, n=1 (2.7%). Preoperative staging data were available for a total of 22 patients, all of whom underwent preoperative endoscopy and computed tomography imaging. Additional imaging with positron emission tomography (PET) was performed in 4 (19.3%) patients and endoscopic ultrasound (EUS) in a single patient (4.5% of the entire patient pool). Most tumors were high stage at the time of diagnosis, with the T4 stage predominating in the patient pool (n=15, 50%), along with a high percentage of lymphatic spread (N positive tumors, n=15, 47%). Only one patient received neoadjuvant chemotherapy, while 20 patients (60%) received postoperative adjuvant chemotherapy, 3 underwent radiation therapy (10%) and 12 patients (40%) did not receive any adjuvant treatment. Patients were followed-up for an average of 30.7±14 months. During the follow up, 11 cases of death and 9 cases of disease recurrence occurred. Table 1 summarizes the above findings. The adjuvant and neoadjuvant regimens utilized are detailed in Supplementary Table 2 (188.3KB, pdf) .
Table 1.
Characteristics of the pooled patient cohort
Survival analysis
The cumulative 3- and 5-year survival probabilities were estimated to be 62.2% and 51.9%, respectively. Regarding disease-free survival, the 3-year probability for being disease free was calculated to be 30.8%. The Kaplan-Meier survival curves for overall and disease-free survivals are depicted in Fig. 2 and 3. In total, 21 patients remained alive at the end of their follow up, with 8 of them (38%) being disease-free.
Figure 2.
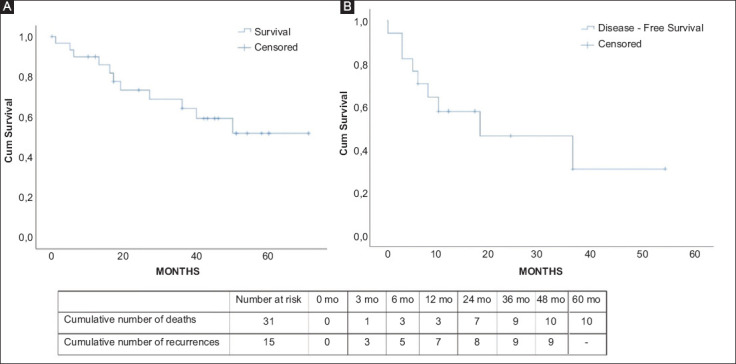
Kaplan-Meier curves of (A) overall survival and (B) disease-free survival
Figure 3.
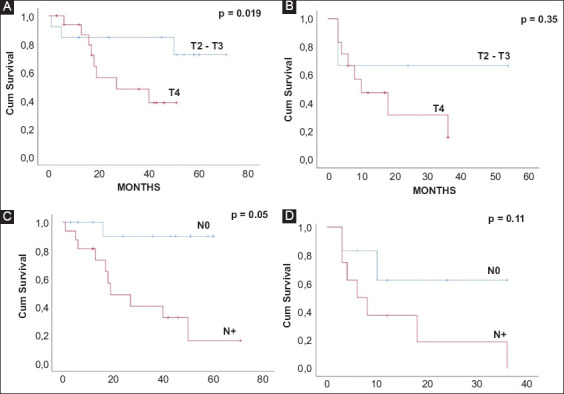
Kaplan-Meier curves of overall survival and disease-free survival stratified by tumor stage (panels A and B) and by lymph node status (panels C and D)
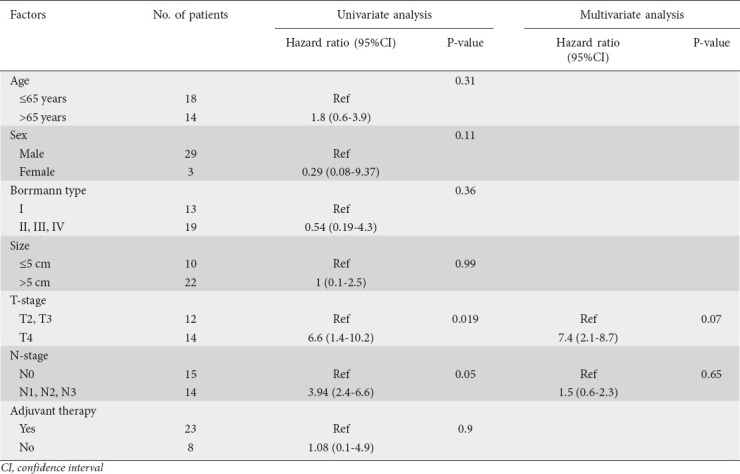
During univariate analysis of factors prognostic for overall survival, only the T4-stage and lymphatic spread were statistically significant predictors of the risk of death from PGSCC (P=0.019 and 0.05, respectively). When introduced into a multivariate model, however, neither of these variables exhibited statistical significance (Table 2).
Table 2.
Univariate and multivariate analyses of prognostic factors for overall survival
Discussion
The paucity of existing literature on PGSCC is a major impediment in the process of understanding this disease and hinders our ability to formulate effective evidence-guided treatment plans. To overcome this obstacle, the present review is the first attempt to summarize all existing literature regarding the outcomes following surgery for PGSCC.
According to our results, PGSCC shows a greater preponderance for male patients, with an estimated 18:1 male: female ratio, and a mean patient age of 61.2 years. These findings suggest that PGSCC is a disease of the 7th decade of life that exhibits an incidence pattern resembling that of other squamous neoplasms of the aerodigestive tract. Although the present patient sample is small, it does suggest that PGSCC shares, at least partly, the same risk factors as esophageal SCC.
The study by Akce et al [3] is, to this date, the largest report on patients with PGSCC, having accumulated 836 patients through national database searching. Their findings with regard to PGSCC demographics are in line with those obtained by the present systematic review, further revealing that the vast majority of gastric SCC cases (73.4%) are not amenable to surgery, and in fact the investigators observed that squamous histology is independently associated with poor patient survival.
Whatever the case may be, it must be acknowledged that PGSCC grows indolently and presents with nonspecific clinical symptoms, not unlike those associated with other gastric malignancies. This is in fact supported by the results of the systematic review, which demonstrated that most PGSCCs are large, averaging 7.1 cm in size, and present at a locally advanced stage (T3-T4, node positive) in their vast majority. Endoscopy is unable to distinguish between PGSCC and gastric adenocarcinoma, because of the macroscopic resemblance between the 2 types of lesion (Borrmann type I being the most common presentation for PGSCC); however, the histopathologic positive staining for p63 and cytokeratins 5, 6, 7 and 20 is unequivocally associated with PGSCC [1].
The treatment paradigm for this rare disease is the same as for other gastric malignancies. Nonetheless, it is uncertain whether oncologic outcomes in cases of localized disease are the same as in localized, resectable adenocarcinomas. Given the paucity of relevant literature on PGSCCs, we constructed a patient cohort from isolated cases to extrapolate survival data following surgical resection of these lesions. Kaplan-Meier curve analysis demonstrated 3- and 5-year overall survival to be approximately 62.2% and 51.9%, respectively, with even lower 3-year disease-free survival at 38% following curative intent surgery with or without adjuvant therapy. Akce et al [3], in their study, quote inferior 5-year overall survival rates of 14.7%, in a patient population that was largely managed non-operatively, mainly because of the presence of metastatic disease at presentation. These observations suggest that PSGCC amenable to surgical resection is likely to be associated with enhanced survival and metastatic disease is possibly an important factor influencing long-term oncologic outcomes.
Furthermore, T4 and node-positive stages were found to be significant negative predictors of survival in univariate but not in multivariate analysis. It is possible that this may be due to a type II statistical error related to the small accrued patient sample, although it is also possible that advanced T-stage correlates strongly with lymphatic spread and therefore cannot be assessed independently. Notwithstanding this limitation, survival following resection of non-metastatic PGSCC appears to be roughly equal to that following resection of gastric adenocarcinoma [33], and therefore surgery should be aggressively pursued whenever disease clearance is technically feasible, irrespective of T and N stage, in the effort to optimize oncologic outcomes.
As far as adjuvant therapy is concerned, no standardized treatment regimens have been established as yet, with most authors reporting the use of 5-FU or cisplatin-based drug combinations [14,16,27]. In our analysis, the use of adjuvant therapy was not associated with prolonged survival, nevertheless, given the lack of data, many factors were left unaccounted for, most importantly the performance status and comorbidities of included patients. In addition, radiotherapy was scarcely used: only in 2 patients of the entire cohort [20,23], for whom no follow-up data are available, and therefore no assumptions on its usefulness in the setting of PGSCC can be made. Taking into consideration the radiosensitivity of other squamous neoplasms of the digestive tract, we speculate that radiation therapy, in addition to chemotherapy, may be more effective in improving disease-free and overall survivals.
It must be emphasized that the present review inherently bears significant limitations that preclude the generalizability of the obtained results. More significantly, the small patient sample drawn from isolated case reports or small case series is thus highly subject to publication and indication biases. Moreover, a large number of patient and disease-related confounding factors were missing from the extracted reports (most importantly, patient comorbidities and overall status, lack of standardized adjuvant treatment protocols and limited follow-up length), and this, when taken together with the possibility of selection bias, reduces the likelihood that the presented outcomes are an accurate cross-sectional portrayal of PGSCC. Instead, they are a reflection of all existing literature, aiming to provide a rough assessment of survival following curative-intent surgical management of PGSCC.
In conclusion, PGSCC is commonly diagnosed in advanced stages, yet survival appears to be on a par with that observed for the most commonly encountered gastric adenocarcinomas. Surgery, when applicable, is the mainstay of treatment and, when coupled with adjuvant therapy, may lead to survival rates of up to 50%. More frequent and thorough reporting of the outcomes of PGSCC patients is still required to elucidate the role of chemoradiotherapy in these particular neoplasms.
Summary Box.
What is already known:
Primary gastric squamous cell carcinoma (PGSCC) is a rare subtype of gastric cancer estimated to comprise around 0.04-0.5% of all gastric malignancies
PGSCC is hypothesized to arise either from metaplastic foci surfacing as a response to injurious insults, or from gastric pluripotent stem cells undergoing malignant transformation
The prognosis of this gastric cancer subtype is poor, with expected survival rates of 17-50%, depending on stage and surgical respectability
What the new findings are:
Through systematic literature searching we identified 23 unique case reports and 1 case series of patients with PGSCC undergoing curative intent surgery
Evaluated patients had predominantly T4 tumors, with a high likelihood of lymphatic spread
Extrapolated survival data after curative-intent surgery indicated projected 3- and 5-year overall survival rates of 62.2% and 51.9%, respectively
The estimated 3-year probability for being disease-free after curative-intent surgery was 30.8%
Biography
National and Kapodistrian University of Athens, Laikon General Hospital; National and Kapodistrian University of Athens, Attikon University Hospital, Greece
Footnotes
Conflict of Interest: None
References
- 1.Guzman Rojas P, Parikh J, Vishnubhotla P, Oharriz JJ. Primary gastric squamous cell carcinoma. Cureus. 2018;10:e2389–e2389. doi: 10.7759/cureus.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.González-Sánchez JA, Vitón R, Collantes E, Rodríguez-Montes JA. Primary squamous cell carcinoma of the stomach. Clin Med Insights Oncol. 2017;11:1179554916686076. doi: 10.1177/1179554916686076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akce M, Jiang R, Alese OB, et al. Gastric squamous cell carcinoma and gastric adenosquamous carcinoma, clinical features and outcomes of rare clinical entities:a National Cancer Database (NCDB) analysis. J Gastrointest Oncol. 2019;10:85–94. doi: 10.21037/jgo.2018.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boswell JT, Helwig EB. Squamous cell carcinoma and adenoacanthoma of the stomach. A clinicopathologic study. Cancer. 1965;18:181–192. doi: 10.1002/1097-0142(196502)18:2<181::aid-cncr2820180209>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Eaton H, Tennekoon GE. Squamous carcinoma of the stomach following corrosive acid burns. Br J Surg. 1972;59:382–387. doi: 10.1002/bjs.1800590514. [DOI] [PubMed] [Google Scholar]
- 6.Vaughan WP, Straus FH, 2nd, Paloyan D. Squamous carcinoma of the stomach after luetic linitis plastica. Gastroenterology. 1977;72:945–948. [PubMed] [Google Scholar]
- 7.Straus R, Heschel S, Fortmann DJ. Primary adenosquamous carcinoma of the stomach. A case report and review. Cancer. 1969;24:985–995. doi: 10.1002/1097-0142(196911)24:5<985::aid-cncr2820240518>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Snietura M, Waniczek D, Piglowski W, et al. Potential role of human papilloma virus in the pathogenesis of gastric cancer. World J Gastroenterol. 2014;20:6632–6637. doi: 10.3748/wjg.v20.i21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang SH, Lee JH, Kim K, et al. Primary squamous cell carcinoma of the stomach:A case report. Oncol Lett. 2014;8:2122–2124. doi: 10.3892/ol.2014.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng Y, Zhang J, Wang H, et al. Poorer prognosis in patients with advanced gastric squamous cell carcinoma compared with adenocarcinoma of the stomach:Case report. Medicine (Baltimore) 2017;96:e9224. doi: 10.1097/MD.0000000000009224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Zhu H, Xu F, et al. Clinicopathological characteristics, treatment, and prognosis of 21 patients with primary gastric squamous cell carcinoma. Gastroenterol Res Pract. 2016;2016:3062547. doi: 10.1155/2016/3062547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement:an updated guideline for reporting systematic reviews. PLOS Med. 2021;18:e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao L, Tang X, Qu H, et al. Primary gastric squamous cell carcinoma presenting as a large submucosal mass:A case report and literature review. Medicine (Baltimore) 2020;99:e22125–e22125. doi: 10.1097/MD.0000000000022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamagata Y, Saito K, Ban S, Fujii A, Oya M. The origin of p40-negative and CDX2-positive primary squamous cell carcinoma of the stomach:case report. World J Surg Oncol. 2019;17:53. doi: 10.1186/s12957-019-1594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vailas MG, Syllaios A, Hasemaki N, et al. A type of neoplasia deadlier than gastric adenocarcinoma?Report of a case of primary gastric squamous cell carcinoma. World J Surg Oncol. 2019;17:113. doi: 10.1186/s12957-019-1657-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang YS, Kim MS, Kim DH, et al. Primary squamous cell carcinoma of the remnant stomach after subtotal gastrectomy. J Gastric Cancer. 2016;16:120–124. doi: 10.5230/jgc.2016.16.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao S, Chen D, Huang L, Dai R, Shan Y. Primary squamous cell carcinoma of the stomach:a case report and literature review. Int J Clin Exp Pathol. 2015;8:9667–9671. [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G, Chen P, Shi L, Zhao W. Squamous cell carcinoma arising from an unknown primary site metastasizing to the stomach:a case report. Oncol Lett. 2014;7:1063–1066. doi: 10.3892/ol.2014.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakabayashi H, Matsutani T, Fujita I, et al. A rare case of primary squamous cell carcinoma of the stomach and a review of the 56 cases reported in Japan. J Gastric Cancer. 2014;14:58–62. doi: 10.5230/jgc.2014.14.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gülçiçek OB, Solmaz A, Özdoğan K, et al. Primary squamous cell carcinoma of the stomach. Ulus cerrahi Derg. 2015;32:221–223. doi: 10.5152/UCD.2015.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patnayak R, Reddy V, Radhakrishnan, Jena A. Primary squamous cell carcinoma of stomach:a rare entity - case report and brief review of literature. J Surg Tech Case Rep. 2015;7:45–47. doi: 10.4103/2006-8808.185656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modi Y, Shaaban H, Parikh N, Guron G, Maroules M. Primary pure squamous cell carcinoma of the stomach treated with neoadjuvant chemotherapy and surgical resection. Indian J Cancer. 2015;52:145. doi: 10.4103/0019-509X.175570. [DOI] [PubMed] [Google Scholar]
- 23.von Waagner W, Wang Z, Picon AI. A rare case of a primary squamous cell carcinoma of the stomach presenting as a submucosal mass. Case Rep Surg. 2015;2015:482342. doi: 10.1155/2015/482342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X-D, Zhou Y, Fan R-G, Zhou B, Shi Q, Jia J. Primary squamous cell carcinoma of the stomach presenting as a huge retroperitoneal tumor:a case report. Rev Esp Enferm Dig. 2016;108:283–284. doi: 10.17235/reed.2015.3795/2015. [DOI] [PubMed] [Google Scholar]
- 25.Mardi K, Mahajan V, Sharma S, Singh S. Primary squamous cell carcinoma of stomach:A rare case report. South Asian J Cancer. 2013;2:199. doi: 10.4103/2278-330X.119897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little M, Munipalle PC, Viswanath YKS. Primary squamous cell carcinoma of the stomach:a rare entity. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-009706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tokuhara K, Nakano T, Inoue K, Nakane Y, Kwon A-H. Primary squamous cell carcinoma in the gastric remnant. Surg Today. 2012;42:666–669. doi: 10.1007/s00595-012-0144-6. [DOI] [PubMed] [Google Scholar]
- 28.Callacondo D, Ganoza-Salas A, Anicama-Lima W, Quispe-Mauricio A, Longacre TA. Primary squamous cell carcinoma of the stomach with paraneoplastic leukocytosis:a case report and review of literature. Hum Pathol. 2009;40:1494–1498. doi: 10.1016/j.humpath.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Choi S-B, Park S-S, Oh S-Y, et al. Primary squamous cell carcinoma of the stomach that developed with Menetrier's disease. Dig Dis Sci. 2007;52:1722–1724. doi: 10.1007/s10620-006-9191-4. [DOI] [PubMed] [Google Scholar]
- 30.Yildirim Y, Akcali Z, Bilezikci B, Ozyilkan O. Primary squamous cell carcinoma of the stomach:a case report. Tumori. 2005;91:440–442. doi: 10.1177/030089160509100513. [DOI] [PubMed] [Google Scholar]
- 31.Hara J, Masuda H, Ishii Y, et al. Exophytic primary squamous cell carcinoma of the stomach. J Gastroenterol. 2004;39:299–300. doi: 10.1007/s00535-003-1294-5. [DOI] [PubMed] [Google Scholar]
- 32.Karaca G, Pekcici MR, Özer H, et al. Primary squamous cell carcinoma of the stomach in a 68-years-old man. Geriatr Gerontol Int. 2011;11:119–120. doi: 10.1111/j.1447-0594.2010.00642.x. [DOI] [PubMed] [Google Scholar]
- 33.Asplund J, Kauppila JH, Mattsson F, Lagergren J. Survival trends in gastric adenocarcinoma:a population-based study in Sweden. Ann Surg Oncol. 2018;25:2693–2702. doi: 10.1245/s10434-018-6627-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


