Abstract
Over 20 years ago, it was first proposed that autoinflammation underpins a handful of rare monogenic disorders characterized by recurrent fever and systemic inflammation. The subsequent identification of novel, causative genes directly led to a better understanding of how the innate immune system is regulated under normal conditions, as well as its dysregulation associated with pathogenic mutations. Early on, IL-1 emerged as a central mediator for these diseases, based on data derived from patient cells, mutant mouse models and definitive clinical responses to IL-1 targeted therapy. Since that time, our understanding of the mechanisms of autoinflammation has expanded beyond IL-1 to additional innate immune processes. However, the number and complexity of IL-1-mediated autoinflammatory diseases has also multiplied to include additional monogenic syndromes with expanded genotypes and phenotypes, as well as more common polygenic disorders seen frequently by the practising clinician. In order to increase physician awareness and update rheumatologists who are likely to encounter these patients, this review discusses the general pathophysiological concepts of IL-1-mediated autoinflammation, the epidemiological and clinical features of specific diseases, diagnostic challenges and approaches, and current and future perspectives for therapy.
Subject terms: Rheumatic diseases, Inflammasome
In this Review, the authors summarize the pathophysiological mechanisms of IL-1-mediated autoinflammation. They describe the epidemiological and clinical features of autoinflammatory diseases, challenges associated with diagnostics and disease management, and current and future therapies for targeting the IL-1 pathway.
Key points
IL-1α, IL-1β and IL-1RA are highly regulated inflammatory mediators involved in damage- and pathogen-associated molecular pattern (DAMPs and PAMPs), and cell death pathways.
Patients with evidence of systemic inflammation without persistent infection or autoantibodies should raise suspicion of an IL-1-mediated autoinflammatory disorder.
Rare monogenic and common polygenic diseases with neutrophilia and inflammation might respond to targeting the IL-1 pathway.
Genetic testing confirms IL-1-driven autoinflammatory disorders, yet new disease phenotype–genotype correlations continue to be identified.
IL-1-targeted therapies are highly effective and safe; new therapeutics focus on targets independent of IL-1 receptor binding, including NLRP3, caspases, IRAK4 and MK2.
Introduction
In the 1970s, searches for the underlying mechanisms of fever led to the discovery of a class of molecules referred to initially as leukocytic pyrogens1. These secreted proteins gained notoriety for their potency in triggering inflammation, and were ultimately given the names IL-1α and IL-1β upon their purification and cloning in the 1980s2,3 (Fig. 1). Since that time, these two unique inflammatory mediators, both of which share a common IL-1 receptor (IL-1R1), became recognized as members of a much larger IL-1 family of cytokines, including 11 pro-inflammatory and anti-inflammatory cytokines that share a common inactive precursor structure and 10 multi-chain cytokine receptors. The IL-1 family also includes two decoy receptors, an inhibitory binding protein and two receptor antagonists, the most well-known of which is IL-1 receptor antagonist (IL-1RA), a natural inhibitor of IL-1R1 activation4. The presence of many proteins within this family, often with synergistic or opposing functions, suggests important biological roles requiring tight regulation. Although the IL-1 family includes disease-relevant cytokines IL-18, IL-33 and IL-36 (reviewed in Mantovani et al.5), for the purposes of this Review on autoinflammatory diseases, we focus on IL-1β, IL-1α and IL-1RA.
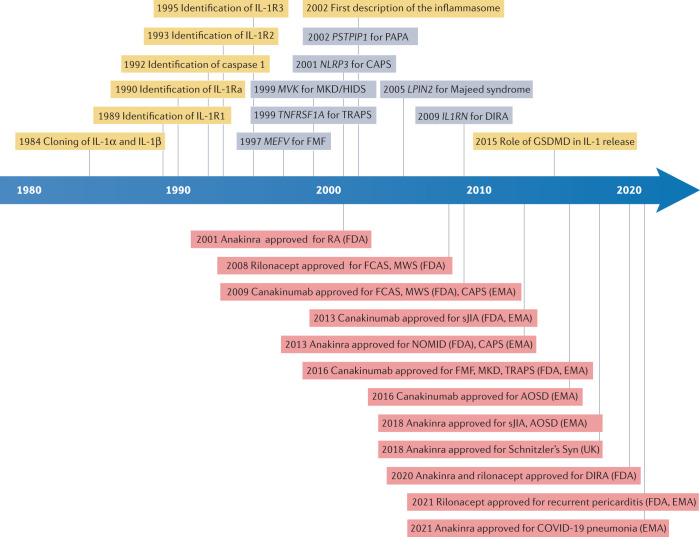
Fig. 1. Timeline of key events leading to current understanding and treatment of IL-1-mediated autoinflammatory diseases.
First described as pyrexin in 1943, and subsequently termed lymphocyte-activating factor and leukocytic pyrogen in the 1970s1, the introduction of interleukin nomenclature united these secreted macrophage products as IL-1. The timeline shows scientific advances in yellow, the first identification of a specific gene as the cause of a given autoinflammatory syndrome in grey, and the initial approval for IL-1-targeted therapies (with agency) in red. AOSD, adult-onset Still disease; CAPS, cryopyrin-associated periodic syndromes; DIRA, deficiency of IL1 receptor antagonist; FCAS, familial cold autoinflammatory syndrome; FMF, familial Mediterranean fever; GSDMD, gasdermin D; HIDS, hyper IgD syndrome; MKD, mevalonate kinase deficiency; MWS, Muckle–Wells syndrome; NOMID, neonatal-onset multisystem inflammatory disease; PAPA, pyogenic arthritis, pyoderma gangrenosum and acne syndrome; RA, rheumatoid arthritis; sJIA, systemic juvenile idiopathic arthritis; Syn, syndrome; TRAPS, tumour necrosis factor receptor-associated periodic syndrome; UK, Medicines and Healthcare Products Regulatory Agency of the United Kingdom.
The most consistent clinical feature of autoinflammation mediated by IL-1, besides fever, is episodic or chronic systemic and/or tissue inflammation. Common areas that are affected include the skin and musculoskeletal system, although certain inflammatory sites are unique to specific IL-1-related disorders, such as serous membranes (pleura and peritoneum), the central nervous system (CNS) and conjunctiva. Patients also frequently complain of fatigue or malaise, symptoms that are commonly associated with chronic inflammation. In general, laboratory evaluation of patients, either during symptoms and sometimes between episodes, reveals elevated acute phase markers and neutrophilia in blood and tissue. Chronic systemic inflammation can lead to anaemia of chronic disease and tissue damage, such as amyloid A amyloidosis.
The first autoinflammatory disorders described were monogenic hereditary fever disorders caused by mutations in single genes encoding for inflammasome-related proteins. This classification has since been expanded to include other monogenic autoinflammatory diseases that result in IL-1-mediated inflammation and putative polygenic disorders that appear to be mediated by IL-1, as indicated by clinical response to IL-1-targeted therapies. The monogenic disorders are generally rare, with a prevalence ranging from 1 in millions to 1 in tens of thousands, although some of these diseases, particularly specific autosomal-recessive conditions, may be observed more frequently in specific isolated populations (such as familial Mediterranean fever (FMF)), likely because of selective infectious pressures occurring centuries ago6. Founder mutations have been described as underpinning several of these disorders in different parts of the world; however, de novo mutations are often identified in many of the autosomal-dominant diseases. In this Review, we describe the molecular biology underlying IL-1-mediated inflammation and its role in autoinflammatory disorders. We highlight pertinent clinical features relevant to the practising rheumatologist, diagnostic and management considerations, and ongoing challenges faced by physicians and patients.
The complex biology of IL-1
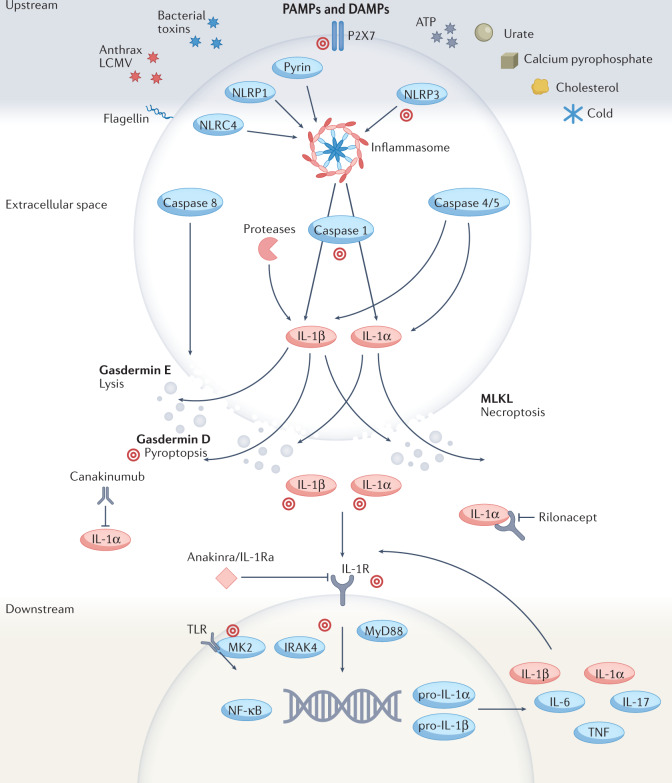
IL-1β, often referred to as IL-1, is expressed primarily in myeloid cells (such as monocytes, macrophages and neutrophils) in a highly controlled fashion involving numerous regulatory mechanisms7,8 (Fig. 2). IL-1β is expressed as an inactive precursor (pro-IL-1β) that is cleaved to its active form by several proteases, including caspase 1 and neutrophil- and microorganism-derived proteases9. Caspase 1-dependent cleavage is driven primarily by activation of large intracellular multi-protein complexes known as inflammasomes, which consist of a sensor protein (such as NLRP3 or pyrin) and an adaptor protein ASC that oligomerize to form polymeric caspase 1 cleavage platforms. In addition to cleaving pro-IL-1β, caspase 1 cleaves and thereby activates gasdermin D, which allows it to form membrane pores enabling the release of IL-1β from the cell and mediating pyroptosis, a pro-inflammatory form of cell death10 (Fig. 2). IL-1β can also be released by a caspase 8 and gasdermin E-mediated lytic process11, by mixed lineage kinase domain-like protein (MLKL)-mediated necroptosis, and likely by other mechanisms that remain to be elucidated12. After IL-1β is released from the cell, the pro-inflammatory effects are exerted through binding to IL-1R1 on the same cell or nearby cells, activating intracellular signalling pathways involving IRAK4, MK2 and NF-κB that ultimately lead to further expression of inflammatory cytokines and inflammasome proteins13. In this manner, IL-1β is a potent activator of its own expression and release, leading to an amplified, autoinflammatory response.
Fig. 2. Mechanisms of IL-1-mediated inflammation and targets for therapy.
The expression, release and functional consequences of IL-1β and IL-1α are intertwined and highly regulated at multiple levels. Many upstream mechanisms, which can be grouped into damage- and pathogen-associated molecular patterns (DAMPs and PAMPs), trigger the activation of the inflammasome, an intracellular complex of multiple proteins (such as NLRC4, NLRP1, pyrin and NLRP3). Activation of the inflammasome, in turn, activates the caspase enzymes 1, 4 and 5, and other proteases, which process (cleave) and activate IL-1β and IL-1α. Non-inflammasome pathways can also activate caspase 8. Upon activation, IL-1β and IL-1α are released from the cell via a gasdermin E lytic process, gasdermin D pore formation and pyroptosis, or mixed lineage kinase domain-like pseudokinase (MLKL)-mediated necroptosis. IL-1β and IL-1α can then bind and activate IL-1R on nearby cells, leading to downstream intracellular signalling, and the expression, processing and release of additional IL-1 proteins, ultimately causing a positive, autoinflammatory feedback loop, and recruitment of neutrophils and other inflammatory cells. The three approved IL-1-targeted biologic therapies prevent IL-1R activation (represented by T-ended arrows). Numerous other drugs, at various stages of development, target inflammasome components and upstream and downstream pathways (target symbols).
Though less frequently recognized, IL-1α is constitutively expressed in all cells as an active pro-form and is unique in that it localizes to the nucleus, cytoplasm and cell membrane, where it has site-specific functions (Fig. 2). IL-1α shuttles between the nucleus and cytoplasm depending on cellular conditions, such as homeostasis or infection. In the nucleus, it binds to chromatin and functions as a transcription factor regulating cytokine expression downstream of NF-κB and AP-1 including IL-6 and IL-8, whereas in the cytoplasm it binds to mitochondrial cardiolipin to regulate NLRP3 inflammasome function14. At the cell membrane, IL-1α can bind to and activate IL-1R1 on adjacent cells, or can be released in membrane-bound apoptotic bodies, leading to further local and systemic inflammation5. Although IL-1α can be cleaved by several proteases, it does not require cleavage for biologic activity or for secretion. The major mechanism of IL-1α release appears to be through lytic cell death (pyroptosis or necroptosis); it is thus considered an alarmin that triggers sterile inflammation locally, such as during ischaemia. Moreover, through binding to IL-1R1, IL-1α acts as a potent driver of neutrophil recruitment in several tissues including the skin and lungs15. Interestingly, IL-1β can bind to IL-1α and serve as a shuttle for its secretion16, suggesting an additional layer of complexity to the regulation of IL-1 release.
A third important member of the IL-1 family, IL-1 receptor antagonist (IL-1RA), appears to be a predominantly anti-inflammatory cytokine. IL-1RA is expressed in all cells and tissues, and is induced by several inflammatory stimuli, as evidenced by high levels of IL-1RA in serum from patients with inflammatory diseases. It is a natural inhibitor of IL-1-mediated inflammation, acting by competitively binding to IL-1R1 and thereby preventing the binding of both IL-1α and IL-1β. Although this function of IL-1RA is well established, it has more than one isoform that may have additional functions17. Moreover, IL-1RA has anti-apoptotic effects via an intracellular non-receptor-mediated mechanism18. Although a complete understanding of IL-1RA biology remains to be reached, its clinical importance became evident with the discovery of patients with deficiency of IL-1RA (DIRA), discussed below, and the widespread utility of recombinant IL-1RA (anakinra) as a therapeutic for IL-1-driven autoinflammatory disease.
IL-1 and autoinflammation
Our appreciation of the complexity of IL-1 biology originally stemmed from attempts to understand the mechanisms of fever. As such, it is fitting that the rekindling of interest in IL-1-related mechanisms at the turn of the century occurred with the discovery of the molecular pathways underlying the pathology of several rare hereditary fever disorders19–22. These conditions are all characterized by recurrent or chronic systemic and tissue inflammation combined with fever, rash and musculoskeletal symptoms. The identification of gene mutations responsible for these immunodysregulation diseases led to the introduction of an entirely new disease classification known as autoinflammation, which encompasses inflammatory disorders driven by innate immunity in the absence of high-titre autoantibodies or antigen-specific T lymphocytes22. Each discovered autoinflammatory disease revealed new mechanisms of dysregulated IL-1 biology, such as intrinsic unregulated inflammasome function, extrinsic mechanisms of inflammasome activation, and ineffective or absent IL-1 regulatory pathways. All of these mechanisms have broader implications for our understanding of innate immune regulation.
The innate immune system requires highly regulated mechanisms to prevent constitutive or uncontrolled responses that could harm the host, while allowing for rapid inflammatory responses that are selective for pathogen- and damage-associated molecular patterns (PAMPs and DAMPs) (Fig. 2). Inflammasome proteins are generally inactive under normal conditions but are activated by specific triggers, such as pathogen toxins23. Uniquely, the NLRP3 inflammasome is activated by numerous triggers including ATP and several crystals24. Gain-of-function mutations in MEFV and NLRP3, which encode pyrin and cryopyrin, respectively, result in intrinsic constitutive activation of the inflammasome, or a reduced threshold for its activation. This mechanism is responsible for the inflammatory phenotype observed in patients with FMF or cryopyrin-associated periodic syndromes (CAPS)19–21. Other hereditary fever disorders, such as mevalonate kinase deficiency (MKD)25,26, TNF-associated periodic syndrome (TRAPS)22, and pyogenic arthritis, pyoderma gangrenosum and acne (PAPA) syndrome27, are caused by mutations in genes that encode proteins with extrinsic effects on inflammasome activation, such as MVK, TNFRSF1A and PSTPIP1, rather than the inflammasome-encoding genes themselves. Although the mechanisms of autoinflammation extend beyond IL-1 to other cytokines and immune pathways28, the study of IL-1-mediated autoinflammatory diseases remains an exciting field that is highly relevant to the practicing rheumatologist.
Aetiology of autoinflammatory diseases
The description and characterization of autoinflammatory diseases has long been linked to the identification of the underlying genetic and molecular basis for inflammation. Although phenotypically distinct patients are typically characterized first in the literature, the description of additional patients with shared genotypes has resulted in a much broader disease spectrum than was initially appreciated. Currently, more than 30 genetically defined autoinflammatory diseases, many with direct and indirect links to the IL-1 pathway, have been described and listed in the registry of hereditary autoinflammatory disorder mutations29–32.
Monogenic autoinflammatory disorders are caused by mutations in single genes related to control of inflammation (Table 1). Despite the rarity of these syndromes, their shared features of fever, arthralgias and rashes suggest that many will present to the rheumatology clinic. Mechanistically, monogenic autoinflammatory disorders lead to persistent activation of the NLPR3 or pyrin inflammasomes and subsequent activation of caspase 1, resulting in IL-1 release and autoinflammation. Other monogenic disorders, including those caused by mutations in TNFRSF1A, MVK, PSTPIP1 and CDC42, lead to IL-1 activation through pathways other than direct inflammasome activation, such as by the accumulation of intracellular stress triggers, enhanced binding to known intracellular sensors, or by affecting immune signalling pathways33–39. Autoinflammatory diseases caused by other members of the IL-1 cytokine family (Supplementary Table 1) are beyond the scope of this review.
Table 1.
Monogenic autoinflammatory diseases driven by IL-1
| Disease | Gene | Presentation | Therapeutic target |
|---|---|---|---|
| FCAS | NLRP3 | Cold urticaria, chills, conjunctivitis, myalgia/arthralgia, fever | IL-1 |
| MWS | NLRP3 | Sensorineural hearing loss, urticarial rash, conjunctivitis, myalgia/arthralgia, fever | IL-1 |
| NOMID | NLRP3 | CNS inflammation (chronic aseptic meningitis, vision loss, hearing loss), knee arthropathy, urticarial rash, fever | IL-1 |
| FMF | MEFV | Serosal pain (abdominal, chest), arthralgia, erysipeloid rash, fever | IL-1 |
| PAAND | MEFV | Sterile skin abscesses, myalgia, myositis, rash, fever | IL-1, TNF |
| HIDS | MVK | Triggered by vaccination, abdominal pain, vomiting, rash, myalgia/arthralgia, aphthous ulcers, fever | IL-1, TNF |
| MA | MVK | Developmental delay, FTT, dysmorphic features, recurrent fever | IL-1, TNF |
| TRAPS | TNFRSF1A | Painful centrifugal rash, periorbital oedema, prolonged fever, abdominal pain, headache, conjunctivitis, myalgia/arthralgia | IL-1, TNF |
| DIRA | IL1RN | Pustular rash, sterile osteomyelitis, periostitis, hepatosplenomegaly, fever | IL-1 |
| PAPA | PSTPIP1 | Pyoderma gangrenosum, arthritis, acne | IL-1, TNF |
| Hz/Hc | PSTPIP1 | Rash, FTT, hepatosplenomegaly, neutropenia | IL-1, TNF |
| CDC42/NOCARH | CDC42 | Pancytopenia, neurodevelopmental defects, facial dysmorphism, recurrent infection, rash, MAS/HLH, fever | IL-1 |
| Majeed syndrome | LPIN2 | Osteomyelitis, dyserythropoietic anaemia, rash, fever | IL-1 |
CNS, central nervous system; DIRA, deficiency of IL1 receptor antagonist; FCAS, familial cold autoinflammatory syndrome; FMF, familial Mediterranean fever; FTT, failure to thrive; HIDS, hyper IgD syndrome; Hz/Hc, hyperzincaemia/hypercalprotectinaemia; HLH, haemophagocytic lymphohistiocytosis; MA, mevalonic aciduria; MAS, macrophage activation syndrome; MKD, mevalonate kinase deficiency; MWS, Muckle–Wells syndrome; NOCARH, neonatal-onset cytopenia with dyshaematopoiesis, autoinflammation, rash, and HLH; NOMID, neonatal-onset multisystem inflammatory disease; PAAND, pyrin-associated autoinflammation with neutrophilic dermatosis; PAPA, pyogenic arthritis, pyoderma gangrenosum and acne; TNF, tumour necrosis factor; TRAPS, tumour necrosis factor receptor-associated periodic syndrome.
IL-1-driven autoinflammatory diseases
NLRP3 spectrum disease
CAPS, also known as cryopyrinopathies, represent a disease continuum caused by gain-of-function mutations in NLRP3. These heterozygous mutations were first described in families with familial cold autoinflammatory syndrome and Muckle–Wells syndrome20, and then subsequently identified in patients with neonatal-onset multisystem inflammatory disease (NOMID; also known as chronic infantile neurological, cutaneous and articular syndrome (CINCA))40. Across the spectrum of severity, patients with CAPS share symptoms of recurrent fever, urticaria-like rash with neutrophilic infiltration, headaches, joint pain and conjunctivitis, as well as serological evidence of systemic inflammation. However, unique clinical features exist and a fairly consistent genotype–phenotype correlation can be used to define where patients fall on the CAPS disease spectrum41. Emphasis on the full spectrum is now reinforced by a newly proposed taxonomy that classifies these disorders as mild, moderate and severe NLRP3-associated autoinflammatory disease (NLRP3-AID)42. For historical context, and consistency with international drug approvals, this Review uses both the old and the new taxonomy.
At the mild end of the CAPS spectrum, patients with familial cold autoinflammatory syndrome (mild NLRP3-AID) experience brief flares, often less than 24 h in duration, induced by exposure to cold temperatures20. More severe clinical features on the CAPS spectrum include longer duration of episodes and stronger neurological symptoms due to CNS inflammation. In the moderate NLRP3-AID phenotype, Muckle–Wells syndrome, patients experience longer episodes of 2–3 days and often develop a progressive sensorineural hearing loss beginning in the first or second decade of life. The most severe NLRP3-AID phenotype, NOMID, is characterized by nearly persistent systemic inflammation with additional neurological symptoms including chronic aseptic meningitis and cognitive impairment43,44. In these patients, increased intracranial pressure may lead to papillary oedema and optic disc atrophy45. Moreover, skeletal abnormalities, including frontal bossing and a distinctive distal femur overgrowth, are also typically observed46,47.
The constellation of symptoms, combined with frequent genetic testing, has also resulted in the identification of NLRP3 variants in other related syndromes. For example, somatic variants in NLRP3 have been described in some patients with Schnitzler syndrome48. CAPS-like phenotypes have also been observed in patients with variants in other autoinflammatory genes including NLRP12 (ref.49), NLRC4 (ref.50) and F12 (Factor XII)51. Thus, the phenotypic spectrum and genotypic aetiology of inflammasome-mediated diseases remain active areas of clinical and translational research.
MEFV spectrum disease
Mutations in MEFV, which encodes pyrin, underlie two distinct autoinflammatory syndromes: pyrin-associated autoinflammatory diseases-FMF and pyrin-associated autoinflammation with neutrophilic dermatosis (PAAND). FMF, likely the most well-known of the autoinflammatory syndromes, is characterized by discrete episodes of fever with serositis, synovitis and rash21. The most frequently reported symptom is abdominal pain, which can resemble an acute abdomen in presentation and on physical examination52. Although the disease phenotype is classically described as inherited in an autosomal-recessive fashion, there are increasing reports of patients with only one identifiable heterozygous MEFV mutation, with clear autosomal-dominant inheritance53,54. Murine studies have confirmed that MEFV mutations observed in patients are gain-of-function, consistent with inflammatory phenotypes in patients with a single identified heterozygous variant55. The most concerning consequence of uncontrolled inflammation in FMF is the development of systemic amyloid A (SAA) amyloidosis. Owing to the prevalence of MEFV mutations in certain parts of the world, it is also evident that specific variants may carry a greater risk of SAA amyloidosis dependent on the underlying genetic background. For example, patients with homozygosity for the M694V missense mutation may experience particularly severe disease with increased frequency of attacks, associated co-morbidities and reduced response to therapy56,57. The goal of therapy for any patient with FMF is reduction of inflammation and ultimately prevention of amyloidosis.
More recently, PAAND has been described as a distinct, autosomal-dominant syndrome caused by mutations in MEFV58,59. Affected patients experience recurrent inflammatory episodes with fever, neutrophilic dermatosis, arthralgia, myalgia and myositis beginning in early childhood. Elevation in serum acute-phase reactants is observed during inflammatory episodes. To date, two families with PAAND have been identified, exhibiting decreased 14-3-3 binding to pyrin, resulting in inflammasome activation and IL-1β and IL-18 secretion, as well as pyroptotic cell death58,59.
Deficiency of the IL-1 receptor antagonist
Autosomal-recessive missense mutations and large deletions in IL1RN were more recently described in patients with deficiency of the IL-1 receptor antagonist (IL-1RA), and are primarily due to founder effects60–63. In the case of deletion mutations, the size of the deletion may affect the phenotype, as has been observed in Puerto Rican patients with DIRA, where a 175-kb genomic deletion eliminates not only IL1RN, but also five IL-1-related genes60,62,64. In all cases, the result is an absent or truncated IL-1RA protein that is not secreted, and ultimately unable to inhibit IL-1α and IL-1β inflammatory responses. Uniquely, patients with DIRA have systemic inflammation with neutrophilia and elevations in serum inflammatory markers, although fever may be absent. Patients present near birth with a neutrophilic pustular rash that can be triggered by mechanical stress. Skin biopsy samples show neutrophilic infiltration of the dermis and epidermis, superficial folliculitis with pustule formation along hair follicles, acanthosis and hyperkeratosis60,62. Osteopenia with sterile lytic bone lesions, epiphyseal ballooning of the long bones and widening of the anterior rib ends, periosteal reaction, joint swelling and fusion of the cervical vertebrae have also been described in the majority of patients60–63,65. Infants frequently demonstrate hypoxaemia and dyspnoea due to interstitial pneumonia, localized ground-glass opacities and areas of atelectasis or gastrointestinal reflux. Thrombosis has also been described.
IL-1-associated autoinflammatory diseases
TNF-associated periodic syndrome
TRAPS is an autosomal-dominant disease caused by mutations in the TNFRSF1A gene. Symptoms of TRAPS include long episodes of fever (>7 days), a tender centripetal migratory skin rash and abdominal pain. Periorbital oedema and musculoskeletal symptoms, especially myalgia of the lower extremities, have also been documented. The high severity of symptoms has resulted in the misdiagnosis of acute abdomen and unnecessary surgical intervention in some patients66. As in the cryopyrinopathies, the TRAPS phenotype severity spectrum is determined to some degree by the type of TNFRSF1A mutations67.
The mechanisms underlying the extended febrile episodes in TRAPS have long been subject to investigation. Initial studies suggested that mutations affecting amino acid residues critical for protein folding lead to a failure of TNF receptor shedding68,69, a cellular process that desensitizes cells to TNF action. However, more recent data support a role for NLRP3 inflammasome activation through a number of potential mechanisms, including intracellular accumulation of misfolded mutant TNF receptor protein, leading to elevated generation of reactive oxygen species, induced cell death or impaired autophagy37,70. This proposed mechanism is supported by the successful treatment of patients with TRAPS with IL-1-targeted therapy71,72.
MVK spectrum disease
MKD results from autosomal-recessive, loss-of-function mutations in the MVK gene encoding mevalonate kinase25,26. It is a disease spectrum, comprising hyperimmunoglobulinaemia D and periodic fever syndrome (HIDS) at the mild end, and mevalonic aciduria at the severe end73. Patients with the milder phenotype are characterized by early onset episodes of fever, rash (macular–papular, urticarial, nodular or petechial), abdominal pain, oral ulcers and adenopathy, lasting nearly a week on average. Those with the more severe mevalonic aciduria phenotype also have recurrent febrile episodes, but additionally present with severe developmental disabilities. Inflammatory flares in MKD may be triggered by routine immunization, infection or physical stress74.
MVK mutations result in reduced or absent enzymatic activity of mevalonate kinase, the first enzyme in the 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase pathway required for cholesterol and isoprenoid synthesis75. Lack of mevalonate kinase function results in a shortage of isoprenoid lipid precursors34, which are required to diminish the inflammatory response, even in response to mild stimuli. Consistently, in vitro studies of cells from patients with HIDS demonstrate a reduced ability to clear antioxidant stress, as well as mitochondrial dysfunction and deficiency in autophagy, all of which are implicated in inflammasome activation35. More recent evidence in human and murine models suggests that the post-translational modification and subsequent attachment of isoprenoids to GTPases, including the Rho, Rac and Rap families of GTPases, are significantly affected by reduced mevalonate kinase activity76, ultimately leading to increased IL-1β secretion from monocytes, likely in a pyrin inflammasome-dependent fashion36,77. The successful use of IL-1-targeted therapy in HIDS is evidence that supports these pathogenic mechanisms71.
PSTPIP1 spectrum disease
PAPA syndrome is an autosomal-dominant disorder resulting from gain-of-function mutations in PSTPIP1, which encodes proline–serine–threonine phosphatase-interacting protein 1 (PSTPIP1). Mechanistically, disease-associated variants result in hyperphosphorylation of PSTPIP1 and enhanced assembly of the pyrin inflammasome, with subsequent uncontrolled IL-1β release78. Symptom onset is prior to 10 years of age, typically with the appearance of sterile pyogenic arthritis first, followed by dermatological features including pyoderma gangrenosum and acne.
Similar to the MEFV spectrum, mutations in PSTPIP1 result in a spectrum of disease beyond PAPA syndrome. Hyperzincaemia and hypercalprotectinaemia (also known as PSTPIP1-associated myeloid-related proteinemia inflammatory syndrome) is characterized by severe systemic and cutaneous inflammation, hepatosplenomegaly, arthritis, pancytopenia and failure to thrive owing to the accumulation of zinc79. Dermatological symptoms share clinical and histopathological similarities to pyoderma gangrenosum and include vasculitis, furuncle-like ulcers, and eczematous and necrotic lesions that typically affect the lower limbs symmetrically, although eyelids may also be involved80. In contrast to PAPA, the hyperzincaemia and hypercalprotectinaemia/PSTPIP1-associated myeloid-related proteinemia inflammatory phenotype has only been associated with a single amino acid change in PSTPIP1, altering the electrostatic potential and enhancing binding to pyrin79.
CDC42-associated autoinflammatory disease
Recently, patients with NOMID-like disease have been reported in small cohorts, underpinned by mutations in CDC42 (ref.38), a small Rho-family GTPase that regulates intercellular adhesion, cytoskeleton formation, cell cycle and cell proliferation81. Patients present near birth with growth restriction, recurrent febrile episodes, urticaria-like rashes, multiple cytopenias and hepatosplenomegaly38. The presence of aseptic meningitis, papilloedema and facial dysmorphisms, including mild frontal bossing, is reminiscent of NOMID presentation. Consistently, skin biopsy samples demonstrate perivascular lymphocytic and neutrophilic infiltration without vasculitis38.
Although the first patients described with CDC42-associated autoinflammatory disease had a remarkable response to IL-1 blockade38, more recent reports have noted that treatment with high dose IL-1 inhibition may not be sufficient to completely resolve symptoms, especially in the face of infection-driven macrophage activation syndrome39. In contrast to other autoinflammatory diseases, patients described to date have elevations in IL-18, suggesting a role for either the pyrin82–84 or NLRC4 inflammasome in CDC42-mediated disease. Even though fewer than 10 cases have been described to date, the severity of disease phenotypes highlights the importance of an accurate diagnosis, and the name neonatal-onset cytopenia with dyshaematopoiesis, autoinflammation, rash and haemophagocytic lymphohistiocytosis syndrome has been proposed for CDC42-associated autoinflammatory diseases39.
NLRP1 spectrum disease
Although NLRP1 was the first NOD-like receptor (NLR) reported to form an inflammasome23, its genetic variation and relationship to disease remains unclear85. Recently, patients carrying gain-of-function mutations in NLRP1 have been described, with some variability in phenotype depending on the mutated domain. Mutations in the PYRIN domain have been described in two families with autosomal-dominant corneal dyskeratosis86,87. By contrast, more distal mutations have been associated with systemic skin and mucosal symptoms (including respiratory or laryngeal papillomatosis, warts, exfoliation and plaque development), as well as inflammatory arthritis88–90. Studies of patient serum, peripheral blood mononuclear cells and primary keratinocytes have demonstrated increased levels of IL-1β and IL-18, consistent with autoinflammatory inflammasome activation86,87,89. At least two patients with NLRP1 spectrum disease have demonstrated some benefit to IL-1 blockade86,89, although the response was not as uniform as for other disorders, perhaps suggesting a role for IL-18 in disease pathogenesis.
Of note, polymorphisms in NLRP1 have been linked to increased risk of several autoimmune disorders, including rheumatoid arthritis, psoriasis, vitiligo and type 1 diabetes (reviewed in Yu et al.91). Further study is needed to determine the functional consequences of these genetic variants and whether IL-1-targeted therapy is appropriate.
Polygenic autoinflammatory disorders
The prominent features of fever, neutrophilia and elevated inflammatory markers observed in the monogenic autoinflammatory disorders, have fuelled a search for the genetic aetiologies behind related inflammatory disorders seen frequently by rheumatologists. Unlike monogenic diseases, many have multiple genetic aetiologies identified by case–control studies and genome-wide association studies of IL-1-related pathways, although it is often other shared clinical signs and symptoms and a lack of autoantibodies that suggest a mechanism related to IL-1-mediated disease pathways. This apparent IL-1 clinical signature suggests that targeted therapy may be effective in some patients with polygenic autoinflammatory disorders (Table 2), as shown in small cohorts and case reports92. Equally evident is the recognition of partial responses to IL-1 blockade, consistent with the complex aetiology underlying these disorders. Nonetheless, the potential for pathway-specific therapy has been observed in some polygenic rheumatological disorders, further discussed below. Using a case-by-case approach, IL-1 blockade may be considered in these conditions.
Table 2.
Summary of polygenic autoinflammatory diseases with a role for IL-1
| Disease | Genetic associations linked with phenotype | Presentation | Therapeutic targets |
|---|---|---|---|
| sJIA/AOSD | Class II HLA locus, HDAC9 (ref.172), LACC1/FAMIN173 | Fever, arthritis, arthralgia, evanescent rash | IL-1, IL-6, TNF, IL-18, IFN-γ |
| Schnitzler syndrome | NLRP3 (ref.48) | Chronic urticaria, fever, arthralgia, lymphadenopathy, bone pain, skeletal hyperostosis | IL-1, TNF |
| Gout | ADRB3, MTHFR, SLC22A11, SLC22A12, SLC2A9, SLC2A12, ABCG2, SLC17A1, SLC17A3, ALC16A9, GCKR, LRRC16A, PDZK1, R3HDMZ, RREB1, ALDH16A1 (ref.174), MAF175, URAT1 (ref.176) | Recurrent flares of inflammatory arthritis, chronic arthropathy, tophaceous deposits, uric acid nephrolithiasis | IL-1 |
| Recurrent pericarditis | MEFV177, HLA B14, DRB1*01, DQB1*0202 (ref.178) | Pleuritic chest pain, pericardial rub, ECG changes, pericardial effusion | IL-1 |
| CRMO | IL1RN95 | Recurrent fever, arthritis, multifocal bone inflammation | IL-1, TNF |
| Hidradenitis suppurativa | PSENEN, NCSTN, PSEN1 (ref.179) | Inflammatory nodules, sinus tracts and open comedones in intertriginous areas | TNF, IL-1 |
| PASH | MEFV, NOD2, NLRP3, IL1RN, PSTPIP1, PSMB8 (ref.180), NCSTN181 | HS lesions, pyoderma gangrenosum, acne | IL-1, IL-18 |
| PAPASH | PSTPIP1 (ref.182) | HS lesions, pyogenic sterile arthritis, pyoderma gangrenosum, acne | IL-1, TNF, IL-17A, IL-18 |
| PASS | Unknown | HS lesions, pyoderma gangrenosum, acne vulgaris, ankylosing spondylitis | IL-1, IL-18 |
| AHLE | CFI183 | Fever, neurological dysfunction, seizures, CSF pleocytosis | IL-1 |
| PFAPA | IL12A, STAT4, IL10, CCR1–CCR3 (ref.109), CARD8 (ref.184), ALPK1 (ref.185) | Recurrent fever with regular periodicity, aphthous stomatitis, exudative pharyngitis, cervical adenitis | IL-1 |
| Kawasaki disease | FCGR2A, BLK, CD40 (refs186,187), ITPKC, CASP3 (ref.188) | Fever, conjunctivitis, mucositis, rash, cervical lymphadenopathy, coronary artery dilatation | TNF, IL-1 |
| Behçet disease | IL12A, IL10, STAT4, CCR1–CCR3, IL23R–IL12RB2, FUT2 (refs109,189), HLAB51/B5 (ref.190) | Recurrent genital aphthae, uveitis or retinal vasculitis, nodular or papulopustular skin lesions, positive pathergy | IL-1, TNF |
AHLE, acute haemorrhagic leukoencephalitis; AOSD, adult onset Still disease; CNS, central nervous system; CRMO, chronic recurrent multifocal osteomyelitis; CSF, cerebrospinal fluid; ECG, electrocardiogram; HS, hidradenitis suppurativa; PAPASH, pyogenic arthritis, acne, pyoderma gangrenosum, and suppurative hidradenitis; PASH, pyoderma gangrenosum, acne, suppurative hidradenitis; PASS, pyoderma gangrenosum, acne and suppurative hidradenitis; PFAPA, periodic fever, aphthous stomatitis, pharyngitis, adenitis; sJIA, systemic juvenile idiopathic arthritis; TNF, tumour necrosis factor.
Chronic recurrent multifocal osteomyelitis
Chronic recurrent multifocal osteomyelitis (CRMO) is characterized by chronic, relapsing sterile bone inflammation. The most commonly affected areas are the knee, ankle or wrist, although the vertebrae, pelvis and clavicle may also be affected. Several genes have been linked to CRMO, including FGR and FBLIM1 (refs93,94). The recent identification of IL1RN variants in patients with CRMO-like presentations could have implications for treatment strategies, such as the use of anakinra, although this needs further exploration95. The association of CRMO with congenital dyserythropoietic anaemia, known as Majeed syndrome, is caused by autosomal-recessive mutations in LPIN2. This rare syndrome has been reported in only three families to date96.
Hidradenitis suppurativa spectrum
Hidradenitis suppurativa is a chronic inflammatory disease affecting the epithelium of the hair follicles, whereby recurrent suppurative lesions lead to tissue destruction and fibrosis, specifically in the intertriginous areas. The pathogenesis of hidradenitis suppurativa is thought to be due to a combination of genetic and environmental factors, although a positive family history is present in nearly 30% of patients, suggesting an autosomal-dominant inheritance97. More recently, hidradenitis suppurativa has been linked to a spectrum of autoinflammatory syndromes including pyoderma gangrenosum, acne, suppurative hidradenitis and ankylosing spondylitis (PASS)98; pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH); pyogenic arthritis, pyoderma gangrenosum, acne and suppurative hidradenitis (PAPASH); and psoriatic arthritis, pyoderma gangrenosum, acne, suppurative hidradenitis (PsAPASH)99. Although all of these syndromes share the sterile neutrophilic inflammatory lesions of hidradenitis suppurativa, the varied joint manifestations are consistent with a complex aetiology.
Other rheumatological diseases
More common rheumatological disorders with autoinflammatory features and IL-1 signatures include systemic juvenile idiopathic arthritis, adult-onset Still disease, recurrent pericarditis and microcrystalline arthropathies, such as gout and calcium pyrophosphate deposition disease. Despite complex genetics, the cardinal features of systemic juvenile idiopathic arthritis and adult-onset Still disease of fever, arthritis, rash and systemic inflammation are commonly also observed in the monogenic autoinflammatory diseases, and it is therefore not surprising that IL-1-targeted therapies are effective in these diseases100–102. Recurrent pericarditis is a clinical feature observed in some patients with monogenic autoinflammatory disorders and may be the initial presentation (FMF with pericarditis). IL-1 pathway-targeted therapies, including colchicine and some IL-1 biologics, are currently approved for use in patients with pericarditis, with or without mutations in MEFV103. Similarly, gout is characterized by recurrent attacks of fever and joint involvement that self-resolve. Given the role of the NLRP3 inflammasome in the recognition and perpetuation of monosodium urate- and calcium pyrophosphate-mediated inflammation24 (Fig. 2), it is unsurprising that IL-1-targeted therapies have been used successfully in gout104 and in calcium pyrophosphate deposition disease105. For other rheumatological diseases, including the seronegative spondyloarthropathies such as ankylosing spondylitis and psoriatic arthritis, IL-1 blockade has modest benefit in some patients106–108, but may be considered in patients with refractory disease. Together, these disorders indicate that much remains to be learned regarding the genetic and environmental drivers of IL-1-mediated inflammation. Nonetheless, the availability of targeted therapies provides symptomatic relief for appropriate patients.
In paediatric patients, clinical presentations of fever and varied mucosal signs of inflammation have a broad differential diagnosis and can be seen in Kawasaki disease, periodic fever, aphthous stomatitis, pharyngitis, adenitis (PFAPA) syndrome and Behçet disease, and the latter two disorders are now postulated to exist on a genetic susceptibility spectrum109 (Table 2). PFAPA syndrome is characterized by recurrent episodes of fever, with set periodicity associated with non-infectious exudative pharyngitis, aphthous ulcers and cervical lymphadenopathy. Given the similarities with recurrent febrile episodes and mucosal inflammation, together with some shared genetic association, PFAPA has been proposed to be in a disease continuum with Behçet disease that is uniquely characterized by genitourinary ulcers and uveitis109. Colchicine and IL-1 inhibitors have been used successfully in some patients on this syndromic spectrum110, but incomplete responses suggest that mechanisms beyond IL-1 might play a role in disease pathogenesis. The self-limited Kawasaki disease shares several clinical features with other autoinflammatory diseases, including fever, inflammation of the skin, conjunctiva and joints, as well as pronounced neutrophilic inflammation. Interestingly, Kawasaki presentations might be the initial sign of autoinflammatory disorders111. Although the aetiology of Kawasaki disease remains a source of active investigation, gene expression data and recent therapeutic success with IL-1 blockers support a major role for the IL-1 pathway in disease pathophysiology112,113. Recent studies support a complex genetic aetiology underlying these disorders, but ongoing research offers opportunities for pharmaceutical intervention.
Management of autoinflammatory diseases
Diagnostic approach
Given the rarity of autoinflammatory diseases, many patients experience delays in diagnosis that could result in the development of complications due to uncontrolled inflammation. Failure to achieve a timely diagnosis may take a toll physically and socially on patients, further reducing quality of life. Lack of timely diagnosis is often due to poor awareness by physicians of autoinflammatory diseases; this is therefore an area in which rheumatologists can provide a patient-centred, comprehensive, team-based clinical experience and fill an unmet need.
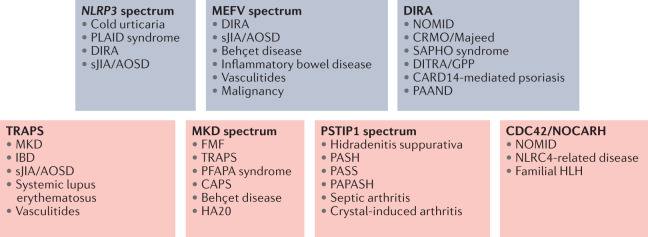
Recognition of systemic signs, symptoms and flare patterns remains the cornerstone of autoinflammatory disease diagnosis. For many disorders, the differential diagnosis includes other autoinflammatory diseases, as well as systemic inflammatory disorders in the rheumatology realm (Fig. 3). In general, non-specific inflammatory markers are elevated in autoinflammatory diseases, but there are few specific tests to define the diagnosis other than molecular genetic evaluations. Consequently, advances in sequencing techniques over the past few decades, including the availability of next generation approaches and commercialization of gene panels for disease classes, have improved the rate of diagnosis and dramatically increased the identification of variants in autoinflammatory disorder genes. However, although heralded as the gold standard, genetic testing is not without challenges. Increasing recognition of low penetrance variants114, oligogenic or digenic presentations115, somatic mosaicism116–119 and ‘mutation-negative’ patients, continues to be clinically challenging with regard to both disease diagnosis and long-term therapeutic management.
Fig. 3. Differential diagnostic considerations for monogenic disorders.
Certain clinical features are shared among autoinflammatory disorders and may be considered in the differential diagnosis of monogenic disorders directly driven by IL-1 (blue) or associated with IL-1 (orange). The lists shown here are not exhaustive and other disorders may be considered based on individual patient clinical presentations. For an overview of CARD14-mediated psoriasis, see ref.191. AOSD, adult onset Still disease; CAPS, cryopyrin-associated periodic syndromes; CARD14-mediated psoriasis191; CRMO, chronic recurrent multifocal osteomyelitis; DIRA, deficiency of IL-1 receptor antagonist; DITRA, deficiency of IL-36 receptor antagonist192,193; FMF, familial Mediterranean fever; GPP, generalized pustular psoriasis; HA20, haploinsufficiency of A20 (ref.194); HLH, hemophagocytic lymphohistiocytosis; MKD, mevalonate kinase deficiency; NLRC4, NLR family CARD domain containing 4 (ref.195); NOCARH, neonatal-onset cytopenia with dyshaematopoiesis, autoinflammation, rash, and HLH; NOMID, neonatal-onset multisystem inflammatory disease; PAAND, pyrin-associated autoinflammation with neutrophilic dermatosis; PAPASH, pyogenic arthritis, acne, pyoderma gangrenosum, and suppurative hidradenitis; PASH, pyoderma gangrenosum, acne, suppurative hidradenitis; PASS, pyoderma gangrenosum, acne and suppurative hidradenitis; PFAPA, periodic fever, aphthous stomatitis, pharyngitis, adenitis; PLAID, PLCγ2-associated antibody deficiency and immune dysregulation196,197; SAPHO, synovitis, acne, pustulosis, hyperostosis, osteitis; sJIA, systemic juvenile idiopathic arthritis; TRAPS, tumour necrosis factor receptor-associated periodic syndrome.
Treat-to-target approaches
As many autoinflammatory diseases present in early childhood, increased emphasis is placed on the long-term management and quality of life, including other determinants of health, growth and development, school or work attendance and social activities120. The ultimate goal of therapy is to obtain clinical control of symptoms with normalization of laboratory biomarkers of systemic inflammation, such as C-reactive protein and SAA, by using a treat-to-target approach, individualized for any given patient121,122. This approach is aimed at minimizing or preventing the development of organ damage in patients, including hearing123 and vision loss, CNS inflammation124 and amyloidosis125. It further underscores the need for ongoing evaluations, in order to make dose adjustments for weight gain and growth, changes in metabolism and the need for a multidisciplinary team to improve patient overall wellbeing126.
Vaccination
Immunizations are an important component of ongoing health care. For most patients with autoinflammatory disorders, normal vaccination schedules are recommended; however, some may have exaggerated inflammatory responses to vaccines. This fact is clearly the case in patients with MKD, who characteristically (and often diagnostically) have disease flares triggered by vaccinations74. In addition, patients with CAPS have been reported to have large local or systemic reactions to pneumococcal vaccinations, particularly the polysaccharide-conjugated form127. It is customarily recommended for patients receiving most forms of immunosuppressive therapy, including the IL-1 inhibitors, to avoid live viral vaccines, although there is little evidence supporting this practice. There is also a theoretical risk of decreased vaccine effectiveness when patients are receiving some anti-inflammatory therapies, as it could affect the normal host immune response to vaccines. Optimization of vaccinations is therefore recommended prior to starting treatment when possible. Specifically, pneumococcal vaccines are particularly important for patients who will be on IL-1 inhibitors, as there is an increased risk of streptococcal disease.
With the exception of MKD, in which immunization is a known trigger for flares74, most patients with IL-1-mediated autoinflammatory diseases tolerate routine vaccination, with no or mild symptoms128. With specific regard to SARS-CoV-2 immunization, patients with autoinflammatory disorders studied to date have tolerated the SARS-CoV-2 vaccines well, with symptoms similar to those observed in large cohorts of healthy individuals, such as local arm pain, myalgia and headache. It is noteworthy, however, that although no flaring of their disease requiring hospitalization has been observed, two patients with NOMID receiving SARS-CoV-2 vaccines reported worsening of headache, leading to a temporary increase in the dose of their anti-IL-1 therapy129. Clearly, longer follow-up and additional studies are needed to determine how to best balance SARS-CoV-2 vaccination and therapeutic dosing in this population.
Treatment of autoinflammatory diseases
Current therapies
The earliest and most widely utilized therapy for IL-1-mediated autoinflammatory disorders is colchicine, a plant-based medicine that was discovered serendipitously in the 1970s as a successful prophylactic treatment for patients with FMF. Colchicine seems to work by disrupting microtubules130, which mediate intracellular organelle and vesicle movement, cytokine secretion, cell division and migration, and regulation of gene expression131,132. The identification of the pyrin inflammasome more than 20 years later, and the demonstration that colchicine blocks pyrin inflammasome activation133, might explain why it is not as effective in the non-pyrin-mediated monogenic autoinflammatory disorders. However, there are some patients with FMF that do not respond to colchicine and others who cannot tolerate the gastrointestinal side effects or adhere adequately to a daily maintenance therapy134. These patients usually respond to IL-1-targeted biologics, although the prevention of amyloidosis has not been as well established as it has with colchicine135. It was the pharmacological development of the IL-1R antagonist, anakinra, initially for the treatment of sepsis, that enabled a direct means of evaluating the role of IL-1 in human diseases. This was a major advance, given that IL-1β levels are low, and IL-1α is typically below the level of detection, in the serum of patients with even the most severe autoinflammatory disease136. Although the sepsis trials failed, FDA approval for anakinra in rheumatoid arthritis in 2001 enabled investigators to test this antagonist in patients137. Since then, two additional IL-1-targeted therapies have been developed and approved, including rilonacept, a recombinant IL-1R that binds to and inhibits IL-1α, IL-1β and IL-1RA, and canakinumab, a human monoclonal antibody that binds specifically to IL-1β92,136. The different mechanisms of action of these three compounds should enable the unique functions of specific IL-1 family members in human disease to be understood, although the actual clinical experience has not necessarily been that clear, likely because of complex intra IL-1 family member regulatory mechanisms. The clinical success of IL-1 blockade (targeting IL-1α or IL-1β, or both) provides the most convincing evidence of a role for IL-1 in autoinflammatory disease pathogenesis136. To that end, empiric trials of anti-IL-1 therapies might be useful in treating patients with either genetically undefined autoinflammatory symptoms or with prominent non-infectious neutrophilia and elevated C-reactive protein or SAA138. All three approved IL-1-targeting therapies (Supplementary Table 2) have similar safety profiles, with the primary adverse effect being increased risk of non-opportunistic infections, which tend to be mild and can often be treated without withdrawing therapy139. There are limited head-to-head trials of the different direct IL-1 inhibitors140,141, and the general consensus among treating experts is that these biologic therapies have equivalent efficacy if used at appropriate doses with sufficient adherence. Nonetheless, there are clear pharmacodynamic differences between direct IL-1 inhibitors, as evidenced by the different dosing frequencies required41. There is likely an advantage of anakinra and rilonacept over canakinumab in treating DIRA given that they block IL-1α and IL-1β, rather than IL-1β alone95. This fact might also be the case with recurrent pericarditis and hidradenitis suppurativa. Diseases with CNS inflammation might respond better to anakinra because of its smaller molecular size and resulting potential to penetrate the blood–brain barrier more efficiently than rilonacept and canakinumab142. There are some patient groups that require higher or more frequent dosing, including patients with more severe autoinflammatory symptoms (such as NOMID and MKD), younger patients, and patients with atypical presentations or low penetrance mutations. Therapy during pregnancy remains an understudied cohort143. Even for DIRA, which may be the most obvious example of links between genetics, disease and pathogenesis, patient-to-patient variability in therapeutic response still exists60. Similarly, there is increasing recognition of a role for inflammasome-driven IL-18 in some diseases, such as PAPA syndrome84, which may explain a lack of uniform responses to IL-1 blockade. Despite the general success in treating autoinflammation, the targeting of such a key mediator of inflammation, as well as the injectable nature of these therapeutics, has resulted in a search for additional therapies.
New therapies targeting the IL-1 pathway
The rapid approval of rilonacept and canakinumab for CAPS144,145 caught the attention of the pharmaceutical industry. However, it was the CANTOS trial146 that confirmed that targeting the IL-1 pathway can have broader implications in more common diseases not traditionally considered as autoinflammatory, such as cardiovascular disease (Box 1). Although the initial focus has been on targeting single proteins in the IL-1 pathway (IL-1β, IL-1R and IL-1α) with predicted pharmacokinetic advantages, the current trend is to either combine protein targets (such as IL-1β and IL-18) or to develop small molecule inhibitors aimed at various steps in the IL-1 pathway (Table 3).
Table 3.
Drugs in development over the past decade targeting the IL-1 pathway
| Drug | Target | Mechanism | Clinical Trial |
|---|---|---|---|
| CE-224535 | P2X7 | Selective P2X(7) receptor antagonist | NCT00628095 |
| AZD9056 | P2X7 | Selective P2X(7) receptor antagonist | NCT00520572 |
| BMS-986299 | NLRP3 | Agonist | NCT03444753 |
| Dapansutrile | NLRP3 | Small molecular inhibitor | NCT03595371 |
| IZD334 | NLRP3 | Small molecule inhibitor | NCT04086602 |
| ZYIL1 | NLRP3 | Small molecule inhibitor | NCT04731324 |
| IZD174 | NLRP3 | Small molecule inhibitor, CNS penetrant | NCT04338997 |
| AC-201 | NLRP3 | Small molecule inhibitor | NCT02287818 |
| VX-765 | Caspase 1 | Small molecule inhibitor | NCT00205465 |
| Emricasan | Caspase 1 | Pan caspase inhibitor | NCT04803227 |
| Disulfiram | GSDMD | Gasdermin D inhibitor | NCT04485130 |
| Bermekimab | IL-1α | Anti-IL-1α monoclonal antibody | NCT03512275 |
| Gevokizumab | IL-1β | Anti-IL-1β monoclonal antibody | NCT01211977 |
| LY2189102 | IL-1β | Anti-IL-1β humanized monoclonal immunoglobulin G4 | NCT00380744 |
| CYT013-IL1bQb | IL-1β | Vaccine to IL-1β | NCT00924105 |
| Lutikizumab | IL-1α/IL-1β | Dual affinity monoclonal antibody to IL-1α/IL-1β | NCT01668511 |
| MAS825 | IL-1β/IL-18 | Bispecific IL-1β and IL-18 monoclonal antibody | NCT04641442 |
| sc-rAAV2.5IL-1Ra | IL-1R1 | Self-complementing, recombinant AAV carrying IL-1RA cDNA | NCT02790723 |
| EBI-005 | IL-1R1 | IL-1β and IL-1 receptor antagonist fusion protein | NCT04121442 |
| HL2351 | IL-1R1 | Human IL-1Ra-hyFc | NCT02853084 |
| MEDI8968 | IL-1R1 | Anti-IL-1R1 human monoclonal antibody | NCT01838499 |
| AMG108 | IL-1R1 | Anti-IL-1R1 monoclonal antibody | NCT00110942 |
| EBI-005 | IL-1R1 | Chimeric IL-1RA- IL-1β | NCT02082899 |
| KT-474 | IRAK4 | Oral heterobifunctional small molecule IRAK4 degrader | NCT04772885 |
| ATI-450 | MK2 | Oral small molecule MAPKAPK2 (MK2) inhibitor | NCT04524858 |
Identified based on search terms IL-1, inflammasome, NLRP3, at clinicaltrials.gov and Dinarello et al.92.
The most prevalent small molecule target for treating autoinflammatory diseases has been NLRP3, beginning with the early development of CRID3 (now known as MCC950)147; furthermore, multiple companies are currently developing, acquiring or studying their own NLRP3 inhibitors for various diseases, including some monogenic autoinflammatory disorders148. Directly inhibiting a protein that is altered in a monogenic disease is theoretically a more targeted approach than use of the currently available biologics against downstream cytokines. Small molecules could also be designed to have better blood–brain barrier penetration, which would have implications for diseases with marked CNS inflammation. One study found that blocking NLRP3 decreased the risk of specific infections associated with broader IL-1 inhibitors149. However, it is also conceivable that small molecules designed to bind to wild-type protein might have less avidity for mutant protein, thereby reducing clinical efficacy in patients150.
Small molecule inhibitors with targets upstream and downstream of the inflammasome pathway are similarly under investigation. Two of the earliest pathway component inhibitors to be developed were compounds targeting P2X7, the receptor for ATP responsible for the potassium flux that activates NLRP3, and caspase 1, the common effector enzyme for inflammasomes (Fig. 2). In addition to non-IL-1 pathway functions, P2X7 inhibitors might not affect intrinsically dysregulated inflammasomes that could be activated independently of P2X7. Specific caspase 1 inhibitors were developed and studied in several diseases, including autoinflammatory diseases, but pharmacodynamic issues and unexpected side effects resulted in their discontinuation. Finally, there are other drugs in development that block downstream IL-1R signalling, including IRAK4 and MK2. Inhibiting these targets could be very effective at blocking the effects of IL-1 in addition to other cytokine receptors, which could have advantages and disadvantages in terms of applicability to other diseases, and as yet unknown adverse effects.
Box 1 Expanded spectrum of diseases under investigation for anti-IL-1 therapy.
Musculoskeletal
Osteoarthritis, anterior cruciate ligament injuries, rheumatoid arthritis, polymyositis, dermatomyositis, inclusion body myositis, subacromial bursitis
Neurological
Multiple sclerosis, chronic fatigue syndrome, autoimmune neurosensory hearing loss, autoimmune inner ear disease
Inflammatory
Scleroderma, pustular psoriasis, palmoplantar pustulosis, urticarial vasculitis, pyoderma gangrenosum, sarcoidosis (cardiac and pulmonary)
Renal/metabolic
End-stage renal disease, chronic kidney disease, type 2 diabetes mellitus
Cardiovascular
Giant cell arteritis, acute myocarditis, heart failure, atherosclerosis
Ophthalmological
Blepharokeratoconjunctivitis, uveitis
These diseases were identified based on the search terms anakinra, rilonacept, canakinumab and IL-1 at clinicaltrials.gov.
Ongoing challenges and future perspectives
The identification of variants of unknown significance, which have neither been previously identified in disease cohorts nor rigorously studied, has introduced further challenges to our understanding of the complex genetic underpinnings of autoinflammatory diseases. The same is true for low penetrance variants, defined as likely benign variants that might be present at low frequency in the general population, but which may nonetheless contribute to IL-1-driven inflammation. Importantly, patients carrying these variants might present with clinical manifestations different from typical disease, and consequently might respond differently to therapeutics.
The most well-known IL-1-associated low penetrance variants are found in NLRP3, MEFV and TNFRSF1A. Patients with low penetrance variants in NLRP3 (including V198M, R488K and Q703K) have a spectrum of disease ranging from asymptomatic, classic CAPS-associated features, or atypical presentation including gastrointestinal symptoms114 or more severe neurological symptoms151. In vitro studies suggest an intermediate phenotype with increased caspase 1 activity and IL-1β secretion compared with wild-type versions of these proteins, but with markedly less secretion than classic disease-causing mutations114. Patients respond at least partially to IL-1 blockade, suggesting that other mechanisms beyond IL-1 may contribute to disease pathogenesis. Nonetheless, in the small cohorts described to date, nearly half of patients experienced complete disease remission, suggesting that IL-1 inhibitors should be trialled in this unique population114.
In TRAPS, the most highly contested variant is traditionally known as R92Q (also known as R121Q), with a population frequency greater than 1%. Similar to the low penetrance variants in NLRP3, many R92Q carriers present with mild TRAPS symptoms with febrile episodes lasting approximately 1 week and in vitro studies demonstrating intermediate function, with reduced cytokine release from transfected cells, compared with cysteine mutations that have a more severe clinical phenotype152. Therapeutic responses to corticosteroids and colchicine have been described, as well as IL-1 blockade, with improvement in inflammatory episodes152,153.
The high prevalence of MEFV variants in certain populations adds to the challenges of variants of unknown significance and low penetrance mutations and their relationship to FMF6. Variants such as E148Q have been described in up to 50% of different Jewish ethnic groups154. Although population frequencies of some pathogenic MEFV variants (such as V726A and M694V) are likely due to positive selection in Eastern Mediterranean populations, by conferring resistance to Yersinia pestis infection155, there might be an unknown heterozygote advantage to the high frequency of carriers156,157. However, the presence of low-penetrance heterozygous variants has led to complex clinical phenotypes including PFAPA syndrome presentations158, Behçet disease159 and risk of amyloidosis160. These varied clinical presentations further demonstrate the need to address patients on an individual basis.
Treating patients with autoinflammatory phenotypes with no identifiable disease-causing mutation is also challenging. Moreover, a subset of patients might have somatic mutations in an autoinflammatory gene that are restricted to a small (4%) proportion of a specific cell population. Such patients might have mild, delayed118,119 or atypical presentations116 or complete disease161. However, a growing number of patients have no clear genetic aetiology. Frequently referred to as syndrome of undifferentiated recurrent fever138,162 or undifferentiated systemic autoinflammatory disease163, choosing therapy for these patients is challenging. Fortunately, for patients with features of IL-1 presentations, treatment with IL-1 blockade and colchicine are successful in a significant proportion of patients138,163.
For patients with a poor response to IL-1 blockade due to complex genetics or lack of access to biologic therapeutics, haematopoietic stem cell transplantations have been attempted164. Although theoretically curative by replacing the haematopoietic cells that drive disease, the identification of patients with gain-of-function somatic mutations is instructive that only a small population of cells can drive disease phenotypes. The optimal treatment for these patients remains an active area of discussion.
Finally, the COVID-19 pandemic has brought systemic inflammatory disease into mainstream attention, with a variety of attempts to quell the cytokine storm and reduce morbidity and mortality. In the paediatric population, a multi-organ hyperinflammatory response to SARS-CoV-2 infection was described to occur weeks after infection, and is now known as multisystem inflammatory syndrome associated with coronavirus disease 2019 or paediatric multi-inflammatory syndrome temporally associated with COVID-19 (refs165–167). Initially thought to be an atypical form of Kawasaki disease, it was quickly recognized that affected children were more likely to have signs of shock, gastrointestinal symptoms and coagulopathy165–167. Immunomodulatory therapy with intravenous immunoglobulin and glucocorticoids appears to be the most effective therapy to date, with a role for IL-1 blockade in refractory cases168–170.
The investigation of autoinflammatory disease and the inflammasome has involved the confluence of phenotyped patients and scientific studies at the bench. Over the past 30 years, such clinic–laboratory cooperation has been instrumental to the development of novel, targeted therapies for such patients171. The elucidation of inflammasome-mediated molecular pathways drove our understanding of basic innate immune mechanisms, solved several questions regarding the mechanisms of IL-1β release and led to successful therapy of many of the monogenic autoinflammatory diseases with biologics directed at the IL-1 pathway. A better understanding of the genetic and molecular mechanisms of autoinflammation and novel targeted therapies, such as biologics blocking more than IL-1 cytokine or small molecule inhibitors targeting upstream or downstream pathway elements, could lead to more effective personalized management of patients with this fascinating and sometimes frustrating family of immune dysregulation disorders.
Supplementary information
Acknowledgements
We acknowledge the American Academy of Allergy, Asthma and Immunology Foundation (L.B.), and the NIH National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK113592-01 (L.B., H.M.H.) and R01 HL140898, RO1 AI15586901 (H.M.H.) for invaluable funding.
Glossary
- Pyroptosis
Inflammatory form of cell death mediated by caspase 1 and gasdermin D.
- Necroptosis
Inflammatory form of necrotic cell death that is caspase independent.
- Neutrophilic dermatosis
Inflammatory skin disorder characterized by predominant neutrophilic infiltrate.
Author contributions
The authors contributed equally to all aspects of the article.
Peer review
Peer review information
Nature Reviews Rheumatology thanks E. Demirkaya, S. Masters, who co-reviewed with T. Reygaerts, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
L.B. is a site PI for Novartis, Inc. H.M.H. is a consultant for Novartis and Kiniksa, H.M.H. has research collaborations with Regeneron, Inc.; Jecure, Inc., Zomagen, Inc., Takeda.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Registry of hereditary auto-inflammatory disorders mutations: https://infevers.umai-montpellier.fr/web/
Contributor Information
Lori Broderick, Email: lbroderick@health.ucsd.edu.
Hal M. Hoffman, Email: hahoffman@health.ucsd.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41584-022-00797-1.
References
- 1.Dinarello CA, Goldin NP, Wolff SM. Demonstration and characterization of two distinct human leukocytic pyrogens. J. Exp. Med. 1974;139:1369–1381. doi: 10.1084/jem.139.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auron PE, et al. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc. Natl Acad. Sci. USA. 1984;81:7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PubMed] [Google Scholar]
- 3.Lomedico PT, et al. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. Nature. 1984;312:458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat. Rev. Rheumatol. 2019;15:612–632. doi: 10.1038/s41584-019-0277-8. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity. 2019;50:778–795. doi: 10.1016/j.immuni.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aksentijevich I, et al. Mutation and haplotype studies of familial Mediterranean fever reveal new ancestral relationships and evidence for a high carrier frequency with reduced penetrance in the Ashkenazi Jewish population. Am. J. Hum. Genet. 1999;64:949–962. doi: 10.1086/302327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi L, et al. IL-1 transcriptional responses to lipopolysaccharides are regulated by a complex of RNA binding proteins. J. Immunol. 2020;204:1334–1344. doi: 10.4049/jimmunol.1900650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sneezum L, et al. Context-dependent IL-1 mRNA-destabilization by TTP prevents dysregulation of immune homeostasis under steady state conditions. Front. Immunol. 2020;11:1398. doi: 10.3389/fimmu.2020.01398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA. Inflammasome-independent regulation of IL-1-family cytokines. Annu. Rev. Immunol. 2015;33:49–77. doi: 10.1146/annurev-immunol-032414-112306. [DOI] [PubMed] [Google Scholar]
- 10.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/S0966-842X(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhou B, Abbott DW. Gasdermin E permits interleukin-1 beta release in distinct sublytic and pyroptotic phases. Cell Rep. 2021;35:108998. doi: 10.1016/j.celrep.2021.108998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hildebrand JM, Murphy JM. The highway to hell: a RIP kinase-directed shortcut to inflammatory cytokine production. Immunity. 2016;45:1–3. doi: 10.1016/j.immuni.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 13.Li S, et al. IL-1α and IL-1β promote NOD2-induced immune responses by enhancing MAPK signaling. Lab. Invest. 2019;99:1321–1334. doi: 10.1038/s41374-019-0252-7. [DOI] [PubMed] [Google Scholar]
- 14.Dagvadorj J, et al. Recruitment of pro-IL-1α to mitochondrial cardiolipin, via shared LC3 binding domain, inhibits mitophagy and drives maximal NLRP3 activation. Proc. Natl Acad. Sci. USA. 2021 doi: 10.1073/pnas.2015632118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik A, Kanneganti TD. Function and regulation of IL-1α in inflammatory diseases and cancer. Immunol. Rev. 2018;281:124–137. doi: 10.1111/imr.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fettelschoss A, et al. Inflammasome activation and IL-1β target IL-1α for secretion as opposed to surface expression. Proc. Natl Acad. Sci. USA. 2011;108:18055–18060. doi: 10.1073/pnas.1109176108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu. Rev. Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 18.Vecile E, et al. Intracellular function of interleukin-1 receptor antagonist in ischemic cardiomyocytes. PLoS One. 2013;8:e53265. doi: 10.1371/journal.pone.0053265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.French FMF Consortium. A candidate gene for familial Mediterranean fever. Nat. Genet. 1997;17:25–31. doi: 10.1038/ng0997-25. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International FMF. Consortium. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell. 1997;90:797–807. doi: 10.1016/S0092-8674(00)80539-5. [DOI] [PubMed] [Google Scholar]
- 22.McDermott MF, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/S0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 23.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 24.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 25.Drenth JP, et al. Mutations in the gene encoding mevalonate kinase cause hyper-IgD and periodic fever syndrome. International Hyper-IgD Study Group. Nat. Genet. 1999;22:178–181. doi: 10.1038/9696. [DOI] [PubMed] [Google Scholar]
- 26.Houten SM, et al. Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome. Nat. Genet. 1999;22:175–177. doi: 10.1038/9691. [DOI] [PubMed] [Google Scholar]
- 27.Wise CA, et al. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum. Mol. Genet. 2002;11:961–969. doi: 10.1093/hmg/11.8.961. [DOI] [PubMed] [Google Scholar]
- 28.Nigrovic PA, Lee PY, Hoffman HM. Monogenic autoinflammatory disorders: conceptual overview, phenotype, and clinical approach. J. Allergy Clin. Immunol. 2020;146:925–937. doi: 10.1016/j.jaci.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milhavet F, et al. The infevers autoinflammatory mutation online registry: update with new genes and functions. Hum. Mutat. 2008;29:803–808. doi: 10.1002/humu.20720. [DOI] [PubMed] [Google Scholar]
- 30.Sarrauste de Menthiere, C. et al. INFEVERS: the Registry for FMF and hereditary inflammatory disorders mutations. Nucleic Acids Res. 2003;31:282–285. doi: 10.1093/nar/gkg031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Touitou I, et al. Infevers: an evolving mutation database for auto-inflammatory syndromes. Hum. Mutat. 2004;24:194–198. doi: 10.1002/humu.20080. [DOI] [PubMed] [Google Scholar]
- 32.Van Gijn ME, et al. New workflow for classification of genetic variants’ pathogenicity applied to hereditary recurrent fevers by the International Study Group for Systemic Autoinflammatory Diseases (INSAID) J. Med. Genet. 2018;55:530–537. doi: 10.1136/jmedgenet-2017-105216. [DOI] [PubMed] [Google Scholar]
- 33.Shoham NG, et al. Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc. Natl Acad. Sci. USA. 2003;100:13501–13506. doi: 10.1073/pnas.2135380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munoz MA, et al. Defective protein prenylation is a diagnostic biomarker of mevalonate kinase deficiency. J. Allergy Clin. Immunol. 2017;140:873–875.e6. doi: 10.1016/j.jaci.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 35.van der Burgh R, et al. Unprenylated RhoA contributes to IL-1β hypersecretion in mevalonate kinase deficiency model through stimulation of Rac1 activity. J. Biol. Chem. 2014;289:27757–27765. doi: 10.1074/jbc.M114.571810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jurczyluk J, et al. Mevalonate kinase deficiency leads to decreased prenylation of Rab GTPases. Immunol. Cell Biol. 2016;94:994–999. doi: 10.1038/icb.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon A, et al. Concerted action of wild-type and mutant TNF receptors enhances inflammation in TNF receptor 1-associated periodic fever syndrome. Proc. Natl Acad. Sci. USA. 2010;107:9801–9806. doi: 10.1073/pnas.0914118107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gernez Y, et al. Severe autoinflammation in 4 patients with C-terminal variants in cell division control protein 42 homolog (CDC42) successfully treated with IL-1β inhibition. J. Allergy Clin. Immunol. 2019;144:1122–1125.e6. doi: 10.1016/j.jaci.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam MT, et al. A novel disorder involving dyshematopoiesis, inflammation, and HLH due to aberrant CDC42 function. J. Exp. Med. 2019;216:2778–2799. doi: 10.1084/jem.20190147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldmann J, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 2002;71:198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Booshehri LM, Hoffman HM. CAPS and NLRP3. J. Clin. Immunol. 2019;39:277–286. doi: 10.1007/s10875-019-00638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ben-Chetrit E, et al. Consensus proposal for taxonomy and definition of the autoinflammatory diseases (AIDs): a Delphi study. Ann. Rheum. Dis. 2018;77:1558–1565. doi: 10.1136/annrheumdis-2017-212515. [DOI] [PubMed] [Google Scholar]
- 43.Kitley JL, Lachmann HJ, Pinto A, Ginsberg L. Neurologic manifestations of the cryopyrin-associated periodic syndrome. Neurology. 2010;74:1267–1270. doi: 10.1212/WNL.0b013e3181d9ed69. [DOI] [PubMed] [Google Scholar]
- 44.Kilic H, et al. Spectrum of the neurologic manifestations in childhood-onset cryopyrin-associated periodic syndrome. Eur. J. Paediatr. Neurol. 2019;23:466–472. doi: 10.1016/j.ejpn.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Aksentijevich I, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caroli F, et al. Clinical and genetic characterization of Italian patients affected by CINCA syndrome. Rheumatology. 2007;46:473–478. doi: 10.1093/rheumatology/kel269. [DOI] [PubMed] [Google Scholar]
- 47.Houx L, et al. Musculoskeletal symptoms in patients with cryopyrin-associated periodic syndromes: a large database study. Arthritis Rheumatol. 2015;67:3027–3036. doi: 10.1002/art.39292. [DOI] [PubMed] [Google Scholar]
- 48.de Koning HD, et al. Myeloid lineage-restricted somatic mosaicism of NLRP3 mutations in patients with variant Schnitzler syndrome. J. Allergy Clin. Immunol. 2015;135:561–564. doi: 10.1016/j.jaci.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 49.Jeru I, et al. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc. Natl Acad. Sci. USA. 2008;105:1614–1619. doi: 10.1073/pnas.0708616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitamura A, Sasaki Y, Abe T, Kano H, Yasutomo K. An inherited mutation in NLRC4 causes autoinflammation in human and mice. J. Exp. Med. 2014;211:2385–2396. doi: 10.1084/jem.20141091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheffel J, et al. Cold-induced urticarial autoinflammatory syndrome related to factor XII activation. Nat. Commun. 2020;11:179. doi: 10.1038/s41467-019-13984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon A, van der Meer JW, Drenth JP. Familial Mediterranean fever — a not so unusual cause of abdominal pain. Best. Pract. Res. Clin. Gastroenterol. 2005;19:199–213. doi: 10.1016/j.bpg.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 53.Mache CJ, Goriup U, Fischel-Ghodsian N, Chen X, Schwingshandl J. Autosomal dominant familial Mediterranean fever-like syndrome. Eur. J. Pediatr. 1996;155:787–790. doi: 10.1007/BF02002908. [DOI] [PubMed] [Google Scholar]
- 54.Booth DR, et al. The genetic basis of autosomal dominant familial Mediterranean fever. Q. J. Med. 2000;93:217–221. doi: 10.1093/qjmed/93.4.217. [DOI] [PubMed] [Google Scholar]
- 55.Chae JJ, et al. Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1beta activation and severe autoinflammation in mice. Immunity. 2011;34:755–768. doi: 10.1016/j.immuni.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akpolat T, Ozkaya O, Ozen S. Homozygous M694V as a risk factor for amyloidosis in Turkish FMF patients. Gene. 2012;492:285–289. doi: 10.1016/j.gene.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Grossman C, Kassel Y, Livneh A, Ben-Zvi I. Familial Mediterranean fever (FMF) phenotype in patients homozygous to the MEFV M694V mutation. Eur. J. Med. Genet. 2019;62:103532. doi: 10.1016/j.ejmg.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 58.Masters SL, et al. Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Sci. Transl. Med. 2016;8:332ra345. doi: 10.1126/scitranslmed.aaf1471. [DOI] [PubMed] [Google Scholar]
- 59.Moghaddas F, et al. A novel Pyrin-associated autoinflammation with neutrophilic dermatosis mutation further defines 14-3-3 binding of pyrin and distinction to familial Mediterranean fever. Ann. Rheum. Dis. 2017;76:2085–2094. doi: 10.1136/annrheumdis-2017-211473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aksentijevich I, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N. Engl. J. Med. 2009;360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jesus AA, et al. A novel mutation of IL1RN in the deficiency of interleukin-1 receptor antagonist syndrome: description of two unrelated cases from Brazil. Arthritis Rheum. 2011;63:4007–4017. doi: 10.1002/art.30588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reddy S, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N. Engl. J. Med. 2009;360:2438–2444. doi: 10.1056/NEJMoa0809568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stenerson M, et al. The first reported case of compound heterozygous IL1RN mutations causing deficiency of the interleukin-1 receptor antagonist. Arthritis Rheum. 2011;63:4018–4022. doi: 10.1002/art.30565. [DOI] [PubMed] [Google Scholar]
- 64.Brau-Javier CN, Gonzales-Chavez J, Toro JR. Chronic cutaneous pustulosis due to a 175-kb deletion on chromosome 2q13: excellent response to anakinra. Arch. Dermatol. 2012;148:301–304. doi: 10.1001/archdermatol.2011.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Altiok E, et al. A novel mutation in the interleukin-1 receptor antagonist associated with intrauterine disease onset. Clin. Immunol. 2012;145:77–81. doi: 10.1016/j.clim.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen YJ, et al. Recurrent abdominal pain as the presentation of tumor necrosis factor receptor-associated periodic syndrome (TRAPS) in an Asian girl: a case report and review of the literature. J. Microbiol. Immunol. Infect. 2014;47:550–554. doi: 10.1016/j.jmii.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 67.Gaggiano C, et al. Clinical features at onset and genetic characterization of pediatric and adult patients with TNF-α receptor-associated periodic syndrome (TRAPS): a series of 80 cases from the AIDA network. Mediators Inflamm. 2020;2020:8562485. doi: 10.1155/2020/8562485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aderka D, et al. Shedding kinetics of soluble tumor necrosis factor (TNF) receptors after systemic TNF leaking during isolated limb perfusion. Relevance to the pathophysiology of septic shock. J. Clin. Invest. 1998;101:650–659. doi: 10.1172/JCI694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xanthoulea S, et al. Tumor necrosis factor (TNF) receptor shedding controls thresholds of innate immune activation that balance opposing TNF functions in infectious and inflammatory diseases. J. Exp. Med. 2004;200:367–376. doi: 10.1084/jem.20040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bulua AC, et al. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J. Exp. Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Benedetti F, et al. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N. Engl. J. Med. 2018;378:1908–1919. doi: 10.1056/NEJMoa1706314. [DOI] [PubMed] [Google Scholar]
- 72.Cudrici C, Deuitch N, Aksentijevich I. Revisiting TNF receptor-associated periodic syndrome (TRAPS): current perspectives. Int. J. Mol. Sci. 2020;21:3263. doi: 10.3390/ijms21093263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frenkel J, et al. Clinical and molecular variability in childhood periodic fever with hyperimmunoglobulinaemia D. Rheumatology. 2001;40:579–584. doi: 10.1093/rheumatology/40.5.579. [DOI] [PubMed] [Google Scholar]
- 74.Drenth JP, Haagsma CJ, van der Meer JW. Hyperimmunoglobulinemia D and periodic fever syndrome. The clinical spectrum in a series of 50 patients. International Hyper-IgD Study Group. Medicine. 1994;73:133–144. doi: 10.1097/00005792-199405000-00002. [DOI] [PubMed] [Google Scholar]
- 75.van der Meer JW, et al. Hyperimmunoglobulinaemia D and periodic fever: a new syndrome. Lancet. 1984;1:1087–1090. doi: 10.1016/S0140-6736(84)92505-4. [DOI] [PubMed] [Google Scholar]
- 76.Politiek FA, Waterham HR. Compromised protein prenylation as pathogenic mechanism in mevalonate kinase deficiency. Front. Immunol. 2021;12:724991. doi: 10.3389/fimmu.2021.724991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park YH, Wood G, Kastner DL, Chae JJ. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat. Immunol. 2016;17:914–921. doi: 10.1038/ni.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waite AL, et al. Pyrin modulates the intracellular distribution of PSTPIP1. PLoS One. 2009;4:e6147. doi: 10.1371/journal.pone.0006147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holzinger D, et al. Single amino acid charge switch defines clinically distinct proline-serine-threonine phosphatase-interacting protein 1 (PSTPIP1)-associated inflammatory diseases. J. Allergy Clin. Immunol. 2015;136:1337–1345. doi: 10.1016/j.jaci.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Resende LO, Jorge MFS, Schmitt JV. Extensive pyoderma gangrenosum-like lesions revealing a case of hyperzincemia and hypercalprotectinemia: when to suspect it? Bras. Dermatol. 2019;94:713–716. doi: 10.1016/j.abd.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu J, et al. The role of cell division control protein 42 in tumor and non-tumor diseases: a systematic review. J. Cancer. 2022;13:800–814. doi: 10.7150/jca.65415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim ML, et al. Aberrant actin depolymerization triggers the pyrin inflammasome and autoinflammatory disease that is dependent on IL-18, not IL-1β. J. Exp. Med. 2015;212:927–938. doi: 10.1084/jem.20142384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nishitani-Isa M, et al. Aberrant localization of CDC42 C-terminal variants to the Golgi apparatus drives pyrin inflammasome-dependent autoinflammation. bioRxiv. 2021 doi: 10.1101/2021.09.03.458902. [DOI] [Google Scholar]
- 84.Stone DL, et al. Excess serum interleukin-18 distinguishes patients with pathogenic mutations in PSTPIP1. Arthritis Rheumatol. 2022;74:353–357. doi: 10.1002/art.41976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Masters SL, et al. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity. 2012;37:1009–1023. doi: 10.1016/j.immuni.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Herlin T, et al. Autoinflammatory disease with corneal and mucosal dyskeratosis caused by a novel NLRP1 variant. Rheumatology. 2020;59:2334–2339. doi: 10.1093/rheumatology/kez612. [DOI] [PubMed] [Google Scholar]
- 87.Zhong FL, et al. Germline NLRP1 mutations cause skin inflammatory and cancer susceptibility syndromes via inflammasome activation. Cell. 2016;167:187–202.e17. doi: 10.1016/j.cell.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 88.Drutman SB, et al. Homozygous NLRP1 gain-of-function mutation in siblings with a syndromic form of recurrent respiratory papillomatosis. Proc. Natl Acad. Sci. USA. 2019;116:19055–19063. doi: 10.1073/pnas.1906184116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grandemange S, et al. A new autoinflammatory and autoimmune syndrome associated with NLRP1 mutations: NAIAD (NLRP1-associated autoinflammation with arthritis and dyskeratosis) Ann. Rheum. Dis. 2017;76:1191–1198. doi: 10.1136/annrheumdis-2016-210021. [DOI] [PubMed] [Google Scholar]
- 90.Sureja NP, Rajasekhahr L. Autoinflammation with arthritis and dyskeratosis an inflammasomopathy: case report and review of literature. Indian. J. Rheumatol. 2021 doi: 10.4103/injr.injr_17_21. [DOI] [Google Scholar]
- 91.Yu CH, Moecking J, Geyer M, Masters SL. Mechanisms of NLRP1-mediated autoinflammatory disease in humans and mice. J. Mol. Biol. 2018;430:142–152. doi: 10.1016/j.jmb.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 92.Dinarello C, Simon A, van der Meer J. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cox AJ, Zhao Y, Ferguson PJ. Chronic recurrent multifocal osteomyelitis and related diseases-update on pathogenesis. Curr. Rheumatol. Rep. 2017;19:18. doi: 10.1007/s11926-017-0645-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abe K, et al. Gain-of-function mutations in a member of the Src family kinases cause autoinflammatory bone disease in mice and humans. Proc. Natl Acad. Sci. USA. 2019;116:11872–11877. doi: 10.1073/pnas.1819825116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuemmerle-Deschner JB, et al. New variant in the IL1RN-gene (DIRA) associated with late-onset, CRMO-like presentation. Rheumatology. 2020;59:3259–3263. doi: 10.1093/rheumatology/keaa119. [DOI] [PubMed] [Google Scholar]
- 96.Ferguson PJ, et al. Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome) J. Med. Genet. 2005;42:551–557. doi: 10.1136/jmg.2005.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Von Der Werth JM, Williams HC, Raeburn JA. The clinical genetics of hidradenitis suppurativa revisited. Br. J. Dermatol. 2000;142:947–953. doi: 10.1046/j.1365-2133.2000.03476.x. [DOI] [PubMed] [Google Scholar]
- 98.Leuenberger M, et al. PASS syndrome: an IL-1-driven autoinflammatory disease. Dermatology. 2016;232:254–258. doi: 10.1159/000443648. [DOI] [PubMed] [Google Scholar]
- 99.Saternus R, Schwingel J, Muller CSL, Vogt T, Reichrath J. Ancient friends, revisited: systematic review and case report of pyoderma gangrenosum-associated autoinflammatory syndromes. J. Transl. Autoimmun. 2020;3:100071. doi: 10.1016/j.jtauto.2020.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Giacomelli R, et al. The treatment of adult-onset Still’s disease with anakinra, a recombinant human IL-1 receptor antagonist: a systematic review of literature. Clin. Exp. Rheumatol. 2021;39:187–195. doi: 10.55563/clinexprheumatol/fsq5vq. [DOI] [PubMed] [Google Scholar]
- 101.Toplak N, Blazina S, Avcin T. The role of IL-1 inhibition in systemic juvenile idiopathic arthritis: current status and future perspectives. Drug Des. Devel. Ther. 2018;12:1633–1643. doi: 10.2147/DDDT.S114532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vastert SJ, et al. Anakinra in children and adults with Still’s disease. Rheumatology. 2019;58:vi9–vi22. doi: 10.1093/rheumatology/kez350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buckley LF, Viscusi MM, Van Tassell BW, Abbate A. Interleukin-1 blockade for the treatment of pericarditis. Eur. Heart J. Cardiovasc. Pharmacother. 2018;4:46–53. doi: 10.1093/ehjcvp/pvx018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Galozzi P, Bindoli S, Doria A, Oliviero F, Sfriso P. Autoinflammatory features in Gouty arthritis. J. Clin. Med. 2021 doi: 10.3390/jcm10091880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thomas M, Forien M, Palazzo E, Dieude P, Ottaviani S. Efficacy and tolerance of anakinra in acute calcium pyrophosphate crystal arthritis: a retrospective study of 33 cases. Clin. Rheumatol. 2019;38:425–430. doi: 10.1007/s10067-018-4272-2. [DOI] [PubMed] [Google Scholar]
- 106.Haibel H, Rudwaleit M, Listing J, Sieper J. Open label trial of anakinra in active ankylosing spondylitis over 24 weeks. Ann. Rheum. Dis. 2005;64:296–298. doi: 10.1136/ard.2004.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jung N, et al. An open-label pilot study of the efficacy and safety of anakinra in patients with psoriatic arthritis refractory to or intolerant of methotrexate (MTX) Clin. Rheumatol. 2010;29:1169–1173. doi: 10.1007/s10067-010-1504-5. [DOI] [PubMed] [Google Scholar]
- 108.Tan AL, et al. Efficacy of anakinra in active ankylosing spondylitis: a clinical and magnetic resonance imaging study. Ann. Rheum. Dis. 2004;63:1041–1045. doi: 10.1136/ard.2004.020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Manthiram K, et al. Common genetic susceptibility loci link PFAPA syndrome, Behcet’s disease, and recurrent aphthous stomatitis. Proc. Natl Acad. Sci. USA. 2020;117:14405–14411. doi: 10.1073/pnas.2002051117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fabiani C, et al. The presence of uveitis is associated with a sustained response to the interleukin (IL)-1 inhibitors anakinra and canakinumab in Behcet’s disease. Ocul. Immunol. Inflamm. 2020;28:298–304. doi: 10.1080/09273948.2018.1511810. [DOI] [PubMed] [Google Scholar]
- 111.Broderick L, Tremoulet AH, Burns JC, Bastian JF, Hoffman HM. Recurrent fever syndromes in patients after recovery from Kawasaki syndrome. Pediatrics. 2011;127:e489–e493. doi: 10.1542/peds.2010-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fury W, et al. Transcript abundance patterns in Kawasaki disease patients with intravenous immunoglobulin resistance. Hum. Immunol. 2010;71:865–873. doi: 10.1016/j.humimm.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marrani E, Burns JC, Cimaz R. How should we classify Kawasaki disease? Front. Immunol. 2018;9:2974. doi: 10.3389/fimmu.2018.02974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kuemmerle-Deschner JB, et al. Clinical and molecular phenotypes of low-penetrance variants of NLRP3: diagnostic and therapeutic challenges. Arthritis Rheumatol. 2017;69:2233–2240. doi: 10.1002/art.40208. [DOI] [PubMed] [Google Scholar]
- 115.Schnappauf O, Aksentijevich I. Current and future advances in genetic testing in systemic autoinflammatory diseases. Rheumatology. 2019;58:vi44–vi55. doi: 10.1093/rheumatology/kez294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kawasaki Y, et al. Identification of a high-frequency somatic NLRC4 mutation as a cause of autoinflammation by pluripotent cell-based phenotype dissection. Arthritis Rheumatol. 2017;69:447–459. doi: 10.1002/art.39960. [DOI] [PubMed] [Google Scholar]
- 117.Kontzias A, et al. Somatic mosaicism in adult-onset TNF receptor-associated periodic syndrome (TRAPS) Mol. Genet. Genom. Med. 2019;7:e791. doi: 10.1002/mgg3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mensa-Vilaro A, et al. Brief report: late-onset cryopyrin-associated periodic syndrome due to myeloid-restricted somatic NLRP3 mosaicism. Arthritis Rheumatol. 2016;68:3035–3041. doi: 10.1002/art.39770. [DOI] [PubMed] [Google Scholar]
- 119.Rowczenio DM, et al. Late-onset cryopyrin-associated periodic syndromes caused by somatic NLRP3 mosaicism — UK single center experience. Front. Immunol. 2017;8:1410. doi: 10.3389/fimmu.2017.01410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Erbis G, et al. Living with autoinflammatory diseases: identifying unmet needs of children, adolescents and adults. Pediatr. Rheumatol. Online J. 2018;16:81. doi: 10.1186/s12969-018-0300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Caorsi R, et al. The schedule of administration of canakinumab in cryopyrin associated periodic syndrome is driven by the phenotype severity rather than the age. Arthritis Res. Ther. 2013;15:R33. doi: 10.1186/ar4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kuemmerle-Deschner JB, et al. Real-life effectiveness of canakinumab in cryopyrin-associated periodic syndrome. Rheumatology. 2016;55:689–696. doi: 10.1093/rheumatology/kev416. [DOI] [PubMed] [Google Scholar]
- 123.Kuemmerle-Deschner JB, et al. Early detection of sensorineural hearing loss in Muckle-Wells-syndrome. Pediatr. Rheumatol. Online J. 2015;13:43. doi: 10.1186/s12969-015-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sibley CH, et al. Sustained response and prevention of damage progression in patients with neonatal-onset multisystem inflammatory disease treated with anakinra: a cohort study to determine three- and five-year outcomes. Arthritis Rheum. 2012;64:2375–2386. doi: 10.1002/art.34409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ter Haar N, et al. Treatment of autoinflammatory diseases: results from the Eurofever Registry and a literature review. Ann. Rheum. Dis. 2013;72:678–685. doi: 10.1136/annrheumdis-2011-201268. [DOI] [PubMed] [Google Scholar]
- 126.Chuamanochan M, et al. State of care for patients with systemic autoinflammatory diseases — results of a tertiary care survey. World Allergy Organ. J. 2019;12:100019. doi: 10.1016/j.waojou.2019.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Walker UA, Hoffman HM, Williams R, Kuemmerle-Deschner J, Hawkins PN. Brief report: severe inflammation following vaccination against streptococcus pneumoniae in patients with cryopyrin-associated periodic syndromes. Arthritis Rheumatol. 2016;68:516–520. doi: 10.1002/art.39482. [DOI] [PubMed] [Google Scholar]
- 128.Jeyaratnam J, et al. The safety of live-attenuated vaccines in patients using IL-1 or IL-6 blockade: an international survey. Pediatr. Rheumatol. Online J. 2018;16:19. doi: 10.1186/s12969-018-0235-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alehashemi S, van Gelderen E, Rastegar A, de Jesus AA, Goldbach-Mansky R. Post-SARS-CoV-2 vaccine monitoring of disease flares in autoinflammatory diseases. J. Clin. Immunol. 2022 doi: 10.1007/s10875-022-01225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Leung YY, Yao Hui LL, Kraus VB. Colchicine — update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015;45:341–350. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Caviston JP, Holzbaur EL. Microtubule motors at the intersection of trafficking and transport. Trends Cell Biol. 2006;16:530–537. doi: 10.1016/j.tcb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 132.Dalbeth N, Lauterio TJ, Wolfe HR. Mechanism of action of colchicine in the treatment of gout. Clin. Ther. 2014;36:1465–1479. doi: 10.1016/j.clinthera.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 133.Gao W, Yang J, Liu W, Wang Y, Shao F. Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proc. Natl Acad. Sci. USA. 2016;113:E4857–E4866. doi: 10.1073/pnas.1601700113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ozen S, Kone-Paut I, Gul A. Colchicine resistance and intolerance in familial Mediterranean fever: definition, causes, and alternative treatments. Semin. Arthritis Rheum. 2017;47:115–120. doi: 10.1016/j.semarthrit.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 135.El Hasbani G, Jawad A, Uthman I. Update on the management of colchicine resistant familial Mediterranean fever (FMF) Orphanet J. Rare Dis. 2019;14:224. doi: 10.1186/s13023-019-1201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lachmann HJ, et al. In vivo regulation of interleukin 1β in patients with cryopyrin-associated periodic syndromes. J. Exp. Med. 2009;206:1029–1036. doi: 10.1084/jem.20082481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cavalli G, Dinarello CA. Anakinra therapy for non-cancer inflammatory diseases. Front. Pharmacol. 2018;9:1157. doi: 10.3389/fphar.2018.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Papa R, et al. Syndrome of undifferentiated recurrent fever (SURF): an emerging group of autoinflammatory recurrent fevers. J. Clin. Med. 2021 doi: 10.3390/jcm10091963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Walker UA, et al. Long-term safety and effectiveness of canakinumab therapy in patients with cryopyrin-associated periodic syndrome: results from the β-Confident Registry. RMD Open. 2021 doi: 10.1136/rmdopen-2021-001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sibley CH, et al. A 24-month open-label study of canakinumab in neonatal-onset multisystem inflammatory disease. Ann. Rheum. Dis. 2015;74:1714–1719. doi: 10.1136/annrheumdis-2013-204877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kuemmerle-Deschner JB, et al. Treatment of Muckle-Wells syndrome: analysis of two IL-1-blocking regimens. Arthritis Res. Ther. 2013;15:R64. doi: 10.1186/ar4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goldbach-Mansky R, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1β inhibition. N. Engl. J. Med. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chang Z, et al. Anakinra use during pregnancy in patients with cryopyrin-associated periodic syndromes (CAPS) Arthritis Rheumatol. 2014;66:3227–3232. doi: 10.1002/art.38811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hoffman HM, et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum. 2008;58:2443–2452. doi: 10.1002/art.23687. [DOI] [PubMed] [Google Scholar]
- 145.Lachmann HJ, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N. Engl. J. Med. 2009;360:2416–2425. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 146.Ridker PM, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 147.Coll RC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mangan MSJ, et al. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018;17:688. doi: 10.1038/nrd.2018.149. [DOI] [PubMed] [Google Scholar]
- 149.LaRock CN, et al. IL-1β is an innate immune sensor of microbial proteolysis. Sci. Immunol. 2016 doi: 10.1126/sciimmunol.aah3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vande Walle L, et al. MCC950/CRID3 potently targets the NACHT domain of wild-type NLRP3 but not disease-associated mutants for inflammasome inhibition. PLoS Biol. 2019;17:e3000354. doi: 10.1371/journal.pbio.3000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mulazzani E, et al. Neurological phenotypes in patients with NLRP3-, MEFV-, and TNFRSF1A low-penetrance variants. J. Neuroinflamm. 2020;17:196. doi: 10.1186/s12974-020-01867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Grandemange S, et al. Clinical dose effect and functional consequences of R92Q in two families presenting with a TRAPS/PFAPA-like phenotype. Mol. Genet. Genom. Med. 2017;5:110–116. doi: 10.1002/mgg3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Camprubi D, Mitjavila F, Arostegui JI, Corbella X. Efficacy of anakinra in an adult patient with recurrent pericarditis and cardiac tamponade as initial manifestations of tumor necrosis factor receptor-associated periodic syndrome due to the R92Q TNFRSF1A variant. Int. J. Rheum. Dis. 2017;20:510–514. doi: 10.1111/1756-185X.13029. [DOI] [PubMed] [Google Scholar]
- 154.Stoffman N, et al. Higher than expected carrier rates for familial Mediterranean fever in various Jewish ethnic groups. Eur. J. Hum. Genet. 2000;8:307–310. doi: 10.1038/sj.ejhg.5200446. [DOI] [PubMed] [Google Scholar]
- 155.Park YH, et al. Ancient familial Mediterranean fever mutations in human pyrin and resistance to Yersinia pestis. Nat. Immunol. 2020;21:857–867. doi: 10.1038/s41590-020-0705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fumagalli M, et al. A population genetics study of the familial Mediterranean fever gene: evidence of balancing selection under an overdominance regime. Genes Immun. 2009;10:678–686. doi: 10.1038/gene.2009.59. [DOI] [PubMed] [Google Scholar]
- 157.Gul A. Selective pressures for the high prevalence of MEFV variants induced by smallpox infection in the “Old World”: a hypothesis. Clin. Exp. Rheumatol. 2006;24:213. [PubMed] [Google Scholar]
- 158.Yildiz M, et al. The role of Mediterranean fever gene variants in patients with periodic fever, aphthous stomatitis, pharyngitis, and adenitis syndrome. Eur. J. Pediatr. 2021;180:1051–1058. doi: 10.1007/s00431-020-03840-z. [DOI] [PubMed] [Google Scholar]
- 159.Touitou I, et al. MEFV mutations in Behcet’s disease. Hum. Mutat. 2000;16:271–272. doi: 10.1002/1098-1004(200009)16:3<271::AID-HUMU16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 160.Topaloglu R, et al. E148Q is a disease-causing MEFV mutation: a phenotypic evaluation in patients with familial Mediterranean fever. Ann. Rheum. Dis. 2005;64:750–752. doi: 10.1136/ard.2004.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tanaka N, et al. High incidence of NLRP3 somatic mosaicism in chronic infantile neurological cutaneous and articular syndrome patients: the results of an international multicenter collaborative study. Arthritis Rheum. 2011;63:3625–3632. doi: 10.1002/art.30512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Luu I, et al. Undifferentiated recurrent fevers in pediatrics are clinically distinct from PFAPA syndrome but retain an IL-1 signature. Clin. Immunol. 2021;226:108697. doi: 10.1016/j.clim.2021.108697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Papa R, et al. Next generation sequencing panel in undifferentiated autoinflammatory diseases identifies patients with colchicine-responder recurrent fevers. Rheumatology. 2020;59:458. doi: 10.1093/rheumatology/kez376. [DOI] [PubMed] [Google Scholar]
- 164.Chan AY, et al. Hematopoietic cell transplantation in patients with primary immune regulatory disorders (PIRD): a primary immune deficiency treatment consortium (PIDTC) survey. Front. Immunol. 2020;11:239. doi: 10.3389/fimmu.2020.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cheung EW, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York city. JAMA. 2020;324:294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Verdoni L, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Feldstein LR, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McArdle AJ, et al. Treatment of multisystem inflammatory syndrome in children. N. Engl. J. Med. 2021;385:11–22. doi: 10.1056/NEJMoa2102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Son MBF, et al. Multisystem inflammatory syndrome in children — initial therapy and outcomes. N. Engl. J. Med. 2021;385:23–34. doi: 10.1056/NEJMoa2102605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Holzinger D, Kessel C, Omenetti A, Gattorno M. From bench to bedside and back again: translational research in autoinflammation. Nat. Rev. Rheumatol. 2015;11:573–585. doi: 10.1038/nrrheum.2015.79. [DOI] [PubMed] [Google Scholar]
- 172.Ombrello MJ, et al. Genetic architecture distinguishes systemic juvenile idiopathic arthritis from other forms of juvenile idiopathic arthritis: clinical and therapeutic implications. Ann. Rheum. Dis. 2017;76:906–913. doi: 10.1136/annrheumdis-2016-210324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rabionet R, et al. Biallelic loss-of-function LACC1/FAMIN mutations presenting as rheumatoid factor-negative polyarticular juvenile idiopathic arthritis. Sci. Rep. 2019;9:4579. doi: 10.1038/s41598-019-40874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Reginato AM, Mount DB, Yang I, Choi HK. The genetics of hyperuricaemia and gout. Nat. Rev. Rheumatol. 2012;8:610–621. doi: 10.1038/nrrheum.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Okada Y, et al. Meta-analysis identifies multiple loci associated with kidney function-related traits in east Asian populations. Nat. Genet. 2012;44:904–909. doi: 10.1038/ng.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tin A, et al. Genome-wide association study for serum urate concentrations and gout among African Americans identifies genomic risk loci and a novel URAT1 loss-of-function allele. Hum. Mol. Genet. 2011;20:4056–4068. doi: 10.1093/hmg/ddr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kees S, et al. Attacks of pericarditis as a manifestation of familial Mediterranean fever (FMF) QJM. 1997;90:643–647. doi: 10.1093/qjmed/90.10.643. [DOI] [PubMed] [Google Scholar]
- 178.Brucato A, et al. Idiopathic recurrent acute pericarditis: familial Mediterranean fever mutations and disease evolution in a large cohort of Caucasian patients. Lupus. 2005;14:670–674. doi: 10.1191/0961203305lu2197oa. [DOI] [PubMed] [Google Scholar]
- 179.Wang B, et al. Gamma-secretase gene mutations in familial acne inversa. Science. 2010;330:1065. doi: 10.1126/science.1196284. [DOI] [PubMed] [Google Scholar]
- 180.Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am. J. Clin. Dermatol. 2017;18:555–562. doi: 10.1007/s40257-017-0265-1. [DOI] [PubMed] [Google Scholar]
- 181.Garcovich SG, Genovese G, Moltrasio C, Malvaso D, Marzano AV. PASH, PAPASH, PsAPASH, and PASS: the autoinflammatory syndromes of hidradenitis suppurativa. Clin. Dermatol. 2020 doi: 10.1016/j.clindermatol.2020.10.016. [DOI] [PubMed] [Google Scholar]
- 182.Marzano AV, et al. Pyogenic arthritis, pyoderma gangrenosum, acne, and hidradenitis suppurativa (PAPASH): a new autoinflammatory syndrome associated with a novel mutation of the PSTPIP1 gene. JAMA Dermatol. 2013;149:762–764. doi: 10.1001/jamadermatol.2013.2907. [DOI] [PubMed] [Google Scholar]
- 183.Broderick L, et al. Mutations of complement factor I and potential mechanisms of neuroinflammation in acute hemorrhagic leukoencephalitis. J. Clin. Immunol. 2013;33:162–171. doi: 10.1007/s10875-012-9767-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cheung MS, et al. Periodic fever with aphthous stomatitis, pharyngitis, and cervical adenitis syndrome is associated with a CARD8 variant unable to bind the NLRP3 inflammasome. J. Immunol. 2017;198:2063–2069. doi: 10.4049/jimmunol.1600760. [DOI] [PubMed] [Google Scholar]
- 185.Sangiorgi E, et al. Rare missense variants in the ALPK1 gene may predispose to periodic fever, aphthous stomatitis, pharyngitis and adenitis (PFAPA) syndrome. Eur. J. Hum. Genet. 2019;27:1361–1368. doi: 10.1038/s41431-019-0421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lee YC, et al. Two new susceptibility loci for Kawasaki disease identified through genome-wide association analysis. Nat. Genet. 2012;44:522–525. doi: 10.1038/ng.2227. [DOI] [PubMed] [Google Scholar]
- 187.Onouchi Y, et al. A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat. Genet. 2012;44:517–521. doi: 10.1038/ng.2220. [DOI] [PubMed] [Google Scholar]
- 188.Kuo HC, et al. Genome-wide association study identifies novel susceptibility genes associated with coronary artery aneurysm formation in Kawasaki disease. PLoS One. 2016;11:e0154943. doi: 10.1371/journal.pone.0154943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Remmers EF, et al. Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with Behcet’s disease. Nat. Genet. 2010;42:698–702. doi: 10.1038/ng.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.de Menthon M, Lavalley MP, Maldini C, Guillevin L, Mahr A. HLA-B51/B5 and the risk of Behcet’s disease: a systematic review and meta-analysis of case-control genetic association studies. Arthritis Rheum. 2009;61:1287–1296. doi: 10.1002/art.24642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Israel L, Mellett M. Clinical and genetic heterogeneity of CARD14 mutations in psoriatic skin disease. Front. Immunol. 2018;9:2239. doi: 10.3389/fimmu.2018.02239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Marrakchi S, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N. Engl. J. Med. 2011;365:620–628. doi: 10.1056/NEJMoa1013068. [DOI] [PubMed] [Google Scholar]
- 193.Onoufriadis A, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am. J. Hum. Genet. 2011;89:432–437. doi: 10.1016/j.ajhg.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhou Q, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat. Genet. 2016;48:67–73. doi: 10.1038/ng.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Romberg N, Vogel TP, Canna SW. NLRC4 inflammasomopathies. Curr. Opin. Allergy Clin. Immunol. 2017;17:398–404. doi: 10.1097/ACI.0000000000000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gandhi C, Healy C, Wanderer AA, Hoffman HM. Familial atypical cold urticaria: description of a new hereditary disease. J. Allergy Clin. Immunol. 2009;124:1245–1250. doi: 10.1016/j.jaci.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ombrello MJ, et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N. Engl. J. Med. 2012;366:330–338. doi: 10.1056/NEJMoa1102140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.