Abstract
Background
A wide variety of surfactant preparations have been developed and tested including synthetic surfactants and surfactants derived from animal sources. Although clinical trials have demonstrated that both synthetic surfactant and animal derived surfactant preparations are effective, comparison in animal models has suggested that there may be greater efficacy of animal derived surfactant products, perhaps due to the protein content of animal derived surfactant.
Objectives
To compare the effect of animal derived surfactant to protein free synthetic surfactant preparations in preterm infants at risk for or having respiratory distress syndrome (RDS).
Search methods
Searches were updated of the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2014), PubMed, CINAHL and EMBASE (1975 through November 2014). All languages were included.
Selection criteria
Randomized controlled trials comparing administration of protein free synthetic surfactants to administration of animal derived surfactant extracts in preterm infants at risk for or having respiratory distress syndrome were considered for this review.
Data collection and analysis
Data collection and analysis were conducted according to the standards of the Cochrane Neonatal Review Group.
Main results
Fifteen trials met the inclusion criteria. The meta‐analysis showed that the use of animal derived surfactant rather than protein free synthetic surfactant resulted in a significant reduction in the risk of pneumothorax [typical relative risk (RR) 0.65, 95% CI 0.55 to 0.77; typical risk difference (RD) ‐0.04, 95% CI ‐0.06 to ‐0.02; number needed to treat to benefit (NNTB) 25; 11 studies, 5356 infants] and a marginal reduction in the risk of mortality (typical RR 0.89, 95% CI 0.79 to 0.99; typical RD ‐0.02, 95% CI ‐0.04 to ‐0.00; NNTB 50; 13 studies, 5413 infants).
Animal derived surfactant was associated with an increase in the risk of necrotizing enterocolitis [typical RR 1.38, 95% CI 1.08 to 1.76; typical RD 0.02, 95% CI 0.01 to 0.04; number needed to treat to harm (NNTH) 50; 8 studies, 3462 infants] and a marginal increase in the risk of any intraventricular hemorrhage (typical RR 1.07, 95% CI 0.99 to 1.15; typical RD 0.02, 95% CI 0.00 to 0.05; 10 studies, 5045 infants) but no increase in Grade 3 to 4 intraventricular hemorrhage (typical RR 1.08, 95% CI 0.91 to 1.27; typical RD 0.01, 95% CI ‐0.01 to 0.03; 9 studies, 4241 infants).
The meta‐analyses supported a marginal decrease in the risk of bronchopulmonary dysplasia or mortality associated with the use of animal derived surfactant preparations (typical RR 0.95, 95% CI 0.91 to 1.00; typical RD ‐0.03, 95% CI ‐0.06 to 0.00; 6 studies, 3811 infants). No other relevant differences in outcomes were noted.
Authors' conclusions
Both animal derived surfactant extracts and protein free synthetic surfactant extracts are effective in the treatment and prevention of respiratory distress syndrome. Comparative trials demonstrate greater early improvement in the requirement for ventilator support, fewer pneumothoraces, and fewer deaths associated with animal derived surfactant extract treatment. Animal derived surfactant may be associated with an increase in necrotizing enterocolitis and intraventricular hemorrhage, though the more serious hemorrhages (Grade 3 and 4) are not increased. Despite these concerns, animal derived surfactant extracts would seem to be the more desirable choice when compared to currently available protein free synthetic surfactants.
Plain language summary
Animal derived surfactant compared to protein‐free synthetic surfactant preparations in preterm infants that have or are at high risk for respiratory distress syndrome.
Review question: Does the use of animal derived surfactant preparations compared to synthetic surfactant preparations that do not contain protein lead to improved outcome in infants at risk for or having respiratory distress syndrome?
Background: Pulmonary surfactant is a substance that prevents the air sacs of the lungs from collapsing by reducing surface tension. Newborn babies with respiratory distress syndrome (RDS) have immature lungs and are often lacking in pulmonary surfactant. Commercially available surfactant preparations (either animal derived surfactant preparations or synthetic surfactant preparations that may or may not contain protein) can be given to these babies and have been proven to decrease the severity of RDS and increase the survival rates of babies with RDS. However, it is unclear whether significant differences in clinical outcome exist among the available animal derived surfactant preparations or the protein‐free synthetic surfactant preparations.
Study characteristics: Fifteen randomized controlled trials met our inclusion criteria.
Results: This review of trials compared animal derived surfactant extracts with synthetic surfactants that did not contain protein and found a decrease in the risk of pneumothorax (air in the lung cavity) and death in babies receiving animal derived surfactant extracts.
Some evidence that animal derived surfactant extract leads to better outcomes in babies with respiratory distress syndrome compared to synthetic surfactants that do not contains proteins.
Background
Description of the condition
Respiratory distress syndrome (RDS) is primarily a disease of the preterm infant and is due to lung immaturity and a deficiency of endogenous surfactant (Jobe 1993; Warren 2009). The incidence of RDS increases with decreasing gestational age. Sixty per cent of infants born at less than 28 weeks gestation will have RDS, 30% of infants born between 28 and 34 weeks gestation will develop RDS, and less than 5% of infants born after 34 weeks will develop RDS (Warren 2009).
The pathophysiology of RDS is secondary to lung immaturity and surfactant deficiency. Most infants with RDS are born prior to the alveolarization stage of lung development (which occurs at ˜ 32 weeks gestation) (Jobe 2006). At lower gestational ages, pulmonary surfactant is not produced in sufficient amounts or with the correct composition of phospholipids and surfactant proteins due to the immaturity of the type II pneumocyte where surfactant is produced, stored and secreted (Wright 1997; Warren 2009). These cells start to differentiate during the canalicular phase of lung development and reach their final, mature stage by the alveolar phase (˜ 32 weeks gestation) (Jobe 2006).
Surfactant is composed of phospholipids and four surfactant proteins, A, B, C and D (SP A, B, C, D). Endogenous surfactant is packaged in the lamellar bodies of the type II pneumocyte, which secrete the surfactant by exocytosis into the extracellular space. Phosphotidylcholine is the major phospholipid found in surfactant and is responsible for decreasing the surface tension at the air‐water interface in the alveoli (Clements 1977; Jobe 2006). The surfactant proteins all have different functions (Possmayer 1990). SP B and C facilitate the adsorption of the surfactant lipids to the air‐water interface and are essential for the surfactant’s surface tension lowering properties (Gower 2008). SP A and D are involved in innate immunity and immunomodulation (Gower 2008). Genetic SP B deficiency is a cause of fatal neonatal respiratory disease (Nogee 1993; Whitsett 1995; Gower 2008). SP C deficiency is associated with respiratory disease that occurs later in life, but the presentation is variable with some patients presenting in the neonatal period with a rapid deterioration in respiratory status or patients present with interstitial lung disease later in life (Nogee 2004; Gower 2008).
The result of preterm birth is decreased surface area for gas exchange and increased surface tension at the alveolar level resulting in atelectasis and intra‐pulmonary shunting leading to ventilation‐perfusion mismatch and hypoxia with subsequent respiratory failure (Warren 2009). Progressive atelectasis, which results in decreased functional residual capacity, causes further lung injury that leads to exudation of protein into the extracellular space, edema and further inactivation of endogenous pulmonary surfactant (Jobe 1993; Warren 2009).
Clinically, RDS is characterized by increased work of breathing and cyanosis (Warren 2009). Chest radiographs reveal the characteristic 'ground glass' appearance to the lung secondary to atelectasis with superimposed air bronchograms. Without intervention, RDS is progressive and potentially fatal.
Description of the intervention
Pulmonary surfactant decreases alveolar surface tension and prevents alveolar collapse and atelectasis, allowing for increased functional residual capacity, increased compliance, and improved oxygenation and ventilation (Jobe 1993; Warren 2009). In infants that are deficient in surfactant or have surfactant inactivation, surfactant replacement therapy has been shown to improve oxygenation and chest X‐ray (CXR) findings within one hour after administration (Warren 2009).
How the intervention might work
Randomized controlled trials have demonstrated the effectiveness of surfactant therapy in both the prevention and treatment of RDS in infants that are at risk of or with the disease. Surfactant administration decreases the severity of respiratory distress, decreases the frequency of pneumothorax, increases survival without chronic lung disease, and decreases mortality (Soll 1992). Surfactant preparations are now widely used and have been credited with recent improvements in overall infant mortality (Horbar 1993b, Schwartz 1994). A wide variety of surfactant preparations have been developed and tested. These include synthetic surfactants and surfactants derived from animal sources. Synthetic surfactants can be protein free or contain peptides that are thought to mimic proteins found in pulmonary surfactant (see below) (Moya 2005; Pfister 2005). Animal derived surfactants are derived from both bovine and porcine sources, and all contain varying degrees of SP B and C as well as phospholipids (Warren 2009). Although clinical trials have demonstrated that both synthetic surfactants and animal derived surfactant preparations are effective, comparison in animal models has suggested that there may be greater efficacy of animal derived surfactant products, perhaps due to the protein content of these surfactant preparations (Tooley 1987).
Timing of surfactant administration also plays an important role in the therapeutic benefit of surfactant replacement therapy (SRT). Although aggressive prophylactic treatment may no longer be necessary (Rojas‐Reyes 2012), studies have demonstrated that early SRT vs. late, selective SRT improves outcomes with significant reductions in the rates of pneumothorax, pulmonary interstitial emphysema (PIE), mortality, bronchopulmonary dysplasia (BPD) or death (Bahadue 2012).
Why it is important to do this review
Multiple systematic reviews have addressed the use of animal derived surfactant preparations or synthetic surfactant preparations in the prevention or treatment of RDS. Meta‐analyses of the original randomized controlled trials of surfactant for the treatment and prevention of RDS were first published in Effective Care of the Newborn (Soll 1992). Since then, multiple systematic reviews have been published in The Cochrane Library including reviews of protein free synthetic surfactant for the prevention and treatment of RDS (Soll 2000a; Soll 2010) and reviews of animal derived surfactant for the prevention and treatment of RDS (Soll 2000b; Seger 2009).
Systematic reviews that compare different treatment strategies have been published (Stevens 2007; Soll 2009; Bahadue 2012; Rojas‐Reyes 2012).
Other reviews have addressed the method of instillation of surfactant (Abdel‐Latif 2011a; Abdel‐Latif 2011b; Abdel‐Latif 2012) as well as the use of surfactant replacement therapy in conditions other than RDS (El Shahed 2007; Aziz 2012; Tan 2012; Hahn 2013).
This review updates the previous reviews of animal derived surfactant extract vs. synthetic surfactant in the prevention and treatment of established RDS published in The Cochrane Library (Soll 1997; Soll 2001). Clinical trials that compare animal derived surfactant extract to protein free synthetic surfactant in the treatment or prevention of respiratory distress syndrome have been included in this systematic review. Trials that compare animal derived surfactant extract to protein containing synthetic surfactant and trials that compare protein free synthetic surfactant to protein containing synthetic surfactant are addressed by other reviews (Pfister 2007; Pfister 2009).
Objectives
To compare the effect of protein free synthetic surfactant to animal derived surfactant extracts surfactant in preterm infants at risk for or having respiratory distress syndrome (RDS).
Separate analyses were done for trials of prophylaxis and trials of selective treatment (rescue).
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials and quasi‐randomized controlled trials were considered for this review.
Types of participants
Preterm infants (less than 37 weeks gestation) at risk for or having RDS.
Types of interventions
Studies in which preterm infants were randomly allocated to receive an animal derived surfactant extract compared to a protein free synthetic surfactant, using either a prophylactic or selective treatment strategy.
Types of outcome measures
Primary outcomes
Mortality
Bronchopulmonary dysplasia (BPD) (defined as oxygen dependency at 28 to 30 days of age)
Chronic lung disease (CLD) (defined as oxygen dependency at 36 weeks postmenstrual age)
BPD or death
CLD or death
Secondary outcomes
Pneumothorax
Patent ductus arteriosus (PDA) (either clinical diagnosis or need for treatment)
Bacterial sepsis
Necrotizing enterocolitis (NEC) (Bell's stage II or greater) (Bell 1978)
Intraventricular hemorrhage (IVH) [all intraventricular hemorrhage and severe intraventricular hemorrhage (Grades 3 and 4)] (Papile 1978)
Retinopathy of prematurity (ROP) [any stage, severe (stages 3 and 4)](ICCROP 2005)
Search methods for identification of studies
We used the standard search methods of the Cochrane Neonatal Review Group.
Electronic searches
We updated searches of the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2014), PubMed, CINAHL and EMBASE (1975 through November 2014). We did not include any language restrictions. The search terms were: pulmonary surfactant; limits; age groups, infants; publication type, clinical trial.
Searching other resources
We searched previous reviews and cross references, abstracts, conference and symposia proceedings, approached expert informants, and undertook journal handsearching in the English language. The abstracts of the Society for Pediatric Research (USA) (published in Pediatric Research) for the years 1985 to 2010 were searched by hand using the following key words: {surfactant} AND {respiratory distress syndrome}. For abstract books that did not include keywords, the search was limited to relevant sections such as pulmonology and neonatology.
We searched for any on‐going or recently completed and unpublished trials using clinicaltrials.gov, controlled‐trials.com, and who.int/ictrp.
Data collection and analysis
We used the methods of the Cochrane Neonatal Review Group for data collection and analysis.
Selection of studies
We included all randomized and quasi‐randomized controlled trials that fulfilled the selection criteria described in the previous section. RS and SA independently reviewed the results of the updated search and selected studies for inclusion. We resolved any disagreement by discussion.
Data extraction and management
We used a data extraction form specifically designed for the study. Two review authors (SA, RS) separately extracted, assessed and coded all data for each additional included study.
We collected information on clinical outcomes, including the incidence of pneumothorax, PDA, IVH (any IVH and severe IVH, grades 3 or 4), BPD, CLD, ROP, and mortality. We resolved differences in assessment by discussion.
For each study, final data were entered into RevMan by one review author (RS) and then checked for accuracy by a second review author (SA). We resolved discrepancies through discussion or by involving RP as a third assessor.
We attempted to contact authors of the original reports to provide further details when information regarding any of the above was unclear.
Assessment of risk of bias in included studies
We used the standard method of the Cochrane Neonatal Review Group. RS and SA independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving RP as a third assessor.
The methodological quality of the studies was assessed using the following criteria.
-
Sequence generation (checking for possible selection bias). For each included study, we categorized the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number);
unclear risk.
-
Allocation concealment (checking for possible selection bias). For each included study, we categorized the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk.
-
Blinding (checking for possible performance bias). For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods as:
low risk, high risk or unclear risk for participants;
low risk, high risk or unclear risk for personnel;
low risk, high risk or unclear risk for outcome assessors.
-
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total number of randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data);
unclear risk.
-
Selective reporting bias. For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk.
-
Other sources of bias. For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk; high risk; unclear risk.
Overall risk of bias (described in Table 8.5c in the Cochrane Handbook for Systematic Reviews of Interventions).
We made explicit judgements regarding whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses (see 'Sensitivity analysis' below).
Measures of treatment effect
We used the standard methods of the Neonatal Review Group to analyze the data.
We performed statistical analyses using the Review Manager software (RevMan 2011). Dicotomous data were analyzed using relative risk (RR), risk difference (RD) and the number needed to treat to benefit (NNTB) or number needed to treat to harm (NNTH). The 95% confidence interval (CI) was reported on all estimates.
No continuous outcomes were included in this review. If included, we planned to analyze continuous data using weighted mean difference (WMD), or the standardized mean difference (SMD) to combine trials that measured the same outcome but used different methods.
Unit of analysis issues
For clinical outcomes such as episodes of sepsis, we analyzed the data as proportion of neonates having one or more episodes.
Dealing with missing data
For included studies, levels of attrition were noted. The impact of including studies with high levels of missing data in the overall assessment of treatment effect was explored by using sensitivity analysis.
All outcomes analyses were on an intention to treat basis that is we included all participants randomized to each group in the analyses. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We examined heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic. If noted, we planned to explore the possible causes of statistical heterogeneity using pre‐specified subgroup analysis (for example differences in study quality, participants, intervention regimens, or outcome assessments).
Assessment of reporting biases
We assessed possible publication bias and other biases using the symmetry or asymmetry of funnel plots.
For included trials that were recently performed (and therefore prospectively registered) we explored possible selective reporting of study outcomes by comparing the primary and secondary outcomes in the reports with the primary and secondary outcomes proposed at trial registration, using the websites www.clinicaltrials.gov and www.controlled‐trials.com. If such discrepancies were found, we planned to contact the primary investigators to obtain missing outcome data on outcomes pre‐specified at trial registration.
Data synthesis
Where meta‐analysis was judged to be appropriate, the analysis was done using the Review Manager software (RevMan 2011), supplied by The Cochrane Collaboration. We used the Mantel‐Haenszel method for estimates of typical RR and RD. No continuous outcomes were included in this review. We planned to analyze continuous measures using the inverse variance method, if included.
We used the fixed‐effect model for all meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We did separate analyses for trials of prophylaxis and trials of selective treatment (rescue).
Sensitivity analysis
We planned sensitivity analyses for situations that might affect the interpretation of significant results (for example where there was risk of bias associated with the quality of some of the included trials or missing outcome data). None were thought necessary in this review.
Results
Description of studies
The review included the following 15 studies. Prevention trials: Hudak 1997; Moya 2005. Selective treatment trials: Alvarado 1993; Horbar 1993; Pearlman 1993; Sehgal 1994; Hudak 1996; VT Oxford 1996; Modanlou 1997; Murdoch 1998; daCosta 1999; Halahakoon 1999; Lloyd 1999; Ainsworth 2000; Kukkonen 2000.
Prevention trials
Hudak 1997: conducted a multicenter randomized controlled trial to compare the efficacy and safety of a synthetic surfactant colfosceril palmitate (Exosurf Neonatal, Burroughs Wellcome) and a surfactant extract of calf lung lavage (Infasurf, Forest Laboratories) in the prevention of neonatal respiratory distress syndrome. Premature infants less than 29 weeks gestational age were randomly assigned to prophylactic surfactant administration with colfosceril palmitate (n = 438) or Infasurf (n = 433) at birth and, if still intubated, at 12 and 24 hours of age. Crossover treatment was allowed within 72 hours of age if severe respiratory failure persisted after three doses of the assigned surfactant. The clinicians were unaware of treatment assignment. Primary outcome measures included the incidence of RDS, the incidence of death due to RDS, and the incidence of survival without BPD at 28 days after birth. Of 871 randomized infants, 18 infants did not receive treatment with a study surfactant, and 25 infants did not meet all eligibility criteria. The primary analysis of efficacy was performed in the 846 eligible infants and analysis of safety outcomes in the 853 infants who received study surfactant. Demographic characteristics did not differ between the two treatment groups. Compared with colfosceril palmitate, calf lung surfactant treatment resulted in a decrease in the incidence of RDS (16% vs. 42%) and a decrease in death due to RDS (1.7% vs. 5.4%) but did not increase the incidence of survival without BPD at 28 days. Treatment with calf lung surfactant resulted in significant improvement in several secondary outcome measures including lower average FIO2 and lower average mean airway pressure for the first 72 hours of life. Crossover surfactant treatment was significantly less frequent in the animal derived surfactant compared with the colfosceril palmitate group (1% vs. 6%). Animal surfactant‐treated infants had significantly less air leak (8% vs. 14%). Intraventricular hemorrhage (IVH) occurred more frequently in animal surfactant‐treated infants (39.0% vs. 29.9%) but the incidence of severe IVH (Grade 3 and 4) did not differ between the two groups (animal derived 11.8% vs. synthetic 8.3%).
Moya 2005: Moya and coworkers compared the efficacy of lucinactant (Discovery Laboratories, Doylestown, PA), a synthetic surfactant containing a functional SP B mimic, with colfosceril palmitate (GlaxoSmithKline, Brentford, United Kingdom), a non‐protein containing synthetic surfactant, and beractant (Abbott Laboratories, Abbott Park, IL), a bovine derived surfactant, in the prevention of RDS. A total of 1294 very preterm infants weighing 600 to 1250 g and ≤ 32 weeks gestational age were randomly assigned to receive colfosceril palmitate (n = 509), lucinactant (n = 527), or beractant (n = 258) within 20 to 30 minutes after birth. Only the comparison groups receiving colfosceril palmitate and beractant were included in this review. Primary outcome measures were the rates of RDS at 24 hours and the rates of death related to RDS during the first 14 days after birth. All‐cause mortality rates, BPD rates, and rates of other complications of prematurity were pre‐specified secondary outcomes. The primary outcomes, air leaks, and causes of death were assigned, with pre‐specified definitions, by an independent, masked adjudication committee.
Selective treatment trials
Alvarado 1993 (abstract): conducted a randomized controlled trial of the modified bovine surfactant extract beractant (Survanta, Ross Laboratories) with synthetic surfactant colfosceril palmitate (Exosurf Neonatal, Burroughs Wellcome) in the treatment of very low birth weight infants with RDS. Sixty‐six infants with birth weights less than or equal to 1500 g who required assisted ventilation, supplemental oxygen greater than 40%, and had radiographic evidence of RDS were entered in the study. Infants received beractant (n = 33) or colfosceril palmitate (n = 33) by random assignment within 24 hours of birth, and received additional doses at 12 hour intervals (up to four doses) if the infants remained on assisted ventilation and required supplemental oxygen greater than 30%. Infants who received beractant and colfosceril palmitate were similar regarding birth weight (1000 ± 280 g and 990 ± 237 g respectively) and gestational age (27 ± 3 weeks and 27 ± 2 weeks), baseline clinical characteristics and ventilator settings. Mean time from birth to treatment was comparable between the two groups (beractant 5 ± 3 hours, colfosceril palmitate 4 ± 2 hours). The authors reported a significant improvement in the duration of mechanical ventilation, supplemental oxygen and hospital stay associated with beractant treatment.
Horbar 1993: conducted a multicenter randomized controlled trial to compare the efficacy of synthetic surfactant colfosceril palmitate (Exosurf Neonatal, Burroughs Wellcome) and modified bovine surfactant extract beractant (Survanta, Ross Laboratories) for the treatment of neonatal RDS. The study was conducted at 11 tertiary care university neonatal intensive care units participating in the National Institute of Child Health and Human Development Neonatal Research Network. Newborn infants (n = 617) weighing 501 to 1500 g with RDS who were receiving assisted ventilation with 30% oxygen or more within six hours of birth were enrolled. Infants were randomly assigned to receive up to four intratracheal doses of either colfosceril palmitate (n = 309) or beractant (n = 308). During the 72 hours after the first surfactant dose, the average fraction of inspired oxygen (FiO2) (± SEM) was 0.50 ± 0.01 for colfosceril palmitate and 0.42 ± 0.01 for beractant (difference 0.08, 95% CI 0.05 to 0.11); the average MAP (± SEM) was 7.64 ± 0.21 cm H2O for colfosceril palmitate and 6.93 ± 0.21 cm H2O for beractant (difference 0.71 cm H2O, 95% CI 0.13 to 1.29 cm H2O). There was no difference between the groups in the incidence of other neonatal morbidities or in the duration of hospitalization, assisted ventilation, or supplemental oxygen administration.
Pearlman 1993 (abstract): conducted a quasi‐randomized controlled trial of synthetic surfactant colfosceril palmitate (Exosurf Neonatal, Burroughs Wellcome) compared with modified bovine surfactant extract beractant (Survanta, Ross Laboratories) in the treatment of RDS. One hundred and twenty‐one premature infants with evidence of RDS were entered in the study. Infants received beractant (n = 57) or colfosceril palmitate (n = 64) on alternate months. Subsequent doses were given at the discretion of the attending physician. Infants who received beractant and colfosceril palmitate were similar regarding birth weight (1322 ± 751 g and 1229 ± 622 g respectively), gestational age (28.4 ± 4.2 weeks and 28.7 ± 3.6 weeks), and baseline Apgar scores. Each group received an equal number of doses (beractant 1.8 ± 1.0, colfosceril palmitate 2.0 ± 1.0). The authors reported no statistically significant differences in the duration of mechanical ventilation, clinical complications, or mortality.
Sehgal 1994: conducted a prospective, randomized, non‐blinded study to determine whether infants with RDS who were treated with modified bovine surfactant extract beractant (Survanta, Ross Laboratories) had earlier and larger responses in gas exchange when compared with similar infants treated with a synthetic surfactant colfosceril palmitate (Exosurf Neonatal, Burroughs Wellcome). Forty‐one infants weighing between 600 g and 1750 g at birth with RDS of sufficient severity to require assisted ventilation with an FiO2 > 0.39 were enrolled in the study and treated with surfactant from one to eight hours after birth. Infants were randomly selected to receive treatment with either colfosceril palmitate or beractant. Despite randomization, the beractant group was over represented with factors associated with greater severity of RDS (lower birth weight, more males, and fewer African Americans). No statistically significant difference was found in the primary outcome measure (arterial‐alveolar (a/A) PaO2 > 0.3 at 24 hours).
VT Oxford 1996, Vermont‐Oxford Neonatal Network: conducted a multicenter randomized controlled trial to compare the efficacy of synthetic surfactant colfosceril palmitate (Exosurf Neonatal, Burroughs Wellcome Co.) and modified bovine surfactant extract beractant (Survanta, Ross Laboratories) for the treatment of neonatal RDS. Premature infants (n = 1296) weighing 501 to 1500 g with RDS requiring assisted ventilation with 30% oxygen or more were enrolled within six hours of birth at 38 neonatal intensive care units participating in the Vermont Oxford Neonatal Network. Infants were randomly assigned to receive up to four intratracheal doses of the synthetic surfactant colfosceril palmitate (n = 644) or the modified bovine surfactant extract beractant (n = 652). The primary outcome measure was the occurrence of death or chronic lung disease (CLD) 28 days after birth. Death or CLD occurred in 57% of the infants treated with colfosceril palmitate and in 54% of those infants treated with beractant (RR 0.95; 95% CI 0.86 to 1.04). Infants with birth weights of 1001 to 1500 g who received beractant had a significantly lower risk of CLD or death at 28 days (27% vs. 34%; RR 0.78, 95% CI 0.60 to 0.99). Treatment with beractant led to significant improvement in several secondary outcome measures. Beractant treated infants received less supplemental oxygen and had lower mean airway pressures six and 72 hours after treatment. Beractant treated infants had significantly fewer pneumothoraces (9% vs. 15%; RR 0.60, 95% CI 0.44 to 0.81). There were no differences between the groups in the incidence of other neonatal complications.
Hudak 1996: conducted a randomized controlled trial to compare the efficacy and safety of a synthetic surfactant colfosceril palmitate (Exosurf Neonatal, Burroughs Wellcome) and a surfactant extract of calf lung lavage (Infasurf, Forest Laboratories) in the treatment of neonatal RDS. Infants with RDS who were undergoing mechanical ventilation were eligible for treatment with two doses of either a synthetic or natural surfactant if the a/A O2 ratio was less than or equal to 0.22. Crossover treatment was allowed within 96 hours of age if severe respiratory failure (defined as two consecutive a/A oxygen tension ratios ≤ 0.10) persisted after two doses of the randomly assigned surfactant. The trial was conducted at 21 centers. Investigators were unaware of treatment assignment. Primary outcome measures included the incidence of pulmonary air leak, the severity of RDS, the incidence of death from RDS, and the incidence of survival without BPD at 28 days after birth. The primary analysis of efficacy was performed in 1033 eligible infants and an analysis of safety outcomes in the 1126 infants who received study surfactant. Demographic characteristics and respiratory status were similar for the two treatment groups, except for a small but significant difference in mean gestational age (0.5 week) that favored the calf lung surfactant treatment group. Pulmonary air leak occurred in 21% of colfosceril palmitate treated infants and 11% of calf lung surfactant treated infants (adjusted RR 0.53; 95% CI 0.40 to 0.71). During the 72 hours after the initial surfactant treatment, infants who received calf lung surfactant had a lower average fraction of inspired oxygen and a lower average mean airway pressure. The incidence of RDS‐related death, total respiratory death, death prior to discharge, and survival without BPD at 28 days after birth did not differ. The number of days of more than 30% inspired oxygen and of assisted ventilation, but not the duration of hospitalization, were significantly lower in calf lung surfactant treated infants.
Modanlou 1997: conducted a clinical trial to compare the efficacy of a synthetic surfactant colfosceril palmitate (Exosurf Neonatal, Burroughs Wellcome) and a natural surfactant extract beractant (Survanta, Ross Laboratories) on the early course of RDS, arterial blood gases, ventilator support, outcome morbidity rate, and complications of prematurity. The trial included infants treated sequentially with colfosceril palmitate (when this was the only FDA approved surfactant preparation), and infants randomly assigned to either colfosceril palmitate or beractant (once beractant became available as an approved alternative). During the randomized phase of the study 61 infants were randomly assigned to receive colfosceril palmitate and 61 infants were assigned to beractant. Although the two randomized groups were similar in severity of RDS based on FiO2 and ventilator support, a significantly greater improvement in respiratory function (as evidenced by FiO2, mean airway pressure, a/A PaO2 difference, and oxygen index) was observed in the beractant group from 12 hours through 48 hours.
Murdoch 1998: Murdoch and colleagues sought to determine the effects of animal derived surfactant compared to synthetic surfactant on cerebral hemodynamics. Twenty preterm infants receiving mechanical ventilation were randomized to receive poractant alfa (Curosurf) or colfosceril palmitate (Exosurf Neonatal). Anterior cerebral artery blood flow velocity (CABFV) was measured using Doppler ultrasound before and up to two hours after treatment. Following animal surfactant treatment, there was a rapid reduction in CABF, whereas artificial surfactant resulted in a slower rise which was less marked. Animal derived surfactant produced rapid improvements in ventilation that were associated with marked alterations in cerebral haemodynamics. The only reported clinical outcomes were mortality and IVH. In a following study (Murdoch 2000), the investigators evaluated the effect of different surfactants on fluid balance. Data were collected on ventilatory parameters, daily urine output, daily weight, fluid intake and serum electrolytes. Ventilatory requirements decreased more rapidly in infants receiving animal derived surfactant, with significantly greater reductions in mean airway pressure from one to 48 hours. No other clinically relevant outcomes were reported.
daCosta 1999: conducted a randomized clinical trial to compare the effects of a synthetic surfactant colfosceril palmitate (Exosurf Neonatal, Burroughs Wellcome) and the modified natural surfactant extract beractant (Survanta, Ross Laboratories) in infants with neonatal RDS. Eighty‐nine patients were randomly allocated to receive one of the two surfactants. Primary outcome variables included both acute and long‐term effects of the surfactant preparations, specifically the oxygenation index at 24 hours and the combined incidence of CLD or death at 28 days. Oxygenation indices in the colfosceril palmitate and beractant groups at 24 hours were similar. However, the magnitude and rapidity of responses were greater for beractant than for colfosceril palmitate. When a/A oxygen tension ratios were compared, the colfosceril palmitate group had a significantly worse a/A ratio at 24 hours than the beractant group (0.21 vs. 0.37). The combined incidence of death or CLD was not different in the two groups (18.6% colfosceril palmitate vs. 15.2% beractant). There were no statistically significant differences in the incidence of other complications of prematurity.
Halahakoon 1999: As part of her PhD thesis, Halahakoon evaluated the effects of poractant alfa, beractant, and colfosceril palmitate (Exosurf Neonatal) on cerebral function, hypoxanthine levels, and antioxidant levels in 24 to 32 weeks gestation infants with RDS requiring assisted ventilation. In this single center, 39 preterm infants between 24 to 32 week gestation were randomized into three groups: poractant alfa at 100 mg/kg [n = 17, mean (SD) birth weight of 926 (278) gram, mean (SD) gestational age of 26.8 (2.4) weeks], colfosceril palmitate at 67.5 mg/kg [ n = 12, mean (SD) birth weight of 956 (233) gram, mean (SD) gestational age of 26.9 (1.9) weeks] and beractant at 100 mg/kg [n =10, mean (SD) birth weight of 1011 (327) gram, mean (SD) gestational age of 27.3 (2.0) weeks]. The study was initially designed to compare only two surfactants (poractant alfa and colfosceril palmitate) and later included beractant, hence differences in number of patients in each group. For purposes of this review, infants randomized to "any animal derived surfactant" (poractant alfa or beractant) was compared to the synthetic surfactant colfosceril palmitate. Method of randomization and any attempt for blinding was not reported. Envelopes were used for allocation concealment.
Lloyd 1999: conducted a study to determine the phospholipid composition of lung aspirates in infants that received colfosceril palmitate (Exosurf), an artificial surfactant, or beractant (Survanta), an animal derived surfactant, compared to an untreated control group of infants with 'normal lungs'. Infants less than 32 weeks gestation with RDS were randomly assigned to receive either colfosceril palmitate or beractant. Endotracheal or hypopharyngeal aspirates were obtained from these infants and from the control infants to determine the phospholipid composition of the lung fluid. The aspirates were taken prior to and up to 28 days following surfactant administration. The different phospholipids were separated by thin layer chromatography and expressed as a per cent of total phospholipid measured.
Infants with 'normal lungs' had a higher proportion of phosphatidylcholine prior to treatment than those with RDS. The infants with 'normal lungs' had a greater proportion of phosphatidylinositol in their lung aspirates than both treatment groups at 24 hours. Infants in the beractant group had a higher proportion of phosphatidylglycerol at 48 hours than the group with 'normal lungs'. No other differences were found in phospholipid composition up to 28 days. There were no major differences in the phospholipid profile in infants with RDS treated with either colfosceril palmitate or beractant.
Infants that received colfosceril palmitate had increased mean airway pressure and oxygen requirements at 24 hours when compared to beractant (8.1 cmH2O vs. 7.7 cmH2O, P < 0.02 and 34% vs. 28%, P < 0.006 respectively). The incidence of CLD at 36 weeks postconceptual age was not statistically significant between the groups. Neither the clinical differences initially seen between infants treated with colfosceril palmitate or beractant nor the long‐term outcomes could be explained by the phospholipid composition of serial samples of lung aspirates.
Ainsworth 2000: conducted a randomized controlled trial to compare a synthetic surfactant (pumactant, Britannia Pharmaceuticals) with a natural porcine surfactant (poractant alfa, Curosurf). Two hundred and twelve neonates born between 25 weeks and 29 weeks and six days gestation who required intubation were randomly assigned to poractant alfa (n = 105) or pumactant (n = 107). Outcome data were analyzed for 199 babies. The trial was stopped on the recommendation of the data and safety monitoring committee because of concern regarding increased mortality in the group receiving synthetic surfactant (poractant alfa 14% vs. pumactant 31%; odds ratio 0.37; 95% CI 0.18 to 0.76). This difference was sustained after adjustment for center, gestation, birth weight, sex, plurality, and use of antenatal steroids.
Kukkonen 2000: conducted a randomized controlled trial to compare the efficacy of a natural porcine surfactant (poractant, Curosurf) and the synthetic surfactant colfosceril palmitate (Exosurf Neonatal, Burroughs Wellcome). In three neonatal intensive care units, 228 neonates with respiratory distress and an a/A O2 ratio < 0.22 were randomly assigned to receive either poractant (100 mg/kg) or colfosceril palmitate (5 ml/kg). After poractant, the FiO2 was lower from 15 min (0.45 ± 0.22 vs. 0.70 ± 0.22, P = 0.0001) to six hours (0.48 ± 0.26 vs. 0.64 ± 0.23, P = 0.0001) and the mean airway pressure was lower at one hour (8.3 ± 3.2 mm H2O vs. 9.4 ± 3.1 mm H2O, P = 0.01). Thereafter, the respiratory parameters were similar. The duration of mechanical ventilation (median six vs. five days) and the duration of oxygen supplementation (median five vs. four days) were similar for poractant and colfosceril palmitate. An increased risk of bacteremia was associated with poractant treatment (11% vs. 4%; RR 3.17, 95% CI 1.05 to 9.52).
Summary
Timing of treatment
Hudak 1997 reported a comparison of calf lung derived surfactant and colfosceril palmitate in the context of prophylactic surfactant administration. Moya 2005 compared colfosceril palmitate, lucinactant or beractant administered within 20 to 30 minutes after birth. All other studies treated infants with signs and symptoms of RDS. Ainsworth 2000 treated infants requiring intubation for "presumed surfactant deficiency". These infants could be treated within 30 minutes of age. Other treatment studies relied on evidence of established RDS.
Entry criteria
In his prevention trial, Hudak 1997 attempted to enroll infants at high risk of developing RDS. Enrolled infants were less than 29 weeks gestation. Moya 2005 enrolled at risk infants between 24 and 32 weeks gestation with birth weights between 600 and 1250 g that had undergone endotracheal intubation.
In the treatment trials, Alvarado 1993, Horbar 1993, VT Oxford 1996 and Modanlou 1997 all studied infants with birth weight < 1500 g. The trials of Hudak 1996 and Pearlman 1993 studied premature infants without a specific birth weight limitation. Sehgal 1994 studied infants between birth weights 600 to 1750 g. Ainsworth studied infants born between 25 and 29 + 6 weeks gestation. All studies required that the infants be on assisted ventilation and have RDS. Ainsworth 2000 required only the clinician's assessment that the infant required intubation and had signs of respiratory distress. In other treatment studies, a variety of criteria for oxygen requirement at entry were used. Alvarado 1993 and daCosta 1999 required that infants be on supplemental oxygen > 40%. The study of Horbar 1993 and the VT Oxford 1996 study required that infants be on supplemental oxygen > 30%. Hudak 1996, Modanlou 1997, and Kukkonen 2000 required that infants demonstrate an a/A oxygen ratio of less than or equal to 0.22. This corresponded to being on approximately 40% supplemental oxygen. A variety of age criteria were set out by investigators. In the treatment trials, age at entry varied from within 30 minutes of birth (Ainsworth 2000) to 72 hours of age (Hudak 1996).
Surfactant preparation
In 12 of the included studies, the synthetic surfactant studied was colfosceril palmitate (Exosurf Neonatal, Burroughs Wellcome), a synthetic surfactant containing colfosceril palmitate, cetyl alcohol and tyloxapol. Ainsworth 2000 studied pumactant (Britannia Pharmaceuticals), a synthetic surfactant composed of dipalmitoyl phosphatidylcholine and phosphatidylglycerol. In eight of the studies, the animal derived surfactant extract tested was beractant (Survanta, Ross Laboratories), a modified bovine surfactant extract. Hudak 1996 and Hudak 1997 studied the bovine surfactant Infasurf (Forest Laboratories). Infasurf is obtained from lavage of calf lung. Unlike beractant, no supplemental lipids are added to this formulation. Ainsworth 2000 and Kukkonen 2000 studied the porcine surfactant poractant.
Study outcomes
The majority of studies included initial clinical improvement as well as a variety of complications of prematurity including pneumothorax, patent ductus arteriosus (PDA), pulmonary hemorrhage, necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), chronic lung disease (CLD), and mortality. This analysis focused on the major clinical outcomes described in these studies.
Risk of bias in included studies
Only randomized clinical trials which compared the effects of non‐protein containing synthetic surfactant to animal derived surfactant extract in preterm infants at risk for or having RDS were included in the analyses. Twenty‐two potentially relevant trials were identified. Eight trials were excluded from the final analysis. The studies of Cotton 1992, Rollins 1993, Stenson 1994, and Sanghvi 1998 were excluded because they were not randomized trials. Grauaug 1994, Choukroun 1994, and Bassiouny 1997 did not present relevant clinical outcomes for inclusion in the analysis. Sinha 2005 compared animal derived surfactant to protein containing synthetic surfactant. The remaining 14 studies were either random or quasi‐random in treatment assignment and reported on at least one relevant clinical outcome.
Methods of randomization
The methods of randomization were specified in 10 of the studies. Sehgal 1994, Hudak 1996, VT Oxford 1996, Hudak 1997, daCosta 1999, Halahakoon 1999, and Kukkonen 2000 all used sealed envelopes opened by the clinical investigators. Horbar 1993 used randomization lists at study center pharmacies. Modanlou 1997 used shuffled color‐coded cards. Ainsworth 2000 had a central telephone randomization system. Pearlman 1993 used a quasi‐randomized strategy allowing for alternate month treatment. The randomization methods used by Alvarado 1993 were not specified. Moya 2005 randomized all patients after birth in a masked manner using sealed envelopes with randomization codes stratified according to birth weight (600 to 800 g, 801 to 1000 g, or 1001 to 1250 g) that were computer generated by an independent, university based, statistical analysis center. Envelopes were opened sequentially as each new patient was randomized (Moya 2005). Infants were randomized to receive lucinactant, colfosceril palmitate, or beractant in a 2:2:1 ratio (Moya 2005).
Blinding
In both the prevention and treatment trials of the animal derived surfactant Infasurf (Hudak 1997), investigators not involved in clinical care administered the surfactant. In the trial of Alvarado 1993 investigators were "blinded" to treatment assignment; however, the methods of masking treatment were not described. In the other trials, treatment concealment was not attempted.
Effects of interventions
Animal derived surfactant extract versus protein free synthetic surfactant (Comparison 1)
Each of the studies comparing animal derived surfactant extract to synthetic surfactant reported greater improvement in the immediate need for respiratory support associated with treatment with animal derived surfactant extract. Relevant clinical outcomes are noted below.
Primary outcome measures
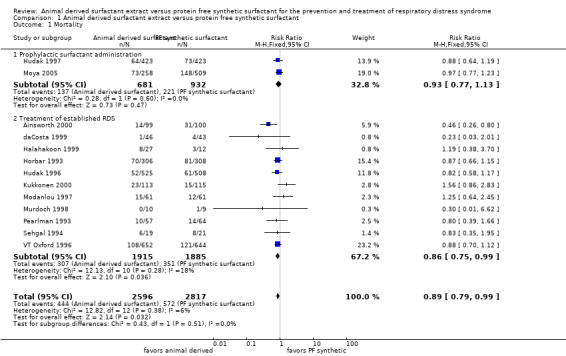
Mortality (Outcome 1.1)(Figure 1):
1.
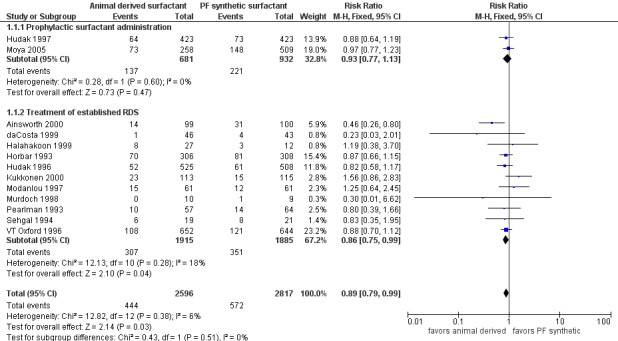
Forest plot of comparison: 1 Animal derived surfactant extract versus protein free synthetic surfactant, outcome: 1.1 Mortality.
Prevention trials
Hudak 1997 reported no decrease in the risk of mortality associated with the prophylactic use of animal derived surfactant (RR 0.88, 95% CI 0.64 to 1.19; RD ‐0.02, 95% CI ‐0.07 to 0.03). Moya 2005 did not find a significant increase in the risk of mortality with either surfactant preparation (RR 0.97, 95% CI 0.77 to 1.23; RD ‐0.01, 95% CI ‐0.08 to 0.06). Meta‐analysis of the prevention trials did not support an increase in mortality risk for either surfactant preparation (typical RR 0.93, 95% CI 0.77 to 1.13; typical RD ‐0.02, CI 95% ‐0.06 to 0.03; 2 studies, 1613 infants).
Treatment trials
Ainsworth 2000 reported a decreased risk of mortality associated with animal derived surfactant extract treatment. For the treatment trials, the meta‐analyses supported a marginal reduction in the risk of mortality (typical RR 0.86, 95% CI 0.75 to 0.99; typical RD ‐0.03, 95% CI ‐0.05 to ‐0.00; 11 studies, 3800 infants).
Overall, the meta‐analyses supported a marginal reduction in the risk of mortality (typical RR 0.89, 95% CI 0.79 to 0.99; typical RD ‐0.02, 95% CI ‐0.04 to ‐0.00; 13 studies, 5413 infants).
Bronchopulmonary dysplasia (BPD) (oxygen requirement at 28 days of life) (Outcome 1.2)(Figure 2):
2.
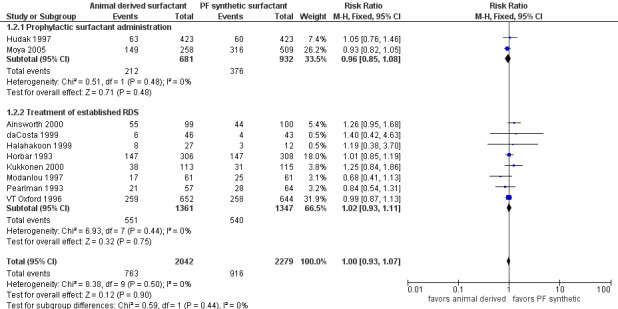
Forest plot of comparison: 1 Animal derived surfactant extract versus protein free synthetic surfactant, outcome: 1.2 Bronchopulmonary dysplasia.
Prevention trials
Hudak 1997 reported no significant effect of surfactant preparation on the risk of BPD (RR 1.05, 95% CI 0.76 to 1.46; RD 0.01, 95% CI ‐0.04 to 0.05). Moya 2005 did not support a significant effect of surfactant preparation on BPD (RR 0.93, 95% CI 0.82 to 1.05; RD ‐0.04, 95% CI ‐0.12 to 0.03). Meta‐analysis of the prophylactic studies did not demonstrate a significant effect of surfactant preparation on BPD (typical RR 0.96, 95% CI 0.85 to 1.08; typical RD ‐0.02, 95% CI ‐0.06 to 0.03; 2 studies, 1613 infants).
Treatment trials
None of the eight treatment trials that reported on the incidence of BPD noted an effect of surfactant preparation on the risk of BPD. The meta‐analysis of the treatment studies demonstrated no effect of surfactant preparation on the risk of BPD (typical RR 1.02, 95% CI 0.93 to 1.11; typical RD 0.01, 95% CI ‐0.03 to 0.04; 8 studies, 2798 infants).
Overall, the meta‐analyses supported no significant effect of surfactant preparation on the risk of BPD (typical RR 1.00, 95% CI 0.93 to 1.07; typical RD 0, 95% CI ‐0.03 to 0.03; 10 studies, 4321 infants).
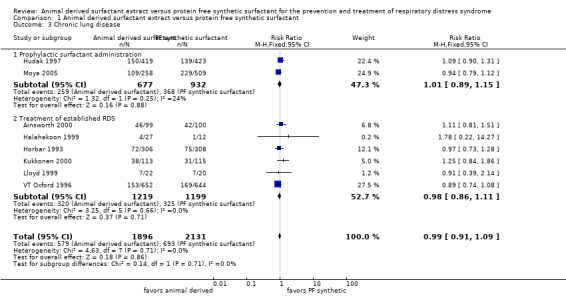
Chronic lung disease (CLD) (oxygen requirement at 36 weeks adjusted age) (Outcome 1.3)(Figure 3):
3.
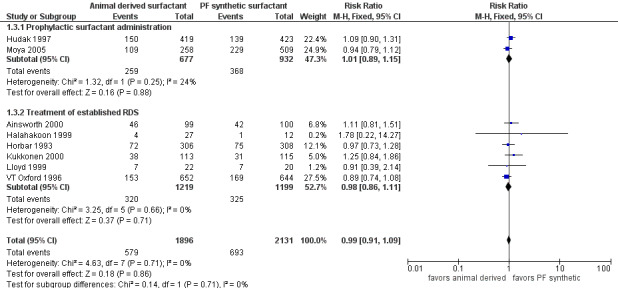
Forest plot of comparison: 1 Animal derived surfactant extract versus protein free synthetic surfactant, outcome: 1.3 Chronic lung disease.
Prevention trials
Hudak 1997 reported no significant effect of surfactant preparation on the risk of CLD (RR 1.09, 95% CI 0.90 to 1.31; RD 0.03, 95% CI ‐0.03 to 0.09). Prophylactic administration of either protein free synthetic surfactant or animal derived surfactant did not affect the risk of CLD in the Moya 2005 trial (RR 0.94, 95% CI 0.79 to 1.12; RD ‐0.03, 95% CI ‐0.10 to 0.05). Meta‐analysis of the prevention trials did not demonstrate any impact on the risk of CLD based on surfactant preparation (typical RR 1.01, CI 95% 0.89 to 1.15; typical RD 0.00, 95% CI ‐0.04 to 0.05; 2 studies, 1609 infants).
Treatment trials
None of the five treatment trials that reported on the incidence of CLD noted an effect of surfactant preparation on the risk of CLD. The meta‐analysis of the treatment studies demonstrated no effect of surfactant preparation on the risk of CLD (typical RR 0.98, 95% CI 0.86 to 1.11; typical RD ‐0.01, 95% CI ‐0.04 to 0.03; 6 studies, 2418 infants).
Overall, the meta‐analyses supported no statistically significant effect of surfactant preparation on the risk of CLD (typical RR 0.99, 95% CI 0.91 to 1.09; typical RD 0.00, 95% CI ‐0.03 to 0.03; 8 studies, 4027 infants).
Bronchopulmonary dysplasia (BPD) or death (Outcome 1.4):
Prevention trials
Hudak 1997 reported no statistically significant effect of surfactant preparation on the risk of BPD or mortality (RR 0.98, 95% CI 0.91 to 1.06; RD ‐0.01, 95% CI ‐0.07 to 0.04). Moya 2005 did not support an increase in the risk of BPD or mortality for either surfactant preparation (RR 0.94, 95% CI 0.83 to 1.06; RD ‐0.04, 95% CI ‐0.11 to 0.04). Meta‐analysis of the prevention trials did not demonstrate an increased risk of BPD or mortality (typical RR 0.96, 95% CI 0.90 to 1.03; typical RD ‐0.02, 95% CI ‐0.07 to 0.02).
Treatment trials
None of the four trials that reported on the incidence of BPD or mortality noted an effect of surfactant preparation on the risk of BPD or mortality. The meta‐analysis of the treatment studies demonstrated no effect of surfactant preparation on the risk of BPD or mortality (typical RR 0.94, 95% CI 0.88 to 1.01; typical RD ‐0.03, 95% CI ‐0.08 to 0.01).
Overall, the meta‐analyses supported a marginal decrease in the risk of BPD or mortality associated with the use of animal derived surfactant preparations (typical RR 0.95, 95% CI 0.91 to 1.00; typical RD ‐0.03, 95% CI ‐0.06 to 0.00).
Chronic lung disease (CLD) or mortality (Outcome 1.5):
Prevention trials
Hudak 1997 reported no significant effect of surfactant preparation on the risk of the combined outcome of CLD or mortality (RR 1.02, 95% CI 0.89 to 1.16; RD 0.01, 95% CI ‐0.06 to 0.08). Moya 2005 did not report any significant effect of surfactant preparation on the combined outcome of CLD or mortality (RR 0.95, 95% CI 0.80 to 1.12; RD ‐0.02, 95% CI ‐0.10 to 0.05). Meta‐analysis of the prevention trials did not demonstrate a significant increase in the risk of CLD or mortality with either synthetic or naturally derived surfactant preparations (typical RR 0.99, 95% CI 0.89 to 1.10; typical RD ‐0.01, 95% CI ‐0.06 to 0.04).
Treatment trials
Kukkonen 2000 noted a marginal increase in the risk of the combined outcome of CLD or mortality (RR 1.35, 95% CI 1.00 to 1.82; RD 0.13, 95% CI 0.00 to 0.26); whereas the Vermont Oxford Trial (VT Oxford 1996) noted a marginal decrease (RR 0.89, 95% CI 0.78 to 1.01; RD ‐0.05, 95% CI ‐0.10 to 0.00). The meta‐analysis of the treatment studies demonstrated no effect of surfactant preparation on the risk of CLD or mortality (typical RR 0.95, 95% CI 0.86 to 1.06; typical RD ‐0.02, 95% CI ‐0.07 to 0.02).
Overall, the meta‐analyses supported no significant effect of surfactant preparation on the risk of the combined outcome of CLD and mortality (typical RR 0.97, 95% CI 0.90 to 1.04; typical RD ‐0.01, 95% CI ‐0.05 to 0.02).
Secondary outcome measures
Pneumothorax (Outcome 1.6)(Figure 4):
4.
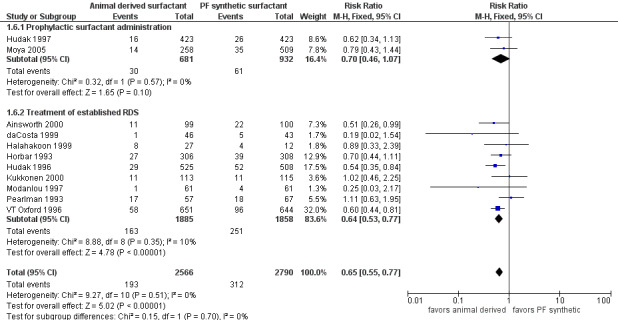
Forest plot of comparison: 1 Animal derived surfactant extract versus protein free synthetic surfactant, outcome: 1.6 Pneumothorax.
Prevention trials
Hudak (Hudak 1997) reported no difference in the risk of pneumothorax associated with the prophylactic use of animal derived surfactant (RR 0.62, 95% CI 0.34 to 1.13; RD ‐0.02, 95% CI ‐0.05 to 0.01). Moya and colleagues (Moya 2005) did not report any significant difference in pneumothorax (RR 0.79, 95% CI 0.43 to 1.44; RD ‐0.01, 95% CI ‐0.05 to 0.02). The meta‐analysis of both of these prophylactic trials (Hudak 1997; Moya 2005) did not show any significant difference in the risk of pneumothorax between the different surfactant preparations (typical RR 0.70, 95% CI 0.46 to 1.07; typical RD ‐0.02, 95% CI ‐0.04 to 0.00; 2 studies, 1613 infants).
Treatment trials
Hudak 1996, VT Oxford 1996, and Ainsworth 2000 reported a decreased incidence of pneumothorax associated with animal derived surfactant extract treatment. For the treatment trials, the meta‐analyses supported a significant reduction in the risk of pneumothorax (typical RR 0.64, 95% CI 0.53 to 0.77; typical RD ‐0.05, 95% CI ‐0.07 to ‐0.03; 9 studies, 3743 infants).
Overall, the meta‐analyses supported a significant reduction in the risk of pneumothorax (typical RR 0.65, 95% CI 0.55 to 0.77; typical RD ‐0.04, 95% CI ‐0.06 to ‐0.02; NNT 25; 11 studies, 5356 infants).
Patent ductus arteriosus (PDA) (Outcome 1.7):
Prevention trials
Hudak 1997 reported no significant effect of surfactant preparation on the risk of PDA (RR 0.97, 95% CI 0.85 to 1.09; RD ‐0.02, 95% CI ‐0.09 to 0.05; 1 study, 846 infants). Moya 2005 did not report on the incidence of PDA in either the animal derived surfactant or non‐protein synthetic surfactant groups.
Treatment trials
Ainsworth 2000 reported a marginal increase in the risk of PDA associated with animal derived surfactant extract treatment (RR 2.02, 95% CI 1.00 to 4.09; RD 0.10, 95% CI 0.00 to 0.20). For the treatment trials, the meta‐analysis demonstrated no effect of surfactant preparation on the risk of PDA (typical RR 0.99, 95% CI 0.90 to 1.08; typical RD ‐0.01, 95% CI ‐0.04 to 0.03; 7 studies, 2476 infants).
Overall, the meta‐analyses supported no significant effect of surfactant preparation on the risk of PDA (typical RR 0.98, 95% CI 0.91 to 1.05; typical RD ‐0.01, 95% CI ‐0.04 to 0.02; 8 studies, 3322 infants).
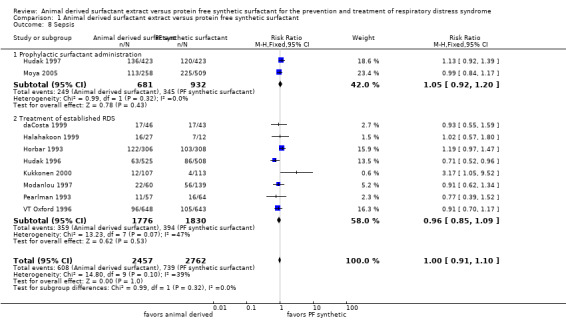
Sepsis (Outcome 1.8):
Prevention trials
Neither the trial of Hudak 1997 (RR 1.13, 95% CI 0.92 to 1.39; RD 0.04, 95% CI ‐0.02 to 0.10) nor the trial of Moya 2005 (RR 0.99, 95% CI 0.84 to 1.17; RD 0.00, 95% CI ‐0.08 to 0.07) reported a significant effect of surfactant preparation on the risk of sepsis. The meta‐analysis did not reveal an association of surfactant preparation and risk of sepsis (typical RR 1.05, 95% CI 0.92 to 1.20; typical RD 0.02, 95% CI ‐0.03 to 0.07; 2 studies, 1613 infants).
Treatment trials
Heterogeneous results were noted in the treatment trials. Hudak 1996 noted a decreased risk of sepsis associated with animal derived surfactant treatment (RR 0.71, 95% CI 0.52 to 0.96; RD ‐0.05, 95% CI ‐0.09 to ‐0.01). Kukkonen 2000 reported an increase in the risk of sepsis associated with animal derived surfactant extract treatment (RR 3.17, 95% CI 1.05 to 9.52; RD 0.08, 95% CI 0.01 to 0.15). For the treatment trials, no overall effect of surfactant preparation on the risk of sepsis was noted (typical RR 0.96, 95% CI 0.85 to 1.09; typical RD ‐0.01, 95% CI ‐0.03 to 0.02; 8 studies, 3606 infants).
Overall, the meta‐analyses supported no significant effect of surfactant preparation on the risk of sepsis (typical RR 1.00, 95% CI 0.91 to 1.10; typical RD 0.00, 95% CI ‐0.02 to 0.02; 10 studies, 5219 infants).
Necrotizing enterocolitis (NEC) (Bell's stage II or greater) (Outcome 1.9):
Prevention trials
Hudak (Hudak 1997) reported no difference in the risk of NEC associated with the prophylactic use of animal derived surfactant compared to protein free synthetic surfactant (RR 1.39, 95% CI 0.83 to 2.34; RD 0.02, 95% CI ‐0.01 to 0.05; 1 study, 846 infants).
Treatment trials
Six of the treatment trials reported on NEC (Horbar 1993; Pearlman 1993; VT Oxford 1996; Modanlou 1997; Ainsworth 2000; Kukkonen 2000). For the treatment trials, the meta‐analyses supported a significant increase in the risk of NEC (typical RR 1.37, 95% CI 1.04 to 1.81; typical RD 0.02, 95% CI 0.00 to 0.04, NNTH 50; 7 studies, 2616 infants).
Overall, the meta‐analyses supported a significant increase in the risk of NEC associated with the use of animal derived surfactants (typical RR 1.38, 95% CI 1.08 to 1.76; typical RD 0.02, 95% CI 0.01 to 0.04; NNTH 50; 8 studies, 3462 infants).
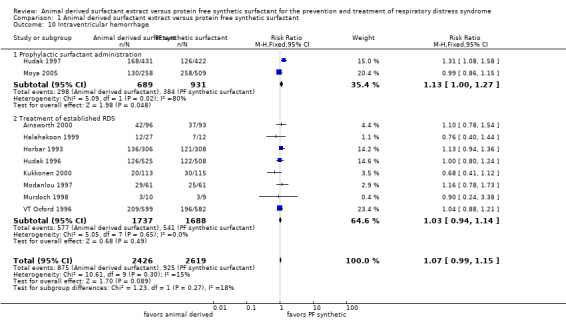
Intraventricular hemorrhage (IVH) (Outcome 1.10):
Prevention trials
Hudak 1997 reported an increase in the risk of IVH associated with animal derived surfactant administration (RR 1.31, 95% CI 1.08 to 1.58; RD 0.09, 95% CI 0.03 to 0.15). Moya 2005 did not support an increased risk of IVH associated with prophylactic administration of either synthetic or animal derived surfactant (RR 0.99, 95% CI 0.86 to 1.15; RD 0.00, 95% CI ‐0.08 to 0.07). The meta‐analyses demonstrated a borderline significance increase in the risk of developing of IVH with the administration of animal derived surfactant (typical RR 1.13, 95% CI 1.00 to 1.27; typical RD 0.05, 95% CI 0.00 to 0.10; 2 studies, 1620 infants).
Treatment trials
None of the six treatment trials that reported on IVH reported an effect of surfactant preparation on IVH. For the treatment trials, the meta‐analysis suggested no effect of surfactant preparation on the risk of IVH (typical RR 1.03, 95% CI 0.94 to 1.14; typical RD 0.01, 95% CI ‐0.02 to 0.04; 8 studies, 3425 infants).
Overall, the meta‐analyses supported a marginal increase in the risk of IVH associated with animal derived surfactant extract treatment (typical RR 1.07, 95% CI 0.99 to 1.15; typical RD 0.02, 95% CI 0.00 to 0.05; 10 studies, 5045 infants).
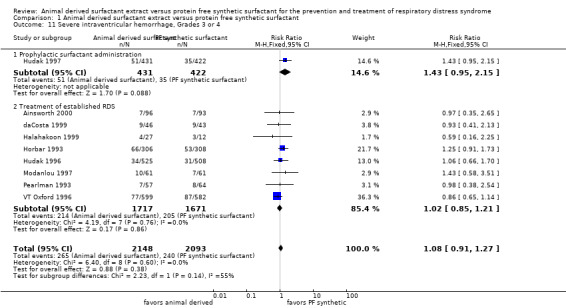
Severe intraventricular hemorrhage (IVH) (Grades 3 or 4) (Outcome 1.11):
Prevention trials
Hudak 1997 reported no significant effect of surfactant preparation on the risk of severe IVH (RR 1.43, 95% CI 0.95 to 2.15; RD 0.04, 95% CI 0.00 to 0.08; 1 study, 853 infants). Moya 2005 did not stratify the data based on severity of IVH.
Treatment trials
None of the seven trials that reported on the incidence of severe IVH noted an effect of surfactant preparation on the risk of severe IVH. For the treatment trials, no effect of surfactant preparation on the risk of severe IVH was noted (typical RR 1.02, 95% CI 0.85 to 1.21; typical RD 0.00, 95% CI ‐0.02 to 0.02; 8 studies, 3388 infants).
Overall, the meta‐analyses supported no significant effect of surfactant preparation on the risk of severe IVH (typical RR 1.08, 95% CI 0.91 to 1.27; typical RD 0.01, 95% CI ‐0.01 to 0.03; 9 studies, 4241 infants).
Retinopathy of prematurity (ROP) (Outcome 1.12):
Prevention trials
Hudak 1997 reported no significant effect of surfactant preparation on the risk of ROP (RR 0.96, 95% CI 0.87 to 1.06; RD ‐0.03, 95% CI ‐0.09 to 0.04). There was no significant difference between the animal derived surfactant and synthetic surfactant groups with respect to ROP in Moya 2005 (RR 0.93, 95% CI 0.72 to 1.20; RD ‐0.02, 95% CI ‐0.08 to 0.05). Meta‐analyses of the trials that used prophylactic surfactant administration did not demonstrate any difference in the risk of ROP (typical RR 0.95, 95% CI 0.86 to 1.05; typical RD ‐0.02, 95% ‐0.07 to 0.02; 2 studies, 1613 infants).
Treatment trials
None of the three trials that reported on ROP noted an association with surfactant preparation. The meta‐analysis of treatment trials demonstrated no effect of surfactant preparation on the risk of ROP (typical RR 0.94, 95% CI 0.86 to 1.03; typical RD ‐0.03, 95% CI ‐0.08 to 0.02; 4 studies, 1589 infants).
Overall, the meta‐analyses supported a trend toward decreased risk of ROP in infants receiving animal derived surfactants (typical RR 0.95, 95% CI 0.89 to 1.01; typical RD ‐0.03, 95% CI ‐0.06 to 0.01; 6 studies, 3202 infants).
Discussion
Non‐protein containing synthetic surfactants and animal derived surfactant extracts have both been proven to be effective in the prevention and treatment of RDS and have become widely available for clinical use (Soll 1992). Although clinical trials have demonstrated that both non‐protein containing synthetic surfactants and animal derived surfactant preparations are effective, comparison in animal models has suggested that there may be greater efficacy of animal derived surfactant products, perhaps due to the protein content of these surfactants (Tooley 1987). The randomized controlled trials that have been conducted comparing animal derived surfactant extract to non‐protein containing synthetic surfactant have universally demonstrated greater improvement in immediate need for ventilator support in infants who receive animal derived surfactant extracts (Soll 1992).
The meta‐analyses support a significant decrease in the risk of pneumothorax (typical RR 0.65, 95% CI 0.55 to 0.77; typical RD ‐0.04, 95% CI ‐0.06 to ‐0.02; NNT 25; 11 studies, 5356 infants) and a marginal decrease in the risk of mortality (typical RR 0.89, 95% CI 0.79 to 0.99; typical RD ‐0.02, 95% CI ‐0.04 to ‐0.00; 13 studies, 5413 infants) associated with animal derived surfactant treatment. In addition, the meta‐analyses support a marginal decrease in the risk of BPD or mortality associated with the use of animal derived surfactant preparations (typical RR 0.95, 95% CI 0.91 to 1.00; typical RD ‐0.03, 95% CI ‐0.06 to 0.00).
However, there is a trend toward an increased risk of IVH (typical RR 1.07, 95% CI 0.99 to 1.15; typical RD 0.02, 95% CI 0.00 to 0.05; 10 studies, 5045 infants) associated with animal derived surfactant extract treatment. The increased risk in overall IVH is not reflected in an increased risk of severe IVH (typical RR 1.08, 95% CI 0.91 to 1.27; typical RD 0.01, 95% CI ‐0.01 to 0.03; 9 studies, 4241 infants).This increased risk was noted in the initial randomized controlled trials and was thought to be related to changes in cerebral blood flow after surfactant administration (Gunkel 1993).
In this updated meta‐analysis, a concern regarding an increase in the risk of NEC is noted in association with animal derived surfactant treatment. However, this risk does not outweigh the more clinically important improvements seen in mortality. A rough estimate from these analyses project that for every 100 infants we treat with an animal derived product as opposed to a protein free synthetic product we see four fewer pneumothoraces, two fewer deaths but two additional cases of NEC and two additional cases of IVH of any grade.
Authors' conclusions
Implications for practice.
Both animal derived surfactant extracts and synthetic surfactant extracts are effective in the treatment and prevention of respiratory distress syndrome. Comparative trials demonstrate greater early improvement in the requirement for ventilator support, fewer pneumothoraces, and fewer deaths associated with animal derived surfactant extract treatment. A trend toward improved survival without bronchopulmonary dysplasia is noted. An increase in the risk of necrotizing enterocolitis is seen with animal derived products. In addition, an increase in the risk of intraventricular hemorrhage is seen with animal derived surfactant extract administration but is only reflected in the lesser grades of hemorrhage. On clinical grounds, animal derived surfactant extracts would seem to be the more desirable choice when compared to currently available synthetic surfactants.
Implications for research.
Clinical trials that compare newer synthetic surfactants such as lucinactant (KL4) to available animal derived surfactant extracts are needed.
What's new
| Date | Event | Description |
|---|---|---|
| 21 August 2015 | Amended | All forest plots changed from RD to RR. |
| 30 November 2014 | New citation required but conclusions have not changed | Search updated November 2014. |
History
Protocol first published: Issue 1, 1997 Review first published: Issue 1, 1997
| Date | Event | Description |
|---|---|---|
| 30 November 2014 | New search has been performed | This updates the review "Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome" published in the Cochrane Database of Systematic Reviews (Soll 2001). Title changed to "Animal derived surfactant extract versus protein free synthetic surfactant for the prevention and treatment of respiratory distress syndrome". Four additional studies added. Outcome of 'necrotizing enterocolitis' added. An increased risk of necrotizing enterocolitis (NEC) is noted in infants who received animal derived surfactant; however, the conclusions are largely unchanged. |
| 27 February 2008 | Amended | Converted to new review format. |
| 9 February 2001 | New search has been performed | Search of literature through December 2000 led to the inclusion of the following additional trials: Prevention: Hudak 1997. Selective treatment: daCosta 1999, Kukkonen 2000, Ainsworth 2000. Subgroup analyses added, by strategy of surfactant use: prophylactic, or selective treatment of infants with established RDS. |
| 9 February 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Dr Soll would like to acknowledge S Hayward for preparation of the review.
Data and analyses
Comparison 1. Animal derived surfactant extract versus protein free synthetic surfactant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 13 | 5413 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.79, 0.99] |
| 1.1 Prophylactic surfactant administration | 2 | 1613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.77, 1.13] |
| 1.2 Treatment of established RDS | 11 | 3800 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.75, 0.99] |
| 2 Bronchopulmonary dysplasia | 10 | 4321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.93, 1.07] |
| 2.1 Prophylactic surfactant administration | 2 | 1613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.85, 1.08] |
| 2.2 Treatment of established RDS | 8 | 2708 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.11] |
| 3 Chronic lung disease | 8 | 4027 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.09] |
| 3.1 Prophylactic surfactant administration | 2 | 1609 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.89, 1.15] |
| 3.2 Treatment of established RDS | 6 | 2418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.11] |
| 4 Bronchopulmonary dysplasia or death | 6 | 3811 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.91, 1.00] |
| 4.1 Prophylactic surfactant administration | 2 | 1613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.90, 1.03] |
| 4.2 Treatment of established RDS | 4 | 2198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.88, 1.01] |
| 5 Chronic lung disease or death | 5 | 3332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.90, 1.04] |
| 5.1 Prophylactic surfactant administration | 2 | 1609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.89, 1.10] |
| 5.2 Treatment of established RDS | 3 | 1723 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.06] |
| 6 Pneumothorax | 11 | 5356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.55, 0.77] |
| 6.1 Prophylactic surfactant administration | 2 | 1613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.46, 1.07] |
| 6.2 Treatment of established RDS | 9 | 3743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.53, 0.77] |
| 7 Patent ductus arteriosus | 8 | 3322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.05] |
| 7.1 Prophylactic surfactant administration | 1 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.85, 1.09] |
| 7.2 Treatment of established RDS | 7 | 2476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.90, 1.08] |
| 8 Sepsis | 10 | 5219 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.91, 1.10] |
| 8.1 Prophylactic surfactant administration | 2 | 1613 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.92, 1.20] |
| 8.2 Treatment of established RDS | 8 | 3606 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.85, 1.09] |
| 9 Necrotizing enterocolitis | 8 | 3462 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.08, 1.76] |
| 9.1 Prophylactic surfactant administration | 1 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.83, 2.34] |
| 9.2 Treatment of established RDS | 7 | 2616 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.04, 1.81] |
| 10 Intraventricular hemorrhage | 10 | 5045 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.99, 1.15] |
| 10.1 Prophylactic surfactant administration | 2 | 1620 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.00, 1.27] |
| 10.2 Treatment of established RDS | 8 | 3425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.94, 1.14] |
| 11 Severe intraventricular hemorrhage, Grades 3 or 4 | 9 | 4241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.91, 1.27] |
| 11.1 Prophylactic surfactant administration | 1 | 853 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.95, 2.15] |
| 11.2 Treatment of established RDS | 8 | 3388 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.21] |
| 12 Retinopathy of prematurity | 6 | 3202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.89, 1.01] |
| 12.1 Prophylactic surfactant administration | 2 | 1613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.86, 1.05] |
| 12.2 Treatment of established RDS | 4 | 1589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.86, 1.03] |
1.1. Analysis.
Comparison 1 Animal derived surfactant extract versus protein free synthetic surfactant, Outcome 1 Mortality.
1.2. Analysis.

Comparison 1 Animal derived surfactant extract versus protein free synthetic surfactant, Outcome 2 Bronchopulmonary dysplasia.
1.3. Analysis.
Comparison 1 Animal derived surfactant extract versus protein free synthetic surfactant, Outcome 3 Chronic lung disease.
1.4. Analysis.

Comparison 1 Animal derived surfactant extract versus protein free synthetic surfactant, Outcome 4 Bronchopulmonary dysplasia or death.
1.5. Analysis.

Comparison 1 Animal derived surfactant extract versus protein free synthetic surfactant, Outcome 5 Chronic lung disease or death.
1.6. Analysis.

Comparison 1 Animal derived surfactant extract versus protein free synthetic surfactant, Outcome 6 Pneumothorax.
1.7. Analysis.

Comparison 1 Animal derived surfactant extract versus protein free synthetic surfactant, Outcome 7 Patent ductus arteriosus.
1.8. Analysis.
Comparison 1 Animal derived surfactant extract versus protein free synthetic surfactant, Outcome 8 Sepsis.
1.9. Analysis.

Comparison 1 Animal derived surfactant extract versus protein free synthetic surfactant, Outcome 9 Necrotizing enterocolitis.
1.10. Analysis.
Comparison 1 Animal derived surfactant extract versus protein free synthetic surfactant, Outcome 10 Intraventricular hemorrhage.
1.11. Analysis.
Comparison 1 Animal derived surfactant extract versus protein free synthetic surfactant, Outcome 11 Severe intraventricular hemorrhage, Grades 3 or 4.
1.12. Analysis.

Comparison 1 Animal derived surfactant extract versus protein free synthetic surfactant, Outcome 12 Retinopathy of prematurity.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ainsworth 2000.
| Methods | Randomized multicenter trial Blinding of randomization: yes (central telephone randomization using sealed envelopes) Blinding of intervention: no Complete follow‐up: no (excluded 13 randomized ineligible infants) Blinding of outcome measurement: no Stratification by referral center | |
| Participants | Gestational age 25‐30 weeks Intubated for presumed surfactant deficiency Clinical signs of RDS No evidence of life‐threatening congenital malformation Pumactant n= 100 Curosurf (poractant alfa) n= 99 | |
| Interventions | Curosurf (poractant alfa) vs. pumactant Multiple doses | |
| Outcomes | PRIMARY: Days spent in "high dependency care" (including assisted ventilation, NCPAP, supplemental oxygen greater than 40%, thoracostomy tube, weight less than 1000 grams) SECONDARY: Neonatal mortality Complications of prematurity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central telephone randomization using sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome measurement: no |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow‐up: no (excluded 13 randomized ineligible infants) |
| Selective reporting (reporting bias) | Low risk | |
Alvarado 1993.
| Methods | Randomized single center trial Blinding of randomization: yes Blinding of intervention: can't tell Complete follow‐up: yes Blinding of outcome measurement: can't tell | |
| Participants | Birth weight less than 1500 grams Assisted ventilation Supplemental oxygen greater than 40% Respiratory distress syndrome Age less than 24 hours Colfosceril palmitate (Exosurf Neonatal) n=33 Beractant (Survanta) n=33 | |
| Interventions | Beractant (Survanta) vs. colfosceril palmitate (Exosurf Neonatal) Multiple doses | |
| Outcomes | Clinical Improvement Days on assisted ventilation Days on supplemental oxygen Days in hospital Mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: can't tell |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome measurement: can't tell |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | |
daCosta 1999.
| Methods | Randomized single center trial Blinding of randomization: yes (Sealed envelopes) Blinding of intervention: no Complete follow‐up: yes Blinding of outcome measurement: no Stratification: none stated | |
| Participants | Gestational age less than 37 weeks Birth weight greater than 999 grams Assisted ventilation Supplemental oxygen greater than or equal to 40% Mean airway pressure greater than or equal to 7.5 cm H2O Respiratory distress syndrome Age less than or equal to 8 hours No evidence of life‐threatening congenital malformation, sepsis, pulmonary hypoplasia, circulatory collapse, pneumothorax, IVH grade 3‐4 Colfosceril palmitate (Exosurf Neonatal) n= 43 Beractant (Survanta) n= 46 | |
| Interventions | Beractant (Survanta) vs. colfosceril palmitate (Exosurf Neonatal) Multiple doses | |
| Outcomes | PRIMARY: Oxygenation Index at 24 hours Death or chronic lung disease at 28 days SECONDARY: Ventilator requirement Complications of prematurity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes (sealed envelopes) Stratification: none stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome measurement: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | |
Halahakoon 1999.
| Methods | As part of her PhD thesis, Halahakoon and colleagues evaluated the effects of poractant, beractant and colfosceril palmitate (Exosurf Neonatal) | |
| Participants | Infants 24 to 32 weeks gestation with RDS requiring assisted ventilation and FiO2 > 0.4 at < 12 hours of age | |
| Interventions | Poractant alfa (n = 17), beractant (n = 10) and colfosceril palmitate (Exosurf neonatal) (n = 12) | |
| Outcomes | Primary outcomes: cerebral function, hypoxanthine levels and antioxidant levels. Also reported on common complications of prematurity and death. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes opened at the time of randomization |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Complete follow‐up: yes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No |
Horbar 1993.
| Methods | Randomized multicenter trial Blinding of randomization: yes (randomization lists at study center pharmacy) Blinding of intervention: no Complete follow‐up: no (3 excluded) Blinding of outcome measurement: no Stratification by birth weight | |
| Participants | Birth weight 501‐1500 grams Assisted ventilation Supplemental oxygen greater than or equal to 30% Respiratory distress syndrome Age less than or equal to 6 hours No mature L/S ratio No evidence of life‐threatening congenital malformation Colfosceril palmitate (Exosurf Neonatal) n= 309 Beractant (Survanta) n= 308 | |
| Interventions | Beractant (Survanta) vs. colfosceril palmitate (Exosurf Neonatal) Multiple doses | |
| Outcomes | PRIMARY: Death or BPD at 28 days Average FiO2, mean airway pressure SECONDARY: Complications of prematurity Complications associated with dosing | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes (randomization lists at study center pharmacy) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome measurement: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes (3 excluded) |
| Selective reporting (reporting bias) | Low risk | |
Hudak 1996.
| Methods | Randomized multicenter trial Blinding of randomization: yes (sealed envelopes) Blinding of intervention: yes Complete follow‐up: no (7 excluded) Blinding of outcome measurement: yes Stratification by birth weight | |
| Participants | Respiratory distress syndrome
Assisted ventilation
a/A ratio less than or equal to 0.22
Age less than 72 hours
No evidence of life‐threatening congenital malformation Colfosceril palmitate (Exosurf Neonatal) n=508 Infasurf n=525 |
|
| Interventions | Infasurf v. colfosceril palmitate (Exosurf Neonatal) Treatment crossover for persistent respiratory insufficiency allowed after second dose | |
| Outcomes | PRIMARY: Pulmonary airleak SECONDARY: Crossover treatment Severity of RDS Mortality Survival without chronic lung disease Complications of prematurity Days on assisted ventilation Days in oxygen Days in hospital | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes (sealed envelopes) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow‐up: no (7 excluded) |
| Selective reporting (reporting bias) | Low risk | |
Hudak 1997.
| Methods | Randomized multicenter trial Blinding of randomization: yes Blinding of intervention: yes Complete follow‐up: no (25 excluded) Blinding of outcome measurement: yes | |
| Participants | Gestational age less than or equal to 29 weeks Intubated and stabilized by 15 minutes of age No life‐threatening congenital anomaly colfosceril palmitate (Exosurf Neonatal) n=423 Infasurf n=423 | |
| Interventions | Infasurf vs. colfosceril palmitate (Exosurf Neonatal) Multiple doses Crossover treatment allowed for persistent respiratory insufficiency | |
| Outcomes | PRIMARY: Incidence of RDS Incidence of death due to RDS Survival without BPD at 28 days SECONDARY: Clinical Improvement Complications of prematurity Mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow‐up: no (25 excluded) |
| Selective reporting (reporting bias) | Low risk | |
Kukkonen 2000.
| Methods | Randomized multicenter trial (3 centers) Blinding of randomization: yes (sealed envelopes) Blinding of intervention: no Complete follow‐up: no (7 randomized infants excluded due to early mortality) Blinding of outcome measurement: no Stratification by birth weight, gender | |
| Participants | Newborn infants Assisted ventilation Respiratory distress syndrome a/A ratio < 0.22 No major congenital anomaly Colfosceril palmitate (Exosurf Neonatal) n=115 Curosurf n=113 | |
| Interventions | Curosurf vs. colfosceril palmitate (Exosurf Neonatal) Multiple doses | |
| Outcomes | PRIMARY: Duration of assisted ventilation Duration of supplemental oxygen SECONDARY: Complications of prematurity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes (sealed envelopes) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome measurement: no |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow‐up: no (7 randomized infants excluded due to early mortality) |
| Selective reporting (reporting bias) | Low risk | |
Lloyd 1999.
| Methods | Randomized controlled trial | |
| Participants | Inborn infants less than 32 weeks gestation with respiratory distress syndrome (RDS) were randomly assigned to receive either colfosceril palmitate (Exosurf Neonatal) an artificial surfactant, or beractant (Survanta) an animal derived surfactant | |
| Interventions | Colfosceril palmitate (Exosurf Neonatal) or beractant (Survanta) | |
| Outcomes | Endotracheal or hypopharyngeal aspirates were obtained from these infants and from control infants who had normal lungs. The aspirates were taken prior to and up to 28 days following surfactant administration. Phospholipids were separated by thin layer chromatography and expressed as a per cent of total phospholipid measured. Infants with normal lungs had a higher proportion of phosphatidylcholine than those with RDS prior to treatment. The infants with normal lungs had a greater proportion of phosphatidylinositol in their lung aspirates than both treatment groups at 24 h. Infants in the beractant (Survanta) group had a higher proportion of phosphatidylglycerol at 48 h than the group with normal lungs. No other differences were found in phospholipid composition up to 28 days. There were no major differences in the phospholipid profile in infants with RDS treated with either colfosceril palmitate (Exosurf Neonatal) or beractant (Survanta). Neither the clinical differences initially seen between infants treated with either colfosceril palmitate (Exosurf Neonatal) or beractant (Survanta) nor the long‐term outcome could be explained by the phospholipid composition of serial samples of lung aspirates | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome measurement: unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | High risk | Many clinically important outcome measures not reported |
Modanlou 1997.
| Methods | Randomized and historical controls (only randomized subjects included in this review), single center trial Blinding of randomization: yes Blinding of intervention: no Complete follow‐up: yes Blinding of outcome measurement: no | |
| Participants | Birth weight 500‐1500 grams Premature infants Assisted ventilation Respiratory distress syndrome Age less than or equal to 8 hours a/A ratio less than or equal to 0.22 or supplemental oxygen greater than or equal to 0.4 No evidence of life‐threatening congenital malformation Randomized infants: Colfosceril palmitate (Exosurf Neonatal) n= 61 Beractant (Survanta) n= 61 | |
| Interventions | Beractant (Survanta) vs. colfosceril palmitate (Exosurf Neonatal) Multiple doses | |
| Outcomes | Average FiO2 Mean airway pressure Duration of ventilation Duration of supplemental oxygen Mortality Complications of prematurity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome measurement: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | |
Moya 2005.
| Methods | Randomized multicenter trial Blinding of randomization: yes Blinding of intervention: yes Complete follow‐up: yes Blinding of outcome measurement: yes | |
| Participants | 1294 very preterm infants, weighing 600 to 1250 g and ≤32 weeks gestational age | |
| Interventions | Colfosceril palmitate (Exosurf Neonatal) (n = 509), lucinactant (n = 527), or beractant (Survanta) (n = 258) within 20 to 30 minutes after birth | |
| Outcomes | Primary outcome measures were the rates of RDS at 24 hours and the rates of death related to RDS during the first 14 days after birth. All‐cause mortality rates, BPD rates, and rates of other complications of prematurity were pre‐specified secondary outcomes. Primary outcomes, air leaks, and causes of death were assigned by an independent, masked, adjudication committee with pre‐specified definitions. The study was monitored by an independent data safety monitoring board | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Infants were randomized in a masked manner to receive colfosceril palmitate (Exosurf Neonatal), lucinactant, or beractant (Survanta) in a 2:2:1 ratio Computer generated randomization sequence Stratified by birth weight: 600‐800g; 801‐1000g; 1001‐1250g Sealed envelopes were provided to centers |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes (use of sealed opaque envelopes) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: partial. Drug administration team. Personel that prepared or administrated the surfactant preparations were not blinded but did not participate in the care of the infants after administration |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome measurement: yes. Only drug administration team aware of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | |
Murdoch 1998.
| Methods | Randomized single center Blinding of randomization: unclear Blinding of intervention: unclear Complete follow‐up: yes Blinding of outcome measurement: unclear | |
| Participants | Study limited to assessing the effects of animal and artificial surfactants on cerebral haemodynamics 20 premature infants born at 25 to 36 weeks gestation receiving mechanical ventilation were randomized to receive Curosurf or colfosceril palmitate (Exosurf Neonatal) surfactant |
|
| Interventions | Poractant (Curosurf) vs. colfosceril palmitate (Exosurf Neonatal) | |
| Outcomes | The primary outcome measured was the anterior cerebral artery blood flow velocity (CABFV) using Doppler ultrasound before and up to 2 hours after administration of either colfosceril palmitate (Exosurf Neonatal) or Curosurf. Secondary outcomes included oxygenation index (OI) | |
| Notes | Following animal surfactant there was a rapid reduction in CABFV (median ‐36%, range ‐43% to +8%, P < 0.01), whereas artificial surfactant resulted in a slower rise which was less marked (median +20%, range ‐7% to +62%, P < 0.05). There were no significant changes in blood pressure. Two hours after administration, the oxygenation index (OI) improved significantly only in babies receiving animal surfactant. In this group there was a significant association between the change in CABFV at 1 min and the change in OI at 2 h (n = 0.66, P < 0.05) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized single center trial |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomization: unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome measurement: unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | |
Pearlman 1993.
| Methods | Quasi randomized (alternate month strategy) Single center trial Blinding of randomization: no Blinding of intervention: no Complete follow‐up: yes Blinding of outcome measurement: no | |
| Participants | Premature infants Respiratory distress syndrome Colfosceril palmitate (Exosurf Neonatal) n=64 Beractant (Survanta) n=57 | |
| Interventions | Beractant (Survanta) vs. colfosceril palmitate (Exosurf Neonatal) | |
| Outcomes | Days on assisted ventilation Pulmonary hemorrhage Mortality Complications of prematurity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi randomized (alternate month strategy) |
| Allocation concealment (selection bias) | High risk | Blinding of randomization: no |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome measurement: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Low risk | |
Sehgal 1994.
| Methods | Randomized single center trial Blinding of randomization: yes (sealed envelopes) Blinding of intervention: no Complete follow‐up: no (1 excluded infant) Blinding of outcome measurement: no | |
| Participants | Birth weight 600 ‐ 1750 grams Assisted ventilation Supplemental oxygen greater than or equal to 40% Respiratory distress syndrome Age less than 8 hours No evidence of life‐threatening congenital malformation Colfosceril palmitate (Exosurf Neonatal) n= 21 Beractant (Survanta) n=19 | |
| Interventions | Beractant (Survanta) vs. colfosceril palmitate (Exosurf Neonatal) Multiple doses | |
| Outcomes | PRIMARY: Initial response (a/A ratio greater than 0.3 at 24 hours) SECONDARY: Complications of prematurity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes (sealed envelopes) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome measurement: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up: 1 excluded infant |
| Selective reporting (reporting bias) | Low risk | |
VT Oxford 1996.
| Methods | Randomized multicenter trial Blinding of randomization: yes (Sealed envelopes) Blinding of intervention: no Complete follow‐up: no (one center omitted from analysis n=22 infants) 32 randomized ineligible infants excluded Blinding of outcome measurement: no Stratification by birth weight | |
| Participants | Birth weight 501‐1500 grams Assisted ventilation Supplemental oxygen greater than or equal to 30% Respiratory distress syndrome Age less than or equal to 6 hours Excluded if known mature L/S ratio Colfosceril palmitate (Exosurf Neonatal) n=644 Beractant (Survanta) n=652 | |
| Interventions | Beractant (Survanta) vs. colfosceril palmitate (Exosurf Neonatal) Multiple doses | |
| Outcomes | PRIMARY: Death or chronic lung disease at 28 days SECONDARY: Complications of prematurity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes (sealed envelopes) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome measurement: no |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow‐up: no (one center omitted from analysis n = 22 infants) 32 randomized ineligible infants excluded |
| Selective reporting (reporting bias) | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bassiouny 1997 | No clinical outcomes given |
| Choukroun 1994 | No clinical outcomes given. Assessment limited to changes in pulmonary function |
| Cotton 1992 | Not assigned treatment by randomization |
| Grauaug 1994 | No clinical outcomes given |
| Rollins 1993 | Not assigned treatment by randomization |
| Sanghvi 1998 | Not assigned treatment by randomization |
| Sinha 2005 | Compares animal derived surfactant to protein containing synthetic surfactant |
| Stenson 1994 | Not assigned treatment by randomization |
Characteristics of studies awaiting assessment [ordered by study ID]
Giannakopoulou 2002.
| Methods | Unclear patient selection. Described as "randomly sampled from register numbers" |
| Participants | The study subjects were 92 premature newborns who had been hospitalized in the Department of Neonatology, of the University of Crete A total of 42 subjects received synthetic surfactant and 50 subjects received animal derived surfactant |
| Interventions | The surfactant was administered in one to three doses, depending on respiratory support requirements |
| Outcomes | The time of administration was a little longer for the animal derived surfactant group. The duration of mechanical ventilatory support, requiring oxygen, the duration of hospitalization and the percentage of increase of arterial alveolar partial pressure oxygen ratio (a/APO2) were slightly higher for the synthetic surfactant group The mortality rate during the neonatal period (28th day) was higher for the synthetic surfactant group than for the animal derived surfactant group (38.1 vs. 24%). A similar tendency was noticed also as regards to complications, e.g. pneumothorax (11.2 vs. 5.2%; relative risk (RR) 0.27) intraventricular hemorrhage (34.6 vs. 21.1%; RR 0.61), septicemia (11.5 vs. 5.2%; RR 0.46) and bronchopulmonary dysplasia (12.5 vs. 2.8%; RR 0.22) |
| Notes | Plan to contact authors re method of treatment assignment |
Peliowski 1998.
| Methods | Randomized controlled trial performed at 10 Canadian neonatal centers |
| Participants | Ventilated premature infants Birth weight 750 to 1250 g a/A ratio < 0.22 Stratified by weight |
| Interventions | Bovine surfactant (bLes) vs. colfosceril palmitate (Exosurf Neonatal) |
| Outcomes | Short term respiratory outcomes (FiO2, MAP, a/A ratio, oxygenation index) Clinical outcomes reported (but no specific data provided) |
| Notes | No data for clinically relevant outcome measures reported in the abstract |
Contributions of authors
Original review: Dr R Soll searched for trials, reviewed trials, excerpted data and wrote text. Dr F Blanco reviewed trials, excerpted data, and reviewed text.
Updated review: Stephanie Ardell (searched for trials, reviewed trials, excerpted data and wrote text); Robert Pfister (reviewed trials and wrote text), R Soll (searched for trials, reviewed trials, excerpted data and wrote text).
Sources of support
Internal sources
No sources of support supplied
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C.
Declarations of interest
Original review:
Dr R Soll has previously acted (over six years ago) as a paid consultant and invited speaker for several of the pharmaceutical companies that manufacture surfactant preparations (Abbott Laboratories, Ross Laboratories, Chiesi Pharmaceuticals, Dey Laboratories, Burroughs Wellcome). Dr. Soll is the principal investigator or co‐principal investigator of two of the randomized controlled trials cited in this review.
Dr F Blanco has no conflict of interests. He was an author of the previous version of this review and did not participate in the update.
Updated review:
Stephanie Ardell has no conflict of interests.
Robert Pfister has no conflict of interests.
Edited (no change to conclusions)
References
References to studies included in this review
Ainsworth 2000 {published data only}
- Ainsworth SB, Beresford MW, Milligan DW, Shaw NJ, Matthews JN, Fenton AC, et al. Pumactant and poractant alfa for treatment of respiratory distress syndrome in neonates born at 25‐29 weeks' gestation: a randomised trial. Lancet 2000;355(9213):1387‐92. [DOI] [PubMed] [Google Scholar]
Alvarado 1993 {published data only}
- Alvarado M, Hingre R, Hakason D, Gross S. Clinical trial of survanta versus exosurf in infants < 1500g with respiratory distress syndrome. Pediatric Research 1993;33:314A. [Google Scholar]
daCosta 1999 {published data only}
- daCosta DE, Pai MG, Al Khusaiby SM. Comparative trial of artificial and natural surfactants in the treatment of respiratory distress syndrome of prematurity: experiences in a developing country. Pediatric Pulmonology 1999;27(5):312‐7. [DOI] [PubMed] [Google Scholar]
Halahakoon 1999 {published data only}
- Halahakoon WL. A study of cerebral function following surfactant treatment for respiratory distress syndrome (Doctoral dissertation). Queen's University of Belfast (UK) 1999.
Horbar 1993 {published data only}
- Horbar JD, Wright LL, Soll RF, Wright EC, Fanaroff AA, Korones SB, et al. A multicenter randomized trial comparing two surfactants for the treatment of neonatal respiratory distress syndrome. National Institute of Child Health and Human Development Neonatal Research Network. Journal of Pediatrics 1993;123(5):757‐66. [DOI] [PubMed] [Google Scholar]
Hudak 1996 {published data only}
- Hudak ML, Farrell EE, Rosenberg AA, Jung AL, Auten RL, Durand DJ, et al. A multicenter randomized masked comparison trial of natural versus synthetic surfactant for the treatment of respiratory distress syndrome. Journal of Pediatrics 1996;128(3):396‐406. [DOI] [PubMed] [Google Scholar]
Hudak 1997 {published data only}
- Hudak ML, Martin DJ, Egan EA, Matteson EJ, Cummings NJ, Jung AL, et al. A multicenter randomized masked comparison trial of synthetic surfactant versus calf lung surfactant extract in the prevention of neonatal respiratory distress syndrome. Pediatrics 1997;100(1):39‐50. [DOI] [PubMed] [Google Scholar]
Kukkonen 2000 {published data only}
- Kukkonen AK, Virtanen M, Jarvenpaa AL, Pokela ML, Ikonen S, Fellman V. Randomized trial comparing natural and synthetic surfactant: increased infection rate after natural surfactant?. Acta Paediatrica 2000;89(5):556‐61. [DOI] [PubMed] [Google Scholar]
Lloyd 1999 {published data only}
- Lloyd J, Todd DA, John E. Serial phospholipid analysis in preterm infants: comparison of Exosurf and Survanta. Early Human Development 1999;54(2):157‐68. [DOI] [PubMed] [Google Scholar]
Modanlou 1997 {published data only}
- Modanlou HD, Beharry K, Padilla G, Norris K, Safvati S, Aranda JV. Comparative efficacy of Exosurf and Survanta surfactants on early clinical course of respiratory distress syndrome and complications of prematurity. Journal of Perinatology 1997;17(6):455‐60. [PubMed] [Google Scholar]
Moya 2005 {published data only}
- Moya F, Sinha S, Gadzinowski J, D'Agostino R, Segal R, Guardia C, et al. One‐year follow‐up of very preterm infants who received lucinactant for prevention of respiratory distress syndrome: results from 2 multicenter randomized, controlled trials. Pediatrics 2007;119(6):e1361‐70. [DOI] [PubMed] [Google Scholar]
- Moya FR, Gadzinowski J, Bancalari E, Salinas V, Kopelman B, Bancalari A, et al. A multicenter, randomized, masked, comparison trial of lucinactant, colfosceril palmitate, and beractant for the prevention of respiratory distress syndrome among very preterm infants. Pediatrics 2005;115(4):1018‐29. [DOI] [PubMed] [Google Scholar]
Murdoch 1998 {published data only}
- Murdoch E, Kempley ST. Randomized trial examining cerebral haemodynamics following artificial or animal surfactant. Acta Paediatrica 1998;87(4):411‐5. [DOI] [PubMed] [Google Scholar]
- Murdoch E, Kempley ST. The effects of synthetic and natural surfactant on fluid balance in acute respiratory distress syndrome. European Journal of Pediatrics 2000;159(10):767‐9 . [DOI] [PubMed] [Google Scholar]
Pearlman 1993 {published data only}
- Pearlman SA, Leef KH, Stefano JL, et al. A randomized trial comparing Exosurf versus Survanta in the treatment of neonatal RDS. Pediatric Research 1993;33:340A. [Google Scholar]
Sehgal 1994 {published data only}
- Sehgal SS, Ewing CK, Richards T, Taeusch HW. Modified bovine surfactant (Survanta) versus a protein free surfactant (Exosurf) in the treatment of respiratory distress syndrome in preterm infants: a pilot study. Journal of the National Medical Association 1994;86(1):46‐52. [PMC free article] [PubMed] [Google Scholar]
VT Oxford 1996 {published data only}
- The Vermont Oxford Neonatal Network. A multicenter randomized trial comparing synthetic surfactant with modified bovine surfactant extract in the treatment of neonatal respiratory distress syndrome. Pediatrics 1996;97(1):1‐6. [PubMed] [Google Scholar]
References to studies excluded from this review
Bassiouny 1997 {published data only}
- Bassiouny MR, Remo C, Cherian E. Comparison of the changes in the a/A oxygen ratio after administration of two surfactants for the treatment of neonatal respiratory distress syndrome. Journal of Tropical Pediatrics 1997;43(1):38‐41. [DOI] [PubMed] [Google Scholar]
Choukroun 1994 {published data only}
- Choukroun ML, Llanas B, Apere H, Fayon M, Galperine RI, Guenard H, et al. Pulmonary mechanics in ventilated preterm infants with respiratory distress syndrome after exogenous surfactant administration: A comparison between two surfactant preparations. Pediatric Pulmonology 1994;18(5):273‐8. [DOI] [PubMed] [Google Scholar]
Cotton 1992 {unpublished data only}
- Cotton RB, Law AB, Lindstrom DP, et al. Differential effects of synthetic and bovine surfactants on lung volume and oxygenation in premature infants with RDS [abstract]. Pediatric Research 1992;31:304A. [Google Scholar]
Grauaug 1994 {published data only}
- Grauaug A, Kohan R, Sly P, et al. Are there advantages of one over the other when used as rescue therapy [abstract]. Pediatric Research 1994;35:335A. [Google Scholar]
Rollins 1993 {published data only}
- Rollins M, Jenkins J, Tubman R, Corkey C, Wilson D. Comparison of clinical responses to natural and synthetic surfactants. Journal of Perinatal Medicine 1993;21(5):341‐7. [DOI] [PubMed] [Google Scholar]
Sanghvi 1998 {published data only}
- Sanghvi KP, Merchant RH. Single dose surfactant rescue therapy in neonatal respiratory distress syndrome. Indian Pediatrics 1998;35(6):533‐6. [PubMed] [Google Scholar]
Sinha 2005 {published data only}
- Sinha SK, Lacaze‐Masmonteil T, Valls i Soler A, Wiswell TE, Gadzinowski J, Hadju J, et al. A multicenter, randomized, controlled trial of lucinactant versus poractant alfa in very premature infants at high risk for respiratory distress syndrome. Pediatrics 2005;115(4):1030‐8. [DOI] [PubMed] [Google Scholar]
Stenson 1994 {published data only}
- Stenson BJ, Glover RM, Pappy GJ, Wilkie RA, Laing IA, Tarnow‐Mordi WO. Static respiratory compliance in the newborn. III. Early changes after exogenous surfactant treatment. Archives of Disease in Childhood 1994;70(1):F19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Giannakopoulou 2002 {published data only}
- Giannakopoulou C, Hatzidaki E, Korakaki E, Christodoulaki M, Margari KM, Mamoulakis D. Comparative randomized study: administration of natural and synthetic surfactant to premature newborns with respiratory distress syndrome. Pediatrics International 2002;44(2):117‐21. [DOI] [PubMed] [Google Scholar]
Peliowski 1998 {published data only}
- Peliowski A, Finer NN for the Canadian Surfactant Study Group. A randomized, blinded, Canadian multicenter trial to compare a bovine surfactant, bLES(R) (b), with a synthetic, Exosurf (E), for the rescue treatment of respiratory distress syndrome (RDS) in premature newborns ≤1250 g [abstract]. Pediatric Research 1998;43:293A. [Google Scholar]
Additional references
Abdel‐Latif 2011a
- Abdel‐Latif ME, Osborn DA. Laryngeal mask airway surfactant administration for prevention of morbidity and mortality in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD008309.pub2] [DOI] [PubMed] [Google Scholar]
Abdel‐Latif 2011b
- Abdel‐Latif ME, Osborn DA. Pharyngeal instillation of surfactant before the first breath for prevention of morbidity and mortality in preterm infants at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD008311.pub2] [DOI] [PubMed] [Google Scholar]
Abdel‐Latif 2012
- Abdel‐Latif ME, Osborn DA. Nebulised surfactant in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD008310.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aziz 2012
- Aziz A, Ohlsson A. Surfactant for pulmonary haemorrhage in neonates. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD005254.pub3] [DOI] [PubMed] [Google Scholar]
Bahadue 2012
- Bahadue FL, Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD001456.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clements 1977
- Clements JA. Functions of the alveolar lining. American Review of Respiratory Disease 1977;115(6 Pt 2):67‐71. [DOI] [PubMed] [Google Scholar]
El Shahed 2007
- Shahed AI, Dargaville P, Ohlsson A, Soll RF. Surfactant for meconium aspiration syndrome in full term/near term infants. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD002054.pub2] [DOI] [PubMed] [Google Scholar]
Gower 2008
- Gower WA, Wert SE, Nogee LM. Inherited surfactant disorders. NeoReviews 2008;9(10):458‐67. [Google Scholar]
Gunkel 1993
- Gunkel JH, Banks PL. Surfactant therapy and intracranial hemorrhage: review of the literature and results of new analyses. Pediatrics 1993;92(6):775‐86. [PubMed] [Google Scholar]
Hahn 2013
- Hahn S, Choi HJ, Soll R, Dargaville PA. Lung lavage for meconium aspiration syndrome in newborn infants. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD003486.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horbar 1993b
- Horbar JD, Wright EC, Onstad L. Decreasing mortality associated with the introduction of surfactant therapy: an observational study of neonates weighing 601‐1300 grams at birth. The Members of the National Institute of Child Health and Human Development Neonatal Research Network. Pediatrics 1993;92(2):191‐6. [PubMed] [Google Scholar]
ICCROP 2005
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology 2005;123(7):991‐9. [DOI] [PubMed] [Google Scholar]
Jobe 1993
- Jobe AH. Pulmonary surfactant therapy. New England Journal of Medicine 1993;328(12):861‐8. [DOI] [PubMed] [Google Scholar]
Jobe 2006
- Jobe A. Why surfactant works for respiratory distress syndrome?. NeoReviews 2006;7:e95‐106. [Google Scholar]
Nogee 1993
- Nogee LM, Mello DE, Dehner LP, Colten HR. Brief Report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. New England Journal of Medicine 1993;328(6):406‐10. [DOI] [PubMed] [Google Scholar]
Nogee 2004
- Nogee LM. Alterations in SP‐B and SP‐C expression in neonatal lung disease. Annual Review of Physiology 2004;66:601‐23. [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [DOI] [PubMed] [Google Scholar]
Pfister 2005
- Pfister RH, Soll RF. New synthetic surfactants: the next generation?. Biology of the Neonate 2005;87(4):338‐44. [DOI] [PubMed] [Google Scholar]
Pfister 2007
- Pfister RH, Soll RF, Wiswell T. Protein containing synthetic surfactant versus animal derived surfactant extract for the prevention and treatment of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006069.pub3] [DOI] [PubMed] [Google Scholar]
Pfister 2009
- Pfister RH, Soll R, Wiswell TE. Protein‐containing synthetic surfactant versus protein‐free synthetic surfactant for the prevention and treatment of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006180.pub2] [DOI] [PubMed] [Google Scholar]
Possmayer 1990
- Possmayer F. The role of surfactant‐associated proteins. American Review of Respiratory Disease 1990;142(4):749‐52. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rojas‐Reyes 2012
- Rojas‐Reyes MX, Morley CJ, Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD000510.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwartz 1994
- Schwartz RM, Luby AM, Scanlon JW, Kellogg RJ. Effect of surfactant on morbidity, mortality and resource use in newborns weighing 500 to 1500 g. New England Journal of Medicine 1994;330(21):1476‐80. [DOI] [PubMed] [Google Scholar]
Seger 2009
- Seger N, Soll R. Animal derived surfactant extract for treatment of respiratory distress syndrome. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007836] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soll 1992
- Soll RF, McQueen MC. Respiratory distress syndrome. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:325‐58. [Google Scholar]
Soll 2000a
- Soll RF. Synthetic surfactant for respiratory distress syndrome in preterm infants. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD001149] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soll 2000b
- Soll RF, Ozek E. Prophylactic natural surfactant extract for preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000511] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soll 2009
- Soll R, Ozek E. Multiple versus single doses of exogenous surfactant for the prevention or treatment of neonatal respiratory distress syndrome. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD000141.pub2] [DOI] [PubMed] [Google Scholar]
Soll 2010
- Soll R, Ozek E. Prophylactic protein free synthetic surfactant for preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD001079.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2007
- Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003063.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2012
- Tan K, Lai NM, Sharma A. Surfactant for bacterial pneumonia in late preterm and term infants. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008155.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tooley 1987
- Tooley WH, Clements JA, Muramatsu K, Brown CL, Schlueter MA. Lung function in prematurely delivered rabbits treated with a synthetic surfactant. American Review of Respiratory Disease 1987;136(3):651‐6. [DOI] [PubMed] [Google Scholar]
Warren 2009
- Warren JB, Anderson JM. Core Concepts: Respiratory Distress Syndrome. NeoReviews 2009;10(7):351‐61. [Google Scholar]
Whitsett 1995
- Whitsett JA, Nogee LM, Weaver TE, Horowitz AD. Human surfactant protein B: structure, function, regulation, and genetic disease. Physiological Reviews 1995;75(4):749‐57. [DOI] [PubMed] [Google Scholar]
Wright 1997
- Wright JR, Clements JA. Metabolism and turnover of lung surfactant. American Review of Respiratory Disease 1987;136(2):426‐44. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Soll 1997
- Soll RF. Natural surfactant extract vs synthetic surfactant in the treatment of established respiratory distress syndrome. Cochrane Database of Systematic Reviews 1997, Issue 3. [DOI: 10.1002/14651858.CD000144] [DOI] [Google Scholar]
Soll 2001
- Soll RF, Blanco F. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD000144] [DOI] [PubMed] [Google Scholar]


