Abstract
OBJECTIVE
Therapeutic inertia threatens the potential long-term benefits of achieving early glycemic control after type 2 diabetes diagnosis. We evaluated temporal trends in second-line diabetes medication initiation among individuals initially treated with metformin.
RESEARCH DESIGN AND METHODS
We included data from 199,042 adults with type 2 diabetes in the U.S. Department of Veterans Affairs health care system initially treated with metformin monotherapy from 2005 to 2013. We used multivariable Cox proportional hazards and linear regression to estimate associations of year of metformin monotherapy initiation with time to second-line diabetes treatment over 5 years of follow-up (primary outcome) and with hemoglobin A1c (HbA1c) at the time of second-line diabetes treatment initiation (secondary outcome).
RESULTS
The cumulative 5-year incidence of second-line medication initiation declined from 47% among metformin initiators in 2005 to 36% in 2013 counterparts (P < 0.0001) despite a gradual increase in mean HbA1c at the end of follow-up (from 6.94 ± 1.28% to 7.09 ± 1.42%, Ptrend < 0.0001). In comparisons with metformin monotherapy initiators in 2005, adjusted hazard ratios for 5-year initiation of second-line diabetes treatment ranged from 0.90 (95% CI 0.87, 0.92) for 2006 metformin initiators to 0.68 (0.66, 0.70) for 2013 counterparts. Among those receiving second-line treatment within 5 years of metformin initiation, HbA1c at second-line medication initiation increased from 7.74 ± 1.66% in 2005 metformin initiators to 8.55 ± 1.92% in 2013 counterparts (Ptrend < 0.0001).
CONCLUSIONS
We observed progressive delays in diabetes treatment intensification consistent with therapeutic inertia. Process-of-care interventions early in the diabetes disease course may be needed to reverse adverse temporal trends in diabetes care.
Introduction
Diabetes affects more than 10% of adults in the U.S. (1,2) and was associated with an estimated $327 billion in direct and indirect costs in 2017 (3). The adverse health effects of diabetes are predominantly related to diabetes complications, including atherosclerotic cardiovascular disease, heart failure, kidney disease, eye disease, and peripheral neuropathy (1). Results of randomized controlled trials suggest that glucose control early in the diabetes disease course has beneficial short- and long-term effects on diabetes complications (4,5), a finding that has been corroborated in observational real-world data (6). Highlighting the importance of early glycemic control, results of randomized trials in patients with long-standing diabetes have suggested less substantial reductions in diabetes complications and potential for harm associated with intensive glycemic control (7–9).
Despite the importance of glycemic control early in the natural history of diabetes, delayed diabetes treatment intensification, so-called “therapeutic inertia,” has been described across multiple health care systems (10–17). Therapeutic inertia can manifest as delays in glycemic surveillance for treatment response (16) and as delays in treatment modification in the face of glucose levels above individualized targets (11,12,15–18). Critically, therapeutic inertia is associated with poor early glycemic control (16,17,19,20), which in turn has been associated with poor long-term glycemia and increased risk for diabetes complications later in the disease course (21,22).
In recent analyses of temporal trends in glycemic control among individuals with diabetes in the U.S., it was found that improvements observed in the 2000s appear to have stagnated (23–29). Similar descriptions of temporal trends in diabetes treatment, particularly early in the disease course, are needed for an understanding of gaps in diabetes care. We performed a retrospective observational cohort study to examine temporal trends in early diabetes treatment patterns for adults receiving care in the U.S. Department of Veterans Affairs health care system (VA), focusing on the transition from monotherapy with metformin, the most common initial medication for type 2 diabetes in the U.S. (30–32), to second-line treatment during the first 5 years of diabetes treatment. We describe trends in glycemic control at the time of metformin and of second-line treatment initiation, and in the time to second-line treatment initiation, among individuals initiating metformin monotherapy between 2005 and 2013 with 5 years of follow-up through 2018.
Research Design and Methods
Study Population
We included individuals receiving routine primary care across the national VA health care system who were diagnosed with diabetes and received initial treatment with a diabetes medication between 2005 and 2013. We limited study entry at 2013 to permit analysis of up to 6 years of follow-up data (through 2019), including the primary 5-year outcome and assessments of glycemia, death, and loss to follow-up through 6 years after initial diabetes treatment. We used a diabetes definition adapted from a validated algorithm for the VA (33) and identified newly diagnosed individuals initiating oral monotherapy for diabetes using an approach adapted from prior studies in the VA (34,35). Briefly, diabetes status was defined as two or more uses of ICD-9, Clinical Modification (ICD-9-CM), diagnosis codes 250.xx or one or more uses of 250.xx associated with a primary care provider visit in conjunction with an outpatient diabetes medication prescription. To be identified as newly diagnosed individuals receiving initial diabetes treatment, participants had to meet two criteria occurring in the 2 years prior to meeting the above diabetes diagnosis definition: 1) a minimum of two clinical encounters in the VA, one of which had to be a primary care provider encounter, without use of an ICD-9-CM diagnosis code for diabetes; and 2) at least one prescription medication filled in the VA for a nondiabetes medication. Thus, those remaining in the sample had data that suggested active VA care with neither recognized diabetes nor diabetes treatment in the 2 years prior to meeting the diabetes diagnostic criteria for inclusion in the study.
Initial diabetes treatment was based on prescription medication fills occurring on or after the first occurrence of a diabetes ICD-9-CM diagnosis code. Supplementary Table 1 lists medications by class that were included in defining diabetes treatment. Individuals prescribed metformin prior to the first occurrence of a diabetes diagnosis code were excluded. Initial metformin monotherapy was defined as a filled prescription for metformin without a filled prescription for any other diabetes medication or metformin combination medication within the subsequent 4 weeks. The date of the initial metformin prescription fill was considered the date of treatment initiation and served as the baseline for the study. We focused on individuals prescribed metformin for initial treatment of diabetes, as they represent 70% of VA patients with diabetes (Supplementary Table 2). The Colorado Multiple Institutional Review Board and the Research & Development Committee of the VA Eastern Colorado Healthcare System provided human subjects oversight and approval.
Outcomes and Predictors
Initiation of a second diabetes medication was the primary outcome of this study. The date of the first prescription filled for a non-metformin diabetes medication was considered the date of second-line treatment initiation. A small proportion of participants who received add-on treatment with multiple medications (N = 1,771, 0.9%) were excluded. Hemoglobin A1c (HbA1c) measurements at baseline (occurring within 1 year before or after metformin initiation), at the time of second-line treatment initiation, and at the end of follow-up were secondary outcomes. HbA1c surveillance during follow-up was an additional secondary outcome. HbA1c surveillance was defined as achieving at least one (“annual”) or two (“semiannual”) HbA1c measurements per year of follow-up between metformin initiation and second-line medication initiation or the end of follow-up—whichever occurred first—among individuals with a minimum of 6 months of follow-up.
The calendar year of metformin initiation was the primary predictor in this study, ranging from 2005 to 2013. For analyses related to second-line treatment initiation, age at baseline, sex, race (classified as Black, White, Hispanic, or other based on VA enrollment records), HbA1c at baseline, BMI at baseline, and creatinine at baseline were included as covariates. The nearest values occurring within 1 year before or after metformin initiation were considered baseline measurements. There was <5% missing data for all of the variables included in analyses (Supplementary Table 3).
Statistical Analysis
Study participant characteristics were summarized with means for continuous variables and proportions for categorical variables. We used multivariable linear regression to compare differences in HbA1c at baseline, at second-line treatment initiation, and at the end of follow-up across metformin initiation years. Analysis of residuals for multivariable linear regression analysis of HbA1c at second-line treatment initiation can be found in Supplementary Fig. 1. We used multivariable Cox proportional hazards regression to test associations of metformin initiation year with time to secondline treatment initiation. We examined second-line treatment initiation within 5 years of metformin, censoring individuals who died prior to second-line treatment initiation. We considered the date of the last HbA1c measurement occurring within 6 years of baseline as the end of follow-up. We visually assessed the proportional hazards assumption by examining a plot of Schoenfeld residuals versus time. The plot had a slope of near-zero (Supplementary Fig. 2), satisfying the proportional hazards assumption, though the P value was < 0.0001 with the Schoenfeld test, likely owing to the large sample size.
All multivariable models were adjusted for age at diabetes diagnosis, race, sex, BMI at baseline, and creatinine at baseline. Baseline HbA1c was an additional covariate in models with examination of HbA1c at and time to second-line treatment initiation. As smoking status and comorbidity burden may influence medication choice and intensity of glycemic control, we performed sensitivity analyses including smoking status and several comorbidities (coronary artery disease, stroke, congestive heart failure, kidney disease, liver disease, chronic obstructive pulmonary disease, and cancer) as covariates in multivariable models. We evaluated differences in the cumulative incidence of second-line treatment over 5 years by metformin initiation year using a log-rank test.
We repeated the multivariable Cox proportional hazards regression including a term for interaction between metformin initiation year and age at diabetes diagnosis (≤55 or >55 years) and after stratifying on age at diabetes diagnosis of 55 years. We selected age 55 years for stratification to capture a younger population of individuals with diabetes who would be <60 years of age at the end of 5 years of follow-up and likely to be eligible for lower individualized glycemic goals for the duration of follow-up. As a sensitivity analysis, we repeated the age-stratified analyses and age interaction term, stratifying at 50 years, 60 years, and 65 years. Finally, we performed a sensitivity analysis including an interaction term between metformin initiation year and cancer status at baseline and after stratifying on presence/absence of a history of cancer at baseline.
We considered P < 0.05 to imply statistical significance in each analysis. All analyses were performed in R 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Data and Resource Availability
The data and all statistical code that support this study are available on reasonable request to the corresponding author and on obtaining required regulatory approvals according to current VA guidelines. Due to the sensitivity of the clinical data collected for this study, data requests must be from qualified researchers with approved human subjects research protocols, and all data will be provided as a deidentified limited data set.
Results
Study Participants and Second-line Diabetes Treatment Patterns
A total of 206,841 individuals with diabetes were initially treated with metformin monotherapy from 2005 to 2013, of whom 199,042 individuals received at most one second-line agent and were included in the study. Median age was 62.6 years, >95% of the sample were men, and the majority were White (69.2%) but with increasing proportions of Black and Hispanic individuals over the period examined (Table 1). At baseline, participants had mildly adverse metabolic characteristics based on BMI, blood pressure, LDL cholesterol, HDL cholesterol, HbA1c, and renal function, and these cardiometabolic variables were relatively stable across the years of metformin initiation (Table 1). Baseline prevalence of coronary artery disease declined from 31 to 24.9%, and prevalence of cancer increased from 28.2 to 37.7%, across consecutive annual cohorts of metformin initiators (Supplementary Table 4). There were smaller changes over time in congestive heart failure, stroke, liver disease, and chronic obstructive pulmonary disease prevalence across consecutive annual cohorts of metformin initiators (Supplementary Table 4). Median follow-up time was 270 weeks or just over 5 years, and 25% of participants had <5 years of follow-up. Individuals who died during follow-up or were lost to follow-up were slightly older and were more likely to have preexisting cardiovascular disease (coronary artery disease, stroke, or heart failure) and chronic obstructive pulmonary disease and less likely to have cancer at baseline (Supplementary Table 5). Among those in the study sample who initiated second-line treatment within 5 years of follow-up, thiazolidinedione medications declined, insulin and dipeptidyl peptidase 4 inhibitor use as second-line diabetes treatment increased, and sulfonylureas were the most frequently used second-line medication class over the study period (Supplementary Fig. 3).
Table 1.
Study participant characteristics
| Total | Metformin start year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | ||
| No. initiating metformin | 199,042 | 17,445 | 20,237 | 22,082 | 22,424 | 22,595 | 23,195 | 24,476 | 23,074 | 23,514 |
| Age (years) | 62.6 [56.3, 69.2] | 61.8 [56.0, 71.1] | 61.8 [56.4, 70.9] | 61.9 [56.9, 70.7] | 62.0 [57.0, 70.2] | 62.1 [56.4, 69.0] | 62.5 [56.1, 68.3] | 63.1 [56.3, 68.3] | 63.4 [56.0, 67.8] | 63.8 [55.8, 67.9] |
| Male sex | 190,710 (95.8) | 16,798 (96.3) | 19,481 (96.3) | 21,259 (96.3) | 21,640 (96.5) | 21,725 (96.1) | 22,165 (95.6) | 23,363 (95.5) | 22,019 (95.4) | 22,259 (94.7) |
| Race | ||||||||||
| Black | 30,935 (15.5) | 2,170 (12.4) | 2,603 (12.9) | 2,876 (13.0) | 3,081 (13.7) | 3,347 (14.8) | 3,838 (16.5) | 4,338 (17.7) | 4,221 (18.3) | 4,461 (19.0) |
| Hispanic | 11,485 (5.8) | 758 (4.3) | 1057 (5.2) | 1198 (5.4) | 1239 (5.5) | 1364 (6.0) | 1402 (6.0) | 1510 (6.2) | 1491 (6.5) | 1466 (6.2) |
| White | 137,799 (69.2) | 12,244 (70.2) | 14,299 (70.7) | 15,677 (71.0) | 15,898 (70.9) | 15,729 (69.6) | 15,913 (68.6) | 16,631 (67.9) | 15,563 (67.4) | 15,845 (67.4) |
| Other | 18,824 (9.5) | 2,273 (13.0) | 2,278 (11.3) | 2,331 (10.6) | 2,206 (9.8) | 2,155 (9.5) | 2,042 (8.8) | 1,997 (8.2) | 1,799 (7.8) | 1,742 (7.4) |
| Smoking status | ||||||||||
| Current | 57,980 (29.1) | 4,197 (24.1) | 5,063 (25.0) | 5,974 (27.1) | 6,439 (28.7) | 6,760 (29.9) | 7,118 (30.7) | 7,568 (30.9) | 7,343 (31.8) | 7,598 (32.3) |
| Former | 94,617 (47.5) | 9,519 (54.6) | 10,662 (52.7) | 10,908 (49.4) | 10,783 (48.1) | 10,443 (46.2) | 10,583 (45.6) | 11,143 (45.5) | 10,252 (44.4) | 10,277 (43.7) |
| Never | 46,329 (23.3) | 3,725 (21.4) | 4,502 (22.2) | 5,188 (23.5) | 5,194 (23.2) | 5,379 (23.8) | 5,486 (23.7) | 5,751 (23.5) | 5,469 (23.7) | 5,618 (23.9) |
| Missing | 116 (0.06) | 2 (0.01) | 1 (0.0) | 4 (0.02) | 8 (0.04) | 12 (0.05) | 19 (0.08) | 16 (0.07) | 25 (0.11) | 29 (0.12) |
| BMI (kg/m2) | 32.5 [29.0, 36.7] | 32.1 [28.7, 36.4] | 32.3 [28.8, 36.4] | 32.2 [28.7, 36.4] | 32.3 [28.8, 36.6] | 32.4 [28.9, 36.6] | 32.6 [29.1, 36.8] | 32.7 [29.1, 36.9] | 32.7 [29.2, 37.0] | 32.9 [29.4, 37.3] |
| SBP (mmHg) | 132 [122, 140] | 134 [123, 143] | 132 [122, 142] | 132 [122, 141] | 131 [122, 140] | 131 [122, 140] | 131 [122, 140] | 131 [122, 140] | 131 [122, 140] | 132 [122, 140] |
| DBP (mmHg) | 78 [70, 84] | 76 [69, 83] | 76 [69, 83] | 77 [70, 83] | 77 [70, 84] | 77 [70, 84] | 78 [70, 84] | 78 [70, 85] | 78 [71, 85] | 78 [71, 85] |
| HDL (mg/dL) | 38 [32, 45] | 38 [32, 45] | 38 [32, 45] | 37 [32, 44] | 37 [32, 44] | 37 [31, 44] | 37 [32, 44] | 38 [32, 45] | 38 [33, 45] | 39 [33, 46] |
| LDL (mg/dL) | 97 [78, 122] | 100 [80, 124] | 100 [80, 124] | 97.4 [78, 122] | 96 [77, 121] | 97 [77, 121] | 97 [77, 122] | 96 [77, 121] | 97 [77, 122.9] | 97 [77, 122] |
| TC (mg/dL) | 172 [148, 201] | 177 [153, 206] | 175 [151, 203] | 172 [148, 201] | 172 [148, 201] | 171 [147, 200] | 171 [147, 200] | 171 [147, 199] | 172 [148, 202] | 173 [148, 202] |
| TG (mg/dL) | 162 [112, 239] | 170 [117, 254] | 161 [111, 236] | 158 [109, 235] | 165 [115, 244] | 163 [113, 240] | 159 [111, 233] | 161 [112, 235] | 162 [113, 238] | 164 [113, 241] |
| FPG (mg/dL) | 137 [118, 166] | 140 [121, 171] | 138 [120, 166] | 138 [120, 165] | 136 [119, 163] | 138 [119, 166] | 136 [117, 165] | 135 [116, 165] | 136 [116, 167] | 135 [116, 166] |
| HbA1c (%) | 7.0 [6.5, 7.6] | 6.9 [6.3, 7.7] | 7.0 [6.4, 7.7] | 6.9 [6.4, 7.6] | 6.9 [6.4, 7.6] | 6.9 [6.4, 7.6] | 7.0 [6.5, 7.6] | 7.0 [6.6, 7.7] | 7.0 [6.6, 7.7] | 7.0 [6.6, 7.7] |
| eGFR (mL/min/1.73 m2) | 77.00 [66.95, 90.12] | 75.30 [65.23, 86.74] | 74.90 [65.07, 86.14] | 74.20 [64.21, 85.55] | 75.95 [66.17, 87.83] | 77.21 [67.27, 89.90] | 79.41 [67.74, 92.89] | 79.82 [68.05, 93.45] | 81.38 [69.31, 94.72] | 80.87 [68.96, 94.05] |
| Serum creatinine (mg/dL) | 1.00 [0.90, 1.10] | 1.00 [0.90, 1.20] | 1.00 [0.90, 1.20] | 1.00 [0.90, 1.20] | 1.00 [0.90, 1.10] | 1.00 [0.90, 1.10] | 1.00 [0.87, 1.10] | 1.00 [0.86, 1.10] | 0.99 [0.85, 1.10] | 0.99 [0.85, 1.10] |
| Follow-up time (weeks) | 269.86 [259.29, 281.57] | 269.14 [255.00, 280.86] | 269.57 [258.00, 281.14] | 269.86 [257.29, 281.57] | 269.71 [257.00, 282.00] | 270.14 [260.86, 282.29] | 270.29 [260.86, 282.29] | 270.14 [260.86, 282.00] | 269.71 [259.14, 281.29] | 269.57 [260.00, 281.00] |
Data are n (%) or median [interquartile range] unless otherwise indicated. DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL, HDL cholesterol; LDL, LDL cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
Temporal Trends in Glycemia
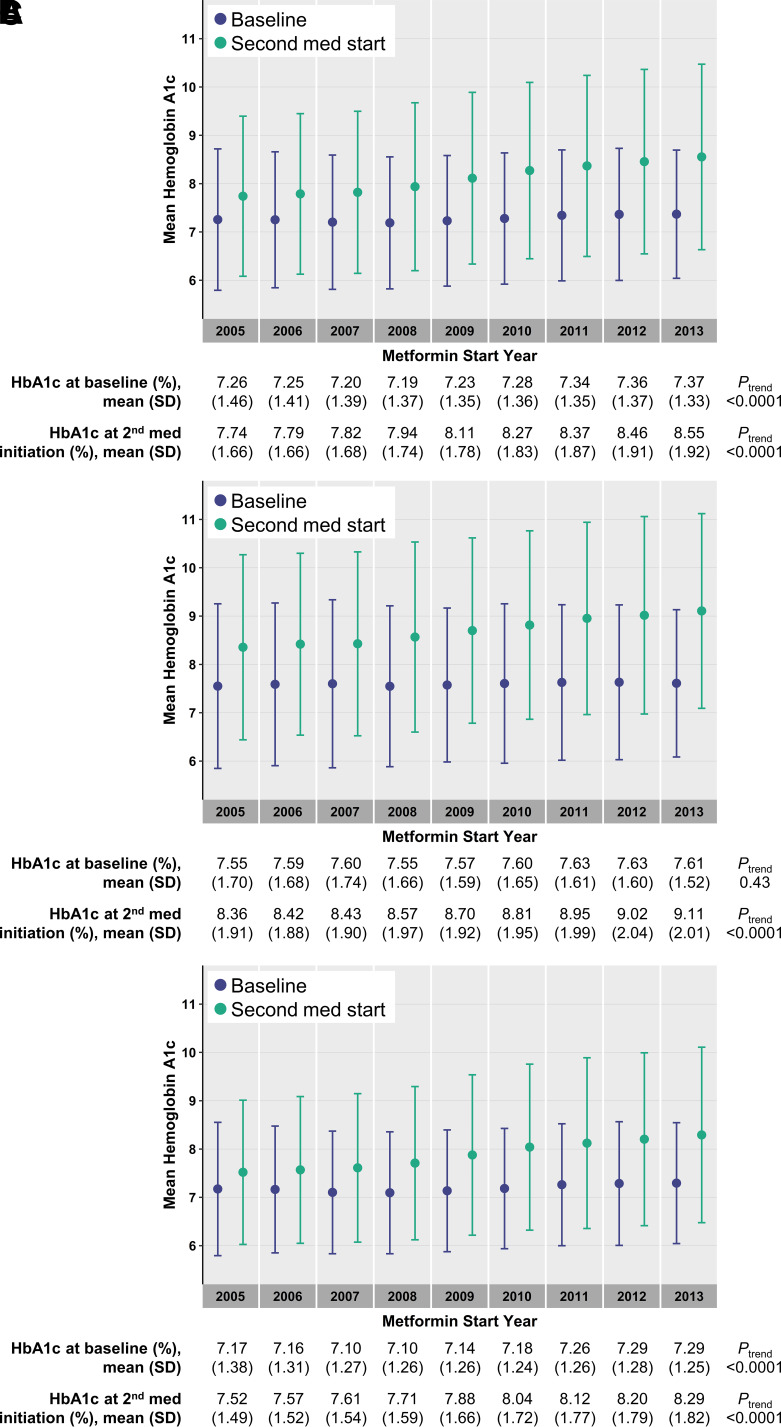
Mean HbA1c at baseline ranged from 7.19 ± 1.37% to 7.37 ± 1.33% (55 ± 10 to 57 ± 10 mmol/mol) with the lowest occurring among metformin initiators in 2008 and the highest for counterparts in 2013. There was a gradual trend of higher baseline HbA1c for each metformin initiation year (0.014% per year higher HbA1c, adjusted Ptrend < 0.0001) (Fig. 1A). Mean HbA1c at initiation of a second diabetes medication ranged from 7.74 ± 1.66% to 8.55 ± 1.92% (61 ± 13 to 70 ± 16 mmol/mol) among metformin initiators in 2005 and 2013, respectively (adjusted difference of 0.64%, P < 0.0001). Metformin initiation year was associated with higher HbA1c at second-line treatment initiation (0.086% per year higher HbA1c, adjusted Ptrend < 0.0001) (Fig. 1A). Among those ≤55 years old at the time of metformin initiation, mean HbA1c at second-line treatment initiation increased from 8.36 ± 1.91% to 9.11 ± 2.01% (68 ± 16 to 76 ± 17 mmol/mol) among metformin initiators in 2005 and 2013 (0.089% per year increase, adjusted Ptrend < 0.0001) (Fig. 1B). Among those >55 years old at the time of metformin initiation, mean HbA1c at second-line treatment initiation increased from 7.52 ± 1.49% to 8.29 ± 1.82% (59 ± 12 to 67 ± 15 mmol/mol) among metformin initiators in 2005 and 2013 (0.085% per year increase, adjusted Ptrend < 0.0001) (Fig. 1C). Similar trends in mean HbA1c at the time of second-line treatment initiation were observed in sensitivity analyses including a range of ages for stratification from 50 years old to 65 years old (Supplementary Table 6). In the full sample and in both age strata, HbA1c at initiation of second-line treatment increased more steeply over time than baseline HbA1c across annual cohorts of metformin monotherapy initiators (Fig. 1). Similar patterns in HbA1c trends were observed in sensitivity analyses with baseline HbA1c defined as values occurring between 1 year before and 1 month after metformin initiation (Supplementary Fig. 4).
Figure 1.
Trends in HbA1c at baseline and at time of initiation of second diabetes medication. Points and vertical bars represent the mean and SD of HbA1c for each year in the full sample (A) and among individuals initially treated with metformin at ages ≤55 years (B) and >55 years (C). Baseline values are shown in purple, and values nearest to the start of second-line diabetes treatment in those who were prescribed second-line treatment within 5 years are shown in green. The mean and SD values corresponding to each plotted point are shown below the plots, along with P values for temporal trends from models with adjustment for sex, race, and baseline age, HbA1c, creatinine, and BMI.
On average, HbA1c at the end of follow-up ranged from 6.94 ± 1.28% to 7.09 ± 1.42% (52 ± 10 to 54 ± 11 mmol/mol) among individuals initiating metformin monotherapy in 2005 and 2013, respectively (Ptrend < 0.0001) (Supplementary Table 7). Metformin initiation year was associated with higher mean HbA1c at the end of follow-up for those who received second-line treatment and for those who did not receive second-line treatment (Ptrend < 0.0001 for both groups) (Supplementary Table 7). Among individuals who did not receive second-line treatment, the proportion with HbA1c <7% at the end of follow-up decreased from 85.6 to 77.1% among metformin monotherapy initiators from 2005 to 2013 and the proportions with HbA1c 7–8% (from 11.6 to 17.6%), HbA1c 8–9% (from 1.8 to 3.6%), and HbA1c ≥9% (from 1.0 to 1.7%) increased (Supplementary Table 7).
Temporal Trends in Time to Second-line Diabetes Treatment
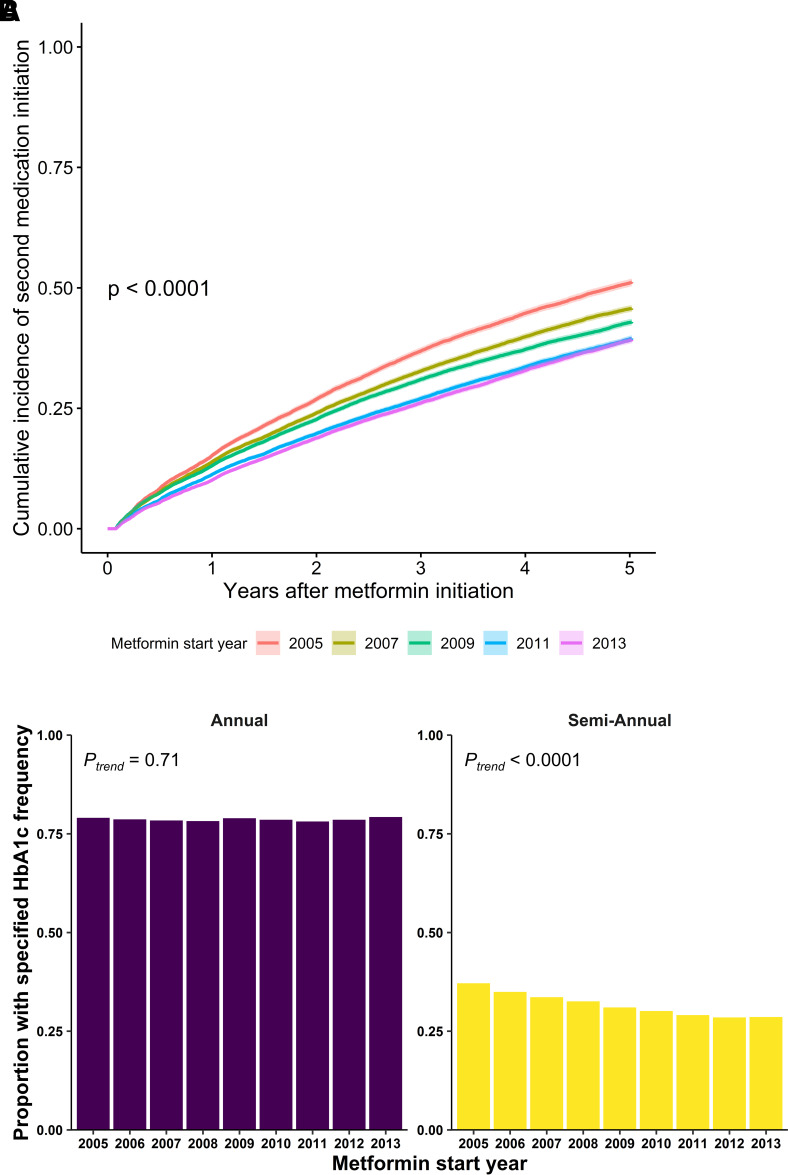
The year of metformin monotherapy initiation was associated with the 5-year cumulative incidence of second-line diabetes treatment initiation, which declined from 47% for metformin monotherapy initiators in 2005 to 36% in 2013 (P < 0.0001) (Table 2, Fig. 2A, and Supplementary Fig. 5). Relative to initiators of metformin monotherapy in 2005, the adjusted hazard ratio (HR) of 5-year second-line treatment initiation declined for each annual cohort of metformin initiators (HR 0.90 [95% CI 0.87, 0.92] in 2006 to 0.68 [95% CI 0.66, 0.70] in 2013) (Table 2). The temporal trends in 5-year second-line diabetes treatment initiation relative to metformin monotherapy initiators in 2005 were similar in additional multivariable models that include smoking status and baseline comorbidities (Supplementary Table 8). The prevalence of cancer at baseline increased from 2005 to 2013 among metformin initiators, providing a possible reason—life-limiting illness—for less intensive diabetes treatment. The temporal trends in 5-year second-line diabetes treatment initiation relative to metformin monotherapy initiators in 2005 were similar for those without and with a history of cancer at baseline (Supplementary Table 9) (Pinteraction = 0.5 between baseline cancer status and metformin initiation year).
Table 2.
Temporal trends in 5-year diabetes treatment intensification among those initially treated with metformin monotherapy
| Metformin start year | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | |
| All participants | |||||||||
| n intensified treatment | 8,245 | 8,831 | 9,347 | 9,000 | 9,005 | 8,899 | 8,971 | 8,286 | 8,547 |
| Total N | 17,445 | 20,237 | 22,082 | 22,424 | 22,595 | 23,195 | 24,476 | 23,074 | 23,514 |
| % intensified treatment | 47.3 | 43.6 | 42.3 | 40.1 | 39.9 | 38.4 | 36.7 | 35.9 | 36.3 |
| Unadjusted model | |||||||||
| HR | Ref. | 0.89 | 0.86 | 0.80 | 0.79 | 0.74 | 0.70 | 0.68 | 0.69 |
| 95% CI | NA | 0.87, 0.92 | 0.83, 0.88 | 0.78, 0.82 | 0.77, 0.82 | 0.72, 0.77 | 0.68, 0.72 | 0.66, 0.70 | 0.67, 0.71 |
| P | NA | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Adjusted model* | |||||||||
| HR | Ref. | 0.90 | 0.87 | 0.83 | 0.81 | 0.75 | 0.70 | 0.67 | 0.68 |
| 95% CI | NA | 0.87, 0.92 | 0.85, 0.90 | 0.80, 0.85 | 0.79, 0.84 | 0.73, 0.78 | 0.68, 0.72 | 0.65, 0.70 | 0.66, 0.70 |
| P | NA | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| ≤55 years old | |||||||||
| n intensified treatment | 2,171 | 2,270 | 2,390 | 2,385 | 2,571 | 2,638 | 2,639 | 2,560 | 2,729 |
| Total N | 3,723 | 4,145 | 4,353 | 4,516 | 4,848 | 5,186 | 5,386 | 5,187 | 5,423 |
| % intensified treatment | 58.3 | 54.8 | 54.9 | 52.8 | 53.0 | 50.9 | 49.0 | 49.4 | 50.3 |
| Unadjusted model | |||||||||
| HR | Ref. | 0.91 | 0.90 | 0.86 | 0.87 | 0.81 | 0.76 | 0.76 | 0.78 |
| 95% CI | NA | 0.86, 0.96 | 0.85, 0.96 | 0.81, 0.92 | 0.82, 0.92 | 0.76, 0.85 | 0.72, 0.80 | 0.71, 0.80 | 0.74, 0.83 |
| P | NA | 0.001 | 0.0008 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Adjusted model* | |||||||||
| HR | Ref. | 0.91 | 0.90 | 0.86 | 0.87 | 0.80 | 0.76 | 0.74 | 0.77 |
| 95% CI | NA | 0.85, 0.96 | 0.85, 0.96 | 0.81, 0.91 | 0.82, 0.92 | 0.76, 0.85 | 0.72, 0.81 | 0.70, 0.78 | 0.73, 0.82 |
| P | NA | 0.002 | 0.0005 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| >55 years old | |||||||||
| n intensified treatment | 6,074 | 6,561 | 6,957 | 6,615 | 6,434 | 6,261 | 6,332 | 5,726 | 5,818 |
| Total N | 13,722 | 16,092 | 17,729 | 17,908 | 17,747 | 18,009 | 19,090 | 17,887 | 18,091 |
| % intensified treatment | 44.3 | 40.8 | 39.2 | 36.9 | 36.3 | 34.8 | 33.2 | 32.0 | 32.2 |
| Unadjusted model | |||||||||
| HR | Ref. | 0.89 | 0.85 | 0.78 | 0.77 | 0.72 | 0.68 | 0.65 | 0.64 |
| 95% CI | NA | 0.86, 0.92 | 0.82, 0.88 | 0.76, 0.81 | 0.74, 0.79 | 0.69, 0.74 | 0.65, 0.70 | 0.62, 0.67 | 0.62, 0.67 |
| P | NA | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Adjusted model* | |||||||||
| HR | Ref. | 0.89 | 0.87 | 0.81 | 0.79 | 0.74 | 0.68 | 0.65 | 0.64 |
| 95% CI | NA | 0.86, 0.93 | 0.84, 0.90 | 0.79, 0.84 | 0.76, 0.82 | 0.71, 0.76 | 0.66, 0.71 | 0.63, 0.67 | 0.62, 0.67 |
| P | NA | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Adjusted models included adjustment for sex, race, baseline age, HbA1c, creatinine, and BMI. NA, not applicable; Ref., referent.
Figure 2.
Trends in time to second-line diabetes treatment initiation and in glycemic surveillance among patients initially treated with metformin monotherapy in 2005–2013. A: Cumulative incidence of initiation of second diabetes medication over 5 years by year of initial metformin monotherapy for diabetes (2005, 2007, 2009, 2011, and 2013). Log-rank test P value for differences in cumulative incidence by initial metformin treatment year is shown. For figure clarity, only odd-numbered years are shown; a similar plot with all years can be found in Supplementary Fig. 3. B: Proportions of patients with diabetes initially treated with metformin with at least one (annual) or two (semiannual) HbA1c measurements per year between metformin initiation and second-line medication initiation or end of follow-up.
We found a significant interaction between age-group and metformin initiation year for the hazard of 5-year second-line diabetes treatment initiation (Pinteraction < 0.0001). The cumulative incidence of 5-year second-line treatment initiation was lower among those ages >55 years (ranging from 44 to 32% of metformin monotherapy initiators in 2005 and 2013, respectively) than among those ages ≤55 years old (ranging from 58 to 50% among metformin monotherapy initiators in 2005 and 2013) (Table 2). The association of metformin initiation year with 5-year second-line treatment initiation was observed in both age strata (P < 0.0001 in both age-groups) (Table 2 and Supplementary Fig. 6). Similar trends in 5-year second-line diabetes treatment initiation relative to metformin initiators in 2005 were observed in sensitivity analyses including a range of ages for stratification from 50 years old to 65 years old, and interactions of dichotomized age with year of metformin initiation were significant irrespective of stratification age (Supplementary Table 10).
Finally, we examined trends in glycemic surveillance in the interval between metformin monotherapy initiation and end of follow-up or second-line treatment initiation. The proportion of individuals with at least one HbA1c measurement per year in the interval between metformin initiation and second-line treatment initiation or end of follow-up was stable among metformin initiators from 2005 (79.0%) to 2013 (79.2%) (Ptrend = 0.71), while the proportion with at least two HbA1c measurements per year declined from 37.1 to 28.6% (Ptrend < 0.0001) (Fig. 2B). Temporal trends in HbA1c surveillance were similar among individuals ≤55 and >55 years old at baseline (Supplementary Fig. 7).
Conclusions
In this retrospective observational study examining second-line diabetes treatment trends in the VA, we found significant increases over time in glycemia at the time of second-line treatment initiation and decreases in cumulative incidence of second-line treatment initiation among individuals initially treated with metformin monotherapy. Temporal variation in second-line treatment initiation was unlikely to be explained solely by changes in patient characteristics over time, as glycemia, lipid levels, blood pressure, and renal function within a year before or after metformin monotherapy initiation were mostly stable over time. In addition, the proportion of individuals with HbA1c <8% at the end of follow-up who did not receive second-line diabetes treatment was stable across consecutive cohorts of metformin initiators, suggesting that improvements in glycemic control on metformin did not explain declines in second-line diabetes treatment initiation. Early glycemic control impacts long-term diabetes-related complications (5,6,21,22). The increases in HbA1c at metformin initiation from 2005 to 2013, at second-line treatment initiation, and in time to second-line treatment initiation over successive years portend potentially adverse long-term population health for veterans with diabetes.
The temporal trend in younger individuals was particularly striking, with the mean HbA1c exceeding 9% at the time of second-line treatment initiation among metformin initiators in 2012 and 2013. Professional society guidelines and the VA clinical practice guidelines for diabetes care recommend individualizing glycemic control targets, including comorbidity burden and life expectancy among the factors used to guide individualization (36–38). In combination with evidence of long-term benefit of early glycemic control (5,6), diabetes care guidelines support more intensive glycemic control for younger patients, making the adverse temporal trends in second-line treatment initiation among younger individuals in our study a potentially concerning indicator of suboptimal diabetes care.
Our findings complement prior descriptions of therapeutic inertia in diabetes care by providing real-world data on temporal trends in treatment patterns early in the diabetes course. Consistent with prior studies of therapeutic inertia (11,12,14,15,17,18,20), we observed second-line treatment initiation at HbA1c levels that exceeded diabetes care guidelines from both the American Diabetes Association and American College of Physicians during the period of care described in our study (39,40)—a pattern that worsened over time. Our observation of consistent temporal trends in glycemic control at all time points and for all subgroups assessed aligns with previous reports documenting that poor glycemic control early persists through the diabetes disease course (16,17,19,20,24). Our results extend recent work describing temporal trends in achieving glycemic, lipid, and blood pressure control in individuals with diabetes in the National Health and Nutrition Examination Survey (NHANES) (28,29). Over the time period examined in our study, data in NHANES suggested stagnant, low rates of risk factor control among people with diabetes in the U.S. with fewer than one-quarter of individuals achieving recommended glycemic, lipid, and blood pressure control goals (28,29).
One potential explanation for the temporal trend is increasing caution with regard to glycemic control after the publication of results of three randomized trials that failed to demonstrate cardiovascular benefit of intensive glycemic control (7–9), in one of which higher all-cause mortality was found to be associated with intensive glycemic control (7). This explanation would be supported by the data showing increasing proportions over time of individuals with HbA1c 7–8% without second-line treatment initiation; however, the temporal trends preceded revised professional society guidelines for diabetes care motivated by the aforementioned trials (37,41). While it is possible that the temporal trends observed in this study reflect time-dependent practice changes toward more cautious individualized glycemic targets, the trends were observed prior to guideline emphasis on flexible glycemic goals. Furthermore, we observed similar trends in younger and older individuals, whereas updated care guidelines emphasizing flexible, individualized glycemic targets urge caution against overly intensive glycemic control particularly for older individuals with existing cardiovascular disease or at high risk and those with potentially life-limiting comorbidities. These same guidelines emphasize the outcomes benefits of tight glucose targets in younger people with longer life expectancy (36,38).
Unfortunately, our data preclude examination of the causes of delays in second-line treatment initiation, particularly whether the care patterns observed reflect patient or provider preferences, repercussions from national VA policy or diabetes-specific guidelines, or other barriers to optimal care. Assessing treatment in the context of individual glycemic goals is necessary to formally evaluate therapeutic inertia, whereas we describe population-level trends. While our study described population patterns that are consistent with therapeutic inertia, we cannot draw firm conclusions regarding inertia without an individual-level analysis that exceeds the scope of this study. That said, we found adverse temporal trends in guideline-recommended semiannual HbA1c measurements (36) that suggest progressive gaps in surveillance may contribute to delays in treatment modification. Irrespective of the cause, the progressive increase in HbA1c at second-line medication initiation to levels exceeding guideline recommendations and the declining rates of recommended semiannual HbA1c surveillance over successive annual cohorts of metformin initiators suggest delayed recognition and treatment modification for those individuals with insufficient glycemic control on metformin monotherapy who are most in need of a change in treatment.
An important limitation of our study pertains to generalizability. The conclusions from our study are limited to individuals receiving care in the VA. Comparisons of VA with non-VA diabetes care have suggested comparable or higher quality by several metrics for individuals receiving care in the VA (42,43), so we do not have reason to suspect that our observations are specific to the VA. Moreover, therapeutic inertia in diabetes care has been described across a diversity of contexts (10–17) and is not specific to the VA. On the other hand, the choice of second-line diabetes medications may differ between VA and non-VA care, as the rates of sulfonylurea selection in our data exceed what has been described in other contexts (44–46). Furthermore, the causes of temporal trends in VA and non-VA samples may differ given variations in access to care, medication cost, and other factors that impact treatment decision-making. Finally, our study sample was predominantly men, possibly limiting generalizability to women, though prior studies do not suggest that women with diabetes would be treated more aggressively than men (47,48). With these factors taken together, we urge caution in generalizing the results until replication in independent non-VA study samples is performed.
There are other limitations of note. First, our study describes temporal trends quantitatively but does not provide causal explanations for the observed treatment patterns. Second, overtreatment and therapeutic inertia in treatment deintensification have also previously been described (49–52); for clarity, we limited our study to addition of second-line therapy. Third, the paradigm of type 2 diabetes treatment has progressed from glycemic targets to medication selection (particularly glucagon-like peptide 1 receptor agonists and sodium–glucose cotransporter 2 inhibitors) informed by risk of specific diabetes complications. The slow uptake of these new classes of medications for indications with robust randomized trial evidence may suggest delays in appropriate treatment initiation and delayed adoption of new therapeutic classes (53,54). Whether there are delays in comorbidity-triggered treatment initiation of glucagon-like peptide 1 receptor agonist and sodium–glucose cotransporter 2 inhibitor medications despite professional society recommendations will need to be evaluated in future work. Finally, we limited our study to individuals initially treated with metformin alone, as this represented most individuals with type 2 diabetes in the VA, and this may limit generalizability to patients with diabetes initially treated with other agents.
We conclude that temporal trends in glycemic control in the first 5 years after type 2 diabetes treatment initiation, in rates of second-line diabetes treatment initiation, in glycemia at the time of second-line treatment initiation, and in rates of guideline-recommended HbA1c surveillance all suggest progressive worsening of management of diabetes early in the disease course in a national integrated health system. Our findings are consistent with worsening therapeutic inertia over time early in the care of patients with diabetes. These trends were similar or sometimes exaggerated among younger individuals, for whom aggressive early glycemic control may be of greatest benefit, indicating missed opportunities for improving diabetes care and preventing long-term complications. Measures are needed to promote earlier guideline-directed diabetes treatment modification in individuals without adequate glycemic control on initial metformin monotherapy.
Article Information
Funding. S.R. receives research support from the U.S. Department of Veterans Affairs (award IK2-CX001907), from the Boettcher Foundation (Webb-Waring Biomedical Research Program), and from the National Institutes of Health (NIH) (award P30DK116073). J.E.B.R. receives research support from the U.S. Department of Veterans Affairs (award CX001532) and from NIH (award P30DK116073). L.S.P. has received research support from the Cystic Fibrosis Foundation. S.R. has previously received research grant funding from the American Heart Association.
The sponsors had no role in the design or conduct of the study; collection, management, analysis, or interpretation of data; or preparation, review, or approval of the manuscript. This work is not intended to reflect the official opinion of the U.S. Department of Veterans Affairs or the U.S. government.
Duality of Interest. Within the past several years, L.S.P. has served on scientific advisory boards for Janssen and the Profil Institute for Clinical Research and has or had research support from Merck, Amylin, Eli Lilly, Novo Nordisk, Sanofi, PhaseBio, Roche, AbbVie, Vascular Pharmaceuticals, Janssen, GlaxoSmithKline, and Pfizer. In the past, he was a speaker for Novartis and Merck, but not for the last 5 years. L.S.P. is also a co-founder, officer and board member, and stockholder of a company, DIASYST, Inc., which is developing software aimed to help improve diabetes management. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. Study design was conceived by S.R., L.S.P., L.C., and J.E.B.R. Data collection and organization were performed by S.R., T.W., W.G.L., K.R., and K.J. Analyses were performed by S.R. All authors participated in interpreting results and manuscript writing and critical revision and all authors approved of the manuscript. S.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 81st Scientific Sessions of the American Diabetes Association (Virtual), 25–29 June 2021.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.19248722.
This article is featured in a podcast available at diabetesjournals.org/journals/pages/diabetes-core-update-podcasts.
References
- 1. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020. Atlanta, GA, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, 2020 [Google Scholar]
- 2. Bullard KM, Cowie CC, Lessem SE, et al. Prevalence of diagnosed diabetes in adults by diabetes type - United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:359–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Diabetes Association . Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 6. Laiteerapong N, Ham SA, Gao Y, et al. The Legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the Diabetes & Aging Study). Diabetes Care 2019;42:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 9. Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 10. Cook CB, Ziemer DC, El-Kebbi IM, et al. Diabetes in urban African-Americans. XVI. Overcoming clinical inertia improves glycemic control in patients with type 2 diabetes. Diabetes Care 1999;22:1494–1500 [DOI] [PubMed] [Google Scholar]
- 11. Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab 2016;18:401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care 2013;36:3411–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med 2001;135:825–834 [DOI] [PubMed] [Google Scholar]
- 14. Shah BR, Hux JE, Laupacis A, Zinman B, van Walraven C. Clinical inertia in response to inadequate glycemic control: do specialists differ from primary care physicians? Diabetes Care 2005;28:600–606 [DOI] [PubMed] [Google Scholar]
- 15. Ziemer DC, Miller CD, Rhee MK, et al. Clinical inertia contributes to poor diabetes control in a primary care setting. Diabetes Educ 2005;31:564–571 [DOI] [PubMed] [Google Scholar]
- 16. Pantalone KM, Wells BJ, Chagin KM, et al. Intensification of diabetes therapy and time until A1C goal attainment among patients with newly diagnosed type 2 diabetes who fail metformin monotherapy within a large integrated health system. Diabetes Care 2016;39:1527–1534 [DOI] [PubMed] [Google Scholar]
- 17. Rajpathak SN, Rajgopalan S, Engel SS. Impact of time to treatment intensification on glycemic goal attainment among patients with type 2 diabetes failing metformin monotherapy. J Diabetes Complications 2014;28:831–835 [DOI] [PubMed] [Google Scholar]
- 18. Fu AZ, Qiu Y, Davies MJ, Radican L, Engel SS. Treatment intensification in patients with type 2 diabetes who failed metformin monotherapy. Diabetes Obes Metab 2011;13:765–769 [DOI] [PubMed] [Google Scholar]
- 19. Desai U, Kirson NY, Kim J, et al. Time to treatment intensification after monotherapy failure and its association with subsequent glycemic control among 93,515 patients with type 2 diabetes. Diabetes Care 2018;41:2096–2104 [DOI] [PubMed] [Google Scholar]
- 20. Mauricio D, Meneghini L, Seufert J, et al. Glycaemic control and hypoglycaemia burden in patients with type 2 diabetes initiating basal insulin in Europe and the USA. Diabetes Obes Metab 2017;19:1155–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Osataphan S, Chalermchai T, Ngaosuwan K. Clinical inertia causing new or progression of diabetic retinopathy in type 2 diabetes: a retrospective cohort study. J Diabetes 2017;9:267–274 [DOI] [PubMed] [Google Scholar]
- 22. Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol 2015;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006-2013. Diabetes Care 2017;40:468–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stone MA, Charpentier G, Doggen K, et al.; GUIDANCE Study Group . Quality of care of people with type 2 diabetes in eight European countries: findings from the Guideline Adherence to Enhance Care (GUIDANCE) study. Diabetes Care 2013;36:2628–2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 2013;368:1613–1624 [DOI] [PubMed] [Google Scholar]
- 26. Kazemian P, Shebl FM, McCann N, Walensky RP, Wexler DJ. Evaluation of the cascade of diabetes care in the United States, 2005-2016. JAMA Intern Med 2019;179:1376–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khunti K, Ceriello A, Cos X, De Block C. Achievement of guideline targets for blood pressure, lipid, and glycaemic control in type 2 diabetes: A meta-analysis. Diabetes Res Clin Pract 2018;137:137–148 [DOI] [PubMed] [Google Scholar]
- 28. Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in U.S. adults, 1999-2018. N Engl J Med 2021;384:2219–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang L, Li X, Wang Z, et al. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999-2018. JAMA 2021;326:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berkowitz SA, Krumme AA, Avorn J, et al. Initial choice of oral glucose-lowering medication for diabetes mellitus: a patient-centered comparative effectiveness study. JAMA Intern Med 2014;174:1955–1962 [DOI] [PubMed] [Google Scholar]
- 31. Desai NR, Shrank WH, Fischer MA, et al. Patterns of medication initiation in newly diagnosed diabetes mellitus: quality and cost implications. Am J Med 2012;125:302.e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hampp C, Borders-Hemphill V, Moeny DG, Wysowski DK. Use of antidiabetic drugs in the U.S., 2003-2012. Diabetes Care 2014;37:1367–1374 [DOI] [PubMed] [Google Scholar]
- 33. Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care 2004;27(Suppl. 2):B10–B21 [DOI] [PubMed] [Google Scholar]
- 34. Wheeler S, Moore K, Forsberg CW, et al. Mortality among veterans with type 2 diabetes initiating metformin, sulfonylurea or rosiglitazone monotherapy. Diabetologia 2013;56:1934–1943 [DOI] [PubMed] [Google Scholar]
- 35. Roumie CL, Hung AM, Greevy RA, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med 2012;157:601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. American Diabetes Association . 6. Glycemic targets: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021;44(Suppl. 1):S73–S84 [DOI] [PubMed] [Google Scholar]
- 37. Qaseem A, Wilt TJ, Kansagara D, et al.; Clinical Guidelines Committee of the American College of Physicians . Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med 2018;168:569–576 [DOI] [PubMed] [Google Scholar]
- 38. The Management of Type 2 Diabetes Mellitus in Primary Care Working Group . VA/DoD Clinical Practice Guideline for the Management of Type 2 Diabetes Mellitus in Primary Care, 2017. Accessed 15 June 2021. https://www.healthquality.va.gov/guidelines/CD/diabetes [PMC free article] [PubMed]
- 39. American Diabetes Association . Standards of medical care in diabetes—2010. In Clinical Practice Recommendations, 2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qaseem A, Vijan S, Snow V, Cross JT, Weiss KB; Clinical Efficacy Assessment Subcommittee of the American College of Physicians . Glycemic control and type 2 diabetes mellitus: the optimal hemoglobin A1c targets. A guidance statement from the American College of Physicians. Ann Intern Med 2007;147:417–422 [DOI] [PubMed] [Google Scholar]
- 41. Fox CS, Golden SH, Anderson C, et al.; American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Quality of Care and Outcomes Research; American Diabetes Association . Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2015;38:1777–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Anhang Price R, Sloss EM, Cefalu M, Farmer CM, Hussey PS. Comparing quality of care in Veterans Affairs and non-Veterans Affairs settings. J Gen Intern Med 2018;33:1631–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kerr EA, Gerzoff RB, Krein SL, et al. Diabetes care quality in the Veterans Affairs Health Care System and commercial managed care: the TRIAD study. Ann Intern Med 2004;141:272–281 [DOI] [PubMed] [Google Scholar]
- 44. Dennis JM, Henley WE, McGovern AP, et al.; MASTERMIND consortium . Time trends in prescribing of type 2 diabetes drugs, glycaemic response and risk factors: a retrospective analysis of primary care data, 2010-2017. Diabetes Obes Metab 2019;21:1576–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Montvida O, Shaw J, Atherton JJ, Stringer F, Paul SK. Long-term trends in antidiabetes drug usage in the U.S.: real-world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care 2018;41:69–78 [DOI] [PubMed] [Google Scholar]
- 46. Wilkinson S, Douglas I, Stirnadel-Farrant H, et al. Changing use of antidiabetic drugs in the UK: trends in prescribing 2000-2017. BMJ Open 2018;8:e022768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gouni-Berthold I, Berthold HK, Mantzoros CS, Böhm M, Krone W. Sex disparities in the treatment and control of cardiovascular risk factors in type 2 diabetes. Diabetes Care 2008;31:1389–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xiang AS, Szwarcbard N, Gasevic D, et al. Trends in glycaemic control and drug use in males and females with type 2 diabetes: results of the Australian National Diabetes Audit from 2013 to 2019. Diabetes Obes Metab 2021;23:2603–2613 [DOI] [PubMed] [Google Scholar]
- 49. Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med 2014;174:259–268 [DOI] [PubMed] [Google Scholar]
- 50. Sussman JB, Kerr EA, Saini SD, et al. Rates of deintensification of blood pressure and glycemic medication treatment based on levels of control and life expectancy in older patients with diabetes mellitus. JAMA Intern Med 2015;175:1942–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lipska KJ, Krumholz H, Soones T, Lee SJ. Polypharmacy in the aging patient: a review of glycemic control in older adults with type 2 diabetes. JAMA 2016;315:1034–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McCoy RG, Lipska KJ, Yao X, Ross JS, Montori VM, Shah ND. Intensive treatment and severe hypoglycemia among adults with type 2 diabetes. JAMA Intern Med 2016;176:969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arnold SV, Inzucchi SE, Tang F, et al. Real-world use and modeled impact of glucose-lowering therapies evaluated in recent cardiovascular outcomes trials: an NCDR Research to Practice project. Eur J Prev Cardiol 2017;24:1637–1645 [DOI] [PubMed] [Google Scholar]
- 54. Nargesi AA, Jeyashanmugaraja GP, Desai N, Lipska K, Krumholz H, Khera R. Contemporary national patterns of eligibility and use of novel cardioprotective antihyperglycemic agents in type 2 diabetes mellitus. J Am Heart Assoc 2021;10:e021084. [DOI] [PMC free article] [PubMed] [Google Scholar]