Abstract
Patients with diabetes mellitus are at elevated risk for secondary complications that result in lower extremity amputations. Standard of care to prevent these complications involves prescribing custom accommodative insoles that use inefficient and outdated fabrication processes including milling and hand carving. A new thrust of custom 3D printed insoles has shown promise in producing corrective insoles but has not explored accommodative diabetic insoles. Our novel contribution is a metamaterial design application that allows the insole stiffness to vary regionally following patient-specific plantar pressure measurements. We presented a novel workflow to fabricate custom 3D printed elastomeric insoles, a testing method to evaluate the durability, shear stiffness, and compressive stiffness of insole material samples, and a case study to demonstrate how the novel 3D printed insoles performed clinically. Our 3D printed insoles results showed a matched or improved durability, a reduced shear stiffness, and a reduction in plantar pressure in clinical case study compared to standard of care insoles.
Keywords: mechanical properties, durability, additive manufacturing, shear stiffness, personalized medicine, plantar pressure, foot ulceration, accommodative foot orthosis
Introduction
Patients with diabetes mellitus and secondary complications to the peripheral nervous system are at increased risk of developing foot ulcers. In the United States, more than 100,000 patients require amputations each year as a result of diabetic foot wounds that have failed to heal, with 60% of these wounds being foot ulcers. This number is expected to increase due to an aging population . Management of the diabetic foot with the goal of avoiding amputation falls into two main categories: wound prevention and wound healing. Prevention strategies focus on patient education and close monitoring, and the prescription of specialized offloading orthotic devices. If a wound occurs, these two strategies are augmented with the prescription of antibiotic and specialized wound healing treatments. The successes of all of these strategies benefit from specialized care through patient-specific strategies [1–3]. Foot ulceration in particular is multifactorial and is commonly correlated with elevated plantar pressure levels [4–6]. Therapeutic offloading devices have been prescribed to reduce plantar pressure and prevent ulceration or promote recovery of existing ulcers. The current standard of care (SoC) includes accommodative shoes and custom insoles designed by specialized clinicians that utilize layers of foam with varying durometers and specially placed additions like heel lifts or metatarsal pads. Patient-specific customized insoles, which rely on clinical expertise and time-consuming manual labor for specific construction materials and shaping techniques, have been shown to be a successful option in reducing and redistributing peak plantar pressures, thereby increasing the ability of the patient to avoid a first ulcer or re-ulceration [7–10].
In recent years, researchers have applied additive manufacturing methods, specifically 3-dimensional (3D) printing, as a viable method for producing custom insoles. One of the most impactful attributes in this space has been the ability to programmatically modulate stiffness in three dimensions through modification of the internal geometry of the 3D printed components [11]. A custom 3D model of an insole can be generated using a 3D scan of a foam crush box impression. The insole can then be manufactured using an appropriate polymeric material with internal geometry strategically designed to create regions of varying stiffness throughout the insole. Different 3D printing methods have been employed to investigate the effect of custom 3D printed insoles. Prior studies have mainly utilized the fused deposition modeling (FDM) method of 3D printing, in which a plastic filament is extruded through a heated nozzle and deposited in layers to form 3D geometry [12–15]. The FDM material used in prior studies has typically been limited to common thermoplastic filaments such as polylactic acid (PLA) or acrylonitrile butadiene styrene (ABS). While it is a ubiquitous and low-cost prototyping method, FDM printing technology currently faces some limitations. FDM printing allows the creation of a fully-geometrically-customized insole; however, materials available using this method under-perform as a final product in terms of durability and mechanical property performance. Specifically, when used with stiffer PLA and ABS materials, FDM printing produces rigid, brittle prints and is typically generated only as proof of concept in the initial stages of prototyping [16–18].
The characteristics of inter-layer adhesion in FDM printing technology results in prints with largely anisotropic mechanical properties, and this may induce early failure in devices fabricated in this manner [16,19]. Prior studies have successfully created insoles for research purposes [12,20,21]; however, the insoles produced using this method have not been evaluated in terms of their ability to match the durability lifetime, the nominal stiffness, nor the level of patient comfort of the SoC accommodative diabetic insoles.
Recently, elastomers have improved and have been specifically designed for 3D printing fabrication methods, as have new methods of 3D print construction [22–24]. New UV-curable resin printing technologies are capable of producing complex print geometries with improved mechanical properties and have demonstrated the ability to produce isotropic materials [25]. Our prior work used parts produced via a UV-cured resin process to investigate the ability to modulate stiffness by lattice geometry design and geometric modification [11]; these elastomers could extend the stiffness modulation beyond the FDM method and show potential in mimicking the material properties of the SoC insoles. Software allows tuning of lattice geometry and prediction of mechanical properties. Furthermore, 3D printing processes that leverage stereolithography have the ability to produce prints with high isotropic material properties [25]. However, the actual performance and longevity of elastomers in 3D printed insoles have not been evaluated.
In this work, the authors set out to accomplish the following goals: to produce a 3D printed lattice material that matches the SoC compressive stiffness; to develop an insole material with improved durability over the SoC material; to reduce the shear stiffness of the insole material over the SoC material; to allow the customization of insole stiffness based on patient plantar pressure data; and to develop a repeatable workflow for the manufacturing of patient-specific 3D printed plantar pressure-offloading insoles and for the rigorous characterization of new 3D printed metamaterial samples for future insole development. In this work, we presented a novel 3D printed insole manufacturing workflow, and we developed two different fully customized patient-specific insole devices through the proposed workflow. The first device was composed of fully-3D printed material, and the second was constructed as a hybrid of 3D printed base material and a bi-laminate foam top layer. We then introduced a testing method to determine the durability performance and shear stiffness of insole material samples. Lastly, we performed an in-vivo case study to demonstrate how the novel 3D printed insole devices applied in a real-life use case in terms of measured plantar pressure during walking.
Methods
Insole Design Process
Current SoC process.
The fabrication of current SoC custom insoles for the treatment of diabetic feet relies heavily on clinical expertise and manual adjustments to accommodate various patient-specific conditions. Typically, a certified orthotist uses a foam crush box to obtain a negative impression of a patient’s foot geometry and then fills the crush box with wet plaster, which, once cured, yields a hard positive model of the patient’s foot. After a series of manually performed modifications to the plaster model, SoC insoles are constructed by heat- and vacuum-forming layers of foam of different compositions and applying an adhesive between the foam sheets to form a layered foam construction. In this approach, the pressure-relieving region may be considered based on clinical judgments and is produced using a range of strategies that include: 1) the addition of material to the positive plantar model to create a manufactured depression in the finished foot orthosis or 2) by insetting a disc of low-density foam or 3) by removing material from the base of the insole in the desired region or 4) some combination of all these strategies. The insole is then manually shaped to ensure proper fit to the patient’s foot and shoes. This entire fabrication process, from generating the foam crush box impression to fitting the insole to the patient and the patient’s shoe, typically requires two patient visits over multiple days, sometimes weeks (Figure 1A).
Figure 1.
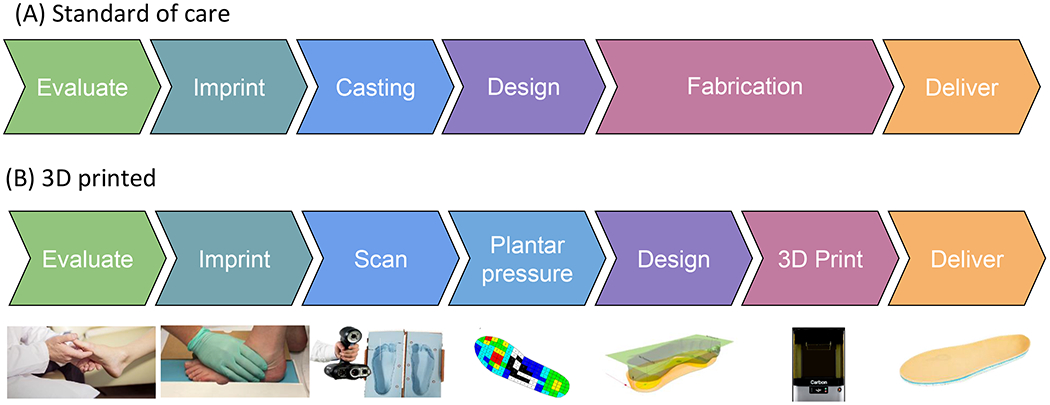
Insole manufacturing workflow
Novel fabrication method.
In this work, our novel fabrication method achieves a fully customized insole that fits the patient’s foot and shoe, as prescribed by an orthotist, and incorporates patient-specific plantar pressure to relieve plantar pressure through targeted offloading regions of insole stiffness reduction. From here on, the insole created using this method will be referred to as the novel insole. We first scanned the foam crush box impression of the patient’s foot generated by an orthotist using a high-precision 3D visible light scanner (Creaform GoScan50, Creaform, Levis, Canada). Then we processed the scanned data using a scan post-processing software (VX Elements 3D, Creaform, Levis, Canada) and exported the impression as an STL file for later patient-specific insole design. Meanwhile, we collected patient-specific plantar pressure using an in-shoe plantar pressure sensor (Pedar-X, novel GMBH, Germany) while walking over flat ground in a laboratory setting in a standardized shoe. The plantar pressure data were calculated using custom MATLAB (The MathWorks Inc., Natick, Massachusetts) algorithm to define the offloading regions using a threshold value of 200 kPa. In the insole model design software (FitFoot360, Fit360 Ltd., Worcestershire, UK), the anatomical landmarks of the foot, including heel and first and fifth metatarsal heads, were manually identified to facilitate proper insole positioning and sizing, and the insole was modeled to match the geometry of the patient’s scanned foot. The resulting one-piece insole model was then exported and further divided into normal-pressure and offloading segments based on a previously defined plantar pressure map using CAD software (Fusion360, Autodesk Inc. San Rafael, California). One version of the model was exported incorporating the full thickness of the model produced through FitFoot360, and a separate model with the top 4 mm of thickness removed was also exported. These two segmented models were then delivered to a third-party company (Carbon, Redwood City, CA) for application of the lattice structure 3D printing.
After extensive testing of a range of elastomers available for various 3D printing technologies, the Carbon® Elastomeric Polyurethane (EPU) 41 material was identified to exhibit the most suitable mechanical properties in terms of elasticity and durability. Furthermore, the lattice design engine (Carbon, Redwood City, CA) enabled us to create a lattice with comparable stiffness to the foam materials used in the SoC insoles by iteratively adjusting the lattice unit size and strut thickness. During the lattice tuning phase, the predicted lattice stiffness was generated by the design engine [26]. Lattice unit size and thickness parameters were tuned until the predicted stiffness resembled the characterized stiffnesses of each of the SoC insole materials. The resulting printed samples were tested for compressive stiffness and the process was repeated until the lattice parameters successfully produced a stiffness closely matching those of the SoC materials. A resulting material with stiffness-matched regions produced according to each individual layer of the SoC insole foam was delivered through the capability of the lattice design engine. Plots of compressive stiffness verification for the final lattice designs compared to the matching SoC foams can be seen in the included supplementary documents, as well as figures detailing the lattice structure. The designs of the full insole models including the latticed structures were then 3D printed on a Carbon L1 3D printer using the EPU41 material. Following the standard Carbon fabrication process for the L1 printing system, once printed, the insoles were spun in a centrifuge to remove excess resin and finished with a heat cure process in an oven. For our hybrid version, only the thin base of the insole was printed using this process, then it is bonded to a Poron-Plastazote bi-laminate top sheet, nominally 4 mm in thickness, to attain the same overall geometry of the fully-3D printed version, and to target a surface feel preferred by patients and clinicians. This Poron-Plastazote bi-laminate top sheet is the same that is used for the SoC customized insoles (Figure 1B).
Material Mechanical Property Characterization
Durability and Compressive Stiffness Characterization
We prepared 3 cylindrical specimens (nominally 31.75 mm in diameter and approximately 8 mm thick, Figure 2A), two of which were samples of the novel insoles, either consisting entirely of EPU41 in lattice geometry manufactured by Carbon or with the additional bi-laminate foam top layer consisting of a soft Plastazote® layer (Zotefoams, Croydon, UK) and a stiffer PORON® layer beneath (Rogers Corporation, Chandler, Arizona). The last specimen was a SoC tri-layer foam (Plastazote, PORON, and EVA 35A) manufactured by Amfit Inc. (Vancouver, Washington). Those specimens underwent a cyclic compressive loading using a material testing machine (Instron, Norwood, Massachusetts, Figure 2A).
Figure 2.
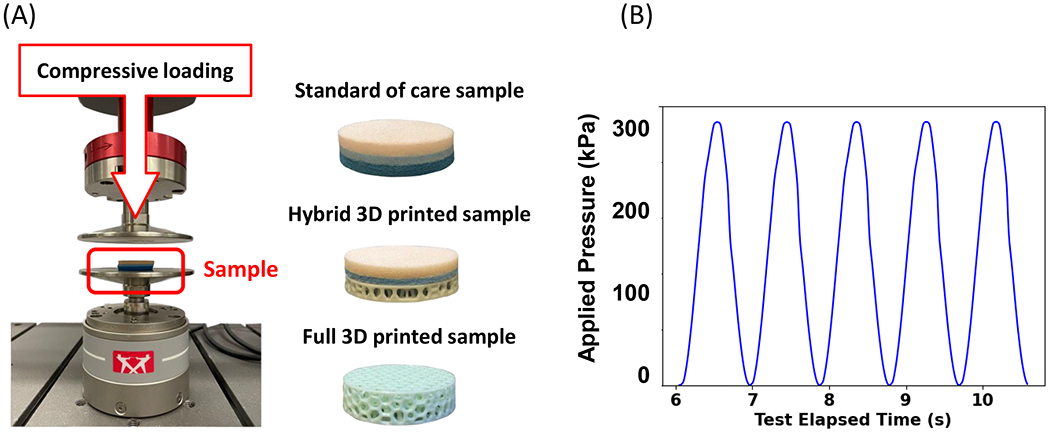
Durability and compressive stiffness characterization. A) testing device setup (Instron), B) loading profile (time/pressure curve)
Each specimen was loaded cyclically using a sinusoidal loading pattern from zero to peak load to simulate an expected walking task. Specifically, a loading protocol with a total cycle count of 1 million at a rate of 1.1 Hz and a compressive pressure of 300 kPa was designed based on the fact that a standard pattern of use was determined to be 5,400 steps per day, or 985.5K steps per insole in 12 months (Figure 2B) [27–29]. Standard of care insoles are prescribed for a maximum use equivalent to 6 months of total wear, however we chose to test all samples for a simulated period of 1 year in order to ensure safety prior to using in a clinical application.
Data were processed to extract stress and strain for each recorded cycle, and the compressive elastic modulus of the sample was determined at each cycle by the following method: the linear region of the stress vs. strain curve was approximated to be contained within the last 25% of the data, then the slope of this linear region was estimated by fitting a first-order polynomial using the Python (Python Software Foundation, Wilmington, Delaware) Numpy package’s polyfit function, which uses a least-squares polynomial fit method. This slope estimation was calculated for each recorded cycle and the change in this slope value was used as a means of comparing the tendency of each insole construction method sample to become stiffer over the course of the loading protocol. We chose to follow this method of characterizing and comparing the compressive modulus because the highest pressures felt by the plantar tissue will be experienced in the last portions of the stress-strain curve. Therefore, this is the most relevant region in which to assess the pressure-reducing ability of a particular insole material. This method was used in several prior works and standardized testing methods [30,31].
Shear Stiffness Characterization
To characterize the novel insole’s ability to mitigate shear stress of the plantar tissue, the shear stiffnesses of the SoC insole and variations of the two 3D printed insole construction methods were characterized using a Mach-1 Mechanical Testing Machine (Biomomentum Inc., Laval, QC, Figure 3A). We prepared cylindrical samples (nominally 31.75 mm in diameter and 8 mm in thickness) for the following insole constructions: 1) SoC EVA with Poron-Plastazote bi-laminate top sheet; 2) 3D printed nominal stiffness lattice-matched to SoC stiffness profile (either fully-3D printed or of hybrid construction with Poron-Plastazote bi-laminate top sheet); 3) 3D printed sparse lattice matching our offloading region stiffness (either fully-3D printed or with Poron-Plastazote bi-laminate top sheet).
Figure 3.
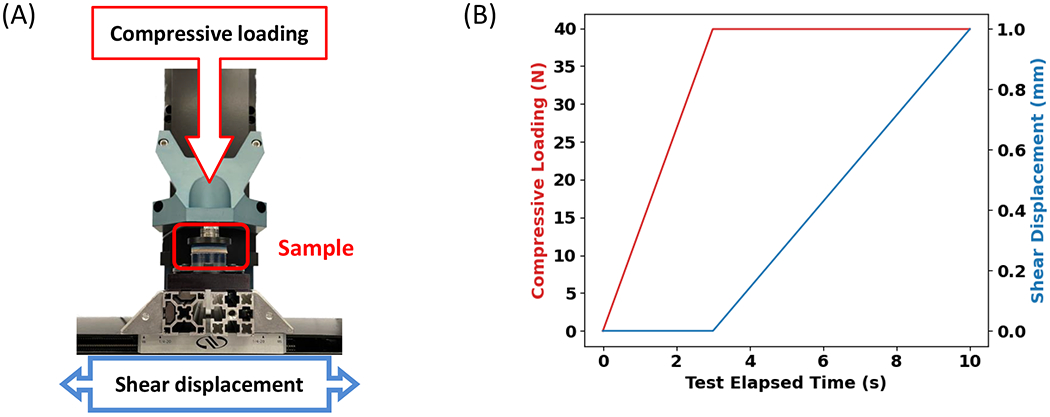
Shear stiffness characterization. A) testing device setup (Biomomentum); B) loading profile, the compressive force of 60 N was applied in the first 3 seconds as a preconditioning, following a shear displacement. The horizontal force at end point was used for shear modulus calculation.
A loading protocol was applied to a previously untested sample of each construction via the Mach-1 Motion Software wherein the sample was loaded in compression to a pressure of 50 kPa, and subsequently loaded cyclically in shear to a strain of 6% for a total of 100 cycles at a rate of 1.1 Hz (Figure 3B). The duration of this preconditioning stage was predetermined by subjecting a prior selection of samples to a total of 1,000 shear loading cycles and calculating the change in shear stiffness versus cycle count. It was found that after 100 cycles, all samples exhibited a change in shear stiffness of less than 1% per cycle, therefore this was set as the threshold for shear preconditioning.
Testing data for the last shear loading cycle of each sample was recorded, including shear load and shear displacement. These data, as well as sample geometry, were used to compare shear stiffnesses (G) between samples. Shear stress was calculated as the ratio of shear load to sample cross-sectional area, and shear strain was calculated as the ratio of change in shear displacement to sample thickness. In order to avoid slight nonlinear stiffness behavior in the initial loading stage, the last 25% of the load cycle was isolated and the slope of the shear strain vs. shear stress data was calculated using the Python Numpy package’s built-in Polyfit function. This method was informed by the same process as described in the compressive stiffness characterization method above.
Application Case Study
A case study with one participant with diabetes without neuropathy or previous ulceration was carried out (male, type II diabetes for 6–7 years, age 71, body height: 199 cm, body mass: 109.4 kg, BMI: 27.6 kg/m2). We followed each of the insole manufacturing procedures detailed above to design three different pairs of insoles for the participant: one SoC, one hybrid 3D printed insole with a bi-laminate foam top layer, and one fully-3D printed insole. An orthotist performed the foot evaluation and foam crush box impression. The foam crush box impression was used for the SoC insole manufacturing by one of the hospital’s existing third-party vendors. In this case, no offloading regions were defined for the SoC insoles based on clinical evaluation and judgments (Figure 4A). The same impression was also 3D scanned for the creation of the hybrid and fully-3D printed insole designs, and the participant underwent an in-shoe plantar pressure measurement. The plantar pressure map was generated, and an offloading region was defined for this case based on the preset cut-off pressure of 200 kPa (Figure 4B and 4C).
Figure 4.
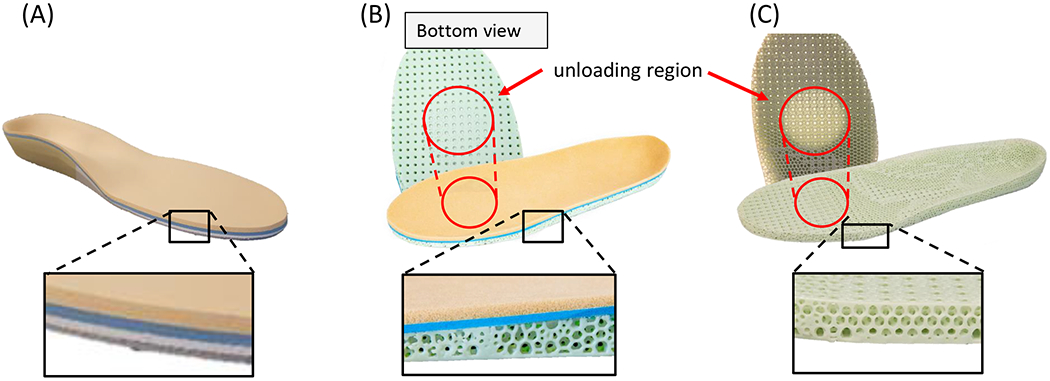
Three pairs of insoles manufactured through our workflow. A) standard of care insole. B) hybrid 3D printed insole with bi-laminate foam top. C) fully-3D printed insole.
After all three pairs of insoles were manufactured, the participant was asked to walk 40 ft in a straight line with one insole type at a time, and the in-shoe plantar pressure measurement was performed again. We collected multiple gait trials until we had 4 successful trials for each insole condition. To avoid any potential effect of fatigue, a rest session and a warm-up session was introduced between each insole condition. Gait speed of each gait trial was recorded using a stopwatch and we used a variation of less than 10% of gait speed to define a successful trial. Each collected set of plantar pressure data was post-processed to segment it into steps. The steps that occurred during gait initiation and termination phases were removed from the analysis. The peak pressure of each sensor cell during each step was found first and then the peak pressures were calculated across all steps in each gait trial. Maximum peak pressure, defined as the highest peak pressure in the offloading region and adjacent region, defined by one cell apart from the offloading region, was calculated from the data to demonstrate the biomechanical effect of SoC and 3D printed insoles.
Results
Material Mechanical Property Characterization
In the durability and compressive stiffness testing, the hybrid 3D printed insole sample closely matched the durability profile of the SoC insole sample, whereas the fully-3D printed insole sample exhibited a lower increase in stiffness over the course of 1 million cycles (Figure 5A). At cycles 1K, 10K, 100K, and 1M, the fully-3D printed sample showed a percent deformation of the original thickness of 3.88%, 4.30%, 5.45%, and 9.49%, respectively. At the same cycle counts, the hybrid sample showed deformation of the original thickness of 9.91%, 20.58%, 24.13%, and 25.20%, respectively. Lastly, for the same cycle counts, the SoC sample showed deformation of the original thickness of 10.74%, 21.08%, 23.59%, and 25.42%, respectively.
Figure 5.
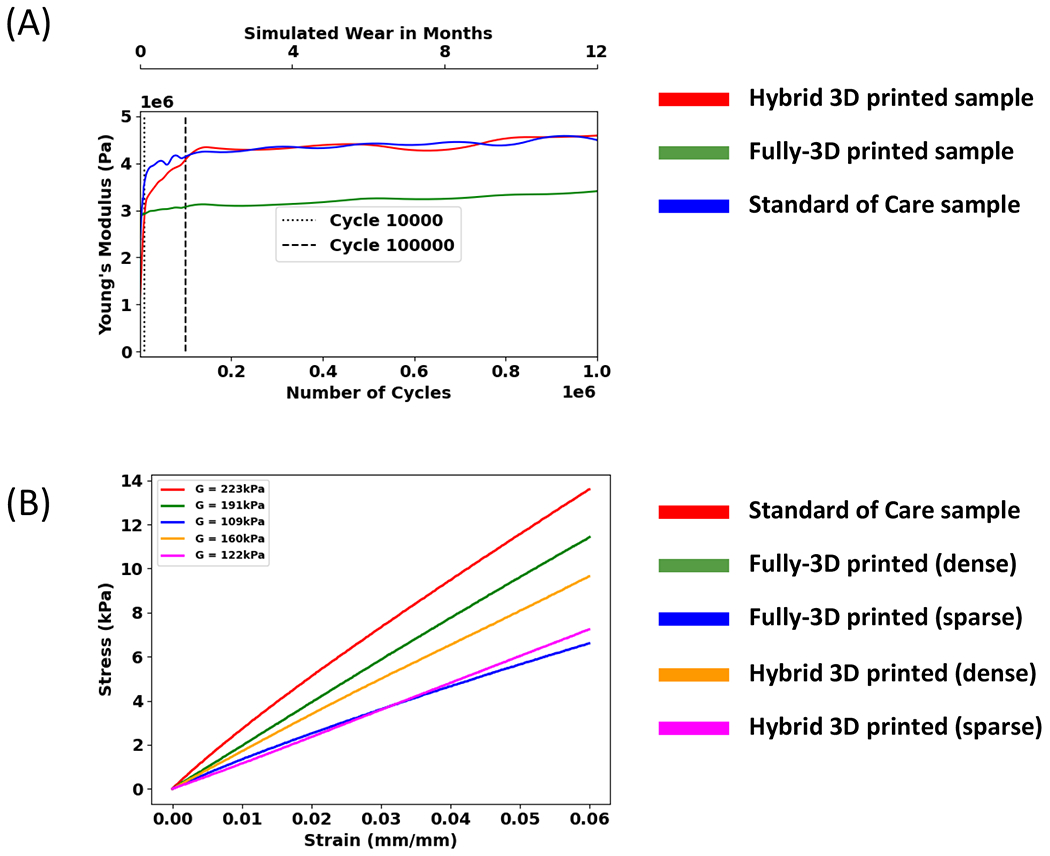
Mechanical properties of the insole samples. A) the young’s modulus over the course of 1 million cycles; B) shear stiffness of the insole samples.
In terms of the shear stiffness (G) for each insole sample, the SoC insole sample had the highest shear stiffness of 223 kPa, followed by the fully-3D printed nominal lattice sample (191 kPa), the hybrid nominal lattice sample (160 kPa), the hybrid sparse lattice sample (122 kPa), and finally the fully-3D printed sparse lattice sample (109 kPa), demonstrating that the SoC had the highest stiffness at a given shear displacement over the range of tested shear strain. (Figure 5B)
Application Case Study
The SoC insole without any offloading region showed an increase in peak plantar pressure compared to the standardized footwear (Mean±Standard deviation: 268.8±7.0 kPa vs. 248.8 ± 9.9 kPa) during walking. The maximum peak plantar pressure values for the hybrid and fully-3D printed insoles in the offloading region were 207.8± 9.6 kPa (~16.5% reduction vs. standardized footwear) and 209.3 ±2.9 kPa (~15.9% reduction vs. standardized footwear) (Figure 6). ln the region adjacent to the offloading region, the SoC reduced the peak pressure during gait to 161.0 ± 4.9 kPa compared to the SoC insole of 186.7 ± 13.8 kPa. The maximum peak plantar pressure values for the hybrid 3D printed, and fully-3D printed insoles were 187.8± 10.0 and 213.5± 15.8 kPa (Figure 6).
Figure 6.
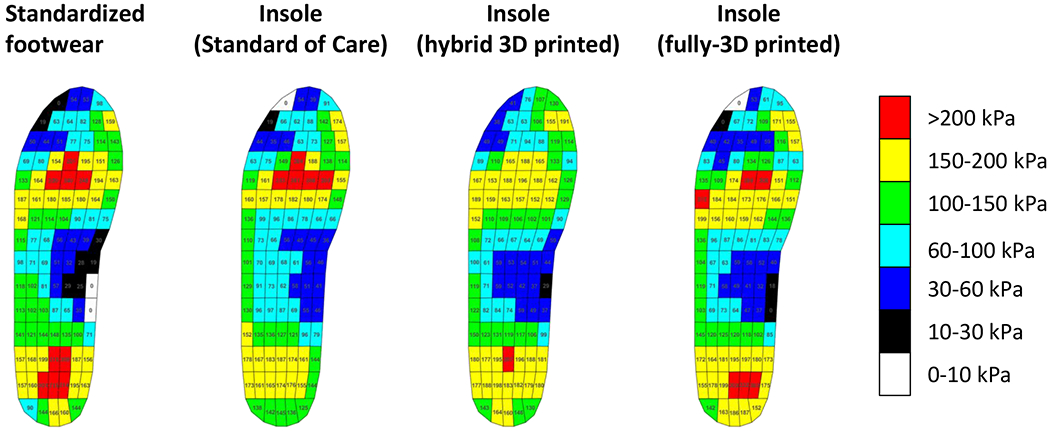
A representative plantar pressure distribution in a patient with diabetes during walking.
Discussion
The goals of this work were the following: to produce a 3D printed lattice material that matched the SoC compressive stiffness for the bilaminate foam and EVA foam; to develop an insole material with improved durability over the SoC material; to reduce the shear stiffness of the insole material over the SoC material; to allow the customization of insole stiffness based on patient plantar pressure data; and to develop a repeatable workflow for the manufacturing of patient-specific 3D printed plantar pressure-offloading insoles and for the rigorous characterization of new 3D printed metamaterial samples for future insole development.
We presented a novel method to design and manufacture 3D printed accommodative insoles with patient-specific metamaterials. We also described a benchtop and human subjects testing framework through which the impact of design parameters on the performance of the device may be assessed in a scientifically rigorous manner. We demonstrated the ability to control nominal lattice geometry and stiffness as well as offloading regional geometry and stiffness. The workflow also facilitated the comparison of printing resins, foam materials, and the combinations of each of these constituent components. We also provided a testing protocol for compressive stiffness, compressive durability, and shear stiffness through which the 3D printed lattice parameters can be evaluated and tuned to produce desirable results. Lastly, we demonstrated the application of a selected elastomeric 3D printing material, EPU41, and a printing process, Carbon DLS™, within our fabrication and material characterization workflow by producing two complete sets of patient-specific insoles (in fully-3D printed and hybrid construction) and comparing the resulting plantar pressure measurements with those of the SoC insole.
Durability of an insole product is a key performance metric regarding its potential clinical effectiveness [32–34]. The ability of an insole device to mitigate plantar pressure will change as the device stiffens, and therefore the ability of an accommodative insole to retain its soft elasticity is critical. The deformation pattern of our 3D printed insole suggests that we could either fully simulate the accommodative behavior with the SoC, such as our hybrid version, or create a different profile, such as our fully-3D printed version. A key difference between the two novel insoles is the presence of the Plastazote top layer, which is specifically intended to compress quickly over areas of high pressure, thereby creating reliefs in areas of peak pressure. The decision to produce a novel insole with Plastazote and one without allowed us to investigate the effect of this material on overall durability performance. Furthermore, prior studies in the area of novel 3D printed insole devices have not demonstrated an evaluation of the insoles’ durability, either alone or in comparison to SoC insoles [12–15,21]. After subjecting representative samples for the two novel insole variations and SoC insole to a 1 million cycle compressive loading test, it has been demonstrated that our two novel insoles were at least as durable as the SoC for a year’s worth of steps for an average patient with diabetes and that the fully-3D printed insole actually demonstrated a lower increase in stiffness, suggesting a higher durability performance. Therefore, the comfort level of the insole from a patient perspective may match or exceed the SoC insole, which may augment patient adherence to a treatment plan that includes regular use of the insole [35–37].
Our 3D printed insole fabrication method and material selection showed the potential to reduce shear stress on the plantar tissue of the wearer by reducing the shear stiffness of the fabricated insole. The SoC construction yielded the highest shear stiffness, followed by the standard-stiffness 3D printed sample and softest-stiffness offloading region 3D printed sample, suggesting that the novel insole product has the ability to regionally control shear stiffness and compressive stiffness to accommodate patient-specific requirements. While plantar shear-offloading for patients with diabetes has been investigated for its potential to reduce rates of plantar ulceration, the clinical effects have not been fully determined [38–40]. The investigation of shear performance warrants future study.
In this study, we focused on controlling several variables within our workflow: printing resin, lattice geometry, offloading region geometry, and offloading region stiffness. We chose to only test one sample of each insole construction method as a proof of concept for our novel fabrication workflow and testing protocols. Durability testing was also performed via a benchtop simulation of 1 year of use instead of a real-life subject use case, which would have taken 1 year to complete but may have been a more realistic depiction of durability performance. In our simulated loading protocol, the compression loading of 1 million cycles equated to a full test time of 10.52 days. Importantly, the standard of care insoles are only prescribed for a use period of 6 months however we chose to test all samples to a simulated period of 1 year in order to ensure the safety of the patients when wearing our novel insoles prior to testing in a clinical application. Therefore, it should be noted that the results presented in this study are limited in that they depict a sample size of 1 for the selected test specimens over the course of a simulated lifetime. Interpretation of the results should only be done with the understanding that we aimed to demonstrate the potential impact of our novel workflow and testing method on the growing field of custom 3D printed insoles. Future work will seek to utilize this workflow in order to assess the impact of design variables, such as 3D printed lattice variables (cell size, infill percentage, printing resin), offloading region design (geometry, nominal-sparse transition method, offloading stiffness), and upper foam selection through clinical evaluation. Also, the insole manufacturing workflow presented in this study could be extended to other foot conditions, such as rheumatoid arthritis, osteoarthritis, plantar fasciitis, and metatarsalgia, that require custom geometry and a mixture of materials to achieve pain relief or correction. This will allow for the further study of each of these variables independently and their respective impact on clinical efficacy for outcome measures of interest.
Our case study results indicate that our 3D printed insole, informed by our plantar pressure mapping workflow, successfully offloaded plantar pressure in the desired region as designed with minimal secondary adjustment or fabrication steps. We believe that this presents an important improvement in clinical applications by shortening the clinical time for the patient to a single visit. In addition, with the 3D printing technology utilized in this work, multiple insoles could be manufactured simultaneously if a patient has multiple types of footwear. Although manually fabricated insoles have shown clinical efficacy, the strength of 3D printing technology enables further redesign and optimization when newer materials are available for clinical use. Our novel workflow has the potential to rigorously assess new 3D printed insole material samples for further development and refinement of this novel 3D printed insole technology.
Supplementary Material
Funding:
This work was supported in part by the Department of Veterans Affairs Rehabilitation Research and Development grants RX002130, A9243C, and RX002357
Footnotes
Competing interests: None declared
Ethical approval: This study has been approved by the Institutional Review Board at the VA Puget Sound Health Care System
References
- [1].Bandyk DF, The diabetic foot: Pathophysiology, evaluation, and treatment, Semin. Vasc. Surg 31 (2018) 43–48. 10.1053/j.semvascsurg.2019.02.001. [DOI] [PubMed] [Google Scholar]
- [2].Lipsky BA, Senneville É, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, Kono S, Lavery LA, Malone M, van Asten SA, Urbančič-Rovan V, Peters EJG, on behalf of the I.W.G. on the D. Foot (IWGDF), Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update), Diabetes Metab. Res. Rev 36 (2020) e3280. 10.1002/dmrr.3280. [DOI] [PubMed] [Google Scholar]
- [3].Del Core MA, Ahn J, Lewis RB, Raspovic KM, Lalli TAJ, Wukich DK, The Evaluation and Treatment of Diabetic Foot Ulcers and Diabetic Foot Infections, Foot Ankle Orthop. 3 (2018) 2473011418788864. 10.1177/2473011418788864. [DOI] [Google Scholar]
- [4].Veves A, Murray HJ, Young MJ, Boulton AJM, The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study, Diabetologia. 35 (1992) 660–663. 10.1007/BF00400259. [DOI] [PubMed] [Google Scholar]
- [5].Bus SA, Armstrong DG, van Deursen RW, Lewis JEA, Caravaggi CF, Cavanagh PR, IWGDF guidance on footwear and offloading interventions to prevent and heal foot ulcers in patients with diabetes, Diabetes Metab. Res. Rev 32 (2016) 25–36. 10.1002/dmrr.2697. [DOI] [PubMed] [Google Scholar]
- [6].Maciejewski ML, Reiber GE, Smith DG, Wallace C, Hayes S, Boyko EJ, Effectiveness of Diabetic Therapeutic Footwear in Preventing Reulceration, Diabetes Care. 27 (2004) 1774–1782. 10.2337/diacare.27.7.1774. [DOI] [PubMed] [Google Scholar]
- [7].Hellstrand Tang U, Zügner R, Lisovskaja V, Karlsson J, Hagberg K, Tranberg R, Comparison of plantar pressure in three types of insole given to patients with diabetes at risk of developing foot ulcers – A two-year, randomized trial, J. Clin. Transl. Endocrinol 1 (2014) 121–132. 10.1016/j.jcte.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Owings TM, Woerner JL, Frampton JD, Cavanagh PR, Botek G, Custom Therapeutic Insoles Based on Both Foot Shape and Plantar Pressure Measurement Provide Enhanced Pressure Relief, Diabetes Care. 31 (2008) 839–844. 10.2337/dc07-2288. [DOI] [PubMed] [Google Scholar]
- [9].Chatzistergos PE, Gatt A, Formosa C, Farrugia K, Chockalingam N, Optimised cushioning in diabetic footwear can significantly enhance their capacity to reduce plantar pressure, Gait Posture. 79 (2020) 244–250. 10.1016/j.gaitpost.2020.05.009. [DOI] [PubMed] [Google Scholar]
- [10].van Netten JJ, Sacco ICN, Lavery LA, Monteiro-Soares M, Rasmussen A, Raspovic A, Bus SA, Treatment of modifiable risk factors for foot ulceration in persons with diabetes: a systematic review, Diabetes Metab. Res. Rev 36 (2020) e3271. 10.1002/dmrr.3271. [DOI] [PubMed] [Google Scholar]
- [11].Johnson LK, Richburg C, Lew M, Ledoux WR, Aubin PM, Rombokas E, 3D Printed lattice microstructures to mimic soft biological materials, Bioinspir. Biomim 14 (2018) 016001. 10.1088/1748-3190/aae10a. [DOI] [PubMed] [Google Scholar]
- [12].Telfer S, Woodburn J, Collier A, Cavanagh PR, Virtually optimized insoles for offloading the diabetic foot: A randomized crossover study, J. Biomech 60 (2017) 157–161. 10.1016/j.jbiomech.2017.06.028. [DOI] [PubMed] [Google Scholar]
- [13].Ma Z, Lin J, Xu X, Ma Z, Tang L, Sun C, Li D, Liu C, Zhong Y, Wang L, Design and 3D printing of adjustable modulus porous structures for customized diabetic foot insoles, Int. J. Lightweight Mater. Manuf 2 (2019) 57–63. 10.1016/j.ijlmm.2018.10.003. [DOI] [Google Scholar]
- [14].Jandova S, Mendricky R, Benefits of 3D Printed and Customized Anatomical Footwear Insoles for Plantar Pressure Distribution, 3D Print. Addit. Manuf (2021). 10.1089/3dp.2021.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Peker A, Aydin L, Kucuk S, Ozkoc G, Cetinarslan B, Canturk Z, Selek A, Additive manufacturing and biomechanical validation of a patient-specific diabetic insole, Polym. Adv. Technol 31 (2020) 988–996. 10.1002/pat.4832. [DOI] [Google Scholar]
- [16].Rankouhi B, Javadpour S, Delfanian F, Letcher T, Failure Analysis and Mechanical Characterization of 3D Printed ABS With Respect to Layer Thickness and Orientation, J. Fail. Anal. Prev 16 (2016). 10.1007/s11668-016-0113-2. [DOI] [Google Scholar]
- [17].Banjanin B, Vladic G, Pál M, Balos S, Dramicanin M, Rackov M, Knezevic I, Consistency analysis of mechanical properties of elements produced by FDM additive manufacturing technology, Matér. Rio Jan 23 (2018). 10.1590/S1517-707620180004.0584. [DOI] [Google Scholar]
- [18].Zou R, Xia Y, Liu S, Hu P, Hou W, Hu Q, Shan C, Isotropic and anisotropic elasticity and yielding of 3D printed material, Compos. Part B Eng 99 (2016) 506–513. 10.1016/j.compositesb.2016.06.009. [DOI] [Google Scholar]
- [19].Lim T, Cheng H, Song W, Lee J, Kim S, Jung W, Simulated and Experimental Investigation of Mechanical Properties for Improving Isotropic Fracture Strength of 3D-Printed Capsules, Materials. 14 (2021) 4677. 10.3390/ma14164677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yildiz K, Medetalibeyoglu F, Kaymaz I, Ulusoy GR, Triad of foot deformities and its conservative treatment: With a 3D customized insole, Proc. Inst. Mech. Eng. [H]. 235 (2021) 780–791. 10.1177/09544119211006528. [DOI] [PubMed] [Google Scholar]
- [21].Teixeira R, Coelho C, Oliveira J, Gomes J, Pinto VV, Ferreira MJ, Nóbrega JM, da Silva AF, Carneiro OS, Towards Customized Footwear with Improved Comfort, Materials. 14 (2021) 1738. 10.3390/ma14071738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hossain M, Navaratne R, Perić D, 3D printed elastomeric polyurethane: Viscoelastic experimental characterizations and constitutive modelling with nonlinear viscosity functions, Int. J. Non-Linear Mech 126 (2020) 103546. 10.1016/j.ijnonlinmec.2020.103546. [DOI] [Google Scholar]
- [23].Morita J, Ando Y, Komatsu S, Matsumura K, Okazaki T, Asano Y, Nakatani M, Tanaka H, Mechanical Properties and Reliability of Parametrically Designed Architected Materials Using Urethane Elastomers, Polymers. 13 (2021) 842. 10.3390/polym13050842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jiang Y, Wang Q, Highly-stretchable 3D-architected Mechanical Metamaterials, Sci. Rep 6 (2016) 34147. 10.1038/srep34147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].DLS 3D Printing Technology, Carbon. (n.d.). https://www.carbon3d.com/carbon-dls-technology/ (accessed February 8, 2022).
- [26].Carbon Design Engine - A New Way to Design 3D Printed Products, Carbon. (n.d.). https://www.carbon3d.com/products/carbon-design-engine/ (accessed February 8, 2022).
- [27].Armstrong DG, Lavery LA, Kimbriel HR, Nixon BP, Boulton AJM, Activity Patterns of Patients With Diabetic Foot Ulceration: Patients with active ulceration may not adhere to a standard pressure off-loading regimen, Diabetes Care. 26 (2003) 2595–2597. 10.2337/diacare.26.9.2595. [DOI] [PubMed] [Google Scholar]
- [28].Brazeau A-S, Hajna S, Joseph L, Dasgupta K, Correlates of sitting time in adults with type 2 diabetes, BMC Public Health. 15 (2015) 793. 10.1186/s12889-015-2086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dasgupta K, Joseph L, Pilote L, Strachan I, Sigal RJ, Chan C, Daily steps are low year-round and dip lower in fall/winter: findings from a longitudinal diabetes cohort, Cardiovasc. Diabetol 9 (2010) 81. 10.1186/1475-2840-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lord JD, Morrell R, Measurement Good Practice Guide No. 98 Elastic Modulus Measurement, (n.d.) 100. [Google Scholar]
- [31].Mihai LA, Goriely A, How to characterize a nonlinear elastic material? A review on nonlinear constitutive parameters in isotropic finite elasticity, Proc. Math. Phys. Eng. Sci 473 (2017) 20170607. 10.1098/rspa.2017.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Faulí AC, Andrés CL, Rosas NP, Fernández MJ, Parreño EM, Barceló CO, Physical Evaluation of Insole Materials Used to Treat the Diabetic Foot, J. Am. Podiatr. Med. Assoc 98 (2008) 229–238. 10.7547/0980229. [DOI] [PubMed] [Google Scholar]
- [33].Paton JS, Stenhouse E, Bruce G, Jones R, A Longitudinal Investigation into the Functional and Physical Durability of Insoles Used for the Preventive Management of Neuropathic Diabetic Feet, J. Am. Podiatr. Med. Assoc 104 (2014) 50–57. 10.7547/0003-0538-104.1.50. [DOI] [PubMed] [Google Scholar]
- [34].Brodsky JW, Pollo FE, Cheleuitte D, Baum BS, Physical Properties, Durability, and Energy-Dissipation Function of Dual-Density Orthotic Materials Used in Insoles for Diabetic Patients, Foot Ankle Int 28 (2007) 880–889. 10.3113/FAI.2007.0880. [DOI] [PubMed] [Google Scholar]
- [35].Bus SA, van Deursen RWM, Kanade RV, Wissink M, Manning EA, van Baal JG, Harding KG, Plantar pressure relief in the diabetic foot using forefoot offloading shoes, Gait Posture. 29 (2009) 618–622. 10.1016/j.gaitpost.2009.01.003. [DOI] [PubMed] [Google Scholar]
- [36].Chantelau E, Kushner T, Spraul M, How Effective is Cushioned Therapeutic Footwear in Protecting Diabetic Feet? A Clinical Study, Diabet. Med 7 (1990) 355–359. 10.1111/j.1464-5491.1990.tb01404.x. [DOI] [PubMed] [Google Scholar]
- [37].Macdonald EM, Perrin BM, Hyett N, Kingsley MIC, Factors influencing behavioural intention to use a smart shoe insole in regionally based adults with diabetes: a mixed methods study, J. Foot Ankle Res 12 (2019) 29. 10.1186/s13047-019-0340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pai S, Ledoux WR, The shear mechanical properties of diabetic and non-diabetic plantar soft tissue, J. Biomech 45 (2012) 364–370. 10.1016/j.jbiomech.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Brady L, Pai S, Iaquinto JM, Wang Y-N, Ledoux WR, The compressive, shear, biochemical, and histological characteristics of diabetic and non-diabetic plantar skin are minimally different, J. Biomech 129 (2021) 110797. 10.1016/j.jbiomech.2021.110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wrobel JS, Ammanath P, Le T, Luring C, Wensman J, Grewal GS, Najafi B, Pop-Busui R, A Novel Shear Reduction Insole Effect on the Thermal Response to Walking Stress, Balance, and Gait, J. Diabetes Sci. Technol 8 (2014) 1151–1156. 10.1177/1932296814546528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


