Abstract
Background
Nowadays, a wide range of wound dressings is already commercially available. The selection of the dressing is of paramount importance as inappropriate wound management and dressing selection can delay the wound healing process. Not only can this be distressing for the patient, but it can also contribute to complications such as maceration and subsequent infection. Many researchers are targeting the design of dressings with superior properties over existing commercial dressings. However, reported results in the state-of-the-art are rarely benchmarked against commercial dressings. The aim of this study was to determine several characteristics of a large variety of the most frequently used commercial wound dressings, providing an overview for both practitioners and researchers.
Methods
For this comparative study, 11 frequently used commercial wound dressings were selected, representing the different types. The morphology was studied using scanning electron microscopy. The dressings were characterized in terms of swelling capacity (water, phosphate buffered saline and simulated wound fluid), moisture vapour transmission rate (MVTR) and moisture uptake capacity (via dynamic vapour sorption) as well as mechanical properties using tensile testing and texturometry.
Results
The selected dressings showed distinctive morphological differences (fibrous, porous and/or gel) which was reflected in the different properties. Indeed, the swelling capacities ranged between 1.5 and 23.2 g/g (water), 2.1 and 17.6 g/g (phosphate buffered saline) or 2.9 and 20.8 g/g (simulated wound fluid). The swelling capacity of the dressings in water increased even further upon freeze-drying, due to the formation of pores. The MVTR values varied between 40 and 930 g/m2/24 h. The maximal moisture uptake capacity varied between 5.8% and 105.7% at 95% relative humidity. Some commercial dressings exhibited a superior mechanical strength, due to either being hydrophobic or multi-layered.
Conclusions
The present work not only offers insight into a valuable toolbox of suitable wound dressing characterization techniques, but also provides an extensive landscaping of commercial dressings along with their physico-chemical properties, obtained through reproducible experimental protocols. Furthermore, it ensures appropriate benchmark values for commercial dressings in all forthcoming studies and could aid researchers with the development of novel modern wound dressings. The tested dressings either exhibited a high strength or a high swelling capacity, suggesting that there is still a strong potential in the wound dressings market for dressings that possess both.
Keywords: Wound dressings, Burns, Exuding wounds, Wound healing, Exudate management, Physico-chemical
Highlights.
This article focuses on the in-depth mechanical and physico-chemical characterization of 11 commercial wound dressings, providing an unprecedented overview, of interest to both researchers and practitioners involved in wound care.
The protocols elaborated herein ensure appropriate benchmark values for commercial dressings.
As demonstrated in this study, the physico-chemical properties can vary widely amongst commercially available dressings, with either a high strength or a high swelling capacity, suggesting the strong potential of dressings combining both.
Background
Wounds are a global medical concern [1]. The international advanced wound care market is expected to exceed $22 billion by 2024. Key factors contributing to the growth include the increasing number of road accidents and chronic diseases such as diabetes as well as an expanding geriatric population [2]. The skin, which is the largest human organ, is an indispensable barrier. It mainly separates the body from the environment, protecting it from thermal, physical and chemical damage and preventing the invasion of most pathogens [3–5]. A wound signifies a breach in the skin due to trauma. Depending on the depth of the wound, the epidermis, dermis and/or hypodermis can be affected and the repair process will be activated [6,7]. The wound healing process is one of the most complicated biological processes that occur in the human body, which makes wound management one of the most essential practices today [8].
Wound dressings are typically used to assist the wound healing process. They are mainly developed to aid re-epithelialization by protecting the wound from further damage and/or infection [1,8]. Modern wound dressings protect the wound from contamination while regulating the exudate to provide a moist healing environment [9]. Nowadays, a large variety of modern wound dressings is already available (Table 1). For both the expert and the non-expert audience, a non-exhaustive list of definitions can be found in Table S1, see online supplementary material. Alginate dressings, hydrocolloids, hydrogels, foams and films all have the capacity to control the level of wound hydration [10–13]. However, selection of the appropriate dressing is of paramount importance in wound management. The choice depends on the wound size and location, the level of exudate and clinician preference. If the dressing is not absorbing enough, it can lead to maceration, which in turn increases the risk of infection. However, a dressing that has a too high absorption capacity can also disrupt the tissue. Inappropriate wound management and dressing selection can thus delay the wound healing process [12,14–16].
Table 1.
Overview of the different types of wound dressings, their (dis)advantages and wound application
| Dressing category | Advantage(s) | Disadvantage(s) | Examples of commercial dressings | Wound types |
|---|---|---|---|---|
| Passive | ||||
| Gauze | Cheap, used as cover | Can stick to and disrupt wound bed upon removal | Sterilux | Minor or superficial clean wounds |
| Tulle | Does not stick to wound surface | Secondary dressing often required | Jelonet, Bactigras | Flat, shallow wounds with minimal to moderate exudate |
| Interactive | ||||
| Semi-permeable films | Allow gaseous exchange of air and water vapour, impermeable for bacteria and fluids, very flexible and conformable | Maceration likely due to limited exudate absorption capacity | DuoDERM | Shallow wounds with low exudate on difficult anatomical sites (e.g. joints) |
| Semi-permeable foams | Designed to absorb large amounts of exudate, can be left in place for several days | Cause dryness and scabbing on low-exuding wounds | Mepilex (Ag) | Moderate to heavily exuding wounds |
| Hydrogels | Maintain moisture, allows vapour and oxygen exchange, aids tissue debridement | Fluid accumulation can lead to maceration or infection of skin, mechanically weak | Hydrosorb | Wounds or cavities with low to medium exudate, necrotic wounds |
| Bioactive | ||||
| Hydrocolloids | Form gel on wound surface maintaining moisture and vapour/fluid exchange | Not suited for infected or heavily exuding wounds | DuoDERM | Light to moderate exuding wounds, sloughing or granulating wounds |
| Hydrofibres | Highly absorbent, non-adherent | Usually requires a secondary dressing | Aquacel (Ag/Extra) | Medium to heavily exuding wounds, burns |
| Alginates | Highly absorbent, optimal moisture level, clotting encouraged | Usually requires a secondary dressing | Kaltostat | Second degree burns, moderate to heavily exuding wounds |
Different wounds require different types of dressings [6,17–22]. They are made with different materials and thus have their own typical characteristics, such as absorption capacity, mechanical strength and moisture vapour transmission rate (MVTR), which all have to be considered for the treatment of a particular type of wound. To choose the optimal dressing, the wound must be evaluated regularly for changes in size and depth, moisture content, necrotic tissue, maceration, infection and patient comfort. The TIME principle (tissue, infection, moisture, edge) offers a systematic approach for clinicians and practitioners dedicated to wound management [23].
Despite the large variety of wound dressings already available, comparative in-depth studies of typical characteristics of commercial wound dressings are scarce in the literature. Furthermore, research is still ongoing to develop new and more advanced wound dressings, including smart polymeric bandages as described in [24–28]. Many researchers are targeting the design of dressings with ‘superior properties’ over existing commercially available dressings. However, the reported results in the state-of-the-art are rarely benchmarked against those of commercial dressings. In view of the lack of comparative studies on the physico-chemical properties of contemporary modern commercial wound dressings, in addition to the current knowledge gap related to the methodological approaches ideally suited to enable an in-depth and reproducible characterization of wound dressings, this article aims to fill the gap and provide an overview for both wound care practitioners and researchers. The selected dressings are shown in Figure 1.
Figure 1.

Overview and classification of the selected wound dressings
This selection includes representatives from the different dressing types (e.g. foams, films, hydrofibres, hydrogels) currently used for the treatment of many different wound types. Within the large range of dressings that are currently available, there is an emphasis on burn wound dressings, antimicrobial dressings and odour-adsorbing dressings as they are used on wounds that are typically concomitant with high levels of exudate. Moreover, the current selection focuses on dressings that are considered the gold standard by the scientific community and that are currently used in the Burn Wound Centre of the Ghent University Hospital (Belgium). In this regard, exploring the differences between the ‘weaker’ (e.g. fibre-based) and ‘stronger’ (e.g. multi-layered) dressings as reported in the literature is especially of interest. Summarizing the above, the following selection gives a strong representation of the wound dressing market.
Kaltostat® Alginate dressing consists of alginate fibres that can form a firm gel/fibre mat upon contact with wound exudate. This dressing can be applied on a variety of wounds that display moderate to high levels of wound exudate, including pressure injuries, venous/arterial/diabetic ulcers and burn wounds. It also helps to control minor bleeding due to the haemostatic properties of alginate [29].
Aquacel® Ag Hydrofiber® dressing is made of sodium carboxymethylcellulose (CMC) in which silver ions are embedded. Upon contact with wound fluid, the hydrofibres become hydrated which leads to the formation of a gel. The dressing can be applied on a variety of acute and chronic wounds including (infected) ulcers and second-degree burns [29].
Aquacel® Extra wound dressing is also a hydrofibre dressing. It is composed of two layers of hydrofibres that are stitched together using strengthening fibres. These strengthening fibres, which are not incorporated in Aquacel® Ag, do not swell upon contact with wound fluid and therefore provide structural integrity. The dressing is mainly applied to moderate to highly exuding wounds [29].
Exufiber® dressing is a nonwoven dressing consisting of poly(vinylalcohol) (PVA) fibres. When the dressing comes into contact with exudate, a gel is formed that conforms closely to the wound bed. It is applied onto moderate to highly exuding wounds, including cavities. The Hydrolock® technology ensures absorption of exudate while also locking it into the fibres [30].
Mepilex® dressing is a foam dressing consisting of polyurethane foam and a silicone wound contact layer. It can be applied onto light to moderate exuding wounds [30].
Mepilex® Ag is identical to Mepilex®, except for the addition of silver nanoparticles that render the dressing antimicrobial. It is indicated for use on (infected) light to moderate exuding wounds and second-degree burns [30].
Hydrosorb® is a transparent hydro-cellular polyurethane-polyurea hydrogel dressing, covered with a semi-permeable outer layer. The aqueous matrix mainly consists of propylene glycol. Its water content of ~60% ensures a moist wound environment, supporting tissue granulation and epithelialization. It also promotes the debridement of necrotic wounds and can be used for treatment of light to moderate exuding wounds [31].
DuoDERM® Extra Thin (ET) is a hydrocolloid dressing that consists of a thin semi-permeable polyurethane film covered with an adhesive layer for the treatment of dry to light exuding wounds [29].
Acticoat◊ dressing is an absorbent wound dressing that ensures a moist wound environment for wound healing. It is mainly designed to control infection of wounds, more specifically burns. The absorbent rayon/polyester layer is sandwiched between two antimicrobial silver-coated poly(ethylene) nets. Upon hydration, silver release is triggered [32].
Carbonet◊ dressing has a composite structure, consisting of a knitted viscose wound contact layer, an absorbing fibrous cellulose pad and an adsorbing activated charcoal layer, which is sandwiched between poly(ethylene) nets, for wound odour. This dressing is used for the treatment of discharging, infected and malodorous wounds [32].
Carboflex® dressing is another multi-layered wound dressing, like the Carbonet◊ dressing, used for the management of malodour associated with exuding malodorous wounds. The absorbent wound-contact layer consists of alginate and a hydrocolloid. The other layers are a central activated charcoal pad and a water-resistant top layer [33,34].
This study aims to compare different frequently used dressings by determining the characteristics of each dressing in terms of swelling, mechanical properties, moisture uptake capacity and MVTR. The current manuscript not only offers insight into a valuable toolbox of suitable wound dressing characterization techniques, but also provides an extensive benchmarking of commercially available dressings along with their physico-chemical properties, obtained through reproducible and standardized experimental protocols. Hence, this paper will provide future opportunities for similar characterization of novel dressings, exploiting the standardized protocols elaborated herein, and thereby ensuring appropriate benchmark values for commercial dressings in all forthcoming studies.
Methods
Materials
Phosphate buffered saline (PBS) tablets, maximum recovery diluent and foetal bovine serum were obtained from Sigma-Aldrich. Hydrosorb® came from Hartmann. DuoDERM® ET, Kaltostat®, Aquacel® Extra, Aquacel® Ag and Carboflex® were purchased from ConvaTec. Mepilex®, Mepilex® Ag and Exufiber® were obtained from Mölnlycke. Carbonet◊ and Acticoat◊ were purchased at Smith&Nephew. All materials were used as received.
Material characterization
Freeze-drying
The materials were freeze-dried in a Christ freeze-dryer alpha 2-4 LSC at −85°C and 0.37 mbar.
Swelling experiments
All swelling tests were performed in triplicate at 20°C. The PBS solution was made by dissolving PBS tablets (1 per 200 mL) in ultrapure water, yielding 0.01 M phosphate buffer, 0.0027 M potassium chloride and 0.137 M sodium chloride with a pH of 7.415. The simulated wound fluid (SWF) was prepared using 50 v/v% foetal bovine serum and 50 v/v% maximum recovery diluent containing 1.0 g L−1 peptic digest of animal tissue and 8.5 g L−1 sodium chloride [35]. This formulation resembling wound exudate was chosen as foetal bovine serum and maximum recovery diluent are both commercially available, thus ensuring easy reproduction and thereby reducing inter-study variability in the field.
When performing the swelling tests, two different methods were used. The first method consisted of first placing the round samples (diameter Ø 12 mm) in ultrapure water for 24 h. Afterwards, the samples were freeze-dried and the dry weight was measured (m0). The samples were then each placed in 10 mL of solution (ultrapure water, PBS or SWF) for 72 h to reach complete swelling equilibrium. The swollen weight was then recorded (m). For the second method, the samples (Ø 12 mm) were weighed (m0) (without leaching and freeze-drying) and directly placed for 72 h into 10 mL of solution. The swollen weight was then also recorded (m). This second method represented the real-life situation when dressings are applied onto patients. The swelling degree S was calculated using equation (1):
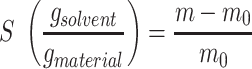 |
(1) |
Tensile testing
Tensile tests were performed 5-fold on a Tinius Olsen 5ST at 20°C and analysed using Horizon software. Two different load cells were used (25 N and 500 N) as the load cell should work between 1 and 100% of its maximal load for a precise measurement. Dog-bone-shaped (50 mm long, 4 mm wide and 1 mm thick) samples were punched from the different wound dressings. The samples were subjected to tensile testing in both the dry and the swollen state, for which the samples were swollen for 72 h in ultrapure water at 20°C before the measurement. Their dimensions were again measured in the swollen state. The tensile tests were performed with a preload of 0.1 N and a speed of 10 mm min−1 until break, which allowed the Young’s modulus to be determined from the slope of the stress–strain curves, the total elongation and the stress at break for each dressing.
Texturometry experiments
Texturometry tests were executed on a TA500 Texture Analyzer from Lloyd Instruments. The results were analysed by Nexygen software. The texturometer was equipped with a 100 N load cell. The samples were placed in ultrapure water for 72 h. Afterwards, they were cut (40 x 40 mm) and fixed onto the flat bottom plate with a round gap (Ø 25 mm) in the middle. An identical plate was placed on top of the sample and secured. A cylindrical plunger (Ø 3 mm) was attached to the load cell for the different experiments. All measurements were performed in triplicate at 20°C.
Fatigue tests subjected the samples to repeated loading, more specifically 15 load cycles. These tests were performed with the cylindrical probe at a rate of 10 mm min−1 and a limit of 2 mm.
For the fracture tests, the cylindrical probe compressed the sample at a rate of 10 mm min−1 until fracture, which was defined as a decrease of the maximum force by 50%.
Scanning electron microscopy
The morphology of the dressings was studied with a Phenom-FEI Desktop scanning electron microscope. The dressing samples were freeze-dried in advance and fixed to the sample holder using double-sided carbon tape. They were gold sputter-coated (20 mA, 60 s, vacuum) using an Automatic Au Sputter Coater EmiTech K550X with a RV3 two-stage rotary vane pump. The fibre diameters of the fibrous dressings or layers were determined with the Fibermetrics software.
MVTR determination
For the determination of the MVTR, samples (Ø 28 mm) were punched from the different dressings. The test dish was filled with 10 mL of ultrapure water. A lightweight grid was placed on the opening of the test dish but underneath the sample to ensure that the sample was fixed. Lastly, the test dish was sealed with an open lid (Ø 25 mm opening). The dish assembly (Figure S1, see online supplementary material) was weighed (m1) and placed in an incubator at 20°C. After 7 days, the dishes were weighed again (m2), which allowed determination of the rate of vapour movement through the sample from the water to the surrounding atmosphere following equation (2):
 |
(2) |
Where t represents the duration of the experiment and A the area of the opening through which the water could evaporate.
Dynamic vapour sorption experiments
Dynamic vapour sorption (DVS) experiments were performed to determine the moisture uptake capacities of the different dressings at varying relative humidities (RH). A Cahn microbalance inside a temperature-controlled cabinet measured both the loaded sample pan and the empty reference pan. Flow controllers regulated the flow of the dry and wet N2 gas. This gravimetric technique allowed control over the temperature and the RH. The DVS experiments were performed with 5–10 mg of the sample at 20°C. As some of the dressings were already humid in the packaging, the first step of 95% RH was necessary to ensure an identical experimental set-up for each wound dressing. The humidity was then varied in steps (95, 90, 60, 30, 0, 30, 60, 90, 95% RH). The subsequent step was initiated when the gravimetric mass change as a function of time was <0.002% min−1.
Fourier transform infrared spectroscopy
The different wound dressing materials were also characterized using Fourier transform infrared spectroscopy (FTIR). FTIR spectra ranging between 4000 and 600 cm−1 were recorded using a PerkinElmer Frontier FTIR/FIR spectrometer, equipped with a MKII Golden Gate Single Reflection ATR system together with a diamond crystal and a sapphire anvil, and analysed using PerkinElmer Spectrum software. The different spectra and an overview of the most characteristic bands can be found in Figure S2 and Table S2, see online supplementary material.
Statistical analysis
All results are displayed as mean ± SD (standard deviation). A two-tailed t-test was performed when two values were compared. If more values were to be compared, statistical analysis was performed using one-way analysis of variance using GraphPad Prism 8. Statistical significance was set at p < 0.05. Statistically significant (*) and non-significant (ns) differences are indicated in all Figures.
Results
In this work, the selected wound dressings were subjected to in-depth characterization. In the first part of the work, the dressings were visualized by scanning electron microscopy (SEM). The swelling capacity in different media was determined, as the absorbance capacity of the dressing plays a crucial role in providing a moist wound-healing environment. In addition, the MVTR was also assessed as it plays a complementary role in moisture control. The dressings were subjected to mechanical analysis both in dry and swollen conditions. The hygroscopic behaviour of the different dressings was further investigated using DVS.
Visualization of wound dressings using SEM
The morphology of the wound dressings was studied using SEM (Figure 2). The fibrous dressings (Figure 2a–d) all contain smooth fibres. The porous nature of the flexible dressings Mepilex® and Mepilex® Ag (Figure 2e, f) displays a strong contrast with the smooth surfaces of Hydrosorb® and DuoDERM® ET (Figure 2g, h). The presence of the silver nanoparticles in Aquacel® Ag (Figure 2b) and Mepilex® Ag (Figure 2f) can be clearly seen in Figure 2. The multi-layered dressings also contain fibrous layers (i–p). Indeed, Carbonet◊ contains a fibrous top, middle and bottom layer whereas Carboflex® has a fibrous top layer (similar to Kaltostat®) and Acticoat◊ a fibrous middle layer. The fibre diameters of the different fibrous dressings or layers in the multi-layered dressings were also determined. As can be seen from Figure 2 (bottom), the diameters of the different fibres range between 11.5 and 20.7 μm.
Figure 2.

Top: SEM images of the commercial dressings. (a) Kaltostat®, (b) Aquacel® Ag, (c) Aquacel® Extra, (d) Exufiber®, (e) Mepilex®, (f) Mepilex® Ag, (g) Hydrosorb®, (h) DuoDERM® ET, (i) Carbonet◊ top, (j) Carbonet◊ middle, (k) Carbonet◊ bottom, (l) Carboflex® top, (m) Carboflex® activated carbon layer, (n) Carboflex® bottom, (o) Acticoat◊ middle and (p) Acticoat◊ cover. Bottom: Fibre diameters of the different fibrous layers determined using SEM. SEM scanning electron microscopy
Swelling properties of wound dressings
The swelling capacities of the different dressings in ultrapure water, PBS and SWF as well as the effect of freeze-drying on the swelling capacity in ultrapure water are shown in Figure 3.
Figure 3.

Swelling capacities of the different wound dressings according to two different methods. (a) The swelling capacity in water (blue), PBS (purple) and SWF (dark blue) without freeze-drying. (b) Comparison of the swelling capacities of the dressings in water with (blue) and without (white) freeze-drying (FD). *Statistically significant. ns non-significant, PBS phosphate buffered saline, SWF simulated wound fluid
As can be seen from Figure 3a, the swelling capacity or absorbency of the different dressings after equilibrium swelling (and without prior freeze-drying, representing the real-life situation) ranges between 1.5 and 23.2 g/g.
Mechanical properties of wound dressings
Results of the mechanical characterization of commercial wound dressings are scarce in the literature [14,36]. However, it can be very useful to quantify the mechanical properties as the dressing will be subjected to multiple tensile and compressive stresses during use. Therefore, in the second part of this work, several mechanical properties were determined using tensile testing and texturometry. Tensile testing is an essential technique in which several properties can be determined by subjecting the sample to controlled tension until failure. The strength and elongation potential of the different commercial wound dressings were assessed.
Texturometry allows the measurement and analysis of several mechanical properties of materials. Samples are compressed by a plunger at a constant rate and temperature. For texture profile analysis, the sample is compressed twice. Fatigue testing allows investigation of changes in elastic behaviour by subjecting the materials to repeated compression. Fracture tests allows determination of the breaking force as the sample is compressed until rupture. The most interesting properties of wound dressings are discussed.
Mechanical properties determined using tensile testing
From the obtained data, it can be observed that the mono-layered dressings (Figure 4a) have a Young’s modulus of <1 MPa, which is much lower compared to the multi-layered dressings (Figure 4b). The Young’s modulus of the fibrous dressings Kaltostat®, Aquacel® Ag, Aquacel® Extra and Exufiber® varies between 0.24 and 0.95 MPa, with the total elongation (Figure 4c) varying between 68 and 134%. When comparing Hydrosorb® and DuoDERM® ET, it can be concluded that their respective mechanical properties do not differ significantly except for the total elongation.
Figure 4.

Mechanical properties of dry wound dressings determined using tensile testing at a traction speed of 10 mm min−1. (a, b) Young’s modulus, (c) total elongation and (d) ultimate stress. The results for the dressings in the swollen state can be found in Table S3, see online supplementary materials. *Statistically significant. ns non-significant, ET Extra Thin
Upon equilibrium swelling, the Young’s moduli significantly decreased for most dressings. The values for DuoDERM® ET and Carbonet◊ are significantly lower in the swollen state compared to their values in the dry state (Table S3, see online supplementary material). The Young’s moduli of Mepilex® and Mepilex® Ag did also significantly decrease to a limited extent upon swelling prior to the tensile test. In contrast, Carboflex® has a significantly higher Young’s modulus in the swollen state. The Young’s modulus of Acticoat◊ did not significantly decrease when swollen. Upon equilibrium swelling, the total elongation dropped significantly for all dressings except Acticoat◊.
The ultimate stress (Figure 4d) of the dry mono-layered dressings does not exceed 0.77 MPa whereas the ultimate stress of the dry multi-layered dressings ranges between 0.17 and 4.60 MPa, with the highest value for Acticoat◊. The fibrous dressings Kaltostat®, Aquacel® Ag, Aquacel® Extra as well as the hydrogel dressing Hydrosorb® exhibit the lowest ultimate stress, ranging between 0.04 and 0.53 MPa.
Texturometry
Fatigue properties
Aquacel® Ag and Aquacel® Extra were too fragile to be tested with texturometry in the swollen state and broke immediately after being clamped. The swollen dressing samples were subjected to 15 compression cycles for fatigue testing. The results are displayed in Figure 5. For most dressings, there is no significant difference between the first and the last cycle load. The extent of plastic deformation of Kaltostat® was studied further by subjecting the dressing to a total of 100 cycles (Figure 3, see online supplementary material). It could be concluded that there was no significant difference between the cycle load after 15 and 100 cycles for this dressing.
Figure 5.

Results of fatigue testing determined using texturometry at a speed of 10 mm min−1. The first cycle load is visualized in light blue, the last cycle load in dark blue. Loads are in Newton (N). *Statistically significant. ET Extra Thin
Fracture behaviour
In a second texturometric experiment, the materials were indented until fracture. The fracture strength can be calculated by dividing the maximal load by the surface area of the plunger (9.8 mm2). As can be observed in Figure 6, the fracture strength is <0.46 MPa for the mono-layered wound dressings whereas the value for the multi-layered dressings varies between 2.02 and 9.03 MPa. The fracture experiments of the fibrous dressings Aquacel® Ag and Aquacel® Extra failed as they already ruptured without a significant impression of the cylinder.
Figure 6.
Results of fracture testing determined via texturometry at a speed of 10 mm min−1. *Statistically significant. ET Extra Thin
MVTR of wound dressings
The MVTR is another important feature of a wound dressing. The MVTR values of the tested commercial dressings (Figure 7) in this work varied between 40 and 930 g/m2/24 h.
Figure 7.
Moisture vapour transmission rates of the selected commercial wound dressings. ns non-significant, MVTR moisture vapour transmission rate, ET Extra Thin
Moisture uptake capacity determination using DVS
As moisture can have a detrimental effect on the shelf-life of healthcare products, the moisture uptake capacity of the dressings was assessed with DVS in order to investigate their sorption behaviour in humid environments. The maximal moisture uptake capacity (Figure 8) varied between 5.8 (DuoDERM® ET) and 105.7% (Hydrosorb®) at 95% RH.
Figure 8.
Moisture absorption of commercial wound dressings at varying relative humidities (RH) determined via dynamic vapour sorption. The subsequent step was initiated when the gravimetric mass change as a function of time was <0.002% min−1, except for Hydrosorb® (0.02% min−1) as no result could be obtained in a comparable timeframe. ET Extra Thin
Discussion
Morphology of the dressings
It was observed that the fibrous and porous morphology of these dressings influences the swelling capacity and MVTR. The thinner the fibre, the higher the surface-to-volume ratio. Aquacel® Ag and Aquacel® Extra have the lowest fibre diameters (Figure 2), leading to a higher surface-to-volume ratio which is also reflected in their higher maximal moisture uptake and swelling capacity compared to Exufiber® (see also further in Figure 3 and Figure 7). There is no significant difference in fibre diameter between Aquacel® Extra and Aquacel® Ag, as expected.
Swelling properties of the dressings
The fibrous dressings Kaltostat®, Aquacel® Ag and Aquacel® Extra and the foam dressing Mepilex® have higher swelling capacities (Figure 3a) compared to the hydrogel and hydrocolloid dressings. Indeed, DuoDERM® ET has a lower swelling capacity of 1.5 g/g due to the presence of the waterproof barrier. The higher swelling capacity of these fibrous and foam dressings can be correlated to their higher surface-to-volume ratio. The multi-layered (three or more layers) dressings Acticoat◊, Carbonet◊ and Carboflex®, despite being multi-layered, exhibit average absorbencies of 3.4–10.3 g/g. Acticoat◊ mainly consists of poly(ethylene) nets, which hardly swell, and Carbonet◊ and Carboflex® both contain a hydrophobic activated-carbon layer [37]. For most materials, the swelling capacity does not differ significantly in the different media. However, Kaltostat® has a significantly lower swelling capacity in PBS compared to ultrapure water. This is probably caused by the partial disintegration of the alginate hydrogel fibres due to the exchange of multivalent Ca2+ with the monovalent ions present in PBS and, to a certain extent, in SWF.
When assessing the swelling properties of the freeze-dried dressings (Figure 3b and Table S4, see online supplementary material), it can be observed that the average swelling capacity is higher for most dressings. This is not unexpected, as freeze-drying can lead to the formation of pores [38] and thus an increased surface-to-volume ratio. This effect is most pronounced and significant for the hydrogel dressing Hydrosorb®, the PVA-based dressing Exufiber® and the CMC-based dressings Aquacel® Ag and Aquacel® Extra.
Mechanical properties of the dressings
The fibrous dressings Kaltostat®, Aquacel® Ag, Aquacel® Extra and Exufiber® have moderate strength and flexibility compared to the other dressings in this study (Figure 4). Mepilex® and Mepilex® Ag have the lowest Young’s moduli in this series, but the highest total elongation, which signifies that they are very flexible. Acticoat◊ has the highest Young’s modulus and the lowest total elongation in this series, rendering it the stiffest dressing of the ones tested. DuoDERM® ET has a high total elongation of 714%, comparable to the foam dressings (814 and 697% respectively for Mepilex® and Mepilex® Ag), implying that DuoDERM® ET is more flexible than Hydrosorb®. When comparing the Young’s moduli of the dressings in the dry to the swollen state (Table S3, see online supplementary material), it can be anticipated that the Young’s modulus will significantly decrease when the material is swollen. This could be observed for several materials. Indeed, the fibrous dressings (Kaltostat®, Aquacel® Ag, Aquacel® Extra and Exufiber®) as well as Hydrosorb® even became too fragile to be subjected to tensile testing. The Young’s modulus of Carboflex® increased upon swelling. As the top layer of this multi-layered dressing is identical to Kaltostat®, it is anticipated that this layer has a larger influence on the Young’s modulus in the dry state compared to the swollen state. Indeed, the values for Carboflex® and Kaltostat® are similar when measured in the dry state. The Young’s modulus of Acticoat◊ did not significantly decrease upon equilibrium swelling, which can be linked to its limited swelling capacity (3.4 g/g).
In contrast to the other dressings, the total elongation of Acticoat◊ did not significantly drop upon swelling, which could again be linked to its limited swelling capacity. As polymer-chain disentanglement occurs due to swelling, the amount of strain required to elongate the swollen materials up to failure decreases [39].
As expected, Mepilex® does not exhibit a significantly higher ultimate stress than Mepilex® Ag. When comparing the ultimate stress in the dry state to the swollen state, it can again be observed that swelling led to a significant decrease for all dressings except for Acticoat◊, which is again linked to its limited swelling, and Carboflex®. The significant increase in ultimate stress of Carboflex® can again be linked to the different influence of the top layer in the swollen state.
Generally, the multi-layered dressings exhibit significantly higher first-cycle loads than the mono-layered dressings (Figure 5), which is in agreement with the above-mentioned trends. Interestingly, Mepilex® exhibits significantly lower cycle load values than Mepilex® Ag, which can be linked to the higher swelling capacity of Mepilex®. Indeed, an increase in swelling leads to an increase in the network mesh size and thus a decrease in its compressive strength [40]. There was no significant decrease in load between the first and last cycle for most dressings, which implies that there is no significant reduction in the force that is necessary for the last compression cycle and thus that there is no significant weakening of the dressing structure after the fatigue test. This signifies that the elastic behaviour hardly changes after repeated loading. Hence, the majority of the materials exhibit no fatigue over 15 cycles. Kaltostat®, however, has a significant decrease in load after only 15 cycles, which could be linked to its strong swelling. There was no significant difference between the cycle load after 15 and 100 cycles for Kaltostat, which implies that the elastic behaviour did not change further after the 15th cycle.
The fracture strength (Figure 6) of the multi-layered dressings is much higher compared to the mono-layered dressings, as they contain strong sublayers (e.g. knitted viscose) that hardly swell and thus keep their strength upon loading.
With regard to the mechanical properties determined both with tensile testing and texturometry, it can be observed that the tested dressings exhibit varying strength and flexibility. To use these dressings for wound healing, sufficient strength is necessary to enable handling by nurses and/or patients, while flexibility contributes to patient comfort. This again indicates that the use of a certain dressing depends strongly on the application and the application area.
MVTR and moisture uptake capacity of the dressings
The higher the MVTR, the more permeable or breathable the dressing. However, a MVTR that is too high can lead to dehydration of the wound area whereas a too low MVTR can cause maceration of the wound. The average MVTR for normal skin equals 204 g/m2/24 h while the MVTR of wounds ranges between 279 (first-degree burn) and 5138 g/m2/24 h (granulating tissue) [41–43]. Whereas the optimal maximal value of the MVTR for moist wound healing used to be defined as 840 g/m2/24 h, more recent publications have shown that dressings with a MVTR up to 2000–2500 g/m2/24 h could still provide a moist environment for optimal healing [8,44,45]. The MVTR values of the commercial dressings (Figure 7) indicate that they can all be used for moist wound healing treatment. High MVTR values are achieved by open pore structures, which can especially be observed for the fibrous and foam dressings [16,17] and could already be anticipated based on their swelling capacities. DuoDERM® ET exhibits the lowest MVTR value in this series, which can be explained by the presence of the waterproof barrier on the dressing. This also affirms that DuoDERM® ET is more suited for lightly exuding wounds, while Hydrosorb®, Mepilex® and Mepilex® Ag can be applied onto lightly to moderately exuding wounds. The fibrous dressings Kaltostat®, Aquacel® Ag, Aquacel® Extra and Exufiber® are all applicable on moderately to highly exuding wounds. Acticoat◊, Carbonet◊ and Carboflex® also display high MVTR values, which mirrors their applications as burn and malodorous wound dressings. These latter dressings thus limit maceration of these typically highly exuding wounds.
Figure 8 demonstrates that only Hydrosorb® can absorb more moisture than its own mass at high RH. The low moisture uptake capacity of DuoDERM® ET and Acticoat◊ was anticipated based on their low swelling capacities even after freeze-drying. The fibrous and foam dressings show a very low degree of hysteresis (Figure S4, see online supplementary material), indicating that all absorbed moisture can be completely desorbed again. This low degree of hysteresis can be linked to the high surface-to-volume ratio of the fibres and pores in these dressings. As all dressings do not display any degree of hysteresis >3% at 60% RH, and based on the type of hysteresis loop, it can be perceived that these materials do not contain ink-bottle pores [46]. This is very interesting as it implies that the dressings can donate all moisture to the surrounding environment when the humidity is lower, such as in a dry wound bed. This can also be linked to the breathability of the dressing. If there is nearly no hysteresis in all dressings, this also allows moisture uptake during the wound healing process and equal release to the environment, which links the results of Figures 7 and 8.
Conclusions
The present work offers a profound overview, characterization and comparison of essential properties of various wound dressings, which could aid researchers with the development of novel modern wound dressings. In general, the multi-layered dressings were mechanically stronger than the mono-layered dressings. However, this is seen in conjunction with lower swelling capacities and, in general, average moisture uptake capacities. On the other hand, Kaltostat® has the highest swelling capacity, being one of the weakest dressings mechanically. The tested commercial dressings thus exhibit a high strength or high swelling, suggesting that there is still a strong potential in the wound dressings market for dressings that exhibit both a high strength and a high swelling capacity. The fibrous and foam dressings exhibited moderate to high swelling and moisture-uptake capacities. Equilibrium swelling of the dressings led to a general decrease in mechanical properties, among which the strongest effect was visible for the Young’s modulus. It was demonstrated that all tested dressings were suitable for moist wound healing treatment based on the MVTR. Their morphological differences, confirmed with SEM, could also be linked to their other properties. As demonstrated herein, the physico-chemical properties can vary widely among commercially available dressings and the different formulations (gels, foams, fibres, etc.) may thus have a different influence on the wound healing process of the different wound types. Indeed, the fibre-based dressings (e.g. Kaltostat®, Aquacel Ag®), due to their high surface area and absorbency, are very suitable for treating burn wounds and are typically paired with high levels of exudate, while film dressings (e.g. DuoDERM® ET) can be applied on difficult anatomical sites (e.g. joints) based on their flexibility. However, due to their low MVTR and absorbency, films should not be placed on heavily exuding wounds due to the risk of maceration. Foam dressings (e.g. Mepilex®) have a more moderate absorbency and MVTR but are mechanically stronger, making them more suitable for wounds in pressure-sensitive areas (e.g. heel). In practice, secondary dressings are still often applied to prevent exudate leakage or to support weaker dressings. Therefore, the design of novel wound dressings (such as smart polymeric bandages) together with the choice of the dressing for a specific treatment requires much consideration and further evaluation. This overview of the characteristics of some of the most frequently used commercial wound dressings can strongly aid in this decision.
Abbreviations
- CMC: carboxymethylcellulose; DVS: Dynamic vapour sorption; FTIR: Fourier transform infrared spectroscopy; MVTR: Moisture vapour transmission rate; PBS: phosphate-buffered saline; PVA: poly(vinylalcohol); RH: Relative humidity; SEM: Scanning electron microscopy; SWF: Simulated wound fluid.
Supplementary Material
Contributor Information
Manon Minsart, Polymer Chemistry & Biomaterials Research Group, Centre of Macromolecular Chemistry, Department of Organic and Macromolecular Chemistry, Ghent University, Krijgslaan 281, Building S4-bis, 9000 Ghent, Belgium.
Sandra Van Vlierberghe, Polymer Chemistry & Biomaterials Research Group, Centre of Macromolecular Chemistry, Department of Organic and Macromolecular Chemistry, Ghent University, Krijgslaan 281, Building S4-bis, 9000 Ghent, Belgium.
Peter Dubruel, Polymer Chemistry & Biomaterials Research Group, Centre of Macromolecular Chemistry, Department of Organic and Macromolecular Chemistry, Ghent University, Krijgslaan 281, Building S4-bis, 9000 Ghent, Belgium.
Arn Mignon, Smart Polymeric Biomaterials Research Group, Biomaterials and Tissue Engineering (SIEM) @ Campus Group T Leuven, Andreas Vesaliusstraat 13, 3000 Leuven, Belgium.
Authors’ contributions
MM performed the research and wrote the manuscript. SVV, PD and AM participated in the research design, implementation and interpretation. PD, SVV and AM reviewed and supervised the writing of the manuscript. All authors read and approved the final manuscript.
Funding
MM would like to thank Fonds Wetenschappelijk Onderzoek (FWO) for financial support (SB PhD fellow at FWO, Grant No. 3SB5619). AM has also received funding from Fonds Wetenschappelijk Onderzoek (Grant No. 12Z2918N).
Conflicts of interest
None declared.
References
- 1. Boateng J, Catanzano O. Advanced therapeutic dressings for effective wound healing - a review. J Pharm Sci. 2015;104:3653–80. [DOI] [PubMed] [Google Scholar]
- 2. Sen CK. Human wounds and its burden: an updated compendium of estimates. Adv Wound Care. 2019;8:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ye H, De S. Thermal injury of skin and subcutaneous tissues: a review of experimental approaches and numerical models. Burns. 2017;43:909–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kolarsick PAJ, Kolarsick MA, Goodwin C. Anatomy and physiology of the skin. J Dermatol Nurses Assoc. 2011;3:203–13. [Google Scholar]
- 5. Mescher AL. Junqueira’s Basic Histology Text and Atlas : Fourteenth edition. Weitz M, Kearns B (eds). USA: McGraw Hill Education, 2016. [Google Scholar]
- 6. Dhivya S, Padma VV, Santhini E. Wound dressings - a review. Biomed. 2015;5:24–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rieger KA, Birch NP, Schiffman JD. Designing electrospun nanofiber Mats to promote wound healing- a review. J Mater Chem B. 2013;1:4531–41. [DOI] [PubMed] [Google Scholar]
- 8. Powers JG, Higham C, Broussard K, Phillips TJ. Wound healing and treating wounds: chronic wound care and management. J Am Acad Dermatol. 2016;74:607–25. [DOI] [PubMed] [Google Scholar]
- 9. Varaprasad K, Jayaramudu T, Kanikireddy V, Toro C, Sadiku ER. Alginate-based composite materials for wound dressing application: a mini review. Carbohydr Polym. 2020;236:116025. 10.1016/j.carbpol.2020.116025. [DOI] [PubMed] [Google Scholar]
- 10. Mir M, Ali MN, Barakullah A, Gulzar A, Arshad M, Fatima S, et al. Synthetic polymeric biomaterials for wound healing: a review. Prog Biomater. 2018;7:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mele E. Electrospinning of natural polymers for advanced wound care: towards responsive and adaptive dressings. J Mater Chem B. 2016;4:4801–12. [DOI] [PubMed] [Google Scholar]
- 12. Jones V, Grey JE, Harding KG. ABC of wound healing: wound dressings. Br Med J. 2006;332:777–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deǧim Z. Use of microparticulate systems to accelerate skin wound healing. J Drug Target. 2008;16:437–48. [DOI] [PubMed] [Google Scholar]
- 14. Uzun M, Anand SC, Shah T. In vitro characterisation and evaluation of different types of wound dressing materials. J Biomed Eng Technol. 2013;1:1–7. [Google Scholar]
- 15. Vowden K, Vowden P. Wound dressings: principles and practice. Surg. 2014;32:462–7. [Google Scholar]
- 16. Boateng JS, Matthews KH, Stevens HNE, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97:2892–923. [DOI] [PubMed] [Google Scholar]
- 17. Falanga V, Brem H, Ennis WJ, Wolcott R, Gould LJ, Ayello EA. Maintenance debridement in the treatment of difficult-to-heal chronic wounds. Recommendations of an expert panel. Ostomy Wound Manage. 2008;Suppl:2–13. [PubMed] [Google Scholar]
- 18. Agarwal A, McAnulty JF, Schurr MJ, Murphy CJ, Abbott NL. Advanced Wound Repair Therapies. Farrar D (ed.). Woodhead Publishing Limited, Elsevier Inc, 2011, Ch 8. [Google Scholar]
- 19. Verbelen J, Hoeksema H, Heyneman A, Pirayesh A, Monstrey S. Aquacel® ag dressing versus Acticoat® dressing in partial thickness burns: a prospective, randomized, controlled study in 100 patients. Part 1: burn wound healing. Burns. 2014;40:416–27. [DOI] [PubMed] [Google Scholar]
- 20. Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: a review of patents and commercial products. Eur Polym J. 2015;65:252–67. [Google Scholar]
- 21. Mogoşanu GD, Grumezescu AM. Natural and synthetic polymers for wounds and burns dressing. Int J Pharm. 2014;463:127–36. [DOI] [PubMed] [Google Scholar]
- 22. Higgins L, Wasiak J, Spinks A, Cleland H. Split-thickness skin graft donor site management: a randomized controlled trial comparing polyurethane with calcium alginate dressings. Int Wound J. 2012;9:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leaper DJ, Schultz G, Carville K, Fletcher J, Swanson T, Drake R. Extending the TIME concept: what have we learned in the past 10 years? Int Wound J. 2012;9:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dong R, Guo B. Smart wound dressings for wound healing. Nano Today. 2021;41:101290. 10.1016/j.nantod.2021.101290. [DOI] [Google Scholar]
- 25. Simoska O, Duay J, Stevenson KJ. Electrochemical detection of Multianalyte biomarkers in wound healing efficacy. ACS Sensors. 2020;5:3547–57. [DOI] [PubMed] [Google Scholar]
- 26. Mostafalu P, Tamayol A, Rahimi R, Ochoa M, Khalilpour A, Kiaee G, et al. Smart bandage for monitoring and treatment of chronic wounds. Small. 2018;14:1–9. [DOI] [PubMed] [Google Scholar]
- 27. Mariani F, Serafini M, Gualandi I, Arcangeli D, Decataldo F, Possanzini L, et al. Advanced wound dressing for real-time pH monitoring. ACS Sensors. 2021;6:2366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tessarolo M, Possanzini L, Gualandi I, Mariani F, Torchia LD, Arcangeli D, et al. Wireless textile moisture sensor for wound care. Front Phys. 2021;9:1–9. [Google Scholar]
- 29. C. Inc. ConvaTec . https://www.convatec.com/wound-skin, accessed: January, 2021.
- 30. M.H.C. AB. Mölnlycke. https://www.molnlycke.com/products-solutions, accessed: January, 2021.
- 31. Paul Hartmann. Hartmann. https://www.hartmann.info/en-au/products/wound-management, accessed: January, 2021.
- 32. Smith & Nephew . https://www.smith-nephew.com/professional/products/advanced-wound-management/, accessed: January, 2021.
- 33. Akhmetova A, Saliev T, Allan IU, Illsley MJ, Nurgozhin T, Mikhalovsky S. Comprehensive review of topical odor-controlling treatment options for chronic wounds. J Wound, Ostomy Cont Nurs. 2016;43:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Illsley MJ, Akhmetova A, Bowyer C, Nurgozhin T, Mikhalovsky SV, Farrer J, et al. Activated carbon-plasticised agarose composite films for the adsorption of thiol as a model of wound malodour. J Mater Sci Mater Med. 2017;28:(10)154. 10.1007/s10856-017-5964-x. [DOI] [PubMed] [Google Scholar]
- 35. Bradshaw CE. An in vitro comparison of the antimicrobial activity of honey, iodine and silver wound dressings. Biosci Horizons. 2011;4:61–70. [Google Scholar]
- 36. Parikh DV, Fink T, Delucca AJ, Parikh AD. Absorption and swelling characteristics of silver (I) antimicrobial wound dressings. Text Res J. 2011;81:494–503. [Google Scholar]
- 37. Minsart M, Mignon A, Arslan A, Allan IU, Van Vlierberghe S, Dubruel P. Activated carbon containing PEG-based hydrogels as novel candidate dressings for the treatment of malodorous wounds. Macromol Mater Eng. 2021;306:1–12. [Google Scholar]
- 38. Grenier J, Duval H, Barou F, Lv P, David B, Letourneur D. Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying. Acta Biomater. 2019;94:195–203. [DOI] [PubMed] [Google Scholar]
- 39. Johnson BD, Beebe DJ, Crone WC. Effects of swelling on the mechanical properties of a pH-sensitive hydrogel for use in microfluidic devices. Mater Sci Eng C. 2004;24:575–81. [Google Scholar]
- 40. Chen H, Xing X, Tan H, Jia Y, Zhou T, Chen Y, et al. Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing. Mater Sci Eng C. 2017;70:287–95. [DOI] [PubMed] [Google Scholar]
- 41. Zahedi P, Rezaeian I, Ranaei-Siadat SO, Jafari SH, Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym Adv Technol. 2010;21:77–95. [Google Scholar]
- 42. Chiu CT, Lee JS, Chu CS, Chang YP, Wang YJ. Development of two alginate-based wound dressings. J Mater Sci Mater Med. 2008;19:2503–13. [DOI] [PubMed] [Google Scholar]
- 43. Liu L, Li X, Nagao M, Elias AL, Narain R, Chung HJ. A pH-indicating colorimetric tough hydrogel patch towards applications in a substrate for smart wound dressings. Polymers (Basel). 2017;9(11):558. 10.3390/polym9110558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu R, Xia H, He W, Li Z, Zhao J, Liu B, et al. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci Rep. 2016;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wlaschin KF, Ninkovic J, Griesgraber GW, Colak Atan S, Young AJ, Pereira JM, et al. The impact of first-aid dressing design on healing of porcine partial thickness wounds. Wound Repair Regen. 2019;27:622–33. [DOI] [PubMed] [Google Scholar]
- 46. Urtasun N, Mignon A, Martínez-Alvarez LM, Baieli MF, Hirsch DB, Cascone O, et al. Synthesis and characterization of chitosan mini-spheres with immobilized dye as affinity ligand for the purification of lactoperoxidase and lactoferrin from dairy whey. Sep Purif Technol. 2021;255:117700. 10.1016/j.seppur.2020.117700. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





