Abstract
Thyroperoxidase (TPO) is an enzyme essential for thyroid hormone (TH) synthesis and a target site for a number of xenobiotics that disrupt TH homeostasis. An in vitro high-throughput screening (HTS) assay for TPO inhibition, the Amplex UltraRed-TPO (AUR-TPO), has been used to screen the ToxCast chemical libraries for this action. Output from this assay would be most useful if it could be readily translated to an in vivo response, namely a reduction of TH in serum. To this end the relationship between TPO inhibition in vitro and serum TH decreases were examined in rats exposed to two classic TPO inhibitors, propylthiouracil (PTU) and methimazole (MMI) for 4,7, or 14 days. Serum and gland PTU, MMI, and TH levels were quantified using tandem liquid chromatography mass spectrometry. TPO activity was determined in thyroid gland microsomes treated with PTU or MMI in vitro and ex vivo from thyroid gland microsomes prepared from exposed animals. A quantitative model was constructed by contrasting in vitro and ex vivo AUR-TPO results and the in vivo time-course and dose-response analysis. In vitro:ex vivo correlations of AUR-TPO outputs indicated that less than 30% inhibition of TPO in vitro was sufficient to reduce serum T4 by 20%, a degree of regulatory significance. Although further testing of model estimates using other TPO inhibitors is essential for verification of these initial findings, the results of this study provide a means to translate in vitro screening assay results into predictions of in vivo serum T4 changes to inform risk assessment.
Keywords: thyroperoxidase, thyroid hormone, mathematical model
INTRODUCTION
Thyroid hormones (THs) critically modulate many physiological processes, including neurodevelopment, metabolism, and cardiac function (Cioffi et al., 2018; Duncan Bassett and Williams, 2018; Gilbert et al., 2012; Oetting and Yen, 2007; Williams, 2008; Yen, 2001; Zoeller, 2007). The endocrine and neurodevelopmental functions of THs have resulted in increased interest in screening for chemicals that may disrupt TH homeostasis via one or more of many possible mechanisms (EFSA et al., 2018; Paul Friedman et al., 2016; USEPA, 2017). One potential target for disruption is thyroperoxidase (TPO). TPO is a membrane bound enzyme that iodinates tyrosine residues of thyroglobulin to form mono-iodotyrosine (MIT) and di-iodotyrosine (DIT) and also couples these molecules to generate the THs tri-iodothyronine (T3) and tetra-iodothyronine (T4 or thyroxine) (Engler et al., 1982a; Taurog, 1976; Taurog et al., 1996). These THs are then released to the serum from the thyroid gland (Engler et al., 1982a; Engler et al., 1982b; Taurog et al., 1996). The role of TPO in the production of thyroid hormone in the gland and its modulation by chemicals that can disrupt TH homeostasis in humans and in rodent models are well-established in the literature (Crofton et al., 2017; Doerge et al., 2002; Pearce, 2006; Zoeller and Crofton, 2005).
Two pharmaceuticals, propylthiouracil (PTU) and methimazole (MMI), are used to treat hyperthyroidism and exert their action by inhibiting TPO and blocking synthesis of thyroid hormones. In a research context, these drugs are often used to investigate developmental and neurological consequences of hypothyroidism (Axelstad et al., 2008; Bernal, 2015; Cooper et al., 1984; Cooper et al., 1983; Gilbert et al., 2014; Gilbert et al., 2016; Hassan et al., 2017b; Hood et al., 1999; Oppenheimer and Schwartz, 1997; Paul et al., 2014; Vickers et al., 2012). Because of their known mechanism(s) and widespread use in in vivo toxicology, PTU and MMI have been used extensively in assay development for TPO inhibition (Cooper et al., 1987; Nagasaka and Hidaka, 1976; Paul et al., 2014). However, other environmentally-relevant chemicals have also been reported to inhibit TPO activity, including the ethylene bisdithiocarbamate pesticides (mancozeb, ziram, and zineb); malachite green; isoflavones; the parasiticide malachite green; and resorcinol (Chang and Doerge, 2000; Divi et al., 1997; Divi and Doerge, 1994; Doerge et al., 1998; Kackar et al., 1997{Marinovich, 1997 #677; Marinovich et al., 1997).
Given the physiological importance of TH homeostasis, and the known importance of the TPO enzyme, high-throughput screening (HTS) assays to identify chemicals that may inhibit TPO and possibly impact TH concentrations in vivo have been developed. For example, the Amplex Ultra Red (AUR)-TPO inhibition assay was developed as part of the ToxCast program to rapidly identify chemicals that may inhibit TPO (Kavlock et al., 2012; Thomas et al., 2019). To date, over 1800 unique chemicals have been screened with the AUR-TPO assay,(Paul Friedman et al., 2016) and the multi-concentration response data are publicly available as part of the ToxCast data release and CompTox Chemicals Dashboard (EPA’s National Center for Computational Toxicology, 2019; Williams et al., 2017). The AUR-TPO assay uses a commercially available fluorogenic substrate, AUR, to detect peroxidase activity in the presence of excess hydrogen peroxide using rat thyroid microsomes as a TPO source (Paul et al., 2014).
The AUR-TPO inhibition assay has identified over 400 xenobiotics as potential TPO inhibitors (Paul Friedman et al., 2016). However, there is an uncertainty in using these data to predict chemicals that may inhibit TPO in vitro and thereby decrease TH synthesis. Moreover, the quantitative relationship between TPO inhibition in the AUR-TPO assay and the magnitude of TH change that would be expected as a result of this inhibition is yet to be elucidated. Therefore, the current study aims to fill this gap by developing a quantitative relationship between in vitro and decrements of serum T4 in vivo. This key quantitative relationship would enable the prioritization of chemicals with sufficient potency and efficacy for further screening in models of greater biological complexity. Translating in vitro findings from the AUR-TPO assay to chemical safety evaluation requires a greater understanding of the magnitude of TPO inhibition required in order to observe TH changes in vivo. Previous studies suggest that a 20% decrease in maternal serum T4 may result in irreversible and adverse neurodevelopmental outcomes for offspring (Haddow et al., 2002; Henrichs et al., 2010; Korevaar et al., 2016; Pop et al., 2003; Pop et al., 1999; Willoughby et al., 2014). However, it is currently unknown what percent change in AUR-TPO screening would be indicative of a possible 20% change in serum T4 in vivo.
The goal of this study was to investigate the applicability of the in vitro AUR-TPO inhibition assay to predict in vivo thyroid hormone levels. First, an in vivo data-set was developed to provide information to construct such a model. Adult male Long-Evans rats were exposed in vivo to three concentrations of PTU or MMI in drinking water. Glandular and serum TH, PTU or MMI concentrations were quantified. AUR-TPO assays were conducted in vitro using thyroid microsomes treated with MMI or PTU as well as ex vivo with thyroid microsomes obtained from the rats orally dosed with PTU or MMI. In vitro and ex vivo potency for TPO inhibition, measured levels of chemical in thyroid glands, and TH levels were then used quantitively to predict in vivo serum hormone concentrations in the rat. The resultant findings support a relationship between in vitro TPO inhibition and predicted TH changes in rats.
MATERIALS AND METHODS
Chemicals
Methimazole (3-methyl-1H-imidazole-2-thione; MMI; CAS No. 60-56-0; purity >98%) and 6-propyl-2-thiouracil (6-propyl-2-sulfanylidene-1H-pyrimidin-4-one; PTU; CAS No. 51-52-5; purity 99.9%) were used in the in vivo dose response studies and analytical grade MMI and PTU (purity 99%) was used for LC/MS/MS and in vitro experiments were obtained from Sigma Chemical Company (St Louis, Missouri). The following stable isotope internal standards, 3,3ʹ-diiodothyronine-13[C6] (T2-13[C6]); 3,3ʹ,5-triiodothyronine-13[C6] HCl (T3-13[C6]); L-thyroxine-13[C6] HCl (T4-13[C6]); 3,3ʹ,5ʹ-triiodothyronine-13[C6] HCl (reverseT3(rT3-13[C6] were purchased from IsoSciences (King of Prussia, PA) and (MIT-13[C6] was purchased from Toronto Research Chemicals (North York, ON, Canada). Thyroid hormone standards 3,3’, 5,5’-tetraiodo-L-thyronine (T4), 3,3’,5-triiodo-L-thyronine (T3) as the sodium salt, 3,3’5’-triiodo-L-thyronine (rT3), 3,5-diiodo-L-thyronine (3,5-T2), 3,3’-diiodo-L-thyronine (3,3’-T2), 3,5-diiodo-L-tyrosine (DIT), and 3-iodo-L-tyrosine (MIT) were purchased as neat materials from Toronto Research Chemicals (North York, ON, Canada) to be used as calibration and spiking standards for the target analytes. Hydrogen peroxide (H2O2, Fisher Chemical) and Amplex UltraRed reagent (Invitrogen, ThermoFisher) were used for the TPO inhibition in vitro assay.
Animal dosing
Adult male (post-natal day 55–60) Long Evans rats (n=64) were obtained from Charles River (Raleigh, NC) and shipped to the animal housing facility at the U.S. EPA. Animal experiments were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and conducted under Institutional Animal Care and Use Committee approved protocol maintained in AALAC accredited facility. On arrival to the facility, animals were individually housed in standard hanging plastic cages and housed under controlled conditions (20–24°C; 40–50% humidity) with 12:12 h light/dark cycle and allowed to acclimate one week before the study began. Rats were fed standard Purina Rat chow 5001 and provided food and water ad libitum. The iodine content in standard Purina rat chow 5001 far exceeds that required for optimal hormone synthesis (1000 vs 275 ng/gm, see Gilbert et al. (2013) and Fisher et al. (2013)) indicating iodine supply is not likely contributing to decrements in hormone synthesis under the conditions of this study. Animals were weight-ranked and randomly assigned to treatment groups and exposed to either PTU (0, 1, 3, and 10 parts per million (ppm) or MMI (0, 3, 30, 200 ppm) in drinking water for 4, 7, or 14 days (n=8 per dose/time point). Animals were weighed three times per week. At the end of each exposure duration, animals were euthanized by decapitation between 0800 and 1200 h and the time of sacrifice was counterbalanced by dose group. Trunk blood was collected, and thyroid glands were removed and weighed. One lobe of each gland was stored in RNALater reagent for gene expression analysis and the other was snap frozen in liquid nitrogen and used for microsomes, hormone, and chemical analysis. Blood was left to clot on ice, centrifuged at 3000 × g at 4 °C for 30 min, and serum was removed and stored at −80 °C.
Quantification of PTU and MMI in the serum and thyroid gland
Serum and thyroid gland samples were prepared and the levels of PTU or MMI were quantified using TSQ Quantum liquid chromatography/tandem mass spectrometry (LC/MS/MS; ThermoFisher) as described in (Hassan et al., 2017a; Woźniak et al., 2014) with minor modification in the quantification method. Briefly, a Shimadzu LC-20AD system and SCIEX 4000 QTrap Mass Spectrometer were used to quantify levels of MMI or PTU in the serum and thyroid gland. Chromatographic separation was performed on a Phenomenex Kinetex HILIC column (2.6 μm, 100 mm × 3 mm) maintained at 30°C. A linear gradient using 15 mM ammonium formate in water (pH 3.2) as mobile phase A and acetonitrile as mobile phase B were used at a flow rate of 0.4 mL min-1. The starting mobile phase composition was 10% A and 90% B. After 2.5 minutes, mobile phase A increased to 90% for 3 minutes. The column was re-equilibrated to starting conditions for 5 minutes. Analytes were detected by positive ion electrospray ionization.
Quality control for chemical measurements:
Matrix-matched calibration standards were used for the quantitation of PTU or MMI levels in thyroid glands. Calibration standards were prepared and analyzed in the same manner as project samples. The calibration curve used a minimum of 5 points over a range of 0.25 to 62.5 ng/mL. The coefficient of determination for the calibration curve was ≥ 0.99. Each sample batch consisted of a matrix-matched method blank and MMI-spiked laboratory control sample and duplicate with a continuing calibration verification sample run after approximately every 20 samples in the batch. All quality control samples were within 80–120% of the nominal spike amount.
Quantification of THs in the serum and thyroid gland
Sample preparation and analysis of serum T3 and T4 and thyroid gland levels of iodotyrosines (MIT, DIT), T3, T4, and reverse T3 (rT3) were based on methods previously described by (Hassan et al., 2017b), with some modifications. Briefly, following solid phase extraction, thyroid hormone extracts were reconstituted with 100 μL of 5% acetonitrile: 95% H2O (v/v) with 0.1% formic acid. The extracts were analyzed using an AB Sciex (Framingham, MA) Exion AC UHPLC-Qtrap 6500+ Linear Ion Trap LC/MS/MS system. Chromatographic separation of the pre-hormones and hormones was performed using a Restek (Bellefonte, PA) Raptor Biphenyl column (2.6 μm, 100 mm × 2.1 mm).
Quality control for hormone measurements:
Single component stock standards were prepared in acetonitrile from neat materials. A mixed intermediate standard with each component present at a concentration of 1 μg/mL was used to prepare a set of 9 intermediate standards by serial dilution over a concentration of 100 to 100,000 pg/mL in methanol. A 100 μL aliquot of each intermediate standard was combined in an autosampler vial with 100 μL of a 100 ng/mL solution of the IS in methanol. Each standard/IS mixture was evaporated under nitrogen on a TurboVap (Caliper Life Sciences, Waltham, MA) set to 35 °C. The dried standard mixture was resuspended in 1 mL of 0.1% formic acid in 95:5 water: methanol to be used for calibration.
Two ion transitions were monitored for each target analyte. The analytes were qualitatively identified based on retention time relative to the internal standard and calibration standard, and the ratio of the peak areas of the monitored ion transitions. The ion ratios of identified target analytes were within 30% of the average ion ratio for standards. Solvent-based calibration standards were used for quantitation of the thyroid hormones in cell media over a range of 10 – 10,000 pg/mL. Each curve used a minimum of five sequential points. The correlation coefficients of the curves were ≥ 0.99. The calibration was verified with a second source standard. The lower limit of quantitation (LLOQ) for each analyte was set to the concentration of the lowest calibration standard that gave an acceptable ion ratio, and acceptable recovery of ±30% of the spike amount. Each sample batch consisted of a method blank, a laboratory control sample (blank spike), and a continuing calibration verification sample prepared in solvent. The calculated concentrations for thyroid hormones in the laboratory control sample and continuing calibration verification sample were within 70–130% of actual spike amount.
Analysis of serum thyroid-stimulating hormone (TSH)
Determination of serum TSH levels was carried out by radioimmunoassay (RIA) based on a method previously reported in (Louis et al., 2017; Stoker et al., 2010; Thibodeaux et al., 2003). The limit of detection for TSH assay was 0.3 ng/ml and intra-assay coefficient of variation was 1.95%.
Thyroid peroxidase inhibition in vitro and ex-vivo with AUR assay
Thyroid gland microsomes (N=8/group pooled) were prepared from rats exposed to PTU or MMI for 4-days and from untreated rats using a method previously described (Paul et al., 2013; Taurog et al., 1996). The mean protein content of the microsomes was determined using Pierce BCA Protein Assay Kit (ThermoFisher) following the manufacture’s protocol. Protein concentration ranged from 0.85 – 1.64 mg/ml and average thyroid gland weight ranged from 6.5–7 mg for PTU dosed animals while average thyroid gland weight for MMI treated rats ranged from 16–19 mg.
The AUR assay was conducted as described by (Paul et al., 2013; Paul et al., 2014) with minor modifications. In brief, the assay was conducted in a 96-well format using rat thyroid microsomes, 200 mM potassium phosphate buffer, AUR reagent diluted from 10 mM AUR DMSO stock diluted in 200 mM phosphate buffer, and H2O2. The reaction was conducted by adding 75μL AUR reagent (25 μM), 10 – 15 μL of microsomal protein (12.5 μM), 25 μL H2O2 (300 μM), and finally 100 μL of 200 mM potassium phosphate buffer. A dose response curve for PTU or MMI was included in each plate as positive control for TPO inhibition in vitro. The standard curve was constructed using untreated rat thyroid microsomes (obtained from thyroid glands of non-dosed animals) exposed in vitro to 0–100 μM PTU or MMI, prepared from a 1 mM stock in DMSO. The assay was also conducted with microsomes prepared ex vivo from PTU or MMI dosed rats. The plates were incubated for 30 minutes at 37°C in the plate reader (BMG Optima FluoStar with excitation/emission of 544/590). The assay was run three separate times on three different days with fresh reagents prepared for each biological replicate (n=3), with technical duplicates in each plate. In vitro concentration that inhibits enzyme activity by 50% (IC50) were calculated using GraphPad Prism (v5.0, LaJolla, California).
RNA extraction and Quantitative RT-PCR (qRT-PCR)
Gene expression analysis was performed on thyroid tissues from PTU or MMI treated rats. RNA was extracted and quantitative real-time polymerase chain reactions (qRT-PCR) was performed according to procedure described by (Gilbert et al., 2016).
Statistical analysis
The dose-response measures of gland and serum PTU, MMI, and thyroid hormone analytes are presented as mean ± standard error (SEM) and were plotted using GraphPad Prism (v5.0, LaJolla, California). PTU, MMI, serum, and gland TH concentrations were evaluated using general linear model analysis of variance (ANOVA) conducted using SAS version 9.2 (Cary, NC). Significant main effects were followed by Dunnett post-hoc multiple comparisons testing with p<0.05 considered statistically significant whereas gene expression significance was set at p<0.01 which was determined by the alpha level by the square root of the number of genes analyzed. For statistical purposes control values of serum and thyroid gland PTU or MMI which were below the detection limit of the assay were set at the LLOQ as follows: thyroid gland PTU and MMI LLOQ = 7 ng/g and 25 ng/g, respectively; serum PTU and MMI LLOQ = 0.38 ng/ml; All the thyroid gland hormone analytes were above the LLOQ = 0.5 ng/ml; and all serum T4 and T3 were above the LLOQ = 0.1 ng/ml, 0.4 ng/ml, respectively.
Quantitative in vitro to in vivo relationship
The quantitative relationships between in vitro derived estimates of TPO inhibition and in vivo serum thyroid hormone levels were examined computationally. Chemical disposition and dosimetry information, and hormone concentrations in the thyroid gland and serum collected in in vivo experiments were used to define two mathematical relationships. First, a relationship between TPO inhibition (as indicated by the AUR-TPO assay) in thyroid gland microsomes from naïve animals and TPO inhibition in microsomes derived ex vivo from exposed animals was defined. Best linear fit for the data from both assays was obtained and correlated. Second, a mathematical relationship between gland concentrations and TPO inhibition was developed; i.e., chemical and hormone concentrations in the gland were coupled with in vitro IC50 to simulate the degree of TPO inhibition. The simulated degree of TPO inhibition was then used to predict serum T4 levels in vivo. This was accomplished by introducing glandular T4, PTU, or MMI concentrations into a one-compartment model to describe TPO inhibition, under steady state condition, as a function of in vitro derived IC50 using the following equation:
The values for Imax (the maximal activity of TPO) and IC50 values (1.2 μM for PTU; 0.11 μM for MMI) were obtained from the in vitro AUR-TPO assay. Ksyn and Kdeg are the basal production and degradation rates of T4, respectively. Their values were obtained from (Silva et al., 1984). Unlike MMI, which remains largely unbound after oral intake, 80 to 90% of PTU remains bound to serum proteins (Jastrzębska, 2015). Therefore, to account for the free portion, the IC50 value for PTU was divided by 0.24, which is the unbound fraction of PTU, obtained from (Kampmann and Mølholm Hansen, 1983).
To relate glandular T4 levels to serum T4, direct mathematical relationships were constructed. First the percent of TPO T4 synthesis inhibition was determined by dividing gland T4 levels at each dose by T4 in the control animals. The percent TPO inhibition was then multiplied by the serum basal level of T4. The resulting predicted T4 serum levels were then plotted against the experimentally determined ones at each dose for both chemicals.
RESULTS
Body and thyroid weights
Body weights were not significantly altered by exposure to PTU or MMI at any time-points (Supplemental Data, Figure 1). Thyroid gland weights were significantly increased 1.3 and 1.6-fold, following 14-days of PTU exposure at doses of 3 and 10 ppm, respectively, [F (3,28) = 11.05; p<0.0001; Figure 1A]. Similarly, 14-day exposure to 30 and 200 ppm MMI significantly increased thyroid gland weights by approximately 1.4 and 1.5-fold compared with controls [ F (3,30) = 5.05, p = 0.006; Figure 1B]. Although 7-day exposure to 30 and 200 ppm MMI also appeared to increase thyroid gland weights, the results were not statistically significant (p=0.052) (Figure 1B).
Figure 1. Thyroid gland weights.
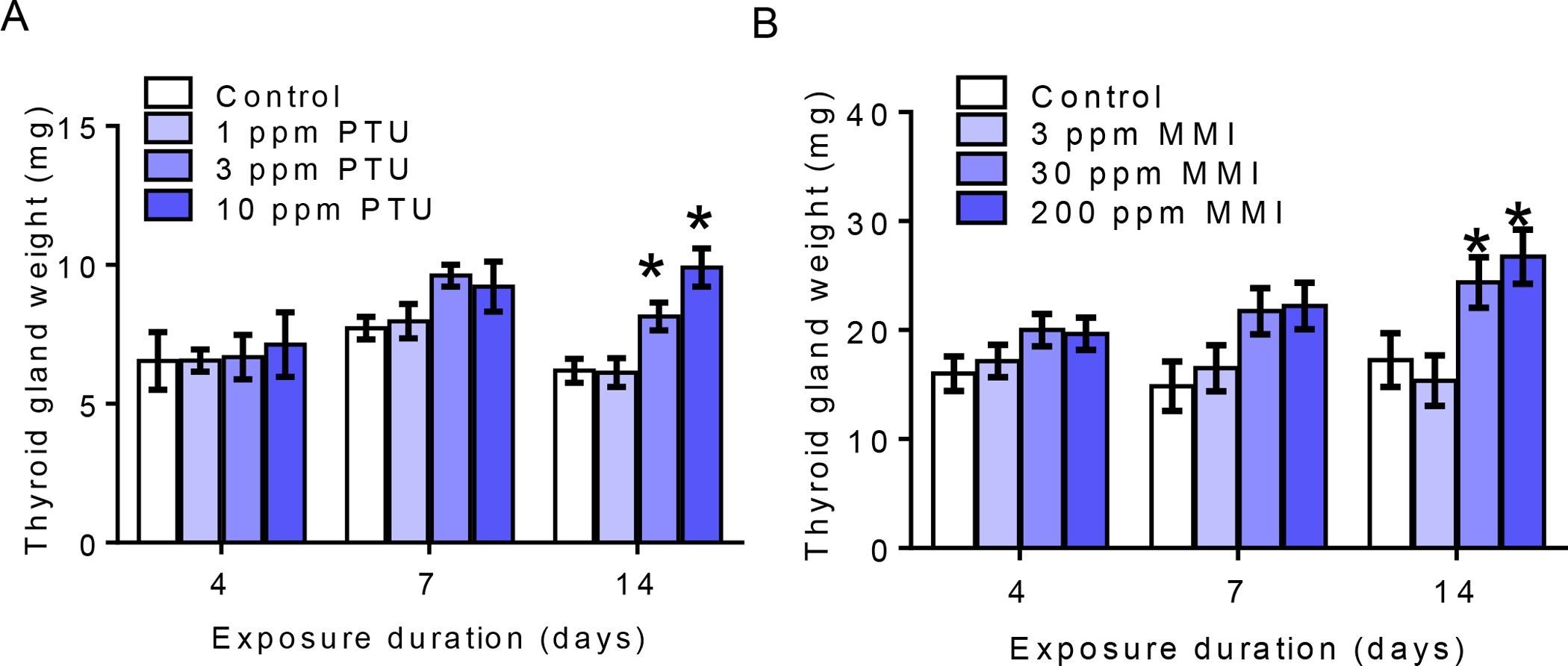
A) Thyroid gland weight was significantly increased with exposure to 3 and 10 ppm PTU for 14 days B) Thyroid gland weight was also increased with exposure to 30 and 200 ppm MMI for 14 days. An N = 8 was analyzed per treatment per timepoint; *represent p<0.01 and bar graph shows mean± SEM.
PTU and MMI concentrations in the thyroid gland and serum of dosed rats
There was a dose-dependent increase in mean PTU concentrations in the thyroid gland and serum at all doses relative to controls with 4, 7, and 14-day exposure to PTU (Table 1, p<0.0001). The levels did not appear to increase with increased exposure duration suggesting that PTU at these concentrations does not accumulate in the gland over time. Similarly, intra-thyroid and serum levels of MMI significantly increased in a dose-dependent manner in animals exposed to 3, 30, and 200 ppm MMI for 4, 7 and 14 days, compared to non-treated controls (p<0.0001; Table 2). Like PTU, MMI did not appear to accumulate in the gland over the duration of exposure. MMI levels at the highest doses were comparable at 4, 7, and 14 days of exposure.
Table 1:
Serum and Thyroid Gland PTU levels and Gland Hormone Concentrations
| PTU exposure duration/doses (ppm) 4 day | Serum PTU (ng/ml) | Gland PTU (ng/g) | Hormone Concentrations in the Thyroid Gland (ng/mg) | ||||
|---|---|---|---|---|---|---|---|
| MIT | DIT | T3 | rT3 | T4 | |||
| 0 | <0.381 | <71 | 35 (9) | 344 (96) | 18 (4) | 10 (2) | 207 (39) |
| 1 | 156 (20) | 4,246 (1170) | 30 (11) | 186 (66) * | 18 (6) | 9 (3) | 186 (30) |
| 3 | 459 (56) | 14,075 (2284) | 32 (7) | 142 (44) * | 13 (2) | 8 (2) | 65 (39) |
| 10 | 1,367 (139) | 20,055 (2725) | 12 (7) * | 94 (34) * | 5 (2) * | 3 (1) * | 48 (19) * |
| 7 day | |||||||
| 0 | <0.381 | <71 | 37 (12) | 640 (113) | 21 (4) | 6 (1) | 168 (27) |
| 1 | 138 (15) | 4,297 (1170) | 9 (1) * | 204 (29) * | 19 (3) | 5 (0.7) | 104 (20) |
| 3 | 412 (41) | 15,037 (2284) | 7 (1) * | 60 (23) * | 6 (1) * | 2 (0.5) | 27 (9) * |
| 10 | 1,589 (131) | 20,542 (2725) | 3 (1) * | 62 (26) * | 3 (1) * | 0.7 (0.3) * | 29 (9) * |
| 14 day | |||||||
| 0 | <0.381 | <71 | 14 (2) | 630 (60) | 24 (2) | 6 (1) | 164 (14) |
| 1 | 175 (49) | 4,867 (1170) | 13 (1) | 148 (36) * | 17 (2) * | 5 (0.8) | 51 (11) * |
| 3 | 453 (50) | 11,438 (2889) | 3 (1) * | 34 (16) * | 2 (0.5) * | 0.7 (0.3) * | 0.6 (0.4) * |
| 10 | 1,626 (113) | 13,800 (2725) | 4 (0.11) * | 6 (1) * | 0.1 (0.02) * | 0.1 (0.02) * | 0.2 (0.04) * |
Serum PTU LLOQ = 0.38 ng/ml; Gland PTU LLOQ = 7 ng/g Gland TH LLOQ = 0.5 ng/ml
indicates significantly different from control group where p<0.05
ppm = parts per million
PTU = 6-propylthiouracil; (n=8/dose/group)
MIT, 3-monoiodotyrosine, DIT, 3,5-diiodotyrosine, rT3, 3,3’,5’-triiodothyronine, T3, 3,3’,5-triiodothyronine, T4, thyroxine.
Table 2:
Serum and Thyroid Gland MMI levels and Gland Hormone Concentrations
| MMI exposure duration/doses (ppm) 4 day | Serum MMI (ng/ml) | Gland MMI (ng/g) | Hormone Concentrations in the Thyroid Gland (ng/mg) | ||||
|---|---|---|---|---|---|---|---|
| MIT | DIT | T3 | rT3 | T4 | |||
| 0 | <0.381 | <251 | 28 (6) | 617 (73) | 36 (4) | 5 (0.6) | 215 (23) |
| 3 | 37 (7) | 1,698 (350) | 25 (6) | 485 (92) | 31 (8) | 5 (0.9) | 190 (50) |
| 30 | 512 (59) | 3,135 (381) | 5 (1) * | 76 (19) * | 3 (0.6) * | 0.8 (0.2) * | 20 (5) * |
| 200 | 2,376 (222) | 3,038 (504) | 9 (3) * | 163 (46) * | 7 (2) * | 1 (0.3) * | 45 (13) * |
| 7 day | |||||||
| 0 | <0.381 | <251 | 23 (7) | 653 (147) | 29 (8) | 9 (2) | 208 (40) |
| 3 | 47 (8) | 1,850 (350) | 14 (0.3) | 266 (54) * | 18 (3) | 3 (0.4) * | 82 (16) * |
| 30 | 517 (64) | 2,871 (430) | 4 (1) * | 41 (18) * | 2 (0.8) * | 0.5 (0.1) * | 12 (4) * |
| 200 | 2,249 (264) | 3,110 (227) | 1 (0.2) * | 12 (3) * | 0.6 (0.1) * | 0.2 (0.1) * | 5 (1) * |
| 14 day | |||||||
| 0 | <0.381 | <251 | 17 (4) | 415 (79) | 32 (7) | 6 (1) | 154 (29) |
| 3 | 24 (6) | 589 (144) | 8 (2) * | 146 (40) * | 18 (2) | 4 (0.4) * | 89 (10) * |
| 30 | 415 (47) | 2,266 (150) | 2 (0.3) * | 13 (2) * | 1 (0.3) * | 0.4 (0.1) * | 7 (1) * |
| 200 | 2,448 (192) | 3,263 (345) | 0.7 (0.1) * | 6 (2) * | 0.4 (0.1) * | 0.1 (0.03) * | 2 (0.6) * |
Serum MMI LLOQ = 0.38 ng/ml; Gland MMI LLOQ = 25 ng/g and thyroid gland LLOQ = 0.1 ng/ml
indicates significantly different from control group where p<0.05
ppm = parts per million
MMI = methimazole; (n=8/dose/group)
MIT, 3-monoiodotyrosine, DIT, 3,5-diiodotyrosine, rT3, 3,3’,5’-triiodothyronine, T3, 3,3’,5-triiodothyronine, T4, thyroxine.
PTU and MMI decreased thyroid hormone levels in the thyroid gland
As shown in Table 1, treatment with PTU for 4 days resulted in a dose-dependent decrease in glandular THs. Exposure to 3 and 10 ppm PTU for 4 days significantly decreased mean gland concentration of THs (p<0.009). In the 1 ppm and 3 ppm PTU exposed groups, DIT was significantly decreased, whereas exposure to 10 ppm PTU reduced all the analytes (p=0.0001). After 7 days of exposure to PTU, a significant decrease in glandular levels of MIT and DIT at all doses was noted while the two highest doses also decreased rT3, T3, and T4 (p<0.0003). Similarly, 14-day exposure significantly decreased DIT, T3, and T4 at all doses and MIT as well as rT3 were also decreased at the two highest doses (p<0.0001).
A similar dose and time-dependent pattern of hormone analytes was also observed in the glands of animals exposed to MMI (Table 2). The two highest doses of MMI significantly decreased MIT, T3, rT3, and T4, whereas DIT was significantly decreased at all doses in the 4-day treated animals (p<0.0001). Decrease in glandular TH levels observed in the 4, 7 and 14-day dosed rats suggested that MMI is a more potent TPO inhibitor than PTU. Significant dose-dependent declines in DIT, rT3, and T4 at all doses were also observed in the 7-day dosed animals and MIT was significantly decreased at the two highest doses only (p<0.001). Similarly, 14-day exposure to 3, 30, and 200 ppm significantly decreased all the analytes except T3 which was decreased at the two highest doses only (p<0.001).
PTU and MMI decreased serum T3, T4, and increased TSH
Mean serum levels of T3 and T4 were moderately to markedly decreased with concomitant increases in TSH as a function of dose and duration of PTU (Figure A-C) exposures. Treatment with doses greater than 1 ppm PTU for 4 and 7 days decreased serum T3 (Figure 2A) and T4 (Figure 2B). Serum T3 declined by 58%, and 84% (p<0.0001) whereas T4 was decreased by 51%, and 58% (p<0.0002) with exposure to 3 and 10 ppm for 4 days, respectively (Figure 2A and B). Exposure to 3 ppm PTU for 7 days decreased serum T3 and T4 by 47% and 61% as well as 89% and 71% in the 10 ppm PTU treated group, and 14-day exposure to PTU resulted in significant declines in serum T3 and T4 at all doses (p<0.0001). Specifically, serum T4 declined by 27%, 71%, and 94%, while T3 was decreased 23%,41%, and 97%, with exposure to 1, 3, and 10 ppm PTU, respectively. Coinciding with declines in serum T4, TSH was increased by 3.5, and 5-fold with exposure to PTU for 7 days and by 5 and 9-fold at the two highest doses with exposure extended to 14 days (p<0.0001, Figure 2C).
Figure 2. Serum thyroid hormones.
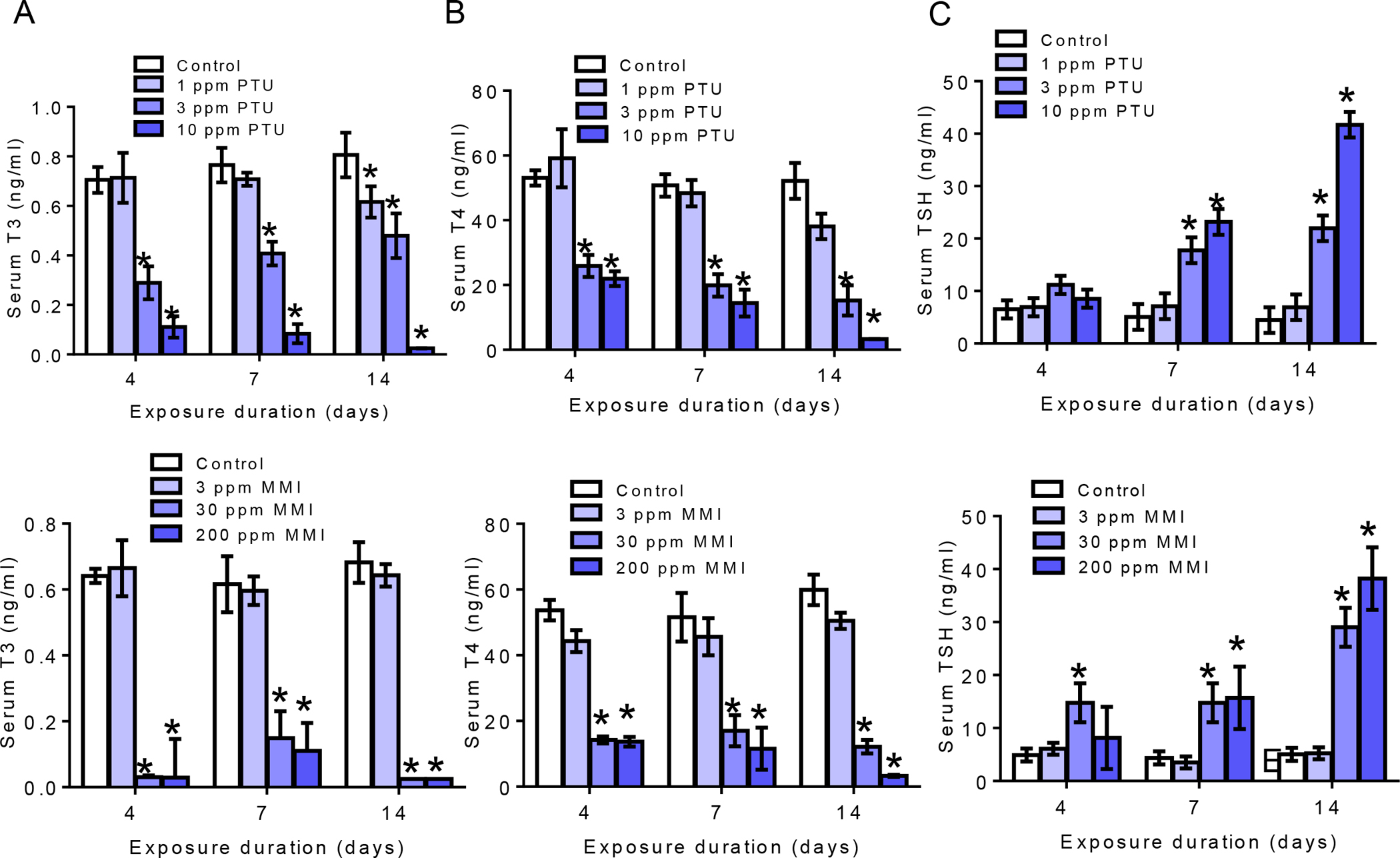
A-C) Serum T3, T4, and TSH levels for PTU treated animals. Both T3 and T4 levels were significantly decreased with exposure to 3 and 10 ppm PTU at all exposure durations with corollary increase in TSH at 3 and 10 ppm at 7 and 14-day exposures only. D-F) T3, T4, and TSH levels in the serum of MMI treated animals. Exposure to 30 and 200 ppm MMI significantly reduced serum T3 and T4 at all exposure durations with significant increase in TSH with exposure to 30 and 200 ppm for 7 and 14 days and 30 ppm only for 4 days. The bar graphs are presented as mean ±SEM and statistically significant is designated as *p<0.05 N=8 per dose/time-point.
Exposure to MMI also significantly decreased serum thyroid hormones. As shown in Figure 2D and 2E, T3 and T4 significantly declined with exposure to doses greater than 3 ppm for 4 days (Figure 2D and E). Unlike PTU however, TSH was simultaneously elevated in animals exposed to 30 ppm MMI after 4-days (Figure 2F). Specifically, TSH levels were significantly increased by 3-fold in rats exposed to 30 ppm for 4-days (p<0.0001). Exposure to 30 and 200 ppm MMI resulted in 3.3 and 3.5-increase in TSH levels in 7-day exposed rats and 5.7 and 7.5-fold increase in 14-day exposed rats (p<0.0001). MMI at the doses used here resulted in severe T3 declines at the two highest doses and at all time points. After 4-day exposure to 30 and 200 ppm MMI, T3 decreased by 95.2% and 95.4%, whereas T4 was declined at all doses by 18%, 73% and 74%, respectively (p<0.0001). Exposure to the two highest MMI doses for 7 days also significantly decreased T3 by 75% and 82% while T4 declined by 67% and 78% (p<0.0001). Similarly, a marked decrease in T3 was noted in 14-day group dosed with 30 and 200 ppm MMI whereas T4 significantly declined at all doses (p<0.0001)
To further characterize the impact of MMI and PTU on thyroid hormone synthesis, glandular mRNA levels of TH regulating genes were determined (Supplemental Data Figure 2). Increased expression of Slc5a5, a transcript encoding for sodium-iodine symporter (NIS), suggests that the gland is responding to the activation of the feedback loop including elevation of serum TSH. Increases in Tpo and Dio1 were seen at the highest dose of PTU and were restricted to the 7-day timepoint.
PTU and MMI inhibit TPO activity in vitro and ex vivo
PTU was less potent inhibitor of TPO in vitro with an IC50 of 1.2 μM compared with 0.11 μM for MMI (Figure 3A and C). Statistically significant inhibition of TPO activity was also seen in microsomes from ex vivo glands from rats exposed to 3 and 10 ppm PTU for 4 days [F (3,20) = 25.83, p<0.0001; Figure 3B], and 3, 30, and 200 ppm of MMI-treated animals [F (3,12) = 14.10, p=0.0003; Figure 3D]. The Z’ factor, a score that distinguishes signal from noise, was calculated for AUR-TPO inhibition assay for both PTU and MMI, and values that exceeded 0.94 were obtained (Zhang et al., 1999).
Figure 3. In vitro and ex vivo TPO inhibition determined by AUR-TPO inhibition.
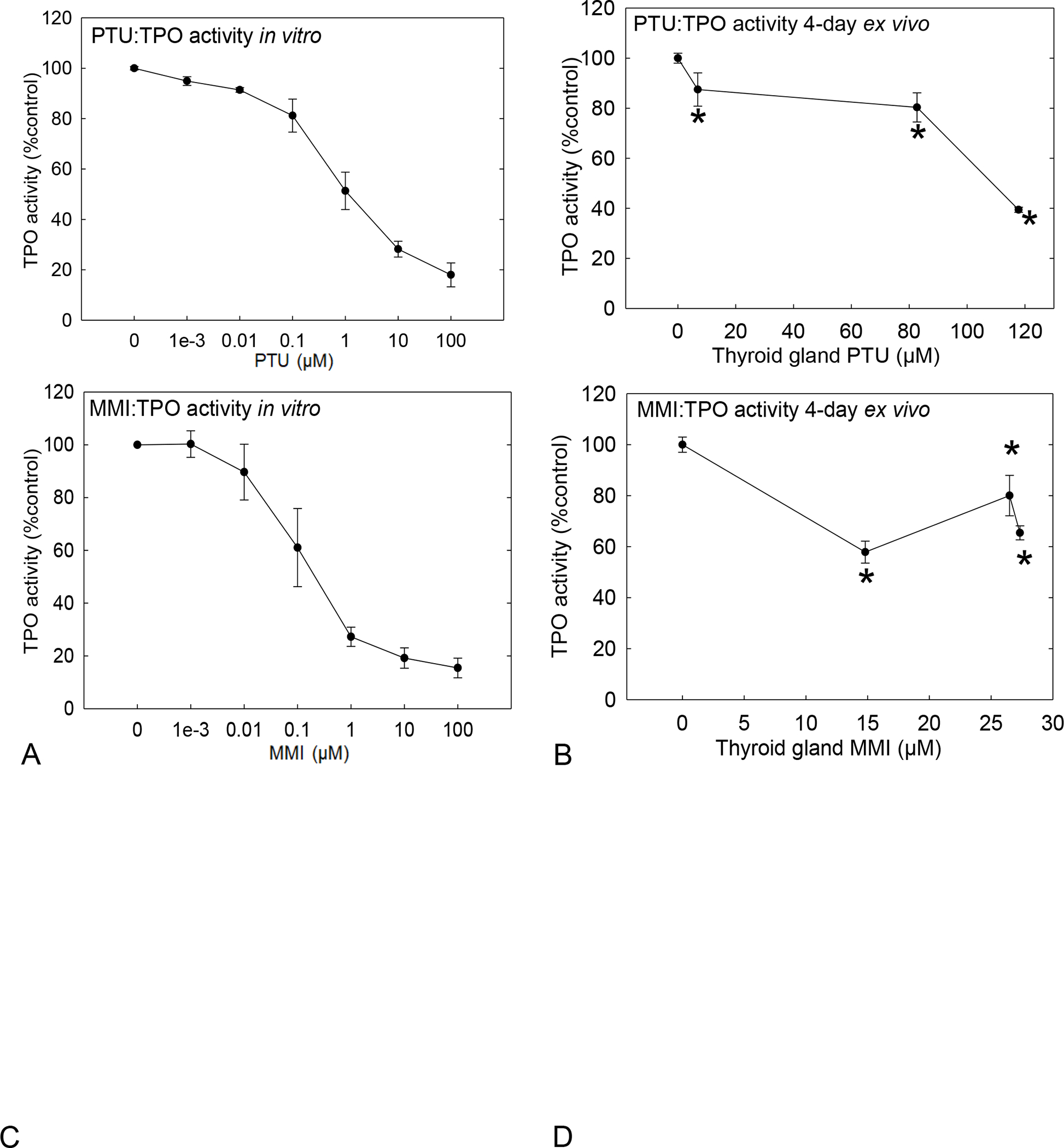
Assay with MMI and PTU in a 96-well format. A) In vitro assay was performed with PTU on rat thyroid microsomes of non-treated rats exposed in vitro to 0–100 μM PTU N=6. IC50 = 1.21μM. B) TPO activity was inhibited at all doses in the ex vivo microsomes obtained from thyroid gland of 4-day PTU exposed animals. C) TPO inhibition measured from MMI exposed rat thyroid microsome in vitro IC50 = 0.11μM. D) TPO inhibition was also demonstrated at all doses in ex vivo microsomes from thyroid gland of 4-day MMI treated animals N=4 Z’ > 0.94. * represent p<0.05, and error bars ± SEM
The quantitative relationship between in vitro and ex vivo measures of TPO activity after 4 days of exposure to PTU or MMI are summarized in Figure 4. For both chemicals, the TPO activity from the in vitro assay was plotted against the ex vivo assay results. The estimated slope for the best linear fit for PTU was 0.89 with an R2 = 0.86 (Figure 4A) and for MMI was 0.84 with an R2 = 0.71 (Figure 4B). For both PTU and MMI, the best fit line approached unity. Based on this in vitro-ex vivo relationship, it was possible to estimate the anticipated in vivo level of reduced TPO activity, permitting the derivation of the corresponding serum T4 level for a given degree of in vitro TPO inhibition. Using the linear correlation equation in Figure 4A or B, TPO activity in vitro was converted to TPO inhibition ex vivo, expressed as a percent of control, and plotted against observed serum T4 levels for both PTU (Figure 4C) and MMI (Figure 4D). This analysis revealed that 10–30% of in vitro TPO inhibition corresponded with a 20% reduction of serum T4.
Figure 4: Quantitative relationships ex vivo and in vitro extrapolation.
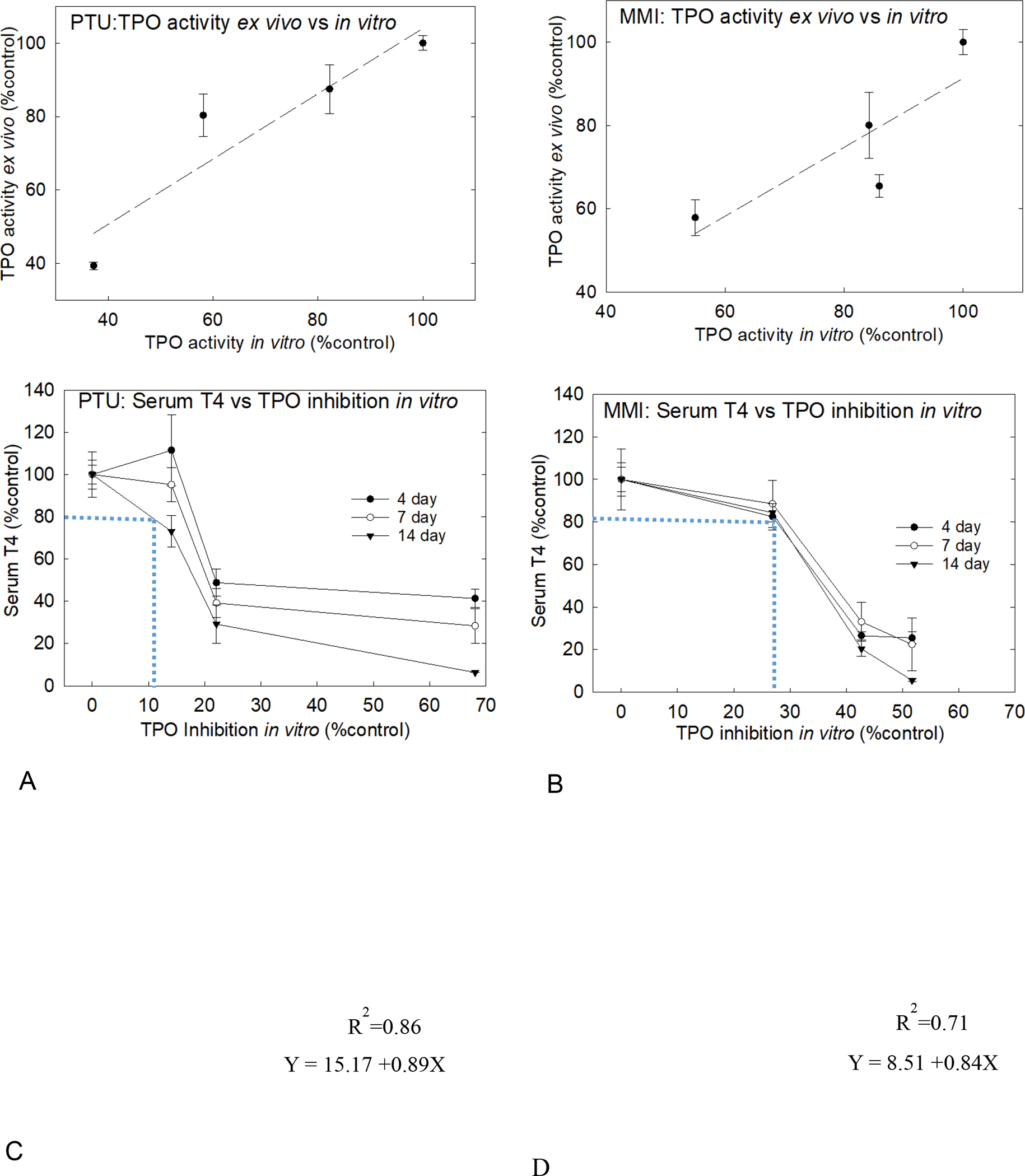
All values plotted as percent control. A) Ex vivo measure of TPO inhibition versus TPO activity for PTU exposure was determined using AUR assay. B) Ex vivo TPO activity correlate well to glandular PTU levels as a linear relationship. C) Examining the relationship between TPO inhibition in vitro, the dotted blue line demonstrates that 10% inhibition in TPO activity in vitro by PTU corresponds to 20% decrement in serum T4 level at 14-day exposure duration. D) For MMI, 20% decline in serum T4 corresponded to 28% TPO inhibition in vitro in the 14-day dosed rats.
Predicting serum T4 with in vitro TPO inhibition
TPO inhibition was simulated using gland concentration of chemical and T4 for all three timepoints to provide a detailed quantitative description of inhibition over time (Figures 5 and 6). Simulation of the 4-day exposure duration resulted in better fit of thyroid gland T4 at lower doses than higher doses for both PTU (Figure 5A) and MMI (Figure 6A). The 7-day exposure duration to PTU (Figure 5A) or MMI (Figure 6A) resulted in the best fits at all doses, while longer exposures of 14 days provided better fits at higher doses. The simulated degree of TPO inhibition was then used to predict the PTU- (Figure 5B) and MMI- driven (Figure 6B) declines in serum T4. In vitro IC50 values of 1.2 μM and 0.11 μM for PTU and MMI, respectively, provided a reasonable fit for the observed data across exposure duration. Furthermore, the simulated percent decline in TPO activity was applied to the basal concentration of serum T4 and was compared with measured serum T4 level for each drug. A simple index value was calculated to quantify the discrepancy between the predicted serum T4 levels and the measured values according to a method published by (Krishnan et al., 1995). The index values for 4, 7, and 14 day PTU exposure was 8, 5, and 2%, respectively while the index values for MMI exposure was 7, 7, and 4% for 4, 7, 14-day treatments, suggesting an increased concordance between predicted serum T4 levels, based on the simulated degree of TPO inhibition, and measured serum T4 levels with increased duration.
Figure 5. PTU computational model and extrapolation of in vitro responses to in vivo serum T4 levels.
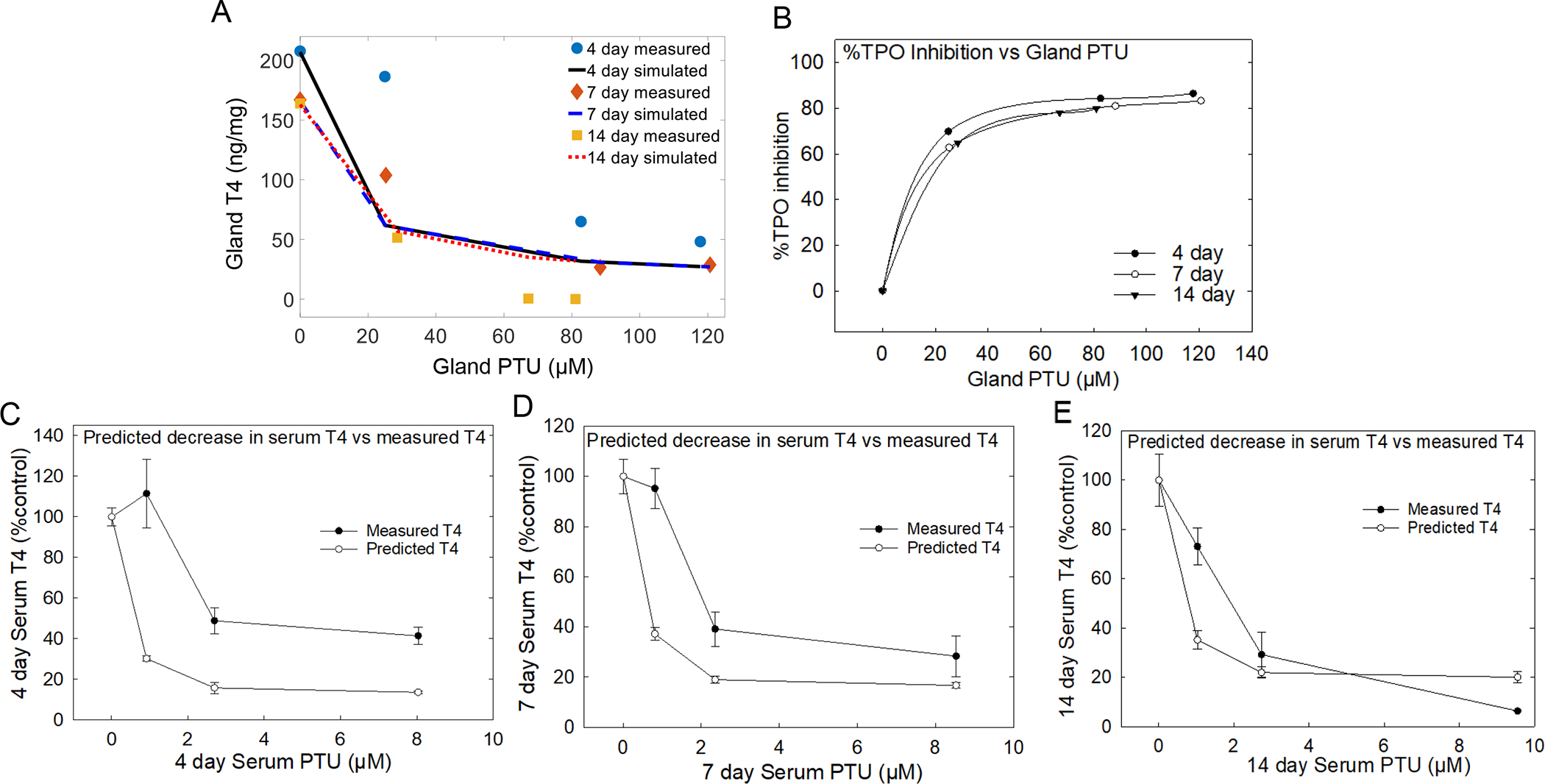
A) in vitro PTU IC50 value of 1.2 μM used to estimate the degree of TPO inhibition for all exposure to points. TPO estimate was better at all doses with 7 and 14-day exposures than 4-day time point and the estimation for 4-day were better at higher PTU doses. B) Degree of TPO inhibition is translated to percent TPO inhibition at all timepoints versus gland PTU levels. C, D, and E) Results demonstrate that the percent TPO decrease, obtained from the simulated degree of TPO inhibition, when applied to serum T4 provides levels that closely aligns with measured serum T4 at 14 days of exposure compared with 4 and 7-day exposures. Error bars represent ± SEM.
Figure 6. MMI computational model and extrapolation of in vitro responses to in vivo serum T4 levels.
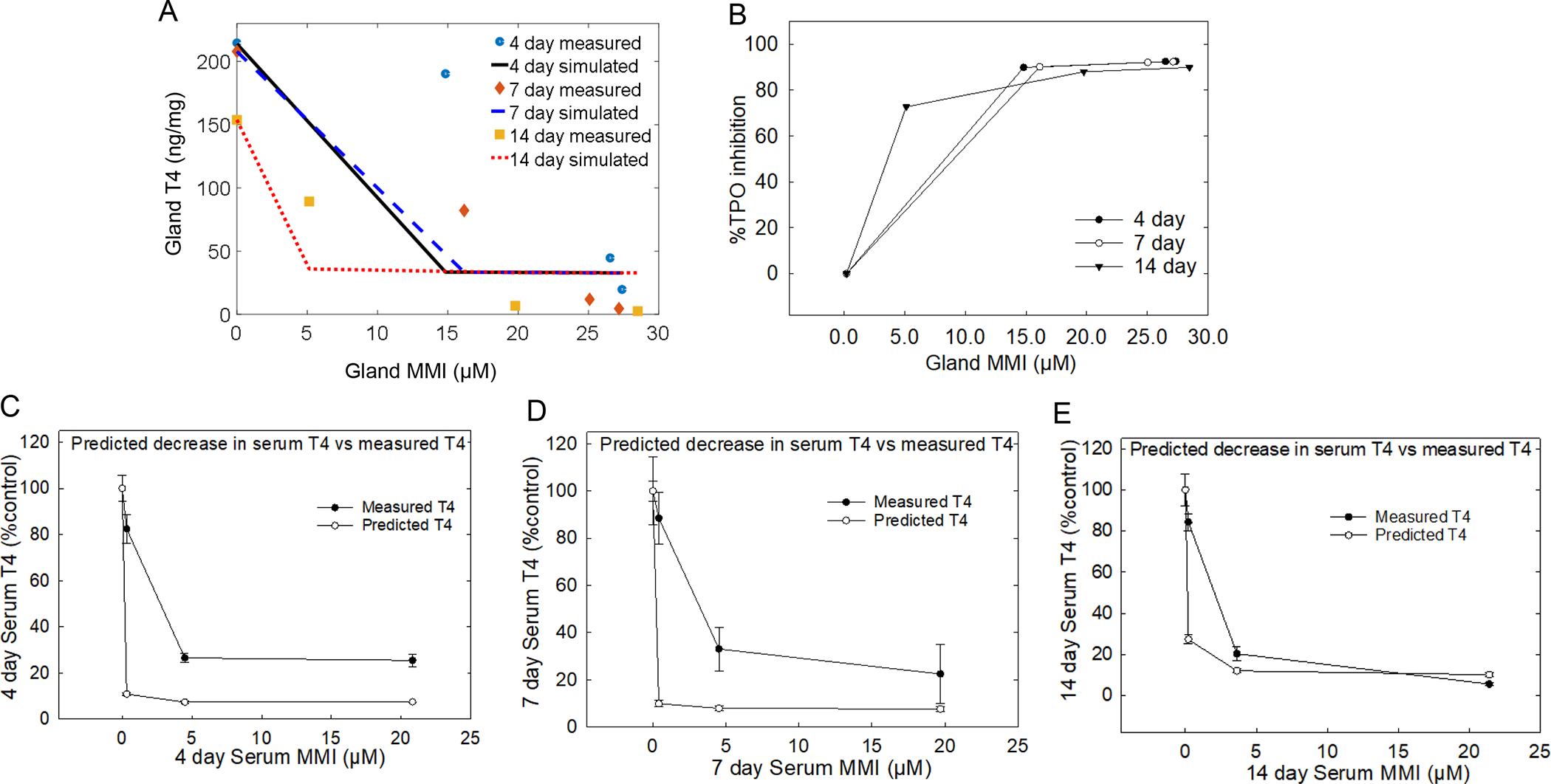
A) Glandular T4 and MMI introduced into an in-silico model to estimate the degree of TPO inhibition resulted in stronger estimation at the lower doses compared with higher doses at all time-points. However, the 7 and 14-day simulations of TPO inhibition provided a better fit of the measured data compared with 4-day estimation of TPO inhibition. B) Translation of the simulated degree of TPO inhibition to %TPO inhibition C, D, and E) finding show when percent TPO inhibition is applied to serum T4 it provides a good prediction of declines in serum T4 compared in the 14-day dosed group compared with the 4 and 7-day dosed groups. Error bars represent ± SEM.
The predicted levels of decline in serum T4 based on serum levels of PTU and MMI for all time-points were 3-fold lower than the measured serum T4 levels for both drugs at the 4- and 7-day exposure durations (Figure 5 C, D; Figure 6 C, D). These differences largely disappear after 14 days of exposure (Figures 5E and 6E). These observations suggest that in addition to concentration of chemical in the gland, kinetic factors including hormone half-life and storage in the gland also contribute to this relationship. The convergence of the predicted and measured serum levels over time may reflect time required to deplete reserved T4 levels in the face of diminished synthesis in the gland (Choksi et al., 2003). Overall, these results suggest that serum T4, a more readily obtainable measure, reflects the TH status in the gland due to TPO inhibition, and it can be calculated using estimates of the degree of the inhibition from in vitro studies when duration of exposure is taken into consideration. These observations may have implications for design of in vivo targeted test strategies, where accuracy of risk estimates is improved with exposure durations that are more than 4 days.
DISCUSSION
Alterations in serum TH concentrations represent a common downstream indicator of xenobiotic-induced thyroid disruption in vivo. HTS assays are increasingly being used to identify and prioritize potential endocrine disrupting chemicals for further testing. Several in vitro assays have been developed to screen for those that interfere with the thyroid axis (Murk et al., 2013; OECD, 2017; Olker et al., 2019; Wang et al., 2018). However, the value of these in vitro experiments would be greatly enhanced if their output could be translated to in vivo measures of regulatory importance. Two major factors influence the fidelity of translating in vitro potency factors to biologically meaningful parameters, and this study addressed both of these challenges for TPO inhibition data generated using the AUR-TPO assay. First, it was important to determine if TPO inhibition observed in the in vitro assay could be recapitulated in vivo. The second factor was the applicability of the quantitative findings obtained from in vitro measures (TPO inhibition) to those achieved in vivo (serum THs). These factors were directly assessed by comparing in vitro and ex vivo estimates of TPO inhibition in the AUR-TPO assay following exposure to two prototypical TPO inhibitors.
In vivo dose-dependent reductions in thyroid gland concentrations of THs provided quantitative estimates of synthesis inhibition by these well-known TPO inhibitors, PTU and MMI. This action resulted in decreased hormone available for release and drove the subsequent declines in T3 and T4 observed in the serum. Consistent with numerous reports in the literature, effects on serum THs by both drugs occurred at lower doses as exposure duration increased (Cooper et al., 1984; Cooper et al., 1983; Hood et al., 1999). In addition, in the current study, concentrations of PTU and MMI in the serum and the thyroid gland reached steady state by the 4-day timepoint and remained constant over all exposure durations. Therefore, the greater declines in hormones in both gland and serum for MMI and PTU likely reflect the diminution of the reserve capacity of the thyroid gland as exposure duration increases (Choksi et al., 2003). In addition, the half life of serum T4, which is 0.5–1 day, may also pay a role in the T4 decrements observed in the serum when T4 synthesis is inhibited in the gland.
Concentration-dependent reductions in in vitro TPO inhibition assays were also in agreement with previous reports (Paul et al., 2013; Paul et al., 2014). Importantly, consistent with in vitro findings, TPO was also inhibited in microsomes prepared ex vivo following in vivo dosing. The in vitro and ex vivo estimates of TPO inhibition for both PTU and MMI were highly correlated with in vivo measurements of hormone concentrations in the gland and the serum, providing a means to incorporate, in a quantitative manner, chemical action on gland hormone synthesis to a downstream serum hormone response familiar to regulators.
Based on these data, a simple one compartment computational model was constructed to estimate the degree of TPO inhibition in the gland as a function of in vitro IC50 and the internal dose of PTU or MMI, and glandular T4 concentrations. Simulations using glandular T4 to predict serum T4 at the shorter duration exposures to PTU or MMI resulted in drastic declines in the predicted serum T4 compared with measured concentrations. However, after 14 days of treatment, the predicted serum T4 was closely aligned with measured serum T4 in both PTU and MMI models. Incorporating parameters of gland capacity beyond the one compartment model may improve model fit and predictions for shorter exposure durations.
Although the primary action of both chemicals is to inhibit TPO, MMI and PTU differ from one another in two ways that could potentially influence their downstream effects on serum THs. A higher glandular PTU concentration relative to MMI concentration (80 μM vs 25 μM) was required to produce a 60% decrement in gland T4, and is consistent with previous observations that MMI is more potent suppressor of TH production (Suplementary Data Figure 3). This same pattern was also reflected in serum hormone profiles for MMI and PTU. Distinct from MMI, PTU also impacts TH synthesis and regulation through inhibition of deiodinase activity (Dio1). This action within the thyroid gland may negatively influence iodine recycling to reduce TH synthesis with resultant decrements in serum T4 concentrations. In contrast, inhibition of Dio1 in the serum and liver would be expected to augment serum T4 by blocking it’s conversion to T3 (Schneider et al., 2006). However, due to the high iodine content of the Purina rat chow and relatively low doses of PTU administered in the current study, it is likely the primary action of PTU on serum thyroid hormone is through its action on TPO.
Collectively, these findings indicate that when combined with estimates of gland concentration derived from dosimetry models, the output of the in vitro AUR-TPO assay may be used to predict chemical effects on serum TH. Incorporation of the in vitro IC50 to the in silico model over-predicted the decline in serum T4 in the short-term, but it was well aligned with declines in serum T4 observed with 14-days of exposure. Although limited to a single ex vivo assessment, a TPO activity decrease in vitro AUR-TPO assay corresponded to a similar percent activity declines observed ex vivo. The relationship between in vitro AUR-TPO inhibition and ex vivo AUR-TPO inhibition approached unity. Establishing this relationship permitted the derivation of a minimal in vitro estimate of TPO inhibition that would be required to reduce serum T4 in vivo. Based on the associations between decrements in serum T4 in pregnant women and adverse neurodevelopmental outcomes in their children (Haddow et al., 1999; Korevaar et al., 2016; Morreale de Escobar et al., 2000) the US EPA considers 20% decline in circulating T4 levels in animal studies as a regulatory level of concern (US EPA 2011). Our analysis suggests that 10–30% TPO inhibition in vitro may be sufficient to reduce serum T4 by 20% after 7–14 days of exposure. Currently, the threshold for a positive response in the AUR-TPO assay as analyzed for the ToxCast program is 20%; now, for at least two chemicals, we have established that this threshold for a positive seems reasonable in terms of the anticipated in vivo change in serum THs that might occur as a result.
A limitation in interpreting the current work is that the mathematical models developed here to relate TPO inhibition in vitro to rat serum TH in vivo may not apply to all chemicals screened in the AUR-TPO assay. Additional chemicals would have to be examined using a similar paradigm to confirm the relationship between in vitro TPO inhibition and in vivo serum TH changes in a rat model. Indeed, findings in the current study contrast with those reported by Chang and Doerge (2000). Using a guaiacol-based TPO assay, Chang et al. reported comparable levels of TPO inhibition in vitro and ex vivo in response to exposure to the isoflavone genistein, but despite nearly 80% inhibition in the TPO assay, no effects were observed on serum hormones in exposed animals. Genistein was also positive in the TPO-AUR assay results of Paul et al. (2014), but less potent than MMI or PTU, with an IC50 value of 4.5 μM. It is not clear if the results reported for genisten by Chang et al. differ due to mechanistic differences in how genistein inhibits TPO activity, due to differences in the TPO assay technology, or due to some other factor. Thus, other chemicals identified in the AUR-TPO assay would require confirmation of any potential to alter serum hormones, either in biologically complex in vitro models or in vivo models. Additional future work could address the variability in the mathematical relationship between TPO inhibition using the AUR-TPO assay and in vivo serum TH changes in rat models.
In conclusion, herein we explored a quantitative model to support translation of AUR-TPO inhibition assay results for prioritization of putative TH disruptors. In this case study, the dose-dependent declines in gland and serum THs and internal doses of PTU and MMI in the serum and thyroid gland were used to develop a computational model to link in vitro TPO inhibition to serum TH reductions in vivo. This work highlights methodology important for extrapolating in vitro assay data to in vivo effects. Further, this work demonstrates quantitative linkages from the molecular initiating event, to target tissue responses, to measurable events at the organism level within an adverse outcome pathway context (Ankley et al., 2010; Edwards et al., 2016; Villeneuve et al., 2014). Although further testing with other TPO inhibitors is necessary to improve confidence in the generalizability of this model to other chemicals, the approach provides a simple scheme to use in vitro assay results to predict an in vivo endpoint important for regulatory toxicology. On a practical basis, this work provides evidence to support a selection of a conservative threshold for a positive response in the AUR-TPO HTS assay for use in prioritization and screening-level assessment.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Drs. Elaina Kenyon and Michael Hornung of the US EPA for reviewing earlier manuscript drafts. Ms. Joan Hedge, Dr. Kevin Crofton and Dr. Heather Stapleton were instrumental in preliminary studies that inspired this work. We gratefully acknowldege Dr. Steven Simmons of the US EPA for providing training using the protocol for AUR-TPO assay and for helpful scientific discussion. We would also like to thank Dr. Denise MacMillan of the US EPA for technical input and consultation. We would also like to ackwledge Michelle Hotchkiss and Susan Thomas for providing technical assistance with the TSH assay.
FUNDING
This work was funded by the United States Environmental Protection Agency.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
This document has been subjected to review by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
REFERENCES
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, and Villeneuve DL (2010). Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29(3), 730–41, 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- Axelstad M, Hansen PR, Boberg J, Bonnichsen M, Nellemann C, Lund SP, Hougaard KS, and Hass U (2008). Developmental neurotoxicity of propylthiouracil (PTU) in rats: relationship between transient hypothyroxinemia during development and long-lasting behavioural and functional changes. Toxicol Appl Pharmacol 232(1), 1–13, 10.1016/j.taap.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Bernal J (2015). Thyroid Hormones in Brain Development and Function. In Endotext (De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, and Weickert MO, Eds.) doi. MDText.com, Inc., South Dartmouth (MA). [Google Scholar]
- Chang HC, and Doerge DR (2000). Dietary genistein inactivates rat thyroid peroxidase in vivo without an apparent hypothyroid effect. Toxicol Appl Pharmacol 168(3), 244–52, 10.1006/taap.2000.9019. [DOI] [PubMed] [Google Scholar]
- Choksi NY, Jahnke GD, St Hilaire C, and Shelby M (2003). Role of thyroid hormones in human and laboratory animal reproductive health. Birth defects research. Part B, Developmental and reproductive toxicology 68(6), 479–491. [DOI] [PubMed] [Google Scholar]
- Cioffi F, Gentile A, Silvestri E, Goglia F, and Lombardi A (2018). Effect of Iodothyronines on Thermogenesis: Focus on Brown Adipose Tissue. Frontiers in endocrinology 9, 254, 10.3389/fendo.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DS, Bode HH, Nath B, Saxe V, Maloof F, and Ridgway EC (1984). Methimazole pharmacology in man: studies using a newly developed radioimmunoassay for methimazole. The Journal of clinical endocrinology and metabolism 58(3), 473–9, 10.1210/jcem-58-3-473. [DOI] [PubMed] [Google Scholar]
- Cooper DS, Kieffer JD, Halpern R, Saxe V, Mover H, Maloof F, and Ridgway EC (1983). Propylthiouracil (PTU) pharmacology in the rat. II. Effects of PTU on thyroid function. Endocrinology 113(3), 921–8, 10.1210/endo-113-3-921. [DOI] [PubMed] [Google Scholar]
- Cooper DS, Maloof F, and Ridgway EC (1987). Rat thyroid peroxidase (TPO) biosynthesis in vitro: studies using antiserum to porcine TPO. Endocr Res 13(1), 15–29. [DOI] [PubMed] [Google Scholar]
- Crofton K, Gilbert ME, Paul Friendman K, Demeneix B, Marty MS, and Zoeller RT (2017). Inhibition of Thyroperoxidase and Subsequent Adverse Neurodevelopmental Outcomes in Mammals. AOP 42 doi: doi:https://aopwiki.org/aops/42, doi:https://aopwiki.org/aops/42. [Google Scholar]
- Divi RL, Chang HC, and Doerge DR (1997). Anti-thyroid isoflavones from soybean: isolation, characterization, and mechanisms of action. Biochemical pharmacology 54(10), 1087–96, 10.1016/s0006-2952(97)00301-8. [DOI] [PubMed] [Google Scholar]
- Divi RL, and Doerge DR (1994). Mechanism-based inactivation of lactoperoxidase and thyroid peroxidase by resorcinol derivatives. Biochemistry 33(32), 9668–74, 10.1021/bi00198a036. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Chang HC, Divi RL, and Churchwell MI (1998). Mechanism for inhibition of thyroid peroxidase by leucomalachite green. Chem Res Toxicol 11(9), 1098–104, 10.1021/tx970226o. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Churchwell MI, and Beland FA (2002). Analysis of DNA adducts from chemical carcinogens and lipid peroxidation using liquid chromatography and electrospray mass spectrometry. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 20(1), 1–20, 10.1081/GNC-120003925. [DOI] [PubMed] [Google Scholar]
- Duncan Bassett JH, and Williams GR (2018). Analysis of Physiological Responses to Thyroid Hormones and Their Receptors in Bone. Methods in molecular biology (Clifton, N.J.) 1801, 123–154, 10.1007/978-1-4939-7902-8_12. [DOI] [PubMed] [Google Scholar]
- Edwards SW, Tan YM, Villeneuve DL, Meek ME, and McQueen CA (2016). Adverse Outcome Pathways-Organizing Toxicological Information to Improve Decision Making. J Pharmacol Exp Ther 356(1), 170–81, 10.1124/jpet.115.228239. [DOI] [PubMed] [Google Scholar]
- EFSA ECA, Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A, and Van der Linden S (2018). Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. In EFSA Journal (Vol. 16, pp. e05311. European Food Safety Authority with the technical support of the Joint Research Centre (EFSA) and European Chemical Agency (ECA) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Taurog A, and Dorris ML (1982a). Preferential inhibition of thyroxine and 3,5,3’-triiodothyronine formation by propylthiouracil and methylmercaptoimidazole in thyroid peroxidase-catalyzed iodination of thyroglobulin. Endocrinology 110(1), 190–7, 10.1210/endo-110-1-190. [DOI] [PubMed] [Google Scholar]
- Engler H, Taurog A, and Nakashima T (1982b). Mechanism of inactivation of thyroid peroxidase by thioureylene drugs. Biochemical pharmacology 31(23), 3801–6. [DOI] [PubMed] [Google Scholar]
- EPA’s National Center for Computational Toxicology (2019). ToxCast Database (invitroDB). [Google Scholar]
- Fisher JW, Li S, Crofton K, Zoeller RT, McLanahan ED, Lumen A, and Gilbert ME (2013). Evaluation of iodide deficiency in the lactating rat and pup using a biologically based dose-response model. Toxicological sciences : an official journal of the Society of Toxicology 132(1), 75–86, 10.1093/toxsci/kfs336. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Hedge JM, Valentin-Blasini L, Blount BC, Kannan K, Tietge J, Zoeller RT, Crofton KM, Jarrett JM, and Fisher JW (2013). An animal model of marginal iodine deficiency during development: the thyroid axis and neurodevelopmental outcome. Toxicological sciences : an official journal of the Society of Toxicology 132(1), 177–95, 10.1093/toxsci/kfs335. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Ramos RL, McCloskey DP, and Goodman JH (2014). Subcortical band heterotopia in rat offspring following maternal hypothyroxinaemia: structural and functional characteristics. Journal of neuroendocrinology 26(8), 528–41, 10.1111/jne.12169. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Rovet J, Chen Z, and Koibuchi N (2012). Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology 33(4), 842–52, 10.1016/j.neuro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Sanchez-Huerta K, and Wood C (2016). Mild Thyroid Hormone Insufficiency During Development Compromises Activity-Dependent Neuroplasticity in the Hippocampus of Adult Male Rats. Endocrinology 157(2), 774–87, 10.1210/en.2015-1643. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, and Klein RZ (1999). Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. The New England journal of medicine 341(8), 549–55, 10.1056/nejm199908193410801. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, and Williams J (2002). Thyroid-stimulating-hormone concentrations and risk of hypothyroidism. Lancet 360(9350), 2081–2; author reply 2082, 10.1016/s0140-6736(02)11973-8. [DOI] [PubMed] [Google Scholar]
- Hassan I, El-Masri H, Kosian PA, Ford J, Degitz SJ, and Gilbert ME (2017a). Neurodevelopment and Thyroid Hormone Synthesis Inhibition in the Rat: Quantitative Understanding Within the Adverse Outcome Pathway Framework. Toxicological sciences : an official journal of the Society of Toxicology 160(1), 57–73, 10.1093/toxsci/kfx163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan I, El-Masri H, Kosian PA, Ford J, Degitz SJ, and Gilbert ME (2017b). Neurodevelopment and Thyroid Hormone Synthesis Inhibition in the Rat: Quantitative Understanding Within the Adverse Outcome Pathway Framework. Toxicological Sciences doi: 10.1093/toxsci/kfx163, kfx163-kfx163, 10.1093/toxsci/kfx163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, Hooijkaas H, de Muinck Keizer-Schrama SM, Hofman A, Jaddoe VV, Visser W, Steegers EA, Verhulst FC, de Rijke YB, and Tiemeier H (2010). Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. The Journal of clinical endocrinology and metabolism 95(9), 4227–34, 10.1210/jc.2010-0415. [DOI] [PubMed] [Google Scholar]
- Hood A, Liu YP, Gattone VH 2nd, and Klaassen CD (1999). Sensitivity of thyroid gland growth to thyroid stimulating hormone (TSH) in rats treated with antithyroid drugs. Toxicological sciences : an official journal of the Society of Toxicology 49(2), 263–71. [DOI] [PubMed] [Google Scholar]
- Jastrzębska H (2015). Antithyroid drugs. Thyroid Research 8(Suppl 1), A12–A12, 10.1186/1756-6614-8-S1-A12. [DOI] [Google Scholar]
- Kackar R, Srivastava MK, and Raizada RB (1997). Studies on rat thyroid after oral administration of mancozeb: morphological and biochemical evaluations. Journal of applied toxicology : JAT 17(6), 369–75. [DOI] [PubMed] [Google Scholar]
- Kampmann JP, and Mølholm Hansen JE (1983). Serum protein binding of propylthiouracil. British journal of clinical pharmacology 16(5), 549–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R, Chandler K, Houck K, Hunter S, Judson R, Kleinstreuer N, Knudsen T, Martin M, Padilla S, Reif D, Richard A, Rotroff D, Sipes N, and Dix D (2012). Update on EPA’s ToxCast program: providing high throughput decision support tools for chemical risk management. Chem Res Toxicol 25(7), 1287–302, 10.1021/tx3000939. [DOI] [PubMed] [Google Scholar]
- Korevaar TI, Muetzel R, Medici M, Chaker L, Jaddoe VW, de Rijke YB, Steegers EA, Visser TJ, White T, Tiemeier H, and Peeters RP (2016). Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. The lancet. Diabetes & endocrinology 4(1), 35–43, 10.1016/s2213-8587(15)00327-7. [DOI] [PubMed] [Google Scholar]
- Krishnan K, Haddad S, and Pelekis M (1995). A simple index for representing the discrepancy between simulations of physiological pharmacokinetic models and experimental data. Toxicology and industrial health 11(4), 413–22, 10.1177/074823379501100404. [DOI] [PubMed] [Google Scholar]
- Louis GW, Hallinger DR, Braxton MJ, Kamel A, and Stoker TE (2017). Effects of chronic exposure to triclosan on reproductive and thyroid endpoints in the adult Wistar female rat. J Toxicol Environ Health A 80(4), 236–249, 10.1080/15287394.2017.1287029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinovich M, Guizzetti M, Ghilardi F, Viviani B, Corsini E, and Galli CL (1997). Thyroid peroxidase as toxicity target for dithiocarbamates. Archives of toxicology 71(8), 508–12. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, and Escobar del Rey F (2000). Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? The Journal of clinical endocrinology and metabolism 85(11), 3975–87, 10.1210/jcem.85.11.6961. [DOI] [PubMed] [Google Scholar]
- Murk AJ, Rijntjes E, Blaauboer BJ, Clewell R, Crofton KM, Dingemans MM, Furlow JD, Kavlock R, Kohrle J, Opitz R, Traas T, Visser TJ, Xia M, and Gutleb AC (2013). Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals. Toxicology in vitro : an international journal published in association with BIBRA 27(4), 1320–46, 10.1016/j.tiv.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Nagasaka A, and Hidaka H (1976). Effect of antithyroid agents 6-propyl-2-thiouracil and 1-mehtyl-2-mercaptoimidazole on human thyroid iodine peroxidase. The Journal of clinical endocrinology and metabolism 43(1), 152–8, 10.1210/jcem-43-1-152. [DOI] [PubMed] [Google Scholar]
- OECD (2017). New Scoping Document on in vitro and ex vivo Assays for the Identification of Modulators of Thyroid Hormone Signalling. [Google Scholar]
- Oetting A, and Yen PM (2007). New insights into thyroid hormone action. Best practice & research. Clinical endocrinology & metabolism 21(2), 193–208, 10.1016/j.beem.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Olker JH, Korte JJ, Denny JS, Hartig PC, Cardon MC, Knutsen CN, Kent PM, Christensen JP, Degitz SJ, and Hornung MW (2019). Screening the ToxCast Phase 1, Phase 2, and e1k Chemical Libraries for Inhibitors of Iodothyronine Deiodinases. Toxicological sciences : an official journal of the Society of Toxicology 168(2), 430–442, 10.1093/toxsci/kfy302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer JH, and Schwartz HL (1997). Molecular basis of thyroid hormone-dependent brain development. Endocrine reviews 18(4), 462–75, 10.1210/edrv.18.4.0309. [DOI] [PubMed] [Google Scholar]
- Paul Friedman K, Watt ED, Hornung MW, Hedge JM, Judson RS, Crofton KM, Houck KA, and Simmons SO (2016). Tiered High-Throughput Screening Approach to Identify Thyroperoxidase Inhibitors Within the ToxCast Phase I and II Chemical Libraries. Toxicological sciences : an official journal of the Society of Toxicology 151(1), 160–80, 10.1093/toxsci/kfw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, Macherla C, Filer DL, Burgess E, Simmons SO, Crofton KM, and Hornung MW (2013). Cross-species analysis of thyroperoxidase inhibition by xenobiotics demonstrates conservation of response between pig and rat. Toxicology 312, 97–107, 10.1016/j.tox.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, Rotroff DM, Hornung MW, Crofton KM, and Simmons SO (2014). Development of a thyroperoxidase inhibition assay for high-throughput screening. Chem Res Toxicol 27(3), 387–99, 10.1021/tx400310w. [DOI] [PubMed] [Google Scholar]
- Pearce EN (2006). Diagnosis and management of thyrotoxicosis. BMJ (Clinical research ed.) 332(7554), 1369–73, 10.1136/bmj.332.7554.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, and de Vijlder JJ (2003). Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clinical endocrinology 59(3), 282–8. [DOI] [PubMed] [Google Scholar]
- Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, and Vader HL (1999). Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clinical endocrinology 50(2), 149–55. [DOI] [PubMed] [Google Scholar]
- Schneider MJ, Fiering SN, Thai B, Wu SY, St Germain E, Parlow AF, St Germain DL, and Galton VA (2006). Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology 147(1), 580–9, 10.1210/en.2005-0739. [DOI] [PubMed] [Google Scholar]
- Silva JE, Gordon MB, Crantz FR, Leonard JL, and Larsen PR (1984). Qualitative and quantitative differences in the pathways of extrathyroidal triiodothyronine generation between euthyroid and hypothyroid rats. The Journal of clinical investigation 73(4), 898–907, 10.1172/jci111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker TE, Gibson EK, and Zorrilla LM (2010). Triclosan exposure modulates estrogen-dependent responses in the female wistar rat. Toxicological sciences 117(1), 45–53, 10.1093/toxsci/kfq180. [DOI] [PubMed] [Google Scholar]
- Taurog A (1976). The mechanism of action of the thioureylene antithyroid drugs. Endocrinology 98(4), 1031–46, 10.1210/endo-98-4-1031. [DOI] [PubMed] [Google Scholar]
- Taurog A, Dorris ML, and Doerge DR (1996). Mechanism of simultaneous iodination and coupling catalyzed by thyroid peroxidase. Arch Biochem Biophys 330(1), 24–32, 10.1006/abbi.1996.0222. [DOI] [PubMed] [Google Scholar]
- Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, Butenhoff JL, Stevenson LA, and Lau C (2003). Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicological Sciences 74(2), 369–381. [DOI] [PubMed] [Google Scholar]
- Thomas RS, Bahadori T, Buckley TJ, Cowden J, Deisenroth C, Dionisio KL, Frithsen JB, Grulke CM, Gwinn MR, Harrill JA, Higuchi M, Houck KA, Hughes MF, Hunter ES, Isaacs KK, Judson RS, Knudsen TB, Lambert JC, Linnenbrink M, Martin TM, Newton SR, Padilla S, Patlewicz G, Paul-Friedman K, Phillips KA, Richard AM, Sams R, Shafer TJ, Setzer RW, Shah I, Simmons JE, Simmons SO, Singh A, Sobus JR, Strynar M, Swank A, Tornero-Valez R, Ulrich EM, Villeneuve DL, Wambaugh JF, Wetmore BA, and Williams AJ (2019). The Next Generation Blueprint of Computational Toxicology at the U.S. Environmental Protection Agency. Toxicological sciences : an official journal of the Society of Toxicology 169(2), 317–332, 10.1093/toxsci/kfz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA (2011). Integrated Approaches to Testing and Assessment Strategy: Use of New Computational and Molecular Tools, FIFRA SCIENTIFIC ADVISORY PANEL. In (W. D. Office of Pesticide Programs, Ed.) Eds.) doi, https://yosemite.epa.gov/sab/sabproduct.nsf/373C1DB0E0591296852579F2005BECB3/$File/OPP+SAP+document-May2011.pdf [Google Scholar]
- USEPA (2017). Continuing Development of Alternative High-Throughput Screens to Determine Endocrine Disruption, Focusing on Androgen Receptor, Steroidogenesis, and Thyroid Pathways. In (E. D. S. Program, Ed.) Eds.) doi: FIFRA SAP, 2017 ed. U.S. EPA Office of Chemical Safety and Pollution Prevention (OCSPP), U.S. EPA Office of Research and Development (ORD), U.S. EPA Office of Water (OW), NIH National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM), https://www.regulations.gov/document?D=EPA-HQ-OPP-2017-0214-0012. [Google Scholar]
- Vickers AE, Heale J, Sinclair JR, Morris S, Rowe JM, and Fisher RL (2012). Thyroid organotypic rat and human cultures used to investigate drug effects on thyroid function, hormone synthesis and release pathways. Toxicol Appl Pharmacol 260(1), 81–8, 10.1016/j.taap.2012.01.029. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, and Whelan M (2014). Adverse outcome pathway (AOP) development I: strategies and principles. Toxicological sciences : an official journal of the Society of Toxicology 142(2), 312–20, 10.1093/toxsci/kfu199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hallinger DR, Murr AS, Buckalew AR, Simmons SO, Laws SC, and Stoker TE (2018). High-Throughput Screening and Quantitative Chemical Ranking for Sodium-Iodide Symporter Inhibitors in ToxCast Phase I Chemical Library. Environmental science & technology 52(9), 5417–5426, 10.1021/acs.est.7b06145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AJ, Grulke CM, Edwards J, McEachran AD, Mansouri K, Baker NC, Patlewicz G, Shah I, Wambaugh JF, Judson RS, and Richard AM (2017). The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. Journal of cheminformatics 9(1), 61, 10.1186/s13321-017-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GR (2008). Neurodevelopmental and neurophysiological actions of thyroid hormone. Journal of neuroendocrinology 20(6), 784–94, 10.1111/j.1365-2826.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, McAndrews MP, and Rovet JF (2014). Effects of maternal hypothyroidism on offspring hippocampus and memory. Thyroid 24(3), 576–84, 10.1089/thy.2013.0215. [DOI] [PubMed] [Google Scholar]
- Woźniak B, Witek S, Matraszek-Żuchowska I, and Żmudzki J (2014). Determination of the Thyreostats in Animal Feeding Stuffs Using Liquid Chromatography-Tandem Mass Spectrometry 58(3), 413, 10.2478/bvip-2014-0064. [DOI] [Google Scholar]
- Yen PM (2001). Physiological and molecular basis of thyroid hormone action. Physiological reviews 81(3), 1097–142, 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Chung TD, and Oldenburg KR (1999). A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. Journal of biomolecular screening 4(2), 67–73, 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Zoeller RT (2007). Environmental chemicals impacting the thyroid: targets and consequences. Thyroid 17(9), 811–7, 10.1089/thy.2007.0107. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, and Crofton KM (2005). Mode of action: developmental thyroid hormone insufficiency--neurological abnormalities resulting from exposure to propylthiouracil. Crit Rev Toxicol 35(8–9), 771–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


