ABSTRACT
The WalR-WalK two-component regulatory system (TCS) is found in all Firmicutes, in which it regulates the expression of multiple genes required for remodeling the cell envelope during growth and division. Unlike most TCSs, WalRK is essential for viability, so it has attracted interest as a potential antibiotic target. In this study, we used overexpression of WalR and CRISPR interference to investigate the Wal system of Clostridioides difficile, a major cause of hospital-associated diarrhea in high-income countries. We confirmed that the wal operon is essential and identified morphological defects and cell lysis as the major terminal phenotypes of altered wal expression. We also used transcriptome sequencing (RNA-seq) to identify over 150 genes whose expression changes in response to WalR levels. This gene set is enriched in cell envelope genes and includes genes encoding several predicted PG hydrolases and proteins that could regulate PG hydrolase activity. A distinct feature of the C. difficile cell envelope is the presence of an S-layer, and we found that WalR affects expression of several genes which encode S-layer proteins. An unexpected finding was that some Wal-associated phenotypic defects were inverted in comparison to what has been reported for other Firmicutes. For example, downregulation of Wal signaling caused C. difficile cells to become longer rather than shorter, as in Bacillus subtilis. Likewise, downregulation of Wal rendered C. difficile more sensitive to vancomycin, whereas reduced Wal activity is linked to increased vancomycin resistance in Staphylococcus aureus.
IMPORTANCE The WalRK two-component system (TCS) is essential for coordinating synthesis and turnover of peptidoglycan in Firmicutes. We investigated the WalRK TCS in Clostridioides difficile, an important bacterial pathogen with an atypical cell envelope. We confirmed that WalRK is essential and regulates cell envelope biogenesis, although several of the phenotypic changes we observed were opposite to what has been reported for other Firmicutes. We also identified over 150 genes whose expression is controlled either directly or indirectly by WalR. Overall, our findings provide a foundation for future investigations of an important regulatory system and potential antibiotic target in C. difficile.
KEYWORDS: cell envelope, regulation of gene expression, signal transduction
INTRODUCTION
To grow and divide, bacteria must thoroughly remodel their peptidoglycan (PG) sacculus, a large, covalently closed macromolecule that surrounds the cell and provides protection against rupture due to turgor pressure (1). How bacteria remodel the sacculus without making errors that lead to inadvertent lysis is a question that has fascinated microbiologists for decades. The question is also of practical significance because small molecules that undermine normal peptidoglycan biogenesis are among our most effective antibiotics.
An important advance in our understanding of cell envelope biogenesis was made over 20 years ago when Fabret and Hoch identified a Bacillus subtilis two-component system (TCS) that is essential for viability (2). This TCS is now called the Wal system and is known to coordinate expression of multiple cell envelope genes in the Firmicutes. WalRK is essential in all species studied so far, including pathogens such as Staphylococcus aureus (3–5) and Streptococcus pneumoniae (6, 7). Because of its essentiality, the Wal TCS has attracted interest as a potential antibiotic target.
The Wal TCS comprises the bifunctional kinase/phosphatase WalK together with its cognate response regulator, WalR. In addition, wal operons typically include genes for membrane proteins known or presumed to modulate WalK activity, but the accessory proteins differ between organisms (8–10). Recent evidence from B. subtilis indicates that WalK activity is regulated by PG fragments generated by PG hydrolases that open spaces to make room for elongation. These PG fragments bind to WalK’s extracellular Cache domain to modulate the phosphorylation state of WalR (11). Phosphorylated WalR, in turn, binds to various promoters to activate or repress gene expression.
The number of genes in the WalR regulon ranges from about a dozen to over 100 (5, 12–16), depending on the species, and only some of these genes are directly activated or repressed by WalR. Although WalR regulons are diverse, they invariably include multiple proteins that contribute to proper biogenesis of the cell envelope, especially PG hydrolases. Consistent with these themes, the phenotypic defects elicited by artificial up- or downregulation of WalRK signaling include abnormal cell shape, larger cells, smaller cells, ghost cells (lysis), thicker PG, abnormal division septa, and altered sensitivity to antibiotics that target PG biogenesis (8–10). In S. pneumoniae walRK is essential because it activates expression of an essential PG hydrolase gene, pcsB (6, 7). However, the essentiality of walRK in B. subtilis and S. aureus is polygenic in nature, resulting from abnormal expression of multiple genes that are not by themselves essential. In all three organisms, it is possible to bypass the walRK requirement by constitutively expressing one or more Wal regulon PG hydrolase genes, sometimes in combination with deleting genes for hydrolase inhibitors (6, 17, 18).
Here, we studied the WalRK TCS in Clostridioides difficile, an anaerobic, spore-forming member of the Firmicutes responsible for close to a quarter of a million hospitalizations and over 12,000 deaths per year in the United States (19). Unlike the envelope of most Firmicutes in which walRK has been studied previously, the C. difficile envelope has a proteinaceous S-layer whose assembly presumably must be coordinated with PG synthesis (20–22). In addition, the PG itself is somewhat unusual (23). About 90% of the N-acetylglucosamine (GlcNAc) is deacetylated (which has implications for PG hydrolase activity), and about 70% of the peptide cross-links are 3-3 rather than 4-3 (which has implications for the structure of PG fragments thought to bind to the Cache domain of WalK [11]). The C. difficile Wal system includes a unique lipoprotein gene and a noncanonical WalK that lacks an intracellular PER-ARNT-SIM (PAS) signaling domain (Fig. 1) (10). These features distinguish C. difficile’s WalRK TCS from those of previously characterized Firmicutes, although a distantly related wal-like system in Mycobacterium tuberculosis (MtrAB) includes a lipoprotein and a WalK-like histidine kinase without an intracellular PAS domain (10, 24, 25). Finally, our recent development of tools for xylose-inducible gene expression and CRISPR interference (26) helped to overcome some of the challenges inherent in phenotypic analysis of essential genes like walRK in C. difficile.
FIG 1.
The wal operon of C. difficile. Based on studies in other bacteria, WalK (red) is a bifunctional signal-transducing enzyme that can phosphorylate or dephosphorylate WalR (green). WalK is predicted to have an extracellular Cache domain, as well as intracellular HAMP, HisKA (phospho-acceptor), and HATPase domains. Unlike the B. subtilis ortholog (inset), it does not have an intracellular PAS domain. WalR~P binds DNA to activate or repress expression of Wal-regulated genes. The lipoprotein (WalA [blue]), which is unique to C. difficile, may modulate WalK activity and is predicted to have a beta-propeller lyase domain. The truA2 gene codes for a pseudouridylate synthase and is not thought to play a role in Wal signaling. The operon locus is cdr20291_1676-1679 in R20291 and cd630_17810-17840 in 630Δerm.
RESULTS
The wal operon is required for cell viability, proper rod morphology, and intrinsic resistance to some antibiotics.
According to transposon insertion sequencing (Tn-seq), the WalRK TCS is essential in C. difficile (27). To confirm and extend this finding, we leveraged the power of CRISPR interference (CRISPRi) for functional analysis of essential genes/operons (28). Our CRISPRi system comprises a xylose-inducible nuclease-defective Cas9, Pxyl::dCas9, that is targeted to a gene of interest by a single guide RNA (sgRNA) expressed constitutively from the glutamate dehydrogenase promoter, Pgdh::sgRNA (26). Because CRISPRi is polar, we decided to target the first gene in the operon, which encodes a predicted lipoprotein unique to the C. difficile wal operon (Fig. 1). We have named this gene walA. For reproducibility, we designed two guides against walA, sgRNA-walA1 and sgRNA-walA2. Subsequent transcriptome sequencing (RNA-seq) experiments described below confirmed that targeting dCas9 to walA suppressed transcription of the entire operon. As a negative control, we used an sgRNA (sgRNA-neg) that does not have a target anywhere on the C. difficile chromosome. In some experiments the CRISPRi machinery was produced from a plasmid that confers resistance to thiamphenicol (Thi), while in others it was integrated into the chromosome at the pyrE locus in a way that does not leave behind an antibiotic resistance marker (29).
Chromosomal- or plasmid-based CRISPRi knockdown of the wal operon in either 630Δerm or R20291 reduced viability ≥104-fold when cells were plated on tryptone-yeast extract (TY) agar containing 1% xylose (Fig. 2A; see also Fig. S1A in the supplemental material). Additional phenotypes were characterized in TY broth using 630Δerm derivatives with the CRISPRi machinery integrated at pyrE. In the absence of xylose, sgRNA-walA1 and sgRNA-walA2 retarded growth slightly compared to the sgRNA-neg control (Fig. 2B and Fig. S1B). This finding indicates that there is leaky expression of Pxyl::dCas9, which was later confirmed by RNA-seq (see below). Under these conditions, cells were, on average, about 10% longer than the wild type and the fraction of cells with curved or irregular contours increased from ~1% in the sgRNA-neg control to ~20% for sgRNA-walA1 or ~10% for sgRNA-walA2 (Fig. 2C to E and Fig. S1E and F). Further knockdown of the wal operon by addition of xylose exacerbated the growth defect in a dose-dependent manner (Fig. 2B and Fig. S1D). In addition, morphological defects became more pronounced, with cells now averaging ~30% longer than controls and up to ~40% exhibiting curved or irregular contours (Fig. 2D and E and Fig. S1E and F).
FIG 2.
The wal operon is required for viability and normal rod morphology of C. difficile 630Δerm. (A) Viability assay. Serial dilutions of overnight cultures were spotted onto TY plates with or without 1% xylose to induce the expression of dCas9. Plates were photographed after incubation overnight. Strains carry chromosomal copies of the CRISPRi components and the following guides: sgRNA-neg (UM554), sgRNA-walA1 (UM555), and sgRNA-walA2 (UM556). (B) Growth curves in liquid TY containing 0, 0.01, 0.1, or 1% xylose as indicated. For clarity, the sgRNA-neg control strain is graphed only at 0% xylose. Samples were taken at 4 h (arrow) for phase-contrast microscopy (C), cell length measurements (D), and determination of curvature (E). Numbers at the bottom of micrographs in panel C refer to percent xylose. Note cell debris indicative of lysis at 1% xylose. Bar = 10 μm. Cell length and percent curved cells were based on about 500 cells per condition. The sgRNA-walA1 strain is longer than the sgRNA-neg control in all pairwise comparisons as determined by a t test (P < 0.0001).
Partial knockdown of the wal operon in the absence of xylose allowed us to screen for altered sensitivity to antibiotics that target cell envelope biogenesis. We found that the MIC for ampicillin and daptomycin decreased about 2-fold, while the MIC for vancomycin decreased about 4-fold (Table 1). But sensitivity to the other two cell wall-active antibiotics, imipenem and bacitracin, and the gyrase inhibitor novobiocin was not affected (Table 1).
TABLE 1.
Effect of CRISPRi-silencing wal on sensitivity to antibioticsa
| Antibiotic | MIC (μg/mL) |
||
|---|---|---|---|
| sgRNA-neg | sgRNA-walA1 | sgRNA-walA2 | |
| Ampicillin | 6.3 | 3.5 | 3.1 |
| Imipenem | 3.1 | 3.1 | 2.6 |
| Vancomycin | 4.6 | 0.9 | 0.7 |
| Daptomycin | 11.3 | 4.1 | 5.7 |
| Bacitracin | 250 | 250 | 250 |
| Novobiocin | 13 | 17 | 16 |
Results are the averages of measurements done in duplicate on four different days.
Overexpression of WalR impairs growth, alters morphology, and slows autolysis.
We also investigated Wal function in C. difficile by overproducing the response regulator WalR to drive cells into an exacerbated Wal-ON state (5, 30). Plating C. difficile 630Δerm harboring a Pxyl::walR expression plasmid onto TY-Thi containing 1% xylose resulted in a 106-fold loss of viability (Fig. 3A). This strain failed to grow when subcultured directly into TY-Thi broth containing 1% xylose, but waiting until the optical density at 600 nm (OD600) reached ~0.2 before adding 3% xylose resulted in only a subtle growth defect (Fig. 3B). Three hours postinduction, the cells had become strikingly phase bright and resistant to autolysis when suspended in buffer containing 0.01% Triton X-100 (Fig. 3C and D). Triton X-100 is thought to reduce the inhibitory effect of lipoteichoic acids on PG hydrolases (31, 32). Similar results were obtained in the R20291 strain background (Fig. S3). It is unclear what causes cells to turn phase bright upon overexpression of walR. Differential staining with Syto 9 and propidium iodide (LIVE/DEAD staining) indicated that >99% of the cells were alive after overproduction of WalR (Fig. 3B, red arrow).
FIG 3.
Overexpression of walR from a multicopy plasmid impairs growth, alters morphology, and slows autolysis in 630Δerm. (A) Viability assay. Overnight cultures of 630Δerm harboring pCE691 (Pxyl::walR) or the empty vector control plasmid pBZ101 (Pxyl EV) were serially diluted and spotted onto TY-Thi plates with or without 1% xylose. Plates were photographed after incubation overnight. (B) Growth curve of 630Δerm/pCE691 (Pxyl::walR). Duplicate cultures were grown to an OD600 of 0.2, at which time one was induced with 3% xylose (green arrow). Both cultures were harvested after 3 h (red arrow) for analysis by phase-contrast microscopy (C) and a lysis assay (D). Bar in panel C = 10 μm. For the lysis assay, cells were suspended in buffer containing 0.01% Triton X-100 and OD600 was monitored over time. Error bars depict SD of 3 technical replicates. Data shown are representative of results from at least 3 independent experiments.
We repeated these experiments after integrating Pxyl::walR into the chromosome of 630Δerm. As expected, the effects of xylose induction were similar but less pronounced when Pxyl::walR was in single copy (Fig. 4A). The addition of xylose at subculture was now tolerated and reduced growth in a dose-dependent manner. At 3% xylose, cells became slightly phase bright. There were some bent or hooked cells, a defect not observed with the Pxyl::walR plasmid, perhaps because it could only be induced for shorter times (Fig. 4C). After 6 h of induction, cells were harvested and evaluated in the lysis assay. We found that overexpression of walR slowed lysis in a dose-dependent manner (Fig. 4B).
FIG 4.
Prolonged overexpression of walR from a single-copy chromosomal Pxyl::walR allele affects cell shape and slows lysis. (A) Growth curves. An overnight culture of strain UM626 (Pxyl::walR integrated at pyrE in 630Δerm) was subcultured 1:50 into TY with varied xylose concentrations as indicated, grown for 6 h, and then analyzed in a lysis assay (B) or by phase-contrast microscopy (C). For the lysis assay, cells were suspended in buffer containing 0.01% Triton X-100 and OD600 was monitored over time. Data are graphed as the mean and SD of 3 technical replicates, but most error bars are smaller than the symbols. Cells from cultures grown in the presence of 0 to 1% xylose appeared normal and are not shown, but in cultures induced with 3% xylose, about 10% of the cells had irregular or bent morphologies, examples of which are shown in panel C. Bar = 10 μm. Data shown are representative of those from at least 3 independent experiments.
Expression profiling under Wal-ON conditions.
As a step toward understanding the basis of the various Wal-related phenotypes, we sought to identify the genes in the wal regulon. (Note that we use the term regulon for convenience to refer to genes whose expression responds to manipulation of the wal operon regardless of whether these effects are direct or indirect.) We began by performing RNA-seq on two strains that overexpress walR from a single-copy Pxyl::walR construct integrated at pyrE. One of these strains overproduced wild-type WalR; the other overproduced a WalRD54E mutant protein expected to mimic phosphorylated WalR and thus be constitutively active (13, 33) (confusingly, D54 is annotated as D66 in the R20291 genome on the BioCyc website). The two strains behaved similarly with respect to growth and performance in the lysis assay (Fig. S4A and B). The control strain for these experiments had the gene for a red fluorescent protein (rfp) integrated at pyrE (26). Strains were grown overnight in TY and then subcultured into fresh TY at a starting OD600 of ~0.05. When the OD600 reached ~0.2, cultures were induced with 3% xylose and allowed to grow for 3 h (~2 mass doublings), at which time cells were harvested and processed for RNA-seq. At this time point, induction of walR had only a small effect on growth rate (Fig. S4A) and >99% were viable as judged by LIVE/DEAD staining.
Not including walR, which was induced directly from Pxyl, the RNA-seq analysis identified 77 genes whose transcript abundance changed ≥4-fold (P < 0.05) upon overexpression of either walR or walRD54E (Table 2). Of these genes, 44 were induced and 33 were repressed. Induction ratios were very similar in the walR and walRD54E strains with only two exceptions, cd630_03901 and cd630_10631, both of which encode hypothetical proteins. The failure of the D54E substitution to increase gene expression could mean that it does not activate in C. difficile WalR, as reported previously for some other response regulators (34). Alternatively, overexpression of walR is itself activating and might obscure the effects of the substitution. The 77 genes are predicted by BioCyc (35) to be organized into 65 operons that contain 18 additional genes which missed our 4-fold expression change cutoff. Visual inspection of the expression data revealed that these genes almost always trended in the right direction, confirming the predicted structure of most of the operons. We therefore included these 18 genes in what we refer to as the Wal-ON regulon, bringing it to a total of 95 genes in 65 operons (Table 2).
TABLE 2.
Genes affected by Wal-ON conditions
| Gene, changed ≥4-fold | Operona | Gene name | Annotationb | Log2 fold change |
Cell wall associationd | ||
|---|---|---|---|---|---|---|---|
| WalR overexpression |
CRISPRi against walc | ||||||
| WalR | WalRD54E | ||||||
| cd630_07390 | 07390 | Hypothetical protein | 5.26 | 5.70 | −1.38 | SPI | |
| cd630_15220 | 15220 | pgdA | Peptidoglycan deacetylase | 4.02 | 4.26 | −1.09 | SPI |
| cd630_05490 | 05490 | Hypothetical protein | 3.87 | 4.51 | −1.84 | SPI | |
| 17810 | walA | Lipoprotein | NCe | NC | −2.76 | SPII | |
| cd630_17820 | 17820 | walR | TC response regulator | 3.81 | 3.67 | −2.51 | |
| 17830 | walK | TC sensor histidine kinase | NC | NC | −2.33 | Membrane | |
| 17840 | truA2 | tRNA pseudouridine synthase A | NC | NC | −2.34 | ||
| cd630_07380 | 07380 | Hypothetical protein | 3.43 | 4.14 | −1.22 | SPI | |
| cd630_08670 | 08670 | Hypothetical protein | 3.28 | 3.97 | NC | Membrane | |
| cd630_07400 | 07400 | PLP-dependent aminotransferase | 3.21 | 2.38 | NC | ||
| cd630_10880 | 10880 | Hypothetical protein | 2.88 | 3.14 | −1.40 | Membrane | |
| cd630_01660 | 01660 | Peptidase | 2.76 | 2.95 | NC | ||
| 01650 | Amino acid transporter | NC | NC | NC | Membrane | ||
| cd630_27940 | 27940 | cwp12 | Cell wall binding protein | 2.69 | 2.82 | NC | SPI |
| cd630_33090 | 33090 | ligA | DNA ligase | 2.64 | 2.62 | NC | |
| cd630_18250 | 18250 | metY | O-Acetylhomoserine sulfhydrylase | 2.50 | 2.80 | −0.57 | |
| cd630_18260 | 18260 | metA | Homoserine O-succinyltransferase | 2.60 | 2.85 | −0.88 | |
| cd630_14281 | 14281 | Hypothetical protein | 2.59 | 3.22 | NC | ||
| cd630_27860 | 27860 | cwp5 | Cell wall binding protein | 2.51 | 2.65 | NC | SPI |
| cd630_25380 | 25380 | Lipoprotein | 2.47 | 2.70 | −0.70 | SPII | |
| cd630_03910 | 03910 | asnB | Asparagine synthetase | 2.47 | 3.30 | NC | Membrane |
| cd630_14960 | 14960 | Hypothetical protein | 2.37 | 2.86 | −0.74 | SPI | |
| cd630_25370 | 25370 | 5′-Nucleotidase/phosphoesterase | 2.33 | 2.49 | −0.59 | SPI | |
| cd630_27910 | 27910 | cwp2 | Cell wall binding protein | 2.30 | 2.36 | −0.59 | SPI |
| 27900 | LmbE-like deacetylase | 1.56 | 1.46 | −0.55 | |||
| 27890 | cwp66 | Cell wall binding protein | 1.41 | 1.42 | −0.58 | SPI | |
| cd630_28620 | 28620 | Peptidase | 2.29 | 2.24 | −2.53 | ||
| cd630_34880 | 34880 | gtaB1 | UTP-G1P uridylyltransferase | 2.23 | 2.34 | −0.73 | |
| cd630_20580 | 20580 | Hypothetical protein | 2.22 | 2.43 | −1.26 | Membrane | |
| cd630_07410 | 07410 | glpK1 | Glycerol kinase | 2.21 | 0.63 | NC | |
| 07420 | eutH | Ethanolamine utilization protein | 1.46 | NC | NC | Membrane | |
| 10350 | cwp16 | Amidase, cell wall binding protein | 1.24 | 1.33 | NC | SPI | |
| cd630_10360 | 10360 | cwp17 | Amidase, cell wall binding protein | 2.15 | 2.35 | NC | SPI |
| cd630_21170 | 21170 | trxB2 | Thioredoxin reductase | 2.12 | 2.18 | NC | |
| cd630_00210 | 00210 | pycA | Pyruvate carboxylase | 2.10 | 2.16 | −0.46 | |
| cd630_13890 | 13890 | Chloromuconate cycloisomerase | 2.09 | 2.18 | −0.28 | ||
| 13900 | Hypothetical protein | 1.30 | 1.64 | Membrane | |||
| cd630_22490 | 22490 | ATPase | 2.07 | 2.20 | NC | ||
| cd630_22480 | 22480 | Hypothetical protein | 1.81 | 2.04 | NC | ||
| cd630_10890 | 10890 10900 |
rgbR
rgbS |
TC response regulator | 2.04 | 2.24 | −1.13 | |
| 10900 | rgbS | TC sensor HK (fragment) | NC | 1.16 | NC | Membrane | |
| cd630_30930 | 30930 | Aminobutyrate hydrolase | 2.15 | 2.35 | NC | ||
| 30920 | Putative amino acid transporter | 1.52 | 1.02 | NC | Membrane | ||
| cd630_26810 | 26810 | Putative Ca-chelating protein | 2.01 | 2.59 | NC | SPI | |
| cd630_36010 | 36010 | d-Alanyl–d-alanine carboxypeptidase | 1.99 | 2.35 | −1.38 | SPI | |
| cd630_00220 | 00220 | Elongation factor G | 1.97 | 2.09 | NC | ||
| cd630_14290 | 14290 | Acyl-CoA N-acyltransferase | 1.92 | 2.47 | −1.15 | ||
| cd630_22280 | 22280 | Hypothetical protein | 1.83 | 2.05 | −0.70 | Membrane | |
| cd630_01100 | 01100 | Hypothetical protein | 1.81 | 2.02 | NC | Membrane | |
| 01110 | Hypothetical protein | 1.51 | 1.73 | NC | SPI | ||
| 01111 | Hypothetical protein | NC | 1.14 | NC | |||
| cd630_09940 | 09940 | Serine-pyruvate aminotransferase | 1.77 | 2.10 | NC | ||
| cd630_19942 | 19942 | Hypothetical protein | 1.74 | 2.12 | NC | ||
| cd630_05270 | 05270 | Beta-lactamase-like hydrolase | 1.73 | 2.13 | −0.85 | ||
| cd630_22270 | 22270 | Radical SAM protein | 1.67 | 2.02 | −0.55 | SPII | |
| cd630_18070 | 18070 | NADPH-dependent FMN reductase | 1.49 | 2.07 | −1.53 | Membrane | |
| cd630_03901 | 03901 | Hypothetical protein | 0.92 | 2.37 | NC | ||
| cd630_10631 | 10631 | Hypothetical protein | −0.07 | 2.18 | −0.62 | Membrane | |
| cd630_30360 | 30360 | Major facilitator superfamily transporter | −1.59 | −2.13 | 1.11 | Membrane | |
| cd630_10540 | 10540 | bcd2 | Acyl-CoA dehydrogenase | −1.93 | −2.23 | 1.03 | |
| cd630_10550 | 10550 | etfB | Electron transfer flavoprotein beta | −1.78 | −2.11 | 1.07 | |
| cd630_10560 | 10560 | etfA | Electron transfer flavoprotein alpha | −1.95 | −2.18 | 1.10 | |
| cd630_10570 | 10570 | crt2 | 3-Hydroxybutyryl-CoA dehydratase | −1.96 | −2.27 | 1.06 | |
| cd630_31000 | 31000 | C4-dicarboxylate anaerobic carrier | −1.88 | −2.59 | NC | Membrane | |
| cd630_30990 | 30990 | Amidohydrolase | −1.80 | −2.47 | NC | Membrane | |
| cd630_10580 | 10580 | hbd | 3-Hydroxybutyryl-CoA dehydrogenase | −1.86 | −2.05 | 0.89 | SPII |
| cd630_10590 | 10590 | thlA1 | Acetyl-CoA acetyltransferase | −1.96 | −2.16 | 0.77 | |
| 16820 | queK | Queuosine hydrolase monomer | −1.31 | −1.60 | NC | ||
| 16830 | ECF transporter S-comp. | −1.39 | −1.65 | NC | Membrane | ||
| cd630_16840 | 16840 | queL | Radical SAM superfamily protein | −1.90 | −2.06 | NC | |
| 23900 | BlaI-like | −1.47 | −1.65 | NC | |||
| cd630_23890 | 23890 | BlaR-like | −1.94 | −2.26 | 0.80 | Membrane | |
| 23880 | Putative PG hydrolase | −1.50 | −1.77 | NC | SPI | ||
| 36040 | Hypothetical protein | −0.86 | −0.94 | 0.71 | |||
| cd630_36030 | 36030 | Phosphoesterase | −1.97 | −2.01 | 1.09 | Membrane | |
| cd630_14040 | 14040 | Oligopeptide transporter | −2.02 | −2.03 | NC | Membrane | |
| 14041 | Hypothetical protein | −2.02 | −1.88 | NC | |||
| 25120 | PTS, subunit IIA | −1.11 | −1.47 | NC | |||
| 25110 | PTS, antiterminator | −1.45 | −1.46 | NC | |||
| cd630_25100 | 25100 | PTS, subunit IIBC | −2.04 | −2.19 | 0.97 | Membrane | |
| cd630_25320 | 25320 | Alanine-glyoxylate transaminase | −2.05 | −2.34 | 1.41 | ||
| cd630_25310 | 25310 | Hypothetical protein | −2.67 | −2.75 | 1.35 | Membrane | |
| cd630_27680 | 27680 | Cell wall hydrolase | −2.06 | −2.29 | NC | SPI | |
| cd630_29660 | 29660 | adhE1 | Acetaldehyde-CoA/alcohol dehydrogenase | −2.08 | −2.37 | −1.09 | Membrane |
| cd630_18120 | 18120 | Response regulator | −2.08 | −2.28 | NC | ||
| cd630_22600 | 22600 | Major facilitator superfamily transporter | −2.13 | −2.32 | NC | Membrane | |
| cd630_20160 | 20160 | Hypothetical protein | −2.19 | −2.29 | 4.18 | ||
| cd630_25090 | 25090 | Glycoside hydrolase | −2.22 | −2.33 | 1.16 | ||
| cd630_21070 | 21070 | Permease family protein | −2.23 | −2.32 | NC | Membrane | |
| cd630_10220 | 10220 | YczE-like membrane protein | −2.26 | −2.73 | 0.93 | Membrane | |
| 10230 | Transcriptional regulator | −1.70 | −1.94 | 1.63 | |||
| cd630_10240 | 10240 | potA | ABC transport ATP-binding protein | −2.27 | −2.36 | 1.77 | |
| cd630_10250 | 10250 | potB | ABC transport permease | −2.47 | −2.82 | 1.72 | Membrane |
| cd630_10260 | 10260 | potC | ABC transport permease | −2.53 | −2.39 | 1.60 | Membrane |
| cd630_10270 | 10270 | potD | ABC transport substrate binding protein | −2.29 | −2.37 | 1.55 | SPII |
| cd630_26640 | 26640 | murE | UDP-N-acetylmuramoylalanyl-d-glutamate-2,6-diaminopimelate ligase | −2.85 | −3.07 | 0.93 | |
| cd630_26670 | 26670 | ptsG-BC | PTS, subunit IIBC | −3.17 | −3.52 | NC | SPI |
| cd630_26660 | 26660 | ptsG-A | PTS, subunit IIA | −3.12 | −3.51 | NC | |
| cd630_21510 | 21510 | Hypothetical protein | −3.56 | −3.82 | 0.68 | Membrane | |
Lists other operon members in order.
TC, two component; PTS, phosphoenolpyruvate-dependent sugar phosphotransferase system; CoA, coenzyme A.
CRISPRi against wal: fold change comparing sgRNA-neg to sgRNA-walA1 strain in the absence of xylose.
Reports predictions by SignalP (SPI, predicted to be a substrate of signal peptidase I; SPII, predicted to be a substrate of signal peptidase II) and by BUSCA (membrane). The BUSCA prediction is given only for those genes that were not already predicted to be SPI or SPII substrates by SignalP.
NC, no change, i.e., the fold change was close to 1 and the P value was >0.05.
Several of the genes and trends in Table 2 are worth highlighting. Xylose induction resulted in a 14-fold increase in walR mRNA (13-fold for walRD54E), while the levels of walK and other members of the native operon were not affected. This means that induction of Pxyl::walR integrated at pyrE does not lead to induction of the native wal operon, consistent with reports that the wal operon is not autoregulated in other bacteria (8, 16). Another striking feature of Table 2 is the abundance of genes encoding cell envelope proteins. Of the 95 genes in Table 2, 22 (23%) are predicted to encode exported proteins and 29 (31%) are predicted to encode cytoplasmic membrane proteins. For comparison, ~7% of the entire proteome is predicted to be exported and 23% predicted to be membrane proteins (see Materials and Methods). Table 2 also includes 21 hypothetical proteins (22% of the total), many of which are likely to be involved in cell envelope processes based on the presence of predicted transmembrane domains or export signals.
Peptidoglycan-associated genes, especially genes encoding PG hydrolases, are prominent members of the Wal regulon in all Firmicutes examined to date (8, 10), and C. difficile is no exception. Table 2 lists 10 peptidoglycan-associated genes, including the second most highly induced gene, pgdA, which was upregulated 16-fold. PgdA deacetylates N-acetylglucosamine in peptidoglycan and is important for C. difficile’s high lysozyme resistance (36, 37). AsnB (~8-fold induced) is annotated as an asparagine synthetase, but its primary function is to amidate diaminopimelic acid (m-DAP) in PG stem peptides (38). In B. subtilis, amidation of m-DAP inhibits PG hydrolase activity (39). MurE (7-fold repressed) catalyzes one of the cytoplasmic steps in PG synthesis, addition of diaminopimelic acid to the nucleotide-linked PG precursor (40). Downregulation of murE suggests that PG synthesis may be decreased under Wal-ON conditions. Cwp16 and Cwp17 are S-layer proteins with predicted amidase domains (41) and were upregulated about 4-fold. Cell wall amidases cleave the amide bond that links stem peptides to N-acetylglucosamine in PG glycan strands (42). CD630_36010 (4-fold induced) is a predicted d-alanyl–d-alanine carboxypeptidase that removes the terminal d-Ala moiety from peptidoglycan pentapeptide side chains. This might limit 4-3 cross-linking of stem peptides but could favor 3-3 cross-linking. Two proteins of unknown function were upregulated >10-fold, CD630_07390 and CD630_07380. We suspect that both of these proteins are involved in PG metabolism because they are predicted to be exported and also induced by lysozyme (43). Notable among the repressed genes in Wal-ON cells is cd630_27680, which is downregulated about 4-fold and is predicted to encode a cell wall hydrolase from the NlpC/P60 family, a domain common to endopeptidases (42). Interestingly, this gene is preceded by two putative WalR binding sites (Table S4), and it has also been characterized as a sortase substrate (44). Another predicted PG hydrolase, CD630_23880, is mildly repressed (~3-fold). Interestingly, the gene encoding this hydrolase is cotranscribed with an uncharacterized BlaI-BlaR regulatory system, cd630_23890 and cd630_23900. Some BlaIR regulatory systems respond to beta-lactam stress (45, 46).
One of the questions that motivated this study was whether the WalR system regulates genes for S-layer proteins in C. difficile. Indeed, the Wal-ON regulon includes six S-layer protein genes, all of which are upregulated by WalR: cwp2, -5, -12, -16, -17, and -66. Two of these genes were mentioned already, the cell wall amidase genes cwp16 and cwp17. The remaining four are of unknown function, although cwp2 and cwp12 have domains implicated in adhesion or pathogenesis and studies with cwp66 have linked it to adhesion, autolysis, and resistance to antibiotics (41, 47). WalRK has been suggested to regulate an S-layer gene in Bacillus anthracis (48).
Expression profiling under Wal-OFF conditions.
As a complementary approach to identify genes in the WalR regulon, we assessed the effect of CRISPRi knockdown of the wal operon on global gene expression. To this end, we performed RNA-seq on a 630Δerm derivative that has the CRISPRi machinery (Pxyl::dCas9 and Pgdh::sgRNA-walA1) integrated into the chromosome at pyrE. The control strain was identical except that it expressed an innocuous sgRNA that does not target anywhere in the C. difficile genome (Pgdh::sgRNA-neg). Two induction conditions were used. One of these, called “no xylose,” relied on leaky expression of dCas9. For this, overnight cultures were diluted into fresh TY to a starting OD600 of 0.03 and harvested when they reached an OD600 of ~0.7 (Fig. S4C). For the other condition, called “low xylose,” cultures in exponential growth were induced with 0.1% xylose at an OD600 of 0.1 and incubated for 2.5 h (~2 mass doublings) before harvest. This induction regimen reduced cell density only modestly (Fig. S4D).
There are 49 genes, organized in 27 operons, that shift 4-fold or more under Wal-OFF conditions (Table 3). The 27 operons contain an additional 14 genes that did not meet our 4-fold cutoff, all but 3 of which nevertheless trended in the right direction. The three exceptions include a pseudogene (cd630_05260) that was not detected by our RNA-seq analysis tool and two membrane protein genes (cd630_05290 and 05280) whose expression was unchanged. Thus, for the purposes of this analysis, CRISPRi knockdown of the wal operon changed the expression of 60 genes, of which 44 were induced and 16 were repressed. Curiously, for most of these genes the fold change was larger in the absence of xylose, suggesting that many of the effects on gene expression might be indirect.
TABLE 3.
Genes affected by Wal-OFF conditions
| Gene, changed ≥4-fold | Operona | Gene name | Annotation | Log2 fold change |
Cell wall associationc | ||
|---|---|---|---|---|---|---|---|
| CRISPRi |
WalR OEb | ||||||
| No Xyl | Low Xyl | ||||||
| cd630_05140 | 05140 | cwpV | Hemagglutinin/adhesin | 4.69 | 2.74 | NCd | SPI |
| cd630_20160 | 20160 | Hypothetical protein | 4.18 | 1.65 | −2.19 | ||
| cd630_11700 | 11700 | Hypothetical protein | 3.52 | 1.83 | NC | ||
| cd630_11710 | 11710 | etfB4 | Electron transfer flavoprotein alpha | 3.30 | 1.40 | NC | |
| cd630_11720 | 11720 | etfA4 | Electron transfer flavoprotein alpha | 2.58 | 1.24 | NC | |
| cd630_11730 | 11730 | FAD-linked oxidase | 2.17 | 1.08 | NC | ||
| cd630_08320 | 08320 | aksA | trans-Homoaconitate synthase | 3.11 | 2.02 | NC | |
| cd630_08330 | 08330 | acnB | Aconitate hydratase | 3.06 | 2.05 | NC | |
| cd630_08340 | 08340 | icd | Isocitrate dehydrogenase | 3.07 | 2.16 | NC | |
| cd630_17290 | 17290 | Sodium:phosphate symporter | 2.76 | 0.45 | −1.46 | Membrane | |
| cd630_13840 | 13840 | LamB/YcsF family protein | 2.17 | 0.88 | NC | ||
| cd630_13850 | 13850 | Hypothetical protein | 2.36 | 1.15 | NC | Membrane | |
| cd630_13860 | 13860 | Allophanate hydrolase subunit 1 | 2.52 | 1.28 | NC | ||
| cd630_13870 | 13870 | Allophanate hydrolase subunit 2 | 2.60 | 1.14 | NC | ||
| cd630_24260 | 24260 | buk1 | Butyrate kinase | 2.45 | NC | −1.41 | |
| 24250 | ptb2 | Phosphate butyryltransferase | 1.65 | NC | |||
| cd630_24240 | 24240 | Pyridoxal phosphate-dependent transferase | 2.55 | NC | −1.15 | ||
| 24230 | Putative transporter | 1.17 | NC | Membrane | |||
| cd630_07650 | 07650 | srlEa | PTS, IIB N-terminal component | 2.17 | NC | NC | |
| cd630_07660 | 07660 | srlEb | PTS, IIB C-terminal component | 2.29 | NC | NC | Membrane |
| cd630_07670 | 07670 | srlB | PTS, IIA subunit | 2.27 | NC | NC | Membrane |
| cd630_07680 | 07680 | srlD | Sorbitol-6-phosphate dehydrogenase | 2.35 | NC | NC | |
| 00350 | Transcriptional regulator | 1.46 | 0.55 | −0.86 | |||
| cd630_00360 | 00360 | acoA | Acetoin dehydrogenase E1 alpha | 2.20 | 0.94 | NC | |
| cd630_00370 | 00370 | acoB | Acetoin dehydrogenase E1 beta | 2.29 | 0.91 | NC | |
| cd630_00380 | 00380 | acoC | Acetoin dehydrogenase E2 | 2.20 | 0.72 | NC | |
| cd630_00390 | 00390 | acoL | Acetoin dehydrogenase E3 | 2.04 | 0.83 | −0.57 | |
| cd630_30010 | 30010 | Hydrolase/isomerase/hydratase | 2.25 | NC | NC | ||
| cd630_23920 | 23920 | tRNA-binding protein | 2.15 | 1.65 | −1.77 | ||
| cd630_30960 | 30960 | bglA3 | 6-Phospho-beta-glucosidase | 2.13 | NC | NC | |
| cd630_30950 | 30950 | bglA2 | 6-Phospho-beta-glucosidase | 2.07 | NC | NC | |
| cd630_25150 | 25150 | Aspartate aminotransferase | 2.12 | 2.38 | −1.76 | ||
| 24291 | Flavo/ferredoxin oxidoreductase delta | 1.19 | NC | NC | |||
| cd630_24290 | 24290 | Flavo/ferredoxin oxidoreductase alpha | 2.11 | NC | −0.91 | ||
| 24280 | Flavo/ferredoxin oxidoreductase beta | 1.66 | NC | NC | |||
| 24270 | Flavo/ferredoxin oxidoreductase gamma | 1.50 | NC | NC | |||
| cd630_30040 | 30040 | kdgT2 | 2-Keto-3-deoxygluconate permease | 2.08 | 0.33 | 0.93 | Membrane |
| cd630_30020 | 30020 | uxaA | d-Galactate dehydratase/altronate hydrolase | 2.06 | 0.25 | NC | |
| cd630_32630 | 32630 | pstC | Phosphate ABC transporter, PstC | 2.03 | NC | NC | Membrane |
| 32620 | pstA | Phosphate ABC transporter, PstA | 1.03 | NC | NC | Membrane | |
| 32610 | pstB | Phosphate ABC transporter, ATP binding | 1.80 | NC | NC | ||
| 32600 | phoU | Phosphate uptake regulator | 1.25 | NC | NC | ||
| 30980 | bglG1 | PTS, antiterminator | 0.95 | NC | NC | ||
| cd630_30970 | 30970 | bglF2 | PTS, subunit IIABC | 2.01 | NC | −0.76 | Membrane |
| cd630_28540 | 28540 | dltD | d-Alanine transferase | −1.52 | −2.41 | 1.03 | Membrane |
| cd630_28530 | 28530 | dltA | d-Ala–d-ala carrier protein ligase 1 | −1.34 | −2.27 | 0.84 | |
| cd630_28520 | 28520 | dltB | d-Alanyl transferase | −1.34 | −2.23 | 0.49 | Membrane |
| 28510 | dltC | d-Ala–d-ala carrier protein ligase 2 | −1.27 | −1.89 | NC | ||
| cd630_09000 | 09000 | opuCA | ABC transporter ATP-binding protein | −1.69 | −2.36 | 1.46 | Membrane |
| cd630_09010 | 09010 | opuCC | ABC transporter permease | −1.68 | −2.40 | 1.27 | |
| cd630_05190 | 05190 | Hypothetical protein | −2.00 | −2.59 | 0.57 | Membrane | |
| 05260 | Aminobenzoyl-glutamate transporter | NDe | ND | ND | |||
| cd630_05250 | 05250 | Peptidase | −2.15 | −1.08 | NC | ||
|
cd630_17671
cd630_17680 |
17671 17680 |
Hypothetical protein Membrane protein |
−2.18 −2.23 |
−1.99 −2.18 |
NC 0.56 |
Membrane | |
| cd630_17810 | 17810 | walA | Lipoprotein | −2.76 | −5.14 | NC | SPII |
| cd630_17820 | 17820 | walR | TC response regulator | −2.51 | −3.91 | 3.81 | |
| cd630_17830 | 17830 | walK | TC sensor histidine kinase | −2.33 | −4.03 | NC | Membrane |
| cd630_17840 | 17840 | truA2 | tRNA pseudouridine synthase A | −2.34 | −3.92 | NC | |
| cd630_05300 | 05300 | Membrane protein | −2.47 | NC | NC | Membrane | |
| 05290 | Membrane protein | NC | NC | NC | Membrane | ||
| 05280 | Amidohydrolase | NC | NC | 0.92 | |||
| cd630_28620 | 28620 | Peptidase | −2.53 | −1.43 | 2.29 | ||
Lists other operon members in order.
Fold change comparing WalR overexpression to negative-control strain.
Predictions by SignalP (SPI, predicted to be a substrate of signal peptidase I; SPII, predicted to be a substrate of signal peptidase II) and by BUSCA (membrane). The BUSCA prediction is given only for those genes that were not already predicted to be SPI or SPII substrates by SignalP.
NC, no change, i.e., the fold change was close to 1 and the P value was >0.05.
ND, CD630_05260 is a pseudogene that was not detected by the RNA-seq analysis tool.
The CRISPRi guide targets the first gene in the operon, walA, which was repressed 6.8-fold in the absence of xylose and 35-fold with low xylose. As expected, polarity resulted in repression of the downstream genes as well, ~5-fold with leaky CRISPRi, increasing to ~15-fold with low xylose. The observed polarity confirms the predicted operon structure, including that a seemingly unrelated (and, according to Tn-seq, nonessential) gene for a tRNA modification enzyme is indeed part of the operon.
Unexpectedly, the Wal-OFF gene set comprises mostly transporters and genes for various metabolic functions. Not counting the members of the wal operon, only 1 of the genes is predicted to be exported and 15 genes (25%) are expected to be membrane protein genes, a percentage comparable to the genome as a whole (see Materials and Methods). Remarkably, the Wal-OFF regulon does not include a single annotated PG metabolism gene. However, we observed ~5-fold repression of the dltDABC operon, which is responsible for the addition of d-alanine to teichoic acids (49). We identified a predicted WalR binding site immediately upstream of dltDABC, suggesting direct regulation by WalR. This putative WalR binding site had previously been recognized as one of two direct repeat sequences in the dlt promoter region (49) (Table S4). d-Alanylation of teichoic acids affects PG hydrolase activity (50, 51) and vancomycin resistance (52, 53) in some bacteria. In C. difficile, a dltD insertion mutant is sensitized to vancomycin and some antimicrobial peptides, but the effects are <2-fold (52). Only one gene in Table 3 codes for an S-layer protein—the hemagglutinin/adhesin gene cwpV, which was ~16-fold induced in no xylose. But this gene is subject to phase variation (54), leading us to suspect that it may not be a true member of the WalR regulon.
Comparison of the Wal-ON and the Wal-OFF gene sets reveals little overlap but opposing trends.
There are only two proteins common to the Wal-ON and the Wal-OFF gene sets, cd630_20160 (hypothetical protein) and cd630_28620 (annotated as a membrane peptidase). However, a large fraction of the 79 Wal-ON genes that met the 4-fold cutoff trended in the opposite direction when evaluated under Wal-OFF conditions, even though the magnitude of change is not as large (Fig. 5A and Table 2). A similar observation was made when genes affected 4-fold or more under Wal-OFF conditions were examined under Wal-ON conditions (Fig. 5B and Table 3).
FIG 5.

Comparison of transcript changes between Wal-ON and Wal-OFF conditions. (A) Heat map showing the 79 genes whose transcript abundance changed ≥4-fold when walR was overexpressed (Wal-ON) in comparison to when the wal operon was silenced with CRISPRi (Wal-OFF). (B) Heat map showing the 49 genes whose transcript abundance changed ≥ 4-fold when the wal operon was silenced (Wal-OFF) in comparison to when walR was overexpressed (Wal-ON). Green, induced; red, repressed.
Selected members of the WalR regulon were confirmed by plasmid-based reporter fusions.
To confirm results of the RNA-seq analysis, we constructed rfp transcriptional fusions (55) to the promoter regions for seven genes from the Wal-ON and Wal-OFF gene sets. Four genes were chosen primarily because they were highly induced when WalR was overexpressed: cd630_07390, pgdA, cd630_07380, and cd630_08670 (Table 2). Additional considerations included that pgdA has an obvious connection to PG biogenesis (36, 37), while cd630_08670 has a predicted WalR-binding site (Table S4). Two genes were chosen because they were the most strongly repressed upon CRISPRi knockdown of the wal operon and are preceded by a predicted WalR-binding site: cd630_28620 and cd630_05300 (Table 3 and Table S4). In addition, cd630_28620 is one of only two genes that made the 4-fold cutoff under both Wal-ON and Wal-OFF conditions. The final gene chosen for confirmation with an rfp reporter was dltD, which incorporates d-alanine into teichoic acids (49) and was modestly downregulated by CRISPRi knockdown of the wal operon. dltD stood out because it has a predicted WalR-binding site and is one of the few cell envelope-associated genes that was at least 4-fold repressed under Wal-OFF conditions (Table 3 and Table S4).
Reporter plasmids were constructed by PCR amplifying ~300 bp upstream of the start codon for each selected gene. These fragments contain transcriptional start sites for five of the seven genes (Clost-Base database [56]); start sites for the remaining two genes have not been mapped. The PCR products were cloned into an RFP reporter plasmid. The resulting plasmids were conjugated into the appropriate Wal-ON and Wal-OFF strain pairs: 630Δerm versus 630Δerm Pxyl::walR and 630Δerm with sgRNA-walA1 versus 630Δerm sgRNA-neg. Reporter strains were grown under the same conditions as used for the RNA-seq experiments, except that the Wal-OFF condition was limited to growth in the absence of xylose, i.e., assaying the effect of leaky dCas9 expression on the reporter gene. Harvested cells were fixed and transferred to aerobic conditions to allow RFP to mature. Fluorescence was quantified by flow cytometry.
We found that all RFP reporters responded in the expected direction when walR expression was manipulated, thus confirming the RNA-seq results by an orthogonal method (Fig. 6). However, the fold changes were uniformly about 4-fold greater by RNA-seq than by RFP fluorescence. The reasons for this difference are not known. As an aside, having RNA-seq and RFP fluorescence data for the same genes enabled us to show that these are correlated, i.e., baseline RFP fluorescence was higher for genes that returned more reads in RNA-seq experiments (Fig. S5A). This correlation was expected and further indicates that our RNA-seq data are robust.
FIG 6.
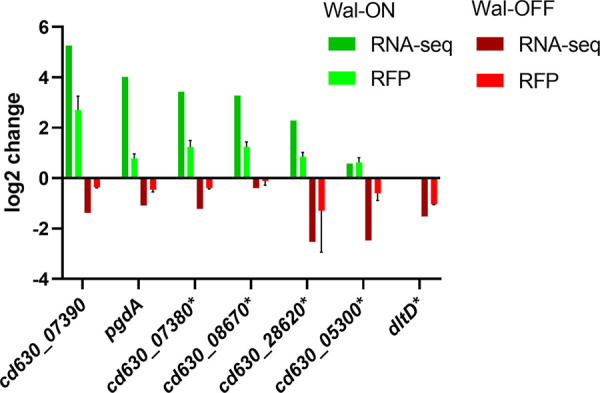
Confirmation of RNA-seq results with RFP reporter fusions to selected genes. Cells harboring plasmids with transcriptional fusions of the red fluorescent protein mCherryOpt to the indicated promoters were grown as per RNA-seq conditions, either Wal-ON (green) or Wal-OFF (red). Red fluorescence was measured by flow cytometry and is graphed as the mean log2 fold change from two independent experiments with two technical replicates. Error bars represent the SD of all four measurements. For comparison, the mean log2 fold change of the same genes as determined by RNA-seq is also shown. The dltD reporter was graphed only under Wal-OFF conditions because this fusion was induced by xylose even in the absence of Pxyl::walR. Genes with asterisks represent fusions that were constructed to the corresponding promoter regions from strain R20291.
Altering the expression level of selected regulon genes does not induce the wal reporter.
In B. subtilis, the WalR regulon can be induced or repressed by manipulating expression of walR-regulated genes involved in PG metabolism (11, 57). We therefore asked whether manipulating expression of wal regulon genes in C. difficile would induce or repress expression of a chromosomal Pcd630_07390::rfp reporter. The reporter was chosen because this promoter was highly induced under Wal-ON conditions as determined by RNA-seq (38-fold) or a plasmid-based RFP reporter fusion (7-fold). The chromosomal Pcd630_07390::rfp reporter was validated by confirming ~10-fold-increased red fluorescence upon overexpression of walR from a Pxyl plasmid (Fig. S5B). Next, we introduced a panel of plasmids that allowed us to directly target 13 WalR regulon genes by overexpression and/or CRISPRi. These 13 genes were chosen for various reasons, including large fold changes in expression, relevance to cell wall biogenesis, and the presence of WalR binding sites (Table S3). Of note, the gene set included genes encoding putative cell wall amidase and the predicted PG hydrolase with an NlpC/P60 domain. Unfortunately, neither overexpression nor CRISPRi knockdown of the selected wal regulon genes altered expression of the Pcd630_07390::rfp reporter. Further work will be needed to determine whether these negative results reflect technical difficulties or more fundamental differences in Wal signaling between B. subtilis and C. difficile.
Perturbation of selected Wal regulon genes individually does not lead to any Wal-associated phenotypic defects.
As noted above, one motivation for determining the Wal regulon was to identify genes that contribute to Wal phenotypes. Recall that induction of Pxyl::walR decreases viability, causes cells to become phase bright, and reduces autolysis in buffer containing 0.01% Triton X-100 (Fig. 3). Two of the genes most highly induced under Wal-ON conditions encode the hypothetical proteins CD630_07380 and CD630_07390. However, overproduction of these proteins either individually or from Pxyl plasmids did not result in any obvious phenotypic changes (Fig. 7). Because these genes are transcribed divergently (35), we needed to reverse the direction of one gene to express them together and decided to clone both possible arrangements: cd630_07380-cd630_07390 and cd630_07390-cd630_07380. Likewise, a CRISPRi plasmid with an sgRNA that targets cd630_27680 had no effects on growth, morphology, or lysis (Fig. 7); this gene encodes PG hydrolase with two predicted WalR-binding sites and is among the most strongly repressed genes under Wal-ON conditions.
FIG 7.
Perturbation of individual Wal-ON regulon genes does not replicate the Wal-ON phenotype. Overnight cultures of 630Δerm harboring overexpression plasmids were subcultured into TY-Thi to an OD600 of 0.03, grown to an OD600 of 0.2, and induced with 3% xylose for 3 h. Overnight cultures of 630Δerm harboring CRISPRi plasmids were subcultured into TY-Thi containing 1% xylose to an OD600 of 0.03 and grown to an OD600 of ~0.8. Each culture was then examined by microscopy (bar = 5 μm) (A) and tested in the lysis assay (B). Induction of walR is the only condition that achieved phase-bright cells and slowed lysis. The plasmids used were pBZ101 (EV), pCE691 (Pxyl::walR), pIA112 (Pxyl::cd630_07380), pIA113 (Pxyl::cd630_07390), pIA114 (Pxyl::cd630_07380-07390), pIA115 (Pxyl::cd630_07390-07380), pIA34 (sgRNA-neg), pCE744 (sgRNA-cd630_27680-1), and pCE745 (sgRNA-cd630_27680-2).
CRISPRi silencing of the wal operon is lethal, and the terminal phenotypes include lysis and loss of rod shape as reflected by an abundance of curved cells (Fig. 2). However, none of these phenotypes were observed when we used a Pxyl::cwpV plasmid to overexpress the most highly induced gene from the Wal-OFF RNA-seq data set, which we tested even though cwpV is subject to phase variation and might not be part of the WalR regulon. We also observed no effect when we used CRISPRi to knock down expression of dltD, which was among the more strongly repressed Wal-OFF genes and is preceded by a putative WalR-binding site (Fig. 8). While this study was not an exhaustive evaluation of all regulon members, our findings suggest that the phenotypic defects observed upon perturbation of the Wal system are not due to changes in expression of any single gene but are cumulative in nature, much like what has been observed in B. subtilis and S. aureus (8–10, 12).
FIG 8.

Perturbation of individual Wal-OFF regulon genes does not replicate the Wal-OFF phenotype. (A) Phase-contrast microscopy of strains harboring overexpression plasmids or CRISPRi plasmids after induction with xylose as described in the legend to Fig. 7. Bar = 5 μm. (B) Viability assay. Overnight cultures were serially diluted and spotted onto TY plates with or without 1% xylose. Plates were photographed after incubation overnight. CRISPRi knockdown of the wal operon was the only condition that resulted in curved cells, lysis, or a viability defect. The plasmids used were pBZ101 (EV), pCE791 (Pxyl::cwpV), pIA34 (sgRNA-neg), pIA50 (sgRNA-walA1), and pCE738 (sgRNA-dltD).
DISCUSSION
In C. difficile the WalRK TCS is predicted to reside in a four-gene operon: walA-walR-walK-truA2. We altered expression of the WalRK regulon by complementary approaches: depleting C. difficile of WalRK by CRISPRi knockdown of the entire operon and overproduction of WalR under Pxyl control. RNA-seq revealed that the wal operon is not autoregulated in C. difficile, as evidenced by a lack of induction when WalR (or WalRD54E) was ectopically expressed from a Pxyl promoter. Wal operons are not autoregulated in other Firmicutes either. RNA-seq also confirmed that the genes constitute an operon, because CRISPRi targeting walA resulted in roughly equivalent knockdown of the three downstream genes. Further studies will be needed to establish the specific role of each gene in WalRK signaling. We suspect that walA is a bona fide part of the Wal system because all wal operons studied to date include accessory genes for membrane proteins that modulate WalK signaling (8, 10). But a rationale for why truA2, which encodes a nonessential tRNA modification enzyme, should have come to reside in the wal operon is not obvious. This might be the result of evolutionary happenstance.
As expected, extensive knockdown of the wal operon with CRISPRi and strong overproduction of WalR from a Pxyl plasmid were both lethal. Phenotypic defects depended on how walRK expression was manipulated, but they included elongation, loss of rod shape (curved or wavy cells), phase-bright cells, enhanced or reduced autolysis, and altered sensitivity to antibiotics that target PG synthesis. Similar phenotypes have been observed upon manipulating Wal signaling in other organisms (2, 6, 7, 9, 10, 12, 17, 18). But there are some intriguing differences. In B. subtilis, in which phosphorylated WalR promotes elongation, artificial upregulation of the Wal system causes cells to become elongated, while artificial downregulation causes cells to become short (10, 18). In C. difficile, however, overexpression of WalR from Pxyl had no obvious effect on cell length, while CRISPRi knockdown of the wal operon led to elongation.
Vancomycin resistance presents another example of an inverse phenotype. In S. aureus, downregulation of walRK expression increases resistance to vancomycin while upregulation of walRK decreases vancomycin resistance (58, 59). Vancomycin-intermediate S. aureus (VISA) strains, both laboratory derived (60) and isolated in the clinic (61), often have mutations in walR or walK that downregulate the system (62). In contrast, we found that partial CRISPRi knockdown of the wal operon in C. difficile had the opposite effect, namely, a modest increase in sensitivity to vancomycin. Vancomycin is a front-line treatment for C. difficile infections, so our findings suggest that a small molecule that interferes with Wal signaling might enhance the efficacy of vancomycin therapy.
RNA-seq identified over 150 genes whose expression responds to manipulation of Wal signaling in C. difficile. Although this gene set is diverse, there are themes: many cell envelope proteins, many hypothetical proteins, and about 10 proteins with various connections to PG metabolism. There are also 7 genes for S-layer proteins, consistent with the need to coordinate S-layer assembly and remodeling with growth and turnover of the PG sacculus. The PG hydrolase genes are of particular interest because they are prominent members of the WalR regulon in other Firmicutes (5, 12–16) and ectopic expression of one or more of the corresponding enzymes can render walRK no longer essential regulators (6, 17, 18). In C. difficile, Wal-ON conditions induced two putative cell wall amidases (cwp16 and cwp17) and repressed one potential d,l-endopeptidase (cd630_27680). Direct control of cd630_27680 by WalR is suggested by the presence of two matches to the WalR-binding site consensus sequence about 100 nucleotides upstream of the start codon (Table S4). The importance of these genes for any Wal-related phenotype is unclear, however, because overexpression and CRISPRi knockdown experiments did not result in any phenotypic changes or alter expression of an RFP reporter that is induced under Wal-ON conditions. In addition to the putative hydrolase genes, several genes in the WalR regulon may play a role in controlling PG hydrolase activity. Deacetylation of PG N-acetylglucosamine (GlcNAc) (63), m-DAP amidation (39), and d-alanylation of teichoic acids (32, 50, 51, 64) have all been shown to affect hydrolase activity in other organisms.
S. pneumoniae is so far the only organism in which a single essential gene, the PG hydrolase gene pcsB, has been identified to be responsible for the essentiality of the wal system (6, 7). In B. subtilis, essentiality of the Wal system arises from the cumulative impact of aberrant expression of multiple genes involved in turnover of PG (5, 10, 11). Wal essentiality is also polygenic in nature in S. aureus (8). This seems to be the case in C. difficile as well. Although CRISPRi silencing of the wal operon leads to cell lysis, the Wal-OFF gene set (Table 3) does not include any genes determined to be essential by transposon mutagenesis (27). In contrast, the Wal-ON regulon (Table 2) includes 3 essential genes. Two of these are induced by WalR, genes for a DNA ligase (ligA, cd630_33090) and a putative calcium-chelating protein (cd630_26810), and one is repressed, the gene for a PG precursor synthesis protein MurE (cd630_26640). It is possible that repression of murE contributes to death during overexpression of WalR but not during CRISPRi knockdown of the wal operon, because murE expression did not change under that condition. Overall, our findings suggest that essentiality of WalRK is driven by a deleterious imbalance of multiple processes. (As an aside, it is worth noting that repression of murE under Wal-ON conditions is not what one would expect if WalR promotes increased PG synthesis, as in B. subtilis [11, 57].)
Here, we have provided an initial delineation of Wal phenotypes and the WalR regulon in C. difficile. Our findings raise a number of exciting questions. Is the protein WalA involved in WalRK signaling, and if so, how? What signals are sensed by WalK? Of note, owing to differences in cross-linking (23), the PG hydrolysis products implicated in regulating WalK activity in B. subtilis (11) are not very abundant in C. difficile. Moreover, C. difficile WalK is missing an intracellular PAS domain found in most other WalK proteins, suggesting that C. difficile WalK senses different signals and/or transmits those signals differently. Another open question is which WalR regulon genes are controlled directly by WalR. It will also be important to determine the functions of the WalR-regulated genes, especially those encoding the many hypothetical proteins. Answers to these questions are likely to provide novel insights into cell wall biogenesis and might point the way toward improved therapies against this important pathogen.
MATERIALS AND METHODS
Strains, media, and growth conditions.
Bacterial strains are listed in Table 4. C. difficile strains used in this study were derived from either 630Δerm or R20291, both of which have been sequenced. C. difficile was routinely grown in tryptone-yeast extract (TY) medium, supplemented as needed with thiamphenicol at 10 μg/mL (TY-Thi). TY medium consisted of 3% tryptone, 2% yeast extract, and 2% agar (for plates). Brain heart infusion (BHI) medium was prepared per the manufacturer’s (Difco) instructions. C. difficile strains were maintained at 37°C in an anaerobic chamber (Coy Laboratory Products) in an atmosphere of 10% H2, 5% CO2, and 85% N2. Escherichia coli strains were grown in LB medium at 37°C with chloramphenicol at 10 μg/mL (Cam10) or ampicillin at 100 μg/mL (Amp100) as needed. LB medium contained 1% tryptone, 0.5% yeast extract, 0.5% NaCl, and 1.5% agar (for plates). OD600 measurements were made with the WPA Biowave CO8000 tube reader in the anaerobic chamber.
TABLE 4.
Strains used in this study
| Strain | Genotype and/or description | Source or reference(s) |
|---|---|---|
| E. coli | ||
| OmniMAX-2 T1R | F′ [proAB+ lacIq lacZΔM15 Tn10 (Tetr) Δ(ccdAB)] mcrA Δ(mrr-hsdRMS-mcrBC) φ80(lacZ)ΔM15 Δ(lacZYA-argF) U169 endA1 recA1 supE44 thi-1 gyrA96 relA1 tonA panD | Invitrogen |
| HB101/pRK24 | F− mcrB mrr hsdS20(rB− mB−) recA13 leuB6 ara-14 proA2 lacY1 galK2 xyl-5 mtl-1 rpsL20 | 66, 67 |
| C. difficile | ||
| R20291 | Wild-type C. difficile strain from UK outbreak (ribotype 027) | |
| 630Δerm | Spontaneous erythromycin-sensitive derivative of strain 630 (ribotype 012) | 76 |
| CRG1496 | 630Δerm ΔpyrE | 29 |
| UM275 | 630Δerm with Pveg::rfp downstream of pyrE | 26 |
| UM554 | 630Δerm with Pxyl::dCas9 Pgdh::sgRNA-neg downstream of pyrE | This study |
| UM555 | 630Δerm with Pxyl::dCas9 Pgdh::sgRNA-walA-1 downstream of pyrE | This study |
| UM556 | 630Δerm with Pxyl::dCas9 Pgdh::sgRNA-walA-2 downstream of pyrE | This study |
| UM626 | 630Δerm with Pxyl::walR downstream of pyrE | This study |
| UM628 | 630Δerm with Pxyl::walRD54E downstream of pyrE | This study |
| UM926 | 630Δerm with Pcd630_0739::mCherryOpt downstream of pyrE | This study |
Plasmid and strain construction.
All plasmids are listed in Table 5; an expanded version of this table which includes additional information relevant to plasmid assembly is provided in Table S2A in the supplemental material. Plasmids were constructed by isothermal assembly (65) using reagents from New England Biolabs (Ipswich, MA). Regions of plasmids constructed using PCR were verified by DNA sequencing. The oligonucleotide primers used in this work were synthesized by Integrated DNA Technologies (Coralville, IA) and are listed in Table S2B. All plasmids were propagated using OmniMax 2-T1R as the cloning host, transformed into HB101/pRK24 (66, 67), and then introduced into C. difficile strains by conjugation. Chromosomal fusions at the pyrE locus in 630Δerm were constructed by allelic exchange (29) using C. difficile CRG1496 (630Δerm ΔpyrE) as a pyrE-deficient recipient. The allelic exchange restored a functional pyrE gene.
TABLE 5.
Plasmids used in this study
| Plasmid | Relevant features | Reference or comment |
|---|---|---|
| pAP114 | Pxyl::mCherryOpt catP | 26 |
| pBZ101 | Pxyl empty vector | This study |
| pCE636 | PdltD::mCherryOpt catP | This study |
| pCE738 | Pxyl::dCas9-opt Pgdh::sgRNA-dltD-1 catP | 37 |
| pCE691 | Pxyl::walR catP | This study |
| pCE738 | Pxyl::dCas9-opt Pgdh::sgRNA-dltD-1 catP | 37 |
| pCE741 | Pxyl::cdr2656 | This study |
| pCE744 | Pxyl::dCas9-opt Pgdh::sgRNA-cd630_27680-1 catP | This study |
| pCE745 | Pxyl::dCas9-opt Pgdh::sgRNA-cd630_27680-2 catP | This study |
| pCE789 | Pxyl::dCas9-opt Pgdh::cwpV-1 catP | This study |
| pCE791 | Pxyl::cwpV | This study |
| pDSW1728 | Ptet::mCherryOpt catP | 55 |
| pDSW2037 | Pxyl in integration vector pMTL-YN1C catP | This study |
| pDSW2053 | Pxyl::dCas9opt-Pgdh::sgRNA-neg in pMTL-YN1C catP | This study |
| pDSW2055 | Pxyl::dCas9opt-Pgdh::sgRNA-cd630_17810-1 in pMTL-YN1C catP | This study |
| pDSW2057 | Pxyl::dCas9opt-Pgdh::sgRNA-cd630_17810-2 in pMTL-YN1C catP | This study |
| pIA33 | Pxyl::dCas9-opt Pgdh::sgRNA-rfp catP | 26 |
| pIA34 | Pxyl::dCas9-opt Pgdh::sgRNA-neg catP | 26 |
| pIA50 | Pxyl::dCas9-opt Pgdh::sgRNA-walA-1 catP | This study |
| pIA51 | Pxyl::dCas9-opt Pgdh::sgRNA-walA-2 catP | This study |
| pIA75 | Pxyl::walR in pMTL-YN1C catP | This study |
| pIA76 | Pxyl::walRD54E in pMTL-YN1C catP | This study |
| pIA79 | Pxyl::dCas9-opt Pgdh::cd630_25040-1 catP | This study |
| pIA80 | Pxyl::dCas9-opt Pgdh::cd630_25040-2 catP | This study |
| pIA81 | Pxyl::dCas9-opt Pgdh::cd630_36010-1 catP | This study |
| pIA93 | Pcdr_0665::mCherryOpt catP | This study; cdr_0665 is ortholog of cd630_07380 |
| pIA95 | Pcdr_0796::mCherryOpt catP | This study; cdr_0796 is ortholog of cd630_08670 |
| pIA97 | Pcdr_2753::mCherryOpt catP | This study; cdr_2753 is ortholog of cd630_28620 |
| pIA98 | Pcdr_0455::mCherryOpt catP | This study; cdr_0455 is ortholog of cd630_53000 |
| pIA100 | Pcd630_0739::mCherryOpt catP | This study |
| pIA101 | Pcd630_0739::mCherryOpt in pMTL-YN1C catP | This stduy |
| pIA102 | Pxyl::dCas9-opt Pgdh::cd630_05490 catP | This study |
| pIA103 | Pxyl::dCas9-opt Pgdh::cd630_27940 catP | This study |
| pIA104 | Pxyl::dCas9-opt Pgdh::cd630_27860 catP | This study |
| pIA105 | Pxyl::dCas9-opt Pgdh::cd630_27910 catP | This study |
| pIA106 | Pxyl::dCas9-opt Pgdh::cd630_10360 catP | This study |
| pIA107 | PpgdA::mCherryOpt catP | This study |
| pIA108 | Pxyl::dCas9-opt Pgdh::cd630_07380-1 catP | This study |
| pIA109 | Pxyl::dCas9-opt Pgdh::cd630_07380-2 catP | This study |
| pIA110 | Pxyl::dCas9-opt Pgdh::cd630_07390-1 catP | This study |
| pIA111 | Pxyl::dCas9-opt Pgdh::cd630_07390-2 catP | This study |
| pIA112 | Pxyl::cd630_0738 | This study |
| pIA113 | Pxyl::cd630_0739 | This study |
| pIA114 | Pxyl::cd630_0738-cd630_0739 | This study |
| pIA115 | Pxyl::cd630_0739-cd630_0738 | This study |
| pMTL-YN1C | E. coli-C. difficile shuttle vector for inserting genes into C. difficile chromosome while restoring pyrE; colE1 RP4oriT-TraJ CB102ori-repH′ catP | 29 |
Conjugation into C. difficile.
Our experiments required conjugating plasmids into C. difficile strains R20291 and 630Δerm. In the case of R20291, we encountered problems with low conjugation efficiencies even with heat shock as described previously (68). After testing several modifications of the procedure, we found that performing conjugations on filters reliably increased efficiency more than 10-fold (Fig. S6). We now routinely use filters for all our conjugations. Briefly, the E. coli donor strain (an HB101/pRK24 derivative harboring the cargo plasmid) was grown overnight in LB Amp100 Cam10. The donor strain was collected gently by centrifugation of a 0.5-mL aliquot at 5,000 × g for 1 min and then washed with 1 mL of TY and pelleted again. The washed cell pellet was moved into the anaerobic chamber. R20291 was prepared for conjugation by a heat shock step (68). For this, a 200-μL aliquot of the overnight culture of the R20291 recipient was transferred to a 1.5-mL microcentrifuge tube and incubated at 48°C for 5 min in a Fisherbrand dry bath (with water-filled wells). Then 100 μL of heat-shocked R20291 was used to take up the pellet of E. coli donor cells. The strain mixture was then pipetted onto a 25-mm-diameter, 0.45-μm-pore-size Millipore mixed-cellulose filter (HAWP02500) placed on BHI plates. After incubation for 24 h at 37°C, cells were flushed from the membrane with 500 μL of TY and then 200 to 500 μL of the resulting bacterial slurry was plated onto TY amended with thiamphenicol (10 μg/mL), kanamycin (50 μg/mL), and cefoxitin (8 μg/mL) to select for exconjugants. Conjugations into 630Δerm were done identically except that the heat shock step was omitted.
Viability assay.
The effect of CRISPRi silencing on plating efficiency was evaluated by making a 10-fold serial dilution of a culture grown overnight in TY-Thi and spotting 5 μL of each dilution onto TY-Thi agar with and without 1% xylose. Plates were photographed after overnight incubation (~18 h).
Microscopy.
Cells were immobilized using thin agarose pads (1%). Phase-contrast and fluorescent micrographs were recorded on an Olympus BX60 microscope equipped with a 100× UPlanApo objective (numerical aperture, 1.35). Micrographs were captured with a Hamamatsu Orca Flash 4.0 V2+ complementary metal oxide semiconductor (CMOS) camera. The image analysis tool MicrobeJ (69) was used to measure cell length and sinuosity, which is the length of the cell axis divided by the distance between the poles. A perfectly straight rod has a sinuosity value of 1, while a curved or wavy cell has a larger value. We classified cells as curved if their sinuosity was ≥1.03 (see Fig. S2 for examples). Viability of cells was assessed with LIVE/DEAD stain (Molecular Probes; L7012). A 1-mL culture sample was pelleted, washed with phosphate-buffered saline (PBS), and resuspended in 100 μL of PBS with 5 μM Syto 9 and 30 μM propidium iodide. Cells were incubated with the dyes for 15 min, then removed from the anaerobic chamber, and immediately imaged by microscopy. For propidium iodide red fluorescence we used filter set 41004 (Chroma Technology) with a 538- to 582-nm excitation filter, 595-nm dichroic mirror (long pass), and a 582- to 682-nm emission filter. Syto 9 green fluorescence was captured with filter set 41017 (Chroma Technology Corp.) with a 450- to 490-nm excitation filter, a 495-nm dichroic mirror (long pass), and a 500- to 550-nm emission filter.
Fixation protocol.
A 500-μL aliquot of cells in growth medium was added directly to a microcentrifuge tube containing 120 μL of a 5× fixation cocktail: 100 μL of a 16% (wt/vol) paraformaldehyde aqueous solution (Alfa Aesar, Ward Hill, MA) and 20 μL of 1 M NaPO4 buffer (pH 7.4). The sample was mixed, incubated in the anaerobic chamber at 37°C for 30 min and then on ice 30 min, and removed from the chamber. The fixed cells were washed twice with 1 mL of PBS, resuspended in 50 μL of PBS, and left in the dark for 18 h to allow for chromophore maturation.
MIC determination.
Antibiotic sensitivity was determined in 96-well plates. A 2-fold dilution series of selected antibiotics was prepared in 50 μL TY medium. Wells were then inoculated with 50 μL of a diluted culture suspension (106 CFU/mL; calculated OD600, ~0.005). Plates were imaged and evaluated after 17 h at 37°C.
Lysis assay.
Cell cultures (1 mL) were removed from the anaerobic chamber, pelleted, and resuspended in 700 μL of 0.01% Triton X-100 in 50 mM NaPO4 buffer (pH 7.4). Of this 700 μL, three 200-μL replicates were pipetted into wells of a clear, flat-bottom 96-well plate. The turbidity was measured at 600 nm every 15 min for 10 h in a plate reader (Tecan Infinite M200 Pro).
Flow cytometry.
Cells were analyzed at the Flow Cytometry Facility at the University of Iowa using the Becton, Dickinson LSR II instrument with a 561-nm laser, a 610/20-nm-band-pass filter, and a 600 LP dichroic filter. Data were analyzed using BD FACSDiva software.
Culture growth for RNA-seq samples.
To obtain robust, quality data, all experiments were performed on three different days for biological replicates. Cultures were grown in 100 mL of TY as follows. For the Wal-ON condition, to characterize the effect of overexpressing walR, strains were subcultured to an OD600 of ~0.05, grown to an OD600 of 0.2, induced with xylose to 3%, and grown for an additional 3h (Fig. S4A). For the Wal-OFF condition, to characterize the effect of partial knockdown of the wal operon under leaky CRISPRi conditions, strains were subcultured to an OD600 of ~0.03 and grown to an OD600 of ~0.7 before harvesting (Fig. S4C). To test the effect of stronger knockdown of the wal operon, growth conditions were identified that allowed exposing cells to moderate levels of xylose (0.1%) without a significant loss of biomass. Strains were subcultured to an OD600 of ~0.03, grown to an OD600 of 0.3, diluted back to an OD600 of ~0.1, induced with xylose to a final concentration of 0.1%, and harvested after an additional 2.5 h of growth (Fig. S4D). From each condition, 25 mL of culture was fixed by rapidly mixing with 25 mL of ice-cold 1:1 ethanol-acetone that had been brought into the anaerobic chamber on dry ice. Fixed cells were stored at −80°C until further workup.
RNA isolation and RNA-seq.
Fixed culture samples were thawed and then pelleted for 10 min at 8,000 × g and 4°C, and pellets were washed once with 500 μL of 1% β-mercaptoethanol (β-ME) in water. Subsequent steps were an adaptation of the Qiagen RNeasy minikit procedure. The washed pellet was resuspended in 1 mL of the Qiagen-provided RLT buffer amended with 1% β-ME and transferred to a 2-mL screw-cap tube filled with glass beads (0.1-mm diameter) to a height of ~2 to 3 mm. Cells were lysed with the FastPrep-24 homogenizer with two cycles at 6 m/s for 45 s, resting on ice for 3 min between cycles. The homogenized material was pelleted at 14,000 × g for 15 min at 4°C. The supernatant was removed, adjusted to 900 μL with RLT buffer/β-ME, mixed with 500 μL of ethanol, and loaded onto the Qiagen RNeasy spin column. Subsequent steps followed the manufacturer’s protocol. The RNA was eluted with 50 μL of water and DNase treated twice (Ambion Turbo DNA free). Absence of DNA was evaluated by amplifying a 364-bp region of mldA with Taq DNA polymerase for 30 cycles. The RNA integrity number (RIN) was determined with an Agilent Bioanalyzer at the Iowa Institute for Human Genetics, Genomics Division. All samples had a RIN of 9.4 or higher. Typical yields from 25 mL of culture were 8 to 20 μg of RNA at 200 to 500 ng/μL.
RNA samples were submitted to the Microbial Genome Sequencing Center (MiGS) in Pittsburgh, PA. MiGS performed Illumina stranded RNA library preparation paired with RiboZero Plus (per the manufacturer’s specifications) and sequenced on a NextSeq 500 using a 75cyc high-output flow cell. Fastq files were trimmed and filtered using a combination of Trimmomatic (70) and FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Alignment, normalization, and differential expression were analyzed with SeqMan NGen version 17.2 (DNASTAR, Madison, WI). Annotation was imported from NCBI with additional information obtained from progenomes (71). All subsequent analysis was performed in Excel and GraphPad Prism 9.
Bioinformatics.
Gene sequences and operon organization were obtained from the BioCyc data collection (35, 72). SignalP 5.0 (73) was used to search for type I and type II signal peptides. BUSCA (74) was used to predict protein cellular location. When all C. difficile proteins were run through the prediction programs, SignalP predicted ~7% to be exported. BUSCA predicted ~4% to exported and ~25% to be membrane associated. Cell wall association in Tables 2 and 3 was reported as follows: (i) signal peptidase substrate if predicted by SignalP and (ii) for all remaining genes, membrane protein if predicted by BUSCA.
Putative WalR binding sites were identified with Virtual Footprint (75) by querying with (i) the B. subtilis consensus motif, TGTWAH-N5-TGTWAH (14), (ii) the same motif, but allowing one mismatch, or (iii) TGTNDH-N5-BKBWRN (8). Searches were limited to the intergenic region of the genome and generated 29, 522, and 684 hits, respectively.
Data availability.
RNA-seq data were submitted to the NCBI GEO repository and assigned accession number GSE200346.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R01AI087834 (C.D.E.) and R01AI155492 (C.D.E. and D.S.W.) from the National Institute of Allergy and Infectious Diseases. Cell fluorescence was quantitated at the Flow Cytometry Facility, which is a Carver College of Medicine/Holden Comprehensive Cancer Center core research facility at the University of Iowa. The facility is funded through user fees and the generous financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veteran’s Administration Medical Center. RNA integrity was characterized by the Genomics Division of the Iowa Institute of Human Genetics, which is supported, in part, by the University of Iowa Carver College of Medicine.
We thank Nigel Minton for pMTL-YN1C, Brianne Zbylicki for plasmid pBZ101, and members of the Ellermeier and Weiss laboratories for helpful discussions.
Footnotes
Supplemental material is available online only.
Contributor Information
Craig D. Ellermeier, Email: craig-ellermeier@uiowa.edu.
David S. Weiss, Email: david-weiss@uiowa.edu.
Laurie E. Comstock, University of Chicago
REFERENCES
- 1.Rohs PDA, Bernhardt TG. 2021. Growth and division of the peptidoglycan matrix. Annu Rev Microbiol 75:315–336. 10.1146/annurev-micro-020518-120056. [DOI] [PubMed] [Google Scholar]
- 2.Fabret C, Hoch JA. 1998. A two-component signal transduction system essential for growth of Bacillus subtilis: implications for anti-infective therapy. J Bacteriol 180:6375–6383. 10.1128/JB.180.23.6375-6383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin PK, Li T, Sun D, Biek DP, Schmid MB. 1999. Role in cell permeability of an essential two-component system in Staphylococcus aureus. J Bacteriol 181:3666–3673. 10.1128/JB.181.12.3666-3673.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubrac S, Msadek T. 2004. Identification of genes controlled by the essential YycG/YycF two-component system of Staphylococcus aureus. J Bacteriol 186:1175–1181. 10.1128/JB.186.4.1175-1181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubrac S, Boneca IG, Poupel O, Msadek T. 2007. New insights into the WalK/WalR (YycG/YycF) essential signal transduction pathway reveal a major role in controlling cell wall metabolism and biofilm formation in Staphylococcus aureus. J Bacteriol 189:8257–8269. 10.1128/JB.00645-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng WL, Robertson GT, Kazmierczak KM, Zhao J, Gilmour R, Winkler ME. 2003. Constitutive expression of PcsB suppresses the requirement for the essential VicR (YycF) response regulator in Streptococcus pneumoniae R6. Mol Microbiol 50:1647–1663. 10.1046/j.1365-2958.2003.03806.x. [DOI] [PubMed] [Google Scholar]
- 7.Ng WL, Kazmierczak KM, Winkler ME. 2004. Defective cell wall synthesis in Streptococcus pneumoniae R6 depleted for the essential PcsB putative murein hydrolase or the VicR (YycF) response regulator. Mol Microbiol 53:1161–1175. 10.1111/j.1365-2958.2004.04196.x. [DOI] [PubMed] [Google Scholar]
- 8.Dubrac S, Bisicchia P, Devine KM, Msadek T. 2008. A matter of life and death: cell wall homeostasis and the WalKR (YycGF) essential signal transduction pathway. Mol Microbiol 70:1307–1322. 10.1111/j.1365-2958.2008.06483.x. [DOI] [PubMed] [Google Scholar]
- 9.Dubrac S, Msadek T. 2008. Tearing down the wall: peptidoglycan metabolism and the WalK/WalR (YycG/YycF) essential two-component system. Adv Exp Med Biol 631:214–228. 10.1007/978-0-387-78885-2_15. [DOI] [PubMed] [Google Scholar]
- 10.Takada H, Yoshikawa H. 2018. Essentiality and function of WalK/WalR two-component system: the past, present, and future of research. Biosci Biotechnol Biochem 82:741–751. 10.1080/09168451.2018.1444466. [DOI] [PubMed] [Google Scholar]
- 11.Dobihal GS, Brunet YR, Flores-Kim J, Rudner DZ. 2019. Homeostatic control of cell wall hydrolysis by the WalRK two-component signaling pathway in Bacillus subtilis. Elife 8:e52088. 10.7554/eLife.52088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bisicchia P, Noone D, Lioliou E, Howell A, Quigley S, Jensen T, Jarmer H, Devine KM. 2007. The essential YycFG two-component system controls cell wall metabolism in Bacillus subtilis. Mol Microbiol 65:180–200. 10.1111/j.1365-2958.2007.05782.x. [DOI] [PubMed] [Google Scholar]
- 13.Delauné A, Dubrac S, Blanchet C, Poupel O, Mader U, Hiron A, Leduc A, Fitting C, Nicolas P, Cavaillon JM, Adib-Conquy M, Msadek T. 2012. The WalKR system controls major staphylococcal virulence genes and is involved in triggering the host inflammatory response. Infect Immun 80:3438–3453. 10.1128/IAI.00195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howell A, Dubrac S, Andersen KK, Noone D, Fert J, Msadek T, Devine K. 2003. Genes controlled by the essential YycG/YycF two-component system of Bacillus subtilis revealed through a novel hybrid regulator approach. Mol Microbiol 49:1639–1655. 10.1046/j.1365-2958.2003.03661.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Gallay C, Kjos M, Domenech A, Slager J, van Kessel SP, Knoops K, Sorg RA, Zhang JR, Veening JW. 2017. High-throughput CRISPRi phenotyping identifies new essential genes in Streptococcus pneumoniae. Mol Syst Biol 13:931. 10.15252/msb.20167449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng WL, Tsui HC, Winkler ME. 2005. Regulation of the pspA virulence factor and essential pcsB murein biosynthetic genes by the phosphorylated VicR (YycF) response regulator in Streptococcus pneumoniae. J Bacteriol 187:7444–7459. 10.1128/JB.187.21.7444-7459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delaune A, Poupel O, Mallet A, Coic YM, Msadek T, Dubrac S. 2011. Peptidoglycan crosslinking relaxation plays an important role in Staphylococcus aureus WalKR-dependent cell viability. PLoS One 6:e17054. 10.1371/journal.pone.0017054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takada H, Shiwa Y, Takino Y, Osaka N, Ueda S, Watanabe S, Chibazakura T, Su'etsugu M, Utsumi R, Yoshikawa H. 2018. Essentiality of WalRK for growth in Bacillus subtilis and its role during heat stress. Microbiology (Reading) 164:670–684. 10.1099/mic.0.000625. [DOI] [PubMed] [Google Scholar]
- 19.CDC. 2019. Antibiotic resistance threats in the United States, 2019. CDC, Atlanta, GA. [Google Scholar]
- 20.Fagan RP, Albesa-Jove D, Qazi O, Svergun DI, Brown KA, Fairweather NF. 2009. Structural insights into the molecular organization of the S-layer from Clostridium difficile. Mol Microbiol 71:1308–1322. 10.1111/j.1365-2958.2009.06603.x. [DOI] [PubMed] [Google Scholar]
- 21.Fagan RP, Fairweather NF. 2014. Biogenesis and functions of bacterial S-layers. Nat Rev Microbiol 12:211–222. 10.1038/nrmicro3213. [DOI] [PubMed] [Google Scholar]
- 22.Kirk JA, Banerji O, Fagan RP. 2017. Characteristics of the Clostridium difficile cell envelope and its importance in therapeutics. Microb Biotechnol 10:76–90. 10.1111/1751-7915.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peltier J, Courtin P, El Meouche I, Lemee L, Chapot-Chartier MP, Pons JL. 2011. Clostridium difficile has an original peptidoglycan structure with a high level of N-acetylglucosamine deacetylation and mainly 3-3 cross-links. J Biol Chem 286:29053–29062. 10.1074/jbc.M111.259150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen HT, Wolff KA, Cartabuke RH, Ogwang S, Nguyen L. 2010. A lipoprotein modulates activity of the MtrAB two-component system to provide intrinsic multidrug resistance, cytokinetic control and cell wall homeostasis in Mycobacterium. Mol Microbiol 76:348–364. 10.1111/j.1365-2958.2010.07110.x. [DOI] [PubMed] [Google Scholar]
- 25.Winkler ME, Hoch JA. 2008. Essentiality, bypass, and targeting of the YycFG (VicRK) two-component regulatory system in gram-positive bacteria. J Bacteriol 190:2645–2648. 10.1128/JB.01682-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müh U, Pannullo AG, Weiss DS, Ellermeier CD. 2019. A xylose-inducible expression system and a CRISPRi-plasmid for targeted knock-down of gene expression in Clostridioides difficile. J Bacteriol 201:e00711-18. 10.1128/JB.00711-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dembek M, Barquist L, Boinett CJ, Cain AK, Mayho M, Lawley TD, Fairweather NF, Fagan RP. 2015. High-throughput analysis of gene essentiality and sporulation in Clostridium difficile. mBio 6:e02383-14. 10.1128/mBio.02383-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA. 2013. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152:1173–1183. 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng YK, Ehsaan M, Philip S, Collery MM, Janoir C, Collignon A, Cartman ST, Minton NP. 2013. Expanding the repertoire of gene tools for precise manipulation of the Clostridium difficile genome: allelic exchange using pyrE alleles. PLoS One 8:e56051. 10.1371/journal.pone.0056051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukuchi K, Kasahara Y, Asai K, Kobayashi K, Moriya S, Ogasawara N. 2000. The essential two-component regulatory system encoded by yycF and yycG modulates expression of the ftsAZ operon in Bacillus subtilis. Microbiology (Reading) 146:1573–1583. 10.1099/00221287-146-7-1573. [DOI] [PubMed] [Google Scholar]
- 31.Camiade E, Peltier J, Bourgeois I, Couture-Tosi E, Courtin P, Antunes A, Chapot-Chartier MP, Dupuy B, Pons JL. 2010. Characterization of Acp, a peptidoglycan hydrolase of Clostridium perfringens with N-acetylglucosaminidase activity that is implicated in cell separation and stress-induced autolysis. J Bacteriol 192:2373–2384. 10.1128/JB.01546-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuhaus FC, Baddiley J. 2003. A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol Mol Biol Rev 67:686–723. 10.1128/MMBR.67.4.686-723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan CY, Igo MM. 1998. Differential expression of the OmpF and OmpC porin proteins in Escherichia coli K-12 depends upon the level of active OmpR. J Bacteriol 180:171–174. 10.1128/JB.180.1.171-174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JG, Latiolais JA, Guanga GP, Pennington JD, Silversmith RE, Bourret RB. 2004. A search for amino acid substitutions that universally activate response regulators. Mol Microbiol 51:887–901. 10.1046/j.1365-2958.2003.03882.x. [DOI] [PubMed] [Google Scholar]
- 35.Caspi R, Billington R, Ferrer L, Foerster H, Fulcher CA, Keseler IM, Kothari A, Krummenacker M, Latendresse M, Mueller LA, Ong Q, Paley S, Subhraveti P, Weaver DS, Karp PD. 2016. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res 44:D471–D480. 10.1093/nar/gkv1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coullon H, Rifflet A, Wheeler R, Janoir C, Boneca IG, Candela T. 2020. Peptidoglycan analysis reveals that synergistic deacetylase activity in vegetative Clostridium difficile impacts the host response. J Biol Chem 295:16785–16796. 10.1074/jbc.RA119.012442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaus GM, Snyder LF, Müh U, Flores MJ, Popham DL, Ellermeier CD. 2020. Lysozyme resistance in Clostridioides difficile is dependent on two peptidoglycan deacetylases. J Bacteriol 202:e00421-20. 10.1128/JB.00421-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ammam F, Patin D, Coullon H, Blanot D, Lambert T, Mengin-Lecreulx D, Candela T. 2020. AsnB is responsible for peptidoglycan precursor amidation in Clostridium difficile in the presence of vancomycin. Microbiology (Reading) 166:567–578. 10.1099/mic.0.000917. [DOI] [PubMed] [Google Scholar]
- 39.Dajkovic A, Tesson B, Chauhan S, Courtin P, Keary R, Flores P, Marlière C, Filipe SR, Chapot-Chartier MP, Carballido-Lopez R. 2017. Hydrolysis of peptidoglycan is modulated by amidation of meso-diaminopimelic acid and Mg(2+) in Bacillus subtilis. Mol Microbiol 104:972–988. 10.1111/mmi.13673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eveland SS, Pompliano DL, Anderson MS. 1997. Conditionally lethal Escherichia coli murein mutants contain point defects that map to regions conserved among murein and folyl poly-gamma-glutamate ligases: identification of a ligase superfamily. Biochemistry 36:6223–6229. 10.1021/bi9701078. [DOI] [PubMed] [Google Scholar]
- 41.Bradshaw WJ, Roberts AK, Shone CC, Acharya KR. 2018. The structure of the S-layer of Clostridium difficile. J Cell Commun Signal 12:319–331. 10.1007/s12079-017-0429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Layec S, Decaris B, Leblond-Bourget N. 2008. Diversity of Firmicutes peptidoglycan hydrolases and specificities of those involved in daughter cell separation. Res Microbiol 159:507–515. 10.1016/j.resmic.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Ho TD, Williams KB, Chen Y, Helm RF, Popham DL, Ellermeier CD. 2014. Clostridium difficile extracytoplasmic function sigma factor sigmaV regulates lysozyme resistance and is necessary for pathogenesis in the hamster model of infection. Infect Immun 82:2345–2355. 10.1128/IAI.01483-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donahue EH, Dawson LF, Valiente E, Firth-Clark S, Major MR, Littler E, Perrior TR, Wren BW. 2014. Clostridium difficile has a single sortase, SrtB, that can be inhibited by small-molecule inhibitors. BMC Microbiol 14:219. 10.1186/s12866-014-0219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amoroso A, Boudet J, Berzigotti S, Duval V, Teller N, Mengin-Lecreulx D, Luxen A, Simorre JP, Joris B. 2012. A peptidoglycan fragment triggers β-lactam resistance in Bacillus licheniformis. PLoS Pathog 8:e1002571. 10.1371/journal.ppat.1002571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandhu BK, Edwards AN, Anderson SE, Woods EC, McBride SM. 2019. Regulation and anaerobic function of the Clostridioides difficile β-lactamase. Antimicrob Agents Chemother 64:e01496-19. 10.1128/AAC.01496-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Q, Rao F, Chen Z, Cheng Y, Zhang Q, Zhang J, Guan Z, He Y, Yu W, Cui G, Qi X, Hong W. 2022. The cwp66 gene affects cell adhesion. Microbiol Spectr 10:e02704-21. 10.1128/spectrum.02704-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhiman A, Bhatnagar S, Kulshreshtha P, Bhatnagar R. 2014. Functional characterization of WalRK: a two-component signal transduction system from Bacillus anthracis. FEBS Open Bio 4:65–76. 10.1016/j.fob.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woods EC, Nawrocki KL, Suárez JM, McBride SM. 2016. The Clostridium difficile Dlt pathway is controlled by the extracytoplasmic function sigma factor σv in response to lysozyme. Infect Immun 84:1902–1916. 10.1128/IAI.00207-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer W, Rösel P, Koch HU. 1981. Effect of alanine ester substitution and other structural features of lipoteichoic acids on their inhibitory activity against autolysins of Staphylococcus aureus. J Bacteriol 146:467–475. 10.1128/jb.146.2.467-475.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wecke J, Perego M, Fischer W. 1996. D-alanine deprivation of Bacillus subtilis teichoic acids is without effect on cell growth and morphology but affects the autolytic activity. Microb Drug Resist 2:123–129. 10.1089/mdr.1996.2.123. [DOI] [PubMed] [Google Scholar]
- 52.McBride SM, Sonenshein AL. 2011. Identification of a genetic locus responsible for antimicrobial peptide resistance in Clostridium difficile. Infect Immun 79:167–176. 10.1128/IAI.00731-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peschel A, Vuong C, Otto M, Götz F. 2000. The D-alanine residues of Staphylococcus aureus teichoic acids alter the susceptibility to vancomycin and the activity of autolytic enzymes. Antimicrob Agents Chemother 44:2845–2847. 10.1128/AAC.44.10.2845-2847.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sekulovic O, Ospina Bedoya M, Fivian-Hughes AS, Fairweather NF, Fortier LC. 2015. The Clostridium difficile cell wall protein CwpV confers phase-variable phage resistance. Mol Microbiol 98:329–342. 10.1111/mmi.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ransom EM, Ellermeier CD, Weiss DS. 2015. Use of mCherry red fluorescent protein for studies of protein localization and gene expression in Clostridium difficile. Appl Environ Microbiol 81:1652–1660. 10.1128/AEM.03446-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuchs M, Lamm-Schmidt V, Sulzer J, Ponath F, Jenniches L, Kirk JA, Fagan Rp Barquist L, Vogel J, Faber F. 2021. An RNA-centric global view of Clostridioides difficile reveals broad activity of Hfq in a clinically important gram-positive bacterium. Proc Natl Acad Sci USA 118:e2103579118. 10.1073/pnas.2103579118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dobihal GS, Flores-Kim J, Roney IJ, Wang X, Rudner DZ. 2022. The WalR-WalK signaling pathway modulates the activities of both CwlO and LytE through control of the peptidoglycan deacetylase PdaC in Bacillus subtilis. J Bacteriol 204:e00533-21. 10.1128/jb.00533-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jansen A, Türck M, Szekat C, Nagel M, Clever I, Bierbaum G. 2007. Role of insertion elements and yycFG in the development of decreased susceptibility to vancomycin in Staphylococcus aureus. Int J Med Microbiol 297:205–215. 10.1016/j.ijmm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 59.McEvoy CR, Tsuji B, Gao W, Seemann T, Porter JL, Doig K, Ngo D, Howden BP, Stinear TP. 2013. Decreased vancomycin susceptibility in Staphylococcus aureus caused by IS256 tempering of WalKR expression. Antimicrob Agents Chemother 57:3240–3249. 10.1128/AAC.00279-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu J, Zhang X, Liu X, Chen C, Sun B. 2015. Mechanism of reduced vancomycin susceptibility conferred by walK mutation in community-acquired methicillin-resistant Staphylococcus aureus strain MW2. Antimicrob Agents Chemother 59:1352–1355. 10.1128/AAC.04290-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peng H, Hu Q, Shang W, Yuan J, Zhang X, Liu H, Zheng Y, Hu Z, Yang Y, Tan L, Li S, Hu X, Li M, Rao X. 2017. WalK(S221P), a naturally occurring mutation, confers vancomycin resistance in VISA strain XN108. J Antimicrob Chemother 72:1006–1013. 10.1093/jac/dkw518. [DOI] [PubMed] [Google Scholar]
- 62.Hu Q, Peng H, Rao X. 2016. Molecular events for promotion of vancomycin resistance in vancomycin intermediate Staphylococcus aureus. Front Microbiol 7:1601. 10.3389/fmicb.2016.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mesnage S, Dellarole M, Baxter NJ, Rouget JB, Dimitrov JD, Wang N, Fujimoto Y, Hounslow AM, Lacroix-Desmazes S, Fukase K, Foster SJ, Williamson MP. 2014. Molecular basis for bacterial peptidoglycan recognition by LysM domains. Nat Commun 5:4269. 10.1038/ncomms5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rice KC, Bayles KW. 2008. Molecular control of bacterial death and lysis. Microbiol Mol Biol Rev 72:85–109. 10.1128/MMBR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gibson DG, Young L, Chuang R-Y, Venter JC, Hutchison CA, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 66.Dineen SS, Villapakkam AC, Nordman JT, Sonenshein AL. 2007. Repression of Clostridium difficile toxin gene expression by CodY. Mol Microbiol 66:206–219. 10.1111/j.1365-2958.2007.05906.x. [DOI] [PubMed] [Google Scholar]
- 67.Trieu-Cuot P, Carlier C, Poyart-Salmeron C, Courvalin P. 1991. Shuttle vectors containing a multiple cloning site and a lacZ alpha gene for conjugal transfer of DNA from Escherichia coli to gram-positive bacteria. Gene 102:99–104. 10.1016/0378-1119(91)90546-N. [DOI] [PubMed] [Google Scholar]
- 68.Kirk JA, Fagan RP. 2016. Heat shock increases conjugation efficiency in Clostridium difficile. Anaerobe 42:1–5. 10.1016/j.anaerobe.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ducret A, Quardokus EM, Brun YV. 2016. MicrobeJ, a tool for high throughput bacterial cell detection and quantitative analysis. Nat Microbiol 1:16077. 10.1038/nmicrobiol.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mende DR, Letunic I, Huerta-Cepas J, Li SS, Forslund K, Sunagawa S, Bork P. 2017. proGenomes: a resource for consistent functional and taxonomic annotations of prokaryotic genomes. Nucleic Acids Res 45:D529–D534. 10.1093/nar/gkw989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caspi R, Altman T, Billington R, Dreher K, Foerster H, Fulcher CA, Holland TA, Keseler IM, Kothari A, Kubo A, Krummenacker M, Latendresse M, Mueller LA, Ong Q, Paley S, Subhraveti P, Weaver DS, Weerasinghe D, Zhang P, Karp PD. 2014. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res 42:D459–D471. 10.1093/nar/gkt1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Almagro Armenteros JJ, Tsirigos KD, Sonderby CK, Petersen TN, Winther O, Brunak S, von Heijne G, Nielsen H. 2019. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol 37:420–423. 10.1038/s41587-019-0036-z. [DOI] [PubMed] [Google Scholar]
- 74.Savojardo C, Martelli PL, Fariselli P, Profiti G, Casadio R. 2018. BUSCA: an integrative web server to predict subcellular localization of proteins. Nucleic Acids Res 46:W459–W466. 10.1093/nar/gky320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Münch R, Hiller K, Grote A, Scheer M, Klein J, Schobert M, Jahn D. 2005. Virtual Footprint and PRODORIC: an integrative framework for regulon prediction in prokaryotes. Bioinformatics 21:4187–4189. 10.1093/bioinformatics/bti635. [DOI] [PubMed] [Google Scholar]
- 76.Hussain HA, Roberts AP, Mullany P. 2005. Generation of an erythromycin-sensitive derivative of Clostridium difficile strain 630 (630Δerm) and demonstration that the conjugative transposon Tn916ΔE enters the genome of this strain at multiple sites. J Med Microbiol 54:137–141. 10.1099/jmm.0.45790-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download jb.00121-22-s0001.xlsx, XLSX file, 0.6 MB (661KB, xlsx)
Tables S2 to S4, Fig. S1 to S6. Download jb.00121-22-s0002.pdf, PDF file, 0.8 MB (813.7KB, pdf)
Data Availability Statement
RNA-seq data were submitted to the NCBI GEO repository and assigned accession number GSE200346.







