Abstract
Background
(R,S)-ketamine, an N-methyl-D-aspartate receptor antagonist, is frequently used as an anesthetic and as a rapid-acting antidepressant. We and others have reported that (R,S)-ketamine is prophylactic against stress in adult mice but have yet to test its efficacy in adolescent or aged populations.
Methods
Here, we administered saline or (R,S)-ketamine as a prophylactic at varying doses to adolescent (5-week-old) and aged (24-month-old) 129S6/SvEv mice of both sexes 1 week before a 3-shock contextual fear-conditioning (CFC) stressor. Following CFC, we assessed behavioral despair, avoidance, perseverative behavior, locomotion, and contextual fear discrimination. To assess whether the prophylactic effect could persist into adulthood, adolescent mice were injected with saline or varying doses of (R,S)-ketamine and administered a 3-shock CFC as a stressor 1 month later. Mice were then re-exposed to the aversive context 5 days later and administered behavioral tests as aforementioned. Brains were also processed to quantify Cyclooxygenase 2 expression as a proxy for inflammation to determine whether the prophylactic effects of (R,S)-ketamine were partially due to changes in brain inflammation.
Results
Our data indicate that (R,S)-ketamine is prophylactic at sex-specific doses in adolescent but not aged mice. (R,S)-ketamine attenuated learned fear and perseverative behavior in females, reduced behavioral despair in males, and facilitated contextual fear discrimination in both sexes. (R,S)-ketamine reduced Cyclooxygenase 2 expression specifically in ventral Cornu Ammonis region 3 of male mice.
Conclusions
These findings demonstrate that prophylactic (R,S)-ketamine efficacy is sex, dose, and age dependent and will inform future studies investigating (R,S)-ketamine efficacy across the lifespan.
Keywords: (R,S)-ketamine; adolescence; aging
Significance Statement.
(R,S)-ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist, is used as an anesthetic and rapid-acting antidepressant. We and others have reported that (R,S)-ketamine is prophylactic against stress in adult mice, but have yet to test its efficacy in adolescent or aged populations. Here, we report that prophylactic (R,S)-ketamine administration during adolescence was efficacious in attenuating learned fear and perseverative behaviors in females and at reducing behavioral despair in males. Importantly, the prophylactic effect could persist into adulthood: prophylactic (R,S)-ketamine administration reduced fear expression in males and decreased behavioral despair and perseverative behavior in females. These results show for the first time, to our knowledge, that there is a long-lasting, prophylactic effect of (R,S)-ketamine on separate stress-induced behaviors in adolescent males and females. Notably, prophylactic (R,S)-ketamine was not effective in aged mice. Overall, our findings underscore the need for age- and sex-specific dosing to increase the efficacy of (R,S)-ketamine treatment in adolescent and aged subjects.
Introduction
(R,S)-ketamine, an N-methyl-D-aspartate receptor antagonist, has emerged as a rapid-acting and long-lasting antidepressant (Berman et al., 2000; Zarate et al., 2006; Kavalali et al., 2015; Carreno et al., 2016). While traditional antidepressants take weeks to reach efficacy and require daily administration to maintain therapeutic efficacy, (R,S)-ketamine often acts within 2 hours following a single dose (Autry et al., 2011; Murrough et al., 2013) in patients with major depressive disorder (MDD). Moreover, (R,S)-ketamine effects can last 1–2 weeks (Zarate et al., 2006; Murrough et al., 2013). We have previously reported that a single injection of (R,S)-ketamine administered 1 week before stress protects against stress-induced behavioral despair and attenuates learned fear in adult mice (Brachman et al., 2016; McGowan et al., 2017). (R,S)-ketamine’s prophylactic effect has been replicated by several other laboratories (Amat et al., 2016; Dolzani et al., 2018) and has been shown to be effective in the clinic (McGhee et al., 2008; Ma et al., 2019), demonstrating its promising potential as a preventative therapy.
Despite its potential as a prophylactic, (R,S)-ketamine has primarily been tested preclinically in adult mice. (R,S)-ketamine is used for short-term procedural sedation in the emergency department setting in a wide age range, starting at 3 months of age (Rosenbaum et al., 2021). Yet, even with a high prevalence of (R,S)-ketamine being administered as an anesthetic in children (Green et al., 2004; Dolansky et al., 2008), few studies have systematically evaluated the behavioral effects of (R,S)-ketamine administration in children (Papolos et al., 2013; Dwyer et al., 2017; Cullen et al., 2018; Zarrinnegar et al., 2019). Moreover, the long-term effects of adolescent (R,S)-ketamine administration later in life, or in males vs females, have not yet been investigated. Whether there is a sex-specific effect and/or an interaction of (R,S)-ketamine and stress during adolescence is also as of yet unknown, making it essential that the behavioral consequences associated with (R,S)-ketamine administration in adolescent populations be characterized.
Similarly, (R,S)-ketamine is used as an anesthetic in older adults (Maneglia and Cousin, 1988; Green and Krauss, 2004), often in combination with other drugs (Willman et al., 2007; Andolfatto et al., 2012). However, there are minimal data about (R,S)-ketamine efficacy as an antidepressant in elderly patients (i.e., >60 years old) (Szymkowicz et al., 2014; Heard et al., 2017; Medeiros et al., 2017; Bahr et al., 2019; Bryant et al., 2019; Pennybaker et al., 2021). Only 2 studies showed that (R,S)-ketamine successfully decreased depressive symptomatology (Srivastava et al., 2015; Medeiros da Frota Ribeiro and Riva-Posse, 2017). Two other studies showed that, although the geriatric population responded to an acute infusion of (R,S)-ketamine, they were unable to maintain an antidepressant response to i.v. (R,S)-ketamine over time, suggesting that (R,S)-ketamine has low efficacy for the elderly (George et al., 2017; Bryant et al., 2019). However, it is important to note that in these studies, (R,S)-ketamine was often given in addition to other antidepressants, in patients with co-morbid disorders, and without taking into account sex differences. Most critically, there are no preclinical studies on (R,S)-ketamine’s prophylactic effects in aged mice.
Studying sex and age is crucial given that brain maturation is affected by both variables. The mouse life-cycle is characterized by the following stages: adolescence (4–7 weeks), adulthood (3–6 months), and aging (18–24 months) (Flurkey et al., 2007). Sex steroids increase during adolescence, enhancing neuronal myelination (Martini and Melcangi, 1991) and modulating the development of neurocircuitry underlying high-order cognition, reward, and emotional processing until adulthood (Flurkey et al., 2007). These neurodevelopmental changes are necessary for developing adult behaviors (Flurkey et al., 2007), but also make the adolescent brain highly susceptible to stress. Thus, adolescence is the time when symptoms of a variety of mental illnesses often emerge (Kessler et al., 2005). Conversely, with aging biomarkers such as sex steroids, energy metabolism, synaptic transmission, cell membrane turnover, and neurogenesis become altered (Duarte et al., 2015). Therefore, behavioral and drug responses may be drastically different in adolescent vs adult or aged populations.
Recent studies showed that inflammatory markers, such as cytokines, enzymes, and metabolites levels, could be used as biomarkers for depressive behavior (Wright et al., 2005; Haroon et al., 2012; Strawbridge et al., 2017). For example, Cyclooxygenase 2 (Cox-2), an enzyme involved in inflammation by synthesizing prostaglandins, has been shown to be increased in depressed patients (Galecki et al., 2012). (R,S)-ketamine has been shown to have anti-inflammatory properties in depressed patients with peripheral inflammation (De Kock et al., 2013; Zanos et al., 2018; Verdonk et al., 2019). Interestingly, (R,S)-ketamine has been shown to interfere with Cox-2 without blunting the local inflammatory process (De Kock et al., 2013). It is still unknown if prophylactic (R,S)-ketamine alters Cox-2 expression in adolescent or aged mice.
Here, to better understand (R,S)-ketamine’s prophylactic efficacy in adolescent and aged populations, male and female adolescent or aged mice were injected with saline or (R,S)-ketamine at 1 of varying doses 1 week before a contextual fear-conditioning (CFC) stressor. Prophylactic (R,S)-ketamine attenuated learned fear in adolescent female mice and decreased behavioral despair in adolescent male mice, but had no effects in aged mice. Prophylactic (R,S)-ketamine also facilitated contextual fear discrimination (CFD) in adolescent but not aged mice. (R,S)-ketamine administration specifically reduced the expression of Cox-2 in ventral Cornu Ammonis region 3 CA3 (vCA3) in adolescent male mice. In summary, by showing that prophylactic (R,S)-ketamine efficacy is sex, dose, and age dependent, this and our prior studies aim to inform future clinical studies investigating (R,S)-ketamine efficacy across the lifespan.
METHODS
For a full description of Methods, please refer to the supplemental Methods and supplemental Table 1.
Mice
For adolescent studies, male and female 129S6/SvEvTac mice were purchased from Taconic (Hudson, NY, USA) at 4 weeks of age, equivalent to 13–17 years of age in humans (Newman et al., 1996; Pattwell et al., 2012). Mice were housed in the vivarium for 1 week to allow for an adjustment.
For aged studies, 129S6/SvEvTac mice were purchased from Taconic and bred in-house. The pups were then aged to 24 months of age, equivalent to 56–69 years of age in humans (Flurkey et al., 2007). Mice were housed 5 per cage in a 12-hour (6:00 am–6:00 pm) light/-dark colony room at 22ºC.
In contrast to other mouse strains, 129S6/SvEvTac mice show greater susceptibility to stress (Chan et al., 2017) and are ideal to model stress-induced psychopathology (Anisman and Matheson, 2005; Ducottet and Belzung, 2005). This mouse strain has also been used in previous studies of (R,S)-ketamine’s prophylactic effects (Brachman et al., 2016; McGowan et al., 2017; Mastrodonato et al., 2018; Mastrodonato et al., 2020; Chen et al., 2020).
Food and water were provided ad libitum. Behavioral testing was performed during the light phase. All experiments were approved by the Institutional Animal Care and Use Committee at the New York State Psychiatric Institute.
Drugs
A single injection of saline (0.9% NaCl) or (R,S)-ketamine (Fort Dodge Animal Health, Fort Dodge, IA, USA) (10, 30, or 100 mg/kg) was administered at 5 weeks or 24 months of age once during the course of each experiment. All drugs were prepared in physiological saline and administered i.p. in volumes of 0.1 cc/10 mg body weight.
Statistical Analysis
All data were analyzed using Prism 8.0 (Graphpad Software, La Jolla, CA, USA). Alpha was set to 0.05 for all analyses. Overall, the effect of age, time, and drug was analyzed using ANOVA, using repeated measures where appropriate. Post-hoc Dunnett’s and Sidak’s tests were used where appropriate. The effect of drug on Cox-2 expression (mean intensity) was analyzed using Student’s t tests. All statistical tests and P values are listed in supplementary Table 2. A summary of behavioral findings is listed in supplementary Table 3.
RESULTS
(R,S)-Ketamine Is Prophylactic in Adolescent but Not Aged Mice
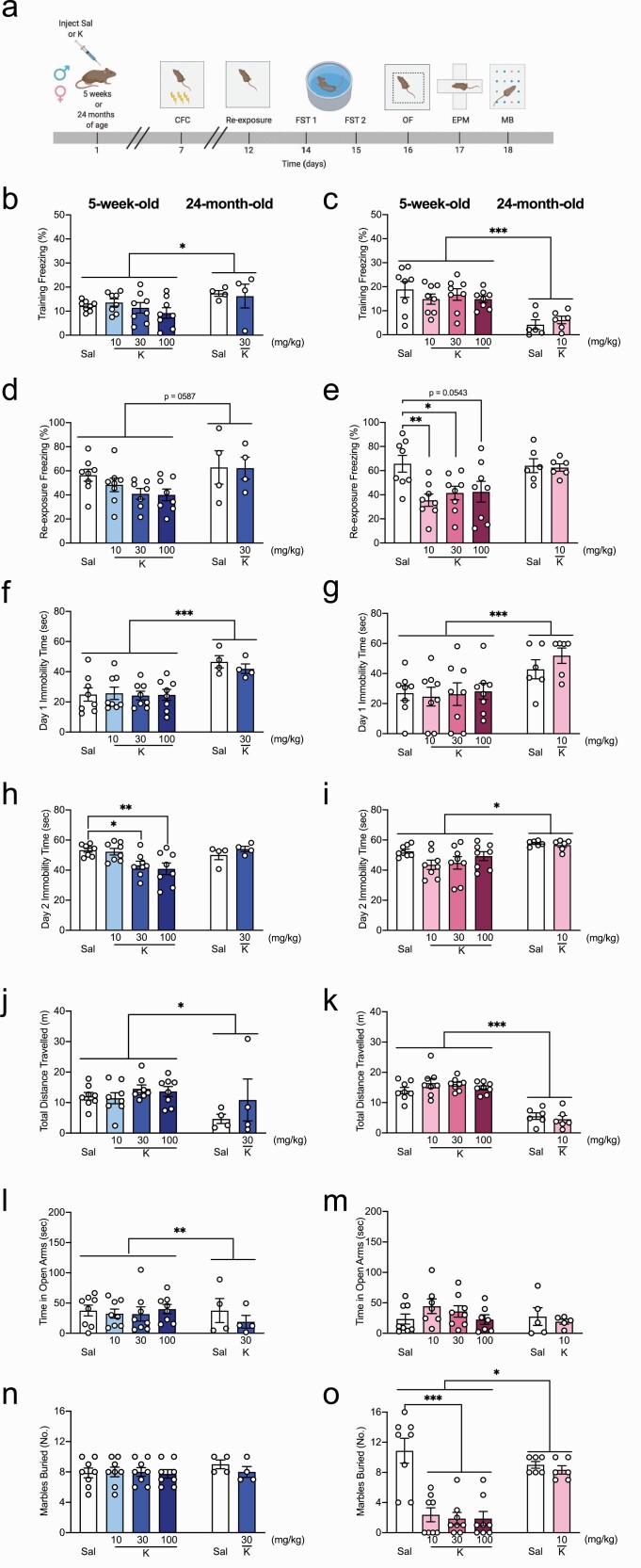
We have previously shown that (R,S)-ketamine is prophylactic against stress-induced behavioral despair in adult mice (Brachman et al., 2016; McGowan et al., 2017; Mastrodonato et al., 2018) but have yet to determine its prophylactic efficacy in adolescent or aged mice. Here, male and female adolescent (5-week-old) and aged (24-month-old) 129S6/SvEv mice were injected with saline or (R,S)-ketamine at varying doses (Fig. 1A). Doses were chosen on previous studies in adolescent (Parise et al., 2021) and adult mice (Brachman et al., 2016; McGowan et al., 2017; Mastrodonato et al., 2018; Chen et al., 2020). Furthermore, since aged mice were bred in-house, and therefore, only a limited number of mice were available for each experiment, only the previously identified effective doses for adult mice (30 mg/kg for males and 10 mg/kg for females) were chosen for these studies.
Figure 1.
Prophylactic (R,S)-ketamine is effective against stress in adolescent but not aged mice. (A) Experimental design. (B) Five-week-old male mice exhibited lower levels of freezing compared with 24-month-old male mice on CFC training (age: P = .030). (R,S)-ketamine did not impact freezing levels in either group (drug: P = .674). (C) Five-week-old female mice exhibited higher level of freezing compared with 24-month-old female mice during CFC training (age: P < .0001). (R,S)-ketamine did not impact freezing levels in either group (drug: P = .735). (D) During CFC re-exposure, 5-week-old mice tended to freeze less than 24-month-old male mice (age: P = .058). (E) Five-week-old female mice exhibited similar freezing levels to 24-month-old female mice (age: P = .086). Five-week-old female mice administered (R,S)-ketamine at 10 or 30 mg/kg, but not 100 mg/kg, exhibited significantly less freezing than female mice administered saline (P = .009; P = .042; P = .054, respectively). (F,G) Five-week-old male and female mice administered saline or (R,S)-ketamine exhibited lower immobility time on day 1 of the FST compared with 24-month-old mice (age: P < .0001; P = .0008, respectively). (H) Five-week- and 24-month-old male mice showed similar immobility time on day 2 of the FST (age: P = .418). Five-week-old male mice administered (R,S)-ketamine at 30 or 100 mg/kg, but not 10 mg/kg, exhibited significantly less immobility time compared with mice administered saline on day 2 of the FST (P = .036 and P = .007, respectively). (I) Five-week-old female mice exhibited significantly less immobility time than 24-month-old female mice (age: P = .005). (R,S)-ketamine did not impact behavioral despair in either group (drug: P = .305). (J, K) Both groups of 24-month-old male and female mice traveled significantly less than 5-week-old male and female mice (age: P = .039; P < .0001, respectively). (R,S)-ketamine did not impact the distance traveled in any groups (drug: P = .119; P = .694, respectively). (L) Five-week-old mice spent more time in the open arms than 24-month-old male mice (age: P = .0003). (R,S)-ketamine increased the time spent in the open arms (drug: P = .0002). (M) Five-week-old female mice spent a similar amount of time in the open arms compared with 24-month-old female mice (age: P = .672). In 5-week-old mice, there was no effect of drug (P = .719). (N) During the MB, all groups of 5-week-old and 24-month-old male mice buried a comparable number of marbles (P = .357). (O) All groups of 5-week-old female mice administered (R,S)-ketamine buried significantly fewer marbles compared with female mice administered saline (P < .0001). Both groups of 24-month-old female mice buried a comparable number of marbles, but significantly higher than 5-week-old mice (P = .028). (n = 4–8 mice per group). Error bars represent + SEM. *P < .05. **P < .01. ***P < .0001. CFC, contextual fear conditioning; EPM, elevated plus maze; FST, forced swim test; K, (R,S)-ketamine; MB, marble burying; OF, open field; Sal, saline; sec, second.
One week later, mice were administered a 3-shock CFC paradigm. During CFC training, all groups of adolescent male mice exhibited lower levels of freezing compared with aged male mice (Fig. 1B). However, all groups of adolescent female mice exhibited higher levels of freezing compared with aged female mice (Fig. 1C). (R,S)-ketamine did not impact freezing levels in any group. Five days later, mice were re-exposed to the CFC context. Adolescent male mice tended to freeze less than aged male mice (P = .06), and there was no effect of drug (Fig. 1D). In females, there was an effect of drug, but not of age, and there was a drug × age interaction. Adolescent (R,S)-ketamine-injected (10 or 30 mg/kg) female mice exhibited significantly less freezing than saline-injected mice (Fig. 1E). Adolescent (R,S)-ketamine-injected (100 mg/kg) mice tended to freeze less than saline-injected mice (P = .05). Saline- and (R,S)-ketamine-injected aged female mice exhibited comparable levels of freezing.
During day 1 of the forced swim test (FST), male and female adolescent mice had lower immobility time than male and female aged mice (Fig. 1F–G). During day 2 of the FST, adolescent (R,S)-ketamine-injected (30 or 100 mg/kg) male mice exhibited significantly less immobility time than saline-injected male mice (Fig. 1H). Saline- and (R,S)-ketamine-injected aged male mice exhibited comparable immobility time. Adolescent female mice exhibited significantly less immobility time than aged female mice (Fig. 1I). (R,S)-ketamine did not impact behavioral despair in adolescent or aged female mice.
In the open field (OF) test, adolescent male and female mice traveled significantly more than aged male and female mice (Fig. 1J–K). (R,S)-ketamine did not impact the distance traveled in any of the groups. In the elevated plus maze (EPM), adolescent male mice spent significantly more time in the open arms than aged male mice (Fig. 1L). There was a significant effect of (R,S)-ketamine on the time spent in the open arms in adolescent male but not female mice (Fig. 1L–M). Adolescent female and aged mice spent a comparable time in the open arms (Fig. 1M). In the marble-burying task, all groups of adolescent and aged male mice buried a comparable number of marbles (Fig. 1N). However, adolescent female mice buried a significantly lower number of marbles than aged female mice, indicating decreased perseverative behavior in these mice (Fig. 1O). All groups of adolescent female mice administered (R,S)-ketamine buried significantly fewer marbles than female mice administered saline. Saline- and (R,S)-ketamine-injected aged female mice buried a comparable number of marbles (Fig. 1O). These data indicate that while ineffective in aged mice, prophylactic (R,S)-ketamine administration decreases stress-induced behavioral despair in adolescent male mice and decreases fear behavior and compulsive behavior in adolescent female mice.
Prophylactic (R,S)-Ketamine Has Long-Lasting Effects in Adolescent Mice
It remained to be determined how long the prophylactic effects of (R,S)-ketamine could persist in adolescent mice. We previously showed that prophylactic (R,S)-ketamine was effective when administered 1 week but not 1 month before stress (McGowan et al., 2018). However, it remained to be determined if prophylactic (R,S)-ketamine followed that same window in adolescent mice. Male and female 5-week-old mice were injected with saline or (R,S)-ketamine (Fig. 2A). One month later, mice were administered a 3-shock CFC paradigm. During CFC training, all groups of mice exhibited comparable levels of freezing (Fig. 2B–C). During CFC context re-exposure, male mice administered (R,S)-ketamine exhibited significantly less freezing than male mice administered saline (Fig. 2D). Female mice administered (R,S)-ketamine tended to exhibit less freezing than female mice administered saline (Fig. 2E).
Figure 2.
Prophylactic (R,S)-ketamine has long-lasting effects in adolescent mice. (A) Experimental design. (B, C) Five-week-old male and female mice administered saline or (R,S)-ketamine froze comparably during CFC training (P = .210, P = .694, respectively). (D) Male mice administered (R,S)-ketamine exhibited significantly less freezing than male mice administered saline during CFC re-exposure (P = .022). (E) In female mice, (R,S)-ketamine–injected mice tended to freeze less than saline-injected mice during CFC re-exposure (P = .053). (F) Male mice administered saline or (R,S)-ketamine exhibited similar immobility time on day 1 of FST (P = .920). (G) Female mice administered saline or (R,S)-ketamine exhibited similar immobility time on day 1 of FST (P = .186). (H) Male mice administered saline or (R,S)-ketamine exhibited similar immobility during day 2 of the FST (P = .444). (I) Female mice administered (R,S)-ketamine exhibited significantly less immobility time compared with female mice administered saline during day 2 of the FST (P = .015). (J, K) Male and female mice administered saline or (R,S)-ketamine traveled a comparable distance during the OF (P = .111, P = .826, respectively). (L, M) Male and female mice administered saline or (R,S)-ketamine spent a comparable amount of time in the open arms of the EPM (P = .428, P = .997, respectively). (N) Male mice administered saline or (R,S)-ketamine buried a comparable number of marbles in the MB (P = .968). (O) Female mice administered (R,S)-ketamine buried significantly fewer marbles than mice administered saline in the MB (P = .002). (n = 6-8 mice per group). Error bars represent + SEM. *P < .05. **P < .01. CFC, contextual fear conditioning; EPM, elevated plus maze; FST, forced swim test; K, (R,S)-ketamine; MB, marble burying; OF, open field; Sal, saline; sec, second.
During day 1 of the FST, all groups of mice had comparable immobility time (Fig. 2F–G). During day 2 of the FST, both groups of male mice had comparable immobility time (Fig. 2H). In female mice, (R,S)-ketamine–injected mice exhibited significantly less immobility time than saline-injected mice (Fig. 2I).
All groups of mice traveled a comparable distance during the OF (Fig. 2J–K) and spent a comparable amount of time in the open arms of the EPM (Fig. 2L–M). During the marble burying task, both groups of male mice buried a comparable number of marbles (Fig. 2N). However, (R,S)-ketamine–injected female mice buried significantly fewer marbles than saline-injected female mice (Fig. 2O). In contrast to our adult male prophylactic (R,S)-ketamine study, these data indicate that (R,S)-ketamine administration has long-lasting prophylactic effects against fear in adolescent male mice and against stress-induced behavioral despair and compulsive behavior in adolescent female mice that can last up to 1 month following a single injection.
Prophylactic (R,S)-Ketamine Facilitates CFD in Adolescent but Not Aged Mice
We next investigated whether the prophylactic (R,S)-ketamine effect was specific for fear expression or could also impact other types of fear learning, such as fear discrimination. We previously reported that (R,S)-ketamine was effective at facilitating fear discrimination in adult male mice (Mastrodonato et al., 2018). Here, adolescent and aged mice were injected with saline or (R,S)-ketamine and administered a CFD paradigm 1 week later (Fig. 3A). In adolescent male mice, saline-injected mice discriminated between the 2 contexts starting on day 5 (Fig. 3B and K). (R,S)-ketamine–injected mice (10 or 100 mg/kg) discriminated between the 2 contexts starting on day 6 (Fig. 3C, E, and K). (R,S)-ketamine–injected mice (30 mg/kg) could discriminate starting on day 4 (Fig. 3D and K). By day 10, only (R,S)-ketamine–injected mice (30 mg/kg) showed an increased discrimination ratio compared with saline-injected mice (Fig. 3J).
Figure 3.
Prophylactic (R,S)-ketamine accelerates contextual fear discrimination in adolescent but not aged mice. (A) Experimental design. (B) Saline-injected male mice could discriminate between the 2 contexts starting from day 5 (context: P < .0001). (C) (R,S)-ketamine–injected male mice (10 mg/kg) could discriminate between the 2 contexts starting from day 6 (context: P < .0001). (D) (R,S)-ketamine–injected male mice (30 mg/kg) could discriminate starting on day 4 (Context: P < .0001). (E) (R,S)-ketamine-injected male mice (100 mg/kg) could discriminate between the 2 contexts starting from day 6 (context: P = .0006). (F, G) Saline- or (R,S)-ketamine–injected female mice (10 mg/kg) could discriminate between the 2 contexts starting on day 5 (context: P < .0001 for both groups). (H, I) (R,S)-ketamine-injected female mice (30 or 100 mg/kg) could discriminate starting on day 7 (context: P < .0001; context: P = .0017, respectively). (J) (R,S)-ketamine-injected male mice (30 mg/kg) exhibited improved discrimination on day 10 (drug: P = .037). (K) Saline- or (R,S)-ketamine–injected male mice could not discriminate between the 2 contexts on day 2 (context: P = .682; drug: P = .161). (R,S)-ketamine–injected male mice (30 mg/kg) could discriminate between the 2 contexts on day 6 (P = .001). All groups of mice could discriminate between the 2 contexts on day 10 (context: P < .0001; drug: P = .327). (L) All groups of female mice exhibited comparable discrimination ratios on day 10 (drug: P = .263). (M) Saline- or (R,S)-ketamine–injected female mice could not discriminate between the 2 contexts on day 2 (drug: P = .010; context: P = .679). (R,S)-ketamine–injected female mice (10 mg/kg) could discriminate between the 2 contexts on day 6 (P = .0004). All groups of mice could discriminate between the 2 contexts on day 10 (context: P < .0001; drug: P = .240). (N, O) Twenty-four-month-old male saline or (R,S)-ketamine–injected mice could not discriminate between the 2 contexts over the 10 days (context: P = .370, P = .969, respectively). (P) Both groups of mice exhibited comparable discrimination ratios on day 10 (drug: P = .622). (Q) On days 2, 6, and 10, all groups exhibited comparable freezing. (R) Twenty-four-month-old saline-injected female mice could not discriminate between the 2 contexts over the 10 days (context: P = .900). (S) (R,S)-ketamine–injected female mice could discriminate between the 2 contexts on day 8 (context: P = .047). (T) Both groups of mice exhibited comparable discrimination ratios on day 10 (drug: P = .571). (U) On days 2, 6, and 10, all groups exhibited comparable freezing (n = 5–10 mice per group). Error bars represent + SEM. ***P < .0001. K, (R,S)-ketamine; Sal, saline.
In adolescent female mice, saline- or (R,S)-ketamine–injected (10 mg/kg) mice discriminated between the 2 contexts starting on day 5 (Fig. 3F, G, and M). In contrast, (R,S)-ketamine–injected mice (30 or 100 mg/kg) discriminated between the 2 contexts starting on day 7 (Fig. 3H, I, and M). By day 10, all groups of female mice administered showed similar discrimination ratios (Fig. 3L).
Both groups of aged male and female mice could not discriminate between the 2 contexts over the course of CFD, and (R,S)-ketamine did not improve this behavioral phenotype (Fig. 3N–U). Although there was an overall effect of context in the female aged mice administered (R,S)-ketamine, only day 8 reached significance (Fig. 3S). These data indicate that prophylactic (R,S)-ketamine administration is effective at facilitating CFD in adolescent but not aged mice.
Prophylactic (R,S)-Ketamine Decreases Cox-2 Expression in vCA3 of Adolescent but Not Aged Mice
We next investigated whether the sex- and age-specific effects of (R,S)-ketamine on behavior could be paralleled by changes in the expression of Cox-2 (Fig. 4A–C), a marker of inflammation (De Kock et al., 2013). Only the previously identified effective (R,S)-ketamine doses for adult mice (30 mg/kg for males and 10 mg/kg for females) were chosen for these studies. In the dorsal hippocampus (HPC), 5-week-old male mice tended to have lower levels of Cox-2 expression than 24-month-old male mice in the dentate gyrus (DG) (P = .05) (Fig. 4D). Five-week-old male mice had similar levels of Cox-2 expression compared with 24-month-old male mice in the CA3 (Fig. 4E). (R,S)-ketamine did not impact Cox-2 expression in either group. Five-week-old female mice had a lower level of Cox-2 expression than 24-month-old female mice in the DG and CA3 (Fig. 4F–G). (R,S)-ketamine did not impact Cox-2 expression in either group.
Figure 4.
Prophylactic (R,S)-ketamine selectively decreases Cox-2 expression in ventral CA3 of adolescent male mice. (A) Experimental design. (B, C) Cox-2 expression in the dorsal and ventral hippocampus. Scale bar = 250 µm and 25 µm (inset). (D) Twenty-four-month-old male mice tended to show increased Cox-2 expression in the dorsal DG compared with 5-week-old mice (age: P = .053). (R,S)-ketamine did not impact Cox-2 expression (drug: P = .866). (E) Twenty-four-month-old male mice had similar Cox-2 expression in the dorsal CA3 compared with 5-week-old mice (age: P = .422). (R,S)-ketamine did not impact Cox-2 expression (drug: P = .505). (F, G) Twenty-four-month-old female mice had increased Cox-2 expression in the dorsal DG and dorsal CA3 compared with 5-week-old mice (age: P = .010; P = .003, respectively). (R,S)-ketamine did not impact Cox-2 expression in either region (drug: P = .968; P = .444, respectively). (H, I) Twenty-four-month-old male mice trended to have and had increased Cox-2 expression in the ventral DG and in the ventral CA3 compared with 5-week-old mice (age: P = .059; P = .036, respectively). (R,S)-ketamine did not impact Cox-2 expression in the ventral DG (drug: P = .603). However, in 5-week-old male mice, (R,S)-ketamine decreased Cox-2 expression in ventral CA3 (drug: P = .043). (J, K) All groups of female mice showed lower level of Cox-2 expression in the ventral DG and ventral CA3 (age: P = .024; P = .002, respectively). (R,S)-ketamine did not impact Cox-2 expression (drug: P = .267; P = .773, respectively). Error bars represent + SEM. *P < .05, **P < .01, ***P < .001. CA3, Cornu Ammonis region 3; DG, dentate gyrus; iDISCO, immunolabeling-enabled three-dimensional imaging of solvent-cleared organs; K, (R,S)-ketamine; Sal, saline.
In the ventral HPC, 5-week-old male mice tended to have or had a lower level of Cox-2 expression than 24-month-old male mice in the DG and CA3, respectively (Fig. 4H–I). (R,S)-ketamine did not impact Cox-2 expression in either group in the ventral DG. However, 5-week-old male mice administered (R,S)-ketamine had lower levels of Cox-2 expression than saline-treated mice in vCA3 (Fig. 4I). (R,S)-ketamine did not impact Cox-2 expression in 24-month-old mice in vCA3. Five-week-old female mice had a lower level of Cox-2 expression compared with 24-month-old female mice in the ventral DG and vCA3 (Fig. 4J–K). (R,S)-ketamine did not impact Cox-2 expression in any groups. These data suggest that (R,S)-ketamine effects might be partially mediated by Cox-2 expression in vCA3 in 5-week-old male mice. Furthermore, in line with our previous data (Mastrodonato et al., 2018), these data suggest that vCA3 is a key node in prophylactic (R,S)-ketamine efficacy.
Discussion
Here, we report for the first time, to our knowledge, that a single injection of (R,S)-ketamine, administered prophylactically, is effective against selective stress-induced behaviors in adolescent, but not aged, mice depending on sex, dose, and time of administration. Specifically, we found that prophylactic (R,S)-ketamine attenuated learned fear and perseverative behavior in adolescent female mice and decreased behavioral despair in adolescent male mice. Importantly, a single injection of (R,S)-ketamine during adolescence attenuated fear in males and behavioral despair in females during adulthood, suggesting that (R,S)-ketamine can provide long-lasting protection against stress. These findings underscore the need for age- and sex-specific dosing to increase the efficacy of (R,S)-ketamine treatment in adolescent and aged subjects. (R,S)-ketamine facilitated CFD in both sexes in adolescent but not aged mice. Lastly, we found that the levels of the marker of inflammation Cox-2 were increased in aged but not adolescent mice. Prophylactic (R,S)-ketamine decreased the level of Cox-2 specifically in vCA3 of adolescent male mice.
Our study demonstrates that during CFC training, aged male mice froze more than adolescent male mice, while aged female mice froze less than aged female mice. Sex differences in fear-related behaviors have been widely reported by several studies. For instance, males have generally been shown to exhibit more fear behaviors than females (e.g., lower ambulation and rearing, reduced open-arm activity in the EPM) as well as behavioral inhibition after previous aversive stimulation (Archer, 1975; Johnston and File, 1991; Gruene et al., 2015). Females, however, more readily show active responding (e.g., darting) and rapid fear extinction (Archer, 1975; Johnston and File, 1991; Gruene et al., 2015). Human studies have also shown sex-specific symptoms of fear-related disorders in men and women. While women diagnosed with MDD experience higher rates of comorbid anxiety disorders and greater suicidal ideation, men are at greater risk of comorbid substance abuse (Altemus et al., 2014). Overall, these studies suggest that males and females have unique fear strategies that, from an evolutionary standpoint, may be differentially advantageous for either sex.
We next showed that prophylactic (R,S)-ketamine attenuated learned fear in adolescent female mice and decreased behavioral despair in adolescent male mice. Intriguingly, these results do not directly parallel what we have found in adult mice, where prophylactic (R,S)-ketamine attenuates learned fear in males (McGowan et al., 2017) and decreases behavioral despair in both sexes (Brachman et al., 2016; Mastrodonato et al., 2018; Chen et al., 2020). Developmental changes in fear neural circuits, such as the amygdala-HPC-prefrontal cortex circuit, during adolescence may result in different behavioral phenotypes and drug responses in adolescent compared with adult mice (Casey et al., 2015; Pattwell et al., 2016). Future studies will investigate how the brain circuits change during development to result in age- and sex-specific behaviors.
Interestingly, we found that aged mice showed increased avoidance behavior compared with adolescent mice only in males. In line with this, recent studies suggest that generalized anxiety disorder is more common in older than in younger adults (Sable and Jeste, 2001; Lenze and Wetherell, 2011). However, these works have not considered sex as a variable in the study of anxiety behavior across the lifespan. It remains to be determined if hormonal differences might be responsible for the increased avoidance behavior that we observed in aged male but not female mice. Consistent with previous studies showing the lack of an anxiolytic effect with (R,S)-ketamine (Autry et al., 2011; Brachman et al., 2016), (R,S)-ketamine did not affect avoidance behavior. Moreover, we report that at all doses tested, (R,S)-ketamine reduced perseverative behavior only in adolescent female mice. These data indicate that (R,S)-ketamine may be protective to attenuate perseverative behavior (i.e., obsessive compulsive disorder) in adolescent females, which has only been reported in clinical studies so far (Rodriguez et al., 2013, 2015; Thompson et al., 2020).
Here, we also report that 1 month after adolescent (R,S)-ketamine administration, male mice exhibit reduced fear expression, and female mice exhibit decreased behavioral despair and decreased perseverative behavior. These results show for the first time, to our knowledge, that there is a long-lasting prophylactic effect of (R,S)-ketamine on separate stress-induced behaviors in males and females. These data are in contrast with our previous work in adult mice showing that prophylactic (R,S)-ketamine is not effective at reducing fear behavior when administered 1 month before CFC (McGowan et al., 2017) and suggest that there are different mechanisms regulating the long-lasting effects of (R,S)-ketamine in adolescents compared with adult mice. Notably, a recent study in mice has shown that (R,S)-ketamine in adolescents does not cause long-lasting detrimental drug-seeking behavior, providing evidence about the safety of (R,S)-ketamine exposure during adolescence as a treatment for stress-induced illnesses (Garcia-Carachure et al., 2020).
Since impaired CFD is a symptom often observed in anxiety disorders, here we assessed if the (R,S)-ketamine was effective against CFD in adolescent and aged mice (Lissek et al., 2010). We report that a similar phenotype in prophylactic (R,S)-ketamine (30 mg/kg)-administered adolescent male mice and (R,S)-ketamine (10 mg/kg)-administered adolescent female mice discriminated faster than their saline-injected controls. Overall, these data indicate that (R,S)-ketamine is effective at mitigating fear overgeneralization in both adolescent and adult mice in a dose- and sex-dependent manner. These data are of clinical relevance because they suggest using (R,S)-ketamine to treat pathological fear generalization in anxiety disorders, which are known to be more prevalent during adolescence than during adulthood (Kessler et al., 2005). Our data also suggest that the dose of (R,S)-ketamine will strongly determine whether there is a positive outcome.
In the CFD experiment, aged mice could not discriminate, which is in accord with a previous study showing that aged mice show a profound impairment in CFD compared with young mice (Wu et al., 2015). (R,S)-ketamine did not facilitate CFD in aged mice. Our results are consistent with recent studies showing that Spravato (esketamine) is less effective in older adults (i.e., >60 years old) (Bahr et al., 2019) and that (R,S)-ketamine infusions are not effective in geriatric patients suffering from treatment-resistant depression (Szymkowicz et al., 2014; da Frota Ribeiro and Riva-Posse, 2017; Heard et al., 2017; Bryant et al., 2019; Pennybaker et al., 2021). These studies, therefore, indicate the necessity of testing different (R,S)-ketamine doses and protocols of administration in the geriatric population. Future studies will expand on our study and investigate a variety of doses and/or combined drug administration in aged mice.
Emerging evidence suggests that peripheral inflammation is a major risk factor for MDD (Haroon et al., 2012). Moreover, patients with significantly elevated levels of pro-inflammatory cytokines at baseline are more likely to develop MDD and less likely to respond to classical antidepressants. In addition to aberrant cytokine levels, the effects of inflammation on mood are mediated by the indoleamine 2,3-dioxygenase (IDO) pathway. This pathway metabolizes tryptophan into kynurenine instead of serotonin (Maes et al., 2007), leading to the production of metabolites that induce neurotoxicity and depressive symptoms. IDO levels can be reduced through the inhibition of Cox-2 (Iachininoto et al., 2013). While the exact molecular mechanism is unknown, our work shows that (R,S)-ketamine reduces Cox-2 expression, suggesting that decreased IDO-induced neurotoxicity may be responsible for its beneficial effects on mood.
Here, we found that Cox-2 is significantly increased in the HPC of aged mice, which aligns with mice and human data showing that several inflammatory markers increase with aging (Chung et al., 2019). Notably, we found that (R,S)-ketamine selectively decreased Cox-2 expression in vCA3 of adolescent male mice. These experiments indicate that Cox-2 expression in vCA3 may contribute to the sex-specific effects of (R,S)-ketamine in adolescent mice. Therefore, these findings indicate that it could be possible to track and/or target inflammation in ventral CA3 to replicate (R,S)-ketamine effects. Moreover, these results could be translatable in the clinic because they suggest that in patients showing high level of Cox-2, using pharmacological interventions to lower these levels might be able to alleviate stress-induced behaviors.
We previously reported that prophylactic (R,S)-ketamine alters nucleotide and neurotransmitter metabolism in both the brain and plasma (McGowan et al., 2018), suggesting novel target candidates for prophylactic (R,S)-ketamine efficacy. Future studies will expand to other markers to assess whether inflammatory activity can predict and/or correlate with (R,S)-ketamine efficacy. This will not only strengthen the translational value of our basic research, but will be an important step towards identifying clinically relevant neural mechanisms by which (R,S)-ketamine can buffer stress responses and/or can be useful in patient populations suffering from peripheral inflammation.
In summary, the present study demonstrates that (R,S)-ketamine administration induces distinct behavioral effects in a sex-, dose-, and age-specific manner. Our results indicate the necessity of including sex and age effects in pharmacological studies in both pre-clinical and clinical settings. Future studies will need to investigate how the brain circuits change across the lifespan to result in specific behavioral effects.
Supplementary Material
Acknowledgments
We thank Drs Jay Gingrich, Francis Lee, and Holly Hunsberger and members of the Denny laboratory for insightful comments on this project and paper.
This work was supported by the Sackler Award from the Sackler Institute for Developmental Psychobiology, the Janssen Fellowship in Translational Neuroscience from Columbia University, and the Alexander Bodini Fellowship in Adolescent Psychiatry at the Italian Academy at Columbia University to A.M.; the New York State Stem Cell Science (NYSTEM C-029157 to I.P.); the retention package from the New York State Psychiatric Institute, a gift from For the Love of Travis, the National Institute of Child Health and Human Development (NICHD R01 HD101402 to C.A.D.), the National Institute on Aging (NIA R56 AG058661 and R21 AG064774 to C.A.D.), and the National Institute of Neurological Disorders and Stroke (NINDS R21 NS114870 to C.A.D. and F99 NS124182 to J.C.M.); the National Institute of Mental Health (NIMH F31 MH122187-01 to J.C.M. and R01 MH108032 to J.J.M.); and the American Foundation for Suicide Prevention (LSRG-09-091-17 to J.J.M.).
Contributor Information
Alessia Mastrodonato, Division of Systems Neuroscience, Research Foundation for Mental Hygiene, Inc./New York State Psychiatric Institute, New York, New York, USA; Department of Psychiatry, Columbia University Irving Medical Center, New York, New York, USA.
Ina Pavlova, Division of Systems Neuroscience, Research Foundation for Mental Hygiene, Inc./New York State Psychiatric Institute, New York, New York, USA; Department of Psychiatry, Columbia University Irving Medical Center, New York, New York, USA.
Noelle C Kee, Barnard College, New York, New York, USA.
Van Anh Pham, Division of Systems Neuroscience, Research Foundation for Mental Hygiene, Inc./New York State Psychiatric Institute, New York, New York, USA.
Josephine C McGowan, Neurobiology and Behavior Graduate Program, Columbia University, New York, New York, USA.
J John Mann, Molecular Imaging and the Neuropathology Division/Department of Psychiatry, Columbia University Irving Medical Center, New York, New York, USA.
Christine A Denny, Division of Systems Neuroscience, Research Foundation for Mental Hygiene, Inc./New York State Psychiatric Institute, New York, New York, USA; Department of Psychiatry, Columbia University Irving Medical Center, New York, New York, USA.
Interest Statement
A.M., I.P., and N.K. have no competing interests. C.A.D. and J.C.M. are named on patents for the prophylactic use of (R,S)-ketamine and other compounds against stress-related psychiatric disorders. J.J.M. receives royalties for commercial use of the C-SSRS from Research Foundation for Mental Hygiene. A.M., C.A.D., and J.J.M. contributed to the conception and design of the work; A.M., N.K., I.P., and V.A.P. contributed to the acquisition of data. A.M. and C.A.D. contributed to the analysis and interpretation of data for the work, drafting the work and revising it critically for important intellectual content; A.M., C.A.D., N.K., I.P., V.A.P., J.C.M., and J.J.M. approved the version of the manuscript to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Altemus M, Sarvaiya N, Neill Epperson C (2014) Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol 35:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Dolzani SD, Tilden S, Christianson JP, Kubala KH, Bartholomay K, Sperr K, Ciancio N, Watkins LR, Maier SF (2016) Previous ketamine produces an enduring blockade of neurochemical and behavioral effects of uncontrollable stress. J Neurosci 36:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolfatto G, Abu-Laban RB, Zed PJ, Staniforth SM, Stackhouse S, Moadebi S, Willman E (2012) Ketamine-propofol combination (ketofol) versus propofol alone for emergency department procedural sedation and analgesia: a randomized double-blind trial. Ann Emerg Med 59:504–12.e1. [DOI] [PubMed] [Google Scholar]
- Anisman H, Matheson K (2005) Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev 29:525–546. [DOI] [PubMed] [Google Scholar]
- Archer J (1975) Rodent sex differences in emotional and related behavior. Behav Biol 14:451–479. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr R, Lopez A, Rey JA (2019) Intranasal esketamine (SpravatoTM) for use in treatment-resistant depression in conjunction with an oral antidepressant. P T 44:340–375. [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Brachman RA, McGowan JC, Perusini JN, Lim SC, Pham TH, Faye C, Gardier AM, Mendez-David I, David DJ, Hen R, Denny CA (2016) Ketamine as a prophylactic against stress-induced depressive-like behavior. Biol Psychiatry 79:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant KA, Altinay M, Finnegan N, Cromer K, Dale RM (2019) Effects of repeated intravenous ketamine in treatment-resistant geriatric depression: a case series. J Clin Psychopharmacol 39:158–161. [DOI] [PubMed] [Google Scholar]
- Carreno FR, Donegan JJ, Boley AM, Shah A, DeGuzman M, Frazer A, Lodge DJ (2016) Activation of a ventral hippocampus-medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine. Mol Psychiatry 21:1298–1308. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Glatt CE, Lee FS (2015) Treating the developing versus developed brain: translating preclinical mouse and human studies. Neuron 86:1358–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JC, Houghton AB, Bale TL (2017) Strained in planning your mouse background? Using the HPA stress axis as a biological readout for backcrossing strategies. Neuropsychopharmacology 42:1749–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BK, Luna VM, LaGamma CT, Xu X, Deng SX, Suckow RF (2020) Sex-specific neurobiological actions of prophylactic (R,S)-ketamine, (2R,6R)-hydroxynorketamine, and (2S,6S)-hydroxynorketamine. Neuropsychopharmacology 45:1545–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HY, Kim DH, Lee EK, Chung KW, Chung S, Lee B, Seo AY, Chung JH, Jung YS, Im E, Lee J, Kim ND, Choi YJ, Im DS, Yu BP (2019) Redefining chronic inflammation in aging and age-related diseases: proposal of the senoinflammation concept. Aging Dis 10:367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Amatya P, Roback MG, Albott CS, Westlund Schreiner M, Ren Y, Eberly LE, Carstedt P, Samikoglu A, Gunlicks-Stoessel M, Reigstad K, Horek N, Tye S, Lim KO, Klimes-Dougan B (2018) Intravenous ketamine for adolescents with treatment-resistant depression: an open-label study. J Child Adolesc Psychopharmacol 28:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kock M, Loix S, Lavand’homme P (2013) Ketamine and peripheral inflammation. CNS Neurosci Ther 19:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolansky G, Shah A, Mosdossy G, Rieder M (2008) What is the evidence for the safety and efficacy of using ketamine in children? Paediatr Child Health 13:307–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolzani SD, Baratta MV, Moss JM, Leslie NL, Tilden SG, Sorensen AT (2018) Inhibition of a descending prefrontal circuit prevents ketamine-induced stress resilience in females. eNeuro 5:ENEURO.0025-18.2018.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducottet C, Belzung C (2005) Correlations between behaviours in the elevated plus-maze and sensitivity to unpredictable subchronic mild stress: evidence from inbred strains of mice. Behav Brain Res 156:153–162. [DOI] [PubMed] [Google Scholar]
- Duarte-Guterman P, Yagi S, Chow C, Galea LA (2015) Hippocampal learning, memory, and neurogenesis: Effects of sex and estrogens across the lifespan in adults. Horm Behav 74:37–52. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, Beyer C, Wilkinson ST, Ostroff RB, Qayyum Z, Bloch MH (2017) Ketamine as a treatment for adolescent depression: a case report. J Am Acad Child Adolesc Psychiatry 56:352–354. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Currer JM, Harrison DE. (2007) The mouse in aging research. In: The mouse in biomedical research. 2nd ed (Foster H ed.), pp637–672. Burlington, MA: Elsevier. [Google Scholar]
- Galecki P, Gałecka E, Maes M, Chamielec M, Orzechowska A, Bobińska K, Lewiński A, Szemraj J (2012) The expression of genes encoding for COX-2, MPO, iNOS, and sPLA2-IIA in patients with recurrent depressive disorder. J Affect Disord 138:360–366. [DOI] [PubMed] [Google Scholar]
- Garcia-Carachure I, Flores-Ramirez FJ, Castillo SA, Themann A, Arenivar MA, Preciado-Piña J, Zavala AR, Lobo MK, Iñiguez SD (2020) Enduring effects of adolescent ketamine exposure on cocaine- and sucrose-induced reward in male and female C57BL/6 mice. Neuropsychopharmacology 45:1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D, Gálvez V, Martin D, Kumar D, Leyden J, Hadzi-Pavlovic D, Harper S, Brodaty H, Glue P, Taylor R, Mitchell PB, Loo CK (2017) Pilot randomized controlled trial of titrated subcutaneous ketamine in older patients with treatment-resistant depression. Am J Geriatr Psychiatry 25:1199–1209. [DOI] [PubMed] [Google Scholar]
- Green SM, Krauss B (2004) Clinical practice guideline for emergency department ketamine dissociative sedation in children. Ann Emerg Med 44:460–471. [DOI] [PubMed] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM (2015) Sexually divergent expression of active and passive conditioned fear responses in rats. Elife 4:e11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH (2012) Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 37:137–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard E, Sohail Y, Nagar A, Glass OM, Hermida AP (2017) Ketamine in late life treatment-resistant depression. Am J Geriat Psychiatry 25:S66. [Google Scholar]
- Iachininoto MG, Nuzzolo ER, Bonanno G, Mariotti A, Procoli A, Locatelli F, De Cristofaro R, Rutella S (2013) Cyclooxygenase-2 (COX-2) inhibition constrains indoleamine 2,3-dioxygenase 1 (IDO1) activity in acute myeloid leukaemia cells. Molecules 18:10132–10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AL, File SE (1991) Sex differences in animal tests of anxiety. Physiol Behav 49:245–250. [DOI] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM (2015) How does ketamine elicit a rapid antidepressant response? Curr Opin Pharmacol 20:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005) Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze EJ, Wetherell JL (2011) A lifespan view of anxiety disorders. Dialogues Clin Neurosci 13:381–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Rabin S, Heller RE, Lukenbaugh D, Geraci M, Pine DS, Grillon C (2010) Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am J Psychiatry 167:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JH, Wang SY, Yu HY, Li DY, Luo SC, Zheng SS, Wan LF, Duan KM (2019) Prophylactic use of ketamine reduces postpartum depression in Chinese women undergoing cesarean section. Psychiatry Res 279:252–258. [DOI] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Ruyter MD, Kubera M, Bosmans E (2007) The immune effects of TRYCATs (tryptophan catabolites along the IDO pathway): relevance for depression - and other conditions characterized by tryptophan depletion induced by inflammation. Neuro Endocrinol Lett 28:826–831. [PubMed] [Google Scholar]
- Maneglia R, Cousin MT (1988) A comparison between propofol and ketamine for anaesthesia in the elderly. Haemodynamic effects during induction and maintenance. Anaesthesia 43 Suppl:109–111. [DOI] [PubMed] [Google Scholar]
- Martini L, Melcangi RC (1991) Androgen metabolism in the brain. J Steroid Biochem Mol Biol 39:819–828. [DOI] [PubMed] [Google Scholar]
- Mastrodonato A, Martinez R, Pavlova IP, LaGamma CT, Brachman RA, Robison AJ, Denny CA (2018) Ventral CA3 activation mediates prophylactic ketamine efficacy against stress-induced depressive-like behavior. Biol Psychiatry 84:846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrodonato A, Cohensedgh O, LaGamma CT, McGowan JC, Hunsberger HC, Denny CA (2020) Prophylactic (R,S)-ketamine selectively protects against inflammatory stressors. Behav Brain Res 378:112238. [DOI] [PubMed] [Google Scholar]
- McGhee LL, Maani CV, Garza TH, Gaylord KM, Black IH (2008) The correlation between ketamine and posttraumatic stress disorder in burned service members. J Trauma 64:S195–8; Discussion S197. [DOI] [PubMed] [Google Scholar]
- McGowan JC, LaGamma CT, Lim SC, Tsitsiklis M, Neria Y, Brachman RA, Denny CA (2017) Prophylactic ketamine attenuates learned fear. Neuropsychopharmacology 42:1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan JC, Hill C, Mastrodonato A, LaGamma CT, Kitayev A, Brachman RA, Narain NR, Kiebish MA, Denny CA (2018) Prophylactic ketamine alters nucleotide and neurotransmitter metabolism in brain and plasma following stress. Neuropsychopharmacology 43:1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros da Frota Ribeiro C, Riva-Posse P (2017) Use of ketamine in elderly patients with treatment-resistant depression. Curr Psychiatry Rep 19:107. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV (2013) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DL, Moffitt TE, Caspi A, Magdol L, Silva PA, Stanton WR (1996) Psychiatric disorder in a birth cohort of young adults: prevalence, comorbidity, clinical significance, and new case incidence from ages 11 to 21. J Consult Clin Psychol 64:552–562. [PubMed] [Google Scholar]
- Papolos DF, Teicher MH, Faedda GL, Murphy P, Mattis S (2013) Clinical experience using intranasal ketamine in the treatment of pediatric bipolar disorder/fear of harm phenotype. J Affect Disord 147:431–436. [DOI] [PubMed] [Google Scholar]
- Parise EM, Parise LF, Sial OK, Cardona-Acosta AM, Gyles TM, Juarez B, Chaudhury D, Han MH, Nestler EJ, Bolaños-Guzmán CA (2021) The resilient phenotype induced by prophylactic ketamine exposure during adolescence is mediated by the ventral tegmental area-nucleus accumbens pathway. Biol Psychiatry 90:482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, Ruberry EJ, Powers A, Mehta N, Yang RR, Soliman F, Glatt CE, Casey BJ, Ninan I, Lee FS (2012) Altered fear learning across development in both mouse and human. Proc Natl Acad Sci U S A 109:16318–16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Liston C, Jing D, Ninan I, Yang RR, Witztum J, Murdock MH, Dincheva I, Bath KG, Casey BJ, Deisseroth K, Lee FS (2016) Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat Commun 7:11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennybaker S, Roach BJ, Fryer SL, Badathala A, Wallace AW, Mathalon DH, Marton TF (2021) Age affects temporal response, but not durability, to serial ketamine infusions for treatment refractory depression. Psychopharmacology 238:3229–3237. [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, Flood P, Simpson HB (2013) Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology 38:2475–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Kegeles LS, Levinson A, Ogden RT, Mao X, Milak MS, Vermes D, Xie S, Hunter L, Flood P, Moore H, Shungu DC, Simpson HB (2015) In vivo effects of ketamine on glutamate-glutamine and gamma-aminobutyric acid in obsessive-compulsive disorder: proof of concept. Psychiatry Res 233:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum SB, Gupta V, Palacios JL (2021) Ketamine. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. [PubMed] [Google Scholar]
- Sable JA, Jeste DV (2001) Anxiety disorders in older adults. Curr Psychiatry Rep 3:302–307. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Gangwar RS, Kumar A (2015) Safety and efficacy of ketamine infusion in late onset depression, and conversion to treatment response. Indian J Psychiatry 57:328–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge R, Young AH, Cleare AJ (2017) Biomarkers for depression: recent insights, current challenges and future prospects. Neuropsychiatr Dis Treat 13:1245–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymkowicz SM, Finnegan N, Dale RM (2014) Failed response to repeat intravenous ketamine infusions in geriatric patients with major depressive disorder. J Clin Psychopharmacol 34:285–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SL, Welch AC, Iourinets J, Dulawa SC (2020) Ketamine induces immediate and delayed alterations of OCD-like behavior. Psychopharmacology 237:627–638. [DOI] [PubMed] [Google Scholar]
- Verdonk F, Petit AC, Abdel-Ahad P, Vinckier F, Jouvion G, de Maricourt P, De Medeiros GF, Danckaert A, Van Steenwinckel J, Blatzer M, Maignan A, Langeron O, Sharshar T, Callebert J, Launay JM, Chrétien F, Gaillard R (2019) Microglial production of quinolinic acid as a target and a biomarker of the antidepressant effect of ketamine. Brain Behav Immun 81:361–373. [DOI] [PubMed] [Google Scholar]
- Willman EV, Andolfatto G (2007) A prospective evaluation of “ketofol” (ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med 49:23–30. [DOI] [PubMed] [Google Scholar]
- Wright CE, Strike PC, Brydon L, Steptoe A (2005) Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun 19:345–350. [DOI] [PubMed] [Google Scholar]
- Wu MV, Luna VM, Hen R (2015) Running rescues a fear-based contextual discrimination deficit in aged mice. Front Syst Neurosci 9:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA Jr, Gould TD (2018) Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev 70:621–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zarrinnegar P, Kothari J, Cheng K (2019) Successful use of ketamine for the treatment of psychotic depression in a teenager. J Child Adolesc Psychopharmacol 29:472–473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.