Abstract
Mouse double minute 2 (MDM2) is an E3 ubiquitin ligase which effectively degrades tumor suppressor p53. In the past two decades, many MDM2 inhibitors that disrupt the MDM2-p53 binding have been discovered and developed. Given that the MDM2-p53 forms auto-regulatory loop in which p53 is a substrate of MDM2 for targeted degradation, while MDM2 is a p53 target for transcriptional upregulation, these MDM2 inhibitors have limited efficacy due to p53 degradation by accumulated MDM2 upon rapid in vivo clearance of the MDM2 inhibitors. Fortunately, proteolysis targeting chimeras (PROTACs), a novel therapeutic strategy, overcome the limitations of MDM2 inhibitors. Some of MDM2 inhibitors developed in the past two decades have been used in PROTAC technology for two applications: 1) as component 1 to bind with endogenous MDM2 as a target for PROTAC-based degradation of MDM2; and 2) as component 2 to bind with endogenous MDM2 as a PROTAC E3 ligand for PROTAC-based degradation of other oncogenic proteins. In this review, we summarize current progress in the discovery and development of MDM2-based PROTAC drugs with future perspectives and challenges for their applications in effective treatment of human cancer.
Keywords: MDM2; E3 ligase ligand; PROTAC; Degradation; Human cancer, drug discovery
1. Introduction
The p53 tumor suppressor is a potent transcription factor that plays key roles in cancer prevention1, 2. Upon activation by a variety of stresses internally or externally, p53 induces growth arrest, if damage is repairable or apoptosis to eliminate unrepairable cells3, 4. The loss of p53 tumor-suppressor activity by point mutations or gene deletion, which is most frequently seen in many human cancers, escapes the p53 role as a genome guardian and allows abnormal proliferation of cells carrying damaged genome5. Such uncontrolled proliferation leads to cancer development. Thus, restoring p53 activity or function in cancer cells has been a long-term goal in the field of cancer drug discovery6.
MDM2 has been well characterized as a negative regulator of p53 by targeting p53 via direct binding7. A variety of stress signals disrupt the MDM2-p53 interaction, leading to p53 activation and subsequent cellular responses, such as growth arrest and apoptosis induction8, 9. MDM2 effective inactivation of p53 through three general mechanisms10–12: 1) MDM2 directly binds to the transcriptional activation domain of p53, thus inhibiting p53-mediated transcriptional activation; 2) MDM2 contains a nuclear export signal sequence, that induces p53 nuclear export upon binding to prevent p53 from binding to the target DNAs; 3) most effectively, MDM2 is an E3 ubiquitin ligase that promotes ubiquitylation and degradation of p53. Thus, the disruption of the MDM2-p53 interaction had become an effective strategy in the past two decades for the discovery and development of potent MDM2 inhibitors that disrupt the MDM2-p53 binding, leading to stabilization and restoration of p53 function for the treatment of human cancers, harboring a wild-type p5313–16.
In the past two decades, PROTAC strategies have gained momentum and shown promise in the discovery and development of new types of small-molecule therapeutics by inducing targeted protein degradation17–22. A PROTAC molecule consists of three components23–27: 1) a small molecule that specifically binds to targeted proteins; 2) another small molecule or peptide that binds to an E3 ligase as an E3 ligand; and 3) a chemical linker the connects the first two components. To date, PROTAC technology has been used to target various proteins, including transcription factors, skeleton proteins, nuclear receptors, enzymes, and regulatory proteins28–37. In the cancer therapy, many studies have shown that degrading a protein is more effective than inhibiting it38–41.
In this review, we will introduce a battery of MDM2 inhibitors, and then describe how some of these inhibitors are used to build-up several MDM2-recruiting PROTAC degraders (Table 1) for 1) the disruptors of the MDM2-p53 binding to stabilize p53, and 2) acting as E3 ligand component of PROTAC for degradation of other targeted oncogenic proteins (Figure 1).
Table 1.
The summary of MDM2 inhibitors and degraders
| No. | Name | Structure | Category | E3 ligase ligand | Target protein |
|---|---|---|---|---|---|
| 1 | Nutlin-3 |
|
Inhibitor | --a | -- |
| 2 | RG7388 |
|
Inhibitor | -- | -- |
| 3 | RG7112 |
|
Inhibitor | -- | -- |
| 4 | MI-77301 / SAR405838 |
|
Inhibitor | -- | -- |
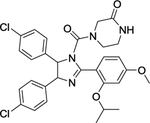
| 5 | HDM201 |
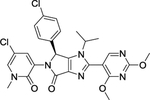
|
Inhibitor | -- | -- |
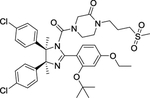
| 6 | DS-3032b |
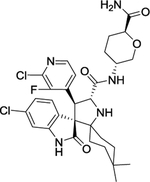
|
Inhibitor | -- | -- |
| 7 | APG-115 |
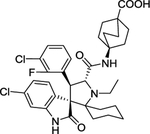
|
Inhibitor | -- | -- |
| 8 | MK-8242 |
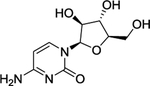
|
Inhibitor | -- | -- |
| 9 | NVP-CGM097 |
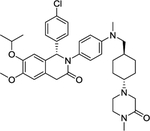
|
Inhibitor | -- | -- |
| 10 | AMG-232 |
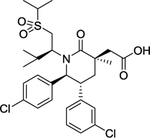
|
Inhibitor | -- | -- |
| 11 | WB214 |

|
Degrader | CRBN | MDM2 |
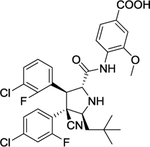
| 12 | TW-32 |
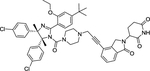
|
Degrader | CRBN | MDM2 |
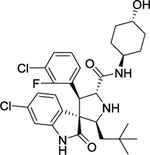
| 13 | MD-224 |
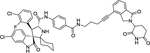
|
Degrader | CRBN | MDM2 |
| 14 | MG-277 |
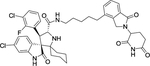
|
Degrader | CRBN | MDM2 |
| 15 | -- |
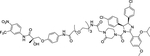
|
Degrader | Nutlin-3 | AR |
| 16 | A1874 |

|
Degrader | RG-7388 | BRD4 |
| 17 | -- |
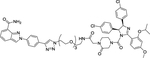
|
Degrader | Nutlin-3 | PARP1 |
| 18 | -- |
|
Degrader | Nutlin-3 | MDM2 |
| 19 | -- |
|
Degrader | RG-7388 | EGFR |
| 20 | -- |
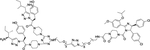
|
Degrader | Nutlin-3 | TRKC |
Not Applicable
Figure 1.
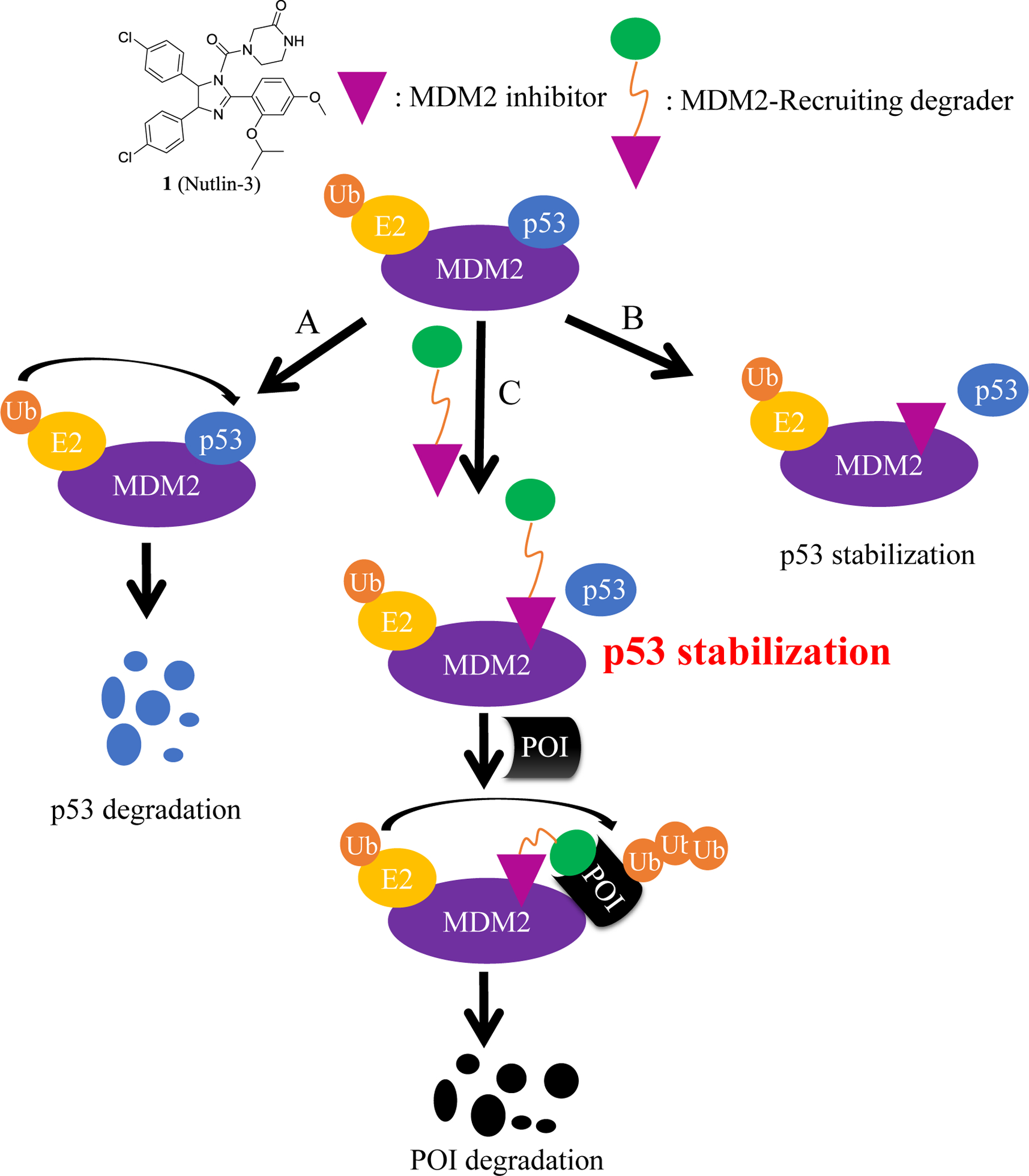
Working model of MDM2-recruiting PROTAC degraders. A) MDM2 promotes the degradation of p53 with overexpression of MDM2. B) MDM2 inhibitors block the protein-protein interaction of MDM2-p53. C) MDM2 degraders block the protein-protein interaction of MDM2-p53 and degrade the POI (protein of interest).
2. MDM2 inhibitors
The MDM2 E3 ubiquitin ligase was found overexpression in a number of human cancers, particularly in soft tissue sarcomas42–45. The main function of MDM2 is to ubiquitylate tumor suppressor p53 for proteasomal degradation, thus acting as an oncogenic protein to promote tumorigenesis46. Thus, targeting MDM2 via disrupting the MDM2-p53 binding was an effective approach for p53 activation and targeted anti-cancer therapy47. Toward this end, many small-molecule inhibitors targeting the p53-MDM2 protein-protein interactions have been discovered in past two decades with Nutlin-3 as the first one2, and few are currently in clinical development may be effective in the treatment of cancer and other related diseases, including RG7388 (NCT02407080)48, RG7112 (NCT01605526, NCT01143740, NCT01677780)49, 50, SAR405838 (NCT01636479, NCT01985191)5, 51, 52, HDM201 (NCT02143635, NCT02343172)53–55, DS-3032b (NCT01877382, NCT02579824, NCT02319369, NCT03634228)56, 57, APG-115 (NCT03781986, NCT02935907)58, 59, MK-8242 (NCT01451437, NCT01463696)60–62, NVP-CGM097 (NCT01760525)63–65, and AMG-232 (NCT03217266, NCT03107780, NCT03041688, NCT03031730)66–68 (Figure 2 and Table 1).
Figure 2.
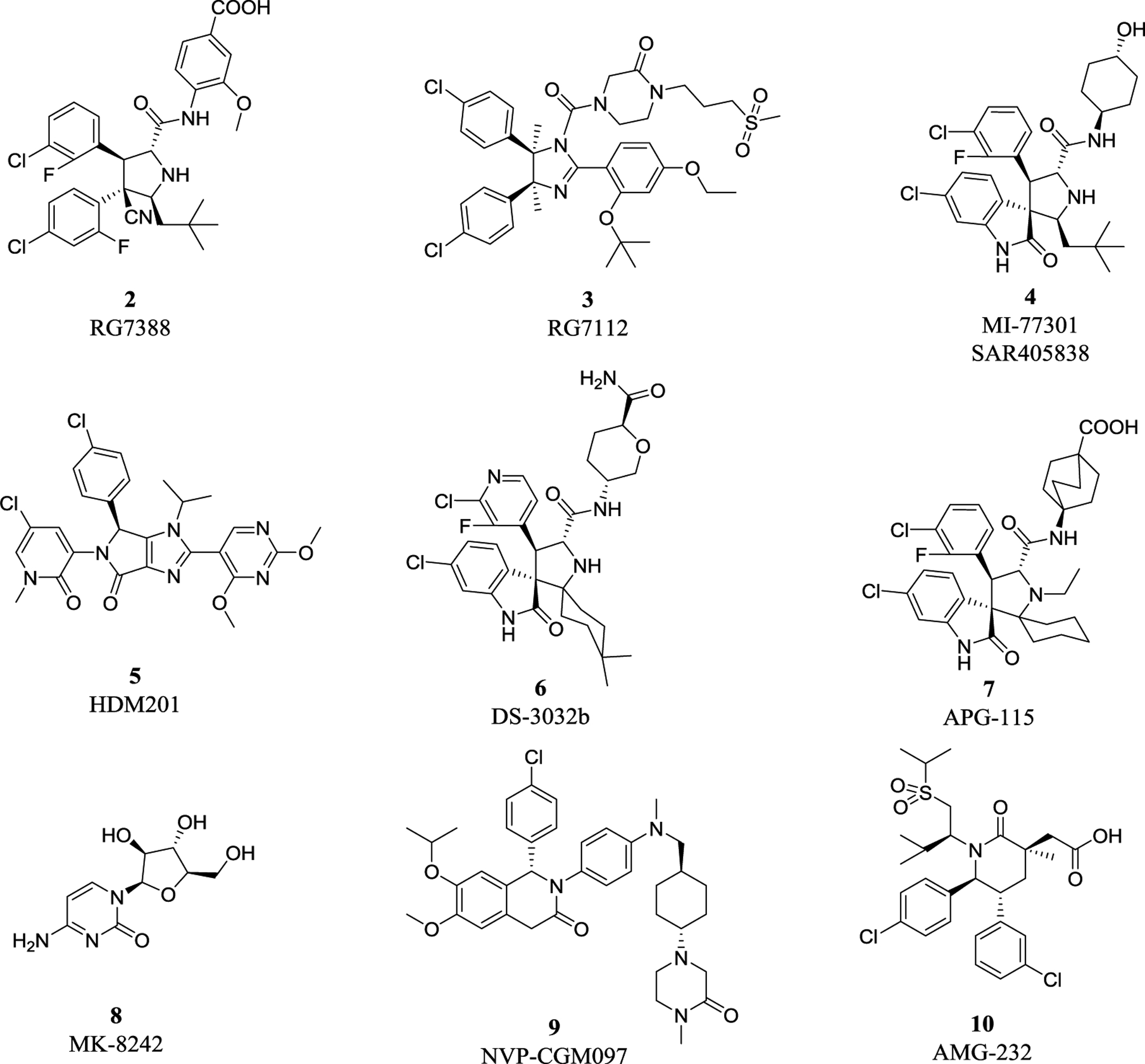
Representative MDM2-p53 inhibitors in clinical trials.
While p53 is a substrate of MDM2 for targeted degradation, MDM2 itself is a p53 target, subjected to p53 upregulation69. This auto-regulatory feedback loop produces a potential side-effect for MDM2 inhibitors, which disrupt the MDM2-p53 binding to free up p53, accumulated p53 then transactivates MDM2 to cause MDM2 accumulation, leading to p53 degradation when MDM2 inhibitors are rapidly cleared in vivo, thus compromising the therapeutic effect of MDM2 inhibitors. In fact, studies have shown that p53 protein accumulates in xenograft tumor tissue for only several hours after a single dose of MDM2 inhibitor is administered. Furthermore, the accumulation of MDM2 protein in normal tissues may have deleterious effects, because MDM2 is itself oncogenic5. To overcome these potential drawbacks of MDM2 inhibitors, new strategies are needed to target MDM2 more effectively.
3. PROTACs and MDM2-associated degraders
Targeted protein degradation (TPD) via the ubiquitin proteasome system has received substantial interest among medicinal chemists and biologists, and is an emerging direction in the field of drug development70–73. Toward this end, the molecules, designated as proteolysis-targeting chimeras (PROTACs), have been developed, which are bi-functional molecules that hijack cellular ubiquitin proteasome system to achieve the degradation of a disease-related target protein74–77. Structurally, PROTACs are new chimeric molecules, consisting of three components: one end binds to the targeted protein, also known as protein of interest (POI), and the other end is a ligand for recruitment of an E3 ubiquitin ligase, and a linker in between to connect both end (Figure 3)78, 79.
Figure 3.

Mode of action of PROTAC degraders.
After multiple rounds of recruiting ubiquitin to the target protein to form polyubiquitinated target, the PROTAC-target molecule is recognized by the 26S proteasome for target degradation80. PROTAC molecule is then recycled for next round of POI targeting. Thus, PROTACs target POIs through an “event-driven” mode rather than an “occupation-driven” mode for small-molecule drugs81, 82. The advantage of PROTAC molecules include the ability to overcome drug resistance and target undruggable targets with low toxicity, high efficiency and selectivity83, 84. It is worth-noting that drug resistance develops to varying degrees after a certain period of clinical use of almost all traditional small-molecule drugs. PROTACs effectively overcome this problem via degrading the target proteins. Furthermore, whereas most traditional small-molecule drugs work by binding to the active site of an enzyme or receptor, PROTACs work efficiently as long as there is effective binding with POI at essentially any site (Figure 3).
Human genome encodes more than 600 E3 ligases, but only a small fraction has been used in designing PROTACs to date, including Cereblon (CRBN), von Hippel-Lindau (VHL), MDM2, and cellular IAP1 (cIAP1)85–87. Two types of MDM2-related PROTAC degraders have been developed. One type is to recruit MDM2 inhibitor as MDM2 binding partner for MDM2 degradation. The second type is to recruit MDM2 as E3 ligand to target other POIs for degradation, although it is less effective than those recruiting CRBN and VHL.
4. PROTAC degraders targeting MDM2
The first type PROTACs, based on nutlin or idasanutlin (RG7388), have been developed with representative CRBN-based MDM2 degraders shown in Figure 4. However, the number of these MDM2 degraders is still limited, owing to their challenging physicochemical profiles and limited degradation activities (e.g. WB214 and TW-32)88. Recently, Wang group disclosed a series potent PROTAC MDM2 degraders, including MD-224 as a lead that targets MDM2 protein to CRBN for degradation (Figure 4)89. MD-224 is effectively in rapidly degrading MDM2 in leukemia cells. Intravenous administration of MD-224 was found to achieve complete and durable tumor regression in a RS4–11 xenograft model. Moreover, MD-224 inhibits the growth of only leukemia cells carrying wild-type p53 but not p53 mutants. On the basis of the previously reported PROTAC MDM2 degrader MD-224, Wang group have disclosed additional analogues. Interestingly and unexpectedly, MG-277, an analogues of MD-224, showed conversion of the PROTAC into a “molecular glue” (Figure 4)90. MG-277 induced only moderate MDM2 degradation and failed to activate wild-type p53. However, it shows highly potent inhibition of tumor cell growth in a p53-independent manner. This compound provides the first example demonstrating that a simple structural modification can turn a bona fide PROTAC degrader into a molecular glue compound90.
Figure 4.
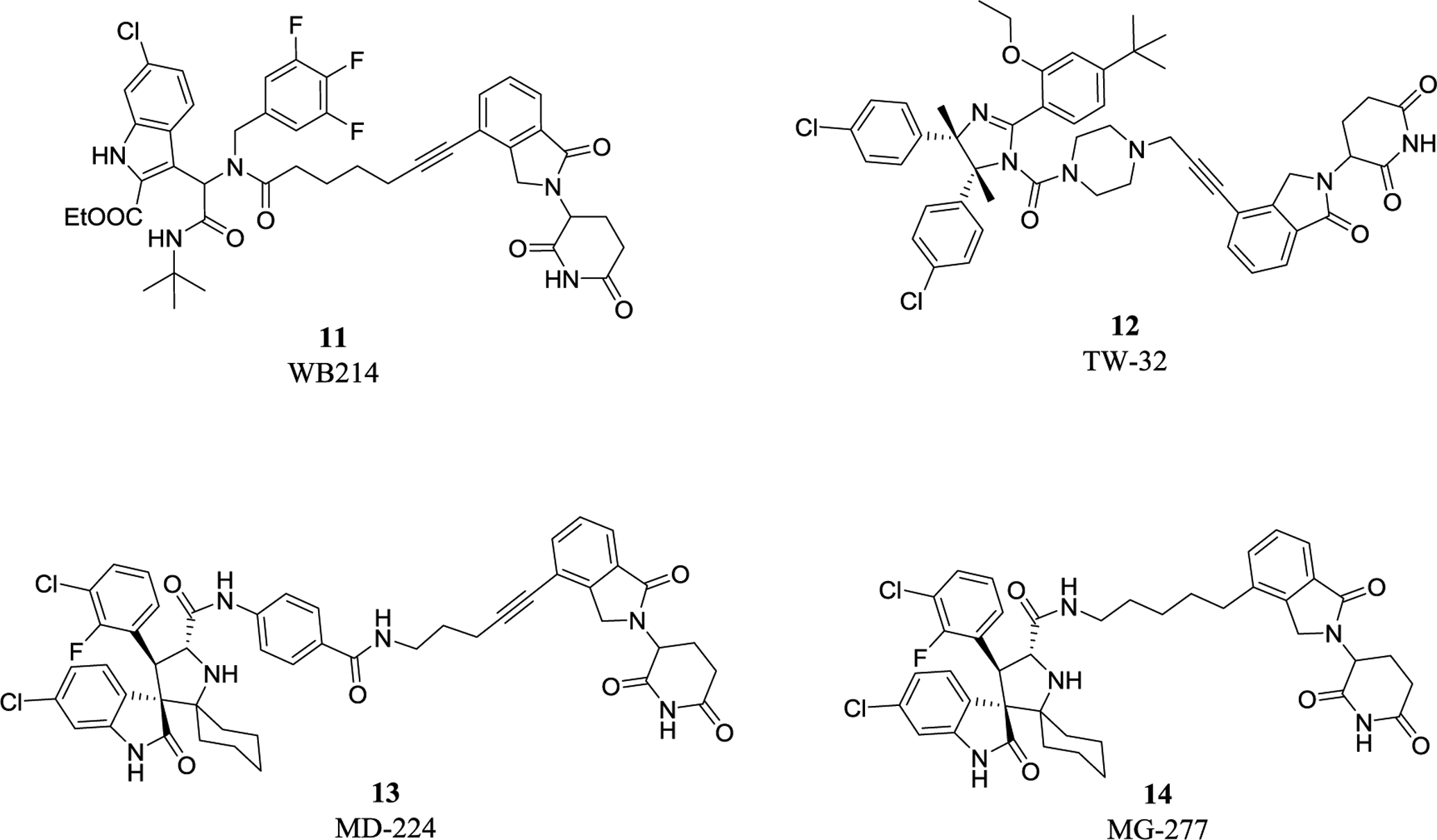
Representative CRBN-based MDM2 degraders and molecular glue.
5. PROTAC degraders recruiting MDM2 to target other POIs
5.1. MDM2-based PROTAC AR degrader
Nutlin-3 is a potent MDM2 inhibitor that bind to the p53-binding pocket of MDM291. Interestingly, Nutlin-3 were also used for the design of the second type PROTACs to recruit endogenous MDM2 for targeting androgen receptor (AR) by Crews group in 200892. This was the first report of an all-small-molecule PROTAC degrader, consisting of an AR antagonist and the nutlin motif, that disrupted the interaction of MDM2 with p53 without affecting the E3 ligase activity of MDM2. Specifically, this first synthetic all-small-molecule PROTAC AR degrader was a heterobifunctional compound consisting of a bicalutamide analogue (non-steroidal androgen receptor ligand) and MDM2 inhibitor joined by a PEG-based linker (Figure 5, compound 15). This cell permeable PROTAC successfully recruits the androgen receptor to MDM2 for ubiquitination and proteasomal degradation, but with weak potency92.
Figure 5.
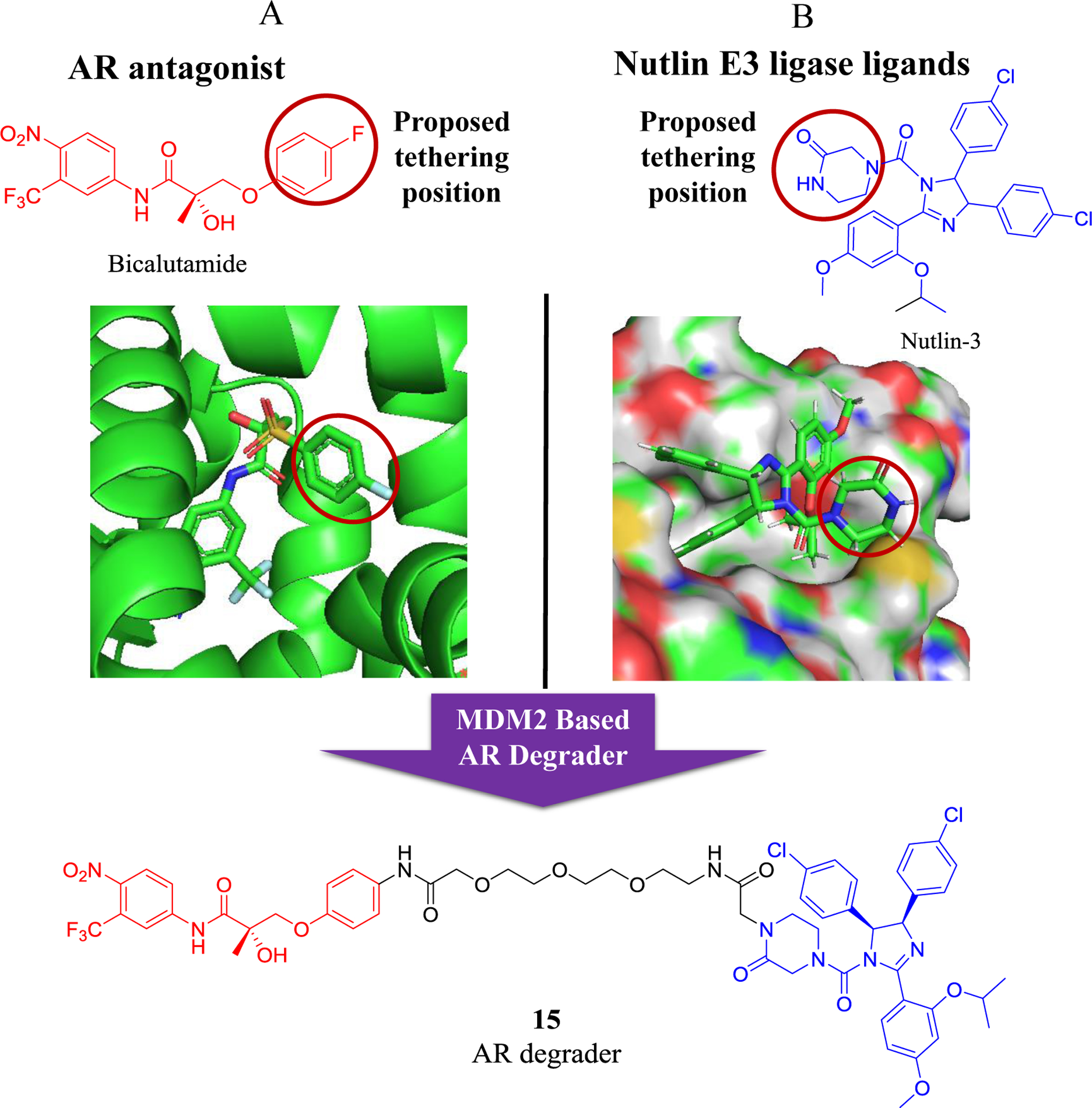
Schematic design of an MDM2 (nutlin-3)-based PROTAC AR degrader. A) Structure of the AR antagonist bicalutamide and its working model with androgen receptor (AR). B) Structure of the MDM2 inhibitor nutlin-3 and its crystal structure with MDM2.
RG7388, another typical MDM2-p53 inhibitor, was also found to bind to MDM2 and used in PROTACs design93. However, the poor physiochemical properties of nutlin-3 were exacerbated after incorporation into PROTACs. Recent efforts have identified MDM2-p53 PPI inhibitors with better solubility and activity may increase the applications of MDM2 in PROTACs (Figure 6)93–95.
Figure 6.
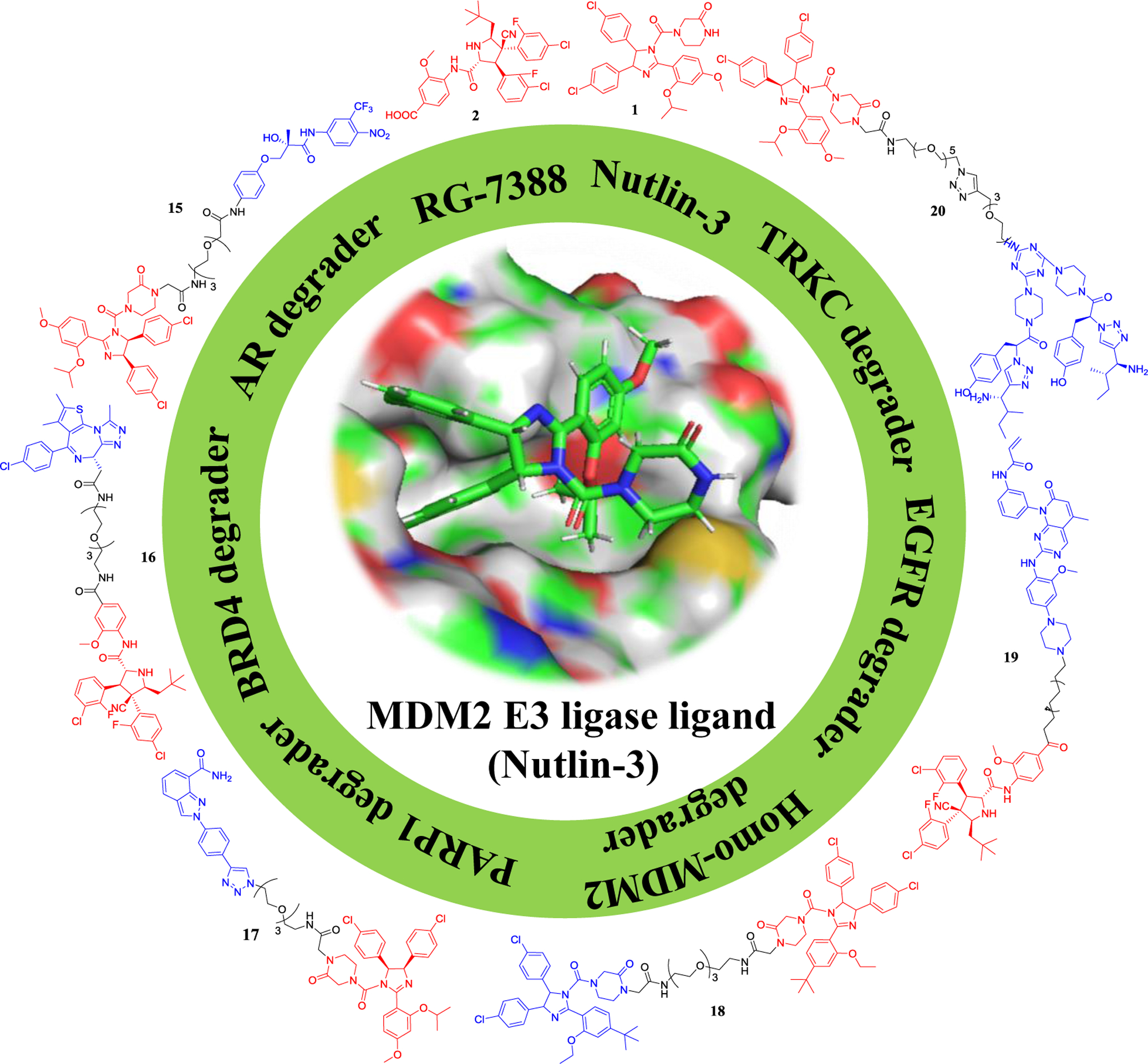
Representative MDM2 E3 ligase ligands and the corresponding PROTACs.
5.2. MDM2-based PROTAC BRD4 degrader
Recently, numerous BET PROTAC degraders have been generated and tested in vitro and in vivo96, 97. Various E3 ligases, including VHL, CRBN, IAP, MDM2, aryl hydrocarbon receptor (AHR), DDB1-cullin 4 associated factor 16 (DCAF16), RING finger protein 114 (RNF114), and RNF4, have been used as E3 ligands to degrade BET proteins98–105. In 2019, Crews group reported an MDM2/nutlin-based BRD4 PROTAC (compound 15, A1874)93, which not only degrades BRD4 protein but also stabilizes p53 gene, and thus exhibiting strong anti-proliferative effects in several tumor cell lines, such as myeloid leukemia cells (Figure 7). Moreover, it increases p53 levels in HCT116 colon cancer cells harboring a wild-type p53 due to the activity of RG7338 against MDM2. A1874 potently inhibits the proliferation of p53-wild-type cancer cells, presumably through dual inhibition of BRD4 and MDM2.
Figure 7.
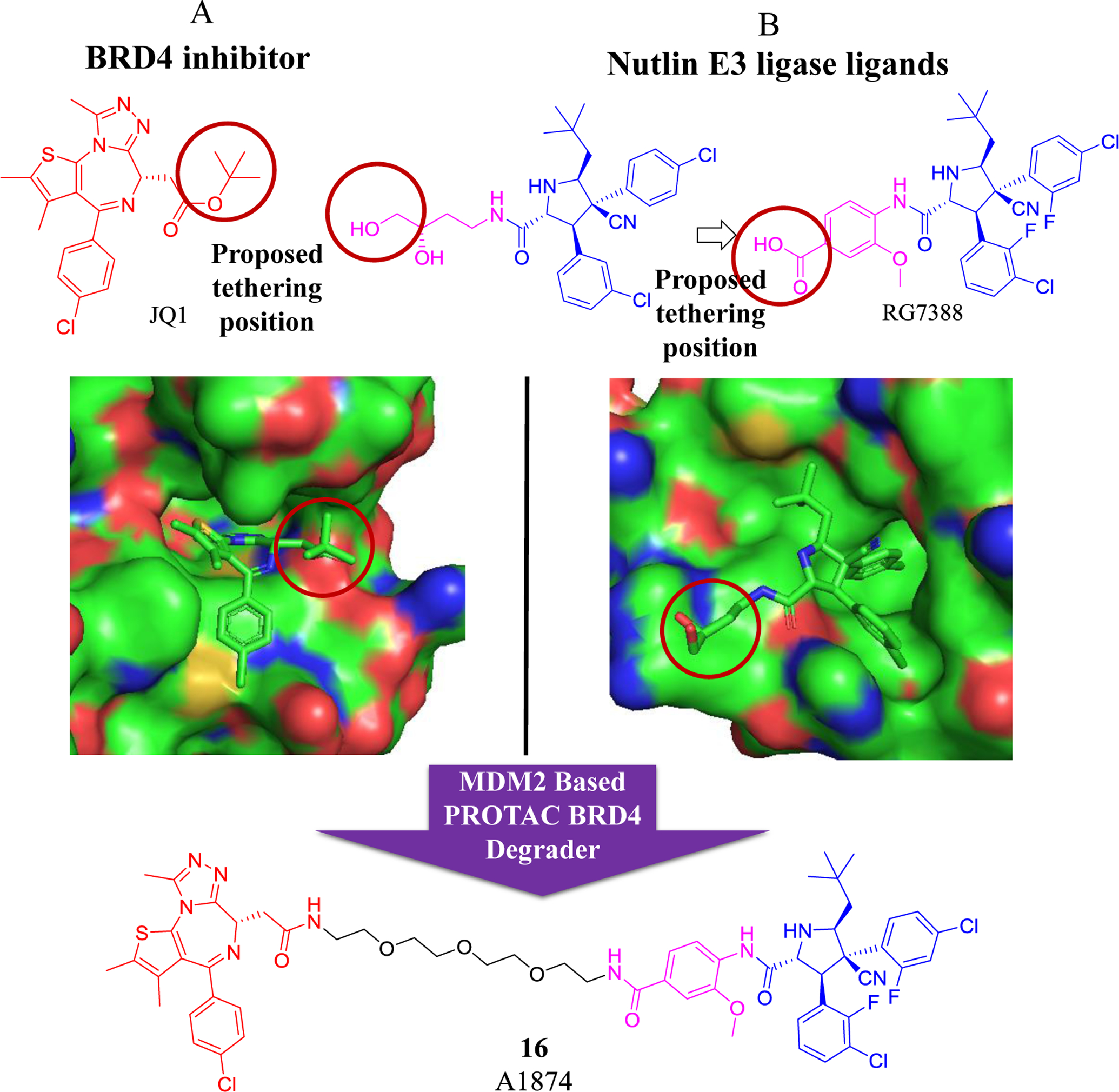
Schematic design of an MDM2 (nutlin-3)-based PROTAC BRD4 degrader. A) Structure of the BRD4/BET inhibitor JQ1 and its crystal structure with BRD4. B) Structure of the MDM2 inhibitor Idasanutlin (RG7388) and its crystal structure with MDM2.
5.3. MDM2-based PROTAC PARP1 degrader
The PARP1 poly(ADP-ribose) polymerase is a ubiquitously expressed DNA-dependent nuclear poly(ADP-ribosyl)transferase that regulates multiple nuclear events, such as transcription, rRNA biogenesis, and DNA repair106, 107. Owing to its essential role in the DNA-damage response, PARP1 is considered a potent cancer therapeutic target. Several PARP1 inhibitors, such as niraparib, iniparib and olaparib, are in various stages of clinical development; however, cytotoxicity and drug resistance are the primary problems that restrict their use in patients108, 109. Thus, additional therapeutic methods to overcome these obstacles are demanded.
In 2018, Rao group reported the first PARP1-targeting PROTAC by linking the PARP1 inhibitor niraparib and the MDM2 inhibitor nutlin-3. Compound 17 was obtained after detailed degradation screening in several triple-negative breast cancer cell lines (Figure 8)95. Impressively, the compound 17 selectively induced significant PARP1 degradation and cell apoptosis in MDA-MB-231 cells with fivefold more potent in antiproliferative activity than the PARP1 inhibitors niraparib, olaparib, and veliparib, while showing no cytotoxicity in normal cells.
Figure 8.
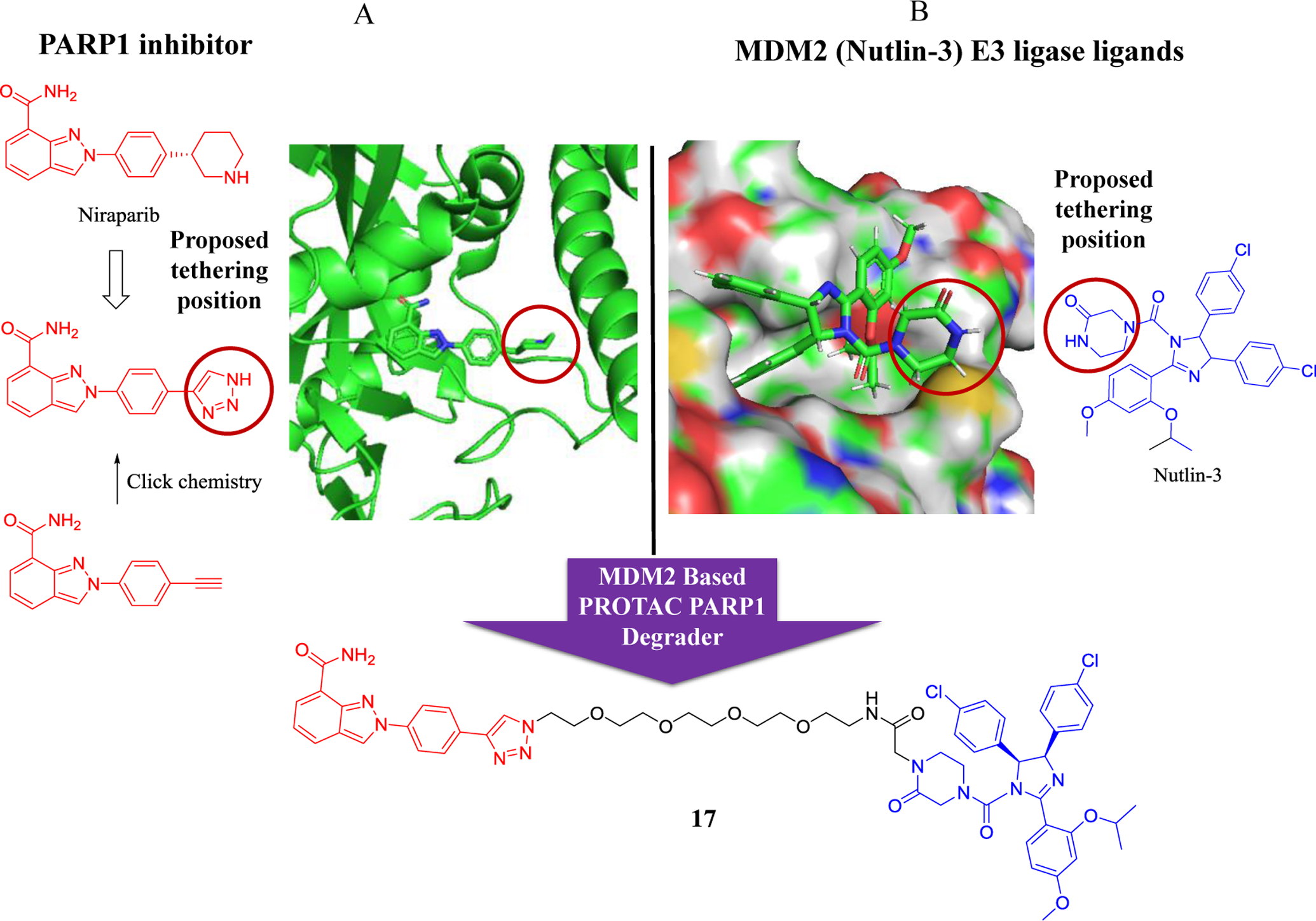
Schematic design of an MDM2 (nutlin-3a)-based PROTAC PARP1 degrader. A) Structure of the PARP1 inhibitors and the crystal structure of niraparib bound to the PARP1 catalytic domain (PDB code: 4R6E). A click chemistry strategy was used to construct the PROTAC candidates targeting PARP1. B) Structure of the MDM2 inhibitor nutlin-3a and its crystal structure with MDM2.
5.4. MDM2-based PROTAC homo-MDM2 degrader
Inspired by previous efforts to design small molecules targeting the MDM2-p53 interaction, the first homo-PROTAC, targeting MDM2 by inducing its self-degradation was recently reported by Sheng group (Figure 9)94. The compound 18 efficiently induced MDM2 dimerization with highly competitive binding activity, and induced proteasome-dependent self-degradation of MDM2 in A549 non-small cell lung cancer (NSCLC) cells. Impressively, compound 18 effectively inhibited the growth of tumor in a xenograft mouse model derived from A549 cells, the first demonstration of the in vivo efficacy of homo-PROTAC, which could be an alternative therapeutic tool for effective targeting human cancers with overexpressed MDM2.
Figure 9.
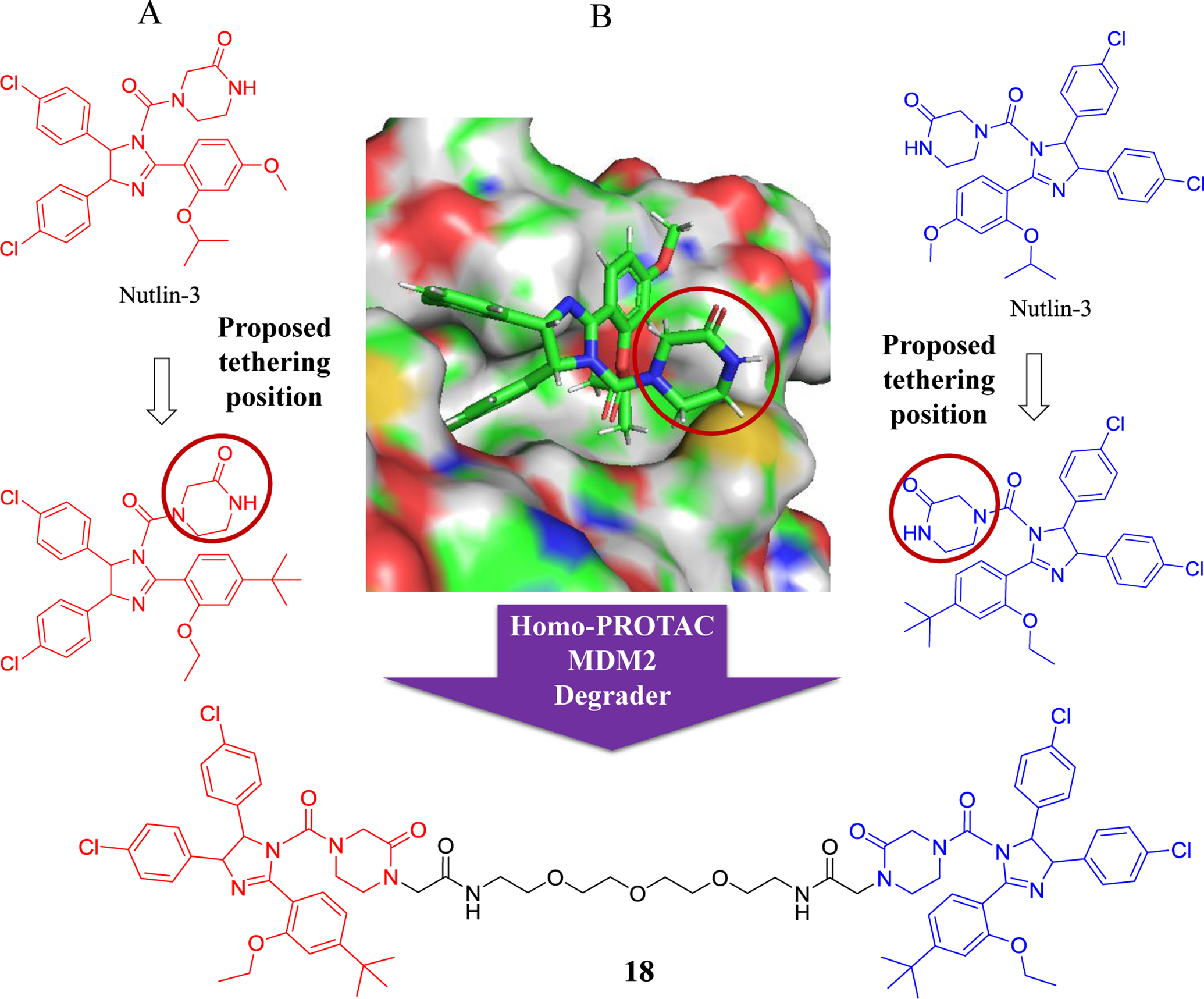
Homo-PROTAC design strategy: A) Chemical structures of nutlin-3. B) Binding model of compound nutlin-3 with MDM2 (PDB: 4IPF).
5.5. MDM2-based PROTAC EGFR degrader and TrkC degrader
In 2020, Ding group reported a series of PROTAC EGFR degraders based on different E3 ligase ligands, one of which was MDM2 inhibitor RG7388110. The compound 19 has moderate degradation on EGFRL858R/T790M mutant (Table 1). In 2019, Burgess group reported a class of TrkC-targeted kinase PROTACs by linking the TrkC-targeted kinase inhibitor IY−IY and the MDM2 ligand nutlin-3111. However, compound 20 has very weak potency in degrading TrkC protein (Table 1).
All 20 compounds described here are summarized in Table 1.
6. Summary and outlook
As we enter the third decade of TPD strategy, which was first reported in 2001112, PROTACs will remain at the forefront of research for targeted degradation of POIs, and have begun to move from vertical to horizontal development. A variety of developed TPD technologies have been discovered and developed, including photo‐controlled PROTACs, homo‐PROTACs, covalent PROTACs, dual-PROTACs, antibody‐PROTACs, lysosome‐targeting chimeras (LYTACs), autophagy‐targeting chimeras (AUTACs) and peptide-based PROTACs113–118. MDM2-based PROTACs selectively binds to the p53 site on the surface of MDM2 stabilizing p53 and degrading target protein with better anti-cancer activity. These PROTACs enable the degradation of a variety of undruggable proteins. However, the major bottleneck in the development of effective PROTAC drugs lies in achieving good oral bioavailability, given that PROTAC molecules are “beyond the rule of 5” for small molecule inhibitors due to their higher molecular weight (M.W.), poorer solubility (LogP), higher topological polar surface area (tPSA) and other poor physicochemical properties. Compounds with favorable physiochemical properties, such as lower M.W. (<800 Da), good water solubility, ideal tPSA, and fewer aromatic groups, must be discovered to increase oral bioavailability. The application of molecule glue could be an effective approach119, 120. The second challenge is to develop more effective E3 ligase, acting as the ligands for PROTACs, given human genome encodes more than 600 E3 ligase. Currently several oral-available PROTAC drugs are in few clinical trials for cancer treatment19, 21, 22, 121, 122. The knowledge gained from basic research and clinical trials in combination of technology development will certainly advance the field of cancer therapy using PROTAC strategy.
Acknowledgements
This work is supported in part by the National Key R&D Program of China (2021YFA1101000 to YS); Zhejiang Provincial Natural Science Foundation of China (LD22H300003 to YS) and US National Institutes of Health (NIH) grants to W.W. (R01CA177910).
Footnotes
Declaration of competing interests
The authors declare that they have no conflicts of interest in this work.
REFERENCES
- 1.Mohammad RM; Wu J; Azmi AS; Aboukameel A; Sosin A; Wu S; Yang D; Wang S; Al-Katib AM, An MDM2 antagonist (MI-319) restores p53 functions and increases the life span of orally treated follicular lymphoma bearing animals. Mol Cancer 2009, 8, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vassilev LT; Vu BT; Graves B; Carvajal D; Podlaski F; Filipovic Z; Kong N; Kammlott U; Lukacs C; Klein C; Fotouhi N; Liu EA, In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303 (5659), 844–848. [DOI] [PubMed] [Google Scholar]
- 3.Shangary S; Wang S, Targeting the MDM2-p53 interaction for cancer therapy. Clin Cancer Res 2008, 14 (17), 5318–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shangary S; Qin DG; McEachern D; Liu ML; Miller RS; Qiu S; Nikolovska-Coleska Z; Ding K; Wang GP; Chen JY; Bernard D; Zhang J; Lu YP; Gu QY; Shah RB; Pienta KJ; Ling XL; Kang SM; Guo M; Sun Y; Yang DJ; Wang SM, Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. P Natl Acad Sci USA 2008, 105 (10), 3933–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang SM; Sun W; Zhao YJ; McEachern D; Meaux I; Barriere C; Stuckey JA; Meagher JL; Bai LC; Liu L; Hoffman-Luca CG; Lu JF; Shangary S; Yu SH; Bernard D; Aguilar A; Dos-Santos O; Besret L; Guerif S; Pannier P; Gorge-Bernat D; Debussche L, SAR405838: An Optimized Inhibitor of MDM2-p53 Interaction That Induces Complete and Durable Tumor Regression. Cancer Res 2014, 74 (20), 5855–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chene P, Inhibiting the p53-MDM2 interaction: An important target for cancer therapy. Nature Reviews Cancer 2003, 3 (2), 102–109. [DOI] [PubMed] [Google Scholar]
- 7.Fang Y; Liao G; Yu B, Small-molecule MDM2/X inhibitors and PROTAC degraders for cancer therapy: advances and perspectives. Acta Pharm Sin B 2020, 10 (7), 1253–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumari S; Sharma V; Tiwari R; Maurya JP; Subudhi BB; Senapati D, Therapeutic potential of p53 reactivation in prostate cancer: Strategies and opportunities. Eur J Pharmacol 2022, 919, 174807. [DOI] [PubMed] [Google Scholar]
- 9.Nag S; Zhang X; Srivenugopal KS; Wang MH; Wang W; Zhang R, Targeting MDM2-p53 interaction for cancer therapy: are we there yet? Curr Med Chem 2014, 21 (5), 553–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrero AB; Rojas EA; Misiewicz-Krzeminska I; Krzeminski P; Gutierrez NC, Molecular Mechanisms of p53 Deregulation in Cancer: An Overview in Multiple Myeloma. Int J Mol Sci 2016, 17 (12), 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao CCK, Mechanisms of p53 degradation. Clin Chim Acta 2015, 438, 139–147. [DOI] [PubMed] [Google Scholar]
- 12.Hu J; Cao J; Topatana W; Juengpanich S; Li S; Zhang B; Shen J; Cai L; Cai X; Chen M, Targeting mutant p53 for cancer therapy: direct and indirect strategies. J Hematol Oncol 2021, 14 (1), 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rusiecki R; Witkowski J; Jaszczewska-Adamczak J, MDM2-p53 Interaction Inhibitors: The Current State-of-Art and Updated Patent Review (2010-Present). Recent Pat Anticancer Drug Discov 2019, 14 (4), 324–369. [DOI] [PubMed] [Google Scholar]
- 14.Burgess A; Chia KM; Haupt S; Thomas D; Haupt Y; Lim E, Clinical Overview of MDM2/X-Targeted Therapies. Front Oncol 2016, 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao YJ; Aguilar A; Bernard D; Wang SM, Small-Molecule Inhibitors of the MDM2-p53 Protein-Protein Interaction (MDM2 Inhibitors) in Clinical Trials for Cancer Treatment. J Med Chem 2015, 58 (3), 1038–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang SY; Lou JF; Li YF; Zhou FL; Yan ZQ; Lyu XL; Zhao YJ, Recent Progress and Clinical Development of Inhibitors that Block MDM4/p53 Protein-Protein Interactions. J Med Chem 2021, 64 (15), 10621–10640. [DOI] [PubMed] [Google Scholar]
- 17.Myung J; Kim KB; Crews CM, The ubiquitin-proteasome pathway and proteasome inhibitors. Med Res Rev 2001, 21 (4), 245–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z; Sun Y; Ni Z; Yang C; Tong Y; Liu Y; Li H; Rao Y, Merging PROTAC and molecular glue for degrading BTK and GSPT1 proteins concurrently. Cell Res 2021, 31 (12), 1315–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garber K, The PROTAC gold rush. Nat Biotechnol 2022, 40 (1), 12–16. [DOI] [PubMed] [Google Scholar]
- 20.He S; Dong G; Cheng J; Wu Y; Sheng C, Strategies for designing proteolysis targeting chimaeras (PROTACs). Med Res Rev 2022, 42(3), 1280–1342. [DOI] [PubMed] [Google Scholar]
- 21.Bekes M; Langley DR; Crews CM, PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov 2022, 21(3), 181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neklesa T; Snyder LB; Willard RR; Vitale N; Pizzano J; Gordon DA; Bookbinder M; Macaluso J; Dong HQ; Ferraro C; Wang G; Wang J; Crews CM; Houston J; Crew AP; Taylor I, ARV-110: An oral androgen receptor PROTAC degrader for prostate cancer. J Clin Oncol 2019, 37 (7). [Google Scholar]
- 23.Han X; Zhao L; Xiang W; Qin C; Miao B; McEachern D; Wang Y; Metwally H; Wang L; Matvekas A; Wen B; Sun D; Wang S, Strategies toward Discovery of Potent and Orally Bioavailable Proteolysis Targeting Chimera Degraders of Androgen Receptor for the Treatment of Prostate Cancer. J Med Chem 2021, 64 (17), 12831–12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiang WG; Zhao LJ; Han X; Qin C; Miao BKY; McEachern D; Wang Y; Metwally H; Kirchhoff PD; Wang L; Matvekas A; He M; Wen B; Sun DX; Wang SM, Discovery of ARD-2585 as an Exceptionally Potent and Orally Active PROTAC Degrader of Androgen Receptor for the Treatment of Advanced Prostate Cancer. J Med Chem 2021, 64 (18), 13487–13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pal P; Thummuri D; Lv D; Liu X; Zhang P; Hu W; Poddar SK; Hua N; Khan S; Yuan Y; Zhang X; Zhou D; Zheng G, Discovery of a Novel BCL-XL PROTAC Degrader with Enhanced BCL-2 Inhibition. J Med Chem 2021, 64 (19), 14230–14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu X; Xu J; Xie L; Wang L; Shen Y; Cahuzac KM; Chen X; Liu J; Parsons RE; Jin J, Design, Synthesis, and Evaluation of Potent, Selective, and Bioavailable AKT Kinase Degraders. J Med Chem 2021, 64 (24), 18054–18081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henning NJ; Manford AG; Spradlin JN; Brittain SM; Zhang E; McKenna JM; Tallarico JA; Schirle M; Rape M; Nomura DK, Discovery of a Covalent FEM1B Recruiter for Targeted Protein Degradation Applications. J Am Chem Soc 2022, 144 (2), 701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han X; Zhao LJ; Xiang WG; Qin C; Miao BKY; Xu TF; Wang M; Yang CY; Chinnaswamy K; Stuckey J; Wang SM, Discovery of Highly Potent and Efficient PROTAC Degraders of Androgen Receptor (AR) by Employing Weak Binding Affinity VHL E3 Ligase Ligands. J Med Chem 2019, 62 (24), 11218–11231. [DOI] [PubMed] [Google Scholar]
- 29.Zhao LJ; Han X; Lu JF; McEachern D; Wang SM, A highly potent PROTAC androgen receptor (AR) degrader ARD-61 effectively inhibits AR-positive breast cancer cell growth in vitro tumor growth in vivo. Neoplasia 2020, 22 (10), 522–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kregel S; Wang C; Han X; Xiao LB; Fernandez-Salas E; Bawa P; McCollum BL; Wilder-Romans K; Apel IJ; Cao XH; Speers C; Wang SM; Chinnaiyan AM, Androgen receptor degraders overcome common resistance mechanisms developed during prostate cancer treatment. Neoplasia 2020, 22(2), 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han X; Wang C; Qin C; Xiang WG; Fernandez-Salas E; Yang CY; Wang M; Zhao LJ; Xu TF; Chinnaswamy K; Delproposto J; Stuckey J; Wang SM, Discovery of ARD-69 as a Highly Potent Proteolysis Targeting Chimera (PROTAC) Degrader of Androgen Receptor (AR) for the Treatment of Prostate Cancer. J Med Chem 2019, 62 (2), 941–964. [DOI] [PubMed] [Google Scholar]
- 32.Salami J; Alabi S; Willard RR; Vitale NJ; Wang J; Dong HQ; Jin MZ; McDonnell DP; Crew AP; Neklesa TK; Crews CM, Androgen receptor degradation by the proteolysis-targeting chimera ARCC-4 outperforms enzalutamide in cellular models of prostate cancer drug resistance. Commun Biol 2018, 1, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y; Yang J; Aguilar A; McEachern D; Przybranowski S; Liu L; Yang CY; Wang M; Han X; Wang S, Discovery of MD-224 as a First-in-Class, Highly Potent, and Efficacious Proteolysis Targeting Chimera Murine Double Minute 2 Degrader Capable of Achieving Complete and Durable Tumor Regression. J Med Chem 2019, 62 (2), 448–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu JT; Hu B; Wang ML; Xu FM; Miao BY; Yang CY; Wang M; Liu ZM; Hayes DF; Chinnaswamy K; Delproposto J; Stuckey J; Wang SM, Discovery of ERD-308 as a Highly Potent Proteolysis Targeting Chimera (PROTAC) Degrader of Estrogen Receptor (ER). J Med Chem 2019, 62 (3), 1420–1442. [DOI] [PubMed] [Google Scholar]
- 35.Khan S; Zhang X; Lv D; Zhang Q; He Y; Zhang P; Liu X; Thummuri D; Yuan Y; Wiegand JS; Pei J; Zhang W; Sharma A; McCurdy CR; Kuruvilla VM; Baran N; Ferrando AA; Kim YM; Rogojina A; Houghton PJ; Huang G; Hromas R; Konopleva M; Zheng G; Zhou D, A selective BCL-XL PROTAC degrader achieves safe and potent antitumor activity. Nat Med 2019, 25 (12), 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou H; Bai L; Xu R; Zhao Y; Chen J; McEachern D; Chinnaswamy K; Wen B; Dai L; Kumar P; Yang CY; Liu Z; Wang M; Liu L; Meagher JL; Yi H; Sun D; Stuckey JA; Wang S, Structure-Based Discovery of SD-36 as a Potent, Selective, and Efficacious PROTAC Degrader of STAT3 Protein. J Med Chem 2019, 62 (24), 11280–11300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai L; Zhou H; Xu R; Zhao Y; Chinnaswamy K; McEachern D; Chen J; Yang CY; Liu Z; Wang M; Liu L; Jiang H; Wen B; Kumar P; Meagher JL; Sun D; Stuckey JA; Wang S, A Potent and Selective Small-Molecule Degrader of STAT3 Achieves Complete Tumor Regression In Vivo. Cancer Cell 2019, 36 (5), 498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou Y; Ma D; Wang Y, The PROTAC technology in drug development. Cell Biochem Funct 2019, 37 (1), 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raina K; Lu J; Qian Y; Altieri M; Gordon D; Rossi AM; Wang J; Chen X; Dong H; Siu K; Winkler JD; Crew AP; Crews CM; Coleman KG, PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proc Natl Acad Sci U S A 2016, 113 (26), 7124–7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang CY; Qin C; Bai L; Wang S, Small-molecule PROTAC degraders of the Bromodomain and Extra Terminal (BET) proteins - A review. Drug Discov Today Technol 2019, 31, 43–51. [DOI] [PubMed] [Google Scholar]
- 41.Schneekloth JS Jr.; Fonseca FN; Koldobskiy M; Mandal A; Deshaies R; Sakamoto K; Crews CM, Chemical genetic control of protein levels: selective in vivo targeted degradation. J Am Chem Soc 2004, 126 (12), 3748–3754. [DOI] [PubMed] [Google Scholar]
- 42.Yu S; Qin D; Shangary S; Chen J; Wang G; Ding K; McEachern D; Qiu S; Nikolovska-Coleska Z; Miller R; Kang S; Yang D; Wang S, Potent and orally active small-molecule inhibitors of the MDM2-p53 interaction. J Med Chem 2009, 52 (24), 7970–7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernard D; Zhao Y; Wang S, AM-8553: a novel MDM2 inhibitor with a promising outlook for potential clinical development. J Med Chem 2012, 55 (11), 4934–4935. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Y; Yu S; Sun W; Liu L; Lu J; McEachern D; Shargary S; Bernard D; Li X; Zhao T; Zou P; Sun D; Wang S, A potent small-molecule inhibitor of the MDM2-p53 interaction (MI-888) achieved complete and durable tumor regression in mice. J Med Chem 2013, 56 (13), 5553–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zafar A; Wang W; Liu G; Xian W; McKeon F; Zhou J; Zhang R, Targeting the p53-MDM2 pathway for neuroblastoma therapy: Rays of hope. Cancer Lett 2021, 496, 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W; Hu Y, Small molecule agents targeting the p53-MDM2 pathway for cancer therapy. Med Res Rev 2012, 32 (6), 1159–1196. [DOI] [PubMed] [Google Scholar]
- 47.Popowicz GM; Domling A; Holak TA, The structure-based design of Mdm2/Mdmx-p53 inhibitors gets serious. Angew Chem Int Ed Engl 2011, 50 (12), 2680–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding Q; Zhang Z; Liu JJ; Jiang N; Zhang J; Ross TM; Chu XJ; Bartkovitz D; Podlaski F; Janson C; Tovar C; Filipovic ZM; Higgins B; Glenn K; Packman K; Vassilev LT; Graves B, Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J Med Chem 2013, 56 (14), 5979–5983. [DOI] [PubMed] [Google Scholar]
- 49.Tovar C; Graves B; Packman K; Filipovic Z; Higgins B; Xia M; Tardell C; Garrido R; Lee E; Kolinsky K; To KH; Linn M; Podlaski F; Wovkulich P; Vu B; Vassilev LT, MDM2 small-molecule antagonist RG7112 activates p53 signaling and regresses human tumors in preclinical cancer models. Cancer Res 2013, 73 (8), 2587–2597. [DOI] [PubMed] [Google Scholar]
- 50.Vu B; Wovkulich P; Pizzolato G; Lovey A; Ding Q; Jiang N; Liu JJ; Zhao C; Glenn K; Wen Y; Tovar C; Packman K; Vassilev L; Graves B, Discovery of RG7112: A Small-Molecule MDM2 Inhibitor in Clinical Development. ACS Med Chem Lett 2013, 4 (5), 466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Jonge M; de Weger VA; Dickson MA; Langenberg M; Le Cesne A; Wagner AJ; Hsu K; Zheng W; Mace S; Tuffal G; Thomas K; Schellens JHM, A phase I study of SAR405838, a novel human double minute 2 (HDM2) antagonist, in patients with solid tumours. Eur J Cancer 2017, 76, 144–151. [DOI] [PubMed] [Google Scholar]
- 52.Jung J; Lee JS; Dickson MA; Schwartz GK; Le Cesne A; Varga A; Bahleda R; Wagner AJ; Choy E; de Jonge MJ; Light M; Rowley S; Mace S; Watters J, TP53 mutations emerge with HDM2 inhibitor SAR405838 treatment in de-differentiated liposarcoma. Nat Commun 2016, 7, 12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Konopleva M; Martinelli G; Daver N; Papayannidis C; Wei A; Higgins B; Ott M; Mascarenhas J; Andreeff M, MDM2 inhibition: an important step forward in cancer therapy. Leukemia 2020, 34 (11), 2858–2874. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y; Wang XH; Wang G; Yang YH; Yuan Y; Ouyang L The past, present and future of potential small-molecule drugs targeting p53-MDM2/MDMX for cancer therapy. Eur. J. Med. Chem, 2019, 176, 92–104. [DOI] [PubMed] [Google Scholar]
- 55.Stachyra-Valat T; Baysang F; D’Alessandro AC; Dirk E; Furet P; Guagnano V; Kallen J; Leder L; Mah R; Masuya K; Stutz S; Vaupel A; Hofmann F; Cherie P; Jeay S; Holzer P, NVP-HDM201: Biochemical and biophysical profile of a novel highly potent and selective PPI inhibitor of p53-Mdm2. Cancer Res 2016, 76, 14 Suppl (abstract 1225). [Google Scholar]
- 56.Gounder MM; Bauer TM; Schwartz GK; Masters T; Carvajal RD; Song S; Kumar P; Gajee R; Zernovak O; Rosen MM; Kochan JP; Chen SQ; Hyman DM; Gokmen S; Meric-Bernstam F; LoRusso P; Somaiah N; Weise AM; Hong DS, A phase 1 study of the MDM2 inhibitor DS-3032b in patients (pts) with advanced solid tumors and lymphomas. J Clin Oncol 2016, 34, 15 Suppl (abstract 2581). [Google Scholar]
- 57.DiNardo CD; Rosenthal J; Andreeff M; Zernovak O; Kumar P; Gajee R; Chen SQ; Rosen M; Song S; Kochan J; Limsakun T; Olin R, Phase 1 Dose Escalation Study of MDM2 Inhibitor DS-3032b in Patients with Hematological Malignancies - Preliminary Results. Blood 2016, 128 (22), 593. [Google Scholar]
- 58.Zhang L; Luo QY; Yan XL; Wan XP; Yuan LP; Zhang YX; Zhou SN; Pan WT; Pan MX; Qiu MZ; Mai SJ; Yang DJ, A novel small molecule inhibitor of MDM2-p53 (APG-115) has antitumor activity in gastric adenocarcinoma. Cancer Res 2019, 79 (13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yi HJ; Yan XL; Luo QY; Yuan LP; Li BX; Pan WT; Zhang L; Chen HB; Wang J; Zhang YB; Zhai YF; Qiu MZ; Yang DJ, A novel small molecule inhibitor of MDM2-p53 (APG-115) enhances radiosensitivity of gastric adenocarcinoma. J Exp Clin Canc Res 2018, 37 (1), 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kang MH; Reynolds CP; Kolb EA; Gorlick R; Carol H; Lock R; Keir ST; Maris JM; Wu J; Lyalin D; Kurmasheva RT; Houghton PJ; Smith MA, Initial Testing (Stage 1) of MK-8242-A Novel MDM2 Inhibitor-by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer 2016, 63 (10), 1744–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wagner AJ; Banerji U; Mahipal A; Somaiah N; Hirsch H; Fancourt C; Johnson-Levonas AO; Lam R; Meister AK; Russo G; Knox CD; Rose S; Hong DS, Phase I Trial of the Human Double Minute 2 Inhibitor MK-8242 in Patients With Advanced Solid Tumors. J Clin Oncol 2017, 35 (12), 1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ravandi F; Gojo I; Patnaik MM; Minden MD; Kantarjian H; Johnson Levonas AO; Fancourt C; Lam R; Jones MB; Knox CD; Rose S; Patel PS; Tibes R, A phase I trial of the human double minute 2 inhibitor (MK-8242) in patients with refractory/recurrent acute myelogenous leukemia (AML). Leuk Res 2016, 48, 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holzer P; Masuya K; Furet P; Kallen J; Valat-Stachyra T; Ferretti S; Berghausen J; Bouisset-Leonard M; Buschmann N; Pissot-Soldermann C; Rynn C; Ruetz S; Stutz S; Chene P; Jeay S; Gessier F, Discovery of a Dihydroisoquinolinone Derivative (NVP-CGM097): A Highly Potent and Selective MDM2 Inhibitor Undergoing Phase 1 Clinical Trials in p53wt Tumors. J Med Chem 2015, 58 (16), 6348–6358. [DOI] [PubMed] [Google Scholar]
- 64.Valat T; Masuya K; Baysang F; Albrecht G; Buschmann N; Erdmann D; Furet P; Gabriel T; Gessier F; Hofmann F; Holzer P; Kallen J; Pissot-Solderman C; Stutz S; Chene P; Jeay S, Mechanistic study of NVP-CGM097: a potent, selective and species specific inhibitor of p53-Mdm2. Cancer Res 2014, 74 (19). [Google Scholar]
- 65.Gessier F; Kallen J; Jacoby E; Chene P; Stachyra-Valat T; Ruetz S; Jeay S; Holzer P; Masuya K; Furet P, Discovery of dihydroisoquinolinone derivatives as novel inhibitors of the p53-MDM2 interaction with a distinct binding mode. Bioorg Med Chem Lett 2015, 25 (17), 3621–3625. [DOI] [PubMed] [Google Scholar]
- 66.Canon J; Osgood T; Olson SH; Saiki AY; Robertson R; Yu D; Eksterowicz J; Ye Q; Jin L; Chen A; Zhou J; Cordover D; Kaufman S; Kendall R; Oliner JD; Coxon A; Radinsky R, The MDM2 Inhibitor AMG 232 Demonstrates Robust Antitumor Efficacy and Potentiates the Activity of p53-Inducing Cytotoxic Agents. Mol Cancer Ther 2015, 14 (3), 649–658. [DOI] [PubMed] [Google Scholar]
- 67.Sun D; Li Z; Rew Y; Gribble M; Bartberger MD; Beck HP; Canon J; Chen A; Chen X; Chow D; Deignan J; Duquette J; Eksterowicz J; Fisher B; Fox BM; Fu J; Gonzalez AZ; Gonzalez-Lopez De Turiso F; Houze JB; Huang X; Jiang M; Jin L; Kayser F; Liu JJ; Lo MC; Long AM; Lucas B; McGee LR; McIntosh J; Mihalic J; Oliner JD; Osgood T; Peterson ML; Roveto P; Saiki AY; Shaffer P; Toteva M; Wang Y; Wang YC; Wortman S; Yakowec P; Yan X; Ye Q; Yu D; Yu M; Zhao X; Zhou J; Zhu J; Olson SH; Medina JC, Discovery of AMG 232, a potent, selective, and orally bioavailable MDM2-p53 inhibitor in clinical development. J Med Chem 2014, 57 (4), 1454–1472. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y; Zhu J; Liu JJ; Chen X; Mihalic J; Deignan J; Yu M; Sun D; Kayser F; McGee LR; Lo MC; Chen A; Zhou J; Ye Q; Huang X; Long AM; Yakowec P; Oliner JD; Olson SH; Medina JC, Optimization beyond AMG 232: discovery and SAR of sulfonamides on a piperidinone scaffold as potent inhibitors of the MDM2-p53 protein-protein interaction. Bioorg Med Chem Lett 2014, 24 (16), 3782–3785. [DOI] [PubMed] [Google Scholar]
- 69.Picksley SM; Lane DP, The p53-mdm2 autoregulatory feedback loop: a paradigm for the regulation of growth control by p53? Bioessays 1993, 15 (10), 689–690. [DOI] [PubMed] [Google Scholar]
- 70.Burslem GM; Crews CM, Small-Molecule Modulation of Protein Homeostasis. Chem Rev 2017, 117 (17), 11269–11301. [DOI] [PubMed] [Google Scholar]
- 71.Toure M; Crews CM, Small-Molecule PROTACS: New Approaches to Protein Degradation. Angew Chem Int Edit 2016, 55 (6), 1966–1973. [DOI] [PubMed] [Google Scholar]
- 72.Lai AC; Toure M; Hellerschmied D; Salami J; Jaime-Figueroa S; Ko E; Hines J; Crews CM, Modular PROTAC Design for the Degradation of Oncogenic BCR-ABL. Angew Chem Int Edit 2016, 55 (2), 807–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winter GE; Buckley DL; Paulk J; Roberts JM; Souza A; Dhe-Paganon S; Bradner JE, Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 2015, 348 (6241), 1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cromm PM; Crews CM, Targeted Protein Degradation: from Chemical Biology to Drug Discovery. Cell Chem Biol 2017, 24 (9), 1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lai AC; Crews CM, Induced protein degradation: an emerging drug discovery paradigm. Nat Rev Drug Discov 2017, 16 (2), 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kronke J; Fink EC; Hollenbach PW; MacBeth KJ; Hurst SN; Udeshi ND; Chamberlain PP; Mani DR; Man HW; Gandhi AK; Svinkina T; Schneider RK; McConkey M; Jaras M; Griffiths E; Wetzler M; Bullinger L; Cathers BE; Carr SA; Chopra R; Ebert BL, Lenalidomide induces ubiquitination and degradation of CK1alpha in del(5q) MDS. Nature 2015, 523 (7559), 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Inuzuka H, Liu J, Wei WY, Rezaeian AH. PROTAC technology for the treatment of Alzheimer’s disease: advances and perspectives. Acta Mater Medica. 2022, 1(1): 24–41. DOI: 10.15212/AMM-2021-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J; Chen H; Liu Y; Shen Y; Meng F; Kaniskan HU; Jin J; Wei W, Cancer Selective Target Degradation by Folate-Caged PROTACs. J Am Chem Soc 2021, 143 (19), 7380–7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Testa A; Lucas X; Castro GV; Chan KH; Wright JE; Runcie AC; Gadd MS; Harrison WTA; Ko EJ; Fletcher D; Ciulli A, 3-Fluoro-4-hydroxyprolines: Synthesis, Conformational Analysis, and Stereoselective Recognition by the VHL E3 Ubiquitin Ligase for Targeted Protein Degradation. J Am Chem Soc 2018, 140 (29), 9299–9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Slabicki M; Yoon H; Koeppel J; Nitsch L; Roy Burman SS; Di Genua C; Donovan KA; Sperling AS; Hunkeler M; Tsai JM; Sharma R; Guirguis A; Zou C; Chudasama P; Gasser JA; Miller PG; Scholl C; Frohling S; Nowak RP; Fischer ES; Ebert BL, Small-molecule-induced polymerization triggers degradation of BCL6. Nature 2020, 588 (7836), 164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gadd MS; Testa A; Lucas X; Chan KH; Chen WZ; Lamont DJ; Zengerle M; Ciulli A, Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat Chem Biol 2017, 13 (5), 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu F; Cai M; Shao L; Zhang JH, Targeting Protein Kinases Degradation by PROTACs. Front Chem 2021, 9, 679120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gabizon R; Shraga A; Gehrtz P; Livnah E; Shorer Y; Gurwicz N; Avram L; Unger T; Aharoni H; Albeck S; Brandis A; Shulman Z; Katz BZ; Herishanu Y; London N, Efficient Targeted Degradation via Reversible and Irreversible Covalent PROTACs. J Am Chem Soc 2020, 142 (27), 11734–11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li H, Wei WY, Xu HX. Drug discovery is an eternal challenge for the biomedical sciences. Acta Mater Medica. 2022, 1(1): 1–3. DOI: 10.15212/AMM-2022-1001 [DOI] [Google Scholar]
- 85.Bond MJ; Crews CM, Proteolysis targeting chimeras (PROTACs) come of age: entering the third decade of targeted protein degradation. RSC Chem Biol 2021, 2 (3), 725–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Groppe JC, Induced degradation of protein kinases by bifunctional small molecules: a next-generation strategy. Expert Opin Drug Discov 2019, 14 (12), 1237–1253. [DOI] [PubMed] [Google Scholar]
- 87.Sun X; Gao H; Yang Y; He M; Wu Y; Song Y; Tong Y; Rao Y, PROTACs: great opportunities for academia and industry. Signal Transduct Target Ther 2019, 4, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang B; Liu J; Tandon I; Wu S; Teng P; Liao J; Tang W, Development of MDM2 degraders based on ligands derived from Ugi reactions: Lessons and discoveries. Eur J Med Chem 2021, 219, 113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li YB; Yang JL; Aguilar A; McEachern D; Przybranowski S; Liu L; Yang CY; Wang M; Han X; Wang SM, Discovery of MD-224 as a First-in-Class, Highly Potent, and Efficacious Proteolysis Targeting Chimera Murine Double Minute 2 Degrader Capable of Achieving Complete and Durable Tumor Regression. J Med Chem 2019, 62 (2), 448–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang JL; Li YB; Aguilar A; Liu ZM; Yang CY; Wang SM, Simple Structural Modifications Converting a Bona fide MDM2 PROTAC Degrader into a Molecular Glue Molecule: A Cautionary Tale in the Design of PROTAC Degraders. J Med Chem 2019, 62 (21), 9471–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang B; Golding BT; Hardcastle IR, Small-molecule MDM2-p53 inhibitors: recent advances. Future Med Chem 2015, 7 (5), 631–645. [DOI] [PubMed] [Google Scholar]
- 92.Schneekloth AR; Pucheault M; Tae HS; Crews CM, Targeted intracellular protein degradation induced by a small molecule: En route to chemical proteomics. Bioorg Med Chem Lett 2008, 18 (22), 5904–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hines J; Lartigue S; Dong HQ; Qian YM; Crews CM, MDM2-Recruiting PROTAC Offers Superior, Synergistic Antiproliferative Activity via Simultaneous Degradation of BRD4 and Stabilization of p53. Cancer Res 2019, 79 (1), 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He S; Ma J; Fang Y; Liu Y; Wu S; Dong G; Wang W; Sheng C, Homo-PROTAC mediated suicide of MDM2 to treat non-small cell lung cancer. Acta Pharm Sin B 2021, 11 (6), 1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao Q; Lan T; Su S; Rao Y, Induction of apoptosis in MDA-MB-231 breast cancer cells by a PARP1-targeting PROTAC small molecule. Chem Commun (Camb) 2019, 55 (3), 369–372. [DOI] [PubMed] [Google Scholar]
- 96.Qin C; Hu Y; Zhou B; Fernandez-Salas E; Yang CY; Liu L; McEachern D; Przybranowski S; Wang M; Stuckey J; Meagher J; Bai LC; Chen Z; Lin M; Yang JL; Ziazadeh DN; Xu FM; Hu JT; Xiang WG; Huang LY; Li SW; Wen B; Sun DX; Wang SM, Discovery of QCA570 as an Exceptionally Potent and Efficacious Proteolysis Targeting Chimera (PROTAC) Degrader of the Bromodomain and Extra-Terminal (BET) Proteins Capable of Inducing Complete and Durable Tumor Regression. J Med Chem 2018, 61 (15), 6685–6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bai LC; Zhou B; Yang CY; Ji J; McEachern D; Przybranowski S; Jiang H; Hu JT; Xu FM; Zhao YJ; Liu L; Fernandez-Salas E; Xu J; Dou YL; Wen B; Sun DX; Meagher J; Stuckey J; Hayes DF; Li SQ; Ellis MJ; Wang SM, Targeted Degradation of BET Proteins in Triple-Negative Breast Cancer. Cancer Res 2017, 77 (9), 2476–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ward CC; Kleinman JI; Brittain SM; Lee PS; Chung CYS; Kim K; Petri Y; Thomas JR; Tallarico JA; McKenna JM; Schirle M; Nomura DK, Covalent Ligand Screening Uncovers a RNF4 E3 Ligase Recruiter for Targeted Protein Degradation Applications. ACS Chem Biol 2019, 14 (11), 2430–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spradlin JN; Hu XR; Ward CC; Brittain SM; Jones MD; Ou LS; To M; Proudfoot A; Ornelas E; Woldegiorgis M; Olzmann JA; Bussiere DE; Thomas JR; Tallarico JA; McKenna JM; Schirle M; Maimone TJ; Nomura DK, Harnessing the anti-cancer natural product nimbolide for targeted protein degradation. Nat Chem Biol 2019, 15 (7), 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang XY; Crowley VM; Wucherpfennig TG; Dix MM; Cravatt BF, Electrophilic PROTACs that degrade nuclear proteins by engaging DCAF16. Nat Chem Biol 2019, 15 (7), 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ohoka N; Tsuji G; Shoda T; Fujisato T; Kurihara M; Demizu Y; Naito M, Development of small molecule chimeras that recruit aryl hydrocarbon receptor (AhR) E3 ligase to induce degradation of target proteins. Mol Cancer Ther 2019, 18 (12). [DOI] [PubMed] [Google Scholar]
- 102.Bai L; Zhou B; Yang CY; Ji J; McEachern D; Przybranowski S; Jiang H; Hu J; Xu F; Zhao Y; Liu L; Fernandez-Salas E; Xu J; Dou Y; Wen B; Sun D; Meagher J; Stuckey J; Hayes DF; Li S; Ellis MJ; Wang S, Targeted Degradation of BET Proteins in Triple-Negative Breast Cancer. Cancer Res 2017, 77 (9), 2476–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zengerle M; Chan KH; Ciulli A, Selective Small Molecule Induced Degradation of the BET Bromodomain Protein BRD4. ACS Chem Biol 2015, 10 (8), 1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gadd MS; Testa A; Lucas X; Chan KH; Chen W; Lamont DJ; Zengerle M; Ciulli A, Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat Chem Biol 2017, 13 (5), 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chan KH; Zengerle M; Testa A; Ciulli A, Impact of Target Warhead and Linkage Vector on Inducing Protein Degradation: Comparison of Bromodomain and Extra-Terminal (BET) Degraders Derived from Triazolodiazepine (JQ1) and Tetrahydroquinoline (I-BET726) BET Inhibitor Scaffolds. J Med Chem 2018, 61 (2), 504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li F; Wu X; Fu X; Liu J; Song W; Xiao GG; Lu A; Zhang G, Poly (ADP-ribose) polymerase 1 (PARP1) inhibition promotes pulmonary metastasis of osteosarcoma by boosting ezrin phosphorylation. Int J Biol Sci 2022, 18 (3), 1238–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Challa S; Kraus WL, Two birds, one stone: Non-canonical therapeutic effects of the PARP inhibitor Talazoparib. Cell Chem Biol 2022, 29 (2), 171–173. [DOI] [PubMed] [Google Scholar]
- 108.Mekhaeil M; Dev KK; Conroy MJ, Existing Evidence for the Repurposing of PARP-1 Inhibitors in Rare Demyelinating Diseases. Cancers (Basel) 2022, 14 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jia Y; Wang M; Sang X; Liu P; Gao J; Jiang K; Cheng H, Phenethyl Isothiocyanate Enhances the Cytotoxic Effects of PARP Inhibitors in High-Grade Serous Ovarian Cancer Cells. Front Oncol 2021, 11, 812264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang X; Xu F; Tong L; Zhang T; Xie H; Lu X; Ren X; Ding K, Design and synthesis of selective degraders of EGFR(L858R/T790M) mutant. Eur J Med Chem 2020, 192, 112199. [DOI] [PubMed] [Google Scholar]
- 111.Zhao B; Burgess K, TrkC-Targeted Kinase Inhibitors And PROTACs. Mol Pharm 2019, 16 (10), 4313–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sakamoto KM; Kim KB; Kumagai A; Mercurio F; Crews CM; Deshaies RJ, Protacs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. P Natl Acad Sci USA 2001, 98 (15), 8554–8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zheng M; Huo J; Gu X; Wang Y; Wu C; Zhang Q; Wang W; Liu Y; Liu Y; Zhou X; Chen L; Zhou Y; Li H, Rational Design and Synthesis of Novel Dual PROTACs for Simultaneous Degradation of EGFR and PARP. J Med Chem 2021, 64 (11), 7839–7852. [DOI] [PubMed] [Google Scholar]
- 114.Guo WH; Qi X; Yu X; Liu Y; Chung CI; Bai F; Lin X; Lu D; Wang L; Chen J; Su LH; Nomie KJ; Li F; Wang MC; Shu X; Onuchic JN; Woyach JA; Wang ML; Wang J, Enhancing intracellular accumulation and target engagement of PROTACs with reversible covalent chemistry. Nat Commun 2020, 11 (1), 4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maneiro MA; Forte N; Shchepinova MM; Kounde CS; Chudasama V; Baker JR; Tate EW, Antibody-PROTAC Conjugates Enable HER2-Dependent Targeted Protein Degradation of BRD4. ACS Chem Biol 2020, 15 (6), 1306–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takahashi D; Moriyama J; Nakamura T; Miki E; Takahashi E; Sato A; Akaike T; Itto-Nakama K; Arimoto H, AUTACs: Cargo-Specific Degraders Using Selective Autophagy. Mol Cell 2019, 76 (5), 797–810. [DOI] [PubMed] [Google Scholar]
- 117.Banik SM; Pedram K; Wisnovsky S; Ahn G; Riley NM; Bertozzi CR, Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature 2020, 584 (7820), 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma B; Feng H; Feng C; Liu Y; Zhang H; Wang J; Wang W; He P; Niu F, Kill Two Birds with One Stone: A Multifunctional Dual-Targeting Protein Drug to Overcome Imatinib Resistance in Philadelphia Chromosome-Positive Leukemia. Adv Sci (Weinh) 2022, e2104850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nishiguchi G; Keramatnia F; Min J; Chang Y; Jonchere B; Das S; Actis M; Price J; Chepyala D; Young B; McGowan K; Slavish PJ; Mayasundari A; Jarusiewicz JA; Yang L; Li Y; Fu X; Garrett SH; Papizan JB; Kodali K; Peng J; Pruett Miller SM; Roussel MF; Mullighan C; Fischer M; Rankovic Z, Identification of Potent, Selective, and Orally Bioavailable Small-Molecule GSPT1/2 Degraders from a Focused Library of Cereblon Modulators. J Med Chem 2021, 64 (11), 7296–7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pei J; Wang G; Feng L; Zhang J; Jiang T; Sun Q; Ouyang L, Targeting Lysosomal Degradation Pathways: New Strategies and Techniques for Drug Discovery. J Med Chem 2021, 64 (7), 3493–3507. [DOI] [PubMed] [Google Scholar]
- 121.Halford B, Arvinas unveils PROTAC structures. Chem Eng News 2021, 99 (14), 5–5. [Google Scholar]
- 122.Mullard A, First targeted protein degrader hits the clinic. Nature Rev Drug Discovery 2019, 18 (4), 237–239. [DOI] [PubMed] [Google Scholar]


