Abstract
Background
Literature that addresses the role of Medical Science Liaison (MSL) is currently limited. To the best of our knowledge, this is the first survey in Spain that gathers opinions from professionals whose work is either in or related to the medical departments from pharmaceutical companies.
Methods
A survey delivered by the SurveyMonkey online platform was completed by 101 pharmaceutical industry professionals (medical advisor/manager, 31.7%; medical director, 26.7%; medical information, 12.9%; and MSL manager, 11.9%).
Results
The median score for the global impression of the MSL was 8.7. The collaboration goes beyond formation since 85.1% of the respondents believed that MSLs should actively collaborate in both clinical trials and investigator-initiated studies, they should have more involvement with market access (51.5%), and they play an important role in compliance (94.0%). There was a tendency toward granting MSLs more responsibilities in terms of budget (73.3%) and their participation in the elaboration of the Medical Plan (91.1%). This position was considered as a strategic partner both internally and externally (76.2%).
Conclusion
MSL is a well-known field-based profile with increasing importance and responsibilities. MSLs represent a strategic position as internal collaborators in the company, whose success relies on cross-functional work. The MSL’s profile is in constant development, currently facing challenges such as adapting to remote interactions, or providing a clear definition with the best metrics to measure their performance as a non-promotional position.
Keywords: Medical Science Liaison, Pharmaceutical industry, Survey, Opinion, Spain
Introduction
The Medical Science Liaison (MSL) is a specialized role within pharmaceutical, biotechnological, medical device, or diagnostic companies in the healthcare industry [1]. The MSL is a field-based role that gives full support to the needs of healthcare professionals, by employing the deep knowledge they possess about the diseases in question and the company’s products, as well as their competence in a determined therapeutic area [2]. It is important to highlight that this role is non-promotional; it is different and separate from commercial functions [3]. All interactions are characterized by being patient-centered, ethical, fair, and balanced in accordance with local and international regulations [3]. In recent years, the number of MSLs has considerably increased, establishing this position as a strategic role in the medical affairs department [1]. The MSL’s interactions with the rest of the company also offer added value. This role is entitled to chair advisory boards and manage continuous medical education during clinical development and even engage with advocacy groups in collaboration with drug safety [4]. To date, the published literature on the role of the MSL is scarce, and most of the information focuses on either insight provided by these professionals [2, 5, 6] or by managers of MSL programs [7], or it addresses the opinion of health care professionals (HCPs) [8, 9]. In this scenario, the MSL task force of the Association of Medicine of the Pharmaceutical Industry in Spain (AMIFE, Asociación de Medicina de la Industria Farmacéutica en España) developed the project “The MSL’s role in Spain.” This project aimed to explore the opinion of diverse stakeholders about the MSL position through four different surveys directed to the following: the MSLs themselves [10], professionals within or related to the medical department, marketing/sales department professionals, and HCPs [11]. This study focuses on understanding the value of the role of MSL from the perspective of the medical department, medical information, clinical research, market access, and pharmacovigilance.
Methods
Study Design
This survey was specifically developed by the MSL task force of AMIFE with the contribution of the authors. The questionnaire was delivered by the SurveyMonkey online platform, and it was distributed to participants through AMIFE, social networks (LinkedIn), and several companies, including ours. It consisted of 24 questions which can be grouped in diverse general topics (Table 1): respondent details (items 1–3: current position, size, and type of company); objective data regarding MSL role (items 4–6: antiquity of the role and coverage of therapeutic areas); subjective opinion toward the role (items 7–10: necessary number of MSLs, professional category, time to master the position, and need for continuous formation); internal collaboration within the company (items 11–15: internal formation, support in clinical development, remote interactions, market access and compliance); opinion about its strategic position (items 16–18: questions regarding budget and involvement in Medical Plans); functions and metrics (items 19–23: most valuable functions, desirable future competencies and daily reports); and, finally, a global rating of the role (item 24). The respondents were current employees from the pharmaceutical and biotechnological industry, working in diverse roles within or related to the medical department. Their positions could be either managerial or non-managerial roles.
Table 1.
Survey questions
| 1 | What position do you occupy in your department? |
| 2 | What is the size of your company in Spain? |
| 3 | What type of company do you work for? |
| 4 | For how long has the MSL position been established in your company? |
| 5 | Does your company have MSLs for all therapeutic areas (in Spain)? |
| 6 | What specialty do MSL work for in your company? |
| 7 | Does the number of MSLs working in your company seem adequate to you? |
| 8 | What professional category do you think an MSL should have? |
| 9 | What is the average time you consider necessary to master the MSL position? |
| 10 | Do you consider it necessary for MSLs to receive continuous training? |
| 11 | Do you consider it appropriate for MSLs to provide internal training for other company employees? |
| 12 | Do you think MSLs should actively collaborate in the development of clinical trials / investigator-initiated studies? |
| 13 | Do you find it positive that MSLs engage in remote interactions with healthcare professionals? |
| 14 | Do you consider that the MSL has a relevant role in Compliance? |
| 15 | Do you think MSLs and market access should collaborate? |
| 16 | Do you think MSLs should have their own budget? |
| 17 | Do you think the MSLs should be involved in the development of the Medical Plan? |
| 18 | Do you consider that the MSL has a strategic position in the company? |
| 19 | What do you think is the most important daily activity that an MSL performs? |
| 20 | What aspect do you value most about the MSL position? |
| 21 | Which competences do you think the MSL position should have in future? |
| 22 | Do you know if the MSLs make any reports (METRICS) of their daily activity? |
| 23 | What reporting system (METRICS) do you think best measures their work? |
| 24 | What is your overall MSL rating from 1 to 10? |
Quantitative Assessments
Most of the items had closed responses, in which the respondent had to select a response from among diverse options. Only two questions did not follow that pattern: question 21 had a multiple-choice format, and in the final question 24 (regarding the global rating of the MSL), the respondent was asked to provide a numeric score (from 1 to 10).
Statistical Analysis
A descriptive statistical analysis was carried out for all variables. Categorical variables were described using absolute and relative frequencies, while continuous variables used the mean and standard deviation (SD). Comparisons of variables were made using the chi-square test or the student's t test for independent data/analysis of the variance (ANOVA) procedure, as appropriate. For all comparisons, a two-sided level of statistical significance of 0.05 was considered. All tables were analyzed for the total of participants and according to their type of position (managerial / non-managerial positions). The statistical analysis followed the principles specified in the ICH E9 guidelines as well as the rules of good clinical practice. All statistical analyses were carried out using SAS 9.4 software.
Results
Overall
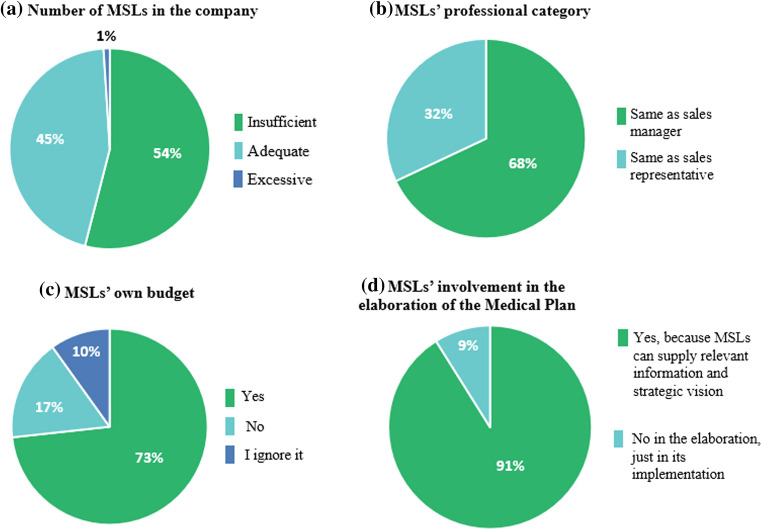
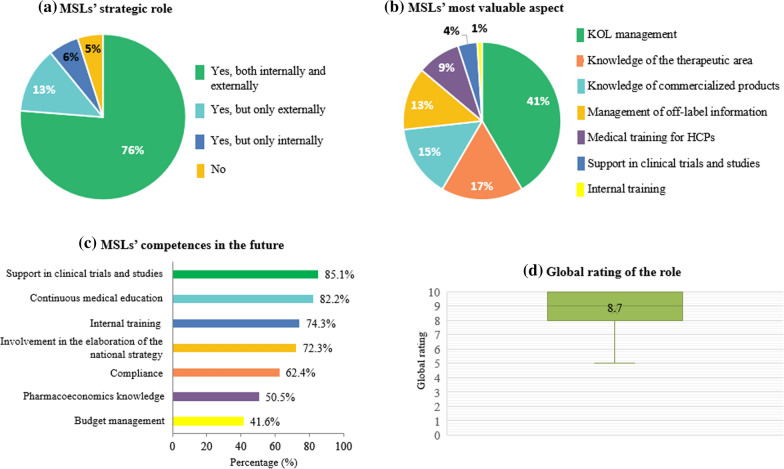
The survey was completed by a total of 101 professionals belonging to or related to the medical department (medical advisor/manager, 31.7%; medical director, 26.7%; medical information, 12.9%; and MSL manager, 11.9%; clinical operations, 4.0%; clinical research associate, 4.0%; clinical trials project manager, 3.0%; and pharmacovigilance, 2.0%; Table 2). There was also a small representation of market access (4.0%). In general, the size of the company was large (> 250 employees), as 59.4% of the respondents work mainly in pharmaceutical or biotechnological companies (76.2% and 8.9%, respectively; Table 2). Regarding the antiquity of the MSL role in the company, there was a distribution from 0 to 1 year (4.0%) to up to more than 10 years (21.0%); however, in 13.0% of the cases, the role of the MSL had not been established (Table 2). This latter situation included consultancy firms, contract research organizations (CROs), a medical device company, and companies categorized in “others.” All biotechnological companies had MSLs and only two pharmaceutical companies did not have this position in their staff. The majority of respondents’ companies (39.6%) had this professional role in every therapeutic area (Table 2). When participants were asked for the most relevant specialty in which these professionals work, the response was oncology, with more MSLs, followed by central nervous system/neurology, cardiovascular, and hematology specialties (Table 2). Moreover, 54.0% of the respondents thought that the number of MSLs in their company was insufficient, while 45.0% responded it was adequate and 1.0% considered it was excessive (Fig. 1a). The participants believed that the professional category of the MSL should be similar to a sales manager (68.0%, Fig. 1b), and the roles that most supported this opinion were medical directors (74.1%), medical advisors (68.8%), and MSL leads (75.0%) (Table 5). Around 65% of the participants considered that 2–3 years was needed to master the position (Table 3). Therefore, on the training items, there was a consensus that continuous formation is necessary (99.0%; Table 3). This role was also thought to have the competence to provide internal training by 90.1% (Table 3). MSLs were considered as important collaborators for both clinical trials and investigator-initiated studies (85.1%; Table 3). The survey also evidenced that the virtual interaction with HCPs is contemplated as positive by the vast majority of the respondents (94.1%; Table 4). Almost all participants indicated that the role of the MSL is relevant in compliance issues (94.0%; Table 4). A total of 51.5% pointed out that MSLs should collaborate more with market access, while for 33.7% of the participants, the interaction was enough (Table 4). In agreement with this data, 73.3% of the participants thought that MSLs should have their own budget (Fig. 1c). Furthermore, up to 91.1% stated that this role can supply relevant information and provide a strategic vision of the different regions, so respondents stated that they should be involved both in the elaboration and implementation of the Medical Plan (Fig. 1d). When asked if the MSL is a strategic position, 76.2% agreed to the statement, both at an internal and external levels, while 12.9% thought this was only true in an external context and 6% thought this was only in the internal context (Fig. 2a). This strategic value is mostly supported among medical advisors, MSL leads and medical directors who believed MSLs should have their own budget (68.8%, 91.7%, and 85.2%, respectively), and they should collaborate in the elaboration of the Medical Plan (87.5%, 100.0%, and 96.3%) and that the MSL is a strategic role (90.6%, 91.7% and 70.4%; Table 5). Face-to-face discussions were considered the most relevant activity (85.1%; Table 3). In this item, only the most relevant option could be selected from among the various options. When asked about the most valuable aspects of the position, with the possibility of selecting multiple answers, key opinion leader (KOL) management was primarily chosen (41.6%), followed by knowledge of the therapeutic area/commercialized products and management of off-label information 16.8%/14.9%, and 12.9%, respectively; Fig. 2b). Regarding future competences that MSLs should have, clinical trials/studies support and continuous medical education to HCPs were the most chosen options by those in the survey (Fig. 2c). When asked about how MSL performance is measured, up to 22.8% ignored the metrics (Table 4). A total of 44.6% stated that the reports are quantitative (N-metrics) and qualitative (Q-metrics), while their general opinion is that Q-metrics should be used (72.3%; Table 4). It is important to highlight that MSLs represent an added value, with a global rating of 8.7 (SD, 1.3; Fig. 2d).
Table 2.
Distribution of the participating professionals by department, size, and type of company; MSLs’ role antiquity; and distribution among therapeutic areas
| Respondent’s current position in the department, n (%) | |
| Clinical operations | 4 (4.0) |
| Clinical research associate (CRA) | 4 (4.0) |
| Clinical trials project manager | 3 (3.0) |
| Medical director | 27 (26.7) |
| Medical information | 13 (12.9) |
| Market access (field) | 2 (2.0) |
| Market access (office) | 2 (2.0) |
| Medical advisor/manager | 32 (31.7) |
| MSL lead/head/manager/coordinator | 12 (11.9) |
| Pharmacovigilance technician | 2 (2.0) |
| Size of the company in Spain, n (%) | |
| Small (11–50 employees) | 7 (6.9) |
| Medium (51–250 employees) | 34 (33.7) |
| Large (> 250 employees) | 60 (59.4) |
| Type of company, n (%) | |
| Biotechnological company | 9 (8.9) |
| Consulting | 2 (2.0) |
| CRO (Contract MSL Organization) | 4 (4.0) |
| Pharmaceutical company | 77 (76.2) |
| Medical Devices | 4 (4.0) |
| Others* | 5 (5.0) |
| Antiquity of MSL role, n (%) ** | |
| 0–1 year | 4 (4.0) |
| 2–4 years | 21 (21.0) |
| 5–7 years | 28 (28.0) |
| 8–10 years | 13 (13.0) |
| > 10 years | 21 (21.0) |
| No MSL in the company | 13 (13.0) |
| MSLs’ distribution among therapeutic areas, n (%) | |
| No MSLs in none of them | 16 (15.8) |
| Only in some of them | 20 (19.8) |
| In most of them | 25 (24.8) |
| In all of them | 40 (39.6) |
| Specialty MSLs work for in the company, n (%) *** | |
| Oncology | 26 (26.8) |
| SNC / Neurology / Neurosciences | 11 (11.3) |
| Cardiovascular / Thrombosis | 6 (6.2) |
| Respiratory | 6 (6.2) |
| Hematology | 5 (5.2) |
| Rare / orphan diseases | 4 (4.1) |
| Gastroenterology | 4 (4.1) |
| Arthritis / Musculoskeletal / Rheumatology | 3 (3.1) |
| Dermatology | 3 (3.1) |
| Immunology | 2 (2.1) |
| Autoimmune diseases | 2 (2.1) |
| Infectious diseases | 2 (2.1) |
| Addictions | 1 (1.0) |
| Allergy | 1 (1.0) |
| Medical devices / Diagnosis | 1 (1.0) |
| Genetic diseases | 1 (1.0) |
| Ophthalmology | 1 (1.0) |
| Women’s health | 1 (1.0) |
| Mental health | 1 (1.0) |
| Urology | 1 (1.0) |
| Others | 15 (15.5) |
*Pharmaceutical company with healthcare, life science and performance materials business; diagnostic imaging; distributor of sanitary technology; cooperative group; advertising and marketing: each 1 (20,0%)
Data missing from: **one medical director, ***four participants
Fig. 1.
Participants’ opinion about the adequacy of the number of MSLs, their professional category, budget disposal, and participation in the Medical Plan development
Table 5.
Comparison of opinion between managerial and non-managerial roles
| Total (n = 101) |
Managerial role (n = 57) |
Non-managerial role (n = 44) |
p value | |
|---|---|---|---|---|
| MSLs involved in the elaboration of the Medical Plan, n (%) | ||||
| Yes, because MSLs can supply relevant information and a strategic vision from different regions | 92 (91.1) | 49 (86.0) | 43 (97.7) | 0.0397 |
| No, MSLs should be in charge of the territorial implementation of Medical Plan, without participating in the elaboration | 9 (8.9) | 8 (14.0) | 1 (2.3) | |
| MSLs should have their own budget, n (%) | ||||
| Yes | 74 (73.3) | 38 (66.7) | 36 (81.8) | 0.2332 |
| No | 17 (16.8) | 12 (21.1) | 5 (11.4) | |
| I ignore it | 10 (9.9) | 7 (12.3) | 3 (6.8) | |
| Professional category MSL should have, n (%) * | ||||
| Same as a sales representative | 32 (32.0) | 20 (35.7) | 12 (27.3) | 0.3690 |
| Same as a sales manager | 68 (68.0) | 36 (64.3) | 32 (72.7) | |
| Average time necessary to master the MSL position, n (%) * | ||||
| 1 year | 25 (25.0) | 15 (26.8) | 10 (22.7) | 0.1189 |
| 2 years | 37 (37.0) | 22 (39.3) | 15 (34.1) | |
| 3 years | 28 (28.0) | 11 (19.6) | 17 (38.6) | |
| 4 years | 10 (10.0) | 8 (14.3) | 2 (4.5) | |
*One data missing from management role
Table 3.
Participants’ opinion about MSLs’ activity
| Average necessary time to master the MSL position, n (%)* | |
| 1 year | 25 (25.0) |
| 2 years | 37 (37.0) |
| 3 years | 28 (28.0) |
| 4 years | 10 (10.0) |
| MSLs’ necessity to receive continuous training, n (%) | |
| Agreement | 100 (99.0) |
| Disagreement | 1 (1.0) |
| Appropriateness for MSLs to provide internal training for other company employees, n (%) | |
| Agreement | 91 (90.1) |
| Disagreement, it is more adequate that other members of the company provide this training | 7 (6.9) |
| Disagreement, it is more adequate that external staff provide this training | 3 (3.0) |
| MSL involvement in the development of clinical trials/investigator-initiated studies, n (%) | |
| Only in clinical trials | 2 (2.0) |
| Only in investigator-initiated studies | 9 (8.9) |
| In both | 86 (85.1) |
| In none | 4 (4.0) |
| Most important daily activity of the MSL, n (%) | |
| Support to trials and studies | 5 (5.0) |
| Face-to-face discussions | 86 (85.1) |
| Clinical sessions | 10 (9.9) |
Data missing from: *one participant
Table 4.
Participants’ opinion about MSLs and remote interactions, compliance, market access, and metrics
| Total (n = 101) | |
|---|---|
| Engagement of MSLs in remote interactions with HCPs, n (%) | |
| Agreement | 95 (94.1) |
| Disagreement | 6 (5.9) |
| Role relevance of MSLs in compliance, n (%)* | |
| Relevant | 94 (93.1) |
| Non-relevant | 6 (5.9) |
| Collaboration between MSLs and market access, n (%) | |
| MSLs should have more involvement with market access | 52 (51.5) |
| MSLs and market access already collaborate enough | 34 (33.7) |
| I don’t know | 15 (14.9) |
| MSLs’ reports (METRICS) of their daily activity, n (%) | |
| Qualitative only (Q-metrics) | 4 (4.0) |
| Quantitative only (N-metrics) | 26 (25.7) |
| Both reports | 45 (44.6) |
| No report | 3 (3.0) |
| I ignore it | 23 (22.8) |
| The reporting system (METRICS) that best measures MSLs’ work, n (%) | |
| Qualitative (Q-metrics) | 73 (72.3) |
| Quantitative (N-metrics) | 28 (27.7) |
*Data missing from one participant
Fig. 2.
Participants’ opinion about present and future MSL competencies, their strategic position, and overall MSL rating
Managerial Roles Versus Non-managerial Roles
Results were stratified according to the managerial role of the participants. All respondents held the MSL position in high regard, especially those in managerial positions. The trend was constant through various opinion items due to the statistically significant differences (Table 5). Most in managerial roles (97.7%) agreed that MSLs should be included in the elaboration of Medical Plans, while this number decreased to 86.0% when those in non-managerial positions were asked (p = 0.040). This tendency was also supported by the fact that 81,8% of managerial profiles support MSLs having their own budget vs. 66.7% who shared the same opinion in the non-managerial group (p = 0.233). Also, when asked about the professional category of the role, 72.7% of participants in charge of staff considered that MSLs should have the same category as the sales managers (vs. 64.3% of non-managerial participants) (p = 0.369).
Discussion
As part of four surveys depicting the opinion of important stakeholders in the pharmaceutical and healthcare sector, our study provides evidence of the knowledge of medical/clinical departments and market access regarding the MSL position. Overall, the role is positively considered across the healthcare industry, and it is felt that MSLs should assume a step forward in responsibility, especially taking into account that, with the passing of time, their competencies are increasingly strategic instead of just being the “executor” of national Medical Plans [10].
This survey was conducted prior to the COVID-19 pandemic, in a context where scientific exchange was already carried out through diverse platforms including face-to-face visits, teleconference, telephone, e-mail, and so on. Nevertheless, according to a survey, we conducted previously [10] with 179 participants who, at that moment, were working as MSLs, 58% of them spent 61–80% of their time out in the field. In this scenario, where face-to-face visits were common, 65% of the contestants stated that they already used remote tools for interactions between MSLs and KOLs. However, 13% of them believed they were not satisfactory, mostly because of technical problems and KOLs lack of familiarity with new technologies. The pandemic has radically altered the scientific interactions, as shown by the MSL Society, which developed an online survey focused on knowing the preferences of KOLs to engage with MSLs during the epidemic in USA [12]. Prior to the COVID-19 situation, 31% and 22% of KOLs would hold one and two in-person meetings per month, respectively. However, these visits became teleconferences during the pandemic as predominantly reported by 31 and 12% of KOLs, respectively.
In our survey, we have shown that 85.1% of the participants consider that the most important daily activity of MSLs is face-to-face scientific exchange. Indeed, this responsibility is fundamental to KOLs, as shown by our previous survey [11]. Overall, 79.2% of HCPs considered that the utility of individual meetings with MSLs was either high or moderate (41.7% and 37.5%, respectively). Additionally, the MSLs were highly appreciated due to their credibility and the added value they provided [11]. Moss et al. conducted a survey with 116 KOLs on the role of the MSL in the diabetes area [8]. KOLs perceived that the MSLs are important since they can engage in intellectual conversations, provide networking opportunities, are aware of emerging products, and are reliable when providing information about unsolicited questions (unbiased product comparisons, address safety issues, and provide value at every meeting) [8]. In a previous survey we conducted, it was identified that updated knowledge about commercialized products (mostly safety, efficacy, and off-label use) and information about investigational products were the main points exchanged with HCPs [10]. MSLs can be an added value not only to KOLs but also within the company by providing effective internal collaboration. For instance, these professionals can support the clinical research department, giving advice on choosing sites or researchers, boosting the investigator’s interest to participate in a trial, or supporting the initial visits, etc. [13]. Moreover, MSLs play a key role in supplying medical information, providing answers to unsolicited requests, or developing approved medical responses to frequently asked question [14]. Overall, MSLs are the scientific reference of the company.
Our series of surveys allowed a comparison to be made about the point of view of MSLs vs. their professional colleagues regarding the metrics employed [10]. In total, 56% of the MSLs reported disagreement with the current performance metrics (29% used only N-metrics, 5% only Q-metrics, and 59% employed a combination of both), with a clear majority (90%) considering that the best method to measure their performance is Q-metrics [10]. It is important to highlight that the survey forced a choice between Q-metrics or N-metrics, without the possibility of selecting a mixed model. This time, when participants of the current survey were asked about the MSL’s metrics, 25.7% believed that the reports are only N-metrics, 4% only Q-metrics, and 44.6% both of them, but 22.8% did not know the report process. When respondents were asked about the best report system, 72.3% chose Q-metrics, so overall, in both surveys, there is an agreement toward measuring the performance by Q-metrics, which is in line with the non-promotional character of the MSL role. In concordance with this, we have recently reported a consensus on the optimum manner to measure the performance over different metrics from the point of view of 28 experts from 19 different companies.
In the present survey, the relationship between market access and MSLs was especially evaluated. A total of 33.7% of the participants believed that this internal collaboration is already enough, while 14.9% ignored the answer. The shift toward evidence-based medicine and value-based pricing means that Market Access and Reimbursement players must deepen their knowledge on patient outcome data and clinical evidence that supports the product [15]. Therefore, internal collaboration is essential since MSLs can provide responses to unsolicited queries along the pre-launch journey, or manage scientific content with regard to price and assist in reimbursement. Nevertheless, the meeting agenda must set out the discussion of scientific topics, not promotional/business issues led by commercial colleagues [16].
While scientific dissemination and interactions with stakeholders remain relevant, emerging responsibilities are gaining importance [16]. Our results show that managerial positions are in favor of MSLs acquiring more responsibilities. There is an agreed opinion on the participation of MSLs in the elaboration of Medical Plans and that MSLs should have their own budget. It is believed that it takes a long time to master the role, and that with the responsibilities should come an upgrade to a superior professional category, above the current one. This trend is in accordance with the tendency seen in preceding years in the industry, as already reported by MSLs in our previous data [10]. Furthermore, the pandemic has forced companies to re-evaluate the MSL’s functions, with tactics and strategies shifting to accommodate changing times, as seen in the expansion and integration of the role in the overall Medical Plan. Our survey showed an increase in the participation of MSLs in the Medical Plan’s preparation/implementation (52%), as well as the execution of clinical trials, and the support of investigator-initiated studies. In addition, 43% of surveyed MSLs managed their own budget, giving the MSL more leeway to select the activities rather than relying on the budget of sales force [10]. The findings by Chicharro et al. [2] support our results, as it was already evident that the MSL role has a wide range of responsibilities to comply with the following: KOL relationship management, continuing medical education activities, scientific advice activities, company trial support, congress speaker training, sales force support and training, investigator-initiated trial research, delivery of scientific presentations, promo speaker training, participating in advisory boards, health economics and outcomes research presentations, etc.
One of the main limitations of our present study was the intrinsic subjective nature of a survey, in providing the opinion of participants. In our case, since recruitment was mostly performed through social networks, the number of companies surveyed could be under-represented. To the best of our knowledge, this is the first survey that reports the opinion from other professionals of the industry, rather than from MSLs or healthcare professionals.
Conclusion
MSL is a well-known field-based profile with increasing importance and responsibilities growing over recent years. MSLs do not only provide scientific exchange with KOLs but also represent a strategic profile and act as an internal collaborator in the company, whose success relies on cross-functional work. MSL is a profile in constant development, now facing the challenges of adapting to remote interactions, and re-evaluating its functions due to the pandemic, as well as the commission to clearly define the best metrics to measure the MSL performance as a non-promotional position.
This survey has established that although all respondents had a positive impression of the MSL; the managerial positions were especially in favor of giving more responsibility to MSLs, especially regarding the elaboration of Medical Plans, taking part in research projects (such as clinical trials and investigator-initiated trials), and assuming their own budget. In accordance with this higher responsibility, their professional category should be in line with their function and performance.
Author Contributions
All authors contributed to the design of the work, the data acquisition, its interpretation, and manuscript revision, and they have read and approved the submitted version.
Funding
No special funding was received for this article. The authors would like to thank AMIFE (Medicine Association of the Pharmaceutical Industry, Madrid, Spain) and Meisys (pharmaceutical consultancy, Madrid, Spain) for the support in writing the article.
Declarations
Conflict of interest
The authors of this publication have no conflict of interest to disclose. They are not speaking on behalf of their current companies or any previous companies.
References
- 1.Medical Science Liaison Society. What is a Medical Science Liaison? Available from: https://www.themsls.org/what-is-an-msl. Accessed 25 Oct 2021.
- 2.Chicharro A, Losada E, Marin H, et al. A survey of medical scientific liaisons in the pharmaceutical industry in Spain. J Med Market. 2017;16:4–9. doi: 10.1177/1745790417717086. [DOI] [Google Scholar]
- 3.Koot D, McMaster A, Nel M, et al. Medical science liaisons (MSL) in Africa: a perspective. Pan Afr Med J. 2019;33:313. doi: 10.11604/pamj.2019.33.313.15143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutherford P, Smith NJ. QuintilesIMS. Medical Science Liaisons: A key to driving patient access to new therapies. 2016. https://www.iqvia.com/-/media/library/white-papers/medical-science-liaisons.pdf. Accessed 25 Oct 2021.
- 5.Bass JL, CM, Klinger CK. Survey of medical liaison practices Assessing practice trends across the pharmaceutical industry. Drug Inf J. 2010;44(5):535–549.
- 6.Marrone CM, Bass JL, Klinger CK. Survey of medical liaison practices across the pharmaceutical industry. Drug Inf J. 2007;41:457–470. doi: 10.1177/009286150704100404. [DOI] [Google Scholar]
- 7.Marrone CM, Bass JL, Klinger C. Survey of medical liaison practices No. 2: assessing training techniques and demonstrating value of medical Liaisons. Drug Inf J. 2008;42(1):67–80. doi: 10.1177/009286150804200111. [DOI] [Google Scholar]
- 8.Moss RJ, Black J. Health Care Professionals’ Expectations of the Medical Science Liaison: a blinded survey. Ther Innov Regul Sci. 2013;47(2):203–208. doi: 10.1177/2168479012470649. [DOI] [PubMed] [Google Scholar]
- 9.Moss RJ, Smith EB, Anderson G, et al. A survey of key opinion leaders to support curriculum development in advanced Medical Science Liaison Training. Ther Innov Regul Sci. 2015;49(1):45–49. doi: 10.1177/2168479014549859. [DOI] [PubMed] [Google Scholar]
- 10.Sastre V, Matesanz-Marín A, García C, et al. The medical science liaison role in Spain: a nationwide survey. Perspect Clin Res. 2021;13(1):48. doi: 10.4103/picr.PICR_53_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.González Del Castillo A, García C, Matesanz-Marín A, et al. The medical science liaison role in Spain: a survey about the opinion of healthcare professionals. Ther Innov Regul Sci. 2021;56(1):96–103. doi: 10.1007/s43441-021-00333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Medical Science Liaison Society. How KOLs prefer to engage with MSLs during the COVID-19 pandemic. Available from: https://www.themsls.org/covid-19-kol-engagement-survey/. Accessed 25 Oct 2021
- 13.PMFarma. MSL & Operaciones Clínicas: hacia una colaboración efectiva. http://www.pmfarma.es/colaboradores/sdg/2889-msl-operaciones-clinicas-hacia-una-colaboracion-efectiva.html. Accessed 25 Oct 2021.
- 14.How does an MSL interact with other functions within the pharma company? https://www.mslconsultant.com/post/how-does-an-msl-interact-with-other-functions-within-the-pharma-company. Accessed 25 Oct 2021.
- 15.Medical Affairs' changing role in market access and reimbursement. https://www.pharmaceuticalcommerce.com/opinion/medical-affairs-changing-role-in-market-access-and-reimbursement/. Accessed 25 Oct 2021.
- 16.Medical Science Liaison Society. Medical Science Liaison guidelines. 2018. https://www.themsls.org/msl-guidelines/. Accessed 25 Oct 2021.