Abstract
The modulation of reaction kinetics with horseradish peroxidase (HRP)-catalyzed crosslinking of proteins remains a useful strategy to modulate hydrogel formation. Here, we demonstrate that the presence of positively charged lysines in silk elastin-like polymers (SELPs) impact the thermal transition temperature of these proteins, while the location in the primary sequence modulates the reactivity of the tyrosines. The positively charged lysine side chains decreased pi-pi interactions among the tyrosines and reduced the rate of formation and number of HRP-mediated dityrosine bonds, dependent on the proximity of the charged group to the tyrosine. The results suggest that the location of repulsive charges can be used to tailor the reaction kinetics for enzymatic crosslinking, providing further control of gelation rates for in situ gel formation, as well as the resulting protein-based gel characteristics.
Keywords: silk-elastin copolymer, tyrosine, peroxidase, crosslinking
INTRODUCTION
Control over the rate at which polymer crosslinks under physiological conditions to form hydrogels with different mechanical properties is important in many fields of research, including regenerative medicine1, fibrosis, tumorogenesis2, cell proliferation, drug and cell delivery, among other needs3–4. There are many strategies utilized to crosslink protein polymers5, mainly focused on cysteine and lysine side chain reactivities6. These strategies can present challenges, as in natural and bioengineered protein designs these amino acids can also have alternative functions apart from crosslinking7–8. Tyrosine plays numerous roles in protein functions, such as antibody recognition via the formation of pi-pi and cation-pi interactions9, in transduction cascades10, or for crosslinking to contribute to tissue stability (e.g., insect resilin, human aorta, bovine skin)11–12.
In vitro, the enzymatic crosslinking of tyrosine (dityrosine formation) is a promising strategy for crosslinking natural and recombinant proteins leading to gel formation11, 13. Horseradish peroxidase (HRP) is an Fe containing protein activated with H2O2 to form tyrosine free radicals that subsequently crosslink to form dityrosine bonds14. These reactions are relatively fast, take place in aqueous solvents under physiological pH and temperature with no toxic side products, which allows the incorporation of cells during the crosslinking reaction15 and the in vivo formation of cell-loaded hydrogels16–18. Strategies to control of the reactivity and crosslinking kinetics with the tyrosines in these biopolymers remains to be more fully addressed. Some strategies have focused on the chemical modification of the enzyme itself by covalently attaching anthraquinone 2-carboxylic acid to the surface-exposed lysine residues to increase enzyme stability and activity19. Others have focused on controlling the reaction kinetics by changing ligand density15, pH20 or enzyme/H2O2 concentrations21. These strategies, while valuable in reaction control in vitro, are difficult to apply in vivo. For example, pH is usually limited to near physiological values, in natural proteins ligand density is limited and invariable and in the body enzyme activity is difficult to modify in situ, although nonnatural enzymes can be used as an alternative22–23.
Silk elastin-like polymers (SELPs) are genetically engineered proteins that combine thermoresponsive (GXGVP) pentapeptide domains found in the intrinsically disordered regions of tropoelastin24–25 (where X stands for any amino acid except proline); and (GAGAGS) motifs found in the crystallizable (beta sheet) domains of the silkworm fibroin heavy chain protein from the silkworm Bombyx mori. Recombinant expression of these proteins offers control over molecular weight, composition, and allows the introduction of systematic modifications in structure. These features allow control of the protein chains and characteristics, thus, empowering SELPs as a valuable tool in fundamental studies of protein folding26–28, for biomaterial scaffolding29 and in drug or gene delivery30, among other technological uses31–32. However, the control of reactive moieties in SELPs towards crosslinking has not been extensively explored.
It seems reasonable thus to crosslink this material using HRP15, 26, and exploit control over structure to tune both the crosslinking rates and the final mechanical properties of the hydrogels. The findings point out that the electrostatic complementary between enzymes and substrates are of crucial importance and determine the effectiveness of the reaction33, 34. Based on the idea that complementary charges facilitate the enzymatic reaction by lowering the energy barrier33, it is reasonable that charges of the same sign would hinder the enzymatic reaction and other possible interactions. Thus, this provides an understanding of repulsive electrostatic forces as a mechanism to increase the activation energy of the enzymatic reaction or interactions.
In this work, we present a systematic study of an efficient charge-mediated method to modulate three aspects of the assembly or properties of genetically engineered SELPs: (i) pi-pi interactions, (ii) HRP-mediated crosslinking kinetics and (iii) mechanics of the gels. This methodology, if applied in vivo, could also be useful to control reactions that take place with injected sol-gel biomaterials, such as for crosslinking or peptide cleavage in situ. Such control could be utilized to regulate the release of a bioactive peptide or modulate the durability or lifetime of the protein materials during tissue remodeling. This strategy could also be useful for modulating tyrosine interactions, such as between these bioengineered materials and cell receptors, to tailor binding kinetics to enable control of bioactivity34.
MATERIAL AND METHODS
SELP PRODUCTION AND PURIFICATION
Gene synthesis, cloning and expression were carried out following our standard protocols35. Briefly, SELPs genes were built through the assembly of oligos (synthetized by Genescript Biotech, Piscataway, New Jersey), subsequently cloned into a pET-29b(+) expression vector lacking the C’-terminal histidine tag and expressed in BLR(DE3) E. coli grown in autoinduction terrific broth (TB) medium (Grisp Research Solutions, Porto, Portugal) in a 2 liter bioreactor at 37°C (New Brunswick, BioFlo 3000) for 18 hours. SELPs were purified based on their thermoresponsive properties35, with an average yield of around 350 mg of pure SELP per liter. Purity and amino acid composition were confirmed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE), matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) and proton nuclear magnetic resonance (1H-NMR).
DIFFERENTIAL SCANNING CALORIMETRY (DSC) AND TEMPERATURE MODULATED DIFFERENTIAL SCANNING CALORIMETRY (TMDSC)
Thermal analysis was performed with a TA Q-100 DSC (TA instruments, New Castle, Delaware, USA) under constant nitrogen flow (50 mL/min) using hermetically sealed Tzero aluminum pans and lids (TA instruments, New Castle, Delaware, USA). For each SELP, a volume of 25 μL (100 mg/mL in PBS, pH 7) was used, with a reference consisting of an equal volume of PBS. For DSC experiments, samples were heated from 15 to 60°C at 2°C/min. For TMDSC, a sinusoidal signal (0.32°C amplitude, 60 s period) was superimposed on the heating ramp. At least two independent experiments were performed for each SELP, and the values presented are the average of those measurements.
CROSSLINKING REACTIONS
For crosslinking reactions experiments, 50 mg/mL SELP solution in PBS (pH 7) was mixed with 10 U/μL of horseradish peroxidase (283 U/mg, SigmaAldrich, Ref: P8375–2KU). The reaction, performed at 37°C, was initiated by the addition of 1% v/v of a 0.75% v/v H2O2 solution.
FLUOROMETRIC ANALYSIS OF THE CROSSLINKING REACTION
Fluorometric experiments were carried out in a Synergy H1 Hybrid Multi-Mode Readerplate reader (BioTek, Agilent) using a 96 clear bottom black plate (Costar) at 37°C. The excitation wavelength was 315 nm; emission wavelength 405 nm for detection of di-tyrosine36. As negative controls, SELPs without HRP and without HRP or H2O2 were used. Error bars represent the standard deviation of three independent measurements.
RHEOLOGY OF THE CROSSLINKING REACTION
Rheology measurements were carried out in an oscillatory rheometer (TA Instruments AR2000ex, New Castle, Delaware, USA) with SELP (50 mg/mL), HRP and H2O2 concentrations as above, using a 25 mm parallel plate geometry and a 1 mm gap. Prior to gelation, frequency and strain sweeps were performed to determine the linear viscoelastic region of the SELPs. Gelation experiments were carried out at 1 Hz frequency, 0.2% strain and 37°C. As a negative control the SELP without tyrosines (S2E8) was used (see Supporting Information).
RESULTS AND DISCUSSION
SELP PRODUCTION
Five different SELPs were produced (Table 1): one without tyrosines (S2E8) and four others (S2E8Y35, S2E8Y-K1, S2E8Y-K3 and S2E8Y-K6) presenting a (GYGVP) pentapeptide motif regularly positioned in the SELP primary sequence. For those labelled with a K (-K1, -K3 and –K6), a positively charged lysine was placed before the first, third and sixth elastin-like pentapeptides in the sequences, respectively, thus a total of 8 lysines were present in these three variants, with none in S2E8 or S2E8Y. The SELPs were characterized by SDS-PAGE and 1H-NMR prior to their use (see Supporting Information).
Table 1.
Name, amino acid composition, molecular weight and isoelectric point (calculated with ProtParam, www.expasy.org) of the SELPS used in this work.
| SELP | AMINO ACID COMPOSITION | Mw (kDa) | pI |
|---|---|---|---|
| S2E8 | MAMGGGA- [(GAGAGS)2(GVGVP)8]8-G | 32.63 | 5.52 |
| S2E8Y | MAMGGGA- [(GAGAGS)2(GVGVP)(GVGVP)(GVGVP)(GVGVP) (GYGVP)(GVGVP)3]8-G | 33.14 | 5.52 |
| S2E8Y-K1 | MAMGGGA- [(GAGAGS)2K(GVGVP)(GVGVP)(GVGVP)(GVGVP) (GYGVP)(GVGVP)3]8-G | 34.17 | 9.89 |
| S2E8Y-K3 | MAMGGGA- [(GAGAGS)2(GVGVP)(GVGVP)K(GVGVP)(GVGVP) (GYGVP)(GVGVP)3]8-G | 34.17 | 9.89 |
| S2E8Y-K6 | MAMGGGA- [(GAGAGS)2(GVGVP)(GVGVP)(GVGVP)(GVGVP) (GYGVP)K(GVGVP)3]8-G | 34.17 | 9.89 |
DIFFERENTIAL SCANNING CALORIMETRY (DSC)
DSC experiments (see Supporting Information) showed typical endotherms associated with the reversible phase transition of the SELPs26, which arises from the loss of the clathrate-like (ordered) structures that hydrate the hydrophobic domains in the SELPs. The control SELP, S2E8 (i.e., lacking tyrosine and charged lysines), exhibited the highest Tt, above 41°C, in good agreement with the literature and the effect of the presence of silk domains on Tt37–38. The SELP with tyrosines and without charge (S2E8Y) exhibited the lowest Tt and the highest enthalpy, corresponding to the presence of tyrosine, a hydrophobic guest amino acid39. The SELPs bearing positive charges exhibit higher Tt’s and a lower enthalpy (Table 2). The presence of charged/hydrophilic moieties in the SELP backbone induced the displacement of Tt towards higher values, as the overall hydrophilicity of the SELP increased40. The decrease in enthalpy was also related to the presence of these charged (lysine) moieties, as their hydrophilic nature reduces the number of water molecules that hydrate the SELP backbone41.
Table 2.
Transition temperatures and enthalpy values for the different transitions observed with the SELPs using DSC and TMDSC.
| SELP | DSC | TMDSC | |||||
|---|---|---|---|---|---|---|---|
| TOTAL | REV. HEAT FLOW | NON-REV HEAT FLOW | |||||
| Tt (°C) | ΔH | Tt (°C) | Tt (°C) | ΔH | Tt (°C) | ΔH | |
| S2E8 | 41.1 | 1.3 | 41.1 | 41.2 | 1.5 | 41.0 | −0.2 |
| S2E8Y | 30.2 | 1.2 | 30.2 | 30.4 | 0.8 | 29.7 | 0.5 |
| S2E8Y-K1 | 35.2 | 0.9 | 34.2 | 34.4 | 0.7 | 33.8 | 0.2 |
| S2E8Y-K3 | 35.3 | 0.8 | 34.2 | 34.5 | 0.7 | 33.8 | 0.1 |
| S2E8Y-K6 | 36.6 | 0.7 | 35.4 | 35.2 | 0.6 | 35.4 | 0.1 |
TEMPERATURE MODULATED DIFFERENTIAL SCANNING CALORIMETRY (TMDSC)
The endotherms with conventional DSC were the result of the sum of the different complex thermal processes, each following different kinetics. TMDSC experiments permitted the decomposition of the total heat flow observed by conventional DSC as the sum of non-reversible and reversible heat flows42, each of these associated with two different phenomena occurring simultaneously during SELP temperature-induced phase transitions. Non-reversible heat flow is related to the destruction of water clathrates that hydrate the SELP backbone, while reversible heat flow is related to the phase transition of the SELP backbone itself43–44. For the tyrosine-lacking control SELP (S2E8), the non-reversible heat flow exhibited an endotherm peak, as a result of the destruction of ordered water structures that hydrate the SELP back bone; and an exothermic reversible heat flow, as a result of the reversible folding of the SELP44.
For the SELPs bearing tyrosines, both non-reversible and reversible heat flow exhibited an endotherm peak. As previously reported43, for SELPs a reversible heat flow endotherm is related to the destruction of ordered structures as result of the temperature increase. By comparison, among the tyrosine-containing SELPs and the control, the presence of the tyrosines capable of pi-stacking (i.e., forming ordered structures) were associated with the endotherm peak observed in the reversible heat flow45–46.
The reversible heat flows of the different SELPs bearing tyrosines, as well as the effect from the location of the positively charged residue within the primary sequence of the SELPs was evaluated. When compared to the control, the presence of charged residues reduced the enthalpy associated with the reversible heat flow, with the effect more noticeable the closer the charged residue was to the tyrosine (Table 2). This result suggests that the closer the electrostatic repulsions are to the tyrosine the greater the reduction in pi-stacking of the tyrosines (thus weaker the pi-pi interactions).
FLUOROMETRIC ANALYSIS OF THE CROSSLINKING REACTION
We next studied HRP-mediated crosslinking of the SELPs (Figure 1). The onset of the sharp increase in fluorescence signal related to tyrosine crosslinking occurred at different time points depending on the SELP sequence, starting at shorter times for the S2E8Y (1 minute), with longer time frames for the SELPs bearing positive charges (3.25, 2.75 and 4.50 minutes for the S2E8Y-K1, -K3 and –K6, respectively). Interestingly, the maximum fluorescence found for the different SELPs was dependent on the presence and location of the positive charges. The S2E8Y fluorescence reached 5*105 while for the S2E8Y-K1 and S2E8Y-K3, bearing positive charges distant to the tyrosine, the fluorescence values reached 3.5*106 and 2.3*106, respectively. For S2E8Y-K6, the fluorescence dropped to 1.5*106. These results suggest the active influence of the repulsive positive charges on the HRP mediated crosslinking of the SELPs.
Figure 1.
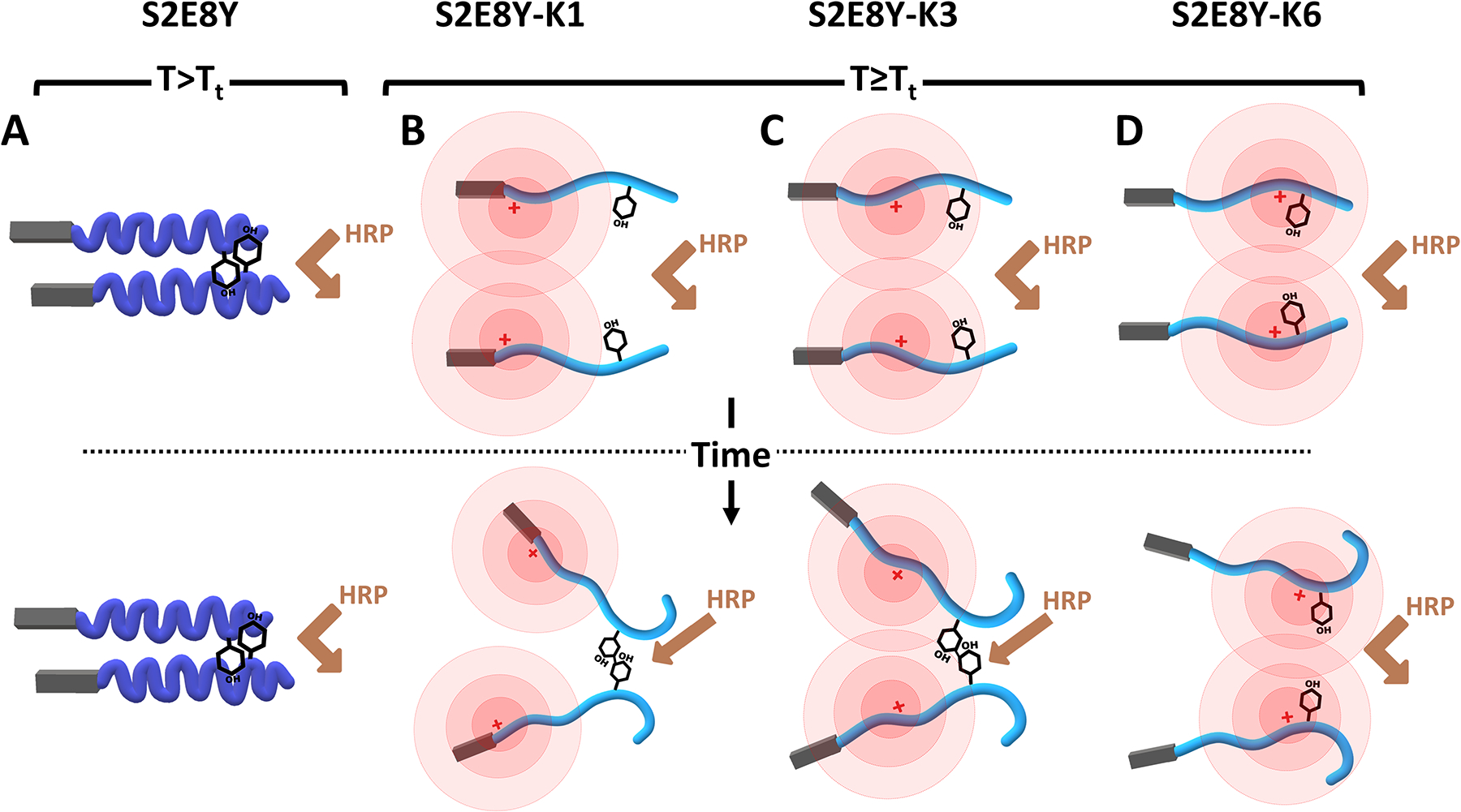
Schematic representation of the effect of positive charges in the HRP-mediated crosslinking of the four SELPs used in this study: (A) S2E8Y, with a Tt far below 37°C, completely phase-transitioned and formed hydrophobic clusters that prevent HRP to access to crosslink tyrosines. For S2E8Y-K1 (B) and S2E8Y-K3 (C) the presence of positively charged lysines raises the Tt of these SELPs to 35°C, preventing full phase transitions. With time, the electrostatic repulsion between lysines and the molecular flexibility of the SELPs further exposes tyrosines for crosslinking. For (D) S2E8Y-K6, the repulsion between positively charged residues and their proximity to tyrosines prevents HRP-mediated crosslinks.
The early onset and the lower fluorescence values for S2E8Y are explained by the absence of positively charged motifs. The lack of positive charges in this SELP prevents electrostatic repulsion among molecules that otherwise hinder the formation of dityrosine bonds. However, the absence of lysines lowers the Tt of the S2E8Y (30.16°C) when compared to the rest of SELPs. This lower Tt supports the complete phase transition at 37°C for this SELP, leaving most of the tyrosines within the hydrophobic clusters formed by the phase transitioned elastin motifs and thus, inaccessible to the HRP.
The distance between the positively charged lysines and tyrosines has a significant impact over the crosslinking reaction. Charges located the farthest from the tyrosine (as in S2E8Y-K1 and –K3) exert the smallest effect over the initiation of crosslinking, while the effect was more prominent the closer the charge was to the tyrosine. Charges located adjacent to the tyrosine (as in S2E8Y-K6) delayed the onset of the reactions the most. When positive charges were located further to the tyrosines (S2E8Y-K1 and -K3), despite the delay in the onset of fluorescence, greater fluorescence values were attained. Interestingly, fluorescence values for S2E8Y-K1 and –K3 followed a similar two-phase increase, explained as result of the combination of three factors: increased Tt, repulsive effects of the positive charges and the high molecular flexibility (associated with the intrinsically disordered origin of SELPs). The increased Tt (≈35°C) for these SELPs means that they are not fully phase transitioned at 37°C, which would facilitate accessibility of the tyrosines to the HRP reaction. Thus, the first increase in fluorescence would result from the crosslinking of these accessible tyrosines. The second sharp increase (at 18 and 10 minutes, for S2E8Y-K1 and –K3, respectively) would be a combination of the repulsion between charges of the same sign and the intrinsic flexibility of the SELPs47. Charge repulsion would be responsible of the molecular rearrangements that further expose tyrosines for HRP crosslinking, which could only be accommodated due to the intrinsic flexibility of the SELP backbone. If charges were located adjacent to the tyrosine, as in S2E8Y-K6, they exert an inhibitory effect over the HRP crosslinking reaction, when compared to the other lysine bearing SELPs. Repulsion between charges maintains the segregation of the tyrosines, hindering crosslinking, slowing the reaction rate, and limiting the number of crosslinks.
Fluorometric measurements of tyrosine crosslinking demonstrated that the presence of positively charged residues had a direct effect over crosslinking rate and the number of crosslinks formed, and this effect was modulated by the distance between the positive charges and the tyrosines. Charges far away from tyrosines had a small effect, while if adjacent, they were inhibitory. Intermediate location of the charges slows the reaction rate but permitted the molecular rearrangements that led to a higher degree of crosslinking.
RHEOLOGY OF CROSSLINKING REACTIONS
The HRP-mediated gelation of SELPs can be seen in Figure 2. For S2E8Y, the increase in mechanical properties was the slowest and the mechanical properties at the end of the experiment were the weakest of the SELPs studied (Table 3). For the SELPs bearing lysines, the increase in mechanical properties was faster and more significant than for S2E8Y. For S2E8Y-K1, the elastic modulus (G’) surpassed the loss modulus (G”) in the initial minutes of the experiment and subsequently, after 21 minutes, sharply increased to reach a plateau. For S2E8Y-K3 we observed similar behavior, the second sharp increase in mechanical properties was delayed when compared to S2E8-K1 (34 minutes). For S2E8-K6, mechanical properties increased with time following a logarithmic-like trend. Interestingly, for all four SELPs, the evolution of mechanical properties, as measured by rheometry and fluorometric signals related to the tyrosine crosslinks, followed similar trends. The lowest fluorescence corresponded with the lowest mechanical properties, as seen with S2E8Y, whereas the highest fluorescence values were obtained with S2E8Y-K1 and -K3, which corresponded to the highest mechanical properties. There is a correspondence in the trends between the fluorometric and the rheological values. For example, both S2E8Y-K1 and -K3 exhibited a 2-step increase in fluorescence, similar to that seen in rheological measurements. Moreover, the sharp increase in fluorescence and mechanical properties occurred at similar time points. This was also the case for S2E8Y-K6, which exhibited a similar logarithmic-like trend for the fluorometric and rheological values. These results corroborate our hypothesis on the effect of the positive charges over HRP-mediated gelation of SELPs.
Figure 2.
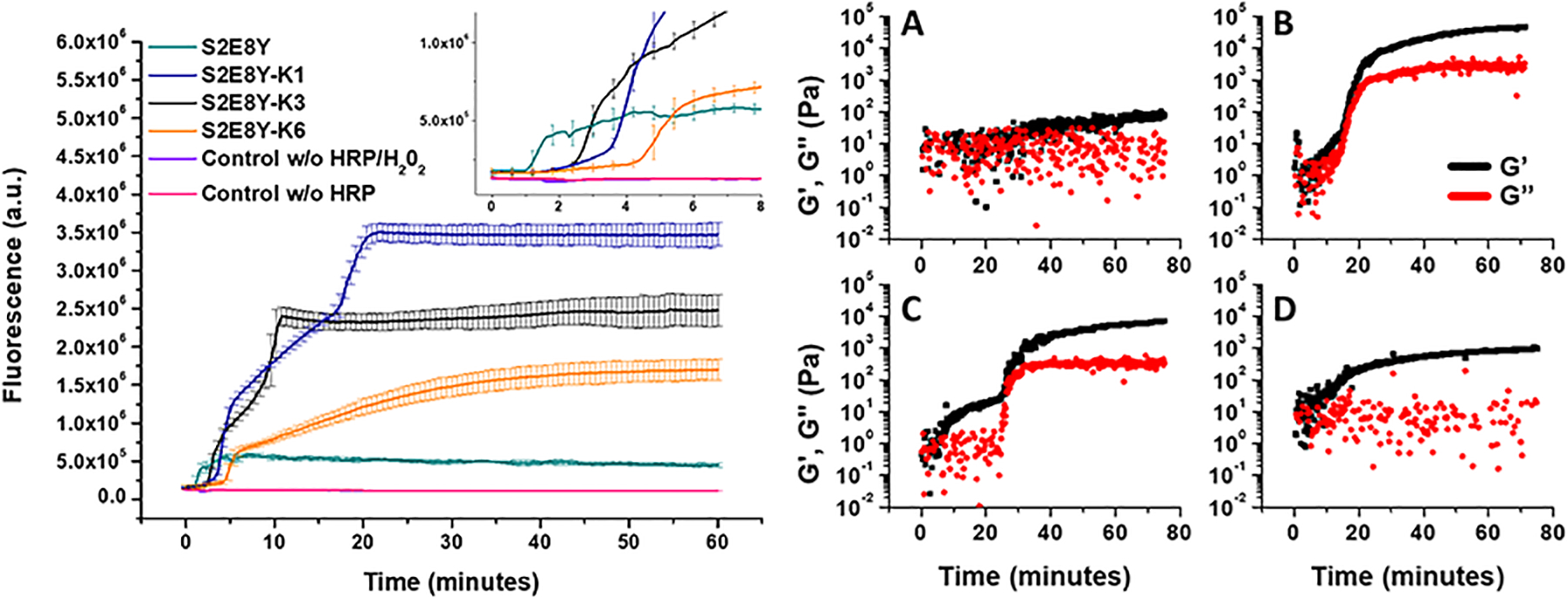
(Left) Fluorometric measurements of HRP-mediated tyrosine crosslinking for the 4 SELPs and their controls, SELPs without HRP and without HRP or H2O2, were used (purple and pink line, respectively). Error bars represent the standard deviation of three independent measurements. (Right) Rheological measurements of the HRP-mediated gelation of the (A) S2E8Y, (B) S2E8Y-K1, (C) S2E8Y-K3 and (D) S2E8Y-K6. In black, the elastic modulus (G’) and in red the loss modulus (G”).
Table 3.
Mechanical properties and fluorescence values at the end of the experiment for the 4 SELPs studied.
| SELP | G’ (Pa) | G” (Pa) | Fluorescence (a.u.) |
|---|---|---|---|
| S2E8Y | 86.7 | 5.2 | 0.5*106 |
| S2E8Y-K1 | 47124.3 | 2922.1 | 3.5*106 |
| S2E8Y-K3 | 7080.8 | 361.6 | 2.3*106 |
| S2E8Y-K6 | 970.9 | 19.5 | 1.5*106 |
CONCLUSIONS
Here we demonstrated that the presence of charged residues within SELPs had a direct effect over the transition temperature and the enthalpy of phase transition. Moreover, the deconvolution of the total heat flow of the phase transition into reversible and non-reversible heat flows by TMDSC allowed us to identify the effect of the positively charged lysines over the pi-stacking capacity of the tyrosines present in the SELPs. This effect was inhibitory and more prominent the closer the charges were to the tyrosine. In addition, the presence of the lysines also had an effect on the HRP-mediated tyrosine crosslinking reaction rate, slowing it in the initial stages, but improving the overall performance of the reaction in all cases when compared to the control SELP. The increased transition temperature of the SELP variants bearing a lysine, in combination with the intrinsically flexible character of the SELPs, further exposed the tyrosines, resulting in higher degrees of crosslinking. The increased number of HRP-mediated crosslinks was dependent on the location of the lysine with respect to the tyrosine. These results suggest that the precise location of charges could be used as strategy (in vitro or in vivo) to control the biological accessibility of tyrosines in these types of protein polymers. Moreover, this regulatory effect could be extended to other enzymatic reactions, where repulsion among charges close to the reactive species or between the enzyme and the biomaterial could be used to tailor the speed at which the reaction takes place, leading, for example, to controlled in vivo hydrogel formation (or peptide cleavage) by enzymes in the tissue.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the ARO, the AFOSR (FA9550-20-1-0363) and the NIH (P41EB027062) for support of these studies.
Footnotes
Supporting Information:
SELP characterization: SDS-PAGE and 1H-NMR
SELP thermal characterization: differential scanning calorimetry (DSC) and temperature modulated differential scanning calorimetry (TMDSC).
Rheology of the negative control S2E8
References:
- 1.Gjorevski N; Sachs N; Manfrin A; Giger S; Bragina ME; Ordóñez-Morán P; Clevers H; Lutolf MP, Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539 (7630), 560–564. [DOI] [PubMed] [Google Scholar]
- 2.Arkenberg MR; Moore DM; Lin C-C, Dynamic control of hydrogel crosslinking via sortase-mediated reversible transpeptidation. Acta Biomater. 2019, 83, 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sackett SD; Tremmel DM; Ma F; Feeney AK; Maguire RM; Brown ME; Zhou Y; Li X; O’Brien C; Li L; Burlingham WJ; Odorico JS, Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci. Rep 2018, 8 (1), 10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J; Mooney DJ, Designing hydrogels for controlled drug delivery. Nat. Rev. Mater 2016, 1 (12), 16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garavand F; Rouhi M; Razavi SH; Cacciotti I; Mohammadi R, Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol 2017, 104, 687–707. [DOI] [PubMed] [Google Scholar]
- 6.Hu J; Wang G; Liu X; Gao W, Enhancing Pharmacokinetics, Tumor Accumulation, and Antitumor Efficacy by Elastin-Like Polypeptide Fusion of Interferon Alpha. Adv. Mater 2015, 27 (45), 7320–4. [DOI] [PubMed] [Google Scholar]
- 7.Heck T; Faccio G; Richter M; Thony-Meyer L, Enzyme-catalyzed protein crosslinking. Appl. Microbiol. Biotechnol 2013, 97 (2), 461–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salazar VS; Gamer LW; Rosen V, BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol 2016, 12 (4), 203–21. [DOI] [PubMed] [Google Scholar]
- 9.Peng H-P; Lee KH; Jian J-W; Yang A-S, Origins of specificity and affinity in antibody–protein interactions. Proc. Natl. Acad. Sci 2014, 111 (26), E2656–E2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter T, The genesis of tyrosine phosphorylation. Cold Spring Harb Perspect Biol 2014, 6 (5), a020644–a020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Partlow BP; Applegate MB; Omenetto FG; Kaplan DL, Dityrosine Cross-Linking in Designing Biomaterials. ACS Biomater. Sci. Eng 2016, 2 (12), 2108–2121. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee S; Kapp EA; Lothian A; Roberts AM; Vasil’ev YV; Boughton BA; Barnham KJ; Kok WM; Hutton CA; Masters CL; Bush AI; Beckman JS; Dey SG; Roberts BR, Characterization and Identification of Dityrosine Cross-Linked Peptides Using Tandem Mass Spectrometry. Anal. Chem 2017, 89 (11), 6136–6145. [DOI] [PubMed] [Google Scholar]
- 13.Lee J; Ju M; Cho OH; Kim Y; Nam KT, Tyrosine-Rich Peptides as a Platform for Assembly and Material Synthesis. Adv. Sci 2019, 6 (4), 1801255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunford HB; Stillman JS, On the function and mechanism of action of peroxidases. Coord. Chem. Rev 1976, 19 (3), 187–251. [Google Scholar]
- 15.Partlow BP; Hanna CW; Rnjak-Kovacina J; Moreau JE; Applegate MB; Burke KA; Marelli B; Mitropoulos AN; Omenetto FG; Kaplan DL, Highly Tunable Elastomeric Silk Biomaterials. Adv. Funct. Mater 2014, 24 (29), 4615–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo K-C; Lin R-Z; Tien H-W; Wu P-Y; Li Y-C; Melero-Martin JM; Chen Y-C, Bioengineering vascularized tissue constructs using an injectable cell-laden enzymatically crosslinked collagen hydrogel derived from dermal extracellular matrix. Acta Biomater. 2015, 27, 151–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoppel WL; Gao AE; Greaney AM; Partlow BP; Bretherton RC; Kaplan DL; Black LD 3rd, Elastic, silk-cardiac extracellular matrix hydrogels exhibit time-dependent stiffening that modulates cardiac fibroblast response. J Biomed Mater Res A 2016, 104 (12), 3058–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y; Raia N; Peterson A; Kaplan DL; House M, Injectable Silk-Based Hydrogel as an Alternative to Cervical Cerclage: A Rabbit Study. Tissue Eng Part A 2020, 26 (7–8), 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mogharrab N; Ghourchian H; Amininasab M, Structural stabilization and functional improvement of horseradish peroxidase upon modification of accessible lysines: experiments and simulation. Biophys J 2007, 92 (4), 1192–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes GR; Pinto DCGA; Silva AMS, Horseradish peroxidase (HRP) as a tool in green chemistry. RSC Adv. 2014, 4 (70), 37244–37265. [Google Scholar]
- 21.Rodríguez-López JN; Lowe DJ; Hernández-Ruiz J; Hiner ANP; García-Cánovas F; Thorneley RNF, Mechanism of Reaction of Hydrogen Peroxide with Horseradish Peroxidase: Identification of Intermediates in the Catalytic Cycle. J. Am. Chem. Soc 2001, 123 (48), 11838–11847. [DOI] [PubMed] [Google Scholar]
- 22.Ravikumar Y; Nadarajan SP; Hyeon Yoo T; Lee C.-s.; H. Yun, Unnatural amino acid mutagenesis-based enzyme engineering. Trends Biotechnol. 2015, 33 (8), 462–470. [DOI] [PubMed] [Google Scholar]
- 23.Gao W; Cho E; Liu Y; Lu Y, Advances and Challenges in Cell-Free Incorporation of Unnatural Amino Acids Into Proteins. Front. Pharmacol 2019, 10 (611). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarakanova A; Yeo GC; Baldock C; Weiss AS; Buehler MJ, Molecular model of human tropoelastin and implications of associated mutations. Proc. Natl. Acad. Sci 2018, 115 (28), 7338–7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeo GC; Tarakanova A; Baldock C; Wise SG; Buehler MJ; Weiss AS, Subtle balance of tropoelastin molecular shape and flexibility regulates dynamics and hierarchical assembly. Sci. Adv 2016, 2 (2), e1501145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang W; Tarakanova A; Dinjaski N; Wang Q; Xia X; Chen Y; Wong JY; Buehler MJ; Kaplan DL, Design of Multistimuli Responsive Hydrogels Using Integrated Modeling and Genetically Engineered Silk-Elastin-Like Proteins. Adv. Funct. Mater 2016, 26 (23), 4113–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarakanova A; Huang W; Qin Z; Kaplan DL; Buehler MJ, Modeling and Experiment Reveal Structure and Nanomechanics across the Inverse Temperature Transition in B. mori Silk-Elastin-like Protein Polymers. ACS Biomater. Sci. Eng 2017, 3 (11), 2889–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeo J; Huang W; Tarakanova A; Zhang Y-W; Kaplan DL; Buehler MJ, Unraveling the molecular mechanisms of thermo-responsive properties of silk-elastin-like proteins by integrating multiscale modeling and experiment. J. Mater. Chem. B 2018, 6 (22), 3727–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acosta S; Quintanilla-Sierra L; Mbundi L; Reboto V; Rodríguez-Cabello JC, Elastin-Like Recombinamers: Deconstructing and Recapitulating the Functionality of Extracellular Matrix Proteins Using Recombinant Protein Polymers. Adv. Funct. Mater 2020, 30, 1909050. [Google Scholar]
- 30.Huang W; Rollett A; Kaplan DL, Silk-elastin-like protein biomaterials for the controlled delivery of therapeutics. Expert Opin Drug Deliv 2015, 12 (5), 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng L; Jiang L; Teng W; Cappello J; Zohar Y; Wu X, Engineering Aqueous Fiber Assembly into Silk-Elastin-Like Protein Polymers. Macromol. Rapid Commun 2014, 35 (14), 1273–1279. [DOI] [PubMed] [Google Scholar]
- 32.Casal M; Cunha A; Machado R, Future Trends for Recombinant Protein-Based Polymers: The Case Study of Development and Application of Silk-Elastin-Like Polymers. In Bio-Based Plastics: Materials and Applications, Kabasci S, Ed. John Wiley & Sons, Ltd: 2013; pp 311–329. [Google Scholar]
- 33.Warshel A; Sharma PK; Kato M; Xiang Y; Liu H; Olsson MHM, Electrostatic Basis for Enzyme Catalysis. Chem. Rev 2006, 106 (8), 3210–3235. [DOI] [PubMed] [Google Scholar]
- 34.Koide S; Sidhu SS, The importance of being tyrosine: lessons in molecular recognition from minimalist synthetic binding proteins. ACS Chem Biol 2009, 4 (5), 325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia XX; Xu Q; Hu X; Qin G; Kaplan DL, Tunable self-assembly of genetically engineered silk--elastin-like protein polymers. Biomacromolecules 2011, 12 (11), 3844–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGill M; Coburn JM; Partlow BP; Mu X; Kaplan DL, Molecular and macro-scale analysis of enzyme-crosslinked silk hydrogels for rational biomaterial design. Acta Biomater. 2017, 63, 76–84. [DOI] [PubMed] [Google Scholar]
- 37.Zhao B; Li NK; Yingling YG; Hall CK, LCST Behavior is Manifested in a Single Molecule: Elastin-Like polypeptide (VPGVG)n. Biomacromolecules 2016, 17 (1), 111–118. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Colino A; Arias FJ; Alonso M; Rodríguez-Cabello JC, Self-Organized ECM-Mimetic Model Based on an Amphiphilic Multiblock Silk-Elastin-Like Corecombinamer with a Concomitant Dual Physical Gelation Process. Biomacromolecules 2014, 15 (10), 3781–3793. [DOI] [PubMed] [Google Scholar]
- 39.Urry DW, Molecular Machines: How Motion and Other Functions of Living Organisms Can Result from Reversible Chemical Changes. Angew. Chem. Int. Ed 1993, 32 (6), 819–841. [Google Scholar]
- 40.Christensen T; Hassouneh W; Trabbic-Carlson K; Chilkoti A, Predicting Transition Temperatures of Elastin-Like Polypeptide Fusion Proteins. Biomacromolecules 2013, 14 (5), 1514–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribeiro A; Arias FJ; Reguera J; Alonso M; Rodríguez-Cabello JC, Influence of the amino-acid sequence on the inverse temperature transition of elastin-like polymers. Biophys J 2009, 97 (1), 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang Z; Imrie CT; Hutchinson JM, An introduction to temperature modulated differential scanning calorimetry (TMDSC): a relatively non-mathematical approach. Thermochim. Acta 2002, 387 (1), 75–93. [Google Scholar]
- 43.Reguera J; Urry DW; Parker TM; McPherson DT; Rodriguez-Cabello JC, Effect of NaCl on the exothermic and endothermic components of the inverse temperature transition of a model elastin-like polymer. Biomacromolecules 2007, 8 (2), 354–8. [DOI] [PubMed] [Google Scholar]
- 44.Rodrıguez-Cabello JC; Reguera J; Alonso M; Parker TM; McPherson DT; Urry DW, Endothermic and exothermic components of an inverse temperature transition for hydrophobic association by TMDSC. Chem. Phys. Lett 2004, 388 (1), 127–131. [Google Scholar]
- 45.Partlow BP; Bagheri M; Harden JL; Kaplan DL, Tyrosine Templating in the Self-Assembly and Crystallization of Silk Fibroin. Biomacromolecules 2016, 17 (11), 3570–3579. [DOI] [PubMed] [Google Scholar]
- 46.McDaniel JR; Weitzhandler I; Prevost S; Vargo KB; Appavou M-S; Hammer DA; Gradzielski M; Chilkoti A, Noncanonical Self-Assembly of Highly Asymmetric Genetically Encoded Polypeptide Amphiphiles into Cylindrical Micelles. Nano Lett. 2014, 14 (11), 6590–6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia Quiroz F; Li NK; Roberts S; Weber P; Dzuricky M; Weitzhandler I; Yingling YG; Chilkoti A, Intrinsically disordered proteins access a range of hysteretic phase separation behaviors. Sci. Adv 2019, 5 (10), eaax5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


