Abstract
Background
Chronic inflammation plays a key role in the pathophysiology of frailty and loss of physical performance, which are closely associated with sarcopenia. In women, the decline in muscle mass and strength is accelerated after menopause. Thus, we examined the association between high sensitivity C-reactive protein (hs-CRP) and relative handgrip strength (HGS) in postmenopausal women.
Methods
This cross-sectional study included 2171 postmenopausal women aged ≥45 years who participated in the Korean National Health and Nutrition Survey (KNHNES) between 2015 and 2018. Relative HGS was categorized into quartiles as follows: Q1, <0.810 (kg/BMI); Q2, 0.810–0.968 (kg/BMI); Q3, 0.969–1.119 (kg/BMI); Q4, >1.119 (kg/BMI). The odds ratios (ORs) and 95% confidence intervals (95% CIs) for high hs-CRP (>1.0 mg/L, 75 percentile of the current samples) were calculated across relative HGS quartiles using multiple logistic regression analysis.
Results
The prevalence of high hs-CRP decreased with relative HGS quartiles. Compared to the highest quartile, the OR (95% CI) of the lowest relative HGS quartile for high hs-CRP was 3.266 (2.227–4.789) after adjusting for age, hypertension, diabetes mellitus, dyslipidemia, education, household income, physical activity, strength exercise, smoking, and alcohol ingestion.
Conclusion
Serum hs-CRP level was inversely and independently associated with relative HGS. Our findings indicate that low-grade inflammation is inversely associated with muscle strength in postmenopausal women.
Keywords: C-reactive protein, hand strength, menopause, inflammation, sarcopenia
Introduction
Increased levels of inflammatory markers and acute-phase protein are a common feature of the aging process. First mentioned by Franceschi et al in 2000, “Inflamm-aging” describes a chronic low-grade systemic inflammation of aging without overt infection.1 Sources of inflamm-aging include the following factors: 1) self-debris from damaged macromolecules and cells accumulated with aging, 2) harmful products produced by the microbial constituents such as oral or gut microbiota, 3) increased activation of the coagulation system with age, 4) cellular senescence which is a cellular response to damage and stress.2,3 Also, intra-abdominal adiposity generally increases in postmenopausal women and can result in chronic low-grade inflammation through adipocytokines.4 Recent studies have shown that sustained low-grade pro-inflammatory state is associated with important age-related diseases, such as cardiovascular diseases (CVD), diabetes mellitus, metabolic syndrome, and several cancers.5,6 Therefore, early detection of low-grade inflammation is important to prevent these age-related diseases. C-reactive protein (CRP), which is produced in the liver, is the most widely used inflammatory marker.7 An acute response to injury or infection may increase CRP,7 but recent studies have also shown elevation in CRP with chronic low-grade inflammatory responses in the presence of cardiometabolic diseases, such as CVD, diabetes mellitus, and metabolic syndrome.8
Decreased estrogen levels in postmenopausal women lead to sarcopenia, which is defined as a decline in muscle mass and muscle strength, as well as redistribution of subcutaneous fat to visceral fat.9 Sarcopenia status in postmenopausal can lead to functional limitations, fractures, disability, and premature mortality.10,11 Therefore, sarcopenia in postmenopausal women is important to note in terms of public health as well. Recently, handgrip strength (HGS) has emerged as a proxy for muscle strength measurement due to its convenience and economic advantages. Therefore, various organizations defining sarcopenia accepted HGS as one of the most reliable tools for diagnosing sarcopenia.12–14 However, the cut-off values of HGS defining low muscle strength varied among different studies. A review paper on sarcopenia suggested that muscle strength measured by handgrip strength should be stratified by body mass index (BMI).15 Taking this into account, several studies have shown that relative HGS adjusted for BMI instead of absolute HGS are inversely related to various age-related diseases, such as metabolic syndrome, diabetes mellitus, CVD and chronic kidney disease.16,17 In recent nationwide population-based studies, relative HGS showed a stronger correlation with cardiovascular biomarkers than absolute HGS and dominant HGS.18,19
Recent studies have shown that inflammation plays an important role directly or indirectly in imbalance of skeletal muscle and in pathophysiology of frailty.20–22 Previous studies have already shown that CRP is positively associated with relative HGS.23,24 Thus, we examined the association of chronic low-grade inflammation indexed by high-sensitivity CRP (hs-CRP) with relative HGS in postmenopausal women using large sample data.
Materials and Methods
Survey Overview and Study Population
This study used data from the Korean National Health and Nutrition Survey (KNHANES) from 2015 to 2018. The KNHANES is a nationally representative dataset for comprehensively understanding the health and nutrition status of the Korean people and consists of independent circulation samples every three years with a yearly survey system. Participants were proportionally distributed with multistage stratification according to region, age, and sex. The survey consists of three categories: health interview, nutrition survey, and health examination survey. Among 31,649 subjects who participated in the 2015–2018 KNHANES, 5881 were selected as relatively healthy postmenopausal women of age ≥40 years. Women who answered “Natural menopause” to the question “Status of menstruation” were referred to as postmenopausal. The following participants were excluded: individuals with an hs-CRP ≥ 10 mg/L (n = 66); presence of cancer or arthritis (osteoarthritis, rheumatoid arthritis) (n = 915); unwell in the last two weeks (acute disease or worsening of chronic diseases) (n = 1446); and those whose data were unavailable to evaluate HGS and hs-CRP (n = 1283). After these exclusions were made, there were 2171 participants eligible for this study. All participants in the survey signed an informed written consent form when the 2015–2018 KNHANES was conducted according to the ethical principles of the Declaration of Helsinki. The study protocol was reviewed and approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (approval no. 2018-1-03-P-A) and Yonsei University Health System (No. Y-2019-0195).
Data Collection
Information on health-related behaviors, such as alcohol consumption, smoking habit, physical activity, strength exercise, education level, and household income, was collected by self-report questionnaires. One was defined as a smoker if they answered “I smoke every day” or “I sometimes smoke” to the question “Are you a current smoker?” and reported that they had smoked more than 5 packs (100 cigarettes) in their lifetime. Alcohol consumption was defined as drinking at least once a month in recent years. Korean version of International Physical Activity Questionnaire (IPAQ) was used to evaluate physical activity.25 This questionnaire asks about the duration (minutes per day) and frequency (days per week) according to each exercise intensity and walking activities, including the following question: “how many days did you perform vigorous physical activities such as heavy lifting, heavier gardening or construction work, chopping wood, aerobics, jogging/running, or fast bicycling during the last 7 days? How much time did you spend performing vigorous physical activities on average per day?” Participants who had performed moderate-intensity activity greater than or equal to 2 hours 30 minutes or high-intensity activity greater than or equal to 1 hour 15 minutes per week were defined as physically active. Moderate-intensity activity refers to activity that causes an increase in breathing or increase in heart rate, and high-intensity refers to intense physical activity that causes a decrease in breathing or a rapid increase in heart rate. The strength exercise group included participants who performed strength exercise three times per week or more according to question “How many days did you do strength exercises such as push-up, sit-up, dumbbell, iron bar, etc. in the last 7 days?”. Level of education was classified into elementary school, middle school, high school, and college. Household income was divided into quartiles and marital status was classified into single, married, or others such as separated or divorced. All blood samples were obtained from the antecubital vein after a 12 hour overnight fast. The Hitachi 7600–110 automated chemistry analyzer was used to measure fasting plasma glucose, triglycerides, and high-density lipoprotein (HDL) cholesterol levels (Hitachi Co., Tokyo, Japan). Hs-CRP level was measured using Cobas immunoturbidimetry (Roche Co., Berlin, Germany). Patient height and weight were measured by experienced medical staff to the nearest 0.1 cm and 0.1 kg, respectively. BMI was calculated as weight in kilograms divided by square of height in meters (kg/m2). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured manually by the stand methods using mercury sphygmomanometers (Baumanometer Wall unit 33; W.A. Baum, Copiague, NY) with the patient in the sitting position. Blood pressure was measured three times for all patients at five-minute intervals. The average of the second and third blood pressure readings was calculated as the final blood pressure values. HGS was measured using a digital grip strength dynamometer (TKK 5401; Takei Scientific Instruments Co., Ltd., Tokyo, Japan). Each participant was measured by educated experts in a standing position with their forearm fully extended sideways away from the body at the level of the thigh three times for each hand and allowed 30 seconds of rest to recover between measurements. The participants were asked to squeeze the dynamometer with as much force as possible, for <3 seconds. HGS was defined as the maximally measured grip strength of the dominant hand. Relative HGS was defined as grip strength per unit of BMI.
High hs-CRP
American Heart Association and Centers for Disease Control and Prevention recommend hs-CRP cutoff values of >3 mg/L for high risk, but these cut-off values are based on Western population. A previous study conducted by our research team showed that CRP level was lower in Korean adults compared to the Western population.26 Although approximately 30% of the US population has CRP concentrations >3 mg/L, only 8.7% of men and 5.9% of women have CRP concentrations >3.0 mg/L. As an alternative, we used an hs-CRP >1.0mg/L as the cutoff value corresponding to the 75th percentile of the current samples. This cut-off value is in line with previous studies conducted in Asian population.27,28
Statistical Analyses
Participants were categorized into quartiles based on relative HGS (Q1, <0.810 kg/BMI; Q2, 0.810–0.968 kg/BMI; Q3, 0.969–1.119 kg/BMI; Q4, >1.119 kg/BMI) and absolute HGS (Q1, <22.0 kg; Q2, 22.0–27.6 kg; Q3, 27.7)-. The results are presented as means ± standard errors (SEs) or percentages (SEs). Differences between groups were evaluated using two-tailed analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables. Pearson’s correlation analysis was performed to calculate the r coefficient between the log-transformed hs-CRP and the relative HGS. The odds ratios (ORs) and 95% CIs for high hs-CRP were calculated using multiple logistic regression analysis after adjusting for confounding variables (age, hypertension, diabetes mellitus, dyslipidemia, education, household income, physical activity, strength exercise, smoking, and alcohol ingestion) across relative HGS quartiles. All analyses were conducted using SPSS statistical software, version 25 (SPSS Institute Inc.) and a P-value of <0.05 was considered statistically significant.
Results
Table 1 shows the clinical characteristics of the participants according to relative HGS quartiles. In the highest relative HGS quartile, the mean values of age, BMI, waist circumference, systolic blood pressure, fasting plasma glucose, and triglycerides were the lowest, whereas HDL-cholesterol level was the highest. Also, the proportion of hypertension and diabetes mellitus were highest in the lowest quartile, whereas individuals with regular physical activity, strength exercise, and alcohol ingestion were most prevalent in the highest quartile of relative HGS.
Table 1.
Clinical Characteristics of Study Population by Relative Handgrip Strength Quartiles
| Relative Hand Grip Strength (kg/BMI) Quartile | |||||
|---|---|---|---|---|---|
| Q1, <0.810 | Q2, 0.810–0.968 | Q3, 0.969–1.119 | Q4, >1.119 | P-value | |
| N | 542 | 543 | 543 | 543 | |
| Age | 66.8± 0.5 | 61.9 ± 0.4 | 59.6 ±0.4 | 56.9 ± 0.3 | <0.001 |
| Body mass index (kg/m2) | 25.8±0.2 | 24.4±0.1 | 23.3±0.1 | 21.8±0.1 | <0.001 |
| Waist circumference (cm) | 86.2±0.4 | 82.4±0.4 | 79.4±0.4 | 75.8±0.3 | <0.001 |
| Systolic blood pressure (mmHg) | 126.5 ±0.8 | 124.5±0.9 | 122.1±0.8 | 118.1±0.8 | <0.001 |
| Diastolic blood pressure (mmHg) | 75.1±0.5 | 75.7±0.6 | 76.3±0.5 | 75.1±0.5 | 0.008 |
| Fasting plasma glucose (mg/dL) | 109.8±1.5 | 103.2±1.11 | 100.8±0.9 | 98.8 ±0.9 | <0.001 |
| Total cholesterol (mg/dL) | 195.6±2.2 | 204.5±2.2 | 205.8±1.9 | 206.3±1.8 | 0.001 |
| HDL-cholesterol (mg/dL) | 49.4±0.6 | 52.1±0.6 | 53.9±0.6 | 56.9±0.7 | <0.001 |
| Triglyceride (mg/dL) | 142.4±3.8 | 133.9±4.2 | 126.6±3.8 | 118.3±4.8 | <0.001 |
| Creatinine (mg/dL) | 0.8±0.01 | 0.7±0.05 | 0.7±0.01 | 0.7±0.01 | 0.002 |
| Current smoking (%) | 6.4 (1.2) | 5.3 (1.3) | 7.2 (1.3) | 7.8 (1.4) | 0.569 |
| Alcohol ingestion (%) | 28.2 (2.2) | 34.1 (2.5) | 33.8 (2.3) | 39.4 (2.3) | 0.009 |
| Physical activity (%) | 28.6 (2.3) | 36.9 (2.6) | 46.8 (2.5) | 50.8 (2.3) | <0.001 |
| Strength exercise (%) | 6.9 (1.3) | 10.9 (1.6) | 10.6 (1.4) | 15.3 (1.7) | 0.001 |
| Hypertension (%) | 46.5 (2.5) | 37.6 (2.3) | 25.2 (2.1) | 19.2 (1.9) | <0.001 |
| Dyslipidemia (%) | 30.8 (2.0) | 30.3 (2.3) | 33.3 (2.2) | 19.1 (1.7) | <0.001 |
| Diabetes mellitus (%) | 22.6 (2.1) | 10.0 (1.0) | 11.7 (1.6) | 6.3 (1.2) | <0.001 |
| Education (%) | <0.001 | ||||
| ≤ Elementary school | 56.6 (2.5) | 33.8 (2.2) | 23.6 (2.1) | 14.8 (1.6) | |
| Middle school | 16.6 (1.9) | 17.9 (2.0) | 19.2 (2.0) | 15.9 (1.9) | |
| High School | 18.6 (2.2) | 31.4 (2.2) | 37.6 (2.4) | 43.5 (2.4) | |
| ≥ College | 8.3 (1.4) | 16.9 (1.9) | 19.7 (2.0) | 25.8 (2.2) | |
| Household income (%) | <0.001 | ||||
| Quartile 1 (lowest) | 37.2 (2.5) | 23.2 (2.0) | 15.7 (1.9) | 10.1 (1.3) | |
| Quartile 2 | 27.2 (92.2) | 27.8 (2.2) | 26.5 (2.3) | 25.0 (2.2) | |
| Quartile 3 | 20.1 (2.3) | 22.5 (2.0) | 27.7 (2.2) | 24.9 (2.1) | |
| Quartile 4 (highest) | 15.5 (1.8) | 26.5 (2.2) | 30.1 (2.2) | 40.0 (2.5) | |
Note: Data presented as mean (SE) or percentages, unless otherwise indicated. P-values were calculated by weighted ANOVA test for continuous variables and weighted chi-square for categorical variables.
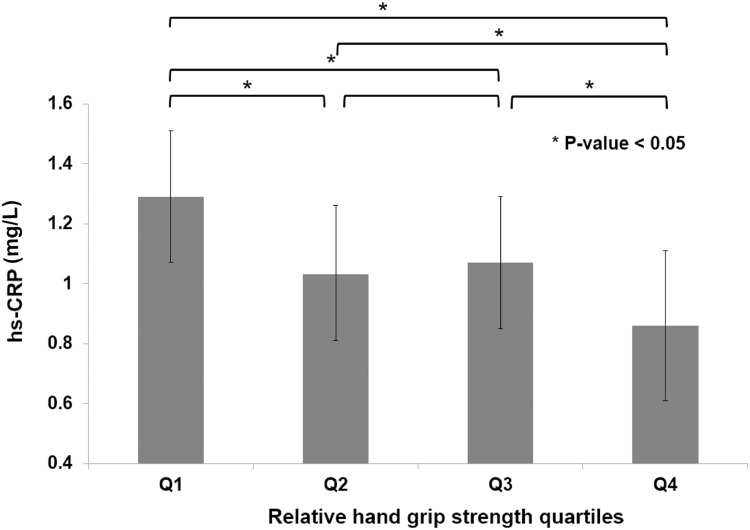
The relative HGS was inversely correlated with the log-transformed hs-CRP with r value 0.224 (p < 0.001). Figure 1 illustrates hs-CRP values according to relative HGS quartiles after adjusting for confounding factors. Mean hs-CRP gradually decreased in accordance with relative HGS quartiles. Table 2 presents the results of multiple logistic regression analysis to assess the ORs for predicting high hs-CRP in terms of relative HGS quartile. Compared to the highest quartile, the ORs (95% CIs) for high hs-CRP of the lowest quartile were 3.266 (2.227–4.789) after adjusting for age, history of hypertension, diabetes mellitus, and dyslipidemia, cigarette smoking, alcohol ingestion, physical activity, strength exercise, household income, and education level. Supplement Tables 1 and 2 show the association between relative HGS and hs-CRP based on menopausal age and BMI group.
Figure 1.
Mean hs-CRP value according to relative handgrip strength quartiles. P value was calculated using the ANOVA test.
Table 2.
Odds Ratios and 95% Confidence Intervals for High hs-CRP According to Relative Hand Grip Strength Quartiles in Postmenopausal Women
| Relative Hand Grip Strength (kg/BMI) Quartile | ||||
|---|---|---|---|---|
| Q1, <0.810 | Q2, 0.810–0.968 | Q3, 0.969–1.119 | Q4, >1.119 | |
| Unadjusted | 3.148 (2.240–4.424) | 1.893 (1.349–2.655) | 1.745 (1.240–2.456) | 1 |
| Model 1 | 3.318 (2.295–4.798) | 1.921 (1.360–2.714) | 1.824 (1.294–2.569) | 1 |
| Model 2 | 3.266 (2.227–4.789) | 1.834 (1.291–2.605) | 1.841 (1.308–2.592) | 1 |
Notes: Model 1: Adjusted for age, hypertension, diabetes mellitus, and dyslipidemia. Model 2: Adjusted for age, hypertension, diabetes mellitus, dyslipidemia, education, household income, physical activity, strength exercise, smoking, and alcohol ingestion.
Discussion
In this nationally representative cross-sectional study, low-grade inflammation, as indexed by serum hs-CRP, showed inverse association with relative HGS in postmenopausal women, independent of age, hypertension, diabetes mellitus, dyslipidemia, health behaviors (smoking status, alcohol consumption, physical activity, and strength exercise) and socioeconomic status. Our results are consistent with previous studies reporting an inverse relationship between hs-CRP and muscle strength. Several previous studies have shown that elevated hs-CRP is related to low physical performance.20,29 These findings suggest that chronic inflammation is involved in loss of physical performance, including muscle mass and strength. Inflammation may affect physical performance through microvascular impairments, hormonal changes, insulin resistance, and impairment of the central nervous system motor control.22 Also, pro-inflammatory cytokines accumulate excessive free-radical species in skeletal muscles.30 It is well known that high levels of free-radicals can damage skeletal muscle force generation.31
A more recent meta-analysis also showed that sarcopenia is associated with elevated CRP levels.32 In contrast, Kim et al reported that the inverse relationship between hs-CRP and HGS was only in older men, not in postmenopausal women.33 However, Smith et al showed inverse associations between HGS and inflammatory markers in women only, not in men.34 These discrepancies in the results may have existed for several reasons, including using different HGS indexes, differences in ethnicity, sex, and body size. However, most of the previous reports did not use relative HGS. Relative HGS has been suggested as a new marker for muscle strength because it minimizes the confounding effect of body size. Choquette et al suggested that relative HGS is superior to absolute HGS as a marker of frailty and functional decline and is a more sensitive index that can explain low physical performance.16 Furthermore, low relative HGS represents not only poor muscle strength but also high level of fat mass, which is closely related to sustained low-grade inflammation. Therefore, relative HGS seems to be more appropriate as an inflammatory related marker. In this regard, we used relative HGS and were able to demonstrate an inverse relationship between hs-CRP and muscle strength in postmenopausal women.
Loss of muscle mass and strength is a natural phenomenon of aging, but it does not progress at the same rate in men and women.35 Women tend to have a sharp decline in muscle strength in the 5th and 6th decades of age, especially after menopause.36 Previous studies have documented that several factors including physical inactivity, low-grade inflammation, protein malnutrition, and oxidative stress may contribute to sarcopenia in postmenopausal women.9,37 It has been postulated that the underlying mechanism, which could explain the association between high hs-CRP and HGS is chronic low-grade inflammation. Chronic low-grade inflammation is well known to impair muscle health through direct or intermediary mechanisms. The ubiquitin proteasome system, which is the predominant pathway to induce protein-degrading in muscle, is activated by chronic low-grade inflammation.38 Furthermore, muscle regenerative capacity is diminished with chronic inflammation because intramuscular inflammatory signaling could disrupt satellite cells, which play a key role in restoring injured muscles.39 In addition, oxidative stress might increase nuclear factor-κB (NF-κB) activity, which is involved in chronic inflammation-induced skeletal muscle degeneration.40
Low-grade chronic inflammation has been considered to play a key role in the pathogenesis of age-related diseases, such as CVD, metabolic syndrome, and cancers. Inflammatory cytokines and markers are often used to detect chronic low-grade inflammation, but laboratory testing is not always possible in some clinical settings. Our study suggests that relative HGS could be a simple and useful indicator that might help predict low-grade chronic inflammation in postmenopausal women.
Limitations of the study should be acknowledged. Our study is of cross-sectional design, which does not provide a causal explanation for the results. Therefore, long-term cohort studies are required to identify any cause-and-effect relationship between hs-CRP and relative HGS. Second, since the KNHANES was observational and collected using a cross-sectional design, other inflammatory markers such as interleukin-6 and tumor necrosis factor-α could not be evaluated. Evaluating the relationship between muscle strength and other inflammatory cytokines may help increase the reliability of the results. In addition, since various rheumatic diseases were not excluded from the study sample due to limitation of data, this may act as a confounding variable. Fourth, some potential residual confounding factors such as medication (aspirin or NSAIDs) history that affect hs-CRP level were not considered in the multiple logistic regression analysis model due to lack of information. Finally, we stratified relative HGS according to BMI, but it is still correlated with body size indexes, such as weight, height, and BMI. Other strategies are required to develop new HGS indices that are not correlated with body size instead of relative HGS. Nevertheless, this study is meaningful in that it shows a strong negative association between inflammatory marker and relative HGS adjusted for BMI.
Conclusion
In conclusion, relative HGS was inversely and independently associated with high hs-CRP level, suggesting it could be a useful tool for assessing low-grade inflammation in postmenopausal women. Relative HGS is easily measured and can be used as a quantitative tool for physicians to regularly monitor inflamm-aging and sarcopenia in postmenopausal women.
Abbreviations
CI, confidence interval; CRP, c-reactive protein; CVD, cardiovascular diseases; HDL, high-density lipoprotein; HGS, relative handgrip strength; Hs-CRP, high sensitivity C-reactive protein; KNHANES, Korean National Health and Nutrition Survey; ORs, odds ratios; SE, standard error.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Franceschi C, Bonafè M, Valensin S, et al. Inflamm‐aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908(1):244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- 2.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol a Biol Sci Med Sci. 2014;69:S4–S9. doi: 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 3.Biagi E, Candela M, Franceschi C, Brigidi P. The aging gut microbiota: new perspectives. Ageing Res Rev. 2011;10(4):428. doi: 10.1016/j.arr.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 4.Blomquist C, Alvehus M, Burén J, et al. Attenuated low-grade inflammation following long-term dietary intervention in postmenopausal women with obesity. Obesity. 2017;25(5):892–900. doi: 10.1002/oby.21815 [DOI] [PubMed] [Google Scholar]
- 5.Cevenini E, Monti D, Franceschi C. Inflamm-ageing. Curr Opin Clin Nutr Metab Care. 2013;16(1):14–20. doi: 10.1097/MCO.0b013e32835ada13 [DOI] [PubMed] [Google Scholar]
- 6.Sanada F, Taniyama Y, Muratsu J, et al. Source of chronic inflammation in aging. Front cardiovasc med. 2018;5:12. doi: 10.3389/fcvm.2018.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guarner V, Rubio-Ruiz ME. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. Interdiscip Top Gerontol. 2015;40:99–106. [DOI] [PubMed] [Google Scholar]
- 9.Geraci A, Calvani R, Ferri E, Marzetti E, Arosio B, Cesari M. Sarcopenia and menopause: the role of estradiol. Front Endocrinol. 2021;12:588. doi: 10.3389/fendo.2021.682012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J. 2016;25(11):3424–3431. doi: 10.1007/s00586-015-3805-5 [DOI] [PubMed] [Google Scholar]
- 11.Sjoblom S, Suuronen J, Rikkonen T, Honkanen R, Kroger H, Sirola J. Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas. 2013;75(2):175–180. doi: 10.1016/j.maturitas.2013.03.016 [DOI] [PubMed] [Google Scholar]
- 12.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. doi: 10.1016/j.jamda.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 14.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol a Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L-K, Lee W-J, Peng L-N, et al. Recent advances in sarcopenia research in Asia: 2016 update from the Asian working group for sarcopenia. J Am Med Dir Assoc. 2016;17(8):767 e1–767 e7. doi: 10.1016/j.jamda.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 16.Choquette S, Bouchard DR, Doyon CY, Sénéchal M, Brochu M, Dionne IJ. Relative strength as a determinant of mobility in elders 67–84 years of age. a nuage study: nutrition as a determinant of successful aging. J Nutr Health Aging. 2010;14(3):190–195. doi: 10.1007/s12603-010-0047-4 [DOI] [PubMed] [Google Scholar]
- 17.Yi DW, Khang AR, Lee HW, Son SM, Kang YH. Relative handgrip strength as a marker of metabolic syndrome: the Korea national health and nutrition examination survey (KNHANES) VI (2014–2015). Diabetes Metab Syndr Obes. 2018;11:227–240. doi: 10.2147/DMSO.S166875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawman HG, Troiano RP, Perna FM, Wang CY, Fryar CD, Ogden CL. Associations of relative handgrip strength and cardiovascular disease biomarkers in US adults, 2011–2012. Am J Prev Med. 2016;50(6):677–683. doi: 10.1016/j.amepre.2015.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee WJ, Peng LN, Chiou ST, Chen LK. Relative handgrip strength is a simple indicator of cardiometabolic risk among middle-aged and older people: a nationwide population-based study in Taiwan. PLoS One. 2016;11(8):e0160876. doi: 10.1371/journal.pone.0160876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niu K, Hozawa A, Guo H, et al. Serum C-reactive protein even at very low (<1.0 mg/l) concentration is associated with physical performance in a community-based elderly population aged 70 years and over. Gerontology. 2008;54(5):260–267. doi: 10.1159/000134286 [DOI] [PubMed] [Google Scholar]
- 21.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119(6):526 e9–526 e17. doi: 10.1016/j.amjmed.2005.10.049 [DOI] [PubMed] [Google Scholar]
- 22.Tuttle CS, Thang LA, Maier AB. Markers of inflammation and their association with muscle strength and mass: a systematic review and meta-analysis. Ageing Res Rev. 2020;64:101185. doi: 10.1016/j.arr.2020.101185 [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, Jung JH, Park S. Changes in high-sensitivity C-reactive protein levels and metabolic indices according to grip strength in Korean postmenopausal women. Climacteric. 2021;25(3):306–310. doi: 10.1080/13697137.2021.1965116. [DOI] [PubMed] [Google Scholar]
- 24.Kim BM, Yi YH, Kim YJ, et al. Association between relative handgrip strength and dyslipidemia in Korean adults: findings of the 2014–2015 Korea national health and nutrition examination survey. Korean J Fam Med. 2020;41(6):404. doi: 10.4082/kjfm.19.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 26.Lee Y-J, Lee J-H, Shin Y-H, Kim J-K, Lee H-R, Lee D-C. Gender difference and determinants of C-reactive protein level in Korean adults. Clin Chem Lab Med. 2009;47(7):863–869. doi: 10.1515/CCLM.2009.196 [DOI] [PubMed] [Google Scholar]
- 27.Park S-W, Park -S-S, Kim E-J, Sung W-S, Ha I-H, Jung B. Sex differences in the association between self-rated health and high-sensitivity C-reactive protein levels in Koreans: a cross-sectional study using data from the Korea National Health And Nutrition Examination Survey. Health Qual Life Outcomes. 2020;18(1):1–10. doi: 10.1186/s12955-020-01597-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Momiyama Y, Kawaguchi A, Kajiwara I, et al. Prognostic value of plasma high-sensitivity C-reactive protein levels in Japanese patients with stable coronary artery disease: the Japan NCVC-collaborative inflammation cohort (JNIC) study. Atherosclerosis. 2009;207(1):272–276. doi: 10.1016/j.atherosclerosis.2009.04.015 [DOI] [PubMed] [Google Scholar]
- 29.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: macArthur studies of successful aging. J Gerontol a Biol Sci Med Sci. 2000;55(12):M709–M715. doi: 10.1093/gerona/55.12.M709 [DOI] [PubMed] [Google Scholar]
- 30.Supinski GS, Callahan LA. Free radical-mediated skeletal muscle dysfunction in inflammatory conditions. J Appl Physiol. 2007;102(5):2056–2063. doi: 10.1152/japplphysiol.01138.2006 [DOI] [PubMed] [Google Scholar]
- 31.Reid MB. Nitric oxide, reactive oxygen species, and skeletal muscle contraction. Med Sci Sports Exerc. 2001;33(3):371–376. doi: 10.1097/00005768-200103000-00006 [DOI] [PubMed] [Google Scholar]
- 32.Bano G, Trevisan C, Carraro S, et al. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. 2017;96:10–15. doi: 10.1016/j.maturitas.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 33.Kim BJ, Lee SH, Kwak MK, Isales CM, Koh JM, Hamrick MW. Inverse relationship between serum hsCRP concentration and hand grip strength in older adults: a nationwide population-based study. Aging. 2018;10(8):2051–2061. doi: 10.18632/aging.101529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith L, Yang L, Hamer M. Handgrip strength, inflammatory markers, and mortality. Scand J Med Sci Sports. 2019;29(8):1190–1196. doi: 10.1111/sms.13433 [DOI] [PubMed] [Google Scholar]
- 35.Maltais ML, Desroches J, Dionne IJ. Changes in muscle mass and strength after menopause. J Musculoskelet Neuronal Interact. 2009;9(4):186–197. [PubMed] [Google Scholar]
- 36.Carville SF, Rutherford OM, Newham DJ. Power output, isometric strength and steadiness in the leg muscles of pre- and postmenopausal women; the effects of hormone replacement therapy. Eur J Appl Physiol. 2006;96(3):292–298. doi: 10.1007/s00421-005-0078-4 [DOI] [PubMed] [Google Scholar]
- 37.Zacarías-Flores M, Sánchez-Rodríguez MA, García-Anaya OD, Correa-Muñoz E, Mendoza-Núñez VM. Relationship between oxidative stress and muscle mass loss in early postmenopause: an exploratory study. Endocrinología Diabetes Nutrición. 2018;65:328–334. [DOI] [PubMed] [Google Scholar]
- 38.Cao PR, Kim HJ, Lecker SH. Ubiquitin-protein ligases in muscle wasting. Int J Biochem Cell Biol. 2005;37(10):2088–2097. doi: 10.1016/j.biocel.2004.11.010 [DOI] [PubMed] [Google Scholar]
- 39.Ogura Y, Mishra V, Hindi SM, Kuang S, Kumar A. Proinflammatory cytokine tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK) suppresses satellite cell self-renewal through inversely modulating notch and NF-kappaB signaling pathways. J Biol Chem. 2013;288(49):35159–35169. doi: 10.1074/jbc.M113.517300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scicchitano BM, Pelosi L, Sica G, Musarò A. The physiopathologic role of oxidative stress in skeletal muscle. Mech Ageing Dev. 2018;170:37–44. doi: 10.1016/j.mad.2017.08.009 [DOI] [PubMed] [Google Scholar]