Abstract
Bacterial isolates were obtained from pine (Pinus sylvestris L.) tissue cultures and identified as Methylobacterium extorquens and Pseudomonas synxantha. The existence of bacteria in pine buds was investigated by 16S rRNA in situ hybridization. Bacteria inhabited the buds of every tree examined, primarily colonizing the cells of scale primordia and resin ducts.
Microorganisms are common companions to plant roots, but there have been fewer reports describing their existence in the aerial parts of plants. For this reason the plant shoot tissues, especially the meristems, have widely been considered sterile. Nevertheless, the vascular tissues are regularly colonized by bacteria (1, 2, 11, 12), and some fungi are known to inhabit the leaves of plants (5, 13, 24, 25).
Plant meristematic tissues are generally utilized as starting material in plant tissue culture. However, the tissue cultures are frequently occupied by different bacteria, fungi, or yeasts which may not become visible at any stage of the culture. The presence of microorganisms is usually explained as being the result of an insufficient surface sterilization process or of laboratory contamination (22), although some scientists have suggested that they are endophytes (17, 18). Microorganisms are also found in the tissue cultures of Scotch pine (Pinus sylvestris L.) (15). We considered the presence of bacteria to be one potential cause of the low regeneration capacity of mature trees. Therefore, we decided to study these bacteria in detail.
A total of 160 buds were collected from mature, healthy trees on a natural stand in northern Finland. The buds were surface sterilized for 1 min in 70% ethanol and for 20 min in 6% calcium hypochlorite, which was found to be effective (reference 29 and data not shown). After being rinsed, the buds were aseptically peeled and placed on a modified Murashige and Skoog medium (15, 26). Of the tissue cultures, 16.7% were found to be inhabited by microorganisms. We obtained five bacterial isolates from 1- to 2-week-old tissue cultures and during protoplast isolation (16) from 2- to 3-week-old calli. The isolates were transferred onto Luria broth plates for further cultivation.
The 16S rRNA genes (rDNAs) from bacterial DNAs (3, 4) were amplified by using universal primers (19). The PCR products were cloned (28) and sequenced (ABI Prism 377 DNA sequencer; Perkin-Elmer). The 16S rDNA sequences were aligned with all accessible sequences, obtained through the Ribosomal Database Project (RDP), with the program Sequence Alignment (23). Four sequences (G, H, I, and J) were identical and aligned with that of Pseudomonas azotoformans. One sequence (F) aligned with Methylobacterium sp. strain GK 101 (14). Members of these genera had not been cultured in our laboratory before, and the tissue culture procedures were performed aseptically to prevent contamination of the cultures. No bacterial growth was observed in mock cultures comprising all the media and chemicals used in the tissue culture and protoplast isolation procedures. Isolates F and G were chosen for further analysis.
Oligonucleotide probes complementary to the 16S rDNAs of the isolates were designed (MB, 5′-AGCGCCGTCGGGTAAGA-3′, for Methylobacterium, corresponding to Escherichia coli positions 1388 to 1371; and PS5, 5′-GCAGAGTATTAATCTACAACC-3′, for Pseudomonas synxantha and related species of the Pseudomonas fluorescens subgroup, E. coli positions 468 to 448) by using the programs Sequence Match and Probe Match (RDP) (23). Also, a probe that hybridized to eubacterial but not chloroplast or mitochondrial rRNA sequences (E11, 5′-AGCCATGCAGCACCTGTCTC-3′, E. coli positions 1065 to 1045) was created. Probes MB and PS5 showed at least one mismatch with all accessible 16S rRNA sequences obtained through the RDP. The oligonucleotides were labeled with digoxigenin (Boehringer Mannheim).
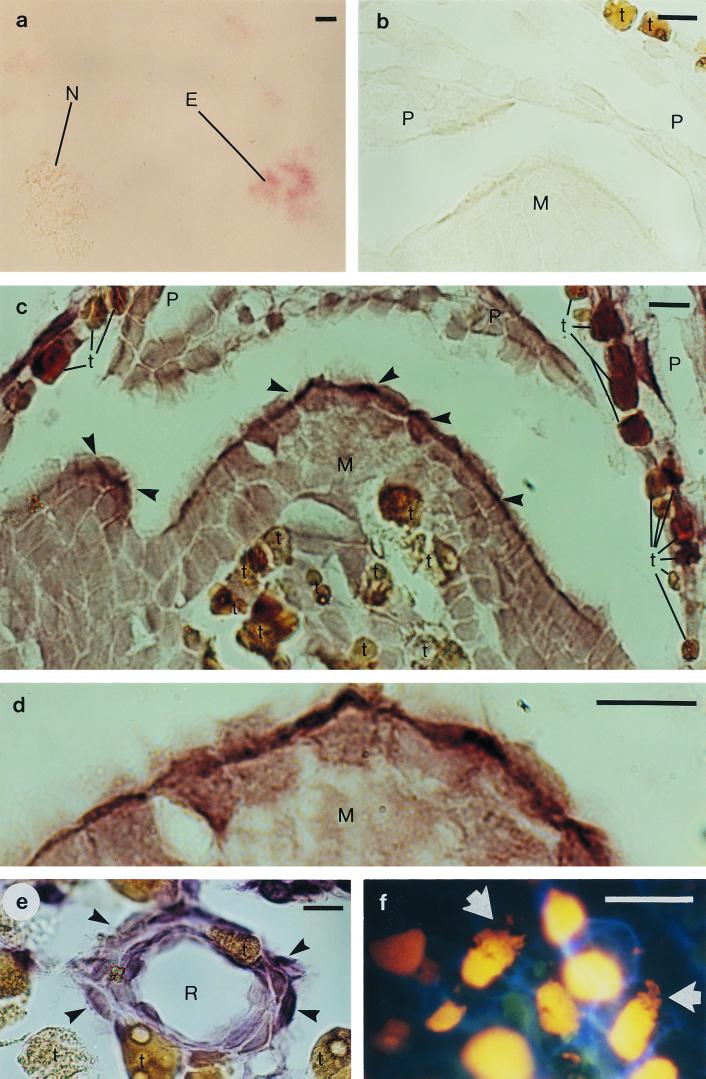
Stringent conditions for the in situ hybridization were determined by hybridizing the probes with pine chloroplasts (27), cells of pine-associated bacteria (the bacteria under study and Mycobacterium sp. [21]), and two control bacteria (E. coli DH5α and Lactobacillus delbrueckii subsp. lactis). The chloroplasts and cells were fixed (6) and attached to slides coated with 3-aminopropyltriethoxysilane (Sigma) by baking at 55°C overnight. The stringency of the hybridization was adjusted by gradually increasing the formamide concentration (31) in the hybridization buffer, which contained 3× SET (6), Denhardt's solution (28), 0.02% tRNA (Sigma), 0.02% poly(A) (Sigma), 10% dextran sulfate (Merck), 50 mM dithiothreitol (Calbiochem), and the probe (0.5 ng/μl). Hybridization was performed at 38°C overnight, after which the slides were washed with 2× SET at room temperature for 15 min and with 0.1× SET at 53°C for 15 min. Detection was performed with the DIG nucleic acid detection kit (Boehringer Mannheim). For E11, MB, and PS5, high levels of specificity were obtained at formamide concentrations of 20, 30, and 35%, respectively. Probe E11 was found to hybridize to eubacterial but not pine chloroplast or mitochondrial rRNA (Fig. 1a).
FIG. 1.
In situ hybridization of pine (Pinus sylvestris L.) bud tissue with digoxigenin-labeled oligonucleotides complementary to bacterial 16S rRNA sequences, and DNA staining of pine bud tissue. (a) Pine needle chloroplasts, mitochondria, and E. coli cells, hybridized with the eubacterial probe E11. The signal is detected in the E. coli cells (E) but not in the chloroplasts or mitochondria (N). (b) A negative control: apical meristem (M) and scale primordia (P), hybridized without a probe. (c) Apical meristem and scale primordia, hybridized with probe E11. The strongest hybridization signal was detected in the outermost cells of the meristem (arrowhead). For differentiation from positively hybridized cells, the naturally brown cells of the pine tissue, which contain tannins and other phenolic compounds, are marked with a t. (d) A magnification of the apical meristem from panel c. (e) A resin duct (R), hybridized with Methylobacterium-specific probe MB. The hybridization signal was detected in the cells surrounding the resin duct (arrowhead). (f) Ethidium bromide staining, revealing irregular structures (arrow) inside the cells, in addition to nuclei (yellow), surrounded by the cell walls (blue). Scale bar = 20 μm.
The specificities of the probes were confirmed by dot blot hybridization. DNA samples, extracted from pine buds (20), E. coli, the Mycobacterium sp., and the two bacteria under study (3, 4), were immobilized on a nitrocellulose membrane (Amersham) and hybridized overnight at 38°C in a buffer comprising 3× SET, 0.1% sodium lauroyl sarcosine (Sigma), 0.02% sodium lauryl sulfate (Sigma), 1% blocking reagent (Boehringer Mannheim), 0.01% poly(A), formamide at a probe-specific concentration, and the probe (1 μg/ml). The membrane was washed and its contents were detected as described above. Probes MB and PS5 hybridized specifically to their targets and also to the DNA isolated from pine buds (data not shown).
Probes E11, MB, and PS5 were then hybridized into pine buds under aseptic conditions. The buds (five, from separate branches of a tree) were collected from mature, healthy trees (three per area) at three different locations in northern Finland (Oulu, Tyrnävä, and Sodankylä). Surface sterilized, peeled buds were fixed in a solution containing 0.1 M NaH2PO4-Na2HPO4 (pH 7.4), 2% paraformaldehyde, and 2.5% glutaraldehyde at 4°C overnight, dehydrated, cleared through an ethanol–t-butanol series, and embedded in paraffin (Merck). The paraffin sections were baked on silane-coated slides; then the paraffin was removed. Altogether, 45 buds were examined after preparation of one to four samples per bud. For each experiment, all three probes, E11, MB, and PS5, were hybridized as described above, and a control without a probe (Fig. 1b) was included every time.
The eubacterial probe E11 hybridized to every bud specimen, particularly in the cells of scale primordia (Fig. 1c). Bacterial rRNA was less abundant in the apical meristem itself, but a signal was usually detected in its outermost cells (Fig. 1c and d) and in the cells of the developing stem, right below the apical meristem. Bacterial rRNA was also abundant in the epithelial cells of the resin ducts (Fig. 1e), but it was detected less frequently in the vascular tissue and in the intercellular spaces. Negative-control hybridizations with sense-strand probes did not result in signal detection. When an RNA probe targeted to 25S rRNA of pea (Pisum sativum) (32) served as the positive control for pine cytoplasmic ribosomes, the hybridization pattern was completely different from that detected with the bacterial probes (data not shown).
DNA staining of the pine bud tissue was performed to support our results. RNase A-treated thin cuts (Sigma; 50 μg/ml in 10 mM Tris-HCl [pH 7.5]–1 mM EDTA for 30 min at 37°C) were rinsed in water, air dried, and stained with ethidium bromide (10 μg/ml). The slides were then viewed immediately under immersion oil (fluor objectives, episcopic-fluorescence attachment EF-D, filter set UV-1A; Nikon). A pronounced distribution of stained DNA in the cytosol of some distinct cells was observed in areas indicated by the in situ hybridization, supporting the presence of bacteria in the tissue (Fig. 1f).
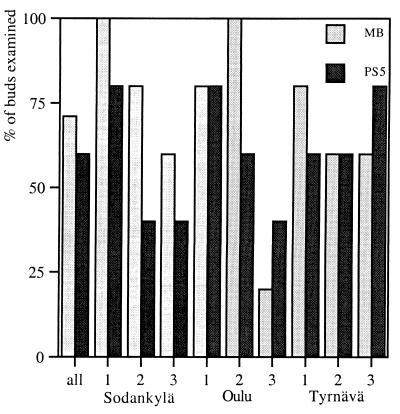
Probes MB (specific for isolate F and other methylobacteria) and PS5 (specific for isolate G and related species) hybridized to the same parts of the pine bud tissue as did the eubacterial probe E11. The two genera inhabited every tree examined; typically, there were MB- or PS5-specific transcripts in three or four buds per tree. MB and PS5 hybridized with 71 and 60% of the specimens, respectively (Fig. 2). Since bacterial expression was found with E11 but not with MB or PS5 in some specimens, other bacteria must be present in the pine buds.
FIG. 2.
Hybridization of MB (specific for Methylobacteria) (gray bars) and PS5 (specific for P. synxantha and relatives) (black bars) into bud specimens collected from three trees in the three sample areas, expressed as a percentage of total number of buds collected (five buds per tree).
Physiological tests for the identification of the bacterial isolates were performed by Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (Braunschweig, Germany). The cellular fatty acid profile and the physiological test results for isolate F were typical of M. extorquens (Table 1), and the partial sequence of the 16S rDNA was 98.4% similar to the 16S rDNA of M. extorquens. The cellular fatty acids of isolate G were typical of the fluorescing RNA group I pseudomonads. The partial sequence of the 16S rDNA was most similar to those of P. azotoformans (99.7%), P. synxantha (99.4%), and P. mucidolens (99.4%). The physiological characteristics pointed to P. synxantha (Table 1).
TABLE 1.
Physiological characteristics of isolates F and G
| Test | Result for isolatea:
|
|
|---|---|---|
| F | G | |
| Shape of cells | Irregular rods | Rods |
| Width of cells (μm) | 0.8–1.0 | 0.5–0.6 |
| Length of cells (μm) | 1.5–3.0 | 1.8–2.5 |
| Motility | ND | + |
| No. of flagella | ND | >1 |
| Gram reaction | − | − |
| Lysis by 3% KOH | w | + |
| Aminopeptidase (Cerny) | + | + |
| Oxidase | + | + |
| Catalase | + | + |
| Color of colonies | Red | Transparent, bluish |
| Pigment fluorescence | ND | + |
| Pyocyanin | ND | − |
| Arginine dihydrolase | − | + |
| NO3 utilization | − | − |
| Denitrification | − | − |
| Urease | ||
| 24 h | − | − |
| 5 days | + | ND |
| Hydrolysis of: | ||
| Gelatin | + | + |
| Esculin | ND | − |
| Levan from sucrose | ND | + |
| Lecithinase | ND | + |
| Indol reaction | − | ND |
| Growth at: | ||
| 41°C | ND | − |
| 37°C | ND | − |
| Utilization of: | ||
| N-Acetylglucosamine | ND | + |
| Adipate | ND | − |
| Arabinose | − | + |
| l-Aspartate | + | ND |
| Betaine | + | ND |
| Caprate | − | + |
| Citraconate | ND | − |
| Citrate | − | + |
| Erythritol | ND | + |
| Ethanol | + | ND |
| Fructose | − | ND |
| Gluconate | − | + |
| Glucose | − | + |
| m-Inositol | ND | + |
| Malate | + | + |
| Maltose | − | ND |
| Mannitol | − | + |
| Mannose | − | ND |
| Methanol | + | ND |
| Phenylacetate | − | − |
| Sebacinate | − | ND |
| Sorbitol | ND | + |
| Trehalose | ND | + |
| Trimethylamine | − | ND |
| d-Xylose | − | ND |
ND, not detected; +, positive; w, weakly positive; −, negative traces
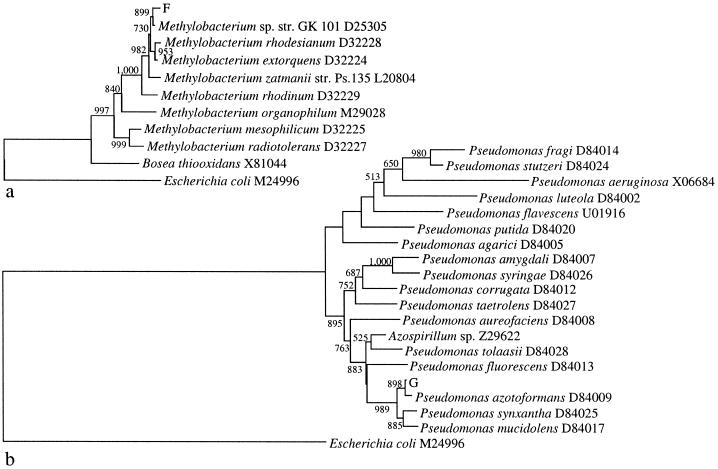
The phylogenetic analysis was performed first with all sequences of the genera available through the RDP and then with the best-characterized strains. The sequences were aligned by using ClustalW (30), and gap positions were excluded manually. A distance matrix was created with the program DNADIST of the Phylip software (8), from which the tree topology was built in NEIGHBOR. Figure 3 contains two neighbor-joining trees showing the phylogenetic positions of bacterial isolates F and G. Isolate F formed a cluster with Methylobacterium sp. strain GK 101 (14), supported by a bootstrap value of 899. Isolate G formed a cluster with P. azotoformans, P. synxantha, and P. mucidolens, supported by a bootstrap value of 989.
FIG. 3.
Phylogenetic positions of pine bud tissue-associated bacterial isolates F and G. The 16S rDNA sequences of the two isolates, E. coli (outgroup), and representatives of the genera Methylobacterium (a) and Pseudomonas (b) were analyzed by the neighbor-joining method. Values from 1,000 bootstrap repeats are presented if support was >50%. All sequences were obtained from the RDP, and the species' names are followed by the corresponding strain GenBank accession numbers.
Taken together, the bacteria isolated from the pine buds were classified as new strains of M. extorquens and P. synxantha. They might be pathogens, but to our knowledge, phytopathogenic strains of P. synxantha or Methylobacterium have not been identified (22). Instead, several members of the genus Methylobacterium produce cytokinins (17, 18), and M. extorquens has been found to participate in the flavor biosynthesis of the strawberry (33).
Pseudomonads and methylobacteria are regularly found in plant tissue cultures (22). To our knowledge, the origin of the bacteria in the plant tissue has not yet been studied. We used a 16S rRNA in situ hybridization method which has often been employed in studying endosymbionts of invertebrates (7, 9, 10), and our results provide the first direct evidence that bacteria exist in pine bud cells. Bacteria appear to be regular inhabitants of pine bud tissue, since we found bacteria in every bud examined. Investigations of bacterial metabolites and studies of bacterial colonization in other tissues would yield more knowledge of the role of the microbes.
Nucleotide sequence accession numbers. M. extorquens (isolate F) and P. synxantha (isolate G) were deposited in DSMZ under accession numbers DSM 13060 and DSM 13080, respectively, and the 16S rDNA sequences were submitted to GenBank under accession numbers AF267912 and AF267911, respectively.
Acknowledgments
We thank M.-A. Alenius for providing the contaminated tissue cultures, L. Räisänen for the Lactobacillus strain, R. Ruonala for technical advice, and C. Ruuskanen for revision of the English in the manuscript.
This work was supported by the Marjatta and Eino Kolli Foundation, the Ahti Pekkala Foundation, and the Tauno Tönning Foundation.
REFERENCES
- 1.Baldani J I, Caruso L, Baldani V L D, Goi S R, Döbereiner J. Recent advances in BNF with non-legume plants. Soil Biol Biochem. 1997;29:911–922. [Google Scholar]
- 2.Bell C R, Dickie G A, Harvey W L G, Chan J W Y F. Endophytic bacteria in grapevine. Can J Microbiol. 1995;41:46–53. [Google Scholar]
- 3.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bollet C, Gevaudan M J, de Lamballerie X, Zandotti C, de Micco P. A simple method for the isolation of chromosomal DNA from gram positive or acid-fast bacteria. Nucleic Acids Res. 1991;19:1955. doi: 10.1093/nar/19.8.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll G C, Carroll F E. Studies on the incidence of coniferous needle endophytes in the Pacific Northwest. Can J Bot. 1978;56:3034–3043. [Google Scholar]
- 6.DeLong E F, Wickham G S, Pace N R. Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells. Science. 1989;243:1360–1363. doi: 10.1126/science.2466341. [DOI] [PubMed] [Google Scholar]
- 7.Dubilier N, Giere O, Distel D L, Cavanaugh C M. Characterization of chemoautotrophic bacterial symbionts in a gutless marine worm (Oligochaeta, Annelida) by phylogenetic 16S rRNA sequence analysis and in situ hybridization. Appl Environ Microbiol. 1995;61:2346–2350. doi: 10.1128/aem.61.6.2346-2350.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felsenstein J. Phylip—phylogeny inference package. Cladistics. 1989;5:164–166. [Google Scholar]
- 9.Fritsche T R, Horn M, Seyedirashti S, Gautom R K, Schleifer K-H, Wagner M. In situ detection of novel bacterial endosymbionts of Acanthamoeba spp. phylogenetically related to members of the order Rickettsiales. Appl Environ Microbiol. 1999;65:206–212. doi: 10.1128/aem.65.1.206-212.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukatsu T, Nikoh N. Two intracellular symbiotic bacteria from the mulberry psyllid Anomoneura mori (Insecta, Homoptera) Appl Environ Microbiol. 1998;64:3599–3606. doi: 10.1128/aem.64.10.3599-3606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner J M, Feldman A W, Zablotowicz R M. Identity and behavior of xylem-residing bacteria in rough lemon roots of Florida citrus trees. Appl Environ Microbiol. 1982;43:1335–1342. doi: 10.1128/aem.43.6.1335-1342.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallman J, Quadt-Hallman A, Mahaffee W F, Kloepper J W. Bacterial endophytes in agricultural crops. Can J Microbiol. 1997;43:895–914. [Google Scholar]
- 13.Hata K, Futai K. Variation in fungal endophyte populations in needles of the genus Pinus. Can J Bot. 1996;74:103–114. [Google Scholar]
- 14.Hiraishi A, Kaneko M. Use of polymerase chain reaction-amplified 16S rRNA gene sequences to identify pink-pigmented bacteria found in a potable water treatment system. Bull Jpn Soc Microb Ecol. 1994;9:55–65. [Google Scholar]
- 15.Hohtola A. Seasonal changes in explant viability and contamination of tissue cultures from mature Scots pine. Plant Cell Tissue Organ Cult. 1988;15:211–222. [Google Scholar]
- 16.Hohtola A, Kvist A-P. Preparation of protoplasts from callus derived from buds of mature Scots pine and subsequent induction of cell proliferation. Tree Physiol. 1991;8:423–428. [Google Scholar]
- 17.Holland M A. Occams razor applied to hormonology. Are cytokinins produced by plants? Plant Physiol. 1997;115:865–868. doi: 10.1104/pp.115.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holland M A, Polacco J C. PPFMs and other covert contamination: is there more to plant physiology than just plant? Annu Rev Plant Physiol Plant Mol Biol. 1994;45:197–209. [Google Scholar]
- 19.Jalava J, Kotilainen P, Nikkari S, Skurnik M, Vanttinen E, Lehtonen O P, Eerola E, Toivanen P. Use of the polymerase chain reaction and DNA sequencing for detection of Bartonella quintana in the aortic valve of a patient with culture-negative infective endocarditis. Clin Infect Dis. 1995;21:891–896. doi: 10.1093/clinids/21.4.891. [DOI] [PubMed] [Google Scholar]
- 20.Jobes D V, Hurley D L, Thien L B. Plant DNA isolation: a method to efficiently remove polyphenolics, polysaccharides, and RNA. Taxon. 1995;44:379–386. [Google Scholar]
- 21.Laukkanen, H., H. Soini, S. Kontunen-Soppela, A. Hohtola, and M. Viljanen. Tree Physiol., in press. [DOI] [PubMed]
- 22.Leifert C, Morris C E, Waites W M. Ecology of microbial saprophytes and pathogens in tissue culture and field-grown plants: reasons for contamination problems in vitro. Crit Rev Plant Sci. 1994;13:139–183. [Google Scholar]
- 23.Maidak B L, Cole J R, Parker C T, Jr, Garrity G M, Larsen N, Li B, Lilburn T G, McCaughey M J, Olsen G J, Overbeek R, Pramanik S, Schmidt T M, Tiedje J M, Woese C R. A new version of the RDP (Ribosomal Database Project) Nucleic Acids Res. 1999;27:171–173. doi: 10.1093/nar/27.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miles C O, di Menna M E, Jacobs S W L, Garthwaite I, Lane G A, Prestidge R A, Marshall S L, Wilkinson H H, Schardl C L, Ball O J-P, Latch G C M. Endophytic fungi in indigenous Australasian grasses associated with toxicity to livestock. Appl Environ Microbiol. 1998;64:601–606. doi: 10.1128/aem.64.2.601-606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller M M, Hallaksela A-M. Diversity of Norway spruce needle endophytes in various mixed and pure Norway spruce stands. Mycol Res. 1998;102:1183–1189. [Google Scholar]
- 26.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 27.Oku T, Tomita G. Photoactivation of oxygen-evolving system in dark-grown spruce seedlings. Physiol Plant. 1976;38:181–185. [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Schultz B, Wanke U, Draeger S, Aust H-J. Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilization methods. Mycol Res. 1993;97:1447–1450. [Google Scholar]
- 30.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner M, Erhart R, Manz W, Amann R, Lemmer H, Wedi D, Schleifer K-H. Development of an rRNA-targeted oligonucleotide probe specific for the genus Acinetobacter and its application for in situ monitoring in activated sludge. Appl Environ Microbiol. 1994;60:792–800. doi: 10.1128/aem.60.3.792-800.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanner L, Gruissem W. Expression dynamics of the tomato rbcS gene family during development. Plant Cell. 1991;3:1289–1303. doi: 10.1105/tpc.3.12.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zabetakis I. Enhancement of flavour biosynthesis from strawberry (Fragraria × ananassa) callus cultures by Methylobacterium species. Plant Cell Tissue Organ Cult. 1997;50:179–183. [Google Scholar]