Abstract
Background
Fractures of the proximal humerus, often termed shoulder fractures, are common injuries, especially in older people. The management of these fractures varies widely, including in the use of surgery. This is an update of a Cochrane Review first published in 2001 and last updated in 2015.
Objectives
To assess the effects (benefits and harms) of treatment and rehabilitation interventions for proximal humeral fractures in adults.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, trial registries, and bibliographies of trial reports and systematic reviews to September 2020. We updated this search in November 2021, but have not yet incorporated these results.
Selection criteria
We included randomised and quasi‐randomised controlled trials that compared non‐pharmacological interventions for treating acute proximal humeral fractures in adults.
Data collection and analysis
Pairs of review authors independently selected studies, assessed risk of bias and extracted data. We pooled data where appropriate and used GRADE for assessing the certainty of evidence for each outcome. We prepared a brief economic commentary for one comparison.
Main results
We included 47 trials (3179 participants, mostly women and mainly aged 60 years or over) that tested one of 26 comparisons. Six comparisons were tested by 2 to 10 trials, the others by small single‐centre trials only. Twelve studies evaluated non‐surgical treatments, 10 compared surgical with non‐surgical treatments, 23 compared two methods of surgery, and two tested timing of mobilisation after surgery. Most trials were at high risk of bias, due mainly to lack of blinding. We summarise the findings for four key comparisons below.
Early (usually one week post injury) versus delayed (after three or more weeks) mobilisation for non‐surgically‐treated fractures
Five trials (350 participants) made this comparison; however, the available data are very limited. Due to very low‐certainty evidence from single trials, we are uncertain of the findings of better shoulder function at one year in the early mobilisation group, or the findings of little or no between‐group difference in function at 3 or 24 months. Likewise, there is very low‐certainty evidence of no important between‐group difference in quality of life at one year. There was one reported death and five serious shoulder complications (1.9% of 259 participants), spread between the two groups, that would have required substantive treatment.
Surgical versus non‐surgical treatment
Ten trials (717 participants) evaluated surgical intervention for displaced fractures (66% were three‐ or four‐part fractures). There is high‐certainty evidence of no clinically important difference between surgical and non‐surgical treatment in patient‐reported shoulder function at one year (standardised mean difference (SMD) 0.10, 95% confidence interval (CI) ‐0.07 to 0.27; 7 studies, 552 participants) and two years (SMD 0.06, 95% CI ‐0.13 to 0.25; 5 studies, 423 participants). There is moderate‐certainty evidence of no clinically important between‐group difference in patient‐reported shoulder function at six months (SMD 0.17, 95% CI ‐0.04 to 0.38; 3 studies, 347 participants). There is high‐certainty evidence of no clinically important between‐group difference in quality of life at one year (EQ‐5D (0: dead to 1: best quality): mean difference (MD) 0.01, 95% CI ‐0.02 to 0.04; 6 studies, 502 participants). There is low‐certainty evidence of little between‐group difference in mortality: one of the 31 deaths was explicitly linked with surgery (risk ratio (RR) 1.35, 95% CI 0.70 to 2.62; 8 studies, 646 participants). There is low‐certainty evidence of a higher risk of additional surgery in the surgery group (RR 2.06, 95% CI 1.21 to 3.51; 9 studies, 667 participants). Based on an illustrative risk of 35 subsequent operations per 1000 non‐surgically‐treated patients, this indicates an extra 38 subsequent operations per 1000 surgically‐treated patients (95% CI 8 to 94 more). Although there was low‐certainty evidence of a higher overall risk of adverse events after surgery, the 95% CI also includes a slightly increased risk of adverse events after non‐surgical treatment (RR 1.46, 95% CI 0.92 to 2.31; 3 studies, 391 participants).
Open reduction and internal fixation with a locking plate versus a locking intramedullary nail
Four trials (270 participants) evaluated surgical intervention for displaced fractures (63% were two‐part fractures). There is low‐certainty evidence of no clinically important between‐group difference in shoulder function at one year (SMD 0.15, 95% CI ‐0.12 to 0.41; 4 studies, 227 participants), six months (Disability of the Arm, Shoulder, and Hand questionnaire (0 to 100: worst disability): MD ‐0.39, 95% CI ‐4.14 to 3.36; 3 studies, 174 participants), or two years (American Shoulder and Elbow Surgeons score (ASES) (0 to 100: best outcome): MD 3.06, 95% CI ‐0.05 to 6.17; 2 studies, 101 participants). There is very low‐certainty evidence of no between‐group difference in quality of life (1 study), and of little difference in adverse events (4 studies, 250 participants) and additional surgery (3 studies, 193 participants).
Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty
There is very low‐certainty evidence from two trials (161 participants with either three‐ or four‐part fractures) of no or minimal between‐group differences in self‐reported shoulder function at one year (1 study) or at two to three years' follow‐up (2 studies); or in quality of life at one year or at two or more years' follow‐up (1 study). Function at six months was not reported. Of 10 deaths reported by one trial (99 participants), one appeared to be surgery‐related. There is very low‐certainty evidence of a lower risk of complications after RTSA (2 studies). Ten people (6.2% of 161 participants) had a reoperation; all eight cases in the hemiarthroplasty group received a RTSA (very low‐certainty evidence).
Authors' conclusions
There is high‐ or moderate‐certainty evidence that, compared with non‐surgical treatment, surgery does not result in a better outcome at one and two years after injury for people with displaced proximal humeral fractures. It may increase the need for subsequent surgery. The evidence is absent or insufficient for people aged under 60 years, high‐energy trauma, two‐part tuberosity fractures or less common fractures, such as fracture dislocations and articular surface fractures.
There is insufficient evidence from randomised trials to inform the choices between different non‐surgical, surgical or rehabilitation interventions for these fractures.
Plain language summary
What are the best ways of treating adults with a fractured (broken) shoulder?
Key messages
‐ There is not enough evidence to tell whether early movement of the arm after one week in a sling makes a difference to long‐term shoulder function or the development of shoulder problems compared with supporting the arm in a sling for three or more weeks.
‐ Patients report that surgery does not result in better shoulder function for most types of displaced fractures (where the broken parts have moved apart) than non‐surgical treatment. However, it may result in a higher risk of follow‐up surgery for complications.
‐ If surgery is undertaken, there is not enough evidence to say what is the best method.
What are proximal humeral fractures?
The proximal humerus is the top end of the upper arm bone. Fracture of the proximal humerus is a common and serious injury in older people. It is often called a broken (fractured) shoulder. It can take several months for people to recover the use of their arm. Some restrictions in movement and pain are common long‐term problems.
What are the usual ways of treating these fractures?
Treatments include:
‐ non‐surgical treatment: the injured arm is supported in a sling for one or more weeks;
‐ surgery: used for ‘displaced’ fractures, where the broken parts have moved apart. Surgery may involve bringing the parts back in place and fixing these with screws in a metal plate or with a nail placed in the bone marrow. Alternatively, in old people, half or all of the ball and socket shoulder joint might be replaced with a metal implant. In hemiarthroplasty, just the ball (humeral head) of the shoulder joint is replaced. The use of reverse total shoulder arthroplasty (RTSA) is increasing. As well as replacing the whole joint, the positions of the ball and the socket joint are reversed in RTSA. After surgery, the injured arm is initially supported in a sling.
All treatments are followed by rehabilitation.
What did we want to find out?
We wanted to find out the best ways of restoring shoulder function and avoiding harmful effects of treatment in adults with shoulder fractures.
What did we do?
We searched medical databases for studies looking at the management of shoulder fractures in adults. We then summarised the results for different comparisons and rated our confidence in the evidence, based on factors such as study quality and size.
What did we find?
We found 47 studies that involved 3179 adults with a shoulder fracture. The studies were conducted in 21 countries. Most studies followed people for at least one year. Most people were aged 60 years and above; over two‐thirds were women. Twelve studies evaluated non‐surgical treatment; 10 studies compared surgical with non‐surgical treatment; 23 compared two methods of surgery; and 2 studies tested timing of mobilisation after surgery.
Main results
Here we focus on three key questions.
1. Is it better to move the shoulder within a week of fracture or delay movement for three or more weeks?
Due to limited evidence from five non‐surgical studies, we are unsure whether early movement of the arm improves or makes no difference to long‐term shoulder function or the development of shoulder problems.
2. Is surgery better than non‐surgical treatment for most types of displaced fractures?
Ten studies tested whether surgery for adults with most types of displaced fractures gave a better result than non‐surgical treatment. There was strong evidence of no important differences between surgical and non‐surgical treatment in patient‐reported shoulder function at 1 and 2 years, and probably at 6 months too. There is strong evidence of no important difference between the two treatments in quality of life at 1 year. Thirty‐one people in the studies died, but only 1 death was linked with surgery. Surgery may result in a higher risk of needing additional surgery and a higher risk of complications. There is, however, also a small possibility of more shoulder problems after non‐surgical treatment.
3. What is the best method of surgery?
We selected two key comparisons. ‐ Four studies compared a plate with a nail for surgical fixation after the bone has been put back together. The choice of surgery may make no difference to shoulder function. The very limited evidence means we are unsure if the choice of surgery affects quality of life, harmful effects or need for additional surgery.
‐ Two studies comparing an RTSA with hemiarthroplasty found shoulder function was improved to a similar extent, but that additional surgery was less frequent after RTSA. However, there is not enough evidence overall to tell whether one type of replacement is better than the other.
What are the limitations of the evidence?
We are confident of the findings of no difference in function or quality of life between surgery and non‐surgical treatment for most types of displaced fractures. Otherwise, we are unsure of other findings, usually because there was not enough evidence.
How up to date is the evidence?
This review updates our previous review published in 2015. The evidence is up to date to September 2020.
Summary of findings
Background
Description of the condition
Proximal humeral fractures account for approximately six per cent of all adult fractures (Court‐Brown 2006). Their incidence rapidly increases with age, and women are affected between two and three times as often as men (Court‐Brown 2006; Launonen 2015a; Lind 1989; Sumrein 2017). The majority of people who sustain proximal humeral fractures are 60 years or older and their bones are osteoporotic. Court‐Brown 2001 found that 87% of these fractures in adults resulted from falls from standing height. Palvanen 2006 found that the annual incidence of osteoporotic‐related fractures of the proximal humerus in Finland had tripled between 1970 and 2002 to 105 per 100,000 people aged 60 or above. Reporting an epidemiological study of proximal humeral fractures in adults in Tampere, Finland, between 2006 to 2010, Launonen 2015b found an overall incidence of 82 per 100,000 person years with an incidence of 204 per 100,000 person years for people aged 60 or above. Notably, Launonen 2015b included both inpatient (hospital discharge) and outpatient data. A registry‐based study of these fractures in Sweden between 2001 and 2012 that also included both inpatient and outpatient data found a 44% increase from 92.7 per 100,000 person years in 2001 to 121.9 per 100,000 person years in 2012 (Sumrein 2017). An epidemiological study of upper‐limb fractures occurring in 2009 in the USA reported an annual incidence of 60 proximal humeral fractures per 100,000 people overall, with four‐fold increased incidence of 253 per 100,000 in those aged 65 or older (Karl 2015).
Most proximal humeral fractures are closed fractures in that the overlying skin remains intact. The most commonly used classification of shoulder fractures is that of Neer, which has 16 categories (Neer 1970). Neer considered four anatomical segments of the proximal humerus ‐ the articular part, the greater tuberosity, the lesser tuberosity and the humeral shaft. These may be affected by fracture lines but are only considered as a 'part' if displaced by more than one centimetre or 45 degrees angulation from each other. Fractures, regardless of the number of fracture lines present, which did not meet the criteria for displacement of any one segment with respect to the others were considered 'minimally displaced'; these are sometimes referred to as one‐part fractures. Neer's categories, two‐part, three‐part and four‐part fractures, all involve the displacement or angulation of some or all of the above four segments. Some fracture categories feature an anterior or posterior humeral head dislocation, or involve the articular surface.
Another widely‐used classification system for these fractures is the Arbeitsgemeinschaft fur Osteosynthesefragen (AO) classification system (Müller 1991). This system was updated in conjunction with the Orthopaedic Trauma Association (OTA) classification in 2007 (Marsh 2007). There are three main types (A, B, C), which in turn are further divided into three groups, each with a further three subgroups. The vascularity (blood supply) of the humerus head is a primary focus of the AO classification system. Type A fractures are "extra‐articular, unifocal, with intact vascular supply"; type B fractures are "extra‐articular, bifocal, with possible vascular compromise"; and type C fractures are "articular, with a high likelihood of vascular compromise" (Robinson 2008).
Many proximal humeral fractures are only minimally displaced. Neer's estimate that approximately 85% of all proximal humeral fractures are minimally displaced (Neer 1970), in that no bone fragment is displaced by more than one centimetre or angulated by more than 45 degrees, is often cited (Koval 1997). However, a much lower figure of 49% was reported in a prospective consecutive series of over 1000 proximal humeral fractures (Court‐Brown 2001). Launonen 2015b reported only 13% of their cohort had minimally displaced (one‐part) fractures.
Irrespective of the extent of displacement or severity of the fracture, the immediate consequences to the individual are substantial. There is an increased risk of death, greatest in the first month but persisting at least to one year, in older people, more so in males (Bergdahl 2020). Recovery takes several months and poor shoulder function and pain are common long‐term outcomes, regardless of treatment. Many people, even those with less serious injuries, continue to report some or worse disability at two years (Hodgson 2007).
Description of the intervention
Non‐surgical ('conservative') treatment is generally the accepted treatment option for minimally displaced fractures, and is often also generally used for older people with displaced fractures. Non‐surgical treatment usually involves a period of immobilisation, such as in an arm sling, for one week or usually longer. This is followed by physiotherapy and exercises. Various aspects of non‐surgical treatment, such as the arm sling and collar and cuff, are illustrated online (Jaeger 2015). Older types of bandages, such as the Desault and Velpeau, are illustrated in Brorson 2011.
There is great variation both internationally and within nations in the use of surgery. However, surgery is typically used for displaced and unstable fractures and those with more complicated fracture patterns. Surgical interventions include:
closed reduction and percutaneous stabilisation using pins;
external fixation;
open reduction and internal fixation with plating; for example, buttress plates, angle blade plates and locking plates;
open reduction and fixation using a tension‐band principle;
open reduction and intramedullary nailing, either antegrade (nail inserted from above and driven down through the medullary canal) or, more rarely, retrograde (nail inserted from below and driven up through the medullary canal) insertion. Intramedullary nails usually offer the option of locking screws to enhance fracture stability;
hemiarthroplasty (replacement of the humeral head);
total shoulder replacement (replacement of the entire joint; both the 'ball' (humeral head) and 'socket' (glenoid)). There are two distinct types: anatomical and reverse shoulder arthroplasty. In reverse arthroplasty, the joint polarity is reversed such that the ball is on the glenoid side and the socket (fixed on a stem) on the humeral side. (Anatomical total shoulder arthroplasties are commonly used in the treatment of osteoarthritis but rarely in fracture treatment.)
Postoperative treatment generally involves a period of immobilisation in an arm sling followed by physiotherapy and exercises.
How the intervention might work
Immobilisation of the injured limb provides support and pain relief in the initial healing period. However, there is a risk of the shoulder becoming stiff and painful with substantial reduction of function. Subsequent physiotherapy and exercises aim to restore function and mobility of the injured arm. Malunion is inevitable in adults with displaced fractures treated non‐surgically; theoretically, this can compromise shoulder function. If non‐surgical treatment fails or the individual acquires a complication, such as symptomatic head necrosis or non‐union, surgery can be performed later on. Persistent pain and painful joint stiffness can be indications for subsequent surgery in people initially treated non‐surgically. These complications may also appear after surgery.
After closed or open reduction (repositioning to restore anatomy) of the fractured parts, surgical fixation using various techniques aims to stabilise the reduced fracture and restore joint integrity. Surgical stabilisation of the fracture may also allow earlier movement of the shoulder and elbow, reducing stiffness. Humeral head replacement avoids the risk of avascular necrosis of the humeral head. However, the assessment of the risk of serious vascular compromise according to fracture pattern is an ongoing topic of research, with many different radiographic measures being proposed as prognostic. Additionally, there is not a direct link between radiologically‐detected avascular necrosis and poor clinical outcome or shoulder function. Overall, bone quality is the key consideration in judging the appropriateness of any intervention in terms of healing and the potential for fixation failure. Furthermore, the individual's frailty may lead to a low rehabilitation drive and delay any recovery from both the initial trauma and any subsequent management.
Why it is important to do this review
Proximal humeral fractures are increasing in incidence, particularly as a result of the ageing populations in many countries. The short‐ and long‐term consequences for individuals with these injuries and for society are substantial (Palvanen 2006). There is considerable variation in practice, both in terms of treatment (such as surgical treatment for displaced fractures (Guy 2010)) and rehabilitation (Hodgson 2006). For example, Han 2015 reported that 67% of Medicare patients with proximal humeral fractures in the USA had non‐surgical treatment between 2005 and 2012; and Launonen 2015a reported that 78% of people with these fractures in Tampere, Finland, had non‐surgical treatment between 2006 and 2010. A study of hospital episode statistics (HES) data for people admitted into hospital for these fractures in England found that over a five‐year period (2007/8 to 2011/12), there were 22,084 final consultant episodes (FCEs; essentially, admissions for these fractures) covering all possible treatments of which 9555 (43%) FCEs were for operations (surgery). For patients aged 65 years or above, there were 8821 (18.5%) FCEs for operations out of the 44,466 FCEs for all treatments (Handoll 2015a; Chapter 8). The same study also noted differences in surgical activity between different hospitals. In their Swedish registry‐based study, Sumrein 2017 reported a 75% increase in surgery from 11.6 per 100,000 person years in 2001 (12.5% operated on) to 20.3 per 100,000 person years in 2012 (16.7% operated on). The greatest rate of increase was in those aged 50 years and older, which mainly reflected a greatly increased use of open reduction and plate fixation (e.g. 12‐fold overall; 19‐fold in women aged 60 to 69 years). Variation in practice includes that of the uptake of new implants, typically before their effectiveness has been evaluated, as illustrated for reverse shoulder arthroplasty in the USA (Schairer 2015). A study of patients aged 65 years or above with newly diagnosed proximal humeral fractures in South Korea between 2008 and 2016 also showed increased surgery, which rose from 24.6% in 2008 to 36.8% in 2016 (Jo 2019). Jo 2019 found similar trends for increased open reduction and internal fixation and, for arthroplasty, the proportion of reverse shoulder arthroplasty rising from 8.2% of the overall arthroplasty procedures in 2008 to 52.0% in 2016.
Proximal humeral fractures constitute a large economic burden globally. The costs of treating these fractures are also substantial and growing, and exert pressure on health care systems. The direct healthcare costs, adjusted to 2007 prices, in the Netherlands of upper arm fractures, mainly proximal humeral fractures, were EUR (euro) 4440 per case, with an overall annual cost of approximately EUR 40 million (Polinder 2013). Polinder 2013 suggested that the increase in cost of fracture care in 'elderly women’ from a previous report of costs in the Netherlands was partly because of a higher number receiving surgery. The trend for increased surgery also applies in other countries, such as the USA (Bell 2011). A later report by the same team in the Netherlands estimated the medical costs per case in 2012 at EUR 11,224; these costs included hospitalisation, rehabilitation, nursing care, physical therapy and home care costs (Mahabier 2015). Mahabier 2015 showed medical costs increased with age, with rehabilitation, nursing care and home care costs exceeding hospital costs in patients aged 70 or over. The estimated costs for lost productivity, including time off work, and other costs for those in work was EUR 20,374 per case in 2012.
These costs also vary considerably per practice and geographical location. Sabharwal 2016a estimated the average inpatient cost of surgical treatment in 2014 as GBP (pounds sterling) 3282, with hemiarthroplasty at the highest end of the range at GBP 4679.31. The main cost drivers were implants, theatre consumables (both at 41%) and theatre staff (20%). A retrospective analysis carried out in the UK reported median costs of implants in 2014 for plate fixation, intramedullary nail fixation, hemiarthroplasty and reverse shoulder arthroplasty as GBP 783, 476, 2129 and 2800, respectively (Dean 2016). Schairer 2015 found that the estimated mean hospital costs in 2011 in the USA were significantly higher for reverse shoulder arthroplasty than for hemiarthroplasty (USD (US dollar) 21,723 versus USD 18,122); a difference which was almost three times greater when it came to mean hospital charges (USD 75,849 versus USD 65,477). Sabesan 2015 reported the fixed standard charges in an American hospital (presumably at 2014 costs) as USD 27,876 for hemiarthroplasty and USD 29,523 for open reduction and internal fixation (ORIF); inpatient charge was USD 2428 per day, which applied to non‐surgically‐treated patients.
As well as costs associated with initial treatment, studies have shown that complications and hospital readmission, such as for secondary surgery, add to the increasing costs associated with proximal humeral fracture (Rosas 2018; Thorsness 2016).
The increasing incidence of these fractures, the often poor treatment outcome, the increasing use of surgery, the high treatment costs and variations in practice all endorse the need for this review update (Han 2015). Also relevant is the notable trend to an increased use of RTSA and the growing numbers of studies focusing on this intervention. The previous two versions of this review noted the insufficiency of the evidence to inform practice, but also located ongoing trials that could potentially help to address this deficiency (Handoll 2012; Handoll 2015b). This update continues the systematic review of the evidence for managing these fractures.
Objectives
To assess the effects (benefits and harms) of treatment and rehabilitation interventions for proximal humeral fractures in adults.
We defined a priori the following broad objectives:
to compare different methods of non‐surgical treatment (including rehabilitation);
to compare surgical versus non‐surgical treatment;
to compare different methods of surgical treatment;
to compare different methods of rehabilitation after surgical treatment.
We aimed to prepare a brief economic commentary summarising the principal findings of relevant economic evaluations for the surgical versus non‐surgical treatment comparison.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised or quasi‐randomised (where the method of allocating participants to a treatment is not strictly random; e.g. by hospital record number) controlled trials which compared two or more interventions in the management of fractures of the proximal humerus in adults.
In preparing this review update, we indicated that we would defer inclusion of pilot or feasibility studies (where recruitment was being extended for a main study), and place any such studies in Studies awaiting classification. We took this decision to avoid the known problems of interim analysis.
Types of participants
We included adults with a fracture of the proximal humerus. We planned for stratification by fracture pattern, primarily whether the fracture was minimally displaced or displaced (according to the Neer classification (Neer 1970)), and by age (under versus over 65 years), if possible. We included trials involving children, provided either separate data for skeletally mature participants were available or the proportion of children was small and, preferably, balanced in intervention groups. Prior to this review update, we clarified that we were not including trials that focused on treating the sequelae of unsuccessful treatment of these fractures, such as for non‐union.
Types of interventions
We included non‐surgical and surgical interventions, as exemplified in Description of the intervention, used in the primary treatment and rehabilitation of fractures of the proximal humerus. We excluded pharmacological trials. As established in our 2020 update of the review protocol, we also excluded trials testing biological interventions, such as autologous bone marrow‐derived and blood‐derived biological therapies. Consistent with our intention to focus on the main categories of interventions described in Description of the intervention, we have deferred the inclusion of trials testing acupuncture and variants thereof, as well as Chinese traditional medicine. This decision also reflects our limited experience of these interventions.
Types of outcome measures
The primary focus is on long‐term functional outcome, preferably measured at one year or more.
Primary outcomes
Functional outcomes: patient‐reported measures of upper‐limb function (e.g. the Disability of the Arm, Shoulder, and Hand questionnaire (DASH), the Oxford Shoulder Score (OSS; Dawson 1996; Dawson 2009), and other validated shoulder rating scales)
Activities of daily living and health‐related quality‐of‐life scores (e.g. European Quality of Life‐5 Dimensions (EuroQol (EQ-5D); the 36‐item Short‐Form Health Survey (SF‐36) and 12‐item Short‐Form Health Survey (SF‐12) (Ware 1996)
Serious adverse events (e.g. death, deep infection, avascular necrosis, complex regional pain syndrome type 1) and need for substantive treatment, such as an operation
Secondary outcomes
Composite scores of subjectively‐ and objectively‐rated function and overall outcome (e.g. the Constant score; also called the Constant and Murley's score or the Constant Shoulder Score (Constant 1987); Neer's rating (Neer 1970))
Pain
Upper‐limb strength and range of movement
Less serious complications/adverse events of limited duration and impact (e.g. superficial infection, transient paraesthesia, skin irritation)
Patient satisfaction with treatment, including cosmetic outcomes
Anatomical outcomes (e.g. radiological deformity)
Economic outcomes
Costs, cost effectiveness and impact of interventions on resource use
Minimal clinically important differences
We based our judgement of clinically important between‐group mean differences in measures of pain and function using the following minimal clinically important differences (MCID); alternative sources are listed after the main selected item in bold.
American Shoulder and Elbow Surgeons score (ASES; 0 to 100: best outcome) (rotator cuff disease): 12.01 (function‐based) (Tashjian 2010).
Constant score (0 to 100: best outcome) (proximal humeral fracture): 11.6 (anchor‐based), 5.1 (distribution‐based) (Van de Water 2014); (upper limb proximal diagnosis): MCID 10.2 (Schmitt 2004).
Disability of the Arm, Shoulder, and Hand questionnaire (DASH; 0 to 100: worst outcome) (proximal humeral fracture): 13.0 (anchor‐based), 8.1 (distribution‐based) (Van de Water 2014); 15 recommended in DASH/QuickDASH.
EQ‐5D (0 to 1: best outcome) (proximal humeral fracture): 0.12 (assessed in relation to a DASH MCID of 10) (Olerud 2011c).
Oxford Shoulder Score (OSS; 0 to 48: best outcome) (proximal humeral fracture): 11.4 (anchor‐based), 5.1 (distribution‐based) (Van de Water 2014).
QuickDASH (0 to 100: worst outcome): 16 in DASH/QuickDASH; 8 (shoulder pain) (Mintken 2009).
SF‐12‐PCS (0 to 100: best outcome) (physical component score) (upper limb proximal diagnosis): MCID 6.5 (Schmitt 2004).
Simple Shoulder Test (SST; 0 to 12: best outcome) (rotator cuff disease): 2.05 (Tashjian 2010).
University of California‐Los Angeles (UCLA; 2 to 35: best outcome) (proximal humeral fracture): 2.4 (anchor‐based), 2.0 (distribution‐based) (Van de Water 2014).
The change score should exceed 2.8 points for the SST, 16.3 points for the DASH, 17.1 points for the QuickDASH and 6.0 points for the OSS to have a clinically relevant change on a patient‐reported outcome measure (PROM) that is not due to measurement error (Van Kempen 2013).
Search methods for identification of studies
We ran the searches for relevant studies in three stages: the first search was run in October 2019; the second search update was run in September 2020; and the third search, which was a 'top‐up' search, was run in November 2021. We have not incorporated the results of the 'top‐up' search in the review. We ran an additional search for the brief economic commentary in November 2019.
Electronic searches
Up to September 2020, we searched the Cochrane Central Register of Controlled Trials (CENTRAL, 23 September 2020, Issue 9) via the Cochrane Register of Studies (CRS‐Web), MEDLINE (Ovid MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions 1946 to 21 September 2020), and Embase (Ovid, 1980 to 23 September 2020).
Up to October 2019, we searched CINAHL (Cumulative Index to Nursing and Allied Health Literature; EBSCO, 1937 to 08 October 2019), AMED (Allied and Complementary Medicine; Ovid, 1985 to 08 October 2019), and PEDro - Physiotherapy Evidence Database (08 October 2019).
In MEDLINE, we combined subject‐specific terms with the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011) (Appendix 1). Search strategies for CENTRAL, Embase, CINAHL, AMED and PEDro can also be found in Appendix 1. For this update, the search results were limited from 2014 onwards. Details of the search strategies used for previous versions of the review are given in Handoll 2007, Handoll 2010, Handoll 2012 and Handoll 2015b. We applied no language or publication restrictions.
We searched the WHO International Clinical Trials Registry Platform Search Portal and ClinicalTrials.gov to identify ongoing and recently completed trials (23 September 2020) (see Appendix 1).
Top‐up search conducted 9 November 2021
We updated the search of CENTRAL, MEDLINE, Embase, the WHO International Clinical Trials Registry Platform Search Portal and ClinicalTrials.gov on 9 November 2021.
Searches for the brief economic commentary
On 12 November 2019 we performed additional searches for the brief economic commentary (BEC). We searched MEDLINE (Ovid MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions 1946 to 11 November 2019) and Embase (1980 to 12 November 2019) for cost‐of‐illness studies ('search 1'). We searched MEDLINE (2014 to 11 November 2019), EMBASE (2010 to 12 November 2019), CINAHL (2009 to 12 November 2019) and the National Health Service Economic Evaluation Database (NHS EED; no date limit) for economic evaluations ('search 2'). We applied no language or publication restrictions. The dates for the economic evaluations studies were limited to the last date NHS EED stopped including studies from each database.
We combined subject‐specific terms from the original search strategies (effectiveness review) with filters for cost‐of‐illness and economic evaluations for databases except NHS EED since this database only contains economic evaluation citations. The searches included all interventions for proximal humeral fractures. Details of the searches can be found in Appendix 2.
Searching other resources
We searched the reference lists of newly included trial reports and reviews identified up to September 2020. We searched The Bone and Joint Journal Orthopaedic Proceedings from November 2014 (on 08 October 2019; see Appendix 1). We searched abstracts of the British Elbow and Shoulder Society (BESS) annual meetings (abstracts 2014 to 2019 published in BESS's journal Shoulder & Elbow), and the British Trauma Society annual scientific meetings (2015, 2016, 2018, 2019 preliminary programmes).
Data collection and analysis
The first protocol for this review was published in 1996 (Thomas 1996), and amended and republished with new authors in 2000 (Gibson 2000). Intended changes to protocol have always been established before conducting review updates and, where these changes are more substantial, have been submitted for editorial comments and approval. Key changes to protocol are summarised in Differences between protocol and review.
Selection of studies
For this update, pairs of three review authors (SB, JE, HH) independently screened search results and assessed potentially eligible studies for inclusion. In case of disagreements, a third review author adjudicated. We based initial decisions about trial eligibility on citations and, where available, abstracts and indexing terms. We obtained full articles and, where necessary to ascertain trial methods and status, one review author (HH) sent requests for information to trial investigators. Final study inclusion was by consensus. Titles of journals, names of authors or supporting institutions were not masked at any stage. Other review authors performed independent study selection for those trials for which a review author was an investigator.
Data extraction and management
Pairs of review authors (SB, JE, HH, TT) independently completed a data extraction tool, which had been used in the previous version of the review, for each newly included trial. We recorded details of the study methods, participants, interventions and outcome assessment and results. Review authors discussed any data extraction differences that were clearly not transcription errors, with final checks made by HH before data entry. Data management and entry into Review Manager (Review Manager 2014) was mainly by one review author (HH), with substantive contribution by a second review author (JE) and checks made by all review authors. When necessary, we requested additional details of trial methodology or data, or both, from trialists.
Assessment of risk of bias in included studies
Pairs of review authors (SB, JE, HH, TT) independently assessed risk of bias for newly included trials, without masking the source and authorship of the trial reports. We resolved all inter‐rater differences through discussion. One review author (HH) checked between‐rater and between‐versions consistency in assessment at data entry. We used the tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008a). This tool incorporates assessment of randomisation (sequence generation and allocation concealment), blinding (of participants, treatment providers and outcome assessment), completeness of outcome data, selection of outcomes reported and other sources of bias. We considered subjective and functional outcomes (e.g. functional outcomes, pain, clinical outcomes, complications) and 'hard' outcomes (e.g. death, reoperation) separately in our assessment of blinding and completeness of outcome data. We assessed two additional sources of bias: bias resulting from major imbalances in key baseline characteristics (e.g. age, gender, type of fracture); and performance bias such as that resulting from lack of comparability in the experience of care providers.
Additionally, we assessed four other aspects of trial quality and reporting that would help us judge the applicability of the trial findings. The four aspects were: definition of the study population; description of the interventions; definition of primary outcome measures; and length of follow‐up.
Measures of treatment effect
For each trial, we calculated risk ratios (RR) and 95% confidence intervals (CIs) for dichotomous outcomes, and mean differences (MD) and 95% CIs for continuous outcomes. We used standardised mean differences (SMD) rather than mean differences when pooling data from continuous outcome measures based on different scoring schemes.
Unit of analysis issues
We remained aware of potential unit of analysis issues arising from inclusion of participants with bilateral fractures, and presentation of outcomes, such as total complications, by the number of outcomes rather than participants with these outcomes. There was just one participant with bilateral fractures (Kristiansen 1988), but there was insufficient information to quantify the small difference this would have made to study findings. We avoided the second described unit of analysis problem, mainly by reporting on incidences of individual complications.
Dealing with missing data
We contacted trialists for missing information, including for denominators and standard deviations. We performed intention‐to‐treat analyses where possible. Where there were missing standard deviations, we calculated these from other data (standard errors, 95% CIs, exact P values) where available. We did not impute missing standard deviations.
Assessment of heterogeneity
We assessed heterogeneity for pooled data from comparable trials by visual inspection of the analyses, along with consideration of the Chi² test for heterogeneity and the I² statistic (Higgins 2003). We based the main quantitative assessment of heterogeneity on the I² statistic, where the following interpretation from the Cochrane Handbook for Systematic Reviews of Interventions was used: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
There are insufficient data thus far (a minimum of 10 trials is required) to merit the production of funnel plots to explore publication bias. The search for trials via conference proceedings and trial registration, together with the contacting of authors for information of trial status and progress, has provided some insights on unpublished trials, which were often abandoned because of poor recruitment.
Data synthesis
Where the data allowed, we pooled the results of comparable groups of trials using both fixed‐effect and random‐effects models. The selection of the model for presentation was determined by the consideration of the extent of the clinical heterogeneity.
Subgroup analysis and investigation of heterogeneity
We set out a priori two subgroup analyses: by age (primarily, under versus over 65 years) and by types of fracture (primarily, minimally displaced versus displaced, based on the Neer classification). To test whether the subgroups are statistically significantly different from one another, we planned to inspect the overlap of confidence intervals and perform the test for subgroup differences available in Review Manager.
Sensitivity analysis
We considered sensitivity analyses based on aspects of trial and review methodology, including the effects of missing data, the inclusion of studies at high or unclear risk of bias (primarily, selection bias with reference to allocation concealment), the inclusion of studies only reported in abstracts, and using fixed‐effect versus random‐effects models for pooling. However, for this version of our review, we conducted sensitivity analyses for the lattermost only for primary outcomes of a few multi‐trial analyses. Although we considered sensitivity analyses for exploring the effects of missing dichotomous data, we did not conduct these routinely nor establish a formal process beforehand.
Summary of findings and assessment of the certainty of the evidence
We produced summary of findings tables only for four key comparisons where a more substantive body of evidence had accrued. The four comparisons are: early versus delayed mobilisation for non‐surgically treatment; surgical versus non‐surgical treatment; locking plate versus locking intramedullary nail; and reverse total shoulder arthroplasty versus hemiarthroplasty.
We used the GRADE approach to assess the certainty of evidence related to each of the key outcomes listed in the Types of outcome measures for each comparison (see the Cochrane Handbook for Systematic Reviews of Interventions Section 12.2, Schünemann 2011). We selected the following outcomes for presentation in the summary of findings tables:
functional outcomes relating to the upper limb and shoulder, measured at one year, three or six months, and two years' follow‐up. We selected the earlier follow‐up of around three months for non‐surgical comparisons, and six months for comparisons involving surgery in at least one intervention group;
activities of daily living and health‐related quality‐of‐life scores at one or two years, dependent on the assessed reliability of the data;
mortality;
serious adverse events (e.g. death, deep infection, avascular necrosis, complex regional pain syndrome type 1); and
need for substantive treatment, such as an operation.
Incorporating economic evidence
We developed a brief economic commentary to summarise the availability and principal findings of the economic evaluations captured as part of this review. This included evaluations alongside trials and model‐based evaluations. We developed and carried out the commentary in accordance with current guidance. While we searched for all published full economic evaluations, we restricted the commentary to those testing the surgical versus non‐surgical comparison. This commentary focused on the extent to which principal findings of eligible economic evaluations indicate that an intervention might be judged favourably (or unfavourably) from an economic perspective when implemented in different settings. We carried out a supplementary search to identify economic studies according to Cochrane Economics Methods guidelines (Aluko 2021).
Results
Description of studies
Results of the search
For this update, after deduplication, we screened a total of 2030 records from the following databases up to 23 September 2020: CENTRAL (934), MEDLINE (394), Embase (767), WHO Trials Registry (164), and ClinicalTrials.gov (89); and from the following databases up to 9 October 2019: CINAHL (158), AMED (9) and PEDro (86). In addition, from searches conducted up to 9 October 2019, we also identified six potentially eligible studies from other sources (The Bone and Joint Journal Orthopaedic Proceedings (3); British Elbow and Shoulder Society annual meetings (2014 to 2019; 3 eligible, total abstracts not counted), and British Trauma Society Annual Scientific Meeting (2015, 2016, 2018, 2019; none eligible, total abstracts not counted)). We did not identify any extra trial reports from checking reference lists. We received reports for three other trials from trial investigators.
Overall, we identified 73 new studies (90 references). Of these:
10 newly included studies (15 references) were entirely new to the review: Biermann 2020 (2 references, including a trial registration); Carbone 2017; Helfen 2020 (3 references, including a trial registration and protocol); Jonsson 2020 (3 references, including a trial registration and preproof trial report); Lopiz 2019; Plath 2019 (2 references, including a trial registration); Sohn 2017; Tousignant 2020 (3 references, including a trial registration and protocol); Zhang 2019; and Ziegler 2019 (2 references, including a trial registration);
33 studies (35 references) were excluded;
7 studies awaiting classification (7 references for full articles) are new to this version of the review (Baring 2017; Chen 2016; Chengjin 2017; Liu 2014; Paladini 2019; Peng 2017; Zhang 2016).
23 new ongoing studies (30 references). With the exception of Hakim 2018, which was reported in a conference abstract only, these trials were represented by trial registrations. In addition, protocols were available for Howard 2018, Launonen 2019, Nerz 2017, ReShAPE and Wu 2016 (2 protocols). An unpublished abstract was made available for NCT03217344.
We obtained further references (46 references) for 24 studies:
11 references were obtained for four already included studies. These comprised a trial registration document for Agorastides 2007; a conference abstract for Hodgson 2003a; eight reports (including a long term follow‐up report) for ProFHER 2015; and a commentary on Sebastiá‐Forcada 2014;
3 references were obtained for one study out of the 38 already excluded studies. This study was renamed from NCT02122315 to Arias‐Buria 2015. One previously excluded study, a commentary by Zuckerman 2012 on Olerud 2011b, was transferred to the latter and deleted from the excluded studies list;
-
31 references were obtained for 18 of the 21 previously ongoing studies. Of these:
17 references for six previously ongoing trials resulted in their move to included studies: DelPhi 2020 (formerly Delphi; 2 extra references); Gracitelli 2016 (formerly NCT01984112; 2 new references); Hengg 2019 (formerly NCT01847508; 3 new references); HURA 2020 (formerly HURA, 6 new references); Launonen 2019a (formerly one of the studies in TPHF; 3 new references); and Ring 2019 (formerly NCT00438633; 1 new reference);
8 references, all updates of trial registration documentation, were obtained for the seven trials that remained ongoing (NCT00999193; NCT01524965; NCT01557413; NCT02075476; NTR3859 (was NTR4019); SHeRPA; TPHF (second study)). A conference abstract was found for NCT01557413.
3 references were obtained for three trials that were transferred to studies awaiting classification: NCT01113411 (no new reference); NTR3208 (revised trial registration for new trial number NTR3060); ProCon 2010 (1 conference abstract and revised trial registration for new trial number NTR1923 was 2040);
3 references were obtained for six trials that were excluded: ACTRN12610000730000 (no new reference); HOMERUS (revised trial registration for new trial number NTR2354, was 2461); NCT00818987 (no new reference); NCT01086202 (updated trial registration); ROTATE 2019 (updated trial registration); Torrens 2015 (no new reference).
1 new reference was obtained for one of the seven studies previous awaiting classification. This resulted in the exclusion of NCT02052206.
In all, 47 trials are now included, 77 trials are excluded, 30 trials are listed as ongoing and 16 are in Studies awaiting classification. A flow diagram summarising the study selection process is shown in Figure 1.
1.
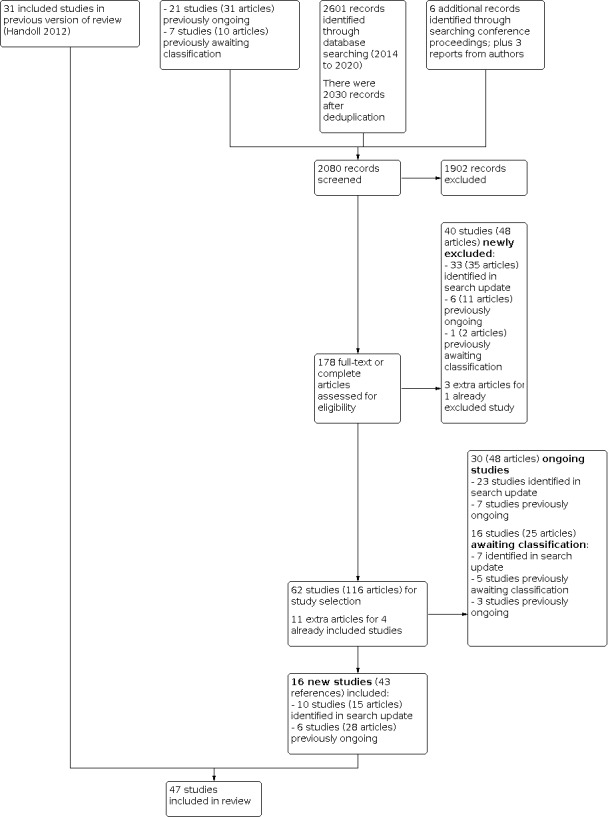
Study flow diagram for update (September 2020)
We have not included successful personal communications in the above tally of references, but these were invaluable for information on trial status, including notification of publication and provision of reports, and for some studies, clarification of methods and provision of additional data. In this update, we obtained further information, data or both from the trial contacts or associates of three newly included studies (Jonsson 2020; Plath 2019; Tousignant 2020); four now excluded studies (HOMERUS; NCT00818987; NCT03017105; Torrens 2015); one study awaiting classification (Brorson 2009); and two ongoing studies (NCT03217344; NTR3859).
Results of a top‐up search conducted 9 November 2021
After screening 529 references, we identified 13 trials (15 references) for which results are newly available, 6 new ongoing trials and 2 new references (an erratum and a paper exploring the external validity of trial results) for DelPhi 2020, an already included trial. We placed the study IDs of the first two groups in Studies awaiting classification. In order to distinguish the trials found in the top‐up search from the others found previously, we prefixed the first group, where there are results, with "Z‐TUp" and the second group of new ongoing trials with "Z‐TUpx". We placed the two extra references in Additional references. These trials are not included in the tallies or results. However, in the following, we note the five instances where trial results are now available for studies listed as ongoing and the one instance where results are now available for a study awaiting classification. A full account of the results of the top‐up search is given in Appendix 3.
Results of the search for the brief economic commentary
From the search conducted on 12 November 2019, we screened a total of 74 records from MEDLINE (20) and Embase (54) for cost‐of‐illness studies. We screened a total of 206 records from MEDLINE (58), Embase (91), CINAHL (50) and NHS EED (7) for economic evaluations. The brief economic commentary appears in Agreements and disagreements with other studies or reviews.
Included studies
Of the 47 included trials, 45 were published as full reports in journals, their availability ranging from 1979 (Lundberg 1979) to 2020 (Biermann 2020; Helfen 2020; HURA 2020; Jonsson 2020; Tousignant 2020). Of the remaining two trials, the results for Ring 2019 were available only in the trial registration site, and Torrens 2012 was published as a conference abstract only. This update features 16 newly included studies, including a total of 1247 participants (Biermann 2020; Carbone 2017; DelPhi 2020; Gracitelli 2016; Helfen 2020; Hengg 2019; HURA 2020; Jonsson 2020; Launonen 2019a; Lopiz 2019; Plath 2019; Ring 2019; Sohn 2017; Tousignant 2020; Zhang 2019; Ziegler 2019). Additional information, which frequently preceded the availability of the main trial report, via other publications, conference abstracts, trial registration details and communications from trial investigators, was available for 30 trials. Details of study methods, participants, interventions and outcome measurements for the individual studies are provided in the Characteristics of included studies and summarised below.
Design
With the exception of Rommens 1993, which was a quasi‐randomised trial using alternation for treatment allocation, all included trials were described as randomised clinical trials. However, seven trials provided no details of their method of randomisation and thus the use of quasi‐randomised methods for sequence generation cannot be ruled out (Cai 2012; Hoellen 1997; Kristiansen 1988; Kristiansen 1989; Lundberg 1979; Stableforth 1984; Wirbel 1999). All trials were parallel design with two intervention groups. Of note is that the design of ProFHER 2015, a multicentre trial that compared surgical versus non‐surgical treatment, was purposefully pragmatic, such as in the requirement for individual surgeons to use surgical methods and implants with which they were familiar. The choice of RTSA prosthesis was left to the treating surgeon in Jonsson 2020. Two types of RTSA were used in DelPhi 2020 (centre dependent) and Lopiz 2019.
Sample sizes
The 47 included trials involved a total of 3179 participants. Study size ranged from 20 participants (Bertoft 1984) to 250 participants (ProFHER 2015). The median study size is 61 participants. One trial included one person with bilateral fractures (Kristiansen 1989); the treatment allocation for this participant is unclear.
Setting
Of the 47 included trials, 41 were single‐centre studies conducted in 18 different countries: Austria (1 trial); Belgium (1); Brazil (1); Canada (1); China (4); Czech Republic (1); Denmark (2); Egypt (1); France (1); Germany (9); Italy (1); the Netherlands (1); Norway (1); Spain (4); South Korea (1); Sweden (6); UK (4); and USA (1). (Though essentially a single‐centre trial, the interventions in Hodgson 2003a were undertaken at two centres within an NHS Trust in the UK.) Four of the six multicentre trials were conducted in single countries: DelPhi 2020 (7 centres in Norway); HURA 2020 (2 centres in Canada); Jonsson 2020 (8 centres in Sweden); and ProFHER 2015 (33 centres in the UK). Both of the other two multicentre trials were conducted in four European countries. Hengg 2019 (8 centres) was conducted in Austria, Belgium, Germany and Switzerland; and Launonen 2019a (6 centres) was conducted in Denmark, Estonia, Finland and Sweden.
Details of the timing and duration, or both, of trial recruitment were provided for 42 trials (see Characteristics of included studies). The earliest start date of recruitment was 1970 for Stableforth 1984, and the latest start date was October 2016 (Ziegler 2019). At 11 years, Stableforth 1984 remains the trial with the longest known period of recruitment. Recruitment lasted five or more years for seven other trials (Boons 2012; Cai 2012; Fjalestad 2010a; Launonen 2019a; Olerud 2011a; Olerud 2011b; Ring 2019). For two trials (Hoellen 1997; Ockert 2010), subsequent publications indicated extended recruitment to that reported in the primary trial reports. Notably, where prospective, trial registration has often shown that recruitment periods have been extended beyond those originally planned in order to meet or attempt to achieve the target sample sizes.
Participants
All but Sohn 2017 provided information on gender. Overall, over two‐thirds of trial participants were women. Just two trials recorded more men: Ring 2019 (28% were female) and Soliman 2013 (29% were female). Most participants were aged 60 and above; two trials included a small number of children (Livesley 1992; Wirbel 1999). Where provided, the mean ages of trial participants ranged from 52 years in Soliman 2013 to 83.5 years in Lopiz 2019. Thirty trials set lower age limits, that by default excluded children. In 14 of these (Boons 2012; Cai 2012; DelPhi 2020; Fjalestad 2010a; Helfen 2020; Hengg 2019; Hoellen 1997; Jonsson 2020; Launonen 2019a; Lopiz 2019; Plath 2019; Sebastiá‐Forcada 2014; Voigt 2011; Zhang 2019), the age limit restricted the population to older adults (aged 60 or above). The three most extreme were Jonsson 2020 and Sebastiá‐Forcada 2014, where only people who were 70 years or over were included, and Lopiz 2019, where the minimum age was 80 years. Zyto 1997 specified that participants should be "elderly". Upper age limits (79 to 85 years) were set in four trials (DelPhi 2020; Gracitelli 2016; Smejkal 2011; Zhang 2019). Where recorded, the youngest participant was aged 6 years in Wirbel 1999, and the oldest was 100 years in Rommens 1993. Exceptionally, the participants of Soliman 2013 were aged between 45 to 60 years, with the majority (71%) being male.
Five trials included only minimally displaced fractures (Bertoft 1984; Hodgson 2003a; Livesley 1992; Lundberg 1979; Revay 1992), whereas 34 selected only people with displaced fractures. Most fractures were minimally displaced in Kristiansen 1989 and Rommens 1993. Carbone 2017 included "stable impacted" fractures, without further description. Lefevre‐Colau 2007 included either minimally displaced or "stable" impacted fractures; the latter included two‐part and three‐part fractures. Torrens 2012 included either minimally displaced or displaced fractures (two‐part or three‐part fractures were reported). In three trials, fractures were only defined in terms of their definitive treatment, this being non‐surgical in Ring 2019 and Tousignant 2020, and surgical in Ziegler 2019.
In 39 trials, fractures were or appeared to be graded using the Neer classification system (Neer 1970). This system was used, together with the AO classification system (Jaeger 2015; Müller 1991), in Fialka 2008, Fjalestad 2010a, Helfen 2020, Lefevre‐Colau 2007, Smejkal 2011 and Zyto 1997 (separate publications). The AO classification only was used in DelPhi 2020 and Wirbel 1999, where a modification of the AO classification system was described. No specific classification system was referred to in the remaining six trials (Bertoft 1984; Carbone 2017; Ring 2019; Rommens 1993; Tousignant 2020; Ziegler 2019).
Interventions
Fourteen trials evaluated non‐surgical treatment; however, this was post‐surgical treatment in two of these. Ten trials compared surgical with non‐surgical treatment. Of the 23 trials comparing two methods of surgery, 10 compared different categories of surgical intervention and 13 compared different methods of performing, or different types of, an intervention in the same category. A list of the comparisons, associated trials and numbers of trial participants, grouped according to the main review objectives presented in the Objectives, is given below.
Methods of non‐surgical management (including rehabilitation)
Initial treatment, including immobilisation
-
Early (up to one week post injury) versus delayed (after three or more weeks) mobilisation. Although five trials compared early versus delayed mobilisation, the timing of the start of early mobilisation varied, as did the nature and intensity of the physiotherapy provided (where it was described).
"Immediate" physiotherapy within one week of fracture versus delayed physiotherapy after three weeks of immobilisation in a collar and cuff sling: Hodgson 2003a (86 participants).
Immobilisation in sling and body bandage for one week versus three weeks: Kristiansen 1989 (85 participants).
Physiotherapy (pendulum movements) started immediately after diagnosis of injury versus physiotherapy delayed until three weeks: Ring 2019 (63 participants).
Physiotherapy started within three days of fracture versus delayed physiotherapy after three weeks of immobilisation in a sling: Lefevre‐Colau 2007 (74 participants).
Immobilisation in sling for one week versus four weeks; all followed same "progressive rehabilitation" regimen: Torrens 2012 (42 participants).
Early intensive mobilisation (10 sessions in two weeks) versus early less intensive mobilisation (10 sessions in five weeks) started one week after the fracture, during sling use: Carbone 2017 (80 participants).
Gilchrist arm sling versus "classic" Desault bandage: Rommens 1993 (28 participants).
Continuing management (rehabilitation) after initial sling immobilisation
Instructed self‐exercise versus conventional physiotherapy: Bertoft 1984 (20 participants); Lundberg 1979 (42 participants).
Swimming pool treatment plus self‐training versus self‐training alone: Revay 1992 (48 participants).
Telerehabilitation versus face‐to‐face rehabilitation: Tousignant 2020 (30 participants)
Pulsed electromagnetic high frequency energy (PHFE) versus placebo (dummy apparatus): Livesley 1992 (48 participants).
Surgical treatment versus non‐surgical treatment
The 10 currently available trials fall into three subcategories but are all treated together in Effects of interventions.
Fracture fixation versus non‐surgical treatment
Percutaneous reduction and external fixation versus closed manipulation and sling: Kristiansen 1988 (30 participants).
Internal fixation using surgical tension band or cerclage wiring versus sling: Zyto 1997 (40 participants; three more were recorded in Tornkvist 1995, another report of Zyto 1997).
Surgery involving open reduction and fixation with a locking plate and metal cerclages versus non‐surgical treatment starting with immobilisation of the injured arm in a modified Velpeau bandage: Fjalestad 2010a (50 participants).
Surgery involving open reduction and fixation with a Proximal Humerus Internal Locking System (PHILOS) plate and non‐absorbable sutures versus non‐surgical treatment starting with arm immobilisation in a sling: Olerud 2011a (60 participants).
Surgery involving open reduction or a minimally invasive approach and fixation using the PHILOS locking plate versus non‐surgical treatment using a collar and cuff or sling support: Launonen 2019a (88 participants).
Arthroplasty versus non‐surgical treatment
Hemiarthroplasty using the Neer prosthesis versus closed manipulation and sling: Stableforth 1984 (32 participants).
Humeral head replacement with the Global Fx prosthesis versus non‐surgical treatment starting with arm immobilisation in a sling: Olerud 2011b (55 participants).
Humeral head replacement with the Global Fx prosthesis versus arm immobiliser alone: Boons 2012 (50 participants)
Reverse total shoulder arthroplasty (RTSA) versus non‐surgical treatment starting with sling immobilisation: Lopiz 2019 (62 participants). Either the Delta XTEND or SMR was used for RTSA.
Surgery (surgeon's choice of method according to their experience) versus non‐surgical treatment
Surgery involving internal fixation (primarily locking plate fixation, most commonly PHILOS plate) or hemiarthroplasty versus sling: ProFHER 2015 (250 participants).
Different methods of surgical management
Comparisons of different categories of surgical intervention (10 trials)
Open reduction with internal fixation using a locking plate (Locking Proximal Humeral Plate (LPHP) or PHILOS) versus a locking intramedullary nail (Centronail, Locking Blade Nail (LBN), MultiLoc, or Proximal Humeral Nail (PHN)): Gracitelli 2016 (72 participants); Helfen 2020 (60 participants); Plath 2019 (81 participants); Zhu 2011 (57 participants). The PHILOS plate was augmented with bone cement in Helfen 2020.
Open reduction with internal fixation using a locking plate versus minimally invasive fixation with distally inserted intramedullary K‐wires (Kirschner wires): Smejkal 2011 (61 participants).
Hemiarthroplasty (DuPuy prosthesis) versus open reduction and locking plate fixation (PHILOS): Cai 2012 (32 participants)
Hemiarthroplasty (Global prothesis) versus tension band wiring: Hoellen 1997 (30 participants); an additional nine participants were reported in another report of this trial (Holbein 1999).
Reverse total shoulder arthroplasty (RTSA) versus locking plate fixation (PHILOS): DelPhi 2020 (124 participants). Either the Delta Xtend Reverse Total Shoulder Arthroplasty (DePuy Synthes) or the Promos Reverse Prosthesis (Smith & Nephew) was used for RTSA.
Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty: Jonsson 2020 (99 participants) and Sebastiá‐Forcada 2014 (62 participants). The choice of prosthesis was left to the 17 treating surgeons, based at eight hospitals, in Jonsson 2020. Sebastiá‐Forcada 2014 compared the SMR Reverse prosthesis with the SMR Trauma prosthesis.
Comparisons of different methods of performing, or different types of, an intervention in the same category (13 trials)
Plate: deltoid‐split approach with a less or 'minimal' invasive approach versus deltopectoral approach for plate fixation: Buecking 2014 (120 participants), HURA 2020 (85 participants) and Sohn 2017 (107 participants). Sohn 2017 explicitly framed their question in terms of it being a minimally invasive plate osteosynthesis (MIPO) versus open reduction and plate fixation; a 3.5 mm proximal humerus anatomical locking plate (PHILOS; Synthes, Paoli, PA) was used in both groups.
Plate: polyaxial versus monoaxial locking plate fixation. Non‐Contact Bridging – Proximal Humerus (NCB‐PH) plate versus PHILOS plate: Ockert 2010 (76 participants; 124 in a later report of this trial (Ockert 2014)); and Humeral SuturePlate (HSP) plate versus PHILOS plate: Voigt 2011 (56 participants).
Plate: CFR‐PEEK plate (made of polyetheretherketone reinforced with carbon fibres) versus the titanium PHILOS plate: Ziegler 2019 (76 participants).
Locking plate: additional glenohumeral joint lavage (PHILOS locking plate): Biermann 2020 (72 participants).
Locking plate: use of medial support locking screws (PHILOS locking plate): Zhang 2011 (72 participants).
Locking plate: in combination with allogeneic femoral head bone grafts (PHILOS locking plate): Zhang 2019 (80 participants).
Locking plate: cement augmentation of the screw tips (PHILOS locking plate): Hengg 2019 (67 participants).
Nail: MultiLoc proximal humeral nail (MPHN) ‐ a straight nail ‐ versus Polarus humeral nail ‐ a curved nail: Lopiz 2014 (54 participants).
Hemiarthroplasty: EPOCA prosthesis (Argomedical) versus HAS prosthesis (Stryker): Fialka 2008 (40 participants).
Hemiarthroplasty: tenodesis of the long head of the biceps (LHB) versus LHB tendon left intact: Soliman 2013 (45 participants).
Continuing management (including rehabilitation) after surgical intervention (2 trials)
Immobilisation in sling for one week versus three weeks after percutaneous fixation: Wirbel 1999 (77 participants).
Early active‐assisted mobilisation (after two weeks) versus late mobilisation (after six weeks) after cemented hemiarthroplasty: Agorastides 2007 (59 participants).
Categories of comparisons tested in the 16 studies newly included in this update
Of the 16 newly included trials, three trials (174 participants) compared different methods of non‐surgical treatment (Carbone 2017; Ring 2019; Tousignant 2020); two trials (150 participants) compared surgical with non‐surgical treatment (Launonen 2019a; Lopiz 2014); and the other 11 trials (950 participants) compared different methods of surgery. Of the latter, five compared different categories of surgery (DelPhi 2020; Gracitelli 2016; Helfen 2020; Jonsson 2020; Plath 2019), and six compared different methods of performing an intervention in the same category (Biermann 2020; Hengg 2019; HURA 2020; Sohn 2017; Zhang 2019; Ziegler 2019).
Outcomes
Many trials in previous versions of this review preceded the availability of validated patient‐reported outcome measures (e.g. DASH, Oxford Shoulder Score (Dawson 1996)) for assessing function. Data for these types of outcomes have become available from a growing number of trials, which now amount to 22 in total. All included trials except Ockert 2010 assessed functioning and pain, but often reported these as part of a combined overall assessment, such as that of Neer (Neer 1970) and Constant and Murley (Constant 1987), that included other measures. Except for Livesley 1992, Revay 1992 and Tousignant 2020, all trials reported on adverse events or complications. Exceptionally, Fjalestad 2010a and ProFHER 2015 reported on costs; reports on costs are pending for DelPhi 2020 and Tousignant 2020. Livesley 1992 did not provide outcomes split by treatment group.
Funding and conflicts of interest
Sixteen trials reported one or more sources of funding (support). Nine of these were externally funded to various extents via public, private foundation or insurance company funds (Cai 2012; Fjalestad 2010a; Hodgson 2003a; Launonen 2019a; Lefevre‐Colau 2007; Lopiz 2019; Olerud 2011a; Olerud 2011b; ProFHER 2015); and five received funds from industry (implant manufacturers) (DelPhi 2020; Hengg 2019; HURA 2020; Voigt 2011; Ziegler 2019). The remaining two trials referred to internal support from their institutions (Revay 1992; Tousignant 2020). Four trials stated explicitly that they received no external funding or that the study was not funded (Plath 2019; Soliman 2013; Zhang 2011; Zhu 2011). The other 27 trials did not provide information on funding.
Twenty‐three trials declared an absence of conflicts of interest and 15 provided no statements on this aspect. Boons 2012 declared there were no conflicts of interest for the authors but recognised there was institutional funding from industry. One or more authors from the other eight trials referred to personal funding for consultancy or other services, or both, to commercially‐relevant industry (DelPhi 2020; Hengg 2019; HURA 2020; Jonsson 2020; Launonen 2019a; Plath 2019; ProFHER 2015; Ziegler 2019). However, direct commercial funding of the trial linked with author payments or employment associated with the funder occurred in only three of these (DelPhi 2020; Hengg 2019; Wirbel 1999).
Excluded studies
We give brief details and reasons for the exclusion of 77 studies in the Characteristics of excluded studies. We excluded 36 studies because they were not RCTs or quasi‐RCTs. Of note is that several of the newly excluded studies were only described as randomised in the abstract, a claim that was contradicted in the main text, study design keywords or both. NCT02052206, which was listed in Studies awaiting classification in Handoll 2015b, features in this group because the study design changed to a single cohort study. We excluded 10 studies primarily because of an ineligible study population, such as humeral shaft fractures or old proximal humeral fractures (Arias‐Buria 2015; Bolano 1995; Chapman 1997; ChiCTR‐TRC‐14004213; Chiu 1997; NCT03804853; NCT04285606; Rodriguez‐Merchan 1995; Shah 2018; Wan 2005). The 'intervention' was not in our review scope in six studies (EUCTR 2015‐001820‐51; Ge 2019; Jin 2016; NCT00384852; NCT01532076; You 2016. Consistent with our revised scope, we excluded three of these six trials because the intervention was biological; however, NCT01532076 was also terminated due to very low recruitment. For reasons summarised in the Characteristics table, we excluded four trials because they had major design or reporting flaws (Chen 2019; Li 2015; Rasool 2015; Zhao 2017).
Most of the 21 other excluded trials had been registered but had either not started or had been abandoned. We reported on 12 of these in Handoll 2015b as follows:
"It is noteworthy that 11 excluded studies were registered (usually in the now archived National Research Register, UK) but either did not take place (Mechlenburg 2009), were abandoned due to lack of or poor recruitment (Brownson 2001; Dias 2001; Flannery 2006; Hems 2000; ISRCTN32335957; Wallace 2000; Kulkarni 2000), or perhaps both of these (Pullen 2007); or were not put forward for publication due to compromised methods or data (Bing 2002; Martin 2000). Edelson 2008 also reported an abandoned randomised trial because of lack of patient consent" (Handoll 2015b).
Seven of the remaining 10 excluded studies were previously listed as ongoing in Handoll 2015b: ACTRN12610000730000 and NCT00818987 were probably abandoned and may not have started; HOMERUS and ROTATE 2019 were stopped because of poor recruitment; NCT01086202 remains unpublished and anyway will have had very few fracture patients; and Torrens 2015 appears not to have existed, having resulted from a miscommunication. Two newly identified trials have also been abandoned because of very low recruitment (NCT02597972; NCT03017105). The final trial is Shah 2003, which we excluded in Handoll 2012 but failed to include in the list of abandoned studies in Handoll 2015b.
Ongoing studies
There are 30 ongoing studies, seven of which remain in this category from the 2015 version of the review (NCT00999193; NCT01524965; NCT01557413; NCT02075476; NTR3859 (was NTR4019); SHeRPA; TPHF (second study)). The other 23 studies appearing in this category are new to this version of the review. Details of the individual ongoing trials are given in the Characteristics of ongoing studies.
We summarise the comparisons tested in the 30 studies below. Four trials, all with three interventions under test, appear in several comparisons (ChiCTR1900022553; ISRCTN76296703; NCT00999193; TPHF).
-
Five studies (aim 685 participants in total) compare different interventions for non‐surgically‐treated patients.
Two studies compare early versus late mobilisation: NCT03217344 (one versus three weeks immobilisation; 130 target); NCT03786679 (rehabilitation started one week post trauma versus after four weeks immobilisation; 400 target).
One study compares physiotherapist‐supervised training plus home‐based training versus home‐based training alone: NCT03498859 (70 target).
One study compares scapula mobilisation with shoulder range of motion (ROM) exercises versus shoulder ROM exercises only: NCT02467803 (50 target).
One study compares interferential versus sham current therapy during rehabilitation: NCT04553497 (35 target).
-
Ten studies (aim 1173 participants in total) compare surgical versus non‐surgical treatment; four are multicentre trials (ISRCTN76296703; Launonen 2019; ReShAPE; TPHF; 764 target overall).
Surgical intervention involves internal fixation in five studies: locking plate is specified in four studies (Howard 2018; NCT00999193; NCT02913378; TPHF); whereas the fixation method is by surgeon's choice in NCT04106674.
Surgical intervention involves arthroplasty in seven studies: hemiarthroplasty is specified in three studies (ISRCTN76296703; NCT00999193; TPHF); and reverse shoulder arthroplasty (RSA) specified in five studies (ISRCTN76296703; Launonen 2019; NCT03599336; NCT03610113; ReShAPE).
-
Nine studies are comparing different categories of surgical intervention.
Three studies (aim 262 participants in total) are comparing plating versus nailing (NCT01557413; NCT02944058; NTR3859). Note that a conference abstract for NCT01557413 reported for 75 rather than 80 participants (Boyer 2019).
Three studies (aim approximately 174 participants in total) are comparing hemiarthroplasty versus plating (ChiCTR1900022553; NCT00999193; TPHF).
One study (aim approximately 60 participants) is comparing reverse shoulder arthroplasty versus plating (ChiCTR1900022553).
Five studies (aim 481 participants in total) are comparing reverse shoulder arthroplasty versus hemiarthroplasty (ChiCTR1900022553; Hakim 2018; ISRCTN76296703; NCT02075476; SHeRPA).
-
Four studies are comparing different methods of performing an intervention in the same category.
One study (aim 82 participants) is comparing two types of plates, both of which are being inserted in a minimally invasive technique (Wu 2016).
One study (aim 100 participants) is testing adding allogeneic fibula intramedullary implantation to locking plate fixation (ChiCTR‐IOR‐16008817).
One study (aim 60 participants) is comparing two designs of reverse shoulder arthroplasty (DRKS00011581).
One study (aim 80 participants) is comparing deltopectoral versus lateral approaches for reverse shoulder arthroplasty (NCT03694457).
-
Four studies are comparing different interventions after surgery.
One study (aim 100 participants) is comparing early versus standard mobilisation begun at three weeks after open reduction and plate fixation (NCT01524965).
One study (aim 70 participants) is comparing task‐oriented exercises and occupational therapy versus general physiotherapy post surgery (ISRCTN17996552).
One study (aim 60 participants) is comparing training with an Armeo Spring arm robot versus standard physiotherapy post surgical fixation (DRKS00009990).
One study (aim 48 participants) is comparing robot‐assisted training using the Armeo Spring device added to conventional occupational and physical therapy versus conventional occupational and physical therapy post surgery (Nerz 2017).
Notes from top‐up search conducted in November 2021
Full trial reports, as listed in Studies awaiting classification with study IDs starting 'Z‐TUp', were identified for four trials listed as ongoing:
ISRCTN17996552 was reported in Z‐TUp Monticone 2021;
NCT01557413 was reported in Z‐TUp Boyer 2021;
NCT03217344 was reported in Z‐TUp Martinez 2021;
Nerz 2017 was reported in Z‐TUp Kröger 2021.
Study results were posted for NCT02075476, also listed as ongoing, in Z‐TUp Alvarez 2020.
Studies awaiting classification
Sixteen studies await classification, six of which were in the same category in the 2015 version of the review (Battistella 2011; Brorson 2009; Liu 2011; Luo 2008; Wang 2013; Zhu 2014), and three of which were formerly listed under ongoing (NCT01113411; NTR3208; ProCon 2010). The other seven studies appearing in this category are new to this version of the review (Baring 2017; Chen 2016; Chengjin 2017; Liu 2014; Paladini 2019; Peng 2017; Zhang 2016). We provide details of the 16 studies in Characteristics of studies awaiting classification, and summarise the reasons for these studies being placed in this category below.
Three studies test interventions currently not covered in this review: Luo 2008 (acupuncture); Peng 2017 (non‐biological bone regeneration product adjuvant to locking plate); Zhang 2016 (Chinese traditional medicine).
We have been unsuccessful in obtaining information on the trial status of three studies: NCT01113411 (early versus standard rehabilitation after locking plate fixation); NTR3208 (RTSA versus hemiarthroplasty); ProCon 2010 (hemiarthroplasty versus non‐surgical treatment).
We have been unsuccessful in obtaining clarification on study design, data or both for five trials: three tested bone grafts or substitutes (Liu 2011; Wang 2013; Zhu 2014); one compared hemiarthroplasty versus plate fixation (Chen 2016); and one compared plate fixation versus hollow screw fixation versus band anchorage fixation for greater tuberosity fractures (Chengjin 2017).
Brorson 2009, which compared hemiarthroplasty versus plate fixation versus non‐surgical treatment was stopped after recruiting 25 participants; the use of these data continues to be under discussion.
There were insufficient data and details of methods in the conference abstract reports of three trials: Baring 2017 (interim analysis of "feasibility" trial comparing two types of immobilisation); Battistella 2011 (two different surgical approaches for plate fixation); and Paladini 2019 (different plate materials).
We were unable to obtain the full text copy of Liu 2014, which compared two types of plate.
Notes from top‐up search conducted in November 2021
We identified a full trial report for NTR3208, which was reported in Z‐TUp Laas 2021.
Risk of bias in included studies
We summarise the risk of bias judgements on nine items for the individual trials in Figure 2 and described these in the risk of bias tables in the Characteristics of included studies. A 'Yes' (+) judgement means that the review authors considered there was a low risk of bias associated with the item, whereas a 'No' (‐) means that there was a high risk of bias. Frequently, assessments resulted in an 'Unclear' (?) verdict; this often reflected a lack of information upon which to judge the item (see Figure 3). However, lack of information on blinding for functional outcomes was always taken to imply that there was no blinding and rated as a 'No'.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Based on reported methods, we judged 20 trials to be at low risk of selection bias resulting from adequate sequence generation and allocation concealment (Bertoft 1984; Boons 2012; Buecking 2014; DelPhi 2020; Fjalestad 2010a; Gracitelli 2016; Helfen 2020; HURA 2020; Launonen 2019a; Lefevre‐Colau 2007; Lopiz 2014; Olerud 2011a; Olerud 2011b; ProFHER 2015; Ring 2019; Sebastiá‐Forcada 2014; Smejkal 2011; Sohn 2017; Tousignant 2020; Voigt 2011). A further five trials also took adequate measures to safeguard allocation concealment (Hengg 2019; Hodgson 2003a; Jonsson 2020; Livesley 1992; Lopiz 2019).
We judged Rommens 1993 to be at high risk of selection bias as it was a quasi‐randomised trial using alternation. We judged the remaining trials as having an unclear risk of bias for either sequence generation or for both domains. This judgement was usually based on insufficient details on the methods of sequence generation or of allocation concealment, such as whether envelopes were sealed or opaque. Seven trials provided no information at all (Cai 2012; Hoellen 1997; Kristiansen 1988; Kristiansen 1989; Lundberg 1979; Stableforth 1984; Voigt 2011). The post‐randomisation application of exclusion criteria for Ockert 2010 left us uncertain about the risk of selection bias for this trial.
Blinding
We judged the following three trials to be at low risk of detection bias for functional outcomes resulting from assessor and participant blinding: Biermann 2020, where blinding for outcome assessment was clearly maintained; Livesley 1992, which used sham controls; and Soliman 2013, where the intervention was very likely to have remained unknown to the blinded assessor of Constant scores. While several other trials reported blinded assessors, the lack of reporting of adequate safeguards and the lack of blinding of participants or care providers meant that we considered the risk of bias as unclear. A high risk of bias, reflecting no reporting or indication of blinding, was likely in 31 trials. However, we rated ProFHER 2015, which did not blind trial participants, personnel or outcome assessment, as having an 'unclear' risk of bias because statistical tests showed a lack of a significant effect of baseline patient preferences on the primary outcome results (Oxford Shoulder Score).
We judged most trials as having an unclear risk of detection bias for the 'hard' outcomes of death and reoperation, where these outcomes were reported. However, we considered Buecking 2014 to be at high risk of detection bias because it seemed very likely that the decision for reoperation would have been intervention‐dependent. We considered eight trials to be at low risk for this item (Bertoft 1984; Biermann 2020; DelPhi 2020; Launonen 2019a; ProFHER 2015; Sebastiá‐Forcada 2014; Zhang 2011; Ziegler 2019).
Incomplete outcome data
We judged twelve trials to be at low risk of bias from the incompleteness of data on functional outcomes (Boons 2012; Helfen 2020; Hodgson 2003a; Lopiz 2019; Olerud 2011a; Olerud 2011b; ProFHER 2015; Sebastiá‐Forcada 2014; Torrens 2012; Tousignant 2020; Zhang 2019; Zhu 2011). Conversely, we deemed 16 trials to be at high risk of bias, usually reflecting large losses to follow‐up and post‐randomisation exclusions. We rated the remaining 19 trials as having an unclear risk of bias for this aspect. We judged 24 trials to be at low risk of bias from the incompleteness of data on the 'hard' outcomes of death and reoperation, where these outcomes were reported. We considered five trials to be at high risk of attrition bias for these outcomes (Kristiansen 1989; Plath 2019; Ring 2019; Smejkal 2011; Zyto 1997). We rated the remaining 18 trials as having an unclear risk of bias for this aspect.
Selective reporting
The lack of trial registration details and protocols often hindered the appraisal of the risk of bias from selective reporting. We considered 12 trials to be at high risk of selective reporting bias, often because expected outcomes were not reported or reported for the whole population only (Agorastides 2007; Biermann 2020; Gracitelli 2016; Hoellen 1997; Livesley 1992; Ockert 2010; Plath 2019; Rommens 1993; Soliman 2013; Zhang 2019; Ziegler 2019; Zyto 1997). We assessed five trials to be at low risk of this bias (Cai 2012; DelPhi 2020; Launonen 2019a; ProFHER 2015; Tousignant 2020). We rated the remaining 30 trials as having an unclear risk of bias for this aspect.
Other potential sources of bias
Baseline characteristics
We considered only the Hengg 2019 trial to be at high risk of bias because of confounding resulting from major imbalances in baseline characteristics; in this case, the type of fracture. We judged 17 trials to be at low risk of bias for this aspect (Boons 2012; Buecking 2014; Carbone 2017; DelPhi 2020; Helfen 2020; HURA 2020; Kristiansen 1988; Launonen 2019a; Lefevre‐Colau 2007; Lopiz 2014; Lopiz 2019; Lundberg 1979; Olerud 2011a; Olerud 2011b; ProFHER 2015; Wirbel 1999; Zyto 1997). We rated the remaining 29 trials as having an unclear risk of bias.
Care programmes
We judged 27 trials to be at low risk of performance bias from important differences in care programmes other than the trial interventions, or differences in the experience of care providers, and 19 trials to have an unclear risk, usually because of inadequate information. We considered only the Zhang 2019 trial to be at high risk of performance bias: mainly because the surgical approach differed in the two groups, although there were also insufficient details relating to rehabilitation.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings 1. Early versus delayed mobilisation for non‐surgically‐treated proximal humeral fractures.
| Early (up to one week post injury) versus delayed (after three or more weeks) mobilisation for non‐surgically‐treated proximal humeral fractures | ||||||
|
Patient or population: adults with minimally displaced or displaced (2‐part or 3‐part) proximal humeral fractures treated non‐surgically (5 trials)
Settings: various, including fracture clinics and physiotherapy Intervention: early (within or at one week) mobilisationa Comparison: delayed (usual) mobilisation or physiotherapy after three or four weeks immobilisation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 3 to 4 weeks immobilisation | Early mobilisation (up to 1 week) | |||||
|
Self‐reported shoulder function: Croft Shoulder Disability Score (1 or more problems)
Follow‐up: 1 year |
725 per 1000b | 428 per 1000 (290 to 638) | RR 0.59 (0.40 to 0.88) | 82 participants (1 study) |
⊕⊝⊝⊝
Very lowc |
Early mobilisation resulted in 297/1000 fewer people with one or more problems at 1 year (95% CI 87 fewer to 435 fewer) |
|
Self‐reported shoulder function: DASH(0 to 100: worst outcome) Follow‐up: 3 months |
The mean DASH score in the delayed group was 24d | The mean DASH score in the early group was 9.00 higher (2.33 lower to 20.33 higher) | 50 participants (1 study) | ⊕⊝⊝⊝
Very lowe |
As well as the 95% CI crossing the line of no effect (or difference), the result is unlikely to be clinically important given the MCID for the DASH score is 13.0. | |
|
Self‐reported shoulder function: Croft Shoulder Disability Score (1 or more problems)
Follow‐up: 2 years |
595 per 1000b | 435 per 1000 (274 to 685) | RR 0.73 (0.46 to 1.15) | 74 participants (1 study) |
⊕⊝⊝⊝
Very lowc |
Early mobilisation resulted in 160/1000 fewer people with one or more problems at 2 years (95% CI 321 fewer to 90 more) |
|
Quality of life: EQ‐5D (0: dead to 1: best quality of life) Follow‐up: 1 year |
The mean EQ‐5D score in the delayed group was 0.76d | The mean EQ‐5D score in the delayed group was 0.09 lower (0.21 lower to 0.03 higher) | 39 participants (1 study)f |
⊕⊝⊝⊝
Very lowe |
The mean difference is lower than the MCID for the EQ‐5D of 0.12. Although an overall SF‐36 score was not provided by another trial (80 participants), this found no evidence of a clinically important difference at one year between the two groups in the SF‐36 scores for each of 8 dimensions (physical and mental). |
|
|
Mortality Follow‐up: 6 months to 1 year |
See comment | ‐ |
92 participants (2 studies) |
⊕⊝⊝⊝
Very lowg |
There was one reported death. | |
|
Adverse events: shoulder complications Follow‐up: 1 year |
See comment | ‐ | 259 participants (4 studies) | ⊕⊝⊝⊝ Very lowg | Five serious shoulder complications were reported (2/127 in the early group versus 3/132 in the delayed group). These were: frozen shoulder (1 case), complex regional pain syndrome type 1 (2 cases) and treated subacromial impingement (2 cases). There were no cases of non‐union and the three cases of fracture redisplacement (not included here) that occurred in one trial (42 participants) did not require surgery. | |
|
Additional surgery or substantive treatment Follow‐up: 1 year |
See comment | ‐ | 259 participants (4 studies) | ⊕⊝⊝⊝ Very lowg | All 5 serious complications listed above would have required substantive treatment, that may have included surgery. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DASH: Disability of the Arm, Shoulder and Hand questionnaire; EQ‐5D: European Quality of Life‐5 Dimensions; MCID: minimal clinically important difference; RR: risk ratio; SF‐36: 36‐item Short‐Form Health Survey | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aWhere described, the intensity of early mobilisation and physiotherapy provided to both groups varied among the trials, from gradual assisted movements of the upper limb, such as pendulum exercises, to two hours of physiotherapy five times a week. bControl risk based on study data. cEvidence was downgraded one level for serious study limitations, one level for serious imprecision (single small trial) and one level for serious indirectness (questions over outcome measure's validity; the individual importance of the 22 problems covered in the Croft measure will vary). dData from control group of study. eEvidence was downgraded by one level for serious study limitations and two levels for serious imprecision (single small trial, wide confidence interval). fEvidence from a trial comparing 1 versus 4 weeks of immobilisation for predominantly displaced fractures. gThe available data for this outcome are too limited to draw any conclusions or useful analysis. Nominally, we downgraded the evidence by one level for serious study limitations and two levels for very serious imprecision (very few events).
Summary of findings 2. Surgical versus non‐surgical treatment for proximal humeral fractures.
| Surgical versus non‐surgical treatment for proximal humeral fractures | ||||||
|
Patient or population: (mainly older) adults with most types of displaced proximal humeral fracturesa (10 trials) Settings: hospital (tertiary care) Intervention: surgery, various: mainly open reduction and internal fixation (ORIF) with locking plate or hemiarthroplasty; reverse total shoulder arthroplasty used in 1 trial Comparison: non‐surgical treatment, mainly sling 'immobilisation'; more rarely, closed reduction/manipulation of the fracture (2 trials) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐surgical treatment | Surgical treatment | |||||
|
Functional scoresb(higher = better outcome) Follow‐up: 1 year |
The mean difference in function (overall) in the surgery groups was 0.10 standard deviations higher (0.07 lower to 0.27 higher) |
SMD 0.10 (‐0.07 to 0.27) | 552 participants (7 studies) |
⊕⊕⊕⊕
Highc |
This does not represent a clinically important difference:
|
|
|
Functional scoresd(higher = better outcome) Follow‐up: 6 months |
The mean difference in function (overall) in the surgery groups was 0.17 standard deviations higher (0.04 lower to 0.38 higher) |
SMD 0.17 (‐0.04 to 0.38) | 347 participants (3 studies) | ⊕⊕⊕⊝ Moderatee | This does not represent a clinically‐important difference.
|
|
|
Functional scoresf(higher = better outcome) Follow‐up: 2 years |
The mean difference in function (overall) in the surgery groups was 0.06 standard deviations higher (0.13 lower to 0.25 higher) |
SMD 0.06 (‐0.13 to 0.25) | 423 participants (5 studies) |
⊕⊕⊕⊕ Highc | This does not represent a clinically‐important difference.
|
|
|
Quality of life: EQ‐5D (0: dead to 1: best quality of life) Follow‐up: 1 yearg |
The mean EQ‐5D score ranged across control groups from 0.65 to 0.90 | The mean EQ‐5D score in the surgery groups was 0.01 higher (0.02 lower to 0.04 higher) | MD 0.01 (‐0.02 to 0.04) | 502 participants (6 studies) |
⊕⊕⊕⊕
Highc |
The MCID of 0.12 was outside the 95% CI at this time period. This applied also to 24 months (MD 0.01, 95% CI ‐0.02 to 0.05; 5 studies, 426 participants).h |
|
Mortality Follow‐up: up to 2 years |
40 per 1000i | 54 per 1000 (28 to 105) |
RR 1.35 (0.70 to 2.62) |
646 participants (8 studies) |
⊕⊕⊝⊝
Lowj |
Surgery resulted in 14 per 1000 more deaths up to 2 years (95% CI 12 fewer to 65 more). Where details were provided, only one of the 31 reported deaths was deemed related to their fracture or treatment. This was an early death due to venous thromboembolism in the surgical group of one trial. |
|
Additional surgery (reoperation or secondary surgery) Follow‐up: up to 2 years |
35 per 1000i | 73 per 1000 (43 to 129) | RR 2.06 (1.21 to 3.51) | 667 participants (9 studies) |
⊕⊕⊝⊝
Lowk |
Additional surgery was reported for 54 participants in total. Surgery resulted in 38 per 1000 more patients having additional surgery up to 2 years (95% CI 8 to 94 more). One trial (250 participants) also reported on additional shoulder‐related therapy (7/1254 versus 4/125; RR 1.75 favouring non‐surgical therapy, 95% CI 0.53 to 5.83). |
|
Adverse events / complications ‐ Number of participants with complications Follow‐up: 2 years |
122 per 1000l | 179 per 1000 (113 to 282) | RR 1.46 (0.92 to 2.31) | 391 participants (3 studies) |
⊕⊕⊝⊝ Lowm | Surgery resulted in 57 per 1000 more patients having adverse events up to 2 years (95% CI 9 fewer to 160 more). All 10 trials reported on individual complications, the pattern of distribution generally reflecting the expected: e.g. infection 8/348 cases after surgery versus 0/352 cases after non‐surgical treatment . |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EQ‐5D: European Quality of Life‐5 Dimensions; MCID: minimal clinically important difference; MD: mean difference; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe inclusion/exclusion criteria varied among the trials: one (88 participants) included only 2‐part fractures that involved the surgical neck; one (30 participants) included 2‐, 3‐ or 4‐part fractures; one (60 participants) included only 3‐part fractures that included surgical neck; three (152 participants) included 3‐ or 4‐part fractures; and three (137 participants) included only 4‐part fractures. The final trial (250 participants) included "displaced fracture of the proximal humerus that involved the surgical neck", resulting in a few 1‐part (but confirmed as still "displaced") as well as 2‐, 3‐ and 4‐part fractures. The majority of the fractures (146/250 = 58.4%) in the largest trial were either 2‐part (128) or 1‐part (18) fractures. Several trials included further criteria; for example, the largest trial explicitly excluded fracture dislocations (i.e. fractures with an associated dislocation of the injured shoulder joint). Consideration is also needed of other inclusion and exclusion criteria, including multiple trauma, clear indications for surgery (severe soft‐tissue compromise), and comorbidities precluding surgery or anaesthesia. bPatient‐reported functional scores were the American Shoulder and Elbow Surgeons score (ASES; 1 trial), the Disability of the Arm, Shoulder and Hand questionnaire (DASH; 4 trials), the Oxford Shoulder Score (OSS; 1 trial); and the Simple Shoulder Test (SST; 1 trial). cAlthough the evidence was first downgraded by one level for serious study limitations, reflecting a potentially high risk of performance bias relating to lack of blinding in four single‐centre trials, the consistency in the results of these and the fifth and largest trial, where additional analysis indicated that the study design limited the risk of bias relating to the inevitable lack of blinding, resulted in an upgrade. dPatient‐reported functional scores were the ASES (1 trial), the DASH (1 trial), and the OSS (1 trial).
eFurther to considerations summed in footnote 'c', the evidence was downgraded one level for serious imprecision. fPatient‐reported functional scores were the ASES (1 trial), the DASH (3 trials), and the OSS (1 trial). gFollow‐up time selected as evidence available from the largest number of trials and participants. hThe evidence was moderate certainty at 24 months, downgraded by one level for serious inconsistency reflecting moderate statistical heterogeneity (Chi² = 9.08, degrees of freedom (df) = 4 (P = 0.06); I² = 56%); the 95% CIs of two trials (102 participants) from the same centre included the MCID favouring surgery. iAssumed control risk is the median control group risk across studies. jThe evidence was downgraded two levels for very serious imprecision because of the low number of events and wide confidence interval crossing the line of no effect. kThe evidence was downgraded one level for serious imprecision and one level for serious inconsistency, especially for trials with two years' follow‐up (heterogeneity: Chi² = 8.21, df = 4 (P = 0.08); I² = 51%). At two years, four trials (242 participants) reported more additional surgery in the surgery group, but the trial (250 participants) contributing 58.2% of the weight of the evidence recorded equal numbers of participants (11 versus 11) undergoing additional surgery. lThe control risk was the pooled control risk. mThe evidence was downgraded one level for serious imprecision and one level for serious indirectness as the definition, measurement and severity of adverse outcomes varied between trials. The evidence was dominated by that of one trial (250 participants) that explicitly collected data on a broader spectrum of complications.
Summary of findings 3. Locking plate versus locking intramedullary nail for surgical fixation of proximal humeral fractures.
| Locking plate versus locking intramedullary nail for surgical fixation of proximal humeral fractures | ||||||
|
Patient or population: adults undergoing surgery for displaced proximal humeral fracturesa (4 trials) Settings: hospital (tertiary care) Intervention: open reduction and internal fixation (ORIF) with locking plate Comparison: open reduction and internal fixation (ORIF) with locking intramedullary nail | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Locking nail | Locking plate | |||||
|
Self‐reported shoulder function: functional scoresb(higher = better outcome) Follow‐up: 1 year |
The mean difference in function (overall) in the plate groups was 0.15 standard deviations higher (0.12 lower to 0.41 higher) |
SMD 0.15(‐0.12 to 0.41) | 227 participants (4 studies) |
⊕⊕⊝⊝ Lowc | This is unlikely to represent a clinically important difference:
|
|
|
Self‐reported shoulder function: DASH (0 to 100: higher = worse disability)b Follow‐up: 6 months |
The mean DASH score in the nail groups ranged from 18.4 to 44.97 | The mean DASH score was 0.39 lower in the plate groups (4.14 lower to 3.36 higher) | ‐ | 174 (3 studies) | ⊕⊕⊝⊝ Lowd | The MCID of 13.0 is greater than the 95% CI at this time period. |
|
Self‐reported shoulder function: ASES (0 to 100: best outcome)b Follow‐up: 2 or 3 years |
The mean ASES score in the nail groups ranged from 73.5 to 90 | The mean ASES score was 3.06 higher in the plate group (0.05 lower to 6.17 higher) | ‐ | 101 participants (2 studies) |
⊕⊕⊝⊝ Lowe | The MCID of 12.01 is greater than the 95% CI at this time period. |
| Quality of life: SF‐36 (0 to 100: best outcome) Follow‐up: 1 year | The mean SF‐36 score in the nail group was 71.7 | The mean SF‐36 score was 2.6 higher in the plate group (2.38 lower to 7.59 higher) | ‐ | 53 participants (1 study) | ⊕⊝⊝⊝ Very lowf | We have not identified an MCID for the SF‐36. It may be comparable to that for the SF‐12 at 6.5. |
|
Mortality Follow‐up: 1 to 3 years |
See comment | ‐ | 176 participants (3 studies) |
⊕⊝⊝⊝ Very lowg | There was no report of any fracture or surgery‐related death. Four of the 11 reported deaths were reported explicitly as having been from unrelated causes. | |
|
Adverse events: number of participants with complications Follow‐up: 1 to 3 years |
217 per 1000h | 241 per 1000 (152 to 380) | RR 1.11 (0.70 to 1.75) | 250 participants (4 studies) |
⊕⊝⊝⊝ Very lowi | By illustration, using a plate resulted in 24/1000 fewer participants having a complication up to 3 years (95% CI 65 fewer to 163 more). All 3 trials reported on individual complications: screw penetration into the humeral head, reported by all 4 trials, was the most common (occurring in 35% of those with complications). |
|
Additional surgery (reoperation or secondary surgery) Follow‐up: 1 year |
139 per 1000h | 102 per 1000 (46 to 224) | RR 0.73 (0.33 to 1.61) | 193 participants (3 studies) |
⊕⊝⊝⊝ Very lowj | By illustration, using a plate resulted in 37/1000 fewer participants having additional surgery up to 1 year (95% CI 93 fewer to 85 more). However, these results are based on a total of 22 events in all. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ASES: American Shoulder and Elbow Surgeons; CI: confidence interval; DASH: Disability of the Arm, Shoulder and Hand questionnaire; MCID: minimal clinically important difference; RR: risk ratio; SF‐36: 36‐item Short‐Form Health Survey; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe inclusion/exclusion criteria varied among the trials: one (72 participants) included 2‐ or 3‐part surgical neck fractures; one (81 participants) included 2‐, 3‐ and 4‐part fractures; and two (117 participants) included 2‐part surgical neck fractures only. Of the 250 recorded fractures, 63% were 2‐part and 33% were 3‐part. bThe two patient‐reported functional scores used in this analysis were the Disability of the Arm, Shoulder and Hand questionnaire (DASH; 2 trials), and the American Shoulder and Elbow Surgeons score (ASES; 2 trials). cThe evidence was downgraded by one level for serious study limitations, primarily reflecting a high risk of performance and detection bias relating to lack of blinding, and one level for serious inconsistency (heterogeneity: Chi² = 6.89, df = 3 (P = 0.08); I2 = 56%). dThe evidence was downgraded one level for serious study limitations, reflecting a high risk of performance and detection bias relating to lack of blinding and selective reporting bias, and one level for serious inconsistency (heterogeneity: Chi² = 4.42, df = 2 (P = 0.11); I2 = 55%). eThe evidence was downgraded one level for serious study limitations, primarily reflecting a high risk of performance and detection bias relating to lack of blinding, and one level for serious imprecision as the evidence was from just two small studies. fThe evidence was downgraded one level for serious study limitations, primarily reflecting a high risk of performance and detection bias relating to lack of blinding, and two levels for very serious imprecision as the evidence was from just one small study. gWith just 11 events, none of which appeared or were confirmed as being related to the fracture and treatment, the available data for this outcome are too limited to draw any conclusions or useful analysis. Nominally, we downgraded the evidence by one level for serious study limitations and two levels for very serious imprecision (very few events). hAssumed risk is the median control group risk across studies. iThe evidence was downgraded one level for serious risk of bias, one level for serious imprecision given the wide confidence interval, and one level for serious inconsistency (I2 = 54%). jThe evidence was downgraded one level for serious risk of bias, one level for serious imprecision given the few events, and one level for serious inconsistency (I2 = 53%).
Summary of findings 4. Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty for treating displaced proximal humeral fractures.
| Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty for treating displaced proximal humeral fractures | ||||||
|
Patient or population: adults undergoing surgery for displaced proximal humeral fracturesa (2 trials) Settings: hospital (tertiary care) Intervention: reverse total shoulder arthroplasty (RTSA) Comparison: hemiarthroplasty (HA) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Hemiarthroplasty | RTSA | |||||
|
Self‐reported shoulder function: % of normal shoulder function based on WOOS score (higher = better outcome) Follow‐up: 1 year |
The mean relative WOOS in the hemiarthroplasty group was 74.5% | The mean relative WOOS was 2.50% higher in the RTSA group (8.25% lower to 13.25% higher) | ‐ | 65 participants (1 study) | ⊕⊝⊝⊝ Very lowb | |
|
Self‐reported shoulder function Follow‐up: 6 months |
See comment | ‐ | ‐ | ‐ | Outcome not reported at the time | |
|
Self‐reported shoulder function: QuickDASH (0 to 55: worst disability); % of normal shoulder function based on WOOS score (higher = better outcome) Follow‐up: 2 or 3 years |
The mean QuickDASH score in the hemiarthroplasty group was 24.4 | The mean QuickDASH score was 6.90 lower in the RTSA group (10.81 to 2.99 lower) | ‐ | 61 participants (1 study) | ⊕⊝⊝⊝ Very lowc | Since pooling of the two results showed significant heterogeneity (I2 = 78%), the separate results are shown here. Neither result is clinically important. Notably, the MCID of 8.8 (adjusted for scale) for the QuickDASH is greater than best estimate (6.9). |
| The mean relative WOOS in the hemiarthroplasty group was 74.5% | The mean relative WOOS was 2.80% higher in the RTSA group (6.90% lower to 12.50% higher) | ‐ | 81 participants (1 study) |
|||
| Quality of life: EQ‐5D (0: dead to 1: best quality of life) Follow‐up: minimum 2 years (mean 2.4 years) | The mean EQ‐5D score in the hemiarthroplasty group was 0.83 | The mean EQ‐5D score was 0.1 higher in the RTSA group (0.05 lower to 0.07 higher) | ‐ | 83 participants (1 study) |
⊕⊝⊝⊝ Very lowb | The mean difference is lower than the MCID for the EQ‐5D of 0.12. A similar lack of difference was found at 1 year by the same study: MD 0.01, 95% CI ‐0.04 to 0.06; 66 participants |
|
Mortality Follow‐up: minimum 2 years |
See comment | ‐ | 161 participants (2 studies) |
⊕⊝⊝⊝ Very lowd | One trial (99 participants) reported 10 deaths: 1 from pneumonia at 8 days after surgery (related); and 9 that occurred subsequently from unrelated causes. There were no deaths reported in the second trial (62 participants). | |
|
Adverse events: number of participants with complications Follow‐up: minimum 2 years |
206 per 1000e | 75 per 1000 (29 to 194) | RR 0.36 (0.14 to 0.94) | 160 participants (2 studies) |
⊕⊝⊝⊝ Very lowf | By illustration, using an RTSA resulted in 131/1000 fewer patients having additional surgery at a minimum of 2 years (95% CI 12 to 177 fewer). The two trials reported on a total of 19 complications: symptomatic proximal migration of the humeral head was the most common (occurring in 7 of those with complications; all had a reoperation), followed by periprosthetic fracture (4 cases). |
|
Additional surgery (reoperation or secondary surgery) Follow‐up: minimum 2 years |
See comment | ‐ | 161 participants (2 studies) |
⊕⊝⊝⊝ Very lowg | 10 participants (6.2%) had a reoperation (2/79 RTSA versus 8/82 HA). This comprised RTSA in one of the RTSA group cases and all of the HA group cases. The other operation in the RTSA group was open reduction and internal fixation of a periprosthetic fracture. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DASH: Disability of the Arm, Shoulder and Hand questionnaire; EQ‐5D: European Quality of Life‐5 Dimensions; MCID: minimal clinically important difference; MD: mean difference; RR: risk ratio; SMD: standardised mean difference; WOOS: Western Ontario Osteoarthritis Shoulder score | ||||||
| GRADE Working Group grades of evidence all High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aBoth trials restricted their populations to people aged at least 70 years with 3‐ or 4‐part fractures. bThe evidence was downgraded by one level for serious study limitations, primarily reflecting a high risk of performance and detection bias relating to lack of blinding, and two levels for very serious imprecision (wide confidence interval crossing line of no effect, 1 small study). cThe evidence was downgraded by one level for serious study limitations, primarily reflecting a high risk of performance and detection bias relating to lack of blinding, one level for serious imprecision (data from single small studies) and, although not pooled, one level for serious inconsistency (heterogeneity: Chi² = 4.63, df = 1 (P = 0.03); I² = 78%). dWith just 10 events, only one of which appeared related to the fracture and treatment, the available data for this outcome are too limited to draw any conclusions or useful analysis. Nominally, we downgraded the evidence by one level for serious study limitations and two levels for very serious imprecision (very few events). eAssumed risk is the median control group risk across studies. fThe evidence was downgraded one level for serious study limitations, primarily reflecting a high risk of performance and detection bias relating to lack of blinding, and two levels for very serious imprecision (only 19 events; evidence was from two small studies only). There were also indications of inconsistency (heterogeneity: Chi² = 1.88, df = 1 (P = 0.17); I² = 47%) that reflected the greater number of complications in the hemiarthroplasty group of one study. gWith just 10 events, the available data for this outcome are too limited to draw any conclusions or useful analysis. Nominally, we downgraded the evidence by one level for serious study limitations and two levels for very serious imprecision (very few events).
Where available, we present outcome data reported at final follow‐up for individual trials in the analyses. We have distinguished primary and secondary outcomes. We report the absence of primary outcomes, but not the absence of secondary outcomes.
We based our judgement of clinically important between‐group mean differences in the various patient‐reported outcome measures (PROMs) using the minimal clinically important differences (MCIDs) listed in bold in Types of outcome measures. We decided that we would rescale MCIDs where a scoring system was rescaled but would not use these where the scoring instruments were modified, such as question removal.
Across all comparisons, there were insufficient data available to conduct our two subgroup analyses, based on age and fracture type (see Subgroup analysis and investigation of heterogeneity).
Methods of non‐surgical management
Initial treatment, including immobilisation
Seven trials reported outcomes following initial treatment for non‐surgically managed proximal humeral fractures (Carbone 2017; Hodgson 2003a; Kristiansen 1989; Lefevre‐Colau 2007; Ring 2019; Rommens 1993; Torrens 2012). All or most fractures were described as minimally displaced in three of these trials (Hodgson 2003a; Kristiansen 1989; Rommens 1993). Both Lefevre‐Colau 2007 and Torrens 2012 included displaced (two‐ or three‐part) fractures; these were described as "stable" in Lefevre‐Colau 2007 while Torrens 2012 put an upper limit to fracture displacement. Carbone 2017 included stable impacted osteoporotic proximal humeral fractures and Ring 2019 implied that their focus was on fractures with limited displacement "and fractures that occur in older, less active or infirm patients"; both groups being treated non‐operatively.
Early (up to one week post injury) versus delayed (after three or more weeks) mobilisation
Although five trials compared early versus delayed mobilisation (Hodgson 2003a; Kristiansen 1989; Lefevre‐Colau 2007; Ring 2019; Torrens 2012), the timing of the start of early mobilisation varied as did the nature and intensity of the physiotherapy provided, where described. Notable is the long (two hours) duration of individual physiotherapy sessions of Lefevre‐Colau 2007. Table 5 presents a summary of the characteristics of the five trials. With a few exceptions, the lack of comparable outcome measurements and data precluded data pooling. Most of the data came from Hodgson 2003a. The results for the Ring 2019 trial, newly added in this review update, are available only in the trial registration document. The evidence for all outcomes was rated as very low, generally being downgraded for study limitations and very serious imprecision.
1. Early versus delayed mobilisation for non‐surgically‐treated fractures: brief characteristics.
| Study | Participants (Neer classification if parts are described) | Early mobilisation (at up to 1 week from injury) | Delayed mobilisation (after 3 or 4 weeks) | Physiotherapy | Follow‐up |
| Hodgson 2003a | 86 participants (81% female, mean age 70 years) with minimally displaced fractures (Neer) with two parts; this included isolated fractures of the greater tuberosity (UK) |
"Immediate" physiotherapy (gradual assisted movements of the upper limb) within one week of fracture | Delayed physiotherapy after three weeks of immobilisation in a collar and cuff sling | Both groups received same rehabilitation programme. | 2 years |
| Kristiansen 1989 | 85 participants (71% female, mean age 72 years) with fractures (74% minimally displaced (Neer)) (Denmark) | Immobilisation in sling and body bandage for one week | Immobilisation in sling and body bandage for three weeks | At the end of immobilisation, instructions were given to perform pendulum exercises as well as active movements of the elbow and hand. | 2 years |
| Lefevre‐Colau 2007 | 74 participants (68% female, mean age 63 years) with impacted ("stable") fractures: 34 minimally displaced; 40 2‐or 3‐part (surgical neck or greater tuberosity (France) | Physiotherapy started within 3 days of fracture: 2‐hour sessions supervised by a physiotherapist, 5 times a week. Then twice a week after 3 weeks |
Physiotherapy started after 3 weeks of immobilisation in a sling: 2‐hour sessions supervised by a physiotherapist, 4 times a week for 4 weeks |
Differences in schedule with similar number of sessions in each group by 6 months: 32 versus 33 until 6 months | 6 months |
| Ring 2019 | 63 participants (of 50: 28% female, mean age 63 years) with non‐operatively treated fractures. No description but implied inclusion was "limited displacement and fractures that occur in older, less active or infirm patients" (USA) | Physiotherapy (pendulum movements) started immediately after diagnosis of injury | Physiotherapy delayed until three weeks | No information | 6 months |
| Torrens 2012 | 42 participants (76% female, mean age 70 years) with displaced (2‐ and 3‐part) or non‐displaced (8 in all) proximal humeral fractures that were not considered for surgery or patient refused surgery (Spain) | Immobilisation in sling for one week | Immobilisation in sling for four weeks | All followed same "progressive rehabilitation" regimen | 1 year |
Primary outcomes
Patient‐reported shoulder function or disability was reported using the DASH (0 to 100: worst outcome; MCID = 13.0) in Ring 2019 and the Croft Shoulder Disability Questionnaire (Croft 1994) in Hodgson 2003a. There is very low‐certainty evidence, downgraded by one level for serious risk of bias and two levels for very serious imprecision, of no important difference between early and delayed mobilisation in DASH scores at three months (MD 9.00, 95% CI ‐2.33 to 20.33; 50 participants) or at six months (MD 4.00, 95% CI ‐1.40 to 9.40; 50 participants, data imputed for 20 participants); Analysis 1.1. There is very low‐certainty evidence, downgraded one level for serious risk of bias, one level for serious imprecision and one level for serious indirectness, of fewer people having any disability (one or more problems listed in the Croft Shoulder Disability Questionnaire) at one year (18/42 versus 29/40; RR 0.59, 95% CI 0.40 to 0.88; Analysis 1.2). There is very low‐certainty evidence, downgraded for the same reasons as above, of little between‐group difference in the same measure at two years (RR 0.73, 95% CI 0.46 to 1.15; 74 participants). Similarly, there is very low‐certainty evidence of little between‐group difference in the incidence of severe disability (5 or more problems) at one year (13/42 versus 17/40; RR 0.73, 95% CI 0.41 to 1.30) or two years; Analysis 1.2. It is notable that, overall, a substantial proportion of participants (34%) continued to report some (51%) or severe (34%) disability at two years. Results at two years for 8 of the 22 questions of the Croft questionnaire are shown in Analysis 1.3. These are presented to give an indication of the variety of problems experienced by the trial participants and the variation in the responses. There was some indirect evidence supporting a quicker recovery in the early group as trial participants given early physiotherapy attended fewer treatment sessions until they and their physiotherapists agreed that independent shoulder function had been achieved (mean difference (MD) ‐5.00 sessions; 95% CI ‐8.25 to ‐1.75; very low‐certainty evidence; evidence downgraded one level for serious risk of bias, one for serious imprecision and one for serious indirectness; Analysis 1.4).
1.1. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 1: Disability of the Arm, Shoulder, and Hand (DASH) score (0 to 100: worst disability)
1.2. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 2: Shoulder function: Croft Shoulder Disability Score
1.3. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 3: Croft Shoulder Disability Score: individual problems at 2 years
1.4. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 4: Number of treatment sessions (until independent function achieved)
Patient‐reported health‐related quality of life was reported separately for eight dimensions of the SF‐36 in Hodgson 2003a and the EQ‐5D in Torrens 2012. As can be seen in Analysis 1.5, participants of the early group had better health‐related quality‐of‐life scores at 16 weeks in two dimensions of the SF‐36 (role limitation physical: MD 22.20, 95% CI 3.82 to 40.58; and pain: MD 12.10, 95% CI 3.26 to 20.94; 81 participants). These results are consistent with the earlier recovery of independent function as indicated by the need for fewer treatment sessions in the early mobilisation group as judged by the physiotherapists. There is, however, no evidence of differences between the two treatment groups in the other six dimensions of the SF‐36 at 16 weeks nor in all eight dimensions at one year; Analysis 1.6. Quality‐of‐life scores (EQ‐5D) favoured the participants in the four‐week group of Torrens 2012 at 3, 6 and 12 months of follow‐up but the 95% CIs crossed the line of no effect for all follow‐ups; Analysis 1.7. Overall, there is very low‐certainty evidence, downgraded one level for serious risk of bias and two levels for very serious imprecision, of little or no difference in quality of life between early and delayed mobilisation at one year.
1.5. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 5: Quality of life: SF‐36 scores (8 dimensions) at 16 weeks
1.6. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 6: Quality of life: SF‐36 scores (8 dimensions) at 1 year
1.7. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 7: Quality of life: EQ‐5D (0: dead to 1: best quality)
There were five serious adverse events distributed between the two treatment groups: 2/127 early versus 3/132 delayed; Analysis 1.8. These comprised one frozen shoulder in Hodgson 2003a, two complex regional pain syndrome type 1 (CRPS‐1) in Kristiansen 1989, and two treated subacromial impingement in Lefevre‐Colau 2007. Hodgson 2003a noted there were no complications arising from fracture displacement. Torrens 2012 reported no complications aside from noting that the three participants (2 early mobilisation versus 1 four‐weeks immobilisation) experiencing a "significant displacement" of their fracture did not require surgical treatment (see Analysis 1.8). Ring 2019 reported there were no adverse events but it was unclear if shoulder complications would have been counted in the definition used. Two trials reporting on fracture complications found no cases of non‐union (Lefevre‐Colau 2007; Torrens 2012). Overall there is very low‐certainty evidence, downgraded one level for serious risk of bias and two levels for very serious imprecision given the very few events, of little or no difference between the two groups in serious shoulder complications (4 trials, 259 participants) or fracture complications (2 trials, 106 participants). The only reported death occurred in the four‐weeks immobilisation group of Torrens 2012.
1.8. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 8: Adverse events
Secondary outcomes
Shoulder function, measured using the Constant score (Constant 1987), was reported relative to the unaffected shoulder in Hodgson 2003a (see Analysis 1.10), and as Constant scores in Lefevre‐Colau 2007 and Torrens 2012 (Analysis 1.11). In Hodgson 2003a, results were better in the early group at 8 and at 16 weeks (mean difference in ratio affected/unaffected arm 0.16; 95% CI 0.07 to 0.25; 83 participants). The between‐group differences were smaller at one year and the confidence interval crossed the line of no effect (MD 0.07, 95% CI ‐0.03 to 0.17; 82 participants). Data for Constant scores (0 to 100: best outcome) at three and six months favoured the early mobilisation group, but while the result at three months may include a clinically relevant effect (MD 6.53, 95% CI 0.77 to 12.30; I2 = 38%; 2 studies, 106 participants), this was not the case at six months (MD 3.39, 95% CI ‐1.46 to 8.24; I2 = 42%; 2 studies, 105 participants). Torrens 2012 did not find a between‐group difference at 12 months. Kristiansen 1989 reported that function, assessed using Neer score without the anatomic section, was similar in both groups at six months and over.
1.10. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 10: Constant score (ratio of affected/unaffected arm)
1.11. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 11: Constant score (0 to 100: best outcome)
There was no evidence of between‐group difference in pain scores (0 to 100: worst pain) at 3 months (MD ‐5.13, 95% CI ‐14.76 to 4.50; I2 = 68%; 2 studies, 106 participants), 6 months (MD 4.29, 95% CI ‐5.48 to 14.07; I2 = 0%; 2 studies, 105 participants) or at 12 months; Analysis 1.12. Ring 2019 found no difference between the two groups at either three or six months; see Analysis 1.13. Pain change data from Lefevre‐Colau 2007 are presented in Analysis 1.14: as in Analysis 1.12, results at three months for Lefevre‐Colau 2007 potentially favoured early mobilisation. Other reports of pain outcome were from Hodgson 2003a, where participants of the early group had better SF‐36 pain dimension results at 16 weeks but not at one year subsequently; and Kristiansen 1989, where the authors reported that participants who started early mobilisation at one week suffered less pain in the first three months than those who kept their bandaging for three weeks; while pain at six months and over was similar in both groups.
1.12. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 12: Pain VAS (0 to 100: worst pain)
1.13. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 13: Pain: Likert scale (0 to 10: severe pain)
1.14. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 14: Changes in pain intensity (mm) from baseline: 100 mm visual analogue scale (positive change = less pain)
No trial reported on strength; which would have been measured as part of the Constant score. There was no evidence of clinically important range of motion differences between the two groups at three and six months from Lefevre‐Colau 2007 and Ring 2019; Analysis 1.15. Kristiansen 1989 also reported findings of similar mobility in the two groups at six months and over.
1.15. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 15: Range of motion at 3 and 6 months (degrees)
All participants of Lefevre‐Colau 2007 attended at least 70% of the supervised physiotherapy sessions; only three participants expressed dissatisfaction with their treatment (see Analysis 1.16). The evidence from Torrens 2012 did not indicate differences between the two groups in patient satisfaction at any of the three follow‐ups (see Analysis 1.17).
1.16. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 16: Patient dissatisfied with treatment
1.17. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 17: Patient satisfaction (0 to 10: higher scores ‐ greater satisfaction)
Early intensive mobilisation versus early less intensive mobilisation
Carbone 2017 compared early intensive mobilisation (10 sessions in two weeks) versus early less intensive mobilisation (10 sessions in five weeks), started one week after the fracture, in 80 people with stable impacted osteoporotic proximal humeral fractures. A removable arm sling was prescribed for the first three weeks after fracture. All participants underwent a total of 20 sessions using the same protocol. Results were reported at 3, 6 and 12 months. The evidence for all reported outcomes was rated as very low, with downgrading for serious study limitations and very serious imprecision.
Primary outcomes
There was no evidence of a difference between the two groups in participant judgement of the performance of the injured shoulder compared with a normal shoulder at all three follow‐ups (Analysis 2.1). Carbone 2017 did not report on quality of life. One participant in the intensive group had a non‐union and one in the less intensive group had loss of reduction (Analysis 2.2). Reflecting low subjective assessment of performance and low Constant scores, the participant with non‐union had surgery using a reverse total shoulder arthroplasty.
2.1. Analysis.

Comparison 2: Early intensive mobilisation versus early less intensive mobilisation, Outcome 1: Subjective shoulder value (0 to 100: % of normal shoulder)
2.2. Analysis.

Comparison 2: Early intensive mobilisation versus early less intensive mobilisation, Outcome 2: Adverse events
Secondary outcomes
Although the Constant scores were lower in the intensive group at all three follow‐ups, between‐group differences (4.0 or 5.0) were small and unimportant clinically given that all were less than the MCID of 11.6 (Analysis 2.3). Four (10% of 40) versus one (2.5% of 40) participants were reported as not complying with the rehabilitation.
2.3. Analysis.

Comparison 2: Early intensive mobilisation versus early less intensive mobilisation, Outcome 3: Constant score (0 to 100: best outcome)
Gilchrist arm sling versus "classic" Desault bandage
In a quasi‐RCT, Rommens 1993 compared the use of two types of immobilisation, the Gilchrist arm sling versus the Desault bandage, worn for two to three weeks in 28 participants with mainly minimally displaced fractures. Results were reported up to fracture consolidation. The evidence for all outcomes was rated as very low certainty, generally being downgraded for serious study limitations and very serious imprecision.
Primary outcomes
Self‐reported function and quality‐of‐life outcomes were not reported by Rommens 1993. There was no report of secondary treatment or serious adverse events.
Secondary outcomes
Rommens 1993 reported, without presenting data, that they had found no differences in the end result, either in terms of functional outcome or fracture healing at fracture consolidation. Pain during immobilisation was reported to be greater in the Desault group but was not reported at fracture consolidation. There was one case of severe skin irritation (7.1% of 14) in the Gilchrist group and three cases (21.4% of 14) in the Desault group; this prompted the premature removal of the bandage in two people of the latter group (Analysis 3.1). More people found the initial application of a Desault bandage uncomfortable, and none of the Gilchrist group versus two of the Desault group gave a poor rating to their assigned bandage at fracture consolidation (Analysis 3.2). Analysis 3.2 also presents a sensitivity analysis where it is presumed that the two participants who had premature removal of their bandage also would have given a poor rating. Slight and inconsequential displacement of the fracture in the first week was reported in two participants of the Gilchrist group (Analysis 3.3).
3.1. Analysis.

Comparison 3: Gilchrist bandage versus 'classic' Desault bandage, Outcome 1: Adverse events (problems with bandages)
3.2. Analysis.

Comparison 3: Gilchrist bandage versus 'classic' Desault bandage, Outcome 2: Participant assessment of comfort and bandage
3.3. Analysis.

Comparison 3: Gilchrist bandage versus 'classic' Desault bandage, Outcome 3: Fracture displacement by 3 weeks
Continuing management (rehabilitation) after initial sling immobilisation
Instructed self‐exercise versus conventional physiotherapy
Two small trials compared self‐directed exercise following a course of instruction versus conventional physiotherapy during the 12 weeks following trauma in a total of 62 participants with minimally displaced fractures (Bertoft 1984; Lundberg 1979). No data pooling was possible. The evidence for all reported outcomes was of very low certainty, downgraded at least one level for serious risk of bias and two levels for very serious imprecision. Bertoft 1984 followed participants for one year and Lundberg 1979 for three months.
Primary outcomes
Neither trial recorded validated measures of patient‐reported function or quality of life. Of the four adverse events reported in Lundberg 1979, there were three cases of frozen shoulder and one of unexplained prolonged pain (1/20 versus 3/22; Analysis 4.1). Bertoft 1984 reported, without providing data, no significant between‐group differences in subjectively‐rated overall activities of daily living based on four activities.
4.1. Analysis.

Comparison 4: Instructed self‐exercise versus conventional physiotherapy, Outcome 1: Adverse events
Secondary outcomes
In both trials, there was no evidence of differences between those receiving instruction for exercises at home and those undergoing supervised physiotherapy in Neer's scores (Analysis 4.2), pain (Analysis 4.3, Analysis 4.4), requested change in treatment (Analysis 4.5), or active glenohumeral elevation (Analysis 4.6). It should be noted that, since Lundberg 1979 did not report whether there had been any loss to long‐term follow‐up at an average of 16 months, the results for Neer's score presented in Analysis 4.2 are for illustrative purposes only.
4.2. Analysis.

Comparison 4: Instructed self‐exercise versus conventional physiotherapy, Outcome 2: Neer's rating (0 to 100: best) at mean 16 months (exploratory analysis)
4.3. Analysis.

Comparison 4: Instructed self‐exercise versus conventional physiotherapy, Outcome 3: Pain at one year (scale 0 to 8: maximum pain)
4.4. Analysis.

Comparison 4: Instructed self‐exercise versus conventional physiotherapy, Outcome 4: Severe or moderate pain at 3 months
4.5. Analysis.

Comparison 4: Instructed self‐exercise versus conventional physiotherapy, Outcome 5: Requested change of therapy
4.6. Analysis.

Comparison 4: Instructed self‐exercise versus conventional physiotherapy, Outcome 6: Active glenohumeral elevation (degrees)
Swimming pool treatment plus self‐training versus self‐training alone
Revay 1992, which included 48 participants with minimally displaced fractures, reported that the addition of supervised exercises in a swimming pool to self‐treatment (at home) did not enhance long‐term outcome. Follow‐up was at 1, 2, 3 and 12 months. No data were available for presentation in the analyses. Thus, the evidence should be considered very low certainty.
Primary outcomes
Revay 1992 did not record validated measures of patient‐reported function or quality of life and did not report on adverse events. Based on participant assessment of nine activities of daily living, Revay 1992 reported better results (higher scores) for these in the control group (self‐treatment only) at two and three months' follow‐up but minimal between‐group differences at one year.
Secondary outcomes
Similarly, participants of the control group were reported as having better functional movements and joint mobility at two and three months' follow‐up, but with minimal between‐group differences at one year. There were no between‐group differences in pain at any follow‐up. Revay 1992 suggested that those using the pool may have neglected their home exercises, but the authors did not evaluate compliance.
Telerehabilitation versus face‐to‐face rehabilitation
Tousignant 2020 provided an eight‐week training programme, starting on average 27 days after injury, to 31 participants (data for 30) with a non‐surgically treated proximal humeral fracture who had returned home after discharge from the emergency ward (or hospital). The supervised sessions were via telerehabilitation or provided face to face in a clinic. The evidence for all reported outcomes was of very low certainty, being downgraded one level for serious risk of bias and two levels for very serious imprecision.
Primary outcomes
At the end of the intervention, there were no between‐group differences found in DASH scores (Analysis 5.1). Tousignant 2020 did not report on quality of life or adverse events.
5.1. Analysis.

Comparison 5: Telerehabilitation versus face‐to‐face rehabilitation (non‐surgical treatment), Outcome 1: Disability of the Arm, Shoulder, and Hand (DASH) score (0 to 100: worst disability) at 8 weeks
Secondary outcomes
At the end of the intervention, there were no between‐group differences found in Constant scores (Analysis 5.2), active ranges of motion (Analysis 5.3) or participant satisfaction with the healthcare provided (Analysis 5.4).
5.2. Analysis.

Comparison 5: Telerehabilitation versus face‐to‐face rehabilitation (non‐surgical treatment), Outcome 2: Constant score (0 to 100: best outcome) at 8 weeks
5.3. Analysis.

Comparison 5: Telerehabilitation versus face‐to‐face rehabilitation (non‐surgical treatment), Outcome 3: Active range of motion (degrees) at 8 weeks
5.4. Analysis.

Comparison 5: Telerehabilitation versus face‐to‐face rehabilitation (non‐surgical treatment), Outcome 4: Satisfaction with healthcare service provided (0 to 100: most satisfied) at 8 weeks
Pulsed electromagnetic high frequency energy (PHFE) versus placebo
Livesley 1992, which included 48 participants with minimally displaced fractures, reported that there was no difference in outcome between the two groups (pulsed electromagnetic high frequency energy (PHFE) versus placebo) at any stage of the trial, but provided no quantitative data for follow‐ups at one, two and six months. The implied evidence for this comparison should be considered very low certainty.
Primary outcomes
Livesley 1992 did not record validated measures of patient‐reported function or quality of life. There was no report of adverse outcomes.
Secondary outcomes
Livesley 1992 assessed function via the "European Shoulder Association assessment charts", pain, range of movement, muscle wasting and strength, and subjective opinion of treatment. All trial participants were reported as achieving a "good" result as opposed to a "poor" one.
Surgical treatment versus non‐surgical treatment
Ten heterogeneous trials, with a total of 717 participants and 718 fractures, evaluated surgical intervention for displaced fractures, of which over 66% were three‐ or four‐part fractures (Neer classification). Table 6 gives a brief summary of their characteristics. The methods of surgery varied between the trials, being restricted to internal fixation in four trials (Fjalestad 2010a; Launonen 2019a; Olerud 2011a; Zyto 1997), external fixation in Kristiansen 1988, hemiarthroplasty in three trials (Boons 2012; Olerud 2011b; Stableforth 1984), and RTSA in Lopiz 2019. Most surgery involved internal fixation in ProFHER 2015, where the surgeons used methods with which they were experienced. Usually, non‐surgical treatment started with sling immobilisation (supporting the arm in a sling); this was preceded by closed manipulation in all participants in two trials (Kristiansen 1988; Stableforth 1984) and in eight participants in Fjalestad 2010a.
2. Surgical versus non‐surgical treatment trials: brief characteristics.
| Study | Participants (Neer classification) | Surgery | Non‐surgical (starting with) | Follow‐up |
| Boons 2012 | 50 participants (94% female, mean age 78 years) with 4‐part fractures (The Netherlands) | Humeral head replacement with the Global prostheses; cemented | Sling immobilisation | 1 year |
|
Fjalestad 2010a |
50 participants (88% female, mean age 73 years) with 3‐ or 4‐part fractures (Norway) |
Open reduction and fixation with an interlocking plate device and metal cerclages | Immobilisation of the injured arm in a modified Velpeau bandage. Closed reduction in 8 participants | 2 years |
|
Kristiansen 1988 |
30 participants (71% female, age range 30 to 91 years) with 31 2‐, 3‐ or 4‐part fractures. Included 7 2‐part, 19 3‐part and 5 4‐part fractures (Denmark) | Percutaneous reduction and external fixation |
Closed manipulation and sling immobilisation | 2 years |
| Launonen 2019a | 88 participants (91% female, mean age 72.5 years) with 2‐part surgical neck fractures (Denmark, Estonia, Finland, Sweden) | Open reduction and fixation with a PHILOS plate; preliminary reduction with sutures | Collar and cuff or sling support | 2 years |
| Lopiz 2019 | 62 participants (of 59: 86% female, mean age 83.5 years) with 3‐ or 4‐part fractures | Reverse total shoulder arthroplasty | Sling immobilisation | 1 year |
| Olerud 2011a | 60 participants (of 59: 86% female, mean 83.5 years) with 3‐part fractures (all had displaced surgical neck fracture) (Sweden) | Open reduction and fixation with a PHILOS plate and non‐absorbable sutures | Sling immobilisation | 2 years |
| Olerud 2011b | 55 participants (85% female, mean age 77 years) with 4‐part fractures (Sweden) | Humeral head replacement with the Global Fx prosthesis | Sling immobilisation | 2 years |
| ProFHER 2015 | 250 participants (77% female, mean age 66 years) with "displaced fracture of the proximal humerus that involved the surgical neck". Included 18 1‐part (but confirmed as still "displaced"), 128 2‐part, 93 3‐part and 11 4‐part fractures (UK) |
Either internal fixation (majority were PHILOS plates) or joint replacement (hemiarthroplasty) Pragmatic trial ‐ choice based on surgeon's experience with method |
Sling immobilisation | 2 years |
| Stableforth 1984 | 32 participants (78% female, mean age 68 years) with 4‐part fracture (UK) | Hemiarthroplasty | Closed manipulation and sling | 6 months |
| Zyto 1997 | 40 participants (88% female, mean age 74 years) with 3‐ or 4‐part fractures (3 others excluded) (Sweden) | Internal fixation using surgical tension band or cerclage wiring | Sling immobilisation | 50 months |
PHILOS: Proximal Humerus Internal Locking System
Primary outcomes
Patient‐reported function
There is high‐certainty evidence of no important clinical difference between the two interventions in patient‐reported function, measured using four different scores (ASES, DASH, OSS and SST), at one year follow‐up: standardised mean difference (SMD) 0.10, 95% CI ‐0.07 to 0.27; I2 = 0%; 7 studies, 552 participants; evidence not downgraded; Analysis 6.1 and Figure 4). Similar findings of no important clinical difference between the two interventions in patient‐reported function apply at six months (SMD 0.17, 95% CI ‐0.04 to 0.38; I2 = 0%; 3 studies, 347 participants; moderate‐certainty evidence, downgraded one level for serious imprecision; Analysis 6.2). Given the availability of three‐ to four‐month follow‐up data from four trials, which also showed no difference between the two groups (SMD ‐0.02, 95% CI ‐0.28 to 0.24; I2 = 4%; 4 studies, 229 participants; low‐certainty evidence, downgraded one level for serious study limitations and one level for imprecision), we conducted an exploratory analysis subgrouped by timing of follow‐up. We found no evidence of subgroup differences between the results at three to four months and those at six months (test for subgroup differences: Chi² = 1.32, degrees of freedom (df) = 1 (P = 0.25), I² = 24.3%; Analysis 6.3). Based on data for three scores reported by five trials, there is high‐certainty evidence of no important clinical difference between the two interventions at 24 months (SMD 0.06, 95% CI ‐0.13 to 0.25; I2 = 0%; 5 studies, 423 participants; evidence not downgraded; Analysis 6.4).
6.1. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 1: Functional scores at 12 months (higher = better outcome)
4.

Forest plot of comparison 4: surgical versus non‐surgical treatment; outcome 4.1: functional scores at 12 months (higher = better outcome)
6.2. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 2: Functional scores at 6 months (higher = better outcome)
6.3. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 3: Functional scores subgrouped by 3 to 4 versus 6 months (higher = better outcome)
6.4. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 4: Functional scores at 24 months (higher = better outcome)
Pooled or, where no pooling occurred, individual trial results for the difference scores also provided no evidence of clinically‐important between‐group differences. Analysis 6.5 presents the pooled Oxford Shoulder Score (OSS; 0 to 48: best function) results from Launonen 2019a and ProFHER 2015 at 6, 12 and 24 months, and Analysis 6.6 presents the primary analysis OSS results for ProFHER 2015 over two years (MD 0.75, 95% CI ‐1.68 to 3.18; P = 0.55; 231 participants) and at 6, 12, 24, 36, 48 and 60 months. None of these showed clinically important (the MCID for the OSS was set at 5 points in ProFHER 2015) differences between the two groups; and all confidence intervals crossed the line of no effect. DASH scores were reported by four trials (Launonen 2019a; Lopiz 2019; Olerud 2011a; Olerud 2011b). Although the best estimates at all four follow‐ups (3 to 4, 6, 12 and 24 months) numerically favoured surgery, all confidence intervals crossed the line of no effect, and all best estimates were lower than the MCID (10 points) for DASH (0 to 100: worst function); for example, pooled findings at 12 months were: MD ‐3.83 (95% CI ‐8.77 to 1.12; I2 = 0%; 4 studies, 238 participants). Fjalestad 2010a found no significant differences between the two groups in the American Shoulder and Elbow Surgeons (ASES; 0 to 24: best function) scores at either 6, 12 or 24 months of follow‐up (see Analysis 6.8). Boons 2012 found no significant differences between the two groups in the Simple Shoulder Test scores at 3 or 12 months (Analysis 6.9). Zyto 1997 provided no evidence of between‐group differences in subjective assessment of function at either one or three years (Analysis 6.10).
6.5. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 5: Oxford Shoulder Score (OSS) (0 to 48: best outcome)
6.6. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 6: Oxford Shoulder Score (OSS) (0 to 48: best outcome): ProFHER trial primary analysis
6.8. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 8: American Shoulder and Elbow Surgeons (ASES) score (0 to 24: best)
6.9. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 9: Simple Shoulder Test (SST) (0 to 12: best function)
6.10. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 10: Activities of daily living
Health‐related quality of life
All of the pooled between‐group differences in health‐related quality‐of‐life scores measured using the EQ‐5D (0: dead to 1: best quality) at four follow‐up times (3 to 4, 6, 12 and 24 months) were clinically unimportant, being smaller than the MCID of 0.12, as well as the 95% CIs crossing the line of no effect (Analysis 6.11). The most data were available at one year follow‐up: MD 0.01, 95% CI ‐0.02 to 0.04; I2 = 0%; 6 studies, 502 participants. Similar findings of no or minimal difference between groups applied at 3 to 4 months (MD 0.01, 95% CI ‐0.01 to 0.04; I2 = 0%; 5 studies, 442 participants), 6 months (MD 0.02, 95% CI ‐0.01 to 0.05; I2 = 35%; 5 studies, 458 participants) and 24 months (MD 0.01, 95% CI ‐0.02 to 0.05; I2 = 56%; 5 studies, 426 participants). We rated the evidence as high certainty for the first three follow‐up times but downgraded the evidence at 24 months one level for serious inconsistency, reflecting moderate statistical heterogeneity and that the 95% CIs of two trials (102 participants) from the same centre included the MCID favouring surgery.
6.11. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 11: Quality of life: EQ‐5D (0: dead to 1: best quality)
Analysis 6.12 presents the EQ‐5D data at seven follow‐ups, from 3 to 60 months, from a multilevel regression analysis from ProFHER 2015; there is no evidence of between‐group differences at any follow‐up time. A separate breakdown of the results from Fjalestad 2010a, which include the number of quality‐adjusted life years (QALYs), showed no differences in any quality‐of‐life outcomes for this trial, including with additional 15D (Sintonen 2001) data from Launonen 2019a at 12 and 24 months (Analysis 6.13). Lopiz 2014 found no between‐group difference at 12 months in the EuroQol‐VAS scores (Analysis 6.14). Based on data from ProFHER 2015, with additional data at one year follow‐up from Lopiz 2019, the SF‐12 physical component scores (0 to 100: best outcome) were slightly higher in the surgery group at all three follow‐ups (Analysis 6.15); conversely, the SF‐12 mental component scores (0 to 100: best outcome) were slightly higher in the non‐surgical treatment group at all three follow‐ups (Analysis 6.16). All confidence limits crossed the line of no effect and all are less than the minimal clinically important difference (6.5 for the physical component).
6.12. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 12: Quality of life: EQ‐5D (0: dead to 1: best health): ProFHER multilevel regression analysis
6.13. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 13: Quality of life: 15D score and number of quality‐adjusted life years (QALYs)
6.14. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 14: Quality of life: EuroQol‐VAS (0 to 100: best quality)
6.15. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 15: Quality of life: SF‐12 Physical Component Score (0 to 100: best)
6.16. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 16: Quality of life: SF‐12 Mental Component Score (0 to 100: best)
Mortality
There was low‐certainty evidence of no or little difference between the two groups in mortality at up to two years' follow‐up (18/322 versus 13/324; RR 1.35, 95% CI 0.70 to 2.62; I2 = 0%; 8 studies, 646 participants; evidence downgraded two levels for very serious imprecision; Analysis 6.17). Where reported, none of the deaths was related to participants' fracture or treatment, with the exception of one early death due to venous thromboembolism in the surgical group of ProFHER 2015. Notably, the two deaths that occurred within three months of surgery in Fjalestad 2010a were people with underlying health problems. In Zyto 1997, 8 out of 43 participants had died at 50 months, but no information on group allocation or causes of death was provided.
6.17. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 17: Mortality
Separate mortality data were not available for Stableforth 1984, which reported that fewer participants of the prosthesis group needed some help with activities of daily living or had died by six months (Analysis 6.18: 2/16 versus 9/16; RR 0.22, 95% CI 0.06 to 0.87).
6.18. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 18: Dependent in activities of daily living (or dead) at 6 months
Additional surgery
Pooled data from nine studies showed a higher risk of additional or secondary surgery in the surgery group (37/332 versus 17/335; RR 2.06, 95% CI 1.21 to 3.51; I2 = 23%; 9 studies, 667 participants; low‐certainty evidence, downgraded one level for serious imprecision and one level for serious inconsistency, especially for the pooled two‐year follow‐up data (I2 = 51%); Analysis 6.19). Only Zyto 1997 did not report this outcome. Details of the reasons for additional surgery are listed below.
6.19. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 19: Additional surgery (reoperation or secondary surgery)
Boons 2012: one surgical group participant underwent revision surgery after one week because of head‐stem separation. A non‐surgically treated participant in Boons 2012 who had surgery at 13 months ‐ thus outside the trial's follow‐up period ‐ because of shoulder pain and impairment was not included in this analysis.
Fjalestad 2010a: treatment failure resulting in an operation occurred in eight surgical group participants, one of whom had refixation plus bone grafting at six months, and seven whose implants were removed because of screw penetration into the joint space; and one non‐surgically treated participant, who had surgery because of fracture redisplacement at two weeks.
Kristiansen 1988: the three cases of treatment failure were the removal of pins due to infection in one surgical group participant, and a change of method resulting from a poor initial fracture reduction in two non‐surgical group participants.
Launonen 2019a: of the three surgical group participants having additional surgery, two had surgery to replace proximal screws that had migrated into the joint and one had a long anatomical locking plate after incurring a peri‐implant fracture distal to the tip of their plate from a fall.
Lopiz 2019: one dropout, who withdrew consent, from non‐surgical group received an RTSA.
Olerud 2011a: the reasons for reoperations in the surgical group were deep infection (two cases), non‐union (one case), impingement (two cases), avascular necrosis (one case), screw penetration into joint (one case) and stiffness (two cases). One non‐surgically‐treated participant in Olerud 2011a had surgery because of impingement. Not included in this analysis is another non‐surgically‐treated participant with non‐union, who abstained from surgery partly because of a late diagnosis of axillary nerve palsy.
Olerud 2011b: the reasons for additional surgery were: screw penetration of the joint (for one participant treated with a locking plate), stiffness and impingement and displaced greater tuberosity, respectively, in three surgical group participants, and for complete displacement of the humeral shaft without bony contact in one non‐surgically‐treated participant. Not included in this analysis is another non‐surgically‐treated participant who refused surgery for a non‐union.
ProFHER 2015: reasons for further surgery in the surgical group were avascular necrosis (two cases), metalwork problems (seven cases) and post‐traumatic stiffness (two cases). The reasons for subsequent surgery in the non‐surgical treatment group of ProFHER 2015 were avascular necrosis (one case), malunion (two cases), non‐union (four cases), post‐traumatic stiffness (one case), rotator cuff tear (one case), severe pain (one case) and not‐reported (one case).
Stableforth 1984: one surgical group participant had their prosthesis removed because of a deep infection.
Only ProFHER 2015 reported on additional shoulder‐related therapy, which occurred in slightly more participants of the surgery group (7/125 versus 4/125; RR 1.75, 95% CI 0.53 to 5.83; P = 0.36; Analysis 6.20).
6.20. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 20: Adverse events / complications
Adverse events or complications
The numbers of people in each group with one or more adverse events or complications were reported in three trials (Launonen 2019a; Lopiz 2019; ProFHER 2015). There is low‐certainty evidence, downgraded one level for imprecision and one level for indirectness, of a higher risk of complication with surgery (35/194 versus 24/197; RR 1.46, 95% CI 0.92 to 2.31; I2 = 0%; 3 studies, 391 participants; Analysis 6.20).
Analysis 6.20 also presents the available data for individual complications. Unsurprisingly, surgery‐related complications (e.g. infection and screw penetration of the joint) were predominant in the surgery treatment group. Whilst radiologically‐detected outcomes, such as non‐union (RR 0.42, 95% CI 0.19 to 0.94; I2 = 0%; 8 studies, 582 participants) and avascular necrosis (RR 0.52, 95% CI 0.33 to 0.81; I2 = 50%; 8 studies, 572 participants), were more common in the non‐surgical treatment group, the clinical implications of these radiological complications are unclear. Some of these outcomes were without symptoms or minor in extent. For instance, in Fjalestad 2010a, both cases of non‐union in the non‐surgical treatment group were without symptoms, and 22 of the 27 participants with radiologically‐detected avascular necrosis were asymptomatic. Neither the one case of non‐union nor the 17 cases of osteonecrosis in the non‐surgical group of Lopiz 2019 were described as complications. Of note is that surgical replacement of the humeral head, as in Boons 2012, Olerud 2011b and Lopiz 2019, precludes avascular necrosis.
Secondary outcomes
Where data were available from one or two small single‐centre trials only, we rated the evidence for secondary outcomes as very low certainty, downgrading for very serious imprecision and usually study limitations. The differences between the two groups in the Constant scores (0 to 100: best outcome) at five different time points (3 to 4, 6, 12, 24 and 50 months) were all small and clinically unimportant (e.g. the most data were for 12 months: MD 3.78, 95% CI 0.19 to 7.37; I2 = 0%; 6 studies, 330 participants; moderate‐certainty evidence, downgraded one level for serious study limitations; Analysis 6.21). The same lack of differences between the two groups applied to the Constant scores of the injured arm in Fjalestad 2010a at 6, 12 and 24 months of follow‐up (see Analysis 6.22). At one‐year follow‐up in Kristiansen 1988, slightly fewer participants of the surgical group had a poor or unsatisfactory rating of function, assessed using the Neer score (3/11 versus 6/10; RR 0.45, 95% CI 0.15 to 1.35; Analysis 6.23).
6.21. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 21: Constant score (overall: 0 to 100: best score)
6.22. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 22: Constant score (difference between injured and uninjured shoulder): Normal = 0.
6.23. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 23: Poor or unsatisfactory function at 1 year (Neer rating)
Boons 2012 reported similar results in the two groups for patient‐assessed disability, based on a 0 to 100 VAS scale, where the maximum score equated to "no restrictions". The clinical relevance of the results, which favoured the surgical group, is uncertain (Analysis 6.24).
6.24. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 24: Visual Analogue Scale (VAS) disability (0 to 100: no restrictions)
Boons 2012 reported lower pain scores, measured using VAS (0 to 100: higher scores mean worse pain), in the hemiarthroplasty group at three months (MD ‐18.00, 95% CI ‐29.03 to ‐6.97; 49 participants; Analysis 6.25) than in the non‐surgical group; this difference is likely to be clinically important. In contrast, there were similar results in the two groups at 12 months (median 23 in the surgery group versus 25 in the non‐surgical group; reported P = 0.725). This tallies with very low or low‐certainty evidence of no or little difference between the two groups in pain at later follow‐up times (e.g. at 24 months: MD ‐2.67, 95% CI ‐8.37 to 3.03; I2 = 25%; 3 studies, 173 participants; low‐certainty evidence, downgraded one level for serious study limitations and one level for serious imprecision; Analysis 6.25). Zyto 1997, which provided a breakdown of the Constant score into the separate components (activities of daily living, pain, range of motion, strength), did not confirm a significant difference between the two groups in the pain component, which was in favour of the non‐surgical treatment group, at 50 months (Analysis 6.26). Nearly all trial participants in Stableforth 1984 had shoulder pain, but fewer in the prosthesis group reported constant pain that impaired sleep or function (Analysis 6.27: 2/15 versus 9/15; RR 0.22, 95% CI 0.06 to 0.86). The categorisation of pain is not clear in the trial report, nor whether pain was assessed for all participants. Assuming the latter is the case, the difference between the two groups is less marked when all those with more than occasional pain are included (4/15 versus 9/15; RR 0.44, 95% CI 0.17 to 1.13; analysis not shown).
6.25. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 25: Pain: VAS (0 to 100: worst pain)
6.26. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 26: Constant score at 50 months: overall and components
6.27. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 27: Constant (often severe) pain at 6 months
Reduced muscle strength and restricted mobility were less frequent in the prosthesis group of Stableforth 1984 (Analysis 6.28 and Analysis 6.29) than in the group receiving closed manipulation and sling. Zyto 1997 found no difference between the two groups in strength ('power') at 50 months' follow‐up. The clinical relevance of the three‐point difference in the range of motion component of the Constant score in favour of non‐surgical treatment is questionable (Analysis 6.26). In Boons 2012, abductor strength, reported as a percentage of the opposite shoulder, was lower in the surgery group at both 3 months (median values: 20% versus 30%; reported P = 0.015) and 12 months (median values: 24% versus 42%; reported P = 0.008). Boons 2012 also found that forward flexion (median 68 versus 88 degrees; reported P = 0.001) and abduction (median 61 versus 78 degrees; reported P = 0.02) were worse in the surgery group at three months. There were no between‐group differences in external rotation and internal rotation at this time, nor for all four range of motion measures at 12 months.
6.28. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 28: Failure to recover 75% muscle power relative to other arm at 6 months
6.29. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 29: Range of movement impairments in survivors at 6 months
Fjalestad 2010a found no differences at one year between the two groups in costs (Analysis 6.30 and Analysis 6.31). The base case economic analysis of ProFHER 2015 showed that at two years, the cost of surgical intervention was, on average, GBP 1780.73 more per patient (95% CI GBP 1152.71 to GBP 2408.75). We comment on the economic evaluations of these two studies as part of the brief economic commentary, presented in Agreements and disagreements with other studies or reviews.
6.30. Analysis.
Comparison 6: Surgical versus non‐surgical treatment, Outcome 30: Costs at 1 year (Euros in 2005)
| Costs at 1 year (Euros in 2005) | ||||
| Study | Measure | Surgery | Non‐surgical treatment | Difference (conclusion) |
| Fjalestad 2010a | Total health‐care costs | mean = 10,367 | mean = 10,946 | Abstract: "the mean difference in total health‐care costs was 597 Euros in favour of surgery (95% CI = ‐5291, 3777)". No significant difference. |
| Health‐care + indirect costs | mean = 23,953 | mean = 21,878 | Reformatted text: "Including indirect costs... the difference [was] 2,075 (95% CI = ‐15,949 to 20,100)". No significant difference, but favours the non‐surgical group. | |
6.31. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 31: Total costs including indirect costs (Euros) at 1 year
Different methods of surgical management
Comparisons of different categories of surgical intervention
Ten trials compared one of six different comparisons of different methods of surgical management (Cai 2012; DelPhi 2020; Gracitelli 2016; Helfen 2020; Hoellen 1997; Jonsson 2020; Plath 2019; Sebastiá‐Forcada 2014; Smejkal 2011; Zhu 2011).
Open reduction and internal fixation using a locking plate versus a locking nail
Open reduction with internal fixation using a locking plate versus a locking nail was compared in four trials that involved a total of 270 participants (Table 7). Gracitelli 2016 compared the PHILOS locking plate versus the Centronail locking nail in 72 participants with two‐part surgical neck fractures or three‐part surgical neck and greater tuberosity fractures. Helfen 2020 compared the PHILOS locking plate with bone cement augmentation versus the multiplanar intramedullary nail (MultiLoc) in 60 participants with two‐part surgical neck fractures. Plath 2019 compared the PHILOS locking plate versus the LBN locking nail in 81 participants with two‐, three‐ or four‐part fractures; isolated tuberosity fractures were excluded. Zhu 2011 compared the LPHP or PHILOS locking plates versus the PHN locking nail in 57 participants with two‐part surgical neck fractures. Details of the surgical approaches used in the four trials are also provided in Table 7.
3. Locking plate versus locking intramedullary nail for surgical fixation (all open reduction): brief characteristics.
| Study | Participants (Neer classification) | Locking plate (and surgical approach) | Locking nail (and surgical approach) | Follow‐up |
| Gracitelli 2016 | 72 participants (of 65: 75% female, mean age 65 years) with 2‐part surgical neck fractures or 3‐part surgical neck and greater tuberosity fractures (Brazil) | PHILOS locking plate (deltopectoral approach) | Centronail locking nail (anterolateral transdeltoid approach) | 1 year |
| Helfen 2020 | 60 participants (67% female, mean age 75 years) with 2‐part surgical neck fractures (Germany) |
PHILOS locking plate with bone cement augmentation (deltopectoral approach) | Multiplanar intramedullary nail (MultiLoc) (mini deltoid approach) | 2 years |
| Plath 2019 | 81 participants (of 68: 75% female, mean age 76 years) with 2‐, 3‐ or 4‐part fractures; isolated tuberosity fractures were excluded (Germany) | PHILOS locking plate (deltopectoral or lateral transdeltoid approach) | Locking Blade Nail (LBN) locking nail (anterolateral transdeltoid approach) |
1 year |
| Zhu 2011 | 57 participants (of 51: 67% female, mean age 53 years) with 2‐part surgical neck fractures (China) | Locking Proximal Humeral Plate (LPHP) or PHILOS locking plates (seems to be the deltoid‐split approach; probably similar to 'lateral transdeltoid' in Plath 2019) | Proximal Humeral Nail (PHN) locking nail (deltopectoral approach) | 3 years |
PHILOS: Proximal Humerus Internal Locking System
Primary outcomes
There is low‐certainty evidence of no difference between the two interventions in patient‐reported function, measured using two different scores (ASES and DASH), at one‐year follow‐up (SMD 0.15 favouring locking plate, 95% CI ‐0.12 to 0.41; I2 = 56%; 4 studies, 227 participants; evidence downgraded one level for serious risk of bias and one level for serious inconsistency; Analysis 7.1). The separate results for the two scores for various follow‐up times, including at one year, are shown in Analysis 7.2 for the ASES (0 to 100: best outcome), and in Analysis 7.3 for the DASH (0 to 100: worst disability). Analysis 7.4 also presents the published results for the DASH scores for Plath 2019. The absolute values of the 95% CI for the pooled ASES scores at one year (MD 2.79 favouring locking plate, 95% CI ‐0.73 to 6.31; I2 = 73%; 2 studies, 108 participants; Analysis 7.2) are smaller than the MCID (12.01) for this score. Similarly, the absolute values of the 95% CI for the pooled DASH results at one year (MD ‐2.24, 95% CI ‐5.96 to 1.48; I2 = 40%; 3 studies, 172 participants; Analysis 7.3) are smaller than the MCID (13.0) for this score. There is low‐certainty evidence of no difference between the two interventions in patient‐reported function assessed using the DASH at six months (MD ‐0.39, 95% CI ‐4.14 to 3.36; I2 = 55%; 3 studies, 174 participants; evidence downgraded one level for serious risk of bias and one level for serious inconsistency; Analysis 7.3). There is very low‐certainty evidence from two studies of no clinically important difference in ASES scores at two or three years (MD 3.06, 95% CI ‐0.05 to 6.17; I2 = 0%; 2 studies, 101 participants; evidence downgraded one level for serious risk of bias and one level for serious imprecision as the evidence was from two small studies; Analysis 7.2). All of the between‐group differences at four follow‐up times in the Oxford Shoulder Score reported by Helfen 2020 are less than the MCID (11.4) for this score (Analysis 7.5).
7.1. Analysis.

Comparison 7: Locking plate versus locking intramedullary nail, Outcome 1: Functional scores at 1 year (higher = better outcome)
7.2. Analysis.

Comparison 7: Locking plate versus locking intramedullary nail, Outcome 2: American Shoulder and Elbow Surgeons (ASES) score (0 to 100: best)
7.3. Analysis.

Comparison 7: Locking plate versus locking intramedullary nail, Outcome 3: Disability of the Arm, Shoulder, and Hand (DASH) score (0 to 100: worst disability)
7.4. Analysis.
Comparison 7: Locking plate versus locking intramedullary nail, Outcome 4: Disability of the Arm, Shoulder, and Hand (DASH) scores in published report (Plath 2019)
| Disability of the Arm, Shoulder, and Hand (DASH) scores in published report (Plath 2019) | ||||
| Study | Follow‐up | Locking plate | Locking nail | Reported significance |
| Plath 2019 | 3 months | median = 52 SD (range) = 17.8 (29 to 93) n = 27 | median = 51 SD (range) = 17.0 (32 to 89) n = 26 | P = 0.505 |
| 6 months | median = 45 SD (range) = 16.0 (26 to 89) n = 26 | median = 41 SD (range) = 14.8 (27 to 85) n = 26 | P = 0.110 | |
| 12 months | median = 42 SD (range) = 19.1 (24 to 84) n = 27 | median = 34 SD (range) = 17.8 (22 to 85) n = 27 | P = 0.042 | |
7.5. Analysis.

Comparison 7: Locking plate versus locking intramedullary nail, Outcome 5: Oxford Shoulder Score (OSS) (0 to 48: best function)
Quality‐of‐life scores measured using the SF‐36 (0 to 100: best outcome) were higher in the locking plate group at four follow‐up times in Helfen 2020; see Analysis 7.7. There is very low‐certainty evidence of no difference between the two interventions in SF‐36 at one year (MD 2.60 favouring plate, 95% CI ‐2.39 to 7.59; 53 participants; evidence downgraded one level for serious risk of bias and two levels for very serious imprecision as the evidence was from one small study only).
7.7. Analysis.

Comparison 7: Locking plate versus locking intramedullary nail, Outcome 7: Quality of life: SF‐36 (0 to 100: best outcome)
The results for mortality, overall and individual complications and reoperation are shown in Analysis 7.6. There is insufficient evidence to determine the relative effects of the two interventions on mortality (3/127 versus 8/130; RR 0.46, 95% CI 0.14 to 1.46; I2 = 0%; 4 studies, 257 participants; very low‐certainty evidence downgraded one level for serious risk of bias and two levels for very serious imprecision). There was no report of fracture or surgery‐related death; 4 of the 11 reported deaths were reported explicitly as having died from unrelated causes (Plath 2019; Zhu 2011).
7.6. Analysis.

Comparison 7: Locking plate versus locking intramedullary nail, Outcome 6: Death, reoperation and adverse events
There is very low‐certainty evidence of little difference between the two interventions in the numbers of participants incurring any complication (29/124 versus 27/126; RR 1.11, 95% CI 0.70 to 1.75; I2 = 54%; 4 studies, 250 participants; evidence downgraded one level for serious risk of bias, one level for serious imprecision, and one level for serious inconsistency; Analysis 7.6). Pooled data from three studies also showed little difference in the number of reoperations (9/95 versus 13/98; RR 0.73 favouring plate, 95% CI 0.33 to 1.61; I2 = 53%; 193 participants; very low‐certainty evidence downgraded one level for serious risk of bias, one level for serious imprecision, and one level for serious inconsistency; Analysis 7.6). The data were incomplete for Zhu 2011, which reported only that five participants in the plate group had a reoperation for screw penetration into the articular surface of the humeral head. Analysis 7.6 shows the available data for individual complications, of which screw penetration into the humeral head was the most common (15/124 versus 3/126; RR 3.82 favouring nail, 95% CI 1.39 to 10.48; I2 = 0%; 4 studies, 250 participants; very low‐certainty evidence).
Secondary outcomes
The differences between the two groups in the Constant scores (0 to 100: best outcome) at four different time points (3, 6, 12 and 24 or 36 months) were all small and, given all of the 95% CIs' absolute values were less than the 11.6 MCID, none was clinically important. The most data were for 12 months (MD 2.47 favours plate, 95% CI ‐0.46 to 5.41; I2 = 0%; 4 studies, 227 participants; low‐certainty evidence, downgraded two levels for very serious risk of bias; Analysis 7.8).
7.8. Analysis.

Comparison 7: Locking plate versus locking intramedullary nail, Outcome 8: Constant score (0 to 100: best)
There was very low‐certainty evidence from Gracitelli 2016 (55 participants) of little difference between the two interventions in pain at 3, 6 and 12 months; see Analysis 7.9. Similar findings applied from evidence from the other two studies of little between‐group differences in pain at either six months or one year for Plath 2019, or one or three years for Zhu 2011; see Analysis 7.10.
7.9. Analysis.

Comparison 7: Locking plate versus locking intramedullary nail, Outcome 9: Pain (VAS 0 to 10: worst pain)
7.10. Analysis.
Comparison 7: Locking plate versus locking intramedullary nail, Outcome 10: Pain (VAS: 0 to 10: worst)
| Pain (VAS: 0 to 10: worst) | ||||
| Study | Follow‐up | Locking plate | Locking nail | Reported significance |
| Plath 2019 | Pain at 6 months | median = 3 SD (range) = 1.3 (0 to 5) n = 26 | median = 2 SD = (range) = 1.6 (0 to 5) n = 26 | P = 0.186 |
| Pain at 1 year | median = 1 SD = (range) = 1.6 (0 to 5) n = 27 | median = 0 SD = (range) = 1.8 (0 to 5) n = 27 | P = 0.766 | |
| Zhu 2011 | Pain at 1 year | median = 0.5 interquartile range: 1.8 n = 29 |
median = 1.0 interquartile range = 1.0 n = 26 |
P = 0.042 |
| Pain at 3 years | median = 0 interquartile range = 0.8 n = 26 |
median = 0 interquartile range = 1.0 n = 25 |
P = 0.642 | |
For completeness, the available data for range of motion outcomes at different follow‐up times are shown in Analysis 7.11, Analysis 7.12 and Analysis 7.13; and for muscle strength in Analysis 7.14.
7.11. Analysis.

Comparison 7: Locking plate versus locking intramedullary nail, Outcome 11: Range of movement (flexion and abduction) degrees
7.12. Analysis.

Comparison 7: Locking plate versus locking intramedullary nail, Outcome 12: Active range of motion (at 3 years)
7.13. Analysis.
Comparison 7: Locking plate versus locking intramedullary nail, Outcome 13: Range of movement: internal rotation (level on spine)
| Range of movement: internal rotation (level on spine) | ||||
| Study | Follow‐up | Locking plate | Locking nail | Reported significance |
| Zhu 2011 | At 1 year | mean location = T8 range = T4 to L2 n = 29 |
mean location = T9 range = T2 to buttock n = 26 |
P = 0.443 |
| At 3 years | mean location = T8 range = T2 to buttock n = 26 |
mean location = T8 range = T2 to buttock n = 25 |
P = 0.636 | |
7.14. Analysis.

Comparison 7: Locking plate versus locking intramedullary nail, Outcome 14: Strength of supraspinatus (relative to opposite side) % at 3 years
Open reduction with internal fixation using a locking plate versus minimally invasive fixation with distally inserted intramedullary K‐wires
Smejkal 2011 compared open reduction and internal fixation using a PHILOS plate versus the Zifko method of minimally invasive fixation with distally inserted intramedullary K‐wires (Kirschner wires) in 61 participants with two‐ or three‐part fractures. The evidence is very low certainty for all sought and available outcomes, being downgraded one level for serious risk of bias and two levels for very serious imprecision as the evidence was from one small study only.
Primary outcomes
Smejkal 2011 did not report patient‐reported function or activities of daily living. The account of the complications seemed incomplete, with no indication of how many required a reoperation, but this may be partly due to difficulties in translation from Czech to English. There is no evidence of a difference between the two groups in the overall numbers of participants incurring a complication (11/28 versus 9/27; RR 1.18, 95% CI 0.58 to 2.38; Analysis 8.1). The recorded nature of the complications reflected the type of implant, with four cases of screw protrusion in the plate group that resulted in impingement and migration of K‐wires, a distal humeral fracture and a nerve injury in the Zifko group.
8.1. Analysis.

Comparison 8: Locking plate versus intramedullary K‐wires, Outcome 1: Complications
Secondary outcomes
Smejkal 2011 found no difference between the two groups in Constant scores relative to the healthy limb at a mean two years' follow‐up (MD ‐0.81%, 95% CI ‐7.45% to 5.83%; see Analysis 8.2). Three participants of each group had a 'poor' Constant score. Analysis 8.3 shows there was no evidence of differences between the two groups in time to union (MD 2.10 weeks, 95% CI ‐2.25 to 6.45 weeks) or in a vaguely‐described measure of time to recover normal upper‐limb function (27.2 versus 21.4 weeks; MD 5.80 weeks; 95% CI ‐0.16 to 11.76 weeks). Smejkal 2011 suggested that the greater time to recover in the plate group reflected a greater impact of complications in this group.
8.2. Analysis.

Comparison 8: Locking plate versus intramedullary K‐wires, Outcome 2: Constant score (% of healthy limb) at mean 2 years
8.3. Analysis.

Comparison 8: Locking plate versus intramedullary K‐wires, Outcome 3: Time to union and time to recover upper‐limb function (weeks)
Hemiarthroplasty versus open reduction and locking plate fixation
Cai 2012, which compared hemiarthroplasty with open reduction and PHILOS plate fixation in 32 participants with four‐part fractures, reported outcome at 4, 12 and 24 months. The evidence is very low certainty for all available outcomes, being downgraded one level for serious risk of bias and two levels for very serious imprecision as the evidence was from one small study only.
Primary outcomes
Although DASH scores at one and two years favoured the hemiarthroplasty group, the mean differences were smaller than the MCID of 13 for DASH (at 12 months: MD ‐7.30, 95% CI ‐16.70 to 2.10; 28 participants; at 24 months: MD ‐6.10, 95% CI ‐11.03 to ‐1.17; 27 participants; Analysis 9.1). There was no evidence of differences between the two groups in quality of life measured via the EQ‐5D at any of the three follow‐up times (Analysis 9.2). One person in the hemiarthroplasty group had died by two years (Analysis 9.3). Reoperations were reported for three participants in the hemiarthroplasty group (one dislocation, one prosthesis loosening, one infection) and three participants in the fixation group (one non‐union, two fixation failure) (see Analysis 9.3).
9.1. Analysis.

Comparison 9: Hemiarthroplasty versus plate fixation (4‐part fractures), Outcome 1: Disability of the Arm, Shoulder, and Hand (DASH) score (0 to 100: worst disability)
9.2. Analysis.

Comparison 9: Hemiarthroplasty versus plate fixation (4‐part fractures), Outcome 2: Quality of life: EQ‐5D score (0: dead to 1: best quality of life)
9.3. Analysis.

Comparison 9: Hemiarthroplasty versus plate fixation (4‐part fractures), Outcome 3: Death or reoperation at 2 years
Secondary outcomes
The Constant scores were higher in the hemiarthroplasty group at all three follow‐ups; in particular, the 95% confidence interval at two years included a small clinically important effect (MD 12.20, 95% CI 2.85 to 21.55; 27 participants; Analysis 9.4). There was no evidence of differences between the two groups at two years in pain (Analysis 9.5) or range of motion (Analysis 9.6).
9.4. Analysis.

Comparison 9: Hemiarthroplasty versus plate fixation (4‐part fractures), Outcome 4: Constant score (0 to 100: best score)
9.5. Analysis.

Comparison 9: Hemiarthroplasty versus plate fixation (4‐part fractures), Outcome 5: Pain VAS (0 to 100: worst pain) at 2 years
9.6. Analysis.

Comparison 9: Hemiarthroplasty versus plate fixation (4‐part fractures), Outcome 6: Range of motion at 2 years
Hemiarthroplasty versus tension band wiring
Hoellen 1997 compared hemiarthroplasty versus reduction and stabilisation of the fracture using tension band wiring. All 30 participants reported in Hoellen 1997 had four‐part fractures. However, patients with three‐part fractures were also eligible, according to a later report of the trial (Holbein 1999), which reported on 39 participants. However, unless further information materialises, we will continue to report the results from Hoellen 1997. The evidence is very low certainty for the few reported outcomes, being downgraded at minimum one level for serious risk of bias and two levels for very serious imprecision as the evidence was from one small study only.
Primary outcomes
Hoellen 1997 did not assess patient‐rated function or quality of life. Hoellen 1997 reported no serious perioperative or postoperative complications, such as pulmonary embolism, in either group, but noted three cases of postoperative delirium (treatment group not reported). There were two cases of haematoma in the tension band wiring group; both required revision surgery (Analysis 10.1). After hospital discharge, no participants of the hemiarthroplasty group required further surgery compared with five participants of the wiring group (the wires displaced in four participants and the fracture completely dislocated in one participant); see Analysis 10.2. None of the three deaths were reported as being directly related to the injury or treatment (Analysis 10.3).
10.1. Analysis.

Comparison 10: Hemiarthroplasty versus tension band wiring (4‐part fractures), Outcome 1: Haematoma
10.2. Analysis.

Comparison 10: Hemiarthroplasty versus tension band wiring (4‐part fractures), Outcome 2: Reoperation post discharge
10.3. Analysis.

Comparison 10: Hemiarthroplasty versus tension band wiring (4‐part fractures), Outcome 3: Death
Secondary outcomes
Results for only 18 of the 30 trial participants were available at one year for Constant scores (minus the power component) and pain. The mean Constant scores for the 18 people available at one‐year follow‐up were similar in the two groups: 48 versus 49 points (out of a maximum of 75).
Reverse total shoulder arthroplasty (RTSA) versus locking plate fixation
Primary outcomes
DelPhi 2020 compared RTSA (two types were used) versus PHILOS plate fixation in 124 participants. It reported function‐related outcomes at two years in 104 participants. Patient‐reported shoulder function was measured at 3, 6, 12 and 24 months using the Oxford Shoulder Score (0 to 48: best result). A figure in the paper showed higher OSS scores in the RTSA group at all four time points. The 95% CIs at three months (estimated MD < 5.0) did not overlap; however, the 95% CIs clearly overlapped at both six months (estimated MD < 4.0) and 12 months (estimated MD < 2.0). All three mean differences are less than the MCID for this outcome. The same observation applies to the results at final follow‐up (MD 4.30, 95% CI 1.18 to 7.42; low‐certainty evidence, downgraded one level for serious risk of bias and one level for serious imprecision given the results are from one study; Analysis 11.1). DelPhi 2020 did not report on quality of life; reporting of this outcome may be waiting on the cost‐analysis report. The evidence for death, complications and reoperations is very low certainty, reflecting downgrading by three levels for very serious imprecision given the very few events and wide confidence intervals that cross the line of no effect. All five deaths (1/4 versus 4/60) occurred after the six‐month follow‐up and there was no report of a direct link with treatment (Analysis 11.2). Slightly fewer participants in the RTSA group incurred a complication (7/64 versus 11/60; RR 0.60, 95% CI 0.25 to 1.44) or had revision surgery (4/64 versus 7/60); see Analysis 11.3 The four operations in the RTSA group comprised two changes of implant components and two revision surgeries for periprosthetic fracture. Four of the seven people having reoperations in the plate group had conversions to RTSA and three had implant removal. These seven participants all were diagnosed with screw penetration, which, at nine cases, was the most commonly recorded individual complication (Analysis 11.3).
11.1. Analysis.

Comparison 11: Reverse total shoulder arthroplasty (RTSA) versus open reduction and internal fixation (ORIF) using a plate, Outcome 1: Oxford Shoulder Score (0 to 48: best outcome) at 2 years
11.2. Analysis.

Comparison 11: Reverse total shoulder arthroplasty (RTSA) versus open reduction and internal fixation (ORIF) using a plate, Outcome 2: Death
11.3. Analysis.

Comparison 11: Reverse total shoulder arthroplasty (RTSA) versus open reduction and internal fixation (ORIF) using a plate, Outcome 3: Complications
Secondary outcomes
The Constant score favoured the RTSA group at both one and two years; the best estimate for mean difference did not exceed the MCID (11.6) for this score at one year (MD 8.50, 95% 0.50 to 16.50) but it did at two years (MD 13.40, 95% CI 6.12 to 20.68); see Analysis 11.4. This analysis also shows data for the pain and power components of the Constant score. However, we decided against presenting the findings for range of motion and activities of daily living as these were further split up into the four separate sub‐components in each group. The main message from inspecting the components at two years is that main differences favouring RTSA were in 'movement' (MD 2.0 out of 10 points); power (MD 3.0 out of 25 points) and range of motion for flexion (MD 2.0 out of 10 points), abduction (MD 2.0 out of 10 points) and external rotation (MD 2.6 out of 10 points).
11.4. Analysis.

Comparison 11: Reverse total shoulder arthroplasty (RTSA) versus open reduction and internal fixation (ORIF) using a plate, Outcome 4: Constant score: overall and some components
The results for radiological findings other than screw penetration and non‐union at two years are presented in Analysis 11.5. These outcomes are generally exclusive to one or the other group; no clinical consequences were described.
11.5. Analysis.

Comparison 11: Reverse total shoulder arthroplasty (RTSA) versus open reduction and internal fixation (ORIF) using a plate, Outcome 5: Radiological assessment findings at 2 years
Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty
Two trials, Jonsson 2020 and Sebastiá‐Forcada 2014, compared RTSA with hemiarthroplasty in 161 participants, all aged 70 years or above, with either three‐ or four‐part fractures, some of which included dislocation. Minimum follow‐up was two years in both trials, with a mean follow‐up of 2.4 years in Jonsson 2020 and a longest follow‐up of 49 months in Sebastiá‐Forcada 2014. The evidence for all outcomes is very low certainty, reflecting downgrading for serious study limitations and very serious imprecision.
Primary outcomes
Patient‐reported upper‐limb function pain was reported as a percentage of normal shoulder function as assessed using the Western Ontario Osteoarthritis Shoulder (WOOS) score in Jonsson 2020 and using the QuickDASH in Sebastiá‐Forcada 2014. Analysis 12.1 presents the results for percentage of normal shoulder function based on WOOS scores at one year (MD 2.50%, 95% CI ‐8.25% to 13.25%; 55 participants) and two years of follow‐up (MD 2.80%, 95% CI ‐6.90% to 12.50%; 81 participants). These show no evidence of a between‐group difference at either time point. We did not use the pooled results from the two trials at two years' follow‐up because of clearly significant heterogeneity (I2 = 78%); Analysis 12.2. Although the QuickDASH scores (0 to 55: worst outcome) were superior in the reverse arthroplasty group (MD ‐6.90, 95% CI ‐10.81 to ‐2.99; Analysis 12.3), the best estimate is below the scale‐adjusted MCID of 8.8.
12.1. Analysis.

Comparison 12: Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty (HA), Outcome 1: Shoulder function score: WOOS (% of normal shoulder function)
12.2. Analysis.

Comparison 12: Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty (HA), Outcome 2: Shoulder function scores at 24 to 49 months (exploratory analysis)
12.3. Analysis.

Comparison 12: Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty (HA), Outcome 3: Shoulder function: QuickDASH score (0 to 55: worst outcome) at 24 to 49 months
Results from Jonsson 2020 showed no evidence of a difference in quality of life assessed using the EQ‐5D (0: dead to 1: best quality) at one year (MD 0.01, 95% CI ‐0.04 to 0.06; 66 participants) or two years (MD 0.01, 95% CI ‐0.05 to 0.07; 83 participants); see Analysis 12.4.
12.4. Analysis.

Comparison 12: Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty (HA), Outcome 4: Quality of life: EQ‐5D (0: dead to 1: best outcome)
In Jonsson 2020, one participant in the RTSA group died from pneumonia eight days after surgery, whilst still in hospital. The other deaths occurred between 0.2 and 3.0 years after surgery and were not related to the proximal humeral fracture or its treatment. No deaths occurred in Sebastiá‐Forcada 2014; Analysis 12.5.
12.5. Analysis.

Comparison 12: Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty (HA), Outcome 5: Death
The two trials reported on a total of 19 complications, more of which occurred in the hemiarthroplasty group (5/79 RTSA versus 14/81 hemiarthroplasty; RR 0.36, 95% CI 0.14 to 0.94; I2 = 47%; 2 studies, 160 participants; Analysis 12.6). The trials also reported on individual complications: the seven cases of symptomatic proximal migration of the humeral head after hemiarthroplasty was the most common adverse event and all resulted in a reoperation to RTSA.
12.6. Analysis.

Comparison 12: Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty (HA), Outcome 6: Complications
Ten participants (6.2%) had a reoperation (2/79 RTSA versus 8/82 hemiarthroplasty; RR 0.26, 95% CI 0.06 to 1.15; I2 = 0%; 2 studies, 161 participants; Analysis 12.7). This comprised replacement RTSA for all of the hemiarthroplasty group cases. The reoperations in the RTSA group comprised a RTSA because of deep infection and open reduction and internal fixation of a periprosthetic fracture of the distal humerus.
12.7. Analysis.

Comparison 12: Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty (HA), Outcome 7: Reoperation
Secondary outcomes
At a minimum of two years, University of California‐Los Angeles scores and Constant and adjusted Constant scores all favoured the RTSA group, the best estimates for mean differences exceeding the MCIDs for both scores (Analysis 12.8). A similar finding in favour of RTSA applied to pain, range of motion, power and activities of daily living components of the Constant score (Analysis 12.9). However, the two trials differed in their findings for the pain component (I2 = 87%): Sebastiá‐Forcada 2014 found a large difference favouring the RTSA group but Jonsson 2020 found no evidence of a between‐group difference. The latter was consistent with findings of no between‐group differences in separately measured average pain scores at one and two years (Analysis 12.10). Both trials found RTSA resulted in superior range of motion in terms of flexion and abduction (Analysis 12.11). Jonsson 2020 found this did not apply to external rotation, which favoured hemiarthroplasty, nor internal rotation, which was reported not to differ between the two groups (reported P = 0.47; Analysis 12.12).
12.8. Analysis.

Comparison 12: Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty (HA), Outcome 8: Composite (objective and subjective) shoulder function scores at 24 to 49 months
12.9. Analysis.

Comparison 12: Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty (HA), Outcome 9: Constant score at 24 to 49 months: overall and components
12.10. Analysis.

Comparison 12: Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty (HA), Outcome 10: Pain (on average) VAS (0 to 100: worst pain)
12.11. Analysis.

Comparison 12: Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty (HA), Outcome 11: Range of motion (degrees) at 24 to 49 months
12.12. Analysis.
Comparison 12: Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty (HA), Outcome 12: Internal rotation at minimum 2 years
| Internal rotation at minimum 2 years | |||
| Study | Anatomical reach at back | RTSA: N (out of 41) | HA: N (out of 43) |
| Jonsson 2020 | None | 0 | 2 |
| Buttocks | 9 | 5 | |
| Sacroiliac joint | 8 | 6 | |
| Waist level | 14 | 15 | |
| 12th thoracic vertebra | 8 | 13 | |
| Interscapular level | 2 | 2 | |
Although Jonsson 2020 found no difference between the two groups in patient satisfaction with their shoulder (VAS 0 to 100: completely satisfied) at one year, satisfaction scores were higher in the RTSA group at two years (MD 16.00, 95% CI 4.06 to 27.94; 84 participants; Analysis 12.13).
12.13. Analysis.

Comparison 12: Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty (HA), Outcome 13: Patient satisfaction with shoulder VAS (0 to 100: total satisfaction)
The findings of radiological assessment (Analysis 12.14) did not confirm a difference between the two groups in malunion or resorption of tuberosities in Sebastiá‐Forcada 2014. Both trials reported that the scapular notching found in a total of 9 cases in the RTSA group was without clinical consequence, as were the 11 cases of heterotopic ossification and observation of radiolucent lines round the implant stem.
12.14. Analysis.

Comparison 12: Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty (HA), Outcome 14: Radiological assessment findings
Comparisons of different methods of performing an intervention in the same category
Thirteen trials tested one of 10 comparisons of different types or methods in the same intervention category (e.g. plating): Biermann 2020; Buecking 2014; Fialka 2008; Hengg 2019; HURA 2020; Lopiz 2014; Ockert 2010; Sohn 2017; Soliman 2013; Voigt 2011; Zhang 2011; Zhang 2019; Ziegler 2019.
Plate: deltoid‐split with minimal invasive plate fixation versus the deltopectoral approach for plate fixation
Deltoid‐split approach with a less or 'minimal' invasive approach versus the deltopectoral approach was tested in three trials, including a total of 312 people with Neer two‐, three‐ or four‐part fractures (Buecking 2014; HURA 2020; Sohn 2017). Final follow‐up was one year in Buecking 2014, on average 26 months (range 12 to 92 months) in HURA 2020, and on average 15 months in Sohn 2017. Pooling of data was possible for only a few outcomes. The evidence for all reported outcomes for this comparison is very low certainty, being downgraded one or two levels for study limitations reflecting a serious or very serious risk of bias, and two levels for very serious imprecision reflecting low numbers of events and participants.
Primary outcomes
HURA 2020 found higher (worse) QuickDASH scores (0 to 100: worst disability) in the deltoid‐split group (MD 14.00, 95% CI 5.14 to 22.86; 69 participants; Analysis 13.1). The 95% CI includes the MCID (16) for the QuickDASH score, indicating potentially a clinically better outcome in the deltopectoral group but also includes no clinically important difference. HURA 2020 found lower (worse) SF‐12 physical component scores (0 to 100: best) in the deltoid‐split group (MD ‐4.00, 95% CI ‐8.49 to 0.49; 69 participants; Analysis 13.2); again the 95% CI includes the MCID (6.5) with a similar interpretation as for the QuickDASH scores. Buecking 2014 reported results for activities of daily living at 6 and 12 months based on a score by Lawton (Lawton 1969). However, the trialists appear not to have used the scoring system correctly and reported scores that are greater than the maximum score of 8. They reported no statistically significant between‐group differences in the mean scores at 6 or 12 months (18 for the deltoid‐split versus 17 for the deltopectoral) but the clinical relevance of these scores is questionable.
13.1. Analysis.

Comparison 13: Plate: deltoid‐split approach and minimally invasive plate osteosynthesis (MIPO) versus deltopectoral approach and open plate fixation, Outcome 1: Shoulder function: QuickDASH score (0 to 100: worst disability) at 26 months (mean)
13.2. Analysis.

Comparison 13: Plate: deltoid‐split approach and minimally invasive plate osteosynthesis (MIPO) versus deltopectoral approach and open plate fixation, Outcome 2: Quality of life: SF‐12 at 26 months (mean)
Overall, there were five deaths (2.4% of 205 participants) at one year reported for Buecking 2014 and HURA 2020, and five deaths (5.9% of 85) at an average of 26 months in HURA 2020; Analysis 13.3. There was no mention of fracture or treatment‐related mortality. Based on relatively few events, there was no evidence of between‐group differences in individual or total complications; Analysis 13.4. In Sohn 2017, unit of analysis problems, where participants had more than one complication, are likely for overall complications (16/45 versus 18/45) and possible for implant‐related complications too (seven in each group). A similar finding of no evidence of between‐group differences applied to reoperations for a complication or a fall (17/104 versus 12/98; RR 1.34, 95% CI 0.67 to 2.66; I2 = 0%; 2 studies, 202 participants; Analysis 13.5). All reported complications in Buecking 2014 resulted in a reoperation. Similar numbers of participants requested plate removal in Buecking 2014.
13.3. Analysis.

Comparison 13: Plate: deltoid‐split approach and minimally invasive plate osteosynthesis (MIPO) versus deltopectoral approach and open plate fixation, Outcome 3: Mortality
13.4. Analysis.

Comparison 13: Plate: deltoid‐split approach and minimally invasive plate osteosynthesis (MIPO) versus deltopectoral approach and open plate fixation, Outcome 4: Complications
13.5. Analysis.

Comparison 13: Plate: deltoid‐split approach and minimally invasive plate osteosynthesis (MIPO) versus deltopectoral approach and open plate fixation, Outcome 5: Reoperation
Secondary outcomes
Results from Buecking 2014 and Sohn 2017 do not show evidence of a difference in Constant scores or, for Sohn 2017, in UCLA scores at follow‐up (Analysis 13.6). Similarly, Buecking 2014 did not find evidence of a difference in pain scores at either 6 or 12 months (Analysis 13.7). Although HURA 2020 found higher (worse) pain scores in the deltoid‐split group at 26 months, the difference is of no or little clinical importance (Analysis 13.7). There is no evidence of a between‐group difference in range of motion or patient assessment of the resulting scar; Analysis 13.8 and Analysis 13.9.
13.6. Analysis.

Comparison 13: Plate: deltoid‐split approach and minimally invasive plate osteosynthesis (MIPO) versus deltopectoral approach and open plate fixation, Outcome 6: Composite (objective and subjective) shoulder function scores: Constant and UCLA
13.7. Analysis.

Comparison 13: Plate: deltoid‐split approach and minimally invasive plate osteosynthesis (MIPO) versus deltopectoral approach and open plate fixation, Outcome 7: Pain (VAS 0 to 10: intolerable pain)
13.8. Analysis.

Comparison 13: Plate: deltoid‐split approach and minimally invasive plate osteosynthesis (MIPO) versus deltopectoral approach and open plate fixation, Outcome 8: Range of motion (degrees) at 26 months
13.9. Analysis.

Comparison 13: Plate: deltoid‐split approach and minimally invasive plate osteosynthesis (MIPO) versus deltopectoral approach and open plate fixation, Outcome 9: Patient scar assessment scale (6 to 60: worst outcome) at 26 months
Plate: polyaxial versus monoaxial locking plate fixation
Two trials made this comparison (Ockert 2010; Voigt 2011). We rated all available evidence for this comparison as very low certainty, being downgraded one level for study limitations reflecting a serious risk of bias, and two levels for very serious imprecision as there were few events and the evidence for individual outcomes was typically from one small study only.
Primary outcomes
Ockert 2010, which reported on outcome for participants (66 participants in their 2010 publication; 124 participants in their later publication (Ockert 2014)) with Neer two‐, three‐ or four‐part fractures, did not report on functional outcome. Voigt 2011 found evidence of no between‐group differences at one year (48 participants with Neer three‐ or four‐part fractures) between the two groups in their DASH scores (RR 2.10, 95 CI ‐6.24 to 10.44; Analysis 14.1), nor at 3, 6 or 12 months in the Simple Shoulder Test results (Analysis 14.2). Neither trial assessed quality of life.
14.1. Analysis.

Comparison 14: Plate: polyaxial versus monoaxial screw insertion, Outcome 1: Disability of the Arm, Shoulder, and Hand (DASH) score at 12 months (0 to 100: greatest disability)
14.2. Analysis.

Comparison 14: Plate: polyaxial versus monoaxial screw insertion, Outcome 2: Simple Shoulder Test (SST) (0 to 12: best outcome)
Since the extended trial report of Ockert 2010 (Ockert 2014) reported only on reoperation at 12 months, the data from the more detailed report of reoperations and complications occurring up to six months from the 2010 publication are also presented in the following. Neither trial found evidence of differences between the two groups in participants having a reoperation, both at six months (data from Ockert 2010: 2/29 versus 3/37) or at one year (15/83 versus 16/97; RR 1.10, 95% CI 0.58 to 2.08; Analysis 14.3). In the initial six months' follow‐up report for Ockert 2010, one participant of the polyaxial group had a loosened screw taken out at 10 weeks; one participant of each group had early hardware removal (at five months) because of subacromial impingement from poor plate positioning; and two monoaxial group participants had early hardware removal and a revision, respectively, because of intra‐articular screw protrusion. In the recruitment and follow‐up extension of Ockert 2010 (Ockert 2014), five polyaxial group versus nine monaxial group participants had revision because of secondary varus displacement with subsequent intra‐articular screw protrusion; four versus two participants had revision because of subacromial impingement; and one monoaxial group participant had revision surgery because of an infection. In Voigt 2011, one person in each group had an early "prosthetic replacement" and three participants in the polyaxial group and one in the monoaxial group had refixation. The two other reoperated polyaxial group participants of Voigt 2011 had a corrective osteotomy and a screw removal, respectively, while two other reoperated monoaxial group participants both had early implant removals. Voigt 2011 reported on two deaths in the polyaxial group (Analysis 14.4). Neither trial found differences between the two groups in the number of participants with any or individual complications detected radiologically (Analysis 14.5).
14.3. Analysis.

Comparison 14: Plate: polyaxial versus monoaxial screw insertion, Outcome 3: Reoperation
14.4. Analysis.

Comparison 14: Plate: polyaxial versus monoaxial screw insertion, Outcome 4: Dead at 1 year
14.5. Analysis.

Comparison 14: Plate: polyaxial versus monoaxial screw insertion, Outcome 5: Complications (radiological assessment)
Secondary outcomes
Voigt 2011 found no evidence of between‐group differences in the Constant score relative to the uninjured arm (Analysis 14.6) or range of motion (Analysis 14.7).
14.6. Analysis.

Comparison 14: Plate: polyaxial versus monoaxial screw insertion, Outcome 6: Constant score at 12 months (% of contralateral limb)
14.7. Analysis.

Comparison 14: Plate: polyaxial versus monoaxial screw insertion, Outcome 7: Range of motion (degrees) at 12 months
Plate: CFR‐PEEK plate (made of polyetheretherketone reinforced with carbon fibres) versus the titanium PHILOS plate
Ziegler 2019 made this comparison in 76 people "with proximal humerus fractures requiring surgery", 63 of whom were available at six months' final follow‐up. We rated all available evidence for this comparison as very low certainty, being downgraded one level for study limitations reflecting a serious risk of bias, and two levels for very serious imprecision as there were few events and the evidence was from one small study only.
Primary outcomes
There were no between‐group differences at any follow‐up time (6 and 12 weeks; 6 months) in any of the three measures of shoulder function: DASH (Analysis 15.1), the OSS (Analysis 15.2) and the Simple Shoulder Test (Analysis 15.3). For example, there was no evidence of a difference between the two groups in the DASH scores (0 to 100: worst disability) at six months: MD ‐1.00, 95% CI ‐10.49 to 8.49. Quality‐of‐life data were not reported. There were few complications, with two reoperations being required in the titanium group for an unspecified new injury (Analysis 15.4). One participant in each group did not receive their allocated plate for medical reasons; these are labelled treatment failure in Analysis 15.4.
15.1. Analysis.

Comparison 15: Plate: CFR‐PEEK plate versus titanium (PHILOS) plate, Outcome 1: Disability of the Arm, Shoulder, and Hand (DASH) score (0 to 100: worst disability)
15.2. Analysis.

Comparison 15: Plate: CFR‐PEEK plate versus titanium (PHILOS) plate, Outcome 2: Oxford Shoulder Score (OSS) (0 to 48: best outcome)
15.3. Analysis.

Comparison 15: Plate: CFR‐PEEK plate versus titanium (PHILOS) plate, Outcome 3: Simple Shoulder Test (SST) (0 to 100: best score)
15.4. Analysis.

Comparison 15: Plate: CFR‐PEEK plate versus titanium (PHILOS) plate, Outcome 4: Complications and reoperations
Secondary outcomes
No secondary outcomes were reported.
Locking plate: additional glenohumeral joint lavage
Biermann 2020 tested the addition of glenohumeral joint lavage after PHILOS locking plate fixation in 72 participants with Neer two‐, three‐ or four‐part fractures. They reported results for 62 participants at one year. We rated all available evidence for this comparison as very low certainty, being downgraded one level for study limitations reflecting a serious risk of bias, and two levels for very serious imprecision as there were very few events and the evidence was from one small study only.
Primary outcomes
Three participants died within one year, all from unrelated causes. There were few complications, with three participants in each group incurring either avascular necrosis (three cases), secondary displacement (two cases) or adhesive capsulitis (one case); Analysis 16.1.
16.1. Analysis.

Comparison 16: Locking plate: glenohumeral joint lavage versus no lavage, Outcome 1: Adverse events
Secondary outcomes
There was no evidence of a clinically important difference (MCID of 11.6) between the two groups in the Constant scores (0 to 100: higher scores are better) at 12 months (MD ‐3.00, 95% CI ‐9.97 to 3.97) or at other intermediate times; Analysis 16.2. A similar finding applied to four range of motion measures at 12 months; Analysis 16.3.
16.2. Analysis.

Comparison 16: Locking plate: glenohumeral joint lavage versus no lavage, Outcome 2: Constant score (0 to 100: best outcome)
16.3. Analysis.

Comparison 16: Locking plate: glenohumeral joint lavage versus no lavage, Outcome 3: Range of motion at 12 months (degrees)
Locking plate: use of medial support locking screws
Zhang 2011 tested the use of medial support locking screws in 72 people with Neer two‐, three‐ or four‐part fractures treated with open reduction with internal fixation using the PHILOS locking plate. They reported results for 68 participants. In the medial support group, locking screws were introduced through the plate so as to run up the inferior portion of the humeral neck providing support to the calcar. In the control group, these screw holes were left empty. We rated all available evidence for this comparison as very low certainty, being downgraded one level for study limitations reflecting a serious risk of bias, and two levels for very serious imprecision as there were very few events and the evidence was from one small study only.
Primary outcomes
Zhang 2011 did not report on patient‐rated function or quality of life. One participant in the medial screw group had early failure of fixation due to plate breakage compared with nine participants with early fixation failure (six varus collapse; three screw penetration) in the control group; however, the 95% CI crossed the line of no effect (RR 0.15, 95% CI 0.02 to 1.11; Analysis 17.1). Seven of these participants, including the participant in the medial screw group, consented to have a reoperation (RR 0.22; 95% CI 0.03 to 1.11). One participant in the medial screw group had asymptomatic osteonecrosis.
17.1. Analysis.

Comparison 17: Locking plate: medial support screws versus control, Outcome 1: Adverse events
Secondary outcomes
The medial screw group had significantly higher Constant scores (0 to 100: best score) at 31 months of follow‐up (MD 9.00, 95% CI 2.41 to 15.59; Analysis 17.2), but only part of the 95% CI includes the MCID (11.6).
17.2. Analysis.

Comparison 17: Locking plate: medial support screws versus control, Outcome 2: Constant score (0 to 100: best) at 2.5 years
Locking plate: in combination with allogeneic femoral head bone grafts
Zhang 2019 tested the use of allogeneic (sourced from other people) bone grafts in combination with PHILOS locking plates in 80 people with Neer three‐ or four‐part fractures. Overall, follow‐up was for three months only. We rated all available evidence for this comparison as very low certainty, being downgraded two levels for study limitations reflecting a very serious risk of bias, two levels for very serious imprecision as there were very few events and the evidence was from one small study only, and ‐ for the Neer's rating and adverse events ‐ one level for serious indirectness reflecting the inappropriately short length of follow‐up.
Primary outcomes
Zhang 2019 did not report patient‐rated function or quality of life, or on the numbers of participants with any complication. For individual complications, there were slightly (one to three) fewer in the bone graft group up to three months (Analysis 18.1).
18.1. Analysis.

Comparison 18: Locking plate: with allogeneic bone grafts versus not (control), Outcome 1: Adverse events
Secondary outcomes
One participant in the bone graft group and three in the control group had a poor outcome based on their Neer shoulder function score at one month (Analysis 18.2).
18.2. Analysis.

Comparison 18: Locking plate: with allogeneic bone grafts versus not (control), Outcome 2: Poor Neer shoulder function scores at 1 month
Locking plate: cement augmentation of the screw tips
Hengg 2019 tested the use of polymethylmethacrylate screw‐tip cement augmentation in 67 people treated with PHILOS plate fixation. Although the trial planned to include only people with Neer three‐ or four‐part fractures, six participants had two‐part fractures. One participant of each group did not receive PHILOS plate fixation and three allocated augmentation (9% of 33) did not receive this because of positive leakage tests. Hengg 2019 reported intention‐to‐treat analysis for up to 58 participants at 12 months' follow‐up except for adverse events, where they reported results according to the actual intervention received for 65 participants. We rated all available evidence for this comparison as very low certainty, being downgraded two levels for study limitations reflecting a very serious risk of bias, and two levels for very serious imprecision as there were very few events and the evidence was from one small study only.
Primary outcomes
There were no significant between‐group differences in functional outcome at 6 or 12 months measured using either the QuickDASH (Analysis 19.1) or SPADI (Shoulder Pain and Disability Index) (Analysis 19.2). Although the QuickDASH scores favoured the non‐augmented group at three months, the difference is of marginal clinical importance and was not sustained: MD 11.90, 95% CI 1.94 to 21.86. There were no significant between‐group differences in health‐related quality of life at any of the follow‐up times (6 weeks, 3, 6 and 12 months), measured using either the EQ‐5D index or the EQ‐5D VAS health state; see Analysis 19.3 and Analysis 19.4. Hengg 2019 reported similar numbers had "mechanical failure" (5/31 versus 4/27; RR 1.09, 95% CI 0.32 to 3.65; Analysis 19.5). There were nine reoperations, four of which were for humeral head necrosis, but the number in each group was not stated. Although presented by treatment received instead of treatment allocated, the numbers of participants with any adverse event were similar in both groups and those for individual events were very low in number anyway; see Analysis 19.5 and Analysis 19.6. There were no sudden deaths and just one direct complication of the cement (leakage into the joint).
19.1. Analysis.

Comparison 19: Locking plate: cement augmentation of screw tips versus none (PHILOS plate fixation), Outcome 1: Shoulder function: QuickDASH score (0 to 100: worst disability)
19.2. Analysis.

Comparison 19: Locking plate: cement augmentation of screw tips versus none (PHILOS plate fixation), Outcome 2: Shoulder Pain and Disability Index (SPADI) score (0 to 100: worst outcome)
19.3. Analysis.

Comparison 19: Locking plate: cement augmentation of screw tips versus none (PHILOS plate fixation), Outcome 3: Quality of life: EQ‐5D index (0 (dead) to 1 (perfect health))
19.4. Analysis.

Comparison 19: Locking plate: cement augmentation of screw tips versus none (PHILOS plate fixation), Outcome 4: Quality of life: EQ‐5D VAS health state (0 to 100: best health state)
19.5. Analysis.

Comparison 19: Locking plate: cement augmentation of screw tips versus none (PHILOS plate fixation), Outcome 5: Adverse events (AEs) and treatment failure (analysed by treatment received)
19.6. Analysis.

Comparison 19: Locking plate: cement augmentation of screw tips versus none (PHILOS plate fixation), Outcome 6: Death and systemic complications (safety data)
Secondary outcomes
There were no significant between‐group differences in the Constant scores at 3, 6 and 12 months (Analysis 19.7).
19.7. Analysis.

Comparison 19: Locking plate: cement augmentation of screw tips versus none (PHILOS plate fixation), Outcome 7: Constant score (0 to 100: best outcome)
Nail: MultiLoc proximal humeral nail (MPHN) ‐ a straight nail ‐ versus Polarus humeral nail ‐ a curved nail
Lopiz 2014 compared these two types of intramedullary nails in 54 people with Neer two‐ or three‐part fractures, reporting results at a mean of 14 months (range 6 to 22 months). Of the two excluded participants, who were both in the MPHN group, one had died and one was lost to follow‐up. We rated all available evidence for this comparison as very low certainty, being downgraded one level for study limitations reflecting a serious risk of bias, and two levels for very serious imprecision as there were very few events and the evidence was from one small study only.
Primary outcomes
Patient‐reported outcome measures were not reported in this trial. Adverse events, including reoperations, are presented in Analysis 20.1. Fewer participants in the MPHN group had a reoperation (3/26 versus 11/26; RR 0.27 favouring MPHN, 95% CI 0.09 to 0.87; P = 0.03). All reoperations involved hardware removal of either a loose screw (one versus seven) or the whole nail (two versus four). One participant of the Polarus group had a non‐union; subsequent to nail removal, this participant had a reverse shoulder arthroplasty. Fewer participants in the MPHN group had rotator cuff symptoms (9/26 versus 19/26; RR 0.47, 95% CI 0.27 to 0.84) or shoulder impingement (2/26 versus 5/26; RR 0.40, 95% CI 0.09 to 1.88).
20.1. Analysis.

Comparison 20: Nail: MultiLoc Proximal Humeral Nail (MPHN) versus Polarus humeral nail, Outcome 1: Adverse events
Secondary outcomes
Both the unadjusted and age‐ and sex‐adjusted Constant scores were higher in the MPHN group (e.g. adjusted Constant score: MD 10.60, 95% CI 1.71 to 19.49; Analysis 20.2). Although the MDs were a little smaller than the MCID (11.2) for the Constant score, the 95% CIs included a clinically relevant difference in favour of the MPHN. There were no significant between‐group differences in range of shoulder motion (Analysis 20.3).
20.2. Analysis.

Comparison 20: Nail: MultiLoc Proximal Humeral Nail (MPHN) versus Polarus humeral nail, Outcome 2: Constant score (0 to 100: best outcome) at 14 months (6 to 22 months)
20.3. Analysis.

Comparison 20: Nail: MultiLoc Proximal Humeral Nail (MPHN) versus Polarus humeral nail, Outcome 3: Range of shoulder motion (degrees)
Hemiarthroplasty: EPOCA prosthesis versus HAS prosthesis
Fialka 2008 compared two types of hemiarthroplasty, the EPOCA prosthesis versus the HAS prosthesis, which differ in a number of ways, including the method of fixation of the tuberosities. Fialka 2008 reported results at one year for 35 of the 40 trial participants. The treatment allocations of three participants who had died and two who were lost to follow‐up were not reported. We rated all available evidence for this comparison as very low certainty, being downgraded two levels for study limitations reflecting a very serious risk of bias, and two levels for very serious imprecision as there were very few events and the evidence was from one small study only.
Primary outcomes
Fialka 2008 did not report on validated patient‐reported measures of function or quality of life. Reported complications were two participants with deep infection in the EPOCA group, two participants with persistent pain scheduled for a reoperation in the HAS group (Analysis 21.1), and a periprosthetic fracture that occurred in one of the three participants who had died by one year.
21.1. Analysis.

Comparison 21: Hemiarthoplasty: EPOCA prosthesis versus HAS prosthesis, Outcome 1: Adverse events
Secondary outcomes
Significantly better functional results, including range of motion, at one year were reported for the EPOCA prosthesis group. The relative (compared with the participant's uninjured shoulder) individual Constant score results were 70.4% (range 38% to 102%) for the EPOCA group versus 46.2% (range 15% to 80%) for the HAS group (reported P = 0.001). Results for range of motion are tabulated in Analysis 21.2; these favoured the EPOCA group. Radiological findings, except for heterotopic ossification where there were contradictory data, are shown in Analysis 21.3. These tended to favour the EPOCA prosthesis. Fialka 2008 noted some association between the bony resorption of the tuberosities and a decreased Constant score.
21.2. Analysis.
Comparison 21: Hemiarthoplasty: EPOCA prosthesis versus HAS prosthesis, Outcome 2: Range of motion results at one year (degrees)
| Range of motion results at one year (degrees) | ||||
| Study | Measure |
EPOCA prosthesis n = 18 |
HAS prosthesis n = 17 |
Reported significance |
| Fialka 2008 | Active forward flexion | mean = 109° range = 30° to 150° | mean = 62° range = 20° to 110° | P < 0.001 |
| Active abduction | mean = 101° range = 30° to 150° | mean = 62° range = 30° to 100° | P = 0.001 | |
| Active external rotation in 90° abduction | mean = 30° range = 0° to 60° | mean = 17° range = 0° to 40° | P = 0.01 | |
| Active external rotation in 90° abduction | mean = 45° range = 0° to 70° | mean = 13° range = 0° to 40° | P = 0.001 | |
21.3. Analysis.

Comparison 21: Hemiarthoplasty: EPOCA prosthesis versus HAS prosthesis, Outcome 3: Radiological assessment findings
Hemiarthroplasty: tenodesis of the long head of the biceps (LHB) versus LHB tendon left intact
Soliman 2013 compared tenodesis of the LHB versus leaving the LHB tendon intact in 45 people undergoing hemiarthroplasty. We rated all available evidence for this comparison as very low certainty, being downgraded two levels for study limitations reflecting a very serious risk of bias, and two levels for very serious imprecision as there were very few events and the evidence was from one small study only.
Primary outcomes
By deduction from the study report, four participants in each group were excluded because they had a complication within three months of follow‐up. These were reported to be tuberosity malposition (three participants); inferior subluxation of the prosthesis (two participants); loss of reduction of the greater tuberosity (two participants); and deep infection that required surgical debridement (one participant). Data for complications split by treatment group are shown in Analysis 22.1. Of these complications, only deep infection resulted in further surgery.
22.1. Analysis.

Comparison 22: Hemiarthroplasty: tenodesis of long head of biceps (LHB) versus LHB tendon left intact, Outcome 1: Complications and further surgery
Secondary outcomes
At two years, the difference between the two groups in the Constant scores in favour of the tenodesis group was below the MCID and thus unlikely to be clinically important (MD 4.60, 95% CI 0.38 to 8.82; Analysis 22.2). Three participants reported mild pain in the tenodesis group and six participants reported pain (four mild and two moderate pain) in the tendon intact group (3/19 versus 6/18; RR 0.47, 95% CI 0.14 to 1.62; Analysis 22.3). Both participants with moderate pain went on to have a mini‐open biceps tenodesis at 18 and 28 months after diagnosis of an inflamed and scarred biceps tendon. There was no difference between the two groups in active shoulder elevation results at two years (Analysis 22.4).
22.2. Analysis.

Comparison 22: Hemiarthroplasty: tenodesis of long head of biceps (LHB) versus LHB tendon left intact, Outcome 2: Constant score (0 to 100: best function) at 2 years
22.3. Analysis.

Comparison 22: Hemiarthroplasty: tenodesis of long head of biceps (LHB) versus LHB tendon left intact, Outcome 3: Shoulder pain at 2 year follow‐up
22.4. Analysis.

Comparison 22: Hemiarthroplasty: tenodesis of long head of biceps (LHB) versus LHB tendon left intact, Outcome 4: Active shoulder elevation (degrees) at 2 years
Continuing management (including rehabilitation) after surgical treatment
We rated all available evidence for these comparisons as very low certainty, being downgraded one or two levels for study limitations reflecting a serious or very serious risk of bias, and two levels for very serious imprecision as there were few events and the evidence was from one small study only in each comparison.
Immobilisation in sling for one week versus three weeks after percutaneous fixation
Wirbel 1999 tested the duration of immobilisation (one week versus three weeks) before starting physiotherapy after closed reduction and percutaneous fixation of displaced fractures in 77 participants.
Primary outcomes
Wirbel 1999 did not report on validated patient‐reported measures of function or quality of life. The report on complications and reoperations was incomplete. There were no cases of avascular necrosis at two years, nor infection or haematoma. Premature removal of Kirschner wires because of loosening occurred in five people in each group (Analysis 23.1); these results, however, were not provided for the whole study population. Additionally not reported were the treatment groups of five people who underwent open revision or hemiarthroplasty. Though similar numbers (three versus two) of people underwent removal of screws due to subacromial impingement after six months, the numbers of people in each group whose displaced tuberosity fractures were fixed with cannulated screws were not reported.
23.1. Analysis.

Comparison 23: Postoperative: immobilisation in sling for 1 versus 3 weeks after percutaneous fixation, Outcome 1: Adverse events
Secondary outcomes
Wirbel 1999 reported minimal differences between the two trial groups in their functional results, assessed using the Neer score at three and six months, or at an average of 14.2 months (Analysis 23.2).
23.2. Analysis.
Comparison 23: Postoperative: immobilisation in sling for 1 versus 3 weeks after percutaneous fixation, Outcome 2: Neer scores (0 to 100: higher score = better function)
| Neer scores (0 to 100: higher score = better function) | ||||
| Study | Follow‐up | 1 week | 3 weeks | Reported significance |
| Wirbel 1999 | 3 months | Mean = 76.4; N = 32 | Mean = 78.5; N = 32 | Not significant |
| 6 months | Mean = 81.2; N = 32 | Mean = 83.1; N = 32 | Not significant | |
| Mean 14.2 months | Mean = 82.3; N = 29 | Mean = 83.6; N = 30 | Not significant | |
Early active‐assisted mobilisation (after two weeks) versus late mobilisation (after six weeks) after cemented hemiarthroplasty
Agorastides 2007 reported the findings of this comparison in 49 of the 59 participants recruited in their trial.
Primary outcomes
At one‐year follow‐up, there were no differences between the two groups in function as rated by the Oxford Shoulder Score (MD ‐6.0, 95% CI ‐16.53 to 4.53; scale was 0 to 100; Analysis 24.1). Agorastides 2007 did not report on quality of life. Agorastides 2007 excluded one participant who developed a deep wound infection in second week after surgery and who required a secondary procedure. All four cases with greater tuberosity migration detected at six weeks developed either non‐union with bone resorption (two cases) or malunion (two cases), and subsequently, superior prosthetic subluxation at three months. All four cases had severe pain at 6 and 12 months. A further three cases in each group developed superior prosthetic subluxation; three of these appeared to be linked with initial rotator cuff injury.
24.1. Analysis.

Comparison 24: Postoperative: early (2 weeks immobilisation) versus late (6 weeks) mobilisation after cemented hemiarthroplasty, Outcome 1: Oxford Shoulder Score (OSS) at 1 year (adjusted: 0 to 100 best)
Secondary outcomes
At one‐year follow‐up, there were no differences between the two groups in function as rated using the Constant score (Analysis 24.3). Similarly, there were minimal and clinically insignificant differences between the two groups at one year in elevation and external rotation (Analysis 24.4).
24.3. Analysis.

Comparison 24: Postoperative: early (2 weeks immobilisation) versus late (6 weeks) mobilisation after cemented hemiarthroplasty, Outcome 3: Constant shoulder score (at 1 year)
24.4. Analysis.

Comparison 24: Postoperative: early (2 weeks immobilisation) versus late (6 weeks) mobilisation after cemented hemiarthroplasty, Outcome 4: Range of motion at 1 year
Discussion
Summary of main results
This review, the scope of which covers most of the non‐pharmacological treatment and rehabilitation interventions for proximal humeral fractures in adults, now includes 47 trials involving a total of 3179 participants. ProFHER 2015, a multicentre trial of 250 participants, remains the largest trial. Including ProFHER 2015, there are now six multicentre trials, two of which were each conducted in four European countries (Hengg 2019; Launonen 2019a). With the increased availability of trials, we have been able to undertake further pooling of data compared with previous versions of the review, but any pooling is still limited to six out of 26 comparisons. We have undertaken substantive pooling in only one comparison, that of surgical versus non‐surgical treatment, including for patient‐reported outcome measures of function and quality of life. As well as this key comparison, we identified three other main comparisons for which we prepared summary of findings tables. These four comparisons are indicated with a bracketed asterisk (*) in the following discussion. Where data allow, we have given the main results of individual comparisons in terms of the listed Primary outcomes, but we have included evidence from secondary outcomes where it seemed helpful to do so.
Methods of non‐surgical management (including rehabilitation)
Non‐surgical management, generally involving a period of arm immobilisation followed by physiotherapy, of (mainly) minimally displaced fractures, was the focus of 12 trials, which tested seven comparisons. There was a general recognition of the impaired function and serious complications, such as complex regional pain syndrome, that could follow a proximal humeral fracture.
Initial treatment, including immobilisation
When considering the extent and duration of initial immobilisation after a fracture, a balance is needed between the advantages of pain relief and potential avoidance of further injury, and the consequences of immobilisation, notably joint stiffness and muscle atrophy.
Early (up to one week post injury) versus delayed (after three or more weeks) mobilisation for non‐surgically treated fractures (*)
Of the five heterogeneous trials (350 participants) comparing early (up to one week from injury) versus delayed (after three or four weeks) mobilisation for non‐surgically treated fractures (Hodgson 2003a; Kristiansen 1989; Lefevre‐Colau 2007; Ring 2019; Torrens 2012), only limited data, mainly for secondary outcomes, could be pooled. Table 5 summarises the main participant characteristics, the interventions, the physiotherapy provided to both groups, and lengths of follow‐up of the individual trials.
Table 1 summarises the data relating to primary outcome measures for early versus delayed mobilisation in non‐surgically treated fractures. With the exception of adverse event data provided by four trials, most of these data are from single studies, especially Hodgson 2003a. There was very low‐certainty evidence (82 participants) of better shoulder function (fewer people with one or more shoulder problems) at one year in the early mobilisation group. However, there is very low‐certainty evidence of little or no between‐group difference in function (DASH) at three months (50 participants) and function (fewer people with one or more shoulder problems) at 24 months (74 participants). There was very low‐certainty evidence of no important between‐group difference in quality of life measured via the EQ‐5D (39 participants) or separate physical and pain aspects of quality of life (80 participants) at one year. There was one reported death and the five serious shoulder complications (1.9% of 259 participants), spread between the two groups, would have required substantive treatment.
Early intensive mobilisation versus early less intensive mobilisation
One trial, Carbone 2017, compared early intensive mobilisation (10 sessions in two weeks) versus early less intensive mobilisation (10 sessions in five weeks) started one week after the fracture in 80 people with stable impacted osteoporotic proximal humeral fractures. There is very low‐certainty evidence of little or no between‐group difference in participant judgement of the performance of the injured shoulder compared with a normal shoulder at 3, 6 and 12 months. One non‐union and one loss of reduction was reported.
Gilchrist arm sling versus "classic" Desault bandage
One quasi‐randomised trial (Rommens 1993: 28 participants with mainly minimally displaced fractures), which reported outcome at fracture consolidation, provided very low‐certainty evidence on the relative effects of two types of bandages: the Gilchrist arm sling versus the Desault body bandage. There was no report of patient‐reported outcome measures (PROMs), nor data to support the claims of no between‐group differences in functional outcome or fracture healing. Severe skin irritation prompted the premature removal of the Desault bandage in two cases. More participants found the arm sling comfortable and acceptable compared with the body bandage.
Continuing management (rehabilitation) after initial treatment involving sling immobilisation
Instructed self‐exercise versus conventional physiotherapy
Two small trials including a total of 62 participants with minimally displaced fractures compared home exercises after receiving instructions versus supervised physiotherapy (Bertoft 1984; Lundberg 1979). Neither trial reported on PROMs for function or quality of life. There was very low‐certainty evidence from single trials of little difference between the two groups in adverse events (frozen shoulder; prolonged unexplained pain), pain, change of therapy and range of motion at one year.
Swimming pool treatment plus self‐training versus self‐training alone
The trial making this comparison in 48 participants with minimally displaced fractures did not provide evidence that could be presented or tested in the analyses (Revay 1992). Thus, the evidence should be considered very low certainty. Revay 1992 claimed that the self‐treatment group had better activities of daily living and joint mobility in the first two to three months but that the two groups had similar results at one year. Revay 1992 suggested that the supervised group had neglected their home exercises, which, if true, effectively undermined the aim of this trial.
Telerehabilitation versus face‐to‐face rehabilitation
There is very low‐certainty evidence from one trial (Tousignant 2020; data for 30 participants with a non‐surgically treated proximal humeral fracture) of no differences at nine weeks in function (DASH or Constant scores), active range of motion, or participant satisfaction after eight weeks of supervised rehabilitation sessions conducted via telerehabilitation compared with being provided face to face in a clinic.
Pulsed electromagnetic high frequency energy (PHFE) versus placebo
Livesley 1992 hypothesised that pain was associated with contracture of the capsule of the glenohumeral joint and that PHFE would reduce inflammation and swelling, improving the end functional result. However, the trial (48 participants with minimally displaced fractures) failed to provide any quantitative data to support or refute this hypothesis.
Surgical treatment versus non‐surgical treatment (*)
Ten heterogeneous trials, with a total of 717 participants and 718 predominantly displaced fractures, evaluated surgical interventions for displaced fractures, of which 66% (477) were three‐ or four‐part fractures (Neer classification). Of note is that all 88 fractures in Launonen 2019a were two‐part fractures, and the majority of the fractures (146/250 = 58.4%) in ProFHER 2015 were either two‐part (128) or one‐part (18) fractures; the other seven two‐part fractures were included in Kristiansen 1988. Table 6 summarises the main participant characteristics, the interventions and lengths of follow‐up of the individual trials. Eight trials specifically limited their trial populations to older people, although the minimum age varied from 55 years in Olerud 2011a and Olerud 2011b to 80 years in Lopiz 2019. Although ProFHER 2015 recruited adults of any age, the majority of trial participants were over 65 years (142/250 = 57%). Data for patient‐reported functional scores and quality‐of‐life scores were available from the seven more recent trials that are thus more likely to represent current practice. The main results of this comparison are presented in Table 2. The results apply to the large majority of displaced proximal humeral fractures involving the surgical neck, but note should be taken of clear exceptions, such as where surgery is required for severe soft‐tissue compromise, as well as the exclusion of fracture‐dislocations, in ProFHER 2015.
There is high‐certainty evidence of no clinically important difference in patient‐reported shoulder and upper‐limb function (various measures) at one‐year (7 studies, 552 participants) and two‐year (5 studies, 423 participants) follow‐up between surgical (primarily locking plate fixation or hemiarthroplasty) and non‐surgical treatment (sling 'immobilisation') for displaced proximal humeral fractures. There is moderate‐certainty evidence of no clinically important between‐group difference in patient‐reported shoulder and upper‐limb function at six months (3 studies, 347 participants). There is high‐certainty evidence of no clinically important between‐group difference in quality of life at one year (6 studies, 502 participants). Reflecting moderate statistical heterogeneity, a similar finding of no clinically important between‐group difference at two years was rated as moderate‐certainty evidence (6 studies, 426 participants). There is low‐certainty evidence (8 studies, 646 participants) of little between‐group difference in mortality. Although there were slightly more deaths in the surgery group, the 95% confidence interval also included the potential for a higher mortality after non‐surgical treatment. Also of note is that, where reported, only one of the 31 reported deaths was explicitly linked with treatment (surgery). There is low‐certainty evidence (9 studies, 667 participants) of a higher risk of additional surgery in the surgery group: based on an illustrative risk of 35 subsequent operations per 1000 non‐surgically‐treated patients, this amounts to an extra 38 subsequent operations per 1000 surgically‐treated patients (95% CI 8 to 94 more). There is also low‐certainty evidence (3 studies, 391 participants) of a higher overall risk of adverse events after surgery; however, the 95% confidence interval also included the potential for a slightly increased risk of adverse events after non‐surgical treatment. Of note is that the clinical and functional consequences of several radiologically‐detected adverse events, such as avascular necrosis, were often unclear or not reported.
Two included studies, Fjalestad 2010a and ProFHER 2015, performed economic evaluations, identified as cost‐utility analyses in the brief economic commentary (BEC); see Agreements and disagreements with other studies or reviews. Fjalestad 2010a found no significant differences in costs between the two groups (Fjalestad 2010b), whereas ProFHER 2015 found surgery was more costly over two years (Corbacho 2016).
Different methods of surgical management
Comparisons of different categories of surgical intervention
Ten trials evaluated one of six different comparisons of different categories of surgical intervention (Cai 2012; DelPhi 2020; Gracitelli 2016; Helfen 2020; Hoellen 1997; Jonsson 2020; Plath 2019; Sebastiá‐Forcada 2014; Smejkal 2011; Zhu 2011).
Open reduction and internal fixation using a locking plate versus a locking intramedullary nail (*)
This comparison was made in four trials that involved a total of 270 participants (Gracitelli 2016; Helfen 2020; Plath 2019; Zhu 2011). Table 7 summarises the main participant characteristics, the interventions and lengths of follow‐up of the individual trials. Of the 250 recorded fractures, 63% were two‐part and 33% were three‐part fractures.
Table 3 summarises the data relating to primary outcome measures for this comparison. There is low‐certainty evidence of no clinically important between‐group difference in shoulder function (ASES and DASH) at one year (4 studies, 227 participants), six months (3 studies, 174 participants) and at two years (2 studies, 101 participants). There is very low‐certainty evidence of no between‐group difference in quality of life at one year (1 study, 53 participants), and little difference in adverse events (4 studies, 250 participants) and additional surgery at one year (3 studies, 193 participants).
Open reduction with internal fixation using a locking plate versus minimally invasive fixation with distally inserted intramedullary K‐wires
There is very low‐certainty evidence from one trial (Smejkal 2011: 61 participants with two‐ or three‐part fractures) of no difference between these two interventions for numbers of participants incurring a complication or in the Constant scores at two years' follow‐up.
Hemiarthroplasty versus internal fixation
With minimal opportunity for pooling, data from two small heterogeneous trials testing this comparison are presented separately.
Hemiarthroplasty versus open reduction and locking plate fixation
The very low‐certainty evidence from one trial (Cai 2012: 32 participants with four‐part fractures) of lower DASH scores (better function) and slightly higher EQ‐5D scores (better quality of life) at one and two years may not equate to clinically important differences in either of these outcomes between hemiarthroplasty and locking plate fixation. Three participants in each group had a reoperation.
Hemiarthroplasty versus tension band wiring
There is very low‐certainty evidence from one trial (Hoellen 1997: 30 participants with four‐part fractures) of a higher risk of reoperation after tension band wiring at one year. There is very low‐certainty evidence of no differences between the two groups in the Constant scores or pain at one year (18 participants).
Reverse total shoulder arthroplasty (RTSA) versus locking plate fixation
This comparison was tested in one trial (DelPhi 2020: function reported for 104 participants with severely displaced fractures). There is low‐certainty evidence of no clinically important difference in patient‐reported shoulder function (OSS) at 24 months. DelPhi 2020 did not report on quality of life. There is very‐low certainty evidence of little between‐group differences in death, complications and reoperations.
Reverse total shoulder arthroplasty versus hemiarthroplasty (*)
Table 4 summarises the data relating to primary outcome measures for this comparison. There is very low‐certainty evidence from two trials (Jonsson 2020 and Sebastiá‐Forcada 2014: 161 participants aged over 70 years with either three‐ or four‐part fractures) of no or minimal between‐group differences in self‐reported shoulder function at one year (WOOS relative to normal shoulder; 1 study, 65 participants) or at two to three years' follow‐up (QuickDASH and WOOS relative to normal shoulder; 2 studies, 142 participants); or in quality of life (EQ‐5D) at one year (1 study, 83 participants) or at a minimum of two years' follow‐up (1 study, 66 participants). Function at six months was not reported. There were 10 deaths reported by one trial (99 participants) of which one death appeared to be related to surgery. There is very low‐certainty evidence of a lower risk of complications after RTSA (2 studies, 160 participants). Ten people (6.2% of 161) had a reoperation; all eight cases in the hemiarthroplasty group received an RTSA (very low‐certainty evidence).
Comparisons of different methods of performing an intervention in the same category
Thirteen trials tested one of 10 comparisons of different types or methods in the same intervention category (e.g. plating): Biermann 2020; Buecking 2014; Fialka 2008; Hengg 2019; HURA 2020; Lopiz 2014; Ockert 2010; Sohn 2017; Soliman 2013; Voigt 2011; Zhang 2011; Zhang 2019; Ziegler 2019.
Plate: deltoid‐split with minimal invasive plate osteofixation versus the deltopectoral approach for plate fixation
This comparison was tested in three trials that included 312 people with Neer two‐, three‐ or four‐part fractures (Buecking 2014; HURA 2020; Sohn 2017). Pooling of data was possible for only a few outcomes. Due to very low‐certainty evidence, we are uncertain of the finding from one trial of potentially a clinically worse outcome in the deltoid‐split plus MIPO group for function (QuickDASH) and quality of life (SF‐12 physical component) at an average follow‐up of 26 months; both results also included the possibility of no important difference. We are also uncertain of the findings of little or no between‐group differences in complications or reoperations, or function measured using Constant or UCLA scores or pain at one year or longer.
Plate: polyaxial versus monoaxial locking plate fixation
Although two trials (Ockert 2010 and Voigt 2011: 180 participants with two‐, three‐ or four‐part fractures) made this comparison, most of the data were from Voigt 2011 (48 participants for function) and only data for reoperation were pooled. There is very low‐certainty evidence of no between‐group differences at one year in function (DASH and Simple Shoulder Test scores), reoperations and complications.
Plate: CFR‐PEEK plate (made of polyetheretherketone reinforced with carbon fibres) versus the titanium PHILOS plate
There is very low‐certainty evidence from one trial (Ziegler 2019: 63 people "with proximal humerus fractures requiring surgery" at six months' follow‐up) of no between‐group differences at any follow‐up time (6 and 12 weeks; 6 months) in any of the three measures of shoulder function (DASH, OSS, SST). There were few complications and just two reoperations for an unspecified new injury.
Locking plate: additional glenohumeral joint lavage
There is very low‐certainty evidence from one trial (Biermann 2020: 62 participants with two‐, three‐ or four‐part fractures with data at one year) of little or no difference from glenohumeral joint lavage after PHILOS locking plate fixation in the numbers of complications, in function (Constant scores) or in range of motion at one year.
Locking plate: use of medial locking screws
There is very low‐certainty evidence from one trial (Zhang 2011: results for 68 participants with two‐, three‐ or four‐part fractures) of fewer early losses of fixation and reoperations with medial locking screws, although the 95% CI results also included a higher risk of both in the medial locking screws group. There is very low‐certainty evidence of a marginally better function reflecting the higher Constant scores with medial screws at 31‐month follow‐up; however, only part of the 95% CI included the MCID.
Locking plate: in combination with allogeneic femoral head bone grafts
There is very low‐certainty evidence from one trial (Zhang 2019: 80 participants with three‐ or four‐part fractures) with short‐term follow‐up of little difference from the use of allogeneic bone grafts in combination with PHILOS locking plates in the numbers of participants with complications, or with poor outcome based on Neer shoulder function score at one month.
Locking plate: cement augmentation of the screw tips
There is very low‐certainty evidence from one trial (Hengg 2019: most results for 58 participants, mainly with three‐ or four‐part fractures) of little or no difference at one year in function (QuickDASH, SPADI), quality of life (EQ‐5D), adverse events (treatment group data were not available for secondary surgery), and Constant scores, from the use of polymethylmethacrylate screw‐tip cement augmentation in people treated with PHILOS plate fixation.
Nail: MultiLoc proximal humeral nail (MPHN) ‐ a straight nail ‐ versus Polarus humeral nail ‐ a curved nail
There is very low‐certainty evidence from one trial (Lopiz 2014: 54 participants with two‐ or three‐part fractures) of fewer adverse events, including reoperations and impingement, for the MPHN nail compared with the Polarus nail. There is also very low‐certainty evidence of half as many participants with rotator cuff symptoms in the MPHN group, as well as higher Constant scores at an average of 14 months in this group; findings which the authors considered were linked.
Hemiarthroplasty: EPOCA prosthesis versus HAS prosthesis
There is very low‐certainty evidence from one trial (Fialka 2008: 35 people with four‐part fractures) of better function (Constant scores) and range of motion at one year for the EPOCA prosthesis when compared with the HAS prosthesis. Two participants in each group had a serious complication or pain requiring further treatment.
Hemiarthroplasty: tenodesis of the long head of the biceps (LHB) versus LHB tendon left intact
There is very low‐certainty evidence from one trial (Soliman 2013: 45 people with four‐part fractures undergoing hemiarthroplasty) of little or no between‐group differences in complications at three months' follow‐up, in function (Constant score), in the numbers of participants with shoulder pain or in active shoulder elevation.
Continuing management (including rehabilitation) after surgical treatment
The need for and duration of immobilisation before commencing physiotherapy after surgery for displaced fractures was tested separately in two small heterogeneous trials for fixation and hemiarthroplasty, respectively. For both comparisons, the evidence is very low certainty and incomplete.
Immobilisation in sling for one week versus three weeks after percutaneous fixation
We are uncertain of the findings from Wirbel 1999 (64 participants at six months) relating to the premature removal of K‐wires or later reoperations, or of the minimal differences between the two groups in function assessed using the Neer outcome score at three, six and an average of 14.2 months.
Early active‐assisted mobilisation (after two weeks) versus late mobilisation (after six weeks) after cemented hemiarthroplasty
We are uncertain of the findings from Agorastides 2007 (49 participants) of little or no difference between participants mobilised after two weeks (which was current practice) after hemiarthroplasty versus those mobilised after six weeks in patient‐rated function (Oxford Shoulder Score), adverse effects that focused on radiological outcomes, the Constant score, and range of motion at one year.
Overall completeness and applicability of evidence
Overall completeness
As summarised above, the included trials (mean recruitment 68 participants) evaluated one of 26 comparisons. Pooling was possible in only six comparisons. We present the evidence from four of these six comparisons in separate summary of findings tables. These tables reveal the limitations and incompleteness of the evidence, including for the comparison of surgical versus non‐surgical treatment. Of a total of 717 participants recruited into the 10 trials making this comparison, pooled data for PROMs measuring function at one year were available for only 552 participants (77%); quality of life data for only 502 participants (70%); mortality for 646 participants (90%); and additional surgery for 667 participants (93%).
Applicability
Overall considerations
To inform consideration of applicability of the evidence from individual trials, we give quite extensive details in the Characteristics of included studies on the study populations and interventions. Notably, over two‐thirds of trial participants were women and aged 60 years or over; this fits with the general characteristics of people presenting with these injuries. Additionally, Table 8 shows our assessments for each trial of four aspects relevant to ascertaining external validity: definition of the study population, description of the interventions, definition of primary outcome measures and length of follow‐up. Clearly unhelpful is where there are incomplete descriptions of study inclusion (16 trials) and interventions (six trials). Eight trials had less than one year of follow‐up: Lefevre‐Colau 2007 (six months), Livesley 1992 (six months), Ockert 2010 (six months), Ring 2019 (six months), Rommens 1993 (until fracture consolidation ‐ time unspecified), Tousignant 2020 (eight weeks, corresponding to the end of intervention), Zhang 2019 (three months) and Ziegler 2019 (six months). Additionally, the minimum follow‐up was six months in Lopiz 2014. Despite the claims of longer follow‐up, the results seemed to apply to six months at most in Stableforth 1984. In Wirbel 1999, though follow‐up of 21 participants was more than two years, the main results applied to the set follow‐up at six months. Our setting of our criterion to one‐year follow‐up as acceptable is arbitrary and mainly reflects a reasonable timing for assessment of function. However, it should be noted that, in terms of a full outcome assessment, data at one‐year follow‐up must be considered preliminary results only, given that complications such as avascular necrosis and device failure may not become evident until later and functional recovery can still be ongoing. The extended follow‐up findings, where surgery was mainly plate fixation, of ProFHER 2015 provide some reassurance that a two‐year follow‐up should suffice (Handoll 2017). However, in a commentary on DelPhi 2020, Cole points to a need to a longer follow‐up for arthroplasty, specifically RTSA in this trial, given that failure and replacement for these occur on a longer timescale (Cole 2020).
4. Assessment of items relating to applicability of trial findings.
| Clearly defined study population? | Interventions sufficiently described? | Main outcomes sufficiently described? | Appropriate timing of outcome measurement? (Yes = 1 year or more) | |
| Agorastides 2007 | Partial: exclusions not specified upfront | Yes | Yes | Yes: 1 year |
| Bertoft 1984 | Partial: no exclusion criteria given (e.g. ability to understand instructions for exercises) | Yes | Yes | Yes: 1 year |
| Biermann 2020 | Partial: mismatch between trial registration and trial report | Yes | Partial: insufficient description of measurement procedures | Yes: 1 year |
| Boons 2012 | Yes | Yes | Yes | Yes: 1 year |
| Buecking 2014 | Partial: indication for hemiarthroplasty poorly defined (27 excluded before randomisation because "implantation of a prosthesis was planned") | Yes | Yes | Yes: 1 year |
| Cai 2012 | Partial: unclear definition of 4‐part fractures | Yes: however, time to surgery not reported | Yes | Yes: 2 years |
| Carbone 2017 | Yes | Yes | Yes | Yes: 1 year |
| DelPhi 2020 | Yes | Yes | Yes | Yes: 2 years |
| Fialka 2008 | Yes | Yes | Yes | Yes: 1 year |
| Fjalestad 2010a | Yes | Yes | Yes | Yes: 2 years |
| Gracitelli 2016 | Yes | Yes | Yes | Yes: 1 year |
| Helfen 2020 | Yes | Yes | Yes | Yes: 2 years |
| Hengg 2019 | Yes | Yes | Yes | Yes: 1 year |
| Hoellen 1997 | Yes: but some question over fracture type in that the Holbein 1999 report included 3‐part fractures too | Yes | Yes | Yes: 1 year |
| Hodgson 2003a | Yes | Yes | Yes | Yes: 2 years |
| HURA 2020 | Yes | Yes | Yes | Yes: 2 years |
| Jonsson 2020 | Yes | Yes | Yes | Yes: 2 years |
| Kristiansen 1988 | Partial: no exclusion criteria given | Partial: incomplete description of timing of sling use and care of external fixator pin sites | Partial: no description of measurement procedures | Yes: 1 year |
| Kristiansen 1989 | Partial: no exclusion criteria given | Partial: although sling and body bandage are common expressions, some variation possible | Partial: no description of measurement procedures | Yes: 2 years |
| Launonen 2019a | Yes | Yes | Yes | Yes: 2 years |
| Lefevre‐Colau 2007 | Yes | Yes | Yes | Partial: 6 months |
| Livesley 1992 | Yes: although this included 4 participants under 20 years with epiphyseal fractures | Yes | Yes | Partial: 6 months |
| Lopiz 2014 | Partial: insufficient criteria given in terms of suitability for surgery | Yes | Yes | Partial: minimum 6 months |
| Lopiz 2019 | Yes | Yes | Yes | Yes |
| Lundberg 1979 | Partial: no exclusion criteria given (e.g. ability to understand instructions for exercises) | Yes | Yes | Yes: 1 year or above (mean: 16 months) |
| Ockert 2010 | Partial: exclusion criteria described in context of post‐randomisation exclusions | Yes | Yes | Partial: 6 months |
| Olerud 2011a | Yes | Yes | Yes | Yes: 2 years |
| Olerud 2011b | Yes | Yes | Yes | Yes: 2 years |
| Plath 2019 | Partial: fracture types for inclusion not specified | Yes | Yes | Yes: 1 year |
| ProFHER 2015 | Yes | Yes In the context of this being a pragmatic trial |
Yes | Yes: 2 years |
| Revay 1992 | Yes | Partial: frequency of swimming sessions not stated | Yes | Yes: 1 year |
| Ring 2019 | Partial: fracture types for inclusion not sufficiently defined, although most were non or minimally displaced | Partial: no information on sling or other method of immobilisation | Yes | Partial: 6 months |
| Rommens 1993 | Yes: but to note that other fractures including rib (3 participants) were included | Yes | Partial: functional outcome assessment not described (sufficiently) | No: only until fracture consolidation |
| Sebastiá‐Forcada 2014 | Yes | Yes | Yes | Yes: minimum 2 years |
| Smejkal 2011 | Yes | Partial: only minimal intraoperative details given and nothing regarding postoperative management including rehabilitation | Partial: this may have been ‘lost in translation’ (Czech article) | Yes: mean 2 years but range not stated (probably most/all > 1 year as recruitment had finished January 2010) |
| Sohn 2017 | Yes | Yes for interventions although no details on rehabilitation | Partial: no details on assessment of functional assessment (Constant score) | Yes: 12 months minimum |
| Soliman 2013 | No: no explanation given for a younger population; insufficient criteria given in terms of suitability for hemiarthroplasty | Yes | Partial: incomplete description of pain categories; no clarification of modification to Constant score | Yes: minimum 21 months follow‐up |
| Stableforth 1984 | Yes | Yes | Partial: no description of measurement procedures, incomplete description of pain categories | Partial: up to 6 months, then between 18 months to 12 years. This is too spread out. Most results applied to the 6‐month follow‐up. |
| Torrens 2012 | Partial: the < 1.5 cm criterion for posterior displacement of the greater tuberosity is unusual and no justification was given by the authors | Partial: incomplete description of accompanying "progressive rehabilitation program" | Partial: incomplete description of measurement procedures | Yes: 1 year |
| Tousignant 2020 | Yes (although fracture classification not reported) | Yes | Yes | No: only 8 weeks, at end of the intervention |
| Voigt 2011 | Yes | Yes | Yes | Yes: 1 year |
| Wirbel 1999 | Yes | Yes | Partial: no description of measurement procedures | Partial: between 9 and 36 months; < 1 year in 10 participants. Main results applied to 6 months |
| Zhang 2011 | Yes | Yes | Partial: insufficient information on measurement of complications and timing of their measurement | Yes: All over 25 months (mean 30.8 months) |
| Zhang 2019 | Partial: fractures poorly described, as are the contraindications for surgery | Yes | No: poor descriptions of outcomes and their measurement | No: maximum 3 months |
| Ziegler 2019 | Partial: fracture types, inclusion criteria and indication for operation not clear | Yes | Yes | Partial: 6 months (too short for late complications after plating) |
| Zhu 2011 | Yes | Yes | Yes | Yes: 1 and 3 years |
| Zyto 1997 | Yes | Yes | Yes | Yes: 1 year, and 3 to 5 years |
The measurement of outcome was variable, though generally comprehensive. In most of the older trials, there was frequent use of non‐validated or, at best, partly‐validated scoring systems, such as the Neer (Neer 1970) and Constant and Murley (Constant 1987) systems, but also of simple rating systems for individual outcomes. Validated schemes, such as the Oxford Shoulder Score (Dawson 1996) and Shoulder Rating Questionnaire (L'Insalata 1997), for subjective assessment of symptoms and function were not available at the time for the trials in earlier versions of this review. Nonetheless, some consideration of inter‐observer reproducibility and other aspects of validity was evident in the establishment of the Constant score in two trials (Lundberg 1979; Zyto 1997). Non‐validated outcome assessment schemes, often with arbitrary criteria for grading overall outcome (excellent, good, fair, poor), are probably best viewed as 'blunt' and flawed instruments. This needs to be noted when viewing the results of many of the older included trials; in particular Kristiansen 1989, whose outcome assessment is almost completely based on the Neer scoring system. As noted also in our 2012 and 2015 updates, more recent trials continue to be better in this respect. In the 2015 update, four of the eight newly included trials reported validated PROMs for function. In this update, 13 of 16 newly included trials reported PROMs for function, mainly DASH or QuickDASH; the exceptions were Biermann 2020, Carbone 2017 and Zhang 2019. As noted in Handoll 2015b, the Constant score, which requires additional clinical examinations, remains in common use, being reported by 13 of the newly included trials. In their choice of the Neer 1970 tool, Zhang 2019 is an exception, using neither a validated PROM or the Constant score.
In a systematic review, Brorson 2019 reported a lack of consensus in the use of terms for and definitions of complications after non‐surgical management. This situation was replicated for surgery‐related complications (Alispahic 2020). This has implications for applicability, and the reason for downgrading for indirectness for adverse events (complications) for the surgical versus non‐surgical treatment comparison was in part the variety in the definition, measurement and severity of adverse outcomes between trials. Additionally, the clinical and functional implications of many radiologically‐detected outcomes, such as avascular necrosis, are generally unclear.
The majority (39 out of 47) of the trials used Neer's fracture classification (Neer 1970). Problems, such as poor inter‐observer reproducibility and intra‐observer reliability, with the classification of fractures according to the Neer and AO systems have been shown for both radiographs and computerised tomographic scans (Bernstein 1996; Brorson 2008; Sidor 1993; Siebenrock 1993; Sjoden 1997). This variation in the classification of fractures, and hence diagnosis, needs to be considered when interpreting the results of trials, both in respect to the comparability and composition of the intervention groups and in the applicability of the trial's findings. The limitations of the Neer classification scheme were also demonstrated by the identification of the valgus impacted four‐part fracture as a separate category with a lower risk of avascular necrosis (Jakob 1991). Ideally, a fracture classification system should act as a guide to treatment as well to enable the comparison of results from studies of people with similar fracture patterns. However, other factors, such as osteoporotic bone, associated soft‐tissue injury and the individual's overall health and motivation, will also influence treatment choices and outcome. Brorson 2012, which looked at the agreement of surgeons' treatment recommendations in conjunction with the Neer classification, concluded that the low observer agreement on the Neer classification may have less clinical importance than previously assumed. However, Brorson and colleagues noted that inter‐observer agreement on treatment did not exceed moderate levels.
The approaches taken in two surgical versus non‐surgical trials warrant further attention. The purposefully pragmatic inclusion criteria used in ProFHER 2015 is noteworthy in this regard. These stipulated that the degree of displacement had to be sufficient for the treating surgeon to consider surgical intervention but did not have to meet the displacement criteria of Neer for inclusion in the trial. Post‐recruitment classification by two independent surgeons of the baseline X‐rays resulted in the identification of 18 one‐part fractures (see Table 6). Nonetheless, these exceptions were judged sufficiently displaced that they would have been considered for surgical intervention in practice, where the exact observation of Neer's arbitrary criteria is rare. Notably, Brorson 2002 found only moderate agreement (kappa for agreement was 0.41) for displacement versus non‐displacement in a study of inter‐observer agreement among 24 orthopaedic surgeons. A publication detailing the practicalities of defining the fracture population, based on the Neer classification, within ProFHER 2015 found that the disparity between the categorisation at baseline (based on tuberosity involvement) and Neer classification as assessed by two raters, who had undergone training and used a detailed pro forma, did not appear to affect trial findings, specifically in terms of influencing the effect of treatment on the primary outcome (OSS) of the trial (Handoll 2016a). Mindful of the issues relating to Neer's classification, Launonen 2019a adopted a rigorous approach, which included the use of computed tomography, where two upper‐extremity trauma surgeons reviewed each patient before randomisation to confirm the classification.
In a study of the death rates in 18,452 patients with a proximal humeral fracture recorded in the Swedish Fracture Register (2011 to 2017), Bergdahl 2020 reported deaths of 310 (1.68%) within 30 days, 615 (3.33%) within 90 days, and 1445 (7.83%) within one year after the injury. Comparing with mortality in the general population in terms of age, sex, and year of follow‐up, the standardised mortality ratios were 5.2 (times more likely to die) at 30 days, 3.4 at 90 days and 2.0 at one year. These data put the populations in the included trials into a different context; thus, generally, these trials are less likely to include the more frail patients or people with other fractures.
While it is possible that all 47 trials are relevant to current practice somewhere in the world, it is likely that some interventions are now rarely used. These include body bandages as tested in Rommens 1993. Nowadays, it is much more common practice to use either a 'collar and cuff' sling or a 'poly‐sling' (these incorporate a chest strap that can be passed around the body). Additionally, the applicability of the findings from older trials, such as Stableforth 1984, is potentially less than that for newer trials, given subsequent changes in practice, including the availability of new implants. These include locking plates, which remain a mainstay for these fractures in many countries (Hasty 2017;Thanasas 2009). In Handoll 2012, we noted that the increasing use of locking plates for these fractures was reflected in the use of locking plates in more recently included trials. This observation still applies, although there has been renewed interest in locking nails. As noted in Handoll 2015b, a more recent development has been the use of reverse total shoulder arthroplasty (RTSA), typically for four‐part fractures in older people. The use of RTSA continues to grow clinically, in particular for people aged 70 years and above; this also reflects a decline in the use of hemiarthroplasty (Han 2015; Hasty 2017). In Handoll 2015b, RTSA featured only in Sebastiá‐Forcada 2014, where it was compared with hemiarthroplasty. In this update, three more RTSA trials are included: DelPhi 2020 (formerly ongoing in Handoll 2015b), which compared RTSA with plate fixation; Jonsson 2020, which compared RTSA with hemiarthroplasty; and Lopiz 2019, whose comparator was non‐surgical treatment. Notably, there are currently five ongoing trials comparing RTSA versus non‐surgical treatment (ISRCTN76296703; Launonen 2019; NCT03599336; NCT03610113; ReShAPE).
COVID‐19 pandemic
All included trials were conducted before the onset of the COVID‐19 pandemic. In our view, the basic characteristics of proximal humeral fractures in terms of population, interventions and outcomes remain as before. However, in some countries, where COVID‐19 has largely been contained, care of people with these fractures may have continued as previously, but in others, such as the UK, there has been a greater emphasis on non‐surgical treatment and treatment as day cases, including discharge on the same day following surgery (Wallace 2020). Overall, the impact of COVID‐19 on health and social care systems has been immense. The pandemic inevitably has and will continue to impact access to appropriate care for patients. The World Health Organization estimates that existing rehabilitation services in 60% to 70% of countries have been disrupted due to the COVID‐19 pandemic (WHO 2021). COVID‐19 has also interrupted research; for example, recruitment was suspended for a period in 2020 for ISRCTN76296703 (PROFHER‐2) but has now resumed.
Comments on individual comparisons
This is restricted to the four main comparisons presented in the summary of findings tables. Commentary on other comparisons, some of it revised from Handoll 2015b, is presented in Appendix 4.
Early (up to one week post injury) versus delayed (after three or more weeks) mobilisation for non‐surgically treated fractures
Most of the evidence for the comparison of early versus delayed mobilisation came from Hodgson 2003a, and thus applies primarily to minimally displaced two‐part fractures. A survey sent to senior hospital physiotherapists working directly with orthopaedic patients revealed large variation in rehabilitation, in particular with regards to routine immobilisation, duration of immobilisation and timing of first contact with a physiotherapist, within and between hospitals in the UK (Hodgson 2003b [pers comm]; Hodgson 2006). A survey sent to the participating centres of ProFHER 2015, which included displaced fractures, found the recommended duration of arm immobilisation for non‐surgically treated patients ranged from two to six weeks, with 29 (91%) of 32 UK hospitals recommending immobilisation of three weeks or over (Handoll 2015a). This variation also needs to be viewed in the context of the type of arm immobilisation used, as methods such as collar and cuff provide support rather than rigid immobilisation. As noted by McKee 2007 in his commentary on Lefevre‐Colau 2007, the applicability of this trial is limited by the intensive physiotherapy regimen used in both groups. Both practically and financially, the 32 two‐hour sessions of physiotherapy may be difficult for patients and healthcare providers; notably, 10 participants withdrew from the trial because of difficulties in attending. In contrast, the mean numbers of treatment sessions in Hodgson 2003a were 9 and 14, respectively, in the two groups. There was incomplete information from Ring 2019 to judge the applicability of the limited additional evidence provided by this newly included trial.
Surgical treatment versus non‐surgical treatment
In our commentary for this comparison in Handoll 2012, we noted that "Trials comparing surgical versus non‐surgical interventions, or indeed different surgical interventions, risk losing currency as different implants and methods become available and fashionable." We also noted the impact of surgical decision‐making in favour of locking plating systems, which allow for stronger constructs and fixation of more complex fracture patterns in osteopenic bone, with the potential for less soft‐tissue stripping and compromise to the blood supply (Thanasas 2009). As noted above and in Handoll 2015b, there is increasing use of reverse shoulder arthroplasty for more complex (predominantly four‐part) fractures, especially in older people, for instance in the USA (Han 2015; Schairer 2015). These illustrate how evolving technology (and marketing forces) mitigates against applying the findings of older types of trials. This is, however, even less of a concern in this update. Trials representative of current practice dominate the evidence, including that for patient‐reported outcome measures of function and of quality of life. This includes the evidence from the two newly included trials: Launonen 2019a, testing locking plate fixation for two‐part fractures in 88 participants aged 60 years or above; and Lopiz 2014, testing RTSA for three‐ or four‐part fractures (85% of total) in 62 participants aged 80 years or above. When considering the validity and applicability of surgical trials, account needs to be taken also of fundamental variations in surgical practice, including facilities and operator expertise. In particular, operator expertise and the linked issue of the surgical learning curve, play a pivotal role in the validity and applicability of surgical trial findings. Awareness of these issues was behind the pragmatic decision in ProFHER 2015 for surgeons to use methods with which they are familiar rather than stipulate the type of surgery. Indeed, the pragmatic multicentre design of ProFHER 2015, including the constant emphasis on good standard practice and surgery by experienced surgeons (predominantly consultants), continues to mean that its results apply at least in the setting where it was conducted (UK National Health Service (NHS) trauma hospitals) and most likely in many other countries with similar surgical practice.
Because of the continuing dominance of the evidence from ProFHER 2015, particular note should be taken of its exclusion criteria (such as of open fractures, fracture dislocations and two‐part greater tuberosity fractures and other patterns not involving the surgical neck) and its study population, the composition of which shows the treatment uncertainty covered by this trial applied to the large majority of displaced fractures of the proximal humerus involving the surgical neck. Additionally, the lack of subgroup differences in ProFHER 2015, either for age (threshold of 65 years) or fracture type (tuberosity involvement or not; or Neer one‐ or two‐part versus three‐ or four‐part) strengthens the case for not differentiating treatment (use of surgery) on the basis of these characteristics. Neither of the two newly included trials, which boosted the evidence for two‐part fractures and for four‐part fractures, reported clinically important differences between the two groups in shoulder function or quality of life. Importantly, in all the trials, eight of which purposefully excluded younger adults, the evidence is predominantly from older people. This reflects the population distribution for these fractures (Karl 2015), but also the population for which the main treatment uncertainty applies, and for which the increased use of surgery was greatest (Sumrein 2017).
Of the 31 deaths over two years (4.8% of 646 participants) reported in the trials, only one was explicitly linked with surgery. This low incidence is likely to have reflected study inclusion decisions. For example, in ProFHER 2015, comorbidities that preclude surgery or anaesthesia was the most common specific clinical reason for excluding patients prior to randomisation.
Open reduction and internal fixation using a locking plate versus a locking nail
For consistency, we kept the order of the two interventions the same as in previous versions of the review; thus, still implying that the more 'experimental' intervention was the locking plate. However, this is no longer the case. The locking plate is now the established device and more recent studies, including the three newly included studies for this comparison, have framed their question in terms of the locking nail being the experimental group. As pointed out in Plath 2019, intramedullary humeral nails, together with locking, have evolved considerably over the years, and there is interest in increasing their indication from two‐part surgical neck fractures to more complex fractures. Both Gracitelli 2016 and Plath 2019 included three‐part fractures involving the surgical neck, Plath 2019 extending this further to also include four‐part fractures. Overall, of the 250 recorded fractures, 63% were two‐part and 33% were three‐part. The three newly included trials also included older participants (mean ages 65, 75 and 76 years), whereas Zhu 2011 had a mean age of 53 years, which makes it less representative in terms of the osteoporotic population for which locking plates have presumed advantages. Of note are the extra measures used for stabilising the implant. Helfen 2020 used cement augmentation for locking plate screws, whereas tension‐band sutures were used in both groups of Gracitelli 2016 and in the nail group of Plath 2019; sutures were also used in the plate group of this trial. Also of note is the variation in the surgical approaches used.
Reverse total shoulder arthroplasty versus hemiarthroplasty
As implied above, this comparison ‐ tested in two trials ‐ is very topical (Jonsson 2020; Sebastiá‐Forcada 2014). In our previous commentary on Sebastiá‐Forcada 2014, we pointed to it being limited in terms of applicability because it was a single‐centre trial with the participants being operated on by two surgeons. We also noted that the prostheses compared within Sebastiá‐Forcada 2014 came from only one manufacturer, and that prostheses made by different manufacturers will differ to some extent. However, we expected the variation between prostheses from different manufacturers is likely to be of lesser importance clinically than the large differences between RTSA and hemiarthroplasty. In contrast, in the multicentre study conducted in Sweden by Jonsson 2020, the choice of prosthesis was by the treating surgeon, which resulted in four different RTSA implants and eight different hemiarthroplasty implants. This pragmatic approach is likely to enhance applicability of the trial findings. Both trials included people aged 70 years or over with three‐ or four‐part fractures.
Quality of the evidence
We have now framed this in terms of 'certainty' of evidence, with the same components for assessment as before: study limitations (risk of bias), inconsistency, indirectness, imprecision and publication bias.
Our assessments of the certainty of the evidence for each comparison for which data are available are stated in Effects of interventions. Additionally, those for four comparisons are detailed in summary of findings tables: Table 1 (Early versus delayed mobilisation); Table 2 (Surgical treatment versus non‐surgical treatment); Table 3 (Open reduction with internal fixation using a locking plate versus a locking nail); and Table 4 (RTSA versus hemiarthroplasty). With the exception of the evidence for the comparison of surgical versus non‐surgical treatment, most of the GRADE assessments for the evidence from other comparisons were very low certainty. This typically reflects the insufficiency of the evidence from the majority of comparisons that were tested by small single trials only. As well as being marked down for study limitations, we downgraded the findings from these comparisons for serious or generally very serious imprecision. As ever, this relates to the clear need for caution in interpreting the results of small trials which demonstrate 'no evidence of an effect' rather than 'evidence of no effect'.
Study limitations
As noted in Handoll 2012 and Handoll 2015b, and continues to apply in this update, more recent trials generally have better study design (e.g. they have appropriate random sequence generation and allocation concealment, and thus are at low risk of selection bias) and reporting (e.g. including participant flow diagrams). Nonetheless, as shown by Figure 2, it remains the case that many of the included trials had serious shortcomings and are at high risk of bias that could affect the validity of their findings. The main, but generally unavoidable, shortcoming in trials testing physical and surgical interventions was the lack of blinding; however, measures were taken by some trials to reduce detection bias. We considered 31 trials to be at high risk of outcome assessment bias for function and other subjective outcomes. Another common source of bias was that resulting from a high loss to follow‐up or exclusion of participants from the analyses: we considered this risk high in 16 trials, three of which were new to this version (HURA 2020; Plath 2019; Ring 2019).
It is encouraging that 12 of the 16 newly included trials had prospective trial registration and, further, a published protocol was available for two of these (DelPhi 2020; Tousignant 2020). Additionally, five of the newly included 16 trials were multicentre.
Inconsistency
Limited pooling opportunities meant that we rarely assessed inconsistency. We downgraded evidence by one level for serious inconsistency for moderate heterogeneity for two outcomes (quality of life at 24 months and additional surgery) for the surgical versus non‐surgical treatment comparison, and for four outcomes (self‐reported shoulder function at six months and two or three years, adverse events and additional surgery) for the locking plate versus locking nail comparison.
Indirectness
Although mindful of this in terms of the incomplete information and limited outcomes collected for some comparisons, we rarely downgraded evidence for indirectness. In the surgical versus non‐surgical treatment comparison, we downgraded the evidence for adverse events one level for serious indirectness because of the wide variation in the definition of adverse events amongst the trials reporting this. In the early versus delayed mobilisation comparison, we downgraded the evidence for function at one year, as this was measured by an outcome tool of uncertain validity. Finally, we downgraded the evidence for all outcomes of Zhang 2019, which tested the use of allogeneic femoral head bone grafts for locking plate fixation, because of its inappropriately short follow‐up: a maximum of three months.
Imprecision
As indicated already, we frequently downgraded for serious or very serious imprecision. For this update, we tended to downgrade more severely for outcomes where the numbers of events were low or really not sufficient to be certain of the result.
Publication bias
We did not downgrade or test for publication bias. We note the minimum threshold for examining this is 10 trials, which was met only for infection for the 'surgical treatment versus non‐surgical treatment' comparison, for which five trials reported no events (Analysis 6.20.3).
Potential biases in the review process
Given this is clearly an active area of research, conducted in an increasing number of countries, it is likely that we have failed to identify some randomised trials, particularly those reported only in abstracts or in non‐English language publications. We may also have overlooked mixed‐population trials that included proximal humeral fractures as a subgroup.
Additionally, while our search was comprehensive, our last formal searches of the main databases occurred in September 2020. Given this, we conducted a top‐up search at editorial review, on 9 November 2021. As before, two review authors (JE and HH) independently screened the search results and obtained full reports before study selection, again conducted independently of each other. We added the references of the 13 studies for which results had now been reported and the six newly registered trials to 'studies awaiting classification', and completed the characteristics table for each trial. In order to keep these trials distinct from the other studies awaiting assessment, we prefixed the study IDs with Z‐TUp for the studies with new results and Z‐TUpx for new ongoing studies, and, where possible, made clear that the results of the top‐up search were reported separately in Appendix 3, and not within the main results. We assessed each of the 13 studies in terms of their readiness for inclusion into the review. We note that none of these trials compared the key comparison of surgical versus non‐surgical treatment. We found that the two studies reported only in conference abstracts would not have been included at all (Z‐TUp Borda 2019; Z‐TUp Hachem 2021). Only three studies appeared to present a sufficiently full account that they would not have prompted a return to authors for clarification, especially relating to incomplete or inconsistent data, should we have processed these in our usual way (Z‐TUp Kröger 2021; Z‐TUp Monticone 2021; Z‐TUp Vijan 2020). All three small trials each tested a new comparison. Additionally, the populations of both Z‐TUp Kröger 2021 and Z‐TUp Monticone 2021 were younger and distinctly different than those of the rest of the review; and Z‐TUp Vijan 2020 was a quasi‐RCT reporting limited outcomes. Although the fact that there are 11 studies for which final data are available which have not yet been incorporated is a source of potential bias, we consider it is inappropriate to speculate on the impact of these data on the findings and conclusions of our review. Nonetheless, we hope that the account of our top‐up search presented in Appendix 3 provides a helpful summary of what has become available since September 2020.
As in all versions of this review, we used systematic processes throughout, including contacting trial investigators for clarification and missing data. All versions have been protocol driven, with an emphasis on setting out important changes to protocol before starting on an update, including those commensurate with evolving methodology, and listing these in Differences between protocol and review. Mindful of the problems of selective outcome reporting in reviews as well as in trials, we set out the outcomes for presentation in the summary of findings tables beforehand in this update. One key source of discussion amongst the review authors on this was the timing of outcome measurement (three versus six months) for our newly included outcome of short‐term function. Although we considered that functional outcome after three months may have some relevance for non‐surgical comparisons, where there are less serious fractures, we considered that it was too early for comparisons involving surgical intervention in terms of the timing of recovery, and thus, selected six months for these comparisons. This decision only had implications for the surgical versus non‐surgical treatment comparison, and a sensitivity analysis did not throw up any concerns (Analysis 6.3).
The decision to pool data from clearly clinically heterogeneous trials ‐ in particular, the surgical treatment versus non‐surgical treatment comparison ‐ inevitably presents a dilemma. However, we kept the overall question in mind and carefully considered the applicability of the results (see above section).
In assessing the clinical importance of continuous outcome scores, such as DASH and OSS, we set out what we would consider were the MCIDs beforehand. While this prespecification is good practice, the use of single‐value MCIDs as a cut‐off should be viewed as a best estimate. Considerations of measurement error for scoring systems are also important, as examined for four shoulder scoring scales for shoulder problems in Van Kempen 2013. This found the smallest detectable changes were 2.8 points for the SST, 16.3 points for the DASH, 17.1 points for the QuickDASH, and 6.0 points for the OSS. Thus to show a relevant change that is not due to measurement error, the change scores or mean differences values would need to be higher than these. These are again estimates and, while noting they are higher than the MCIDs we selected for these three scores, we did not adjust our criteria.
Finally, as we were not able to conduct sensitivity analysis related to missing data, the impact of missing data on the robustness of the results is still unclear.
Agreements and disagreements with other studies or reviews
In this section, we first discuss 18 systematic reviews published since 2015 and then, as per guidance in Aluko 2021, we present our brief economic commentary relating to the surgical versus non‐surgical treatment comparison.
Other systematic reviews
Given the broad scope of our review and the considerable increase in the number of systematic reviews of interventions (usually restricted to single comparisons) in this area published since 2015, we took a decision to select clearly systematic as opposed to narrative reviews. Further, we restricted our discussion to those found via our search that are published in English language journals, for which (with one exception) we could obtain a copy of the full‐text article. With the exception of Navarro 2018, which reported a comprehensive health technology assessment and had a broader remit, the systematic reviews of interventions tested in primary studies covered either a single comparison or a subset of interventions. We present the 18 reviews in Appendix 5, which lists the search date, the types of studies included, presents quoted summaries of the findings and conclusions usually extracted from the published abstract, and a commentary on each review, mainly relating to the currency of the search and new or missing RCTs. Of the 18 reviews, an updated review examined the effects of exercise in people with select upper‐limb fractures, including proximal humeral fractures (Bruder 2017); five reviews primarily compared surgical versus non‐surgical treatment (Beks 2018; Launonen 2015a; Navarro 2018; Sabharwal 2016b; Xie 2016); two reviews were network meta‐analyses that included non‐surgical treatment, ORIF (locking plate), hemiarthroplasty and RTSA (Du 2017; Orman 2020); four reviews compared locking plates versus intramedullary nailing (Li 2018; Shi 2019; Sun 2018; Wang 2015); two reviews compared RTSA versus hemiarthroplasty (Austin 2019; Wang 2016); one review compared the deltoid‐split approach versus the deltopectoral approach for plate fixation (Xie 2019); two reviews compared minimally invasive plate osteosynthesis versus open plating (Li 2019; Zang 2018); and one review compared acute RTSA for fracture and delayed RTSA for fracture sequelae (Torchia 2019). The comparison in the final review falls outside the scope of our review, but nonetheless provides some reassurance that outcome after secondary RTSA is not markedly worse. In considering the messages from our checking of these reviews, we concluded that our more up‐to‐date search, that generally included further RCTs, and our comprehensive scope meant that this updated review continues to serve a unique role, by systematically identifying, appraising and interpreting the evidence from RCTs (and quasi‐RCTs) for all key comparisons for treating proximal humeral fractures in adults.
The most popular topic covered in reviews was surgical versus non‐surgical treatment, a finding reinforced in Fang 2019, which identified 10 meta‐analyses on this topic. Notably, all five reviews found the overall evidence supported non‐surgical treatment for the majority of displaced fractures in older adults but, exceptionally, based on a flawed subgroup analysis, Sabharwal 2016b concluded they had demonstrated that "differences in the type of fracture and surgical treatment result in outcomes that are distinct from those generated from analysis of all types of fracture and surgical treatments grouped together". A letter from one of us and colleagues (Handoll 2016b), which was initially published separately but is now subsumed together with the authors' response into supplementary files for Sabharwal 2016b, points out the major problems with the methods and conclusions of this review. Subgroup analyses in ProFHER 2015 by fracture type (one‐ and two‐part versus three‐ and four‐part) did not find a difference in treatment effect (patient‐reported function measured via the Oxford Shoulder Score). Moreover, individually neither Launonen 2019a (two‐part fractures) nor Lopiz 2019 (four‐part fractures), both published subsequently to Sabharwal 2016b, found a clinically important difference in shoulder function (Analysis 6.1). These observations again support our pooling of data from the 10 clinically heterogeneous trials testing this comparison.
Although Slobogean 2015 is dated, the key findings of their scoping review of the existing proximal humeral fracture literature reporting primary research up to 30 October 2012, are likely to apply and mirror some of our findings. Firstly, though far more RCTs are being conducted, published RCTs are still very much in the minority of the literature (33 (3.1%) out of 1051 studies in Slobogean 2015). Secondly, as observed by Slobogean 2015, the numbers of studies focusing on surgical treatment (708 (67.4%)) vastly outweigh those on either non‐surgical management (45 (4.3%)) or rehabilitation (17 (1.6%)). Notably, the focus on surgery is dominant in this review too, and continues in this update where 11 (950 participants) of the 16 new trials (1247 participants) compared different methods of surgery. As observed by Slobogean 2015, the deficiency in the research literature on the "outcomes of non‐operative management" is "a noticeable weakness given that the overwhelming majority of proximal humerus fractures are treated without surgery". This once again is reflected in our suggestions for future research (Implications for research).
Brief economic commentary (BEC)
To supplement this main comparison, we sought economic evaluations of surgical and non‐surgical treatments for adults with a proximal humeral fracture. We identified eight potentially eligible studies from the search carried out on 12 November 2019. These comprised three cost analysis (Rosas 2018; Sabharwal 2016a; Solomon 2016), three cost‐utility analysis (Corbacho 2016; Fjalestad 2010b; Nwachukwu 2016), and two cost‐effectiveness analysis studies (Osterhoff 2017; Thorsness 2018). See Appendix 6 for definitions of different types of economic evaluations. We included the three cost‐utility analyses because they are full economic evaluations which directly compared surgical with non‐surgical interventions in the treatment of proximal humeral fractures in adults. Corbacho 2016 and Fjalestad 2010b were trial‐based economic evaluations of ProFHER 2015 and Fjalestad 2010a; and Nwachukwu 2016 was a model‐based economic evaluation. We present commentaries on these three economic evaluations in Appendix 7, which also features an addendum that reports on the findings of an additional search on 20 May 2021.
Summary
Our search for the BEC, carried out on 12 November 2019, identified three full economic evaluations, all cost‐utility analyses (CUAs), that compared surgical with non‐surgical interventions. One trial‐related CUA found no significant differences in costs between the two groups (Fjalestad 2010b), whereas the other trial‐related CUA found surgery was more costly over two years (Corbacho 2016). The third study, a model‐based CUA, found non‐surgical intervention is more cost‐effective compared with surgical interventions; however, no cost was assigned to the non‐surgical intervention which resulted in a biased result (Nwachukwu 2016). It is clear that the three eligible economic evaluations are not directly comparable due to differences in methodology, time horizons and setting. Moreover, the results of the different studies are not consistent. Within the remit of the BEC criteria and methodology, we have not sought to determine what might be the potential reasons results differ between studies, nor have we conducted any critical appraisal. Consequently, we do not attempt to draw any firm or general conclusions regarding the cost‐effectiveness of non‐surgical with non‐surgical interventions as the main aims of BEC are to summarise the availability and principal findings of identified eligible economic evaluations.
Authors' conclusions
Implications for practice.
There is high‐ or moderate‐certainty evidence that, compared with non‐surgical treatment, surgery does not result in a better outcome at one and two years after injury for people with the large majority of displaced proximal humeral fractures. It may increase the need for subsequent surgery. The available evidence does not cover the treatment of two‐part tuberosity fractures and is insufficient to draw conclusions on fractures in younger adults (e.g. under 60 years), on high‐energy trauma, or for less common fractures, such as fracture dislocations and articular surface fractures.
There is insufficient evidence from randomised controlled trials to inform the choices between different non‐surgical interventions, different surgical interventions, or different rehabilitation interventions for these fractures.
Implications for research.
The increasing availability of high‐quality evidence, including from sufficiently powered multicentre randomised trials (Launonen 2019a; ProFHER 2015), is the key reason why this review can now inform on the use of surgery for the large majority of displaced fractures. There is a need for similar trials to help address other key treatment uncertainties, which should include questioning where there is increased use of surgery, such as for younger adults. Decisions on priority topics should also consider the coverage of the current evidence base, as well as the topics covered by the ongoing trials. Of particular note is the focus of research on reverse total shoulder arthroplasty (RTSA), with four ongoing trials comparing RTSA versus non‐surgical treatment, four comparing RTSA versus hemiarthroplasty, and one comparing RTSA versus non‐surgical treatment versus hemiarthroplasty. Only three of these ongoing trials have follow‐ups of over two years: one is checking for further surgery at five years, and two have final follow‐ups at 10 years. This should be viewed in the context that the long‐term consequences for patients and society of the increasing use of RTSA are unknown, especially for those patients with longer life expectancy. Further, revision surgery can be difficult and salvage procedures are few. This points to the need for the systematic and prospective collection of data, such as via topic‐specific registries, on revision surgery for this intervention.
Although the identification of priority topics requires input from others, including patients, we suggest that research should be focused primarily on optimising non‐surgical treatment. Where randomised trials are warranted, these should use standard and validated outcome measures, including patient‐reported measures of functional outcome and quality of life, and also assess resource implications. They should also meet the Consolidated Standards of Reporting Trials (CONSORT) criteria for design and reporting of non‐pharmacological studies (Boutron 2017), and the adequate reporting of interventions (Hoffmann 2014). Consideration should be given to enhancing the potential applicability of trial findings at the study design stage and, at completion, this aspect examined and reported.
With 26 comparisons, this Cochrane Review has become unwieldy and hard to keep up to date, including in the context of changing methodology and reporting standards. Rather than another update, an evaluation of the scope or scopes of reviews that cover the key comparisons in this area is needed. Anticipated benefits of this approach include the production of published protocols that meet current methodological requirements.
What's new
| Date | Event | Description |
|---|---|---|
| 8 June 2021 | New citation required and conclusions have changed | The changes to the conclusions reflected our re‐evaluation of the certainty of the evidence in the light of new evidence. Three new authors were added to the byline. |
| 8 June 2021 | New search has been performed | In this update, published in 2022, the following changes were made. 1. The subject specific context was updated, particularly in the Background and Discussion sections, and enhanced with additional insights relating to the cost burden of these fractures. 2. We added clarifications relating to our inclusion criteria, notably in relation to the explicit exclusion of pharmacological and biological interventions. 3. The full search was updated to September 2020. 4. We included 16 new trials (1247 participants): Biermann 2020; Carbone 2017; DelPhi 2020; Gracitelli 2016; Helfen 2020; Hengg 2019; HURA 2020; Jonsson 2020; Launonen 2019a; Lopiz 2019; Plath 2019; Ring 2019; Sohn 2017; Tousignant 2020; Zhang 2019; Ziegler 2019. These gave rise to seven new comparisons and augmented the evidence for five existing comparisons. 3. We explicitly specified the summary of findings table outcome measures in Methods and revised the two previous tables in the light of these revisions and new evidence. We also reframed the evidence in terms of certainty, rather than quality, and adjusted the GRADE ratings, especially in relation to imprecision, according to revised guidance. We prepared two further summary of findings tables based on an assessment of the importance of the comparisons and data availability. 6. We added a brief economic commentary relating to the surgical versus not‐surgical treatment comparison. 7. We added in citations to previous versions of the review and protocols. Several of these are no longer published but are available on request (https://bjmt.cochrane.org/). |
History
Protocol first published: Issue 1, 1996 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 30 October 2015 | New search has been performed | In this update, published in Issue 11, 2015, the following changes were made: 1. The full search was updated to November 2014. 2. Overall, 32 new studies were identified. Of these, eight were included, 12 were excluded, eight were placed in ongoing trials and four await classification. Two further reports were identified for two already included trials. Upon identification of a trial registration document and published protocol, one study previously awaiting classification was moved to ongoing. Published protocols were found for two ongoing trials; and additional information from updated trial registration documentation added in for seven ongoing trials. Additional information for one ongoing trial resulted in its transfer from ongoing to studies awaiting classification. 3. Quality of the evidence was assessed using GRADE; two 'Summary of findings' tables added and the Discussion revised and updated. 4. Changes made to the conclusions. |
| 30 October 2015 | New citation required and conclusions have changed | 1. Changed conclusions for the surgical versus non‐surgical intervention comparison. 2. Conclusions changed to accommodate findings of the new comparisons. 3. Change in authorship. |
| 22 October 2012 | New citation required and conclusions have changed | 1. Conclusions changed to accommodate findings of the new comparisons. 2. Change in authorship. |
| 22 October 2012 | New search has been performed | In this update, published in Issue 12, 2012, the following changes were made: 1. The full search was updated to January 2012, with other searches extended to June 2012. 2. Eighteen new studies were identified. Of these, seven were included, four were excluded, five were placed in ongoing studies and two await assessment. A new report was available for one already included trial, and contact with a trialist resulted in the exclusion of one study that had been previously listed as ongoing. In the absence of further information after attempts at contact, consideration of one former ongoing study and two studies formerly in 'Studies awaiting assessment' led to their exclusion. 3. Discussion revised and updated. 4. Changes made to the conclusions. |
| 1 November 2010 | New citation required and conclusions have changed | 1. Conclusions changed to accommodate findings of the new comparisons. 2. Change in authorship. |
| 1 November 2010 | New search has been performed | In this update, published in Issue 12, 2010, the following changes occurred: 1. The full search was updated to March 2010; with other searches extended to August 2010. 2. Sixteen new studies were identified, of which one was included, four were excluded, 10 were placed in ongoing trials and one awaits assessment. New reports or information resulted in the inclusion of three more trials and the exclusion of two studies that had been identified previously. 3. Review methods and formatting were updated. 4. Background and Discussion revised and updated. 5. Changes made to the conclusions. |
| 5 August 2008 | Amended | Converted to new review format. |
| 28 September 2007 | New search has been performed | The fourth update (Issue 2, 2007) included the following: 1. Trial search extended from May 2003 to September 2006. 2. Identification of six new studies: one of which was placed in 'Ongoing studies', one was excluded and the other four are in 'Studies awaiting assessment', pending further information or publication. 3. Various adjustments were made to text, tables and presentation of the analyses to conform to revised methodology and the Cochrane Style Guide: the 'Synopsis' was amended to a 'Plain language summary'; the 'Abstract' was shortened; the 'Objectives' were reworded; methodological quality scores of individual criteria are no longer summed; all totals were removed from the Analyses (Forest plots) and the number of Analyses were reduced by presenting similar outcome measures (e.g. complications) together from the same trial. There was no change to the conclusions of the review. |
| 12 August 2003 | New search has been performed | The third update (Issue 4, 2003) included the following: 1. Trial search extended from November 2001 to May 2003. 2. Inclusion of two new trials, one of which had been listed as ongoing. 3. Inclusion of two new ongoing trials. 4. Exclusion of four trials previously listed as ongoing. 5. One trial, previously in pending, was excluded. 6. Addition of limited findings from newly identified trial reports of an already included trial. 7. The conclusions of the review were slightly modified to include the possibility of immediate physiotherapy, without immobilisation, for some types of undisplaced fractures. |
| 8 May 2002 | Amended | The second update (Issue 3, 2002) included some changes to the Discussion in response to comments received from an external reviewer. |
| 15 February 2002 | New search has been performed | The first update (Issue 2, 2002) included the following: 1. Trial search extended from July 2000 to November 2001. 2. Inclusion of one new trial. 3. Inclusion of one new ongoing trial. 4. Exclusion of four trials previously listed as ongoing. 5. One trial excluded and another placed in pending. 6. Addition of material from a newly available epidemiological study and commentary on a newly available systematic review. There was no change to the conclusions of the review. |
Acknowledgements
We are grateful to Maria Clarke for running the search updates and deduplicating the search results.
We are grateful to the following trial investigators for providing information, including notice of trial publications: Jenna Bardsley, Jeroen Bransen, Stig Brorson, Ron Diercks, Tore Fjalestad, Per Olerud, Catherine Page, Johannes Plath, Darren Roffey and Carlos Torrens. We thank Yuan Chi for translating Li 2015.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
The acknowledgements for former versions of the review are presented in Appendix 8.
Editorial and peer‐reviewer contributions
The Cochrane Bone, Joint and Muscle Trauma (BJMT) Group supported the authors in the development of this Cochrane Review. None of the authors, four (SB, JE, HH, TT) of whom are members of the Cochrane BJMT Group and one (PA) of the Cochrane Incontinence Group, were involved in the editorial process or decision‐making for this review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Michael Brown, Michigan State University College of Human Medicine;
Managing Editors (selected peer reviewers, provided comments, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Lara Kahale and Helen Wakeford, Cochrane Editorial and Methods Department;
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Editorial and Methods Department;
Copy Editor (copy‐editing and production): Faith Armitage, Copy Edit Support team
Peer reviewers (provided comments and recommended an editorial decision): Jennifer Hilgart, Cochrane Editorial and Methods Department (methods review); Robin Featherstone, Cochrane Editorial and Methods Department (search review); Amin Sharifan, Department of Pharmaceutical Care, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran (consumer reviewer); and two additional clinical experts whose names were provided to the authors but who did not want to be acknowledged formally.
Appendices
Appendix 1. Search strategies January 2014 to September 2020
CENTRAL (CRS‐Web)
The CENTRAL search was run in two stages: the first search was run in October 2019 and a second search was run in September 2020.
Search 1
1 MESH DESCRIPTOR Shoulder Fractures EXPLODE ALL AND CENTRAL: TARGET(108) 2 MESH DESCRIPTOR Humeral Fractures EXPLODE ALL AND CENTRAL: TARGET(139) 3 MESH DESCRIPTOR Humerus EXPLODE ALL AND CENTRAL: TARGET (86) 4 (shoulder* or humer*): AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL: TARGET (13601) 5 (fract*): AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL: TARGET(53399) 6 #3 OR #4 (13634) 7 #1 OR #2 (230) 8 #5 AND #6 (993) 9 #7 OR #8 (1083) 10 01/10/2014_TO_01/10/2019:CRSCREADTED AND CENTRAL: TARGET (774360) 11 (#9 AND #10) (734)
Search 2
10 01/10/2019_TO_23/09/2020:CRSCREATED AND CENTRAL:TARGET (159816) 11 #9 AND #10 (200)
MEDLINE (Ovid Online)
The MEDLINE search was run in two stages: the first search was run in October 2019 and a second search was run in September 2020.
Search 1
1 Shoulder Fractures/ (3193) 2 Humeral Fractures/ (7333) 3 ((humer* or shoulder*) adj10 (fracture* or fixat*)).tw. (9928) 4 or/2‐3 (12426) 5 (proximal or neck*1 or sub?capital).tw. (352621) 6 and/4‐5 (3386) 7 or/1,6 (4758) 8 randomized controlled trial.pt. (490139) 9 controlled clinical trial.pt. (93281) 10 randomized.ab. (394169) 11 placebo.ab. (182420) 12 drug therapy.fs. (2143135) 13 randomly.ab. (272007) 14 trial.ab. (410508) 15 groups.ab. (1686833) 16 or/8‐15 (4165889) 17 exp animals/ not humans.sh. (4622931) 18 16 not 17 (3557351) 19 7 and 18 (613) 20 (201410* or 201411* or 201412* or 2015* or 2016* or 2017* or 2018* or 2019*).ed,dt. (4338808) 21 19 and 20 (265)
Search 2
20 (201910* or 201911* or 201912* or 2020*).ed,dt. (2018706) 21 19 and 20 (129)
Embase (Ovid Online)
The Embase search was run in two stages: the first search was run in October 2019 and a second search was run in September 2020.
Search 1
1 Humerus Fracture/ (8691) 2 ((humer* or shoulder*) adj10 (fract* or fixat*)).tw. (13051) 3 1 or 2 (15939) 4 (proximal or neck*1 or sub?capital).tw. (520431) 5 3 and 4 (4985) 6 Randomized controlled trial/ (571370) 7 Controlled clinical study/ (465674) 8 Random*.ti,ab. (1452635) 9 randomization/ (84464) 10 intermethod comparison/ (254908) 11 placebo.ti,ab. (290925) 12 (compare or compared or comparison).ti. (472398) 13 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. (1992940) 14 (open adj label).ti,ab. (74943) 15 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. (217938) 16 double blind procedure/ (164012) 17 parallel group*1.ti,ab. (24433) 18 (crossover or cross over).ti,ab. (98367) 19 ((assign* or match or matched or allocation) adj5 (alternate or group*1 or intervention*1 or patient*1 or subject*1 or participant*1)).ti,ab. (312904) 20 (assigned or allocated).ti,ab. (367022) 21 (controlled adj7 (study or design or trial)).ti,ab. (328700) 22 (volunteer or volunteers).ti,ab. (233122) 23 trial.ti. (277325) 24 or/6‐23 (4403091) 25 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5832735) 26 24 not 25 (3800078) 27 5 and 26 (1022) 28 (2014* or 2015* or 2016* or 2017* or 2018* or 2019*).dc,yr. (9569161) 29 27 and 28 (559)
Search 2
28 (2019* or 2020*).dc,yr. (3443991) 29 27 and 28 (208)
CINAHL (EBSCO)
The CINAHL search was run in October 2019.
S1 (MH "Shoulder Fractures") (990) S2 (MH "Humeral Fractures") (1,817) S3 (MH "Humerus/IN/SU") (715) S4 TX ( ( humer* or shoulder* ) ) AND TX ( ( fracture* or fixat* ) ) (6,319) S5 S2 or S3 or S4 (6,611) S6 TX proximal or neck or subcapital or sub‐capital (77,412) S7 S5 and S6 (1,769) S8 S1 or S7 (2,166) S9 MH randomized controlled trials (86,778) S10 MH double‐blind studies (42,531) S11 MH single‐blind studies (12,824) S12 MH random assignment (56,166) S13 MH pretest‐posttest design (38,901) S14 MH cluster sample (3,897) S15 TI (randomised OR randomized) (93,260) S16 AB (random*) (269,847) S17 TI (trial) (95,297) S18 MH (sample size) AND AB (assigned OR allocated OR control) (3,700) S19 MH (placebos) (11,452) S20 PT (randomized controlled trial) (86,754) S21 AB (control W5 group) (94,625) S22 MH (crossover design) OR MH (comparative studies) (236,134) S23 AB (cluster W3 RCT) (301) S24 MH animals+ (85,551) S25 MH (animal studies) (107,377) S26 TI (animal model*) (2,781) S27 S24 OR S25 OR S26 (185,184) S28 MH (human) (1,984,251) S29 S27 NOT S28 (163,310) S30 S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 (620,521) S31 S30 NOT S29 (593,461) S32 S8 AND S31 (271) S33 EM 2014 OR EM 2015 OR EM 2016 OR EM 2017 OR EM 2018 OR EM 2019 (2,219,736) S34 S32 AND S33 (158)
AMED (Ovid Online)
The AMED search was run in October 2019.
1 exp Shoulder/ (1386) 2 exp Humerus/ (158) 3 Fractures Bone/ (1422) 4 (fract* or break* or broken or ruptur*).tw. (11959) 5 1 or 2 (1517) 6 3 or 4 (11959) 7 5 and 6 (76) 8 ((humer* or shoulder*) adj10 (fracture* or fixat*)).tw. (151) 9 7 or 8 (167) 10 (proximal or neck*1 or sub?capital).tw. (6075) 11 9 and 10 (48) 12 (2014* or 2015* or 2016* or 2017* or 2018* or 2019*).up,yr. (58621) 13 11 and 12 (9)
Other searches
PEDro
The PEDro search was run in October 2019.
Simple search
1. proximal AND humer* (27 records)
2. neck AND humer* = (11 records)
Advanced search
3. Abstract and title: fracture*
Body part: upper arm, shoulder or shoulder girdle
Method: Clinical trial (48 records)
The Bone and Joint Journal Orthopaedic Proceedings
proximal humer* AND random*
limited to Orthopaedic Proceedings
limited to Nov 2014 to Oct 2019 (3 records)
WHO International Clinical Trials Registry Platform Search Portal 1. proximal and humer* (147 records)
2. neck and humer* (17 records)
ClinicalTrials.gov
(proximal OR neck) AND humerus | First posted from 11/01/2014 to 10/08/2019 (74 records) (proximal OR neck) AND humerus | First posted from 10/08/2019 to 09/23/2020 (15 records)
British Elbow and Shoulder Society
Annual meetings (2014 to 2019) published in Shoulder & Elbow. Searched "random" and "proximal" separately. (Three abstracts found)
British Trauma Society
For 2015, 2016, 2018, 2019 (preliminary programme only) searched "humer" and "random" separately. (No trials identified)
Appendix 2. Search strategies conducted on 12 November 2019 for the brief economic commentary
MEDLINE (OVID Online)
We used two different search strategies for the MEDLINE search. We combined the subject‐specific terms from the original search strategy with a filter for cost‐of‐illness (search 1) and for economic evaluation (search 2).
Search 1
1 Shoulder Fractures/ (3212) 2 Humeral Fractures/ (7364) 3 ((humer* or shoulder*) adj10 (fracture* or fixat*)).tw. (11705) 4 or/2‐3 (14207) 5 (proximal or neck*1 or sub?capital).tw. (399671) 6 and/4‐5 (4052) 7 or/1,6 (5426) 8 (cost? adj2 (illness or disease or sickness)).tw. (3790) 9 (burden? adj2 (illness or disease? or condition? or economic*)).tw. (34288) 10 ("quality‐adjusted life years" or "quality adjusted life years" or QALY?).tw. (11750) 11 Quality‐adjusted life years/ (11565) 12 "cost of illness"/ (25948) 13 Health expenditures/ (19399) 14 (out‐of‐pocket adj2 (payment? or expenditure? or cost? or spending or expense?)).tw. (4394) 15 (expenditure? adj3 (health or direct or indirect)).tw. (8326) 16 ((adjusted or quality‐adjusted) adj2 year?).tw. (19429) 17 or/8‐16 (103012) 18 7 and 17 (20)
Search 2
1 Shoulder Fractures/ (3212) 2 Humeral Fractures/ (7364) 3 ((humer* or shoulder*) adj10 (fracture* or fixat*)).tw. (11705) 4 or/2‐3 (14207) 5 (proximal or neck*1 or sub?capital).tw. (399671) 6 and/4‐5 (4052) 7 or/1,6 (5426) 8 Economics/ (27101) 9 exp "costs and cost analysis"/ (229952) 10 Economics, Dental/ (1908) 11 exp economics, hospital/ (23997) 12 Economics, Medical/ (9038) 13 Economics, Nursing/ (3995) 14 Economics, Pharmaceutical/ (2897) 15 (economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (751042) 16 (expenditure$ not energy).ti,ab. (28457) 17 value for money.ti,ab. (1619) 18 budget$.ti,ab. (28079) 19 or/8‐18 (896857) 20 ((energy or oxygen) adj cost).ti,ab. (3978) 21 (metabolic adj cost).ti,ab. (1349) 22 ((energy or oxygen) adj expenditure).ti,ab. (24174) 23 or/20‐22 (28542) 24 19 not 23 (890294) 25 letter.pt. (1050308) 26 editorial.pt. (508248) 27 historical article.pt. (355078) 28 or/25‐27 (1894601) 29 24 not 28 (855263) 30 exp animals/ not humans/ (4641968) 31 29 not 30 (800992) 32 bmj.jn. (78053) 33 "cochrane database of systematic reviews".jn. (15281) 34 health technology assessment winchester england.jn. (1282) 35 or/32‐34 (94616) 36 31 not 35 (794730) 37 limit 36 to yr="2014 ‐Current" (285727) 38 7 and 37 (58)
EMBASE (OVID Online)
We used two different search strategies for the Embase search. We combined the subject‐specific terms from the original search strategy with a filter for cost‐of‐illness (search 1) and for economic evaluation (search 2).
Search 1
1 Humerus Fracture/ (8539) 2 ((humer* or shoulder*) adj10 (fract* or fixat*)).tw. (12926) 3 1 or 2 (15767) 4 (proximal or neck*1 or sub?capital).tw. (520799) 5 3 and 4 (4934) 6 (cost? adj2 (illness or disease or sickness)).tw. (5796) 7 (burden? adj2 (illness or disease? or condition? or economic*)).tw. (54086) 8 ("quality‐adjusted life years" or "quality adjusted life years" or QALY?).tw. (20942) 9 Quality‐adjusted life years/ (24959) 10 "cost of illness"/ (18722) 11 exp "health care cost"/ (279524) 12 (out‐of‐pocket adj2 (payment? or expenditure? or cost? or spending or expense?)).tw. (6128) 13 (expenditure? adj3 (health or direct or indirect)).tw. (10593) 14 ((adjusted or quality‐adjusted) adj2 year?).tw. (28157) 15 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 (368802) 16 5 and 15 (54)
Search 2
1 Humerus Fracture/ (8539) 2 ((humer* or shoulder*) adj10 (fract* or fixat*)).tw. (12926) 3 1 or 2 (15767) 4 (proximal or neck*1 or sub?capital).tw. (520799) 5 3 and 4 (4934) 6 Health Economics/ (28160) 7 exp Economic Evaluation/ (293350) 8 exp Health Care Cost/ (279524) 9 pharmacoeconomics/ (7124) 10 6 or 7 or 8 or 9 (511509) 11 (econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (981741) 12 (expenditure$ not energy).ti,ab. (37293) 13 (value adj2 money).ti,ab. (2292) 14 budget$.ti,ab. (35620) 15 11 or 12 or 13 or 14 (1015176) 16 10 or 15 (1229319) 17 letter.pt. (1053036) 18 editorial.pt. (622127) 19 note.pt. (768426) 20 17 or 18 or 19 (2443589) 21 16 not 20 (1129985) 22 (metabolic adj cost).ti,ab. (1421) 23 ((energy or oxygen) adj cost).ti,ab. (3981) 24 ((energy or oxygen) adj expenditure).ti,ab. (30323) 25 22 or 23 or 24 (34685) 26 21 not 25 (1123053) 27 animal/ (1311897) 28 exp animal experiment/ (2324474) 29 nonhuman/ (5972745) 30 (rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (5164395) 31 27 or 28 or 29 or 30 (8177791) 32 exp human/ (19776059) 33 human experiment/ (469746) 34 32 or 33 (19777315) 35 31 not 34 (5931203) 36 26 not 35 (1018226) 37 0959‐8146.is. (52424) 38 (1469‐493X or 1366‐5278).is. (22358) 39 1756‐1833.en. (30651) 40 37 or 38 or 39 (95400) 41 36 not 40 (1011411) 42 conference abstract.pt. (3624952) 43 41 not 42 (821083) 44 limit 43 to yr="2010 ‐Current" (412472) 45 5 and 44 (91)
CINAHL (EBSCO)
For the CINAHL search we combined the subject‐specific terms from the original search strategy with a filter for economic evaluation (search 2).
Search 2 (only)
S1 (MH "Shoulder Fractures") (991) S2 (MH "Humeral Fractures") (1,825) S3 (MH "Humerus/IN/SU") (720) S4 TX ( ( humer* or shoulder* ) ) AND TX ( ( fracture* or fixat* ) ) (6,376) S5 S2 or S3 or S4 (6,668) S6 TX proximal or neck or subcapital or sub‐capital (78,291) S7 S5 and S6 (1,788) S8 S1 or S7 (2,185) S9 MH "Economics+" (765,134) S10 MH "Financial Management+" (60,025) S11 MH "Financial Support+" (475,033) S12 MH "Financing, Organized+" (141,115) S13 MH "Business+" (142,126) S14 S10 OR S11 OR S12 OR S13 (760,990) S15 S9 NOT S14 (95,472) S16 MH "Health Resource Allocation" (8,455) S17 MH "Health Resource Utilization" (16,602) S18 S16 OR S17 (24,594) S19 S15 OR S18 (112,467) S20 TI (cost or costs or economic* or pharmacoeconomic* or price* or pricing*) OR AB (cost or costs or economic* or pharmacoeconomic* or price* or pricing*) (201,884) S21 S19 OR S20 (270,169) S22 PT editorial (263,516) S23 PT letter (277,014) S24 PT commentary (264,477) S25 S22 OR S23 OR S24 (643,394) S26 S21 NOT S25 (252,710) S27 MH "Animal Studies" (108,366) S28 (ZT "doctoral dissertation") or (ZT "masters thesis") (24,901) S29 S26 NOT (S27 OR S28) (249,494) S30 PY 2009‐ (4,202,479) S31 S8 AND S29 AND S30 (50)
Appendix 3. Screening and selection results of the top‐up search conducted on 9 November 2021
For the top‐up search, we screened a total of 529 (post deduplication) records from the following databases up to 9 November 2021: CENTRAL (218), MEDLINE (169), Embase (277), WHO Trials Registry (187), and ClinicalTrials.gov (23).
After screening, we identified 13 trials (15 references) for which results are newly available, 6 new ongoing trials and 2 new references (an erratum and a paper exploring the external validity of trial results) for DelPhi 2020, an already included trial. We placed the study IDs of the first two groups in Studies awaiting classification. In order to distinguish the trials found in the top‐up search from the others found previously, and clump these together, we prefixed the first group, where there are results, with "Z‐TUp" and the second group of new ongoing trials with "Z‐TUpx ". We placed the two extra references for DelPhi 2020 in Additional references (Erratum DelPhi; Tallay 2021).
Trials with new results
The table inserted in this appendix is ordered by the 10 comparisons tested in the 13 trials with results reported since September 2020. Seven studies were newly identified and the other six trials are either listed as an ongoing study or awaiting classification in this review (Z‐TUp Alvarez 2020; Z‐TUp Boyer 2021; Z‐TUp Kröger 2021; Z‐TUp Laas 2021; Z‐TUp Martinez 2021; Z‐TUp Monticone 2021). The third column of the table presents brief details and comments on each trial in terms of data completeness and discrepancies. Of note is that four trials used quasi‐randomised methods for allocation (Z‐TUp Batar 2020; Z‐TUp Izquierdo‐Fernández 2021; Z‐TUp Laas 2021; Z‐TUp Vijan 2020). Additionally,two trials were reported inadequately in abstracts only (Z‐TUp Borda 2019; Z‐TUp Hachem 2021). The final column of the table gives an assessment of whether the trial is reported sufficiently well that a return to authors or other measures were not needed before we would proceed to data extraction and assessment of risk of bias. This assessment resulted in three trials, each testing a new comparison to those tested already in the review (Z‐TUp Kröger 2021; Z‐TUp Monticone 2021; Z‐TUp Vijan 2020).
| Study ID | Links with studies in review | Brief details and commentary | Ready for inclusion? |
| Methods of non‐surgical management (including rehabilitation) | |||
| Initial treatment, including immobilisation | |||
| Immobilisation in sling for one week versus three weeks | |||
| Z‐TUp Martinez 2021 | NCT03217344 (ongoing study) | RCT 143 participants Of 111; mean age 70 years Discrepancies between full report and abstract | No. Return to authors needed. |
| Continuing management (rehabilitation) after initial sling immobilisation | |||
| Pulsed electromagnetic field versus sham therapy | |||
| Z‐TUp Borda 2019 | New | RCT 62 participants, mean age 69 years Incompletely reported | No. Abstract only. Need to get full report. Return to authors needed. |
| Comparisons of different categories of surgical intervention | |||
| Locking plate versus locking nail | |||
| Z‐TUp Boyer 2021 | NCT01557413 (ongoing study) | RCT 99 participants Of 85, mean age 74 years Missing data for interim follow‐ups | No. Return to authors needed. |
| Z‐TUp Li 2020 | New | RCT?
37 participants, mean age 66 years Some contradictions |
No. Better translation required. Return to authors probably needed |
| Locking plate versus wire fixation | |||
| Z‐TUp Vijan 2020 | New | Quasi‐RCT 30 participants, mean age 52 years Limited outcomes | Yes. New comparison (different wiring method). |
| Reverse total shoulder arthroplasty versus hemiarthroplasty | |||
| Z‐TUp Alvarez 2020 | NCT02075476 (ongoing study) | RCT 40 participants; mean age 76 years Small discrepancies | No. Return to authors needed. |
| Z‐TUp Batar 2020 | New | Quasi‐RCT 80 participants Of 58 in analysis, mean age 71 years. Serious discrepancies | No. Return to authors needed. |
| Z‐TUp Laas 2021 | NTR3208 (study awaiting classification) | Multicentre RCT 33 participants, mean age 75 years Missing data including denominators | No. Return to authors needed. |
| Comparisons of different methods of performing, or types of, an intervention in the same category | |||
| Deltoid‐split approach versus deltopectoral approach for plate fixation | |||
| Z‐TUp Bhayana 2021 | New | Quasi‐RCT 84 participants, mean age 45 years Missing data | No. Return to authors needed. |
| Anterosuperior approach versus deltopectoral approach for reverse total surgery arthroplasty | |||
| Z‐TUp Izquierdo‐Fernández 2021 | New | Quasi‐RCT 40 participants Of 32, mean age 74 years Missing data | No. Return to authors needed. |
| Reverse total shoulder arthroplasty with anatomic stem angle 135 versus 155 degrees | |||
| Z‐TUp Hachem 2021 | New | RCT
78 participants
Of 40, mean age 74 years Interim analysis |
No. Abstract report of first 40 cases. |
| Continuing management (including rehabilitation) after surgical intervention | |||
| Supervised rehabilitation of job‐specific task‐orientated exercises and occupational therapy versus general physiotherapy | |||
| Z‐TUp Monticone 2021 | ISRCTN17996552 (ongoing study) | RCT 70 'working' participants, mean age 49 years | Yes. New comparison. |
| Robot‐assisted training plus conventional occupational and physical therapy versus conventional therapy only | |||
| Z‐TUp Kröger 2021 | Nerz 2017 (ongoing study) | Multicentre RCT 48 participants, mean age 55 years | Yes. New comparison. |
New ongoing trials
The comparisons tested, under test or to be tested in the six newly identified ongoing trials are listed below. Two trials appear twice in the following: Z‐TUpx NCT04651543 because its population will include both non‐surgically‐treated and surgically‐treated participants; and Z‐TUpx ISRCTN85422168, which has three intervention groups.
Methods of non‐surgical management (including rehabilitation)
X‐ray taken at one week versus not taken in non‐surgically‐treated participants in Z‐TUPx NCT04572022 (90 participants).
Mobile health rehabilitation video instruction module plus standard care versus standard care only in Z‐TUpx NCT04651543 (60 participants).
Surgical treatment versus non‐surgical treatment
Surgery involving reverse shoulder arthroplasty with a 155° inclination angle versus reverse shoulder arthroplasty with a 135° inclination angle versus non‐surgical treatment in Z‐TUpx ISRCTN85422168 (90 participants).
Comparisons of different categories of surgical intervention
Locking plate versus intramedullary nail in Z‐TUpx Song 2020 (64 participants).
Comparisons of different methods of performing an intervention in the same category
Deltopectoral versus antero‐superior approach for inserting reverse shoulder arthroplasty in Z‐TUPx NCT04405947 (100 participants).
Through the rotator cuff versus through the rotator cuff interval for inserting intramedullary nails in Z‐TUpx NCT04917536 (80 participants).
Reverse shoulder arthroplasty with a 155° inclination angle versus reverse shoulder arthroplasty with a 135° inclination angle in Z‐TUpx ISRCTN85422168 (60 participants).
Continuing management (including rehabilitation) after surgical intervention
Mobile health rehabilitation video instruction module plus standard care versus standard care only in Z‐TUpx NCT04651543 (60 participants).
Appendix 4. Commentary on applicability of comparisons not presented in summary of findings tables
Methods of non‐surgical management (including rehabilitation)
Aside from the comparison of early versus delayed mobilisation (covered in Overall completeness and applicability of evidence), there were six, with one exception, single‐trial comparisons relating to initial treatment, including immobilisation, or continuing management (rehabilitation). Two comparisons were new in this update.
Carbone 2017, conducted in Italy, compared early intensive mobilisation (10 sessions in two weeks) versus early less intensive mobilisation (10 sessions in five weeks) started one week after the fracture in 80 people with stable impacted osteoporotic proximal humeral fractures. All participants were scheduled 20 one‐hour sessions each, which is likely to be unattainable in many countries.
The Desault body bandage tested in Rommens 1993, which compared this with the Gilchrist arm sling, is rarely used in practice. The survey of practice carried out as part of ProFHER 2015 confirms this in the UK, where 'collar and cuff' slings, poly‐slings and more rarely broad‐arm slings are used (Handoll 2010).
The three trials that examined supervised versus home exercises, in two comparisons, were based in Sweden, and possible differences in conventional physiotherapy regimens within and between countries, then and now, need to be taken into account when considering the application of trial findings. If they work, self‐instruction and home‐based exercise programmes are attractive for patients and conserve health care resources. There is some evidence from a Cochrane Review on fall prevention that older people, if well instructed and with intensive support (regular phone calls etc.), can maintain a home‐based exercise programme (Gillespie 2003; Gillespie 2009). However, there will still be some patients with insufficient understanding or motivation to perform the required exercises.
Bringing treatment into the digital age was Tousignant 2020 that compared telerehabilitation versus face‐to‐face rehabilitation. There is substantial investment and need for good internet connectivity to facilitate the intensive real‐time sessions. Such requirements and the ability of the patient to engage are major limitations to general applicability.
Livesley 1992 tested the use of pulsed electromagnetic high frequency energy (PHFE) for resolving pain associated with contracture of the joint capsule but provided no data. A Cochrane Review on electrotherapy modalities for adhesive capsulitis (frozen shoulder) found two small placebo‐control studies testing pulsed electromagnetic field therapy, one of which did not report outcome data (Page 2014). They concluded that, based on very low‐certainty evidence, they were uncertain whether this intervention is more or less effective than placebo.
Comparisons of different categories of surgical intervention
The variety of available implants, and auxiliary interventions, in the same category can limit the applicability and usefulness of trials comparing different categories of surgical intervention by comparisons of specific implants. Two of the comparisons (locking plate versus locking nail; reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty) are discussed in Overall completeness and applicability of evidence. The other four comparisons, each represented by a single study, and one of which is new this update, are commented on below.
The comparison by Smejkal 2011 of a locking plate versus minimally invasive fixation with distally inserted multiple intramedullary K‐wires (the Zifko method of minimally invasive fixation) may be of decreasing relevance to current practice in the context of the increased focus on locking nails.
The trial comparing hemiarthroplasty versus open reduction and locking plate fixation was too small to inform practice (Cai 2012). The absence of intraoperative conversions for the open reduction and internal fixation (ORIF) group to hemiarthroplasty or early failures are notable for a series of 13 displaced four‐part fractures and fracture‐dislocations, and may indicate differences in assessing and dealing with problematic or failed fixation in this centre compared with other centres.
Hoellen 1997, a flawed trial with only one‐year follow‐up, considered only one of several shoulder prostheses now available (the prosthesis was cemented in place) in their comparison with tension band wiring.
Reverse total shoulder arthroplasty (RTSA) versus locking plate fixation was tested in DelPhi 2020 for older participants, aged over 65, with severely displaced fractures. Although the displacement criteria (> 45 degrees valgus or > 30 degrees varus in a true anteroposterior projection, > 45 degrees angulation in a scapular Y projection with the arm in neutral rotation, or > 50% displacement of the humeral head against the metaphysis) for inclusion were likely to have some comparability with those of Neer, the fractures were categorised using the OA system and were not described using the Neer system. The trial population and the multicentre nature of this trial conduced in Norway enhance its applicability. Although only the two‐year follow‐up is available at present; the five‐year follow‐up results are pending.
Comparisons of different methods of performing an intervention in the same category
Thirteen trials tested one of 10 comparisons of different types or methods in the same category; five were new to this update.
Three trials, Buecking 2014, HURA 2020 and Sohn 2017, compared the deltoid‐split approach with minimal invasive plate osteofixation (MIPO) versus the deltopectoral approach for plate fixation in 312 people with Neer two‐, three‐ or four‐part fractures. In Handoll 2015b, we described the comparison in Buecking 2014 as the deltoid‐split approach versus the deltopectoral approach for plate fixation; however, in further consideration of actual interventions prompted by the inclusion of HURA 2020 and Sohn 2017, we noted the MIPO aspect applied to the deltoid‐split group of all three trials. The trial report of HURA 2020 also framed the question in terms of the approach but at trial registration, the emphasis was on the "lateral minimally invasive approach" in the report‐described deltoid‐split group. In Sohn 2017, which was framed as a MIPO versus ORIF trial, the differences in approach (deltoid‐split versus deltopectoral) were subsidiary. We consider that composite comparison helps to bring out the characteristics of the interventions being compared in these three trials. As pointed out previously, Buecking 2014 had two notable limitations in terms of external validity. These are the absence of criteria for excluding patients for whom hemiarthroplasty was planned, and the inappropriate interpretation of the Lawson quality‐of‐life score. Sohn 2017 was also limited in terms of external validity, with no details on postop care or rehabilitation and inadequate details on outcome assessment.
The two trials comparing 'polyaxial' (where surgeons had greater control in their positioning of screws into the bone) versus 'monoaxial' locking plate fixation found no difference between the two methods (Ockert 2010; Voigt 2011). With no report of functional outcome, Ockert 2010 contributed relatively little to this question. Voigt 2011, which was a stronger trial but still insufficient to be conclusive, pointed out that the "majority of surgeons chose the same screw directions for the polyaxial screws as already exist in the fixed angle plate". In their 2014 publication, Ockert 2010 also found that polyaxial screws were placed similarly to monoaxial screws. Of note also is the differences in the types of screws in the two implants in Voigt 2011, which could in some respects alter the question.
Ziegler 2019, which compared the CFR‐PEEK plate (made of polyetheretherketone reinforced with carbon fibres) versus the titanium PHILOS plate did not describe their study population aside from "requiring surgery", and additionally, follow‐up was limited to six months. This was a study funded by the manufacturer of the CFR‐PEEK plate and thus this comparison awaits independent assessment.
Biermann 2020 tested the use of glenohumeral joint lavage after PHILOS locking plate fixation. Their hypothesis was that glenohumeral joint lavage would reduce the risk of post‐traumatic arthrofibrosis leading to a stiff shoulder and thus might improve range of motion and shoulder function. Although the evidence of no difference from lavage was rated very low certainty, we suggest that rather than more invasive procedures, the investigation of post‐surgical rehabilitation is likely to be more fruitful.
While Zhang 2011 did not provide conclusive evidence of clinical benefit of the enhanced stabilisation of this commonly used plate, the direction of effect is consistent with the theoretical advantages of medial support screws.
Zhang 2019 tested the use of allogeneic bone grafts in combination with locking plates but provided an insufficient description of trial inclusion criteria and its overall follow‐up was too short at three months. For a more recent trial, it is unusual in its use of the dated and non‐validated Neer shoulder function score and, moreover, reporting this outcome at one month only.
Cement augmentation of the screw tips for locking plate fixation was tested by Hengg 2019, a multicentre and international trial that was terminated early and substantially underpowered. Of interest is the speculation by the authors of an unexpectedly low mechanical failure rate at six weeks that this reflected good surgical techniques but also, perhaps, a Hawthorne effect in that the surgeons may have paid more attention to surgical details. We are unsure about the latter and consider that six weeks is too early to ascertain the full extent of mechanical failure. Additionally, as detailed in the Notes section of the Characteristics of included studies table entry for this trial, there were 14 protocol violations and an additional three participants from the augmented group were crossed over to the control group due to positive leakage tests. The need for a leakage test would need to be considered when using this intervention.
In their comparison of the MultiLoc Proximal Humeral Nail (MPHN) versus the Polarus nail, Lopiz 2014 found the newer "straight" nail (the MPHN) resulted in fewer adverse events (screw loosening, impingement, rotator cuff symptoms) than the "curved" Polarus nail. This is plausible given the different design features of the MPHN that attempt to avoid the various problems, including impingement, that have been identified when using the Polarus nail. However, some consideration is also required of the rather high incidence of adverse events for the Polarus nail and the general inadequacies of tests for rotator cuff symptoms (Hanchard 2013).
Fialka 2008 compared two shoulder prostheses but although the authors ascribed the different functional outcomes to tuberosity fixation, it is possible that other design differences may also affect outcome.
The study population of Soliman 2013, which compared tenodesis of the long head of the biceps (LHB) versus LHB tendon left intact in people undergoing hemiarthroplasty, was exceptional in being younger (aged 45 to 60 years) than all other trial populations in this review and younger than the population for whom hemiarthroplasty is more typically used. Although the inclusion criteria included more severe injuries, such as head‐splitting fractures, Soliman 2013 provided insufficient criteria on which to judge participant suitability for hemiarthroplasty.
Continuing management (including rehabilitation) after surgical intervention
The need for and duration of immobilisation before commencing physiotherapy after surgical treatment is likely to depend on the type of surgery and other factors, such as bone quality. There is a tension between starting mobilisation to start restoring function and potentially reduce the risks of 'immobilisation' such as shoulder stiffness, and avoiding destabilisation of the fracture fixation after internal fixation or tuberosity fixation after hemiarthroplasty. However, currently the evidence, limited to that from two small heterogeneous trials, Agorastides 2007 and Wirbel 1999, is insufficient to inform on this issue.
Appendix 5. Summaries of other systematic reviews on interventions for treating proximal humeral fractures
| Systematic review on the effects of exercise in people with upper‐limb fractures | |||||
| Review ID | Search date | Studies | Findings | Conclusion | Comment |
| Bruder 2017 | January 2011 and July 2016 Updated review for Bruder 2011 |
22 RCTs (1299 participants with an upper‐limb fracture); 6 RCTs with proximal humeral fracture: Agorastides 2007; Bertoft 1984; Hodgson 2003a; Lefevre‐Colau 2007; Lundberg 1979; Revay 1992 |
"There was insufficient evidence from 13 trials to support or refute the effectiveness of home exercise therapy compared with therapist‐supervised exercise or therapy that included exercise following distal radius or proximal humeral fractures. There was insufficient evidence from three trials to support or refute the effectiveness of exercise therapy compared with advice/no exercise intervention following distal radius fracture. There was moderate evidence from five trials (one examining distal radius fracture, one radial head fracture, and three proximal humeral fracture) to support commencing exercise early and reducing immobilisation in improving activity during upper limb rehabilitation compared with delayed exercise and mobilisation... Less than 40% of included trials reported adequate exercise program descriptions to allow replication according to the TIDieR checklist." | "There is emerging evidence that current prescribed exercise regimens may not be effective in reducing impairments and improving activity following an upper limb fracture. Starting exercise early combined with a shorter immobilisation period is more effective than starting exercise after a longer immobilisation period." | Separate subgroup analysis for proximal humeral fractures.
The study questions were recast in terms of exercise and thus different from comparisons in our review; we also separated primary non‐surgical treatment / rehabilitation and post‐surgical rehabilitation. Out‐of‐date search. Missing RCTs for early versus delayed mobilisation: Kristiansen 1989; Ring 2019; Torrens 2012 |
| Systematic reviews of surgical versus non‐surgical treatment (main focus) | |||||
| Review ID | Search date | Studies | Findings (data not included) | Conclusion | Comment |
| Beks 2018 | September 2017 | 22 studies (1743 participants): 7 RCTs: Boons 2012; Fjalestad 2012; Olerud 2011a; Olerud 2011b; ProFHER 2015; Stableforth 1984; Zyto 1997, and 15 observational studies |
"There was no difference in functional outcome between operative and nonoperative treatment, ... . Major reinterventions occurred more often in the operative group. Pooled effects of RCTs were similar to pooled effects of observational studies for all outcome measures." | "We recommend nonoperative treatment for the average elderly patient (aged > 65 years) with a displaced proximal humeral fracture. Pooled effects of observational studies were similar to those of RCTs, and including observational studies led to more generalizable conclusions." | Separate analysis for different study designs Out‐of‐date search Missing new RCTs: Launonen 2019a; Lopiz 2019 Also missing old RCT: Kristiansen 1988 |
| Launonen 2015a | April 2014 | 9 studies (409 participants): 8 RCTs Boons 2012; Fjalestad 2010a (separate publications counted twice); Fialka 2008; Olerud 2011a; Olerud 2011b; Voigt 2011; Zyto 1997; and one controlled clinical trial (Carbone 2012) | "No statistically significant differences were found between nonoperative treatment and operative treatment with a locking plate for any disability, for quality‐of‐life score, or for pain, in patients with 3‐ or 4‐part fractures. In 4‐part fractures, 2 trials found similar shoulder function between hemiarthroplasty and nonoperative treatment. 1 trial found slightly better health‐related quality of life (higher EQ‐5D scores) at 2‐year follow‐up after hemiarthroplasty. Complications were common in the operative treatment groups (10–29%)." | "Nonoperative treatment over locking plate systems and tension banding is weakly supported. 2 trials provided weak to moderate evidence that for 4‐part fractures, shoulder function is not better with hemiarthroplasty than with nonoperative treatment. 1 of the trials provided limited evidence that health‐related quality of life may be better at 2‐year follow‐up after hemiarthroplasty. There is a high risk of complications after operative treatment." | No meta‐analysis.
Contradictory regarding scope and inclusion: included trials comparing different surgical interventions (e.g. Fialka 2008).
Out‐of‐date search Missing RCTs: Launonen 2019a; Lopiz 2019; ProFHER 2015; Stableforth 1984 Also missing old RCT: Kristiansen 1988 |
| Navarro 2018 | December 2016 | 18 RCTs and 21 non‐randomised studies on humerus fractures. RCTs for proximal fractures: Agorastides 2007; Boons 2012 Buecking 2014; Chen 2016 (in our studies awaiting assessment: SAA); Fialka 2008; Fjalestad 2010a (2012 and 2014 papers treated as if separate); Gracitelli 2016; Liu 2011 (in our SAA); Lopiz 2014; Olerud 2011a; Olerud 2011b; ProFHER 2015); Sebastiá‐Forcada 2014; Voigt 2011; Zhang 2011; Zyto 1997 |
"For hemiarthroplasty (HA) and non‐operative treatment, there was no clinically important difference for moderately displaced PHF at one‐year follow‐up regarding patient rated outcomes, (...). The intervention cost for HA was at least USD 5500 higher than non‐surgical treatment. The trend in Sweden is that surgical treatment of PHF is increasing. When functional outcome of percutaneous fixation/plate fixation/prosthesis surgery and non‐surgical treatment was compared for PHF there were no clinically relevant differences, (...). There was not enough data for interpretation of quality of life or complications. Evidence was scarce regarding comparisons of different surgical options for humerus fracture treatment." | "There is moderate/low certainty of evidence that surgical treatment of moderately displaced PHF in elderly patients has not been proven to be superior to less costly non‐surgical treatment options. Further research of humerus fractures is likely to have an important impact." | "The objective of this Health Technology Assessment was to evaluate effectiveness, complications and cost‐effectiveness of surgical or non‐surgical treatment for proximal, diaphyseal or distal fractures of the humerus in elderly patients." Just the surgical versus non‐surgical treatment comparison was considered to have enough evidence. Out‐of‐date search Missing new RCTs: Launonen 2019a and Lopiz 2019 Also missing old RCTs: Kristiansen 1988 and Stableforth 1984 |
| Sabharwal 2016b | 1 May 2015 | 7 RCTs (528 participants): Boons 2012; Fjalestad 2010a; Olerud 2011a; Olerud 2011b; ProFHER 2015; Stableforth 1984; Zyto 1997 | "The overall meta‐analysis found that there was no difference in clinical outcomes. However, subgroup and sensitivity analyses found improved patient outcomes for more complex fractures managed surgically. Four‐part fractures that underwent surgery had improved long‐term health utility scores (...). They were also less likely to result in osteoarthritis, osteonecrosis and non/malunion (...). Another significant subgroup finding was that secondary surgery was more common for patients that underwent internal fixation compared with conservative management within the studies with predominantly three‐part fractures (...)." | "This meta‐analysis has demonstrated that differences in the type of fracture and surgical treatment result in outcomes that are distinct from those generated from analysis of all types of fracture and surgical treatments grouped together. This has important implications for clinical decision making and should highlight the need for future trials to adopt more specific inclusion criteria". | Over‐interpretation of subgroup analysis. Letter from Handoll et al in 2016 pointing out this and other major flaws of this review and authors' response are available in review supplementary materials (Handoll 2016b). Out‐of‐date search. Missing new RCTs: Launonen 2019a; Lopiz 2019 Also missing old RCT: Kristiansen 1988 |
| Xie 2016 | July 2015 | 7 RCTs (518 participants): Boons 2012; Fjalestad 2010a; Olerud 2011a; Olerud 2011b; ProFHER 2015; Stableforth 1984; Zyto 1997 |
"No statistical differences were found between operative and non‐operative treatment in CS [Constant] scores at 12 mo (months) [...] and 24 mo [...]. There are also no statistical differences between operative and non‐operative treatment in DASH scores at 12 mo [...] and 24 mo [...]. Statistical differences were found between operative and non‐operative treatment in total complication rates [...]. Statistical differences in EQ‐5D at 24 mo [...] were found between operative and non‐operative treatment but no statistical differences were found in ED‐5D at 12 mo [...], 15D at 12 mo [...] and 15D at 24 mo [...]" | "Operative treatments did not significantly improve the functional outcome and healthy‐related quality of life in elderly patients. Instead, Operative treatment for [complex proximal humeral fractures]CPHFs led to higher incidence of postoperative complications." | Out‐of‐date search. Missing new RCTs: Launonen 2019a; Lopiz 2019 Also missing old RCT: Kristiansen 1988 |
| Network meta‐analyses (NMAs) including non‐surgical treatment (NST), ORIF (locking plate), hemiarthroplasty and RTSA [RSA] (limited to 3‐part or 4‐part fractures) | |||||
| Review ID | Search date | Studies | Findings (data not included) | Conclusion | Comment |
| Du 2017 | July 2017 | 7 RCTs (347 participants): Boons 2012; Cai 2012; Fjalestad 2010a; Olerud 2011a; Olerud 2011b; Sebastiá‐Forcada 2014; Zyto 1997 | "The rank probability plot of Constant score showed that the RSA had significantly the highest Constant score and lower reoperation than other treatments. The other way around, the efficacy of ORIF was the poorest. The rank for the Constant score was: RSA, HA, nonoperation and ORIF. The rank for the reduction in total reoperation rates was: RSA, nonoperation, HA and ORIF." | "The statistical result suggested that RSA has become a beneficial choice to treat displaced 3‐ or 4‐part fracture in elderly patients, that might result in more favorable clinical outcomes and reduction of reoperation rates than other methods performed for the same indication. But the ORIF is the worst." | Over‐interpretation of evidence from just 31 participants with RSA.
Out‐of‐date search Key missing RCT within search date: ProFHER 2015 (pragmatic trial and thus does not readily fit into an NMA) Also other missing RCTs, e.g.: DelPhi 2020; Jonsson 2020; Launonen 2019a; Lopiz 2019 |
| Orman 2020 | September 2016 | 8 RCTs (364 participants): Boons 2012; Cai 2012; Chen 2016; Fjalestad 2010a; Olerud 2011a; Olerud 2011b; Sebastiá‐Forcada 2014; Zyto 1997 | "Non‐surgical treatment was associated with a lower rate of additional surgery and adverse events compared to open reduction internal fixation. Reverse total shoulder arthroplasty resulted in fewer adverse events and a better clinical outcome score than hemiarthroplasty. Non‐surgical treatment produced similar clinical scores, adverse event rates, and additional surgery rates to hemiarthroplasty and reverse total shoulder arthroplasty." | "Non‐surgical treatment results in fewer complications and additional surgeries compared to open reduction internal fixation. Preliminary data supports reverse total shoulder arthroplasty over hemiarthroplasty, but more evidence is needed to strengthen this conclusion." | Out‐of‐date search Key missing RCT within search date: ProFHER 2015 (pragmatic trial and thus does not readily fit into an NMA) Also other missing RCTs, e.g.: DelPhi 2020; Jonsson 2020; Launonen 2019a; Lopiz 2019 We placed Chen 2016 in Studies awaiting assessment as we had concerns over Methods. |
| Systematic reviews on locking plate versus intramedullary nail | |||||
| Review ID | Search date | Studies | Findings (data not included) | Conclusion | Comment |
| Li 2018 | December 2016 | 20 studies (1384 participants): 3 listed RCTs: Gracitelli 2016; Zhu 2011 and Tian 2016 (see Comment); and 17 retrospective studies | "Analyses showed that intramedullary nails were superior to locking plates in incision length, peri‐operative bleeding time, operation time and fracture healing time. However, there were no differences between treatments in Constant score or post‐operative complications." | "The intramedullary nail is superior to locking plate in reducing the total complication, intraoperative blood loss, operative time, postoperative fracture healing time and postoperative humeral head necrosis rate of PHF. Due to the limitations in this meta‐analysis, more large‐scale, multicenter, and rigorous designed RCTs should be conducted to confirm our findings." | Mixed study meta‐analysis Out‐of‐date search. Missing RCTs: Helfen 2020 and Plath 2019 Note: we did not identify Tian 2016 (60 participants) in our search; nor in our initial check of the reference list of this review. Based on the English abstract, this study seems to report mainly on short‐term perioperative outcomes and radiological outcomes. It also does not appear to contribute data to the meta‐analyses of the review. Hence, even if it is an RCT (see Comment for Shi 2019), it seems unlikely it would contribute much evidence for this comparison. |
| Shi 2019 | July 2018 | 38 "retrospective" studies (2699 participants) reported in the Abstract. However, 2 RCTs included and described in a table: Gracitelli 2016; Zhu 2011 |
"Meta‐analysis results show that the intramedullary nails in the treatment of proximal humeral fractures are superior to locking plates in terms of intraoperative blood loss, operative time, fracture healing time, postoperative complications, and postoperative infection. But there is no significance in constant, neck angle, VAS, external rotation, antexion, intorsion pronation, abduction, NEER, osteonecrosis, additional surgery, impingement syndrome, delayed union, screw penetration, and screw back‐out." | "The intramedullary nail is superior to locking plate in reducing the total complication, intraoperative blood loss, operative time, postoperative fracture healing time and postoperative humeral head necrosis rate of PHF. Due to the limitations in this meta‐analysis, more large‐scale, multicenter, and rigorous designed RCTs should be conducted to confirm our findings." | Inconsistently reported Mixed study meta‐analysis Out‐of‐date search Missing RCTs: Helfen 2020 and Plath 2019 Note: Tian 2016 was described as retrospective here. |
| Sun 2018 | April 2017 | 13 studies (958 participants): 3 RCTs: Gracitelli 2016; Smejkal 2011; Zhu 2011; and 10 comparative studies | "A significantly greater external rotation (...) and a significantly higher penetration rate (...) were observed in the locking plate group compared with the intramedullary nail group. Constant‐Murley scores, DASH scores and total complication rate were comparable between the two groups. Moreover, there were no significant differences in forward elevation, VAS scores, and other complications." | "Current evidence indicates that locking plates and intramedullary nails have similar performance in terms of the functional scores and total complication rate. No superior treatment was suggested between locking plates and intramedullary nails for displaced proximal humeral fractures." | Mixed study meta‐analysis Out‐of‐date search. Missing RCTs: Helfen 2020 and Plath 2019 We considered Smejkal 2011 was not a locking nail and made this a separate comparison. |
| Wang 2015 | October 2014 | 8 studies (615 fractures): 2 RCTs: Smejkal 2011; Zhu 2011; and 6 observational studies | "Similar Constant scores were observed between the locking plate and intramedullary nail both in randomized controlled trials (RCTs) (...) and observational studies (...). Only one RCT provided American Shoulder and Elbow Surgeons Standardized scores indicating that the locking plate was better than the intramedullary nail (...). The total complication rate did not specifically favor the locking plate or intramedullary nail both in the RCTs (...) and observational studies (...)." | "In the existing literature, limited evidence suggests that the locking plate and intramedullary nail are both valuable options for the treatment of proximal humeral fractures. Because of the observed heterogeneity and variance between the subgroups, more RCT are needed to be able to definitively recommend a locking plate or intramedullary nail for specific fracture patterns." | Separate analysis for different study designs.
Out‐of‐date search.
Missing RCTs: Gracitelli 2016, Helfen 2020 and Plath 2019 We considered Smejkal 2011 was not a locking nail and made this a separate comparison. |
| Systematic reviews on RTSA versus hemiarthroplasty | |||||
| Review ID | Search date | Studies | Findings | Conclusion | Comment |
| Austin 2019 | October 2017 | 17 studies: 1 RCT: Sebastiá‐Forcada 2014; and 16 comparative cohort studies 15 studies included in the meta‐analysis (913 participants) |
"We found that RSA is associated with improvements in forward flexion, clinical outcome scores [Constant and Dash etc pooled], and risk of reoperation, with no differences in external rotation, tuberosity healing, and deep infection rate." | "In conclusion, the results of our meta‐analysis suggest that RSA for the treatment of acute proximal humerus fractures in patients older than 65 years of age should be strongly considered as the first‐line arthroplasty option. Our results are only applicable for the short and medium term, and thus further work will be required to determine the long‐term outcomes of RSA for proximal humerus fractures." | Mixed study meta‐analysis. Out‐of‐date search. Missing RCT: Jonsson 2020 |
| Wang 2016 | December 2014 | 8 studies (581 participants): no RCTs, 7 were retrospective | "Compared with HA, RSA was associated with a lower rate of total complications, higher American Shoulder and Elbow Surgeons (ASES) score, more healed tuberosities and improved active forward elevation. Both treatments were comparable in term of revision surgeries, mortality, subjective satisfaction and active external rotation." | "The present evidence from this meta‐analysis suggested that RSA was a more advantaged method for the treatment of complex proximal humeral fractures. Clinical decision should be preferred to RSA on the condition that patients’ medical conditions are indicated." | Mixed study meta‐analysis, mostly retrospective studies. Out‐of‐date search. Missing RCTs: Jonsson 2020 and Sebastiá‐Forcada 2014 (published before their search date) |
| Systematic review on deltoid‐split approach versus deltopectoral approach for plate fixation | |||||
| Review ID | Search date | Studies | Findings | Conclusion | Comment |
| Xie 2019 | December 2017 | 6 studies (426 participants in the analyses): 3 RCTs: Buecking 2014; Martetschlager 2012 (see Comment); Zhao 2017 (see Comment); and 3 non‐random prospective comparative studies. | "The meta‐analysis showed that the DS group had a significantly low humeral head necrosis rate and short operation time. No significant difference was found in total complication rate, functional outcome, and other Perioperative parameters between DS and DP groups." | "The prospective evidence suggested that DS approach for proximal humerus fractures had less humeral head necrosis and short operation time than DP approach. Both DS and DP approach had similar results in functional outcomes, total complication, VAS, and hospital stay." | Mixed study meta‐analysis.
Out‐of‐date search.
Missing RCT: HURA 2020
We excluded 2 trials included in Xie 2019: Martetschlager 2012 wasn't an RCT and Zhao 2017 had major flaws. We considered that the less or minimal invasive feature of surgery in the deltoid‐split group changed the comparison, in which Sohn 2017 also fitted. |
| Systematic reviews on minimally invasive plate osteosynthesis versus open plating | |||||
| Review ID | Search date | Studies | Findings | Conclusion | Comment |
| Li 2019 | April 2019 | 16 studies (1050 participants): 2 RCTs: Sohn 2017; Zhao 2017 (see Comment); and 14 non‐RCTs | "According to the meta‐analysis, MIPO was superior to ORIF in operation time, blood loss, postoperative pain, fracture union time, and constant score. However, MIPO was associated with more exposure to radiation and axillary nerve injury. No significant differences were found in length of hospital stays and complication except for axillary nerve injury." | "The present evidence indicates that compared to ORIF, MIPO had advantages in functional outcomes, operation time, blood loss, postoperative pain, and fracture union time for the treatment of PHFs. However, the MIPO technique had a higher rate of axillary nerve injury and longer radiation time compared to ORIF." | Mixed study meta‐analysis.
Out‐of‐date search.
We excluded Zhao 2017 as it had major flaws. We note that the deltoid‐split approach was used in the MIPO group and the deltopectoral approach was used in the ORIF group of Sohn 2017. We reflected this in a composite comparison that also included Buecking 2014 and HURA 2020. |
| Zang 2018 | Not known | 7 studies (unknown number of participants). I RCT found in reference list: Sohn 2017 | "Meta‐analysis showed the significant differences in terms of blood loss, operative time, length of hospital stays and constant score between two groups. No significant differences were found in time to union, the union rate and complications." | "Minimally invasive plate osteosynthesis in proximal humeral fractures provided significantly shorter operative times, length of hospital stays, less blood loss and better clinical outcomes without increasing complications." | Article not obtained as behind very expensive paywall. However, reference list was available. Mixed study meta‐analysis. Out‐of‐date search. |
| Acute RTSA for fracture and delayed RTSA for fracture sequelae | |||||
| Review ID | Search date | Studies | Findings | Conclusion | Comment |
| Torchia 2019 | January 2018 | 16 studies (322 participants), 4 comparative (46 participants) whereas 12 were case series (276 participants). | "Among studies directly comparing acute versus delayed RTSA, no differences in forward flexion (P = .72), clinical outcome scores (P = .78), or all‐cause reoperation (P = .92) were found between the 2 groups. Patients undergoing delayed RTSA achieved 6° more external rotation than those undergoing acute RTSA; this difference was significant (P = .01)." | "Given the risks associated with surgery in the elderly population, consideration may be given to an initial trial of nonoperative treatment in these patients, saving RTSA for those in whom nonoperative treatment fails without compromising the ultimate outcome." | Preliminary data only but reassuring. This comparison is not in the scope of our review, which focuses on acute treatment. |
Appendix 6. Glossary of terms for health economics and systematic review (source: CCEMG website)
The following definitions of terms are extracted from the Campbell & Cochrane Economics Methods Group (CCEMG) website: methods.cochrane.org/economics/sites/methods.cochrane.org.economics/files/public/uploads/ccemg_website_glossary.pdf
Cost benefit analysis (CBA) is a form of economic evaluation whereby both costs and benefits of an intervention are measured in commensurate, normally monetary units to assess whether an intervention is worthwhile. A full or an ideal CBA (where all the benefits can be valued in monetary units and all alternative uses, opportunity costs, are incorporated) can be used to address the question of allocative efficiency. Benefits in a CBA are valued in monetary units usually by using stated preference (see willingness to pay, contingent valuation, discrete choice experiment or conjoint analysis) or revealed preference (see hedonic models/pricing, travel cost models, defensive behaviour and damage cost methods) approaches.
Cost consequence analysis (CCA) is an economic evaluation whereby an array of health and potentially other outcome measures are enumerated alongside costs. This is distinct from cost‐effectiveness analysis where there is a single summary (health) outcome measure. In cost consequence analysis, an overall valuation of the bundle of outcome measures is not attempted but rather left to the decision‐maker to choose which outcome measure suits the decision‐making context.
Cost‐effectiveness analysis (CEA) is a form of economic evaluation that is best suited to addressing questions of technical efficiency. Comparisons are limited to services or treatment options that produce the same type of benefit, which is valued strictly in one dimensional, natural unit.
Cost‐effectiveness ratio (CER) refers to the ratio of the difference between two programmes’ mean costs to the difference between two programmes’ mean effects. Cost‐effectiveness thresholds help a decision‐maker make judgements about the opportunity costs of an intervention. If an intervention has an incremental cost‐effectiveness ratio below a given threshold, it is assumed that resources can be reallocated from interventions that have an incremental cost‐effectiveness ratio above that threshold.
Cost minimisation analysis (CMA) is a special type of cost‐effectiveness analysis, which is possible only if it has been determined (or more often assumed) that there are no differences in benefits between the alternate interventions compared and thus the evaluation.
Cost utility analysis (CUA) is a variant of cost‐effectiveness analysis where the health outcome measure of interest is usually expressed as a quality adjusted life year, a single index that combines the length of life and a quality adjustment for less than perfect health (i.e. the utility score).
Incremental cost‐effectiveness ratio (ICER) is the ratio of the difference in costs between an intervention and a specified comparator to the difference in effectiveness between that intervention and the specified comparator. From the results of a cost‐effectiveness analysis, an incremental cost‐effectiveness ratio can be calculated that depicts the extra cost per unit of the outcome obtained, in comparing one treatment option to another. In this case, a value judgement will be required to assess whether the extra unit of outcome is worthwhile (see cost‐benefit analysis).
Appendix 7. Brief economic commentary: commentaries on three economic evaluations
Economic evaluation 1
The cost‐utility analysis (CUA) carried out by Corbacho 2016 was from the perspective of the UK National Health Service (NHS) (health payer). It utilised data from ProFHER 2015, which recruited 250 adults, mean age 66 years, from NHS hospitals between September 2008 and April 2011. The costs and outcomes were expressed in the 2012 UK pound sterling (GBP) and quality‐adjusted life‐years (QALYs), respectively, at a discount of 3.5%. The costs and resources used included those of the staff involved in the treatment and rehabilitation, type of implant and disposables used and length of stay. The results of ProFHER 2015, which found no clinically important or statistically significant between‐group difference in the primary outcome at two years' follow‐up (Oxford Shoulder Score (0 to 48 best outcome): mean difference (MD) 0.75, 95% confidence interval (CI) ‐1.33 to 2.84) are presented above. Of the 109 participants allocated surgery who received primary surgery, 90 (82.6%) had locking plates, 10 (9.2%) had hemiarthroplasty, 4 (3.7%) had intramedullary nails and 5 (4.6%) had other types of surgery.
The mean operating time for surgery was 144 minutes and the mean length of stay was 3.8 days. The mean cost of surgery in the trial was GBP 3053 per participant. At three months, the mean costs of surgery were GBP 2767 while that for non‐surgery was GBP 694. The main cost drivers were costs of the surgery and follow‐up treatment for the surgical arm, while subsequent hospital admissions were the main cost driver for the non‐surgical arm. Participants in the surgical arm had higher outpatient visits but fewer inpatient admissions. The study also found that a high proportion of the cost in the surgical arm was incurred within the first three months.
The mean European Quality of Life‐5 Dimensions (EQ‐5D) score of surgical group participants at baseline was 0.43 (standard deviation (SD) = 0.37) while that of the non‐surgical group participants was 0.38 (SD = 0.37). There was little difference in the EQ‐5D score at the second year (0.67 for surgery versus 0.69 non‐surgery; Analysis 6.11). However, participants in the surgical group gained less QALY than those in the non‐surgical group with an incremental QALY of ‐0.066 (95% CI ‐0.186 to 0.054). The incremental costs over two years of surgery compared with non‐surgery was an average of GBP 1758 per participant (95% CI 1126 to 2389).
In both the base case and sensitivity analyses, surgery was not cost‐effective. Some limitations highlighted by the author include time horizon, which was considered too short (2 years) to cover potential functional deterioration and reduced quality of life from later complications. However, it was noted that most complications occurred in the first year of the surgery. Moreover, it was considered unlikely that there would be any significant difference in the QALYs beyond two years. This supposition was corroborated in a five‐year follow‐up of the trial where utility scores were collected at three, four and five years (Handoll 2017). Cost data were not collected as it was reported not to have any impact on the findings from the main trial. The results of extended follow‐up study found that the analyses of the utility values for the five‐year period were consistent with the main trial analyses, which had little differences in the utility scores between both groups at the end of the second year of treatment. Nevertheless, participants in the non‐surgical group had a marginally higher QALY gain with the QALY gained maintained over time when compared with the surgical group.
Corbacho 2016 concluded surgical treatment was not cost effective in treating the majority of displaced proximal humeral fractures because at a National Institute for Health and Care Excellence (NICE) willingness‐to‐pay threshold of GBP 20,000 per QALY gained, the probability of surgery being cost‐effective was less than 10%.
Economic evaluation 2
The CUA reported in Fjalestad 2010b was conducted from a Norwegian societal perspective. It used data from Fjalestad 2010a, which recruited 50 participants, mean age 73 years, between May 2003 and May 2008. The surgery used was open reduction and internal fixation with an interlocking plate. The outcome measures were costs and quality of life (QOL) captured over a 12‐month time horizon. The cost was measured in 2005 Norwegian kroner (NOK) but expressed in euros (EUR) while outcome was expressed in QALYs. The health utility economic instrument (15D) was used in estimating utility values.
Costs were determined through hospital accounts, patient interviews and a fee schedule. The direct costs included operating room procedures, personnel (operating surgeon, assistant surgeon, surgery nurse and anaesthesia nurse) according to wage rates, implant costs, operating room sterile‐covering equipment, radiographic services, hospital and rehabilitation stay, transport costs, general practitioner and household task assistance. The indirect costs included productivity loss from sick leave, family use of time and transportation. The costs and outcomes were not discounted due to the short time horizon (12 months).
Fjalestad 2010b found no significant between‐group difference in the mean 15D score before the fracture: surgical group 0.877, non‐surgical group 0.830; MD 0.047, 95% CI ‐0.013 to 0.112, reported P = 0.123. There was also no significant between‐group difference at 12 months follow‐up (MD 0.022, 95% CI ‐0.038 to 0.081; reported P = 0.436). The 15D data at 3, 6 and 12 months' follow‐ups are presented in Analysis 6.13. There was no significant between‐group difference in the QALY gained at 12 months: 0.027, 95% CI ‐0.025 to 0.078; reported P = 0.417. The data provided for the individual groups, which probably excluded the two people who died in the surgery group, are presented in Analysis 6.13. Fjalestad 2010b reported using linear regression to account for the unbalanced baseline 15D score. This resulted in a QALY difference of 0.009 (95% CI = ‐0.025 to 0.042), that was reportedly “in favour of conservative treatment”.
The mean hospital stay was 8 days (excluding a participant who died after 61 days, but 10.1 days when included) for the surgical group and 5.5 days for the non‐surgical group. This resulted in mean total direct health care costs of EUR 10,367 for the surgical group and EUR 10,946 for the non‐surgical group (MD 597 euros, 95% CI 5116 euros to 1888 euros). When indirect costs were included, the mean total costs were EUR 23,953 for the surgical group and EUR 21,878 for the non‐surgical group (MD ‐2.075 euros, 95% CI 15,949 euros to 20,100 euros). Fjalestad 2010b found that the costs of surgical treatment were higher during the hospital stay (MD 1573 euros) but this was offset by the rehabilitation costs associated with non‐surgical treatment (EUR 2086). The resulting incremental cost‐effectiveness ratio (ICER) was EUR 230,556 per QALY gained from a societal perspective.
In the sensitivity analysis carried out, there was no significant between‐group difference in the costs or QALYs.
Although results from Fjalestad 2010b suggest that surgical and non‐surgical treatment had similar outcomes and costs, there were several limitations highlighted by the author. The study was not blinded, and the patient sample size was small. In addition, the authors suggested that the 12‐month follow‐up was too short to assess the full impact of the treatment differences, especially in relation to complications such as avascular necrosis that could develop subsequently. Hence, these short‐term results should be interpreted with care. Subsequently, Fjalestad 2010a reported a two‐year follow‐up that found a slight increase between one and two years in quality of life according to 15D scores for both surgically‐ and conservatively‐treated participants, with no significant between‐group difference at two years (Analysis 6.13). Although there were six extra cases of radiologically‐diagnosed avascular necrosis between one and two years, most remained asymptomatic (Fjalestad 2014a).
Economic evaluation 3
Nwachukwu 2016, which was conducted from a United States' payer and hospitals perspective, confined its scope to surgery comprising either hemiarthroplasty (HA) or reverse total shoulder arthroplasty (RTSA), and non‐surgical treatment.
The cost utility analysis by Nwachukwu 2016 used the Markov model to compare non‐surgical treatment, HA, and RTSA for complex proximal humeral fractures in older people from the health care perspective (US payer and hospitals). Nwachukwu 2016 used data from published RCTs and followed a lifetime horizon. It included patients with complex fractures that require surgery but are not amenable to fixation. Nwachukwu 2016 assumed a base case of a 70‐year old patient with a complex fracture that could be treated surgically or non‐surgically.
The costs and outcomes were expressed in 2013 US dollars (USD) and QALYs, respectively, and discounted at 3%. The Markov model consisted of three treatment strategies with patients treated surgically assigned two health states: non‐surgical fracture management and death. Both HA and RTSA were assigned seven health states: (1) success after primary HA, (2) failure after primary HA, (3) success after revision HA, (4) failure after revision HA, (5) successful salvage RTSA for failed HA, (6) lifetime limited benefit RTSA, and (7) dead. For RTSA, the seven health states were: (1) success after primary RTSA, (2) failure after primary RTSA, (3) success after revision RTSA, (4) failure after revision RTSA, (5) success after repeated revision RTSA, (6) lifetime limited benefit RTSA, and (7) dead.
Probabilities were derived from published literatures with a preference for systematic reviews. Utility values for non‐surgical and HA were derived from published 2‐year follow‐up RCT data in Olerud 2011b, while RTSA was based on published QOL data (Garrigues 2012).
Due to difficulties in obtaining representative costs estimates, average national payer and hospital cost sources were used.
The utility values in the first year for non‐surgical treatment, RTSA and HA were 0.59, 0.77 and 0.73, respectively, while the utility values beyond two years were 0.65, 0.85 and 0.81 for non‐surgical treatment, RTSA and HA, respectively.
For the base analysis, the discounted lifetime costs of HA and RTSA were USD 15,300 and USD 18,000, respectively, for the payer while the hospital analysis resulted in lifetime costs of USD 63,600 and USD 92,200 for HA and RTSA, respectively. The lifetime discounted QALY gains for non‐surgical intervention, HA and RTSA were 7.91, 9.64 and 10.18, respectively. The costs of RTSA reimbursement and hospital charges were estimated as USD 13,093.50 and USD 66,482.50, respectively, while the cost of HA reimbursement and hospital charges were estimated as USD 11,063 and USD 44,558.50, respectively.
When RTSA and HA were selected over non‐surgical management, the QALY gains were 2.27 and 1.73, respectively.
From the payer perspective, HA was found to be associated with a higher ICER and less effective than RTSA when both treatments were compared with non‐surgical intervention. Hence, HA was dominated by both non‐surgical treatment and RTSA, so HA was eliminated from the comparison. When RTSA was compared with HA, the ICER was USD 8100 per QALY gained. Comparing HA with non‐surgical intervention yielded an incremental cost‐effectiveness ratio of USD 36,700 per QALY gained. When RTSA was preferred over HA, the incremental benefit of RTSA over HA resulted in an ICER of USD 57,400.
At a willingness‐to‐pay (WTP) of USD 100,000 per QALY gained, RTSA was determined by the study authors to be an optimal intervention from the hospital perspective.
In the sensitivity analysis carried out, the model was sensitive to QALY gained, operative costs, patient age and success probabilities. However, when analysis was carried out from the payer perspective, only the costs of primary RTSA was sensitive to change, with HA becoming dominant and preferred when RTSA costs exceeded USD 65,200.
At a threshold of USD 100,000 per QALY gained, the probabilities of RTSA, HA and non‐surgical intervention being cost‐effective was 61.0%, 34.7%, and 4.3% of payer analyses, and 53.8%, 37.4%, and 8.8% of hospital analyses, respectively.
Some of the limitations associated with the study included assigning a zero cost to non‐operative treatment due to incomplete national database evidence which may potentially favour non‐surgical intervention. The author concluded that RTSA can be cost‐effective when the WTP is below USD 100,000 per QALY, regardless of the perspective. The author also concluded that HA can be cost‐effective, depending on the cost perspective. Based on the authors results, non‐surgical intervention is more cost‐effective compared with surgical interventions, although no cost was assigned to the non‐surgical intervention which resulted in a biased result.
Addendum
We screened a further 51 records after deduplication of 85 records (MEDLINE (34), Embase (38), and CINAHL (13)) for economic evaluations from an additional search on 20 May 2021. We identified two potentially eligible economic evaluations: Austin 2020, a CUA which compared RTSA and open reduction and internal fixation (ORIF), and Wu 2020, a cost‐minimisation analysis, which compared non‐surgical treatment, open reduction and internal fixation, reverse total shoulder arthroplasty and hemiarthroplasty. While we have not prepared a BEC on Wu 2020, which used a US private‐payer claims database of 22 million patient records from 2007 to 2016 for their comparison, we note that they reported that "[n]onsurgical treatment was associated with lower average total costs compared with surgical intervention".
Appendix 8. Previous acknowledgements and contribution of authors
Acknowledgements
We thank Panos Thomas for his contribution to the protocol, and Linda Digance, Christopher Muller and Sonia Stewart for foreign translations. We thank Bill Gillespie, Peter Herbison, Leeann Morton, David Sonnabend, John Stothard, Marc Swiontkowski and Janet Wale for their help at editorial review of the first version. We thank David Sonnabend for sharing his observations on the second version, and Bill Gillespie for his suggestions for incorporating these into the third version. We thank Lesley Gillespie, Peter Herbison, Nicola Maffulli and Janet Wale for their help at editorial review of the fourth version. We thank Joanne Elliott, Bill Gillespie, Lindsey Shaw and Janet Wale for their contributions at editorial review of the fifth version. We thank also Lesley Gillespie for her help in developing the revised search strategy and trial retrieval for the updates and advice on presentation. We thank Laurent Audige of the AO‐ASIF Foundation and Anette Blümle of the German Cochrane Centre for the supply of several trial reports.
For the sixth version: we thank Nigel Hanchard, Annie Herbert and David Limb for their helpful feedback at editorial review. We thank Joanne Elliott for patiently supplying several search downloads for this version and Lindsey Elstub for her editorial support. We thank Ling‐Hsiang Chuang for her help with translations from Chinese. We thank Graham Tytherleigh‐Strong for his early input on this update.
For the seventh version: we thank Xavier Griffin, David Limb and Yemisi Takwoingi for their helpful feedback at editorial review. We thank Catherine Deering for patiently supplying several search downloads for this version, Joanne Elliott for advice on searching and Lindsey Elstub for her editorial support.
For the eighth version: we thank Mario Lenza and David Limb for their helpful feedback at editorial review. We thank Lindsey Elstub for her editorial support. We thank Joanne Elliott for patiently supplying several search downloads for this version and for her advice on searching other databases.
We thank Alastair Gibson for his major contributions to the first four versions of this review and Rajan Madhok for his major contributions to the first five versions of the review. We thank Benjamin Ollivere for his major contribution to the six and seventh version of the review and Katie Rollins for her major contribution to the seventh version of this review.
We thank the following for further information on their research in this area before the current update: Alison Armstrong, Harm Boons, Cathy Booth, Stig Brorson, Peter Brownson, Piet de Boer, Joe Dias, Tore Fjalestad, Mark Flannery, Tim Hems, Stephen Hodgson, Roo Kulkarni, Inger Mechlenburg, Per Olerud, Shea Palmer, Rajiv Sharma, Matt Smith, Omar Soliman, Carlos Torrens, Robin Turner, Christine Voigt, Lei Yang and Lei Zhang.
Helen Handoll's work on the first version of the review was supported by the Chief Scientist Office, Department of Health, The Scottish Office, UK. Her work on the first and second updates was supported by the East Riding and Hull Health Authority, UK.
Contribution of authors
Rajan Madhok (RM) and Panos Thomas initiated the review and wrote the protocol. Helen Handoll (HH) searched for trials and provided a set of potential studies for inclusion. Alastair Gibson (JNAG) and HH assessed trial quality, tabulated the data and were the main authors of the first published version of the review. JNAG, HH and RM contributed to the final manuscript.
For the first and third (both substantive) updates, Helen Handoll initiated the update by extending the search for trials and relevant materials, contacting trialists and preparing the first drafts. JNAG, HH and RM assessed the newly identified trials and contributed to the final manuscripts. All authors contributed to rewording of the discussion in the second minor update (amendment).
For the fourth (minor) update, Helen Handoll initiated the update by extending the search for trials and relevant materials, contacting trialists and preparing the first drafts. RM performed study selection and contributed to the final manuscript.
For the fifth update (sixth version), Helen Handoll initiated the update by extending the search for trials and relevant materials, contacted trialists, revised text and tables to conform to new methodology and formatting requirements, performed risk of bias assessment for already included trials and prepared the first full draft. Both authors piloted forms, performed study selection, and assessed risk of bias and extracted data for the newly included trials. Benjamin Ollivere provided feedback on interim drafts and contributed to the final manuscript.
For the sixth update (seventh version), Helen Handoll initiated the update by extending the search for trials and relevant materials, contacted trialists, performed most of the data entry, and prepared the first full draft. All three authors screened and selected studies, and assessed risk of bias and extracted data for the newly included trials. Katie Rollins also entered data into Review Manager. Both Benjamin Ollivere and Katie Rollins provided feedback on interim drafts and contributed to the final manuscript.
For the seventh update (eighth version), Helen Handoll initiated the update by extending the search for trials and relevant materials, contacted trialists, performed most of the data entry and prepared the first full draft. Both authors screened and selected studies, assessed risk of bias and extracted data for the newly included trials, and assessed the quality of the evidence using GRADE. Stig Brorson provided feedback on interim drafts and contributed to the final manuscript.
Appendix 9. Numbers and status of studies in the published versions of the review
| Version | Trial status | Changes |
| Ist version
Issue 1, 2001 Gibson 2001 |
The original review had 9 included studies, 4 excluded studies and 6 studies listed as ongoing. | |
| 2nd version (substantive update)
Issue 2, 2002 Gibson 2002a |
This update had 10 included studies, 9 excluded studies, 3 studies listed as ongoing and 1 study awaiting assessment. | Of the four newly identified studies, one was included (Stableforth 1984), one was excluded (Warnecke 1999), one listed as ongoing (Dias 2001), and the final one was placed in 'Studies awaiting assessment' (Martin 2000). Further information obtained from trialists resulted in the exclusion of four trials that had been previously listed as ongoing studies. Three of these had been set up as a multicentre study to test the Halder nail (Halder 2001) (Brownson 2001; Hems 2000; Wallace 2000), and one had been set up to compare surgical with conservative treatment (Kulkarni 2000). |
| 3rd version (minor update)
Issue 3, 2002 Gibson 2002b |
As above | Note: this update included some changes to the Discussion in response to comments received from an external reviewer. |
| 4th version (substantive update)
Issue 4, 2003 Handoll 2003a |
This update had 12 included studies, 11 excluded studies, and 4 studies listed as ongoing. | Of four newly identified studies, one was included (Wirbel 1999), one excluded (de Boer 2003), and two (Frostick 2003; Shah 2003) are listed as ongoing. The other newly included trial was formerly listed as an ongoing trial (Hodgson 2003a). A trial (Martin 2000), previously in 'Studies awaiting assessment', was excluded. Limited additional findings from newly identified trial reports were included for Hoellen 1997. |
| 5th version (minor update)
Issue 2, 2007 Handoll 2007 |
This update had 12 included studies, 12 excluded studies, 5 studies listed as ongoing and 4 pending assessment. | Six new studies were identified: one was listed as ongoing (Fjalestad 2007), one was excluded (Flannery 2006), and the other four were placed in 'Studies awaiting assessment', pending further information. |
| 6th version
(new citation update)
Issue 12, 2010 Handoll 2010 |
This update had 16 included studies, 18 excluded studies, 11 studies listed as ongoing and 4 pending assessment. | Sixteen new studies were identified. Of these, one was included (Fialka 2008), four were excluded (Gradl 2009; Mechlenburg 2009; Wan 2005; Yang 2006), 10 were placed in ongoing trials (Brorson 2009; HURA 2020; Liverpool (renamed as ISRCTN32335957 in the next update); NCT00438633; NCT00818987; NCT00999193; NCT01086202; NCT01113411; ProCon 2010; ProFHER 2015), and one awaits assessment (Luo 2008). New reports or information resulted in the inclusion of three more trials (Agorastides 2007: former ongoing study Frostick 2003; Fjalestad 2010a: former ongoing study Fjalestad 2007; and Lefevre‐Colau 2007: formerly Lefevre‐colau 2006, a study awaiting assessment); and the exclusion of two studies (Bing 2002: former ongoing trial Sharma 2000; Dias 2001: former ongoing trial Dias 2001 and study awaiting assessment Der Tavitian 2006). |
| 7th version (new citation update) Issue 12, 2012 Handoll 2012 |
This update had 23 included studies, 26 excluded studies, 14 studies listed as ongoing and 3 pending assessment. | Overall, 18 new studies were identified. Of these, seven were included (Ockert 2010; Olerud 2011a; Olerud 2011b; Smejkal 2011; Voigt 2011; Zhang 2011; Zhu 2011), four were excluded (Carbone 2012; Edelson 2008; Liao 2009; Zhang 2010), five were placed in ongoing trials (ACTRN12610000730000; HOMERUS; NCT01557413; NTR3208; TPHF), and two await assessment (Battistella 2011; 'Fjalestad (RCT proposal)'. Further information was obtained for several studies in the previous version (Handoll 2010); this included the one‐year follow‐up report of functional outcome for Fjalestad 2010a, and information resulting in the exclusion of ISRCTN32335957, a former ongoing study. Further consideration of Shah 2003, which was listed as an ongoing study, and Pullen 2007, which was awaiting classification, led to their exclusion: it is very unlikely that any further information will be obtained for these trials, including whether they started. Also excluded was Parnes 2005, another study awaiting classification in Handoll 2010; there is currently insufficient evidence to support this being a randomised trial. |
| 8th version (new citation update) Issue 11, 2015 Handoll 2015b |
This update had 31 included studies, 38 excluded studies, 21 studies listed as ongoing and 7 awaiting classification. | Overall, 32 new studies were identified. Of these, eight were included (Boons 2012; Buecking 2014; Cai 2012; Lopiz 2014; ProFHER 2015 (5 references, including 1 trial registration and trial protocol); Sebastiá‐Forcada 2014; Soliman 2013 (2 references, including 1 trial registration); Torrens 2012 (1 reference and unpublished data)); 12 were excluded (Cigni 2012; Elidrissi 2013; Erdoğan 2014; Fan 2012; IRCT2013052313435N1; Maniscalco 2014a (2 references); Martetschlager 2012; NCT00384852; NCT01532076; NCT02122315; NTR2186; Zuckerman 2012); eight were placed in ongoing trials (NCT01524965; NCT01847508; NCT01984112; NCT02075476; NTR3895; ROTATE 2019 (2 references, including 1 trial registration); SHeRPA; Torrens 2015 2015a); and four await classification (Liu 2011 (2 papers); NCT02052206; Wang 2013; Zhu 2014). Further information was obtained for several studies in the previous version (Handoll 2012); this included the two‐year follow‐up report (Fjalestad 2014a) of functional outcome for Fjalestad 2010a, and an additional article (Ockert 2014), which reported on 48 additional participants for Ockert 2010. A trial registration document and published protocol (Fjalestad 2014b) were found for a newly designated ongoing trial (DELPHI), previously 'Fjalestad (RCT proposal)', in studies awaiting classification. Published protocols were also found for two ongoing trials (HOMERUS (Verbeek 2012); TPHF (Launonen 2012)). Additional information from updated trial registration documentation was added in for seven ongoing trials (HURA; NCT00438633; NCT00818987; NCT00999193; NCT01113411; NCT01557413; TPHF). Additional information was also available for Brorson 2009, which was moved from ongoing to studies awaiting classification. |
| 9th version (new citation update) Issue 5, 2022 | This update had 47 included studies, 77 excluded studies, 30 studies listed as ongoing and 16 awaiting classification. | Overall, 73 new studies (90 references) were identified. Of these:
Further references (46 references) were obtained for 24 studies:
We obtained further information, data or both from the trial contacts of three newly included studies (Jonsson 2020; Plath 2019; Tousignant 2020), three now excluded studies (HOMERUS; NCT03017105; Torrens 2015); one study awaiting classification (Brorson 2009), and two ongoing studies (NCT03217344; NTR3859). |
Data and analyses
Comparison 1. Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Disability of the Arm, Shoulder, and Hand (DASH) score (0 to 100: worst disability) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.2 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Shoulder function: Croft Shoulder Disability Score | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 Disability (1 or more problems) at 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2.2 Severe disability (5 or more problems) at 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2.3 Disability (1 or more problems) at 2 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2.4 Severe disability (5 or more problems) at 2 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Croft Shoulder Disability Score: individual problems at 2 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.1 Pain on movement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.2 Bathing difficulties | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.3 Change position at night more often | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.4 Disturbed sleep | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.5 No active pastimes or usual physical recreation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.6 Lifting problems | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.7 Help needed | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.8 More accidents (e.g. dropping things) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 Number of treatment sessions (until independent function achieved) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5 Quality of life: SF‐36 scores (8 dimensions) at 16 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5.1 Physical functioning (0 to 100: excellent) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5.2 Social functioning (0 to 100: excellent) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5.3 Role limitation physical (0 to 100: none) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5.4 Role limitation emotional (0 to 100: none) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5.5 Pain (0 to 100: none) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5.6 Mental health (0 to 100: none) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5.7 Vitality (0 to 100: best) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5.8 General health perception | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6 Quality of life: SF‐36 scores (8 dimensions) at 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.1 Physical functioning (0 to 100: excellent) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.2 Social functioning (0 to 100: excellent) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.3 Role limitation physical (0 to 100: none) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.4 Role limitation emotional (0 to 100: none) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.5 Pain (0 to 100: none) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.6 Mental health (0 to 100: none) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.7 Vitality (0 to 100: best) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6.8 General health perception | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.7 Quality of life: EQ‐5D (0: dead to 1: best quality) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.7.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.7.2 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.7.3 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.8 Adverse events | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.8.1 Frozen shoulder | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 1.8.2 Fracture displacement | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.22, 22.45] |
| 1.8.3 Non‐union | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.8.4 Complex regional pain syndrome type 1 | 2 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.07, 16.71] |
| 1.8.5 Treated (injection) subacromial impingement | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.07, 15.30] |
| 1.8.6 Shoulder complications | 4 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.15, 3.63] |
| 1.8.7 Fracture complications | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.22, 22.45] |
| 1.8.8 Adverse events ('serious' and 'other') | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.9 Mortality | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.02, 8.48] |
| 1.10 Constant score (ratio of affected/unaffected arm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.10.1 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.10.2 16 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.10.3 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.11 Constant score (0 to 100: best outcome) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.11.1 At 6 weeks | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 10.10 [2.02, 18.18] |
| 1.11.2 At 3 months | 2 | 106 | Mean Difference (IV, Fixed, 95% CI) | 6.53 [0.77, 12.30] |
| 1.11.3 At 6 months | 2 | 105 | Mean Difference (IV, Fixed, 95% CI) | 3.39 [‐1.46, 8.24] |
| 1.11.4 At 12 months | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 1.46 [‐7.05, 9.97] |
| 1.11.5 6 months: subjective assessment (0 to 35: best) | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐0.54, 4.34] |
| 1.11.6 6 months: objective assessment range of motion and strength (0 to 65: best) | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 4.10 [‐0.62, 8.82] |
| 1.12 Pain VAS (0 to 100: worst pain) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.12.1 At 6 weeks | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐20.76, 13.56] |
| 1.12.2 At 3 months | 2 | 106 | Mean Difference (IV, Fixed, 95% CI) | ‐5.13 [‐14.76, 4.50] |
| 1.12.3 At 6 months | 2 | 105 | Mean Difference (IV, Fixed, 95% CI) | 4.29 [‐5.48, 14.07] |
| 1.12.4 At 12 months | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 10.80 [‐4.59, 26.19] |
| 1.13 Pain: Likert scale (0 to 10: severe pain) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.13.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.13.2 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.14 Changes in pain intensity (mm) from baseline: 100 mm visual analogue scale (positive change = less pain) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.14.1 At 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.14.2 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.14.3 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.15 Range of motion at 3 and 6 months (degrees) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.15.1 Abduction at 3 months | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 14.00 [‐9.29, 37.29] |
| 1.15.2 Abduction at 6 months | 2 | 114 | Mean Difference (IV, Fixed, 95% CI) | 7.57 [‐0.85, 15.98] |
| 1.15.3 Anterior elevation at 6 months | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 3.60 [‐2.77, 9.97] |
| 1.15.4 External rotation at 3 months | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 9.00 [‐2.33, 20.33] |
| 1.15.5 External (lateral) rotation at 6 months | 2 | 114 | Mean Difference (IV, Fixed, 95% CI) | 3.38 [‐3.06, 9.83] |
| 1.15.6 Forward flexion at 6 months | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 4.00 [‐9.32, 17.32] |
| 1.16 Patient dissatisfied with treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.17 Patient satisfaction (0 to 10: higher scores ‐ greater satisfaction) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.17.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.17.2 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.17.3 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
1.9. Analysis.

Comparison 1: Early (up to 1 week post injury) versus delayed (after three or more weeks) mobilisation, Outcome 9: Mortality
Comparison 2. Early intensive mobilisation versus early less intensive mobilisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Subjective shoulder value (0 to 100: % of normal shoulder) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1.2 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1.3 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2.1 Fracture non‐union | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2.2 Loss of reduction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 Constant score (0 to 100: best outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3.2 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3.3 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Gilchrist bandage versus 'classic' Desault bandage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Adverse events (problems with bandages) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.1 Severe skin irritation during immobilisation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.2 Premature bandage removal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Participant assessment of comfort and bandage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.1 Application of bandage was uncomfortable | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.2 Poor rating at fracture consolidation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.3 Poor rating at fracture consolidation: sensitivity analysis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3 Fracture displacement by 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. Instructed self‐exercise versus conventional physiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.2 Neer's rating (0 to 100: best) at mean 16 months (exploratory analysis) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.3 Pain at one year (scale 0 to 8: maximum pain) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.4 Severe or moderate pain at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.5 Requested change of therapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.6 Active glenohumeral elevation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. Telerehabilitation versus face‐to‐face rehabilitation (non‐surgical treatment).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Disability of the Arm, Shoulder, and Hand (DASH) score (0 to 100: worst disability) at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.2 Constant score (0 to 100: best outcome) at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3 Active range of motion (degrees) at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3.1 Flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3.2 Extension | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3.3 Internal rotation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3.4 External rotation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3.5 Abduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.4 Satisfaction with healthcare service provided (0 to 100: most satisfied) at 8 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. Surgical versus non‐surgical treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Functional scores at 12 months (higher = better outcome) | 7 | 552 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.07, 0.27] |
| 6.1.1 DASH (0 to 100: worst disability) (reversed) | 4 | 238 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.07, 0.44] |
| 6.1.2 ASES (0 to 24: best) | 1 | 48 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.67, 0.46] |
| 6.1.3 SST (0 to 12: best) | 1 | 47 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.46, 0.69] |
| 6.1.4 OSS (0 to 48: best) | 1 | 219 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.22, 0.31] |
| 6.2 Functional scores at 6 months (higher = better outcome) | 3 | 347 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.04, 0.38] |
| 6.2.1 DASH (0 to 100: worst disability) (reversed) | 1 | 73 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.33, 0.59] |
| 6.2.2 ASES (0 to 24: best) | 1 | 48 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.55, 0.58] |
| 6.2.3 OSS (0 to 48: best) | 1 | 226 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.04, 0.48] |
| 6.3 Functional scores subgrouped by 3 to 4 versus 6 months (higher = better outcome) | 6 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.3.1 3 to 4 months | 4 | 229 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.28, 0.24] |
| 6.3.2 6 months | 3 | 347 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.04, 0.38] |
| 6.4 Functional scores at 24 months (higher = better outcome) | 5 | 423 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.13, 0.25] |
| 6.4.1 DASH (0 to 100: worst disability) (reversed) | 3 | 171 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.11, 0.49] |
| 6.4.2 ASES (0 to 24: best) | 1 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.62, 0.59] |
| 6.4.3 OSS (0 to 48: best) | 1 | 210 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.30, 0.24] |
| 6.5 Oxford Shoulder Score (OSS) (0 to 48: best outcome) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.5.1 At 6 months | 2 | 303 | Mean Difference (IV, Fixed, 95% CI) | 1.86 [‐0.31, 4.04] |
| 6.5.2 At 12 months | 2 | 291 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐1.80, 2.39] |
| 6.5.3 At 24 months | 2 | 282 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐2.73, 1.57] |
| 6.6 Oxford Shoulder Score (OSS) (0 to 48: best outcome): ProFHER trial primary analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.6.1 Over 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.6.2 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.6.3 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.6.4 At 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.6.5 at 36 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.6.6 at 48 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.6.7 at 60 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.7 Disability of the Arm, Shoulder, and Hand (DASH) score (0 to 100: worst disability) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.7.1 at 3 to 4 months | 3 | 180 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐7.53, 4.13] |
| 6.7.2 at 6 months | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐10.52, 5.72] |
| 6.7.3 at 12 months | 4 | 238 | Mean Difference (IV, Fixed, 95% CI) | ‐3.83 [‐8.77, 1.12] |
| 6.7.4 at 24 months | 3 | 171 | Mean Difference (IV, Fixed, 95% CI) | ‐3.75 [‐10.03, 2.53] |
| 6.8 American Shoulder and Elbow Surgeons (ASES) score (0 to 24: best) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.8.1 at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.8.2 at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.8.3 at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.9 Simple Shoulder Test (SST) (0 to 12: best function) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.9.1 at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.9.2 at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.10 Activities of daily living | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.10.1 Unable to manage personal hygiene at 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.10.2 Unable to comb hair at 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.10.3 Unable to sleep on fractured side at 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.10.4 Unable to carry 5 kg at 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.10.5 Unable to manage personal hygiene at 50 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.10.6 Unable to comb hair at 50 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.10.7 Unable to sleep on fractured side at 50 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.10.8 Unable to carry 5 kg at 50 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.11 Quality of life: EQ‐5D (0: dead to 1: best quality) | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.11.1 at 3 to 4 months | 5 | 442 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.01, 0.04] |
| 6.11.2 at 6 months | 5 | 458 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.01, 0.05] |
| 6.11.3 at 12 months | 6 | 502 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.02, 0.04] |
| 6.11.4 at 24 months | 5 | 426 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.02, 0.05] |
| 6.12 Quality of life: EQ‐5D (0: dead to 1: best health): ProFHER multilevel regression analysis | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.12.1 at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.12.2 at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.12.3 at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.12.4 at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.12.5 at 36 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.12.6 at 48 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.12.7 at 60 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.13 Quality of life: 15D score and number of quality‐adjusted life years (QALYs) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.13.1 15D at 3 months (0: death; 1: perfect health) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.04, 0.04] |
| 6.13.2 15D at 6 months | 2 | 125 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.02, 0.04] |
| 6.13.3 15D at 12 months | 2 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 6.13.4 number of QALYs at 1 year | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.02, 0.06] |
| 6.13.5 15D at 24 months | 2 | 114 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.05] |
| 6.14 Quality of life: EuroQol‐VAS (0 to 100: best quality) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.14.1 at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.15 Quality of life: SF‐12 Physical Component Score (0 to 100: best) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.15.1 at 6 months | 1 | 216 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐0.24, 5.44] |
| 6.15.2 at 12 months | 2 | 277 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐1.10, 3.49] |
| 6.15.3 at 24 months | 1 | 210 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐1.99, 4.19] |
| 6.16 Quality of life: SF‐12 Mental Component Score (0 to 100: best) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.16.1 at 6 months | 1 | 216 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐3.57, 2.37] |
| 6.16.2 at 12 months | 2 | 277 | Mean Difference (IV, Fixed, 95% CI) | ‐1.83 [‐4.28, 0.62] |
| 6.16.3 at 24 months | 1 | 210 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐4.33, 1.53] |
| 6.17 Mortality | 8 | 646 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.70, 2.62] |
| 6.18 Dependent in activities of daily living (or dead) at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.19 Additional surgery (reoperation or secondary surgery) | 9 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.21, 3.51] |
| 6.19.1 at 6 to 12 months | 4 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.29, 3.74] |
| 6.19.2 at 2 years | 5 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.31, 4.28] |
| 6.20 Adverse events / complications | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.20.1 Number of patients with complications | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.92, 2.31] |
| 6.20.2 Additional shoulder‐related therapy | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.53, 5.83] |
| 6.20.3 Infection | 10 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.31 [1.11, 16.74] |
| 6.20.4 Nerve injury / palsy | 5 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.54, 4.22] |
| 6.20.5 Non‐union | 8 | 582 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.19, 0.94] |
| 6.20.6 Avascular necrosis | 8 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.81] |
| 6.20.7 Symptomatic malunion | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.22, 2.91] |
| 6.20.8 Screw penetration into joint | 4 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.97 [2.39, 41.58] |
| 6.20.9 Metalwork (internal fixation) problems | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 21.00 [1.24, 354.53] |
| 6.20.10 Wire penetration at 1 year | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 69.31] |
| 6.20.11 Redisplacement resulting in an operation | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.22] |
| 6.20.12 Implant‐related (hemiarthroplasty) failure | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.00 [0.45, 35.18] |
| 6.20.13 Secondary dislocation or resorption of the greater tuberosity | 2 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.15 [1.78, 96.90] |
| 6.20.14 Tuberosity displacement at 50 months | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.71] |
| 6.20.15 Fixation failure resulting in an operation | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 70.30] |
| 6.20.16 Refracture | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.92] |
| 6.20.17 Post‐traumatic stiffness | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.38, 3.83] |
| 6.20.18 Impingement | 2 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.18, 5.62] |
| 6.20.19 Rotator cuff tear | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.48, 18.73] |
| 6.20.20 Post‐traumatic stiffness | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.38, 3.83] |
| 6.20.21 CRPS or severe pain | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.18, 21.78] |
| 6.20.22 Dislocation or instability | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
| 6.20.23 Heterotopic ossification | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 6.20.24 Post‐traumatic osteoarthritis (signs of) | 4 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.27, 1.70] |
| 6.21 Constant score (overall: 0 to 100: best score) | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.21.1 at 3 to 4 months | 3 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐7.35, 1.56] |
| 6.21.2 at 6 months | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐6.39, 10.79] |
| 6.21.3 at 12 months | 6 | 330 | Mean Difference (IV, Fixed, 95% CI) | 3.78 [0.19, 7.37] |
| 6.21.4 at 24 months | 4 | 215 | Mean Difference (IV, Fixed, 95% CI) | 0.52 [‐4.75, 5.79] |
| 6.21.5 at 50 months | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐5.00 [‐17.52, 7.52] |
| 6.22 Constant score (difference between injured and uninjured shoulder): Normal = 0. | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.22.1 at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.22.2 at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.22.3 at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.23 Poor or unsatisfactory function at 1 year (Neer rating) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.24 Visual Analogue Scale (VAS) disability (0 to 100: no restrictions) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.24.1 at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.24.2 at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.25 Pain: VAS (0 to 100: worst pain) | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.25.1 at 3 months | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐18.00 [‐29.03, ‐6.97] |
| 6.25.2 at 6 months | 1 | 77 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐6.10, 9.90] |
| 6.25.3 at 12 months | 2 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐3.64 [‐9.79, 2.51] |
| 6.25.4 at 24 months | 3 | 173 | Mean Difference (IV, Fixed, 95% CI) | ‐2.67 [‐8.37, 3.03] |
| 6.26 Constant score at 50 months: overall and components | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.26.1 Overall score (0‐100: best score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.26.2 Pain (maximum score 15) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.26.3 Range of motion (maximum score 40) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.26.4 Power (maximum score 25) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.26.5 Activities of daily living (maximum score 20) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.27 Constant (often severe) pain at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.28 Failure to recover 75% muscle power relative to other arm at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.28.1 Flexion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.28.2 Abduction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.28.3 Lateral rotation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.29 Range of movement impairments in survivors at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.29.1 Flexion < 45 degrees | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.29.2 Unable to place thumb on mid spine (T12) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.29.3 Lateral rotation < 5 degrees | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.30 Costs at 1 year (Euros in 2005) | 1 | Other data | No numeric data | |
| 6.31 Total costs including indirect costs (Euros) at 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
6.7. Analysis.

Comparison 6: Surgical versus non‐surgical treatment, Outcome 7: Disability of the Arm, Shoulder, and Hand (DASH) score (0 to 100: worst disability)
Comparison 7. Locking plate versus locking intramedullary nail.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Functional scores at 1 year (higher = better outcome) | 4 | 227 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.12, 0.41] |
| 7.1.1 ASES (0 to 100: best outcome) | 2 | 108 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐0.05, 0.72] |
| 7.1.2 DASH (0 to 100: worst disability) (reversed) | 2 | 119 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.38, 0.34] |
| 7.2 American Shoulder and Elbow Surgeons (ASES) score (0 to 100: best) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.2.1 At 3 months | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [‐3.57, 6.97] |
| 7.2.2 At 6 months | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐5.13, 5.93] |
| 7.2.3 At 1 year | 2 | 108 | Mean Difference (IV, Fixed, 95% CI) | 2.79 [‐0.73, 6.31] |
| 7.2.4 At 2 or 3 years | 2 | 101 | Mean Difference (IV, Fixed, 95% CI) | 3.06 [‐0.05, 6.17] |
| 7.3 Disability of the Arm, Shoulder, and Hand (DASH) score (0 to 100: worst disability) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.3.1 at 3 months | 3 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐0.72 [‐5.06, 3.61] |
| 7.3.2 at 6 months | 3 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐4.14, 3.36] |
| 7.3.3 at 12 months | 3 | 172 | Mean Difference (IV, Fixed, 95% CI) | ‐2.24 [‐5.96, 1.48] |
| 7.3.4 at 24 months | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐5.20 [‐10.20, ‐0.20] |
| 7.4 Disability of the Arm, Shoulder, and Hand (DASH) scores in published report (Plath 2019) | 1 | Other data | No numeric data | |
| 7.5 Oxford Shoulder Score (OSS) (0 to 48: best function) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.5.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.5.2 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.5.3 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.5.4 At 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.6 Death, reoperation and adverse events | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.6.1 Death | 4 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.14, 1.46] |
| 7.6.2 Any complication | 4 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.70, 1.75] |
| 7.6.3 Reoperation | 3 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.33, 1.61] |
| 7.6.4 Screw penetration into humeral head | 4 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.82 [1.39, 10.48] |
| 7.6.5 Screw protrusion | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.04, 2.95] |
| 7.6.6 Implant loosening | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.66] |
| 7.6.7 Infection | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.66] |
| 7.6.8 Heterotopic ossification | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.19, 20.12] |
| 7.6.9 Osteonecrosis | 3 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.24, 12.48] |
| 7.6.10 Insufficient reduction | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.12, 68.95] |
| 7.6.11 Loss of reduction of greater tuberosity | 2 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.33, 29.30] |
| 7.6.12 Malposition of implants | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.10, 1.70] |
| 7.6.13 Head migration | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.11, 2.87] |
| 7.6.14 Loss of humeral head reduction | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [0.75, 6.77] |
| 7.6.15 Non‐union | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 7.6.16 Secondary varus collapse | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 7.6.17 Degenerative change of glenohumeral joint | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 7.6.18 Axillary nerve lesion | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.36 [0.14, 79.76] |
| 7.6.19 Complex regional pain syndrome | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.66] |
| 7.6.20 Shoulder stiffness | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.10, 2.47] |
| 7.6.21 Complete rotator cuff tears | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.10, 2.47] |
| 7.6.22 Adhesive capsulitis | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.36 [0.14, 79.76] |
| 7.6.23 Re‐fracture | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 6.88] |
| 7.7 Quality of life: SF‐36 (0 to 100: best outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.7.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.7.2 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.7.3 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.7.4 At 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.8 Constant score (0 to 100: best) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.8.1 At 3 months | 3 | 177 | Mean Difference (IV, Fixed, 95% CI) | ‐3.45 [‐7.28, 0.39] |
| 7.8.2 At 6 months | 3 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐1.28 [‐5.17, 2.60] |
| 7.8.3 At 1 year | 4 | 227 | Mean Difference (IV, Fixed, 95% CI) | 2.47 [‐0.46, 5.41] |
| 7.8.4 At 2 or 3 years | 2 | 101 | Mean Difference (IV, Fixed, 95% CI) | 2.26 [‐0.52, 5.03] |
| 7.9 Pain (VAS 0 to 10: worst pain) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.9.1 at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.9.2 at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.9.3 at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.10 Pain (VAS: 0 to 10: worst) | 2 | Other data | No numeric data | |
| 7.11 Range of movement (flexion and abduction) degrees | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.11.1 Flexion at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.11.2 Flexion at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.11.3 Flexion at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.11.4 Abduction at 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.11.5 Abduction at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.11.6 Abduction at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.12 Active range of motion (at 3 years) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.12.1 Forward elevation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.12.2 External rotation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.13 Range of movement: internal rotation (level on spine) | 1 | Other data | No numeric data | |
| 7.14 Strength of supraspinatus (relative to opposite side) % at 3 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.14.1 At 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.14.2 At 3 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 8. Locking plate versus intramedullary K‐wires.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1.1 Any complication | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1.2 Malunion (usually slight) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.2 Constant score (% of healthy limb) at mean 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.3 Time to union and time to recover upper‐limb function (weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.3.1 Time to radiographic union | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.3.2 Time to recover normal upper‐limb function | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 9. Hemiarthroplasty versus plate fixation (4‐part fractures).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Disability of the Arm, Shoulder, and Hand (DASH) score (0 to 100: worst disability) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1.1 At 4 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1.2 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1.3 At 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.2 Quality of life: EQ‐5D score (0: dead to 1: best quality of life) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.2.1 At 4 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.2.2 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.2.3 At 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.3 Death or reoperation at 2 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.3.1 Dead | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.3.2 Reoperation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.4 Constant score (0 to 100: best score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.4.1 At 4 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.4.2 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.4.3 At 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.5 Pain VAS (0 to 100: worst pain) at 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.6 Range of motion at 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.6.1 Flexion (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.6.2 Abduction (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 10. Hemiarthroplasty versus tension band wiring (4‐part fractures).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Haematoma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.2 Reoperation post discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.3 Death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 11. Reverse total shoulder arthroplasty (RTSA) versus open reduction and internal fixation (ORIF) using a plate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Oxford Shoulder Score (0 to 48: best outcome) at 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.2 Death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3 Complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3.1 Any complication | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3.2 Revision surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3.3 Perioperative glenoid fracture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3.4 Fracture: periprosthetic or distal to plate | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3.5 Nerve injury (transient) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3.6 Deep wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3.7 Screw penetration (problematic) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3.8 Non‐union | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3.9 Rotator cuff rupture | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.4 Constant score: overall and some components | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.4.1 Overall score (0 to 100: best score) at 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.4.2 Overall score (0 to 100: best score) at 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.4.3 Pain (maximum score 15) at 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.4.4 Power (maximum score 25) at 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.5 Radiological assessment findings at 2 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.5.1 Resorption of greater tuberosity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.5.2 Scapular notching | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.5.3 Avascular necrosis 50% or greater | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.5.4 Greater tuberosity displacement 10 mm or greater | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.5.5 Peri‐articular ossification | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 12. Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty (HA).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Shoulder function score: WOOS (% of normal shoulder function) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1.1 At 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1.2 At 2+ years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.2 Shoulder function scores at 24 to 49 months (exploratory analysis) | 2 | 142 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.77, ‐0.09] |
| 12.2.1 QuickDASH score (0 to 55: worst outcome) | 1 | 61 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐1.40, ‐0.35] |
| 12.2.2 WOOS (% of normal shoulder function) | 1 | 81 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.56, 0.31] |
| 12.3 Shoulder function: QuickDASH score (0 to 55: worst outcome) at 24 to 49 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.4 Quality of life: EQ‐5D (0: dead to 1: best outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.4.1 At 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.4.2 At 2 years or more | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.5 Death | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.21, 2.36] |
| 12.6 Complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.6.1 Any complication | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.14, 0.94] |
| 12.6.2 Intraoperative fracture | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.63] |
| 12.6.3 Periprosthetic fracture | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.29] |
| 12.6.4 Proximal migration of implant | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.98] |
| 12.6.5 Deep infection | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.12, 68.66] |
| 12.6.6 Superficial infection | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.63] |
| 12.6.7 Haematoma | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.12, 68.66] |
| 12.6.8 Neurological complications | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.63] |
| 12.6.9 Severe stiffness | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.63] |
| 12.6.10 Complex regional pain syndrome | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.18 [0.13, 76.31] |
| 12.6.11 Pneumonia | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.18 [0.13, 76.31] |
| 12.7 Reoperation | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.06, 1.15] |
| 12.8 Composite (objective and subjective) shoulder function scores at 24 to 49 months | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.8.1 UCLA score (0 to 35: best outcome) | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 8.00 [3.47, 12.53] |
| 12.8.2 Constant score (0 to 100: best outcome) | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | 13.15 [7.23, 19.07] |
| 12.8.3 Constant score % relative to opposite side | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 23.90 [10.37, 37.43] |
| 12.9 Constant score at 24 to 49 months: overall and components | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.9.1 Overall score (0 to 100: best score) | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | 13.15 [7.23, 19.07] |
| 12.9.2 Pain (maximum score 15) | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | 1.61 [0.05, 3.18] |
| 12.9.3 Range of motion (maximum score 40) | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | 6.33 [3.67, 8.99] |
| 12.9.4 Power (maximum score 25) | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | 2.37 [1.26, 3.49] |
| 12.9.5 Activities of daily living (maximum score 20) | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | 3.08 [1.46, 4.70] |
| 12.9.6 Overall score (0 to 100: best score) at 1 year | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 9.70 [1.18, 18.22] |
| 12.10 Pain (on average) VAS (0 to 100: worst pain) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.10.1 At 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.10.2 At 2 years or more | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.11 Range of motion (degrees) at 24 to 49 months | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.11.1 Anterior forward or flexion | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | 36.53 [24.44, 48.62] |
| 12.11.2 Abduction | 2 | 145 | Mean Difference (IV, Fixed, 95% CI) | 30.86 [19.29, 42.42] |
| 12.11.3 External rotation | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐9.00 [‐16.91, ‐1.09] |
| 12.12 Internal rotation at minimum 2 years | 1 | Other data | No numeric data | |
| 12.13 Patient satisfaction with shoulder VAS (0 to 100: total satisfaction) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.13.1 At 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.13.2 At 2 years or more | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.14 Radiological assessment findings | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.14.1 Malunion of tuberosities | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.45, 4.64] |
| 12.14.2 Resorption of tuberosities | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.20, 1.42] |
| 12.14.3 Scapular notching | 2 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.53 [1.25, 72.80] |
| 12.14.4 Heterotopic ossification | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.28, 2.45] |
| 12.14.5 Glenoid erosion | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.93] |
| 12.14.6 Greater tuberosity not healed | 2 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.60, 1.52] |
| 12.14.7 Radiolucent lines round stem | 2 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.42, 5.43] |
| 12.15 Length of surgery (minutes) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
12.15. Analysis.

Comparison 12: Reverse total shoulder arthroplasty (RTSA) versus hemiarthroplasty (HA), Outcome 15: Length of surgery (minutes)
Comparison 13. Plate: deltoid‐split approach and minimally invasive plate osteosynthesis (MIPO) versus deltopectoral approach and open plate fixation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Shoulder function: QuickDASH score (0 to 100: worst disability) at 26 months (mean) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.2 Quality of life: SF‐12 at 26 months (mean) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.2.1 Physical component score (0 to 100: best) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.2.2 Mental component score (0 to 100: best) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.3 Mortality | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.3.1 At 1 year | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.05, 2.02] |
| 13.3.2 At 26 months (mean) | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.73 [0.43, 31.99] |
| 13.4 Complications | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.4.1 Overall complications | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.52, 1.51] |
| 13.4.2 Total with one of 5 complications (see footnote) | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.65, 3.38] |
| 13.4.3 Implant‐related complications | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.38, 2.62] |
| 13.4.4 Axillary nerve damage | 2 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 13.4.5 Infection | 2 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.02] |
| 13.4.6 Fixation failure | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.59 [0.28, 23.88] |
| 13.4.7 Screw perforation or cut‐out | 3 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.58, 5.58] |
| 13.4.8 Implant (head or shaft) loosening | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.27, 2.58] |
| 13.4.9 Screw loosening | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.19, 21.28] |
| 13.4.10 Humeral head necrosis | 3 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.29, 4.56] |
| 13.4.11 Non‐union | 2 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.07 [0.32, 113.84] |
| 13.4.12 Loss of reduction (> 10 degrees) | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.58, 3.59] |
| 13.4.13 Varus collapse | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.26, 8.55] |
| 13.4.14 Heterotopic ossification | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.19, 4.03] |
| 13.4.15 Greater tuberosity migration | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.15, 6.79] |
| 13.4.16 Stiff shoulder | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.23, 2.79] |
| 13.4.17 Injurious fall on shoulder | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.62] |
| 13.5 Reoperation | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.5.1 For complication (or a fall) | 2 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.67, 2.66] |
| 13.5.2 Plate removal by patient request | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.42, 2.32] |
| 13.6 Composite (objective and subjective) shoulder function scores: Constant and UCLA | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.6.1 Constant score (0 to 100: best outcome) at 6 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 4.00 [‐4.28, 12.28] |
| 13.6.2 Constant score (0 to 100: best outcome) at 12 or mean 15 months | 2 | 180 | Mean Difference (IV, Fixed, 95% CI) | 2.46 [‐2.29, 7.22] |
| 13.6.3 UCLA score (0 to 35: best outcome) at a mean 15 months | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐2.69, 0.69] |
| 13.7 Pain (VAS 0 to 10: intolerable pain) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.7.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.7.2 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.7.3 At 26 months (mean) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.8 Range of motion (degrees) at 26 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.8.1 External rotation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.8.2 Flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.9 Patient scar assessment scale (6 to 60: worst outcome) at 26 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 14. Plate: polyaxial versus monoaxial screw insertion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Disability of the Arm, Shoulder, and Hand (DASH) score at 12 months (0 to 100: greatest disability) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.2 Simple Shoulder Test (SST) (0 to 12: best outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.2.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.2.2 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.2.3 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.3 Reoperation | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.3.1 By 6 months | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.15, 4.76] |
| 14.3.2 By 1 year | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.58, 2.08] |
| 14.4 Dead at 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.5 Complications (radiological assessment) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.5.1 Any complication | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.5.2 Primary implant malposition | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.5.3 Secondary loss of reduction and screw perforation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.5.4 Non‐union / delayed union due to osteonecrosis (6 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.5.5 Avascular necrosis at 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.5.6 Varus deformity (> 10 / ≥20 degrees) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.5.7 Greater tuberosity displacement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.5.8 Screw cut‐out (intra‐articular) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.6 Constant score at 12 months (% of contralateral limb) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.7 Range of motion (degrees) at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.7.1 Flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.7.2 Abduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.7.3 External rotation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.7.4 Internal rotation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 15. Plate: CFR‐PEEK plate versus titanium (PHILOS) plate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Disability of the Arm, Shoulder, and Hand (DASH) score (0 to 100: worst disability) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1.1 At 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1.2 At 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1.3 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.2 Oxford Shoulder Score (OSS) (0 to 48: best outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.2.1 At 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.2.2 At 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.2.3 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.3 Simple Shoulder Test (SST) (0 to 100: best score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.3.1 At 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.3.2 At 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.3.3 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.4 Complications and reoperations | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.4.1 Infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.4.2 Non‐union | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.4.3 Screw perforation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.4.4 Displacement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.4.5 Reoperation (for new injury) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.4.6 Treatment failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 16. Locking plate: glenohumeral joint lavage versus no lavage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.1.1 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.1.2 Total with complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.1.3 Primary screw penetration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.1.4 Plate malposition | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.1.5 Avascular necrosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.1.6 Secondary displacement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.1.7 Adhesive capsulitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.2 Constant score (0 to 100: best outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.2.1 At 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.2.2 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.2.3 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.2.4 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.3 Range of motion at 12 months (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.3.1 Forward flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.3.2 Abduction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.3.3 External rotation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.3.4 Internal rotation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 17. Locking plate: medial support screws versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 17.1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17.1.1 Early loss of fixation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17.1.2 Reoperation for early failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17.1.3 Osteonecrosis (asymptomatic) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17.2 Constant score (0 to 100: best) at 2.5 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 18. Locking plate: with allogeneic bone grafts versus not (control).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 18.1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18.1.1 Infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18.1.2 Humeral head necrosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18.1.3 Screw cut‐out | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18.1.4 Varus humeral head | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18.1.5 Delayed fracture healing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18.2 Poor Neer shoulder function scores at 1 month | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 19. Locking plate: cement augmentation of screw tips versus none (PHILOS plate fixation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 19.1 Shoulder function: QuickDASH score (0 to 100: worst disability) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.1.1 At 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.1.2 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.1.3 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.1.4 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.2 Shoulder Pain and Disability Index (SPADI) score (0 to 100: worst outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.2.1 At 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.2.2 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.2.3 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.2.4 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.3 Quality of life: EQ‐5D index (0 (dead) to 1 (perfect health)) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.3.1 At 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.3.2 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.3.3 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.3.4 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.4 Quality of life: EQ‐5D VAS health state (0 to 100: best health state) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.4.1 At 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.4.2 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.4.3 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.4.4 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.5 Adverse events (AEs) and treatment failure (analysed by treatment received) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.1 Mechanical failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.2 Any adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.3 Any intraoperative AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.4 Poor intra‐op reduction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.5 Screw/plate malpositioning | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.6 Cement leakage into joint | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.7 Any post‐operative AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.8 Screw perforation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.9 Screw/plate loosening | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.10 Implant failure/breakage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.11 Non‐union | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.12 Malunion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.13 Loss of reduction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.14 Humeral head necrosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.15 Humeral head impaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.16 Other fracture‐related AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.17 Deep wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.18 Superficial wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.19 Wound dehiscence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.20 Haematoma (requiring revision) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.21 Other wound tissue AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.22 Impingement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.23 Nerve injury | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.24 Other soft‐tissue skeletal AE | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.5.25 Allergic reaction to cement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.6 Death and systemic complications (safety data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.6.1 Sudden death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.6.2 Stroke | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.6.3 Thromboembolic complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.6.4 Sepsis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.6.5 Pneumonia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.6.6 Renal insufficiency | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.6.7 Other systemic adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.7 Constant score (0 to 100: best outcome) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.7.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.7.2 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.7.3 At 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 20. Nail: MultiLoc Proximal Humeral Nail (MPHN) versus Polarus humeral nail.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 20.1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20.1.1 Reoperation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20.1.2 Post‐op impingement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20.1.3 Screw loosening | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20.1.4 Non‐union | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20.1.5 Rotator cuff symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20.1.6 Intraoperative complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20.1.7 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20.1.8 Radiographic malunion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20.2 Constant score (0 to 100: best outcome) at 14 months (6 to 22 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.2.1 Unadjusted Constant score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.2.2 Adjusted Constant score | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.3 Range of shoulder motion (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.3.1 Lateral elevation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.3.2 Forward flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.3.3 External rotation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 21. Hemiarthoplasty: EPOCA prosthesis versus HAS prosthesis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 21.1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21.1.1 Deep infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21.1.2 Persistent pain ‐ scheduled for reoperation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21.2 Range of motion results at one year (degrees) | 1 | Other data | No numeric data | |
| 21.3 Radiological assessment findings | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21.3.1 Resorption of tuberosities | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21.3.2 Secondary dislocation of tuberosities | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21.3.3 Superior migration of prosthesis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21.3.4 Anterior subluxations | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21.3.5 Glenoid erosion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21.3.6 Aseptic loosening of stem | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 22. Hemiarthroplasty: tenodesis of long head of biceps (LHB) versus LHB tendon left intact.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 22.1 Complications and further surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22.1.1 Any complication | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22.1.2 Further surgery for listed complications | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22.1.3 Deep infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22.1.4 Tuberosity malunion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22.1.5 Inferior subluxation of prosthesis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22.1.6 Loss of reduction of greater tuberosity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22.2 Constant score (0 to 100: best function) at 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 22.3 Shoulder pain at 2 year follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22.4 Active shoulder elevation (degrees) at 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 23. Postoperative: immobilisation in sling for 1 versus 3 weeks after percutaneous fixation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 23.1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 23.1.1 Premature removal of Kirschner wires | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 23.1.2 Avascular necrosis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 23.1.3 Infection or haematoma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 23.2 Neer scores (0 to 100: higher score = better function) | 1 | Other data | No numeric data |
Comparison 24. Postoperative: early (2 weeks immobilisation) versus late (6 weeks) mobilisation after cemented hemiarthroplasty.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 24.1 Oxford Shoulder Score (OSS) at 1 year (adjusted: 0 to 100 best) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 24.2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 24.2.1 Greater tuberosity migration (all had severe pain at 6 & 12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 24.2.2 Non‐union (with bone resorption) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 24.2.3 Malunion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 24.2.4 Superior luxation of prosthesis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 24.3 Constant shoulder score (at 1 year) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 24.3.1 Overall score (0 to 100: best) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 24.3.2 Pain component (0 to 15: best)) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 24.3.3 Activities of daily living component (0 to 25: best) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 24.3.4 Mobility component (0 to 40: best) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 24.3.5 Strength component (0 to 25: best) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 24.4 Range of motion at 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 24.4.1 Elevation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 24.4.2 External rotation (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
24.2. Analysis.

Comparison 24: Postoperative: early (2 weeks immobilisation) versus late (6 weeks) mobilisation after cemented hemiarthroplasty, Outcome 2: Adverse events
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agorastides 2007.
| Study characteristics | ||
| Methods | Randomised using sequentially‐numbered, sealed envelopes Assessor blinding: stated for Constant and Oxford scores at 6 and 12 months Loss to follow‐up at 1 year: 10 (all exclusions: 4 wrong prosthesis; 1 pathological fracture; 1 deep infection requiring further procedure; 2 initial greater tuberosity malpositioning; 2 did not attend follow‐up visits) | |
| Participants | Royal Liverpool Hospital, Liverpool, UK Period of study recruitment: October 2002 to October 2003 59 participants with displaced proximal humeral fractures, 3‐part or 4‐part or articular fractures who were treated with cemented hemiarthroplasty. Isolated non‐pathologic fractures < 6 weeks old. Physiologically old participants with poor bone quality. Informed consent Exclusion criteria: no extra information Of 49: 39 female, 10 male; mean age 70 years, range 34 to 85 years | |
| Interventions | Intervention started post surgery (mean 10 days; range 1 to 30 days after injury)
1. Early active‐assisted mobilisation (after 2 weeks). Arm kept in sling in neutral rotation for 2 weeks; only pendulum and elbow exercises allowed. Between weeks 3 and 6, progressed to active‐assisted exercises; from week 7, to active exercises.
2. Late mobilisation (after 6 weeks). Arm kept in sling in neutral rotation for 6 weeks; only elbow exercises allowed. From week 7 to week 12, progressed from pendulum to active‐assisted exercises; from week 13, to active exercises. Both mobilisation protocols were supervised by a team of specialist shoulder physiotherapists. |
|
| Outcomes | Length of follow‐up: 1 year; also assessed at 2 and 6 weeks, and 3 and 6 months (coinciding with outpatient visits) Oxford Shoulder Score Constant score (mobility, strength, pain, activities of daily living) Range of motion: elevation, external and internal rotation Complications Radiological assessment: greater tuberosity migration; superior luxation of prosthesis | |
| Funding and conflict of interest statements | There was no statement on funding. There was no statement on conflicts of interest. | |
| Notes | The early mobilisation regimen represented normal practice at the hospital. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of method: "Patients were randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | "Randomization took place in the operating theater after the procedure, by use of sequentially numbered, sealed envelopes." |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | "At the 6‐ and 12‐month visits, an independent blinded observer completed the Constant Shoulder Assessment and Oxford scores." However, care providers and participants were not blind to allocation, and assessment of complications was also not blinded. |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Incomplete account of participant flow, with exclusion of 10 participants from the analyses. |
| Selective reporting (reporting bias) | High risk | No protocol available. May have been stopped early; greater tuberosity migration not specifically listed in brief trial entry in the National Research Register (UK). |
| Balance in baseline characteristics? | Unclear risk | Incomplete data to back up claims of lack of baseline differences as these given only for 49 (10 excluded) but a 5‐year difference in mean age (72 versus 67 years). |
| Free from performance bias? | Unclear risk | Although 3 upper‐limb surgeons performing the operations agreed to the same procedures, a different uncemented prosthesis was used in 4 subsequently excluded participants. "Both mobilization protocols were supervised by a team of specialist shoulder physiotherapists." |
Bertoft 1984.
| Study characteristics | ||
| Methods | Use of permutation table, single‐blind, independently administered Assessor blinded Loss to follow‐up at 1 year: 7/20 (2 excluded) | |
| Participants | Central hospital, Vasteras, Sweden Period of study recruitment: not stated 20 participants with non or minimally displaced proximal humeral fractures (7 had fracture of the greater tubercle); sling for 10 days. Exclusion criteria: no information 17 female, 3 male; mean age 64 years, range 50 to 75 years | |
| Interventions | Interventions started 10 to 12 days post injury, after removal of sling. 1. Instructed self‐exercise: participants instructed to train 5 to 10 minutes, 4 to 5 times daily. They had three training sessions (day 1, weeks 3 & 8 post injury) 2. Conventional physiotherapy: 9 sessions (average 20 to 30 minutes), 1 to 2 times each week, over 10 to 12 weeks. No thermoelectrotherapy. Assigned: 10/10 Completed ( > 1 year): 7/6 | |
| Outcomes | Length of follow‐up: 1 year; also assessed at 3, 8, 16 & 24 weeks Range of motion: forward flexion (graph), abduction, internal & external rotation Functional movements: placing hand on neck, placing hand on back Pain: when placing hand on neck: combing hair (graph) Isometric muscle strength: vertical & horizontal pushing Change of treatment requested | |
| Funding and conflict of interest statements | There was no statement on funding. There was no statement on conflicts of interest. | |
| Notes | The 2 excluded participants were in the control group: 1 died and 1 underwent an operation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mention of "permutation table" and "randomized controlled" trial |
| Allocation concealment (selection bias) | Low risk | "A third person was responsible for the randomization procedure and kept the key to the permutation table" |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | "A second physiotherapist examined the patients. She did not know to which group the patient belonged, and the patients were instructed not to tell her." However, there is no guarantee of blinding and, for practical reasons, neither participants nor care provider were blinded. |
| Blinding (performance bias and detection bias) Death, reoperation | Low risk | Lack of blinding unlikely to affect assessment of these outcomes |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Participant flow provided but large loss to follow‐up (7/20 = 35%). |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Participant flow provided |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge this. |
| Balance in baseline characteristics? | Unclear risk | Incomplete data to back up claims of lack of baseline differences but a 4‐year difference in mean age between groups (66 versus 62 years). |
| Free from performance bias? | Low risk | No indication of performance bias. |
Biermann 2020.
| Study characteristics | ||
| Methods | Method of randomisation: computer‐generated randomisation sequence; closed envelopes Assessor blinding: blinded Loss to follow‐up at 12 months: 10/72 (1 lost to follow‐up; 3 died; 6 excluded as biceps tenodesis performed) |
|
| Participants | University Hospital, Munich, Germany
Period of study recruitment: January 2016 to April 2018 72 participants with displaced (> 1 cm or > 45 degrees angulation) intracapsular proximal humeral fractures. Age 18+ years. Written informed consent. Exclusion criteria: previous fracture of the proximal humerus, other injuries to the same proximal humerus requiring surgery, polytrauma, concomitant nerve injury, known rotator cuff tear, alcoholism or drug use, non‐statutory independent person, dementia, non‐command of written or spoken German language, open fracture, medical conditions that precluded surgery Post‐randomisation exclusion: biceps tenodesis performed due to concomitant injury of the long head of the biceps tendon (dislocation, instability, subluxation, and/or tear): this occurred in 3 participants of each group Of 62: 41 female, 21 male; mean age 65 years, range not given |
|
| Interventions | Surgery was within 5 days of fracture. Used deltopectoral approach with the patient placed in the beach chair position. General anaesthesia. Open reduction and internal fixation with a locking (PHILOS) plate placed 5 mm lateral to the bicipital groove and 5 mm inferior to the most lateral insertion of the supraspinatus tendon. Full‐thickness rotator cuff tears were treated if present. Tuberosity sutures used in all cases. Two locking screws were placed in the inferior third of the humeral head (calcar screws). 1. Additional glenohumeral joint lavage. A 1.8 x 43 mm paracentesis needle was placed in the anterior glenohumeral joint space in between the humeral head and the glenoid rim superior to the palpated subscapularis tendon and inferior to the supraspinatus tendon. A 50‐mL syringe filled with sterile Ringer solution was connected via a luer lock adapter; the fluid was gently infused inside the capsule and then aspirated. After aspiration, the humerus was abducted and rotated in order to mobilise clotted hematoma. A newly filled syringe was used for every flushing process. This procedure was repeated until no remnant of the hemarthrosis was detected, followed by the aspiration of residual fluid only. During movements of the arm, the tip of the needle was pulled back just enough to remain inside the capsule in order to prevent damage to the glenohumeral cartilage. 2. No additional glenohumeral joint lavage Sling for postoperative comfort and pain management during the first week. Passive‐ and active‐assisted abduction and elevation until 90o from the first day after surgery. Assistive rotation was allowed up to 20o postoperatively, progressing to 40o "after 6 weeks". After 6 weeks, no further restrictions. Rehabilitation was monitored and followed 2 to 3 times a week until 6 months from surgery. Assigned: 36/36 Completed (1 year): 31/31 |
|
| Outcomes | Length of follow‐up: 1 year; also assessed at 6 weeks and 3 & 6 months Constant score Shoulder range of motion Complications: total, screw penetration (0), plate malposition (0), avascular necrosis, secondary displacement, adhesive capsulitis Mortality |
|
| Funding and conflict of interest statements | There was no statement on direct funding of the trial. The authors declared that they and their families had no commercial conflict of interests. |
|
| Notes | Previously ongoing Date of first enrolment: 31 January 2019 Dr Ben Ockert Ludwig‐Maximilians‐Universität München Nußbaumstr. 20, 80336 Munich, Germany E‐mail: ben.ockert at med.uni‐muenchen.de |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was accomplished by an external operating system ‘‘randoulette’’ from the Institute of Biometry and Epidemiology of our institution." |
| Allocation concealment (selection bias) | Unclear risk | "Randomization was concealed by the use of a closed envelope technique, which was opened by the surgeon just before performing the operation" No mention of the envelopes being opaque or sequentially numbered. |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | Low risk | "Randomization was ... kept blinded to the patient and the staff assessing the clinical follow‐up for the whole study period." |
| Blinding (performance bias and detection bias) Death, reoperation | Low risk | "Randomization was ... kept blinded to the patient and the staff assessing the clinical follow‐up for the whole study period." |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Participant flow diagram provided. Losses, including post‐randomisation exclusions based on intraoperative findings, were the same in each group: 5/36 (14%). |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Participant flow diagram provided. Losses, including post‐randomisation exclusions based on intraoperative findings, were the same in each group: 5/36 (14%). |
| Selective reporting (reporting bias) | High risk | Retrospective trial registration. Incomplete definition of study inclusion in trial report. No patient‐reported outcome measures (PROMs). |
| Balance in baseline characteristics? | Unclear risk | Baseline characteristics provided only for those in the analyses. No major imbalances in these but distribution of surgically‐treated rotator cuff tears not reported. |
| Free from performance bias? | Low risk | Surgery was by 3 experienced senior trauma surgeons who used the same operating procedures in both groups. There was a common rehabilitation protocol in both groups. |
Boons 2012.
| Study characteristics | ||
| Methods | Method of randomisation: computer‐generated randomisation sequence; sealed opaque envelopes stored in statistician's room Assessor blinding: not blinded Loss to follow‐up at 12 months: 3/50 (2 withdrawn; 1 died) |
|
| Participants | Rijnstate Hospital, Arnhem, the Netherlands
Period of study recruitment: June 2004 to July 2009
50 participants with an acute displaced (based on Neer's criteria) 4‐part proximal humeral fractures (8, 4 in each group, had valgus impacted fractures; no mention of fracture‐dislocations). Age 65 or older. Informed consent.
Exclusion criteria: pre‐existing mental disorders (dementia) or unable to provide informed consent or answer the questionnaires; disabling disorder or additional trauma to the affected arm; pathological or open fracture; associated neurovascular injury; pre‐existing impairment of the contralateral shoulder; unable to understand Dutch; unable to participate in the rehabilitation protocol; contraindicated for surgery (American Society of Anesthesiologists (ASA) physical status 4). 47 female, 3 male; mean age 78 years, range not stated |
|
| Interventions | Randomisation was performed in the first week after fracture.
1. Surgery: operation within 7 days of injury. Under general anaesthesia. Humeral head replacement using a deltopectoral approach with the Global Fx shoulder fracture endoprosthesis (DePuy, Leeds, UK). All prostheses were cemented. Cancellous bone graft from the head fragment was applied on the proximal stem before restoration of the tuberosities. Non‐absorbable sutures used to encircle the tuberosities to "enhance anatomic restoration". (All participants had prophylactic antibiotics.) Post surgery: shoulder immobiliser for 6 weeks.
2. Non‐surgical treatment: shoulder immobiliser for 6 weeks. Rehabilitation was same in both groups: experienced shoulder physical therapists instructed the participants for 40‐minute sessions three times a week up to 12 weeks. Up to 2 weeks light passive range of motion (ROM) movements; between 2 and 6 weeks, passive ROM up to 45 degrees forward flexion and abduction and active ROM up to 30 degrees forward flexion and abduction were allowed if pain control adequate; no external rotation. After 6 weeks, unlimited passive glenohumeral exercise, with active ROM up to 90 degrees in forward flexion and abduction. External rotation was allowed up to 30 degrees. After the 3‐month visit, participants were seen by the physical therapist every month until the 12‐month follow‐up, with an emphasis on maximizing ROM, strength and return to daily activities. Assigned: 25/25 Completed (at 1 year): 23/24 (based on text account) |
|
| Outcomes | Length of follow‐up: 1 year; also assessed at 1 & 6 weeks and 3 months
Constant score (contralateral shoulder measured at 1 week follow‐up as reference)
SST (Simple Shoulder Test)
Pain (visual analogue scale (VAS)) Disability (VAS) Abduction strength (contralateral shoulder measured at 1 week follow‐up as reference) Range of motion: abduction, flexion, external rotation, internal rotation (lumbar level) Complications: non‐union, osteonecrosis, pain and impingement, heterotopic ossification, infection, implant dislocation (head stem separation), secondary migration of greater tuberosity, secondary rotator cuff tear (from migration of hemiarthroplasty), non‐union of greater tuberosity Subsequent surgery (reasons: head stem separation and pain and impingement) |
|
| Funding and conflict of interest statements | There was no statement on direct funding of the trial. The institution of 4 authors received funding from the St Elisabeth Research Foundation, connected with DePuy Orthopaedics, Inc. The authors declared that, individually, they had no commercial associations, such as consultancies, that might pose a conflict of interest. | |
| Notes | Additional information on group of a withdrawn participant (had deteriorating condition) and SST scores (incorrect in Table 2 in article) requested from Dr van Loon (14 Feb 2015). Response from Dr Boons (12 March 2015) identified group of the withdrawn participant (hemiarthroplasty) and provided the raw data for SST. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[P]atients were randomly allocated to nonoperative treatment or hemiarthroplasty. The randomization list was generated by an independent statistician". "A computer‐generated variable block schedule was used..." |
| Allocation concealment (selection bias) | Low risk | "[T]he resulting treatment allocations were stored in sealed opaque envelopes in the statistician’s room." |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No mention of independent or blinded assessment. Initial care providers could not be blinded for these contrasting interventions. |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Lack of blinding unlikely to affect assessment of these outcomes |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Low risk | Active and systematic surveillance. Low loss to follow‐up (6%). Author's response resolved problem over contradictory statements in trial report on group allocation for one participant. |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Active and systematic surveillance. Low loss to follow‐up (6%). Author's response resolved problem over contradictory statements in trial report on group allocation for one participant. |
| Selective reporting (reporting bias) | Unclear risk | No protocol. Outcome measures well described but the problems with the SST scores (incorrect data and direction of effect) gave slight cause for concern |
| Balance in baseline characteristics? | Low risk | No difference in baseline characteristics, including in numbers of valgus impacted (4 versus 4) fractures |
| Free from performance bias? | Low risk | Standard procedure performed by two experienced shoulder surgeons. Same rehabilitation protocols including during shoulder immobilisation |
Buecking 2014.
| Study characteristics | ||
| Methods | Method of randomisation: block randomisation stratified by type of fracture; pre‐sealed randomisation envelope given by study staff to surgeon before surgery Assessor blinding: not blinded (independent observer) Loss to follow‐up at 12 months: 13/120 (9 lost to follow‐up; 4 died) |
|
| Participants | University Hospital of Giessen and Marburg, Marburg, Germany
Period of study recruitment: December 2009 to November 2011
120 participants with displaced proximal humeral fractures (Neer 2‐part; and 3‐ or 4‐part). Age 18 or older. Written consent.
Exclusion criteria: glenohumeral dislocation, concomitant ipsilateral fractures of the arm or forearm, malignancy‐related fractures, multiple trauma, other surgery planned (prosthesis or a longer plate) 92 female, 28 male; mean age 68 years, range 63 to 72 |
|
| Interventions | Randomisation was performed prior to surgery; timing not stated All participants received a plate osteosynthesis with the non‐contact bridging plate for the proximal humerus. In addition to the plate, a cable wire was used to fix the greater tuberosity in 3‐ and 4‐part fractures. All participants received a single‐shot antibiotic. 1. Deltoid‐split approach: anterolateral 3 cm deltoid split with two small incisions for the three locking screws in the humeral shaft. 2. Deltopectoral approach: fracture was exposed through a classical anterior approach; a 10 to 12 cm incision was begun at the tip of the coracoid process and run medially in the direction of the deltoid muscle. Rehabilitation after surgery was same in both groups. Operated shoulder immobilised for first 2 days; then passive and limited active motion started. For 3‐ and 4‐part fractures, only limited assisted abduction up to 90º was allowed for first 6 weeks. Assigned: 60/60 Completed (at 1 year): 48/42 |
|
| Outcomes | Length of follow‐up: 1 year; also assessed at 6 weeks and 6 months
Constant score (normalised)
Activities of daily living (Lawton 1969)
Pain (VAS) Complications: humeral head necrosis (0), axillary nerve damage (0), deep infection, screw perforations, implant loosening at head or shaft, inadequate reduction, implant failure from subsequent fall Change during primary operation: primary prosthesis inserted Reoperations (for complications and at request of participant) Fluoroscopy use Length of surgery Hospital stay |
|
| Funding and conflict of interest statements | There was no statement on direct funding of the trial. The authors confirmed that they had no funding or commercial associations that might pose a conflict of interest in connection with the submitted article. | |
| Notes | The range of scores for Lawton's instrumental activities of daily living score is from 0 to 8 (best score); those reported in this trial report include mean scores of 11 up to 21. Since there is no explanation for this in the trial report, the meaning of the data is unclear and they are not presented in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "With the use of a block randomization stratified by type of fracture (two‐part fractures versus three‐ and four part fractures), patients were randomized to either the deltoid‐split or the deltopectoral approach." Not quite enough detail to be certain, although likely |
| Allocation concealment (selection bias) | Low risk | "Presealed randomization envelopes were given by the study staff to the attending surgeon before surgery." |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | "Standardised follow‐up examinations were performed at 6 weeks, 6 months, and 12 months after surgery by the same independent observer..." However, the incision would still have been obvious. |
| Blinding (performance bias and detection bias) Death, reoperation | High risk | Unclear if choices for primary or secondary surgery were influenced by prior knowledge. No revisions to hemiarthroplasty in the deltoid‐split group could indicate that reoperation was only considered if a deltopectoral approach could be enlarged. |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Inappropriate post‐randomisation exclusions: primary prosthesis (2 versus 4), secondary prosthesis (5 versus 2) and enlarged deltopectoral approach during revision surgery (0 versus 4). |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Inappropriate post‐randomisation exclusions: primary prosthesis (2 versus 4), secondary prosthesis (5 versus 2) and enlarged deltopectoral approach during revision surgery (0 versus 4). However, these were known for these outcomes. Similar numbers were not reachable (4 versus 5) |
| Selective reporting (reporting bias) | Unclear risk | No protocol. Although systematic data collection and all outcomes reported, the disparity between the reported data for the Lawton instrumental activities of daily living score (maximum 8) and the actual scores presented in the text is of some concern |
| Balance in baseline characteristics? | Low risk | Good balance in baseline characteristics |
| Free from performance bias? | Low risk | Operations were performed by 3 senior surgeons who were trained in both techniques. Equivalent care programmes in both groups |
Cai 2012.
| Study characteristics | ||
| Methods | Method of randomisation: no details Assessor blinding: not blinded (independent observer) Loss to follow‐up at 24 months: 5/32 (4 lost to follow‐up; 1 died) |
|
| Participants | Shanghai Tenth People’s Hospital of Tongji University, Shanghai, China Period of study recruitment: April 2005 to March 2010 32 participants with acute displaced 4‐part proximal humeral fracture of the surgical neck (Neer classification). At least one tubercle needed to be displaced more than 10 mm in relation to the head fragment but the other did not need to meet this criterion (thus 3‐part fractures were also acceptable); see Notes. Age 67 or older with low‐energy trauma. Independent living conditions (not institutionalised), and no severe cognitive dysfunction (3 or more correct answers on a 10‐item Short Portable Mental Status Questionnaire (SPMSQ)) Exclusion criteria: completely displaced shaft in relation to the head fragment, such as a fracture without bony contact; valgus impacted fracture, previous shoulder problems 27 female, 5 male; mean age 72 years, range 67 to 86 |
|
| Interventions | Randomisation was performed after clearance by an anaesthetist prior to surgery; timing not stated. All participants received a single dose of antibiotic preoperatively. 1. Hemiarthroplasty using the DuPuy prosthesis with suturing of tuberosities. Cemented stem. Bone graft from removed humeral head used to restore the humeral offset 2. Open reduction and internal fixation with PHILOS plate. Suturing of tuberosities Postoperative arm sling for 4 weeks (optional thereafter). All participants referred to physiotherapy. Pendulum exercises and passive elevation/abduction up to 90° were started on postoperative day 1. After 4 weeks, the participants were allowed free active range of motion. Assigned: 19/13 Completed (at 2 years): 15/12 |
|
| Outcomes | Length of follow‐up: 2 years; also assessed at 4 and 12 months
DASH Constant score Pain (VAS) Complications (relating to reoperations): non‐union, fixation failure, dislocation, infection, prosthesis loosening Reoperations (for complications) Length of surgery |
|
| Funding and conflict of interest statements | The study was supported by the National Science Foundation for Distinguished Young Scholars of China (30901529) and Research Fund for the Doctoral Program of Higher Education (20090072120021) and the Bureau of Public Health, China. There was no statement on conflicts of interest. |
|
| Notes | One participant initially had a 3‐part greater tuberosity fracture but at surgery, the lesser tuberosity was also found to be displaced > 1 cm. Hence all had 4‐part fractures. Three of 32 participants had fracture dislocations. Sent email to Dr Li requesting details of the randomisation method and clarification on deaths on 24 May 2015: no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[T]he patients were randomized". No other details |
| Allocation concealment (selection bias) | Unclear risk | "[T]he patients were randomized". No other details |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Not blinded even though there was some independent assessment at final follow‐up: "Final 24‐month follow‐up was performed by an independent orthopedic surgeon (K.T.) not involved in treatment." |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Lack of blinding less likely to affect assessment of these outcomes. Standardisation of assessment |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Active and systematic surveillance and clear participant flow diagram. However, more participants lost to follow‐up in the hemiarthroplasty group (4 (21%) versus 1 (8%)). There are also some incorrect percentages that give rise to concern. |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Active and systematic surveillance and clear participant flow diagram. It is likely that participants with complications would have returned. |
| Selective reporting (reporting bias) | Low risk | No protocol. However, systematic data collection and reporting of all outcomes. |
| Balance in baseline characteristics? | Unclear risk | Where reported, the baseline characteristics were balanced in the two groups. However, the baseline distribution of the fracture types, which included three 4‐part fracture dislocations, was not reported. |
| Free from performance bias? | Low risk | "All patients underwent surgery performed by 1 of 2 orthopedic surgeons (M.C., S.L.), both experienced in shoulder surgery." Same rehabilitation. |
Carbone 2017.
| Study characteristics | ||
| Methods | Method of randomisation: use of random blocks Assessor blinding: the Constant score was measured by a physiatrist blinded to randomisation Loss to follow‐up at 24 months: 5/32 (4 lost to follow‐up; 1 died) | |
| Participants | Ospedale San Camillo de Lellis, Rieti, Italy Period of study recruitment: June 2014 to December 2015 80 people with stable impacted (any part of metaphysis or head was impacted into the shaft) osteoporotic (cortical bone thickness lower than 6 mm) proximal humeral fracture presenting at the emergency department; signed informed consent Exclusion criteria: fractures showing metaphyseal comminution and a fifth fragment. By implication, hospitalised for medical comorbidities or concomitant proximal femur or wrist fracture 60 female, 20 male; mean age 74 years, range 57 to 86 |
|
| Interventions | Randomisation was performed after assessment of X‐rays and a computed tomography (CT) scan indicated suitable fracture and informed consent obtained. In both groups, the arm sling was prescribed for the first 3 weeks, with removal of the sling for the rehabilitation time and for hygienic care. 1. Rehabilitation began 7 days after the fracture. It consisted of one hour sessions under the supervision of a physiotherapist, five times a week, with the oral analgesics (etoricoxib 90 mg or paracetamol 1000 mg) prescribed 2 hours before the session. Treatment consisted of:
After 10 sessions, active motion was started for 10 further sessions, with a frequency of three times a week, 1 hour per session. Then, participants were advised to perform daily exercises at home. 2. The same protocol was adopted, with a frequency of twice a week sessions in the first 10 sessions. Participants underwent a total of 20 sessions in both groups. Assigned: 40/40 Completed (at 1 year): 36/39 |
|
| Outcomes | Length of follow‐up: 1 year; also assessed at 3 and 6 months Subjective shoulder value Constant score Loss of reduction and non‐union Reoperations (for non‐union) Non‐compliance with rehabilitation programme |
|
| Funding and conflict of interest statements | There was no statement on funding. The authors declared that they had no conflicts of interest. | |
| Notes | The TECAR acronym is based on Spanish for Capacitive Resistive Electric Transfer (Transferencia Eléctrica Capacitiva Resistiva.) This refers to a non‐invasive radio frequency treatment that results in localised heating. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients ... were randomly allocated in one of the two groups by an independent researcher using random blocks". Insufficient detail to judge |
| Allocation concealment (selection bias) | Unclear risk | "Patients ... were randomly allocated in one of the two groups by an independent researcher using random blocks". Insufficient detail to judge |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | The Constant score was “measured by a physiatrist blinded to randomization”. However, this does not apply to other outcomes and there is some risk. |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Lack of blinding less likely to affect assessment of these outcomes. |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | 4 (10%) versus 1 (2.5%). Overall this is quite a low loss to follow‐up although different in the two groups; unlikely to affect outcome although not intention‐to‐treat as these are excluded for non‐compliance |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | As above, but outcomes less susceptible as very few. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or published protocol. Seems OK but some complications might be unreported (as not sought?) |
| Balance in baseline characteristics? | Low risk | Sufficiently balanced in key characteristics. Some imbalance in integrity of medial hinge (12 versus 18) but unlikely to make a difference here. |
| Free from performance bias? | Unclear risk | Insufficient information on care providers to judge. |
DelPhi 2020.
| Study characteristics | ||
| Methods | Method of randomisation: secure web‐based randomisation program Assessor blinding: single‐blinded (physiotherapists for measuring the Constant score) Loss to follow‐up at 2 years: 20/124 (15 withdrew; 5 died) | |
| Participants | 7 hospitals, Norway
Period of study recruitment: 1 January 2013 to 1 June 2017
124 people, aged 65 years to 85 years, admitted in hospital with a severely displaced proximal humeral fracture of type B2 or C2 (OTA/AO 2007 revision). Severe displacement was defined as > 45° valgus or > 30° varus in a true anteroposterior projection, > 45° angulation in a scapular Y projection with the arm in neutral rotation, or > 50% displacement of the humeral head against the metaphysis. The degree of tubercle displacement was not critical for inclusion. Written consent. Exclusion criteria: previous injury or illness of the injured or contralateral shoulder, concomitant injury to the ipsilateral or contralateral upper extremity, alcohol or other substance abuse, dementia or neurological disease, non‐Norwegian‐speaking patients, glenoid fracture or deformity, or patients who were deemed noncompliant to rehabilitation. Head‐split fractures, fracture‐dislocations, and high‐energy trauma. 111 female, 13 male; mean age 75 years, range 64.8 to 85.8 years |
|
| Interventions | Preoperative radiographs and CT scans examined by dedicated orthopaedic surgeon at recruiting hospitals and verified by coordinating surgeon at Oslo University Hospital. Surgery at mean 5.4 days since injury. 1. Reversed total shoulder prosthesis. Use of a deltopectoral approach, resection of the supraspinatus tendon and insertion of a monobloc cemented reversed total shoulder arthroplasty. Delta Xtend Reverse Total Shoulder Arthroplasty (DePuy Synthes) (6 hospitals, 52 participants) or the Promos Reverse Prosthesis (Smith & Nephew) (1 hospital, 12 participants). Then, physical therapy with active‐assisted exercises for the first 6 weeks with restrictions relating to external rotation of the shoulder. Activating the deltoid muscle with assisted physiotherapy was considered equally important. 2. ORIF with PHILOS angular stable plate (Synthes, Switzerland). Deltopectoral approach. Thread cerclages used to secure the tubercles and rotator cuff insertions to the plate. If judged necessary by the surgeon, a bone substitute or bone graft could have been used to enhance stability of the fracture (Norian® or autologous bone graft from the iliac crest). Exercises were started immediately after the surgical procedure, with limitations of resistance exercises in the first 6 weeks. Both groups started standardised patient exercises and supported physiotherapy during the first 3 postoperative days. Differences in timing of active exercises were tabulated but with some potential inconsistencies with the text: functional exercises (6 versus 4 weeks); dynamic strengthening exercises. Isometric resistance exercises (8 versus 6 weeks); and progressive strengthening exercises (12 versus 8 weeks). Assigned: 64/60 Completed (at 2 years): 57/47 |
|
| Outcomes | Follow‐up: 3, 6, 12 and 24 months (5 years planned in protocol) Oxford Shoulder score Complications (total, nerve injury, screw penetration, deep wound infection, periprosthetic fracture or fracture distal to plate, glenoid fracture, rotator cuff rupture) Reoperations Mortality Quality of life measured with the 15D score Constant score Radiographic results Health economic outcomes (not reported yet; planned in protocol) |
|
| Funding and conflict of interest statements | Funding: Sophies Minde Ortopedi AS https://sophiesminde.no) contributed to the study with research grants. (Grant for general research in collaboration with Oslo University Hospital; co‐ordinating centre.) Potential conflicts of interest: two authors indicated that they had a relevant financial relationship in the biomedical arena (Sophies Minde Ortopedi AS) outside the submitted work |
|
| Notes | This appeared as an ongoing trial in the 2015 version of the review as DELPHI. Final report provided to HH by Dr Tore Fjalestad; now published. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized using a secured web solution, NTNU WebCRF" |
| Allocation concealment (selection bias) | Low risk | "Patients were randomized using a secured web solution, NTNU WebCRF" |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Participants and care providers not blinded. Some between‐group differences in the staging of rehabilitation could have reinforced this. Blinding only for assessment of Constant scores. Unavoidable risk of bias. |
| Blinding (performance bias and detection bias) Death, reoperation | Low risk | Lack of blinding unlikely to affect assessment of these outcomes in this trial. |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Participant flow provided. Some imbalance between losses to follow‐up between the two groups at 2 years (7/64 (11%) versus 13/60 (22%)) that could have influenced the results. |
| Incomplete outcome data (attrition bias) Death, reoperation | Unclear risk | Participant flow provided. Some imbalance between losses to follow‐up between the two groups at 2 years (7/64 (11%) versus 13/60 (22%)) that could have influenced the results. |
| Selective reporting (reporting bias) | Low risk | Study protocol available and outcomes are prespecified except for the planned intention to report the difference between the Constant scores of the injured and the uninjured shoulder and the Constant score adjusted for age and gender. Instead of the latter, the authors stratified on fracture type and age. Although these are changes to the protocol, the reported outcomes are valid and clearly defined. |
| Balance in baseline characteristics? | Low risk | Baseline characteristics were balanced except for diabetes (RSA 8, ORIF 1) and hand dominance (RSA1, ORIF 8). We considered these imbalances were unlikely to importantly affect the trial results. |
| Free from performance bias? | Low risk | Efforts made to standardise treatment and outcome assessment including documents on the trial website. All physiotherapists took part in workshops before the start of the trial. All attending surgeons were consultant orthopaedic surgeons experienced with both RTSA and plate fixation. The surgeons attended meetings on technical standardisation of the procedures, and the senior author (TF) took part in the first operations in the attending hospitals. |
Fialka 2008.
| Study characteristics | ||
| Methods | Method of randomisation: referral to random list and randomisation timed at surgery Assessor blinding: no Loss to follow‐up at 1 year: 5/40 (3 deaths, 2 lost to follow‐up) | |
| Participants | Vienna General Hospital, Austria Period of study recruitment: not stated ‐ lasted 22 months 40 participants with acute 4‐part (Neer) proximal humeral fractures (type C: AO/ASIF classification), aged > 50 years, no history of previous problems in either shoulder, informed consent Exclusion criteria: concomitant vascular or neurological injuries of involved limb; prior operative procedures; neurologic or mental disorders; or drug abuse 30 female, 10 male; mean age 75 years; of 35: range 56 to 88 years | |
| Interventions | Surgery started 7.3 days after injury (0 to 26 days). General anaesthesia used in all cases. Stems were cemented in place and bone grafting was performed using cancellous bone from patient's humeral head. 1. Hemiarthroplasty using EPOCA prosthesis (Argomedical: see Notes). Fixation of tuberosities using wire cables threaded through a medial and lateral hole in the stem. 2. Hemiarthroplasty using HAS prosthesis (Stryker). Fixation of tuberosities using transosseous braided sutures tied to lateral fin of the stem. Same general rehabilitation protocol used for both groups: shoulder kept for 2 weeks in immobiliser to prevent active external rotation, passive movement for 15 minutes per day by physiotherapist to avoid contractures and shoulder stiffness. Then, active range of motion increased to horizontal level. Active external rotation initiated after another 2 weeks. Assigned: number in each group not known Completed (at 1 year): 18/17 | |
| Outcomes | Length of follow‐up: 1 year; also assessed at 12 days, 3 and 6 weeks, and 6 months Functional assessment (individual Constant score, where results were relative to participant's unaffected shoulder) Range of motion (active forward flexion, abduction, external rotation) Radiological assessment: resorption of tuberosities, superior migration of prosthesis, anterior subluxations, glenoid erosion, aseptic stem loosening, secondary dislocation of the tuberosities, heterotopic ossification Deep infection Periprosthetic fracture Reoperation and scheduled for reoperation (persistent pain) Mortality | |
| Funding and conflict of interest statements | There was no statement on funding. There was no statement on conflicts of interest. | |
| Notes | Differences between the two prostheses include the type and position of fixation of the tuberosities and the volume of the stem in the metaphyseal area, thus allowing different amounts of additional (autologous) cancellous bone grafting.
The data for heterotopic ossification were contradictory and not used here.
Request for information sent to contact trialist on 19 February 2010: no response. In trying to locate the two devices, we found that "Synthes GmbH took over the EPOCA Shoulder Prosthesis System and all the respective rights from Argomedical AG" in 2007: Argomedical website: www.argomedical.online/products/shoulder-surgery.html (accessed 2 May 2022). We were unable to find any reference to the HAS prosthesis on the Stryker website. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The random list was designed to finally produce 2 groups of equal size." |
| Allocation concealment (selection bias) | Unclear risk | "Each surgeon was informed at the beginning of the operation as to which implant had randomly been selected." |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No blinding. |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Lack of blinding unlikely to affect assessment of these outcomes. Standardisation of assessment. |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | High risk | The group allocation and baseline data were not provided for 5 participants: 2 lost to follow‐up and 3 who had died. Standard deviations not provided. |
| Incomplete outcome data (attrition bias) Death, reoperation | Unclear risk | Group allocation not provided for those who had died. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge this. |
| Balance in baseline characteristics? | Unclear risk | Incomplete baseline data (5 excluded) to confirm baseline comparability of those in analysis. |
| Free from performance bias? | Low risk | No indication of performance bias: a "general rehabilitation protocol was used for all patients regardless of the type of implant."; each of the 4 participating surgeons was experienced in joint replacement surgery. |
Fjalestad 2010a.
| Study characteristics | ||
| Methods | Method of randomisation: use of computer software by independent hospital statistician; block size 12; use of numbered opaque sealed envelopes Assessor blinding: no, but assessment by two independent physiotherapists Loss to follow‐up at 1 year: 2/50 (2 deaths); at 2 years: 8/50 (3 deaths) | |
| Participants | Olso University Hospital, Oslo, Norway Period of study recruitment: May 2003 to May 2008 50 participants with displaced proximal humeral fractures, AO group B2 or C2 (displaced 3‐part and 4‐part fractures) who were admitted to hospital. Malposition was at least 45° angular deviation in the true frontal (inclination) or transthoracic radiographic projections, regardless of whether the fracture was impacted or not. The greater or lesser tuberosity had to be displaced at least 10 mm. Furthermore, the displacement between the head and metaphyseal/diaphyseal main fragments should not exceed 50% of the diaphyseal diameter. Age 60 years or over. Written informed consent. Resident in Oslo. Exclusion criteria: non‐Scandinavian ethnicity, previous history of injury or illness of the injured or contralateral shoulder, injury of the other part of the humerus or the contralateral upper extremity, alcohol or drug abuse, dementia or neurological disease or severe cardiovascular disease that would contraindicate surgery. 44 female, 6 male; mean age 73 years, range 60 to 88 years | |
| Interventions | Interventions (and randomisation) started after hospital admission. (On admission to the hospital, all patients were immobilised in a modified Velpeau bandage.) 1. Surgery: operation occurred within the first week after admission to hospital. Open reduction and fixation using a minimally open deltopectoral approach with an interlocking plate device (Locking Compression Plate (LCP) of the AO basic type, Synthes, Switzerland) and metal cerclages to secure the tuberosities. Surgery was performed under fluoroscopic control. Then immobilisation in a modified Velpeau bandage until self‐exercises and instructed physiotherapy was started on the third postoperative day. 2. Non‐surgical treatment: all participants stayed in the hospital for at least 1 day and received the same instructions from the physiotherapist as those allocated to surgery. If the displacement between the head and metaphyseal fragment (main fragments) exceeded 50% of the diaphyseal diameter (subsequent to randomisation), closed reduction was performed in the operating room under general anaesthesia within 48 hours of admission. Immobilisation in a modified Velpeau bandage for 2 weeks before self‐exercises and instructed physiotherapy started on day 15. The same self‐training programme and instructed physiotherapy programme used for both groups, although the non‐surgical treatment group started 12 days later. Both groups progressed to strengthening exercises at the 6‐week time point. Physical therapy and self‐exercise were recommended for at least 6 months. Assigned: 25/25 Completed (at 1 year): 23/25 | |
| Outcomes | Length of follow‐up: 2 years; also assessed at 2, 8, 12, 26 and 52 weeks Constant Score (both shoulders) (3, 6, 12 and 24 months) ASES (American Shoulder and Elbow Surgeons) questionnaire (sports domains not included ‐ maximum 24 points) (6, 12 and 24 months) Quality of life score: Harri Sintonen 15D instrument (sexual function domain not included) Mortality Fixation failure or redisplacement ‐ subsequent operation Radiographic outcomes including avascular necrosis (score 2 = no changes; 1 = changes to normal trabecular organisation < 50% of humeral head; 0 = > 50% or partial collapse); and post‐traumatic glenohumeral osteoarthritis Check for axillary nerve injury Health economic outcomes, including direct (cost of surgery; cost of hospital stays) and indirect costs (sick leave, family use of time to assist patient) Length of stay in acute hospital and hospital rehabilitation centre | |
| Funding and conflict of interest statements | Funding was provided by Helse Sor‐Ost (Regional Hospital and Health Services in Norway): this paid for 50% salary of a physiotherapist during the study period. The authors declared that they had no conflicts of interest. | |
| Notes | Information on the trial received December 2006 from Dr Tore Fjalestad.
Initially, only some results for one year follow‐up were published. Communication from Dr Tore Fjalestad in April 2010 indicated that the two‐year follow‐up was likely to be finished during 2010. Further information from Dr Tore Fjalestad in April 2012 indicated that the two‐year follow‐up had been submitted to another journal (estimated publication during 2012). More details on non‐surgical treatment were provided in Fjalestad 2012. Tore Fjalestad also provided in an email (April 2012) the following clarification on the use of closed reduction for 8 non‐surgically treated participants (this had not been described in the protocol): "The primary X‐rays were assessed for classification and decision‐making for closed reduction. Those eight patients had a new radiographic examination after allocation to non‐surgical treatment and after the procedure in the operating room, to confirm an acceptable position of the fragments. If not acceptable, the patients had to be treated with ORIF. Surprisingly, only one patient demonstrated unacceptable re‐displacement after two weeks, and was analyzed according to intention‐to‐treat principle in the non‐surgical group at one year." Two‐year follow‐up results published in 2014 (Fjalestad 2014a). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The [randomisation] procedure was designed by the statistician at the hospital research centre using the computer software S‐PLUS 6.0 for Windows 2002 ... Randomisation was based on equal blocks of length 12, with the exception of the last one, which was interrupted due to 50 patients." |
| Allocation concealment (selection bias) | Low risk | "Randomisation was performed by means of consecutively numbered and sealed non‐translucent envelopes containing each participant’s allocation to surgery or to non‐surgical treatment." Independent statistician. |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Two trained physiotherapists performed the 15D interviews. The physiotherapists were not blinded to group assignment. No provider or participant blinding. |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Lack of blinding unlikely to affect assessment of these outcomes, but may affect decisions for subsequent surgery. |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Participant flow diagram provided and intention‐to‐treat analysis conducted. However, reported in 2014: "Missing data were handled according to single imputation by the last observation for each individual." Also, some imbalance in loss to follow‐up: 2 (8%) (surgery) versus 6 (24%) (non‐surgical). |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Participant flow diagram provided and intention‐to‐treat analysis conducted. |
| Selective reporting (reporting bias) | Unclear risk | Trial registered after completion. Small discrepancies in trial inclusion criteria. |
| Balance in baseline characteristics? | Unclear risk | Statistically non‐significant imbalance in gender (5 females versus 1 male) and baseline quality‐of‐life scores (higher in surgical group). |
| Free from performance bias? | Low risk | All the operations were performed by three surgeons experienced in the procedure performed. |
Gracitelli 2016.
| Study characteristics | ||
| Methods | Method of randomisation: use of computer software with allocation concealed with password‐protected database Assessor blinding: yes, evaluations were performed by a physiotherapist Loss to follow‐up at 1 year: 7/72 (4 deaths) | |
| Participants | University Hospital, São Paulo, Brazil Period of study recruitment: May 2011 to March 2014 72 participants with closed displaced surgical neck proximal humeral fractures, without or with involvement of the greater tuberosity (2‐part or 3‐part surgical neck fractures, abiding by the Neer criteria of displacement ≥ 1 cm or ≥ 45 degrees angulation), aged 50 to 85 years, treated surgically ≤ 21 days of injury. Written informed consent. Exclusion criteria: isolated fracture of the greater or lesser tuberosity, Neer 4‐part fractures, fracture involving the articular surface of the humeral head, fracture‐dislocation of the proximal humerus, neurological injuries in the affected limb, previous surgery on the affected shoulder, associated fractures in the affected limb, pathologic fractures, psychiatric illnesses or inability to understand preoperative questionnaires, active infection or previous infection on the shoulder. (Post‐randomisation exclusion criteria listed: loss to follow‐up before the first clinical assessment at 3 months, irreparable tendon tears of the rotator cuff.) Of 65: 47 female, 18 male; mean age 65 years, range not stated | |
| Interventions | Interventions started: surgery on average 9.7 days after injury (randomisation after anaesthesia). All had general anaesthesia with an interscalene brachial plexus block and were positioned in dorsal decubitus with 30 degrees elevation. 1. Internal fixation using a locking plate: PHILOS (DePuy‐Synthes, Solothurn, Switzerland), deltopectoral approach. The plate was placed 1 cm inferior to the upper portion of the greater tuberosity and 1 cm lateral to the long head of the biceps tendon. Three guidewires were inserted through the plate proximal holes, followed by distal fixation with a cortical or locking screw under radioscopic imaging. At least 5 locking screws were inserted proximally, followed by 2 more distal locking screws, with a minimum of 3 distal screws. Tension‐band sutures were placed in the proximal plate holes, with non‐absorbable sutures passed through the rotator cuff tendons. 2. Internal fixation using a locking nail: Centronail (Orthofix, Verona, Italy), 3 proximal locking screws, longitudinal anterolateral transdeltoid approach. Supraspinatus and infraspinatus tendons sutured with non‐absorbable sutures. Greater tuberosity fractures were reduced before the intramedullary nail was inserted. The humeral head was reduced and provisionally fixed with Kirschner wires. A longitudinal 1.5‐cm incision was made in the supraspinatus tendon. The nail was placed 5 mm below the cartilage and proximally fixed with 3 screws and distally fixed with 2 screws. Tension‐band sutures were placed around the proximal screws, with non‐absorbable sutures passed through the rotator cuff tendons. The supraspinatus tendon was repaired with non‐absorbable sutures. Slings were used for 4 weeks. Physical therapy with passive‐ and active‐assisted exercises was started 14 days after surgery. Active and active resisted exercises were started 30 and 60 days after surgery, respectively. Assigned: 36/36 Completed (at 1 year): 29/32 but analysed at 32/33 (data used from 3‐ or 6‐month follow‐up for 3 and 1 participants) |
|
| Outcomes | Length of follow‐up: 1 year (also 3 and 6 months) DASH score Constant score (absolute and relative to opposite shoulder) Pain (VAS) Mortality Complications (overall number of participants with a complication, varus malunion (not reported); osteonecrosis, non‐union, hardware problems, refracture, shoulder stiffness, infection, neurologic injury, rotator cuff tendon tears) Reoperation Radiographic outcomes, including avascular necrosis, union, and loss of reduction of humeral head, loss of reduction of the greater tuberosity, insufficient reduction | |
| Funding and conflict of interest statements | Funding: not reported The authors declared no benefits in any form were received from a commercial entity. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was generated on the website “http://www.randomization.com”, with random blocks, and stratified in 2 levels corresponding to the Neer classification." |
| Allocation concealment (selection bias) | Low risk | "Allocation was performed after anesthesia by using a password‐protected database." Adequate safeguards seem likely. |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | "Outcome evaluations were performed by a physical therapist who did not participate in the rehabilitation of patients and who was blinded to their allocation. The evaluations were performed with the patients wearing clothes to preclude the visualization of the surgical wound." However, participants and personnel were not blinded. |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Lack of blinding unlikely to affect assessment of these outcomes, but may affect decisions for subsequent surgery. |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Participant flow described. Comparable losses to follow‐up in the two groups in terms of the analyses: 4 for the plate group (2 died); and 3 for the nail group (1 died). However, 1 participant in the plate group and 3 in the nail group did not undergo the 12‐month assessment but data from 3 or 6 months were included in the final analysis. Thus, overall 7 (19.4%) versus 4 (11.1%) were lost to follow‐up. |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Participant flow described and comparable between‐group losses. One conversion from nail to plate analysed as allocated. These outcomes are unlikely to have occurred without detection. |
| Selective reporting (reporting bias) | High risk | Trial registration before analysis. However, there were differences in outcomes reported: the UCLA score is not reported but described at trial registration; VAS pain is reported but not described at trial registration. |
| Balance in baseline characteristics? | Unclear risk | Balanced baseline characteristics for those in the analyses. However, baseline characteristics missing for 8 participants. |
| Free from performance bias? | Low risk | All surgery was done by the principal investigator ‐ no indication of experience with the two operations but expected. Other co‐interventions ‐ anaesthesia and rehabilitation ‐ were similar in the two groups. |
Helfen 2020.
| Study characteristics | ||
| Methods | Single‐centre, parallel group, randomised controlled trial. Single‐blinded (participant). | |
| Participants | University Hospital, Munich, Germany
Period of study recruitment: November 2015 to December 2017 60 participants ≥ 60 years or postmenopausal female with a 2‐part surgical neck fracture of the proximal humerus (AO type 11‐A3), signed informed consent Exclusion criteria: not independent, had dementia and/or was institutionalised, did not understand written and spoken guidance in German, pathologic fracture or a previous fracture of the same proximal humerus, alcoholism or drug addiction, other injury to the same upper limb requiring surgery, major nerve injury (e.g. complete radial or axillary nerve palsy), rotator cuff tear arthropathy, open fracture, multi‐trauma or ‐fractured patient, fracture dislocation or head‐splitting fracture, non‐displaced fracture, isolated fracture of the major or minor tubercle, any medical condition that excludes surgical treatment, pregnant 40 female, 20 male; mean age 75 years, range not given |
|
| Interventions | Interventions started: surgery on average 2.7 days after injury. All fractures initially immobilised with a Gilchrist sling and patients admitted to the trauma ward.
1. Augmented plate fixation (PHILOS with augmentation). Deltopectoral approach. Angle stable 3‐hole plate fixation system PHILOS with augmentation (Depuy‐Synthes). For plate fixation, screws are set in a standardised fashion: 7 screws placed subcortical in row A, B, D and F as well as 3 screws bicortical in the shaft (1 proximal non‐locking and 2 distal locking screws). Whenever possible (according to contrast dye testing) the screws in row A and E were augmented with 0.5 mL Traumacem V+ (Depuy‐Synthes). Alternatively screws in row B and D were used 2. Multiplanar intramedullary nail group (MultiLoc). Mini deltoid approach. MultiLoc nail length 160 mm with diameter according to the humeral shaft (Depuy‐Synthes). Three 4.5 mm MultiLoc screws at levels A, B and D were inserted as standard. Two additional 3.5 mm screw‐in screws were optionally used at levels A and B and aimed posteriorly. In the shaft, one ascending calcar screw and two multi‐planar distal locking screws were implanted in all participants. Post‐surgical rehabilitation protocol allowed passive‐ and active‐assisted motion exercises, supervised by a physical therapist, beginning immediately on day one after surgery. Abduction and elevation were limited to 60°, without forced external rotation for the first 6 weeks, followed by active exercises with full range of motion and increasing strength exercises. Assigned: 30/30 Completed (at 2 years): 25/25 |
|
| Outcomes | Follow‐up: 2 years (also 3 weeks, 6 weeks, 3 months, 6 months and 1 year) DASH American Shoulder and Elbow Score (ASES) Oxford Shoulder Score (OSS) 36‐item Short‐Form Health Survey (SF‐36) Constant score Complications (implant malposition, secondary screw penetration, secondary trauma) Revision surgery Mortality |
|
| Funding and conflict of interest statements | Funding: not reported The authors declared they had no competing financial interests or personal relationships that would have influenced the trial. | |
| Notes | (Previously ongoing ‐ but new this update.) All had CT scans to confirm fracture type. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sequence generation by on‐line Statistical Computing Web Program: www.randomization.com" |
| Allocation concealment (selection bias) | Low risk | "[P]articipants were included and randomized by sealed envelope (opaque, not resealable) drawing". Screening and randomisation by one of the study investigators. Adequate safeguards seem likely in this trial |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No provider, participant or outcome assessment blinding. |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Lack of blinding unlikely to affect assessment of these outcomes, but may affect decisions for subsequent surgery. |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Low risk | Participant flow diagram available. Loss to follow‐up acceptable (17%) and balanced between the two groups (5 in each) and unlikely to be related to outcome. |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Participant flow diagram available. Loss to follow‐up acceptable (17%) and balanced between the two groups (5 in each) and unlikely to be related to outcome. |
| Selective reporting (reporting bias) | Unclear risk | Prospective trial registration and protocol published before trial recruitment ended. Barthel index and range of motion planned at trial registration but not reported in protocol or trial report. However, range of motion is part of the Constant score. The final report does not cite the protocol. The careful classification of complications described in the protocol was not mentioned in the trial report. Thus, there are grounds for a little uncertainty for this domain. |
| Balance in baseline characteristics? | Low risk | Baseline characteristics appeared balanced in the two groups. |
| Free from performance bias? | Low risk | All 4 surgeons were senior trauma surgeons who were experienced with both devices for treating proximal humeral fractures. The same rehabilitation protocol was applied. |
Hengg 2019.
| Study characteristics | ||
| Methods | Method of randomisation: stratified block randomisation and opaque sealed envelopes Assessor blinding: no mention Loss to follow‐up at 1 year: 10/67 (8 withdrawals and 2 lost to follow‐up) (see Notes) | |
| Participants | 8 European centres: Innsbruck, Austria; Leuven, Belgium; Aachen, Freiburg, Homburg, Ludwigshafen and Tubingen, Germany; Lucerne and Zurich, Switzerland Period of study recruitment: January 2014 to April 2016 67 participants aged 65 years and older, diagnosed radiographically with an acute (≤ 10 days), closed, displaced or unstable 3‐ or 4‐part proximal humeral fracture sustained after low‐energy trauma, and scheduled for primary fracture treatment with a PHILOS plate. (See Notes.) Informed consent. Exclusion criteria: bilateral or previous proximal humeral fracture, cuff‐arthropathy on either side, head‐splitting or impression fracture of the humeral head, or associated nerve or vessel injuries. Known clotting disorders, severe cardiac or pulmonary insufficiencies, severe systemic diseases (American Society of Anesthesiologists (ASA) class IV to VI), or not medically managed severe systemic diseases classified as ASA class III. Known hypersensitivity or allergy to any of the components of the Traumacem V+ cement Kit. Prisoners or recent history of substance abuse. Participation in any other medical device or medicinal product study within the previous month. Report stated that "patients were excluded before randomization if they received implants other than PHILOS or PHILOS screw augmentation"; however, in 2 cases this appears to be post‐randomisation. 55 female, 12 male; mean age 77 years, range 65 to 92 years | |
| Interventions | Interventions and randomisation at surgery (timing of surgery was meant to be within 10 days)
1. Screw tip augmentation with high viscous polymethylmethacrylate (PMMA) cement. PHILOS plate applied according to manufacturer’s manual and screw augmentation using Traumacem V+ cement kit. Leakage tests were performed with an injection of cement (≤ 0.5 mL) under image intensifier control. Each participant in the augmented group must have 2 to 4 screws augmented. 2. PHILOS plate applied according to manufacturer’s manual. All the postoperative treatments were done according to the standard of care at the investigating sites. Shoulder sling use. Assigned: 33/34 (see Notes regarding protocol violations) Completed (at 1 year): 30/27 but analysed according to intention‐to‐treat analysis at 31/27 (or according to data availability for individual outcomes). Per‐protocol analysis: 23/27; not reported in the review |
|
| Outcomes | Length of follow‐up: 1 year (also 6 weeks, and 3 and 6 months)
QuickDASH score
Shoulder Pain and Disability Index (SPADI)
Constant score (absolute and relative to contralateral shoulder)
EQ‐5D index and health state
Mortality ("sudden death" = 0) Mechanical failures (broadly included loss of reduction (varus malposition and a relative displacement of the greater or lesser tuberosity), humeral head impaction, screw/plate loosening, and secondary screw perforation) Complications: overall number of participants with any adverse event (local and general); these included implant‐related problems, cement leakage, nerve injury, systemic events such as stroke, wound‐related problems such as haematoma; impingement, etc.) Reoperation (data not split by treatment group) Radiographic outcomes, including loss of reduction of humeral head, cement leakage into joint, humeral head necrosis |
|
| Funding and conflict of interest statements | Funding: The study was supported by the AO Foundation "via the AOTK Trauma and the AOTrauma networks". The AO Clinical Investigation and Documentation (AOCID) was thanked for "conducting the study, including site management, medical and scientific support, statistical analysis, and medical writing services". Open access for the report publication was from the University of Innsbruck and Medical University of Innsbruck. The conflict of interest statement listed one author as a consultant with DePuy‐Synthes who "did not receive personal benefits for the current study"; one author as the chairman of the AO TK‐System during the time of study; and one author as "a member of the AO UEEG". |
|
| Notes | The trial registration document states there were 69 rather than 67 participants; originally 128 was the planned enrolment. There were 14 protocol violations: 11 were deemed ineligible post‐randomisation (6 had 2‐part fractures, 2 had fractures > 10 days old, 1 had a nerve/vessel injury, 2 did not receive PHILOS plates); and 3 were protocol violations due to having more than four screws augmented, not having a leakage test performed before augmentation, and/or receiving screw augmentation despite joint perforation. An additional 3 participants from the augmented group crossed over to the control group due to positive leakage tests. The report presented an intention‐to‐treat analysis and a per‐protocol analysis for most outcomes. Adverse events were reported according to actual intervention received; thus the 3 cross‐overs were reported according to treatment rather than allocation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was stratified for each participating center ... Three block sizes were used, chosen at random." |
| Allocation concealment (selection bias) | Low risk | "Randomization was stratified for each participating center and took place during surgery via opaque sealed envelopes after the fracture reduction was achieved and cannulated locking screws were inserted. Three block sizes were used, chosen at random. To maintain allocation concealment, the pattern of the blocks was kept confidential." |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No mention of blinding. Assessment of mechanical failure was done "by two experienced independent reviewers". It is unclear whether the participants knew of their group but the procedures were different in terms of testing for suitability when allocated to the augmented group. |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Outcome less susceptible and so considered at unclear risk of bias despite clear absence of blinding. |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Participant flow provided and denominators provided for patient‐reported outcomes (intention‐to‐treat analysis). Some imbalances in losses: e.g. losses 4 (12% of 33) versus 8 (23.5% of 34) for QuickDASH at 1 year. The trial registration document states there were 69 enrolled rather than 67 as reported in the trial report. |
| Incomplete outcome data (attrition bias) Death, reoperation | Unclear risk | Although participant flow is provided, data for reoperation were not separated by group. The trial registration document states there were 69 enrolled rather than 67 as reported in the trial report. |
| Selective reporting (reporting bias) | Unclear risk | Trial registration available. Most important outcomes were reported. However, reoperations were not specified according to groups. Trial was stopped prematurely. |
| Balance in baseline characteristics? | High risk | Although there was balance in many characteristics, there were more 4‐part fractures in augmentation group (15 versus 9), and fewer 2‐part fractures (5 versus 1). This is likely to have been a source of bias. |
| Free from performance bias? | Unclear risk | Insufficient information to judge this |
Hodgson 2003a.
| Study characteristics | ||
| Methods | Randomised using sequentially numbered sealed envelopes Assessor blinding: yes, on review of participants at home or clinic appointment Loss to follow‐up at 1 year: 4 (1 death); at 2 years: 12 (3 deaths) | |
| Participants | Royal Hallamshire Hospital, Sheffield, UK Period of study recruitment: November 1998 to April 2000 86 participants, over 40 years old, with minimally displaced fractures (in 2 parts), including isolated fractures of the greater tuberosity Exclusion criteria: inability to understand written or verbal information 70 female, 16 male; mean age 70 years | |
| Interventions | Intervention started: at arrival at A&E (i.e. hospital emergency care). 1. Early physiotherapy (within 1 week of the fracture). Most participants were seen by a physiotherapist at clinic the day after their fracture. Participants received a sling for comfort but were instructed to take their arm out of the sling and to perform gradual, assisted movements of the upper limb. 2. Late physiotherapy after 3 weeks of immobilisation in a collar and cuff sling. Both groups received same rehabilitation programme. First 2 weeks: education and instruction for home exercises; weeks 2 to 4: progression to full passive flexion and light functional exercises; week 4: start of progressive functional exercises. Discharge when both participant and physiotherapist thought independent shoulder function was achieved. | |
| Outcomes | Length of follow‐up: 2 years, also 8 and 16 weeks and 1 year Functional assessment (Constant score) Patients' perceived health status: SF‐36 (physical function, physical role limitation, pain); Croft Shoulder Disability Questionnaire Complications Number of physiotherapy treatment sessions | |
| Funding and conflict of interest statements | The research was funded by a grant from the Trent Research Scheme (UK). The authors declared no benefits in any form were received from a commercial party. |
|
| Notes | Information on this trial received from Mr Hodgson on several occasions. This included draft report of the 2‐year follow‐up and notice of their plan to extend follow‐up to 5 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details: "using sequentially numbered sealed envelopes we randomly allocated patients" |
| Allocation concealment (selection bias) | Low risk | "[U]sing sequentially numbered sealed envelopes we randomly allocated patients". Also from phone conversation (8 August 2001): "physio opened envelopes when details entered on envelope" |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Blinded assessor of function but participants and care providers were not blinded |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Lack of blinding unlikely to affect assessment of these outcomes |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Low risk | A full account of loss to follow‐up provided. While 14% at 2 years (12/86), it was under 5% (4/86) at 1 year |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Participant flow provided |
| Selective reporting (reporting bias) | Unclear risk | Trial registration was incomplete and differed slightly from final reports |
| Balance in baseline characteristics? | Unclear risk | More males in the early mobilisation group (11 versus 5) |
| Free from performance bias? | Low risk | Performance bias seemed unlikely. |
Hoellen 1997.
| Study characteristics | ||
| Methods | Randomisation method unknown Assessor blinding: not stated Loss to follow‐up at 1 year: 12/30 (3 deaths) | |
| Participants | University Clinic Ulm, Germany Period of study recruitment: 1 December 1994 to 30 June 1996 in Hoellen 1997 report (to 31 August 1998 in Holbein 1999 report) 30* participants with 4‐part fractures (Neer). *See Notes Exclusion criteria: age < 65 years, > 14 days since fracture, rheumatoid arthritis, previous shoulder injury, terminally ill 24 female, 6 male; mean age 74 years | |
| Interventions | Interventions started within 14 days of fracture. 1. Hemiarthroplasty (Global prosthesis, DePuy, US) ‐ cemented 2. "Minimal osteosynthesis": tension band wiring ‐ 2 pins + figure of 8 wire All were given low‐dose heparin for deep‐vein thombosis (DVT) prophylaxis. The same postoperative treatment was used in both groups. A Gilchrist bandage was used for temporary rests. Passive moving exercises started from first postoperative day, with active exercises postponed until after 6 weeks. Referral to rehabilitation clinic for 3 to 4 weeks post discharge. Assigned: 15/15 Completed (1 year): 9/9 | |
| Outcomes | Length of follow‐up: 1 year Functional assessment (Constant score) Mobility (component of Constant score) Pain (component of Constant score) Power Haematoma Infection Implant failure Medical complications Reoperation Time on ward Discharge location Mortality | |
| Funding and conflict of interest statements | There was no statement on funding. There was no statement on conflicts of interest. | |
| Notes | The plan for longer term follow‐up was announced in the Hoellen 1997 trial report. Further abstracts and a trial report (Holbein 1999) were identified for the review update (Issue 4, 2003). Holbein 1999 reported on 39 participants (19 versus 20), with 3‐ and 4‐part fractures, 31 (number in each group not known) of whom had been followed up for 1 year and 24 (number in each group not known) for 2 years. Requests (June 2003) for further information, including for denominators, resulted in the discovery that both Dr Holbein and Dr Hoellen were no longer at Ulm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details: prospective randomised trial |
| Allocation concealment (selection bias) | Unclear risk | No details: prospective randomised trial |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No blinding |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Lack of blinding unlikely to affect assessment of these outcomes |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Participant flow provided but large loss to follow‐up (12/30 = 40%); and potential exclusions |
| Incomplete outcome data (attrition bias) Death, reoperation | Unclear risk | Participant flow provided but large loss to follow‐up (12/30 = 40%). Serious outcomes, though, are less likely to be missed. |
| Selective reporting (reporting bias) | High risk | Insufficient information to judge this but the pragmatic removal of the power component of the Constant score was post hoc. Also, non addressed difference in trial inclusion criteria between the two reports of this trial. |
| Balance in baseline characteristics? | Unclear risk | No information on baseline characteristics of the two treatment groups but inclusion criteria rule out some confounders. |
| Free from performance bias? | Unclear risk | Same postoperative treatment but in all there is insufficient information to assess performance bias. |
HURA 2020.
| Study characteristics | ||
| Methods | Randomised using numbered sealed black envelopes opened sequentially Assessor blinding: no, except for radiographic assessment performed retrospectively by two independent assessors Loss to follow‐up at 1 year: 9 (1 death); at 2 years: 16 (5 deaths) | |
| Participants | Two university hospitals and Level 1 trauma centres, Montreal and Sherbrooke, Quebec, Canada
Period of study recruitment: November 2007 to August 2015 85 adults over 18 years old (closed physis) with displaced proximal humeral fracture including a fracture line in the humeral neck (Neer II, III, IV valgus type). The classic displacement criteria of 1 cm and/or 45° were used. Available for a minimum of 2 years of follow‐up, understanding English or French. Patient consent Exclusion criteria: open fracture, complex fracture (Neer IV with varus type), fracture dislocation, head split fracture, fracture unfixable according to the surgeon and going to arthroplasty, polytrauma with upper‐limb involvement, neurological injury on the ipsilateral upper limb, previous pathology or surgery to the fracture shoulder, “non‐collaborative patient” (dementia, alcoholic, or other severe mental illness), inflammatory arthritis, active local or systemic infection, participation in any other pharmaceutical, biologic, or medical‐device clinical investigation 66 female, 19 male; mean age 62 years |
|
| Interventions | Randomisation was performed prior to surgery; mean delay to surgery was 5.2 days
All received a proximal humeral fracture locking plate, but of different dimensions in the two groups
1. Deltoid split approach (called the "lateral minimally invasive approach" at trial registration): a proximal skin incision of 4 cm to 5 cm was done on the anterolateral aspect of the shoulder, 1 cm posterior to the anterior acromion corner. Identifying the axillary nerve by digital palpation under the deltoid muscle was essential to avoid injuring it during plate or screw insertion. The proximal window was used for fracture reduction and humeral head assessment. To prevent axillary nerve entrapment, the surgeon inserted a finger under the nerve before sliding the plate between the finger and the bone. A longer 5‐hole plate was chosen for this approach to safely insert the distal screws. A second distal incision allowed screw insertion and distal fixation of the plate.
2. Deltopectoral approach: a classic skin incision of 10 cm or more, starting at the level of the coracoid and aligned with the deltopectoral groove, was used. Standard deep dissection was done by pushing the deltoid and the cephalic vein laterally. Reduction was performed while protecting the circumflex vessels. The plate was applied laterally to the bicipital groove while aiming for the retroverted humeral head. The shorter (3 holes) 3.5 proximal humerus locking plate from Synthes (Synthes USA) was used in all cases. All participants were operated under general anaesthesia and were given antibiotic prophylaxis before the incision. All cases in both groups were assisted by fluoroscopy to achieve adequate fracture reduction and to avoid iatrogenic proximal screw perforation of the humeral head. The type, number and length of the screws were chosen according to surgeon preference. In all participants, after standard wound closure, the arm was placed in a thoraco‐brachial sling. Postoperative rehabilitation consisted of rest in a thoraco‐brachial sling for the first month and then active‐assisted gentle ROM for 1 month. Strengthening and weight bearing were permitted after 6 weeks when radiological signs of bone healing were present. Assigned: 44/41 (although 88 were included, 3 were excluded "per‐op" [sic]) Completed (mean 25.4 months): 35/34 |
|
| Outcomes | Length of follow‐up: 24 months; also assessed at 2 and 6 weeks, 3, 6, 12 and 18 months; reported at 25.4 months (range 12 to 92 months)
QuickDASH
SF‐12 (version 2)
Constant score (in trial registration; not reported in main article) Patient Scar Assessment Scale Complications, including infection, cut‐out, non‐union, fixation failure, reduction loss ≥ 10 degrees, avascular necrosis, heterotopic ossification and reoperation (2 cases of transient axillary nerve impairment also mentioned) Mortality External rotation and flexion Length of surgery Blood loss |
|
| Funding and conflict of interest statements | Departmental funding to the two involved institutions for educational and research purposes for the authors was received from a total of 10 listed companies, including Synthes. The detailed statement on conflicts of interest indicated several consultants acted as consultants for various companies. Specifically, the lead investigator acts as a "consultant with Wright Medical for R&D on proximal humerus fractures", but confirmed that "Wright Medical did not finance this study and was not involved in any aspect of the submitted work". |
|
| Notes | Was ongoing trial in Handoll 2015, identified via trial registration but entry for trial (clinicaltrials.gov) on 10 April 2012 (version 1) indicated that the "recruitment status of this study is unknown because the information has not been verified recently". Entry for trial on 7 August 2020 (version 14) indicated that the anticipated study completion date was December 2020. Conference abstract reports available and trial report published prior to this. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A randomized block design was chosen to prevent bias and achieve a similar number for each surgery in each center, using sealed black envelopes. This method also ensured an even distribution of both techniques over the study period. For each block of 10 envelopes, a research assistant from another research team (spine surgery) randomly mixed 5 DP [deltopectoral] cases and 5 DS [deltoid‐split] cases. Each envelope was given a number and the ranking strictly followed." |
| Allocation concealment (selection bias) | Low risk | "[U]sing sealed black envelopes", and "Each envelope was given a number and the ranking strictly followed." This seems secure as also done independently |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Participants and care providers not blinded, nor outcome assessment, except for retrospective radiographic assessment |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Lack of blinding unlikely to affect assessment of these outcomes |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Loss to follow‐up at final follow‐up (mean 25.4 months) was 16 (18.8%), balanced between the two groups (9 (20.5%) versus 7 (17.1%)). However the spread of follow‐up was large (12 to 92 months) and seemed to differ between the two groups (the trial took a long time to recruit). Hence, the possibility of bias cannot be ruled out. |
| Incomplete outcome data (attrition bias) Death, reoperation | Unclear risk | Reoperations and deaths for 82/88 (96.5%) of participants. However, some discrepancies in the participant flow diagram (2 included not accounted for) and 3 potential post‐randomisation exclusions "per‐op" [sic]; assigned group not stated. Also number recruited stated as 79 in final version of trial registration. |
| Selective reporting (reporting bias) | Unclear risk | Trial registration available. Some minor discrepancies in outcomes listed with main report. However, main issue is the reporting of final outcome at follow‐up between 12 and 92 months, when active follow‐up at 12, 24 months. So, some potential problems. |
| Balance in baseline characteristics? | Low risk | Although data are missing for 8 participants for fracture types, there are no major baseline differences between the two groups. |
| Free from performance bias? | Low risk | Some recognition of surgeon variation: "In order to minimize surgical bias related to the fracture reduction technique, the same group of 7 surgeons performed both approaches". The lengths (number of holes) of the plate differed; consistent with approach. Same preoperative and rehabilitation programme. |
Jonsson 2020.
| Study characteristics | ||
| Methods | Randomised using sequentially numbered, sealed opaque envelopes Assessor blinding: no, although a clinical examination (range of motion, strength) was conducted by an independent physiotherapist Loss to follow‐up at 2 years: 15 (10 deaths) | |
| Participants | Multicentre trial involving 5 university hospitals and 3 county hospitals in Sweden
Period of study recruitment: September 2013 to May 2016 99 independently‐living people aged over 70 years with displaced 3‐ or 4‐part fracture of the proximal humerus resulting from a low‐energy injury. Written informed patient consent. Exclusion criteria: a pre‐existing shoulder condition and comorbidity or concurrent injury considerably affecting shoulder rehabilitation; severe cognitive impairment. In trial registration document: more than 14 days from injury to surgery. Of 84: 76 female, 8 male; mean age 79.5 years |
|
| Interventions | Randomisation was performed prior to surgery; mean delay to surgery was 6.1 days The choice of prosthesis brand was at the discretion of the treating surgeon. 1. Reverse total shoulder arthroplasty. The stem was typically inserted with a retroversion of 20° or slightly less. The supraspinatus tendon was excised and the tuberosities were reduced as anatomically as possible and reattached with non‐absorbable sutures (#2 and #5). The general strategy for placing fixating sutures was as described by Boileau and colleagues (Boileau 2000), but adapted to the particular type of prosthesis and fracture pattern. After RTSA, a sling was worn typically for 2 to 4 weeks. Rehabilitation was generally started with passive‐ and active‐assisted motion, progressing to active exercises approximately 4 to 6 weeks postoperatively. External rotation and internal rotation behind the back were avoided during the first 6 postoperative weeks. Strengthening exercises with light loads were typically initiated 8 to 12 weeks postoperatively. 2. Hemiarthroplasty. The stem was typically implanted in 20° to 30° of retroversion and the humeral head size was chosen to best replicate the patient’s anatomy. The tuberosities were reduced and fixed as for the RTSA, except for Global Fx and Global Unite prostheses, where the tuberosities were fixed as indicated by the manufacturer. Ruptures of the rotator cuff encountered intraoperatively were repaired in a standard way. Rehabilitation was similar to that for RTSA but was progressed more cautiously, including deferring active exercises until around 6 weeks postoperatively. All participants received prophylactic antibiotics perioperatively according to local practice. The patient was placed in the beach‐chair position, and the deltopectoral approach was used for all but two RTSA procedures where an anterosuperior approach was used. Assigned: 48/51 Completed (mean 2.4 years): 41/43 |
|
| Outcomes | Length of follow‐up: minimum 2 years (mean 2.4 years); also assessed at 1 year WOOS (Western Ontario Osteoarthritis of the Shoulder) index Quality of life: EQ‐5D‐3L (3 levels) Constant score Complications: information collected at final follow‐up on four predetermined adverse events (infection, joint dislocation, reoperation and neurologic complications) and others as arose Pain (VAS) Patient satisfaction with shoulder (VAS) Mortality Range of motion: abduction, flexion, external rotation and internal rotation Radiographically‐assessed outcomes: greater tuberosity healing, notching, glenoid erosion Length of surgery |
|
| Funding and conflict of interest statements | Funding: not mentioned Declaration of interest statements. One author is a paid presenter or speaker for three firms that produce orthopaedic implants and receives "consultant fees from Lima outside the submitted work". | |
| Notes | Per Olerud sent preproof trial report, when published, to H Handoll on 15 December 2020. We used this as the basis of our data extraction and critical appraisal. The report was fully published in the May 2021 issue of Journal of Shoulder and Elbow Surgery (citation in the study references: Jonsson 2020). Four different brands of prosthesis were used in the RTSA group (n): Aequalis Reversed FX (2), Comprehensive (6), Delta Xtend (26) and SMR reverse (7). All RTSA stems were fixed with cement, while all glenoid components were non‐cemented. Eight different brands of prosthesis were used in the HA group (n): Aequalis FX (4), Bigliani/Flatow (5), Comprehensive (10), Equinoxe (2), Global Fx (4), Global Unite (6), SMR trauma (11) and SMR reverse (1). The stem was fixed with cement in 32 participants. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was performed in blocks of ten. The blocks were generated by an online computer program (Sealed Envelope Ltd., London, UK)." |
| Allocation concealment (selection bias) | Low risk | "For treatment allocation, sequentially numbered, sealed opaque envelopes were used." |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No blinding of participants and outcome assessors Clinical examination, including range of motion and measurement of strength, was performed by an independent physiotherapist, but no measures to safeguard blinding were reported. |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Lack of blinding unlikely to affect assessment of these outcomes |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Participant flow diagram provided and overall losses were balanced at final follow‐up. The article's Methods indicated that WOOS questionnaires were excluded if half or more of the items in a particular domain were missing or if more than 3 items were missing in total. For questionnaires with 3 or fewer missing items, values were imputed based on the average of the available responses for the particular domain. This led to very few other losses (1 versus 2) at 2+ years. However, the reduction in numbers available at 1 year follow‐up (e.g. 35 versus 30 for WOOS) are not explained. |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Final loss of follow‐up was balanced (7 (4 deaths) versus 8 (6 deaths)) and accounted for. |
| Selective reporting (reporting bias) | Unclear risk | Trial registration published after completion of recruitment. In the trial registration document (December 2017), the primary outcomes were both Constant score and WOOS; however, only the Constant score was selected as the primary outcome in the report; perhaps due to significant results? Secondary outcomes, such as EQ‐5D, pain VAS and shoulder satisfaction VAS, were not specified in protocol. |
| Balance in baseline characteristics? | Unclear risk | Baseline characteristics missing for the 15 lost to follow‐up; 10 of whom had died. The 15 were older (mean 82.4 years) compared with the 84 remaining (79.5 years) and had a lower pre‐injury EQ‐5D index (0.80 vs 0.91) of the presented characteristics. |
| Free from performance bias? | Unclear risk | Seventeen surgeons, all experienced in shoulder replacement, performed the procedures. "The choice of prosthesis brand was at the discretion of the treating surgeon, generally based on local routine." |
Kristiansen 1988.
| Study characteristics | ||
| Methods | Method of randomisation: unknown, "randomly selected" Assessor blinding: unlikely Loss to follow‐up at 1 year: 10/31 (4 failed to attend, 2 died, 4 excluded) | |
| Participants | Rigshospitalet, Copenhagen, Denmark Period of study recruitment: not stated 30 participants with 31 displaced 2‐part (7 fractures), 3‐part (19 fractures) and 4‐part (5 fractures) proximal humeral fractures (Neer). Exclusion criteria: no information (Of 31 fractures) 22 female, 9 male; age range 30 to 91 years | |
| Interventions | Interventions started: not stated 1. Surgery: percutaneous reduction (using Steinmann pin under image intensifier control) and external fixation (2 half pins with continuous threads into humeral head and 2 or 3 pins into the humeral shaft, and neutralising bar applied; Steinmann pin removed) 2. Non‐surgical treatment: closed manipulation under general anaesthesia and sling Assigned: 15/16 Completed (at 1 year): 11/10 | |
| Outcomes | Length of follow‐up: 12 months; also assessed at 3 and 6 months ‘Treatment failure’: poor reduction, pin removal due to loosening non‐union Quality of fracture reduction: good, fair, poor Functional overall score: excellent, satisfactory, unsatisfactory, poor. Neer (without anatomical section) Complications: avascular humeral head necrosis, deep infection, radiographic pseudarthrosis, refracture Reoperations Mortality | |
| Funding and conflict of interest statements | There was no statement on funding. The authors declared that no benefits were or would be received from a commercial party for the trial subject. | |
| Notes | In both groups, functional exercises were started under instruction during the first week. Excluded participants were: 1 treatment failure (deep infection) in the surgical group; and 2 treatment failures (poor reduction) and 1 refracture in the non‐surgical treatment group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details: "randomly selected for treatment" |
| Allocation concealment (selection bias) | Unclear risk | No details: "randomly selected for treatment" |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No blinding reported. |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Unlikely to be affected by lack of blinding |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Exclusion of data for participants with treatment failure and early refracture from 12‐month review. Large loss to follow‐up (10/31 = 32%). |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Participant flow provided |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge this |
| Balance in baseline characteristics? | Low risk | No information on the participant with bilateral fractures but a relatively minor unit of analysis issue |
| Free from performance bias? | Unclear risk | No information on operator competence/expertise |
Kristiansen 1989.
| Study characteristics | ||
| Methods | Method of randomisation: unknown Assessor blinding: yes at 2‐year follow‐up Loss to follow‐up at 2 years: 46/85 (18 deaths, 28 non‐attenders) | |
| Participants | Hvidovre University Hospital, Denmark Period of study recruitment: 1983 85 participants with proximal humeral fractures; 74% minimally displaced (Neer) Exclusion criteria: no information 60 female, 25 male; median age 72 years (1 week group), 70 years (3 weeks group) | |
| Interventions | Interventions started immediately or after closed or open manipulation. 1. One week immobilisation in sling and body bandage 2. Three weeks immobilisation in sling and body bandage At the end of immobilisation, instructions were given to perform Codman's pendulum exercises as well as active movements of the elbow and hand. Assigned: 42/43 Completed (at 2 years): 18/21 | |
| Outcomes | Length of follow‐up: 2 years; also assessed at 1, 3, 6 and 12 months Overall score (Neer without anatomic section) Mobility: overall from Neer score (range of motion: flexion, extension, abduction, internal and external rotation) Function: overall from Neer score (strength, reaching, stability) Pain: overall from Neer score (none to disabling) Complex regional pain syndrome type 1 (this was referred to as reflex sympathetic dystrophy in the trial report) | |
| Funding and conflict of interest statements | There was no statement on funding. There was no statement on conflicts of interest. | |
| Notes | Post immobilisation for both groups: instructions given for Codman’s pendulum exercises as well as active movements of elbow and hand. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details: "Random allocation to immobilization for 1 to 3 weeks was performed" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Only claimed for outcome assessors at final follow‐up: "The 2‐year follow‐up examination was blind, as the examiners had no knowledge of the period of immobilization." |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | No blinding but may not have affected appraisal of mortality (which was not split by treatment group) |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Large loss to follow‐up (46/85 = 54%). Numbers given for those available at follow‐up but incompletely reported data: only medians |
| Incomplete outcome data (attrition bias) Death, reoperation | High risk | Large loss to follow‐up. Although numbers given for those available at follow‐up, only overall mortality data provided (extracted from graph) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge this |
| Balance in baseline characteristics? | Unclear risk | Although there appeared to be comparability between treatment groups in age and gender, the percentage of minimally displaced fractures (79% versus 70%: 33/42 versus 30/43) differed between the two groups and no information was available on the numbers who had open manipulation (thus entailing surgery). |
| Free from performance bias? | Unclear risk | Lack of information to judge on performance bias |
Launonen 2019a.
| Study characteristics | ||
| Methods | Method of randomisation: "random number matrix in block allocation", use of sealed envelopes opened by independent research nurse at the co‐ordinating centre
Assessor blinding: yes, for outcome assessment by trained physiotherapists who otherwise did not take part in the study Loss to follow‐up at 24 months: 16/88 (11 discontinued (withdrew), 5 adverse events including 1 death) |
|
| Participants | 6 hospitals in 4 Northern European countries: 2 in Finland, 2 in Sweden, 1 each in Denmark and Estonia Period of study recruitment: February 2011 to April 2016 88 participants aged 60 years or older with displaced (more than 1 cm or 45 degrees) 2‐part surgical (or anatomical) neck proximal humeral fracture occurring less than 2 weeks before allocation. Written consent Exclusion criteria: not independent, dementia, institutionalised, did not understand written and spoken guidance in either Finnish or Swedish, alcoholism or drug addiction, pathologic fracture or a previous fracture of the same proximal humerus, other injury to the same upper limb requiring surgery, major nerve injury (e.g. complete radial‐ or axillary nerve palsy), rotator cuff tear arthropathy, open fracture, multi‐trauma or ‐fractured patient, fracture dislocation or head‐splitting fracture, isolated fracture of the major or minor tubercle, gross displacement of the fracture fragments (no bony contact between fracture parts or the humerus shaft is in contact with the articular surface), any medical condition that excluded surgical treatment 80 female, 8 male; mean age 72.5 years, range 60 to 90 years |
|
| Interventions | All participants had a computer tomography scan to confirm fracture types before randomisation.
1. Surgery using the PHILOS locking plate system (Synthes, Solothurn, Switzerland). Protocol proposed: beach‐chair position; plexus anaesthesia when possible; deltopectoral, deltoid‐split, or minimally invasive plate osteosynthesis approach; routine inspection of cuff tendons and sutures passed through each tendon to ease the handling of the fragments; the fragments to be preliminarily reduced with sutures and K‐wires and plate placed lateral from the biceps groove and 5 mm distal from the tip of the large tuberosity. Six to eight locking screws were to be placed in the humeral head and three conventional or locking screws in the shaft. The shoulder was to be immobilised with a collar and cuff in the operating theatre. 2. Non‐surgical treatment using collar and cuff or sling support and early physiotherapy. In both groups, participants were instructed by a physiotherapist on joint mobilisation during hospitalisation. Participants received a written aftercare protocol with detailed pictures. A collar and cuff or a sling was used for three weeks for pain relief. During the first three weeks, pendulum exercises were allowed, and free joint mobilisation and normal limb activation throughout the treatment was strongly supported. Active range‐of‐motion exercises, as permitted by pain, begun at three weeks. Physiotherapist contacts were arranged for three and six weeks after the beginning of the treatment, and all participants were scheduled to have five physiotherapist contacts within the first three months. Assigned: 44/44 Completed (at 2 years): 33/39 |
|
| Outcomes | Length of follow‐up: 2 years, also 3, 6 and 12 months
Disabilities of Arm, Shoulder and Hand (DASH) score Oxford Shoulder Score Constant score Pain (VAS) Quality of life questionnaire 15D EQ‐5D Complications, including infection, nerve damage, bleeding, malunion or non‐union, hardware problems Adverse events, including mortality Subsequent referral for operation or substantive treatment |
|
| Funding and conflict of interest statements | Funding was received from the Academy of Finland (www.aka.fi; ref 275481, ML) for the Finland branch of the consortium and the overall maintenance of the trial. The funders had no other role in the trial. Conflict of interest: one author (OW) declared a consultancy for Anatomica, and a paid presentation for DePuy Synthes and Link Sweden. Another author (AR) declared a paid lecture (Orion Ltd.). The other authors declared that they had no competing interests. |
|
| Notes | There were no anatomical neck fractures included. This was an ongoing trial (TPHF) in Handoll 2015b. In the protocol for this trial, 2 'strata' were described (Launonen 2012): 'Stratum I', which comprised 2‐part fractures is reported in this trial; 'Stratum II' comprised 3‐ and 4‐part fractures. Separate reporting was planned for each protocol. As explained in the final report for this trial (stratum I), stratum II is "still in the recruitment phase (159 of 218 patients recruited by 2 April 2019), which is scheduled to be completed during 2019". As participants will then be followed up for 2 years, we have retained TPHF in ongoing studies for this part of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andomized using a random number matrix in block allocation fashion” (protocol). "[T]he pre‐trial randomization sequence was generated with 10 blocks according to center and stratified by age (60 to 70 years, more than 70 years) due to the association between age and the measured outcomes.” Not exactly described but very likely to be computer generated. |
| Allocation concealment (selection bias) | Low risk | “The treatment allocations from the matrix will be sealed in an envelope. After the patient's enrollment in the study has been confirmed, the research physician will contact the research coordinator, who will open the envelope and the randomized treatment will be carried out” (protocol). “After enrollment, patients underwent randomization by means of a telephone call from the treating surgeon to the coordinating center’s research nurse. Sealed envelopes were used” (trial report). The independent procedure means it is very likely that allocation concealment was safeguarded. |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Trial participants and treatment providers were not blinded. However, some attempt was made to blind outcome assessors. "The trial was semi‐blinded, and the outcome assessors—trained physiotherapists who otherwise did not take part in the study—were unaware of which treatment group patients belonged to, and patients were encouraged not to reveal their treatment group. In addition, patients wore a T‐shirt to cover any scars on their shoulder." |
| Blinding (performance bias and detection bias) Death, reoperation | Low risk | “… patients knew their allocation, but the persons who collected and analyzed the data and the authors were unaware of the study‐group allocation of the patients.” |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Participant flow diagram was provided. By 1 year, of 16 (18% of 88) participants lost to follow‐up, the proportion was higher in the surgery group (11 (25%) versus 5 (11%)). It is unclear what effect this imbalance might have had on results. The reliability of analysis based on last observation carried forward for imputing missing values is uncertain. |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Participant flow diagram was provided, with small and comparable losses for these outcomes (4 versus 2). |
| Selective reporting (reporting bias) | Low risk | Prospective trial registration and protocol available. Although the use of imputation for missing data for continuous outcomes was not described, a separate analysis based on available data was reported for the primary outcome (DASH). This indicated comparable findings of no difference and thus we consider that the risk of this bias is low. |
| Balance in baseline characteristics? | Low risk | Baseline characteristics were comparable in the two groups. |
| Free from performance bias? | Low risk | “A specialized upper extremity team at each participating hospital provided the allocated treatment.” Prescribed rehabilitation was the same in both groups. |
Lefevre‐Colau 2007.
| Study characteristics | ||
| Methods | Randomised using block randomisation (under supervision of a statistician) and telephone to an independent researcher with patient details Assessor blinding: yes Loss to follow‐up at 6 months: 10 (all had difficulties in travelling to the hospital for scheduled sessions) | |
| Participants | Cochlin Hospital, Paris, France Period of study recruitment: October 2002 to March 2005 74 participants, over 20 years old, with non‐operatively treated impacted ("stable") fractures, including 34 minimally displaced (1‐part fracture); 16 2‐part (surgical neck or greater tuberosity (1)); and 24 3‐part (surgical neck and greater tuberosity) (Neer). (AO classification also given.) Written consent Exclusion criteria: pre‐existing shoulder pathology, neurological upper‐limb disorder, indication for shoulder surgery, multiple injuries, high‐energy trauma, or difficulties with language or unable to understand rehabilitation programme or other treatment information 54 female, 20 male; mean age 63 years | |
| Interventions | Intervention started within 72 hours after fracture.
1. Early mobilisation: active rehabilitation begun within 72 hours of fracture: 2‐hour sessions supervised by a physiotherapist, 5 times a week. Progressing from physical techniques to manage pain, then passive motion, performed by physiotherapist, in a) abduction, with arm suspension and participant supine (session 1); passive range of motion in forward elevation with the participant in a lateral supine position (session 2), with addition of external rotation with the participant in a seated position at session 8. After 3 weeks, sessions occurred twice a week without arm suspension. Participants wore a sling between sessions for 4 to 6 weeks, depending on the level of pain. After 6 weeks, active range of motion was begun during weekly sessions. Strengthening began at 3 months in twice‐monthly sessions. Participants underwent a total of 32 sessions.
2. Usual care, starting with 3 weeks of sling immobilisation. Then 2‐hour sessions supervised by a physiotherapist 4 times a week for 4 weeks. Passive mobilisation in all planes without arm suspension was performed by physiotherapist. Participants kept their arm in a sling between sessions for 1 to 3 additional weeks, depending on pain level. Then sessions were scheduled 2 times weekly for 5 weeks. Active range‐of‐motion exercises began after 6 weeks. After 9 weeks of rehabilitation, sessions occurred twice monthly until 6 months. Each participant underwent a total of 33 sessions. Participants used oral analgesics to manage pain. After 4 to 6 weeks, participants were advised to perform daily exercises at home. Participants were discharged from the study at 6 months. |
|
| Outcomes | Length of follow‐up: 6 months, also 6 weeks and 3 months Functional assessment (Constant score: split into subjective and objective components) Pain Patient satisfaction Range of motion: abduction, anterior elevation, lateral rotation Complications: non‐union (0); fracture displacement (0); treatment (injection) for subacromial impingement syndrome Compliance | |
| Funding and conflict of interest statements | Funding support for authors to conduct the work was received from the Department of Clinical Research of the Assistance Publique‐Hospitaux de Paris (APHP). The authors stated they had received no funding from a commercial entity in relation to the work, and implied no conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomization involved choosing randomly from among blocks of lengths 4 and 2 to prevent the risk of predictability." |
| Allocation concealment (selection bias) | Low risk | "After completion of the trial entry details, an independent researcher responsible for treatment allocation was contacted by telephone." |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | "Outcome measures were recorded by two physicians, including one of the authors (F.F.), who were blinded to the treatment assignments." However, care providers and participants were not blinded to allocation. |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Data were unavailable for 10 participants (5 in each group) who were lost to follow‐up because of difficulties in travelling to the hospital. Their characteristics were reported not to differ from those who attended. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge this; retrospective trial registration |
| Balance in baseline characteristics? | Low risk | Good balance in baseline characteristics |
| Free from performance bias? | Low risk | Rehabilitation was standardised and "delivered by physiotherapists who were experienced in the field". |
Livesley 1992.
| Study characteristics | ||
| Methods | Method of randomisation: unknown; double‐blind Assessor blinding: likely as code only broken at end of trial Loss to follow‐up at 6 months: 3/48 | |
| Participants | Mansfield District General Hospital, Mansfield, UK Period of study recruitment: November 1988 to May 1990 48 participants with minimally displaced humeral neck fractures (all Neer Group 1); 4 had epiphyseal fractures Exclusion criteria: unable to co‐operate with treatment and attend daily therapy for the first 10 working days. 37 female, 11 male; mean age 62 years, range 11 to 85 years | |
| Interventions | Interventions started on average 8.6 days since injury, upon referral to physiotherapy department. 1. Pulsed high frequency electromagnetic field (‘Curapulse’), 30 minutes/day for first 10 working days. (Intensity setting 3, pulse repetition frequency 35, maximum pulse power 300 watts.) 2. Dummy apparatus (deactivated machine) Assigned: 22/26 Completed (at 6 months): 21/24 | |
| Outcomes | Length of follow‐up: 6 months; also assessed at 1 and 2 months No data provided in report Range of movement of glenohumeral & scapulothoracic joints Pain scores, at rest, on movement, analgesia requirement Muscle wasting and strength Overall functional assessment score Subjective opinion of treatment Overall estimation of treatment (a ‘good result’) Time to discharge | |
| Funding and conflict of interest statements | There was no statement on funding. There was no statement on conflicts of interest. | |
| Notes | All participants received the same standardised physiotherapy regimen. No data provided in report for comparison between the two interventions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided: "patients were randomized into two groups" |
| Allocation concealment (selection bias) | Low risk | "[D]ouble‐blind", and randomisation code was only broken at end of the trial period to permit analyses |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | Low risk | "[D]ouble‐blind", use of sham control |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Although loss to follow‐up reported, no results were presented for the trial groups |
| Selective reporting (reporting bias) | High risk | Results not presented |
| Balance in baseline characteristics? | Unclear risk | Baseline comparability. However, although the article claims "patients ... were referred to the physiotherapy department without delay", the ranges for average time from injury to start treatment were 0 to 17 days (active) and 0 to 27 days (sham). |
| Free from performance bias? | Unclear risk | "Standardized physiotherapy regimen". However, although the article claims "patients ... were referred to the physiotherapy department without delay", the ranges for average time from injury to start treatment were 0 to 17 days (active) and 0 to 27 days (sham). |
Lopiz 2014.
| Study characteristics | ||
| Methods | Method of randomisation: used sequentially numbered, opaque, sealed envelopes Assessor blinding: not done Loss to follow‐up: 2 participants of the MultiLoc nail group were excluded (one died and one was lost to follow‐up) |
|
| Participants | Clinico San Carlos Hospital, Madrid, Spain Period of study recruitment: March 2011 to September 2012 54 participants with displaced Neer 2‐ or 3‐part proximal humeral fractures Exclusion criteria: pathological or open fractures, 4‐part fractures, concomitant fractures in the same upper limb, or the opposite and previous surgery on that shoulder. Lack of consent. Of 52: 41 female, 11 male; mean age 70 years, range 38 to 89 years |
|
| Interventions | All had general anaesthesia and intrascalene block. Mainly minimally invasive (percutaneous) ‐ small deltoid‐splitting incision (5 were open reduction with extended superior incision) 1. MultiLoc proximal humeral nail (MPHN) (Synthes‐DePuy, Solothurn, Switzerland) ‐ a straight nail 2. Polarus humeral nail (Acumed LLC, Hillsboro, OR, USA) ‐ a curved nail Postoperatively, participants were immobilised with a sling. Passive range‐of‐motion exercises were allowed 24 to 48 hours after surgery, followed as soon as possible by active‐assisted motion. Assigned: 28/26 Completed: 26/26 (mean 14 months) |
|
| Outcomes | Length of follow‐up: mean 14 months (6 to 22 months); formally 1, 3, 6 and 12 months Constant score (categories: excellent; good; satisfied; fair; poor) Constant score – adjusted for age and sex Physical tests to assess evidence of rotator cuff disease for entry point morbidity Non‐union, protrusion of the osteosynthesis material (subacromial impingement or articular surface intrusion of the screws), final alignment of the healed fracture (malunion) Reoperation (hardware removal for complications; reverse arthroplasty) Length of operation Intraoperative complications (none) Length of hospital stay Mortality |
|
| Funding and conflict of interest statements | Funding: not mentioned The authors declared that they and any research foundation with which they were affiliated received no financial payments or other benefits from any relevant commercial entity. | |
| Notes | Request for information sent to Dr Lopiz on 20 October 2014 requesting clarification on method of sequence generation, details on the 2 excluded participants, query on tuberosity involvement of 3‐part fractures in the MultiLoc nail group, clarification on whether 1 of 2 participants had a reverse shoulder replacement and length of follow‐up times for each group. No response received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[A]ssistant generated the random allocation sequence, which was concealed from the authors." "Patients were randomly assigned to 2 parallel groups, initially at a 1:1 ratio"; description raises the concern that the sequence may have been predictable (not random) in the early stages ‐ but was probably OK |
| Allocation concealment (selection bias) | Low risk | "Randomization was carried out with use of sequentially numbered, opaque, sealed envelopes." "All patients were randomized by a research co‐ordinator who was not involved subsequently in the study." |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | “The health care providers involved with subsequent patient care were not blinded to the treatment.” No mention of independent or blinded outcome assessment |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | “The health care providers involved with subsequent patient care were not blinded to the treatment.” However, it is unlikely that lack of blinding will affect the reporting of these outcomes. |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Variable follow‐up with no confirmation of similar follow‐ups in the two groups. Additionally, data lost from 2 participants in the Polarus nail group (1 died + 1 lost to follow‐up). |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Data lost from 2 participants in the Polarus nail group (1 died + 1 lost to follow‐up). Unlikely to bias the results |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge this. No protocol found |
| Balance in baseline characteristics? | Low risk | Although baseline data were not presented for 2 participants in the MultiLoc nailing group, there were no major imbalances in baseline characteristics between the two groups: "No statistically significant differences were found between the 2 groups." |
| Free from performance bias? | Low risk | "All surgeries were performed by 1 of the 3 senior trauma surgeons in the unit." Postoperative care was the same in both groups. |
Lopiz 2019.
| Study characteristics | ||
| Methods | Method of randomisation: used sequentially numbered, opaque, sealed envelopes Assessor blinding: not done Loss to follow‐up: 3 participants, 2 withdrew one of whom was excluded and 1 death (unrelated) |
|
| Participants | Clinico San Carlos Hospital, Madrid, Spain Period of study recruitment: May 2014 to January 2018 62 participants aged 80 years or above with Neer 3‐ or 4‐part displaced proximal humeral fractures, available for follow‐up for at least 12 months Exclusion criteria: mental disorders including cognitive impairment, open fracture, pathological fracture, fracture‐dislocation or head‐splitting fracture according to Neer, neurologic disorder, associated ipsilateral or contralateral upper‐ or lower‐limb fracture, prior surgery on the shoulder, or associated comorbidity contraindicating surgery, not autonomous prior to the fracture as determined using the Katz index. Unable to understand or provide written consent Of 59: 51 female, 8 male; mean age 83.5 years, range not stated |
|
| Interventions | Randomised during outpatient consultation the first week after the fracture. 1. Reverse shoulder arthroplasty. Surgery was performed around 7 days after injury by at least 2 of the 3 senior shoulder surgeons. General anaesthesia and interscalene block. Deltopectoral approach. Prosthesis: Delta XTEND or SMR. Tuberosities were reattached. A cerclage suture around the greater tuberosity. The tuberosities were packed with cancellous bone graft. 2. Non‐surgical treatment. See common management and rehabilitation below. The arm was immobilised in a sling for 3 weeks, allowing elbow, wrist, hand and pendulum shoulder movements from the first day after surgery. From the second week, passive‐assisted Codman movements with neutral rotation and less than 90 degrees of anteversion were allowed. Active range of motion started at 6 weeks and gradually progressed until counter‐resistance was felt after 12 weeks to strengthen the musculature. Participants were assessed by a different independent physiotherapist monthly to assess progress. If no improvement noted between 2 visits, formal hospital physiotherapy was discontinued. Assigned: 30/32 Completed: 29/30 (12 months) |
|
| Outcomes | Length of follow‐up: 12 months, also 1, 3, 6 and 12 months Constant score, also adjusted for age and sex DASH Pain (VAS) SF‐12 (physical and mental); EQ‐5D and EuroQol‐VAS Participant satisfaction (would they undergo the same treatment intervention again?) Adverse events: supracapsular nerve injury, prosthesis dislocation (0 cases), periprosthetic fracture (0), acromial stress fracture (0), infection (0) Secondary surgery (dropout from non‐surgery group that had an RSA) Radiological results: non‐union, malunion, osteonecrosis, greater tuberosity non‐anatomic healing or resorption Mortality Range of motion data categories (forward elevation, abduction, external rotation, internal rotation) |
|
| Funding and conflict of interest statements | Funding: work supported by a scientific grant from Mutua Madrileňa Foundation (no. 153192014). The authors declared that they had no conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "Randomization ... was based on sequentially numbered, opaque, sealed envelopes". "The surgeons were not involved in the randomization process". |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Participants and personnel were not blinded; although some blinded assessment was done, safeguards were not reported. "Functional evaluation was performed by 2 independent examiners who were not involved in the surgical procedure and did not know the treatment undergone by the patients". |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | These outcomes were not blinded but are less susceptible to bias. |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Low risk | Dropouts 3/59 (1 death, 2 consent withdrawals including 1 conversion to RSA). Consent withdrawal balanced across groups |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Participant flow provided with few losses |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration available but primary outcome and minimally important clinical difference used from a prior study; and a consistent and systematic approach taken |
| Balance in baseline characteristics? | Low risk | There was a 3‐year difference in mean age at baseline: 82 versus 85 (P = 0.007). Given the balance in other characteristics, we judged this not to be a risk of other bias. |
| Free from performance bias? | Low risk | Identical rehabilitation with independent assessment of progress |
Lundberg 1979.
| Study characteristics | ||
| Methods | Method of randomisation: unknown Assessor blinding: no, but mention of independent assessors Loss to follow‐up at 3 months: 0/42; not known for final assessment | |
| Participants | Gavle, Sweden Period of study recruitment: not stated 42 participants with undisplaced proximal humeral fractures (all Neer Group 1) fixed with a sling; 13 had avulsion of the greater tuberosity Exclusion criteria: no information 37 female, 5 male; mean age 65 years | |
| Interventions | Interventions started 7 days post injury, after removal of sling. 1. Instructed self‐exercise: participants instructed to train 5 to 10 minutes, 4 to 5 times daily. They had 3 visits (day 1, and 1 and 3 months) to physiotherapist for instructions and checks. At 1 month, participants were told how to extend their exercises to same level as in physiotherapy group. 2. Conventional physiotherapy: 9 visits (average 20 to 30 minutes) between 2 to 3 months; participants encouraged to continue exercise at home. At about 4 weeks, treatment was intensified. Assigned: 20/22 Completed (at 3 months): 20/22; (at mean 16 months): number in each group not known | |
| Outcomes | Length of follow‐up: > 1 year (mean 16 months); also assessed at 1 and 3 months Range of movement: abduction, shoulder elevation ‐ active and passive Pain (insignificant, moderate, severe), longstanding Lifting power of shoulder Frozen shoulder (secondary) Neer score (at final evaluation) including failure category Hand grip strength | |
| Funding and conflict of interest statements | There was no statement on funding. There was no statement on conflicts of interest. | |
| Notes | No indication in the report of any loss to follow‐up at last follow‐up (> 1 year), but cannot be assumed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of method: "In all, 42 patients were randomly assigned into two groups." |
| Allocation concealment (selection bias) | Unclear risk | No details of method: "In all, 42 patients were randomly assigned into two groups." |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No blinding, although independent assessment claimed: "Examination was made by physicians and physiotherapists independently at 1 month and 3 months." |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Full data provided for 1 and 3 months' follow‐up; but denominators not stated for long‐term (mean 16 months) follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge this |
| Balance in baseline characteristics? | Low risk | No major imbalances in baseline characteristics |
| Free from performance bias? | Low risk | No indications of performance bias |
Ockert 2010.
| Study characteristics | ||
| Methods | Method of randomisation: used closed envelopes Assessor blinding: unknown Loss to follow‐up (2010 publication): 10 participants excluded from analysis following randomisation; 6 with polytrauma, 2 with neurologic deficiency and 2 (1 versus 1) who were converted to shoulder arthroplasty intraoperatively. There was no mention of group allocation at randomisation or evaluation in the paper ‐ these (8 versus 2) were notified after contact with the lead trial investigator. Loss to follow‐up (2014 publication): not stated |
|
| Participants | Ludwig‐Maximilians University, Munich, Germany Period of study recruitment: August 2006 to July 2008 (extended to February 2010 for 2014 publication) 2010 publication: 76 participants, aged over 18 years, with displaced proximal humeral fractures with displacement > 1 cm and angulation of fragments > 45 degrees (Neer criteria) Exclusion criteria: poly‐traumatised patients, neurologic deficit or intraoperative conversion to shoulder arthroplasty. (Paper noted there were no open or pathological fractures.) Of 66: 48 female, 18 male; mean age 68 years, range 29 to 92 years 2014 publication: 124 participants with displaced proximal humeral fractures with displacement > 1 cm and angulation of fragments > 45 degrees (Neer criteria) Exclusion criteria: open or pathological fractures, poly‐traumatised patients, primary nerve palsy (given as examples) 89 female, 35 male; mean age 71 years, range not given |
|
| Interventions | 1. Polyaxial angular stable plate fixation using a Non‐Contact Bridging – Proximal Humerus (NCB‐PH) plate (Zimmer GmbH). Polyaxial plating allows a range of 0‐ to 15‐degree angle off‐centre. After insertion, a threaded screw cap locks the axis of the screw.
2. Monoaxial angular stable plate fixation with a PHILOS plate (Synthes GmbH). Monoaxial locking plate technique is characterised by fixed divergent and convergent screw orientation due to threaded screw holes. A deltopectoral approach was used for open reduction and internal fixation of all fractures. All participants received prophylactic intravenous antibiotic immediately before surgery. "The postoperative rehabilitation protocol included immediate passive‐ and active‐assisted range of motion (ROM) up to 60‐degree angle of abduction and elevation without forced external rotation for 6 weeks. Full ROM with active exercises was started 6 weeks after operation.” (2010 publication) Assigned: 39/37 (2010 publication); 58/66 (2014 publication but post‐randomisation exclusions may have occurred) Completed: 29/37; 58/66 (2014 publication) |
|
| Outcomes |
2010 publication: Length of follow‐up: 6 months (X‐rays 1 day, 6 weeks, 3 months and 6 months) Secondary varus displacement (> 10 degrees) Delayed union (due to osteonecrosis) Intra‐articular screw cut out Reoperation: revision surgery and early hardware removal Infection (none) Neurovascular injuries (none) 2014 publication: Length of follow‐up: 12 months (X‐rays 1 day, 6 weeks, and 3, 6 and 12 months) Revision surgery (reasons given: secondary varus displacement, subacromial impingement, intra‐articular screw cut out, infection) Screw position in different region of the humeral head |
|
| Funding and conflict of interest statements | There was no statement on funding. A conflict of interest statement in one article stated: "None". | |
| Notes | Request for information sent to Dr Ockert on 2 June 2012. Repeated on 8 June 2012, in email to Peter Biberthaler regarding identification and further information on ongoing trial referred to in conference abstract (Biberthaler 2009) ‐ it seems highly likely that the ongoing trial was this trial. However, this was not clear from email from Ben Ockert on 18 June 2012; this also provided details of the method of randomisation, the numbers allocated and analysed in each group. The 2014 publication of this trial (Ockert 2014) reported on an additional 48 participants, reflecting an extended period of trial recruitment, and a longer follow‐up. Only the revision surgery data from Ockert 2014 were used in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[C]onsecutive patients ... were prospectively randomized". No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | "[C]onsecutive patients ... were prospectively randomized". Contact from trialist revealed they "used closed envelope technique for randomization". (Exclusion criteria appeared to be applied post‐randomisation.) 2014 publication: "Randomization was performed by closed envelope technique." |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No mention of blinding. Radiographic assessment performed by two trained radiologists twice in separate sessions 8 weeks apart. Consensus decision for osteonecrosis and implant‐related failure. Criteria for healing stated |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | No mention of blinding, but unlikely to affect this |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | High risk | "Follow‐up rate was 71% of all radiographs taken 1 day, 6 weeks, 3 months, and 6 months after surgery." Numbers of participants allocated or assessed by intervention group provided after personal communication. Post‐randomisation exclusions (10/76 = 13%) were imbalanced (8 versus 2) and other loss to follow‐up not accounted for. 2014 publication: concerns about post‐randomisation exclusions continue for this publication |
| Incomplete outcome data (attrition bias) Death, reoperation | Unclear risk | As above. Paper described cases of revision surgery and early removal of metalwork; however, group allocation not given. Information provided subsequently |
| Selective reporting (reporting bias) | High risk | No protocol available. The extension of the recruitment, incomplete results and lack of full listing of exclusion criteria are of concern in the Ockert 2014 publication. |
| Balance in baseline characteristics? | Unclear risk | "The fracture types were equally distributed in both study groups." However, this applied to 66 participants. Does not state how many participants in each group or compare demographics Age and gender were comparable in the two groups in the Ockert 2014 publication. There was no mention of fracture type. |
| Free from performance bias? | Low risk | Six experienced surgeons performed the surgery: "In advance of this study, all surgeons were trained in the respective monoaxial and polyaxial locking plate system”. Same antibiotic regimen and postop management |
Olerud 2011a.
| Study characteristics | ||
| Methods | Method of randomisation: opaque, sealed envelopes Assessor blinding: no, but mention of independent surgeon Loss to follow‐up at 24 months: 7/60 (1 excluded themselves; 2 lost; 4 died) | |
| Participants | Stockholm Söder Hospital, Stockholm, Sweden Period of study recruitment: April 2003 to March 2008 60 participants with acute displaced (based on Neer's criteria) 3‐part proximal humeral fractures (all had displaced surgical neck fracture, all bar one had a displaced greater tuberosity; the exception had a displaced lesser tuberosity). Age 55 or older with a fracture sustained after a low‐energy trauma (e.g. a simple fall). Independent living conditions Exclusion criteria: patients with a completely displaced shaft in relation to the head fragment or with a valgus impact fracture. Institutionalised, severe cognitive dysfunction (< 3 correct answers on a 10‐item Short Portable Mental Status Questionnaire). Of 59 (1 participant excluded themselves): 48 female, 11 male; mean age 74 years, range 56 to 92 years (Operations were performed within a mean of 6 (SD 4.1) days after the injury.) | |
| Interventions | Interventions (and randomisation) started after hospital admission. 1. Surgery: operation occurred at mean of 6.1 days of injury. Open reduction and fixation using a deltopectoral approach with a PHILOS plate (Synthes, Stockholm, Sweden) and with non‐absorbable sutures used to fix displaced/unstable lesser and/or greater tuberosity fractures. The reduction and position of the implant was checked with the aid of an X‐ray image intensifier. (All participants had pre‐operative antibiotics.) Post surgery, the arm was placed in a sling and participants were referred to a physiotherapist. The sling was used for 4 weeks; afterwards, the participants were allowed to use it at their own convenience. Pendulum exercises and passive elevation/abduction up to 90 degrees were started from the first postoperative day. After 4 weeks, the participants were allowed a free active range of movement. 2. Non‐surgical treatment: arm immobilisation in a sling for 2 weeks, after which they were allowed to use it at their own convenience. After 2 weeks, the participants were referred to a physiotherapist, and pendulum exercises and passive elevation/abduction up to 90 degrees were started. After 4 weeks, they were allowed a free active range of movement. Assigned: 30/30 Completed (at 2 years): 27/26 | |
| Outcomes | Length of follow‐up: 2 years
Constant score (both shoulders)
DASH (Disabilities of the Arm, Shoulder and Hand) questionnaire
Quality‐of‐life score: EQ‐5D
Mortality
Pain
Range of motion: abduction, flexion
Fixation failure, redisplacement, non‐union, malunion Subsequent surgery (reasons including deep infection, etc) Radiographic outcomes, including avascular necrosis and osteoarthritis |
|
| Funding and conflict of interest statements | Study was partly supported by grants from the Trygg‐Hansa Insurance Company and Stockholm Country Council. Statement confirming that the authors, their immediate families and affiliated research foundations had not received financial payments or related benefits from any commercial entity in relation to the article.(Independence from the funders regarding stages from data collection onwards declared in Olerud 2011b.) | |
| Notes | Trial run concurrently with Olerud 2011b Additional information on randomisation and trial location obtained from Dr Olerud (April 2012). Pain data received May 2012. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After clearance by an anesthetist, the patients were randomized (independently prepared opaque, sealed envelopes) to open reduction and internal fixation with a locking plate or nonoperative treatment." (trial report) "[T]he patients were randomised by numbered sealed opaque envelopes drawn consecutively. The envelopes were independently prepared and thoroughly mixed. After that the envelopes were numbered by another person. At the time of randomisation the envelopes were drawn in numerical order." (personal communication) |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No assessor blinding, although the "final 24‐month follow‐up was performed by an independent orthopaedic surgeon not previously involved in the treatment." No provider or participant blinding |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Lack of blinding unlikely to affect assessment of these outcomes, but may affect decisions for subsequent surgery |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Low risk | "In the outcome analyses, all patients remained in their randomization group regardless of secondary procedures according to the intention‐to‐treat principle." Participant flow provided; no cause for concern |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | As above |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge this. No protocol found |
| Balance in baseline characteristics? | Low risk | No imbalances: baseline comparability |
| Free from performance bias? | Low risk | "All operations in patients randomized to surgery were performed by 1 of 2 orthopaedic surgeons, both well experienced in shoulder surgery." While all surgical patients were referred to a physiotherapist after their surgery and non‐surgically treated patients were referred after 2 weeks, this was unlikely to influence results. Otherwise, similar exercise / rehabilitation schedules |
Olerud 2011b.
| Study characteristics | ||
| Methods | Method of randomisation: opaque, sealed envelopes Assessor blinding: no, but mention of independent surgeon Loss to follow‐up at 24 months: 6/55 (1 lost; 5 died) | |
| Participants | Stockholm Söder Hospital, Stockholm, Sweden Period of study recruitment: April 2003 to March 2008 55 participants with an acute displaced (based on Neer's criteria) 4‐part proximal humeral fractures (all had displaced surgical neck, greater and lesser tuberosity fractures). Age 55 or older with a fracture sustained after a low‐energy trauma (e.g. a simple fall), no previous shoulder problems. Independent living conditions. Exclusion criteria: patients with a completely displaced shaft in relation to the head fragment or with a valgus impact fracture. Institutionalised, severe cognitive dysfunction (< 3 correct answers on a 10‐item Short Portable Mental Status Questionnaire) 47 female, 8 male; mean age 77 years, range 58 to 92 years | |
| Interventions | Interventions (and randomisation) started after hospital admission.
1. Surgery: operation occurred at mean of 6.0 days of injury. Humeral head replacement using a deltopectoral approach with the Global Fx prosthesis (DePuy, Sollentuna, Sweden); this is a modular prosthesis with a fixed angle and a conventional head ‐ it has 3 fins. Heavy non‐absorbable sutures were tagged on the bone tendon interface of both tuberosities. Cancellous bone graft from the head fragment was placed between the shaft and the tuberosities. (All participants had preoperative and 2 doses postoperative antibiotics.) Post surgery, the arm was placed in a sling and participants were referred to a physiotherapist. The sling was used for 6 weeks; afterwards, participants were allowed to use it at their own convenience. Pendulum exercises and passive elevation/abduction up to 90 degrees were started from the first postoperative day. After 6 weeks, the participants were allowed a free active range of movement. Strengthening exercises were begun after 3 months. 2. Non‐surgical treatment: arm immobilisation in a sling for 2 weeks, after which they were allowed to use it at their own convenience. After 2 weeks, the participants were referred to a physiotherapist, and pendulum exercises and passive elevation/abduction up to 90 degrees were started. After 4 weeks, they were allowed a free active range of movement. Assigned: 27/28 Completed (at 2 years): 24/25 |
|
| Outcomes | Length of follow up: 2 years
Constant score (both shoulders)
DASH (Diasabilities of the Arm, Shoulder and Hand) questionnaire
Quality‐of‐life score: EQ‐5D
Mortality
Pain
Range of motion: abduction, flexion
Fixation failure, redisplacement, non‐union, malunion Subsequent surgery (reasons including non‐union, etc) Radiographic outcomes, including avascular necrosis and osteoarthritis |
|
| Funding and conflict of interest statements | Study was partly supported by grants from the Trygg‐Hansa Insurance Company (B1/2006) and Stockholm Country Council (20060292). Statement confirming that the authors, their immediate families and affiliated research foundations had not received financial payments or related benefits from any commercial entity in relation to the article.(Independence from the funders regarding stages from data collection onwards declared.) | |
| Notes | Trial run concurrently with Olerud 2011a. Additional information on randomisation and trial location obtained from Dr Olerud (April 2012). Pain data received May 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "After clearance by an anesthesiologist, the patients were randomized (opaque sealed envelopes prepared independently) to a primary HA or nonoperative treatment." (trial report) "[T]he patients were randomised by numbered sealed opaque envelopes drawn consecutively. The envelopes were independently prepared and thoroughly mixed. After that the envelopes were numbered by another person. At the time of randomisation the envelopes were drawn in numerical order." (personal communication) |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No assessor blinding, although "The final 24‐month follow‐up was performed by an independent orthopaedic surgeon not previously involved in the treatment." No provider or participant blinding |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Lack of blinding unlikely to affect assessment of these outcomes, but may affect decisions for subsequent surgery |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Low risk | "In the outcome analyses, all patients remained in their randomization group regardless of secondary procedures according to the intention‐to‐treat principle." Participant flow provided; no cause for concern |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | As above |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge this. No protocol found |
| Balance in baseline characteristics? | Low risk | No imbalances: baseline comparability |
| Free from performance bias? | Unclear risk | "In patients randomized to surgery, all operations were performed by 1 of 2 orthopedic surgeons, both well experienced in shoulder surgery ..." While all surgical participants were referred to a physiotherapist after their surgery and non‐surgically treated participants were referred after 2 weeks, this was unlikely to influence results. As were the differences in timing for free ROM (6 versus 4 weeks). However, it was only reported for the surgical group that strengthening exercises were begun after 3 months. |
Plath 2019.
| Study characteristics | ||
| Methods | Method of randomisation: random numbers list Assessor blinding: no, but mention of independent surgeon Loss to follow‐up at 12 months: 26/81 (13 post‐randomisation exclusions; 3 died; 7 lost to follow‐up, 3 received RSA) |
|
| Participants | University Hospital of Augsburg, Augsburg, Germany Period of study recruitment: September 2012 to February 2015 81 participants with a proximal humeral fracture. Age 60 or older and able to give written informed consent. (Included Neer 2‐, 3‐ and 4‐part fractures) Exclusion criteria: isolated tuberosity fractures, previous trauma or surgery of the affected shoulder, advanced osteoarthritis, fracture dislocation, pathological fractures, open fractures and neurological disorders. (Post‐randomisation exclusion criteria listed: full thickness rotator cuff tears as well as intraoperative change of treatment due to a fracture line through the nail entry point or where bone quality was considered not amenable to stable fixation with either implants.) Of 68: 51 female, 17 male; mean age 76 years, range 60 to 92 years | |
| Interventions | Interventions started: timing of surgery not stated (randomisation before surgery). Surgery was performed with participants in the beach‐chair position under general anaesthesia. 1. Internal fixation using a locking plate: PHILOS (DePuy‐Synthes, Solothurn, Switzerland), deltopectoral or lateral transdeltoid approach. Closed reduction. In 3‐ and 4‐part fractures, non‐absorbable sutures were used to secure the greater and/or lesser tuberosities to the plate. A minimum of 3 screws were used at the humeral shaft (2 locking and 1 non‐locking screw) and a minimum of 6 locking screws, including 2 calcar support screws, to fix the plate proximally. 2. Internal fixation using a locking nail: Locking Blade Nail, LBN, Marquard Medizintechnik Europe. A triangular construct which aims to add medial calcar support. Anterolateral transdeltoid approach. The supraspinatus tendon is split in the line of its fibres and a guide wire is introduced into the nail entry point at the apex of the humeral head. The medullary canal is opened using a cannulated drill, and the nail inserted over the guide‐wire. Proximal and distal locking is achieved by use of an attached guide. Optional non‐absorbable tension band sutures through the rotator cuff and secured to washers on the proximal screws. Finally, the rotator cuff tendon and deltoid are repaired and the skin closed. In both groups, active and passive range‐of‐motion exercises were initiated on the day following surgery, as pain allowed, without restriction. No immobilisation was used in either group. Participants were instructed not to load‐bear with the affected shoulder for 6 weeks postoperatively wherever possible. Assigned: ?/? (81 overall); baseline for 32/36 (after post‐randomisation exclusions) Completed (at 1 year): 27/28 |
|
| Outcomes | Length of follow‐up: 1 year (also 3 and 6 months)
DASH score
Constant score (absolute and age and adjusted)
Pain (VAS)
Subjective overall condition of the shoulder (1‐6)
Mortality
Complications (overall number of participants with a complication, malposition of implants, screw cut‐out, loss of reduction, osteonecrosis, axillary nerve lesion, adhesive capsulitis, infection (0))
Reoperation (included indication for)
Active shoulder flexion and abduction
Radiographic outcomes including loss of reduction of humeral head, loss of reduction of the greater tuberosity, tuberosity resorption / head migration, osteonecrosis of humeral head Length of surgery Length of hospital stay |
|
| Funding and conflict of interest statements | No external funding Declarations of interest: the first author (JEP) had participated in a shoulder fellowship programme that was sponsored by DePuy Synthes Mitek; the senior author (EM) is a consultant and receives royalties from Marquardt Medizintechnik Europe. All other authors declared that they had no competing interests. | |
| Notes | HH sent request for trial data set or means, standard deviations and number of participants data for Constant and DASH scores to Johannes Plath on 22 April 2020. Reminder sent on 1 May 2020. Response from Johannes Plath with data received 2 May 2020. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[R]andomisation performed using a random numbers list" |
| Allocation concealment (selection bias) | Unclear risk | "One trauma surgeon, who was not directly involved in the surgical intervention enrolled the patients ..." There was, however, no mention of safeguards for blinding. |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | "One trauma surgeon, who was not directly involved in the surgical intervention enrolled the patients and evaluated the outcome measurements." There was no mention of blinding in relation to participants, care providers or outcome assessment. |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | "One trauma surgeon, who was not directly involved in the surgical intervention enrolled the patients and evaluated the outcome measurements." There was no mention of blinding in relation to participants, care providers or outcome assessment. Lack of blinding unlikely to affect assessment of these outcomes, but may affect decisions for subsequent surgery |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | High risk | After randomisation, 13 participants were excluded intraoperatively by the surgeon (7 rotator cuff tears, 4 RSA, 2 fractures at nail entry point). The allocation to the two groups of the randomised 81 participants was not provided. The missing outcome data, however, were balanced between groups for the 68 participants included in the data set. |
| Incomplete outcome data (attrition bias) Death, reoperation | High risk | After randomisation, 13 participants were excluded intraoperatively by the surgeon (7 rotator cuff tears, 4 RSA, 2 fractures at nail entry point). The allocation to the two groups of the randomised 81 participants was not provided. The missing outcome data were balanced between groups for the 68 participants included in the data set. |
| Selective reporting (reporting bias) | High risk | Study registration was registered retrospectively. Unexplained presentation of median together with SD data. |
| Balance in baseline characteristics? | Unclear risk | Balanced baseline characteristics for those in the analyses. However, baseline characteristics missing for 13 participants who were excluded post randomisation |
| Free from performance bias? | Low risk | There is no indication of experience with the two operations but similar experience anticipated. Other co‐interventions ‐ anaesthesia and rehabilitation ‐ were similar in the two groups. |
ProFHER 2015.
| Study characteristics | ||
| Methods | Method of randomisation: remote randomisation computer programme with 1:1 allocation, stratifying by tuberosity involvement (yes or no) and using random block sizes of 4, 8 and 12
Assessor blinding: no, except for blinded independent coding Loss to follow‐up at 24 months (main follow‐up): 32/250 (12 no response, 6 withdrew (+ 1 who died), 14 died) |
|
| Participants | 33 acute UK National Health Service hospitals, UK Period of study recruitment: September 2008 and April 2011 250 participants aged 16 years or older, presenting within 3 weeks after sustaining a displaced fracture of the proximal humerus that involved the surgical neck. The degree of displacement had to be sufficient for the treating surgeon to consider surgical intervention but did not have to meet the displacement criteria of Neer (1 cm or 45° angulation of displaced parts, or both) for inclusion in the trial. Written consent. Exclusion criteria: patients who had associated dislocation of the injured shoulder joint, open fracture, insufficient mental capacity to understand the trial or instructions for rehabilitation, comorbidities precluding surgery or anaesthesia, clear indication for surgery such as severe soft‐tissue compromise, multiple injuries (upper‐limb fractures), pathological fracture (other than osteoporotic), terminal illness, or not resident in the hospital catchment area 192 female, 58 male; mean age 66 years, range 24 to 92 years |
|
| Interventions | Interventions (and randomisation) started after presentation at the hospital 1. Surgery: either internal fixation, such as with plate and screws (majority were PHILOS plates), or joint replacement (hemiarthroplasty) 2. Non‐surgical treatment: participants were given a sling for the injured arm for as long as deemed necessary (3 weeks was suggested), followed by active early rehabilitation. Delivery of care and rehabilitation, which was freely available for all participants, incorporated three set measures to ensure good standards of care within the NHS: provision of an information leaflet on personal care during sling immobilisation; a basic treatment protocol to guide physiotherapy; and promotion of home exercises. Rehabilitation care was provided by physiotherapists in inpatient, outpatient and/or community settings. Assigned: 125/125 Completed (at 2 years): 106/109 (OSS data for 104/106) Completed (at 5 years): 78/75 (OSS data for 76/73); only 88/88 (176) consented to the extended follow‐up. |
|
| Outcomes | Length of follow‐up: 2 years, also 3 (for EQ‐5D), 6 and 12 months; subsequently extended to 5 years, also 3 and 4 years
Oxford Shoulder Score (OSS) SF‐12 (12‐item Short‐Form Health Survey) EQ‐5D Complications, including surgical complications (wound infection, implant failure, shoulder dislocation, septicaemia); early medical complications, i.e. chest infection, confirmed myocardial infarction or stroke, treated deep vein thrombosis and pulmonary embolism Mortality Subsequent referral for operation or substantive treatment Data for economic evaluation: NHS and societal costs |
|
| Funding and conflict of interest statements | The trial was funded by the National Institute for Health Research (UK), Health Technology Assessment Programme (project No. 06/404/53) The authors had no conflict of interests except lead author, Amar Rangan, who reported receiving grants and personal fees from DePuy Ltd; receiving grants from JRI Ltd; and having a UK and European patent pending for a shoulder replacement prosthesis. |
|
| Notes | Published protocol. Five‐year follow‐up results published in 2017: OSS data were presented as part of an extended primary analysis using a multilevel regression model at 6, 12, 24, 36, 48 and 60 months. The OSS data for at least 1 follow‐up time point were available for 237 participants (95%), of which 231 (92%) also had complete covariate data and were included in the primary analysis (114 in the surgical group versus 117 in the non‐surgical group). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done with a computer programme using 1:1 allocation, stratifying by tuberosity involvement (yes or no) and using random block sizes of 4, 8 and 12." |
| Allocation concealment (selection bias) | Low risk | "[R]esearch associates randomly allocated individual patients to surgical or non‐surgical treatment using an independent remote randomization service". |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | "There was no blinding of trial participants, clinicians, or assessment of outcomes." "Coding was performed by at least 2 independent coders blinded to treatment allocation." Discussion: "Although lack of blinding of patient‐reported outcome assessment is unavoidable, similarities in the 2 groups in patient return of questionnaires and baseline characteristics at 24 months, and the lack of a significant effect of baseline patient preferences on the OSS results suggest this did not introduce a bias." |
| Blinding (performance bias and detection bias) Death, reoperation | Low risk | As above. Additionally, lack of blinding unlikely to affect assessment of these outcomes |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Low risk | Loss to follow‐up balanced in the two groups. Trial reports: "Overall, 41 patients (16%) had missing follow‐up data on at least 1 time point. Using complete data derived by multiple imputation resulted in comparable treatment effect estimates to the primary analysis with no overall statistically significant group difference (P = .48). ..... Nonresponse (none or intermittent) was not associated with any demographic or fracture characteristics." |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Very high return of hospital forms: 249 of 250 (99.6%) at 1‐year follow‐up forms and 234 of 250 (93.6%) 2‐year (but 2‐year forms not sent for those who had already died before 1 year) |
| Selective reporting (reporting bias) | Low risk | Prospective trial registration, publication of trial protocol and trial analysis plan. All intended outcomes reported |
| Balance in baseline characteristics? | Low risk | The baseline characteristics were well balanced except for smoking status (there were more smokers in the non‐surgical group). However, this was shown not to impact on the results. |
| Free from performance bias? | Low risk | "It was emphasized that good standards care, both surgical and nonsurgical, should be provided throughout the treatment pathway for the injury, including surgical care or management of the sling, postoperative care, and rehabilitation in both groups. Participating hospitals did not introduce new or experimental interventions for these fractures during the study." "To avoid learning curve problems, surgeons and physiotherapists used surgical interventions and procedures with which they were familiar." "Physiotherapy treatment log data demonstrated equal access and implementation between groups,with similarly high numbers of participants recorded as performing home exercises in both groups." |
Revay 1992.
| Study characteristics | ||
| Methods | Randomisation from closed envelopes Assessor blinded Loss to follow‐up at 1 year: 1/48 | |
| Participants | Danderyd Hospital, Danderyd, Sweden Period of study recruitment: not stated 48 participants with 2‐, 3‐ or 4‐part minimally displaced proximal humeral fractures (< 1 cm or < 45 degrees; Neer Group 1) treated non‐surgically with sling immobilisation for 1 week. Exclusion criteria: patients with skin diseases and/or chlorine allergy, non‐ambulatory 39 female, 9 male; mean age 62 years | |
| Interventions | Interventions started 5 to 10 days post injury after removal of sling. 1. Swimming pool training (30 minutes each session, up to 20 sessions maximum) in groups (6 to 8 participants) plus instructions for self‐training (see below). 2. Instructions for self‐training: exercises to be performed at least 4 times a day for 10 to 15 minutes each time, use of hand on injured side for activities of daily living, advice on relaxation and resting positions. Assigned: 25/23 Completed: number in each group not known | |
| Outcomes | Length of follow‐up: 1 year; also assessed at 3 weeks, 2 and 3 months Pain (analogue scale) Activities of daily living: subjective assessment of 9 activities each rated on a 5‐point scale Functional scale: 6‐point scale Joint movement (abduction, flexion, internal rotation) | |
| Funding and conflict of interest statements | Study was supported by Daneryd Hospital (Sweden) There was no statement on conflicts of interest. | |
| Notes | Means (probably) presented without standard deviations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details: "patients were randomized into two groups" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details of safeguards: "randomized and given instructions in a sealed envelope" |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | "All patients were examined by a physiotherapist who did not know which group each patient belonged to". However, no participant or care provider blinding nor mention of ways to prevent disclosure to assessor |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | High risk | The treatment group of the participant lost to follow‐up was not stated. Standard deviations not provided. Graphs only provided for female participants ‐ denominators not provided for these |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge this |
| Balance in baseline characteristics? | Unclear risk | Baseline data not provided for gender |
| Free from performance bias? | Unclear risk | Uncertainty if any compensatory advice given for the control group |
Ring 2019.
| Study characteristics | ||
| Methods | Method of randomisation: computerised random number generator Assessor blinding: no Loss to follow‐up at 6 months: 33/63 | |
| Participants | Massachusetts General Hospital, Boston, Massachusetts, USA
Period of study recruitment: February 2005 to March 2014 (completion date)
63 participants, aged 18 years or over, with non‐operatively treated proximal humeral fractures
Exclusion criteria: < 18 years, with multiple other fractures, received surgical treatment including closed reduction and percutaneous fixation, open reduction and internal fixation (plates, screws, pins, tension wire bands, cerclage wiring and/or intramedullary nailing) and/or articular shoulder prosthesis 14 female, 36 male (of 50 at 3 months); mean age 63 years |
|
| Interventions | 1. Physical therapy started immediately after diagnosis of injury (immediate rehabilitation with pendulum movements) 2. Physical therapy delayed until 3 weeks after diagnosis of injury | |
| Outcomes | Length of follow‐up: 6 months DASH Shoulder flexion Shoulder pain Likert scores External and internal rotation (internal rotation not reported) Abduction Mortality Serious and other adverse events |
|
| Funding and conflict of interest statements | There was no statement on funding, but the sponsor was the hospital at which the trial took place and which employed the principle investigators. No declarations of interest statements available. | |
| Notes | The trial registration background section states: "Proximal humerus fractures with limited displacement and fractures that occur in older, less active or infirm patients are treated non‐operatively." Thus displaced fractures cannot be ruled out. History This trial was listed as ongoing in the 2015 version of the review. There were, however, some concerns about its study design and status.
Version 11 of the trial registration document (update 21 June 2019) presented Study Results. Dr Neal Chen, the listed contact in the most recent version of the trial registration ‐ which, although including published results still had contradictory information on study design ‐ responded to a query on status and method of treatment allocation on 18 June 2020: "This was a study that David Ring had started when he was here previously. It was submitted for publication, but has not gone forwards for unclear reasons. I believe that it was a randomized controlled trial. I’ll reach out to David to find out details." Dr Chen confirmed 25 June 2020: "After informed consent was obtained by a member of the study staff, participants were randomized 1:1 according to a computerized random number generator by the research assistant to either early (immediate prescription to learn pendulum exercises) or delayed (at least three weeks after injury) exercises. Allocation to treatment groups was concealed. The research assistant responsible for recording the outcome measures was not blinded to the allocation." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After informed consent was obtained by a member of the study staff, participants were randomized 1:1 according to a computerised random number generator by the research assistant. Allocation to treatment groups was concealed. |
| Allocation concealment (selection bias) | Low risk | Allocation to treatment groups was concealed. |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | The research assistant responsible for recording the outcome measures was not blinded to the allocation. |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Unlikely to be affected |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | High risk | 20% loss to follow‐up at 3 months, although reasonably balanced between groups. However, 52% losses at 6 months |
| Incomplete outcome data (attrition bias) Death, reoperation | High risk | As above |
| Selective reporting (reporting bias) | Unclear risk | Internal rotation not reported. Trial not reported sufficiently to tell, even though the trial was registered |
| Balance in baseline characteristics? | Unclear risk | Although apparently balanced, the data only apply to 50 (79% of 63) |
| Free from performance bias? | Unclear risk | Not enough information to judge |
Rommens 1993.
| Study characteristics | ||
| Methods | Method of randomisation: alternation Assessor blinding: unlikely Loss to follow‐up at 3 weeks: 0/28 | |
| Participants | Leuven University Hospital, Belgium Period of study recruitment: 1991 28 participants with acute 2‐ and 3‐part proximal humeral fractures (but most were non‐ or minimally displaced). Exclusion criteria: those indicated for surgical intervention, age < 15 years, with multiple injuries or other fractures at same site 22 female, 6 male; mean age 69 years, range 25 to 100 years | |
| Interventions | Interventions started immediately. 1. Gilchrist bandage, 2 to 3 weeks. The arm was bandaged with mesh type tubing and held by two slings: one round the shoulder and neck and the other which immobilised the distal part of the upper arm. (Bandage allowed wrist and hand exercises.) 2. Desault bandage, 2 to 3 weeks. Arm was immobilised to the chest using a circular elastic body bandage. (Some had one or more strips of plaster to stop the bandage slipping.) Assigned: 14/14 Completed (at fracture consolidation): 14/14 | |
| Outcomes | Length of follow‐up: until fracture consolidation; also assessed at 1 and 3 weeks Functional results: overall result, no data Pain: patient questionnaire, 0 (none) to 100 (significant) scale Displacement of fracture Complication: skin irritation Removal of bandage Surgeon assessment of ease of application of bandage Patient assessment of bandage | |
| Funding and conflict of interest statements | There was no statement on funding. There was no statement on conflicts of interest. | |
| Notes | Two fractures in the Gilchrist group required reduction. Seven participants had other fractures: 3 in group 1 (2 rib, 1 vertebra); 4 in group 2 (1 ankle, 1 hip, 1 rib, 1 vertebra). Trial reports in German; translation obtained. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised: alternation |
| Allocation concealment (selection bias) | High risk | Alternation |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No mention of blinding |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | While all participants were followed up and intention‐to‐treat analyses seemed to have been done, no data on function were presented nor were the criteria for judging fracture consolidation. |
| Selective reporting (reporting bias) | High risk | Insufficient information to judge this, but data not provided on function |
| Balance in baseline characteristics? | Unclear risk | Small discrepancies (e.g. in other injuries or having fracture reduction) can have bigger consequences for small group sizes. |
| Free from performance bias? | Unclear risk | Differences in care programmes cannot be ruled out. |
Sebastiá‐Forcada 2014.
| Study characteristics | ||
| Methods | Method of randomisation: sequentially numbered, opaque, sealed envelopes Assessor blinding: yes, independent surgeons who did not know which type of prothesis was used Loss to follow‐up at minimum 24 months: 1/62 (1 died) | |
| Participants | Hospital Universitario de Elda, Elda, Alicante, Spain
Period of study recruitment: 2009 to 2011 62 older participants with acute complex proximal humeral fractures (Neer's: 3‐part, 4‐part and 4‐part + dislocation). Age > 70 years. Candidate for shoulder arthroplasty: indications for shoulder arthroplasty were complex fractures not amenable to reconstruction, including displaced 4‐part fractures, fracture‐dislocations with 3‐part fractures, and head‐splitting fractures with more than 40% articular surface involvement. (All had computed tomography.) Informed consent. Exclusion criteria: contraindications to surgery, prior surgery in the shoulder, associated ipsilateral upper‐limb fracture and neurologic disorder 53 female, 9 male; mean age 74 years, range 70 to 85 years (operations were performed within a mean of 5.1 (range 1 to 12) days after the injury). |
|
| Interventions | A modular shoulder replacement system (SMR; Lima, Udine, Italy) was used in both groups. The system allows the choice of cementless shoulder prostheses: hemiarthroplasty, reverse and anatomic arthroplasty). A common cementless humeral stem with porous coating titanium was assembled with one of two prostheses. The same deltopectoral approach and basic surgical technique was used at each shoulder; the tuberosities were repositioned as anatomically as possible and reattached with non‐absorbable sutures. Regional anaesthesia. In both groups, a suction drain was placed postoperatively for 24 hours. Standard antibiotic and antithrombotic prophylaxis was given. 1. Reverse shoulder arthroplasty (RSA): an SMR Reverse prosthesis was used in all shoulders. Of note is that the reverse liner of polyethylene (cross‐link) had a chamfer in its inferior portion designed to decrease the risk of impingement and the consequent scapular notching. The proximal humeral body was in titanium alloy with a hole to allow suture of the tuberosities. Shoulders were postoperatively immobilised in sling for 2 weeks in a regimen similar to that of the HA group. Participants then continued with physiotherapy in a rehabilitation centre for at least 4 weeks to perform deltoid activation exercises and activities as tolerated. 2. Hemiarthroplasty: an SMR Trauma prosthesis was implanted. The proximal humeral body had holes to allow suture of the tuberosities to the stem, and the modular head was in titanium alloy. Rotator cuff tears repaired if possible. Sling immobilisation after surgery, gradually discontinued around 3 weeks. Passive mobilisation and pendulum exercises were allowed immediately. At week 2, passive‐ and active‐assisted exercises were allowed in a rehabilitation centre with forward elevation and abduction limited to 100º and external rotation limited to 30º. When consolidation of tuberosities was observed on the radiographs (around 6 weeks), active and resisted exercises were started. Assigned: 31/31 Completed (at 2 years): 31/30 |
|
| Outcomes | Length of follow‐up: mean 28.5 months (range: 24 to 49 months); also followed‐up but no data for 6 weeks, 3, 6 and 12 months (and then yearly)
QuickDASH
University of California‐Los Angeles (UCLA) score
Constant score (absolute and adjusted for age and gender) Mortality Complications (intraoperative fracture, infection, haematoma, neurological, severe stiffness, proximal migration of implant) Reoperations Range of motion (anterior forward; abduction) Tuberosity healing, malunion, non‐union resorption Strength (not reported) Radiographs: acromiohumeral distance; scapular notching, loosening, heterotopic ossification, proximal migration, radiolucent lines |
|
| Funding and conflict of interest statements | There was no statement on funding. Statement confirming that the authors, their immediate families and affiliated research foundations had not received financial payments or related benefits from any commercial entity in relation to the article. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Method not stated, although seems likely that an appropriate method was used |
| Allocation concealment (selection bias) | Low risk | "Randomization to the HA or RSA group was based on sequentially numbered opaque sealed envelopes. The surgeons were not involved in the randomization process." |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | "All postoperative functional evaluation forms were completed at each visit by an independent experienced surgeon (A.L.U.) who had not participated in the surgeries and did not know which type of prosthesis had been used". However, there was no blinding of care providers or participants. |
| Blinding (performance bias and detection bias) Death, reoperation | Low risk | Surgeons were experienced. "[C]linical and radiologic evaluations were performed by independent observers who had not participated in the surgeries". |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Low risk | One loss to follow‐up (death) only. Interim follow‐up data not reported |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | One loss to follow‐up (death) only |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or published protocol. Marginal but some arbitrary definitions of outcomes. No data on interim follow‐ups |
| Balance in baseline characteristics? | Unclear risk | Baseline characteristics were balanced in the two groups. (Characteristics of 1 participant not provided.) Difference in cuff tears between groups accounted for and tested. |
| Free from performance bias? | Low risk | All operations were performed by 2 surgeons experienced in shoulder surgery; the modular shoulder replacement system was already in use at centre before the study. Same approach and operating methods and conditions; regional anaesthesia etc. Comparable rehabilitation ‐ differences appropriate for different procedures |
Smejkal 2011.
| Study characteristics | ||
| Methods | Method of randomisation: computer‐generated block randomisation with sealed envelopes Assessor blinding: no mention in the paper Loss to follow‐up: 4 lost to follow‐up and 2 died of breast cancer during the study period |
|
| Participants | University Hospital in Hradec Králové, Czech Republic Period of study recruitment: January 2006 to January 2010 61 participants with AO type A2, A3, B1 and C1 (2‐part and 3‐part) proximal humeral fractures aged between 18 and 80 years able to give informed consent Exclusion criteria: open fracture, associated injury (Abbreviated Injury Scale (AIS) > 2), open growth plates, or patient's health would limit the extent of surgery Of 55: 45 females, 10 males; mean age 61 years, range 21 to 81 years |
|
| Interventions | Interventions started 0 to 24 days after injury.
1. Open reduction and internal fixation group: consisted of participants undergoing open reduction with angle‐stable osteosynthesis using a PHILOS plate (Synthes, Switzerland). 2. Minimally invasive group: Zifko method of minimally invasive osteosynthesis with intramedullary K‐wire (Kirschner wire) insertion (distally inserted) – figure in article shows 8 wires inserted into humeral head along medullary canal. Assigned: number in each group not known (total 61) Completed: 28/27 |
|
| Outcomes | Length of follow‐up: mean 2 years Days to operation Constant score (relative to healthy limb) Time to recover normal upper‐limb function Complications Time to radiographically‐assessed recovery Anatomical position X‐ray exposure Length of operation Length of hospital stay |
|
| Funding and conflict of interest statements | There was no statement on funding. There was no statement on conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The patients were randomised to the groups by a computer programme which facilitates the maintenance of homogeneity of the groups compared.” Web‐based translation implied use of random numbers and permuted blocks so as to get similar numbers on each group. Produced independently by a statistical company. |
| Allocation concealment (selection bias) | Low risk | The sealed envelopes were created by a professional statistical company (Pharm test s. r. o., Hradec Králové): in accordance with the randomisation sheet, each numbered envelope had sealed inside either "zifko" or "LCP". The sealed envelopes were opened sequentially. |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Not possible to blind participant/providers. No mention of outcome assessment |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | May not affect assessment |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Incomplete data (and group of 6 excluded participants not noted) |
| Incomplete outcome data (attrition bias) Death, reoperation | High risk | Incomplete data (and group of 2 deaths not stated) |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Balance in baseline characteristics? | Unclear risk | Aside from age – no details or confirmation of this |
| Free from performance bias? | Unclear risk | No details – including of surgeon’s experience |
Sohn 2017.
| Study characteristics | ||
| Methods | Method of randomisation: computer‐generated block randomisation with sealed envelopes Assessor blinding: no mention in the paper Loss to follow‐up: 17 lost to follow‐up |
|
| Participants | Either National Medicalk Center or Ewha Womans University Mokdong Hospital, Yangcheon‐gu, Seoul, Republic of Korea Period of study recruitment: August 2010 to May 2014 107 skeletally‐mature participants with unilateral two‐part surgical neck, three or four‐part proximal humeral fractures, previously uninjured humerus, time to surgery within 3 weeks, and 3 dimensional computed tomography (3D CT) evaluation. Written consent. Exclusion criteria: isolated greater or lesser tuberosity fractures, injuries with more than 3 weeks between the injury and surgery, pathologic fractures, open fractures, combined dislocation or scapular fractures, neurovascular compromise from the initial trauma. Also stated: "less than 1 year follow up". Of 90: gender not stated; mean age 61.8 years, range not stated |
|
| Interventions | Interventions started on average 3.8 days after injury. A 3.5 mm proximal humerus anatomical locking plate (PHILOS; Synthes, Paoli, PA) was used in both groups 1. Minimally invasive plate osteosynthesis (MIPO), which reduction is not done under direct view. A deltoid splitting approach was used proximally, starting from the anterolateral corner of the acromion along the anterior raphe of the deltoid muscle. 2. Open reduction and plate fixation was performed through the conventional deltopectoral approach with careful dissection so that the periosteal blood supply was preserved as much as possible. Assigned: 55/52 Completed: 45/45 (12 months) |
|
| Outcomes | Follow‐up: mean 14.65 months; also monthly until bone union was achieved, and at 6 and 12 months Constant score University of California, Los Angeles (UCLA) score Patient satisfaction (VAS) Complications, including from radiological assessment: intra‐articular screw penetration; screw loosening; varus collapse; greater tuberosity migration; stiff shoulder; AVN, axillary nerve injury (0), infection (0), non‐union (0) Length of surgery Time to fracture union |
|
| Funding and conflict of interest statements | There was no statement on funding. The authors declared that they had no conflicts of interest. | |
| Notes | The sample size was calculated based on the interim results for Constant score after 40 participants had been recruited. Request sent by HH on 23 February 2020 to Sang‐Jin Shin asking where trial was conducted, and for brief details of rehabilitation, how many women and men in each group and on secondary surgery for complications split by treatment group: no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was performed by permuted block randomization. The block length was 4, and randomization was performed by means of consecutive numbering. The randomization sequence was created using a web‐based service with a 1:1 balanced allocation." The statement is confusing but we considered that sequence generation was very likely to be random. |
| Allocation concealment (selection bias) | Low risk | "After the patients were transferred to the operating room, an independent assistant oversaw the randomization using the web site. After confirming the allocation of the patient, the independent assistant notified the surgeon about the allocation." |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No mention of blinding for functional outcomes. "Radiographic outcomes were estimated independently and agreed upon by 2 orthopaedic surgeons who were not involved in this study." Not relevant to the outcomes collected here. |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Non‐attendance of 12‐month follow‐up 17/107 = 16%; 10/55 = 18% in MIPO and 13.5% in open group. No information on why these were lost to follow‐up although reasonably balanced losses between groups |
| Selective reporting (reporting bias) | Unclear risk | No trial registration or published protocol. Sample size calculated after interim analysis. Focused on radiographic outcomes; incomplete account of assessment of functional outcomes |
| Balance in baseline characteristics? | Unclear risk | Only age and fracture type data provided and only for the 90 (84%) of 107 randomised. The authors stated without data that there “were no statistically significant differences in other demographic data”. |
| Free from performance bias? | Unclear risk | No information on care provider expertise, on co‐interventions or rehabilitation |
Soliman 2013.
| Study characteristics | ||
| Methods | Method of randomisation: use of computer‐generated random number table Assessor blinding: yes, blinded observer for Constant score, pain and range of motion Loss to follow‐up: 8 post‐randomisation exclusions |
|
| Participants | Cairo University Hospital, Cairo, Egypt Period of study recruitment: 2005 to 2009 45 participants treated with hemiarthroplasty for 4‐part fractures of the proximal humerus, fracture dislocations or head‐splitting fractures presenting within the first five days after injury. Informed consent Exclusion criteria: not available. Exclusion criteria applied to post‐randomisation exclusions of participants with complications. 13 females, 32 males; mean age 52 years (of 37 participants), range 45 to 60 years (Note: this was a young population with very severe injuries. Predominantly males and presumably high‐energy trauma. The biceps tendon is stronger in younger people.) |
|
| Interventions | Interventions started within 5 days after injury. Same prosthesis (Johnson and Johnson) and surgical technique used in both groups. "A standard deltopectoral approach was used and the coracoacromial ligament was preserved in all patients." The operative technique is described at length in the article.
1. Hemiarthroplasty and tenodesis of long head of the biceps (LHB): LHB tendon was divided at its insertion and tenodesed by Ethibond sutures into the insertion of the pectoralis major. 2. Hemiarthroplasty: LHB tendon left intact. Post‐surgery, the arm was immobilised in a position of neutral rotation for 4 weeks. This was followed by the same physiotherapy protocol for all participants. Assigned: 23/22 Completed (2 years): 19/18 |
|
| Outcomes | Length of follow‐up: mean 2 years (range 21 to 27 months) Constant score ("modified"; not clear how) Pain (VAS, then categorised to none, mild, moderate, severe) Reoperation Complications (these were excluded ‐ see Notes) Anterior shoulder elevation |
|
| Funding and conflict of interest statements | Explicit statement of no external funding A conflict of interest statement stated: "None". | |
| Notes | Post‐randomisation exclusions: "Eight patients were excluded from the study within the first 3 months of follow‐up due to tuberosity malposition (three patients), inferior subluxation of the prosthesis (two patients), loss of reduction of the greater tuberosity (two patients) and deep infection, which slowed down the physiotherapy protocol and required surgical debridement (one patient)" (page 262 in report). Contact with the lead author resulted in no clarification of the method of randomisation ("we enrolled the patients in a random number"), but yielded information on the manufacturer of the implant, a breakdown of the numbers of participants with specific complications in each group and clarification that there were no reoperations aside from debridement for a deep infection. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to either hemiarthroplasty or hemiarthroplasty and tenodesis of the LHB, according to a computer‐generated random number sequence." |
| Allocation concealment (selection bias) | Unclear risk | "After enrolment, cases were sequentially arranged and plotted on the random number table to determine to which group they will be assigned". No mention of safeguards |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | Low risk | All patients were evaluated by a blinded observer using the Constant score. |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Lack of blinding unlikely to affect reporting |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Inappropriate exclusion of eight participants. Although the number of post‐randomisation exclusions was four in each group, the loss to follow‐up was 17% (4/23 in the tenodesis group) and 18% (4/22 in the intact LHB tendon group), and all eight participants were more likely to have had poor results. |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Data from author clarified that only the participant with deep infection had subsequent treatment for a complication. |
| Selective reporting (reporting bias) | High risk | Retrospective trial registration. Inadequate description of outcomes, including the categorisation of pain. Strength was measured but not reported. Pain categories and measurement not defined sufficiently |
| Balance in baseline characteristics? | Unclear risk | No separate data aside from age. While balanced for age, these data apply to 37 of 45 participants. No details on fracture severity and cuff integrity, both of which could affect result |
| Free from performance bias? | Low risk | Same surgeon operated with same prosthesis. Same post‐surgical care and rehabilitation |
Stableforth 1984.
| Study characteristics | ||
| Methods | Method of randomisation: unknown; "randomly selected" Assessor blinding: unlikely Loss to follow‐up at 18 months to 12 years: 2/32 (2 deaths) | |
| Participants | Bristol Royal Infirmary, Bristol, UK Period of study recruitment: 1970 to 1981 32 participants with displaced 4‐part proximal humeral fractures (Neer) Exclusion criteria: impacted or minimally displaced fractures 25 female, 7 male; mean age 68 years, range 52 to 88 years | |
| Interventions | Interventions started: within 5 days for surgery. 1. Neer prosthesis, uncemented 2. Non‐surgical treatment: closed manipulation All were placed in sling, mobilisation of hand encouraged, shoulder flexion rotation exercises after 2 to 3 days. Supervised physiotherapy for 3 to 6 months Assigned: 16/16 Completed (at 1 year): 15/15 (but totals given as 16/16 in tables in the trial report) | |
| Outcomes | Length of follow‐up: stated as 18 months to 12 years; but also assessed regularly up to 6 months Dependent in activities of daily living Range of motion (flexion, medial rotation, lateral rotation) Pain Muscle strength (flexion, abduction, lateral rotation) Complications: haematoma, cellulitis, deep sepsis, early shoulder stiffness Mortality | |
| Funding and conflict of interest statements | There was no statement on funding. There was no statement on conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details: "assigned by pre‐arranged random selection" |
| Allocation concealment (selection bias) | Unclear risk | No details: "assigned by pre‐arranged random selection" |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Not blinded |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | No blinding but may not have affected appraisal of mortality |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Large loss to follow‐up (46/85 = 54%). Numbers given for those available at follow‐up but incompletely reported data: only medians |
| Incomplete outcome data (attrition bias) Death, reoperation | Unclear risk | Slight discrepancy in trial report that 2 deaths are reported, one in each group, but long‐term denominators are as at baseline |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge this, but the protracted nature of this trial makes selective reporting more likely |
| Balance in baseline characteristics? | Unclear risk | Surgical group on average 4.5 years younger, but uncertainties mainly reflect Inadequate information in terms of other comorbidities and injuries for this broad category of participants |
| Free from performance bias? | Unclear risk | Inadequate information on care programme comparability especially given the protracted nature of the trial recruitment. However, one surgeon operated throughout. |
Torrens 2012.
| Study characteristics | ||
| Methods | Method of randomisation: use of independently‐produced, computer‐generated random numbers list Assessor blinding: no mention Loss to follow‐up: 3 (1 death) |
|
| Participants | Castelldefels, Barcelona, Spain Period of study recruitment: not known 42 participants with displaced or non‐displaced proximal humeral fractures that were not considered for surgery or patient refused surgery. (Included: 8 non‐displaced fractures, 11 2‐part and 23 3‐part fractures.) Written informed consent Exclusion criteria: incapacity to understand or complete the tests or sign informed consent form; no contact of humeral head and humeral shaft, fracture dislocation, posterior displacement of the greater tuberosity ≥ 1.5 cm 32 female, 10 male; mean age 70 years, range 60 to 80 years | |
| Interventions | Interventions started: probably very soon after participants attended with their fracture 1. Functional one week immobilisation regimen using arm sling in internal rotation 2. Conventional four weeks immobilisation regimen, using arm sling in internal rotation Both groups followed the same progressive rehabilitation programme Assigned: 20/22 Completed (at 1 year): 19/20 |
|
| Outcomes | Length of follow‐up: 1 year (also 1 week, and 3 and 6 months) Pain (VAS: 0 to 10: higher scores = worse pain) Constant score Satisfaction score (VAS: 0 to 10: higher scores = greater satisfaction) EQ‐5D Mortality Secondary surgery and complications Further 'significant' displacement |
|
| Funding and conflict of interest statements | There was no statement on funding. There was no statement on conflicts of interest. | |
| Notes | Conference abstract (2012) presented data for 42 participants (mean age 70 years), 32 of whom had displaced fractures. A query on publication status was sent 23 May 2015, with response from Carlos Torrens received 25 May 2015: "Unfortunately this study was stopped because of lack of money so we just could recruit 40 patients." This included notification of an ongoing trial (Torrens 2015) testing the same comparison. A data collection form sent to Carlos Torrens for this trial on 5 June 2015 was returned completed by him on 10 June 2015. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was done by the Statistics that gave us a computer generated random numbers list" (email 10 June 2015). |
| Allocation concealment (selection bias) | Unclear risk | No mention of safeguards |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No mention of blinding |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Not blinded but less likely that these outcomes would be affected |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Low risk | One lost to follow‐up in each group |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Data provided by trialist. One lost to follow‐up in each group |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration |
| Balance in baseline characteristics? | Unclear risk | There were fewer 'non‐displaced' fractures in the 1 week immobilisation group (1 versus 7). However, this was not statistically significant and the changes in the reported fracture distribution between abstract (4 2‐part; 26 3‐part; 10 non‐displaced) and unpublished data (11 2‐part; 23 3‐part; 8 non‐displaced) may indicate some intra‐ or inter‐rater discrepancies in applying (if applied) the Neer classification system. Abstract reported "no differences as far as age, gender and displacement between conventional and functional groups". Insufficient information to confirm this |
| Free from performance bias? | Unclear risk | There is insufficient information to confirm this. However, it seems likely because both groups followed the same rehabilitation regimen. |
Tousignant 2020.
| Study characteristics | ||
| Methods | Method of randomisation: random number generator with sealed envelopes Assessor blinding: blinded evaluator Loss to follow‐up: 1 (group not known) |
|
| Participants | Centre Hospitalise Universitario de Sherbrooke (CHUS), Sherbrooke, Quebec, Canada Period of study recruitment: June 2015 to January 2017 31 participants (originally 52 were planned) with a non‐surgically treated proximal humeral fracture who: returned home after discharge from the hospital or emergency; were apt to do exercises; had sufficient verbal and written comprehension to participate in the treatment and evaluations; and had access to a high‐speed Internet connection at home. Exclusion criteria: intra‐articular proximal humeral fracture; presence of any other upper‐limb fracture that could interfere with rehabilitation; surgical treatment following the fracture Of 30: 28 female, 2 male; mean age 63 years, range not reported |
|
| Interventions | Trial entry (and baseline evaluation) was on average 27 days after the fracture. 1. Telerehabilitation: at home from a clinic. When the training programme was supervised, thus twice during weeks 1, 3 and 5, and once for the other weeks (2, 4, 6, 7, 8), this was via telerehabilitation. The other sessions were without supervision. Details of the telerehabilitation technological platform: "The patient and clinician systems include a 22″ touch monitor, a mini‐PC (Intel NUC), a pan‐tilt‐zoom (PTZ) camera with embedded h264 video codec, a microphone array and a speaker. The telerehabilitation software, Vigil2, runs on both systems. The software includes functionalities for management (users, systems and sessions), patient status (online, offline, previous sessions, planned sessions), secure video, audio and data transfer over the Internet, and intuitive camera control (point‐and‐click control scheme). It also includes an easy way for the patient to turn on and off the system using the touch screen. Audio, video and sensor data coming from the patient’s home are transferred to the clinician using an application and database server over a secure link, allowing real‐time sessions to occur." 2. Face‐to‐face intervention in a clinic. When the training programme was supervised, thus twice during weeks 1, 3 and 5, and once for the other weeks (2, 4, 6, 7, 8), this was face‐to‐face rehabilitation at the outpatient clinic. The other sessions were without supervision. The rehabilitation programme was identical in both groups, and was dispensed by physical therapists from the same clinic. Thus only the delivery mode differed. The training programme consisted of 30‐ to 45‐minute sessions, twice daily for 8 weeks, with supervision by qualified physical therapists. The exercise programme, based on a post‐prosthesis and post‐fracture rehabilitation programme developed by the orthopaedic surgery division of the CIUSSS de l’Estrie ‐ CHUS, includes stretching, pain management, range of motion and muscular strengthening, in addition to a question period. The attending physical therapist adjusted the exercises according to the progression of each participant’s condition. |
|
| Outcomes | Length of follow‐up: 9 weeks, thus immediately after the 8‐week intervention period DASH Constant score Range of motion User satisfaction (Health Care Satisfaction questionnaire) Cost of services to the public healthcare system (not reported yet) | |
| Funding and conflict of interest statements | Funding: supported by a grant received from the Department of Surgery of the Université de Sherbrooke, plus from the Chair in Telerehabilitation The authors declared that they had no conflicts of interest. | |
| Notes | Published protocol available. Trial registration. Start: June 2015 and actual completed date was 30 March 2017 Request sent on 31 January 2020 by HH for plans for publication of this trial. Response from Catherine Page included URL to recent publication. This trial was preceded by a 'proof‐of‐concept' study that investigated the feasibility of an in‐home telerehabilitation programme for proximal humeral fractures (Tousignant 2014). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From protocol: "Randomization will be done by a random number generator with sealed envelopes." “The randomised list has been generated electronically using block randomization of size 4.” |
| Allocation concealment (selection bias) | Low risk | From protocol: "Randomization will be done by a random number generator with sealed envelopes."
From report (discussion): “Strengths of this study are based on the control of a potential selection bias. Indeed, randomization was blinded to the evaluator and participants’ characteristics.” Comment: although there is insufficient detail on safeguards, the point in the discussion implies this aspect was secure. |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Participants and care providers were not blinded.
“The evaluator is the only one who will be blind to the randomisation.” No indication of safeguards taken to stop the participants telling which group they belonged to during the evaluation |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Low risk | One participant was lost to follow‐up (group unknown). It seems unlikely that this would have affected the results. |
| Selective reporting (reporting bias) | Low risk | Trial registration and protocol available. All outcomes in protocol presented, except for costs |
| Balance in baseline characteristics? | Unclear risk | Although the type of fracture was not given, the other baseline characteristics were reasonably balanced. However, the baseline scores and range of motion values were all higher in the telemedicine group, which aids the impression that the two groups were somewhat different. |
| Free from performance bias? | Unclear risk | The rehabilitation programmes and staff were the same (in intention) aside from the two interventions. However, there is no information about the therapists nor about consistency in the adjustments made to the rehabilitation programme. |
Voigt 2011.
| Study characteristics | ||
| Methods | Method of randomisation: drawing balls from a bag by an independent person Assessor blinding: yes Loss to follow‐up at 12 months: 8/56 (did not complete follow‐up: 2 deaths, 4 dropouts, 2 excluded because of early secondary arthroplasty) |
|
| Participants | Friederikenstift Hospital Hannover, Hannover, Germany Period of study recruitment: conducted over 18 month period (no dates) 56 participants with isolated Neer type 3‐ and 4‐part proximal humeral fractures, aged > 60 years Exclusion criteria: fractures older than 2 weeks, open fractures, pathological fractures, refractures, neurologic disease and patients who would be clearly non‐compliant (e.g. alcoholics, patients of no fixed address) Of 48: 38 female, 10 male; of 56: mean age 74 years, range 60 to 87 years |
|
| Interventions | Interventions started: at surgical fixation (time to surgery from injury not given) 1) Polyaxial locked screws: Humeral SuturePlate™ (HSP) (Arthrex, Naples, Florida, USA) with polyaxially locked screws. Screws were blunt‐ended (considered better in the prevention of glenoid erosions in case of screw perforations). 2) Non‐polyaxial (monoaxial) implant: PHILOS (Synthes, Bettlach, Switzerland) with non‐polyaxially locked screws. (Screws were pointed in the PHILOS plate.) All surgery performed under general anaesthesia using deltopectoral approach. Tuberosity fragments reduced with fibre wire, different approaches for head fragment depending on whether valgus or varus. Allocated plate positioned anatomically and fixed with a shaft screw Participants' shoulders were immobilised in a sling for 2 days. Then, active‐assisted motion beyond 90 degrees flexion and abduction were initiated, avoiding the provocation of pain. At 7 weeks, free range of motion was allowed. Assigned: 25/31 Completed: 20/28 (at 12 months) |
|
| Outcomes | Length of follow‐up: 12 months Simple Shoulder Test (SST) DASH score Constant score (relative to contralateral limb) Death Complications Reoperation Range of active shoulder motion (flexion, abduction, internal rotation, external rotation) Fracture healing ‐ anterioposterior and axillary radiographs Duration of operation Fluoroscopy time |
|
| Funding and conflict of interest statements | No funding was received for the study. The authors thanked the two implant manufacturers for providing the implants: Synthes Bettlach, Switzerland (PHILOS) and Arthrex Karlsfeld, Germany (HSP). Explicit declaration by authors of "no conflicts of interest" | |
| Notes | Additional information and clarification of 8 participants who did not complete follow‐up and gender data for those who completed follow‐up obtained from Dr Voigt (May 2012) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization technique was blinded by drawing balls from a bag: one ball for HSP and the other ball for PHILOS by an independent person.” |
| Allocation concealment (selection bias) | Low risk | “The randomization technique was blinded by drawing balls from a bag: one ball for HSP and the other ball for PHILOS by an independent person.” |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No mention of blinding of participants or personnel other than assessor blinding: "Follow‐up evaluations postoperatively were performed in a standardized fashion by an independent trauma surgeon" |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Unlikely to influence this |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Although clarification on loss to follow‐up (8 participants: 5 versus 3) received from author, the impact on the results for functional outcomes is unclear |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Clarification received from author on the loss to follow‐up: 2 were deaths and 2 were replacement arthroplasty |
| Selective reporting (reporting bias) | Unclear risk | No protocol provided |
| Balance in baseline characteristics? | Unclear risk | Balance in 3‐ versus 4‐part fractures, probably age and preoperative DASH. Incomplete data on gender, 2 versus 6 with diabetes (but no frozen shoulder) |
| Free from performance bias? | Unclear risk | No details of surgeon experience |
Wirbel 1999.
| Study characteristics | ||
| Methods | Method of randomisation: unknown, "random allocation" Assessor blinding: unlikely Loss to follow‐up at 6 months: 13/77; also 14 months (9 to 36 months): 18/77 | |
| Participants | University Hospital, Homburg/Saar, Germany Period of study recruitment: January 1995 to March 1998 77 participants with displaced (separation exceeds 1 cm; fragment angulation > 30 degrees, or when tuberosity fragment is separated by > 3 mm) subcapital humeral fractures of type A1, A3, B and C1 (modified AO classification) treated by closed reduction and percutaneous fixation Exclusion criteria: extensive local skin infection; impacted fractures of type A2 (treated non‐surgically); not fit enough to undergo anaesthesia and X‐ray of affected shoulder in anterior‐posterior plane; closed reduction not feasible 54 female, 23 male; mean age 63 years, range 6 to 89 years | |
| Interventions | Interventions started postoperatively after percutaneous (minimally invasive) fixation (Kirschner wires plus in 38 cases, cannulated screws) 1. 1 week immobilisation in Gilchrist sling 2. 3 weeks immobilisation in Gilchrist sling Active mobilisation of elbow from first postoperative day. Active and passive physiotherapy of the shoulder (optional continuous passive motion) after removal of sling. Removal of Kirschner wires after 4 to 6 weeks, with post‐procedure continuation of active exercises Assigned: 38/39 Completed (at 6 months): 32/32 | |
| Outcomes | Length of follow‐up: 9 to 36 (mean 14 months) months (in 59 participants), but also assessed at 1, 3 and 6 months Neer score Complications: avascular necrosis, local infection/haematoma, premature removal of Kirschner wires, screw removal due to subacromial impingement | |
| Funding and conflict of interest statements | There was no statement on funding. There was no statement on conflicts of interest. | |
| Notes | Short report (1997) from conference proceedings gave interim results for 51 participants. Full report and some results provided by Dr Wirbel (February 2003). Most of the results given in the trial report were either for the whole study population or split by basic AO fracture type. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details: "a random allocation of patients in 2 groups was done" |
| Allocation concealment (selection bias) | Unclear risk | No details: "a random allocation of patients in 2 groups was done" |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No mention of blinding |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | Not blinded but less likely that these outcomes would be affected |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Limited data on function using a non‐validated assessment instrument with a moderate loss to follow‐up at 6 months (13/77 = 17%) |
| Incomplete outcome data (attrition bias) Death, reoperation | Unclear risk | Incomplete data. Although loss to follow‐up reported, reoperations were not sufficiently reported by treatment group |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge this |
| Balance in baseline characteristics? | Low risk | No indication of any major baseline imbalance |
| Free from performance bias? | Low risk | No indication of performance bias from differences in care programmes |
Zhang 2011.
| Study characteristics | ||
| Methods | Method of randomisation: computer‐generated random numbers Assessor blinding: likely; "independent" assessor at follow‐up Loss to follow‐up: 4 participants within the first year after surgery due to moving out of the area and change of telephone number |
|
| Participants | The Third Affiliated Hospital of Whenzou, Whenzou, China Period of study recruitment: October 2007 to September 2008 72 participants aged over 18 years with an acute closed 2‐, 3‐ or 4‐part fracture (Neer classification) of the proximal humerus treated with open reduction and internal fixation using a locking plate. Exclusion criteria: pathological fractures, primary or metastatic tumour and fracture with non‐union Of 68 followed‐up: 46 female, 22 male; mean age 63 years, range 32 to 78 years |
|
| Interventions | Interventions started: both at surgery; time from injury not stated 1. ORIF with PHILOS locking plates (Synthes, Switzerland). Standard deltopectoral approach; reduction enabled with a K‐wire (Kirschner wire) under fluoroscopy. Locking plate was placed 10 mm posterior to the intertubercular groove and 10 mm distal to the tip of greater tubercle. A cortical screw was inserted initially to fix the distal fragment. Four or five locking screws were used for the fixation of the proximal fragment. All proximal screws were inserted 5 mm below subchondral bone. One or two additional locking screws were inserted obliquely into the medio‐inferior region of the humeral head in this group. The tubercular fragments and rotator cuff tendon were fixed using Ethibond sutures. Autograft bone was used in comminuted fractures where there was a mass defect and for reconstruction of the medial support structures. Fracture reduction and screw length were finally assessed with fluoroscopy. 2. As above without medial support locking screws. All participants received prophylactic intravenous antibiotics before the procedure. Passive abduction and clockwise rotation exercises were allowed on the day after surgery. Active rehabilitation was started six weeks postoperatively. Assigned: 32/40 (total: 72) Completed (2+ years): 29/39 |
|
| Outcomes | Length of follow‐up: average 30.8 months (also 4, 8, 12 weeks, 6, 9 and 12 months and yearly) Shoulder function (Constant score) Union Complications: osteonecrosis of the humeral head, early failure and loss of fixation Reoperation |
|
| Funding and conflict of interest statements | Explicit statement that there was no financial support for the study The authors declared that they had no conflict of interest. |
|
| Notes | Personal contact (email 14 May 2012) clarified: method of randomisation: group of participants who were lost to follow‐up; and complications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomized into two groups for study according to computer‐generated random numbers". (Group sizes, however, were unequal.) |
| Allocation concealment (selection bias) | Unclear risk | There were insufficient safeguards (selection according to odd and even random numbers) to confirm allocation concealment. |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | It is possible that the participants did not know which group they were in. The difference between the two interventions was not large. “Complications, shoulder function and radiological measurement were recorded by an independent junior doctor (YJH) who did not participate in the surgery.” |
| Blinding (performance bias and detection bias) Death, reoperation | Low risk | These outcomes are fairly robust regarding blinding. |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Active surveillance but missing data for 4 participants lost to follow‐up. Personal correspondence gave details on complications. |
| Incomplete outcome data (attrition bias) Death, reoperation | Unclear risk | Missing data for 4 participants. Personal correspondence provided information on reoperations. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data to judge this. No protocol available |
| Balance in baseline characteristics? | Unclear risk | Although the baseline characteristics of 68 participants were comparable, data were missing for 4 participants lost to follow‐up. |
| Free from performance bias? | Low risk | “Operations were performed by two senior surgeons.” All participants received same rehabilitation |
Zhang 2019.
| Study characteristics | ||
| Methods | Method of randomisation: use of a random number table Assessor blinding: unlikely, no mention Loss to follow‐up: not stated but short follow‐up |
|
| Participants | Zhuji People’s Hospital of Zhejiang Province, Zhuji, Zhejiang Province, China Period of study recruitment: June 2016 to June 2017 80 participants aged between 60 to 79 years with an acute (within 3 days of fracture) closed 3‐ or 4‐part fracture (Neer classification) of the proximal humerus; osteoporosis (T values < ‐2.5 ); no vascular or nerve injuries; no contraindications to surgery; informed consent of participants and families Exclusion criteria: bone tuberculosis; serious endocrine system diseases; severe cardio‐cerebral vascular diseases; severe liver, kidney, and lung dysfunction; fractures with severe suppuration and infections; autoimmune diseases; previous fracture surgery in the same location; acute or chronic systemic infections; coagulation dysfunction 43 female, 37 male; mean age 71 years, range 60 to 79 years (criteria if not actual) |
|
| Interventions | Interventions started: both at surgery, mean time from injury = 1.9 days 1. ORIF with PHILOS locking plates combined with allogeneic femoral head bone grafts. Deltoid splitting approach. The bone grafts were pruned and implanted into the medullary canal according to the bone defect. 2. ORIF with PHILOS locking plate. Standard deltopectoral approach All participants received a brachial plexus nerve block. All received anti‐infection therapy for 3 days, as well as anti‐osteoporosis therapy, including calcitriol once a day orally; calcium carbonate 2 tablets/time and 2 times/day orally; intramuscular injections of salmon calcitonin injections 1 time/2 days for 1 year. Assigned: 42/38 (total: 80) Completed (3 months): 42/38 (assumed) |
|
| Outcomes | Length of follow‐up: 3 months (also 1 week and 1 month) Shoulder function (Neer shoulder joint function score: includes pain, function, mobility and anatomy) Pain (VAS) Fracture healing time Complications: infection, osteonecrosis of the humeral head, screw cut, delayed fracture healing; varus displacement (head‐shaft angle < 120 degrees) |
|
| Funding and conflict of interest statements | There was no statement on funding. The authors declared that there was no conflict of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eighty patients ... were selected and divided into two groups using a random number table". |
| Allocation concealment (selection bias) | Unclear risk | There was no mention of safeguards for ensuring allocation concealment. |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | There was no mention of blinding, which seems unlikely. |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Low risk | Although participant flow was not reported, losses are unlikely given the short follow‐ups: 1 week for pain, 1 month for function, 3 months for adverse events and fracture union. |
| Selective reporting (reporting bias) | High risk | No trial registration or protocol available or referred to. Follow‐up was inappropriately short, and the Neer shoulder function score is likely to have been categorised into a binary score, perhaps increasing the likelihood of statistically significant results. |
| Balance in baseline characteristics? | Unclear risk | Although the presented characteristics were balanced, the fractures were insufficiently described. |
| Free from performance bias? | High risk | Insufficent details of rehabilitation. The surgical approach differed in the two groups. |
Zhu 2011.
| Study characteristics | ||
| Methods | Method of randomisation: computer‐generated random numbers list reviewed by nurse before surgery Assessor blinding: no, but mention of independent observer Loss to follow‐up at 3 years: 6/57 (5 lost to follow‐up; 1 died) | |
| Participants | Beijing Ji Shui Tan Hospital, Beijing, China Period of study recruitment: November 2004 to December 2006 57 skeletally‐mature participants with an acute 2‐part surgical neck fracture of the proximal humerus (Neer's classification) treated surgically within 21 days of the injury. Participant consent Exclusion criteria: open physes; fracture and displacement involving the greater or lesser tuberosity or extension of the fracture line distally beyond the deltoid tubercle; associated musculoskeletal injuries to the same upper extremity; open fracture; and prior surgery on the affected shoulder Of 51 followed up: 34 female, 17 male; mean age 53 years |
|
| Interventions | Interventions started: surgery on average 9 days after injury (randomisation before surgery). 1. Open reduction with internal fixation using a locking plate: Locking Proximal Humeral Plate (LPHP; Synthes) or PHILOS (Synthes). General anaesthesia combined with an interscalene block. Indirect reduction under image intensifier, with reduced fracture temporarily fixed by a Kirschner wire. After placement, position of the locking plate checked with the image intensifier intraoperatively, and the plate was fixed with locking screws. Finally, a thorough fluoroscopic screening was done to ensure that no screw was penetrating the articular surface of the humeral head. 2. Open reduction with internal fixation using a locking nail: the Proximal Humeral Nail (PHN; Synthes). An interscalene brachial plexus block was used. Nail was inserted under image control without reaming after the fracture was fully reduced. After insertion of the spiral blade and the distal locking screws, an end cap was screwed in to lock the spiral blade. The rotator cuff tendon and the deltoid were carefully repaired during wound closure. The affected extremity was protected by a sling for six weeks postoperatively. Passive range‐of‐motion exercises, supervised by a physical therapist, were initiated on the first postoperative day. Active and active‐assisted exercises began after six weeks, when early callus formation could be seen on radiographs. Strengthening exercises were started three months after the surgery. Assigned: 29/28 Completed (at 3 years): 26/25 |
|
| Outcomes | Length of follow‐up: 3 years (also 1 year) ASES (American Shoulder and Elbow Surgeons) score Constant score (both shoulders) Pain (VAS) Mortality Complications (overall, infection (none), heterotopic ossification, screw penetration, pseudothorax) Reoperation Range of motion (active flexion, external rotation, internal rotation) Strength Duration of surgery Blood loss and transfusion Radiographic outcomes, including avascular necrosis, union, and degenerative change (osteoarthritis) | |
| Funding and conflict of interest statements | Explicit statement that there was no outside funding or grants for the study Statement confirming that the authors or their immediate families had not received financial payments or other benefits from any commercial entity in relation to the article nor had they made any commitments or agreements to provide such benefits |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was accomplished with use of a random numbers list generated by software and kept by the operating room nurse." |
| Allocation concealment (selection bias) | Unclear risk | "Before the surgery, the circulating nurse reviewed the random‐numbers list. Patients who had been assigned an odd number were subsequently treated with a locking nail, and those who had been assigned an even number were managed with a locking plate." |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | No mention of blinding. However: "All of the follow‐up physical examinations and radiographic evaluations were done by the same independent observer." |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | No mention of blinding; lack of blinding less likely to affect these outcomes |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Low risk | Participant flow diagram provided; similar and modest losses in each group |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | Participant flow diagram provided; similar and modest losses in each group: data reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge this |
| Balance in baseline characteristics? | Unclear risk | No indication of any major baseline imbalance in 51 participants followed up at 3 years but no data for 6 participants lost to follow‐up |
| Free from performance bias? | Low risk | All surgical procedures performed by senior surgeon and comparable rehabilitation. Although general anaesthesia used only for the plate group, this was considered unlikely to affect the findings |
Ziegler 2019.
| Study characteristics | ||
| Methods | Method of randomisation: computer‐generated randomised list with allocations placed in consecutively numbered envelopes Assessor blinding: not blinded Loss to follow‐up at 6 months: 13/76 (9 withdrew and 4 exclusions: 2 didn't receive allocated plate and 2 required additional surgery after another injury) | |
| Participants | BG Hospital Tübingen, Tübingen, Germany Period of study recruitment: October 2016 to June 2018 76 participants, aged over 18 years, "with proximal humerus fractures requiring surgery". Participant consent Exclusion criteria: bilateral or previous humerus fractures; head‐split fractures; rotator cuff arthropathy; neural or vascular injury; thrombophilia, severe heart or lung disease; alcohol or drug abuse; hypersensitivity or allergy for plate material. (Also reported as excluded in the trial registration document were American Society of Anesthesiologists (ASA) class 4 to 6 and untreated class 3.) 60 female, 16 male; mean age 60.7 years |
|
| Interventions | Interventions started: not stated; randomisation immediately before surgical procedure 1. ORIF using the CFR‐PEEK (carbon fibre reinforced polyetheretherketone) plate (PEEK Power Humeral Fracture Plate; Arthrex, Naples, Florida, USA). This plate is made of polyetheretherketone reinforced with carbon fibres. It is anatomically preshaped and designed to allow placement of polyaxial locking screws and fixation of stay sutures at the plate. It is radiolucent and designed for use with 3.5 mm and 4.0 mm titanium screws in the shaft and head of the humerus, respectively. 2. ORIF using the titanium PHILOS plate (DePuy Synthes, West Chester, Pennsylvania, USA). The plate is anatomically preshaped and designed to allow placement of locking 3.5 mm titanium screws at the humeral shaft and head as well as fixation to the rotator cuff using stay sutures. Three screws (locking or conventional) can be placed at the humeral shaft and nine screws (locking) at the humeral head. In both groups, the anterolateral approach (Mackenzie) was used with the participant under general anaesthesia and in beach‐chair position. Single‐dose perioperative antibiotic prophylaxis with cefuroxime was also administered. The greater and lesser tubercles of the humerus were looped using non‐absorbable stay sutures. The respective plate was placed 5 to 8 mm distal to the greater tubercle and directly lateral to the bicipital groove and then fixed using a cortical screw and two 3.5 mm locking screws at the shaft. Postoperative rehabilitation comprised using the Gilchrist bandage for 7 to 10 days with pendulum exercises performed with the bandage in place during this period of immobilisation. Subsequently, the range of motion was extended to assisted/active movements up to 60° flexion/abduction and 0° external rotation/extension over a period of 2 weeks. Thereafter, the range of motion was further extended to assisted/active movements up to 90°flexion/abduction and 20° external rotation/extension for another 2‐week period. Finally, at 5 weeks after surgery, the full range of motion was permitted. In addition, the participants were instructed not to expose the operated arm to loads of more than 15 kg for a period of 6 weeks after surgery. Assigned: 37/39 Completed (at 6 months): 32/31 |
|
| Outcomes | Length of follow‐up: 6 months (also 6 and 12 weeks) Disability of the Arm, Shoulder and Hand (DASH) score Oxford Shoulder Score Simple Shoulder Test Complications (recorded no cases of infection, displacement, screw perforation, non‐union) Reoperation (for new injury) Fracture healing: AP and scapular Y radiographs Radiological outcome: neck‐shaft angle Length of inpatient stay in hospital |
|
| Funding and conflict of interest statements | The study was "financially supported by Arthrex" (the manufacturer of the CFR‐PEEK plate). The conflict of interest statement reported that four of the five listed trial authors received "study support (third party funding) from Arthrex". | |
| Notes | The trial registration document reported the intention to follow‐up for 12 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization list was created before the start of the study, using the “Randbetween” function in MS Excel." |
| Allocation concealment (selection bias) | Unclear risk | "The corresponding results (PEEK/titanium) were placed in consecutively numbered envelopes. These were opened by the operating surgeon immediately before the surgical procedure." Comment: It is unclear whether the envelopes were opaque and sealed. |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | There was no blinding of the participants, surgeons or investigators. |
| Blinding (performance bias and detection bias) Death, reoperation | Low risk | Lack of blinding very unlikely to affect assessment of the reported outcome |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Participant flow diagram provided with numbers and reasons. Comparable loss to follow‐up in the 2 groups (5 (13.5%) versus 8 (20.5)) but 2 of the titanium plate group were excluded because of a reoperation. Missing data were not imputed but also it was not made clear whether there were any missing data for the individual outcomes. Hence, we judged this was at an unclear risk of bias. |
| Incomplete outcome data (attrition bias) Death, reoperation | Low risk | The participant flow diagram included the data for reoperation. The loss to follow‐up from participant withdrawals were comparable in the two groups. |
| Selective reporting (reporting bias) | High risk | Trial registration available. Unexplained change of primary outcome from fracture consolidation at trial registration to DASH in the report. Also: reduction in length of follow‐up from 12 months to 6 months. No recording of late complications |
| Balance in baseline characteristics? | Unclear risk | Baseline data split by treatment group provided for the 63 participants in the analyses instead of the 76 randomised participants. Reported characteristics balanced except for fracture type distribution, shown below. CRF‐PEEK: 2‐part: 6 (16.2%); 3‐part: 22 (64.9%); 4‐part: 4 (18.9%) Titanium: 2‐part: 5 (23.0%); 3‐part: 13 (38.5%); 4‐part: 13 (38.5%) |
| Free from performance bias? | Unclear risk | Interventions, including rehabilitation, other than the type of plate, were comparable in intent but not reported in practice. There was an imbalance in the number of operations in the two groups performed by the two surgeons (surgeon 1: 15 CRF‐PEEK and 25 titanium; surgeon 2: 17 CRF‐PEEK and 6 titanium). The effect on performance of the funding of the study by one of the plate manufacturers is unclear. |
Zyto 1997.
| Study characteristics | ||
| Methods | Method of randomisation: sealed envelopes Independent assessor at final follow‐up Loss to follow‐up at 3 years: 14/43 (8 deaths, 2 could not be traced, 1 hemiprosthesis, 3 exclusions) | |
| Participants | Huddinge University Hospital, Stockholm, Sweden Period of study recruitment: April 1990 to February 1993 43 "elderly" participants with proximal humeral fractures (AO classification system: A 8; B 27; C 8) ‐ see Notes In trial report: 40 participants with displaced 3‐ or 4‐part fractures (Neer) Exclusion criteria: pathological fracture, high‐energy trauma, < 30% contact between humeral head and shaft, other fractures, impaired ability of patient to co‐operate, relevant concomitant disease 35 female, 5 male; mean age 74 years | |
| Interventions | Interventions started: surgery within 48 hours. 1. Internal fixation (cerclage wiring (8): or surgical tension band (14)) under general anaesthesia. Antibiotic therapy. Physiotherapy 2. Non‐surgical treatment: sling for 7 to 10 days. Then physiotherapy Assigned: 22/21; (20/20) Completed (50 months): 15/14 | |
| Outcomes | Length of follow‐up: 3 to 5 years (listed as 50 months in trial report; patient questionnaire, clinical and radiological assessment); also after treatment and at 1 year Subjective assessment of function, including ability to carry 5 kg, sleep on injured side, comb hair, perform personal hygiene Constant score: overall shoulder function and components (pain, power, range of motion, activities of daily living) Complications: deep infection, non‐union, pulmonary embolism, avascular necrosis of humeral head Mortality | |
| Funding and conflict of interest statements | No mention of direct funding Statement confirming that no benefits in any form had been received from a commercial entity in relation to the study | |
| Notes | Both groups had the same physiotherapy regimen.
Three participants excluded from 1995 data set (Tornkvist 1995) as, on review by Zyto and a radiologist, these participants did not have 3‐ or 4‐part fractures (personal communication). Zyto's response to a letter from H. A. Karladani admits that there may have been some inaccuracy in their classification of the fracture patterns but stressed that the Neer classification system was flawed and that other factors, such as osteoporotic bone, need to be considered too. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "[R]andomised by sealed envelopes" |
| Allocation concealment (selection bias) | Unclear risk | "[R]andomised by sealed envelopes" (at time of admission); no indication of safeguards |
| Blinding (performance bias and detection bias) Functional outcomes, pain, clinical outcomes, complications | High risk | Some independent assessment by radiographer and potentially by main author but no blinding |
| Blinding (performance bias and detection bias) Death, reoperation | Unclear risk | No blinding but may not have affected appraisal of mortality (which was not split by treatment group) |
| Incomplete outcome data (attrition bias) Functional outcomes, pain, clinical outcomes, complications | Unclear risk | Post‐randomisation exclusions and moderately large loss to follow‐up (14/43 = 32%; (11/40 = 28%)) |
| Incomplete outcome data (attrition bias) Death, reoperation | High risk | Only whole group data presented for deaths out of 40 participants |
| Selective reporting (reporting bias) | High risk | Insufficient information to judge this, but some post‐randomisation exclusions and final follow‐up performed by first author who does not appear in the earlier reports of the trial |
| Balance in baseline characteristics? | Low risk | No important imbalances in baseline characteristics |
| Free from performance bias? | Low risk | No indications of serious performance bias: surgery performed by orthopaedic specialists who were experienced in the surgical technique |
AO: Arbeitsgemeinschaft fur Osteosynthesefragen / Association for the Study of Internal Fixation (or ASIF); AVN: avascular necrosis; A&E: accident and emergency; BMD: bone mineral density; CT: computed tomography; DASH: Disability of the Arm, Shoulder, and Hand questionnaire; EQ‐5D: European Quality of Life‐5 Dimensions; MI: myocardial infarction; ORIF: open reduction and internal fixation; OTA: Orthopaedic Trauma Association; PE: pulmonary embolism; PHILOS: Proximal Humerus Internal Locking System; PROMs: patient‐reported outcome measures; ROM: range of motion; RSA: reverse shoulder arthroplasty; RTSA: reverse total shoulder arthroplasty; SD: standard deviation; SF‐12: 12‐item Short‐Form Health Survey; SF‐36: 36‐item Short‐Form Health Survey; UCLA: University of California‐Los Angeles score; VAS: visual analogue scale; WOOS: Western Ontario Osteoarthritis Shoulder score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12610000730000 | This trial, formerly listed as ongoing, intended to compare minimally invasive versus standard open reduction of proximal humeral fractures. The trial was not recruiting, nor had ethics approval been applied for, at time of registration (2 September 2010). The trial registration has not been updated and we received no response to our request on 31 January 2020 for clarification of the trial status from Mr Jeremy Stanley, Auckland, New Zealand. Inspection of the current interests of Mr Stanley indicate that he has a special interest in sports injuries. We anticipate that this trial was abandoned and may not have started. |
| Arias‐Buria 2015 | This RCT investigated the inclusion of trigger point dry needling in a multimodal physical therapy programme for postoperative shoulder pain. Mixed population; 15 participants had a proximal humeral fracture. However, the single duration intervention was given 5 months post‐surgery. Ineligible participant population. (Also, very short term follow‐up.) |
| Avci 2013 | This study investigated two different surgical drilling techniques. An online translation of the Turkish article indicates that participants "were divided into two groups according to the surgical technique used to determine and send the screw length." Not an RCT. |
| Bai 2014 | Online translation indicates this was a “retrospective case analysis” evaluating the lateral deltoid‐splitting approach for treating proximal humeral fractures. Not an RCT. |
| Bai 2015 | Online translation indicates the participants with proximal humeral fractures “were divided into two groups according to the internal fixation protocol”. Not an RCT. |
| Baudi 2014 | Retrospective study that compared functional and radiographic results of reverse prosthesis versus hemiarthroplasty after complex displaced proximal humeral fractures in elderly people. Ineligible study design. |
| Bing 2002 | This was a randomised clinical trial (sealed envelopes ‐ computer‐generated sequence ‐ held in a box), recruitment 3 November 1997 to 14 January 1999, that compared Rush pins fixation with Polaris nail fixation of displaced two‐part fractures of the proximal humerus. Contact with a Dr Sharma in July 2000 revealed 65 of the 80 participants in the trial had reached 2‐year follow‐up. Abstract by Bing et al published in 2002 indicated 40 participants, of whom 30 had been followed‐up for one year. Information gained via Alison Armstrong from Grahame Taylor (one of the authors of the Bing abstract) indicated that there were some concerns about the extent of missing data. Both groups had a high reoperation rate to remove metalware causing impingement. This trial has been excluded because of insufficient data. It seems very likely, based on location and study dates, that the trial registration, Der Tavitian 2006, formerly awaiting classification, is for this trial. |
| Bolano 1995 | No proximal humeral fractures in a randomised trial of humeral shaft fracture treatment. |
| Brownson 2001 | This is listed in the National Research Register as a multicentre randomised trial of the management of displaced surgical neck and displaced shaft fractures of the humerus with the Halder humeral nail. Contact with Mr Brownson revealed this to be part of the trial run from Nottingham (see Wallace 2000) which had been abandoned. Mr Brownson indicated that the very specific inclusion criteria (2‐part fractures with over 50% displacement) had reduced the potential sample size; participant consent had also been a problem. |
| Caforio 2017 | This trial investigated the effects of an early rehabilitation program in 3‐part proximal humeral fractures treated with endomedullary nailing. Although described as randomised in the abstract, the discussion reports that a limitation of the research was that it was not a randomised controlled study. |
| Caliskan 2019 | The discussion indicates this was a retrospective study that compared open reduction and internal fixation with non‐operative management for proximal humeral fractures. Ineligible study design. |
| Carbone 2012 | This is a prospective comparison of MIROS (Minimally Invasive Reduction and Osteosynthesis System®) versus traditional percutaneous pinning, each intervention being carried out at one of two hospitals in the same town in Italy. Not randomised. |
| Chapman 1997 | No proximal humeral fractures in a randomised trial of humeral shaft fracture treatment. |
| Chen 2014 | Study compared two surgical approaches for treating proximal humeral fractures. The PubMed entry indicates this is a case‐control study. |
| Chen 2019 | Although this study ‐ testing a bespoke sling after plate fixation ‐ met the inclusion criteria for this review, it became clear after data extraction and risk of bias assessment that this poorly conducted and reported small trial had several major flaws and data issues that made the findings unreliable. We received no response to our request sent on 7 February 2020 to Zong‐Sheng Yin for further information on interventions, randomisation, loss to follow‐up and fracture type distribution. In particular, we were uncertain of the Constant scores, which were reported for day 1 pre‐op, day 3 post‐op, 30 days (primary outcome) and final follow‐up. Measurement of Constant scores (0 to 100: best outcome) at the first two times is unusual practice. The high mean scores are unusual for this population and for day 3 seem incorrect, as did the lack of progress over time at 30 days and at final follow‐up. |
| Chen H 2018 | This is a retrospective comparison of people with displaced proximal humeral fractures treated by locking compression plate with or without fibular allograft. |
| Chen JL 2018 | This is a retrospective comparison of two locking plates: PHILOS plate versus Zimmer Periarticular Locking Plate. |
| ChiCTR1900025412 | This study planned to compare different interventions (PHILOS plate, hemiarthroplasty and reverse total shoulder arthroplasty) for treating 3‐ or 4‐part proximal humeral fracture in elderly people. Non‐randomised observational study. |
| ChiCTR‐TRC‐14004213 | The objective of this unpublished study (recruitment period 2014 to 2016) was to investigate when to begin passive motion exercise after operation of old fractures of greater tuberosity. 'Old' not defined but probably surgery on healed fractures of the greater tuberosity causing post‐traumatic impingement. Ineligible study population. |
| Chiu 1997 | No proximal humeral fractures in a quasi‐randomised trial of humeral shaft fracture treatment. |
| Cigni 2012 | This study, published only as a conference abstract, compared two approaches for plate fixation. The abstract did not mention how the 40 participants "were divided in two groups", but we judged it was very unlikely this was through randomisation. |
| De Boer 2003 | This is a multicentre comparative study of locked internal fixators and non‐operative treatment. Not randomised. |
| Dias 2001 | Trial abandoned. This randomised trial (random number sheets that were remotely administered) compared hemiarthroplasty versus fixation (generally, suture reinforced with wires) versus non‐surgical treatment (manipulation, sling for 2 weeks, then mobilisation) for 3‐ and 4‐part fractures of the proximal humerus. Trial started in 2001, with one year follow‐up (outcome was assessed by independent physiotherapists). Aimed for 90 to 100 participants, aged > 45 years. Contact with Alison Armstrong revealed that recruitment stalled at 11 participants (16 refusals) in 2008; centre stopped trial when it became a trial site for the ProFHER trial. |
| Edelson 2008 | Article mentions an abandoned randomised trial comparing "operative versus conservative care" which was unsuccessful "because patients insisted on proactively choosing rather than being assigned to a treatment group by lot"." No other details given. |
| Elidrissi 2013 | Prospective study involving 26 participants with proximal humeral fractures treated with open reduction and internal fixation using an anatomical humeral plate (12 participants) or a palm tree pinning technique of Kapandji (14 participants). Inspection of the full text confirmed this was not a randomised or quasi‐randomised trial. |
| Erdoğan 2014 | This study compared locking plate fixation with or without an inferomedial screw (IMS) in 36 proximal humeral fractures. Inspection of the full text showed this to be a retrospective comparison. |
| EUCTR 2015‐001820‐51 | This randomised study evaluated the effect of implanting bone marrow‐derived mononuclear cells for bone augmentation of plate‐stabilised proximal humeral fractures. Biological intervention outside the scope of our review. |
| Fan 2012 | Translation of this study comparing surgical versus non‐surgical treatment showed this was a retrospective comparison: "To this end, we retrospectively analyzed 2009 1 Month ‐ January 2011 35 cases". |
| Flannery 2006 | This is listed in the National Research Register as a randomised trial comparing non‐surgical treatment and hemiarthroplasty for four‐part fractures of the proximal humerus. Contact with Mr Flannery revealed his centre failed to recruit anyone into the trial. Mr Turner, the lead investigator of the multicentre trial, involving the South Thames Shoulder and Elbow Group, confirmed that the trial was abandoned due to the inability to recruit participants. |
| Ge 2017 | Participants were divided into a plate group (locking plates) or the nail group (intramedullary nails) at discretion of the treating physician and finally of the participant. The participants who declined surgical treatment and only agreed to receive conservative treatment constituted the conservative group. Not an RCT. |
| Ge 2019 | Comparison of 2 types of needle therapy acting as adjuvant analgesic treatment. Intervention not in scope of review. |
| Gradl 2009 | Prospective study involving 152 participants with unilateral displaced and unstable proximal humeral fractures treated either with an antegrade angular and sliding stable proximal interlocking nail or an angular stable plate. Not a randomised or quasi‐randomised trial. |
| Hems 2000 | This is listed in the National Research Register as a randomised trial comparing non‐surgical treatment and the Halder humeral nail for displaced fractures of the surgical neck and shaft of the humerus. Contact with Mr Hems revealed this to be part of the trial run from Nottingham (see Wallace 2000). Mr Hems indicated that they had had considerable difficulty in recruiting participants (only those with proximal humeral fractures were eligible in his centre) and had no results. |
| HOMERUS | This single‐centre trial aimed to compare angle stable locking compression plate osteosynthesis versus hemiarthroplasty for three‐ and four‐part fracture of the proximal humerus in 134 older people. It was listed as ongoing in the 2015 version of the review. Contact with Prof Diercks on the status of the trial revealed that it had been abandoned: "Thank you, sorry not to have informed you but we could not reach the inclusion rate necessary for the power, so the study was stopped some years ago" (13 June 2020). |
| IRCT2013052313435N1 | The trial registration document for this study stated that it was randomised. However, a search identified a journal publication that described "prospective clinical trial, observational ‐ Cohort studying" and gave no mention of random allocation. Hence this is not a randomised trial. |
| ISRCTN32335957 | This was registered as a randomised study of reverse shoulder prosthesis and hemiarthroplasty for elderly people with proximal humeral fractures. However, the principal investigator left the hospital (and country) before it started and a contact at Liverpool (Matthew Smith) confirmed that the study was closed after this. |
| Jin 2016 | This study investigated the clinical effects of the probing method with depth gauge for determining the screw depth of locking proximal humeral plate. Technical detail outside the scope of our review. |
| Kim 2016 | Non‐randomised comparison of open reduction and plate osteosynthesis and minimally invasive plate osteosynthesis for the treatment of 2‐part proximal humeral fractures. Not an RCT. |
| Kim 2018 | Retrospective study evaluating augmented fixation procedures: locking plate fixation and fibular allograft augmentation versus locking plate fixation and additional inferomedial screws. Ineligible study design. |
| Kulkarni 2000 | This is listed in the National Research Register as a randomised comparison of operative and non‐operative management of proximal humeral fractures. This trial was abandoned due to poor recruitment, mainly due to lack of participant consent. |
| Li 2015 | For a trial comparing two surgical procedures (minimally invasive versus open plate fixation), the 8‐week follow‐up is very short. The measurement of individual outcomes is either not or poorly defined. The only outcomes that we would present in the review ‐ complications ‐ are misleadingly reported and the short follow‐up means that these can be considered interim outcomes only. We have excluded this trial because we consider the flawed study design and reporting of this trial mean that it cannot and does not contribute meaningful data for the comparison |
| Liao 2009 | While the English abstract claims that "70 senile patients" were "randomly divided into three groups to receive different surgical methods", the distribution and characteristics (age and fracture type) of the participants in the three groups indicated serious selection bias and implied this was not a randomised trial. For example: "21 patients in the group A receiving Kirschner tension band or screw internal fixation, 37 patients in group B receiving internal fixation of locking proximal humeral plate, and 12 patients in group C receiving humeral head replacement." There was no reply to request for clarification from the lead author. |
| Maniscalco 2014a | This was stated in the title and text of the conference abstract to be a "randomized controlled clinical trial" that compared early rehabilitation with standard rehabilitation after intramedullary nailing with a Diphos nail. However, the two groups were not concurrent and it seems that this was retrospective comparison with an historic control group. A subsequent publication of a cohort study of the nail used in this study makes no mention of this trial and adds support to our interpretation (Maniscalco 2014b). Ineligible study design. |
| Martetschlager 2012 | The choice of intervention (deltopectoral versus anterolateral‐splitting approach for locking plate fixation) was according to surgeon's preference and not "random" as suggested by the study authors. The Discussion referred to "several limitations, including its retrospective study design" of this "current study". Hence this was not a randomised or quasi‐randomised controlled trial. |
| Martin 2000 | Contact with a trialist revealed that, due to the discovery of problems with randomisation, it was decided not to proceed with publication as the trial results could be compromised. |
| Mechlenburg 2009 | This was originally registered as a randomised controlled trial comparing a plate with a hemiarthroplasty. However, it is now registered as a prospective study of fixation with a PHILOS plate. Inger Mechlenburg confirmed that no participants had been included in the trial ‐ the trial was abandoned because no funding was obtained. |
| NCT00205959 | Non‐randomised study comparing three surgical implants for proximal humeral fracture. |
| NCT00384852 | The primary aim of this multicentre randomised trial was to "assess whether fracture union is accelerated in subjects with humeral fractures (proximal, diaphyseal) treated non‐surgically (standard of care) and a single dose of rhBMP‐2/CPM [recombinant human bone morphogenetic protein‐2 (rhBMP‐2)/Calcium Phosphate Matrix] compared to subjects who receive standard of care alone". Its results were reported in a systematic review (ref 69* in Lo 2012). This reported that "[w]hile promising, the published results from the Phase II studies for humeral and femoral fractures showed little enhancement over traditional treatments [69,70]. A positive risk/benefit ratio for these treatments was not demonstrated leading to Pfizer no longer pursuing the clinical development of rhBMP‐2/CPM for these applications." Attempts over several months to obtain the report using the citation provided always met with the claim of 'server maintenance'. Unfortunately, Kevin Lo also could not supply a copy of this article (12 February 2015) and hence the reason for exclusion. *A Phase 2, Multicenter, Double‐blind, Randomized, Stratified, Controlled, Efficacy, Safety and Feasibility Study of Recombinant Human Bone Morphogenetic Protein‐2 (rhBMP‐2)/Calcium Phosphate Matrix (CPM) as an Adjuvant Therapy in Closed Fractures of the Humerus, Pfizer, Inc. Sep 15. 2011 ClinicalStudyResults.org,. http://www.clinicalstudyresults.org/documents/company‐study_11378_0.pdf |
| NCT00818987 | This randomised controlled trial, registered in 2009, intended to compare surgery (internal fixation) versus non‐surgical treatment. Through his inspection in 2020 of the centre research records, Darren Roffey found that the study was terminated in 2013, with no record of participants being recruited. |
| NCT01086202 | The study design for this completed study is unclear. It is labelled as an observational case‐control study but describes in the trial registration that "20 patients will be randomized to the Tornier Reversed shoulder Arthroplasty Medial offset, and 20 will receive the Lateral offset design". It has a mixed population (rotator cuff tear arthroplasty, irreparable rotator cuff tear, significant proximal humeral fracture and malunions, or chronic proximal humerus dislocation) that, in view of the small sample size, means it is unlikely to provide useful data for this review. |
| NCT01532076 | This randomised trial, which compared adipose tissue‐derived mesenchymal stem cells composite graft augmentation versus acellular composite graft augmentation, was terminated early after recruiting only 8 of the planned 290 participants with an isolated proximal humeral fracture. |
| NCT01817933 | This study investigated an integrated rehabilitation program for humeral neck fracture, vertebral fracture, distal radial fracture and hip fractures after surgical fixation. Not an RCT. |
| NCT02052206 | This was originally registered as a randomised trial aiming to compare computer‐assisted 3D (3 dimensional) planning versus conventional 2D (2 dimensional) planning for shoulder replacement surgery in 80 participants with either osteoarthritis of the shoulder or complex proximal humeral fracture. However, the updated entry November 2017 indicated that it was a single intervention cohort in 20 participants. Hence it is excluded because it is not a randomised comparison. |
| NCT02597972 | RCT investigating reverse total shoulder arthroplasty versus open reduction internal fixation. Trial was terminated after enrolling 3 participants. |
| NCT03017105 | Trial examining cross‐education was terminated because of "very low recruitment" (Jenna Bardsley). No publication intended. |
| NCT03804853 | RCT evaluating rehabilitation following reverse total shoulder arthroplasty in people with osteoarthritis: ineligible participant population. |
| NCT04285606 | RCT testing short immobilisation with patient‐directed therapy versus long immobilisation with supervised therapy after reverse shoulder arthroplasty. Ineligible participant population: rotator cuff tear and osteoarthritis. |
| NTR2186 | The registration document of this study, which compared the DePuy Delta Xtend reversed shoulder prosthesis with non‐surgical treatment in the management of 4‐part fractures of the proximal humerus, indicates this is not a randomised trial. The non‐surgical treatment arm was a historical control group. |
| Parnes 2005 | There has been no response from the lead author of this ‘trial’ (last contact attempted 8 June 2012), which appears to have been reported in a conference abstract only. In 2003, 50 participants with 3‐ and 4‐part fractures and fracture dislocations of the proximal humerus were "random selected" for surgery (closed or open reduction and external fixation or hemiarthroplasty) or non‐surgical treatment. The very limited results are split descriptively according to three groups (2 reflecting the 2 different surgical methods). There is currently insufficient evidence to support this being a randomised trial. |
| Pullen 2007 | This is listed in the National Research Register Archive as a randomised trial comparing the T2 proximal humeral nail with the PHILOS plate system in participants with 2‐ or 3‐part proximal humeral fractures. The recruitment target was 100 participants (between 1 September 2005 to 1 September 2007), and follow‐up was 16 weeks. We have not located any report of this study other than the details provided in the National Research Register (UK) by, at that time, a Trauma and Orthopaedic Registrar who has now moved to another hospital. There was no response to a request for further information sent 8 June 2012. There is no indication that this study, which may not have started, will ever be reported. |
| Rasool 2015 | This very inadequately reported and methodologically flawed trial reported range of movement results only. These results included implausible data that were inconsistently reported in the abstract. Our search for the lead author was hampered by variations in their name; the status of the institute where the research was conducted was also uncertain. We received no response to our enquiries. This trial is excluded because the very limited evidence available is invalid. |
| Rodriguez‐Merchan 1995 | No proximal humeral fractures in a quasi‐randomised trial of humeral shaft fracture treatment. |
| Rollo 2019 | Discussion confirmed this was a retrospective study that compared a new design of proximal humeral plate with the most‐used plates in the treatment of proximal humeral fractures. Ineligible study design. |
| ROTATE 2019 | This trial, listed as ongoing in the 2015 version of the review, aimed to compare external rotation of the shoulder in a neutral rotation brace versus polysling holding the proximal humerus in internal rotation after extramedullary plate fixation. It was terminated after recruiting just 2 participants out of the intended 100. |
| Schoch 2019 | Cohort study comparing uncemented versus cemented reverse shoulder arthroplasty for proximal humeral fractures. Not an RCT. |
| Shah 2003 | This is listed in the National Research Register Archive as a multicentre randomised trial of the management of four‐part fractures of proximal humerus that compared hemiarthroplasty versus non‐surgical treatment. The recruitment target was 200 participants, with a one‐year follow‐up using the Constant‐Murley Shoulder Score and Oxford Shoulder Score. The listed start and end dates were 1 January 2003 and 1 February 2005. No details were received of the other centres in the very limited further information received from Mr Shah in April 2003. There was no response to a request for further information sent 13 November 2006. There is no indication that this study, which may not have started, will ever be reported. |
| Shah 2018 | This study investigated the effect of desensitisation methods during the early mobilisation phase in post‐fracture conditions of the upper extremity. Ineligible participant population despite some PHFs in this mixed upper‐extremity fracture population. |
| Torrens 2015 | Clarification was received from Carlos Torrens on 8 July 2020 that this trial, with the same comparison as Torrens 2012, reported as ongoing in the 2015 version of the review, did not in fact take place. |
| Wallace 2000 | This is listed in the National Research Register as a multicentre randomised trial of the management of displaced surgical neck and displaced shaft fractures of the humerus with the Halder humeral nail. Contact with Prof Wallace's secretary revealed that the study had not gone ahead. The secretary mentioned three other sites (Halifax; Liverpool; and one in Scotland). No reason given. See Brownson 2001. |
| Wan 2005 | This is a mixed population trial evaluating additional mobilisation therapy that included other fractures (e.g. clavicular and scapular fractures) as well as proximal humeral fractures. This trial was excluded because separate proximal humeral fracture data were not reported and the contact author is unavailable. |
| Wang 2014 | The keywords describe this as a ‘case control’ study that compared swing shoulder and internal fixation in treating proximal humeral fractures. Not an RCT. |
| Warnecke 1999 | A multicentre prospective study but not a randomised trial. |
| Yang 2006 | Correspondence with the author revealed that this was not a randomised trial. The choice of surgery was dependent on the success of closed reduction. |
| You 2016 | Application of 3D (3 dimensional) printing technology on the treatment of complex proximal humeral fractures. Diagnostic tool tested in a clinical setting, not really an intervention. |
| Zhang 2010 | While the English abstract claims that "58 patients with 3 parts and 4 parts fractures of proximal humerus were randomly treated with AO locked compressive plates (LCP) or humeral head replacement", the characteristics (fracture type) of the participants in the two groups indicated serious selection bias and implied this was not a randomised trial. Thus, 25 of 28 participants in the plate group had 3‐part fractures (1 with a dislocation) and 3 had 4‐part fractures (1 with dislocation) whereas 11 of 30 in the replacement group had 3‐part fractures (2 with dislocation), 16 with 4‐part fractures (4 with dislocation) and the other three had humeral head split fractures. There was no reply to request for clarification from the lead author. |
| Zhao 2017 | Although this study (comparing minimally invasive versus open reduction plate fixation) met the inclusion criteria for this review, it became clear after data extraction and risk of bias assessment that this poorly conducted and reported small trial had several major flaws and data issues that made the findings unreliable. The flaws included: a) an ill‐defined method of randomisation ("randomized block"); b) no account of participant flow including post‐randomisation exclusion of an unknown number of older participants because of "severe systemic diseases"; c) the ill‐defined fracture population; d) the variable and wide length of follow‐up (4 to 24 months); d) the usually high Constant‐Murley and Neer scores with usually narrow standard deviations (e.g. 1.0), especially for data collected at different follow‐up times; and the scores of over 100 (e.g. 129.4) for 36‐item Short‐Form Health Survey (SF‐36) scores (the maximum should be 100). After checking the peer‐reviewer comments in the journal, we considered that it would serve no purpose going back to get clarification. |
| Zhao 2019 | The abstract of this study, which tested the addition of fibular allograft to locking plate fixation, reported that the participants "were randomly divided" but the Methods indicated that it was a retrospective study and the Discussion clarified that "it is not a randomized trial because the two techniques were performed at different times". |
PHILOS: Proximal Humerus Internal Locking System
Characteristics of studies awaiting classification [ordered by study ID]
Baring 2017.
| Methods | Randomised trial ("feasibility study") |
| Participants | 15 (at time of abstract report) participants with 2‐, 3‐ and 4‐part displaced proximal humeral fractures |
| Interventions | With or without surgery, four weeks of immobilisation in one of the following: 1. a neutral rotation brace 2. a simple sling The authors state that "was independent to operative vs non‐operative treatment". |
| Outcomes | DASH score, Oxford Shoulder Score (OSS), Constant score (CS) and range of movement were assessed at 6 weeks, 3 months and 1 year. |
| Notes | Reported in a conference abstract only. Interim findings at 3 months were reported for 15 participants (7 versus 8), five of whom (1 versus 4) had had surgical fixation of their fracture. We are unsure of the status of this small pilot trial and are unable to find email contact details of either author but have not pursued further details on this given the Covid‐19 situation. Specific searches for the authors of this trial have not identified a relevant publication. |
Battistella 2011.
| Methods | "Randomized clinical study" |
| Participants | 54 participants (38 female, 26 male; mean age 61 years) with 2‐part surgical neck fractures or 3‐part valgus impacted fractures |
| Interventions | Surgery involving a titanium plate: 1. Minimally invasive fixation based on anterolateral deltoid split approach and percutaneous reduction 2. Open reduction and internal fixation by standard deltopectoral approach |
| Outcomes | Constant score, instrumental activities of daily living, pain (VAS), range of motion, union, complications |
| Notes | Reported in a conference abstract only. Requests for further information sent to Dr Battistella (8 and 14 May 2012) were unsuccessful. Specific searches for the authors of this trial have not identified a relevant publication. |
Brorson 2009.
| Methods | Multicentre, randomised clinical trial (central randomisation unit) |
| Participants | 25 recruited out of a planned 162 participants with displaced 4‐part fractures of the proximal humerus |
| Interventions | 1. Hemiarthroplasty 2. Fixed‐angle plate osteosynthesis 3. Non‐surgical treatment |
| Outcomes | Follow‐up: 3 years (primary outcome: 1 year) Primary outcome: Constant scale Secondary outcomes: Oxford Shoulder Score, Short Form‐36 |
| Notes |
Request sent by HH to Stig Brorson on status of the data set. Response received 24 January 2020: "The data set has been retained as per the Ethics application but has not been made available at this time." |
Chen 2016.
| Methods | Randomised using a computer‐generated randomisation sequence |
| Participants | 60 participants (32 female, 28 male; mean age 66 years) with acute osteoporotic (bone mineral density (BMD) < ‐3.0) 4‐part proximal humeral fractures with or without fracture dislocation |
| Interventions | 1. Intramedullary fibular allograft (IFA) with locking compression plate (LCP). Rehabilitation not described. 2. Hemiarthroplasty. Prosthesis (LINK, Germany). All had general anaesthesia and a deltopectoral approach to surgery. |
| Outcomes | Follow‐up: mean 36 months (range 24 to 48 months) DASH score, Constant score, subjective rating (excellent, good, fair, poor); pain (VAS) (not reported), complications, secondary surgery, time to union |
| Notes | BMD < ‐3.0 was a criterion for inclusion but mean BMD in both groups was ‐2.5. Thus criterion not followed. A request for further information and clarification sent to P Tang (by email 28 February 2020) was unsuccessful. The request sought details of the rehabilitation for the graft and plate group, checks on Table 4 in the trial report, especially the DASH scores which seem very low % of affected side, and what arthrolysis involved for the 4 participants treated for shoulder stiffness. Concerns over the analyses and data meant that we decided it was best to wait on clarification from the authors before inclusion. |
Chengjin 2017.
| Methods | "randomly divided" |
| Participants | 108 participants (39 female, 69 male, mean age 52 years) with isolated displaced greater tuberosity fracture ("large fissure") of the humerus |
| Interventions | All joint dislocation patients in clinic were reset and those with the greater tuberosity fracture being treated with one of the following. 1. Plate fixation 2. Hollow screw fixation 3. Band anchorage fixation |
| Outcomes | Shoulder function score (probably Constant), intraoperative blood loss, operation time, length of stay, fracture healing time and complications (follow‐up for 2 years) |
| Notes | Queries sent by email (obtained via another publication) to last author on 22 April 2020 on author names, method of allocation, any loss to follow‐up and name of shoulder function score. The email was returned as "undeliverable" on 24 April 2020. Hence, trial remains 'awaiting classification' at this time. The authors are listed as reported in Embase: Chengjin Z, Haibin H, Shengmei S, Kai L. It is possible, however, that they should be listed as Zhao C, Hou H, Shi S, Lv K. |
Liu 2011.
| Methods | "Randomly divided" |
| Participants | 50 participants (36 females, 14 males; mean age 70 years (range 60 to 83)) with 2‐, 3‐ or 4 part proximal humeral fractures |
| Interventions | 1. PHILOS plate augmentation with minimally invasive injectable graft (MIIG) X3 Hivisc
2. PHILOS plate alone Minimally invasive percutaneous plate osteosynthesis used in both groups |
| Outcomes | Follow‐up: mean 18 months (range 12 to 25 months) Neer scoring system, complications, healing time, operative time, blood loss |
| Notes | No response to inquiry on method of allocation sent 7 December 2014; baseline imbalance in type of fracture with more 3‐part and 4‐part fractures in the first group. |
Liu 2014.
| Methods | "Patients were ... divided into 2 groups according to the random number method" |
| Participants | 284 participants with proximal humeral fractures > 60 years of age |
| Interventions | 1. Proximal humerus locking plate (LPHP) 2. PHILOS plate |
| Outcomes | Fracture healing time, pain at 3 months, Neer shoulder function score at 2 years |
| Notes | As of April 2020, we had not been able to obtain a copy of the article: http://rs.yiigle.com/CN112137201447/562382.htm |
Luo 2008.
| Methods | Participants were randomly allocated via a random numbers table. |
| Participants | 60 participants (32 females, 28 males; age range: 39 to 62 years) treated operatively for fracture of the surgical neck of the humerus. |
| Interventions | 1. Acupuncture (electroacupuncture and infrared radiation) plus passive exercise of the shoulder joint
2. Exercises only: passive exercise of the shoulder joint followed by active exercises Treatment lasted 1 month. |
| Outcomes | Follow‐up: 1 month Shoulder pain score (VAS) Shoulder joint activity |
| Notes | Trial in Chinese with English abstract. Translation of methods section (1.1) confirmed that this was a randomised trial. The review authors agreed to defer trials testing acupuncture and variants thereof as well as Chinese traditional medicine. This also reflects our limited experience of these interventions. |
NCT01113411.
| Methods | Randomised clinical trial |
| Participants | 80 participants aged over 18 years treated by PHILOS locked plate system for unstable closed fracture of the proximal humerus (2‐part and 3‐part fractures according to the Neer classification) within 7 days on injury. |
| Interventions | 1. Early and intensive exercise programme
"A thoraco brachial brace will be worn for 48 hours following the surgery and then removed for the remainder of treatment. Patients will then start the intensive rehabilitation programme without physical therapy. The exercise programme will be provided to the patient. The exercises consist of active and active‐assisted movements of the shoulder for a period of six weeks, limiting external rotation to 0°. Patients are encouraged to use their affected limb for daily activities. Strengthening exercises are started the 6th week following surgery and the full programme will be completed three months after surgery. Patients who wish can then continue their rehabilitation with a physiotherapist. The patient will complete a daily diary to validate the frequency and intensity of the exercises." 2. Standard rehabilitation programme "The patient will wear the thoraco brachial brace for a period of four weeks following the surgery. It may be taken off for hygiene purposes and dressing. After the four weeks, the patient will take the brace off permanently and begin an exercise programme, writing down the frequency and intensity of the exercises. Physiotherapy is allowed for the remaining part of the three months rehabilitation programme." |
| Outcomes | Length of follow‐up: 12 months Primary outcome: Constant score (adjusted for age) at 6 months. A difference of 10 points is considered significant (standard deviation of 15 points). Secondary outcomes: reoperation, redisplacement, Constant score at 12 months, DASH, return to professional activities, pain, range of motion |
| Notes | Entry for trial (clinicaltrials.gov) on 19 January 2015, indicated that the "recruitment status of this study is unknown because the information has not been verified recently". Status as "recruiting" had been last verified on December 2012. The study completion date had been changed from December 2012 to December 2013. The start date was December 2009. Email enquiring status sent to PI, Dr Stéphane Pelet, Hopital de l'Enfant‐Jésus, Canada (stephane.pelet.ortho@gmail.com) on 16 July 2020. |
NTR3208.
| Methods | Multicentre, randomised controlled trial. Unblinded |
| Participants | 52 participants aged over 65 years with displaced 3‐ or 4‐part proximal humeral fractures who are candidates for primary shoulder arthroplasty |
| Interventions | 1. Aequalis reverse fracture prosthesis 2. Aequalis fracture prosthesis |
| Outcomes | Follow‐up: 1 year Primary outcome: Constant score Secondary outcomes: DASH (Disabilities of the Arm, Shoulder and Hand) Score, SF‐12 questionnaire, VAS pain |
| Notes | Start date: July 2010 Estimated completion date: December 2014 Trial registration renumbered from NTR3208 to NTR3060 Yde Engelsma was based at Medisch Centrum Alkmaar, the Netherlands at time of registration. Since 2016 is now working in Movement Care Clinic in Naarden. Email querying status sent on 17 July 2020 to gmail address, valid in 2012 in publication, ydedepunt@gmail.com. Top‐up search conducted in November 2021 included the full trial report that reported results for 33 participants (Z‐TUp Laas 2021) |
Paladini 2019.
| Methods | Method of allocation not stated. May not be randomised |
| Participants | 42 participants with proximal humeral fractures |
| Interventions | Open reduction and internal fixation with 1. CRF‐PEEK (carbon fibre reinforced polyetheretherketone) plate 2. Metal plate |
| Outcomes | Follow‐up: minimum 2 years Constant score, Simple Shoulder Test (SST), ROM, VAS and radiographic evaluation |
| Notes | Study reported in a conference abstract only. While unlikely to be randomised, details on study methods required before exclusion |
Peng 2017.
| Methods | Randomised using a "random sampling number table" |
| Participants | 80 participants (48 females, 32 males, mean age 74.3 years) with displaced proximal humeral fractures (Neer 2‐, 3‐ and 4‐part) |
| Interventions | Anatomic locking plates 1. Biomimetic mineralised collagen putty 2. Control (no putty inserted) |
| Outcomes | American Shoulder and Elbow Surgeons (ASES) score at mean between 14 to 24 months, fracture healing time, complication rate. Follow‐up at mean 15.6 months, range 14 to 24 months |
| Notes | Non‐biological bone regeneration product adjuvant to locking plate. This product was judged outside of the interventions considered for the current version of this review. |
ProCon 2010.
| Methods | Randomised trial: "variable block randomisation will be accomplished via a trial website" |
| Participants | Participants (65 years or older) with a comminuted proximal humeral fracture. 80 participants (65 years or older) with a comminuted proximal humeral fracture: 3‐part (Hertel classification type 9, 10, 11), four‐part (Hertel type 12), anatomical neck (Hertel type 2), or split‐head fractures of the humeral head |
| Interventions | 1. Hemiarthroplasty (Affinis Fracture shoulder endoprosthesis) 2. Non‐surgical treatment (collar and cuff for three weeks) |
| Outcomes | Follow‐up: 1, 3 and 6 weeks, and 3, 6, 12 and 24 months Primary outcome (Constant score) and secondary outcomes (DASH, pain, radiographic healing, secondary intervention rates, complication rates, mortality rates, SF‐36, and European Quality of Life‐5 Dimensions (EQ‐5D) Costs for (in)formal healthcare consumption |
| Notes | Start date: 15 June 2009
Planned end date: 31 December 2013 Dennis Den Hartog Department of Surgery‐Traumatology Erasmus MC University Medical Center Rotterdam P.O. Box 2040 3000 CA Rotterdam The Netherlands d.denhartog@erasmusmc.nl Published protocol. Email enquiring status sent to Dennis Den Hartog on 17 July 2020. (Seems to still be there) |
Wang 2013.
| Methods | "Randomly divided" |
| Participants | 80 "elderly" participants (42 females, 38 males; mean age 67 years (range 60 to 83)) with proximal humeral fractures (AO classification: 12 A, 45 B and 23 C fractures) |
| Interventions | 1. Minimally invasive percutaneous insertion of PHILOS plate with injectable bone 2. Minimally invasive percutaneous insertion of PHILOS plate alone |
| Outcomes | Length of follow‐up: unknown Constant score, complications, healing time, bone mineral density, patient satisfaction with results of treatment |
| Notes | No response to inquiry on method of allocation sent 15 January 2014; translated title from Chinese states this is a "case‐control" study. Data extracted from abstract. (Unusually high Constant score in the bone substitute group: 97.2 (SD 4.6)). Translation required if it is considered that the study should be included in future. |
Zhang 2016.
| Methods | Participants were "randomly divided" |
| Participants | 100 participants with proximal humeral fracture |
| Interventions | 1. "TCM [traditional Chinese medicine] fracture three stages therapy" (not described in the English abstract) 2. Conventional therapy (not described in the English abstract) |
| Outcomes | "shoulder function", "total effective rate", complications (hematoma, wound infection, traumatic arthritis) |
| Notes | Article in Chinese with English abstract. Copy of article not obtained. The review authors agreed to defer trials testing Chinese traditional medicine. This also reflects our limited experience of these interventions. |
Zhu 2014.
| Methods | Patients "randomly either underwent ORIF (open reduction and internal fixation) using a fixed‐angle locked plate and iliac crest bone graft ... or only had open reduction and fixed‐angle locked plate fixation ... ." |
| Participants | 40 people with comminuted proximal humeral fractures (however, "patients with evidence of arthrosis or a clinical follow‐up shorter than 12 months were excluded") |
| Interventions | 1. Locking plate and an autologous crest bone graft 2. Locking plate only |
| Outcomes | Length of follow‐up: mean 25 months, range 13 to 48 months Range of motion, pain, SF‐36, return to previous activities or occupation, complications, time to union was also recorded. |
| Notes | Queries on allocation method and donor site complications and morbidity sent 22 May 2015 |
Z‐TUp Alvarez 2020.
| Methods | Randomised controlled trial |
| Participants | 40 participants, aged 70 years or over, with 3‐ or 4‐part proximal humeral fractures Of 39: 27 females, 12 males; mean age 76 years (range 71 to 85 years) |
| Interventions | 1. Reverse shoulder arthroplasty (Reverse Arthroplsty Delta Xtent (DePuy)) 2. Hemiarthroplasty (Hemiarthroplasty Global Fx (DePuy)) |
| Outcomes | Follow‐up: 36 months ASES (American Shoulder and Elbow) score, DASH score, Constant score, complications |
| Notes | Found in November 2021 top‐up search. Also listed as an ongoing study: NCT02075476 Study results were added to the trial registration document in April 2020. Small discrepancy in denominators between baseline and analyses data. |
Z‐TUp Batar 2020.
| Methods | Quasi‐randomised based on last digit (odd or even) of patient's ID |
| Participants | 80 participants with 3‐ or 4‐part proximal humeral fractures aged over 60 years Of 58 participants followed up: 39 females, 19 males, mean age 71 years |
| Interventions | 1. Reverse shoulder arthroplasty (RSA) 2. Hemiarthroplasty |
| Outcomes | Follow‐up: mean 27 months in RSA group and 52 months in hemiarthroplasty group American Shoulder and Elbow Surgeons score (ASES), Constant score, active and passive shoulder ROM, muscle strength, complications and radiographic evaluation of greater tuberosity healing |
| Notes | Found in November 2021 top‐up search. New to review Some issues in the reporting give rise to concern. These include high, incompletely accounted for and imbalanced losses to follow‐up in the two groups (7/40 (17.5%) in Group 1 and 15/40 (37.5%) in Group 2). Major imbalances, age and follow‐up, are also evident in the participant characteristics of the 58 followed up. Return to authors for clarification before a decision can be taken. |
Z‐TUp Bhayana 2021.
| Methods | Quasi‐randomised: "alternately allocated" (odd and even "sequential enrollment" numbers) |
| Participants | 84 participants (39 females, 45 males; mean age 44.6 years) with 3‐part (with greater tuberosity involvement) or 4‐part proximal humeral fractures undergoing locking plate fixation (either PHILOS or "indigenous implant of same design") |
| Interventions | 1. Deltoid‐split approach for plate fixation 2. Deltopectoral approach for plate fixation |
| Outcomes | Follow‐up: mean 23 months (range 19 to 48 months) Relative Constant score, ease of surgical reduction of the greater tuberosity, shoulder ROM, radiological malunion, complications and duration of surgery |
| Notes | Found in November 2021 top‐up search. New to review Unclear whether there was loss to follow‐up ‐ the 84 participants were those available at final follow‐up. |
Z‐TUp Borda 2019.
| Methods | "Randomized controlled study"; "randomly assigned" |
| Participants | 62 women (mean age 69 years) with osteoporotic proximal humeral fractures treated by reduction and immobilisation |
| Interventions | 1. Pulsed electromagnetic field therapy post immobilisation (10 minutes/day for 10 days) 2. Sham therapy |
| Outcomes | Follow‐up: not stated Pain, ROM, functional performance using DASH |
| Notes | Found in November 2021 top‐up search. New to review Conference abstract only. Imbalance in group numbers: 36 versus 26 participants. Insufficient information available for inclusion. Attempt to find other report and contact authors required. |
Z‐TUp Boyer 2021.
| Methods | Single‐centre, randomised controlled trial. Computer randomisation with various block sizes |
| Participants | 99 participants with 3‐ and 4‐part "displaced" acute proximal humeral fractures, aged between 40 and 80 years. Of 85: 61 females, 24 males; mean age 73.7 years |
| Interventions | 1. Intramedullary nail (Multilock, DePuy Synthes) 2. Locking plate (SURFIX, Integra) |
| Outcomes | Follow‐up: mean 66 months (minimum 4 years) American Shoulder and Elbow Surgeons score (ASES), Constant score, Simple Shoulder Test score, complications, revision surgery, shoulder pain |
| Notes | Found in November 2021 top‐up search. Also listed as an ongoing study: NCT01557413. There are differences between the protocol at trial registration ‐ including follow‐up (the primary follow‐up was 1 year) and different outcome measures. There are also abstracts reporting interim results. Although suitable for inclusion, we would defer inclusion to approach the authors to obtain follow‐up data at earlier times and clarification on the follow‐up procedures. |
Z‐TUp Hachem 2021.
| Methods | "[D]ouble‐blinded randomized controlled trial" |
| Participants | 78 participants with displaced and complex proximal humeral fractures in the "elderly" having a reverse shoulder arthroplasty (RSA) Of 40: 32 females, 8 males; mean age 74.4 years |
| Interventions | 1. RSA with anatomic stem angle of 135 degrees 2. RSA with anatomic stem angle of 155 degrees |
| Outcomes | Follow‐up: mean 16.3 months (range 13 to 36 months) QuickDASH, Constant score, ROM, consolidation of the tuberosities |
| Notes | Found in November 2021 top‐up search. New to review Conference abstract only. Reporting results of "a pilot study" of the first 40 cases. Full report awaited |
Z‐TUp Izquierdo‐Fernández 2021.
| Methods | Quasi‐randomised based "on history number of patient" (odd or even) |
| Participants | 40 participants, aged over 65 years, undergoing reverse shoulder arthroplasty (RSA) for complex proximal humeral fractures, including 3‐ and 4‐part fractures Of 32: 29 females, 3 males; mean age 74.1 years (range 65 to 87 years) |
| Interventions | 1. Anterosuperior approach for RSA surgery 2. Deltopectoral approach for RSA surgery |
| Outcomes | Follow‐up: 7 years (also 1 year) Constant score, Hospital for Special Surgery score, ROM, radiological assessment (component loosening, scapular notching, condition of the tuberosities) |
| Notes | Found in November 2021 top‐up search. New to review No baseline characteristics data for the two groups |
Z‐TUp Kröger 2021.
| Methods | Multicentre randomised clinical trial. Randomised using internet‐based sealed envelope software, stratified by centre, fracture location and arm dominance |
| Participants | 48 participants (31 females, 17 males; mean age 55 years) with surgically‐treated proximal humeral fracture. Participants were included between the fourth and seventh weeks after surgery. |
| Interventions | 1. Robot‐assisted training using the ArmeoSpring device added to conventional occupational and physical therapy 2. Conventional occupational and physical therapy Both the intervention group and the control group received physical and occupational therapy for a period of 3 weeks. |
| Outcomes | Follow‐up: 13 months postoperatively DASH, Wolf Motor Function Test–Orthopaedic version, active ROM, grip strength, adverse events, adherence |
| Notes | Found in November 2021 top‐up search. Also listed as an ongoing study: Nerz 2017 |
Z‐TUp Laas 2021.
| Methods | Multicentre, randomised controlled trial: use of a computerised "sequential minimized randomization procedure" |
| Participants | 33 participants aged over 65 years with displaced 3‐ or 4‐part proximal humeral fractures who are candidates for primary shoulder arthroplasty Of 31: 18 females, 13 males; mean age 75 years (range 65 to 92) |
| Interventions | 1. Reverse shoulder arthroplasty (Aequalis reverse fracture prosthesis) 2. Hemiarthroplasty (Aequalis fracture prosthesis) Both had cemented stems. All participants received a shoulder immobiliser for 6 weeks. |
| Outcomes | Follow‐up: 1 year Constant score, DASH score, SF‐12, VAS pain, ROM, radiographic outcomes (healing of tuberosities, heterotrophic ossification, scapular notching), complications |
| Notes | Found in November 2021 top‐up search. Also listed in this section under NTR3208.
Before the publication in May 2021 of the trial report, we anticipated that this trial was abandoned. However, the authors persevered and recruited 33 participants "before termination of inclusion", recruitment being "more difficult than expected". The trialists found "limited number of available patients for inclusion", and "a growing belief among the participating surgeons that RSA was superior to HA". Data for continuous outcomes, such as DASH, SF‐12, pain) are presented as medians and interquartile ranges. Missing data were adjusted for by the use of "linear mixed model analysis" but denominators were not provided, which would prompt a data request being sent to the authors. A commentary by Li was also identified. |
Z‐TUp Li 2020.
| Methods | Use of a "random number table" |
| Participants | 37 older adults (24 females, 13 males; mean age 66 years) with proximal humeral fractures |
| Interventions | 1. Multiloc intramedullary nail 2. Locking plate |
| Outcomes | Follow‐up: 12 months Neer shoulder function score, operation time, blood loss, healing time of fractures, VAS pain, complications |
| Notes | Found in November 2021 top‐up search. New trial Trial reported in Chinese; English abstract. Use of Google translate was practical, although incomplete. A small concern over claim: "A retrospective analysis of the clinical data of" but otherwise appears to have involved random selection. |
Z‐TUp Martinez 2021.
| Methods | Randomised controlled trial: computer‐generated randomised list and sealed envelopes |
| Participants | 143 participants with non‐surgically‐treated acute proximal humeral fractures (> 50% contact between humeral head and shaft): 1‐, 2‐, 3‐ and (only one) 4‐part fractures Of 111 in analysis: 88 females, 23 males; mean age 70.4 years (range 42 to 94 years) |
| Interventions | 1. One week of sling immobilisation
2. Three weeks of sling immobilisation All were instructed to do passive elbow movements from first day after fracture. After immobilisation, all participants followed the same progressive rehabilitation programme. |
| Outcomes | Follow‐up: 24 months Pain VAS, Constant score, Simple Shoulder Test, complications, secondary treatment/surgery, radiographic assessment |
| Notes | Found in November 2021 top‐up search. Also listed as an ongoing study: NCT03217344 Also identified was a published conference abstract reporting 12‐month data. This reported that 146 people had been randomised and different numbers in the two groups of the 111 participants in the analysis. These discrepancies would need clarification. |
Z‐TUp Monticone 2021.
| Methods | "Randomised parallel‐group superiority‐controlled trial". Permuted‐block randomisation procedure |
| Participants | 70 working participants (41 females, 29 males; mean age 49 years) who had surgical treatment (open reduction and plate fixation) for proximal humeral fractures (Arbeitsgemeinschaft fur Osteosynthesefragen (AO) classification) |
| Interventions | 1. Supervised rehabilitation programme of task‐oriented exercises based on participant's specific job activities, and occupational therapy (12 weeks) 2. General physiotherapy, including supervised mobility, strengthening and stretching exercises (12 weeks) Rehabilitation sessions performed three times a week for one hour in the outpatient setting |
| Outcomes | Follow‐up: 1 year DASH, pain intensity (numerical rating scale), SF‐36, patient‐rated efficacy of treatment assessed using the Global Perceived Effect scale, adverse effects, adherence |
| Notes | Found in November 2021 top‐up search. Also listed as an ongoing study: ISRCTN17996552 |
Z‐TUp Vijan 2020.
| Methods | Quasi‐randomised based on alternation |
| Participants | 30 participants (11 females, 19 males; mean age 52.3 years) with 2‐, 3‐ or 4‐part proximal humeral fractures aged over 18 years |
| Interventions | 1. Open reduction and plate (PHILOS) fixation
2. Closed reduction and wire fixation All were given a shoulder immobiliser for 1 month |
| Outcomes | Follow‐up: 12 months Constant score (categories), complications and radiographic evaluation of greater tuberosity healing |
| Notes | Found in November 2021 top‐up search. New to review |
Z‐TUpx ISRCTN85422168.
| Methods | Randomised controlled trial |
| Participants | 90 (target) participants aged above 60 years with complex (3‐ or 4‐part) proximal humeral fractures |
| Interventions | 1. Reverse shoulder arthroplasty with a 155‐degree inclination angle 2. Reverse shoulder arthroplasty with a 135‐degree inclination angle 3. Non‐surgical treatment (rehabilitation only) |
| Outcomes | Follow‐up: 24 months Quality of life measured using the Western Ontario Osteoarthritis score (WOOS); pain, activities of daily living, Constant score (ROM and strength); quality of life measured using the Subjective Shoulder Value (SSV) questionnaire; bone healing response evaluated using x‐ray, complications and revisions reported in medical records |
| Notes | Found in November 2021 top‐up search Ongoing trial. Trial dates: April 2021 to September 2027 Protocol and Patient Information Sheet included in trial registration |
Z‐TUPx NCT04405947.
| Methods | Randomised controlled trial |
| Participants | 100 participants, aged between 18 and 85 years, who are having a reverse shoulder arthroplasty for acute proximal humeral fracture, secondary osteoarthritis of the shoulder (rotator cuff arthropathy) |
| Interventions | 1. Deltopectoral surgical approach for reverse shoulder arthroplasty 2. Antero‐superior approach for reverse shoulder arthroplasty |
| Outcomes | Follow‐up: 2 years Constant score, scapular notching, positioning of the implant |
| Notes | Found in November 2021 top‐up search. Ongoing trial. Recruitment completed. Trial dates: January 2016 to October 2020 Mixed population |
Z‐TUPx NCT04572022.
| Methods | Randomised controlled trial (pilot) |
| Participants | 60 (target) participants between 18 to 58 years of age with trauma‐associated proximal humeral fractures, treated either surgically or non‐surgically |
| Interventions | 1. Mobile health shared step‐wise rehabilitation teaching video instructions module delivered via classic bluetooth technology plus standard care 2. Standard care only |
| Outcomes | Follow‐up: 4 months QuickDASH, Oxford Shoulder Score, participant and doctor satisfaction |
| Notes | Found in November 2021 top‐up search Ongoing trial. Not yet recruiting (July 2021). Trial dates: September 2021 to September 2023. |
Z‐TUpx NCT04651543.
| Methods | Randomised controlled trial |
| Participants | 90 (target) participants, aged between 18 and 85 years, who have non‐surgical treatment for an acute proximal humeral fracture |
| Interventions | 1. X‐ray taken at 1 week 2. No X‐ray taken at 1 week |
| Outcomes | Follow‐up: 2 years Constant score, quality of life, complications |
| Notes | Found in November 2021 top‐up search Ongoing trial. Not yet recruiting (December 2020). Trial dates: December 2021 to June 2022 |
Z‐TUpx NCT04917536.
| Methods | Randomised controlled trial |
| Participants | 80 (target) participants, aged between 10 and 70 years, with a humeral shaft fracture or proximal humeral fracture (Neer 2, 3 or 4); assigned for anterograde nailing |
| Interventions | 1. Rotator cuff split approach: nail inserted through the rotator cuff split, between the supraspinatus tendon and the long part of the biceps 2. Rotator cuff approach: nail inserted through the supraspinatus tendon, which is closed at the end of the surgery |
| Outcomes | Follow‐up: 1 year QuickDASH, Constant score, Simple Shoulder Test, pain (VAS), complications, duration of surgery, duration of sick leave, duration of rehabilitation, radiological assessment of bone healing, haemoglobin levels |
| Notes | Found in November 2021 top‐up search Ongoing trial. Recruiting. Trial dates: June 2021 to June 2024 |
Z‐TUpx Song 2020.
| Methods | Randomised controlled trial: "computer‐created stochastic number table" |
| Participants | 64 (minimum target) participants, aged between 55 and 80 years, treated for 3‐part or 4‐part acute proximal humeral neck fractures |
| Interventions | 1. Locking plate (deltopectoral approach) 2. Intramedullary nail (anterolateral approach) using Proximal Humeral Nail Same postoperative rehabilitation protocol in both groups |
| Outcomes | Follow‐up: "the patients are followed up at 2 weeks, 6 weeks, 12 weeks and every three months thereafter" (final follow‐up not stated) DASH score, Constant score, shoulder ROM, intraoperative blood loss, operation time, postoperative complications, radiological assessment |
| Notes | Found in November 2021 top‐up search Ongoing trial. Published protocol refers to Research Registry 6047 entry but we could not locate this. |
DASH: Disabilities of the Arm, Shoulder and Hand score; PHILOS: Proximal Humerus Internal Locking System; ROM: range of motion; SF‐12: 12‐item Short‐Form Health Survey; SF‐36: 36‐item Short‐Form Health Survey; VAS: visual analogue scale
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1900022553.
| Study name | Clinical outcomes of three‐ or four‐part complex proximal humerus fractures: reverse total shoulder arthroplasty, hemiarthroplasty versus locking plate fixation |
| Methods | "Randomized parallel control" (Google translation): computer generated random numbers |
| Participants | 90 participants with three‐ or four‐part complex proximal humeral fractures Inclusion criteria: closed fracture; affected limb is not accompanied by other multiple injuries; Neer III or IV proximal humeral fractures; participant's body can tolerate surgery and can be combined with functional exercise after surgery Exclusion criteria: unable to tolerate surgery; choose conservative treatment; pre‐injury diagnosis of upper‐limb disability; pathological fracture; malnutrition and metabolic diseases that affect fracture and wound healing |
| Interventions | 1. Reverse total shoulder arthroplasty
2. Hemiarthroplasty 3. Locking steel plate fixation |
| Outcomes | Follow‐up: no information Primary outcomes: haemoglobin, operative time, complications, diabetes (?), surgical bleeding volume, Constant score |
| Starting date | Start: 15 April 2019. Finish: 30 April 2020 |
| Contact information | Research leader: Xiangyang Chen 99 Huaihai Road West, Xuzhou, Jiangsu, China 221002 +86 15905201635 Email: 1173503735@qq.com Affiliated Hospital of Xuzhou Medical University Applicant: Ye Zhang 99 Huaihai Road West, Xuzhou, Jiangsu, China 221002 +86 13023506123 136926070@qq.com Affiliated Hospital of Xuzhou Medical University |
| Notes | Status was "recruiting" on 23 April 2019 Additional information in http://www.chictr.org.cn/showproj.aspx?proj=37955 |
ChiCTR‐IOR‐16008817.
| Study name | Locking plate fixation combined with allogeneic fibula intramedullary implantation for treatment of proximal humerus fracture with comminuted medial column |
| Methods | Randomised: use of "computer random grouping software 1: 1 grouping" |
| Participants | 100 adults with proximal humeral fracture with comminuted medial column Inclusion criteria: age ≥ 18 years; X‐ray and computerised tomography (CT) confirmed proximal humeral fracture with medial column comminution; fresh fracture, occurred within 3 weeks after injury; participant's informed consent; their general condition can tolerate surgery Exclusion criteria: concomitant fractures on the ipsilateral upper limb; fracture extends to the humeral shaft; the ipsilateral upper limb has a history of fracture or surgery or chronic arthritis that affects the function of the upper limb before the injury; pathological fractures |
| Interventions | 1. Locking compression plate (LCP) + allogeneic fibula intramedullary implantation 2. Locking compression plate |
| Outcomes | Follow‐up: 24 months, also 3, 6 and 12 months after operation Primary outcomes: Simplified Chinese version of Oxford Shoulder Score; QuickDASH (Disabilities of the Arm, Shoulder and Hand) Secondary outcomes: Constant score; SF‐36 Health Survey Form; range of shoulder motions; postoperative complications |
| Starting date | Start: 1 August 2016. Planned completion date: 1 August 2019 |
| Contact information | Maimaitiaili Tuerxun 600 Yishan Road, Shanghai, China 200233 +86 15800576712 Email: 602416584@qq.com Shanghai Jiao Tong University Affiliated Sixth People's Hospital Yunfeng Chen 600 Yishan Road, Shanghai, China 200233 +86 13301819858 Email: drchenyf@qq.com Shanghai Jiao Tong University Affiliated Sixth People's Hospital |
| Notes | Status recruiting on 23 November 2016 Additional information in http://www.chictr.org.cn/showproj.aspx?proj=14903 |
DRKS00009990.
| Study name | Robot training assistance in humerus fracture – a multicenter, controlled, randomized interventional trial ‐ RASTA |
| Methods | Randomised controlled trial, not blinded |
| Participants | 60 participants with surgically fixed proximal humeral fractures (classification AO 11) Inclusion criteria: age 35 to 66 years, fracture osteosynthesis stable for movements Exclusion criteria: limited cognition (short orientation memory test > 10); pain during shoulder movement (> 5 visual analogue scale); limited vision (< 20%); limited hearing ability (understanding of talking); isolated greater tuberosity fracture (AO 11 A1); fracture with involvement of glenoid, double fracture; plexus injury or axillar nerve injury; cardiac failure (NYHA classification: III/IV); chronic obstructive pulmonary disease III‐IV; limited walking ability (use of crutches) |
| Interventions | 1. Training with ArmeoSpring arm robot 3 to 5 times a week, duration about 1 hour for 3 weeks 2. Standard physiotherapy for 3 weeks |
| Outcomes | Follow‐up: 3 months, also 3 weeks Primary outcome: DASH score three months after fracture Secondary outcomes: change of Wolf Motor Function Test after Treatment (3 weeks); a standardised function test that assesses 15 daily activities (e.g. lifting a can, folding a towel) regarding time, function, and quality of movement; change of shoulder joint mobility (degrees measured with goniometer) after treatment (3 weeks); change of hand power (kg measured with dynamometer) after treatment (3 weeks) |
| Starting date | Date of first enrolment: 15 February 2016 |
| Contact information | Professor Janina Lackner Professor‐Küntscher‐Str. 8 82418 Murnau Germany Affiliation: Berufsgenossenschaftliche Unfallklinik Murnau Email: janina.lackner@bgu‐murnau.de |
| Notes | Also presented in DRKS00009990 |
DRKS00011581.
| Study name | Clinical, functional and psychosocial outcome of reverse shoulder arthroplasty ‐ a prospective randomized comparison of two types of prostheses after traumatic fracture of the proximal humerus |
| Methods | Randomised comparison, not blinded |
| Participants | 60 people with multi‐fragmentary (comminuted) fractures of the proximal humerus Inclusion criteria: participants with multi‐fragmentary fractures of the proximal humerus and indication for reverse shoulder arthroplasty, age 18 years or over Exclusion criteria: known intolerance for SonoVue, severe heart failure (NYHA classification: III/IV), myocardial infarction within the last 14 days, severe respiratory diseases, pregnancy and breastfeeding |
| Interventions | 1. RSA (reverse shoulder arthroplasty) after traumatic injury ‐ model 'fracture prothesis' (with cavity for bone graft). Alows refixation of the rotator graft 2. RSA after traumatic injury ‐ model 'conventional prothesis' |
| Outcomes | Follow‐up: 6 and 24 months postoperatively Primary outcomes: shoulder function: Constant score, ASES score, DASH score, Simple Shoulder Test; psychosocial outcome: SF‐12; pain VAS; radiological outcome Secondary outcomes: perfusion in deltoid muscle tissue, assessed with contrast‐enhanced ultrasound |
| Starting date | Date of first enrolment: 12 January 2017 |
| Contact information | Dr Christian Alexander Fischer
Universität Heidelberg, Zentrum für Orthopädie, Unfallchirurgie und Paraplegiologie Schlierbacher Landstraße 200a 69118 Heidelberg Germany Email: christian.fischer at med.uni-heidelberg.de |
| Notes | Trial also contains a third non‐randomised arm of RSA for people with rotator cuff arthropathy and other osteoarthritis. It is unclear whether the target size for population includes this control group. Recruitment status: recruitment ongoing on form |
Hakim 2018.
| Study name | Randomised control trial comparing hemiarthroplasty and reverse polarity shoulder replacement for the treatment of proximal humerus fractures in the elderly |
| Methods | Randomised controlled trial using a computer‐generated block technique, blinded collection of range of motion data |
| Participants | 27 participants (interim analysis) with acute proximal humeral fracture suitable for replacement Inclusion criteria: acute, radiologically‐confirmed proximal humeral fracture requiring replacement, age > 65 with capacity to consent Exclusion criteria: pre‐existing shoulder pathology, anaesthetic risk precluding surgery, inflammatory arthritis, ipsilateral elbow pathology, open fractures and neurological injuries |
| Interventions | 1. Primary reverse arthroplasty 2. Hemiarthroplasty |
| Outcomes | Follow‐up: 1 year Oxford Shoulder Score; DASH score; range of flexion, abduction and external rotation |
| Starting date | Not known |
| Contact information | Professor Peter Brownson Consultant orthopaedic shoulder and elbow surgeon The Royal Liverpool and Broadgreen University Hospitals NHS Trust Email: peter.brownson@rlbuht.nhs.uk |
| Notes | Abstract is an interim analysis; no other publication found |
Howard 2018.
| Study name | Open reduction internal fixation vs nonoperative management in proximal humerus fractures: a prospective, randomized controlled trial protocol |
| Methods | Randomised, single‐centre, single‐blind (outcome assessors), clinical trial |
| Participants | 30 participants (actual) aged > 60 years with acute 2‐ or 3‐part proximal humeral fractures, or 4‐part fractures that are deemed amenable to surgical fixation Inclusion criteria: displaced 2‐, 3‐ or 4‐part proximal humeral fractures (Neer classification), > 60 years of age, low‐energy mechanism of injury, acute fracture (< 3 weeks) |
| Interventions | 1. Open reduction and internal fixation (ORIF) with locked plate 2. Non‐operative treatment: sling immobilisation for a period of 6 weeks |
| Outcomes | Follow‐up: 3, 6, 12 and 24 months Primary outcome: Constant score Secondary outcomes: American Shoulder and Elbow Surgeons Standardized Shoulder Assessment, Short Form‐36, complication rates, reoperation rates, radiographic time to union, radiographic malunion, hardware position and evidence of avascular necrosis or post‐traumatic osteoarthritis |
| Starting date | Start date: September 2015 Estimated end date: August 2020 |
| Contact information | Peter Lapner The Ottawa Hospital Ottawa Ontario Canada, K1H8L6 Telephone: 613‐737‐8899 Ext. 78377 Email: plapner@toh.on.ca |
| Notes | Target no. of participants was 160 Discrepancies between the published protocol and the trial registration document:
|
ISRCTN17996552.
| Study name | Rehabilitation for humeral fractures in working ages |
| Methods | "Randomised parallel‐group superiority‐controlled trial". Permuted‐block randomisation procedure |
| Participants | 70 participants aged 20 to 65 years undergoing surgical treatment of proximal humeral fractures, classified as AO Foundation for the Study of Internal Fixation type 11 (2) |
| Interventions | 1. Task‐oriented exercises and occupational therapy (12 weeks) 2. General physiotherapy: passive humeral and upper‐limb mobilisation, strengthening, muscle segmentary stretching, and postural control (12 weeks) Rehabilitation sessions performed three times a week for 1 hour |
| Outcomes | Follow‐up: 1 year Primary outcome: DASH Secondary outcomes: pain intensity; Short‐Form Health Survey (SF‐36); patient‐rated efficacy of treatment assessed using the Global Perceived Effect scale |
| Starting date | 1 May 2012 End date: 30 June 2017 |
| Contact information | Prof Marco Monticone Dept. Medical Sciences and Public Health ‐ Faculty of Medicine Cittadella Universitaria S.S. 554 Bivio Monserrato ‐ Sestu Cagliari 09042 Italy +39 (0)706753109 marco.monticone@unica.it |
| Notes | Retrospectively registered. Top‐up search conducted in November 2021 identified a full trial report that reported results for 70 participants (Z‐TUp Monticone 2021). |
ISRCTN76296703.
| Study name | Effectiveness and cost‐effectiveness of reverse shoulder arthroplasty versus hemiarthroplasty versus non‐surgical care for acute 3 and 4 part fractures of the proximal humerus in participants aged over 65 years – the PROFHER‐2 randomised trial |
| Methods | Multi‐centre randomised controlled superiority pragmatic trial |
| Participants | 380 participants who are aged 65 years or over with radiographically‐confirmed acute three‐part (including surgical neck) or four‐part displaced fracture of the proximal humerus (Neer Classification) including head‐splitting fractures of the humeral head and fracture dislocations; trial interventions can be provided within 5 weeks of injury |
| Interventions | 1. Reverse shoulder arthroplasty (RSA) 2. Hemiarthroplasty (HA) 3. Non‐surgical care: supporting the injured arm in a sling for 3 weeks The study aims to randomise 152 to each of the surgical treatments and 76 to the non‐surgical treatment. |
| Outcomes | Follow‐up: 6, 12 and 24 months (5 years for subsequent surgery)
Primary outcome: Oxford Shoulder Score Secondary outcomes: quality of life measured using EQ‐5D; pain measured using the Patient‐Reported Outcomes Measurement Information System (PROMIS) pain interference; pain measured using a visual analogue pain scale; range of shoulder motion measured at discharge from physiotherapy and independently assessed at 6 months; healing and implant position using X‐rays at 6 months; further procedures and complications; physiotherapy requirements and use (including time to start of physiotherapy; number of sessions; modalities used; and duration of rehabilitation) collected during the trial. Also, associated costs |
| Starting date | Recruitment start date: 01 June 2018 Recruitment end date: 31 May 2021 |
| Contact information | 1. Puvan Tharmanathan (scientific) York Trials Unit Lower Ground Floor ARRC Building Department of Health Science University of York Heslington York YO10 5DD United Kingdom Email: puvan.nathan@york.ac.uk 2. Catherine Arundel (public) Email: catherine.arundel@york.ac.uk |
| Notes | Funding for PROFHER‐2 by the NIHR Health Technology Assessment Programme: HTA 16/73/03. Funder website also includes 2 versions (01 November 2017 and 09 March 2018) of the trial protocol: https://www.fundingawards.nihr.ac.uk/award/16/73/03
Trial website: https://www.york.ac.uk/healthsciences/research/trials/research/trials/profher-2/ Recruitment into the trial was suspended because of COVID‐19. "Internal review. 16/04/2020: Due to current public health guidance, recruitment for this study has been paused.(notified on 5/5/20)". |
Launonen 2019.
| Study name | Nordic Innovative Trials to Evaluate osteoPorotic Fractures (NITEP) Collaboration: The Nordic DeltaCon Trial protocol—non‐operative treatment versus reversed total shoulder arthroplasty in participants 65 years of age and older with a displaced proximal humerus fracture: a prospective, randomised controlled trial |
| Methods | Multicentre and multinational randomised, single‐blind (outcome assessors) clinical trial |
| Participants | 150 participants (estimated) aged 65 to 85 years with a displaced 3‐ and 4‐part proximal humeral fracture |
| Interventions | 1. Reverse total shoulder arthroplasty 2. Non‐operative treatment: immobilisation in a sling for 2 weeks before starting self‐exercises and instructed physiotherapy |
| Outcomes | Follow‐up: 3 months, 1, 2, 5 and 10 years Primary outcome: QuickDASH score measured at 2 years Secondary outcomes: "QuickDASH score after 1, 2 (short term) and 5 years (medium term)", visual analogue scale for pain, grip strength, Oxford Shoulder Score, Constant score, 15D Quality of Life and the number of reoperations and complications |
| Starting date | Start date: October 2018 Estimated end date: January 2027 |
| Contact information | Antti Launonen antti.launonen@pshp.fi Tore Fjalestad, Principal investigator, Consultant Orthopaedic Surgeon, Ph:+4797013389, Oslo University Hospital torfja@online.no / tofjal@ous‐hf.no |
| Notes |
NCT00999193.
| Study name | Proximal humeral comminuted fractures in the elderly ‐ PERCELE Trial Official title: Effectiveness and cost‐effectiveness of conservative and operative treatment of three‐ and four‐part fractures of the proximal humerus. A nested randomised controlled trial and cohort study |
| Methods | Randomised single‐blind (outcomes assessor) |
| Participants | 90 older participants with comminuted, displaced fractures of the proximal humerus Inclusion criteria: age 65 years or older; acute trauma with randomisation within 7 days of injury; 3‐ or 4‐part fracture with > 5 mm dislocation of the anatomic neck (AO classification C1‐2 for non‐luxation fractures; C3 for luxation fractures) |
| Interventions | 1. [PHILOS]* locking plate: open reduction of the fracture (and glenohumeral joint), internal fixation with the [PHILOS]* locking plate. Tuberculum fragments are sutured to the plate with thick non‐absorbable suture. 2. [Global FX]* hemiarthroplasty: replacement of the humeral articular head with hemiprosthesis. Tubercles are sutured to the prosthesis with thick non‐absorbable sutures. 3. Non‐surgical treatment: immobilisation in a supporting brace for 3 weeks, then increasingly active rehabilitation programme supported by a physiotherapist until 12 weeks after the injury. |
| Outcomes | Follow‐up: 24 months Primary outcomes: Pain at rest and activity (Numeric Rating Scale), Constant score Secondary outcomes: Simple Shoulder test (SST), Disabilities of the Arm, Shoulder and Hand (DASH), quality‐of‐life assessment (15D), subjective patient satisfaction, complications and costs |
| Starting date | November 2010 End date: December 2021 (December 2019 = final data collection date for primary outcome) |
| Contact information | Tuomas Lähdeoja, MD: tuomas.lahdeoja@hus.fi
Mika Paavola, MD: mika.paavola@hus.fi Helsinki University, Helsinki, Finland |
| Notes | As of registration update (22 January 2012), the study was recruiting. Start and end dates amended. Entry for trial (clinicaltrials.gov) on 19 January 2015 indicated that this "study is currently recruiting participants"; "Verified October 2014 by Helsinki University". Study completion date changed from November 2014 to December 2018. The estimated enrolment dropped from 150 to 90. Entry for trial (clinicaltrials.gov) on 3 March 2020 indicated that the study was "recruiting" on 6 June 2018. Study completion date changed from December 2018 to December 2021. The estimated enrolment dropped from 150 to 90. *The names of the locking plate and hemiarthroplasty are no longer present in the 6 June 2018 version of the trial registration. |
NCT01524965.
| Study name | The effect of the timing of postoperative mobilisation after locking plate osteosynthesis of fractures of the surgical neck of the humerus |
| Methods | Randomised controlled trial Single‐blind (outcomes assessor) |
| Participants | 100 participants, 18 years or older, with surgery performed within 10 days of injury for a dislocated (> 1 cm or 35 degrees) AO 11‐A2, ‐A3, ‐B1 or ‐B2 fracture of the surgical neck of the proximal humerus with a possible fracture of the greater tuberosity |
| Interventions | 1. Immediate mobilisation after open reduction and PHILOS plate fixation: immediate passive range of motion exercises are begun postoperatively, after 3 weeks, active unloaded mobilisation begins after three weeks and active, loaded use is allowed 6 weeks postoperatively 2. Standard mobilisation after open reduction and PHILOS plate fixation: immediately postoperatively the arm is held in a sling, active mobilisation of healthy joints and pendulum exercises are begun. Passive range of motion exercises of the shoulder are begun 3 weeks postoperatively. Active mobilisation begins after six weeks |
| Outcomes | Follow‐up: 3 and 6 weeks, 3, 6, 12 and 24 months Primary outcome: DASH (Disabilities of the Arm, Shoulder and Hand) score Secondary outcomes: Constant score, Simple Shoulder Test (SST), pain at rest and during motion, subjective satisfaction, quality of life using the 15D instrument, complications |
| Starting date | May 2011 Estimated completed date: December 2020 (primary date); December 2022 (final date) |
| Contact information | Tuomas Lahdeoja, MD Töölö Hospital, Helsinki University Central Hospital Helsinki, Finland, 00029 Email: tuomas.lahdeoja@hus.fi |
| Notes | Verified as recruiting participants in October 2014 by Helsinki University Trial registration document updated 3 January 2017 (version 4 with new completion dates) |
NCT01557413.
| Study name | Randomised study between intramedullary locking nails and locking plates for treatment of proximal humerus fractures (HUMERUS) Official title: Randomised study between intramedullary locking nails and locking plates for treatment of proximal humerus fractures in patients after 40‐year‐old |
| Methods | Single‐centre, randomised controlled trial. Unblinded |
| Participants | 80 participants, aged between 40 and 85 years, with a type III or IV "cephalotuberosity" proximal humeral fracture (classification of Neer and Duparc) |
| Interventions | 1. Intramedullary nail (Multilock, Synthes) 2. Locking plate (SURFIX, Integra) |
| Outcomes | Follow‐up: 1 year Primary outcome: Constant score Secondary outcomes: QuickDASH, complication (malunion, necrosis, infection) |
| Starting date | Start date: February 2012 Completion date: December 2016 |
| Contact information | Dr Patrick Boyer Group Hospitalier Bichat ‐ Claude Bernard 46, rue Henri‐Huchard Paris Ile de France France 75018 patrick.boyer@bch.aphp.fr |
| Notes | Entry for trial (clinicaltrials.gov) on 19 January 2015, indicated that this "study is currently recruiting participants"; "Verified August 2014 by Assistance Publique ‐ Hôpitaux de Paris". Study completion date changed from November 2015 to February 2017. The estimated enrolment dropped from 144 to 84. Entry for trial (clinicaltrials.gov) on 2 March 2020 indicated that study was completed December 2016. The actual enrolment was 80. Request sent to lead author on 2 March 2020 inquiring on publication status; no response received. Conference poster of 2019, with abstract by Boyer and Dukan, was identified and initially we treated this as a separate trial but believe these belong together. Summary below: Participants: 75 patients with 3‐ and 4‐part displaced acute proximal humeral fractures, mean age 69 years Interventions: 1. Locking compression plate: Surfix Plate (Intergra); 2. Intramedullary interlocking nail: Multilock Nail (Synthes). All had early rehabilitation and 6 weeks in a sling. Outcomes: Constant score, American Shoulder and Elbow Surgeons (ASES) score, complications (non‐union, malunion, secondary displacement, avascular necrosis, impingement, hardware removal), forward flexion, abduction, VAS pain, and muscle strength. Notes: Reported in a conference abstract and poster only. Abstract presents results for follow‐up at mean 28 months (24 to 36 months) for 71 participants; poster for 75 participants at mean 34 months follow‐up. Specific searches for the authors of this trial have not identified a relevant publication. Top‐up search conducted in November 2021 identified a full trial report that reported results for 85 participants at a mean of 66 months follow‐up (Z‐TUp Boyer 2021). |
NCT02075476.
| Study name | Prospective, randomized and double blind study of parallel groups for evaluating the effectiveness between two surgical techniques for reconstruction of humeral proximal extremity fractures or fractures luxation in three or four fragments of Neer's classification |
| Methods | Randomised controlled trial |
| Participants | 40 participants, aged 70 years or over, with "humeral proximal extremity fracture or fracture luxation in three or four fragments of Neer's classification" |
| Interventions | 1. Reverse arthroplasty Delta Xtent (DePuy) 2. Hemiarthroplasty Global Fx (DePuy) |
| Outcomes | Follow‐up: 1, 3, 6, 12, 18 and 24 months Primary outcome: ASES (American Shoulder and Elbow Surgeons) score Secondary outcomes: intraoperative complications; postoperative complications, surgical time, recovery time |
| Starting date | May 2013 Estimated completed date: May 2016 (actually completed 6 August 2019) |
| Contact information | Carlos Alvarez, MD Hospital General Universitario Gregorio Marañon Madrid, Spain, 28007 Email: calvargon@gmail.com |
| Notes | Verified as recruiting participants in March 2014 by Hospital General Universitario Gregorio Marañon Verified as completed as posted 7 August 2019 Top‐up search conducted in November 2021 identified updated trial registration with results posted (Z‐TUp Alvarez 2020) |
NCT02467803.
| Study name | Shoulder functional outcomes of patients with proximal humerus fractures: comparison of two different treatment protocol[s] |
| Methods | Randomised cross‐over trial |
| Participants | 50 (target) participants with a radiographically‐proven, closed fracture of the proximal humerus admitted to the emergency department of Hacettepe University Hospital, who were considered suitable for primary non‐operative management by the orthopedic surgeon. Age between 30 to 75 years |
| Interventions | 1. Scapula mobilisation with shoulder ROM exercises 2. Shoulder ROM exercises only |
| Outcomes | Follow‐up: 6 months Primary outcome: Constant score. "The differences between baseline score and at the end of 6 months score will be assessed." Secondary outcomes: DASH scores, range of motion, pain and depression level with Beck Depression Scale. |
| Starting date | June 2015 Estimated primary completion date: January 2016 |
| Contact information | Dr Haney Guney, Hacettepe University, Ankara, Turkey Email: hande.guney@hacettepe.edu.tr |
| Notes | At trial registration the recruitment status was: not yet recruiting Request for information on the status of this trial sent to Haney Guney, Ankara, Turkey on 16 July 2020 |
NCT02913378.
| Study name | Conservative vs surgical treatment for proximal humerus fractures in the elderly |
| Methods | Randomised controlled trial |
| Participants | 58 participants (target) aged more than 60 years with displaced proximal humeral fractures (> 1 cm shaft translation; > 0.5 cm tuberosity displacement; head shaft angulation > 45 degrees; < 30 days from injury |
| Interventions | 1. Surgery: open reduction and locking plate fixation 2. Not surgery: sling and physiotherapy rehabilitation only |
| Outcomes | Follow‐up: 2 years Primary outcome: Constant score relative to the unaffected shoulder Secondary outcomes: ASES (American Shoulder and Elbow Surgeons) shoulder score; Constant score; quality of life SF‐12; VAS pain; complication and reoperation rate; radiographic evaluation (union, head‐shaft angle, tuberosities reduction, medial support and Fjalestad reduction criteria); rotator cuff condition assessed using ultrasound and magnetic resonance |
| Starting date | January 2016 Estimated completion date: January 2020 |
| Contact information | Dr Mauro EC Gracitelli
University of São Paulo, São Paulo, Brazil Email: mgracitelli@gmail.com |
| Notes |
NCT02944058.
| Study name | Plate fixation versus intramedullary nailing of 3 and 4 part proximal humerus fractures |
| Methods | Randomised controlled trial |
| Participants | 90 participants (target) aged 18 or over with 3‐ and 4‐part proximal humeral fractures |
| Interventions | 1. PHILOS plate
2. Multiloc intramedullary nail Deltopectoral or deltoid split as surgical access. All participants will have calcar screws and cuff sutures |
| Outcomes | Follow‐up: 6, 12, 52 and 104 weeks, but also 5 years Primary outcome: DASH (QuickDASH at 6 weeks) Secondary outcomes: Constant score; radiologically‐assessed complications (plate cut‐out, nail migration, screw cut‐out, reduction of head shaft angle / varus subsidence); complications (infection, avascular necrosis, thrombosis, nerve compression or damage, pseudoarthrosis); economics (visits to doctor, physiotherapist, sick leave, reoperations) |
| Starting date | October 2016 Study completion: October 2025 |
| Contact information | Dr Annette KB Wikerøy, University Hospital, Akershus, Norway Email: awikeroy@hotmail.com |
| Notes | Status: recruiting (July 2018) |
NCT03217344.
| Study name | Conservative treatment of proximal humeral fractures |
| Methods | Randomised controlled trial |
| Participants | 130 participants with non‐surgically‐treated proximal humeral fractures |
| Interventions | 1. One week of sling immobilisation 2. Three weeks of sling immobilisation |
| Outcomes | Follow‐up: 1 year (also 3 months) Pain (analogue pain scale), Constant score, secondary surgery Fracture displacement |
| Starting date | 19 October 2015; ended 20 June 2020 |
| Contact information | Carlos Torrens, MD, Castelldefels, Spain Email: CTorrens@parcdesalutmar.cat |
| Notes | The trial registration document was updated on 12 March 2019.
Response received from Carlos Torrens (8 July 2020) to our request for the current status of publication. This confirmed that they "started enrolling patients in a randomized fashion (group I 1‐week immobilization / group II 3‐week immobilization) in October 2015". The last participant was enrolled in May 2018; in total they enrolled "152 patients". They had completed the one‐year follow‐up and sent the abstract to the European Federation of National Associations of Orthopaedics and Traumatology (EFORT) meeting in 2020 (however, this meeting was cancelled due to COVID‐19). In May 2020, they ended the 2‐year follow‐up and were currently updating the Excel database. They hope to submit for publication by October or November 2020. Carlos Torrens provided a copy of an extended abstract and draft results for the 1‐year follow‐up on 10 July 2020; we decided we should wait on the full publication of this trial before inclusion. Top‐up search conducted in November 2021 identified a full trial report and an abstract; both are listed under Z‐TUp Martinez 2021. |
NCT03498859.
| Study name | Efficacy of physiotherapist‐supervised rehabilitation after proximal humerus fracture |
| Methods | Double‐blind randomised controlled trial |
| Participants | 70 participants, aged 60 years or older, with a low‐energy proximal humeral displaced (more than 5 mm or 30 degrees) two‐part fracture where fracture line emerges through the surgical (or anatomical) neck, treated non‐operatively |
| Interventions | 1. Physiotherapist‐supervised training once per week during 10 weeks in addition to home‐based training 2. Ten weeks of home‐based training with no supervision of a physiotherapist |
| Outcomes | Follow‐up: 3 and 12 months Primary outcome: DASH (3 months after fracture) Secondary outcomes: DASH (at 12 months), Constant score, 15‐dimensional health‐related quality‐of‐life instrument, Pain Catastrophizing Scale, General Self‐Efficacy Scale, accelerometer‐based activity in the upper extremity Other outcomes: cost‐effectiveness |
| Starting date | Start: 24 April 2018. Estimated study completion date: October 2021 |
| Contact information | Contact: Helle Østergaard, PhD student +45 61718026 Email: helle.oestergaard@viborg.rm.com |
| Notes | Recruiting at Viborg Regional Hospital, Denmark and Oslo University Hospital, Norway |
NCT03599336.
| Study name | RSA vs. nonop for 3 & 4‐part proximal humerus fractures Official title: Reverse total shoulder arthroplasty versus nonoperative treatment of 3 & 4‐part proximal humerus fractures |
| Methods | Open‐label randomised controlled trial |
| Participants | 100 participants, aged 65 or older, with displaced 3‐ and 4‐part proximal humeral fractures |
| Interventions | 1. Surgical management: ReUnion RSA (Reverse Shoulder Arthroplasty System) 2. Non‐operative treatment: sling immobilisation for 3 weeks. The sling will be removed for elbow, wrist and hand range of motion, hygiene, and dressing only. At 3 weeks passive ROM in external rotation and forward elevation will be added. At 6 weeks from injury, the sling will be removed and stretching in all planes will be allowed. Use of the arm will be up to a fork/knife/toothbrush only. At 3 months from injury, strengthening will be added. Supervised physical therapy will be offered to participants for use at their discretion, as is current practice. |
| Outcomes | Follow‐up: 1 and 2 years Primary outcome: function as described by American Shoulder and Elbow Surgeons (ASES) score at 1 year follow‐up Secondary outcomes: none reported |
| Starting date | Start: 1 August 2018. Estimated study completion date: 1 August 2022 |
| Contact information | Contact: Jonathan Furuseth 507‐293‐6470 Email: furuseth.jonathan@mayo.edu |
| Notes | Recruiting at Mayo Clinic in Rochester, USA |
NCT03610113.
| Study name | Reverse or nothing for complex proximal humeral fractures Official title: Reverse shoulder arthroplasty versus conservative treatment for complex proximal fractures in elderly patients |
| Methods | Double‐blind randomised controlled trial |
| Participants | 81 participants, aged 70 years and older, with a 3‐ or 4‐part proximal humeral fracture |
| Interventions | 1. Reverse shoulder arthroplasty followed by rehabilitation protocol 2. Conservative treatment: use of sling for three weeks, followed by rehabilitation protocol |
| Outcomes | Follow‐up: 1 year Primary outcomes: Constant score Secondary outcomes: number of participants with treatment‐related surgical re‐interventions, treatment‐related shoulder joint infection and treatment‐related implant dislocation |
| Starting date | Start: 1 August 2018. Estimated study completion date: 1 August 2022 |
| Contact information | Contacts: Joan Miquel (Principal Investigator), Consorci Sanitari de l'Anoia, +659133365 Email: joanmiquelnoguera@hotmail.com |
| Notes | Recruiting at Consorci Sanitari de l'Anoia, Spain |
NCT03694457.
| Study name | Comparison between anterior approach (deltopectoral) and lateral approach (deltoid splitting) in shoulder reverse arthroplasty for proximal humerus fracture (DELTOPSUPEX) |
| Methods | Randomised controlled trial |
| Participants | 80 participants, aged over 65 years, with a proximal humeral fracture (Neer 3‐4 and 2 dislocated) |
| Interventions | 1. Reversed total shoulder arthroplasty: deltopectoral (anterior) approach 2. Reversed total shoulder arthroplasty: lateral approach |
| Outcomes | Follow‐up: 3, 6 and 12 months Primary outcomes: Constant score Secondary outcomes: QuickDASH score, SF‐12 score, Alder score, Simple Shoulder Test, WOOS (Western Ontario Osteoarthritis of the Shoulder index), intensity of pain, radiologic evaluation, complications |
| Starting date | Start: 26 November 2018. Estimated study completion date: 30 June 2021 |
| Contact information | Contact: Lise LACLAUTRE 04 73 75 49 63, Email: drci@chu‐clermontferrand.fr |
| Notes | Recruiting at Chu Clermont‐Ferrand, France |
NCT03786679.
| Study name | Non‐operative treatment in Sweden of proximal humeral fractures, a randomised multicentre trial |
| Methods | Randomised trial, with assessors who are not involved in the treatment; radiological assessment of images will be assessed with anonymised images |
| Participants | 400 people age 18 years or over with a proximal humeral fracture no older than 7 to 10 days |
| Interventions | 1. Orthosis group (Ultrasling ER III orthosis). An orthosis with the broken arm in neutral position fixed for four weeks. After four weeks, the participant is instructed to start rehabilitation. 2. Early rehabilitation group. The participant is instructed to start early rehabilitation (early range‐of‐motion exercises) about one week after the trauma. |
| Outcomes | Follow‐up: 12 months Primary outcome: union of fracture Secondary outcomes: Oxford Shoulder Score, numerical pain reporting scale (pain at rest, at night and during activity); QuickDASH, global assessment of improvement, shoulder range of motion (elevation, abduction, internal and external rotation) |
| Starting date | Start: 25 February 2019. Estimated study completion date: 25 February 2024 |
| Contact information | Dr Hanna C Björnsson Hallgren, Linkoeping University, Sweden Email: hanna.bjornsson.hallgren@regionostergotland.se |
| Notes |
NCT04106674.
| Study name | Two‐part proximal humerus ‐ conservative vs operative Official title: conservative versus operative treatment of two‐part proximal humerus fractures |
| Methods | Single‐blind randomised controlled trial |
| Participants | 50 participants, aged 60 to 85 years, with two‐part proximal humeral fractures (more than 50% displacement between head or shaft or 50° angulation of the head against the shaft in Y‐projection or more than 45° valgus or more than 30° varus of the Head Shaft Angle). |
| Interventions | 1. Surgery: open reduction internal stabilisation. Implant choice pragmatic, surgeons' choice 2. Conservative treatment |
| Outcomes | Follow‐up: 1 year Primary outcome: QuickDASH Secondary outcomes: Constant score, EQ‐5D, radiology, visual analogue scale score, number of participants with complications such as infection, avascular necrosis, failure of osteosynthesis, screw cut‐out, nerve damage, deep vein thrombosis |
| Starting date | Start: 1 October 2019. Estimated study completion date: 31 October 2023 |
| Contact information | Contacts: Annette Konstanse Bordewich Wikerøy, +004799717481 Email: awikeroy@hotmail.com |
| Notes | Recruiting at Akershus University Hospital, Norway |
NCT04553497.
| Study name | IFC therapy in proximal humerus fractures Official title: Interferential current provides additional benefit to rehabilitation program for the patients with proximal humeral fractures: a randomized controlled study |
| Methods | Triple‐blind quasi‐randomised controlled trial (participant, investigator, outcomes assessor). Randomised by coin toss |
| Participants | 35 participants, aged 40 to 80 years, proximal humeral fractures that did not require surgery Exclusion criteria: had any surgery due to proximal humerus fracture; any previous experience of any electrotherapy prior to the proximal humeral fracture (to ensure blinding of therapy); any contraindication for interferential current; had experienced a known or suspected joint infection or a specific condition such as peripheral or central nervous system lesions, neoplasm, diabetes mellitus or osteonecrosis; any history of mental impairment or poor general health status that would affect results |
| Interventions | 1. Rehabilitation and interferential current therapy 2. Rehabilitation and sham interferential current therapy |
| Outcomes | Follow‐up: 4 months Primary outcome: Constant score (at week 6) Secondary outcomes: visual analogue scale score, DASH score |
| Starting date | 1 April 2014 End date: 15 October 2015 |
| Contact information | Emine Duran (PI) Ege University, School of Medicine, Physical Medicine and Rehabilitation, Turkey |
| Notes | Trial details submitted 11 September 2020. Observation by HH on 5 May 2022: the results of this trial are now available via the clinicaltrial.gov website: clinicaltrials.gov/ct2/show/study/NCT04553497 |
Nerz 2017.
| Study name | Effectiveness of robot‐assisted training added to conventional rehabilitation in patients with humeral fracture early after surgical treatment: protocol of a randomised, controlled, multicentre trial |
| Methods | Multicentre randomised clinical trial. Single‐blinded (outcomes assessor). Pilot study |
| Participants | 48 participants (actual) aged between 35 and 66 years with proximal humeral fracture (AO 11) and surgical treatment of the shoulder joint. Participants included between the fourth and seventh weeks after surgery. |
| Interventions | 1. Robot‐assisted training using the ArmeoSpring device added to conventional occupational and physical therapy 2. Conventional occupational and physical therapy Both the intervention group and the control group received physical and occupational therapy for a period of 3 weeks. |
| Outcomes | Follow‐up: 3 weeks, 3, 6 and 12 months Primary outcome: DASH Secondary outcomes: Wolf Motor Function Test–Orthopaedic version Outcomes as reported in protocol |
| Starting date | Start date: February 2016 Completion date: December 2019 |
| Contact information | Corinna Nerz Research associate Robert Bosch Gesellschaft für Medizinische Forschung mbH |
| Notes | Target number of participants was 60 Top‐up search conducted in November 2021 identified a full trial report that reported results for 48 participants (Z‐TUp Kröger 2021) |
NTR3859.
| Study name | PROMOTION‐trial: a PROspective randomized Multicenter trial for the treatment of dislocated 3‐part proximal humerus fractures: Open reduction and internal fixaTION versus intramedullary nailing |
| Methods | Randomised controlled trial |
| Participants | 92 participants, aged 18 years or over, with unilateral displaced 3‐part proximal humeral fracture (> 45 degrees or > 0.5 cm displacement between major fracture fragments) |
| Interventions | 1. PHILOS plate (Synthes) 2. PHN (Proximal Humeral Nailing system, Stryker) |
| Outcomes | Follow‐up: 1 year Primary outcome: Constant score Secondary outcomes: DASH score, pain, SF‐12, EQ‐5D, complication and mortality rate |
| Starting date | 1 August 2013 Planned closing date: 31 July 2017 (see Notes) |
| Contact information | Jeroen Bransen Maastrict University Medical Centre Department of Surgery PO Box 5800 6202 AZ Maastricht The Netherlands jeroenbransen@gmail.com |
| Notes | Ethics approval not received for this multicentre trial at time of registration (3 June 2013) Response received from J Bransen on 14 August 2020 on trial status: "Our trial is currently still open, although inclusions are very low. We have not yet decided if it is worthwhile to continue." |
ReShAPE.
| Study name | The ReShAPE trial: Reverse Shoulder Arthroplasty for treatment of Proximal humeral fractures in the Elderly |
| Methods | Multicentre (11 hospitals) randomised and observational study. Using: "Dynamic (adaptive) random allocation stratified by site using minimisation to allow for gender and age (70‐80, over 80)." "Central randomisation by phone" |
| Participants | 72 (target) participants aged 70 years or older with 3‐ or 4‐part proximal humeral fracture according to the Neer classification |
| Interventions | 1. Reverse total shoulder arthroplasty. Postoperatively, the arm will be placed in a shoulder immobiliser (either in internal rotation or some external rotation at the discretion of the treating surgeon). 2. Non‐surgical treatment starting with a shoulder immobiliser All participants will be instructed on elbow, wrist and hand exercises to commence immediately. After two weeks, pendular exercises and passive flexion to 90 degrees and passive external rotation to neutral will be commenced. Unrestricted passive and active‐assisted exercises will be allowed, graduating to active mobilisation (as tolerated) at 6 weeks. Resisted range‐of‐motion exercises will be allowed after 12 weeks. A minimum of 5 physiotherapy one‐on‐one, face‐to‐face contacts within 3 months of treatment will be required. Participants will perform self‐guided exercises every day as instructed in their face‐to‐face sessions. |
| Outcomes | Follow‐up: 2 years (main follow‐up), also 3 and 6 months, and 1, 5 and 10 years Primary outcome: ASES (American Shoulder and Elbow Surgeons) score at 24 months Secondary outcomes: DASH (Disability of the Arm, Shoulder and Hand) score; EQ‐5D and EuroQol‐VAS (quality of life and general health); pain (verbal analogue scale 0 to 10 points); radiological parameters (healing and positioning of tuberosities, scapula notching, prosthetic loosening, alignment); complications after reverse shoulder arthroplasty (repeat shoulder surgery, readmission, infection requiring treatment, neurological deficit, dislocation, death) |
| Starting date | Actual start date (recruitment): 9 March 2016 Anticipated end date (recruitment): 9 March 2018 |
| Contact information | Dr Geoffrey Smith, Sydney Orthopaedic Trauma and Reconstructive Surgery 5/19 Kensington St, Kogarah. NSW 2217, Australia. Email: gcssmith@icloud.com |
| Notes | Non‐consenting patients were to be offered participation in the observational arm of the study. Their treatment was to consist of the same two treatment options as the RCT arm. Treatment being decided by patient preference as per usual practice at each institution. |
SHeRPA.
| Study name | Comparison of two shoulder replacement methods after trauma |
| Methods | Multicentre (5 centres in the UK), randomised controlled trial. Participants are blinded. |
| Participants | 50 participants aged over 65 years with displaced 3‐ or 4‐part proximal humeral fractures; fit for surgery Exclusion criteria: dementia, non‐consent, unfit for reverse shoulder arthroplasty, glenoid fracture, axillary nerve palsy |
| Interventions | 1. Reverse total shoulder arthroplasty 2. Hemiarthroplasty |
| Outcomes | Follow‐up: 2 years, also 6 weeks, 3 and 12 months Primary outcome: Constant score (at 12 months) Secondary outcomes: QuickDASH (Disabilities of the Arm, Shoulder and Hand) Score, Oxford Shoulder Score, ASES score |
| Starting date | Start date: June 2013 Estimated completion date: August 2017; changed to May 2019 |
| Contact information | Prof Adam Watts Wrightington Hospital Hall Lane Appley Bridge Wigan Lancashire WN6 9EP United Kingdom |
| Notes | Retrospectively registered (applied: 13 January 2015) after end of recruitment; end date extended to May 2019 Funder: Tornier UK Limited (probably manufacturer of implants under test) |
TPHF.
| Study name | Treatment of Proximal Humeral Fractures (TPHF) Official title: A national, prospective, randomized, multicenter, controlled head‐to‐head comparison of conservative, plate fixation and prosthesis in treatment of displaced 2‐, 3‐ and 4‐part fractures of proximal humerus of 60 years and older patients |
| Methods | Multicentre, randomised clinical trial. Single‐blinded (outcomes assessor) (conducted as 2 studies; see Notes) |
| Participants | 250 (see Notes; original aim was 290) participants aged over 60 years with displaced 2‐, 3‐ and 4‐part proximal humeral fractures The ongoing study is: 162 (in the protocol the aim was for 198 participants) participants aged over 60 years with displaced 3‐ and 4‐part proximal humeral fractures |
| Interventions | 1. PHILOS locking plate 2. EPOCA prosthesis 3. Non‐surgical management |
| Outcomes | Follow‐up: 2 years Primary outcome: DASH (Disabilities of the Arm, Shoulder and Hand) score Secondary outcomes: EQ‐5D Questionnaire, Oxford Shoulder Score, Constant score, pain (VAS), quality‐of‐life questionnaire 15D, complications |
| Starting date | Start date: January 2011 Recruitment completed: November 2019 |
| Contact information | Antti Launonen Tampere University Hospital Tampere Pirkanmaa Finland, 33521 antti.launonen@pshp.fi |
| Notes | As of registration update (1 February 2011), trial was recruiting in Tampere University Hospital, but recruitment had not started in Oulu or Kuopio. Entry for trial (clinicaltrials.gov) on 19 January 2015, indicated that this "study is currently recruiting participants"; "Verified November 2014 by Tampere University Hospital". End date of study changed from September 2016 to September 2018. In the protocol [17], 2 trial strata were described. Stratum I comprised 2‐part fractures and stratum II comprised 3‐ and 4‐part fractures. It was foreseen in the original protocol that stratum I would commence first, and therefore the reporting was planned separately for each protocol; stratum I is now published: see included trial Launonen 2019a. The report indicated that, at "present, stratum II is still in the recruitment phase (159 of 218 patients recruited by 2 April 2019), which is scheduled to be completed during 2019. The analysis and reporting of these patients will start after the 2‐year follow‐up period." However, the updated trial registration document (update submitted 9 March 2020) indicated that the recruitment stopped in November 2019, with the total = 250 participants. This equates to 162 participants for the ongoing study (stratum II). |
Wu 2016.
| Study name | Minimally invasive treatment of proximal humerus fractures with locking compression plate improves shoulder function in older patients: study protocol for a prospective randomized controlled trial |
| Methods | Single‐centre randomised trial using "random number table" |
| Participants | 82 (actual enrolment) older participants aged over 60 years with Neer 2‐ or 3‐part proximal humeral fractures admitted to hospital within 6 hours of injury |
| Interventions | 1. Locking compression plate fixation using minimally invasive technique
2. Conventional locking plate fixation using minimally invasive technique Closed reduction via shoulder lateral approach was used for both operations. Both plates were purchased from "Xiamen Dabo Yingjing Medical Devices Co., Ltd., Xiamen, China." |
| Outcomes | Follow‐up: 6 months
Primary outcome: "Neer classification system score" at 6 months Secondary outcomes: length of operation; intraoperative blood loss; postoperative hospital stay; pain (postoperative pain relief) assessed by change in VAS at 1 and 3 days, and 1 and 2 weeks post surgery; fracture healing (X‐ray assessment at 0.5, 1, 3 and 6 months); quality of life measured by change in SF‐36 scores at 0.5, 1, 3 and 6 months Recording of adverse events was also planned |
| Starting date | Start date: May 2014 Completed: December 2015 |
| Contact information | Tao Wu, Department of Joint Surgery, Affiliated Hospital of Qinghai University, Xining, Qinghai Province, China |
| Notes | Most of the data are extracted from the trial registration, which was retrospective. The study protocol is available in Chinese and English (http://www.clinicalto.com/text.asp?2016/1/2/51/183002). We have been unable to find a publication for this trial completed in 2015. |
AO: Arbeitsgemeinschaft fur Osteosynthesefragen / Association for the Study of Internal Fixation (or ASIF); ASES: American Shoulder and Elbow Surgeons; DASH: Disabilities of the Arm, Shoulder and Hand score; EQ‐5D: European Quality of Life‐5 Dimensions; LCP: locking compression plate; NRR: National Research Register; NYHA: New York Heart Failure Association; ORIF: open reduction and internal fixation; PHILOS: Proximal Humerus Internal Locking System; ROM: range of motion; SF‐12: 12‐Item Short‐Form Health Survey; SF‐36: 36‐item Short‐Form Health Survey
Differences between protocol and review
Update published in 2022
At the time of the search, CENTRAL was fully up to date with all records from the BJMT Group’s Specialised Register and so we did not search the group's register separately. We did not search CINAHL, AMED and PEDro, or search conference proceedings, beyond October 2019 when we were updating our search to September 2020. This was because, from our assessment of already included studies, the three databases were unlikely to contribute unique eligible trial records to future updates. In addition, we assessed that conference proceedings were unlikely to be the only sources of study reports that would merit trials being included.
Prior to full‐text screening, we made the following clarifications relating to our inclusion criteria.
We would consider deferring inclusion of pilot or feasibility studies ‐ and thus place these in Studies awaiting classification ‐ where recruitment was being extended for a main study. This was to avoid the known problems of interim analysis.
Our focus was on primary treatment of fractures; thus, we would exclude trials comparing interventions for fracture non‐union or other sequelae of unsuccessfully treated fractures (see Types of participants).
We would exclude trials testing biological interventions, such as autologous bone marrow‐derived and blood‐derived biological therapies, that are not primarily designed for treating proximal humeral fractures. Such interventions are applied more generally, typically for promoting bone healing (see Types of interventions).
Consistent with our intention to focus on the main categories of interventions described in Description of the intervention, we have deferred the inclusion of trials testing acupuncture and variants thereof, as well as Chinese traditional medicine. This also reflects our limited experience of these interventions.
We prepared two further summary of findings tables based on an assessment of the importance of the comparisons and data availability. We explicitly specified the summary of findings table outcome measures in Methods, and revised the two previous tables in the light of these revisions and new evidence. We also reframed the evidence in terms of certainty, rather than quality, and adjusted the GRADE ratings, especially in relation to imprecision, according to revised guidance.
We invited Patricia Aluko to enhance the economic perspective of the review and prepare a brief economic commentary, with a specific focus on economic evidence for the surgical versus non‐surgical treatment comparison.
Update published in 2015
The first primary outcome was split into patient‐reported shoulder‐related scores and patient‐reported quality‐of‐life scores (see Types of outcome measures).
A key change in terminology was replacing 'conservative' treatment with 'non‐surgical' treatment.
We assessed the quality of the evidence using GRADE and, where sufficient evidence was available, prepared summary of findings tables.
Update published in 2012
A statement was added to Types of participants, clarifying the inclusion of trials with a small proportion of children.
A new secondary outcome was added to Types of outcome measures (composite scores, whether validated or not, of subjectively‐ and objectively‐rated function and overall outcome). This was to distinguish explicitly between validated measures of patient‐reported function and activities of daily living and other commonly‐used composite scores, such as the Constant score.
Examples of the secondary outcome 'Other complications' were added.
Update published in 2010
Most of the changes to methods in Issue 12, 2010 reflected the uptake of new methodology and reporting, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008b). These included risk of bias assessment and more explicit reporting of data analysis and collection. Types of outcome measures were revised to define primary and secondary outcomes. Patient‐reported measures of upper‐limb function and a separate category for serious adverse events were added.
Update published in 2007
The order of the main categories of outcome measures was altered in Issue 2, 2007 to reflect the greater priority given to functional and clinical outcomes.
Contributions of authors
For the eighth update (ninth version), HH initiated the update and, with discussion with three other authors (SB, JE, TT), revised the protocol which was submitted for editorial approval. JE co‐ordinated study screening and selection, including managing this within Covidence, and the data extraction and risk of bias assessment process. Three review authors (SB, JE, HH) performed study screening and selection, with independent arbitration where required. Four review authors (SB, JE, HH, TT) assessed risk of bias and extracted data for the newly included trials. HH contacted trialists, performed most of the data entry and prepared the first full draft, with contributions on specific sections by JE and feedback on specific clinical areas and interim drafts from SB and TT. All four full review authors commented on the final manuscript.
PA screened the economic searches and wrote the first draft of the brief economic commentary, with interim feedback and reworking by HH in relation to the clinical context.
Helen Handoll is the guarantor of the review.
The summaries of the contributions of authors for previous versions of the review are presented in Appendix 8.
Sources of support
Internal sources
-
University of Teesside, Middlesbrough, UK
As employer of HH up to end of April 2020
-
University of Edinburgh, UK
Email, computing and library support (HH)
-
University of Manchester , UK
Email, computing and library support (JE and HH)
External sources
No sources of support provided
Declarations of interest
Patricia Aluko has no interests to declare. Stig Brorson was the lead investigator on Brorson 2009. No other interests to declare. Joanne Elliott has no interests to declare. Helen Handoll was a member of the trial management group of ProFHER 2015; an independent review of this trial was performed by Stig Brorson. No other interests to declare. Theis Thillemann has no interests to declare.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Agorastides 2007 {published and unpublished data}27032632
- Agorastides I, Sinopidis C, El Meligy M, Yin Q, Brownson P, Frostick SP. Early versus late mobilization after hemiarthroplasty for proximal humeral fractures. Journal of Shoulder and Elbow Surgery 2007;16(3 Suppl):S33-8. [DOI] [PubMed] [Google Scholar]
- Frostick S. Randomised prospective study to evaluate sling immobilisation versus early active assisted mobilisation following hemiarthroplasty for fractures of the proximal humerus. In: National Research Register, Issue 1, 2003. Oxford; Update Software.
- Frostick S. Randomised prospective study to evaluate sling immobilisation versus early active assisted mobilisation following hemiarthroplasty for fractures of the proximal humerus. www.controlled-trials.com/mrct/trial/483871 (accessed 6 August 2010).
- ISRCTN27032632. Randomised prospective study to evaluate sling immobilisation versus early active assisted mobilisation following hemiarthroplasty for fractures of the proximal humerus. www.isrctn.com/ISRCTN27032632 (first posted 12 September 2003).
Bertoft 1984 {published data only}
- Bertoft ES, Lundh I, Ringqvist I. Physiotherapy after fracture of the proximal end of the humerus. Comparison between two methods. Scandinavian Journal of Rehabilitation Medicine 1984;16(1):11-6. [PubMed] [Google Scholar]
Biermann 2020 {published data only}
- Biermann N, Schirren M, Siebenburger G, Fleischhacker E, Helfen T, Bocker W, et al. Glenohumeral joint lavage does not affect clinical outcomes in open reduction and internal fixation of displaced intracapsular proximal humeral fractures: a prospective, randomized, double-blinded trial. Journal of Shoulder and Elbow Surgery 2020;29(9):1758-64. [DOI] [PubMed] [Google Scholar]
- DRKS00017561. The effect of glenohumeral joint lavage in open reduction and internal fixation of displaced proximal humeral fractures (Version 1). who.int/trialsearch/Trial2.aspx?TrialID=DRKS00017561 (first posted 3 July 2019).
Boons 2012 {published data only}
- Boons H. Data for SST scores and group of withdrawn participants [personal communication]. Email to: H Handoll 12 March 2015.
- Boons HW, Goosen JH, Grinsven S, Susante JL, Loon CJ. Hemiarthroplasty for humeral four-part fractures for patients 65 years and older: a randomized controlled trial. Clinical Orthopaedics and Related Research 2012;(470):3483-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buecking 2014 {published data only}
- Buecking B, Mohr J, Bockmann B, Zettl R, Ruchholtz S. Deltoid-split or deltopectoral approaches for the treatment of displaced proximal humeral fractures? Clinical Orthopaedics and Related Research 2014;472(5):1576-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cai 2012 {published data only}
- Cai M, Tao K, Yang C, Li S. Internal fixation versus shoulder hemiarthroplasty for displaced 4-part proximal humeral fractures in elderly patients. Orthopedics 2012;35(9):e1340-6. [DOI] [PubMed] [Google Scholar]
Carbone 2017 {published data only}
- Carbone S, Razzano C, Albino P, Mezzoprete R. Immediate intensive mobilization compared with immediate conventional mobilization for the impacted osteoporotic conservatively treated proximal humeral fracture: a randomized controlled trial. Musculoskeletal Surgery 2017;101(Suppl 2):137-43. [DOI] [PubMed] [Google Scholar]
DelPhi 2020 {unpublished data only}
- Cole PA. A word of caution: success may be limited to 2 years and highly displaced OTA/AO B2 and C2 Injuries: commentary on an article by Alexander Nilsskog Fraser, MD, et al.: "Reverse Total Shoulder Arthroplasty Is Superior to Plate Fixation at 2 Years for Displaced Proximal Humeral Fractures in the Elderly. A Multicenter Randomized Controlled Trial". Journal of Bone and Joint Surgery. American Volume 2020;102(6):e30. [DOI] [PubMed] [Google Scholar]
- Fjalestad T, Iversen P, Hole MO, Smedsrud M, Madsen JE. Clinical investigation for displaced proximal humeral fractures in the elderly: a randomized study of two surgical treatments: reverse total prosthetic replacement versus angular stable plate Philos (The DELPHI-trial). BMC Musculoskeletal Disorders 2014;15(323):[13 p.]. [DOI: 10.1186/1471-2474-15-323] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjalestad T. Plan for trial [personal communication]. Email to: H Handoll 27 April 2012.
- Fraser AN, Bjørdal J, Wagle TM, Karlberg AC, Lien OA, Eilertsen L, et al. Reverse shoulder arthroplasty is superior to plate fixation at 2 years for displaced proximal humeral fractures in the elderly: a multicenter randomized controlled trial. Journal of Bone and Joint Surgery. American Volume 2020;102(6):477-85. [DOI: 10.2106/JBJS.19.01071] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01737060. Displaced proximal humeral fractures: Delta prothesis or Philos plate? (DELPHI) (version 10; submitted 10 September 2019). www.clinicaltrials.gov/show/NCT01737060 (first posted 29 November 2012).
- NCT01737060. Displaced proximal humeral fractures: Delta prothesis or Philos plate? (DELPHI) (version 4; submitted 3 October 1014). www.clinicaltrials.gov/show/NCT01737060 (first posted 29 November 2012).
Fialka 2008 {published data only}
- Fialka C, Stampfl P, Arbes S, Reuter P, Oberleitner G, Vecsei V. Primary hemiarthroplasty in four-part fractures of the proximal humerus: randomized trial of two different implant systems. Journal of Shoulder and Elbow Surgery 2008;17(2):210-5. [DOI] [PubMed] [Google Scholar]
Fjalestad 2010a {published and unpublished data}
- Fjalestad T, Hole M. Displaced proximal humeral fractures: operative versus non-operative treatment−a 2-year extension of a randomized controlled trial. European Journal of Orthopaedic Surgery & Traumatology : Orthopedie Traumatologie 2014;24(7):1067-73. [DOI] [PubMed] [Google Scholar]
- Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. Journal of Orthopaedic Trauma 2012;26(2):98-106. [DOI] [PubMed] [Google Scholar]
- Fjalestad T, Hole MO, Jorgensen JJ, Stromsoe K, Kristiansen IS. Health and cost consequences of surgical versus conservative treatment for a comminuted proximal humeral fracture in elderly patients. Injury 2010;41(6):599-605. [DOI] [PubMed] [Google Scholar]
- Fjalestad T, Stromsoe K, Blucher J, Tennoe B. Fractures in the proximal humerus: functional outcome and evaluation of 70 patients treated in hospital. Archives of Orthopaedic and Trauma Surgery 2005;125(5):310-6. [DOI] [PubMed] [Google Scholar]
- Fjalestad T. Details of intended fracture population [personal communication]. Email to: H Handoll 3 December 2006.
- Fjalestad T. Information on two year follow-up timing and clarification on the use of closed reduction [personal communication]. Email to: H Handoll 12 April 2010.
- Fjalestad T. Notice that two-year follow-up report had been submitted [personal communication]. Email to: H Handoll 27 April 2012.
- Kristiansen IS, Jorgensen JJ, Fjalestad T, Stromsoe K. Fracture of the proximal humerus. Randomised clinical study at the Aker University Hospital - economic evaluation [Fraktur i øvre humuserende. Randomisert klinisk studie ved Aker Universitetssykehus HF- Økonomisk evaluering]. University of Oslo website: www.hero.uio.no/prosjekter/prosjekt6.13.html (accessed 13 November 2006).
- NCT00863473. Comminuted proximal humeral fractures. A randomised study of surgical versus conservative treatment (Version 1). clinicaltrials.gov/ct2/show/record/NCT00863473 (first posted 18 March 2009).
Gracitelli 2016 {published data only}
- Gracitelli ME, Malavolta EA, Assuncao JH, Kojima KE, dos Reis PR, Silva JS, et al. Locking intramedullary nails compared with locking plates for two- and three-part proximal humeral surgical neck fractures: a randomized controlled trial. Journal of Shoulder and Elbow Surgery 2016;25(5):695-703. [DOI] [PubMed] [Google Scholar]
- Gracitelli ME, Malavolta EA, Assuncao JH, Kojima KE, Dos Reis PR, Silva JS, et al. Locking intramedullary nails compared with locking plates for two- and three-part proximal humeral surgical neck fractures: a randomized controlled trial (abstract). Journal of Shoulder and Elbow Surgery 2017;26(5):e149. [DOI] [PubMed] [Google Scholar]
- NCT01984112. Proximal humerus fractures: randomized study between locking nails and locking plates for Neer 2 and 3 parts (Version 1). clinicaltrials.gov/ct2/show/NCT01984112 (first posted 14 November 2013).
Helfen 2020 {published data only}
- Helfen T, Siebenburger G, Fleischhacker E, Gleich J, Bocker W, Ockert B. Operative treatment of 2-part surgical neck type fractures of the proximal humerus in the elderly: cement augmented locking plate PHILOS vs. proximal humerus nail multiloc. Injury 2020;51(10):2245-52. [DOI] [PubMed] [Google Scholar]
- Helfen T, Siebenburger G, Mayer M, Bocker W, Ockert B, Haasters F. Operative treatment of 2-part surgical neck fractures of the proximal humerus (AO 11-A3) in the elderly: cement augmented locking plate Philos vs. proximal humerus nail MultiLoc. BMC Musculoskeletal Disorders 2016;17(448):[7 p.]. [DOI: 10.1186/s12891-016-1302-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02609906. Operative treatment of 2-fragment-fractures (AO 11-A3) of the proximal humerus in the elderly: cement augmented locking plate Philos vs. proximal humerus nail MultiLoc (Version 1). clinicaltrials.gov/ct2/show/NCT02609906 (first posted 20 November 2015).
Hengg 2019 {published data only}
- DRKS00005261. A multicenter randomized controlled trial to investigate the treatment outcome of PHILOS Screw Augmentation compared to PHILOS without augmentation in older adult patients with proximal humerus fractures (Version 2). www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00005261 (first posted 15 January 2014).
- Hengg C, Nijs S, Klopfer T, Jaeger M, Platz A, Pohlemann T, et al. Cement augmentation of the proximal humerus internal locking system in elderly patients: a multicenter randomized controlled trial. Archives of Orthopaedic and Trauma Surgery 2019;139(7):927-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01847508. PHILOS augmented - a multicenter randomized controlled trial (Version 17). clinicaltrials.gov/show/NCT01847508 (first posted 7 May 2013).
- NCT01847508. PHILOS augmented - a multicenter randomized controlled trial (Version 22; 12 June 2019). clinicaltrials.gov/ct2/show/NCT01847508 (first posted 7 May 2013).
Hodgson 2003a {published and unpublished data}ISRCTN87732015
- Campbell M. Early versus late physiotherapy in fractured proximal neck of humerus: a randomised controlled study. In: National Research Register, Issue 3, 2000. Oxford: Update Software.
- Hodgson S, Stanley D. A randomised controlled trial investigating functional outcome, with early and late physiotherapy, on patients sustaining a fractured proximal humerus. In: British Elbow and Shoulder Society (BESS) Annual Scientific Meeting and Instructional Course; 2000 May 4-5; Nottingham (UK). 2000.
- Hodgson S, Stanley S, Mawson S. Timing of physiotherapy in management of fractured proximal humerus: randomised controlled trial (abstract). Physiotherapy 2002;88(12):763. [Google Scholar]
- Hodgson S, Stanley S, Mawson S. Timing of physiotherapy in the management of the fractured proximal humerus: a randomised controlled trial. In: Extending the boundaries. Chartered Society of Physiotherapy Congress 2001; 2001 Oct 19-21; Birmingham (UK). 2001.
- Hodgson SA, Mawson SJ, Saxton JM, Stanley D. Rehabilitation of two-part fractures of the neck of the humerus (two-year follow-up). Journal of Shoulder and Elbow Surgery 2007;16(2):143-5. [DOI] [PubMed] [Google Scholar]
- Hodgson SA, Mawson SJ, Stanley D. Rehabilitation after two-part fractures of the neck of the humerus. Journal of Bone and Joint Surgery. British Volume 2003;85(3):419-22. [DOI] [PubMed] [Google Scholar]
- Hodgson SA. A controlled, randomised study investigating functional outcome, with early and late physiotherapy, on patients following a fractured proximal humerus. In: National Research Register, Issue 3, 2000.
- Hodgson SA. Proximal humerus fracture rehabilitation. Clinical Orthopaedics and Related Research 2006;(442):131-8. [PubMed] [Google Scholar]
- Stanley D. A prospective, controlled, randomised study investigating functional outcome, with early and late physiotherapy, on patients following a fractured proximal humerus. In: National Research Register, Issue 2, 2001. Oxford: Update Software.
- Stanley D. A prospective, controlled randomised study investigating functional outcome, with early and late physiotherapy, on patients following a fractured proximal humerus. www.controlled-trials.com/mrct/trial/485287 (accessed 6 August 2010).
Hoellen 1997 {published data only}
- Bauer G, Hoellen I, Hohlbein O. Primary prosthetic humerus head replacement in dislocated multiple fragment fracture of the humerus head in over 60-year-olds [Der primare prothetische Humeruskopfersatz bei der dislozierten Humeruskopfmehrfragmentfraktur bei uber 60jahrigen]. Hefte zur der Unfallchirurg 1999;272:169-70. [Google Scholar]
- Hoellen IP, Bauer F, Holbein O. Primary endoprosthesis in comminuted fractures of the proximal humerus - an alternative treatment for elderly patients? [Der prothetische humeruskipfersatz bei der dislozierten humerusmehrfragmentfraktur des alten menschen - eine alternative zur minimalosteosynthese]. Zentralblatt fur Chirurgie 1997;122(11):994-1001. [PubMed] [Google Scholar]
- Hoellen IP, Holbein O, Bauer G. Prosthetic humerus head replacement in dislocated humerus head multiple fracture in older people: an alternative to minimal osteosynthesis? [Der prothetische Humeruskopfersatz bei der dislozierten Humeruskopfmehrfragmentfraktur des alten Menschen: Eine Alternative zur Minimalosteosynthese?]. Hefte zur der Unfallchirurg 1997;268:49-51. [PubMed] [Google Scholar]
- Holbein O, Bauer G, Hoellen I, Keppler P, Hehl G, Kinzl L. Is primary endoprosthetic replacement of the humeral head an alternative treatment for comminuted fractures of the proximal humerus in elderly patients? [Stellt die primare Implantation einer Humeruskopfprothese bei der dislozierten Humeruskopfmehrfragmentfraktur des alten Menschen eine Alternative zur Minimalosteosynthese dar?]. Osteosynthese International 1999;7 Suppl 2:207-10. [Google Scholar]
- Holbein O, Hehl G, Keppler P, Kinzl L. Treatment of proximal humerus multiple fracture in old and very old people [Die Behandlung der Mehrfragmentfraktur des proximalen Humerus bei alten und uralten Menschen]. Hefte zur der Unfallchirurg 2000;275:207-8. [Google Scholar]
- Pokar S, Holbein O, Kinzl L, Hehl G. Primary head of humerus prosthetic vs. minimal osteosynthesis in the treatment of head of humerus multiple-fragment fractures in older patients [Primare Humeruskopfprothese vs. Minimalosteosynthese in der Behandlung von Humeruskopfmehrfragmentfrakturen des alteren Patientenatients]. Hefte zur der Unfallchirurg 2001;283:375-6. [Google Scholar]
HURA 2020 {unpublished data only}
- NCT00612391. Lateral mini approach vs anterior approach for plating of proximal humerus fracture (HURA) (version 1). clinicaltrials.gov/show/NCT00612391 (first posted 11 February 2008).
- NCT00612391. Lateral mini approach vs anterior approach for plating of proximal humerus fracture (HURA) (version 11 updated 15 April 2019). clinicaltrials.gov/show/NCT00612391 (first posted 11 February 2008).
- NCT00612391. Lateral mini approach vs anterior approach for plating of proximal humerus fracture (HURA) (version 4 updated 19 August 2014). clinicaltrials.gov/show/NCT00612391 (first posted 11 February 2008).
- Rouleau D, Balg F, Benoit B, Leduc S, Malo M, Vezina F, et al. Delto-pectoral vs deltoid split approach for proximal humerus fracture fixation with locking plate: a prospective randomized study (HURA). Journal of Shoulder and Elbow Surgery 2020;29(4):e162. [DOI] [PubMed] [Google Scholar]
- Rouleau D, Balg F, Benoit B, Leduc S, Malo M, Vezina F, et al. Deltopectoral vs deltoid split approach for proximal humerus fracture fixation with locking plate: a prospective randomized study (HURA study). Journal of Shoulder and Elbow Surgery 2019;3(4):240. [DOI] [PubMed] [Google Scholar]
- Rouleau D, Laflamme GY, Balg F, Benoit B, Malo M, Vezina F, et al. Deltopectoral versus deltoid split approach for proximal humerus fracture fixation with locking plate: a prospective randomized study (HURA Study). In: Orthopaedic Trauma Association Annual Meeting, 2019 September 25-28; Denver (CO). 2019.
- Rouleau D. Delto-pectoral vs deltoid split approach for proximal humerus fracture fixation with locking plate: a prospective randomized study (HURA) (abstract). whenithurtstomove.org/wp-content/uploads/2019-Richards-Award_Rouleau_Summary.pdf (accessed 24 April 2020). [DOI] [PubMed]
- Rouleau DM, Balg F, Benoit B, Leduc S, Malo M, Vezina F, et al. Deltopectoral vs. deltoid split approach for proximal HUmerus fracture fixation with locking plate: a prospective RAndomized study (HURA). Journal of Shoulder and Elbow Surgery 2020;29(11):2190-9. [DOI: 10.1016/j.jse.2020.06.020] [DOI] [PubMed] [Google Scholar]
Jonsson 2020 {published data only}
- Jonsson EÖ, Ekholm C, Salomonsson B, Demir Y, Olerud P, Collaborators in the SAPF Study Group. Reverse total shoulder arthroplasty provides better shoulder function than hemiarthroplasty for displaced 3- and 4-part proximal humeral fractures in patients aged 70 years or older: a multicenter randomized controlled trial. Journal of Shoulder and Elbow Surgery 2021;30(5):994-1006. [DOI: 10.1016/j.jse.2020.10.037] [DOI] [PubMed] [Google Scholar]
- NCT03383991. Reverse total shoulder arthroplasty versus hemiarthroplasty for displaced 3- and 4-part proximal humeral fractures in patients older than 70 years. A multicenter randomized controlled trial (Version 1). clinicaltrials.gov/ct2/show/NCT03383991 (first posted 27 December 2017).
- Olerud P. Preproof trial report published [personal communication]. Email to: H Handoll 15 December 2020.
- Olerud P. Publication status of trial [personal communication]. Email to: H Handoll 14 August 2020.
Kristiansen 1988 {published data only}
- Kristiansen B, Kofoed H. Transcutaneous reduction and external fixation of displaced fractures of the proximal humerus. A controlled clinical trial. Journal of Bone and Joint Surgery. British Volume 1988;70(5):821-4. [DOI] [PubMed] [Google Scholar]
Kristiansen 1989 {published data only}
- Kristiansen B, Angermann P, Larsen TK. Functional results following fractures of the proximal humerus. A controlled clinical study comparing two periods of immobilization. Archives of Orthopaedic and Trauma Surgery 1989;108(6):339-41. [DOI] [PubMed] [Google Scholar]
Launonen 2019a {published data only}
- Launonen AP, Lepola V, Flinkkila T, Strandberg N, Ojanpera J, Rissanen P, et al. Conservative treatment, plate fixation, or prosthesis for proximal humeral fracture. A prospective randomized study. BMC Musculoskeletal Disorders 2012;13(167):[7 p.]. [DOI: 10.1186/1471-2474-13-167] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launonen AP, Sumrein BO, Reito A, Lepola V, Paloneva J, Jonsson KB, et al. Operative versus non-operative treatment for 2-part proximal humerus fracture: A multicenter randomized controlled trial. PLoS Medicine 2019;16(7):e1002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler W. Surgical treatment of displaced 2-fragment fractures of the proximal humerus in older patients is not superior to nonoperative treatment. Results of a prospective randomized multicenter study. Der Unfallchirurg 2020;123(6):501-4. [DOI] [PubMed] [Google Scholar]
- NCT01246167. Treatment of proximal humeral fractures (TPHF) (version 10 submitted 10 April 2019). clinicaltrials.gov/ct2/show/NCT01246167 (first posted 23 November 2010).
- NCT01246167. Treatment of proximal humeral fractures (TPHF) (version 2 submitted 1 February 2011). clinicaltrials.gov/show/NCT01246167 (first posted 23 November 2010).
Lefevre‐Colau 2007 {published and unpublished data}
- Lefevre-Colau MM, Babinet A, Fayad F, Fermanian J, Anract P, Roren A, et al. Immediate mobilization compared with conventional immobilization for the impacted nonoperatively treated proximal humeral fracture. A randomized controlled trial. Journal of Bone and Joint Surgery - American Volume 2007;89(12):2582-90. [DOI] [PubMed] [Google Scholar]
- NCT00326794. Efficacy of shoulder mobilisation versus conventional immobilisation for nonsurgically proximal humerus fracture (version 1). clinicaltrials.gov/show/NCT00326794 (first posted 17 May 2006).
Livesley 1992 {published data only}
- Livesley PJ, Mugglestone A, Whitton J. Electrotherapy and the management of minimally displaced fracture of the neck of the humerus. Injury 1992;23(5):323-7. [DOI] [PubMed] [Google Scholar]
Lopiz 2014 {published data only}
- Lopiz Y, Garcia-Coiradas J, Garcia-Fernandez C, Marco F. Proximal humerus nailing: a randomized clinical trial between curvilinear and straight nails. Journal of Shoulder & Elbow Surgery 2014;23(3):369-76. [DOI] [PubMed] [Google Scholar]
Lopiz 2019 {published data only}
- Lopiz Y, Alcobia-Diaz B, Galan-Olleros M, Garcia-Fernandez C, Picado A L, Marco F. Reverse shoulder arthroplasty versus nonoperative treatment for 3- or 4-part proximal humeral fractures in elderly patients: a prospective randomized controlled trial. Journal of Shoulder and Elbow Surgery. 2019;28(12):2259-71. [DOI] [PubMed] [Google Scholar]
Lundberg 1979 {published data only}
- Lundberg BJ, Svenungson-Hartvig E, Wikmark R. Independent exercises versus physiotherapy in nondisplaced proximal humeral fractures. Scandinavian Journal of Rehabilitation Medicine 1979;11(3):133-6. [PubMed] [Google Scholar]
Ockert 2010 {published data only}
- Biberthaler P, Braunstein V, Kirchoff C, Kroetz M, Kettler M, Mutschler W. Surgical therapy of humeral head fractures with a locking plate: analysis of 176 cases. Journal of Bone & Joint Surgery - British Volume 2009;91(Suppl 1):40. [Google Scholar]
- Ockert B, Braunstein V, Kirchhoff C, Korner M, Kirchhoff S, Kehr K, et al. Monoaxial versus polyaxial screw insertion in angular stable plate fixation of proximal humeral fractures: radiographic analysis of a prospective randomized study. Journal of Trauma 2010;69(6):1545-51. [DOI] [PubMed] [Google Scholar]
- Ockert B, Pedersen V, Geyer L, Wirth S, Mutschler W, Grote S. Position of polyaxial versus monoaxial screws in locked plating for proximal humeral fractures: analysis of a prospective randomized study. European Journal of Orthopaedic Surgery and Traumatology 2014;24(5):747-52. [DOI] [PubMed] [Google Scholar]
- Ockert B. Details of randomisation method and recruitment numbers [personal communication]. Email to: H Handoll 18 June 2012.
Olerud 2011a {published data only}
- Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: a randomized controlled trial. Journal of Shoulder & Elbow Surgery 2011;20(5):747-55. [DOI] [PubMed] [Google Scholar]
- Olerud P. Additional information on randomisation and trial location [personal communication]. Email to: H Handoll 19 April 2012.
- Olerud P. Pain data [personal communication]. Email to: H Handoll 22 May 2012.
Olerud 2011b {published data only}
- Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Hemiarthroplasty versus nonoperative treatment of displaced 4-part proximal humeral fractures in elderly patients: a randomized controlled trial. Journal of Shoulder & Elbow Surgery 2011;20(7):1025-33. [DOI] [PubMed] [Google Scholar]
- Olerud P. Additional information on randomisation and trial location [personal communication]. Email to: H Handoll 19 April 2012.
- Olerud P. Pain data [personal communication]. Email to: H Handoll 22 May 2012.
- Zuckerman JD. Hemiarthroplasty improved health-related quality of life more than nonoperative treatment in older patients with four-part proximal humeral fractures: commentary. Journal of Bone and Joint Surgery - American Volume 2012;94(10):942. [DOI] [PubMed] [Google Scholar]
Plath 2019 {published data only}
- DRKS00015245. Locking nail versus locking plate for proximal humeral fracture fixation in an elderly population: a prospective randomised controlled trial (version 1). drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00015245 (first posted 22 August 2018).
- Plath J, Kerschbaum C, Seebauer T, Holz R, Henderson DJH, Forch S, et al. Locking nail versus locking plate for proximal humeral fracture fixation in an elderly population: a prospective randomised controlled trial. BMC Musculoskeletal Disorders 2019;20(20):[13 p.]. [DOI: 10.1186/s12891-019-2399-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath J. Data for DASH and Constant scores [personal communication]. Email to: H Handoll 2 May 2020.
ProFHER 2015 {published data only}ISRCTN50850043
- Corbacho B, Duarte A, Keding A, Handoll H, Chuang LH, Torgerson D, et al. Cost effectiveness of surgical versus non-surgical treatment of adults with displaced fractures of the proximal humerus: economic evaluation alongside the PROFHER trial. Bone & Joint Journal 2016;98-B(2):152-9. [DOI] [PubMed] [Google Scholar]
- Handoll H, Brealey S, Rangan A, Keding A, Corbacho B, Jefferson L, et al. The ProFHER (PROximal Fracture of the Humerus: Evaluation by Randomisation) trial – a pragmatic multicentre randomised controlled trial evaluating the clinical effectiveness and cost-effectiveness of surgical compared with non-surgical treatment for proximal fracture of the humerus in adults. Health Technology Assessment 2015;19(24):1-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handoll H, Brealey S, Rangan A, Torgerson D, Dennis L, Armstrong A, et al. Protocol for the ProFHER (PROximal Fracture of the Humerus: Evaluation by Randomisation) trial: a pragmatic multi-centre randomised controlled trial of surgical versus non-surgical treatment for proximal fracture of the humerus in adults. BMC Musculoskeletal Disorders 2009;10(140):[11 p.]. [DOI: 10.1186/1471-2474-10-140] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handoll HH, Brealey SD, Jefferson L, Keding A, Brooksbank AJ, Johnstone AJ, et al. Defining the fracture population in a pragmatic multicentre randomised controlled trial: PROFHER and the Neer classification of proximal humeral fractures. Bone and Joint Research 2016;5(10):481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handoll HH, Goodchild L, Brealey SD, Hanchard NC, Jefferson L, Keding A, et al. Developing, delivering and documenting rehabilitation in a multi-centre randomised controlled surgical trial: experiences from the ProFHER trial. Bone & Joint Research 2014;3(12):335-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handoll HH, Keding A, Corbacho B, Brealey SD, Hewitt C, Rangan A. Five-year follow-up results of the PROFHER trial comparing operative and non-operative treatment of adults with a displaced fracture of the proximal humerus. Bone & Joint Journal 2017;99-B(3):383-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN50850043. Multi-centre randomised trial evaluating surgery for displaced fractures of the proximal humerus (version 1). www.controlled-trials.com/ISRCTN50850043 (first assigned 25 March 2008).
- Keding A, Handoll H, Brealey S, Jefferson L, Hewitt C, Corbacho B, et al. The impact of surgeon and patient treatment preferences in an orthopaedic trauma surgery trial. Trials 2019;20(570):[12 p.]. [DOI: 10.1186/s13063-019-3631-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JG, Brealey S, Keding A, Torgerson D, Rangan A. Does time to surgery affect patient-reported outcome in proximal humeral fractures? A subanalysis of the PROFHER randomized clinical trial. Bone and Joint Journal 2020;102-B(1):33-41. [DOI] [PubMed] [Google Scholar]
- Norman JG, Keding A, Brealey S, Torgerson D, Rangan A. Time to surgery: effect on patient-reported outcomes in proximal humeral fractures. A sub-analysis of the PROFHER trial (abstract). Shoulder & Elbow 2018;10(3S):S9. [Google Scholar]
- Rangan A, Brealey S, Handoll H, Keding A, Jefferson L, Corbacho-Martin B, et al. Proximal Fracture of the Humerus: Evaluation by Randomization (ProFHER) Trial (abstract). In: Orthopaedic Trauma Association’s 31st Annual Meeting; 2015 October 7-11; San Diego (CA). OTA, 2015.
- Rangan A, Handoll H, Brealey S, Jefferson L, Keding A, Corbacho Martin B, et al. Surgical vs nonsurgical treatment of adults with displaced fractures of the proximal humerus: the PROFHER randomized clinical trial. JAMA 2015;313(10):1037-47. [DOI] [PubMed] [Google Scholar]
- Rangan A, Handoll H, Brealey S, Jefferson L, Keding A, Martin BC, et al. Surgical and nonsurgical treatment produced similar outcomes for proximal humeral fractures. Journal of Bone and Joint Surgery. American Volume 2015;97(22):1890. [DOI] [PubMed] [Google Scholar]
Revay 1992 {published data only}
- Revay S, Dahlstrom M, Dalen N. Water exercise versus instruction for self-training following a shoulder fracture. International Journal of Rehabilitation Research 1992;15(4):327-33. [DOI] [PubMed] [Google Scholar]
Ring 2019 {unpublished data only}
- Chen N. Intention to seek details on method of treatment allocation from David Ring [personal communication]. Email to: H Handoll 18 June 2020.
- Chen N. Method of treatment allocation [personal communication]. Email to: H Handoll 25 June 2020.
- NCT00438633. Comparison of early and late therapy for adults with non-operatively treated proximal humerus fractures (version 11; record updated 21 June 2019). clinicaltrials.gov/show/NCT00438633 (first posted 22 February 2007).
- NCT00438633. Comparison of early and late therapy for adults with non-operatively treated proximal humerus fractures (version 4). clinicaltrials.gov/show/NCT00438633 (first posted 22 February 2007).
- NCT00438633. Comparison of early and late therapy for adults with non-operatively treated proximal humerus fractures (version 8; record updated 6 April 2015). clinicaltrials.gov/show/NCT00438633 (first posted 22 February 2007).
- Ring D. Trial status [personal communication]. Email to: H Handoll 16 April 2012.
Rommens 1993 {published data only}
- Deldycke J, Rommens PM, Heyvaert G, Broos PL. Conservative treatment of sub-capital humerus fractures. A comparative study between the classical Desault-bandage and the new Gilchrist-bandage [Die konservative Behandlung von subkapitalen Humerusfrakturen. Eine vergleichende Studie zwischen dem klassischen Desault-Verband und der neuen Gilchrist-Bandage]. Hefte zur der Unfallchirurg 1993;232:145-6. [DOI] [PubMed] [Google Scholar]
- Rommens PM, Heyvaert G. Conservative treatment of subcapital humerus fractures. A comparative study of the classical Desault bandage and the new Gilchrist bandage [Die konservative behandlung subkapitaler humerusfrakturen. Eine vergleichende studie zwischen dem klassischen Desault-verband und der neuen Gilchrist-bandage]. Unfallchirurgie 1993;19(2):114-8. [DOI] [PubMed] [Google Scholar]
Sebastiá‐Forcada 2014 {published data only}
- Gulotta LV. Reverse shoulder arthroplasty provided better functional outcomes than hemiarthroplasty for acute proximal humeral fractures (commentary). Journal of Bone and Joint Surgery. American volume 2015;97(10):861. [DOI: 10.2106/JBJS.9710.ebo103] [DOI] [PubMed] [Google Scholar]
- Sebastiá-Forcada E, Cebrián-Gómez R, Lizaur-Utrilla A, Gil-Guillén V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. Journal of Shoulder and Elbow Surgery 2014;23(10):1419-26. [DOI] [PubMed] [Google Scholar]
Smejkal 2011 {published data only}
- Smejkal K, Didek T, Zvak I, Trlica J, Folvarsky J. Operation treatment of 2-3 fragments proximal humeral fractures using Zifko versus Philos [abstract]. European Journal of Trauma & Emergency Surgery 2008;34(Suppl 1):44-5. [Google Scholar]
- Smejkal K, Lochman P, Dedek T, Trlica J, Koci J, Zvak I. Surgical treatment for proximal humerus fracture [Operacni lecba zlomenin proximalniho humeru]. Acta Chirurgiae Orthopaedicae et Traumatologiae Cechoslovaca 2011;78(4):321-7. [PubMed] [Google Scholar]
Sohn 2017 {published data only}
- Sohn HS, Jeon YS, Lee J, Shin SJ. Clinical comparison between open plating and minimally invasive plate osteosynthesis for displaced proximal humeral fractures: a prospective randomized controlled trial. Injury 2017;48(6):1175-82. [DOI] [PubMed] [Google Scholar]
Soliman 2013 {published data only}
- PACTR201205000381245. Proximal humeral fractures treated with hemiarthroplasty. Does tendodesis of the long head of the biceps improve results? apps.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201205000381245 (registered 10 May 2012).
- Soliman O. Comment on when 8 patients were excluded [personal communication]. Email to: H Handoll 6 May 2015.
- Soliman O. Information on implant manufacturer, and complications and reoperations [personal communication]. Email to: H Handoll 3 May 2015.
- Soliman OA, Koptan WMT. Proximal humeral fractures treated with hemiarthroplasty: does tenodesis of the long head of the biceps improve results? Injury 2013;44(4):461-4. [DOI] [PubMed] [Google Scholar]
Stableforth 1984 {published data only}
- Stableforth PG. Four-part fractures of the neck of the humerus. Journal of Bone and Joint Surgery. British Volume 1984;66(1):104-8. [DOI] [PubMed] [Google Scholar]
Torrens 2012 {published data only}
- Torrens C, Perez FS, Rigol P, Puig L. Management of conservatively treated proximal humeral fractures: prospective randomized study [abstract]. American Academy of Orthopaedic Surgeons. 2012 Mar 7-11; San Francisco (CA). www.aaos.org/education/anmeet/prevmeet.asp (accessed 26 February 2013) 2012.
- Torrens C. Additional trial data and information [personal communication]. Email to: H Handoll 10 June 2015.
- Torrens C. Clarification of study numbers [personal communication]. Email to: H Handoll 4 June 2015.
Tousignant 2020 {published data only}
- Cabana F, Page C, Svotelis A, Langlois-Michaud S, Tousignant M. Is an in-home telerehabilitation program for people with proximal humerus fracture as effective as a conventional face-to-face rehabilitation program? A study protocol for a noninferiority randomized clinical trial. BMC Sports Science, Medicine and Rehabilitation 2016;8(27):[8 p.]. [DOI: 10.1186/s13102-016-0051-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02425267. Effectiveness of a home telerehabilitation program for people with proximal humerus fracture (version 5, 23 May 2019). clinicaltrials.gov/show/NCT02425267 (first received 20 April 2015).
- Page C. Publication of full report [personal communication]. Email to: H Handoll 3 February 2020.
- Tousignant M, Cabana F, Langlois-Michaud S, Brière S, Pagé C. In-home telerehabilitation for proximal humerus fractures compared to conventional rehabilitation: a randomized trial. Acta Scientific Medical Sciences 2020;4(2):1-6. [Google Scholar]
Voigt 2011 {published data only}
- Voigt C, Geisler A, Hepp P, Schulz AP, Lill H. Are polyaxially locked screws advantageous in the plate osteosynthesis of proximal humeral fractures in the elderly? A prospective randomized clinical observational study. Journal of Orthopaedic Trauma 2011;25(10):596-602. [DOI] [PubMed] [Google Scholar]
- Voigt C. Additional information and clarification of those not completing follow-up and gender data [personal communication]. Email to: H Handoll 30 May 2012.
Wirbel 1999 {published data only}
- Wirbel R, Knorr V, Saur B, Duhr B, Mutschler W. Minimally invasive fixation of displaced proximal humeral fractures. Orthopaedics and Traumatology 1999;7(1):44-53. [Google Scholar]
- Wirbel RJ, Knorr V, Mutschler W. Minimal invasive therapy in dislocated proximal humerus fractures. Influence of post-operative immobilisation on the function outcome [Minimal invasive Therapie bei dislozierten proximalen Humerusfrakturen. Einfluss der postoperativen Immobilisation auf das funktionelle Ergebnis]. Hefte zur der Unfallchirurg 1997;268:678-81. [Google Scholar]
Zhang 2011 {published data only}
- Zhang D. [personal communication]. Email to: H Handoll 14 May 2012.
- Zhang L, Zheng J, Wang W, Lin G, Huang Y, Zheng J, et al. The clinical benefit of medial support screws in locking plating of proximal humerus fractures: a prospective randomized study. International Orthopaedics 2011;35(11):1655-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2019 {published data only}
- Zhang J, Zhou SJ, Cai F, Ma Y. Efficacy of proximal humeral internal locking system combined with allogeneic femoral head bone grafts for complex osteoporotic proximal humeral fractures. International Journal of Clinical and Experimental Medicine 2019;12(7):8847-54. [Google Scholar]
Zhu 2011 {published data only}
- Zhu Y, Lu Y, Shen J, Zhang J, Jiang C. Locking intramedullary nails and locking plates in the treatment of two-part proximal humeral surgical neck fractures: a prospective randomized trial with a minimum of three years of follow-up. Journal of Bone and Joint Surgery. American Volume 2011;93(2):159-68. [DOI] [PubMed] [Google Scholar]
Ziegler 2019 {published data only}
- DRKS00011376. PEEK (Polyetheretherketon) power plate for the treatment of proximal humerus fractures: a prospective study and comparison to fixation with a conventional locking plate (version 3 submitted 13 August 2018). www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00011376 (first posted 25 November 2016).
- Ziegler P, Maier S, Stöckle U, Gühring M, Stuby FM. The treatment of proximal humerus fracture using internal fixation with fixed-angle plates. Deutsches Ärzteblatt International 2019;116(45):757-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zyto 1997 {published data only}
- Karladani HA. Treatment of displaced proximal humeral fractures in elderly patients [Letter and reply]. Journal of Bone and Joint Surgery. British Volume 1999;81(1):181-2. [PubMed] [Google Scholar]
- Tornkvist H, Ahrengart L, Sperber A. Tension band wiring vs. non-operative treatment of displaced proximal humerus fractures. A prospective randomized study [abstract]. Orthopaedic Transactions 1997;21(2):592. [Google Scholar]
- Tornkvist H, Ahrengart L, Sperber A. Tension band wiring vs. nonoperative treatment of proximal humerus fractures - a prospective randomized study [Abstract]. Acta Orthopaedica Scandinavica. Supplementum 1995;66(265):40-1. [Google Scholar]
- Zyto K, Ahrengart L, Sperber A, Tornkvist H. Treatment of displaced proximal humeral fractures in elderly patients. Journal of Bone and Joint Surgery. British Volume 1997;79(3):412-7. [DOI] [PubMed] [Google Scholar]
- Zyto K. Explanation of reasons for excluding participants [personal communication]. Letter to: H Handoll 21 March 1997.
References to studies excluded from this review
ACTRN12610000730000 {unpublished data only}
- ACTRN12610000730000. Minimally invasive versus standard open reduction of proximal humerus fractures. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=335913 (first posted 1 September 2010).
Arias‐Buria 2015 {published data only}
- Arias-Buria JL, Valero-Alcaide R, Cleland JA, Salom-Moreno J, Ortega-Santiago R, Atin-Arratibel MA, et al. Inclusion of trigger point dry needling in a multimodal physical therapy program for postoperative shoulder pain: a randomized clinical trial. Journal of Manipulative and Physiological Therapeutics 2015;38(3):179-87. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Arias-Buria Jl, Valero-Alcaide R, Cleland JA, Salom-Moreno J, Ortega-Santiago R, Atin-Arratibel MA. Inclusion of trigger point dry-needling in a multimodal physical therapy program for postoperative shoulder pain: a randomised controlled trial. New Zealand Journal of Physiotherapy 2016;44(2):113. [DOI] [PubMed] [Google Scholar]
- NCT02122315. Dry needling in post-operative shoulder pain. www.clinicaltrials.gov/ct2/show/NCT02122315 (first posted 14 April 2014).
- Twaddle R. Inclusion of trigger point dry-needling in a multimodal physical therapy program for postoperative shoulder pain: a randomised controlled trial. New Zealand Journal of Physiotherapy 2016;44(2):113. [Google Scholar]
Avci 2013 {published data only}
- Avci CC, Gulabi D, Saglam N, Kurtulmus T, Saka G. Measurement of screw length through drilling technique in osteosynthesis of the proximal humerus fractures. Eklem Hastalik Cerrahisi [Joint Disease Surgery] 2013;24(3):156-62. [DOI: 10.5606/ehc.2013.34] [DOI] [PubMed] [Google Scholar]
Bai 2014 {published data only}
- Bai LC, Gu S, Xiong Y, Liu BL, Zhao F, Wang DX. Optimal position of locking compression plate for proximal humeral fractures: choice of lateral deltoid splitting approach? Chinese Journal of Tissue Engineering Research 2014;18(9):1453-8. [DOI: 10.3969/j.issn.2095-4344.2014.09.024] [DOI] [Google Scholar]
Bai 2015 {published data only}
- Bai JZ, Hou B, Shi HF, Yang W, Xu G, Liang CG. Prognosis of locking plate versus ordinary steel plate fixation for proximal humeral fractures. Chinese Journal of Tissue Engineering Research 2015;19(26):4213-7. [DOI: 10.3969/j.issn.2095-4344.2015.26.022] [DOI] [Google Scholar]
Baudi 2014 {published data only}
- Baudi P, Campochiaro G, Serafini F, Gazzotti G, Matino G, Rovesta C, et al. Hemiarthroplasty versus reverse shoulder arthroplasty: comparative study of functional and radiological outcomes in the treatment of acute proximal humerus fracture. Musculoskeletal Surgery 2014;98(Suppl 1):19-25. [DOI: 10.1007/s12306-014-0322-3] [DOI] [PubMed] [Google Scholar]
Bing 2002 {unpublished data only}
- Armstrong A. [personal communication]. Email to: H Handoll 17 July 2010.
- Bing AJ, Eastwood G, Sharma R, Cross R, Taylor GS, Harper WM. A randomised prospective trial comparing Polarus nail and Rush pins for fixation of proximal humeral fractures - Poster A5. In: British Orthopaedic Association Annual Congress; 2002 Sept 18-20; Cardiff (UK). London (UK): British Orthopaedic Association, 2002:2.
- Sharma R. A prospective, randomised clinical trial to compare Rush pins fixation with Polaris nail fixation of displaced two part fractures of the proximal humerus. In: National Research Register, Issue 2, 2000. Oxford: Update Software.
Bolano 1995 {published data only}
- Bolano LE. Operative treatment of humeral shaft fractures: a prospective, randomized study of intramedullary nailing versus dynamic compression plating [abstract]. Orthopaedic Transactions 1995;19(1):33. [Google Scholar]
Brownson 2001 {unpublished data only}
- Brownson P. A prospective randomised trial comparing conservative treatment and the Halder Humeral Nail for displaced fractures of the surgical neck and shaft of the humerus. In: National Research Register, Issue 2, 2000. Oxford: Update Software.
Caforio 2017 {published data only}
- Caforio M, Maniscalco P. The importance of early rehabilitation in proximal humeral fracture: a clinical trial of efficacy and safety of a new endomedullary nail. Journal of Back and Musculoskeletal Rehabilitation 2017;30(2):195-202. [DOI: 10.3233/BMR-160732] [DOI] [PubMed] [Google Scholar]
Caliskan 2019 {published data only}
- Caliskan E, Dogan O. PHILOS plate versus nonoperative treatment in 2-, 3-, and 4-part proximal humeral fractures: comparison with healthy control subjects. Journal of Orthopaedic Surgery (Hong Kong) 2019;27(3):[7 p.]. [DOI: 10.1177/2309499019875169] [DOI] [PubMed] [Google Scholar]
Carbone 2012 {published data only}
- Carbone S, Tangari M, Gumina S, Postacchini R, Campi A, Postacchini F. Percutaneous pinning of three- or four-part fractures of the proximal humerus in elderly patients in poor general condition: MIROS® versus traditional pinning. International Orthopaedics 2012;36(6):1267-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman 1997 {published data only}
- Chapman J, Weber TG, Henley B, Benca PJ. Randomized prospective study of humerus fixation: nails vs. plates [abstract]. Orthopaedic Transactions 1997;21(2):594. [Google Scholar]
- Chapman J, Weber TG, Henley MB, Benca P. Randomized prospective study of humerus fixation: nails vs. plates [abstract]. Orthopaedic Transactions 1996;20(1):10. [Google Scholar]
Chen 2014 {published data only}
- Chen Q, Ji L, Pan Z, Zhou X, Zhu J, Cao Z, et al. Treating Neer two- and three-part proximal humeral fractures through anterolateral acromial approach and deltopectoral approach. Zhongguo Gu Shang [China Journal of Orthopaedics and Traumatology] 2014;27(12):991-4. [PubMed] [Google Scholar]
Chen 2019 {published data only}
- Chen X, Yu ZX, Wang HY, Shen F, Lin GB, Wang S, et al. Proximal humeral internal locking plate combined with a custom neutral-position shoulder and elbow sling for proximal humerus fractures: a randomized study. Medicine 2019;98(17):e15271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen H 2018 {published data only}
- Chen H, Yin P, Wang S, Li J, Zhang L, Khan K, et al. The augment of the stability in locking compression plate with intramedullary fibular allograft for proximal humerus fractures in elderly people. BioMed Research International 2018;Sep 16:3130625. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen JL 2018 {published data only}
- Chen JL, Chang FC, Lin SJ, Chuang PY, Peng KT, Huang KC, et al. The outcome of surgical management of proximal humeral fractures using locking plates: comparison between locking plates with different geometry. Journal of Shoulder and Elbow Surgery 2018;27(12):2159-66. [DOI: 10.1016/j.jse.2018.05.033] [DOI] [PubMed] [Google Scholar]
ChiCTR1900025412 {published data only}
- ChiCTR1900025412. Different interventions treating 3- or 4-part proximal humeral fracture in elderly patient: a prospective study. who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1900025412 (first posted 26 August 2019).
ChiCTR‐TRC‐14004213 {published data only}
- ChiCTR-TRC-14004213. Immediate or delayed passive motion after operation of old fractures of greater tuberosity. who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-TRC-14004213 (first posted 19 January 2014).
Chiu 1997 {published data only}
- Chiu FY, Chen CM, Lin CF, Lo WH, Huang YL, Chen TH. Closed humeral shaft fractures: a prospective evaluation of surgical treatment. Journal of Trauma 1997;43(6):947-51. [DOI] [PubMed] [Google Scholar]
Cigni 2012 {published data only}
- Cigni S, Battista E, Chessa A, Pietrogrande L. Stability plate surgery in proximal humerus fractures: deltoidpectoral approach versus direct lateral transdeltoid approach [abstract]. Journal of Orthopaedics and Traumatology 2012;13(Suppl 1):S20. [Google Scholar]
De Boer 2003 {unpublished data only}
- Booth C. Study status [personal communication]. Contact with H Handoll 20 March 2003.
- De Boer P. Plating of proximal humerus fracture: a blind comparative study. In: National Research Register, Issue 1, 2003. Oxford: Update Software.
- De Boer P. Study design [personal communication]. Contact with H Handoll 12 March 2003.
Dias 2001 {unpublished data only}
- Armstrong A. Study stopped [personal communication]. Email to: H Handoll 17 July 2010.
- Der Tavitian J. The management of comminuted proximal humeral fractured, a randomised prospective trial. In: National Research Register, Issue 3, 2006. Oxford; Update Software.
- Dias J. Study status [personal communication]. Contact with H Handoll 16 November 2001.
Edelson 2008 {published data only}
- Edelson G, Safuri H, Salami J, Vigder F, Militianu D. Natural history of complex fractures of the proximal humerus using a three-dimensional classification system. Journal of Shoulder & Elbow Surgery 2008;17(3):399-409. [DOI] [PubMed] [Google Scholar]
Elidrissi 2013 {published data only}
- Elidrissi M, Bensaad S, Shimi M, Elibrahimi A, Elmrini A. Surgical treatment of proximal humeral fractures anatomical plate versus palm tree pinning (26 cases) [Le traitement chirurgical des fractures de l'extrémité supérieure de l'humérus: plaque anatomique versus embrochage en palmier, à propos de 26 cas]. Chirurgie de la Main. 2013;32(1):25-9. [DOI] [PubMed] [Google Scholar]
Erdoğan 2014 {published data only}
- Erdoğan M, Desteli EE, İmren Y, Üztürk A, Kılıç M, Sezgin H. The effect of inferomedial screw on postoperative shoulder function and mechanical alignment in proximal humerus fractures. European Journal of Orthopaedic Surgery & Traumatology 2014;24(7):1055-9. [DOI] [PubMed] [Google Scholar]
EUCTR 2015‐001820‐51 {published data only}
- EUCTR2015-001820-51-DE. BMC2012, Cell based therapy by implanted bone marrow-derived mononuclear cells (BMC) for bone augmentation of plate-stabilized proximal humeral fractures - a randomized, open, multicentric study - phase IIa [BMC2012, Zellbasierte Therapie mit bone marrow-derived mononuclear cells (BMC) zur Knochenaugmentation bei der Osteosynthese proximaler Humerusfrakturen - eine randomisierte, offene, multizentrische Phase IIa Studie]. clinicaltrialsregister.eu/ctr-search/trial/2015-001820-51/DE (first posted 15 October 2015).
- NCT02803177. Cell therapy by autologous BMC for large bone defect repair (BMC2012) (Version 7). clinicaltrials.gov/ct2/show/NCT02803177 (first posted 16 June 2016).
Fan 2012 {published data only}
- Fan Y, Wang S, Luo Y. Effectiveness comparison of operative and non-operative treatment for complex proximal humeral fractures in elderly patients. Chung-Kuo Hsiu Fu Chung Chien Wai Ko Tsa Chih [Chinese Journal of Reparative & Reconstructive Surgery] 2012;26(9):1029-32. [PubMed] [Google Scholar]
Flannery 2006 {unpublished data only}
- Flannery M. A prospective randomised trial for the treatment of four part fractures of proximal humerus: "conservative vs hemiarthroplasty". In: National Research Register, Issue 3, 2006. Oxford; Update Software.
- Flannery M. No recruitment at centre [personal communication]. Email to: H Handoll 16 November 2006.
- Turner R. Study stopped [personal communication]. Contact with H Handoll 19 November 2006.
Ge 2017 {published data only}
- Ge W, Sun Q, Li G, Lu G, Cai M, Li SH. Efficacy comparison of intramedullary nails, locking plates and conservative treatment for displaced proximal humeral fractures in the elderly. Clinical Interventions in Aging 2017;12:2047-54. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ge 2019 {published data only}
- Ge HQ, Bai YY, Tang BB, Guan H. Floating needle therapy for postoperative functional rehabilitation in patients with surgical neck fracture of humerus: a randomized controlled trial. Zhongguo Zhen Jiu [Chinese Acupuncture And Moxibustion] 2019;39(5):473-6. [DOI: 10.13703/j.0255-2930.2019.05.004] [DOI] [PubMed] [Google Scholar]
Gradl 2009 {published data only}
- Gradl G, Dietze A, Kaab M, Hopfenmuller W, Mittlmeier T. Is locking nailing of humeral head fractures superior to locking plate fixation? Clinical Orthopaedics and Related Research 2009;467(11):2986-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hems 2000 {unpublished data only}
- Hems T. A prospective randomised trial comparing conservative treatment and the Halder Humeral Nail for displaced fractures of the surgical neck and shaft of the humerus. In: National Research Register, Issue 3, 2000. Oxford: Update Software.
HOMERUS {unpublished data only}
- Diercks RL. Trial status [personal communication]. Email to: H Handoll 13 June 2020.
- NTR2354. Hemiarthroplasty versus osteosynthesis in humeral fractures (HOMERUS): a multicenter randomized trial (accessed 12 June 2020) (renumbered). www.trialregister.nl/trial/2354 (registered 10 August 2010).
- NTR2461. Hemiarthroplasty versus osteosynthesis in humeral fractures (HOMERUS): a multicenter randomized trial (accessed 10 April 2012). www.trialregister.nl/trialreg/admin/rctview.asp?TC=2461 (registered 10 August 2010).
- Verbeek PA, Van den Akker-Scheek I, Wendt KW, Diercks RL. Hemiarthroplasty versus angle-stable locking compression plate osteosynthesis in the treatment of three- and four-part fractures of the proximal humerus in the elderly: design of a randomized controlled trial. BMC Musculoskeletal Disorders 2012;13(16):[7 p.]. [DOI: 10.1186/1471-2474-13-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT2013052313435N1 {unpublished data only}
- IRCT2013052313435N1. Comparing review of the treatment results based on surgical or nonsurgical methods in the patients with fracture of proximal humerus referred to Al-Zahra and Kashani Hospitals (Isfahan) during 1388 -1392 (years). apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2013052313435N1 (registered 8 August 2013).
- Nouraei MH, Majd DA, Zamani F. Comparing the treatment results of proximal humerus fracture based on surgical or nonsurgical methods. Advanced Biomedical Research 2014;3(253):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN32335957 {published data only}
- ISRCTN32335957. Prospective randomised study of reverse shoulder prosthesis and hemiarthroplasty for elderly patients with proximal humeral fractures. www.controlled-trials.com/ISRCTN32335957 (first registered 14 March 2007).
- Smith M. Study closed [personal communication]. Email to: H Handoll 27 April 2012.
Jin 2016 {published data only}
- Jin L, Guo J, Yin Y, Hou Z, Zhang Y. Clinical effects of the probing method with depth gauge for determining the screw depth of locking proximal humeral plate. Biomed Research International 2016;2016:5898161 PMC-5126400. [DOI: 10.1155/2016/5898161] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2016 {published data only}
- Kim J-W, Oh C-W, Oh J-K, Park K-H. Is minimally invasive plate osteosynthesis helpful in the fixation of 2-part proximal humerus fractures compared to open plating? (abstract). In: Orthopaedic Trauma Association Annual Meeting, 2016 October 5–8; National Harbor (MD). 2016.
Kim 2018 {published data only}
- Kim DS, Lee DH, Chun YM, Shin SJ. Which additional augmented fixation procedure decreases surgical failure after proximal humeral fracture with medial comminution: fibular allograft or inferomedial screws? Journal of Shoulder and Elbow Surgery 2018;27(10):1852-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Kulkarni 2000 {unpublished data only}
- Kulkarni R. Welsh proximal humeral fracture project. In: National Research Register, Issue 2, 2000. Oxford: Update Software.
Li 2015 {published data only}
- Li D, Zhang GW, Liu JB. Minimally invasive and open reduction plate fixation for proximal humerus fractures: range of motion of the shoulder joint. Chinese Journal of Tissue Engineering Research 2015;19(39):6355-9. [DOI: 10.3969/j.issn.2095-4344.2015.39.022] [DOI] [Google Scholar]
Liao 2009 {published data only}
- Liao C, Wang P, Xie Y, Fan T, Li P, Liang W. Different surgical methods for treatment of senile osteoporotic comminuted proximal humerus fracture. Zhongguo Xiufu Chongjian Waike Zazhi [Chinese Journal of Reparative and Reconstructive Surgery] 2009;23(12):1443-6. [PubMed] [Google Scholar]
Maniscalco 2014a {published data only}
- Maniscalco P, Caforio M. The importance of early rehabilitation in proximal humeral fracture: a randomized controlled clinical trial of efficacy and safety of endomedullary Diphos nail [abstract]. Journal of Orthopaedics and Traumatology 2014;15(Suppl 1):S32-3. [Google Scholar]
Martetschlager 2012 {published data only}
- Martetschlager F, Siebenlist S, Weier M, Sandmann G, Ahrens P, Braun K, et al. Plating of proximal humeral fractures. Orthopedics 2012;35(11):e1606-12. [DOI] [PubMed] [Google Scholar]
Martin 2000 {published data only}
- Heath C. The effect of interfential current on pain following proximal fracture of the humerus. A single blind, randomised, controlled clinical trial. In: National Research Register, Issue 4, 2001. Oxford: Update Software.
- Martin D, Palmer S, Heath C. Interferential current as an adjunct to exercise and mobilisation in the treatment of proximal humerus fracture pain: lack of evidence of an additional effect [Abstract]. Physiotherapy 2000;86(3):147. [Google Scholar]
- Palmer S. Compromised trial will not be reported [personal communication]. Email to: H Handoll 9 May 2003.
Mechlenburg 2009 {unpublished data only}
- Mechlenburg IB. Trial abandoned [personal communication]. Email to: H Handoll 25 May 2010.
- NCT00408291. Comparison of the Philos plate and cemented hemiprosthesis (Bigliani) in the treatment of three- and four-part fractures of the proximal humerus: a prospective, randomised migration and bone density study (Version 6). clinicaltrials.gov/show/NCT00408291 (first posted 6 December 2006).
- NCT00408291. Evaluation of the Winsta PH osteosynthesis device in the treatment of three- and four-part fractures of the proximal humerus: a prospective migration and bone density study (Version 8). clinicaltrials.gov/show/NCT00408291 (first posted 6 December 2006).
NCT00205959 {published data only}
- NCT00205959. LPHP-Philos-PHN conservative treatment (version 2; record updated 6 September 2006). clinicaltrials.gov/ct2/show/NCT00205959 (first posted 21 September 2005).
NCT00384852 {unpublished data only}
- Lo K. Study status [personal communication]. Email to: H Handoll 12 February 2015.
- NCT00384852. A study of rhBMP-2/CPM in closed fractures of the humerus (Version 15 posted 28 February 2013). clinicaltrials.gov/ct2/show/NCT00384852 (first posted 6 October 2006).
NCT00818987 {unpublished data only}
- NCT00818987. Operative versus non operative treatment of proximal humerus (shoulder joint) fractures (Version 1). clinicaltrials.gov/show/NCT00818987 (first posted 8 January 2009).
- NCT00818987. Operative versus non operative treatment of proximal humerus (shoulder joint) fractures (Version 4). clinicaltrials.gov/show/NCT00818987 (first posted 8 January 2009).
- Roffey D. Status of trial [personal communication]. Email to: H Handoll 22 July 2020.
NCT01086202 {published data only}
- NCT01086202. Clinical outcome comparison between medial and lateral offset reverse shoulder arthroplasty (version 2; updated 25 March 2010). clinicaltrials.gov/show/NCT01086202 (first posted 15 March 2010).
- NCT01086202. Clinical outcome comparison between medial and lateral offset reverse shoulder arthroplasty (version 6; updated 16 September 2016). clinicaltrials.gov/show/NCT01086202 (first posted 15 March 2010).
NCT01532076 {unpublished data only}
- NCT01532076. Effectiveness of adipose tissue derived mesenchymal stem cells as osteogenic component in composite grafts (ROBUST) (Version 3, updated 17 September 2014). www.clinicaltrials.gov/ct2/show/NCT01532076 (first posted 13 February 2012).
NCT01817933 {published data only}
- NCT01817933. Integrated rehabilitation program for fractures (Version 1). clinicaltrials.gov/ct2/show/NCT01817933 (first posted 26 March 2013).
NCT02052206 {unpublished data only}
- NCT02052206. Accuracy evaluation of shoulder replacement surgery (Version 1). www.clinicaltrials.gov/ct2/show/NCT02052206 (first received 31 January 2014).
- NCT02052206. Reconstruction of complex proximal humeral fractures (version 6, 24 November 2017). www.clinicaltrials.gov/ct2/show/NCT02052206 (first received 31 January 2014).
NCT02597972 {published data only}
- NCT02597972. Reverse total shoulder arthroplasty versus open reduction internal fixation of 3&4 part proximal humerus fractures (Version 7). clinicaltrials.gov/ct2/show/NCT02597972 (first posted 5 November 2015).
NCT03017105 {published data only}
- Bardsley J. Trial status: early termination due to "very low recruitment" [personal communication]. Email to: H Handoll 30 June 2020.
- NCT03017105. Cross-education for proximal humerus fracture rehabilitation (Version 1). clinicaltrials.gov/ct2/show/NCT03017105 (first posted 11 January 2017).
NCT03804853 {published data only}
- NCT03804853. Rehabilitation following reverse total shoulder arthroplasty (Version 3). clinicaltrials.gov/ct2/show/NCT03804853 (first posted 15 January 2019).
NCT04285606 {published data only}
- NCT04285606. Effect of postop rehab methods on outcomes following reverse shoulder arthroplasty (Version 3). clinicaltrials.gov/show/NCT04285606 (first posted 26 February 2020).
NTR2186 {unpublished data only}
- NTR2186. Delta Xtend reversed shoulder prosthesis. apps.who.int/trialsearch/Trial2.aspx?TrialID=NTR2186 (first posted 28 January 2010).
Parnes 2005 {published data only}
- Parnes N, Pritsch T, Mozes G. Is surgery the best choice in the treatment of complex fractures of proximal humerus? Preliminary study on 50 cases [Abstract]. Journal of Bone & Joint Surgery - British Volume 2005;87(Suppl 3):384. [Google Scholar]
Pullen 2007 {unpublished data only}
- Pullen H. Comparison of the T2 procimal humeral nail and PHILOS system in the treatment of 2+3 part proximal humeral fractures. In: National Research Register, Issue 3, 2006. Oxford; Update Software.
Rasool 2015 {published data only}
- Rasool K, Zubair M, Muhammad E, Akhtar S. Outcome of open reduction and internal fixation using plate versus closed reduction and percutaneous fixation using K-wires for treatment of two part proximal humerus fracture. Pakistan Journal of Medical and Health Sciences 2015;9(4):1257-9. [Google Scholar]
Rodriguez‐Merchan 1995 {published data only}
- Rodriguez-Merchan EC. Compression plating versus Hackethal nailing in closed humeral shaft fractures failing nonoperative reduction. Journal of Orthopaedic Trauma 1995;9(3):194-7. [DOI] [PubMed] [Google Scholar]
Rollo 2019 {published data only}
- Rollo G, Porcellini G, Rotini R, Bisaccia M, Pichierri P, Paladini P, et al. A new plate design to treat displaced 3-4 parts proximal humeral fractures in comparison to the most tested and used plate: clinical and radiographic study. Medicinski Glasnik 2019;16(2):284-91. [DOI: 10.17392/1033-19] [DOI] [PubMed] [Google Scholar]
ROTATE 2019 {unpublished data only}38563880
- Kassam AM, Ainsworth B, Hawken R, Ramesh R, Conboy V. Return of function and external rotation post-proximal humerus fracture fixation with neutral rotation brace (abstract). Bone and Joint Journal 2013;95(Suppl 9):31. [Google Scholar]
- NCT02073695. Return of function and external rotation post proximal humerus fracture fixation with neutral rotation brace (ROTATE) (Version 2). clinicaltrials.gov/ct2/show/NCT02073695 (first posted 27 February 2014).
- NCT02073695. Return of function and external rotation post proximal humerus fracture fixation with neutral rotation brace (ROTATE) (Version 4, submitted 17 July 2019). clinicaltrials.gov/ct2/show/NCT02073695 (first posted 27 February 2014).
Schoch 2019 {published data only}
- Schoch B, Aibinder W, Walters J, Sperling J, Throckmorton T, Sanchez-Sotelo J, et al. Outcomes of uncemented versus cemented reverse shoulder arthroplasty for proximal humerus fractures. Orthopedics 2019;42(2):e236-41. [DOI: 10.3928/01477447-20190125-03] [DOI] [PubMed] [Google Scholar]
Shah 2003 {unpublished data only}
- Shah N. Brief detail on study status [personal communication]. Contact with H Handoll 1 April 2003.
- Shah N. Shoulder function following four part fractures of proximal humerus: A prospective randomised trial for treatment of four part fractures of proximal humerus - conservative vs hemiarthroplasty. In: National Research Register, Issue 1, 2003. Oxford: Update Software.
Shah 2018 {published data only}
- Shah PS, Shinde SB. Effect of desensitization methods during the early mobilization phase in post-fracture conditions of upper extremity. Asian Journal of Pharmaceutical and Clinical Research 2018;11(7):93-6. [Google Scholar]
Torrens 2015 {published data only}
- Torrens C. Correction on the status of this trial [personal communication]. Email to: H Handoll 8 July 2020.
- Torrens C. Notice of ongoing trial [personal communication]. Email to: H Handoll 4 June 2015.
- Torrens C. Ongoing trial details [personal communication]. Email to: H Handoll 10 June 2015.
Wallace 2000 {unpublished data only}
- Wallace WA. A prospective randomised trial of the management of displaced surgical neck and displaced shaft fractures of the humerus with the Halder Humeral Nail. In: National Research Register, Issue 3, 2000. Oxford: Update Software.
Wan 2005 {published data only}
- Wan L, Wang G-X. Interventional effect of improved mobilization on dysfunction of fractured shoulder joint. Zhongguo Linchuang Kangfu [Chinese Journal of Clinical Rehabilitation] 2005;9(26):10-1. [Google Scholar]
Wang 2014 {published data only}
- Wang Y, Xu B, Yu Z, Dai S, Li F, Wu Y. Comparison of swing shoulder and internal fixation for the treatment of proximal humeral fractures in elderly. Zhongguo Gu Shang [China Journal of Orthopaedics and Traumatology] 2014;27(12):980-5. [PubMed] [Google Scholar]
Warnecke 1999 {published data only}
- Warnecke J, Jansen T, Oestern H-J. Surgical treatment of proximal humerus fractures [Operative behandlung proximaler humerusfrakturen - AO multicenterstudie]. Deutsche Gesellschaft fur Chirurgie 1999;Suppl Kongressband II:1021-4. [Google Scholar]
Yang 2006 {published data only}
- Yang L, Li B, Pan XY, Li C, Huang JW, Wang ZW, et al. Percutaneous reduction and fixation of osteoporotic fractures for the proximal humerus in a geriatric population. Zhonghua Wai Ke Za Zhi [Chinese Journal of Surgery] 2006;44(12):830-2. [PubMed] [Google Scholar]
- Yang L. [personal correspondence]. Contact with: H Handoll 9 March 2010.
You 2016 {published data only}
- You W, Liu LJ, Chen HX, Xiong JY, Wang DM, Huang JH, et al. Application of 3D printing technology on the treatment of complex proximal humeral fractures (Neer3-part and 4-part) in old people. Orthopaedics & Traumatology, Surgery & Research : OTSR 2016;102(7):897-903. [DOI: 10.1016/j.otsr.2016.06.009] [DOI] [PubMed] [Google Scholar]
Zhang 2010 {published data only}
- Zhang JH, Di ZL, He ZY, Feng JX, Xu RM. Comparison of humeral head replacement and internal fixation for the treatment of 3 parts and 4 parts fractures of proximal humerus in the elderly. Zhongguo Gu Shang [China Journal of Orthopaedics and Traumatology] 2010;23(6):435-9. [PubMed] [Google Scholar]
Zhao 2017 {published data only}
- ChiCTR-INR-17011098. A comparative study on the clinical outcome between MIPPO and conventional ORIF through the deltoid-pectoralis approach for the treatment of proximal humeral fracture in elderly patients. www.chictr.org.cn/showprojen.aspx?proj=18875 (first registered 9 April 2017).
- Zhao L, Yang P, Zhu L, Chen AM. Minimal invasive percutaneous plate osteosynthesis (MIPPO) through deltoid-pectoralis approach for the treatment of elderly proximal humeral fractures. BMC Musculoskeletal Disorders 2017;18:[6 p.]. [DOI: 10.1186/s12891-017-1538-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2019 {published data only}
- Zhao L, Qi YM, Yang L, Wang GR, Zheng SN, Wang Q, et al. Comparison of the effects of proximal humeral internal locking system (PHILOS) alone and PHILOS combined with fibular allograft in the treatment of Neer three- or four-part proximal humerus fractures in the elderly. Orthopaedic Surgery 2019;11(6):1003-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Baring 2017 {published data only}
- Baring T, Desai K. The N-brace trial - a feasibility study (abstract). Shoulder & Elbow 2017;9(1S):S6. [Google Scholar]
Battistella 2011 {published data only}
- Battistella F, Oldani M, Tajana MS, Prestamburgo D. Treatment of proximal humerus fractures with locking plate by minimally invasive technique (abstract). Journal of Orthopaedics and Traumatology 2011;12(Suppl):S15-6. [Google Scholar]
Brorson 2009 {published data only}
- Brorson S, Olsen BS, Frich LH, Jensen SL, Johannsen HV, Sorensen AK, et al. Effect of osteosynthesis, primary hemiarthroplasty, and non-surgical management for displaced four-part fractures of the proximal humerus in elderly: a multi-centre, randomised clinical trial. Trials 2009;10:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brorson S. Availability of trial data [personal communication]. Email to: H Handoll 24 January 2020.
- Brorson S. Trial status [personal communication]. Email to; H Handoll 3 February 2015.
- Brorson S. Trial status [personal communication]. Email to: H Handoll 11 June 2012.
- Brorson S. Trial status [personal communication]. Email to: H Handoll 22 May 2015.
- Brorson S. Trial status [personal communication]. Email to: H Handoll 28 January 2015.
- NCT00835562. Effect of osteosynthesis, primary hemi-arthroplasty, and non-surgical management for displaced four-part fractures of the proximal humerus in elderly: a multi-centre, randomised clinical trial (Version 1). clinicaltrials.gov/ct2/show/record/NCT00835562 (first posted 3 February 2009). [DOI] [PMC free article] [PubMed]
- NCT00835562. Effect of osteosynthesis, primary hemi-arthroplasty, and non-surgical management for displaced four-part fractures of the proximal humerus in elderly: a multi-centre, randomised clinical trial (Version 3, posted 7 June 2012). clinicaltrials.gov/ct2/show/record/NCT00835562 (first posted 3 February 2009).
Chen 2016 {published data only}
- Chen H, Ji X, Gao Y, Zhang L, Zhang Q, Liang X, et al. Comparison of intramedullary fibular allograft with locking compression plate versus shoulder hemi-arthroplasty for repair of osteoporotic four-part proximal humerus fracture: consecutive, prospective, controlled, and comparative study. Orthopaedics & Traumatology, Surgery & Research : OTSR 2016;102(3):287-92. [DOI] [PubMed] [Google Scholar]
Chengjin 2017 {published data only}
- Chengjin Z, Haibin H, Shengmei S, Kai L. Comparison of the effect of the fixation of the articular plate and the hollow screw and the band anchorage in the large nodule of humerus. Biomedical Research (India) 2017;28(18):8138-41. [Google Scholar]
Liu 2011 {published data only}
- Liu ZZ, Zhang GM, Ge T, Yang YF. Proximal humeral internal locking system and injectable calcium sulfate graft for the treatment of osteoporotic proximal humeral fractures in elderly patients. Chinese Journal of Tissue Engineering Research 2012;16(51):9679-83. [Google Scholar]
- Liu ZZ, Zhang GM, Ge T. Use of a proximal humeral internal locking system enhanced by injectable graft for minimally invasive treatment of osteoporotic proximal humeral fractures in elderly patients. Orthopaedic Surgery 2011;3(4):253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2014 {published data only}
- Liu J, Di J, Zhao C, Yao S, Zhu L, Wang B, et al. Clinical efficacies of different internal fixation materials in the treatment of senile proximal humerus fractures. Zhonghua Yi Xue Za Zhi [Chinese Medical Journal] 2014;94(47):3758-60. [PubMed] [Google Scholar]
Luo 2008 {published data only}
- Luo KM, Hou Z, Yang L. Observation on therapeutic effect of electroacupuncture on activity disturbance of the shoulder joint after operation of fracture. Zhongguo Zhen Kiu [Chinese Acupuncture & Moxibustion] 2008;28(10):727-9. [PubMed] [Google Scholar]
NCT01113411 {unpublished data only}
- NCT01113411. Effectiveness of intensive rehabilitation on shoulder function after proximal humerus fracture (Version 5). clinicaltrials.gov/show/NCT01113411 (first posted 29 April 2010).
- NCT01113411. Effectiveness of intensive rehabilitation on shoulder function after proximal humerus fracture (Version 8). clinicaltrials.gov/show/NCT01113411 (first posted 29 April 2010).
NTR3208 {unpublished data only}
- NTR3060. Arthroplasty in three- or four-part proximal humerus fracture: hemi or reverse? (renumbered). www.trialregister.nl/trial/3060 (first posted 18 December 2011).
- NTR3208. Arthroplasty in three- or four-part proximal humerus fracture: hemi or reverse? www.trialregister.nl/trialreg/admin/rctview.asp?TC=3208 (first posted 18 December 2011).
Paladini 2019 {published data only}
- Paladini P, Padolino A, Saporito M, Porcellini G, Merolla G. Clinical and radiographic evaluation of three-four fragments proximal humerus treated with CRF-PEEK or metal plates at two years follow-up. JSES Open Access 2019;3(4):248. [Google Scholar]
Peng 2017 {published data only}
- Peng C, Wang HP, Yan JH, Song TX. Locking system strengthened by biomimetic mineralized collagen putty for the treatment of osteoporotic proximal humeral fractures. Regenerative Biomaterials 2017;4(5):289-94. [DOI: 10.1093/rb/rbx016] [DOI] [PMC free article] [PubMed] [Google Scholar]
ProCon 2010 {published and unpublished data}
- Den Hartog D, Schep N, Mahabier K, Lordens G, De Zwart A, Verhofstad MH, et al. Hemiarthroplasty versus nonoperative treatment of comminuted proximal humeral fractures: results of the ProCon multicenter randomized clinical trial (abstract). In: Orthopaedic Trauma Association Annual Meeting; 2018 Oct 17–20; Kissimmee (FL). 2018.
- Den Hartog D, Van Lieshout EM, Tuinebreijer WE, Polinder S, Van Beeck EF, Breederveld RS, et al. Primary hemiarthroplasty versus conservative treatment for comminuted fractures of the proximal humerus in the elderly (ProCon): a multicenter randomized controlled trial. BMC Musculoskeletal Disorders 2010;11(97):[9 p.]. [DOI: 10.1186/1471-2474-11-97] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTR1923. Primary hemiarthroplasty versus conservative treatment for comminuted fractures of the proximal humerus in the elderly (ProCon) - A multicenter randomized trial (Version 2: renumbered). www.trialregister.nl/trial/1923 (first posted 30 September 2009).
- NTR2040. Primary hemiarthroplasty versus conservative treatment for comminuted fractures of the proximal humerus in the elderly (ProCon) - A multicenter randomized trial. www.trialregister.nl/trialreg/admin/rctview.asp?TC=2040 (first posted 30 September 2009). [DOI] [PMC free article] [PubMed]
Wang 2013 {published data only}
- Wang ZH, Deng D, Chen LQ, Zhang WK, Yan HB, Chen XY, et al. Case-control studies on therapeutic effects of combined methods of minimally invasive percutaneous proximal humerus locked osteosynthesis plate with injectable bone for the treatment of proximal humerus fractures in elderly patients. Zhongguo Gu Shang [China Journal of Orthopaedics and Traumatology] 2013;26(5):404-7. [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- Zhang AF. Discussion on the effect of TCM fracture three stages therapy in treating proximal humerus fractures. Guang Ming Zhong Yi [Guangming Journal of Traditional Chinese Medicine] 2016;31(10):1422-3. [Google Scholar]
Zhu 2014 {published data only}
- Zhu L, Liu Y, Yang Z, Li H, Wang J, Zhao C, et al. Locking plate fixation combined with iliac crest bone autologous graft for proximal humerus comminuted fracture. Chinese Medical Journal 2014;127(9):1672-6. [PubMed] [Google Scholar]
Z‐TUp Alvarez 2020 {published data only}
- NCT02075476. Effectiveness between two surgical techniques for reconstruction of humeral proximal extremity fractures (version 7 updated 1 April 2020). clinicaltrials.gov/ct2/show/NCT02075476 (first posted 3 March 2014).
Z‐TUp Batar 2020 {published data only}
- Batar S, Turkmen I, Celik H, Uzer G, Bilsel K. Improved functional outcomes with reverse shoulder arthroplasty compared to hemiarthroplasty after proximal humeral fractures in the elderly [Lepsi funkcni vysledky reverzni artroplastiky ramena ve srovnani s hemiartroplastikou u zlomenin proximalniho humeru u starsich pacientu]. Acta Chirurgiae Orthopaedicae et Traumatologiae Cechoslovaca 2020;87(4):278-84. [PubMed] [Google Scholar]
Z‐TUp Bhayana 2021 {published data only}
- Bhayana H, Chouhan DK, Aggarwal S, Prakash M, Patel S, Arora C, et al. Outcomes of plate osteosynthesis for displaced 3-part and 4-part proximal humerus fractures with deltopectoral vs. deltoid split approach. European Journal of Trauma and Emergency Surgery 2021 Jul 31 [Epub ahead of print]. [DOI: 10.1007/s00068-021-01761-6] [DOI] [PubMed]
Z‐TUp Borda 2019 {published data only}
- Borda IM, Irsay L, Ciortea V, Onac I, Ungur R. Pulsed electromagnetic field in rehabilitation of proximal humeral osteoporotic fractures (abstract). Osteoporosis International 2019;30(Suppl 2):S765-6. [Google Scholar]
Z‐TUp Boyer 2021 {published data only}
- Boyer P, Couffignal C, Bahman M, Mylle G, Rousseau MA, Dukan R. Displaced three and four part proximal humeral fractures: prospective controlled randomized open-label two-arm study comparing intramedullary nailing and locking plate. International Orthopaedics 2021;45(11):2917-26. [DOI] [PubMed] [Google Scholar]
Z‐TUp Hachem 2021 {published data only}
- Hachem AI, Rius X, Rodriguez-Bascones K, Catasus M, Matellanes J, Verdalet I, et al. Can anatomic stem angles of 135º in reverse shoulder prosthesis obtain better consolidation rates and clinical outcomes than 155º in RSA fracture? A randomized control trial (Abstract). Journal of Shoulder and Elbow Surgery 2021;30(7):e425-6. [Google Scholar]
Z‐TUp Izquierdo‐Fernández 2021 {published data only}
- Izquierdo-Fernández A, Gómez-Rodríguez M, Urbano-Luque M, García-Carmona M, Quevedo-Reinoso R, Minarro JC. Reverse shoulder arthroplasty in complex fractures of the proximal humerus: results after 7 years of follow-up. Journal of Orthopaedica and Traumatology 2021;22(1):38. [DOI: 10.1186/s10195-021-00597-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Z‐TUp Kröger 2021 {published data only}
- Kröger I, Nerz C, Schwickert L, Schölch S, Müßig JA, Studier-Fischer S, et al. Robot-assisted training after proximal humeral fracture: a randomised controlled multicentre intervention trial. Cinical Rehabilitation 2021;35(2):242-52. [DOI] [PubMed] [Google Scholar]
Z‐TUp Laas 2021 {published data only}
- Laas N, Engelsma Y, Hagemans FJ, Hoelen MA, Van Deurzen DF, Burger BJ. Reverse or hemi shoulder arthroplasty in proximal humerus fractures: a single blinded prospective multi-center randomized clinical trial. Journal of Orthopaedic Trauma 2021;35(5):252-8. [DOI] [PubMed] [Google Scholar]
- Li X. In older adults with 3- or 4-part dislocated proximal humeral fractures, reverse shoulder arthroplasty improved anterior elevation and Constant score, but not DASH scores, quality of life, or radiographic outcomes compared with hemiarthroplasty. Journal of Bone and Joint Surgery. American Volume 2021;103(22):2142. [DOI] [PubMed] [Google Scholar]
Z‐TUp Li 2020 {published data only}
- Li G, Wei WF, Liu X, Yang T. Treatment of proximal humeral fractures in elderly patients with intramedullary nail and locking plate. Chung Hua I Hsueh Tsa Chih [Chinese Medical Journal (Taipei)] 2020;100(41):3240-5. [DOI] [PubMed] [Google Scholar]
Z‐TUp Martinez 2021 {published data only}
- Martinez R, Santana F, Pardo A, Torrens C. One versus 3-week immobilization period for nonoperatively treated proximal humeral fractures: a prospective randomized trial. Journal of Bone and Joint Surgery. American Volume 2021;103(16):1491-8. [DOI] [PubMed] [Google Scholar]
- Martinez Torregrosa R, Sabtana Perez F, Torens Canovas C. One or three-weeks immobilization period in proximal humeral fractures conservatively treated. Prospective randomized study (abstract). Journal of Shoulder and Elbow Surgery 2021;30(7):e422. [Google Scholar]
Z‐TUp Monticone 2021 {published data only}ISRCTN17996552
- Monticone M, Portoghese I, Cazzaniga D, Liquori V, Marongiu G, Capone A, et al. Task-oriented exercises improve disability of working patients with surgically-treated proximal humeral fractures. A randomized controlled trial with one-year follow-up. BMC Musculoskeletal Disorders 2021;22(1):293. [DOI: 10.1186/s12891-021-04140-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Z‐TUp Vijan 2020 {published data only}
- Vijan K, Saoji K. Comparison of functional outcome of fracture proximal humerus treated with open reduction and plate osteosynthesis v/s closed reduction and wire fixation. Indian Journal of Forensic Medicine and Toxicology 2020;14(4):6653-7. [Google Scholar]
Z‐TUpx ISRCTN85422168 {published data only}
- ISRCTN85422168. Comparing the outcomes of non-surgical versus surgical treatment of shoulder fractures with different shoulder replacements (date last edited: 1 September 2021). www.isrctn.com/ISRCTN85422168 (Date applied: 3 July 2021). [DOI: ]
Z‐TUPx NCT04405947 {published data only}
- NCT04405947. Influenze of approach in reversed shoulder prosthesis (version 2 updated 30 March 2021). clinicaltrials.gov/show/NCT04405947 (first posted 28 May 2020).
Z‐TUPx NCT04572022 {published data only}
- NCT04572022. Impact of mobile health technology application on proximal humerus fracture care practice (version 2 updated 21 July 2021). clinicaltrials.gov/ct2/show/NCT04572022 (first posted 1 October 2020).
Z‐TUpx NCT04651543 {published data only}
- NCT04651543. X-ray follow-up in proximal humeral fractures conservatively treated. clinicaltrials.gov/ct2/show/NCT04651543 (first posted: 3 December 2020).
Z‐TUpx NCT04917536 {published data only}
- NCT04917536. Comparison of the speed of functional recovery (Constant Score) between two different approaches of humeral nailing in humeral fractures: through the rotator cuff or through the rotator interval split (HUNAAP) (version 2 updated 22 June 2021). clinicaltrials.gov/show/NCT04917536 (first posted 8 June 2021).
Z‐TUpx Song 2020 {published data only}
- Song H, Wang M, Du H, Mu W. Comparison of locking plates and intramedullary nails in treatment of three-part or four-part proximal humeral neck fractures in elderly population: a randomized trial protocol. Medicine (Baltimore) 2020;99(46):e22914. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR1900022553 {published data only}
- ChiCTR1900022553. Clinical outcomes of three- or four-part complex proximal humerus fractures: reverse total shoulder arthroplasty, hemiarthroplasty versus locking plate fixation. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1900022553 (first posted 16 April 2019).
ChiCTR‐IOR‐16008817 {published data only}
- ChiCTR-IOR-16008817. Treatment of proximal humeral fractures with locking plates and intramedullary fibular allograft: prospective randomized controlled study (last refreshed 18 April 2017). apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR-IOR-16008817 (first posted 11 July 2016).
DRKS00009990 {published data only}
- DRKS00009990. Robot training assistance in humerus fracture – a multicenter, controlled, randomized interventional trial (Version 2). who.int/trialsearch/Trial2.aspx?TrialID=DRKS00009990 (first posted 2 February 2016).
DRKS00011581 {published data only}
- DRKS00011581. Clinical, functional and psychosocial outcome of reverse shoulder arthroplasty - a prospective randomized comparison of two types of prostheses after traumatic fracture of the proximal humerus (CEUS #Delta) (Version 1). drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00011581 (first posted 18 January 2017).
Hakim 2018 {published data only}
- Hakim Z, Daw R, Wilkinson P, Brookes-Fazakerley S, Smith M, Kent M, et al. Randomised control trial comparing hemi arthroplasty and reverse polarity shoulder replacement for the treatment of proximal humerus fractures in the elderly - interim analysis (abstract). Shoulder & Elbow 2018;10(3S):S24-5. [Google Scholar]
Howard 2018 {published data only}
- Howard L, Berdusco R, Momoli F, Pollock J, Liew A, Papp S, et al. Open reduction internal fixation vs non-operative management in proximal humerus fractures: a prospective, randomized controlled trial protocol. BMC Musculoskeletal Disorders 2018;19(299):[10 p.]. [DOI: 10.1186/s12891-018-2223-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02362100. Proximal humerus fractures randomized control trial (Version 6). clinicaltrials.gov/ct2/show/NCT02362100 (first posted 12 February 2015).
ISRCTN17996552 {unpublished data only}
- ISRCTN17996552. Rehabilitation for humeral fractures in working ages. www.isrctn.com/ISRCTN17996552 (first posted 16 December 2019).
ISRCTN76296703 {published data only}ISRCTN76296703
- ISRCTN76296703. Effectiveness and cost-effectiveness of reverse shoulder arthroplasty versus hemiarthroplasty versus non-surgical care for acute 3 and 4 part fractures of the proximal humerus in patients aged over 65 years – the PROFHER-2 randomised trial. isrctn.com/ISRCTN76296703 (first posted 5 April 2018).
Launonen 2019 {published data only}
- Launonen AP, Fjalestad T, Laitinen MK, Lahdeoja T, Ekholm C, Wagle T, et al. Nordic Innovative Trials to Evaluate osteoPorotic Fractures (NITEP) Collaboration: the Nordic DeltaCon Trial protocol - Non-operative treatment versus reversed total shoulder arthroplasty in patients 65 years of age and older with a displaced proximal humerus fracture: a prospective, randomised controlled trial. BMJ Open 2019;9(1):e024916. [DOI: 10.1136/bmjopen-2018-024916] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03531463. Nordic DeltaCon Trial: for displaced proximal humeral fractures in elderly (DeltaCon) (Version 6). clinicaltrials.gov/ct2/history/NCT03531463 (first posted 21 May 2018).
NCT00999193 {unpublished data only}
- NCT00999193. Proximal humeral comminuted fractures in the elderly - PERCELE trial (version 8, updated 2 June 2018). clinicaltrials.gov/ct2/show/record/NCT00999193 (first posted 21 October 2009).
- NCT00999193. Treatment of comminuted fractures of the proximal humerus. A randomised, controlled study (version 2, updated 26 May 2010). clinicaltrials.gov/ct2/show/record/NCT00999193 (first posted 21 October 2009).
- NCT00999193. Treatment of comminuted fractures of the proximal humerus. A randomised, controlled study (version 4, updated 22 January 2012). clinicaltrials.gov/ct2/show/record/NCT00999193 (first posted 21 October 2009).
NCT01524965 {unpublished data only}
- NCT01524965. The effect of the timing of postoperative mobilisation after locking plate osteosynthesis of fractures of the surgical neck of the humerus (Version 2). clinicaltrials.gov/ct2/show/NCT01524965 (first posted 2 February 2012).
- NCT01524965. The effect of the timing of postoperative mobilisation after locking plate osteosynthesis of fractures of the surgical neck of the humerus (Version 4; sent 3 January 2017). clinicaltrials.gov/ct2/show/NCT01524965 (first posted 2 February 2012).
NCT01557413 {unpublished data only}
- Boyer P, Dukan R. Displaced 3- and 4-part proximal humeral fractures: a prospective randomized study comparing intramedullary nail and locking plate (abstract). In: American Academy of Orthopaedic Surgeons Annual Meeting; 2019 Mar 12-16; Las Vegas (CA). 2019.
- NCT01557413. Randomised study between intramedullary locking nails and locking plates for treatment of proximal humerus fractures (HUMERUS) (Version 1). clinicaltrials.gov/show/NCT01557413 (first posted 19 March 2012).
- NCT01557413. Randomised study between intramedullary locking nails and locking plates for treatment of proximal humerus fractures (HUMERUS) (Version 4, updated 11 March 2015). clinicaltrials.gov/show/NCT01557413 (first posted 19 March 2012).
- NCT01557413. Randomised study between intramedullary locking nails and locking plates for treatment of proximal humerus fractures (HUMERUS) (Version 6, updated 20 February 2017). clinicaltrials.gov/show/NCT01557413 (first posted 19 March 2012).
NCT02075476 {unpublished data only}
- NCT02075476. The effectiveness between two surgical techniques for reconstruction of humeral proximal extremity fractures or fractures luxation in three or four fragments (version 2 updated 4 March 2014). clinicaltrials.gov/ct2/show/NCT02075476 (first posted 3 March 2014).
- NCT02075476. The effectiveness between two surgical techniques for reconstruction of humeral proximal extremity fractures or fractures luxation in three or four fragments (version 6 updated 6 August 2019). clinicaltrials.gov/ct2/show/NCT02075476 (first posted 3 March 2014).
NCT02467803 {published data only}
- NCT02467803. Shoulder functional outcomes of patients with proximal humerus fractures: comparison of two different treatment protocol (Version 1). clinicaltrials.gov/ct2/show/NCT02467803 (first posted 10 June 2015).
NCT02913378 {published data only}
- NCT02913378. Conservative vs surgical treatment for proximal humerus fractures in the elderly (Version 2; 29 October 2017). clinicaltrials.gov/ct2/show/NCT02913378 (first posted 23 September 2016).
NCT02944058 {published data only}
- NCT02944058. Plate fixation versus intramedullary nailing of 3 and 4 part proximal humerus fractures (Version 2; posted 6 July 2018). clinicaltrials.gov/ct2/show/NCT02944058 (first posted 5 October 2016).
NCT03217344 {published data only}
- NCT03217344. Conservative treatment of proximal humeral fractures (Version 3). clinicaltrials.gov/ct2/show/NCT03217344 (first posted 14 July 2017).
- Torrens C. Information on the trial status [personal communication]. Email to: H Handoll 8 July 2020.
- Torrens C. Provision of unpublished abstract and results table for 1 year follow-up [personal communication]. Email to: H Handoll 10 July 2020.
NCT03498859 {published data only}
- NCT03498859. Efficacy of physiotherapist-supervised rehabilitation after proximal humerus fracture (Version 3). clinicaltrials.gov/ct2/show/NCT03498859 (first posted 17 April 2018).
NCT03599336 {published data only}
- NCT03599336. RSA vs. nonop for 3 & 4-part proximal humerus fractures (Version 3). clinicaltrials.gov/ct2/show/NCT03599336 (first posted 26 July 2018).
NCT03610113 {published data only}
- NCT03610113. Reverse or nothing for complex proximal humeral fractures (Version 3). clinicaltrials.gov/ct2/show/NCT03610113 (first posted 1 August 2018).
NCT03694457 {published data only}
- NCT03694457. Comparison between anterior approach (deltopectoral) and lateral approach (deltoid splitting) in shoulder reverse arthroplasty for proximal humerus fracture (Version 3). clinicaltrials.gov/ct2/show/NCT03694457 (first posted 3 October 2018).
NCT03786679 {published data only}
- NCT03786679. Non-operative treatment in Sweden of proximal humeral fractures (Version 1). clinicaltrials.gov/show/NCT03786679 (first posted 26 December 2018).
NCT04106674 {published data only}
- NCT04106674. Two- part proximal humerus - conservative vs operative (Version 1). clinicaltrials.gov/ct2/show/NCT04106674 (first posted 27 September 2019).
NCT04553497 {published data only}
- NCT04553497. IFC therapy in proximal humerus fractures (Version 1). clinicaltrials.gov/ct2/show/NCT04553497 (first posted 17 September 2020).
Nerz 2017 {published data only}
- NCT03100201. Robotic-assisted training after upper arm fracture (RASTA) (Version 3). clinicaltrials.gov/show/NCT03100201 (first posted 4 April 2017).
- Nerz C, Schwickert L, Becker C, Studier-Fischer S, Mußig JA, Augat P. Effectiveness of robot-assisted training added to conventional rehabilitation in patients with humeral fracture early after surgical treatment: protocol of a randomised, controlled, multicentre trial. Trials 2017;18(1):10.1186/s13063-017-2274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
NTR3859 {unpublished data only}
- Bransen J. Current status of trial [personal communication]. Email to: H Handoll 14 August 2020.
- NTR3859. PROMOTION-trial: a PROspective randomized Multicenter trial for the treatment of dislocated 3-part proximal humerus fractures: Open reduction and internal fixaTION versus intramedullary nailing (version; renumbered). trialregister.nl/trial/3859 (first posted 3 June 2013).
- NTR4019. PROMOTION-trial: a PROspective randomized Multicenter trial for the treatment of dislocated 3-part proximal humerus fractures: Open reduction and internal fixaTION versus intramedullary nailing. apps.who.int/trialsearch/Trial2.aspx?TrialID=NTR4019 (first posted 3 June 2013).
ReShAPE {published data only}
- ACTRN12616000345482. The ReShAPE trial: Reverse Shoulder Arthroplasty for treatment of Proximal humeral fractures in the Elderly (updated version 22 Februrary 2020). anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616000345482 (first posted 16 March 2016).
- Smith GC, Bateman E, Cass B, Damiani M, Harper W, Jones H, et al. Reverse Shoulder Arthroplasty for the treatment of Proximal humeral fractures in the Elderly (ReShAPE trial): study protocol for a multicentre combined randomised controlled and observational trial. Trials 2017;18(1):91. [DOI: 10.1186/s13063-017-1826-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
SHeRPA {published data only}21981284
- ISRCTN21981284. Comparison of two shoulder replacement methods after trauma. www.isrctn.com/ISRCTN21981284 (first posted 27 January 2015).
- ISRCTN21981284. Comparison of two shoulder replacement methods after trauma (record updated 3 December 2015). www.isrctn.com/ISRCTN21981284 (first posted 27 January 2015). [DOI: 10.1186/ISRCTN21981284] [DOI]
TPHF {published data only}
- Launonen AP, Lepola V, Flinkkila T, Strandberg N, Ojanpera J, Rissanen P, et al. Conservative treatment, plate fixation, or prosthesis for proximal humeral fracture. A prospective randomized study. BMC Musculoskeletal Disorders 2012;13(167):[7 p.]. [DOI: 10.1186/1471-2474-13-167] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01246167. Treatment of proximal humeral fractures (TPHF) (Version 11 submitted 9 March 2020). clinicaltrials.gov/show/NCT01246167 (first posted 23 November 2011).
- NCT01246167. Treatment of proximal humeral fractures (TPHF) (Version 2 submitted 1 February 2011). clinicaltrials.gov/show/NCT01246167 (first posted 23 November 2011).
Wu 2016 {published data only}
- NCT02784522. Minimally invasive of proximal humerus fractures with internal fixation improves shoulder function in older patients (Version 1). clinicaltrials.gov/ct2/show/NCT02784522 (first posted 27 May 2016).
- Wu T, Zhang GQ. Minimally invasive treatment of proximal humerus fractures with locking compression plate improves shoulder function in older patients: study protocol for a prospective randomized controlled trial. Chinese Journal of Tissue Engineering Research 2016;20(44):6655-60. [DOI: 10.3969/j.issn.2095-4344.2016.44.017] [DOI] [Google Scholar]
- Wu T, Zhang GQ. Minimally invasive treatment of proximal humerus fractures with locking compression plate improves shoulder function in older patients: study protocol for a prospective randomized controlled trial. Clinical Trials in Orthopaedic Disorders 2016;1:51-7. [Google Scholar]
Additional references
Alispahic 2020
- Alispahic N, Brorson S, Bahrs C, Joeris A, Steinitz A, Audigé L. Complications after surgical management of proximal humeral fractures: a systematic review of event terms and definitions. BMC Musculosketelal Disorders 2020;21(327):[6 p.]. [DOI: 10.1186/s12891-020-03353-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aluko 2021
- Aluko P, Graybill E, Craig D, Henderson C, Drummond M, Wilson EC, et al, on behalf of the Campbell and Cochrane Economics Methods Group. Chapter 20: Economic evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors), Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Austin 2019
- Austin DC, Torchia MT, Cozzolino NH, Jacobowitz LE, Bell JE. Decreased reoperations and improved outcomes with reverse total shoulder arthroplasty in comparison to hemiarthroplasty for geriatric proximal humerus fractures: a systematic review and meta-analysis. Journal of Orthopaedic Trauma 2019;33(1):49-57. [DOI: 10.1097/BOT.0000000000001321] [DOI] [PubMed] [Google Scholar]
Austin 2020
- Austin DC, Torchia MT, Tosteson AN, Gitajn IL, Tapp SJ, Bell JE. The cost-effectiveness of reverse total shoulder arthroplasty versus open reduction internal fixation for proximal humerus fractures in the elderly. Iowa Orthopaedic Journal 2020;40(2):20-9. [PMC free article] [PubMed] [Google Scholar]
Beks 2018
- Beks RB, Ochen Y, Frima H, Smeeing DP, Van der Meijden O, Timmers TK, et al. Operative versus nonoperative treatment of proximal humeral fractures: a systematic review, meta-analysis, and comparison of observational studies and randomized controlled trials. Journal of Shoulder and Elbow Surgery 2018;27(8):1526-34. [DOI: 10.1016/j.jse.2018.03.009] [DOI] [PubMed] [Google Scholar]
Bell 2011
- Bell JE, Leung BC, Spratt KF, Koval KJ, Weinstein JD, Goodman DC, et al. Trends and variation in incidence, surgical treatment, and repeat surgery of proximal humeral fractures in the elderly. Journal of Bone and Joint Surgery. American Volume 2011;93(2):121-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergdahl 2020
- Bergdahl C, Wennergren D, Ekelund J, Möller M. Mortality after a proximal humeral fracture. Bone and Joint Journal 2020;102-B(11):1484-90. [DOI] [PubMed] [Google Scholar]
Bernstein 1996
- Bernstein J, Alder LM, Blank JE, Dalsey RM, Williams GR, Iannotti JP. Evaluation of the Neer system of classification of proximal humeral fractures with computerized tomographic scans and plain radiographs. Journal of Bone and Joint Surgery. American Volume 1996;78(9):1371-5. [DOI] [PubMed] [Google Scholar]
Biberthaler 2009
- Biberthaler P, Braunstein V, Kirchoff C, Kroetz M, Kettler M, Mutschler W. Surgical therapy of humeral head fractures with a locking plate: analysis of 176 cases. Journal of Bone and Joint Surgery. British Volume 2009;91(Suppl 1):40. [Google Scholar]
Boileau 2000
- Boileau P, Walch G, Krishnan SG. Tuberosity osteosynthesis and hemiarthroplasty for four-part fractures of the proximal humerus. Techniques in Shoulder and Elbow Surgery 2000;1(2):96-100. [Google Scholar]
Boutron 2017
- Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P, CONSORT NPT Group. CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Annals of Internal Medicine 2017;167(1):40-7. [DOI] [PubMed] [Google Scholar]
Boyer 2019
- Boyer P, Dukan R. Displaced 3- and 4-part proximal humeral fractures: a prospective randomized study comparing intramedullary nail and locking plate (abstract). In: American Academy of Orthopaedic Surgeons Annual Meeting; 2019 Mar 12-16; Las Vegas (CA). 2019.
Brorson 2002
- Brorson S, Bagger J, Sylvest A, Hróbjartsson A. Low agreement among 24 doctors using the Neer-classification; only moderate agreement on displacement, even between specialists. Internal Orthopaedics 2002;26(5):271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brorson 2008
- Brorson S, Hróbjartsson A. Training improves agreement among doctors using the Neer system for proximal humeral fractures in a systematic review. Journal of Clinical Epidemiology 2008;61(1):7-16. [DOI] [PubMed] [Google Scholar]
Brorson 2011
- Brorson S. Management of proximal humeral fractures in the nineteenth century: an historical review of preradiographic sources. Clinical Orthopaedics and Related Research 2011;469(4):1197-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brorson 2012
- Brorson S, Olsen BS, Frich LH, Jensen SL, Sorensen AK, Krogsgaard MR, et al. Surgeons agree more on treatment recommendations than on classification of proximal humeral fractures. BMC Musculoskeletal Disorders 2012;13(114):[5 p.]. [DOI: 10.1186/1471-2474-13-114] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brorson 2019
- Brorson S, Alispahic N, Bahrs C, Joeris A, Steinitz A, Audige L. Complications after non-surgical management of proximal humeral fractures: a systematic review of terms and definitions. BMC Musculoskeletal Disorders 2019;20(91):[7 p.]. [DOI: 10.1186/s12891-019-2459-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruder 2011
- Bruder A, Taylor NF, Dodd KJ, Shields N. Exercise reduces impairment and improves activity in people after some upper limb fractures: a systematic review. Journal of Physiotherapy 2011;57(2):71-82. [DOI] [PubMed] [Google Scholar]
Bruder 2017
- Bruder AM, Shields N, Dodd KJ, Taylor NF. Prescribed exercise programs may not be effective in reducing impairments and improving activity during upper limb fracture rehabilitation: a systematic review. Journal of Physiotherapy 2017;63(4):205-20. [DOI: 10.1016/j.jphys.2017.08.009] [DOI] [PubMed] [Google Scholar]
Cole 2020
- Cole PA. A word of caution: success may be limited to 2 years and highly displaced OTA/AO B2 and C2 Injuries: commentary on an article by Alexander Nilsskog Fraser, MD, et al.: "Reverse Total Shoulder Arthroplasty Is Superior to Plate Fixation at 2 Years for Displaced Proximal Humeral Fractures in the Elderly. A Multicenter Randomized Controlled Trial". Journal of Bone and Joint Surgery. American Volume 2020;102(6):e30. [DOI] [PubMed] [Google Scholar]
Constant 1987
- Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clinical Orthopaedics and Related Research 1987;(214):160-4. [PubMed] [Google Scholar]
Corbacho 2016
- Corbacho B, Duarte A, Keding A, Handoll H, Chuang LH, Torgerson D, et al. Cost effectiveness of surgical versus non-surgical treatment of adults with displaced fractures of the proximal humerus: economic evaluation alongside the PROFHER trial. Bone & Joint Journal 2016;98-B(2):152-9. [DOI] [PubMed] [Google Scholar]
Court‐Brown 2001
- Court-Brown CM, Garg A, McQueen MM. The epidemiology of proximal humeral fractures. Acta Orthopaedica Scandinavica 2001;72(4):365-71. [DOI] [PubMed] [Google Scholar]
Court‐Brown 2006
- Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury 2006;37(8):691-7. [DOI] [PubMed] [Google Scholar]
Croft 1994
- Croft P, Pope D, Zonca M, O'Neill T, Silman A. Measurement of shoulder related disability: results of a validation study. Annals of the Rheumatic Diseases 1994;53(8):525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dawson 1996
- Dawson J, Fitzpatrick R, Carr A. Questionnaire on the perceptions of patients about shoulder surgery. Journal of Bone and Joint Surgery. British Volume 1996;78(4):593-600. [PubMed] [Google Scholar]
Dawson 2009
- Dawson J, Rogers K, Fitzpatrick R, Carr A. The Oxford shoulder score revisited. Archives of Orthopaedic and Trauma Surgery 2009;129(1):119-23. [DOI] [PubMed] [Google Scholar]
Dean 2016
- Dean BJ, Jones LD, Palmer AJ, Macnair RD, Brewer PE, Jayadev C, et al. A review of current surgical practice in the operative treatment of proximal humeral fractures: does the PROFHER trial demonstrate a need for change? Bone & Joint Research 2016;5(5):178-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Der Tavitian 2006
- Der Tavitian J. The management of comminuted proximal humeral fractured, a randomised prospective trial. In: National Research Register, Issue 3, 2006. Oxford; Update Software.
Du 2017
- Du S, Ye J, Chen H, Li X, Lin Q. Interventions for treating 3- or 4-part proximal humeral fractures in elderly patient: a network meta-analysis of randomized controlled trials. International Journal of Surgery 2017;48:240-6. [DOI: 10.1016/j.ijsu.2017.09.002] [DOI] [PubMed] [Google Scholar]
Fang 2019
- Fang ZR, Jiang T, Hong WW, Huang YQ, Peng JJ, Liu ZT, et al. Multiloc intramedullary nail versus PHILOS locking plate in the treatment of proximal humerus fracture: a meta-analysis. Chinese Journal of Tissue Engineering Research 2019;23(36):5896-904. [Google Scholar]
Fjalestad 2010b
- Fjalestad T, Hole MO, Jorgensen JJ, Stromsoe K, Kristiansen IS. Health and cost consequences of surgical versus conservative treatment for a comminuted proximal humeral fracture in elderly patients. Injury 2010;41(6):599-605. [DOI] [PubMed] [Google Scholar]
Fjalestad 2012
- Fjalestad T, Hole MO, Hovden IA, Blucher J, Stromsoe K. Surgical treatment with an angular stable plate for complex displaced proximal humeral fractures in elderly patients: a randomized controlled trial. Journal of Orthopaedic Trauma 2012;26(2):98-106. [DOI] [PubMed] [Google Scholar]
Fjalestad 2014a
- Fjalestad T, Hole M. Displaced proximal humeral fractures: operative versus non-operative treatment − a 2-year extension of a randomized controlled trial. European Journal of Orthopaedic Surgery & Traumatology 2014;24(7):1067-73. [DOI] [PubMed] [Google Scholar]
Fjalestad 2014b
- Fjalestad T, Iversen P, Hole MO, Smedsrud M, Madsen JE. Clinical investigation for displaced proximal humeral fractures in the elderly: a randomized study of two surgical treatments: reverse total prosthetic replacement versus angular stable plate Philos (The DELPHI-trial). BMC Musculoskeletal Disorders 2014;15:[13 p.]. [DOI: 10.1186/1471-2474-15-323 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garrigues 2012
- Garrigues GE, Johnston PS, Pepe MD, Tucker BS, Ramsey ML, Austin LS. Hemiarthroplasty versus reverse total shoulder arthroplasty for acute proximal humerus fractures in elderly patients. Orthopedics 2012;35(5):e703-8. [DOI] [PubMed] [Google Scholar]
Gillespie 2003
- Gillespie LD. personal communication August 08 2003.
Gillespie 2009
- Gillespie LD, Robertson MC, Gillespie WJ, Lamb SE, Gates S, Cumming RG, et al. Interventions for preventing falls in older people living in the community. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD007146. [DOI: 10.1002/14651858.CD007146.pub2] [DOI] [PubMed] [Google Scholar]
Guy 2010
- Guy P, Slobogean GP, McCormack RG. Treatment preferences for displaced three- and four-part proximal humerus fractures. Journal of Orthopaedic Trauma 2010;24(4):250-4. [DOI] [PubMed] [Google Scholar]
Han 2015
Hanchard 2013
- Hanchard NC, Lenza M, Handoll HH, Takwoingi Y. Physical tests for shoulder impingements and local lesions of bursa, tendon or labrum that may accompany impingement. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No: CD007427. [DOI: 10.1002/14651858.CD007427.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Handoll 2015a
- Handoll H, Brealey S, Rangan A, Keding A, Corbacho B, Jefferson L, et al. The ProFHER (PROximal Fracture of the Humerus: Evaluation by Randomisation) trial – a pragmatic multicentre randomised controlled trial evaluating the clinical effectiveness and cost-effectiveness of surgical compared with non-surgical treatment for proximal fracture of the humerus in adults. Health Technology Assessment 2015;19(24):1-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Handoll 2016a
- Handoll HH, Brealey SD, Jefferson L, Keding A, Brooksbank AJ, Johnstone AJ, et al. Defining the fracture population in a pragmatic multicentre randomised controlled trial: PROFHER and the Neer classification of proximal humeral fractures. Bone & Joint Research 2016;5(10):481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Handoll 2016b
- Handoll HH, Brealey SD, Keding A, Rangan A. Going far beyond the evidence (e-letter, 20 October 2016). Available at: online.boneandjoint.org.uk/doi/suppl/10.1302/2046-3758.510.2000638/suppl_file/handoll%20et%20al%20eletter%20re%202000638.pdf (accessed 7 July 2021).
Handoll 2017
- Handoll HH, Keding A, Corbacho B, Brealey SD, Hewitt C, Rangan A. Five-year follow-up results of the PROFHER trial comparing operative and non-operative treatment of adults with a displaced fracture of the proximal humerus. Bone & Joint Journal 2017;99-B(3):383-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasty 2017
- Hasty EK, Jernigan EW 3rd, Soo A, Varkey DT, Kamath GV. Trends in surgical management and costs for operative treatment of proximal humerus fractures in the elderly. Orthopedics 2017;40(4):e641-7. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008a
- Higgins JP, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies; Table 8.5a. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org.
Higgins 2008b
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org.
Hodgson 2003b [pers comm]
- Hodgson SS. Notice of a survey [personal communication]. Email to: H Handoll 14 May 2003.
Hodgson 2006
- Hodgson S. Proximal humerus fracture rehabilitation. Clinical Orthopaedics & Related Research 2006;(442):131-8. [PubMed] [Google Scholar]
Hodgson 2007
- Hodgson SA, Mawson SJ, Saxton JM, Stanley D. Rehabilitation of two-part fractures of the neck of the humerus (two-year follow-up). Journal of Shoulder & Elbow Surgery 2007;16(2):143-5. [DOI] [PubMed] [Google Scholar]
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI: 10.1136/bmj.g1687] [DOI] [PubMed] [Google Scholar]
Holbein 1999
- Holbein O, Bauer G, Hoellen I, Keppler P, Hehl G, Kinzl L. Is primary endoprosthetic replacement of the humeral head an alternative treatment for comminuted fractures of the proximal humerus in elderly patients? [Stellt die primare Implantation einer Humeruskopfprothese bei der dislozierten Humeruskopfmehrfragmentfraktur des alten Menschen eine Alternative zur Minimalosteosynthese dar?]. Osteosynthese International 1999;7 Suppl 2:207-10. [Google Scholar]
Jaeger 2015
- Jaeger M, Leung F, Li W. Nonoperative treatment. AO Surgery Reference: surgeryreference.aofoundation.org/orthopedic-trauma/adult-trauma/proximal-humerus (accessed 11 September 2015).
Jakob 1991
- Jakob RP, Miniaci A, Anson PS, Jaberg H, Osterwalder A, Ganz R. Four-part valgus impacted fractures of the proximal humerus. Journal of Bone and Joint Surgery. British Volume 1991;73(2):295-8. [DOI] [PubMed] [Google Scholar]
Jo 2019
- Jo YH, Lee KH, Lee BG. Surgical trends in elderly patients with proximal humeral fractures in South Korea: a population-based study. BMC Musculoskeletal Disorders 2019;20:[8 p.]. [DOI: 10.1186/s12891-019-2515-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karl 2015
- Karl JW, Olson PR, Rosenwasser MP. The epidemiology of upper extremity fractures in the United States, 2009. Journal of Orthopaedic Trauma 2015;29(8):e242-4. [DOI] [PubMed] [Google Scholar]
Koval 1997
- Koval KJ, Gallagher MA, Marsicano JG, Cuomo F, McShinawy A, Zuckerman JD. Functional outcome after minimally displaced fractures of the proximal part of the humerus. Journal of Bone and Joint Surgery. American Volume 1997;79(2):203-7. [DOI] [PubMed] [Google Scholar]
L'Insalata 1997
- L'Insalata JC, Warren RF, Cohen SB, Altchek DW, Peterson MG. A self-administered questionnaire for assessment of symptoms and function of the shoulder. Journal of Bone and Joint Surgery. American Volume 1997;79(5):738-48. [PubMed] [Google Scholar]
Launonen 2012
- Launonen AP, Lepola V, Flinkkila T, Strandberg N, Ojanpera J, Rissanen P, et al. Conservative treatment, plate fixation, or prosthesis for proximal humeral fracture. A prospective randomized study. BMC Musculoskeletal Disorders 2012;13:[7 p.]. [DOI: 10.1186/1471-2474-13-167] [DOI] [PMC free article] [PubMed] [Google Scholar]
Launonen 2015a
- Launonen AP, Lepola V, Flinkkila T, Laitinen M, Paavola M, Malmivaara A. Treatment of proximal humerus fractures in the elderly: a systemic review of 409 patients. Acta Orthopaedica 2015;86(3):280-5. [DOI: 10.3109/17453674.2014.999299] [DOI] [PMC free article] [PubMed] [Google Scholar]
Launonen 2015b
- Launonen AP, Lepola V, Saranko A, Flinkkilä T, Laitinen M, Mattila VM. Epidemiology of proximal humerus fractures. Archives of Osteoporosis 2015;10(209):[no pagination]. [DOI: 10.1007/s11657-015-0209-4] [DOI] [PubMed] [Google Scholar]
Lawton 1969
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9(3):176-86. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Li 2018
- Li M, Wang Y, Zhang Y, Yang M, Zhang P, Jiang B. Intramedullary nail versus locking plate for treatment of proximal humeral fractures: a meta-analysis based on 1384 individuals. Journal of International Medical Research 2018;46(11):4363-76. [DOI: 10.1177/0300060518781666] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2019
- Li F, Liu X, Wang F, Gu Z, Tao Q, Yao C, et al. Comparison between minimally invasive plate osteosynthesis and open reduction-internal fixation for proximal humeral fractures: a meta-analysis based on 1050 individuals. BMC Musculoskeletal Disorders 2019;20:[11 p.]. [DOI: 10.1186/s12891-019-2936-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lind 1989
- Lind T, Kroner K, Jensen J. The epidemiology of fractures of the proximal humerus. Archives of Orthopaedic and Trauma Surgery 1989;108(5):285-7. [DOI] [PubMed] [Google Scholar]
Lo 2012
- Lo KW, Ulery BD, Ashe KM, Laurencin CT. Studies of bone morphogenetic protein-based surgical repair. Advanced Drug Delivery Reviews 2012;64(12):1277-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahabier 2015
- Mahabier KC, Hartog DD, Van Veldhuizen J, Panneman MJ, Polinder S, Verhofstad MH, et al. Trends in incidence rate, health care consumption, and costs for patients admitted with a humeral fracture in The Netherlands between 1986 and 2012. Injury 2015;46(10):1930-7. [DOI] [PubMed] [Google Scholar]
Maniscalco 2014b
- Maniscalco P, Caforio M, Del Vecchio EO, D’Ascola J, Crainz E, Ferrata P. Preliminary experience with Diphos Nail in the treatment of proximal humerus fractures [Esperienza preliminare con il chiodo Diphos nail nel trattamento delle fratture prossimali di omero]. Giornale Italiano di Ortopedia e Traumatologia 2014;39(April):48-55. [Google Scholar]
Marsh 2007
- Marsh JL, Slongo TF, Agel J, Broderick JS, Creevey W, DeCoster TA, et al. Fracture and dislocation classification compendium - 2007: Orthopaedic Trauma Association classification, database and outcomes committee. Journal of Orthopaedic Trauma 2007;21(Suppl 10):S1-133. [DOI] [PubMed] [Google Scholar]
McKee 2007
- McKee MD. Commentary and perspective on "Immediate mobilization compared with conventional immobilization for the impacted nonoperatively treated proximal humeral fracture. A randomized controlled trial". JBJS-Online: www.ejbjs.org/Comments/2007/cp_dec07_mckee.dtl (accessed 08 January 2008). [DOI] [PubMed]
Mintken 2009
- Mintken PE, Glynn P, Cleland JA. Psychometric properties of the shortened Disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH) and Numeric Pain Rating Scale in patients with shoulder pain. Journal of Shoulder and Elbow Surgery 2009;18(6):920-6. [DOI] [PubMed] [Google Scholar]
Müller 1991
- Müller M, Allgower M, Schneider R, Willenegger H. Manual of Internal Fixation: Techniques Recommended by the AO-ASIF Group. 3rd edition. Berlin, Germany: Springer-Verlag, 1991. [Google Scholar]
Navarro 2018
- Navarro CM, Brolund A, Ekholm C, Heintz E, Ekstrom EH, Josefsson PO, et al. Treatment of humerus fractures in the elderly: a systematic review covering effectiveness, safety, economic aspects and evolution of practice. PloS One 2018;13(12):e0207815. [DOI: 10.1371/journal.pone.0207815] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neer 1970
- Neer CS. Displaced proximal humeral fractures. Classification and evaluation. Journal of Bone and Joint Surgery. American Volume 1970;52(6):1077-89. [PubMed] [Google Scholar]
Nwachukwu 2016
- Nwachukwu BU, Schairer WW, McCormick F, Dines DM, Craig EV, Gulotta LV. Arthroplasty for the surgical management of complex proximal humerus fractures in the elderly: a cost-utility analysis. Journal of Shoulder and Elbow Surgery 2016;25(5):704-13. [DOI] [PubMed] [Google Scholar]
Ockert 2014
- Ockert B, Pedersen V, Geyer L, Wirth S, Mutschler W, Grote S. Position of polyaxial versus monoaxial screws in locked plating for proximal humeral fractures: analysis of a prospective randomized study. European Journal of Orthopaedic Surgery and Traumatology 2014;24(5):747-52. [DOI] [PubMed] [Google Scholar]
Olerud 2011c
- Olerud P, Tidermark J, Ponzer S, Ahrengart L, Bergstrom G. Responsiveness of the EQ-5D in patients with proximal humeral fractures. Journal of Shoulder and Elbow Surgery 2011;20(8):1200-6. [DOI] [PubMed] [Google Scholar]
Orman 2020
- Orman S, Mohamadi A, Serino J, Murphy J, Hanna P, Weaver MJ, et al. Comparison of surgical and non-surgical treatments for 3- and 4-part proximal humerus fractures: a network meta-analysis. Shoulder & Elbow 2020;12(2):99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osterhoff 2017
- Osterhoff G, O'Hara NN, D'Cruz J, Sprague SA, Bansback N, Evaniew N, et al. A cost-effectiveness analysis of reverse total shoulder arthroplasty versus hemiarthroplasty for the management of complex proximal humeral fractures in the elderly. Value in Health 2017;20(3):404-11. [DOI] [PubMed] [Google Scholar]
Page 2014
- Page MJ, Green S, Kramer S, Johnston RV, McBain B, Buchbinder R. Electrotherapy modalities for adhesive capsulitis (frozen shoulder). Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No: CD011324. [DOI: 10.1002/14651858.CD011324] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palvanen 2006
- Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clinical Orthopaedics and Related Research 2006;(442):87-92. [DOI] [PubMed] [Google Scholar]
Polinder 2013
- Polinder S, Lordens GI, Panneman MJ, Eygendaal D, Patka P, Den Hartog D, et al. Trends in incidence and costs of injuries to the shoulder, arm and wrist in the Netherlands between 1986 and 2008. BMC Public Health 2013;13:[8 p.]. [DOI: 10.1186/1471-2458-13-531] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 2008
- Robinson BC, Athwal GS, Sanchez-Sotelo J, Rispoli DM. Classification and imaging of proximal humerus fractures. Orthopedic Clinics of North America 2008;39(4):393-403. [DOI] [PubMed] [Google Scholar]
Rosas 2018
- Rosas S, Kurowicki J, Yee T, Momoh E, Kalandiak SP, Levy JC. Cost of treatment for proximal humerus fractures: an acute and 90-day cost evaluation. Journal of Long Term Effects of Medical Implants 2018;28(3):173-9. [DOI] [PubMed] [Google Scholar]
Sabesan 2015
- Sabesan VJ, Valikodath T, Childs A, Sharma VK. Economic and social impact of upper extremity fragility fractures in elderly patients. Aging-Clinical & Experimental Research 2015;27(4):539-46. [DOI] [PubMed] [Google Scholar]
Sabharwal 2016a
- Sabharwal S, Carter AW, Rashid A, Darzi A, Reilly P, Gupte CM. Cost analysis of the surgical treatment of fractures of the proximal humerus: an evaluation of the determinants of cost and comparison of the institutional cost of treatment with the national tariff. Bone and Joint Journal 2016;98-B(2):249-59. [DOI] [PubMed] [Google Scholar]
Sabharwal 2016b
- Sabharwal S, Patel NK, Griffiths D, Athanasiou T, Gupte CM, Reilly P. Trials based on specific fracture configuration and surgical procedures likely to be more relevant for decision making in the management of fractures of the proximal humerus: findings of a meta-analysis. Bone and Joint Research 2016;5(10):470-80. [DOI: 10.1302/2046-3758.510.2000638] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schairer 2015
- Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. Reverse shoulder arthroplasty versus hemiarthroplasty for treatment of proximal humerus fractures. Journal of Shoulder and Elbow Surgery 2015;24(10):1560-6. [DOI: 10.1016/j.jse.2015.03.018] [DOI] [PubMed] [Google Scholar]
Schmitt 2004
- Schmitt JS, Di Fabio RP. Reliable change and minimum important difference (MID) proportions facilitated group responsiveness comparisons using individual threshold criteria. Journal of Clinical Epidemiology 2004;57(10):1008-18. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glaziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available at www.cochrane-handbook.org.
Shi 2019
- Shi X, Liu H, Xing R, Mei W, Zhang L, Ding L, et al. Effect of intramedullary nail and locking plate in the treatment of proximal humerus fracture: an update systematic review and meta-analysis. Journal of Orthopaedic Surgery and Research 2019;14(1):285. [DOI: 10.1186/s13018-019-1345-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sidor 1993
- Sidor ML, Zuckerman JD, Lyon T, Koval K, Cuomo F, Schoenberg N. The Neer classification system for proximal humeral fractures. An assessment of interobserver reliability and intraobserver reproducibility. Journal of Bone and Joint Surgery. American Volume 1993;75(12):1745-50. [DOI] [PubMed] [Google Scholar]
Siebenrock 1993
- Siebenrock KA, Gerber C. The reproducibility of classification of fractures of the proximal end of the humerus. Journal of Bone and Joint Surgery. American Volume 1993;75(12):1751-5. [DOI] [PubMed] [Google Scholar]
Sintonen 2001
- Sintonen H. The 15D instrument of health-related quality of life: properties and applications. Annals of Medicine 2001;33(5):328-36. [DOI] [PubMed] [Google Scholar]
Sjoden 1997
- Sjoden GO, Movin T, Guntner P, Aspelin P, Ahrengart L, Ersmark H, et al. Poor reproducibility of classification of proximal humeral fractures. Additional CT of minor value. Acta Orthopaedica Scandinavica 1997;68(3):239-42. [DOI] [PubMed] [Google Scholar]
Slobogean 2015
- Slobogean GP, Johal H, Lefaivre KA, MacIntyre NJ, Sprague S, Scott T, et al. A scoping review of the proximal humerus fracture literature. BMC Musculoskeletal Disorders 2015;16:[10 p.]. [DOI: 10.1186/s12891-015-0564-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 2016
- Solomon JA, Joseph SM, Shishani Y, Victoroff BN, Wilber JH, Gobezie R, Gillespie RJ. Cost analysis of hemiarthroplasty versus reverse shoulder arthroplasty for fractures. Orthopedics 2016;39(4):230-4. [DOI] [PubMed] [Google Scholar]
Sumrein 2017
- Sumrein BO, Huttunen TT, Launonen AP, Berg HE, Felländer-Tsai L, Mattila VM. Proximal humeral fractures in Sweden-a registry-based study. Osteoporosis International 2017;28(3):901-7. [DOI] [PubMed] [Google Scholar]
Sun 2018
- Sun Q, Ge W, Li G, Wu J, Lu G, Cai M, et al. Locking plates versus intramedullary nails in the management of displaced proximal humeral fractures: a systematic review and meta-analysis. International Orthopaedics 2018;42(3):641-50. [DOI: 10.1007/s00264-017-3683-z] [DOI] [PubMed] [Google Scholar]
Tashjian 2010
- Tashjian RZ, Deloach J, Green A, Porucznik CA, Powell AP. Minimal clinically important differences in ASES and simple shoulder test scores after non operative treatment of rotator cuff disease. Journal of Bone and Joint Surgery. American Volume 2010;92(2):296-303. [DOI] [PubMed] [Google Scholar]
Thanasas 2009
- Thanasas C, Kontakis G, Angoules A, Limb D, Giannoudis P. Treatment of proximal humerus fractures with locking plates: a systematic review. Journal of Shoulder and Elbow Surgery 2009;18(6):837-44. [DOI] [PubMed] [Google Scholar]
Thorsness 2016
- Thorsness R, Shields E, Iannuzzi JC, Zhang L, Noyes K, Voloshin I. Cost drivers after surgical management of proximal humerus fractures in Medicare patients. Journal of Orthopaedic Trauma 2016;30(5):262-8. [DOI] [PubMed] [Google Scholar]
Thorsness 2018
- Thorsness RJ, Iannuzzi JC, Shields EJ, Noyes K, Voloshin I. Cost-effectiveness of open reduction and internal fixation compared with hemiarthroplasty in the management of complex proximal humerus fractures. Journal of Shoulder and Elbow Arthroplasty 2018;2:1-9. [Google Scholar]
Tian 2016
- Tian F, Li C, Shao J. Comparison between locking proximal humerus plate and intramedullary nail in the treatment of humerus surgical neck fracture. Chinese Journal of Modern Drug Application 2016;10(14):22-3. [Google Scholar]
Torchia 2019
- Torchia MT, Austin DC, Cozzolino N, Jacobowitz L, Bell JE. Acute versus delayed reverse total shoulder arthroplasty for the treatment of proximal humeral fractures in the elderly population: a systematic review and meta-analysis. Journal of Shoulder and Elbow Surgery 2019;28(4):765-73. [DOI: 10.1016/j.jse.2018.10.004] [DOI] [PubMed] [Google Scholar]
Tornkvist 1995
- Tornkvist H, Ahrengart L, Sperber A. Tension band wiring vs. nonoperative treatment of proximal humerus fractures - a prospective randomized study (abstract). Acta Orthopaedica Scandinavica. Supplementum 1995;66(265):40-1. [Google Scholar]
Tousignant 2014
- Tousignant M, Gigurère A-M, Morin M, Pelletier J, Sheehy A, Cabana F. In-home telerehabilitation for proximal humerus fractures: a pilot study. International Journal of Telerehabilitation 2014;6(2):31-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van de Water 2014
- Van de Water AT, Shields N, Davidson M, Evans M, Taylor NF. Reliability and validity of shoulder function outcome measures in people with a proximal humeral fracture. Disability and Rehabilitation 2014;36(13):1072-9. [DOI] [PubMed] [Google Scholar]
Van Kempen 2013
- Van Kampen DA, Willems WJ, Van Beers LW, Castelein RM, Scholtes VA, Terwee CB. Determination and comparison of the smallest detectable change (SDC) and the minimal important change (MIC) of four-shoulder patient-reported outcome measures (PROMs). Journal of Orthopaedic Surgery and Research 2013;8(40):[9 p.]. [DOI: 10.1186/1749-799X-8-40] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verbeek 2012
- Verbeek PA, Van den Akker-Scheek I, Wendt KW, Diercks RL. Hemiarthroplasty versus angle-stable locking compression plate osteosynthesis in the treatment of three- and four-part fractures of the proximal humerus in the elderly: design of a randomized controlled trial. BMC Musculoskeletal Disorders 2012;13:[7 p.]. [DOI: 10.1186/1471-2474-13-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallace 2020
- Wallace CN, Kontoghiorghe C, Kayani B, Chang JS, Haddad FS. The impact of COVID-19 on trauma and orthopaedic surgery in the United Kingdom. Bone and Joint Open 2020;1(7):420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2015
- Wang G, Mao Z, Zhang L, Zhang L, Zhao Y, Yin P, et al. Meta-analysis of locking plate versus intramedullary nail for treatment of proximal humeral fractures. Journal of Orthopaedic Surgery and Research 2015;10(1):1-9. [DOI: 10.1186/s13018-015-0242-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2016
- Wang J, Zhu Y, Zhang F, Chen W, Tian Y, Zhang Y. Meta-analysis suggests that reverse shoulder arthroplasty in proximal humerus fractures is a better option than hemiarthroplasty in the elderly. International Orthopaedics 2016;40(3):531-9. [DOI: 10.1007/s00264-015-2811-x] [DOI] [PubMed] [Google Scholar]
Ware 1996
- Ware JE, Kosinski N, Keller SD. The 12-Item Short-Form Health Survey (SF-12): construction of scales and preliminary tests of reliability and validity. Medical Care 1996;34(3):220-33. [DOI] [PubMed] [Google Scholar]
WHO 2021
- World Health Organization. Rehabilitation. Fact Sheet. www.who.int/news-room/fact-sheets/detail/rehabilitation (accessed 25 May 2021).
Wu 2020
- Wu EJ, Zhang SE, Truntzer JN, Gardner MJ, Kamal RN. Cost-minimization analysis and treatment trends of surgical and nonsurgical treatment of proximal humerus fractures. Journal of Hand Surgery 2020;45(8):698-706. [DOI] [PubMed] [Google Scholar]
Xie 2016
- Xie L, Xing D, Ding F, Zhao Z. Operative versus non-operative treatment for complex proximal humeral fractures in the elderly patients: a systematic review of overlapping meta-analyses. International Journal of Clinical and Experimental Medicine 2016;9(7):13319-28. [Google Scholar]
Xie 2019
- Xie L, Zhang Y, Chen C, Zheng W, Chen H, Cai L. Deltoid-split approach versus deltopectoral approach for proximal humerus fractures: a systematic review and meta-analysis. Orthopaedics and Traumatology: Surgery and Research 2019;105(2):307-16. [DOI: 10.1016/j.otsr.2018.12.004] [DOI] [PubMed] [Google Scholar]
Zang 2018
- Zang JC, Du JJ, Li C, Wang JB, Ma XL. Comparison between minimally invasive plate osteosynthesis and open plating for proximal humeral fractures: a meta-analysis. Journal of Comparative Effectiveness Research 2018;7(10):1001-8. [DOI: 10.2217/cer-2018-0042] [DOI] [PubMed] [Google Scholar]
Zuckerman 2012
- Zuckerman JD. Hemiarthroplasty improved health-related quality of life more than nonoperative treatment in older patients with four-part proximal humeral fractures: commentary. Journal of Bone and Joint Surgery. American Volume 2012;94(10):942. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gibson 2000
- Gibson JN, Madhok R, Handoll HH. Interventions for treating proximal humeral fractures in adults. Cochrane Database of Systematic Reviews 2000, Issue 1 (No longer published; originally published in Issue 1, 2000 - Issue 4, 2000). [Google Scholar]
Gibson 2001
- Gibson JN, Handoll HH, Madhok R. Interventions for treating proximal humeral fractures in adults. Cochrane Database of Systematic Reviews 2001, Issue 1 (No longer published; originally published in Issue 1, 2001 - Issue 1, 2002). [Google Scholar]
Gibson 2002a
- Gibson JN, Handoll HH, Madhok R. Interventions for treating proximal humeral fractures in adults. Cochrane Database of Systematic Reviews 2002, Issue 2 (No longer published; originally published in Issue 2, 2002). [DOI] [PubMed] [Google Scholar]
Gibson 2002b
- Gibson JN, Handoll HH, Madhok R. Interventions for treating proximal humeral fractures in adults. Cochrane Database of Systematic Reviews 2002, Issue 3 (No longer published; originally published in Issue 3, 2002 - Issue 3, 2003). [Google Scholar]
Handoll 2003a
- Handoll HH, Gibson JN, Madhok R. Interventions for treating proximal humeral fractures in adults. Cochrane Database of Systematic Reviews 2003, Issue 4 (No longer published; originally published in Issue 4, 2003 - Issue 1, 2007). Art. No: CD000434. [DOI: 10.1002/14651858.CD000434] [DOI] [PubMed] [Google Scholar]
Handoll 2007
- Handoll HH, Madhok R. Interventions for treating proximal humeral fractures in adults. Cochrane Database of Systematic Reviews 2003, Issue 4. Art. No: CD000434. [DOI: 10.1002/14651858.CD000434] [DOI] [PubMed] [Google Scholar]
Handoll 2010
- Handoll HH, Ollivere BJ. Interventions for treating proximal humeral fractures in adults. Cochrane Database of Systematic Reviews 2010, Issue 12. Art. No: CD000434. [DOI: 10.1002/14651858.CD000434.pub2] [DOI] [PubMed] [Google Scholar]
Handoll 2012
- Handoll HH, Ollivere BJ, Rollins KE. Interventions for treating proximal humeral fractures in adults. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD000434. [DOI: 10.1002/14651858.CD000434.pub3] [DOI] [PubMed] [Google Scholar]
Handoll 2015b
- Handoll HH, Brorson S. Interventions for treating proximal humeral fractures in adults. Cochrane Database of Systematic Reviews 2015, Issue 11. Art. No: CD000434. [DOI: 10.1002/14651858.CD000434.pub4] [DOI] [PubMed] [Google Scholar]
Thomas 1996
- Thomas P, Kelly SM, Madhok R. Treatment of proximal humeral fractures in skeletally mature individuals. Cochrane Database of Systematic Reviews 1996, Issue 1 (No longer published; originally published in Issue 1, 1996 - Issue 4, 1999). [Google Scholar]


