Abstract
The seasonal dynamics of river biofilm communities in two German rivers, the Elbe and one of its tributaries, the Spittelwasser, were investigated for the first time by using fluorescence in situ hybridization and a standardized biofilm sampling procedure. We show the importance of members of the β subclass of the class Proteobacteria, which formed the largest single group in the massively polluted Spittelwasser at all times. Clear seasonal peaks of abundance were observed for the planctomycetes and the Cytophaga-Flavobacterium cluster.
In recent investigations of microbial communities in rivers in which the 16S ribosomal DNA approach has been used, the workers have focused on very few samples which were analyzed in depth (3, 8) or on succession during the development of a biofilm (13). They did not determine how the communities were affected by the seasonal fluctuations in physical and chemical parameters that typically occur in rivers. The dynamics of rivers make sampling of microbial communities which are representative of a certain geomorpholocial region and time span during the year very difficult. Therefore, we studied sessile microbial communities which grew on glass plates exposed at a defined depth below the river surface. Biofilms were harvested after 1 month of growth in situ. The microbial community composition was determined at the level of the major phylogenetic groups present. Quantification was based on microscopic counts of individual cells that hybridized with fluorescent probes directed to rRNA (fluorescence in situ hybridization [FISH]) (2). The standardized way of obtaining biofilm samples used in this study allowed us for the first time to compare seasonal dynamics of community structure in two rivers with different levels of pollution.
Study site.
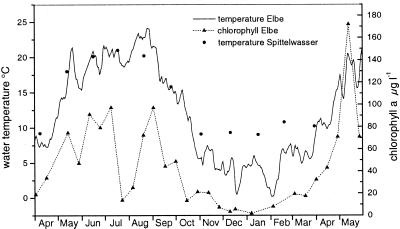
Biofilms were studied at the following two sites: (i) the Elbe River upstream of the city of Magdeburg, at km 320 (11°40′E, 52°4′N), which is one of the big rivers in Europe and has a catchment area of 148,268 km2 and a total length of 1,091 km (http://www.hamburg.de/Umwelt/wge/), and (ii) a massively polluted second-order tributary of the Elbe, the Spittelwasser River, which is close to the industrial center of Bitterfeld (12°17′E, 51°42′N). The Spittelwasser River had high ammonia concentrations in all seasons (average, 2.0 mg/liter, compared to 0.3 mg/liter in the Elbe), a lower oxygen content (average, 6.3 mg/liter, compared to 10.5 mg/liter in the Elbe), and high concentrations of heavy metals (mercury, cadmium, lead, copper, and arsenic), chlorinated aromatic compounds (hexachlorohexane, hexachlorobenzene, dichlorodiphenyl trichloroethane, polychlorinated biphenyl), adsorbable organic halogens, and organotin compounds in the suspended particulate matter which exceeded by far the concentrations in the Elbe. The water temperature of the Spittelwasser River was about 3 to 4°C higher than that of the Elbe from November to April due to discharge of warm factory production wastewater (Fig. 1). Primary production in the Elbe correlated well with the temperature, which started to increase in April and reached low values again at the beginning of October (Fig. 1). During this time microinvertebrates were frequently found in the biofilms.
FIG. 1.
Seasonal fluctuations in the water temperatures in the Elbe and Spittelwasser rivers and in the chlorophyll a concentrations in the Elbe River.
Sampling device and sampling procedure.
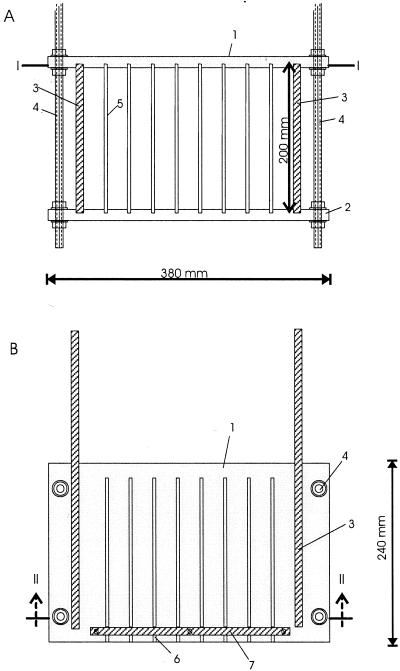
To raise a sufficient amount of natural biofilm in each river, a biofilm collector was constructed (Fig. 2). This device consisted of a Plexiglas frame carrying seven glass plates and two holders for microscopic slides. The frame was mounted on threaded rods. The collector was submerged 20 to 30 cm below the river surface, either by using a floating device (Elbe) or by inserting the rods into the river bottom (Spittelwasser). Biofilms were harvested monthly from May 1994 until September 1994 in the Elbe and in both the Elbe and the Spittelwasser from May 1997 until May 1998. After 35 ± 3 days of exposure to the river water the biofilm collector was removed from the river. The glass plates were taken out, and the biofilm was scraped off with a sterile razor blade. Clean glass plates and slides were inserted into the Plexiglas frame and exposed again in the river. Biofilm samples could not be collected in March and April 1998 due to a period of high water. The communities which developed on the plates can best be compared to the epilithic biofilms described by Lock (10). They were formed by single attached bacteria, algae, and trapped particulate matter glued together by extracellular polysaccharides. Thus, both free-living and particle-attached river bacteria were represented. Subsequently, growing processes, as well as grazing by both protozoans and macroinvertebrates, influenced the development of the biofilms. Biofilms and slides were fixed and then stored for FISH analysis for both gram-negative and gram-positive bacteria as described previously (2, 18) directly on-site by using a mobile laboratory.
FIG. 2.
Sampling device for growing biofilms on glass plates in a river (biofilm collector). (A) Cross-sectional view (line II—II in panel B). (B) Top view (line I—I in panel A). 1 and 2, top and bottom plates with eight grooves for exposed glass plates (380 by 240 by 15 mm); 3, side plate (200 by 400 by 10 mm); 4, threaded metal rod (length, 750 mm); 5, exposed glass plate (200 by 400 by 4 mm); 6, groove for exposed glass plate (4.5 by 5 by 220 mm); 7, stabilizing bar (5 by 10 by 270 mm).
Preparation of samples.
Prior to counting the contents of the samples had to be dispersed. An appropriate amount of each fixed biofilm sample (200 to 1,000 μl, depending on the ratio of fixative to biofilm) was centrifuged (2 min, 7,000 × g). Pellets (approximately 50 μl) were suspended in 950 μl of Na4P2O7 (0.1%, wt/vol) and homogenized by ultrasonification (30 s; 50 W; impulse, 0.6 s; Labsonic U; B. Braun). Approximately 1- to 2-μl portions of the samples were spotted onto gelatinated, Teflon-coated slides (Paul Marienfeld GmbH & Co. KG, Bad Mergentheim, Germany), dried for 20 min at 37°C, and dehydrated (2). This procedure yielded about 100 to 300 DAPI (4′,6′-diamidino-2-phenylindole)-stained cells per counting grid (10 by 10 fields; magnification, ×1,000).
Probes and stringency conditions.
Probes EUB338 (11), ALF1b (11), BET42a (11), GAM42a (11), CF319a (12), PLA46 (15), and HCG69a (18) were used as described previously. Nonfluorescent competitor probes were used in equal amounts with probes ALF1b, BET42a, GAM42a, and HGC69a to obtain optimal stringency conditions. BET42a and GAM42a served as competitors for each other. The competitor for HGC69a was probe NHGC (18). For probe ALF1b we obtained higher specificity by using two competitor probes, NEP (5′CGTTCAYTCTGAGCCAG3′) and NLHGC (5′CGTTCGYCCTGAGCCAG3′). Nonspecific binding was checked with probe NonEUB338 (22). The 5′ ends of the oligonucleotides were labeled with the indocarbocyanine dye Cy3 (TIB MOLBIOL, Berlin, Germany) except for the competitor probes. To check the stringency of hybridization directly for each slide, one well was used as a control; a mixture of easily distinguishable representative strains belonging to target and nontarget groups was applied.
Whole-cell hybridization of samples and DAPI staining.
FISH of whole cells was performed as described previously (2, 11). The concentration of each probe was 5 ng/μl of hybridization solution. Ten microliters of hybridization buffer containing probes was applied to each well. The washing buffer contained concentrations of NaCl which corresponded to the formamide concentrations in the hybridization buffer. The DNA-specific dye DAPI (16) was used to determine the total numbers of cells in the samples. Ten microliters of a DAPI solution (5.5 μg/ml) was added to each well. After 2 min the DAPI was carefully rinsed off with 50 ml of MilliQ water. The slides were air dried, mounted with Citifluor AF1 against bleaching, and sealed with coverslips (24 by 50 mm) and nail polish.
Microscopy and counting.
The hybridized and DAPI-stained samples were examined with an epifluorescence microscope (Axioplan; Zeiss, Oberkochen, Germany) equipped with a Plan-Neofluar objective (magnification, ×100). Excitation was carried out with a 50-W high-pressure mercury bulb (Osram HBO50) and filter sets for DAPI (filter set 01; Zeiss) and Cy3 (filter set 41007a HQ; AHF Analysentechnik, Tübingen, Germany). Two replicate samples were counted for each hybridization procedure. For each sample, 16 to 20 randomly chosen microscopic fields were counted per well by using a counting grid, the data for the two parallel counts were averaged, and the standard deviation was determined. For each grid up to 300 DAPI-stained cells were counted, and for each hybridization more than 4,000 DAPI-stained cells were counted.
Biofilm in situ hybridization.
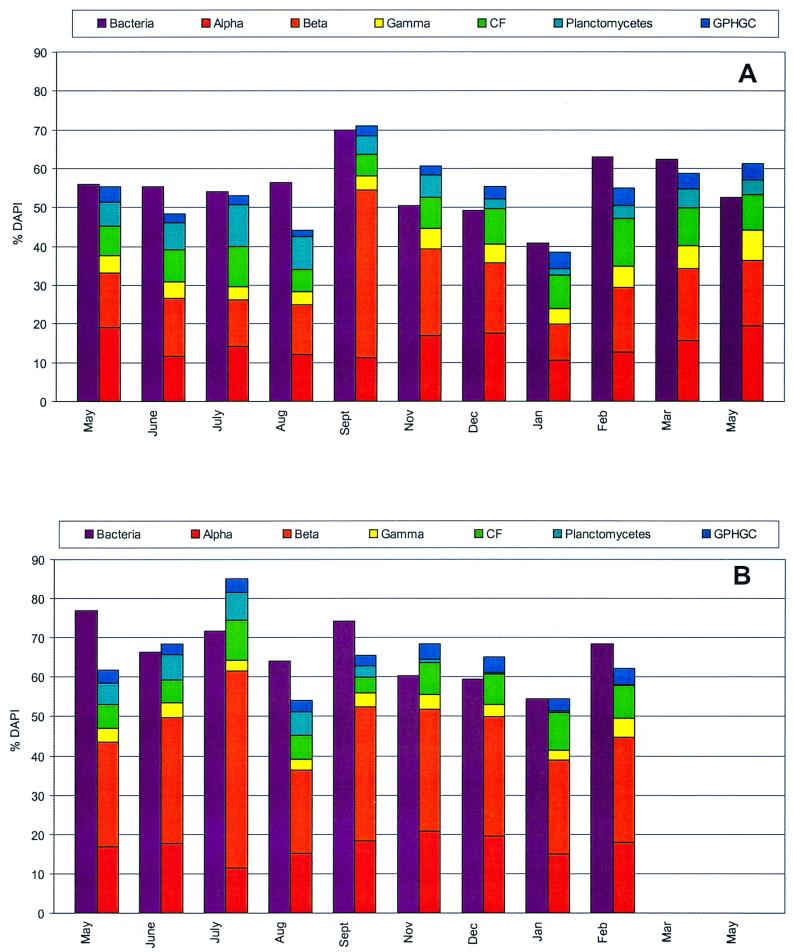
The average staining efficiency of the bacterial probe was 56% for the Elbe biofilms and was consistently 10% higher for the Spittelwasser biofilms. For the Elbe, the highest percentage of EUB338-stained cells was found in September (70.0%) (Fig. 3A); this maximum value corresponded to a bloom of members of the β subclass of the class Proteobacteria (β-Proteobacteria) during this period. The lowest percentage of EUB338-stained cells (40.8%) was observed in January. For the Spittelwasser biofilms (Fig. 3B), a bloom of β-Proteobacteria was observed in July. No pronounced decline in staining efficiency occurred in January. These values are within the range of values frequently observed for aquatic habitats (reviewed in reference 4). Since low-nutrient (oligotrophic) environments are expected to have lower staining efficiencies than high-nutrient (eutrophic) environments (6, 8, 23), the data might indicate that the activity of the Spittelwasser biofilms was higher because of a higher nutrient level. However, some investigators have observed unexpectedly high staining efficiencies for low-nutrient and/or cold habitats, such as an oligotrophic river (13), drinking water biofilms (7), and the winter cover and pelagic layers of an oligotrophic mountain lake (1).
FIG. 3.
Abundance of major phylogenetic groups in river biofilms during one annual cycle as determined by whole-cell hybridization of biofilm samples with rRNA-targeted fluorescent probes. Alpha, α-Proteobacteria; Beta, β-Proteobacteria; Gamma, γ-Proteobacteria; CF, CF cluster; GPHGC, gram-positive bacteria with high G+C contents. (A) Elbe River. (B) Spittelwasser River.
Effect of pollution on river biofilm community structure.
The two rivers which we compared differed with respect to pollution (extreme pollution in the Spittelwasser, medium pollution in the Elbe) and with respect to size and the parameters correlated with it (e.g., flow velocity). However, the major phylogenetic groups of bacteria were present in both rivers at similar abundances (Fig. 3). One significant difference was the large proportion of β-Proteobacteria in the Spittelwasser biofilms; in these biofilms they were the most abundant organisms at all times, accounting for on average 31% of all of the cells (compared to 18% in the Elbe biofilms). The ammonia concentrations were significantly higher in the Spittelwasser River than in the Elbe throughout the year and might have supported a large population of ammonia-oxidizing bacteria, which are a monophyletic assemblage in the β-Proteobacteria (20). The γ-Proteobacteria did not constitute a major component of the community, based on numerical abundance, neither in the Spittelwasser nor in the Elbe biofilms. In the past many biodegrading strains were assigned to the genus Pseudomonas, which forms a tight cluster within the γ-Proteobacteria (14). However, since then several former Pseudomonas species have been redescribed and assigned to members of the β-Proteobacteria (9), and several new biodegrading strains belonging to the β-Proteobacteria have been isolated (21). Thus, some of the diversity of the β-Proteobacteria in the Elbe and particularly in the Spittelwasser River may be due to pollutant-degrading bacteria.
Seasonal fluctuations in the planctomycetes and the Cytophaga-Flavobacterium cluster.
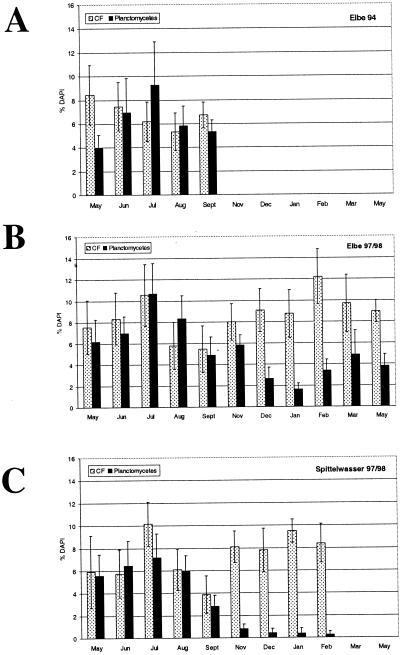
For the Proteobacteria and gram-positive bacteria with high G+C contents, no seasonal pattern was observed, probably because of the great diversity of physiologically different microorganisms in these groups. Another reason might be that despite the open character and dynamics of rivers, there is a certain uniformity in the microbial community. A seasonal cycle of abundance was observed for the planctomycetes and the Cytophaga-Flavobacterium (CF) cluster (Fig. 4). The maximum level of planctomycetes occurred in July, and the abundance decreased during autumn; the densities were especially low in December, January, and February both in the Elbe and in the Spittelwasser River. The July maximum was confirmed by analyzing biofilms which were collected in the Elbe River in 1994. Less pronounced but still significant was the bimodal distribution of the abundance of members of the CF cluster; a maximum occurred in July, and a second maximum occurred in the winter (in February for the Elbe and in January for the Spittelwasser). Thus, in the cold season (Fig. 1), low levels of planctomycetes and high levels of members of the CF cluster were present. These general patterns occurred in both rivers and thus must have been caused by factors common to both. Maximum levels of members of the CF cluster have been observed frequently in the winter in aquatic environments, as determined by both culturing and culture-independent methods (8, 17). Planctomycetes are widespread in aquatic habitats (15) and have been shown to follow or accompany algal or cyanobacterial blooms (for a review, see reference 5). However, as-yet-uncultured members of the planctomycetes might have completely different physiological properties (19).
FIG. 4.
Seasonal variation in abundance of members of the planctomycetes and the CF cluster (CF) in biofilms obtained from the Elbe River in 1994 (A) and 1997–1998 (B) and in biofilms obtained from the Spittelwasser River in 1997–1998 (C).
Our analysis of standardized biofilms by the whole-cell FISH method revealed for the first time that there are seasonal patterns of abundance for the smaller, less diverse phylogenetic groups in rivers and that there may be a relationship between the abundance of β-Proteobacteria and the degree of pollution. Moreover, the observed stability of the overall river biofilm community structure in spite of significant variations in chemical and biological parameters during the year was an interesting finding which needs to be confirmed by analyses that provide higher resolution.
Acknowledgments
We are grateful to S. Wolff and H. Reincke of the ARGE (Cooperative for the Protection of the Elbe), E. Becker of the STAU (Federal Bureau of Environment Protection, Magdeburg, Germany), and D. Spott and H. Guhr of UFZ (Center for Environmental Research Leipzig-Hallebranch Magdeburg) for access to sampling sites, data, and site maps. We thank F. Walkow of the Landratsamt Bitterfeld, V. Harms of the Regierungspräsidium Dessau, H. Meye and A. Schlicht of the STAU Dessau/Wittenberg for providing Spittelwasser data. We especially thank S. Thieme of the measuring station in Magdeburg, Germany, who was always ready to help. Critical comments on the manuscript by Andreas Felske are gratefully acknowledged.
This work was funded by a grant from the Studienstiftung des Deutschen Volkes to Ingrid Brümmer and by a grant from the Niedersächsischer Schwerpunkt Meeresbiotechnologie to Wanda Fehr.
REFERENCES
- 1.Alfreider A, Pernthaler J, Amann R, Sattler B, Glöckner F-O, Wille A, Psenner R. Community analysis of the bacterial assemblages in the winter cover and pelagic layers of a high mountain lake by in situ hybridization. Appl Environ Microbiol. 1996;62:2138–2144. doi: 10.1128/aem.62.6.2138-2144.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann R I. In situ identification of micro-organisms by whole cell hybridization with rRNA-targeted nucleic acid probes. Mol Microb Ecol Man. 1995;3.3.6:1–15. [Google Scholar]
- 3.Crump B C, Armbrust E V, Baross J A. Phylogenetic analysis of particle-attached and free-living bacterial communities in the Columbia River, its estuary, and the adjacent coastal ocean. Appl Environ Microbiol. 1999;65:3192–3204. doi: 10.1128/aem.65.7.3192-3204.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daims H, Brühl A, Schleifer K-H, Wagner M. The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol. 1999;22:434–444. doi: 10.1016/S0723-2020(99)80053-8. [DOI] [PubMed] [Google Scholar]
- 5.Fürst J A. The planctomycetes: emerging models for microbial ecology, evolution and cell biology. Microbiology. 1995;141:1493–1506. doi: 10.1099/13500872-141-7-1493. [DOI] [PubMed] [Google Scholar]
- 6.Glöckner F-O, Fuchs B M, Amann R. Bacterioplankton compositions of lakes and oceans: a first comparison based on fluorescence in situ hybridization. Appl Environ Microbiol. 1999;65:3721–3726. doi: 10.1128/aem.65.8.3721-3726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalmbach S, Manz W, Szewzyk U. Dynamics of biofilm formation in drinking water: phylogenetic affiliation and metabolic potential of single cells assessed by formazan reduction and in situ hybridization. Microb Ecol. 1997;22:265–279. [Google Scholar]
- 8.Kenzaka T, Yamaguchi N, Tani K, Nasu M. rRNA-targeted fluorescent in situ hybridization analysis of bacterial community structure in river water. Microbiology. 1998;144:2085–2093. doi: 10.1099/00221287-144-8-2085. [DOI] [PubMed] [Google Scholar]
- 9.Lessie T G, Hendrickson W, Manning B D, Devereux R. Genomic complexity and plasticity of Burkholderia cepacia. FEMS Microbiol Lett. 1996;144:117–128. doi: 10.1111/j.1574-6968.1996.tb08517.x. [DOI] [PubMed] [Google Scholar]
- 10.Lock M A. Attached microbial communities in rivers. In: Ford T E, editor. Aquatic microbiology—an ecological approach. Oxford, United Kingdom: Blackwell Scientific Publications; 1993. pp. 113–138. [Google Scholar]
- 11.Manz W, Amann R, Ludwig W, Wagner M, Schleifer K-H. Phylogenetic oligodeoxynucleotide probes for the major subclasses of Proteobacteria: problems and solutions. Syst Appl Microbiol. 1992;15:593–600. [Google Scholar]
- 12.Manz W, Amann R, Ludwig W, Vancanneyt M, Schleifer K-H. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga-flavobacter-bacteroides in the natural environment. Microbiology. 1996;142:1097–1106. doi: 10.1099/13500872-142-5-1097. [DOI] [PubMed] [Google Scholar]
- 13.Manz W, Wendt-Pothoff K, Neu T R, Szewzyk U, Lawrence J R. Phylogenetic composition, spatial structure, and dynamics of lotic bacterial biofilms investigated by fluorescent in situ hybridization and confocal laser scanning microscopy. Microb Ecol. 1999;37:225–237. doi: 10.1007/s002489900148. [DOI] [PubMed] [Google Scholar]
- 14.Moore E R B, Mau M, Arnscheidt A, Böttger E C, Hutson R A, Collins M D, Van de Peer A, De Wachter R, Timmis K N. The determination and comparison of the 16S rRNA gene sequences of species of the genus Pseudomonas (sensu strictu) and estimation of the natural intrageneric relationships. Syst Appl Microbiol. 1996;1515:1–15. [Google Scholar]
- 15.Neef A, Amann R, Schlesner H, Schleifer K-H. Monitoring a widespread bacterial group: in situ detection of planctomycetes with 16S rRNA-targeted probes. Microbiology. 1998;144:3257–3266. doi: 10.1099/00221287-144-12-3257. [DOI] [PubMed] [Google Scholar]
- 16.Porter K, Feig Y G. The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr. 1980;25:943–948. [Google Scholar]
- 17.Reichenbach H. The order Cytophagales. In: Balows A, Truper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. A handbook on habitat, isolation and identification of bacteria. Vol. 4. Berlin, Germany: Springer-Verlag; 1992. pp. 3631–3675. [Google Scholar]
- 18.Roller C, Wagner M, Amann R, Ludwig W, Schleifer K-H. In situ probing of Gram-positive bacteria with high DNA G+C content using 23S rRNA-targeted oligonucleotides. Microbiology. 1994;140:2849–2858. doi: 10.1099/00221287-140-10-2849. [DOI] [PubMed] [Google Scholar]
- 19.Strous M, Fuerst J A, Kramer E H M, Logemann S, Muyzer G, Van de Pas-Schoonen K T, Webb R, Kuenen J G, Jetten M S M. Missing lithotroph identified as new planctomycete. Nature. 1999;400:446–449. doi: 10.1038/22749. [DOI] [PubMed] [Google Scholar]
- 20.Teske A, Alm E, Regan J M, Toze S, Rittmann B E, Stahl D A. Evolutionary relationships among ammonia- and nitrite-oxidizing bacteria. J Bacteriol. 1994;176:6623–6630. doi: 10.1128/jb.176.21.6623-6630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Schie P M, Yong L Y. Isolation and characterization of phenol-degrading denitrifying bacteria. Appl Environ Microbiol. 1998;64:2432–2438. doi: 10.1128/aem.64.7.2432-2438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallner G, Amann R, Beisker W. Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry. 1993;14:136–143. doi: 10.1002/cyto.990140205. [DOI] [PubMed] [Google Scholar]
- 23.Weiss P, Schweitzer B, Amann R, Simon M. Identification in situ and dynamics of bacteria on limnetic organic aggregates (Lake Snow) Appl Environ Microbiol. 1996;62:1998–2005. doi: 10.1128/aem.62.6.1998-2005.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]