ABSTRACT
Faropenem (FRPM) is active against extended-spectrum β-lactamase (ESBL)-producing Enterobacterales, but evidence for its efficacy is lacking. This study determined the correlation between the susceptibility by disk diffusion method and the MIC of FRPM for third-generation cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae, and the effectiveness of FRPM for the treatment of urinary tract infection (UTI) caused by these two bacteria in a retrospective cohort analysis. Of the 48 third-generation cephalosporin-resistant clinical isolates tested, 44 isolates produced ESBL, and 8 isolates produced AmpC, including 4 isolates produced both ESBL and AmpC. Thirty-seven isolates had an FRPM MIC of ≤1 mg/L, and seven had an FRPM MIC of 2 mg/L. An FRPM MIC of >2 mg/L was observed with four isolates. In a retrospective cohort analysis, 63 patients with UTI treated with FRPM were identified. All isolates of ESBL-producing E. coli (n = 54) and K. pneumoniae (n = 9) treated with FRPM showed disk diffusion zone diameters larger than 16.0 mm (estimated MIC, 2.2 mg/L). All patients completed the scheduled treatment courses with FRPM, but 28- and 90-day relapses happened in 10 patients (16%) and 16 patients (25%), respectively. No significant risk factors for the 28- and 90-day relapses were found. FRPM can be used according to disk diffusion susceptibility testing in UTI. Further investigations are necessary to assess the clinical breakpoint of FRPM for ESBL-producing Enterobacterales and the candidates most likely to benefit from using FRPM.
KEYWORDS: extended-spectrum β-lactamase, AmpC β-lactamase, Escherichia coli, Klebsiella pneumoniae, faropenem, urinary tract infection
INTRODUCTION
Enterobacterales, such as Escherichia coli, Klebsiella spp., and Proteus spp., produce extended-spectrum β-lactamases (ESBLs), which belong to the A Ambler classification and hydrolyze a wide range of β-lactam antimicrobial agents, including penicillin, oxyimino-cephalosporins, and monobactams (1). The spread of ESBL-producing Enterobacterales in both community and health care settings has become a global health concern (2, 3). In Japan, the rates of third-generation cephalosporin resistance in E. coli and Klebsiella pneumoniae have been increasing since the 2000s (4). Carbapenems, which are stable against hydrolysis by ESBL enzymes, are effective for treating infections caused by ESBL-producing Enterobacterales. This also applies to cephamycins, including cefmetazole (CMZ), which possess clinical and microbiological efficacies comparable to those of carbapenems according to observational studies (5–7). Faropenem (FRPM) is an oral antimicrobial agent with activity against ESBL-producing Enterobacterales; however, clinical evidence regarding its efficacy is lacking. AmpC is another β-lactamase of Enterobacterales belonging to class C of the Ambler classification and hydrolyzes third-generation cephalosporins. Some ESBL-producing Enterobacterales coproduce AmpC. Notably, FRPM is hydrolyzed by AmpC-producing Enterobacterales in vitro (8), but the clinical implications of this phenomenon are unclear.
In this study, we determined the MICs of FRPM for ESBL and AmpC-producing Enterobacterales in clinical isolates of third-generation cephalosporin-resistant E. coli and K. pneumoniae using the broth microdilution method to assess their correlations with disk diffusion testing results. We also retrospectively assessed the 28-day relapse of patients using FRPM and the clinical efficacy of FRPM for urinary tract infections (UTIs) with ESBL-producing Enterobacterales.
RESULTS
Correlation of susceptibility between the broth microdilution and disk diffusion testing methods for FRPM in third-generation cephalosporin-resistant isolates.
The MICs of FRPM determined using the broth microdilution method with profiles of ESBL- and AmpC-production are listed in Table 1. Between 1 January and 30 June 2020, 45 E. coli (ESBL producing, n = 40; AmpC producing, n = 5) and 9 K. pneumoniae (ESBL producing, n = 4; ESBL and AmpC producing, n = 4; and AmpC producing, n = 1) were isolated from 33 urine, 13 blood, 5 sputum, and 3 wound culture specimens. Overall, the MIC50 and the MIC90 of FRPM were 1 and 2 mg/L, respectively. We detected 37 isolates (77%) with an MIC of ≤1 mg/L, including 3 AmpC-producing isolates. Seven isolates had an MIC of 2 mg/L (15%), which included one AmpC-producing isolate. In contrast, all 4 AmpC/ESBL-producing isolates (8%) showed MICs of >2 mg/L. The distributions of MICs for all tested antimicrobials for 54 isolates are shown in Tables S1 to S3 in the supplemental material. All 44 ESBL-producing isolates were susceptible to CMZ, meropenem (MEPM), and imipenem, whereas all 6 AmpC-producing isolates were susceptible to cefepime, MEPM, imipenem, ciprofloxacin, and tobramycin. All four AmpC/ESBL-producing isolates were susceptible to MEPM, imipenem, amikacin, and gentamicin.
TABLE 1.
Antimicrobial activity of FRPM against ESBL- and AmpC-producing Enterobacterales strains classified by phenotype (broth microdilution testing, n = 48)
| Phenotype | No. (%) of strains at various MICs (mg/L) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ≤0.12 | 0.12 | 0.25 | 0.5 | MIC50 | MIC90 | 4 | 8 | >8 | |
| ESBL+ AmpC− (n = 40) | 1 | 14 | 19 | 6 | |||||
| ESBL+ AmpC+ (n = 4) | 2 | 2 | |||||||
| ESBL− AmpC+ (n = 4) | 3 | 1 | |||||||
| Total | 1 (2) | 14 (29) | 22 (46) | 7 (15) | 0 (0) | 2 (4) | 2 (4) | ||
The correlation between the MICs and disk diffusion testing results for FRPM was examined in 48 isolates for which the results of the disk diffusion test were available. As shown in Table 1, all ESBL-producing isolates (36 E. coli and 4 K. pneumoniae isolates) and AmpC-producing isolates (4 E. coli isolates) showed low MICs to FRPM, whereas isolates producing both AmpC/ESBL (4 K. pneumoniae isolates) showed a markedly high MICs to FRPM.
Among the 44 ESBL-producing isolates, 16 (36%) (13 E. coli and 3 K. pneumoniae) possessed the CTX-M-1 group gene, 22 (50%) (21 E. coli and 1 K. pneumoniae) possessed the CTX-M-9 group gene, 10 (23%) (2 E. coli and 8 K. pneumoniae) the SHV gene, and 21 (48%) (17 E. coli and 4 K. pneumoniae) had the TEM gene.
Among the 8 AmpC-producing isolates, 3 isolates had the CIT genotype and 4 isolates had the DHA gene. Although the production of AmpC was detected phenotypically, we did not detect any of the investigated resistant genes in one isolate. Some isolates possessed multiple resistance genes (Table 2).
TABLE 2.
Genotypes of ESBL and AmpC-producing Enterobacterales strains
| Gene(s) | No. (%) of strains |
||
|---|---|---|---|
| E. coli | K. pneumoniae | Total | |
| ESBL-producing Enterobacterales β-lactamase gene combination | n = 36 | n = 8 | n = 44 |
| TEM, SHV, CTX-M-1 | 0 (0) | 3 (38) | 3 (7) |
| TEM, CTX-M-1 | 8 (22) | 0 (0) | 8 (18) |
| TEM, SHV, CTX-M-9 | 0 (0) | 1 (13) | 1 (2) |
| CTX-M-9 | 13 (36) | 0 (0) | 13 (30) |
| TEM, CTX-M-9 | 8 (20) | 0 (0) | 8 (18) |
| SHV | 1 (3) | 4 (50) | 5 (11) |
| TEM, SHV | 1 (3) | 0 (0) | 1 (2) |
| CTX-M-1 | 5 (14) | 0 (0) | 5 (11) |
| AmpC-producing Enterobacterales β-lactamase gene | n = 4 | n = 4 | n = 8 |
| CIT | 3 (75) | 0 (0) | 3 (38) |
| DHA | 1 (25) | 3 (75) | 4 (50) |
| Not detected | 0 (0) | 1 (25) | 1 (13) |
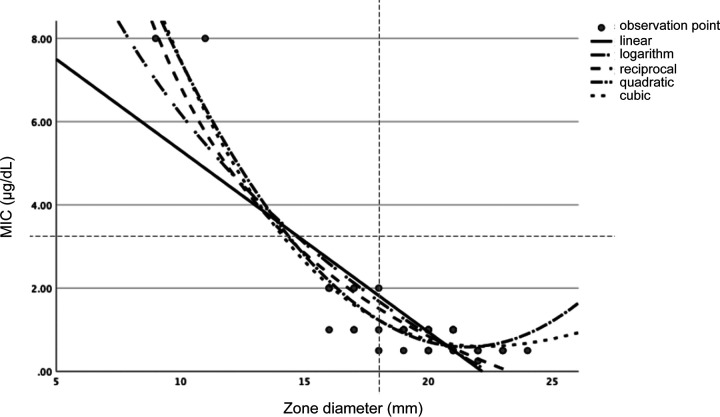
A scatterplot and approximate function for FRPM susceptibility using 48 strains were generated using the disk diffusion testing and broth microdilution testing results, excluding isolates with MICs of >8 mg/L because the precise MIC was not available (Fig. 1).
FIG 1.
Scatterplot of MICs (μg/dL) and inhibition zone diameters (mm) of FRPM for ESBL- and AmpC-producing Enterobacterales isolates. Approximate equations were derived by applying linear, quadratic, cubic, logarithmic, and reciprocal functions.
The correlation analysis yielded the following equations:
We found that the approximate equation of the cubic function had the highest correlation coefficient. Approximate equations for the estimated MIC (eMIC) and disk diffusion testing were used to derive the following reference values: susceptible, eMIC ≤ 2.2 mg/L; intermediate, eMIC = 2.8 to 4.3 mg/L; and resistant, eMIC > 5.2 mg/L.
Clinical course of patients with UTIs treated with FRPM.
Between October 2010 and April 2021, 63 patients with UTI were treated with FRPM at our hospital (Table 3). The median age (interquartile range [IQR]) of the patients was 72 years. The number of males was 24 (38%). The total number of patients with underlying diseases of the urinary system was 23 (37%), including 14 (22%) who underwent post-urological surgery.
TABLE 3.
Clinical characteristics of patients with urinary tract infections treated with faropenema
| Parameter | Result |
|---|---|
| No. of patients | 63 |
| Median age in yrs (IQR) | 72 (23) |
| No. (%) male | 24 (38) |
| No. (%) of underlying diseases of the urinary systemb | 23 (37) |
| No. (%) of subjects needing post-urological surgery | 14 (22) |
| No. (%) of causative pathogens | |
| Escherichia coli | 54 (86) |
| Klebsiella pneumoniae | 9 (14) |
| Median inhibition zone diam (minimum–maximum, IQR) in mm | 20.0 (16.0–26.0, 3.0) |
| eMIC (minimum–maximum) in mg/Lc | 1.0 (1.3–2.2) |
| No. (%) of subjects with complicated UTI | 56 (89) |
| Diagnosis, no. (%) of subjects with | |
| Acute pyelonephritis | 32 (51) |
| Prostatitis | 6 (10) |
| Unclassifiable | 8 (13) |
| Bacteremia | 10 (16) |
| Cystitis | 17 (27) |
| No. (%) of subjects who switched from other antimicrobial agents | |
| As empirical treatment | 31 (49) |
| CMZ | 11 (17) |
| MEPM | 15 (24) |
| PIPC/TAZ | 3 (5) |
| Fluoroquinolone | 2 (3) |
| FOM | 1 (2) |
| No. (%) of subjects on therapy regimen with FRPM | |
| Monotherapy | 36 (57) |
| Combination therapy with FOM | 25 (40) |
| Combination therapy with a fluoroquinolone | 2 (3) |
| Duration of antimicrobial treatment | |
| Total median duration of treatment in days (IQR) | 16.0 (18) |
| Median duration of FRPM treatment in days (IQR) | 14.0 (17) |
| Clinical effectiveness, no. (%) of subjects | |
| 28-day relapse/28-day reinfection after completion of FRPM | 10 (16)/3 (5) |
| 90-day relapse/90-day reinfection after completion of FRPM | 16 (25)/4 (6) |
| All-cause mortality/ICU admission | 0/0 |
CMZ, cefmetazole; eMIC, estimated MIC; FOM, fosfomycin; IQR, interquartile range; MEPM, meropenem; PIPC/TAZ, piperacillin-tazobactam; ICU, intensive care unit.
Including after urological surgery.
The eMIC in the minimum range is more than the median eMIC due to the approximate function calculated by 9 to 24 (mm).
Fifty-four ESBL-producing E. coli (86%) and nine ESBL-producing K. pneumoniae (14%) samples were isolated from the patients. The median zone-of-inhibition diameter was 20.0 mm (IQR, 3.0).
All of the isolates from the patients treated with FRPM showed zone of inhibition diameters of ≥16 mm in FRPM, which was recognized as tentatively susceptible and equivalate with eMIC from the cubic function obtained above as 2.2 mg/L. Of the 63 UTI patients, 56 patients (88.9%) were diagnosed with complicated UTI. Pyelonephritis was the most frequent diagnosis (32 patients, 51%), followed by cystitis (17 patients, 27%), and 10 patients (16%) were diagnosed with bacteremia. Thirty-two patients (51%) switched from other antimicrobial agents, MEPM (n = 15), CMZ (n = 11), piperacillin-tazobactam (n = 3), fluoroquinolone (n = 2), and fosfomycin (FOM) (n = 1). FRPM was used as monotherapy by 36 patients (57%). Twenty-seven patients (43%) were treated with FRPM in combination with oral FOM (n = 25) or oral fluoroquinolone (n = 2). The total median duration of treatment was 16 days (IQR, 18), whereas the median duration of FRPM treatment was 14 (IQR, 17). All patients successfully completed scheduled antimicrobial treatment courses using FRPM, but 10 patients (16%) and 16 patients (25%) experienced 28- and 90-day relapses after the completion of treatment, respectively. No patient was admitted to the intensive care unit, and all-cause mortality did not occur. We extracted baseline characteristics of 16 relapsing patients in Table S4 in the supplemental material. The duration of treatment was more than 7 days for cystitis, more than 14 days for pyelonephritis (11 days in only 1 case of complicated UTI), and more than 35 days for prostatitis, respectively. Blood culture positive were only 3 cases (19%) in pyelonephritis. There were three cases (19%) inserting the urinary catheter and 10 (63%) cases in uncontrolled source due to fistula, cancer, and benign prostate hypertrophy and so on. Bivariate analysis of the 28- and 90-day relapses after completion of treatment in patients with UTI was performed, which revealed no significantly different factors (Tables 4 and 5).
TABLE 4.
Univariate analysis comparing the urinary tract infection patient groups with or without 28-day relapse
| Parametera | Patient groups with or without a 28-day relapse |
P | |
|---|---|---|---|
| Without (n = 53) | With (n = 10) | ||
| Median age in yrs (IQR) | 73.0 (23) | 64.0 (29) | 0.21 |
| Male, no. (%) of subjects | 19 (36) | 5 (50) | 0.31 |
| No. (%) of subjects with underlying urinary diseaseb | 19 (36) | 4 (40) | 1.00 |
| Escherichia coli, no. (%) | 45 (85) | 9 (90) | 1.00 |
| Klebsiella pneumoniae, no. (%) | 8 (15) | 1 (10) | |
| Zone diam in mm (minimum–maximum, IQR)/eMIC(minimum–maximum) in mg/L | 20.0 (16.0–26.0, 3)/1.0 (1.3–2.2) | 20.0 (18.0–22.0, 4)/0.78 (0.9–1.4) | 0.89 |
| No. (%) of subjects with complicated UTI | 48 (91) | 8 (80) | 0.31 |
| No. (%) of subjects with diagnosis of | |||
| Acute pyelonephritis | 28 (53) | 6 (60) | |
| Prostatitis | 5. (9) | 1. (10) | |
| Unclassifiable | 6 (11) | ||
| Cystitis | 14 (26) | 3 (30) | 1.00 |
| FRPM as empirical treatment, no. (%) | 26 (49) | 5 (50) | 1.00 |
| Combination therapy, no. (%) | 21 (40) | 6 (60) | 0.30 |
| Total duration of treatment, days (IQR) | 15 (20) | 26.5 (24) | 0.24 |
| Duration of FRPM treatment, days (IQR) | 14 (18) | 14 (16) | 0.46 |
eMIC, estimated MIC; IQR, interquartile range.
Including post-urological surgery.
TABLE 5.
Univariate analysis comparing the urinary tract infection patient groups with and without 90-day relapse
| Parametera | Patient groups with or without a 90-day relapse |
P | |
|---|---|---|---|
| Without (n = 47) | With (n = 16) | ||
| Median age in yrs (IQR) | 73.0 (24) | 68.0 (26) | 0.47 |
| Male, no. (%) | 16 (34) | 8 (50) | 0.26 |
| No. (%) of subjects with underlying urinary diseaseb | 16 (34) | 7 (44) | 0.49 |
| Zone diam, in mm (minimum–maximum, IQR)/eMIC(minimum–maximum) in mg/L | 20.0 (16.0–26.0, 3)/1.0 (1.3–2.2) | 20.0 (18.0–22.0, 4)/0.78 (0.9–1.4) | 0.68 |
| Escherichia coli, no. (%) | 40 (85) | 14 (88) | 1.00 |
| Klebsiella pneumoniae, no. (%) | 7 (15) | 2 (12) | |
| No. (%) of subjects with complicated UTI | 43 (91) | 13 (81) | 0.36 |
| No. (%) of subjects with diagnosis of | |||
| Acute pyelonephritis | 4 (9) | 9 (56) | |
| Prostatitis | 25 (53) | 2 (13) | |
| Unclassifiable | 5 (11) | 1 (6) | |
| Cystitis | 13 (28) | 4 (25) | 1.00 |
| FRPM as empirical treatment, no. (%) | 23 (49) | 8 (50) | |
| Combination therapy, no. (%) | 18 (38) | 9 (56) | 0.21 |
| Total duration of treatment, days (IQR) | 15 (20) | 23 (23) | 0.21 |
| Duration of FRPM treatment, days (IQR) | 14 (18) | 15 (12) | 0.19 |
eMIC, estimated MIC; IQR, interquartile range.
Including post-urological surgery.
DISCUSSION
FRPM is an oral antimicrobial belonging to the penem class of β-lactam antimicrobials, shares structural similarities with both penicillin and cephalosporin, and has a broad antimicrobial spectrum active against aerobic Gram-positive, Gram-negative, and anaerobic bacteria. Although its in vitro activity against ESBL-producing Enterobacterales has been demonstrated (9), its clinical utility against infections due to ESBL-producing Enterobacterales remains unknown (10). The antimicrobial susceptibility testing for FRPM uses the disk diffusion method because most automated susceptibility testing systems and their test panels do not contain FRPM. The correlation between the disk diffusion method and the broth microdilution method for ESBL-producing Enterobacterales was evaluated using isolates recently collected in Japan (11).
In this study, the most common CTX-M groups among the ESBL-producing isolates were CTX-M-1 in 16 isolates (36%) and CTX-M-9 in 22 isolates (50%). Previous studies in Japan reported the major CTX-M types as CTX-M-14 (36%), CTX-M-27 (22%), and CTX-M-15 (20%); CTX-M-15 belongs to the CTX-M-1 group, and CTX-M-14 and CTX-M-27 belong to the CTX-M-9 group (12, 13). Therefore, our results reflect the common prevalence of CTX-M types among ESBL-producing Enterobacterales in Japan. Regarding non-CTX-M genes, TEM and SHV (TEM, SHV, and TEM/SHV) without CTX-M were identified in 6 ESBL-producing isolates (E. coli [n = 2] and K. pneumoniae [n = 4]) in this study, although TEM and SHV were typically not ESBLs and accompanied by CTX-M in ESBL-producing isolates in Japan (14, 15). Further studies of the resistance mechanisms of phenotypically ESBL-producing isolates without CTX-M genes are necessary.
There is currently no CLSI standard breakpoint for FRPM, and the disk diffusion assay is often used to determine the susceptibility of clinical isolates based on the zone of inhibition diameter values in Japan. The susceptible range of eMIC in Kirby-Bauer disks derived from the approximate equations in this study were ≤2.2 mg/L. A previous study proposed tentative breakpoints (for zones of inhibition) of >16 mm as susceptible (MIC, <2.0 mg/L) and <12 mm as resistant (MIC, >8.0 mg/L) (11). These results were nearly concordant each other.
This study revealed that FRPM was used for to treat UTI caused by Enterobacterales resistant to third-generation cephalosporins and showed a ≥16-mm diameter according to the disk diffusion method (eMIC = 2.2 mg/L) for FRPM, as the breakpoint of ≥16 mm in diameter in the disk diffusion method for FRPM was considered to indicate susceptibility. Few studies have addressed the clinical effectiveness of FRPM against infections caused by ESBL-producing Enterobacterales. In a previous randomized controlled trial of FRPM against cystitis, 3.4% of CTX-M-14 in E. coli isolates was reported. The highest MIC of FRPM was 2 mg/L, and this strain was eradicated after 7 days of treatment (10). FRPM was mainly used as an oral step-down regimen following initial intravenous antimicrobial treatment. All patients in this study completed the treatment course as scheduled. Our results suggest that FRPM can be used to treat UTI caused by Enterobacterales resistant to third-generation cephalosporins when the disk diffusion method shows a diameter of ≥16 mm.
The frequency of relapse after the completion of treatment of UTI with FRPM was also 16%, which may raise another concern regarding using FRPM against ESBL-producing Enterobacteriaceae. There was no significant difference between patients with or without 28- and 90-day relapses in multiple factors, including susceptibility for FRPM, presence of complications in the urinary tract, diagnosis, and treatment regimens used with FRPM. In previous studies, the 28-day relapse rate for pyelonephritis caused by ESBL-producing Enterobacterales and afebrile symptomatic UTI in male patients was approximately 10% (5, 16). Compared to these previous studies, we found that more patients had a history of post-urological surgery or underlying diseases of the urinary system and uncontrolled source due to anatomical problems which may be risk factors for relapse other than FRPM use. Since there was no difference in efficacy in a previous study of complicated UTIs comparing levofloxacin and FRPM with good urinary tract penetration (17), this drug may be safe to use in treating cystitis. Further studies of larger sample sizes are needed to identify patients with UTI who would benefit from FRPM treatment and define an appropriate breakpoint of FRPM.
This study had several limitations. First, sequencing of the CTX-M, TEM, SHV, and AmpC genes was not performed to assess the differences in each isolate. Second, the approximate equation was calculated in the range of 9 to 24 mm for the disk inhibition diameter in the strains; therefore, the only eMIC in this range was calculated for clinical use. Many patients were treated with combination therapies rather than FRPM monotherapy. Therefore, further studies to compare FRPM monotherapy and combination therapy are needed to evaluate the efficacy of FRPM.
Conclusion.
The correlation of the disk diffusion method and broth microdilution method to test the susceptibility of ESBL-producing Enterobacterales was sufficient, and a temporary clinical breakpoint of a 16-mm zone of diameter in disk diffusion is equivalent to an eMIC of 2.2 mg/L. FRPM is an available option for treating UTI, particularly cystitis caused by ESBL-producing Enterobacterales using the current temporary breakpoint; however, identifying risk factors for relapse and patients who may benefit from FRPM require further analysis.
MATERIALS AND METHODS
Correlation of disk diffusion method and broth microdilution method to test susceptibility of faropenem. (i) Collection of isolates resistant to third-generation cephalosporins.
Third-generation cephalosporin-resistant E. coli and K. pneumoniae clinical isolates collected between 1 January and 30 June 2020 at the Department of Clinical Laboratory of St. Luke’s International Hospital in Tokyo, Japan, were used to analyze the correlation of the disk diffusion method with the broth microdilution method to test FRPM susceptibility. As daily clinical tests, microbial identification and antimicrobial susceptibility testing was performed using a MALDI Biotyper (Bruker Daltonik, Bremen, Germany) and MicroScan WalkAway 96 Plus with Neg ID panels (Beckman Coulter, Brea, CA) which did not contain FRPM. Resistance to third-generation cephalosporin was defined as a cefotaxime (CTX) MIC of ≥4 mg/L, a ceftriaxone MIC of ≥4 mg/L, a ceftazidime MIC of ≥16 mg/L, or a cefpodoxime MIC of ≥8 mg/L. Susceptibility tests using the disk-diffusion method for FRPM were performed using Muller Hinton II agar (BD Biosciences, Franklin Lakes, NJ) and Kirby-Bauer disks (Eiken Chimial Co., Inc., Tokyo, Japan). Tentative breakpoints of the FRPM zone of inhibition diameters were adopted from a previous study and the manufacturer’s instructions as follows: susceptible, ≥16 mm; intermediate, 13 to 15 mm; and resistant, ≤12 mm (11).
(ii) Susceptibility tests for FRPM using the broth microdilution method.
The MIC of FRPM was determined using the broth microdilution method with cation-adjusted Mueller-Hinton II broth (BD Biosciences) and the custom-made Dry Plate test system (Eiken Chemical Co., Inc.) containing not only FRPM but also other antimicrobials—cefazolin, CTX, ceftazidime, cefepime, CMZ, ceftibuten, aztreonam, ciprofloxacin, piperacillin-tazobactam, amoxicillin-clavulanate, MEPM, imipenem, trimethoprim-sulfamethoxazole (ST), flomoxef, fosfomycin, gentamicin, amikacin, and tobramycin—for an incubation time of 16 to 20 h at 35°C. The clinical breakpoints endorsed in the Clinical and Laboratory Standards Institute (CLSI) M100-Ed31 document (18) were used to determine the antimicrobial susceptibility, except for those of FRPM and flomoxef, for which clinical breakpoints were not available in the CLSI document.
(iii) Phenotypic identification and genotyping of ESBL- and AmpC-producing Enterobacterales.
ESBL- and AmpC-producing Enterobacterales were screened with a MASTDISCS Combi D68C (Mast Group, Ltd., Bootle, UK) according to the manufacturer’s instructions. DNA for ESBL genotyping was extracted using the boiling method, in which a suspension of bacterial cells was boiled, and the supernatant was used as the template for PCR. Cica Geneus DNA extraction reagent (Kanto Chemical Co., Ltd.) was used for DNA extraction for AmpC genotyping. Multiplex PCR was performed using a Multiplex PCR kit (Qiagen, Hilden, Germany) and PCR primers (19) for ESBL and the Cica Geneus AmpC genotype detection kit (Kanto Chemical Co., Ltd.) for AmpC. The ESBL-producing Enterobacterales were genotyped based on the presence of genes belonging to the following homology groups: blaSHV, blaTEM, blaCTX-M-1, blaCTX-M-2, blaCTX-M-8/25, and blaCTX-M-9. The AmpC-producing Enterobacterales were genotyped based on the presence of genes belonging to the following homology groups: blaCIT, blaFOX, blaACT, blaDHA, blaACC, and blaMOX.
(iv) Correlation of susceptibility between the estimated MIC and disk diffusion testing methods for FRPM.
The correlation between the FRPM zone of inhibition diameters determined in the disk diffusion assay and MICs determined using the broth microdilution method was assessed by applying linear, quadratic, cubic, logarithmic, and reciprocal functions to derive an approximate function using SPSS 19.0 J statistical software (SPSS, Inc., Chicago, IL). The estimated MIC (eMIC) based on the disk inhibitory zone diameter was calculated for each isolate using the approximate function.
Clinical course of patients with ESBL and AmpC-producing Enterobacterales UTIs treated with FRPM.
A retrospective chart review was performed for patients with UTI treated with FRPM between October 2010 and April 2021. This study was approved by the Institutional Review Board of St. Luke’s International Hospital in Tokyo, Japan (number 20-R151). The requirement for patient consent was waived based on the retrospective study type.
The following information was collected: age, sex, diagnosis, concomitant antimicrobial agents, treatment duration, 28-day relapse or reinfection after the completion of antimicrobial treatment, and 90-day relapse or reinfection after the completion of antimicrobial treatment. Relapse was defined as development of a UTI with an ESBL-producing Enterobacterales. Reinfection was defined as the development of a UTI caused by a different organism. Complicated UTI was defined as the UTI of all males, all females older than 50 years, patients with a history of urological surgery, and any anatomical or functional disorders in the urinary tract. The eMIC of each isolate for FRPM was calculated from the inhibitory zone diameter result of FRPM.
Bivariate associations were assessed using χ2 and Fisher exact tests for categorical variables and the Mann-Whitney U test for continuous variables. A significance level of 0.05 was set for bivariate analyses. All analyses were performed using SPSS 19.0 J statistical software.
Data availability.
The anonymized data set supporting these conclusions is available upon reasonable request from the corresponding author.
ACKNOWLEDGMENTS
We thank the staff of the Department of Clinical Laboratory of St. Luke’s International Hospital and Department of Medical Library of St. Luke’s International University for their assistance in conducting this study.
We declare there are no competing interests.
K.I., Y.U., H.G., T.I., Y.M., Sayoko Kawakami, M.S., and Shizuo Kayama carried out experimental analysis. A.S. and Y.D. designed and prepared the Dry Plate panels for susceptibility testing. S.T. and D.K. supervised the statistical analysis. K.I. drafted the manuscript, and Y.U., N.M., and S.N. supervised this research. All authors discussed the results and contributed to the final manuscript.
This study was approved by the Institutional Review Board of St. Luke’s International Hospital in Tokyo, Japan (number 20-R151). The requirement for patient consent was waived based on the retrospective study type.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Ambler RP. 1980. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci 289:321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- 2.Peña C, Gudiol C, Calatayud L, Tubau F, Domínguez MA, Pujol M, Ariza J, Gudiol F. 2008. Infections due to Escherichia coli producing extended-spectrum beta-lactamase among hospitalized patients: factors influencing mortality. J Hosp Infect 68:116–122. doi: 10.1016/j.jhin.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Ami R, Rodríguez-Baño J, Arslan H, Pitout JDD, Quentin C, Calbo ES, Azap ÖK, Arpin C, Pascual A, Livermore DM, Garau J, Carmeli Y. 2009. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing Enterobacterales in nonhospitalized patients. Clin Infect Dis 49:682–690. doi: 10.1086/604713. [DOI] [PubMed] [Google Scholar]
- 4.Japan Nosocomial Infections Surveillance. 2020. JANIS annual open report (all facilities) Japan nosocomial infections surveillance. Japan Ministry of Health, Labor, and Welfare, Tokyo, Japan. https://janis.mhlw.go.jp/english/report/index.html. [Google Scholar]
- 5.Doi A, Shimada T, Harada S, Iwata K, Kamiya T. 2013. The efficacy of cefmetazole against pyelonephritis caused by extended-spectrum beta-lactamase-producing Enterobacterales. Int J Infect Dis 17:e159–e163. doi: 10.1016/j.ijid.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Matsumura Y, Yamamoto M, Nagao M, Komori T, Fujita N, Hayashi A, Shimizu T, Watanabe H, Doi S, Tanaka M, Takakura S, Ichiyama S. 2015. Multicenter retrospective study of cefmetazole and flomoxef for treatment of extended-spectrum-β-lactamase-producing Escherichia coli bacteremia. Antimicrob Agents Chemother 59:5107–5113. doi: 10.1128/AAC.00701-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuchi T, Iwata K, Kobayashi S, Nakamura T, Ohji G. 2016. Cefmetazole for bacteremia caused by ESBL-producing Enterobacterales comparing with carbapenems. BMC Infect Dis 16:427. doi: 10.1186/s12879-016-1770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mushtaq S, Hope R, Warner M, Livermore DM. 2007. Activity of faropenem against cephalosporin-resistant Enterobacterales. J Antimicrob Chemother 59:1025–1030. doi: 10.1093/jac/dkm063. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Komatsu M, Yamasaki K, Fukuda S, Higuchi T, Ono T, Nishio H, Sueyoshi N, Kida K, Satoh K, Toda H, Toyokawa M, Nishi I, Sakamoto M, Akagi M, Mizutani T, Nakai I, Kofuku T, Orita T, Zikimoto T, Natsume S, Wada Y. 2014. Susceptibility of various oral antibacterial agents against extended spectrum beta-lactamase producing Escherichia coli and Klebsiella pneumoniae. J Infect Chemother 20:48–51. doi: 10.1016/j.jiac.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Hamasuna R, Tanaka K, Hayami H, Yasuda M, Takahashi S, Kobayashi K, Kiyota H, Yamamoto S, Arakawa S, Matsumoto T, Japanese Research Group for UTI (JRGU). 2014. Treatment of acute uncomplicated cystitis with faropenem for 3 days versus 7 days: multicentre, randomized, open-label, controlled trial. J Antimicrob Chemother 69:1675–1680. doi: 10.1093/jac/dku014. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs PC, Barry AL, Sewell DL. 1995. Antibacterial activity of WY-49605 compared with those of six other oral agents and selection of disk content for disk diffusion susceptibility testing. Antimicrob Agents Chemother 39:1472–1479. doi: 10.1128/AAC.39.7.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumura Y, Yamamoto M, Nagao M, Tanaka M, Takakura S, Ichiyama S. 2016. In vitro activities and detection performances of cefmetazole and flomoxef for extended-spectrum β-lactamase and plasmid-mediated AmpC β-lactamase-producing Enterobacterales. Diagn Microbiol Infect Dis 84:322–327. doi: 10.1016/j.diagmicrobio.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 13.D’Andrea MM, Arena F, Pallecchi L, Rossolini GM. 2013. CTX-M-type β-lactamases: a successful story of antibiotic resistance. Int J Med Microbiol 303:305–317. doi: 10.1016/j.ijmm.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Yano H, Uemura M, Endo S, Kanamori H, Inomata S, Kakuta R, Ichimura S, Ogawa M, Shimojima M, Ishibashi N, Aoyagi T, Hatta M, Gu Y, Yamada M, Tokuda K, Kunishima H, Kitagawa M, Hirakata Y, Kaku M. 2013. Molecular characteristics of extended-spectrum β-lactamases in clinical isolates from Escherichia coli at a Japanese tertiary hospital. PLoS One 8:e64359. doi: 10.1371/journal.pone.0064359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong Y, Shimoda S, Yakushiji H, Ito Y, Miyamoto T, Kamimura T, Shimono N, Akashi K. 2013. Community spread of extended-spectrum β-lactamase-producing Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis: a long-term study in Japan. J Med Microbiol 62:1038–1043. doi: 10.1099/jmm.0.059279-0. [DOI] [PubMed] [Google Scholar]
- 16.Drekonja DM, Trautner B, Amundson C, Kuskowski M, Johnson JR. 2021. Effect of 7 versus 14 days of antibiotic therapy on resolution of symptoms among afebrile men with urinary tract infection: a randomized clinical trial. JAMA 326:324–331. doi: 10.1001/jama.2021.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muratani T, Iihara K, Nishimura T, Inatomi H, Fujimoto N, Kobayashi T, Yamada Y, Takahashi K, Matsumoto T. 2002. Faropenem 300 mg three times daily versus levofloxacin 100 mg three times daily in the treatment of urinary tract infections in patients with neurogenic bladder and/or benign prostatic hypertrophy. Kansenshogaku Zasshi 76:928–938. doi: 10.11150/kansenshogakuzasshi1970.76.928. [DOI] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. 2021. Performance standards for antimicrobial susceptibility testing, 31st ed. CLSI supplement M100. CLSI, Wayne, PA, [Google Scholar]
- 19.Dallenne C, Da Costa A, Decré D, Favier C, Arlet G. 2010. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacterales. J Antimicrob Chemother 65:490–495. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S4. Download aac.00125-22-s0001.pdf, PDF file, 0.1 MB (153.3KB, pdf)
Data Availability Statement
The anonymized data set supporting these conclusions is available upon reasonable request from the corresponding author.