ABSTRACT
Infections by drug-resistant fungi are increasingly reported worldwide; however, only few novel antifungals are being developed. The old antimicrobial nitroxoline is currently repurposed for oral treatment of bacterial urinary tract infections (UTI). Previously, antifungal activity has been demonstrated and in contrast to many antifungals nitroxoline reaches high urinary concentrations. In this study, the activity of nitroxoline was assessed in vitro in a collection of yeasts from the German National Reference Centre for Invasive Fungal Infections. Susceptibility was determined by broth microdilution (BMD) and disk diffusion (DD). The collection comprised 45 Candida isolates originating from the urinary tract. MICs of amphotericin, anidulafungin and azoles were analyzed using EUCAST BMD. Among the collection isolates, resistance to antifungals was common, e.g., for fluconazole the MIC50/90 was 16/>64 mg/L; in contrast MIC50/90 of nitroxoline was 2/2 mg/L (MIC range 0.25–4 mg/L), which is at least two dilutions below the EUCAST breakpoint for uncomplicated UTI defined for E. coli (susceptible ≤ 16mg/L). Activity of nitroxoline was high irrespective of resistance to other agents. As BMD is labor-intensive, DD was investigated as an alternative method using three different agars. Nitroxoline disks produced large inhibition zones on all agars (≥19mm), but the correlation of MICs and zone diameters was low, with the highest correlation recorded for the CLSI recommended agar for antifungal DD (Pearson’s r = −0,52). In conclusion, isolates of different Candida species are highly susceptible to nitroxoline, which could be a promising antimicrobial to treat candiduria caused by multidrug resistant yeasts.
KEYWORDS: 8-hydroxyquinoline, Candida auris, Candida glabrata, azole, azole-nonsusceptible, candiduria, multidrug resistance, quinolines, urinary tract infection, yeast
INTRODUCTION
Antimicrobial resistance (AMR) is one of the top 10 public health threats facing humanity according to the World Health Organization (WHO) (1). For a long time, the increase of resistant fungi remained unnoticed amid the emergence of resistant bacteria (2). WHO currently compiles a priority list considering among other issues the treatment options for fungal infections with respect to specific species (Candida auris) and emerging antifungal resistance (3).
Candiduria is mostly asymptomatic, and the necessity of antifungal therapy is discussed controversially even in patient populations susceptible for opportunistic infections. Unsatisfactory microbiological clearance rates, a missing impact on survival alongside with a potential of spontaneous recovery without the use of antifungals are valid arguments against candiduria treatment (4). On the other hand, treatment may prevent some patients from developing invasive infections and may be necessary, e.g., in case of symptomatic candiduria (5). For azole-resistant Candida spp. amphotericin B deoxycholate quickly becomes the weapon of last resort, as poor renal clearance of echinocandins leads to subtherapeutic concentrations and selection of echinocandin resistant yeast populations (6).
In the light of the few classes of antifungals, repurposing of approved antimicrobials is warranted (7). Approved drugs possessing antifungal activity that are structurally unrelated to classical antifungal drugs could be used to spare azoles, echinocandins and polyenes and may prevent the selection and spread of resistant fungi. The old antibiotic nitroxoline (8-hydroxy-quinoline) could therefore be an interesting option for the treatment of candiduria, as it has previously demonstrated antifungal activity (8, 9). It is currently recommended among the first line antimicrobials for the treatment of uncomplicated urinary tract infections (uUTI) in Germany (10). EUCAST has introduced a clinical breakpoint limited to uUTI and E. coli (susceptible, ≤16mg/L or inhibition zone diameter [IZD] ≥15mm) in 2016 (11). The mode of action is poorly understood, yet chelation-based antifungal activity has been reported for nitroxoline already decades ago (9). Currently, its clinical use is limited to uUTI treatment, since systemic concentrations are low, compared to the high urinary concentrations after oral administration (8). In a recent study, nitroxoline was proposed as an alternative treatment option in C. auris candiduria (12).
The aim of the present study was to analyze the antifungal activity of nitroxoline in a diverse collection of Candida spp. from the urinary tract with a focus on isolates exhibiting decreased susceptibility to common antifungals. Activity of nitroxoline was compared to the activity of amphotericin, anidulafungin, fluconazole, voriconazole and posaconazole. Nitroxoline is approved in Germany and Poland, which could facilitate further trial development or even compassionate use.
RESULTS
For nitroxoline MIC50/90 was 2/2 mg/L across isolates of all species (MIC range 0.25 mg/L - 4 mg/L), demonstrating high antifungal activity. The recorded MICs are at least two dilutions below the current UTI breakpoint of nitroxoline for E. coli defined by EUCAST (11). The overall highest nitroxoline MICs were displayed for C. glabrata (MIC50/90 2/4 mg/L). Of note, all three isolates with the highest MIC demonstrated within the study (4 mg/L) were C. glabrata isolates. In contrast, C. glabrata quality control strain ATCC 90030 yielded a nitroxoline MIC of 0.5 mg/L.
To compare the activity of nitroxoline to that of classical antifungal drugs, the MICs of amphotericin, anidulafungin and different azoles (fluconazole, voriconazole, posaconazole) for all clinical isolates were determined (Table 1). Acquired resistance or intrinsically decreased susceptibility to azoles was demonstrated in 37/45 isolates, to anidulafungin in 22/45 isolates and to amphotericin in one isolate. Six isolates were resistant to two antifungal classes (Table 1).
TABLE 1.
Susceptibility of clinical Candida spp. to antifungals and nitroxolinea
| Azoles |
Nitroxoline |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Collection ID | AMB | AFG | FLC | VRC | POS | MIC | IZD RPMI | IZD CLSI | IZD MH |
| (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mm) | (mm) | (mm) | ||
| C. glabrata (N = 18) | 14-52 16-167 14-64 17-181 19-568 20-372 19-34 18-320 16-205 16-244 18-370 20-215 15-130 16-49 19-294 19-718 18-431 20-753 |
0.25 0.25 0.5 0.5 0.5 0.25 0.5 0.125 0.5 0.5 0.5 0.5 0.25 0.5 0.25 0.25 0.5 0.25 |
2 2 0.06 0.06 ≤0.016 ≤0.016 4 4 2 2 2 2 0.5 0.03 ≤0.016 ≤0.016 0.03 0.03 |
>64 >64 >64 >64 64 >64 4 16 8 4 4 8 0.5 8 8 4 4 2 |
4 8 4 8 1 2 0.125 0.25 0.25 0.06 0.125 0.5 ≤0.016 0.125 0.5 0.125 0.06 0.06 |
>8 >8 4 >8 2 >8 0.5 1 0.5 0.25 0.5 2 0.06 0.5 2 0.125 2 0.125 |
0.25 1 1 2 2 1 1 2 2 2 1 1 2 4 4 4 2 1 |
33 34 35 36 33 31 31 35 30 32 30 31 32 32 32 29 35 32 |
33 27 28 27 28 22 27 26 22 25 25 25 22 24 28 22 20 26 |
28 31 30 31 27 27 27 26 25 27 24 27 26 21 27 23 19 25 |
| C. albicans (N = 8) | 16-166 17-280 20-175 16-238 20-668 18-435 19-51 19-742 |
0.25 0.25 1 0.5 0.125 0.5 0.25 0.125 |
0.5 0.004 ≤0.016 0.016 ≤0.002 0.03 ≤0.016 ≤0.016 |
>64 >64 >64 16 16 0.25 0.25 0.25 |
>8 >8 >8 0.06 0.06 ≤0.016 0.03 0.03 |
>8 >8 >8 0.06 <0.016 0.03 ≤0.016 ≤0.016 |
0.5 0.5 0.5 1 2 1 1 0.5 |
32 31 32 33 27 33 32 35 |
30 29 25 29 25 29 29 30 |
30 28 28 30 23 28 28 27 |
| C. parapsilosis (N = 7) | 20-389 20-548 19-669 19-84 18-227 19-726 20-294 |
0.25 0.25 0.5 0.5 0.5 0.25 0.5 |
1 1 1 1 2 2 1 |
>64 >64 64 32 1 1 0.5 |
4 4 1 1 0.03 0.03 ≤0.016 |
0.03 ≤0.016 0.06 0.03 ≤0.016 ≤0.016 ≤0.016 |
1 0.5 0.25 0.5 2 0.5 0.25 |
33 35 32 35 29 35 40 |
30 28 30 31 24 25 30 |
28 31 27 31 25 25 28 |
| C. auris (N = 5) | 19-731 20-170 20-742 20-39 20-87 |
2 0.5 1 1 1 |
1 0.5 4 0.125 0.125 |
>64 >64 8 16 16 |
>8 2 0.03 0.125 0.125 |
2 ≤0.016 ≤0.016 ≤0.016 ≤0.016 |
1 1 0.5 0.5 1 |
33 35 34 39 38 |
27 27 25 29 30 |
30 29 29 33 33 |
| C. tropicalis (N = 4) | 19-42 17-182 19-318 16-7 |
0.5 0.5 0.5 0.25 |
0.25 0.125 1 0.06 |
>64 >64 0.5 0.5 |
>8 >8 0.125 0.06 |
>8 >8 0.03 0.03 |
2 2 1 1 |
30 32 32 37 |
23 25 31 28 |
30 30 33 36 |
| C. krusei (N = 2) | 17-243 20-694 |
1 0.5 |
0.125 0.03 |
64 32 |
1 0.25 |
0.5 0.25 |
1 1 |
32 32 |
27 27 |
30 31 |
| C. pararugosa (N = 1) | 17-442 | 0.125 | 0.25 | 8 | 0.25 | 0.25 | 2 | 34 | 26 | 29 |
| Nitroxoline quality control strains (N = 6) | ||||||||||
| ATCC 25922 Escherichia coli ATCC 10231 Candida albicans ATCC 90030 Candida glabrata ATCC 90877 Candida guilliermondii ATCC 6285 Candida krusei ATCC 22019 Candida parapsilosis |
2 0.25 0.5 0.25 0.5 1 |
22 35 34 35 32 29 |
23 32 28 29 29 27 |
23 32 31 31 31 26 |
||||||
Bold numbers indicate MICs in the resistant range based on EUCAST breakpoints (version 10.0) or tentative CDC-breakpoints for C. auris (available at https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html [last accessed 11/2021]). For Nitroxoline quality control and reproduction of nitroxoline susceptibility testing with yeasts elsewhere, test results of six different ATCC strains were included.
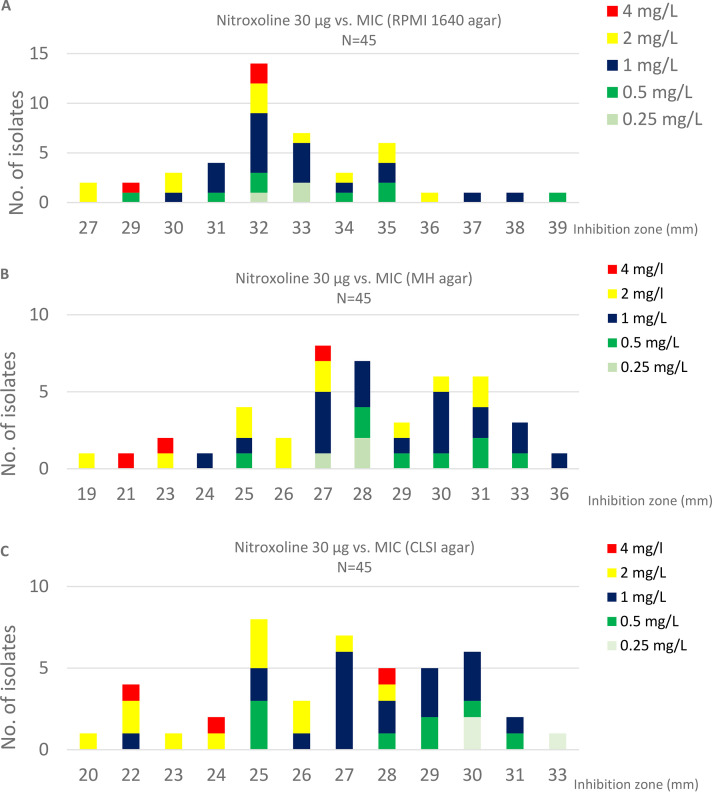
In addition to MIC determination by broth microdilution, disk diffusion testing was carried out using three different agars and results were compared to MIC determination (Fig. 1 and Table 1). Nitroxoline disks produced large inhibition zones on all agars (19–36 mm on MH agar, 20–33 mm on CLSI agar, 27–39 mm on RPMI 1640) (Table 1). However, species-specific analysis of correlation coefficients is limited by the small number of isolates for most species. If unstratified by species, the highest correlation coefficient for all clinical isolates (N = 45) was recorded for CLSI supplemented MH agar with Pearson’s r = −0,52, compared to r = −0,26 for RPMI and r = −0,46 for MH agar.
FIG 1.
Histograms of MICs (broth microdilution) vs. respective inhibition zone by disk diffusion on different agars. (A) RPMI 1640 agar; (B) MH agar; (C) supplemented MH agar according to CLSI for yeast susceptibility testing.
DISCUSSION
Candida spp. are opportunistic pathogens, and antifungal treatment is required only in invasive infections and patient populations susceptible for opportunistic infections (5). Although candiduria is a common entity especially in hospitalized patients exposed to antibacterial therapy and/or indwelling urinary catheterization, the indication for treatment remains controversial (4). According to the Clinical Practice guideline of the Infectious Diseases Association of America (IDSA) (updated 2016) candiduria treatment should be limited to symptomatic patients or asymptomatic patients with specific risk factors (i.e., pregnancy, neutropenia, very-low-birth-weight infants, undergoing surgery of the urinary tract) (5).
Overall data on guideline adherence is scarce and some authors found evidence for poor impact of the clinical decisions in candiduria management on endpoints such as patient mortality, recurrence of candiduria or length of hospital stay (13). However, for some patient populations candiduria treatment is essential, yet few antifungals are suitable for treatment. Especially when fluconazole, the most common drug of choice, cannot be used (e.g., allergy, hepatotoxicity, azole-resistant Candida spp.). In this case, amphotericin B, sometimes in combination with flucytosine, remains the last option for therapy, despite unfavorable side effects (14). Another option is bladder irrigation using amphotericin B. This method reduces amphotericin related systemic side effects, but its use is controversial and limited by the need for continuous catheterization (15).
With increasing antimicrobial resistance, the need for new therapeutic options has led to regained interest in old antimicrobials (7). Nitroxoline has recently shown in vitro activity in different emerging pathogens (16–18) and has been proposed for further evaluation in fungal infections because of its excellent in vitro activity against C. auris (12). In the present study, nitroxoline MICs for all isolates (range 0.5–4 mg/L) were at least two dilutions below the current EUCAST breakpoint for bacterial uUTI (≤16mg/L), demonstrating high antifungal activity in all isolates, including those resistant to azoles and irrespective of species. In contrast for fluconazole the most commonly used drug in candiduria treatment MIC50/90 was 16/>64 mg/L.
For the comparison of the MICs of classical antifungals to nitroxoline the differences in urinary concentrations have to be considered. Among the comparators tested in this study, only fluconazole achieves relevant urinary concentrations in contrast to voriconazole (19), posaconazole (20) and anidulafungin (19). For amphotericin approximately 20% of the dose is excreted via urine, but only if amphotericin deoxycholate is administered (21). Flucytosine reaches high urinary concentrations (19) but is only recommended for combination therapy.
In vivo antimicrobial effects of nitroxoline rely on both conjugated and unconjugated forms of nitroxoline (22). MICs determined in this study (only unconjugated nitroxoline was tested) can be considered promising for therapeutical effects in urine (i.e., concentration of >5 mg/L for unconjugated and >200mg/L for conjugated nitroxoline have been reported in urine from hospitalized patients [23]) and even in the prostate (24, 25). However, as reviewed recently, most data on nitroxoline pharmacokinetics are old and from studies with small sample sizes (8). In addition, more studies focusing on technical aspects of nitroxoline antifungal susceptibility testing (AFST) are warranted. We previously established nitroxoline AFST in a collection of C. auris (N = 35 isolates) (12). While the MICs displayed for C. auris from the previous and the current study (N = 5) are well comparable (highest MIC assessed 1 mg/L) the correlation with disk diffusion was not as high for other Candida spp., possibly as a result of the small number of strains for each species. Additionally, isolates with high MICs/small IZDs for nitroxoline were not present, limiting correlation of disk diffusion and microdilution results. Overall, until disk diffusion breakpoints for different species are established broth microdilution should be used for nitroxoline AFST in future studies. Nevertheless, disk diffusion is an easily implementable technique that can be carried out in most clinical microbiology laboratories. An IZD ≥ 19 mm on commercial MH or CLSI-MH agar or ≥ 27 mm on RPMI 1640 may be used as a tentative screening breakpoint in circumstances when microdilution is not available and no other antifungal options are available.
Our study has several limitations. Most importantly, low MICs do not necessarily translate into microbiological and clinical cure in vivo. For nitroxoline data on clinical success is still scarce despite promising in vitro studies on MICs and biofilms (12, 16–18, 26–30). Treatment failure in bacterial UTI has also been reported (23). Since nitroxoline activity relies on chelation of ions, host factors such as urinary pH and urinary ion concentrations may interfere with the antimicrobial effects of the drug in vivo (8). On the other hand, the MICs observed in this collection of otherwise drug resistant fungi are at least two dilutions below the current uUTI breakpoint for bacteria. Second, the conjugated forms of nitroxoline have also chelating and antimicrobial effects (22). Despite its use for decades nitroxoline resistant microorganisms have rarely been reported thus it remains elusive whether an increase in nitroxoline usage may lead to rapid increase in the prevalence of nitroxoline resistant microorganisms (29, 30). In our challenge collection, no isolates with high nitroxoline MICs were present, although we analyzed different species from all UTI Candida isolates sent to the NRZmyk (N = 45). Of note, UTI isolates are rarely submitted to the NRZMyk, and the most common reason is the detection of a resistant phenotype by the submitting laboratory. Hence only 45 isolates were included (2014–2020), and the high frequency of azole resistance is not representative for overall susceptibility of Candida spp. from urinary specimen.
Overall, nitroxoline has an approved efficacy and safety profile (30) and is already recommended in a national treatment guideline (10), which may facilitate compassionate use and the development of in vivo studies for correlation of in vitro data with clinical endpoints.
To conclude, this study demonstrated excellent in vitro activity of nitroxoline against Candida spp. from the urinary tract with resistance to other antifungals. With respect to increasing AMR, nitroxoline could be an alternative drug for treatment of candiduria sparing classical antifungals for invasive infections.
MATERIALS AND METHODS
Isolates.
To test the activity of nitroxoline against Candida spp. in UTI, all Candida spp. from urine specimen were included which were sent to the German National Reference Center for Invasive Fungal Infections (NRZmyk) between 2014 and 2020 (N = 45). Of the 45 isolates (Candida glabrata (N = 18), Candida albicans (N = 8), Candida parapsilosis (N = 7), Candida auris (N = 5), Candida tropicalis (N = 4), Candida krusei (N = 2) and Candida pararugosa (N = 1)) 37 isolates demonstrated acquired resistance or had intrinsically reduced susceptibility to azoles (e.g., C. auris, C. krusei, C. glabrata), 22 to echinocandines and one to polyenes based on EUCAST breakpoints (31) or tentative CDC breakpoints for C. auris (fluconazole ≥32 mg/L, amphotericin B ≥2 mg/L, anidulafungin ≥ 4 mg/L; available at https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html [last accessed 11/2021]). Species identification was done using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) on a Biotyper system (Bruker Daltonics, Germany).
Susceptibility testing.
Nitroxoline broth microdilution testing was performed as described previously (12), using RPMI 1640 broth (Becton, Dickinson, Heidelberg, Germany). Nitroxoline powder was obtained from the manufacturer (Rosen Pharma, St. Ingbert, Germany). Nitroxoline disk diffusion testing was carried out using 30 μg disks (Oxoid, Wesel, Germany) with commercial MH agar (Oxoid) and RPMI 1640 agar (prepared in-house). Additionally, MH agar (Becton, Dickinson, Germany) supplemented with 0.5 μg/mL methylene blue and 2% glucose according to CLSI for yeast disk diffusion testing was also assessed (32). E. coli ATCC 25922 is the only strain with a defined quality control range for nitroxoline and was therefore used only for nitroxoline quality control purposes alongside a collection of different ATCC yeast strains (ATCC 10231 C. albicans, ATCC 90030 C. glabrata, ATCC 90877 C. guilliermondii, ATCC 6285 C. krusei, ATCC 22019 C. parapsilosis) (12) to facilitate reproduction of nitroxoline susceptibility testing with yeasts elsewhere. Nitroxoline susceptibility testing was performed by two independent examiners, blinded to the results of each other. Susceptibility testing of other antifungals was performed by EUCAST broth microdilution as described previously (33–35).
Statistical analysis.
For correlation of nitroxoline MICs with disk diffusion results Pearson’s correlation coefficient was calculated using GraphPad Prism 8.0 (La Jolla, CA, United States).
ACKNOWLEDGMENTS
F.F. contributed to conceptualization, investigation, validation, writing original draft, reviewing and editing. A.A. contributed to writing the original draft, reviewing and editing, data collection, isolate collection. A.M.H. and O.K. contributed to investigation, data collection, characterization and collection of isolates, writing and reviewing. A.G.H. contributed to conceptualization, investigation, validation, reviewing and editing. G.W. contributed with data collection and isolate collection, reviewing, and editing. All authors contributed to the article and approved the submitted version.
We have no conflict of interest to declare.
Work of the NRZMyk is supported by the Robert Koch Institute from funds provided by the German Ministry of Health (grant 1369-240). F.F. has a clinician scientist position supported by the Deans Office, Medical Faculty, University of Cologne. A.M.H. has a research grant supported by the Medical Faculty, University of Cologne (Maria Pesch foundation).
We thank Marco Schwabe for his support in preparation and validation of the agar plates. Nitroxoline powder was provided free of charge by Rosen Pharma.
We declare that the study was conducted in accordance with guidelines outlined by the Declaration of Helsinki.
REFERENCES
- 1.Mendelson M, Matsoso MP. 2015. The World Health Organization global action plan for antimicrobial resistance. S Afr Med J 105:325. 10.7196/SAMJ.9644. [DOI] [PubMed] [Google Scholar]
- 2.Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. 2017. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis 17:e383–e392. 10.1016/S1473-3099(17)30316-X. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). WHO antifungal expert group on identifying priority fungal pathogens: first meeting report. Geneva, Switzerland: World Health Organization; 2020. https://apps.who.int/iris/bitstream/handle/10665/332309/9789240006355-eng.pdf. Accessed September, 2021. [Google Scholar]
- 4.Alfouzan WA, Dhar R. 2017. Candiduria: evidence-based approach to management, are we there yet? J Mycol Med 27:293–302. 10.1016/j.mycmed.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. 2016. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 62:409–417. 10.1093/cid/civ1194. [DOI] [PubMed] [Google Scholar]
- 6.Biagi MJ, Wiederhold NP, Gibas C, Wickes BL, Lozano V, Bleasdale SC, Danziger L. 2019. Development of high-level echinocandin resistance in a patient with recurrent Candida auris candidemia secondary to chronic candiduria. Open Forum Infect Dis 6:ofz262. 10.1093/ofid/ofz262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miró-Canturri A, Ayerbe-Algaba R, Smani Y. 2019. Drug repurposing for the treatment of bacterial and fungal infections. Front Microbiol 10:41. 10.3389/fmicb.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wijma RA, Huttner A, Koch BCP, Mouton JW, Muller AE. 2018. Review of the pharmacokinetic properties of nitrofurantoin and nitroxoline. J Antimicrob Chemother 73:2916–2926. 10.1093/jac/dky255. [DOI] [PubMed] [Google Scholar]
- 9.Medić-Sarić M, Maysinger D, Movrin M, Dvorzak I. 1980. Antibacterial and antifungal activities of nitroxoline Mannich bases. Chemotherapy 26:263–267. 10.1159/000237915. [DOI] [PubMed] [Google Scholar]
- 10.Kranz J, Schmidt S, Lebert C, Schneidewind L, Vahlensieck W, Sester U, Fünfstück R, Helbig S, Hofmann W, Hummers E, Kunze M, Kniehl E, Naber K, Mandraka F, Mündner-Hensen B, Schmiemann G, Wagenlehner FME. 2017. Epidemiology, diagnostics, therapy, prevention and management of uncomplicated bacterial outpatient acquired urinary tract infections in adult patients: update 2017 of the interdisciplinary AWMF S3 guideline. Urologe A 56:746–758. 10.1007/s00120-017-0389-1. [DOI] [PubMed] [Google Scholar]
- 11.European Committee on Antimicrobial Susceptibility Testing. Nitroxoline: rationale for the clinical breakpoints, version 1.0 2016. http://www.eucast.org. Accessed September, 2021.
- 12.Fuchs F, Hof H, Hofmann S, Kurzai O, Meis JF, Hamprecht A. 2021. Antifungal activity of nitroxoline against Candida auris isolates. Clin Microbiol Infect. 10.1016/j.cmi.2021.06.035. [DOI] [PubMed] [Google Scholar]
- 13.He Z, Huo X, Lei D, Zhao H, Jia K, Wang F. 2021. Management of candiduria in hospitalized patients: a single-center study on the implementation of IDSA guidelines and factors affecting clinical decisions. Eur J Clin Microbiol Infect Dis 40:59–65. 10.1007/s10096-020-03999-1. [DOI] [PubMed] [Google Scholar]
- 14.Fisher JF, Sobel J, Kauffman CA, Newman C. 2011. Candida urinary tract infections—treatment. Clinical Infectious Diseases 52:S457–S466. 10.1093/cid/cir112. [DOI] [PubMed] [Google Scholar]
- 15.Drew RH, Arthur RR, Perfect JR. 2005. Is it time to abandon the use of amphotericin B bladder irrigation? Clin Infect Dis 40:1465–1470. 10.1086/429722. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs F, Hamprecht A. 2019. Susceptibility of carbapenemase-producing Enterobacterales (CPE) to nitroxoline. J Antimicrob Chemother 74:2934–2937. 10.1093/jac/dkz275. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs F, Wille J, Hamprecht A, Parcina M, Lehmann C, Schwarze-Zander C, Seifert H, Higgins PG. 2019. In vitro activity of mecillinam and nitroxoline against Neisseria gonorrhoeae - re-purposing old antibiotics in the multi-drug resistance era. J Med Microbiol 68:991–995. 10.1099/jmm.0.001014. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs F, Becerra-Apericio F, Xanthopoulou K, Seifert H, Higgins PG. 2022. In vitro activity of nitroxoline against carbapenem-resistant Acinetobacter baumannii isolated from the urinary tract. J Antimicrob Chemother in press. [DOI] [PubMed] [Google Scholar]
- 19.Bellmann R, Smuszkiewicz P. 2017. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection 45:737–779. 10.1007/s15010-017-1042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Krekels EHJ, Verweij PE, Buil JB, Knibbe CAJ, Brüggemann RJM. 2020. Pharmacokinetics and pharmacodynamics of posaconazole. Drugs 80:671–695. 10.1007/s40265-020-01306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. 2002. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob Agents Chemother 46:834–840. 10.1128/AAC.46.3.834-840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagenlehner FM, Münch F, Pilatz A, Bärmann B, Weidner W, Wagenlehner CM, Straubinger M, Blenk H, Pfister W, Kresken M, Naber KG. 2014. Urinary concentrations and antibacterial activities of nitroxoline at 250 milligrams versus trimethoprim at 200 milligrams against uropathogens in healthy volunteers. Antimicrob Agents Chemother 58:713–721. 10.1128/AAC.02147-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forstner C, Kwetkat A, Makarewicz O, Hartung A, Pfister W, Fünfstück R, Hummers-Pradier E, Naber KG, Hagel S, Harrison N, Schumacher U, Pletz MW. 2018. Nitroxoline in geriatric patients with lower urinary tract infection fails to achieve microbiologic eradication: a noncomparative, prospective observational study. Clin Microbiol Infect 24:434–435. 10.1016/j.cmi.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Dufour A, Bollack C. 1979. De la pénétration de la nitroxoline dans le parenchyme prostatique. Essai thérapeutique. JUrolNephrol 3:207–212. [PubMed] [Google Scholar]
- 25.Rosen Pharma GmbH. 2012. Fachinformation nitroxolin forte 2021. Rosen Pharma GmbH, Blieskastel, Germany: [in German] available at: https://s3.eu-central-1.amazonaws.com/prod-cerebro-ifap/media_all/77703.pdf. Accessed September, 2021. [Google Scholar]
- 26.Abouelhassan Y, Yang Q, Yousaf H, Nguyen MT, Rolfe M, Schultz GS, Huigens RW. 3rd, 2017. Nitroxoline: a broad-spectrum biofilm-eradicating agent against pathogenic bacteria. Int J Antimicrob Agents 49:247–251. 10.1016/j.ijantimicag.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Sobke A, Makarewicz O, Baier M, Bär C, Pfister W, Gatermann SG, Pletz MW, Forstner C. 2018. Empirical treatment of lower urinary tract infections in the face of spreading multidrug resistance: in vitro study on the effectiveness of nitroxoline. Int J Antimicrob Agents 51:213–220. 10.1016/j.ijantimicag.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Sobke A, Klinger M, Hermann B, Sachse S, Nietzsche S, Makarewicz O, Keller PM, Pfister W, Straube E. 2012. The urinary antibiotic 5-nitro-8-hydroxyquinoline (Nitroxoline) reduces the formation and induces the dispersal of Pseudomonas aeruginosa biofilms by chelation of iron and zinc. Antimicrob Agents Chemother 56:6021–6025. 10.1128/AAC.01484-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kresken M, Körber-Irrgang B. 2014. In vitro activity of nitroxoline against Escherichia coli urine isolates from outpatient departments in Germany. Antimicrob Agents Chemother 58:7019–7020. 10.1128/AAC.03946-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naber KG, Niggemann H, Stein G, Stein G. 2014. Review of the literature and individual patients' data meta-analysis on efficacy and tolerance of nitroxoline in the treatment of uncomplicated urinary tract infections. BMC Infect Dis 14:628. 10.1186/s12879-014-0628-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The European Committee on Antimicrobial Susceptibility Testing. 2020. Breakpoint tables for interpretation of MICs for antifungal agents, version 10.0. http://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals/. Accessed February, 2022.
- 32.CLSI. 2018. Method for antifungal disk diffusion susceptibility testing of yeasts, vol M44ed3E. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 33.Arendrup M.C., Meletiadis J., Mouton J.W., Lagrou K., Hamal P., Guinea J. 2020. Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST), EUCAST DEFINITIVE DOCUMENT E.DEF 7.3.2 method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7.3.2_Yeast_testing_definitive_revised_2020.pdf. Accessed May 21, 2021.
- 34.Aldejohann AM, Herz M, Martin R, Walther G, Kurzai O. 2021. Emergence of resistant Candida glabrata in Germany. JAC Antimicrob Resist 3:dlab122. 10.1093/jacamr/dlab122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamprecht A, Barber AE, Mellinghoff SC, Thelen P, Walther G, Yu Y, Neurgaonkar P, Dandekar T, Cornely OA, Martin R, Kurzai O, German Candida auris Study Group . 2019. Candida auris in Germany and Previous Exposure to Foreign Healthcare. Emerg Infect Dis 25:1763–1765. 10.3201/eid2509.190262. [DOI] [PMC free article] [PubMed] [Google Scholar]