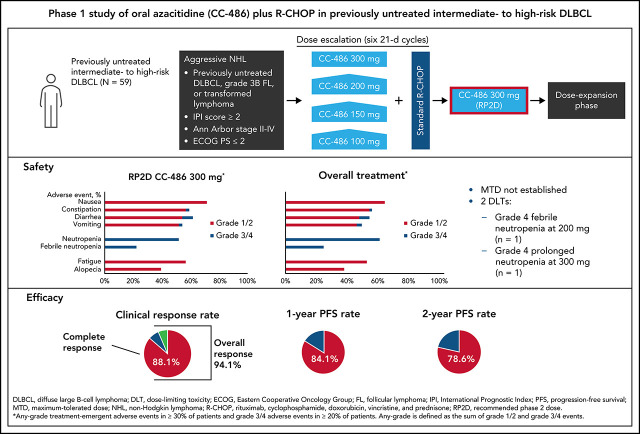
Martin and colleagues report the results of a phase 1b trial of adding oral azacitidine (CC-486) to R-CHOP. “Priming” with CC-486 is shown to cause extensive genomewide hypomethylation in paired tumor biopsies, and the combination demonstrates promising clinical activity in patients with previously untreated intermediate- to high-risk aggressive B-cell lymphomas. This concept is being further explored in a phase 2/3 randomized study of R-miniCHOP with or without CC-486 in older patients.
Key Points
Epigenetic priming with oral azacitidine (CC-486) before R-CHOP demonstrated an acceptable safety profile.
CC-486 plus R-CHOP showed clinical activity in previously untreated intermediate- to high-risk DLBCL or grade 3B/transformed FL.
Visual Abstract
Abstract
Resistance to standard immunochemotherapy remains an unmet challenge in diffuse large B-cell lymphoma (DLBCL), and aberrant DNA methylation may contribute to chemoresistance. Promising early-phase results were reported with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) plus subcutaneous azacitidine, a hypomethylating agent. In this phase 1 study, we evaluated CC-486 (oral azacitidine) plus 6 cycles of R-CHOP in patients with previously untreated intermediate- to high-risk DLBCL or grade 3B/transformed follicular lymphoma. CC-486 doses of 100, 150, 200, or 300 mg given 7 days before cycle 1 and on days 8-21 of cycles 1-5 were evaluated; additional patients were enrolled in the expansion phase to examine preliminary efficacy. The primary objectives were to determine the safety and the maximum tolerated dose (MTD) of CC-486 in combination with R-CHOP. The most common grade 3/4 toxicities were hematologic, including neutropenia (62.7%) and febrile neutropenia (25.4%); grade 3/4 nonhematologic toxicities were uncommon (<7%). The MTD was not established; 2 patients had dose-limiting toxicities (1 with grade 4 febrile neutropenia; 1 with grade 4 prolonged neutropenia). The recommended phase 2 dose was established as 300 mg. The overall response rate was 94.9%, with 52 patients (88.1%) achieving complete responses. With a median follow-up of 28.9 months, estimated 1- and 2-year progression-free survival rates were 84.1% and 78.6%, respectively. Overall, epigenetic priming with CC-486 before R-CHOP can be delivered with acceptable safety to patients with previously untreated intermediate- to high-risk DLBCL or grade 3B/transformed follicular lymphoma. ClinicalTrials.gov: NCT02343536.
Introduction
Rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is the standard first-line treatment of patients with diffuse large B-cell lymphoma (DLBCL) or grade 3B or transformed follicular lymphoma (FL).1 Approximately 75% of unselected DLBCL patients treated with R-CHOP remain progression-free 2 years after treatment, whereas those with International Prognostic Index (IPI) scores of ≥3 fare significantly worse.2 Among patients not cured by R-CHOP, nearly 80% will likely die of progressive lymphoma or complications of subsequent therapies1,3; therefore, the optimal strategy to improve survival is to improve initial therapy and prevent relapse.1,4 Unfortunately, multiple attempts to improve upon R-CHOP, such as changing the chemotherapy backbone, selecting an alternate anti-CD20 antibody, or adding targeted agents, such as bortezomib, enzastaurin, ibrutinib, and lenalidomide, have failed in randomized clinical trials.2,5-13
DNA methylation patterning contains epigenetic information that influences transcriptional programming, which leads to the phenotype of normal and malignant cells. DNA methylation occurs at cytosines in CpG dinucleotides by the enzymes DNMT1, DNMT3a, and DNMT3b. FL and DLBCL cells exhibit aberrant DNA methylation that contributes to disease progression and treatment resistance,14 and can also decrease immune recognition of these cells.15 DNMT inhibitors, or hypomethylating agents (HMAs), can reprogram the phenotype of cancer cells by decreasing aberrant DNA hypermethylation. Although HMAs do not generally induce prominent cytotoxicity, they generate new vulnerabilities that can be therapeutically exploited.14 In previous preclinical and human pilot studies, DLBCL cells exposed to HMAs showed a senescence-like phenotype characterized by reduced tolerance to DNA damage.4 These lymphoma cells upregulated the expression of genes involved in cell-cycle control (eg, p21), microenvironment signaling (eg, SMAD1/TFGB), and immune response (eg, IRF4, HLA-I/II molecules).4,15,16 We capitalized on this newly acquired vulnerability by developing a tolerable regimen based on the combination of subcutaneous azacitidine priming followed by R-CHOP chemoimmunotherapy that resulted in chemo-sensitization.4
CC-486 was recently approved in the United States for treatment of acute myeloid leukemia (AML) in adults who achieved complete response (CR) or CR with incomplete blood count recovery after induction chemotherapy and are not able to complete intensive curative therapy.17,18 This approval was based on results from the QUAZAR AML-001 clinical study, which demonstrated that CC-486 treatment significantly prolonged median overall survival compared with placebo (24.7 months vs 14.8 months; P < .001).17,18 As an oral formulation of azacitidine, CC-486 has the potential to further improve upon R-CHOP because it enables continuous low-dose administration over longer periods of time in the outpatient setting, which appears to augment the extent and duration of epigenetic effects.19 Here, we report results from the phase 1 study of CC-486 in combination with R-CHOP in patients with previously untreated DLBCL, grade 3B FL, or transformed lymphoma.
Materials and methods
Study design
CC-486-DLBCL-001 Alliance Foundation Trials (AFT)-08 (NCT02343536) is a phase 1, open-label, dose-escalation study of CC-486 plus R-CHOP in patients with previously untreated, intermediate- to high-risk DLBCL, grade 3B FL, or transformed lymphoma. The study was conducted at 6 sites across the United States. Institutional review boards and/or ethics committees approved the protocols and amendments (WCM-NYPH IRB: No. 1507016419). Study conduct followed International Conference on Harmonization Guidelines for Good Clinical Practice, including written informed consent from all patients and data monitoring.
The primary objectives were to determine safety per National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.03, and the maximum tolerated dose (MTD) of CC-486 in combination with R-CHOP. Secondary objectives were to characterize the pharmacokinetics (PK) of CC-486 and determine the preliminary efficacy per International Working Group (IWG) criteria.20 Exploratory objectives included evaluating the pharmacodynamic effects of CC-486 and potential predictive or correlative biomarkers for DLBCL subgroups.
Patients
Eligible patients were aged 18-80 years with histologically confirmed, previously untreated DLBCL, including high-grade B-cell lymphoma with BCL2 or BCL6 and/or MYC rearrangements, grade 3B FL, or transformed lymphoma. Patients were required to have measurable disease >1.5 cm in the longest diameter, Ann Arbor stage II-IV disease, an IPI score ≥2 or DLBCL double-positive for BCL2 and MYC by immunohistochemistry or fluorescent in situ hybridization (FISH) based on local pathology laboratory assessment, and an Eastern Cooperative Oncology Group performance status ≤2. Cell of origin (COO) was determined by immunohistochemistry using the Hans algorithm at the local institution and/or by RNA-sequencing algorithm in available cases.
Treatment
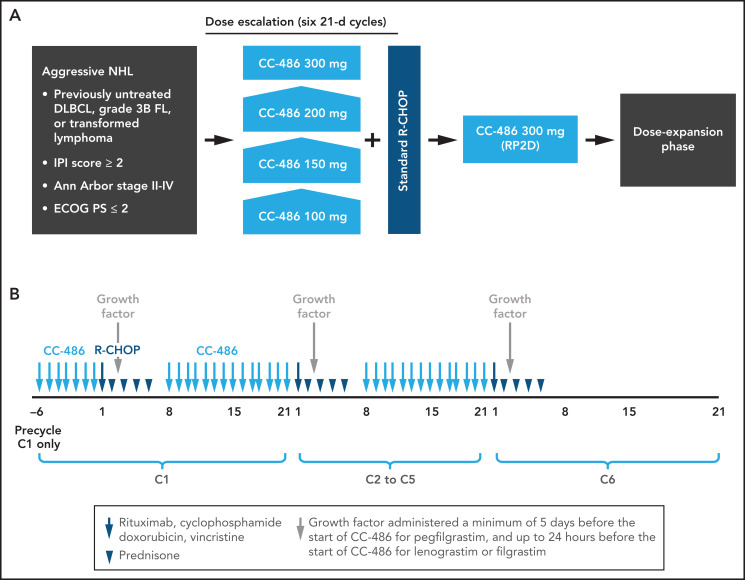
Chemotherapy treatment consisted of six 21-day cycles. In the first cycle, CC-486 was administered orally once daily as a 7-day priming regimen before initiation of R-CHOP on day 1 of cycle 1; thereafter, CC-486 was administered daily for 14 days (days 8-21) during cycles 1-5 of R-CHOP. There was no CC-486 administration during cycle 6. Growth factor was administered ≥5 days before the start of CC-486 for pegfilgrastim and up to 24 hours before the start of CC-486 for lenograstim or filgrastim. A dose of a prophylactic antiemetic given 30 minutes before each dose of CC-486 was strongly recommended for all patients. Prephase treatment with prednisone 1 mg/kg/day, or equivalent, for ≤7 days was allowed prior to day −6 of cycle 1 for patients with bulky disease, systemic symptoms, comprehensive disease, or rapidly progressing adenopathies. In exceptional cases, if clinically indicated, a higher dose of prednisone and/or slightly longer duration was permitted for the purpose of urgent symptom management. The study examined 4 escalating dose levels of CC-486 (100, 150, 200, and 300 mg daily). In order to assign dose levels in a staggered fashion (for patients one by one) without delaying patient enrollment, time-to-event continual reassessment method (TiTE-CRM)21 was used starting at CC-486 100 mg (Figure 1). TiTE-CRM assumed a simple model for the probability of a dose-limiting toxicity (DLT) as a function of the combination and used the occurrence of toxicities in patients enrolled in this study to sequentially determine which combination to be allocated to a new patient. New patients were continuously recruited without pausing for complete follow-up of already enrolled patients. Patients received treatment of 6 cycles unless the outcome of the response evaluation after cycle 3 necessitated a treatment change or until disease progression, unacceptable toxicity, death, or withdrawal of consent from treatment, whichever occurred first.
Figure 1.
CC-486 DLBCL-001 study design. (A) Overall study design. (B) Dosing schedule. DLBCL, diffuse large B-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; FL, follicular lymphoma; IPI, International Prognostic Index; R-CHOP, rituximab plus cyclophosphamide, doxorubicin hydrochloride, vincristine, and prednisolone; RP2D, recommended phase 2 dose.
The DLT period spanned all 6 cycles of therapy. A DLT in any cycle included grade 4 neutropenia with fever, grade ≥3 thrombocytopenia with significant bleeding, any other nonhematologic grade ≥3 toxicity not related to the underlying disease, vomiting or diarrhea of grade ≥2 persisting for 48 hours or worsening despite supportive care, cumulative toxicity-related delays of R-CHOP for >21 days over 6 cycles, a delay of >7 days in the start of R-CHOP in cycle 2, or if patients were ineligible to receive CC-486 within 48 hours of day 8 in >2 out of 5 cycles based on dose modification criteria. Based on early analyses of safety data for multiple cycles at all dose levels in which neutropenia was reported on day 8 but had recovered by day 1 of the next cycle, the hematologic requalification criteria were modified such that CC-486 could be started on day 8 despite any-grade neutropenia, provided the patient was afebrile.
Study assessments
Adverse events were assessed per NCI CTCAE version 4.03 up to 28 days after the last dose of study treatment. Blood samples for PK analysis were collected on days −6 and 8 of cycle 1 at the following timepoints: predose, 0.25, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 6.0, and 8.0 hours postdose. Response was assessed once between days 15 and 21 of cycle 3, at the end of study treatment, then every 6 months for up to 2 years or until disease progression. Responses were assessed per IWG criteria for non-Hodgkin’s lymphoma (NHL)20; positron emission tomography (PET) scans were interpreted according to the Deauville Criteria and were required at baseline and the end-of-treatment only.22,23
The pharmacodynamic effects of CC-486 were evaluated by comparing global cytosine methylation, genome-wide DNA methylation, and transcriptomics in tumor cells, as well as genome-wide DNA methylation in peripheral blood CD3+ T cells. Samples were collected on days −6 and 1 of cycle 1. Genome-wide DNA methylation was measured using the enhanced reduced representation bisulfide sequencing method at the Weill Cornell Medicine Epigenomics Core Facility (New York, NY). Transcriptomic changes were assessed by RNA sequencing using the TruSeq RNA sample kit (Illumina, San Diego, CA). The interferon cytokines IFN-α2a, IFN-β, IFN-γ, and IL-29/IFN-λ1 were quantified in plasma using a multiplexing assay (U-PLEX Interferon Combo, Meso Scale Diagnostics, Rockville, MD). Detailed methods for these analyses are provided in the supplemental Methods.
Statistical analyses
The safety population consisted of all patients who received ≥1 dose of CC-486 or R-CHOP. The efficacy-evaluable population comprised all patients who met all eligibility criteria and had ≥1 tumor response assessment after receiving ≥1 dose of the study drug. The PK population included all patients who received ≥1 dose of CC-486 and had evaluable concentration data to determine the PK parameters. The Kaplan-Meier method was used to analyze progression-free survival (PFS), defined as the time from treatment initiation to the first documented disease progression or death) for each cohort and the overall population. PK parameters were summarized using descriptive statistics for each dose level/cycle-day. All analyses were based on the 29 January 2020 data cutoff date.
Results
Patients
Between July 2015 and October 2017, 59 patients were enrolled; 33 in the dose-escalation phase and 26 in the dose-expansion phase. As of 29 January 2020, 54 patients (91.5%) completed treatment, and 5 (8.5%) did not complete study treatment. Reasons for not completing treatment included treatment-emergent adverse event, treatment-related death, progressive disease, lack of efficacy, and withdrawal of consent (n = 1 each). Additionally, 2 patients discontinued vincristine, and 1 patient discontinued vincristine plus doxorubicin prior to completing 6 cycles of therapy.
Patient baseline characteristics are reported in Table 1. Median age was 66 years (range, 25-80) with 76.3% of patients aged ≥60 years. All but one patient had DLBCL; the remaining patient had grade 3B FL. Ten patients (16.7%) entered the study with transformed DLBCL, 9 from FL and 1 from marginal zone lymphoma. Median time between first diagnosis and first dose was 28 days (range, 3-380). Per Hans COO classification, 25 patients (42.4%) had germinal cell B-cell (GCB) lymphoma, and 18 (30.5%) had non-GCB. Immunohistochemistry for COO was not performed or not reported for 12 patients (20.3%) and was performed but considered indeterminate for 4 patients (6.8%). Most patients had higher-risk disease, with 59.3% having an IPI score ≥3 and 62.7% having Ann Arbor stage IV disease. Among 54 patients with known expression levels, 17 (31.5%) had overexpression of BCL2, 12 (22.2%) had overexpression of MYC, and 7 (13.0%) had double overexpression of BCL2 and MYC. Of 54 patients with available samples for FISH analyses, 2 had double-hit (BCL2 or BCL6 and MYC rearrangements), and 1 had triple-hit (BCL2, BCL6, and MYC rearrangement) disease.
Table 1.
Baseline patient characteristics
| Characteristic | ESCAL | EXP | ESCAL+EXP | |
|---|---|---|---|---|
| Overall (n = 33) |
RP2D 300 mg (n = 26) |
RP2D 300 mg (n = 40) |
Overall (N = 59) |
|
| Median (range) age, y | 65 (25, 80) | 71 (55, 80) | 67 (30, 80) | 66 (25, 80) |
| Age > 60 y, n (%) | 22 (66.7) | 23 (88.5) | 31 (77.5) | 45 (76.3) |
| Male, n (%) | 18 (54.5) | 17 (65.4) | 25 (62.5) | 35 (59.3) |
| ECOG PS, n (%) | ||||
| 0 | 17 (51.5) | 12 (46.2) | 21 (52.5) | 29 (49.2) |
| 1 | 14 (42.4) | 13 (50.0) | 18 (45.0) | 27 (45.8) |
| Ann Arbor disease stage, n (%) | ||||
| II | 2 (6.1) | 2 (7.7) | 2 (5.0) | 4 (6.8) |
| III | 10 (30.3) | 8 (30.8) | 13 (32.5) | 18 (30.5) |
| IV | 21 (63.6) | 16 (61.5) | 25 (62.5) | 37 (62.7) |
| Median time between first diagnosis and first dose (range), d | 28 (7, 380) | 27 (3, 103) | 28 (3, 380) | 28 (3, 380) |
| DLBCL* | 27 (9, 61) | 25 (3, 103) | 24 (3, 103) | 26 (3, 103) |
| IPI score, n (%) | ||||
| Low/low-intermediate (= 2)† | 15 (45.5) | 9 (34.6) | 18 (45.0) | 24 (40.6) |
| High-intermediate/high (≥ 3) | 18 (54.5) | 17 (65.4) | 22 (55.0) | 35 (59.3) |
| Transformed DLBCL, n (%) | 5 (15.2) | 5 (19.2) | 8 (20.0) | 10 (16.9) |
| Cell of origin, n (%)‡ | ||||
| GCB | 13 (39.4) | 12 (46.2) | 16 (40.0) | 25 (42.4) |
| Non-GCB | 9 (27.3) | 9 (34.6) | 14 (35.0) | 18 (30.5) |
| Undetermined | 11 (33.3) | 5 (19.2) | 10 (25.0) | 16 (27.1) |
| Overexpression of BCL2 and/or MYC, n (%) | ||||
| Yes§ | 19/29 (65.5) | 23/25 (92.0) | 32/38 (84.2) | 42/54 (77.8) |
| BCL2 | 9 (31.0) | 8 (32.0) | 13 (34.2) | 17 (31.5) |
| MYC | 7 (24.1) | 5 (20.0) | 9 (23.7) | 12 (22.2) |
| Double expressor | 4 (13.8) | 3 (12.0) | 5 (13.2) | 7 (13.0) |
| Unknown | 4 (12.1) | 1 (3.8) | 2 (5.0) | 5 (8.5) |
| Molecular abnormalities, n (%) | ||||
| Yes‖ | 14/31 (45.2) | 13/23 (56.5) | 21/37 (56.8) | 27/54 (50.0) |
| Double hit | 1 (3.2) | 1 (4.3) | 2 (5.4) | 2 (3.7) |
| Triple hit | 0 | 1 (4.3) | 1 (2.7) | 1 (1.9) |
| Missing | 2 (6.1) | 3 (11.5) | 3 (7.5) | 5 (8.5) |
ESCAL, dose-escalation phase; EXP, expansion phase; DLBCL, diffuse large B-cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; GCB, germinal center B cell; IPI, International Prognostic Index; RP2D, recommended phase 2 dose.
Total number of patients with DLBCL (not transformed follicular lymphoma) was 49 (ESCAL, n = 28; EXP, n = 21; RP2D 300 mg, n = 32).
Includes 1 patient with an IPI score of 0/1 (low) and 23 patients with an IPI score of 2 (low-intermediate).
By Hans algorithm.
Overexpression of BCL2 (≥30%) or MYC (≥40%) was determined by immunohistochemistry based on local pathology laboratory assessment. Double expressors have increased expression of BCL2 and MYC. The denominators are the total number of patients with known expression levels.
Includes all patients with BCL2, BCL6, and/or MYC rearrangements. Double-hit are BCL2 or BCL6 and MYC rearrangements, and triple-hit are BCL2, BCL6, and MYC rearrangements. The denominators are the total number of patients with no missing data.
Treatment exposure
Among all patients treated in both the dose-escalation and expansion phases, 88.1% completed all cycles of CC-486, and 91.5% completed 6 cycles of R-CHOP. Twelve (85.7%) of the 14 patients treated with CC-486 200 mg and 38 (95.0%) of the 40 patients treated with CC-486 300 mg completed 6 cycles of R-CHOP. Five patients completed <6 cycles of R-CHOP; 3 (5.1%) and 2 (3.4%) completed 2 and 5 cycles, respectively. The mean relative dose intensity (RDI) of CC-486 was 81.1% with 36 patients (61.0%) having an RDI >85% (supplemental Table 1). The mean RDI of CC-486 was 76.1% (standard deviation [SD], 23.1%) and 82.2% (SD, 22.6%) among patients treated with CC-486 200 mg and 300 mg, respectively. A similar mean RDI of R-CHOP was observed in patients treated with CC-486 200 mg (96.0%; SD, 4.8%) and 300 mg (96.2%; SD, 6.3%). The overall mean RDI of CC-486 plus R-CHOP was 89.6%, with 41 patients (69.5%) having an RDI >85%. Nineteen patients (32.2%) had a CC-486 dose reduction, 13 (22.0%) had treatment-emergent adverse events (TEAEs) leading to dose reduction, and 27 (45.8%) had a dose interruption. Additionally, 5 patients (8.5%) discontinued R-CHOP or a component of R-CHOP due to TEAEs, and 3 patients discontinued treatment due to progression, lack of efficacy, and withdrawal of consent. Eleven patients (18.5%) had TEAEs leading to R-CHOP interruption, and 3 (5.1%) had a vincristine dose reduction because of a TEAE.
Safety
All patients experienced a TEAE, most commonly nausea (66.1%), neutropenia (62.7%), and constipation (57.6%) (Table 2). Most grade 3/4 TEAEs were hematologic, including neutropenia (62.7%), febrile neutropenia (25.4%), anemia (16.9%), and thrombocytopenia (13.6%). Although growth factor was administered before the start of CC-486, febrile neutropenia occurred during the first 3 cycles of R-CHOP in the 15 patients who experienced this TEAE, with most events occurring in cycle 1 (n = 8). Ten patients had grade 3, and 5 patients had grade 4 febrile neutropenia; 1 of the grade 4 febrile neutropenia events was considered a DLT and 4 were not.
Table 2.
Treatment-emergent adverse events occurring by treatment phase (safety population)*
| ESCAL | ESCAL | ESCAL | ESCAL | ESCAL | EXP | RP2D 300 mg ESCAL+EXP (n = 40) |
Overall ESCAL+EXP (N = 59) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 mg (n = 1) |
150 mg (n = 4) |
200 mg (n = 14) |
300 mg (n = 14) |
Overall (n = 33) |
RP2D 300 mg (n = 26) |
|||||||||||
| Any Grade | Grade 3/4 |
Any Grade | Grade 3/4 |
Any Grade | Grade 3/4 |
Any Grade | Grade 3/4 |
Any Grade | Grade 3/4 |
Any Grade |
Grade 3/4 |
Any Grade |
Grade 3/4 |
Any Grade |
Grade 3/4 |
|
| ≥1 TEAE, n (%) | 1 (100) | 1 (100) | 4 (100) | 4 (100) | 14 (100) | 12 (85.7) | 14 (100) | 10 (71.4) | 33 (100) | 27 (81.8) | 26 (100) | 19 (73.1) | 40 (100) | 29 (72.5) | 59 (100) | 46 (78.0) |
| Gastrointestinal | ||||||||||||||||
| Nausea | 0 | 0 | 2 (50.0) | 0 | 8 (57.1) | 0 | 11 (78.6) | 0 | 21 (63.6) | 0 | 18 (69.2) | 0 | 29 (72.5) | 0 | 39 (66.1) | 0 |
| Constipation | 0 | 0 | 2 (50.0) | 0 | 8 (57.1) | 0 | 9 (64.3) | 0 | 19 (57.6) | 0 | 15 (57.7) | 1 (3.8) | 24 (60.0) | 1 (2.5) | 34 (57.6) | 1 (1.7) |
| Diarrhea | 1 (100) | 0 | 1 (25.0) | 0 | 6 (42.9) | 1 (7.1) | 8 (57.1) | 1 (7.1) | 16 (48.5) | 2 (6.1) | 17 (65.4) | 2 (7.7) | 25 (62.5) | 3 (7.5) | 33 (55.9) | 4 (6.8) |
| Vomiting | 1 (100) | 0 | 0 | 0 | 7 (50.0) | 1 (7.1) | 8 (57.1) | 1 (7.1) | 16 (48.5) | 2 (6.1) | 14 (53.8) | 0 | 22 (55.0) | 1 (2.5) | 30 (50.8) | 2 (3.4) |
| Dyspepsia | 0 | 0 | 1 (25.0) | 0 | 5 (35.7) | 0 | 5 (35.7) | 0 | 11 (33.3) | 0 | 3 (11.5) | 0 | 8 (20.0) | 0 | 14 (23.7) | 0 |
| Stomatitis | 1 (100) | 0 | 1 (25.0) | 0 | 1 (7.1) | 0 | 5 (35.7) | 1 (7.1) | 8 (24.2) | 1 (3.0) | 5 (19.2) | 0 | 10 (25.0) | 1 (2.5) | 13 (22.0) | 1 (1.7) |
| Abdominal pain | 0 | 0 | 2 (50.0) | 0 | 2 (14.3) | 0 | 1 (7.1) | 0 | 5 (15.2) | 0 | 7 (26.9) | 1 (3.8) | 8 (20.0) | 1 (2.5) | 12 (20.3) | 1 (1.7) |
| Hematologic | ||||||||||||||||
| Neutropenia | 1 (100) | 1 (100) | 4 (100) | 4 (100) | 11 (78.6) | 11 (78.6) | 8 (57.1) | 8 (57.1) | 24 (72.7) | 24 (72.7) | 13 (50.0) | 13 (50.0) | 21 (52.5) | 21 (52.5) | 37 (62.7) | 37 (62.7) |
| Febrile neutropenia | 0 | 0 | 2 (50.0) | 2 (50.0) | 4 (28.6) | 4 (28.6) | 2 (14.3) | 2 (14.3) | 8 (24.2) | 8 (24.2) | 7 (26.9) | 7 (26.9) | 9 (22.5) | 9 (22.5) | 15 (25.4) | 15 (25.4) |
| Anemia | 0 | 0 | 2 (50.0) | 2 (50.0) | 2 (14.3) | 2 (14.3) | 2 (14.3) | 1 (7.1) | 6 (18.2) | 5 (15.2) | 7 (26.9) | 5 (19.2) | 9 (22.5) | 6 (15.0) | 13 (22.0) | 10 (16.9) |
| Thrombocytopenia | 0 | 0 | 1 (25.0) | 1 (25.0) | 3 (21.4) | 2 (14.3) | 1 (7.1) | 1 (7.1) | 5 (15.2) | 4 (12.1) | 5 (19.2) | 4 (15.4) | 6 (15.0) | 5 (12.5) | 10 (16.9) | 8 (13.6) |
| Leukopenia | 0 | 0 | 1 (25.0) | 1 (25.0) | 1 (7.1) | 1 (7.1) | 0 | 0 | 2 (6.1) | 2 (6.1) | 1 (3.8) | 1 (3.8) | 1 (2.5) | 1 (2.5) | 3 (5.1) | 3 (5.1) |
| Other | ||||||||||||||||
| Fatigue | 0 | 0 | 3 (75.0) | 0 | 6 (42.9) | 0 | 9 (64.3) | 0 | 18 (54.5) | 0 | 14 (53.8) | 0 | 23 (57.5) | 0 | 32 (54.2) | 0 |
| Alopecia | 1 (100) | 0 | 0 | 0 | 6 (42.9) | 0 | 5 (35.7) | 0 | 12 (36.4) | 0 | 11 (42.3) | 0 | 16 (40.0) | 0 | 23 (39.0) | 0 |
| Decreased appetite | 0 | 0 | 2 (50.0) | 0 | 3 (21.4) | 0 | 4 (28.6) | 0 | 9 (27.3) | 0 | 7 (26.9) | 0 | 11 (27.5) | 0 | 16 (27.1) | 0 |
| Dizziness | 0 | 0 | 0 | 0 | 3 (21.4) | 0 | 4 (28.6) | 0 | 7 (21.2) | 0 | 7 (26.9) | 0 | 11 (27.5) | 0 | 14 (23.7) | 0 |
| Headache | 0 | 0 | 1 (25.0) | 0 | 4 (28.6) | 0 | 5 (35.7) | 0 | 10 (30.3) | 0 | 4 (15.4) | 0 | 9 (22.5) | 0 | 14 (23.7) | 0 |
| Insomnia | 1 (100) | 0 | 0 | 0 | 2 (14.3) | 0 | 5 (35.7) | 0 | 8 (24.2) | 0 | 4 (15.4) | 0 | 9 (22.5) | 0 | 12 (20.3) | 0 |
| Edema peripheral | 1 (100) | 0 | 0 | 0 | 1 (7.1) | 0 | 3 (21.4) | 0 | 5 (15.2) | 0 | 7 (26.9) | 0 | 10 (25.0) | 0 | 12 (20.3) | 0 |
| Pulmonary embolism | 0 | 0 | 0 | 0 | 1 (7.1) | 1 (7.1) | 0 | 0 | 1 (3.0) | 1 (3.0) | 3 (11.5) | 3 (11.5) | 3 (7.5) | 3 (7.5) | 4 (6.8) | 4 (6.8) |
ESCAL, dose-escalation phase; EXP, expansion phase; RP2D, recommended phase 2 dose; TEAE, treatment-emergent adverse event.
Events of any grade reported in ≥20% of patients and grade 3/4 events reported in ≥5% of patients are shown.
All but 1 patient had a TEAE suspected of being related to CC-486, most commonly nausea (57.6%), neutropenia (49.2%), and vomiting (42.4%) (supplemental Table 2). Hematologic events were the only grade 3/4 TEAEs related to CC-486 reported in >2 patients and included neutropenia (49.2%), febrile neutropenia (20.3%), thrombocytopenia (11.9%), anemia (10.2%), and leukopenia (5.1%). All patients had a TEAE suspected of being related to R-CHOP, most commonly neutropenia (61.0%), nausea (54.2%), and fatigue (45.8%) (supplemental Table 3). The incidence of TEAEs decreased over the course of the study, with most events occurring during cycles 1 or 2 (98.3% and 93.2% of patients, respectively).
Twenty-three patients (39.0%) had serious adverse events (SAEs), the most frequent of which was febrile neutropenia (23.7%) (supplemental Table 4). Other SAEs reported in >1 patient were gastrointestinal hemorrhage, pulmonary embolism, cellulitis, and pneumonia (n = 2 [3.4%] each). One patient died during the study; the cause of death was acute respiratory failure likely related to multiple infections.
No DLTs were reported at the 100-mg and 150-mg CC-486 dose levels. One patient receiving the 200-mg dose level had grade 4 febrile neutropenia, and 1 patient receiving the 300-mg dose level had grade 4 neutropenia requiring treatment interruption. The MTD was not reached in this study. However, the TiTE-CRM suggested 300 mg as the recommended phase 2 dose (RP2D).
Efficacy
Among the 59 patients evaluable for efficacy in both the escalation phase and expansion phase, the overall response rate (ORR) was 94.9% (95% CI, 85.9-98.9) (Table 3). CRs were achieved in 52 patients (88.1%), including 35 (87.5%) treated at the 300-mg dose level (Table 3). One patient (who was censored) achieved a CR but discontinued the study and subsequently received other anti-lymphoma therapy. Although sample sizes are small, similar CR rates were observed at all dose levels examined in the escalation phase (100 mg, 100% [n = 1/1]; 150 mg, 100% [n = 4/4]; 200 mg, 85.7% [n = 12/14]; 300 mg, 92.9% [n = 13/14]). Additionally, response rates were similar for patients with IPI scores of 2 and ≥3 (supplemental Table 5). The ORR for patients with IPI score 2 was 95.8% (95% CI, 78.9-99.9), with CRs reported in 22 patients (91.7%). The ORR for patients with IPI ≥3 was 94.3% (95% CI, 80.8-99.3), with 30 patients (85.7%) achieving a CR. Similar ORR and CR rates were reported regardless of COO and transformation origin (supplemental Figure 1).
Table 3.
Efficacy by treatment phase (safety population)
| ESCAL | EXP | RP2D 300 mg ESCAL+EXP (n = 40) |
Overall ESCAL+EXP (N = 59) |
|
|---|---|---|---|---|
| Overall (n = 33) |
RP2D 300 mg (n = 26) |
|||
| ORR, n (%) [95% CI] |
32 (97.0) [84.2-99.9] |
24 (92.3) [74.9-99.1] |
38 (95.0) [83.1-99.4] |
56 (94.9) [85.9-98.9] |
| CR | 30 (90.9) | 22 (84.6) | 35 (87.5) | 52 (88.1) |
| PR | 2 (6.1) | 2 (7.7) | 3 (7.5) | 4 (6.8) |
| SD | 1 (3.0) | 1 (3.8) | 1 (2.5) | 2 (3.4) |
| Median time to response, months* | 2.2 | 2.1 | 2.1 | 2.1 |
| PFS rate at 1 y, % | 96.8 | 69.2 | 77.5 | 84.1 |
| PFS rate at 2 y, % | 89.9 | 65.2 | 72.4 | 78.6 |
ESCAL, dose-escalation phase; EXP, expansion phase; ORR, overall response rate; PFS, progression-free survival; SD, stable disease.
Overall responders only.
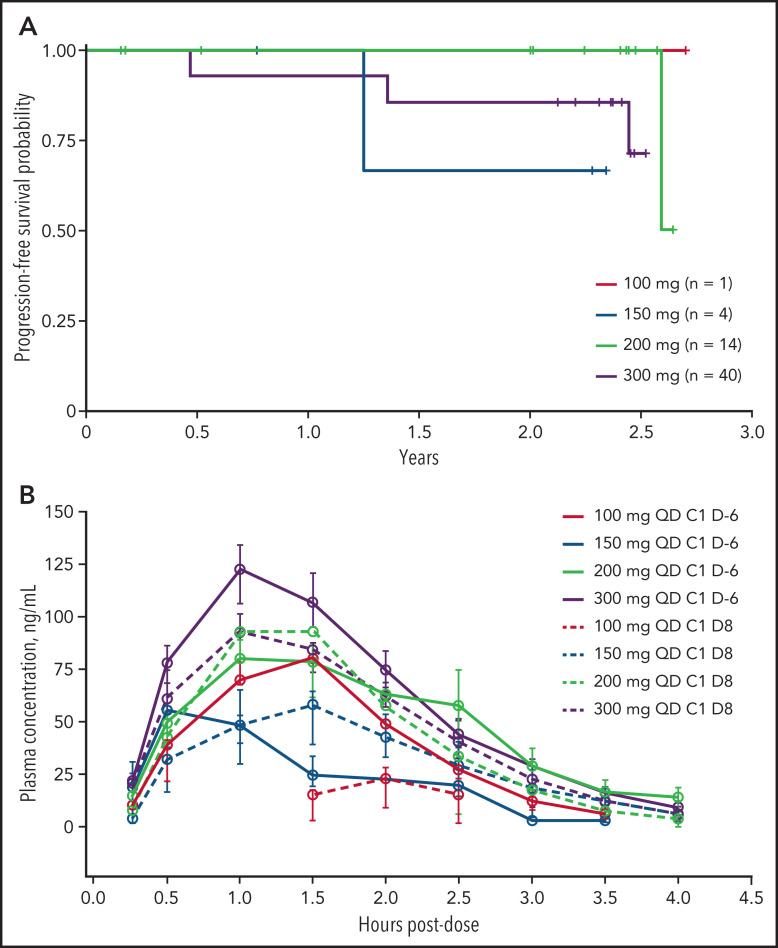
With a median follow-up of 28.9 months, 1- and 2-year PFS rates for the overall population (n = 59) were 84.1% and 78.6%, respectively (Table 3). In the escalation phase, 1- and 2-year PFS rates for the overall population were 96.8% and 89.9%, respectively (100 mg, 100% and 100%; 150 mg, 100% and 66.7%; 200 mg, 100% and 100%; 300 mg, 92.9% and 85.7% for 1- and 2-year PFS rates, respectively). Among 24 patients with IPI score 2, the 1- and 2-year PFS rates were 86.4% and 72.4%. Of 35 patients with IPI scores ≥3, both the 1- and 2-year PFS rates were 82.9% (supplemental Table 5).
Pharmacokinetics
Mean plasma concentration-time profiles of CC-486 alone on day −6 of cycle 1 and in combination with R-CHOP on day 8 of cycle 1 are shown in Figure 2B; plasma PK parameters are summarized in supplemental Table 6. The mean CC-486 plasma concentration versus time profiles are characterized over the 4-hour postdose sampling interval. Following CC-486 administration alone or in combination with R-CHOP, the mean time to maximum CC-486 plasma concentration ranged from 0.6 to 2.2 hours postdose. A dose-proportional increase in CC-486 plasma exposure occurred from the 100-mg to 300-mg dose. CC-486 alone and in combination with R-CHOP had a terminal half-life of 0.3-0.6 hours; the plasma clearance ranged from 812 to 2335 L/hour. The total plasma exposure of CC-486 was comparable when CC-486 was administered as a single agent 6 days prior to cycle 1 of R-CHOP or between cycles of R-CHOP; however, a relatively large interpatient variability for both area under the curve and maximum plasma concentration was noted, based on the geometric coefficient of variation (supplemental Table 6).
Figure 2.
Progression-free survival and exposure of CC-486 plus R-CHOP. (A) Kaplan-Meier curves of progression-free survival in the dose-escalation phase by dose (n = 14). (B) Mean plasma concentration time profiles of CC-486 with and without R-CHOP. Data are presented by dose. For cycle 1, day −6 dose administration, CC-486 was administered alone. For cycle 1, day 8 administration, CC-486 was given in combination with R-CHOP.
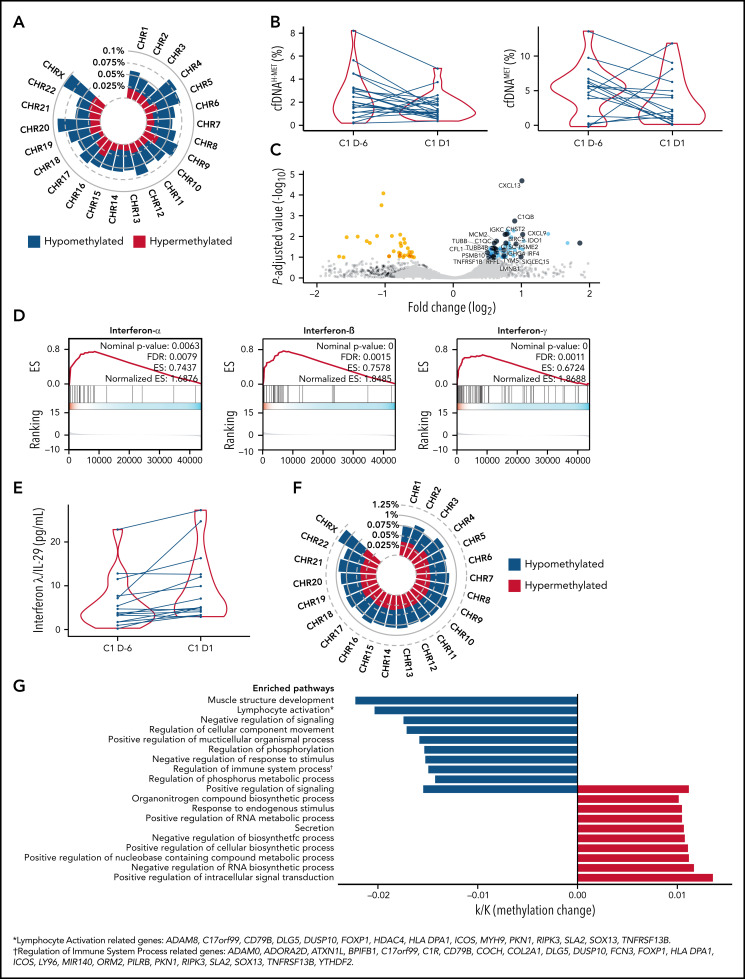
Correlative studies
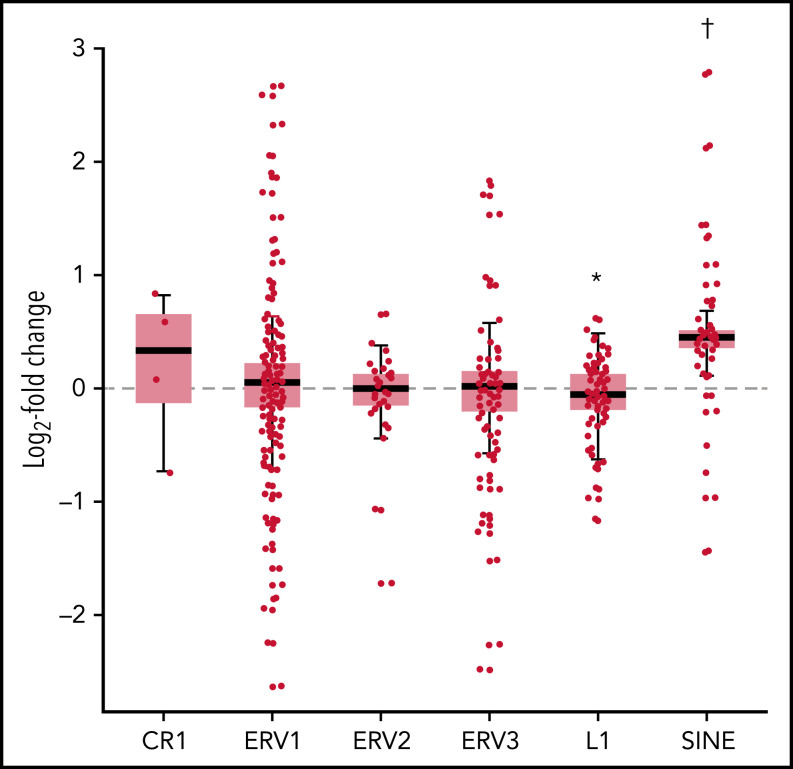
Extensive genome-wide hypomethylation was a prominent effect of CC-486 in the DNA methylome of 5 paired (day −6 vs day 1 of cycle 1) tumor samples (Figure 3A). Pharmacodynamic effects of CC-486 were evaluated in 21 patients through assessment of cytosine hydroxymethylation (cfDNAHMET) and cytosine methylation (cfDNAMET) in cell-free DNA from plasma. Of the 21 patients, 16 had decreased cfDNAMET and/or cfDNAHMET at this time point (Figure 3B). Changes were more prominent in cfDNAMET with approximately a 3.4-fold decrease. Analysis of the transcriptional changes in tumor samples showed increased expression of immune-related genes (Figure 3C), including CXCL9 (T-cell chemotactic molecule), PSME2 and PSMB10 (immunoproteasome components), and IRF4 (interferon responsive gene), that are frequently hypermethylated genes in DLBCL.4 In addition, an upregulation of the tryptophan metabolizing enzyme IDO, a gene associated with suppression of anti-tumor T-cell immunity,24 was observed. Pathway analysis of significantly altered genes indicated the activation of interferon production (Figure 3D). Because no significant overlap was observed between hypomethylated and upregulated genes, nonspecific upregulation of transposable elements and other repeated elements in the genome were investigated. Most transposable elements mobilized by CC-486 have no individual functions, particularly short interspersed nuclear elements (SINEs), and the majority of long interspersed nuclear elements activate common pathways independently of their localization in the genome. Among the evaluated transposable elements, SINEs were substantially upregulated (P < .0001) (Figure 4). The SINEs with the highest upregulation were FLAM and AluY15, the CR1 named L2, and the ERV1 named LTR58, this later SINE specifically expressed in B cells (supplemental Table 7).25 Of the interferon family molecules analyzed (IFN-α2a, IFN-β, IFN-γ, and IL-29/IFN-λ1), IL-29/IFN-λ1 was upregulated (Figure 3E), suggesting the activation of an antiviral-like immune response.26 Differential paired analysis (day −6 vs day 1 of cycle) on DNA methylomes of normal peripheral CD3+ T cells from 3 patients showed both hyper and hypomethylated regions (Figure 3F). Promoter regions showed significant hypomethylation in genes related to “lymphocyte activation” and “regulation of immune system process” among others (Figure 3G).
Figure 3.
Pharmacodynamic and molecular changes associated with CC-486 treatment. (A) Tumor methylation changes (CpGs with >10% of differential methylation and Q value <0.05) upon CC-486 administration by chromosome location in 5 tumors. Hypomethylated and hypermethylated regions are labeled as blue and red, respectively. (B) Changes in cell-free DNA (cfDNA) hydroxymethylation (H-MET) (left) and methylation (MET) (right) as percentage over total cfDNA in 22 patients comparing cycle 1, day −6 (C1 D-6) versus cycle 1, day 1 (C1 D1). (C) Volcano plot of tumor transcriptional changes by RNA-sequencing of 5 lymphoma paired samples. Upregulated genes associated with immune pathways are depicted in dark blue. (D) Gene Set Enrichment Analysis (GSEA) of significantly upregulated genes from panel C (interferon-α, interferon-β, interferon-γ). (E) Interferon-λ/IL-29 concentration in 14 paired plasma samples. (F) Tumor methylation changes (CpGs with >10% of differential methylation and Q value <0.05) upon CC-486 administration by chromosome location in CD3+ T cells from 3 patients. (G) Pathway analysis of differentially methylated gene promoters from CD3+ T cells, including genes associated with “lymphocyte activation” and “regulation of immune system progress” pathways. *Lymphocyte activation related genes are ADAM8, C17orf99, CD79B, DLG5, DUSP10, FOXP1, HDAC4, HLA-DPA1, ICOS, MYH9, PKN1, RIPK3, SLA2, SOX13, and TNFRSF13B. †Regulation of immune system process related genes are ADAM8, ADORA2B, ATXN1L, BP1FB1, C17orf99, CIR, CD79B, COCH, COL2A1, DLG5, DUSP10, FCN3, FOXP1, HLA-DPA1, ICOS, LY96, MIR140, ORM2, PILRB, PKN1, RIPK3, SLA2, SOX13, TNFRSF13B, and YTHDF2.
Figure 4.
Changes in expression of transposable elements in tumor cells. Fold change (log2) of several classes of transposable elements and repeated sequences in lymphoma cells including traces of clades L1 and CR1 of long interspersed elements (LINEs), long-terminal repeat retrotransposons ERV1, ERV2 and ERV3, and short interspersed elements (SINEs). *P < .001. †P < .0001.
Discussion
This phase 1, dose-escalation study demonstrated that CC-486 in combination with R-CHOP can be delivered with acceptable safety in patients with previously untreated intermediate to high-risk DLBCL, grade 3B FL, or transformed lymphoma. The safety profile of the combination was consistent with the known safety profiles of each therapy alone, with the most common TEAEs being hematologic or gastrointestinal.27-29
Neutropenia is an expected toxicity with both azacitidine and R-CHOP treatment. Although the rate of grade 3/4 neutropenia observed here (62.7%) was higher than some reports with either R-CHOP or CC-486 alone,7,30 this difference may be partly related to the frequency and timing of laboratory testing. This is supported by studies with equally frequent laboratory testing, which report similar rates of hematological toxicity (eg, grade 3/4 neutropenia occurred in 57.9% of patients treated with R-CHOP in the PHOENIX trial [NCT01855750]).14 The rate of febrile neutropenia in this study (25.4%) was similar to the rate reported among patients ≥60 years of age treated with R-CHOP plus ibrutinib in the PHOENIX trial (21.4%),6 and was lower than the rate in patients treated with dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R) in the CALGB 50303 trial.2 Although hematologic toxicity was frequent with CC-486, it did not lead to delays in initiating R-CHOP. Most TEAEs were manageable with dose modifications or treatment delays, and few events led to treatment discontinuation. There also did not appear to be a clear relationship between the rates of most TEAEs and the dose of CC-486, although the small number of patients included in each dose cohort may limit interpretation of these results.
Epigenetic priming with CC-486 before R-CHOP demonstrated preliminary clinical activity in this patient population. In both the dose-escalation phase and expansion phase, CRs were reported in most patients at cycle 3 and were maintained until the end of treatment. As patients without progression 2 years after the onset of initial therapy have shown excellent survival outcome, 2-year PFS has become a benchmark for evaluating the success of initial treatment in DLBCL.31 The 2-year PFS rate of 78.6% in this study appears similar to the CALGB 50303 randomized phase 3 trial (NCT00118209), which reported a 2-year PFS rate of 75.5% in patients treated with R-CHOP and 78.9% in patients treated with DA-EPOCH-R, despite the fact that most patients enrolled in the current study had higher-risk disease.2 Furthermore, the CALGB 50303 trial included ≥60% of patients with low/low-intermediate IPI scores.2 However, it is important to note that the median diagnosis-to-treatment interval among patients in the current study was 28 days, suggesting many enrolled patients may not have required urgent symptom control or systemic treatment.32 Although crosstrial comparisons are limited by the small number of patients included in the current trial, our results appear numerically better than the 2-year PFS rate of 65.1% reported in patients with high-intermediate and high-risk IPI scores who received R-CHOP in the PYRAMID trial (NCT00931918), but are similar to the 2-year PFS rate of 72.4% reported in patients with high and high-intermediate IPI scores treated with bortezomib-R-CHOP.5 Moreover, Davies et al reported 30-month PFS rates of 70.1% in R-CHOP-treated patients and 74.3% in bortezomib-R-CHOP-treated patients in the REmoDL-B trial.33 Results from the PYRAMID and REmoDL-B trials suggest that our observed results might fall within the upper bounds reported in those studies.5,33
The MTD of CC-486 combined with R-CHOP was not reached. Based on an overall assessment of the data, the RP2D of CC-486 was determined to be 300 mg. The approved dose of single-agent CC-486 in adult patients with AML in complete remission is 300 mg administered orally once daily for the first 14 days of each 28-day cycle.17 The highest dose to be tested in the present study was 300 mg, and few patients were enrolled in the 100-mg and 200-mg dose groups. Although response rates were similar at the 200-mg and 300-mg dose levels, a longer PFS was observed at the lower, 200-mg dose. This may be due to the higher percentage of patients with intermediate- to high-risk disease being treated with 300 mg (eg, non-GCB subtype, transformed DLBCL, and molecular abnormalities). However, the small sample sizes preclude accurate comparisons of results across dose levels. Similarly, the relatively high PK variability observed across the dose levels examined is most likely due to the limited number of enrolled patients. While taking into account the limited number of patients in each cohort, given the similar safety and efficacy profiles observed in this study, it may be reasonable for future studies to use either 200 mg or 300 mg when combining CC-486 with R-CHOP.
HMAs, including azacitidine and decitabine, have demonstrated diverse immune-modulating activities.34 Changes in immune evasion-related genes, including type III interferon, demonstrated by RNA-sequencing analysis as well as increase tumor infiltration of T cells have been observed with azacitidine treatment in preclinical B-cell lymphoma models.15 Furthermore, HMAs have been reported to upregulate expression of the inhibitory checkpoint receptor programmed cell death protein 1 (PD-1) on T cells and the inhibitory ligands programmed death ligand 2 (PD-L2) and programmed ligand 1 (PD-L1) on tumor cells.34,35 Results from an ongoing phase 1 study show that decitabine in combination with immune checkpoint blockade improved antitumor activity in patients with NHL.36 In support of the hypothesized mechanisms of CC-486, significant correlative changes in gene expression for interferon-related immune responses as well as increases in circulating IFN-λ levels were observed. Furthermore, nonspecific upregulation of transposable elements and SINEs were upregulated. This could indicate that, in contrast to large CpG islands associated with gene promoters, small CpG islands (200-500 bp) that are frequently associated with SINEs are more susceptible to CC-486 and/or that CpG islands associated with SINEs are more frequently hypermethylated in DLBCL. The upregulation of SINEs by the demethylating agent decitabine has shown to⌆form double-stranded RNAs, leading to an endogenous type-1 interferon response in a preclinical study.37 These perhaps explain the overall increased expression of interferon-regulated genes in patients. Overall, these data suggest, in agreement with preclinical data,15 that CC-486-induced hypomethylation does not negatively affect the function of T cells but rather increases the production of IFN-λ that may contribute to an antitumor immunity.
In this study, a TiTE-CRM study design was selected with consideration for the DLT period across 6 full cycles of treatment. The apparent worsening of outcomes in older individuals treated with ibrutinib plus R-CHOP in the PHOENIX trial supports the concept that clinical trials in DLBCL should prioritize completion of planned therapy.6 The occurrence of TEAEs during cycles 1/2 in this trial are well-accepted adverse events in DLBCL therapy, suggesting that extending the DLT period to 6 cycles may not be necessary. Moreover, during rapid enrollment, the dose of CC-486 was escalated in a short period of time, limiting the ability of the model to account for toxicity in later cycles. For future phase 1 trials in DLBCL, it will be necessary to balance the evaluation of DLTs early in therapy with the later toxicities that preclude completion of therapy and practical time period for dose escalation.
In summary, results from this dose-escalation study demonstrated acceptable safety and the preliminary signs of antitumor activity with CC-486 in combination with R-CHOP in patients with previously untreated intermediate- to high-risk DLBCL. The results reported here indicate further exploration of this combination is warranted. A prospective phase 2/3 randomized trial (Southwest Oncology Group [SWOG] S1918) evaluating CC-486 plus rituximab and reduced dose of CHOP in elderly patients with DLBCL will be conducted in 2021 by the SWOG Cancer Research Network Trials.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
We thank all of the CC-486-DLBCL-001 study investigators, patients, and their families. The study was supported by Celgene, a Bristol-Myers Squibb Company, and the Alliance Foundation Trials (https://acknowledgments.alliancefound.org). Medical writing and editorial assistance were provided by Hannah H. Chang, of Bio Connections LLC, and were funded by Bristol Myers Squibb.
Footnotes
RNA-sequencing and DNA methylation sequencing data are deposited in GEO with accession number GSE157302.
The Bristol Myers Squibb company policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.M., J.D., and J.P.L. designed the study; C.W. performed statistical analysis; and P.M., N.B., J.C.C., J.L.R., S.M.S., A.S.L., J.J., J.D., C.W., E.M., M.V.R., L.C., and J.P.L. collected, analyzed, and interpreted data and contributed to the development of the paper.
Conflict-of-interest disclosure: P.M. served as a consultant for Bayer, BeiGene, Celgene (a Bristol Myers Squibb [BMS] Company), Cellectar, I-MAB Biopharma, Janssen, Karyopharm Therapeutics, Kite Pharma, MorphoSys, Regeneron, Sandoz, TeneoBio, and Verastem, received research funding from Karyopharm Therapeutics, and travel support from Janssen; N.L.B. served as a consultant for Seagen Inc., received research funding from ADC Therapeutics, Autolus, BMS, Celgene (a BMS Company), Forty Seven, Genentech, Immune Design, Janssen, Kite Pharma, Merck, Millennium, Pharmacyclics, and Seagen Inc.; J.C.C. served as a consultant for Bayer, Celgene (a BMS Company), Juno Therapeutics (a BMS Company), Karyopharm Therapeutics, Kite/Gilead, MorphoSys, Novartis, Pfizer, TeneoBio, and Verastem, participated in the speakers’ bureau for AstraZeneca, BeiGene, Genentech, and Kite/Gilead, and received research funding from Merck; S.M.S. served as a consultant for Abbvie/Genentech, AstraZeneca, Bayer, BMS. Celgene (a BMS Company), Gilead, Kite Pharma, Pharmacyclics, Portola Pharmaceuticals, Seagen Inc., and TG Therapeutics, and received research funding from Acerta Pharma/AstraZeneca, Celgene (a BMS Company), and Pharmacyclics/Janssen; A.S.L. served as a consultant for Seagen Inc. and BMS, participated in the speakers’ bureau for Research to Practice; J.J. and J.D. are employees and have equity ownership with BMS; C.W. is a former employee of BMS; L.C. received research funding from Celgene (a BMS Company); and J.P.L. served as a consultant for ADC Therapeutics, Akcea Therapeutics, AstraZeneca, Bayer, BMS, Celgene (a BMS Company), Epizyme, Genentech/Roche, Gilead, Karyopharm Therapeutics, MEI Pharma, Merck, Miltenyi, Morphosys, Nordic Nanovector, Regeneron, Sandoz, and Sutro, received research funding from Alliance for Clinical Trials in Oncology, Celgene (a BMS Company), Janssen, National Cancer Institute, Pfizer, and Takeda, and received travel support from BeiGene. The remaining authors declare no competing financial interests.
Correspondence: Peter Martin, Weill Cornell Medical College, New York, NY, 10021, e-mail: pem9019@med.cornell.edu
REFERENCES
- 1.Coiffier B, Sarkozy C. Diffuse large B-cell lymphoma: R-CHOP failure-what to do? Hematology (Am Soc Hematol Educ Program). 2016;2016(1):366-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett NL, Wilson WH, Jung SH, et al. Dose-adjusted EPOCH-R compared with R-CHOP as frontline therapy for diffuse large B-cell lymphoma: clinical outcomes of the phase III intergroup trial Alliance/CALGB 50303. J Clin Oncol. 2019;37(21):1790-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mondello P, Mian M. Frontline treatment of diffuse large B-cell lymphoma: beyond R-CHOP. Hematol Oncol. 2019;37(4):333-344. [DOI] [PubMed] [Google Scholar]
- 4.Clozel T, Yang S, Elstrom RL, et al. Mechanism-based epigenetic chemosensitization therapy of diffuse large B-cell lymphoma. Cancer Discov. 2013;3(9):1002-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leonard JP, Kolibaba KS, Reeves JA, et al. Randomized phase II study of R-CHOP with or without bortezomib in previously untreated patients with non-germinal center B-cell-like diffuse large B-cell lymphoma. J Clin Oncol. 2017;35(31):3538-3546. [DOI] [PubMed] [Google Scholar]
- 6.Younes A, Sehn LH, Johnson P, et al. PHOENIX investigators. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non–germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37(15):1285- 1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitolo U, Trněný M, Belada D, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol. 2017;35(31):3529-3537. [DOI] [PubMed] [Google Scholar]
- 8.Adde M, Enblad G, Hagberg H, Sundström C, Laurell A. Outcome for young high-risk aggressive B-cell lymphoma patients treated with CHOEP-14 and rituximab (R-CHOEP-14). Med Oncol. 2006;23(2):283-294. [DOI] [PubMed] [Google Scholar]
- 9.Delarue R, Tilly H, Mounier N, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol. 2013;14(6):525-533. [DOI] [PubMed] [Google Scholar]
- 10.Chaiwatanatorn K, Stamaratis G, Opeskin K, Firkin F, Nandurkar H. Protein kinase C-beta II expression in diffuse large B-cell lymphoma predicts for inferior outcome of anthracycline-based chemotherapy with and without rituximab. Leuk Lymphoma. 2009;50(10):1666-1675. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz N, Nickelsen M, Ziepert M, et al. ; German High-Grade Lymphoma Study Group (DSHNHL) . Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL 2002-1). Lancet Oncol. 2012;13(12):1250-1259. [DOI] [PubMed] [Google Scholar]
- 12.Crump M, Leppä S, Fayad L, et al. Randomized, double-blind, phase III trial of enzastaurin versus placebo in patients achieving remission after first-line therapy for high-risk diffuse large B-cell lymphoma. J Clin Oncol. 2016;34(21):2484-2492. [DOI] [PubMed] [Google Scholar]
- 13.Vitolo U, Witzig TE, Gascoyne RD, et al. ROBUST: first report of phase III randomized study of lenalidomide/R-CHOP (R2-CHOP) vs placebo/R-CHOP in previously untreated abc-type diffuse large B-cell lymphoma. Hematol Oncol. 2019;37(S2):36-37. [Google Scholar]
- 14.Cerchietti L, Leonard JP. Targeting the epigenome and other new strategies in diffuse large B-cell lymphoma: beyond R-CHOP. Hematology (Am Soc Hematol Educ Program). 2013;2013(1):591-595. [DOI] [PubMed] [Google Scholar]
- 15.Kotlov N, Bagaev A, Revuelta MV, et al. Clinical and biological subtypes of B-cell lymphoma revealed by microenvironmental signatures. Cancer Discov. 2021;11(6):1468-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stelling A, Wu CT, Bertram K, et al. Pharmacological DNA demethylation restores SMAD1 expression and tumor suppressive signaling in diffuse large B-cell lymphoma. Blood Adv. 2019;3(20):3020-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onureg® (azacitidine) [package insert] . Summit, NJ; Celgene, a Bristol Myers Squibb company; 2020. [Google Scholar]
- 18.Wei AH, Döhner H, Pocock C, et al. ; QUAZAR AML-001 Trial Investigators . Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383(26):2526-2537. [DOI] [PubMed] [Google Scholar]
- 19.Laille E, Shi T, Garcia-Manero G, et al. Pharmacokinetics and pharmacodynamics with extended dosing of CC-486 in patients with hematologic malignancies. PLoS One. 2015;10(8):e0135520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheson BD, Fisher RI, Barrington SF, et al. ; United Kingdom National Cancer Research Institute . Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung YK, Chappell R. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics. 2004;56(4):1177-1182. [DOI] [PubMed] [Google Scholar]
- 22.Itti E, Meignan M, Berriolo-Riedinger A, et al. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: comparison between Deauville criteria and ΔSUVmax. Eur J Nucl Med Mol Imaging. 2013;40(9):1312-1320. [DOI] [PubMed] [Google Scholar]
- 23.Meignan M, Barrington S, Itti E, Gallamini A, Haioun C, Polliack A. Report on the 4th international workshop on positron emission tomography in lymphoma held in Menton, France, 3-5 October 2012. Leuk Lymphoma. 2013;55(1):31-37. [DOI] [PubMed] [Google Scholar]
- 24.Liu XQ, Lu K, Feng LL, et al. Up-regulated expression of indoleamine 2,3-dioxygenase 1 in non-Hodgkin lymphoma correlates with increased regulatory T-cell infiltration. Leuk Lymphoma. 2013;55(2):405-414. [DOI] [PubMed] [Google Scholar]
- 25.He J, Babarinde IA, Sun L, et al. Identifying transposable element expression dynamics and heterogeneity during development at the single-cell level with a processing pipeline scTE. Nat Commun. 2021;12(1):1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding S, Khoury-Hanold W, Iwasaki A, Robek MD. Epigenetic reprogramming of the type III interferon response potentiates antiviral activity and suppresses tumor growth. PLoS Biol. 2014;12(1):e1001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng F, Zhong D, Zhang L, Shao Y, Ma Q. Efficacy and safety of rituximab combined with chemotherapy in the treatment of diffuse large B-cell lymphoma: a meta-analysis. Int J Clin Exp Med. 2015;8(10):17515-17522. [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Manero G, Gore SD, Kambhampati S, et al. Efficacy and safety of extended dosing schedules of CC-486 (oral azacitidine) in patients with lower-risk myelodysplastic syndromes. Leukemia. 2015;30(4):889-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Hoff DD, Rasco DW, Heath EI, et al. Phase I study of CC-486 alone and in combination with carboplatin or nab-paclitaxel in patients with relapsed or refractory solid tumors. Clin Cancer Res. 2018;24(17):4072-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savona MR, Kolibaba K, Conkling P, et al. Extended dosing with CC-486 (oral azacitidine) in patients with myeloid malignancies. Am J Hematol. 2018;93(10):1199-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maurer MJ, Habermann TM, Shi Q, et al. Progression-free survival at 24 months (PFS24) and subsequent outcome for patients with diffuse large B-cell lymphoma (DLBCL) enrolled on randomized clinical trials. Ann Oncol. 2018;29(8):1822-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida M, Nakaya Y, Shimizu K, et al. Importance of diagnosis-to-treatment interval in newly diagnosed patients with diffuse large B-cell lymphoma. Sci Rep. 2021;11(1):2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies A, Cummin TE, Barrans S, et al. Gene-expression profiling of bortezomib added to standard chemoimmunotherapy for diffuse large B-cell lymphoma (REMoDL-B): an open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20(5):649-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wrangle J, Wang W, Koch A, et al. Alterations of immune response of non-small cell lung cancer with azacytidine. Oncotarget. 2013;4(11):2067-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daver N, Boddu P, Garcia-Manero G, et al. Hypomethylating agents in combination with immune checkpoint inhibitors in acute myeloid leukemia and myelodysplastic syndromes. Leukemia. 2018;32(5):1094-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C, Liu Y, Zhang W, et al. Phase I study of anti-PD1 in combination with low-dose decitabine in patients with advanced and untreated malignancies. J Clin Oncol. 2017;35(suppl 15):e14555. [Google Scholar]
- 37.Leonova KI, Brodsky L, Lipchick B, et al. p53 cooperates with DNA methylation and a suicidal interferon response to maintain epigenetic silencing of repeats and noncoding RNAs. Proc Natl Acad Sci USA. 2012;110(1):E89-E98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.