Abstract
Purpose
There has been concern that asthma and chronic obstructive pulmonary disease [COPD] increase the risk of developing and exacerbating COVID-19. The effect of medications such as inhaled corticosteroids (ICS) and biologics on COVID-19 is unclear. This systematic literature review analyzed the published evidence on epidemiology and the burden of illness of asthma and COPD, and the use of baseline medicines among COVID-19 populations.
Patients and Methods
Using Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, Embase®, MEDLINE® and Cochrane were searched (January 2019–August 2021). The prevalence of asthma or COPD among COVID-19 populations was compared to the country-specific populations. Odds ratios (ORs) were estimated to compare healthcare resource utilization (HCRU) rates, and meta-analyses of outcomes were estimated from age-adjusted ORs (aORs) or hazard ratios (aHRs). Meta-analyses of COVID-19 outcomes were conducted using random effects models for binary outcomes.
Results
Given the number and heterogeneity of studies, only 183 high-quality studies were analyzed, which reported hospitalization, intensive care unit (ICU) admissions, ventilation/intubation, or mortality. Asthma patients were not at increased risk for COVID-19–related hospitalization (OR = 1.05, 95% CI: 0.92 to 1.20), ICU admission (OR = 1.21, 95% CI: 0.99 to 1.1.48), ventilation/intubation (OR = 1.24, 95% CI: 0.95 to 1.62), or mortality (OR = 0.85, 95% CI: 0.75 to 0.96). Accounting for confounding variables, COPD patients were at higher risk of hospitalization (aOR = 1.45, 95% CI: 1.30 to 1.61), ICU admission (aOR = 1.28, 95% CI: 1.08 to 1.51), and mortality (aOR = 1.41, 95% CI: 1.37 to 1.65). Sixty-five studies reported outcomes associated with ICS or biologic use. There was limited evidence that ICS or biologics significantly impacted the risk of SARS-CoV-2 infection, HCRU, or mortality in asthma or COPD patients.
Conclusion
In high-quality studies included, patients with asthma were not at significantly higher odds for adverse COVID-19–related outcomes, while patients with COPD were at higher odds. There was no clear evidence that baseline medication affected outcomes.
Registration
PROSPERO (CRD42021233963).
Keywords: healthcare resource utilization, mortality, SARS-COV-2, inhaled corticosteroids, biologics
Introduction
The World Health Organization declared severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) a global pandemic in March 2020. Many factors contribute to COVID-19 severity, with age being a major determinant.1 The presence of comorbidities increases the severity of COVID-19.1,2 Chronic obstructive pulmonary disease (COPD) and asthma are chronic lung diseases estimated to affect 251 million and 339 million people worldwide, respectively.3 Since SARS-CoV-2 is a respiratory virus, there has been concern that underlying respiratory conditions pose an increased risk of more severe COVID-19–related disease and mortality. The US Centers for Disease Control and Prevention (CDC) lists COPD and moderate-to-severe and uncontrolled asthma as risk factors for severe COVID-19.4 However, extensive data of variable quality have emerged since the start of the pandemic such that it is now possible to synthesize the data to address these concerns.
Early reports from March 2020 suggested that asthma patients were more likely to have severe COVID-19 requiring intensive care unit (ICU) admission.5,6 Data from the large UK database OPENSafely with data collected from March 2020 to May 2020 showed that those with asthma or COPD taking inhaled corticosteroids (ICS) or oral corticosteroids had higher COVID-19–related mortality than the overall population, but this may have been affected by confounding of the indication for ICS.7–9 Other large database studies that included patients between February and April 2020 or from March to August 2020, respectively, from the UK or USA, showed that while COPD was a risk factor for mortality in univariate analysis, asthma was not associated with increased death.10,11
Another concern is whether patients’ medications affect SARS-CoV-2 infection rates and outcomes from COVID-19 disease. ICS are the mainstay of asthma treatments and are sometimes indicated for patients with COPD at risk of exacerbations. More severe asthma may require biologic agents targeting immunoglobulin E or cytokines.12,13 ICS can suppress host immunity,14 but several studies suggest that they may play a protective role by reducing viral replication or decreasing expression of ACE2, the cell surface protein critical for SARS-CoV-2 host cell entry.15,16 Biologics may also modulate the antiviral immune response by altering the cytokine cascade.17 Although patients are encouraged to continue their prescribed medications, there is no consensus on the effect of these treatments on COVID-19 outcomes.
The perception that asthma and COPD may increase the risk of COVID-19 severity may impact the triage and treatment of patients after SARS-CoV-2 diagnosis. Identification of the risks posed and accurate assessment of SARS-CoV-2 effects in patients with asthma or COPD would facilitate clinical decision-making.
Although several previous systematic literature reviews (SLRs) have assessed how asthma18,19 or COPD20,21 affect COVID-19–related healthcare resource utilization (HCRU) and mortality, these studies are limited by the population size, small number of included studies, lack of adjustment for confounding variables, and the quality of included studies. The objective of the current SLR was to gather and analyze a larger body of published evidence on epidemiology and the burden of asthma and COPD in COVID-19, focusing on high-quality studies. Secondary objectives were to review how severe asthma and the use of ICS and biologics affect COVID-19 outcomes.
Methods
Data Review and Extraction
SLRs of burden of illness (BOI) and baseline therapies were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.22,23 The methods were based on the Cochrane Handbook for Systematic Reviews of Interventions, the Centre for Reviews and Dissemination’s Guidance for Undertaking Reviews in Health Care, and the Methods for the Development of the National Institute for Health and Care Excellence’s Public Health Guidance.23–25
Data Sources
The key biomedical literature databases (MEDLINE®, Embase®) and Cochrane were searched via the Ovid platform (January 2020 to August 6, 2021). MEDLINE® Epub Ahead of Print, In-Process & Other Non-Indexed Citations were searched to retrieve any non-indexed citations. Bibliographies of relevant meta-analyses and SLRs were searched for additional literature.
Search Strategy and Inclusion/Exclusion Material
See the Supplemental File for detailed search strategy and results. The scope of the SLRs was defined by Population, Intervention, Comparators, Outcomes, and Study Design (PICOS) criteria (Supplementary Tables S1–S4). Both SLRs included patients with confirmed COVID-19. Pre-prints and congress abstracts were excluded to ensure high-quality. Publications in the BOI SLR reported on outcomes in patients with either asthma or COPD. Studies that reported asthma and COPD together were excluded. Outcomes included prevalence, HCRU (hospitalization, ICU admission, and invasive ventilation rates), and clinical outcomes (length of stay [LOS], treatment patterns, mortality). Real-world evidence studies were included, and the search was not restricted to English language studies. In studies with <20 relevant patients, only prevalence outcomes were extracted. The anti-asthmatic therapies considered were baseline use of biologics, ICS, or systemic corticosteroids.
Definition of High-Quality Studies
Given the number and heterogeneity of studies reporting COVID-19–related outcomes, studies were analyzed to determine if they were high-quality, as defined by:
Inclusion of a confirmatory diagnosis of COVID-19.
A statement and/or accounting for missing data.
Sample collection that extended beyond 1 month past the first COVID-19 case in each country, as temporal changes in outcomes have been reported.26
Bias assessment used the Newcastle-Ottawa scale; most studies (80%) scored above 7/9.
Meta-Analyses
Meta-analyses calculated asthma and COPD prevalence in patients with COVID-19 and assessed the risk of hospitalization, ICU admission, and mortality associated with each condition, following methods published by the Cochrane Collaboration guidelines, the Agency for Healthcare Research and Quality, and NICE.23–25 To explore the impact of study quality on each outcome, meta-analyses were stratified (high-quality studies vs other). Country-specific 2016 prevalence of asthma and COPD in COVID-19–positive patients were estimated for each country with ≥2 studies and then versus background population prevalence from each country.27 Prevalence rates were measured with no reference to SARS-CoV-2 variants. Odds ratios (OR) were estimated to compare HCRU and outcomes based on studies that reported outcomes in both asthma/COPD and non-asthma/non-COPD. Studies reporting a general COVID-19–positive population and asthma/COPD subgroup were re-calculated into asthma/COPD and non-asthma/non-COPD groups. Means and standard deviations were estimated from medians and interquartile ranges to facilitate meta-analysis of mean differences to compare hospital LOS using previously validated techniques.28 Pooled estimates were calculated overall and stratified by geographic region (US, EU5, China) where sufficient sample size was available using 2016 Global Burden of Disease collaborator data.27 Because age is a factor associated with COPD,29 meta-analyses of hospitalization rates, ICU admission, and mortality were estimated from age-adjusted odds ratios (aORs) or age-adjusted hazard ratios (aHRs), where sufficient sample size was available. Meta-analyses were conducted using the “Meta” package in RStudio (Version 1.2.5033) using a Generalized Linear Mixed Models approach with a logit transformation for binary outcomes, the inverse variance method for OR and HR, and the Clopper–Pearson method for estimation of 95% confidence intervals. Random effects models were used for binary outcomes. Heterogeneity was summarized using I2 for fixed effects models and τ for random effects models. Tests for differences between subgroups were performed using Cochran's Q-test for heterogeneity.
Results
Study Characteristics
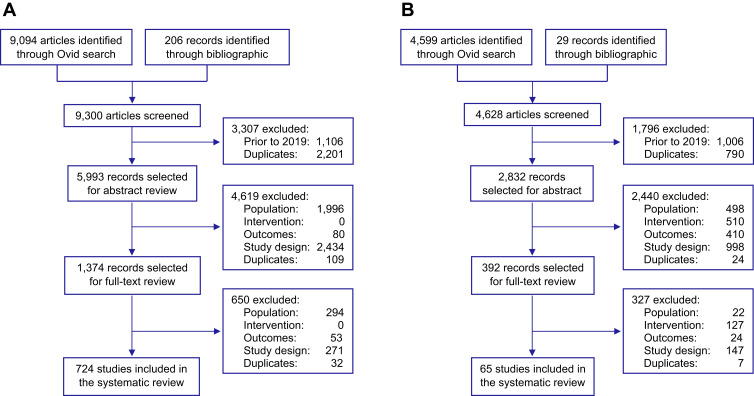
The BOI SLR identified 9094 publications. Following a full-text review, 706 records were included for meta-analysis (Figure 1A). See the Supplementary File for a full list of included studies.
Figure 1.
PRISMA flow diagram for (A) BOI SLR and (B) ICS/Biologics SLR.
Note: Adapted from Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.56
Among COVID-19 populations, 419 studies reported asthma-specific outcomes and 589 studies reported COPD-specific outcomes. These studies covered 61 countries (USA [n = 166]; China [n = 150]; and Italy [n = 60]). Sample sizes ranged from 115 to 211,003, with mean age from 2 to 93 years and male percentages from 0.0% to 94.0% (Supplementary Figure S1A and B). From 183 high-quality studies, 119 reported asthma-specific outcomes; 155 reported COPD-specific outcomes.
Prevalence of Asthma in COVID-19
To understand if those with underlying asthma are at increased risk of infection from SARS-CoV-2, data were pooled from 112 high-quality studies, with asthma prevalence among COVID-19– positive patients ranging from 0.3% to 21.0%. After restricting the sample to countries with ≥2 studies, 89 high-quality studies were included. Among those, USA, UK, and Ghana were the only countries that showed significantly higher pooled asthma prevalence in the COVID-19 population estimates, with a difference in prevalence percentage of 4.40 (95% CI, 2.94 to 6.11, p < 0.001), 4.06 (95% CI, 1.95 to 6.42, p < 0.001) and 1.84 (95% CI, 0.25 to 4.01, p = 0.02) respectively. Korea, Mexico, China, Italy, and Sweden showed lower pooled asthma COVID-19 prevalence rates versus background rates. There were no significant differences between the prevalence rates in the two populations in the other countries (Table 1).
Table 1.
Prevalence of Asthma in General Population and COVID-19 Positive Population
| Country | General Population | COVID-19 Positive Population | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All Studies | High-Quality Studies | ||||||||
| Asthma Prevalence | N | Prevalence (95% CI) | Difference (95% CI) | P | N | Prevalence (95% CI) | Difference (95% CI) | P | |
| Portugal | 8.8 | 2 | 2.37 (0.99 to 5.54) | −6.43 (−7.81 to −3.26) | 0.00 | ||||
| Chile | 7.08 | 2 | 2.60 (0.82 to 7.90) | −4.48 (−6.26 to 0.82) | 0.08 | ||||
| Malaysia | 5.72 | 3 | 1.99 (1.04 to 3.78) | −3.73 (−4.68 to −1.94) | 0.00 | ||||
| Philippines | 6.35 | 2 | 3.23 (2.88 to 3.63) | −3.12 (−3.47 to −2.72) | 0.00 | ||||
| Iran | 5.53 | 10 | 3.41 (2.14 to 5.40) | −2.12 (−3.39 to −0.13) | 0.02 | ||||
| Russia | 4.93 | 2 | 2.18 (1.40 to 3.37) | −2.75 (−3.53 to −1.56) | 0.00 | ||||
| Italy | 4.96 | 13 | 2.99 (1.98 to 4.50) | −1.97 (−2.98 to −0.46) | 0.02 | 3 | 2.81 (1.98 to 3.98) | −2.15 (−2.98 to −0.98) | 0.00 |
| China | 3.01 | 23 | 1.05 (0.69 to 1.60) | −1.96 (−2.32 to −1.41) | 0.00 | 4 | 0.62 (0.55 to 0.70) | −2.39 (−2.46 to −2.31) | 0.00 |
| Korea | 5.51 | 19 | 3.75 (2.65 to 5.28) | −1.76 (−2.86 to −0.23) | 0.03 | 6 | 2.75 (2.53 to 2.98) | −2.76 (−2.98 to −2.53) | 0.00 |
| Oman | 5.38 | 2 | 4.04 (1.81 to 8.76) | −1.34 (−3.57 to 3.38) | 0.47 | ||||
| Mexico | 4.11 | 19 | 2.87 (2.57 to 3.21) | −1.24 (−1.54 to −0.90) | 0.00 | 8 | 3.01 (2.85 to 3.18) | −1.10 (−1.26 to −0.93) | 0.00 |
| Sweden | 8.01 | 5 | 7.00 (6.83 to 7.17) | −1.01 (−1.18 to −0.84) | 0.00 | 5 | 7.00 (6.83 to 7.17) | −1.01 (−1.18 to −0.84) | 0.00 |
| Saudi Arabia | 4.35 | 3 | 3.49 (2.86 to 4.25) | −0.86 (−1.49 t0 −0.10) | 0.03 | ||||
| Netherlands | 6.72 | 3 | 5.93 (2.79 to 12.14) | −0.79 (−3.93 to 5.42) | 0.74 | ||||
| Brazil | 3.86 | 10 | 3.31 (1.64 to 6.56) | −0.55 (−2.22 to 2.70) | 0.67 | 4 | 2.84 (0.80 to 9.64) | −1.02 (−3.06 to 5.78) | 0.63 |
| Canada | 7.21 | 3 | 6.75 (3.30 to 13.29) | −0.46 (−3.91 to 6.08) | 0.85 | 3 | 6.75 (3.30 to 13.29) | −0.46 (−3.91 to 6.08) | 0.85 |
| Spain | 6.43 | 31 | 6.02 (4.62 to 7.79) | −0.41 (−1.81 to 1.36) | 0.62 | 5 | 6.18 (4.95 to 7.70) | −0.25 (−1.48 to 1.27) | 0.73 |
| Japan | 6.01 | 6 | 5.67 (3.78 to 8.42) | −0.34 (−2.23 to 2.41) | 0.77 | ||||
| Australia | 11.03 | 2 | 10.95 (8.30 to 14.31) | −0.08 (−2.73 to 3.28) | 0.96 | ||||
| India | 2.86 | 6 | 3.15 (2.54 to 3.90) | 0.29 (−0.32 to 1.04) | 0.37 | ||||
| Belgium | 5.53 | 2 | 6.22 (3.31 to 11.37) | 0.69 (−2.22 to 5.84) | 0.71 | ||||
| Turkey | 5.89 | 18 | 7.25 (5.45 to 9.59) | 1.36 (−0.44 to 3.70) | 0.15 | ||||
| Ghana | 3.66 | 2 | 5.50 (3.91 to 7.67) | 1.84 (0.25 to 4.01) | 0.02 | 2 | 5.50 (3.91 to 7.67) | 1.84 (0.25 to 4.01) | 0.02 |
| Switzerland | 6.89 | 4 | 9.73 (5.90 to 15.64) | 2.84 (−0.99 to 8.75) | 0.18 | ||||
| France | 6.6 | 11 | 10.04 (6.67 to 14.82) | 3.44 (0.07 to 8.22) | 0.05 | 4 | 7.45 (5.44 to 10.13) | 0.85 (−1.16 to 3.53) | 0.45 |
| UK | 8.85 | 24 | 13.77 (12.38 to 15.30) | 4.92 (3.53 to 6.45) | 0.00 | 12 | 12.91 (10.87 to 15.27) | 4.06 (1.95 to 6.42) | 0.00 |
| USA | 4.72 | 143 | 10.15 (9.25 to 11.13) | 5.43 (4.53 to 6.41) | 0.00 | 32 | 9.12 (7.66 to 10.83) | 4.40 (2.94 to 6.11) | 0.00 |
| Norway | 6.93 | 3 | 12.40 (10.20 to 14.99) | 5.47 (3.27 to 8.06) | 0 | ||||
| Denmark | 5.66 | 5 | 11.85 (7.56 to 18.10) | 6.19 (1.90 to 12.44) | 0.00 | ||||
| Germany | 5.74 | 4 | 12.35 (11.12 to 13.70) | 6.61 (5.38 to 7.96) | 0.00 | ||||
| Finland | 7.96 | 2 | 16.15 (13.44 to 19.28) | 8.19 (5.48 to 11.32) | 0.00 | ||||
| Egypt | 6.84 | 2 | 19.06 (8.73 to 36.69) | 12.22 (1.89 to 29.85) | 0.01 | ||||
Note: Random effects as I2 was high (=99.8%) and countries were excluded when the number of studies was < 2.
Abbreviations: CI, confidence interval; N, number of studies.
Prevalence of COPD in COVID-19
From 151 high-quality studies, COPD prevalence rates among patients with COVID-19 ranged from 0.0% to 32.9%. Restricting the sample to the countries with ≥2 studies yielded 130 high-quality studies. Significantly higher pooled prevalence rates in the COVID-19 population were observed in Denmark, USA, Italy, and Netherlands, while a significantly lower prevalence was seen in Canada and China. There were no significant differences between the prevalence rates in the two populations in the other countries (Table 2).
Table 2.
Prevalence of COPD in General Population and COVID-19 Positive Population
| Country | General population | COVID-19 Positive Population | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All Studies | High-Quality Studies | ||||||||
| COPD Prevalence | N | Prevalence (95% CI) | Difference (95% CI) | P | N | Prevalence (95% CI) | Difference (95% CI) | P | |
| India | 4.18 | 13 | 3.87 (2.51 to 5.95) | −0.31 (−1.67 to 1.77) | 0.74 | 2 | 5.11 (2.46 to 10.33) | 0.93 (−1.72 to 6.15) | 0.59 |
| Saudi Arabia | 1.69 | 2 | 1.28 (0.77 to 2.10) | −0.41 (−0.92 to 0.41) | 0.27 | ||||
| China | 3.92 | 145 | 3.01 (2.59 to 3.49) | −0.91 (−1.33 to −0.43) | 0.00 | 20 | 2.39 (1.53 to 3.70) | −1.53 (−2.39 to −0.22) | 0.03 |
| Mexico | 3.17 | 24 | 2.22 (1.67 to 2.94) | −0.95 (−1.50 to −0.23) | 0.01 | 9 | 2.75 (1.63 to 4.61) | −0.42 (−1.54 to 1.44) | 0.60 |
| Switzerland | 7.15 | 4 | 6.18 (4.94 to 7.70) | −0.97 (−2.21 to 0.55) | 0.20 | ||||
| Kuwait | 2.09 | 2 | 0.43 (0.28 to 0.66) | −1.66 (−1.81 to −1.43) | 0.00 | ||||
| Finland | 4.63 | 2 | 2.61 (1.61 to 4.22) | −2.02 (−3.02 to −0.41) | 0.02 | ||||
| Brazil | 4.32 | 6 | 2.26 (0.92 to 5.46) | −2.06 (−3.40 to 1.14) | 0.15 | 2 | 1.16 (0.15 to 8.15) | −3.16 (−4.17 to 3.83) | 0.19 |
| Australia | 3.81 | 2 | 1.67 (0.80 to 3.45) | −2.14 (−3.01 to −0.36) | 0.03 | ||||
| Canada | 4.09 | 3 | 1.62 (1.02 to 2.56) | −2.47 (−3.07 to −1.53) | 0.00 | 3 | 1.62 (1.02 to 2.56) | −2.47 (−3.07 to −1.53) | 0.00 |
| Korea | 3.57 | 21 | 3.67 (1.99 to 6.69) | 0.10 (−1.58 to 3.12) | 0.93 | 7 | 2.15 (0.88 to 5.12) | −1.42 (−2.69 to 1.55) | 0.26 |
| Russia | 4.15 | 2 | 4.56 (2.60 to 7.86) | 0.41 (−1.55 to 3.71) | 0.74 | ||||
| Sweden | 6.76 | 5 | 7.48 (3.47 to 15.37) | 0.72 (−3.29 to 8.61) | 0.79 | 5 | 7.48 (3.47 to 15.37) | 0.72 (−3.29 to 8.61) | 0.79 |
| Denmark | 6.01 | 8 | 7.09 (4.24 to 11.61) | 1.08 (−1.77 to 5.60) | 0.53 | 2 | 8.48 (7.76 to 9.26) | 2.47 (1.75 to 3.25) | 0.00 |
| Pakistan | 2.48 | 2 | 3.62 (2.01 to 6.41) | 1.14 (−0.47 to 3.93) | 0.20 | ||||
| France | 4.27 | 14 | 5.43 (4.00 to 7.34) | 1.16 (−0.27 to 3.07) | 0.12 | 7 | 5.24 (3.17 to 8.54) | 0.97 (−1.10 to 4.27) | 0.42 |
| Japan | 3.49 | 5 | 4.91 (4.15 to 5.81) | 1.42 (0.66 to 2.32) | 0.00 | 12 | |||
| Spain | 6.34 | 37 | 7.80 (6.28 to 9.66) | 1.46 (−0.06 to 3.32) | 0.06 | 6.50 (5.32 to 7.93) | 0.16 (−1.02 to 1.59) | ||
| Norway | 5.53 | 2 | 7.41 (3.75 to 14.12) | 1.88 (−1.78 to 8.59) | 0.39 | ||||
| Poland | 3.98 | 2 | 6.02 (1.33 to 23.31) | 2.04 (−2.65 to 19.33) | 0.58 | ||||
| Greece | 6 | 2 | 9.14 (5.67 to 14.40) | 3.14 (−0.33 to 8.40) | 0.08 | 28 | 10.17 (8.16 to 12.62) | ||
| USA | 5.16 | 125 | 8.40 (7.37 to 9.56) | 3.24 (2.21 to 4.40) | 0.00 | 3 | 6.80 (2.39 to 17.87) | 5.01 (3.00 to 7.46) | 0.00 |
| Turkey | 3.96 | 29 | 7.29 (5.69 to 9.29) | 3.33 (1.73 to 5.33) | 0.00 | 2.84 (−1.57 to 13.91) | 0.31 | ||
| Iran | 2.23 | 13 | 5.89 (3.55 to 9.63) | 3.66 (1.32 to 7.40) | 0.00 | ||||
| Belgium | 6.66 | 3 | 10.39 (7.31 to 14.56) | 3.73 (0.65 to 7.90) | 0.01 | ||||
| UK | 5.32 | 21 | 10.10 (7.22 to 13.97) | 4.78 (1.90 to 8.65) | 0.00 | 9 | 9.22 (5.08 to 16.19) | 3.90 (−0.24 to 10.87) | 0.07 |
| Italy | 4.41 | 49 | 9.32 (7.61 to 11.36) | 4.91 (3.20 to 6.95) | 0.00 | 18 | 8.14 (6.02 to 10.91) | 3.73 (1.61 to 6.50) | 0.00 |
| Germany | 6.15 | 7 | 13.36 (12.82 to 13.91) | 7.21 (6.67 to 7.76) | 0.00 | ||||
| Egypt | 2.42 | 4 | 12.50 (4.17 to 31.95) | 10.08 (1.75 to 29.53) | 0.00 | ||||
| Netherlands | 5.45 | 5 | 15.81 (13.35 to 18.63) | 10.36 (7.90 to 13.18) | 0.00 | 2 | 18.33 (14.99 to 22.21) | 12.88 (9.54 to 16.76) | 0.00 |
Note: Random effects as I2 was high (=99.8%) and countries were excluded when the number of studies was < 2.
Abbreviations: CI, confidence interval; N, number of studies.
HCRU and Mortality of Asthma Patients with COVID-19
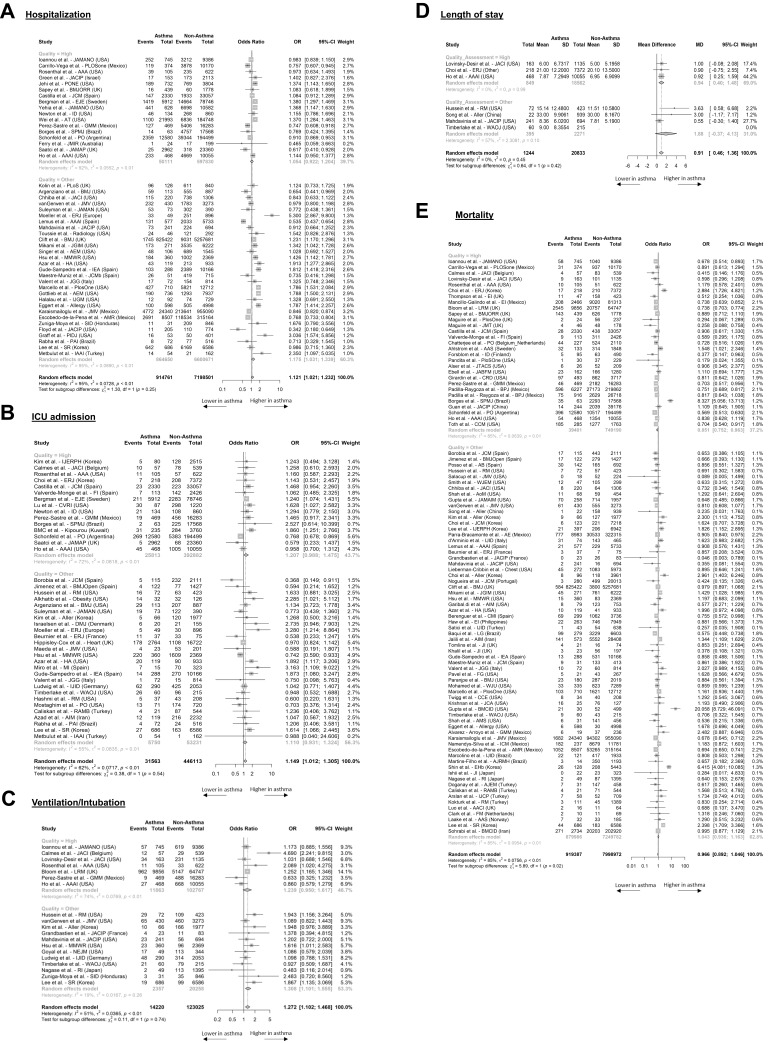
Compared to non-asthma patients, the ORs of hospitalization, ICU admission, and ventilation/intubation were not significantly higher for asthma patients in high-quality studies, although significant differences were found when studies of lower quality were included. The OR of hospitalization in asthma versus non-asthma patients was 1.05 (95% CI, 0.92 to 1.20) in 17 high-quality studies (Figure 2A). The OR for ICU admission was 1.21 (95% CI, 0.99 to 1.48) in 15 high-quality studies (Figure 2B). The OR of ventilation/intubation in asthma versus non-asthma patients was 1.24 (95% CI, 0.95 to 1.62) in seven high-quality studies (Figure 2C). Finally, LOS was longer in asthma patients by almost one day in the high-quality analysis (3 studies; 0.94, 95%: 0.40 to 1.48) (Figure 2D).
Figure 2.
Forest plots of odds ratios of asthma compared to non-asthma patients for COVID-19 related HCRU and mortality. Data is presented for all studies with evaluable evidence and stratified by quality of studies. Outcomes included hospitalization (A), ICU admission (B), ventilation (C), length of stay (D), and mortality (E).
Patients with asthma were at decreased risk of death compared to non-asthma patients with an OR of 0.85 (95% CI, 0.75 to 0.96) in the 29 high-quality studies (Figure 2E). When studies of lower quality were also included, there were no significant geographic trends in HCRU or mortality among asthma patients (Supplementary Figure S2).
Except for the pooled aHRs for ICU admission, all other pooled aHRs or aORs for hospitalization, ICU admission, ventilation/intubation, and mortality remained nonsignificant among high-quality studies (Supplementary Figure S3).
Eight studies reported outcomes associated with asthma severity, using varying definitions of severity. Six studies compared outcomes to non-asthmatics. From the Severe Acute Respiratory and emerging Infection Consortium (ISARIC) WHO Clinical Characterisation Protocol UK (CCP-UK) study of over 70,000 patients, in those between 16 and 49 years, patients with severe asthma had an increased risk of mortality (HR = 1.96, 95% CI: 1.25 to 3.08, p=0.0037) compared to those without asthma.30 Only two studies addressed the risk of mortality between mild and moderate-to-severe asthma, showing no effect of asthma severity on risk of death. A Korean study of 7272 adult COVID-19 patients reported an aOR of 1.33 (95% CI: 0.54 to 3.30, p = 0.5260) comparing moderate-severe asthma to mild asthma.31 In a database study based in England, the risk of ICU admission was higher in those with active asthma (aHR = 1.32, 95% CI: 1.14 to 1.59) or severe asthma (aHR = 1.29, 95% CI = 1.08 to 1.60) compared to 1.07 (95% CI = 0.91 to 1.27) among asthma patients. However, this did not translate into a higher risk for mortality with an aHR for death for active asthma of 1.05 (95% CI: 0.96 to 1.15) and for severe asthma of 1.08 (95% CI: 0.98 to 1.19) compared to 0.99 (95% CI: 0.91 to 1.07) among asthma patients.32
HCRU and Mortality of COPD Patients with COVID-19
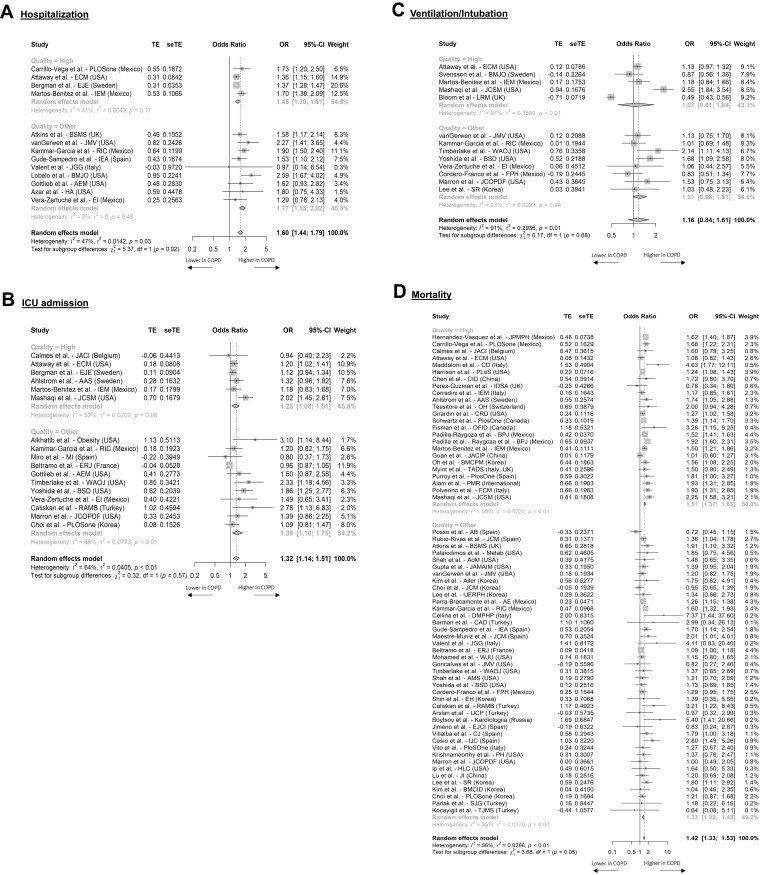
To account for age, aHR or aORs were used in the meta-analyses. Hospitalization, ICU admission, and ventilation/intubation rates were higher in COPD patients versus non-COPD patients. Among four high-quality studies, the aOR was 1.45 (95% CI, 1.30 to 1.61) (Figure 3A), with similar results seen for aHR from four studies (Supplementary Figure S4A). Likewise, the aOR for ICU admission from six high-quality studies was significant with a pooled value of 1.28 (95% CI, 1.08 to 1.51) (Figure 3B), whereas the aOR for ventilation/intubation was non-significant from five high-quality studies 1.31 (95% CI, 0.61 to 1.88) (Figure 3C). Adjusted measures of LOS were not reported.
Figure 3.
Forest plots summarizing adjusted odds ratios for COPD compared to non-COPD patients for COVID-19 related HCRU and mortality. Data is presented for all studies with evaluable evidence. Outcomes included hospitalization (A), ICU admission (B), ventilation (C), and mortality (D).
Higher hospitalization and ICU admission rates translated into higher mortality in COPD. The aOR for mortality from 24 high-quality studies was 1.51 (95% CI, 1.37 to 1.65) (Figure 3D). Similarly, aHR was 1.26 (95% CI, 1.10 to 1.44) based on thirteen high-quality studies (Supplementary Figure S4B). These results suggest that after adjusting for confounding variables, COPD confers a significant risk for adverse COVID-19 outcomes.
Use of Inhaled Corticosteroids or Biologic Therapies and Their Role in COVID-19–Related Outcomes: SLR Results
Following a full-text review, 65 studies were selected for data extraction (Figure 1B). Fifteen countries were represented, with the largest number of studies coming from Spain (n = 15), USA (n = 14), and Italy (n = 8). See the Supplementary File for a full list of included studies.
Based on ten studies that evaluated the risk of COVID-19 infection, neither the use of ICS alone or in combination,33,34 nor the use of biologics33,35 significantly impacted the risk of SARS-CoV-2 infection (Supplementary Figure S5).
Thirty-two studies reported HCRU or mortality outcomes in the context of ICS or biologic use among those with respiratory conditions (Supplementary Table S5). There was no evidence that ICS use impacts COVID-19–related hospitalization, ICU admission, or mortality rates. While a US-based study showed that hospitalized COVID-19 patients had higher baseline use of ICS with long-acting beta agonists (LABA) versus an outpatient group (18.4% vs 14.7%), this was not considered a risk factor (OR = 1.08 [0.73 to 1.59]).36 A Korean study showed that ICS users had higher hospitalization rates versus non-users (13.2% vs 0.5%).34 However, other studies37–39 show lower hospitalization rates among ICS users.
Considering the use of biologics, hospitalization rates varied. Some studies35,36 reported similar hospitalization rates and others reported lower rates,37,39 even when higher hospitalizations were reported; this was not significant in multivariate analysis (OR = 2.36, 95% CI: 0.273 to 20.4), p = 0.43)40, (Supplementary Figure S6A and B). Similarly, there is no definitive evidence that ICS or biologics affects the risk of COVID−19–related ICU admission (Supplementary Figure S6C and D). Beurnier et al show a dose-dependent increase in ICU admission rate with increasing ICS dose.41 Other studies show that after adjusting for age, sex, and asthma severity, the use of ICS alone or in combination was not a significant risk factor for ICU admission,36,42–44 nor is biologic use.44
Mortality rates were 0.05% to 10% among ICS users and 0% to 33% among biologics users (Supplementary Figure S7). Graziani et al showed no difference in ICS use between COVID-19 survivors and non-survivors.45 Although two studies reported that ICS alone or in combination was a mortality risk factor,7,34 risk was accounted for in both studies after considering relevant factors like disease severity. Moreover, most studies reported that ICS was not associated with adverse outcomes.36,42–45
Discussion
COVID-19 Related HCRU and Mortality in Asthma and COPD
The prevalence of asthma and COPD in patients with COVID-19 varied geographically and was higher in the UK and the US than in the general population. Our analyses are limited by the assumption that baseline populations have similar characteristics, particularly age, to the general population. Given the concern that asthma and COPD increase COVID-19 risk, these populations may be more likely to practice infection control measures, such as shielding, masking, and testing,4,46 behaviors unaccounted for in the current studies. Ideal comparator populations would be cohorts matched for age- and sex-matched cohorts, but this was not feasible in this study as there was insufficient information in the studies to allow matching.
Although the symptoms of asthma and COPD are similar and both are characterised by airflow obstruction, their pathogenesis and inflammatory endotypes differ.47 These differences could affect the susceptibility to SARS-CoV-2 infection and the severity of COVID-19 infection.48,49 We found that COPD increases COVID-19–related HCRU and mortality, while overall asthma does not increase the risk of these outcomes. Patients with severe asthma may be at increased risk of hospitalization or ICU admission, but they are not at an increased risk of mortality compared to patients with mild asthma. There was no evidence that ICS or biologics significantly impacts the risk of SARS-CoV-2-infection or clinical outcomes.
Several recent reviews based on more limited data have evaluated the risks posed by asthma and COPD. Terry et al18 reported that asthma did not increase the risk of COVID-19–related hospitalization, disease severity, or mortality. We found an increase in HCRU among asthma patients when low-quality studies were included, but this became nonsignificant when using only high-quality studies. Among high-quality studies, asthma patients had a lower risk of mortality compared to non-asthma patients, confirming the minimal risk in this patient population.
Gerayeli et al20 and Rabbani et al50 report that COPD conferred an approximate 3-fold increase in COVID-19 deaths, but they did not account for age, sex, or other comorbidities. Our study used adjusted HRs or ORs to account for these confounding variables. Differences in the included confounding variables exist between studies, but given that age is a major factor contributing to COVID-19 outcomes,51 these other covariates likely have a more limited impact on outcomes. While our results show a statistically significant increased risk for COPD patients in terms of their COVID-19–related HCRU and mortality, the reported aORs are below 1.6, suggestive of only a moderate risk among this patient population and potentially less than other risk factors such as age. For example, in a UK-based database study, the unadjusted HR for death for COPD compared to non-COPD was 1.68 (95% CI: 1.57 to 1.80), whereas the unadjusted HR for those aged 50–69 was 2.55 (95% CI: 2.06 to 3.17) and among those aged ≥80 13.47 (95% CI: 11.2 to 16.20) compared to those <50 years old.11
The Role of ICS and Biologics in COVID-19 Outcomes
An early SLR52 tried to delineate the role of ICS in COVID-19 outcomes but was challenged by a lack of reliable studies reporting comorbidities. Our SLR encountered similar difficulties. Although 65 studies reported on the use of ICS or biologics among a COVID-19 population, the small evaluable sample size and lack of detail about specific drugs or dosages limited analytical possibilities. Heterogeneity was noted in the baseline populations of extracted studies, including pediatric-only populations,33 mixed asthma/COPD populations,7,53 and populations with other inflammatory conditions.54,55 Finally, the results of meta-analyses comparing ICS/biologics and non-ICS/non-biologics users could be confounded by an underlying condition: treatment allocation may relate to factors like disease severity that affect COVID-19 outcomes. Our review shows that, overall, use of ICS or biologics in patients with asthma or COPD does not significantly affect COVID-19–related HCRU or mortality. Potential confounding in our results stems from the inability to account for how asthma/COPD disease severity, smoking history, or other comorbidities affect COVID-19–related outcomes.
Study Limitations
Significant heterogeneity was observed in the meta-analyses. Some of this heterogeneity can be accounted for by the varied range of sample sizes (19 to 331,298), differences in patient populations (emergency room visits, hospitalized, elderly), how comorbidities were defined, and geographic difference in treatment protocols, including when patients are transferred to the ICU or require intubation.
Another limitation is the reliability of comorbidity identification in studies. Many studies (74%) collected data from chart reviews or medical records with no objective confirmation of disease or linkage to primary care data; others relied on patient surveys (3%). Our analysis also does not account for geographic differences in comorbidity diagnosis. Finally, as studies included data on patients presenting over different time periods and at different stages of the pandemic, including from the start of the pandemic, it was not possible to evaluate temporal trends in COVID-19 outcomes.
To address some of these limitations, we undertook an analysis of studies deemed to be high-quality using pre-defined criteria that address the accuracy in COVID-19 and comorbidity diagnosis. Compared to the lower quality studies, heterogeneity was lower among the 183 high-quality studies. The difference in results between high-quality studies compared to the analysis of all studies highlights the importance of considering quality criteria when evaluating COVID-19 data.
The quickly evolving COVID-19 landscape, as well as the introduction of vaccination, suggests that further analyses will be necessary, particularly as the robustness of diagnosis, recording of comorbidities, and prior medication have been recognized as important and management of COVID-19 is likely to further improve.
Conclusion
When considering high-quality studies, our analysis shows that while COPD confers a moderate but significant risk of adverse COVID-19 outcomes, asthma does not. We have also shown that there is no clear evidence that ICS or biologics pose additional risk for SARS-CoV-2 infection or poorer outcomes. The risk of HCRU or mortality in either asthma or COPD remains modest after accounting for confounding variables.
Disclosure
This study was funded by AstraZeneca (AZ) and Way’s Group. The following AZ and Way’s Group employees were involved in the study design, decision to publish, and preparation of the manuscript: Professor Adrian Rabe and Dr. Wei Jie Loke. Professor Adrian Rabe reports grants from AstraZeneca, during the conduct of the study. Dr. Wei Jie Loke reports personal fees from AstraZeneca UK, during the conduct of the study. Professor David Halpin was an external consultant. Professor David Halpin reports personal fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, GSK, Novartis, Pfizer, Sanofi, outside the submitted work. Stacy Grieve, Patrick Daniele, Sanghee Hwang, and Anna Forsythe are employees of Purple Squirrel Economics, a Cytel Company, which was a paid consultant to AstraZeneca in connection with the development of this manuscript. The authors report no other conflicts of interest in this work.
References
- 1.Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging. 2020;12(13):12493–12503. doi: 10.18632/aging.103579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koh J, Shah SU, Chua PEY, Gui H, Pang J. Epidemiological and clinical characteristics of cases during the early phase of COVID-19 pandemic: a systematic review and meta-analysis. Front Med. 2020;7:295. doi: 10.3389/fmed.2020.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global surveillance, prevention and control of chronic respiratory diseases. Available from: https://www.who.int/publications-detail-redirect/global-surveillance-prevention-and-control-of-chronic-respiratory-diseases. Accessed March 26, 2021.
- 4.CDC. COVID-19 and your health. Centers for disease control and prevention; 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed March 19, 2021. [Google Scholar]
- 5.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin M, Chen C, Huang J, et al. Clinical characteristics of COVID-19 patients with asthma in Wuhan, China: a retrospective cohort study. J Asthma. 2020:1–9. doi: 10.1080/02770903.2020.1850768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultze A, Walker AJ, MacKenna B, et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8(11):1106–1120. doi: 10.1016/S2213-2600(20)30415-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh D, Halpin DMG. Inhaled corticosteroids and COVID-19-related mortality: confounding or clarifying? Lancet Respir Med. 2020;8(11):1065–1066. doi: 10.1016/S2213-2600(20)30447-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newton S, Zollinger B, Freeman J, et al. Factors associated with clinical severity in emergency department patients presenting with symptomatic SARS-CoV-2 infection. J Am Coll Emerg Physicians Open. 2021;2(4):e12453. doi: 10.1002/emp2.12453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross NJ, Barnes PJ. New therapies for asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;195(2):159–166. doi: 10.1164/rccm.201610-2074PP [DOI] [PubMed] [Google Scholar]
- 13.Maselli DJ, Hardin M, Christenson SA, et al. Clinical approach to the therapy of asthma-COPD overlap. Chest. 2019;155(1):168–177. doi: 10.1016/j.chest.2018.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M, Zhang Y, Chen H, Lin J, Zeng J, Xu Z. Inhaled corticosteroids and risk of upper respiratory tract infection in patients with asthma: a meta-analysis. Infection. 2019;47(3):377–385. doi: 10.1007/s15010-018-1229-y [DOI] [PubMed] [Google Scholar]
- 15.Lipworth B, Chan R, Kuo CR. Use of inhaled corticosteroids in asthma and coronavirus disease 2019: keep calm and carry on. Ann Allergy Asthma Immunol. 2020;125(5):503–504. doi: 10.1016/j.anai.2020.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halpin DMG, Faner R, Sibila O, Badia JR, Agusti A. Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med. 2020;8(5):436–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morais-Almeida M, Aguiar R, Martin B, et al. COVID-19, asthma, and biological therapies: what we need to know. World Allergy Organ J. 2020;13(5):100126. doi: 10.1016/j.waojou.2020.100126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terry PD, Heidel RE, Dhand R. Asthma in adult patients with COVID-19: prevalence and risk of severe disease. Am J Respir Crit Care Med. 2021;203:893–905. doi: 10.1164/rccm.202008-3266OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aggarwal AN, Agarwal R, Dhooria S, Prasad KT, Sehgal IS, Muthu V. Impact of asthma on severity and outcomes in COVID-19. Respir Care. 2021;66(12):1912–1923. doi: 10.4187/respcare.09113 [DOI] [PubMed] [Google Scholar]
- 20.Gerayeli FV, Milne S, Cheung C, et al. COPD and the risk of poor outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2021;33:100789. doi: 10.1016/j.eclinm.2021.100789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pardhan S, Wood S, Vaughan M, Trott M. The risk of COVID-19 related hospitalsation, intensive care unit admission and mortality in people with underlying asthma or COPD: a systematic review and meta-analysis. Front Med. 2021;8(101648047):668808. doi: 10.3389/fmed.2021.668808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535–b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NICE. Methods for the development of NICE public health guidance (third edition); 2012. Available from: https://www.ncbi.nlm.nih.gov/books/NBK395862/pdf/Bookshelf_NBK395862.pdf. Accessed December 23, 2019.
- 24.Centre for reviews and dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care; 2009. Available from: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf. Accessed December 23, 2019.
- 25.Higgins J, Thomas J, Chandler J, Cumpston M Cochrane handbook for systematic reviews of interventions | Cochrane training. in: 6.0. John Wiley & Sons; Available from: https://training.cochrane.org/handbook/current. 2019. Accessed December 23, 2019. [Google Scholar]
- 26.Jorge A, D’Silva KM, Cohen A, et al. Temporal trends in severe COVID-19 outcomes in patients with rheumatic disease: a cohort study. The Lancet Rheumatol. 2021;3(2):e131–e137. doi: 10.1016/S2665-9913(20)30422-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLean S, Hoogendoorn M, Hoogenveen RT, et al. Projecting the COPD population and costs in England and Scotland: 2011 to 2030. Sci Rep. 2016;6(1):31893. doi: 10.1038/srep31893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloom CI, Drake TM, Docherty AB, et al. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO clinical characterisation protocol UK. Lancet Respir Med. 2021;9(7):699–711. doi: 10.1016/S2213-2600(21)00013-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SC, Son KJ, Han CH, Jung JY, Park SC. Impact of comorbid asthma on severity of coronavirus disease (COVID-19). Sci Rep. 2020;10(1):21805. doi: 10.1038/s41598-020-77791-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aveyard P, Gao M, Lindson N, et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: a population cohort study. Lancet Respir Med. 2021;9:909–923. doi: 10.1016/S2213-2600(21)00095-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green I, Merzon E, Vinker S, Golan-Cohen A, Magen E. COVID-19 susceptibility in bronchial asthma. J Allergy Clin Immunol Pract. 2020;9:684–692.e1. doi: 10.1016/j.jaip.2020.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi JC, Jung S-Y, Yoon UA, et al. Inhaled corticosteroids and COVID-19 risk and mortality: a nationwide cohort study. J Clin Med. 2020;9(11):1–13. doi: 10.3390/jcm9113406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanon S, Brusselle G, Deschampheleire M, et al. COVID-19 and biologics in severe asthma: data from the Belgian severe asthma registry. Eur Respir J. 2020;56(6):2002857. doi: 10.1183/13993003.02857-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Foer D, Bates DW, Boyce JA, Zhou L. Risk factors for hospitalization, intensive care, and mortality among patients with asthma and COVID-19. J Allergy Clin Immunol. 2020;146(4):808–812. doi: 10.1016/j.jaci.2020.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izquierdo JL, Almonacid C, Gonzalez Y, et al. The impact of COVID-19 on patients with asthma. Eur Respir J. 2020;56. doi: 10.1183/13993003.03142-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chhiba KD, Patel GB, Vu THT, et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146(2):307–314.e4. doi: 10.1016/j.jaci.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Floyd GC, Dudley JW, Xiao R, et al. Prevalence of asthma in hospitalized and non-hospitalized children with COVID-19. J Allergy Clin Immunol Pract. 2021;9(5):2077–2079.e2. doi: 10.1016/j.jaip.2021.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferastraoaru D, Hudes G, Jerschow E, et al. Eosinophilia in asthma patients is protective against severe COVID-19 Illness. J Allergy Clin Immunol Pract. 2021;9(3):1152–1162.e3. doi: 10.1016/j.jaip.2020.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beurnier A, Jutant EM, Jevnikar M, et al. Characteristics and outcomes of asthmatic patients with COVID-19 pneumonia who require hospitalisation. Eur Respir J. 2020;56(5). doi: 10.1183/13993003.01875-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calmes D, Graff S, Maes N, et al. Asthma and COPD are not risk factors for ICU stay and death in case of SARS-CoV2 infection. J Allergy Clin Immunol Pract. 2020;9:160–169. doi: 10.1016/j.jaip.2020.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi YJ, Park JY, Lee HS, et al. Effect of asthma and asthma medication on the prognosis of patients with COVID-19. Eur Respir J. 2020. doi: 10.1183/13993003.02226-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Menaya JM, Cordobes-Duran C, Rangel-Mayoral JF, Garcia-Martin E, Agundez JAG. Outcomes and laboratory and clinical findings of asthma and allergic patients admitted with Covid-19 in a Spanish University Hospital. Front Pharmacol. 2020;11:570721. doi: 10.3389/fphar.2020.570721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graziani D, Soriano JB, Del Rio-Bermudez C, et al. Characteristics and prognosis of COVID-19 in patients with COPD. J Clin Med. 2020;9(10):1–11. doi: 10.3390/jcm9103259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brusselle G, Bracke K. Targeting immune pathways for therapy in asthma and chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(Suppl 5):S322–8. doi: 10.1513/AnnalsATS.201403-118AW [DOI] [PubMed] [Google Scholar]
- 47.Higham A, Mathioudakis A, Vestbo J, Singh D. COVID-19 and COPD: a narrative review of the basic science and clinical outcomes. Eur Respir Rev. 2020;29:200199. doi: 10.1183/16000617.0199-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackson DJ, Busse WW, Bacharier LB, et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146(1):203–206.e3. doi: 10.1016/j.jaci.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guidance for people previously considered clinically extremely vulnerable from COVID-19. GOV.UK. Available from: https://www.gov.uk/government/publications/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19/guidance-on-shielding-and-protecting-extremely-vulnerable-persons-from-covid-19. Accessed January 12, 2022.
- 50.Rabbani G, Shariful Islam SM, Rahman MA, et al. Pre-existing COPD is associated with an increased risk of mortality and severity in COVID-19: a rapid systematic review and meta-analysis. Expert Rev Respir Med. 2021:1–12. doi: 10.1080/17476348.2021.1866547 [DOI] [PubMed] [Google Scholar]
- 51.Kim L, Garg S, O’Halloran A, et al. Impact of 10-valent pneumococcal conjugate vaccine introduction on pneumococcal carriage and antibiotic susceptibility patterns among children aged <5 years and adults with human immunodeficiency virus infection: Kenya, 2009–2013.. Clin Infect Dis. 2020;70:814–826. doi: 10.1093/cid/ciaa1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020;55(5):2001009. doi: 10.1183/13993003.01009-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heffler E, Detoraki A, Contoli M, et al. COVID-19 in severe asthma network in Italy (SANI) patients: clinical features, impact of comorbidities and treatments. Allergy. 2020. doi: 10.1111/all.14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not tnf antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159(2):481. doi: 10.1053/j.gastro.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scire CA, Carrara G, Zanetti A, et al. COVID-19 in rheumatic diseases in Italy: first results from the Italian registry of the Italian Society for Rheumatology (CONTROL-19). Clin Exp Rheumatol. 2020;38(4):748–753. [PubMed] [Google Scholar]
- 56.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700pmid:19622552 [DOI] [PMC free article] [PubMed] [Google Scholar]