Abstract
Background
With progress being made in the treatment of cancer, various clinical and treatment options are being pursued. In China, Traditional Chinese Medicine (TCM) is used widely in the treatment of cancer.
Objective
To estimate TCM treatment preferences and SDM mode of physicians in China.
Methods
This study was conducted among physicians (n=185) from nine tertiary hospitals in China by discrete-choice experiment (DCE) survey and Shared Decision-Making Questionnaire-physician version (SDM-Q-Doc) survey. The DCE was developed with the inclusion of the most relevant attributes at appropriate levels for the TCM treatment of lung cancer. The empirical data analyses of physicians were performed using mixed logit models. Additionally, subgroup analysis was conducted.
Results
In total, 185 respondents completed the questionnaire. All attributes were statistically significant except out-of-pocket costs. Physicians showed the strongest preferences for increasing disease control rate, relieving nausea and vomiting, and reducing the risk of side effects. Most of the physicians (78.38%) self-reported a high willingness to use SDM during the decision-making process. The physicians with a higher SDM-Q-Doc score had more preference for improving all three attributes than those with a lower score. Little variation was found in preferences among the physicians with other sociodemographic characteristics.
Conclusion
In China, physicians considered disease control rate as the most essential attribute in the TCM treatment of lung cancer. The physicians in China mainly preferred SDM, and the preference was different according to SDM mode when involving the TCM therapy for patients with lung cancer. The study findings could inform future TCM therapy for lung cancer and promote SDM.
Keywords: lung cancer, physician preference, discrete-choice experiment, shared decision-making, Traditional Chinese Medicine, China
Introduction
Globally, lung cancer is the most prevalent cancer and the leading cause of cancer-related deaths. It is a multi-step disease with various histological subtypes. An estimated 2.21 million new cases of lung cancer and 1.80 million associated deaths occurred globally in 2020, remaining the leading cause of cancer deaths worldwide.1 Particularly, more than one-third of all newly diagnosed lung cancers and over 40% of all deaths worldwide occurred in China.2 In recent years, lung cancer has become an increasingly prevalent cancer and public health concern in China, resulting in a high economic burden to society.3
Regarding to treatment, lung cancer therapy is based on the diagnosed disease stage. For early-stage lung cancer, surgery is the first-line treatment choice; whereas chemotherapy, chemo-radiotherapy or radiotherapy, molecular targeted therapies, and immunotherapy are common treatments for patients with advanced lung cancer.4,5 Compared with Western Medicine, Traditional Chinese Medicine (TCM) has three main advantages in preventing tumorigenesis, attenuating toxicity and enhancing treatment effect, and reducing tumor recurrence and metastasis at certain stages of cancer treatment. Therefore, TCM plays an important role in the whole course of cancer prevention and treatment.6,7 In China, TCM with other traditional therapies is a common combined medication, which can improve the temporary negative effects and alleviate the symptoms with good safety.8,9 A survey conducted in Southwestern China found that TCM use forms 53.0% of all therapies in cancer treatment, which is much higher than that in other countries.10 However, other studies have raised concerns about the safety and toxicity of TCM in recent years.11,12 Therefore, different TCM regimens may offer different clinical outcomes regarding efficacy, potential risk of toxicity, administration modes, and patient expenditures. In which case, physicians need to consider these aspects and provide the most suitable TCM therapy for patients with lung cancer.
Shared decision-making (SDM) has been regarded as a key element based on the ideal of patient-centered care, especially in the field of oncology.13 SDM is based on the assumption that healthcare involves at least the patient and a health care provider, and it can help patients and physicians reach a consensus on treatment choice in clinical decision-making, improving patient satisfaction and treatment compliance.14 Charles et al15 defined SDM as a two-way exchange of information, including medical and personal information, between the patient and physician. Based on consensus, a treatment option is chosen after discussions on possible options and outcomes.16 SDM has been necessitated in many health systems, obtaining constant attention worldwide with the changes in patients’ expanding knowledge of diseases and treatments, more available treatment options and preferences for more active patient involvement.17 According to existing research, the preferred decision-making mode of patients is correlated to their final decision-making and therapy preference.18,19 Communication between clinicians and patients, including SDM, can improve health outcomes directly and affect health indirectly through affective-cognitive and behavioral outcomes, where preference plays an important role.20–23 Since one important domain of SDM is preference-based discussion, suggesting an important linkage between preference and decision-making mode, understanding of this connection is necessary for better clinician and patient engagement in medical decision-making. During clinician-patient communication and treatment decision-making, physicians have much more control over medical technology use and know its increasing help and harm clearly. Therefore, due to their information superiority, physicians play the same important role as patients. However, few studies focusing on the preference and SDM mode of physicians have been conducted. Therefore, understanding of physicians’ treatment preferences and preferred SDM mode is necessary.24
In China, to the best of our knowledge, this study would be the first study to investigate physician preference when making TCM therapy decisions for patients with lung cancer. Most previous discrete-choice experiment (DCE) studies focused on patients’ treatment preferences, whereas few published studies investigated physicians’ preferences, paradoxically.25–27 Hence, this study sought to identify Chinese physicians’ preferences for TCM therapy decision-making for patients with lung cancer and their SDM mode.
Methods
Study Participants
A multi-center survey among physicians at nine tertiary hospitals from Shanghai, China was conducted in 2019. Altogether, 185 respondents were enrolled in this study, based on the following inclusion criteria: (1) physicians from the oncology, respiratory, TCM, or integrated Traditional Chinese and Western Medicine departments; (2) physicians with experience of more than 1-year lung cancer TCM therapy practice; and (3) physicians informed about the purpose of the survey and rights during the study. Physicians were excluded from the study if they did not complete the questionnaire. These physicians were investigated in-person using the Shared Decision-Making Questionnaire-physician version (SDM-Q-Doc) questionnaire and DCE survey
Ethical approval for the physicians’ preference study was provided by the Human Research Ethics Committee, Fudan University, Shanghai, China, in accordance with the Helsinki Declaration of 1964, as revised in 2013.
Shared Decision-Making Survey
The SDM-Q-Doc is a nine-item tool for measuring the decision-making process during medical encounters from the physician’s perspective. It has good acceptance, feasibility, and reliability.28 The SDM-Q-Doc was developed based on the nine practical steps of the SDM process, which include (1) disclosure that a decision needs to be made; (2) formulation of equality of partners; (3) presentation of treatment options; (4) information on the benefits and risks of options; (5) investigation of patient’s understanding and expectations; (6) identification of both parties’ preferences; (7) negotiation; (8) reaching a shared decision; and (9) arrangement of follow-up.29 The SDM survey on physicians in China was conducted using the SDM-Q-Doc. Physicians responded to questions on a five-point scale that ranged from “completely disagree” (1) to “completely agree” (5). A higher score represented greater satisfaction with the information provided, indicating a higher level of perceived SDM. The SDM survey aimed to assess physicians’ preferences on patient participation in the decision-making process during TCM therapy for lung cancer. The SDM-Q-Doc questionnaire is presented in Table 1.
Table 1.
Physicians’ Shared Decision-Making Mode Study- SDM-Q-Doc
| Item | Completely Disagree | Strongly Disagree | Mediate | Strongly Agree | Completely Agree |
|---|---|---|---|---|---|
| I made clear to my patient that a decision needs to be made. | |||||
| I wanted to know exactly from my patient how he/ she wants to be involved in making the decision. | |||||
| I told my patient that there are different options for treating his/her medical condition. | |||||
| I precisely explained the advantages and disadvantages of the treatment options to my patient. | |||||
| I helped my patient understand all the information | |||||
| I asked my patient which treatment option he/she prefers. | |||||
| My patient and I thoroughly weighed the different treatment options. | |||||
| My patient and I selected a treatment option together. | |||||
| My patient and I reached an agreement on how to proceed. |
Descriptive analyses were conducted for each SDM-Q-Doc item score. A total raw score of 0–45 was calculated by adding the scores of all items, and the mean scores of each item were measured.
Discrete-Choice Experiment Survey
Methodology
DCE, based on the assumptions that individuals’ preferences of healthcare interventions or services can be assessed by several key attributes, depending on the levels of these attributes, is commonly used instrument in health economics.30 A DCE survey was conducted in this study, following the guidance from a report by the ISPOR Conjoint Analysis Good Research Practices Task Force.31 Each respondent was required to make trade-offs between attributes and the levels of the key attributes in the DCE questionnaire.26
Identifying the Attributes of Treatment and Their Levels
To identify the key attributes of TCM therapy in this study, we performed a literature search of Chinese journal literature databases, including CNKI, CBM, and Wanfang, and global databases, such as PubMed, EMBASE, and MEDLINE. The keywords included “lung cancer”, “preference”, “main safety indicators”, “safety”, “effectiveness”, “efficacy”, “toxicity”, “adverse effect” and “Traditional Chinese Medicine”.
Based on the results of the literature search, eight attributes, including disease control rate, nausea and vomiting, thrombocytopenia, leukopenia, peripheral nerve damage, liver function abnormality, quality of life, and joint pain, were initially gathered. Additionally, lung cancer physicians and related experts were consulted based on a semi-structured interview, which helped to clarify the suitable attributes, their exact meanings and responding levels. The interview guide included three main parts: (1) treatment methods, costs, efficacy indicators, and adverse effects of TCM therapy for lung cancer; (2) advantages and disadvantages of TCM therapy for lung cancer; and (3) design of DCE choice set.
Therefore, we identified and summarized four attributes of TCM therapy preference for physicians: disease control rate (DCR), nausea and vomiting, risk of side effects, and out-of-pocket costs to patients. Among these attributes, disease control rate, nausea and vomiting, and risk of side effects were categorical variables assigned three or four levels, whereas out-of-pocket cost to patients was a continuous variable. The list of these attributes and their levels is shown in Table 2.
Table 2.
Attributes and Their Levels in the DCE Questionnaire
| Attributes | Description to Physicians | Levels | Criteria |
|---|---|---|---|
| Disease control rate | Tumor lesions are reduced in size or stable | 30% | |
| 55% | |||
| 80% | |||
| Nausea & vomiting | Various degrees of nausea and vomiting caused by treatment | Strongly Severe | Hard to control |
| Severe | Necessary to treat | ||
| Moderate | Intermittent vomiting | ||
| Mild | Nausea | ||
| Risk of side effects | Decrease in platelets or leukopenia and abnormal liver function caused by treatment | High | Severe side effects |
| Medium | Moderate side effects | ||
| Low | Slight side effects | ||
| Out-of-pocket costs | The monthly medical expenses that patients are willing to pay during the treatment | CNY 9000 | |
| CNY 6000 | |||
| CNY3000 |
Construction of the DCE Questionnaire
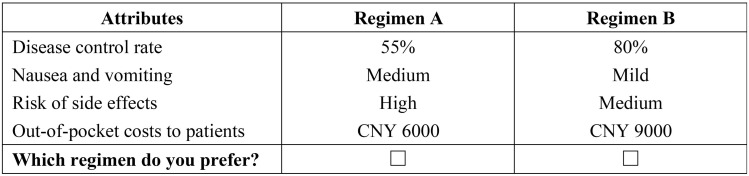
The four attributes and their corresponding levels (three attributes with three levels and one attribute with four levels) resulted in 108 hypothetical scenarios when the D-optimal designs were constructed using the SAS software, version 9.4 (SAS Institute, Cary, NC, USA). We chose 18 choice sets in order to guarantee statistical efficiency and relieve respondents of further burden when completing the questionnaire.32 The ultimate DCE design consisted of 18 choice sets, and each physician respondent needed to answer nine trade-off questions. Figure 1 illustrates an example of a DCE survey choice set. Before the DCE choice sets, Table 2 was presented in the questionnaire to help reduce the cognitive burden of respondents and increase engagement.
Figure 1.
Example of the discrete-choice experiment choice set.
Besides the DCE survey, data on the demographic characteristics of physicians and other factors that may influence the physicians’ preferences for TCM therapy in terms of lung cancer were included in the survey instrument. A single-center pilot survey (n=10) was conducted before the formal survey to improve the questionnaire and study.
Statistical Analysis
After data cleaning, data on choice and demographic characteristics of respondents were imported into STATA version 16 (StataCorp LP, College Station, TX, USA) for analysis. A mixed logit model was used to estimate preference weights for the involved attributes, obtaining the main effects of the model on physicians. The utility function can be specified as:
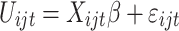 |
where U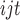 is the utility individual i from choosing alternative j in the choice set t, X is a vector of observed attributes (ie, the TCM therapy characteristics and corresponding levels), β is a vector of coefficients expressing the desirability of the attributes, and
is the utility individual i from choosing alternative j in the choice set t, X is a vector of observed attributes (ie, the TCM therapy characteristics and corresponding levels), β is a vector of coefficients expressing the desirability of the attributes, and 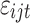 is an error term.
is an error term.
According to the split-sample analysis methods, we conducted a subgroup analysis to estimate potential differences in preference of physician subgroups with different SDM modes self-reported by clinicians, SDM-Q-Doc scores, and several key demographic characteristics. The general characteristics of the physicians were summarized as means and standard deviations or frequencies and percentages.
In the analysis, random effects were included to explain the fact that each physician was answering multiple questions and may have systematic preferences. All DCE responses were analyzed with multiple logistic regression models. Additionally, information criteria (ie, Bayesian information criterion33 and Akaike information criterion34) were evaluated.
The out-of-pocket cost was regarded as a continuous variable in the models, whereas other attributes were encoded as dummy variables, according to their assigned levels. All coefficients of the model were assumed to be normally distributed. In estimating parameters, p <0.05 was considered statistically significant.
Results
Demographics
In this study, 185 eligible physician respondents from nine sample hospitals in Shanghai participated in the questionnaire survey on preference and SDM of TCM therapy for lung cancer. The social demographic characteristics of the respondents are reported in Table 3. Among 185 respondents, 124 (67.03%) respondents were female. The average age of the participants was 36.74±10.26 (with a range of 25–75) years old. Most of the physicians had a graduate degree (76.75%), worked in oncology departments (69.73%), or majored in TCM (65.57%). For the working years for lung cancer treatment practice, 83 (54.86%) physicians had experience of more than 10 years (54.86%).
Table 3.
Demographic Characteristics of the Physician Respondents
| Characteristic | Subjects (n=185) | Proportion (%) |
|---|---|---|
| Gender | ||
| Male | 61 | 32.97 |
| Female | 124 | 67.03 |
| Age | ||
| 20–29 years | 56 | 30.27 |
| 30–39 years | 66 | 35.68 |
| 40–49 years | 43 | 23.24 |
| 50–59 years | 13 | 7.03 |
| 60 years and older | 7 | 3.78 |
| Education level | ||
| Bachelor degree and below | 43 | 23.24 |
| Master degree | 96 | 51.89 |
| Doctor degree | 46 | 24.86 |
| Professional title | ||
| No title | 12 | 6.49 |
| Resident physician | 62 | 33.51 |
| Attending doctor | 67 | 36.22 |
| Deputy chief physician | 25 | 13.51 |
| Chief physician | 19 | 10.27 |
| Years for lung cancer treatment practice | ||
| Less than 10 years | 102 | 55.14 |
| 10–20 years | 50 | 27.03 |
| More than 20 years | 33 | 17.83 |
| Employment | ||
| Budgeted post | 88 | 47.57 |
| Contract appointment | 62 | 33.51 |
| Retirement | 7 | 3.78 |
| Others | 28 | 15.14 |
| Preferred decision-making mode | ||
| Decision made by patient | 2 | 1.08 |
| Decision made by both patient and physician | 145 | 78.38 |
| Decision made by physician | 38 | 20.54 |
SDM-Q-Doc Results
A survey on the respondents’ shared decision-making was conducted using the SDM-Q-Doc instrument. The mean SDM-Q-Doc score of 185 participants was 38.44±9.62 and the median SDM score was 42. The average score of each of the nine items was above four. Most of the physicians (78.38%) self-reported a high willingness to support patient participation in the decision-making process on TCM therapy for lung cancer. Table 4 shows the SDM-Q-Doc scores of the respondents in this study.
Table 4.
SDM-Q-Doc Scores of the Respondents in This Study (n=185)
| Item | Completely Disagree (%) | Strongly Disagree (%) | Mediate (%) | Strongly Agree (%) | Completely Agree (%) | Mean Score (n=185) |
|---|---|---|---|---|---|---|
| 1 | 14 (7.57) | 5 (2.70) | 7 (3.78) | 53 (28.65) | 106 (57.30) | 4.25±1.16 |
| 2 | 14 (7.57) | 5 (2.70) | 9 (4.86) | 42 (22.70) | 115 (62.16) | 4.29±1.17 |
| 3 | 14 (7.57) | 4 (2.16) | 3 (1.62) | 37 (20.00) | 127 (68.65) | 4.40±1.14 |
| 4 | 16 (8.65) | 2 (1.08) | 6 (3.24) | 38 (20.54) | 123 (66.49) | 4.35±1.18 |
| 5 | 15 (8.11) | 4 (2.16) | 14 (7.57) | 51 (27.57) | 101 (54.59) | 4.18±1.18 |
| 6 | 14 (7.57) | 4 (2.16) | 10 (5.41) | 50 (27.03) | 107 (57.84) | 4.25±1.16 |
| 7 | 14 (7.57) | 4 (2.16) | 13 (7.03) | 48 (25.95) | 106 (57.30) | 4.23±1.16 |
| 8 | 11 (5.95) | 6 (2.16) | 12 (6.49) | 57 (30.81) | 99 (53.51) | 4.23±1.10 |
| 9 | 15 (8.11) | 3 (2.16) | 7 (3.78) | 56 (30.27) | 104 (56.22) | 4.25±1.15 |
| Total SDM-Q-Doc score | 38.44±9.62 | |||||
Physician Preferences on TCM Therapy for Lung Cancer
The main effects of the mixed logit model results are presented in Table 5. The parameters of the choice model for each attribute level can be explained as regression coefficients, which reflect the extent to which each attribute influences treatment choice intention. In this study, the coefficients were statistically significant for most attributes, including disease control rate, nausea and vomiting, and risk of side effects (p<0.05). These three attributes proved to be related to the physician TCM therapeutic decision-making process for lung cancer, whereas the monthly out-of-pocket costs for therapy seemed to have no effect on it.
Table 5.
Main Effects of the Mixed Logit Model Results: Physicians Preference for TCM Therapy of Lung Cancers
| Attributes and Levels | Coefficient | SE | SD | SE | 95% CI | |
|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||
| Disease control rate (Ref: 30%) | ||||||
| 55% | 1.217*** | 0.112 | 0.007 | 0.158 | 0.998 | 1.435 |
| 80% | 2.389*** | 0.183 | 1.298*** | 0.169 | 2.029 | 2.748 |
| Nausea & vomiting (Ref: Strongly Severe) | ||||||
| Severe | 1.011*** | 0.153 | 0.002 | 0.206 | 0.711 | 1.312 |
| Moderate | 1.944*** | 0.165 | 0.018 | 0.416 | 1.620 | 2.267 |
| Mild | 2.034*** | 0.169 | 0.395** | 0.291 | 1.702 | 2.366 |
| Risk of side effects (Ref: High) | ||||||
| Medium | 0.592*** | 0.105 | 0.003 | 0.157 | 0.386 | 0.798 |
| Low | 0.781*** | 0.120 | 0.693*** | 0.173 | 0.547 | 1.016 |
| Monthly out-of-pocket costs | −0.0000201 | 0.0000172 | 0.0001069** | 0.0000366 | −0.0000538 | 0.0000135 |
| Sample size | 185 | |||||
| Observation value | 3330 | |||||
| LR chi2 (8) | 56.76 | |||||
| Log likelihood | −729.519 | |||||
| AIC | 1489.039 | |||||
| BIC | 1580.699 | |||||
Notes: **p <0.05; ***p <0.01.
Abbreviations: SE, standard error; SD, standard deviation; Ref, reference; AIC, Akaike information criterion; BIC, Bayesian information criterion.
According to the results, physicians considered disease control rate to be about thrice as important to risk of side effects, and nausea and vomiting almost as important as the disease control rate. Specifically, physicians showed a strong preference for a higher disease control rate (level, 80%) and fewer episodes of nausea and vomiting (level, mild), which were their most valued attributes when making TCM therapeutic decisions.
Subgroup Analysis
Additionally, we conducted a subgroup analysis to estimate the preferences of physicians with different key sociodemographic characteristics. Physicians with a clinical education background emphasized on the improvement of disease control rate and relief of nausea and vomiting. Those who majored in TCM had a stronger preference for reducing the risk of toxic side effects and less for improving disease control rate and relieving nausea and vomiting. Apart from the SDM-Q-Doc score and preferred SDM mode, the preferences for all attributes between different subgroups with other demographic characteristics were relatively similar. Tables 6 and 7 show the results of the mixed logit models among different physician subgroups according to the SDM-Q-Doc score (Median score, 42) and the self-reporting preferred decision-making mode. Physicians with a higher SDM-Q-Doc score emphasized the importance of high disease control rate and relief of nausea and vomiting. Physicians who self-reported preferring a physician decision-making mode indicated high focus on increasing disease control rate and preferred the least level of nausea and vomiting. Physician preferences were different according to the SDM-Q-Doc score and preferred shared decision-making mode.
Table 6.
Results of Mixed Logit Models by Different Subgroups in SDM-Q-Doc Score
| Attributes and Levels | SDM-Q-Doc Score<42 | SDM-Q-Doc Score≥42 | ||
|---|---|---|---|---|
| Coefficient | SE | Coefficient | SE | |
| Disease control rate (Ref: 30%) | ||||
| 55% | 1.020*** | 0.156 | 1.562*** | 0.191 |
| 80% | 2.098*** | 0.256 | 2.976*** | 0.334 |
| Nausea & vomiting (Ref: Strongly Severe) | ||||
| Severe | 0.991*** | 0.219 | 1.091*** | 0.246 |
| Moderate | 1.752*** | 0.236 | 2.437*** | 0.305 |
| Mild | 1.786*** | 0.244 | 2.509*** | 0.299 |
| Risk of side effects (Ref: High) | ||||
| Medium | 0.610*** | 0.150 | 0.577*** | 0.164 |
| Low | 0.595*** | 0.166 | 1.096*** | 0.207 |
| Monthly costs | −0.0000229 | 0.0000263 | −0.0000091 | 0.0000292 |
| Sample size | 88 | 97 | ||
| Observation value | 1584 | 1746 | ||
| LR chi2 (8) | 21.24 | 41.61 | ||
| Log likelihood | −371.233 | −350.109 | ||
| AIC | 774.467 | 732.218 | ||
| BIC | 860.350 | 819.660 | ||
Note: ***p <0.01.
Abbreviations: SE, standard error; SD, standard deviation; Ref, reference; AIC, Akaike information criterion; BIC, Bayesian information criterion.
Table 7.
Results of Mixed Logit Models by Different Subgroups in Preferred Decision-Making Mode
| Attributes and Levels | Preferred Shared Decision-Making | Preferred Physician Decision-Making | ||
|---|---|---|---|---|
| Coefficient | SE | Coefficient | SE | |
| Disease control rate (Ref: 30%) | ||||
| 55% | 1.246*** | 0.134 | 1.164*** | 0.298 |
| 80% | 2.290*** | 0.217 | 3.064*** | 0.529 |
| Nausea & vomiting (Ref: Strongly Severe) | ||||
| Severe | 0.930*** | 0.175 | 1.301*** | 0.429 |
| Moderate | 1.944*** | 0.198 | 2.046*** | 0.444 |
| Mild | 1.954*** | 0.204 | 2.722*** | 0.531 |
| Risk of side effects (Ref: High) | ||||
| Medium | 0.612*** | 0.122 | 0.709*** | 0.274 |
| Low | 0.771*** | 0.136 | 1.204*** | 0.357 |
| Monthly costs | −0.0000168 | 0.0000214 | −0.0000599 | 0.0000462 |
| Sample size | 145 | 36 | ||
| Observation value | 2610 | 648 | ||
| LR chi2 (8) | 44.82 | 5.54 | ||
| Log likelihood | −589.471 | −114.156 | ||
| AIC | 1210.943 | 260.313 | ||
| BIC | 1304.816 | 331.895 | ||
Note: ***p <0.01.
Abbreviations: SE, standard error; SD, standard deviation; Ref, reference; AIC, Akaike information criterion; BIC, Bayesian information criterion.
Discussion
In this study, a multi-center DCE survey was conducted to investigate physician preferences for on TCM therapy for lung cancer, and an SDM-Q-Doc survey was conducted to estimate their perspective of the shared decision-making process in their clinical encounters with patients. To the best of our knowledge, this study is the first study to assess preferences for the TCM therapy of lung cancer in China from the perspective of physicians. According to our findings, most of the physicians showed a relatively high willingness to engage in a shared decision-making process during the TCM treatment for lung cancer. Besides, the results showed that the physicians preferred the TCM therapy for lung cancer with higher disease control rate, fewer nausea and vomiting episodes, and lower risk of side effects. However, treatment costs seemed irrelevant to them. Additionally, preferences for these attributes were similar among physicians with various sociodemographic characteristics, while differences in preference existed among physicians, based on medical majors, SDM-Q-Doc score, and SDM mode.
Sun et al35 reported that Chinese physicians had strong preferences for improvement in lung cancer and reduction of the risk of severe side effects. Additionally, Hauber et al36 found that physicians paid much attention to side effects, such as fatigue, nausea, and risk of febrile neutropenia (fever). Therefore, the findings of our study were basically consistent with those of previous studies on physician preferences for the treatment of lung cancer.
Physicians with different medical educational backgrounds had different preferences for the attributes of the TCM therapy for lung cancer. Unlike the physicians who majored in clinical medicine, those who had TCM educational backgrounds had a stronger preference for reducing the risk of toxic side effects and less preference for improving disease control rate and reducing nausea and vomiting episodes. The difference in preference may be due to the difference in treatment ideas between TCM and Western medicine. Studies have shown that TCM focuses on alleviating clinical symptoms, reducing side effects, and improving the health-related quality of life of patients and their immunity by strengthening the body and removing pathogenic factors, indicating a different ideal from that of Western medicine which adopts the standard disease-targeted approach.37
Generally, physicians are the main source of information on therapeutic options, and they are strongly involved in the decision-making process.38 Discussions between patients and physicians on therapy and its potential adverse effects play an essential role in the treatment of lung cancer, where shared decision-making has proven to improve informed consent and decrease uncertainty about clinical decision-making.39 With the rapid development of health technology, more available treatment options for cancer will emerge, which makes shared decision-making and communication between patients and physicians increasingly important, especially for treating lung cancer with high mortality in China.40,41
In this study, most physicians preferred to use an SDM mode when it came to TCM therapy for lung cancer, which was consistent with the findings reported by Driever et al42 However, Driever et al42 concluded that though most physicians preferred shared decision-making, they commonly reverted to the paternalistic decision-making mode during the decision-making process. Besides, Tariman et al43 indicated that patients with cancer preferred a shared role and increased participation in decision-making than that allowed them in current clinical settings. Additionally, based on the results of the subgroup analysis, physician preferences seemed to be various according to the SDM-Q-Doc score and preferred SDM mode, which was probably due to use of different measurement instruments. The preferred SDM mode was self-reported by physician respondents, which reflected their preference, whereas the SDM-Q-Doc was used in this study to assess physicians’ perspective on the shared decision-making process in clinical encounters, which could reflect the real SDM mode of physicians.28 Further research is needed to demonstrate this difference found in the study.
An unexpected finding of our study was the insignificance of statistical estimates for the out-of-pocket cost attribute. This finding was probably because most physician respondents in the study only focused on the questions on other attributes and assessed the risk-benefits of the TCM therapy without a trade-off in cost. According to previous studies, from the perspective of physicians, they commonly pay much attention to the clinical effect of treatment on patients with lung cancer patients. Such clinical effects include best-case outcome, expected survival, and adverse events.36,44 Besides, several studies revealed that physicians might be influenced by health insurance reimbursement when providing medical services for patients with cancer, incognizant of the patients’ out-of-pocket costs that involved patients’ individual perceptions.45,46 Additionally, since cost of TCM therapy was not a statistically significant factor in this study, though the physician preference seemed to be different according to the SDM mode in the subgroup analysis, it was unable to estimate whether SDM mode influences physician preference through the calculation of willingness-to-pay directly, which might be caused by the small sample size. Therefore, further studies with large sample sizes should be conducted to improve our understanding of this point.
This study had few limitations. First, due to the limitation of DCEs, we could not include all the potentially important factors influencing their preference. However, the attributes included in our study were determined by literature review and expert consultation. Second, the participating physicians were all from the tertiary hospitals in Shanghai, China, which may not be representative of physicians from other countries or hospitals of other levels. Therefore, a large-sample survey in the future will corroborate the findings of the subgroup analysis in this study. Third, given that clinical decision-making is complex and dynamic, particularly with respect to the treatment of lung cancer, the SDM-Q-Doc could probably not comprehensively reflect the preferred decision-making mode of physicians in the real world. Therefore, further studies using more appropriate tools are needed to reflect the actual preferred decision-making mode of physicians.
Conclusion
This study was the first to estimate physicians’ preference for TCM therapy of lung cancer in China. Besides, it assessed their perspective of SDM. Physicians placed a higher value on disease control rate and relief of nausea and vomiting. Most physicians in China preferred SDM with patients with lung cancer during the decision-making process. Additionally, physician preference for TCM therapy was different according to shared decision-making mode. This study provided more information and evidence to improve TCM treatment of lung cancer and SDM in China.
Acknowledgments
This study was supported by the Ministry of education of Humanities and Social Science project (NO.18YJCZH187).
Disclosure
The authors report no conflicts of interest.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Shi J, Wang H, et al. Population-level economic burden of lung cancer in China: provisional prevalence-based estimations, 2017–2030. Chinese J Cancer Res. 2021;33(1):79–92. doi: 10.21147/j.issn.1000-9604.2021.01.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv1–iv21. doi: 10.1093/annonc/mdx222 [DOI] [PubMed] [Google Scholar]
- 5.Ettinger DS, Wood DE, Aggarwal C, et al. NCCN guidelines insights: non–small cell lung cancer, Version 1.2020. J Natl Compr Canc Netw. 2019;17(12):1464–1472. doi: 10.6004/jnccn.2019.0059 [DOI] [PubMed] [Google Scholar]
- 6.Ling C, Yue X, Ling C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. J Integr Med. 2014;12(4):331–335. doi: 10.1016/S2095-4964(14)60038-8 [DOI] [PubMed] [Google Scholar]
- 7.Lv C, Shi C, Li L, et al. Chinese herbal medicines in the prevention and treatment of chemotherapy-induced nausea and vomiting. Curr Opin Support Palliat Care. 2018;12(2):174–180. doi: 10.1097/SPC.0000000000000348 [DOI] [PubMed] [Google Scholar]
- 8.Xue C, Hu Z, Jiang W, et al. National survey of the medical treatment status for non-small cell lung cancer (NSCLC) in China. Lung Cancer. 2012;77(2):371–375. doi: 10.1016/j.lungcan.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 9.Jiao L, Dong C, Liu J, et al. Effects of Chinese medicine as adjunct medication for adjuvant chemotherapy treatments of non-small cell lung cancer patients. Sci Rep. 2017;7(1):1–12. doi: 10.1038/srep46524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Yan J, Liu X, et al. Pharmacovigilance practice and risk control of Traditional Chinese Medicine drugs in China: current status and future perspective. J Ethnopharmacol. 2012;140(3):519–525. doi: 10.1016/j.jep.2012.01.058 [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Titch T, Luo Z, et al. Confirmation of a proarrhythmic risk underlying the clinical use of common Chinese herbal intravenous injections. J Ethnopharmacol. 2012;142(3):829–835. doi: 10.1016/j.jep.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 12.Levit L, Balogh E, Nass S, Ganz PA. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 13.Rockenbauch K, Schildmann J. Shared decision making (SDM): a systematic survey of terminology use and concepts. Gesundheitswesen. 2011;73(7):399–408. doi: 10.1055/s-0030-1262870 [DOI] [PubMed] [Google Scholar]
- 14.Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision making: concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172–1179. doi: 10.1016/j.pec.2015.06.022 [DOI] [PubMed] [Google Scholar]
- 15.Charles C, Gafni A, Whelan T. Decision-making in the physician–patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med. 1999;49(5):651–661. doi: 10.1016/S0277-9536(99)00145-8 [DOI] [PubMed] [Google Scholar]
- 16.Chewning B, Bylund CL, Shah B, et al. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9–18. doi: 10.1016/j.pec.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levinson W, Kao A, Kuby A, et al. Not all patients want to participate in decision making: a national study of public preferences. J Gen Int Med. 2005;20(6):531–535. doi: 10.1111/j.1525-1497.2005.04101.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandelblatt JS, Sheppard VB, Hurria A, et al. Breast cancer adjuvant chemotherapy decisions in older women: the role of patient preference and interactions with physicians. J Clin Oncol. 2010;28(19):3146. doi: 10.1200/JCO.2009.24.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making. 2015;35(1):114–131. doi: 10.1177/0272989X14551638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Street RL Jr, Makoul G, Arora NK, et al. How does communication heal? Pathways linking clinician–patient communication to health outcomes. Patient Educ Counsel. 2009;74(3):295–301. doi: 10.1016/j.pec.2008.11.015 [DOI] [PubMed] [Google Scholar]
- 21.Kreps GL, O’hair DAN, Clowers M. The influences of human communication on health outcomes. Am Behav Sci. 1994;38(2):248–256. doi: 10.1177/0002764294038002006 [DOI] [Google Scholar]
- 22.Fukui S, Matthias MS, Salyers MP. Core domains of shared decision-making during psychiatric visits: scientific and preference-based discussions. Adm Policy Ment Health. 2015;42(1):40–46. doi: 10.1007/s10488-014-0539-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barratt A. Evidence based medicine and shared decision making: the challenge of getting both evidence and preferences into health care. Patient Educ Counsel. 2008;73(3):407–412. doi: 10.1016/j.pec.2008.07.054 [DOI] [PubMed] [Google Scholar]
- 24.Quill TE, Brody H. Physician recommendations and patient autonomy: finding a balance between physician power and patient choice. Ann Int Med. 1996;125(9):763–769. doi: 10.7326/0003-4819-125-9-199611010-00010 [DOI] [PubMed] [Google Scholar]
- 25.Meirelles I, Magliano C. Stated preferences in non-small-cell lung cancer: a discrete choice experiment. Patient Prefer Adherence. 2021;15:911. doi: 10.2147/PPA.S302394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mühlbacher AC, Bethge S. Patients’ preferences: a discrete- choice experiment for treatment of non- small- cell lung cancer. Eur J Health Econ. 2015;16:657–670. doi: 10.1007/s10198-014-0622-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjamin L, Cotté FE, Philippe C, et al. Physicians’ preferences for prescribing oral and intravenous anticancer drugs: a discrete choice experiment. Eur J Cancer. 2012;48(6):912–920. doi: 10.1016/j.ejca.2011.09.019 [DOI] [PubMed] [Google Scholar]
- 28.Scholl I, Kriston L, Dirmaier J, et al. Development and psychometric properties of the Shared Decision-Making Questionnaire–physician version (SDM-Q-Doc). Patient Educ Counsel. 2012;88(2):284–290. doi: 10.1016/j.pec.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 29.Giersdorf N, Loh A, Bieber C, et al. Development and validation of assessment instruments for shared decision making. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2004;47(10):969–976. doi: 10.1007/s00103-004-0905-5 [DOI] [PubMed] [Google Scholar]
- 30.de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. 2012;21:145–172. doi: 10.1002/hec.1697 [DOI] [PubMed] [Google Scholar]
- 31.Hauber AB, González JM, Groothuis-Oudshoorn CGM, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19(4):300–315. doi: 10.1016/j.jval.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 32.Kuhfeld WF. Marketing Research Methods in SAS Experimental Design, Choice, Conjoint and Graphical Techniques. Cary: SAS Institute; 2010. [Google Scholar]
- 33.Hurvich CM, Tsai CL. Regression and time series model selection in small samples. Biometrika. 1989;76(2):297–307. doi: 10.1093/biomet/76.2.297 [DOI] [Google Scholar]
- 34.McLachlan G, Peel D. Finite Mixture Models. New York: John Wiley & Sons; 2000. [Google Scholar]
- 35.Sun H, Wang H, Shi L, et al. Physician preferences for chemotherapy in the treatment of non-small cell lung cancer in China: evidence from multicentre discrete choice experiments. BMJ Open. 2020;10(2):e032336. doi: 10.1136/bmjopen-2019-032336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hauber B, Penrod JR, Gebben D, et al. The value of hope: patients’ and physicians’ preferences for survival in advanced non-small cell lung cancer. Patient Prefer Adherence. 2020;14:2093–2104. doi: 10.2147/PPA.S248295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Zhu X, Yuan P, et al. Efficacy of traditional Chinese medicine combined with chemotherapy in patients with non-small cell lung cancer (NSCLC): a meta-analysis of randomized clinical trials. Support Care Cancer. 2020;28(8):3571–3579. doi: 10.1007/s00520-020-05433-w [DOI] [PubMed] [Google Scholar]
- 38.McMullen S, Hess LM, Kim ES, et al. Treatment decisions for advanced non- squamous non- small cell lung cancer: patient and physician perspectives on maintenance therapy. Patient. 2019;12:223–233. doi: 10.1007/s40271-018-0327-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boss EF, Mehta N, Nagarajan N, et al. Shared decision making and choice for elective surgical care: a systematic review. Otolaryngol-Head Neck Surg. 2016;154(3):405–420. doi: 10.1177/0194599815620558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho JH. Immunotherapy for non-small-cell lung cancer: current status and future obstacles. Immune Netw. 2017;17(6):378–391. doi: 10.4110/in.2017.17.6.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer. 2019;10(1):3–7. doi: 10.1111/1759-7714.12916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Driever EM, Stiggelbout AM, Brand PLP. Shared decision making: physicians’ preferred role, usual role and their perception of its key components. Patient Educ Counsel. 2020;103(1):77–82. doi: 10.1016/j.pec.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 43.Tariman JD, Berry DL, Cochrane B, et al. Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review. Ann Oncol. 2010;21(6):1145–1151. doi: 10.1093/annonc/mdp534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu FX, Witt EA, Ebbinghaus S, et al. Patient and oncologist preferences for attributes of treatments in advanced melanoma: a discrete choice experiment. Patient Prefer Adherence. 2017;11:1389–1399. doi: 10.2147/PPA.S140226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthews BA, Anderson RC, Nattinger AB. Colorectal cancer screening behavior and health insurance status (United States). Cancer Causes Control. 2005;16(6):735–742. doi: 10.1007/s10552-005-1228-z [DOI] [PubMed] [Google Scholar]
- 46.Kim SY, Shin DW, Park B, et al. Cancer cost communication: experiences and preferences of patients, caregivers, and oncologists—a nationwide triad study. Support Care Cancer. 2018;26(10):3517–3526. doi: 10.1007/s00520-018-4201-6 [DOI] [PubMed] [Google Scholar]