Abstract
Leucocin A is a class IIa bacteriocin produced by Leuconostoc spp. that has previously been shown to inhibit the growth of Listeria monocytogenes. A spontaneous resistant mutant of L. monocytogenes was isolated and found to be resistant to leucocin A at levels in excess of 2 mg/ml. The mutant showed no significant cross-resistance to nontype IIa bacteriocins including nisaplin and ESF1-7GR. However, there were no inhibition zones found on a lawn of the mutant when challenged with an extract containing 51,200 AU of pediocin PA-2 per ml as determined by a simultaneous assay on the sensitive wild-type strain. DNA and protein analysis of the resistant and susceptible strains were carried out using silver-stained amplified fragment length polymorphism (ssAFLP) and one- and two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), respectively. Two-dimensional SDS-PAGE clearly showed a 35-kDa protein which was present in the sensitive but absent from the resistant strain. The N-terminal end of the 35-kDa protein was sequenced and found to have an 83% homology to the mannose-specific phosphotransferase system enzyme IIAB of Streptococcus salivarius.
Bacteriocins are proteinaceous antimicrobial peptides synthesized by bacteria and are usually active against strains closely related to the producing organism (13, 21). There is increasing interest in the use of bacteriocins from lactic acid bacteria in foods (20, 22). For example, the class I bacteriocin nisin was approved for use in 1969 and has been applied to several foods (6). Class II bacteriocins are characteristically small, heat-stable peptides (15), some of which contain the consensus motif YGNGV and include leucocin A and pediocin PA-2. These were isolated from food-related lactic acid bacteria and have potential as food preservatives. With the addition of nisin (and other bacteriocins) into the environment, there is a concomitant interest in resistance to these antimicrobial compounds (4, 5, 10, 18, 19, 33). The mechanism of resistance has not been fully established, but has been attributed to cell membrane and S-layer changes.
Leucocin A is produced by several Leuconostoc spp. It inhibits the growth of the important potential food-borne pathogen Listeria monocytogenes (11, 24). Resistance to class IIa bacteriocins has been reported to be a stable phenomenon (8, 30). Transposon-mediated inactivation of ς54 in L. monocytogenes has rendered it resistant to mesentericin Y105 (a class IIa bacteriocin) (31). There is, however, very little known about the molecular basis of resistance in naturally isolated strains.
In an attempt to discover resistance-associated phenomena at both the DNA and proteiomic levels, amplified fragment length polymorphism (AFLP) and two-dimensional (2-D) gel electrophoresis were employed, respectively. AFLP is a genome fingerprinting technique based on the selective amplification of a subset of DNA fragments generated by restriction enzyme digestion (34). 2-D gel electrophoresis is a powerful tool for the analysis of complex protein mixtures (23) and allows for an overall view of proteins and the discovery of proteins that may be induced or repressed in mutant strains that are resistant to an antimicrobial compound.
Bacterial strains and growth conditions.
L. monocytogenes B73 is a food isolate from the laboratory collection at the University of Natal, Pietermaritzburg, South Africa, and was grown on brain heart infusion agar or broth at 30°C for all experiments. A resistant strain was isolated after a single colony of L. monocytogenes B73 was picked for use in an agar overlay for an activity test using leucocin A. No zones were detected on the lawn containing this strain although a high level of activity was previously observed. The strain was selected for further study. The identity was verified to be isogenic to L. monocytogenes B73 by AFLP analysis. The resistant strain was designated L. monocytogenes B73-MR1 and was maintained in the same manner as the parental strain. Pediococcus acidilactici PA-2 was grown in MRS (Biolab) broth supplemented with 0.1% Tween 80 at 30°C.
Bacteriocin preparation.
Leucocin A was synthesized by a solid-phase method using Fmoc-amino acid derivatives on a peptide synthesizer 433A (Applied Biosystems Inc., Foster City, Calif.). A protected peptide resin was treated with reagent K for 3 h at room temperature (14). Crude product was purified by reversed-phase high-performance liquid chromatography on Cosomosil 5C18 AR (Nacalai Tesque, Kyoto, Japan) to yield the reduced form of leucocin A. A dimethyl sulfoxide (DMSO) solution (0.3 ml) of the purified product (0.5 mg) was mixed with 1 M HCl (0.1 ml) and stirred for 2 h at room temperature (32). A product, the native form of leucocin A, was directly isolated from the DMSO solution by using the same high-performance liquid chromatography column as described above and checked by matrix-assisted laser desorption ionization–time of flight mass spectrometry. Lyophilized, purified leucocin A was resuspended in a small volume of 0.1% trifluoroacetic acid in order to give the desired activity units (AU) as described by Hastings et al. (12). Synthesized ESF1-7GR was prepared as previously described for leucocin A (12). The synthetic peptide ESF1-(7GR) is an analog of an α-helical portion of the antimicrobial peptide magainin PGLa, which was isolated from a frog. It mimics the charge distribution of the parent peptide (7). Nisaplin was obtained from Aplin and Barrett Ltd (Beaministeri, United Kingdom) and was prepared by dissolving in 0.2 N HCl. For pediocin PA-2, broth from an overnight culture of P. acidilactici PA-2 was filter sterilized and freeze-dried. The lyophilized supernatant was resuspended in a small volume of 0.1% trifluoroacetic acid. Pronase E (Boehringer Mannheim) inactivation confirmed that the antimicrobial activity observed was due to peptide activity only. All bacteriocin stock solutions were stored at −20°C until used.
MIC and stability of phenotype.
Liquid MIC determination was done using standard methods (www.interchg.ubc.ca/bobh /peptides.htm). Bacteriocin activity was determined using the spot-on-lawn method (12). The resistant phenotype was found to be resistant to levels of leucocin A in excess of 2 mg/ml, which corresponds to 1011 AU/ml. This result was confirmed by the spot-on-lawn assay, as well as by liquid MIC determination (result indicated is an average of three trials for both methods used). This level of resistance is significantly higher than those found previously (4, 8, 16, 30, 33). In order to assess the stability of the phenotypic resistant character, the resistant mutant strain was subcultured 20 times in brain heart infusion broth without bacteriocin. After each subculture, the overnight culture was used as an indicator lawn and its MIC was determined as described above. The high level of resistance found was still present even after subculturing in unsupplemented media for 20 generations. This indicates that the resistant phenotype is not an adaptive response, but rather a spontaneous but stable genetic mutation. The stability of the phenotype is similar to that described in previous reports (30), in which the resistant phenotype was stable for 10 subcultures in the absence of bacteriocin. However, Dykes and Hastings (8) in a previous study found that the resistant phenotype in cocultivation with the sensitive strain had lost its resistant phenotype after 10 transfers in unsupplemented media. This may indicate that there are various modes of resistance to bacteriocins.
Cross-resistance.
The spot-on-lawn assay (12) was used to determine cross-resistance. The L. monocytogenes B73-MR1 culture did not show the same level of sensitivity to nisin (class I) or to the antimicrobial peptide ESF1-(7GR). However, when a crude extract of pediocin PA-2 was tested for activity against both the sensitive and leucocin A-resistant strains, the sensitive strain showed inhibition up to the 512−1 dilution (equivalent to 51,200 AU/ml), whereas the resistant strain showed no zones of inhibition, even in the undiluted sample. This indicates that the mechanism of resistance may not be specific for only leucocin A and that there may be a general mechanism of resistance to class IIa bacteriocins. Previous researchers have found that resistant mutants generated by challenging with a single class IIa bacteriocin resulted in cross-resistance to other bacteriocins within the class (8, 30).
AFLP analysis.
Intact genomic DNA was isolated using the NucleoSpin C + T (Macherey-Nagel) kit according to the manufacturer's instructions with a few minor modifications. (i) A 4-ml overnight culture suspension was used, and (ii) eluted DNA was treated overnight at 37°C with RNase I (Boehringer Mannheim). DNA was quantified electrophoretically using Lambda standards (Boehringer Mannheim) on 0.8% agarose gels. Ligation and preselective PCR was carried out with an AFLP Ligation and Pre-selective amplification kit (Perkin-Elmer, Foster City, Calif.) according to the manufacturer's instructions. AFLP products were run on a 6% denaturing polyacrylamide gel containing ultrapure urea (ICN Biochemicals Inc., Aurora, Ohio). AFLP products were detected using the silver-staining procedure described previously (2) and in the Silver Sequence DNA Sequencing System Technical Manual (Promega), with the following modifications. (i) The gel was fixed and the staining process was stopped using 12% acetic acid, (ii) 2.5 ml of 14.8% formaldehyde (BDH) per liter was used for both the impregnation and developing solution, and (iii) 125 μl of 0.1 M sodium thiosulfate per liter was added to the developer prior to use. No polymorphic bands were detected using ssAFLP (results not shown), indicating that the portions of the genome that were scanned by the AFLP process were identical.
2D sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
L. monocytogenes B73 and B73-MR1 cells were grown for 16 h at 30°C. Bacterial cells were harvested and washed once in 0.01 M phosphate-buffered saline (pH 7.0) and twice in 32 mM Tris-HCl (pH 7.0) (Boehringer Mannheim). The cell pellet was finally resuspended in 1× Tris-EDTA buffer (pH 7.0) containing the mini Complete tablet (Boehringer Mannheim). Cells were sonicated three times in 4-min bursts at power setting 15 on ice (Versonic; The Virtis Company, Inc., Gardiner, N.Y.). The mixture was treated with 5.5 μl of a 10-mg/ml stock DNase I solution (Boehringer Mannheim) and 5.5 μl of RNase I (Boehringer Mannheim) and was incubated at 37°C for 30 min. To the mixture, 9.5 M Ultra pure urea (ICN Biochemicals), 100 mM dithiothreitol (Boehringer Mannheim), 4% Triton X-100 (BDH), 0.4% Ampholine preblended (Pharmacia Biotech, Uppsala, Sweden) (pH 3.5 to 9.5), and 1.6% Ampholine (Pharmacia Biotech) (pH 5 to 7) were added to the final concentrations indicated. In addition, the same solution was incubated at 30°C for 2 h. Insoluble material was removed by centrifugation (30,000 × g) for 45 min. Protein concentration was determined using the modified Bradford assay (29). Aliquots of 12 and 350 μg were applied to the 1-dimensional (1-D) gels for silver and Coomassie staining, respectively. 2-D electrophoresis was performed according to the method described by O'Farrell (23). Gels were silver stained (3) or Coomassie stained, depending on the amount of protein loaded. Samples were isolated in duplicate, and at least three gels of each sample were run before the protein pattern was considered to be reliable. The region of the gel containing a 35-kDa protein that was present only in the protein gel of the sensitive strain was excised immediately after running and was electroblotted onto polyvinylidine difluoride Western blotting membranes (Boehringer Mannheim) as described by Matusdaira (18), except that the electroblotting was carried out for 2 h and not for the 10 to 30 min as described. The sequence of the first 20 amino acids was determined using automated Edman degradation on a 491 Procise automated sequencer (Perkin-Elmer, Applied Biosystems). The identity of the protein was determined using the default settings on the Blast advanced database (1).
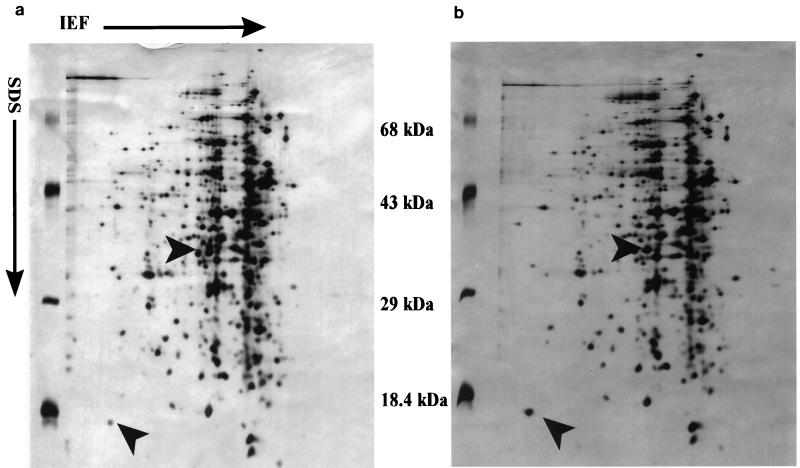
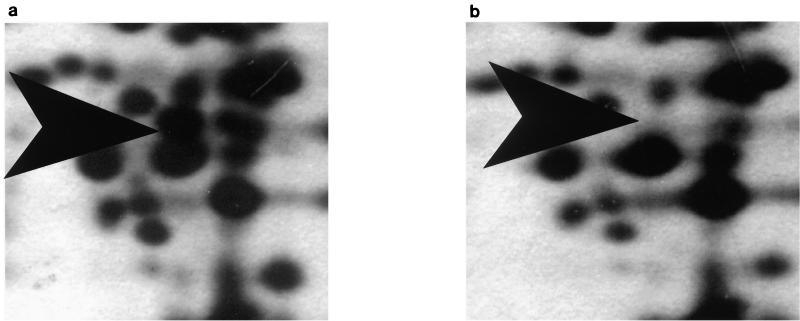
No polymorphic bands (results not shown) were evident after 1-D SDS-PAGE of total cellular protein. However, analysis of 2-D SDS-PAGE of total cellular protein showed one unambiguous difference. A 35-kDa protein was clearly present in the protein gel from the sensitive strain (L. monocytogenes B73) but absent in the gel from the resistant strain (L. monocytogenes B73-MR1) (Fig. 1 and 2). There were some other differences that were not unambiguous, the clearest of which was a difference in expression levels of an 18-kDa protein, which showed a higher intensity in the resistant phenotype than in the sensitive phenotype. The sequence of the 35-kDa protein was MVGIILAT/GHGWFAEGIKQWG, which has a 65% identity and an 83% homology to the mannose-specific phosphotransferase system (PTS) enzyme IIAB of Streptococcus salivarius. The molecular mass of the protein isolated also corresponds to the size of the IIABLman of the S. salivarius subunit with a molecular mass of 35.2 kDa (25), as well as the IIABman domain of Escherichia coli, which has a molecular mass of 35.0 kDa (9).
FIG. 1.
Silver-stained 2-D SDS-PAGE of total cellular proteins extracted from 16-h cultures of L. monocytogenes B73 (a) and L. monocytogenes B73-MR1 (b) grown at 30°C, which were firstly separated by isoelectric focusing (IEF) (1.6% [vol/vol] Ampholine [Pharmacia] [pH 5 to 7] and 0.4% [vol/vol] Ampholine [Pharmacia] [pH 3.5 to 9.5]) in the horizontal direction, followed by SDS-PAGE (16.5% acrylamide-bisacrylamide [44:0.8]) in the vertical direction. Directions of isoelectric focusing and SDS-PAGE are indicated by arrows. Protein differences are indicated by arrow heads.
FIG. 2.
Magnification of a region between 29 and 43 kDa of a silver-stained 2-D SDS-PAGE gel of total cellular proteins extracted from 16-h cultures of L. monocytogenes B73 (a) and L. monocytogenes B73-MR1 (b) grown at 30°C, which were firstly separated by isoelectric focusing (IEF) (1.6% [vol/vol] Ampholine [Pharmacia] [pH 5 to 7] and 0.4% [vol/vol] Ampholine [Pharmacia] [pH 3.5 to 9.5] in the horizontal direction, followed by SDS-PAGE (16.5% acrylamide-bisacrylamide [44:0.8]) in the vertical direction. Directions of isoelectric focusing and SDS-PAGE are indicated by arrows. The protein difference at 35 kDa is indicated by arrow heads.
The results shown in this paper indicate that resistance to leucocin A may be associated with the loss of a putative mannose-specific PTS protein. Whether leucocin A interacts specifically with the mannose PTS as a target is a possibility that requires further study. The putative loss of the mannose PTS due to the absence of the EIIAB component did not appear to affect the growth rate of the resistant strain. The use of glucose as a preferred carbohydrate (28) and/or the ability of a PTS to import more than one carbohydrate source (25, 26, 27) may be a plausible explanation for this. This is the first report linking type IIa bacteriocin activity and resistance to a specific molecule in the membrane of target cells.
ACKNOWLEDGMENTS
This work was partially supported by grants from the National Research Foundation and the Natal University Research Fund.
We thank S. Aimoto of the Institute for Protein Research, Osaka, Japan, for synthesizing Leucocin A and ESF1-7GR and R. Chauhan of the Molecular Biology Unit, University of Natal, for the amino acid sequencing analysis.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped Blast and PSI-BLAST: a new generation of protein data base search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassam B J, Caetano-Anolle's G, Gresshoff P M. Fast and sensitive silver-staining of DNA in polyacrylamide gels. Anal Biochem. 1991;196:80–83. doi: 10.1016/0003-2697(91)90120-i. [DOI] [PubMed] [Google Scholar]
- 3.Blum H, Beier H, Gross H J. Improved silver staining of plant proteins, RNA, and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99. [Google Scholar]
- 4.Crandall A D, Montville T J. Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype. Appl Environ Microbiol. 1998;64:231–237. doi: 10.1128/aem.64.1.231-237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies E A, Falahee M B, Adams M R. Involvement of the cell envelope of Listeria monocytogenes in the acquisition of nisin resistance. J Appl Bacteriol. 1996;31:139–146. doi: 10.1111/j.1365-2672.1996.tb04491.x. [DOI] [PubMed] [Google Scholar]
- 6.Delves-Broughton J. Nisin and its use as a food preservative. Food Technol. 1990;44:100–118. [Google Scholar]
- 7.Dykes G A, Aimoto S, Hastings J W. Modifications of a synthetic antimicrobial peptide for improved inhibitory activity. Biochem Biophys Res Commun. 1998;248:268–272. doi: 10.1006/bbrc.1998.8940. [DOI] [PubMed] [Google Scholar]
- 8.Dykes G A, Hastings J W. Fitness costs associated with class IIa bacteriocin resistance in Listeria monocytogenes B73. Lett Appl Microbiol. 1998;26:5–8. doi: 10.1046/j.1472-765x.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- 9.Erni B, Zanolari B, Graff P, Kocher H P. Mannose permease of Escherichia coli, domain structure and function of the phosphorylating subunit. J Biol Chem. 1989;264:18733–18741. [PubMed] [Google Scholar]
- 10.Harris L J, Fleming H P, Klaenhammer T R. Sensitivity and resistance of Listeria monocytogenes ATCC 1915, Scott A, and UAL 500 to nisin. J Food Prot. 1991;54:836–840. doi: 10.4315/0362-028X-54.11.836. [DOI] [PubMed] [Google Scholar]
- 11.Hastings J W, Sailer M, Johnson K, Roy K L, Vederas J C, Stiles M E. Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene from Leuconostoc gelidum. J Bacteriol. 1991;173:7495–7500. doi: 10.1128/jb.173.23.7491-7500.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hastings J W, Gibson P T, Chauhan R, Dykes G A, von Holy A. Similarity of bacteriocins from meat spoilage lactic acid bacteria. S Afr J Sci. 1996;92:376–380. [Google Scholar]
- 13.Jack R W, Tagg J R, Ray B. Bacteriocins of gram positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King D S, Fields C G, Fields G B. A cleavage method which minimizes side reactions following Fmoc solid phase peptide synthesis. Int J Pept Protein Res. 1990;36:255–266. doi: 10.1111/j.1399-3011.1990.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 15.Klaenhammer T R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12:39–86. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 16.Maisiner-Patin S, Richard J. Cell wall changes in nisin-resistant variants of Listeria innocua grown in the presence of high nisin concentrations. FEMS Microbiol Lett. 1996;140:29–35. doi: 10.1111/j.1574-6968.1996.tb08310.x. [DOI] [PubMed] [Google Scholar]
- 17.Matsudaira P. Sequence from picomole quantities of protein electro blotted onto Polyvinylidene Difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 18.Ming X, Daeschel M A. Nisin resistance of food borne bacteria and specific resistance responses of Listeria monocytogenes Scott A. J Food Prot. 1993;56:944–948. doi: 10.4315/0362-028X-56.11.944. [DOI] [PubMed] [Google Scholar]
- 19.Ming X, Daeschel M A. Correlation of cellular phospholipid content with nisin resistance of Listeria monocytogenes Scott A. J Food Prot. 1995;58:416–420. doi: 10.4315/0362-028X-58.4.416. [DOI] [PubMed] [Google Scholar]
- 20.Montville T J, Winkowski K, Ludescher R D. Models and mechanism for bacteriocin actions and application. Int Dairy J. 1995;5:797–814. [Google Scholar]
- 21.Nes I F, Drep D B, Havarstein L S, Brurberg M B, Eijsink V, Holo H. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:13–128. doi: 10.1007/BF00395929. [DOI] [PubMed] [Google Scholar]
- 22.Nettles C G, Barefoot S F. Biochemical and genetic characteristics of bacteriocins of food-associated lactic acid bacteria. J Food Prot. 1993;56:338–356. doi: 10.4315/0362-028X-56.4.338. [DOI] [PubMed] [Google Scholar]
- 23.O'Farrell H D. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 24.Papathanasopoulos M A, Krier F, Revol-Junelles A, Lefehvre G, LeCaer J P, van Holy A, Hastings J W. Multiple bacteriocin production by Leuconostoc mesenteroides TA 33a and other Leuconostoc/Weissella strains. Curr Microbiol. 1998;35:331–335. doi: 10.1007/s002849900264. [DOI] [PubMed] [Google Scholar]
- 25.Pelletier G, Frenette M, Vadeboncoeur C. Transport of mannose by inducible phosphoenolpyruvate:fructose phosphotransferase system in Streptococcus salivarius. Microbiology. 1994;140:2433–2438. doi: 10.1099/13500872-140-9-2433. [DOI] [PubMed] [Google Scholar]
- 26.Pelletier M, Lortie L-A, Frenette M, Vadeboncoeur C. The phosphoenolpyruvate: mannose phosphotransferase system of Streptococcus salivarius. Functional and biochemical characterization of II ABLMAN and II ABHMAN. Biochemistry. 1998;37:1604–1612. doi: 10.1021/bi9721647. [DOI] [PubMed] [Google Scholar]
- 27.Postma P W, Lengeler J W, Jacobson G R. Phosphoenolpyruvate: carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Premartine R J, Lin W-J, Johnson E A. Development of an improved, chemically-defined, minimal medium for Listeria monocytogenes. Appl Environ Microbiol. 1991;57:3046–3048. doi: 10.1128/aem.57.10.3046-3048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramagli L S, Rodriques L. Quantification of microgram amounts of protein in two-dimensional electrophoresis sample buffer. Electrophoresis. 1998;6:559–563. [Google Scholar]
- 30.Rekhif N, Atrih A, Lefebvre G. Selection and properties of spontaneous mutants of Listeria monocytogenes ATCC15313 resistant to other bacteriocins produced by lactic acid bacteria. Curr Microbiol. 1994;28:237–241. [Google Scholar]
- 31.Robichon D, Gouin E, Debarbouille M, Cossart P, Cenatiempo Y, Hechard Y. The rpoN (ς54) gene from Listeria monocytogenes is involved in resistance to mesentericin Y105, an antibacterial peptide from Leuconostoc mesenteriodes. J Bacteriol. 1997;179:7591–7594. doi: 10.1128/jb.179.23.7591-7594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamamura H, Otaka A, Nakamura J, Okubo K, Koide T, Ikeda K, Ibuka T, Fujii N. Disulfide bond-forming reaction using a dimethyl sulfoxide/aqueous HCL system and its application to regioselective two bond formation. Int J Pept Protein Res. 1995;45:312. doi: 10.1111/j.1399-3011.1995.tb01043.x. [DOI] [PubMed] [Google Scholar]
- 33.Verheul A, Russell N J, Hof R V T, Rombouts F M, Abee T. Modifications of membrane phospholipid composition in nisin-resistant Listeria monocytogenes Scott A. Appl Environ Microbiol. 1997;63:3451–3457. doi: 10.1128/aem.63.9.3451-3457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vos P, Hoger R, Blecker M, Reijans M, van de Lee T, Hornes M, Frjiters A, Pot J, Peleman J, Muiper M, Zabeau M. AFLP: a new concept for DNA fingerprinting. Nucleic Acids Res. 1995;21:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]