Abstract
We demonstrated that oxidative stress plays a role in freeze-thaw-induced killing of Campylobacter coli following analysis of mutants deficient in key antioxidant functions. Superoxide anions, but not H2O2, were formed during the freeze-thaw process. However, a failure to detoxify superoxide anions may lead to spontaneous disproportionation of the radicals to H2O2.
Campylobacter coli and Campylobacter jejuni are the major bacterial causes of food-associated human diarrheal disease in the developed world (2). It is surprising, therefore, that compared to certain other bacteria, little is known about the mechanisms which govern the survival of these important pathogens in food or in the environment.
C. jejuni and C. coli have the unique property, among food-borne bacterial pathogens, of being microaerophilic. Accordingly, they require at least 3% oxygen for growth, but 5 to 7% oxygen is optimal (9). Despite the obvious importance of oxygen and its reactive intermediates in the contamination cycle of campylobacters, the mechanisms of oxygen tolerance and oxygen metabolism are poorly understood. Recently, however, the contribution of a number of key functions in the defense against oxidative stress has been elucidated. A single catalase, encoded by katA, provides protection against oxidative stress by converting H2O2 to H2O and O2 (5). In addition, superoxide dismutase (SOD), which catalyzes the conversion of oxygen radicals to H2O2 and O2, is thought to provide the first line of defense against the toxic effects of reactive oxygen intermediates (13, 14). Unlike the situation in Escherichia coli, which expresses three distinct types of this enzyme, an Fe SOD is the only SOD in C. coli and C. jejuni (12–14). This single enzyme, however, plays a crucial role in defense against oxidative stress in campylobacters, particularly during survival when growth has ceased (14). More recently, an iron-regulated alkyl hydroperoxide reductase (AhpC) has been shown to be important in the resistance of C. jejuni to alkyl hydroperoxides (1).
Freezing and thawing of living cells result in injury, and it has been proposed that the injury is the result of several factors, including ice nucleation and dehydration (10). Recently, however, oxidative damage has been implicated as a mechanism that contributes to freeze-thaw injury since it has been predicted that an oxidative burst occurs upon thawing (6, 11). Indeed, in a recent study researchers demonstrated that oxidative stress contributes to injury of yeast cells during the freeze-thaw process and that SOD is required for resistance to this injury (11). In this study we exploited the availability of mutations in key components of the oxidative stress defense system of C. coli in order to determine the role of oxidative stress in the injury of these cells that occurs during freezing and thawing.
Generation of an SOD-deficient, catalase-deficient double mutant of C. coli.
Mutants derived from C. coli UA585 that are deficient in either SOD activity (CCSD1) or catalase activity (CK100) have been described previously (5, 14). A mutant deficient in both enzymes was generated by inactivating the katA gene of SOD-deficient mutant CCSD1 by allelic exchange as described previously (5), except that the antibiotic marker used was a kanamycin resistance cassette. One kanamycin-resistant transformant generated by this procedure, designated CCKS1, was shown by Southern hybridization to contain an inactivated copy of katA and lacked catalase activity (Park, unpublished data).
Sensitivity of SOD-deficient mutants to freezing.
Previously, we have shown that SOD, but not catalase, is an important determinant in the ability of C. coli to survive following exposure to air (14). In order to assess the effect of the absence of both SOD and catalase, the survival of CCKS1 was assessed under aerobic conditions in Mueller-Hinton broth (MHB) at 25°C as described previously (14). Survival of the SOD-deficient, catalase-deficient double mutant paralleled survival of the SOD-deficient mutant, demonstrating that under the conditions used at least, the presence of an additional katA mutation in a SOD-deficient background did not further sensitize cells to oxidative stress (Park, unpublished data).
It has been shown previously that freezing of campylobacter cultures results in a rapid decrease in viability (7, 8). Furthermore, the results of a recent study carried out with yeast cells provided convincing evidence that reactive oxygen intermediates are generated during the freeze-thaw process and that these intermediates contribute to the lethal damage to the cell (11). To determine whether oxidative stress contributes to the death of C. coli during freezing, the tolerance of C. coli mutants deficient in various antioxidant enzymes was assessed. Cells were grown at 37°C to confluence on Mueller-Hinton agar plates under microaerobic conditions. The growth was then harvested in Mueller-Hinton broth (∼5 × 108 CFU ml−1), and aliquots were stored at −20°C for various periods. When necessary, aliquots were allowed to thaw at room temperature and then immediately refrozen to obtain freeze-thaw cycles. Viability was assessed by plate counting as described previously (14).
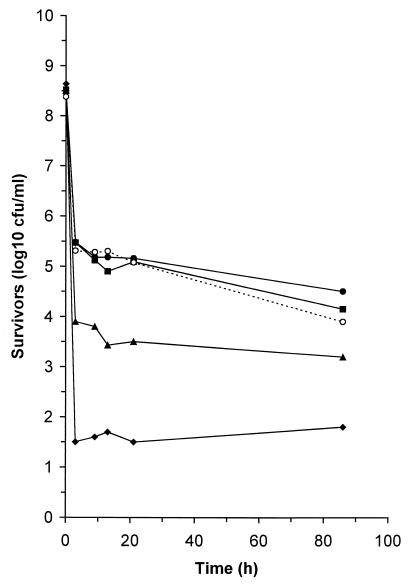
During continuous storage, the general viability profile was the same irrespective of the phenotype of the cells (Fig. 1). Thus, for all cell types the initial sample (taken after 3 h) revealed that there was a dramatic reduction in viability compared to unfrozen cultures, but after this viability declined at a much lower rate. This suggests that freezing or thawing had the greatest impact on viability and that survival is generally independent of the length of the period of exposure to −20°C. The sensitivity of the catalase-deficient mutant, CK100, to freezing was similar to that of the parental strain. In contrast, the viability of the SOD-deficient mutant after the initial 3-h period was 38-fold less than the viability of the wild-type strain after the same period. When the viability of the SOD-deficient, catalase-deficient double mutant was assessed, we found that this strain was even more sensitive to freeze-thaw injury than the sodB mutant was. Accordingly, the number of viable cells detected after 3 h was 251-fold lower than the number of viable cells measured for the SOD-deficient mutant. Generally, the mutants also exhibited the same differential sensitivity to the freeze-thaw process when the stress was generated by sequential cycles of freezing and thawing (Fig. 2). Thus, after two cycles of freezing and thawing, the viable counts of the parental strain and the catalase-deficient strain had decreased by factors of 4.8 × 103 and 2.9 × 103, respectively. In contrast, after the same two cycles of freezing and thawing the viable counts of the SOD-deficient mutant and the catalase-deficient, SOD-deficient double mutant had decreased by 2.5 × 106- and 2.2 × 107-fold, respectively. After three cycles the levels of survival of CCSD1 and CCKS1 were broadly equivalent, but they were still 851-fold less than the levels of survival for the parental strain and the catalase-deficient strain after the same treatment.
FIG. 1.
Sensitivity of SOD-deficient cells of C. coli during frozen storage at −20°C. Cells of C. coli UA585 (●), CK100 (■), CCSD1 (▴), CCKS1 (⧫), and CCSD1 containing pSOD13 (○) were frozen for various periods, and viability was assessed after thawing. Similar results were reproducibly obtained in at least three separate experiments.
FIG. 2.
Sensitivity of SOD-deficient cells to sequential cycles of freezing and thawing. Cells of C. coli UA585 (●), CK100 (■), CCSD1 (▴), CCKS1 (⧫), and CCSD1 containing pSOD13 (○) were subjected to sequential cycles of freezing and thawing, and viability was assessed. Similar results were reproducibly obtained in at least three separate experiments.
Plasmid pSOD13, containing a recombinant copy of sodB, has been used previously to complement the sodB mutation in CCSD1 (14). The presence of this plasmid in SOD-deficient CCSD1 cells restored the viability of this strain during freezing to levels comparable to the parental strain levels (Fig. 1 and 2), which confirmed that the sensitivity of CCSD1 cells to freezing was due to the loss of SOD activity alone.
Involvement of oxygen and its reactive intermediates in freeze-thaw damage.
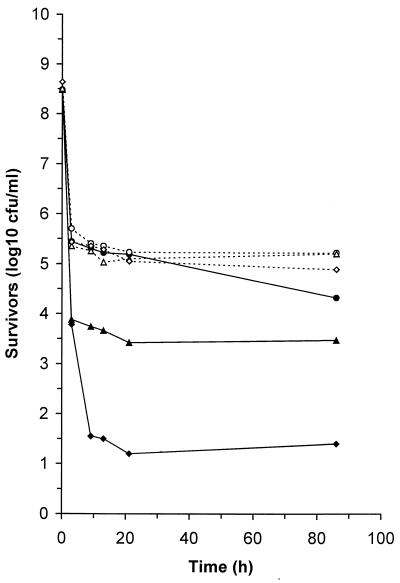
SOD protects cells from the toxic effects of superoxide anions. Consequently, as the SOD-deficient mutant was more sensitive to freezing and thawing, it is likely that reactive oxygen intermediates were generated during this process and that the failure to detoxify these intermediates was responsible for the sensitivity of the sodB mutants. Since generation of superoxide radicals is related to the availability of oxygen, we sought to confirm the role of superoxide radicals in cell injury by assessing the effect of oxygen restriction on viability during frozen storage (Fig. 3). Cells were grown as described above, but harvesting was carried out in an anaerobic atmosphere (10% H2, 10% CO2, 80% N2) generated in a model MACS MG500 workstation (Don Whitley Scientific, Shipley, United Kingdom). The cell suspensions were introduced into screw-top Eppendorf tubes, which were sealed, frozen for various periods, and then thawed with the seal maintained; then viability was assessed as described above. When cells were subjected to freezing and thawing in the absence of oxygen, both the SOD-deficient mutant and the catalase-deficient, SOD-deficient double mutant exhibited a level of freeze-thaw tolerance similar to that of the parental strain (Fig. 3). This demonstrated that oxygen was required for injury and, consequently, that superoxide radicals probably play a role. Although cells of the parental strain frozen in the absence of oxygen were slightly more resistant to freeze-thaw injury than cells exposed to oxygen were, the difference was small, suggesting that other processes, in addition to generation of superoxide ions, also contributed to cell death.
FIG. 3.
Involvement of oxygen in freeze-thaw damage. The effect of oxygen in the freezing medium on freeze-thaw tolerance was assessed by freezing and thawing in the presence (solid symbols) or absence (open symbols) of oxygen and storing cells for various periods at −20°C. Symbols: ○ and ●, C. coli UA585; ▵ and ▴, CCSD1; ◊ and ⧫, CCKS1. Similar results were reproducibly obtained in at least three separate experiments.
Conclusions.
In this study we demonstrate that SOD-deficient mutants are sensitive to freezing and thawing. Since the resistance of the cells to freezing and thawing could be restored by freezing in the absence of oxygen, it is likely that superoxide radicals are generated during this process and that SOD is important in the resistance of the cells to these radicals. Conversely, the freeze-thaw tolerance of cells containing a single deletion in katA was not altered. Since the catalase reaction is the primary route for H2O2 detoxification in C. coli and there is no alternative hydrogen peroxidase activity (5), it is unlikely that H2O2 is generated during freezing and thawing in cells that possess SOD activity. Interestingly, the absence of catalase activity in an SOD-deficient background increased the sensitivity of the cells to freezing and thawing. In this situation, it is possible that the failure to detoxify superoxide anions leads to spontaneous disproportionation of superoxide radicals to H2O2 (3, 4) and that in the absence of catalase accumulation of this agent causes cell death. The sodB katA double mutant did not exhibit increased sensitivity compared to the SOD-deficient mutant when cells were incubated in the presence of air without freezing. However, this finding may be explained by the fact that spontaneous disproportionation generally occurs at low pH values (3, 4) and the fact that drastic changes in the intracellular pH during freezing result in an acidic intracellular environment (15).
Finally, our results further highlight the important role of SOD in the physiology of campylobacters. Previously, it has been shown that SOD provides protection against oxidative stress during survival in food (14), colonization of the gastrointestinal tract of chickens (14), and survival in macrophages (12). In this study we demonstrated for the first time that campylobacters encounter oxidative stress during freezing and thawing and that SOD is important in resistance of cells to this stress.
REFERENCES
- 1.Baillon M L, van Vliet A H, Ketley J M, Constantinidou C, Penn C W. An iron-regulated alkyl hydroperoxide reductase (AhpC) confers aerotolerance and oxidative stress resistance to the microaerophilic pathogen Campylobacter jejuni. J Bacteriol. 1999;181:4798–4804. doi: 10.1128/jb.181.16.4798-4804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaser M J. Epidemiologic and clinical features of Campylobacter jejuni infections. J Infect Dis. 1997;176(Suppl. 2):S103–S105. doi: 10.1086/513780. [DOI] [PubMed] [Google Scholar]
- 3.Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 1989;264:7761–7764. [PubMed] [Google Scholar]
- 4.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 5.Grant K A, Park S F. Molecular characterization of katA from Campylobacter jejuni and generation of a catalase-deficient mutant of Campylobacter coli by intraspecific allelic exchange. Microbiology. 1995;141:1369–1376. doi: 10.1099/13500872-141-6-1369. [DOI] [PubMed] [Google Scholar]
- 6.Hermes-Lima M, Storey K B. Antioxidant defenses in the tolerance of freezing and anoxia by gartersnakes. Am J Physiol. 1993;265:646–652. doi: 10.1152/ajpregu.1993.265.3.R646. [DOI] [PubMed] [Google Scholar]
- 7.Humphrey T J. Injury and recovery in freeze- or heat-damaged Campylobacter jejuni. Lett Appl Microbiol. 1986;3:81–84. [Google Scholar]
- 8.Humphrey T J, Cruickshank J G. Antibiotic and deoxycholate resistance in Campylobacter jejuni following freezing or heating. J Appl Bacteriol. 1985;59:65–71. doi: 10.1111/j.1365-2672.1985.tb01777.x. [DOI] [PubMed] [Google Scholar]
- 9.Luechtefeld N W, Reller L B, Blaser M J, Wang W L. Comparison of atmospheres of incubation for primary isolation of Campylobacter fetus subsp. jejuni from animal specimens: 5% oxygen versus candle jar. J Clin Microbiol. 1982;15:53–57. doi: 10.1128/jcm.15.1.53-57.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazur P. Cryobiology: the freezing of biological systems. Science. 1970;168:939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- 11.Park J I, Grant C M, Davies M J, Dawes I W. The cytoplasmic Cu,Zn superoxide dismutase of Saccharomyces cerevisiae is required for resistance to freeze-thaw stress: generation of free radicals during freezing and thawing. J Biol Chem. 1998;273:22921–22928. doi: 10.1074/jbc.273.36.22921. [DOI] [PubMed] [Google Scholar]
- 12.Pesci E C, Cottle D L, Picket C L. Genetic, enzymatic, and pathogenic studies of the iron superoxide dismutase of Campylobacter jejuni. Infect Immun. 1994;62:2687–2695. doi: 10.1128/iai.62.7.2687-2694.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purdy D, Park S F. Cloning, nucleotide sequence, and characterization of a gene encoding superoxide dismutase from Campylobacter jejuni and Campylobacter coli. Microbiology. 1994;140:1203–1208. doi: 10.1099/13500872-140-5-1203. [DOI] [PubMed] [Google Scholar]
- 14.Purdy D, Cawthraw S, Dickinson J H, Newell D G, Park S F. Generation of a superoxide dismutase (SOD)-deficient mutant of Campylobacter coli: evidence for the significance of SOD in Campylobacter survival and colonization. Appl Environ Microbiol. 1999;65:2540–2546. doi: 10.1128/aem.65.6.2540-2546.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van den Berg L. Physiochemical changes in foods during freezing and subsequent storage. In: Hawthorn J, Rolfe E J, editors. Low temperature biology of foodstuffs. Oxford, United Kingdom: Pergamon Press; 1968. pp. 205–219. [Google Scholar]