Abstract
Dilation of the fluid-filled cerebral ventricles (ventriculomegaly) characterizes hydrocephalus and is frequently seen in autism and schizophrenia. Recent work suggests that the genomic study of congenital hydrocephalus may be unexpectedly fertile ground for revealing insights into neural stem cell regulation, human cerebrocortical development, and pathogenesis of neuropsychiatric disease.
The cerebrospinal fluid (CSF)-filled ventricles of the brain, first described by Aristotle in 350 BCE, are perhaps the largest human anatomical structure for which the function is so poorly understood. This is surprising because neurons of the cerebral cortex are not generated locally in the gray matter but are derived from proliferative neural stem cells (NSCs) that line the embryonic brain ventricles. In fact, crucial steps of cortical neurogenesis begin at the brain-CSF interface where NSCs undergo neurogenic divisions at the ventricle wall to generate progenies that radially migrate toward the cortical plate (Silbereis et al., 2016). The elaboration of the cortex from ventricular wall NSCs is one important example of the developmental and functional relationships that exist between brain parenchyma and the intracranial CSF compartments. It is becoming increasingly clear that a deeper understanding of the CSF-ventricular system is necessary to illuminate gaps in knowledge about human brain development.
Cerebral ventricular dysmorphology is associated with morbid neurological and neurodevelopmental pathologies. Dilation of the cerebral ventricles (ventriculomegaly) characterizes hydrocephalus (Gr., “water on the brain”), one of the oldest known neurological disorders in humans, with archeological records suggesting its recognition already in ancient Egypt. Hydrocephalus can present at any age, although it most commonly appears in early childhood or late adulthood. In newborns and infants, hydrocephalus can arise from secondary causes such as hemorrhage or infection (acquired hydrocephalus) or can be classified as primary or congenital hydrocephalus (CH) in the absence of a known antecedent. CH is a common and morbid brain malformation often associated with poor neurodevelopmental outcomes (see Figure 1A for representative neuroimaging of a pediatric patient with CH).
Figure 1. Cerebral ventricles as windows into human cerebral cortex development and function.
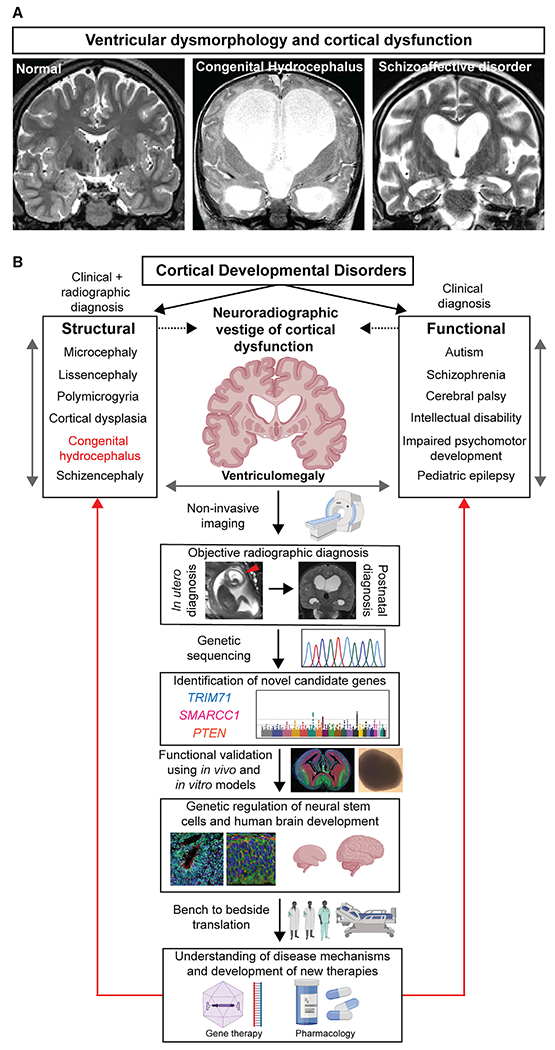
(A) Brain magnetic resonance imaging (MRI) from a neurologically healthy individual compared to a patient with congenital hydrocephalus and a patient with schizoaffective disorder. Brain MRI of normal adult human brain was obtained from OpenNeuro Dataset ds000221. Original de-identified brain MRI scans were obtained from human patients with hydrocephalus or schizoaffective disorder.
(B) Ventriculomegaly as a phenotypic correlate of cortical developmental disorders in humans. Despite the vast heterogeneity among cortical developmental disorders, patients with cerebrocortical disorders often exhibit ventriculomegaly. Thus, ventriculomegaly is potentially a compelling phenotype in a forward genetic screen for novel genetic regulators of human cortical development. In this proposed paradigm, patients diagnosed with ventriculomegaly can be subjected to genetic sequencing to determine potential disease-associated genetic variants. Functional validation of the putative disease genes by study of animal or in vitro organoid models may provide new understanding into the genetic regulation of NSCs and human brain development. This knowledge can inform the convergent disease mechanisms shared by cortical developmental disorders to guide investigation of novel therapies.
Ventricular expansion in hydrocephalus has been largely attributed to CSF overaccumulation stemming from an imbalance of fluid production versus reabsorption or anatomical abnormalities that obstruct CSF flow. In this model, CSF accumulation distends the ventricles and raises intracranial pressure, which compresses overlying brain tissue, presumably leading to brain damage responsible for poor neurodevelopmental outcomes and, if untreated, lethal brain herniation. Consequently, reduction of CSF volume by neurosurgical CSF diversion (shunting and endoscopy) has largely been the standard approach to treat hydrocephalus for the past decades. While this paradigm makes sense for some types of hydrocephalus, and CSF shunts can be lifesaving for many patients, other types of hydrocephalus, including non-obstructive (“communicating”) CH, seem different. Here, intracranial pressure can be paradoxically normal or even low (Pang and Altschuler, 1994), and even after technically successful CSF diversion, ventriculomegaly may persist and neurodevelopmental outcomes can remain poor (Riva-Cambrin et al., 2021). These observations highlight our inadequate taxonomy and incomplete patho-etiological knowledge of this disorder. Moreover, they suggest that current treatments directed at restoring CSF homeostasis while addressing some consequences of the disease do not address the underlying disease mechanism. Indeed, CH often presents with a constellation of other structural brain defects not explicable by altered fluid circulation, including open lip schizencephaly, colpocephaly, and polymicrogyria (Jin et al., 2020). Thus, the hydrocephalus phenotype may arise from developmental defects intrinsic to the cerebral parenchyma rather than from a disturbance of CSF homeostasis.
Emerging studies are shifting an “impaired brain plumbing” view of some forms of pediatric hydrocephalus to a new paradigm of dysregulated NSC fate. We recently performed whole-exome sequencing of case-parent trios to discover that damaging de novo gene mutations account for approximately one-fourth of patients with sporadic CH (Jin et al., 2020). Contrary to the classic belief that hydrocephalus arises from abnormal fluid hydrodynamics, all the genes exhibiting genome-wide significant enrichment of de novo mutations in CH cases are known regulators of NSC fate and prenatal neuro-gliogenesis. The most frequently mutated gene in human sporadic CH, TRIM71, is the prototypical example of NSC involvement in CH. A target of the ancient let-7 microRNA heterochronic pathway, TRIM71 encodes an RNA-binding protein that plays a critical role in guiding the developmental timing of NSC progression. Loss of TRIM71 in the mouse causes premature neuronal differentiation at the expense of NSC expansion (Chen et al., 2012). This developmental pathology affecting NSC progression in the setting of TRIM71 deficiency is likely applicable to other genetic causes of CH because integrative genomic analyses showed that CH risk genes overall were enriched in human cortical neurogenesis elements, including proliferative neuroprogenitors and migrating excitatory neurons (Jin et al., 2020). CH risk genes also converged in a gene co-expression module of the midgestational cortex implicated in other neurodevelopmental disorders such as autism and microcephaly (Jin et al., 2020), which is classically characterized by defective NSC proliferation and neurogenesis. This suggests that genetically encoded dysregulation of NSCs that comprise the germinal neuroepithelium lining the developing brain ventricles, leading to impaired cortical neurogenesis, is a primary mechanism driving pathogenesis of a significant percentage of CH.
Emerging human genetic studies and work in model systems suggest that a “NSC model” of hydrocephalus better explains the data than does the classical “plumbing model” in many pediatric patients, especially those with communicating CH subtypes and potentially even the more common hemorrhagic forms in preemies wherein germinal matrix hemorrhage abutting CSF may impact ventricular wall NSCs. In the NSC model, dysregulated fate of NSCs at the ventricular neuroepithelium results in abnormal prenatal neuro-gliogenesis, with impaired morphological development of the cerebral cortex associated with ventriculomegaly. This model shifts focus from the CSF per se to the dysgenic brain parenchyma as the patho-etiological “scene of the crime.” Perturbed NSC development affects multiple developmental processes that conspire to cause ventricular dilation and neurologic dysfunction. Because multiciliated ependymal cells lining the postnatal ventricle wall are direct descendants of NSCs, loss of NSCs could disrupt ependymogenesis with attendant loss of cilia-generated CSF flow contributing to ventriculomegaly (Rodríguez and Guerra, 2017).
However, another often overlooked parameter in intracranial physiology that also influences ventricular size and intracranial pressure is the cerebral parenchyma compartment itself. Indeed, the production and circulation of CSF in the ventricles generate a constant positive pressure that must be counteracted by the surrounding brain tissue/parenchyma to maintain constant ventricular size (Peña et al., 2002). The absence or reduction of such counteracting forces by neural tissue facilitates passive CSF pooling with secondary ventricular expansion, even in the absence of obstructed CSF flow as predicted by computational models (Peña et al., 2002). Thus, the impact of brain tissue biomechanics on maintaining ventricular size directly links abnormal neurogenesis to the development of hydrocephalus. Innate hypoplasia of the cortical mantle due to reduced neuroprogenitor proliferation and neurogenesis results in a “floppy” cerebral cortex that engenders ventriculomegaly even when intracranial pressure is not increased. Impaired cortical neurogenesis not only accounts for of low- or normal-pressure ventriculomegaly (Pang and Altschuler, 1994) but also may explain neurologic dysfunction that can fail to improve even after technically successful CSF diversion surgery in children with hydrocephalus (Riva-Cambrin et al., 2021). Overall, the “NSC” model of hydrocephalus provides explanatory power for ventricular and cortical malformations as well as the associated clinical endophenotypes.
The recognition of some cases of hydrocephalus as a disorder of ventricular wall NSCs rather than of primary fluid overaccumulation not only has implications for treatment of patients with hydrocephalus but also suggests that the mechanisms underlying the hydrocephalus phenotype may be more generally applicable than previously appreciated. Ventricular dilation is found not only in clinical hydrocephalus but also is a common (if often overlooked) structural finding in multiple developmental neuropsychiatric disorders, including autism, schizophrenia, and bipolar disorder (DeSpenza et al., 2021; Svancer and Spaniel, 2021) (see Figure 1A for example of massive ventriculomegaly observed in a human patient with schizoaffective disorder). In fact, of all structural changes in the brain documented by decades of neuroimaging studies, ventriculomegaly remains the single most robust, consistent, and reproducible morphometric finding in the clinically heterogeneous disease of schizophrenia (Svancer and Spaniel, 2021). Ventriculomegaly in neuropsychiatric diseases has been hypothesized to reflect either neurodevelopmental or neurodegenerative changes in the cerebral cortex (Svancer and Spaniel, 2021), suggesting cerebral ventricular dysmorphology as a neuroradiographic vestige of abnormal cerebral cortex development and dysfunction (Figure 1B). Thus, despite the typical view of these diseases as predominantly functional brain disorders rather than structural malformations, ventriculomegaly still stands out as a convergent correlate of abnormal cerebral cortex physiology. Understanding the onset and progression of ventricular dysmorphology provides hope of finding an objective radiographic “biomarker” of disease states that might guide diagnosis, prognosis, and tailored behavioral and/or pharmacological interventions. However, the pathophysiological mechanisms that produce enlarged ventricles in the setting of neuropsychiatric dysfunction remain poorly understood. Clarification of the mechanisms leading to ventriculomegaly is, thus, an important step toward elucidation of the developmental etiologies leading to cortical dysfunction in neuropsychiatric disorders and has the potential to shift the paradigm for understanding disorders of poorly understood mechanisms such as autism and schizophrenia.
Dysregulation of prenatal NSCs comprising the ventricular neuroepithelium may explain not only ventricular dilation in some types of pediatric hydrocephalus but also structural defects and cortical dysfunction in other developmental disorders that often feature ventriculomegaly. Indeed, integrative analyses have revealed the convergence of hydrocephalus risk genes in human brain transcriptional networks implicated in other cortical developmental disorders such as autism and microcephaly (Jin et al., 2020), suggesting shared biological mechanisms underlying pediatric hydrocephalus and other human neurodevelopmental disorders. Autism has classically been considered a disorder affecting the development and function of post-mitotic neurons or synaptic regulation. However, a recent integrative genomic analysis revealed functional convergence of autism risk genes in not only differentiated neurons but also ventricular zone neuroprogenitors of the developing human neocortex (Willsey et al., 2021). Functional validation in the Xenopus model showed that individual loss of function in ten high-confidence autism risk genes altered telencephalon size and increased the ratio of neuroprogenitors to neurons despite the apparently divergent molecular functions of the genes (Willsey et al., 2021). The functional roles of these autism genes in neurogenesis were also confirmed in human-derived in vitro neuroprogenitor models. A recent study similarly investigated the developmental pathologies of human idiopathic schizophrenia by generating and characterizing cerebral cortical organoids derived from induced pluripotent stem cells of schizophrenic patients (Notaras et al., 2021). The authors found early developmental defects at the level of ventricular zone cortical neuroprogenitors in schizophrenia, marked by the premature death of neuroprogenitors and disrupted neuronal differentiation that together reduced neurogenesis. Contrary to the paradigm that autism and schizophrenia are disorders of post-mitotic neurons, these findings suggest that the pathologies leading to disease arise much earlier in brain development than previously expected. Rather, the formation of the cerebral cortex is affected months before birth and the subsequent emergence of debilitating clinical symptoms in childhood and adolescence, given that the bulk of cortical neurogenesis is finished by the end of the second trimester of gestation (Silbereis et al., 2016). Thus, an effect on NSCs and cortical neurogenesis should be considered as a common developmental pathology in functional cortical developmental disorders classically associated with synaptic dysfunction. Such abnormalities in ventricular wall NSCs may explain both the neurobehavioral manifestations of these disorders and the neuroradiographic finding of ventriculomegaly in autism and schizophrenia (Styner et al., 2005; Svancer and Spaniel, 2021). It is, thus, remarkable that some cortical organoids derived from schizophrenia patients show signs of apparent ventriculomegaly in parallel with reduced neuroprogenitor proliferation (Notaras et al., 2021), although this phenotype requires further study.
NSC involvement in the development of cerebral ventricles and cerebral cortex suggests that detailed genetic and mechanistic studies of the hydrocephalus phenotype will likely illuminate fundamental mechanisms of prenatal NSC regulation in corticogenesis and may reveal the etiologies of human cortical developmental disorders (Figure 1B). A major advantage of studying the cerebral ventricles is that ventricular morphology can be quantitatively assessed by noninvasive imaging early in pregnancy (Figure 1B), whereas the postnatal diagnoses of developmental neuropsychiatric disorders are often challenging because of vague and subjective behavioral symptoms that lack biomarker correlates. Thus, abnormalities in the ventricles and CSF compartments provide a noninvasive window into cortical function and development even months before birth. Studying the genetic causes of ventriculomegaly and hydrocephalus in humans constitutes a clinically relevant forward genetic screening approach to identify novel genetic regulators of cerebral cortex development, starting from the earliest steps of NSC progression. These insights could then inform gene therapy approaches to correct the underlying genetic abnormalities and/or development of targeted pharmacotherapy to modulate convergent molecular defects shared by the various types of cortical developmental disorders (Figure 1B). Understanding the emergence and function of the brain ventricles and CSF spaces, structures historically neglected by neuroscientists, provides a powerful opportunity to understand the mechanisms that regulate cerebral cortex development while advancing precision medicine approaches for common and morbid human brain disorders.
ACKNOWLEDGMENTS
This work was supported by the NIH Medical Scientist Training Program Training Grant T32GM136651 (to P.Q.D.), F30HD106694 (to P.Q.D.), K99/R00 Pathway to Independence Award K99HL143036 (to S.C.J.) and ROOHL143036-02 (to S.C.J.), Hydrocephalus Association Innovator Award (to S.C.J.), NIDA grant DA023999 (to P.R.), R01NS111029-01A1 (to K.T.K.), R01NS109358 (to K.T.K.), and K12228168 (to K.T.K.); and the Rudi Schulte Research Institute (to K.T.K.). Cartoons and diagrams were created using BioRender.com.
Footnotes
DECLARATION OF INTERESTS
D.H.G. and C.A.W. are members of the Neuron advisory board. The other authors declare no competing interests.
REFERENCES
- Chen J, Lai F, and Niswander L (2012). The ubiquitin ligase mLin41 temporally promotes neural progenitor cell maintenance through FGF signaling. Genes Dev. 26, 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSpenza T Jr., Carlson M, Panchagnula S, Robert S, Duy PQ, Mermin-Bunnell N, Reeves BC, Kundishora A, Elsamadicy AA, Smith H, et al. (2021). PTEN mutations in autism spectrum disorder and congenital hydrocephalus: developmental pleiotropy and therapeutic targets. Trends Neurosci. 44, 961–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SC, Dong W, Kundishora AJ, Panchagnula S, Moreno-De-Luca A, Furey CG, Allocco AA, Walker RL, Nelson-Williams C, Smith H, et al. (2020). Exome sequencing implicates genetic disruption of prenatal neuro-gliogenesis in sporadic congenital hydrocephalus. Nat. Med 26, 1754–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaras M, Lodhi A, Dündar F, Collier P, Sayles NM, Tilgner H, Greening D, and Colak D (2021). Schizophrenia is defined by cell-specific neuropathology and multiple neurodevelopmental mechanisms in patient-derived cerebral organoids. Mol. Psychiatry Published online November 12, 2021. 10.1038/s41380-021-01316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang D, and Altschuler E (1994). Low-pressure hydrocephalic state and viscoelastic alterations in the brain. Neurosurgery 35, 643–655, discussion 655–656. [DOI] [PubMed] [Google Scholar]
- Peña A, Harris NG, Bolton MD, Czosnyka M, and Pickard JD (2002). Communicating hydrocephalus: the biomechanics of progressive ventricular enlargement revisited. Acta Neurochir. Suppl. (Wien) 81, 59–63. [DOI] [PubMed] [Google Scholar]
- Riva-Cambrin J, Kulkarni AV, Burr R, Rozzelle CJ, Oakes WJ, Drake JM, Alvey JS, Reeder RW, Holubkov R, Browd SR, et al. (2021). Impact of ventricle size on neuropsychological outcomes in treated pediatric hydrocephalus: an HCRN prospective cohort study. J. Neurosurg. Pediatr Published online November 12, 2021. 10.3171/2021.8.PEDS21146. [DOI] [PubMed] [Google Scholar]
- Rodríguez EM, and Guerra MM (2017). Neural Stem Cells and Fetal-Onset Hydrocephalus. Pediatr. Neurosurg 52, 446–461. [DOI] [PubMed] [Google Scholar]
- Silbereis JC, Pochareddy S, Zhu Y, Li M, and Sestan N (2016). The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron 89, 248–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styner M, Lieberman JA, McClure RK, Weinberger DR, Jones DW, and Gerig G (2005). Morphometric analysis of lateral ventricles in schizophrenia and healthy controls regarding genetic and disease-specific factors. Proc. Natl. Acad. Sci. USA 102, 4872–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svancer P, and Spaniel F (2021). Brain ventricular volume changes in schizophrenia. A narrative review. Neurosci. Lett 759, 136065. [DOI] [PubMed] [Google Scholar]
- Willsey HR, Exner CRT, Xu Y, Everitt A, Sun N, Wang B, Dea J, Schmunk G, Zaltsman Y, Teerikorpi N, et al. (2021). Parallel in vivo analysis of large-effect autism genes implicates cortical neurogenesis and estrogen in risk and resilience. Neuron 109, 788–804.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]


