Abstract
Objective.
To examine glucocorticoid-sparing immunomodulatory medication use in youth with systemic lupus erythematosus (SLE) during their first year of care.
Methods.
We conducted a retrospective cohort study using administrative claims for 2000 to 2013 from Clinformatics DataMart for youth ages 10–24 years with an incident diagnosis of SLE (≥3 International Classification of Diseases, Ninth Revision codes for SLE [710.0], each >30 days apart). We determined the proportion of subjects filling a prescription for immunomodulatory medications within 12 months of the first SLE code (index date). We used multivariable regression to examine associations between demographic/disease factors and time to prescription fill in the first year, and also between prescription fill at any time after the index date.
Results.
We identified 532 youth with an incident SLE diagnosis, of which 413 (78%) had a glucocorticoid-sparing immunomodulatory prescription fill in the first year. Prescriptions for hydroxychloroquine and immunosuppressants were filled in the first year by 366 youth (69%) and by 182 (34%), respectively. Those with adult-onset (versus childhood-onset) disease were less likely to fill an immunomodulatory medication by 12 months. No other statistically significant associations were found, although there was increasing likelihood of immunomodulatory medication fills with each subsequent calendar year.
Conclusion.
Among youth with newly diagnosed SLE, hydroxychloroquine use is prevalent although not universal, and prescription immunosuppressant use is notably low during the first year of care. Further research is needed to identify factors contributing to suboptimal immunomodulatory medication use during the first year of care.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic autoimmune condition characterized by multiorgan inflammation and damage. Children and adolescents with SLE have particularly active disease with a high incidence of nephritis, a steep disease damage trajectory, and an increased risk for mortality compared to adults (1,2). One-third of youth with SLE suffer significant organ damage, including cerebrovascular accidents and renal failure, within the initial years of disease onset (1,3). SLE treatment involves immunomodulatory therapy, including glucocorticoids, hydroxychloroquine, and other immunosuppressant medications. Glucocorticoid-sparing immunomodulatory treatment is important to prevent major organ damage and to help children reduce adverse effects of glucocorticoid exposure, including interference with growth and development. Clinical trials demonstrate reduction of disease activity and overall morbidity with use of hydroxychloroquine and immunosuppressant medications (4). The early severe disease activity and resulting rapid accrual of organ damage in youth with SLE make early effective immunomodulatory therapy important in this vulnerable population.
Increased understanding of medication use early during the disease course could inform interventions to improve care for youth with SLE. This study examined patterns of glucocorticoid-sparing immunomodulatory medication use in youth with newly diagnosed SLE. Specifically, we: 1) determined the proportion of youth filling a prescription for immunomodulatory medication in the first year of care, 2) ascertained the median time to immunomodulatory prescription fill in the first year of care, and 3) estimated the associations between time to immunomodulatory prescription fill and relevant demographic and disease factors.
PATIENTS AND METHODS
Study design, data sources, and sample.
We conducted a retrospective cohort study using a large national administrative database. Institutional review board approval was obtained from The Children’s Hospital of Philadelphia, Vanderbilt University Medical Center, and The Hospital for Sick Children. We extracted administrative health care claims from Clinformatics DataMart, a database of private health insurance and Medicare Advantage (C and D) claims representing 15–20% of the commercially insured US population. The database contains de-identified patient-level information, including demographics, medical diagnoses, and health care use, such as prescription drug use.
The study sample included youth ages 10–24 years with an incident diagnosis of SLE during a fixed time period between 2000 to 2013. An incident diagnosis of SLE was defined as having a minimum of 3 physician visits or hospital discharge claims with an International Classification of Diseases, Ninth Revision (ICD-9) code for SLE (710.0), each documented at least 30 days apart, and having no preceding SLE diagnosis code for at least 1 year of continuous enrollment before the first diagnosis code for SLE (i.e., index date). This validated method has been used to identify incident SLE cases in medical records databases and incident rheumatoid arthritis cases using claims data (5–7). Individuals were excluded from the sample if they were not continuously enrolled for at least 1 year after the index date. Age was determined at the index date. The upper age limit was chosen to capture youth who were transitioning from pediatric to adult health systems, a time of increased risk for suboptimal health care use and adverse outcomes. The lower age limit was imposed to exclude monogenic causes of early-onset SLE that may represent unique phenotypes affecting health outcomes and health care use.
Outcome measures.
The proportions of youth filling a prescription for an immunomodulatory medication within 3, 6, and 12 months of the index date were calculated. The proportions of youth not filling a prescription for an immunomodulatory medication included youth who either did not have a prescription written or who had a prescription written but did not fill it; we were unable to distinguish these individuals within the claims data set. The primary outcome measure was time from the index date to the first prescription fill of an immunomodulatory medication. The secondary outcome measure was the probability of any immunomodulatory prescription fill within 12 months of the index date. Immunomodulatory medications examined included hydroxychloroquine and prescription immunosuppressants (mycophenolate mofetil, azathioprine, leflunomide, methotrexate, calcineurin inhibitors, and cyclophosphamide). Intravenous infusions were not included because they are inadequately captured in claims data.
Covariates.
The following demographic and disease-related covariates were included in the multivariable analysis: age, sex, race/ethnicity, household educational level, geographic region of residence, index year, and the presence/absence of nephritis or seizure/stroke during the enrollment period. We modeled age both as a continuous variable and binary variable (10–17 versus 18–24 years) to examine potential differences by childhood-onset and adult-onset disease. The data set derived household education levels from US census data and race/ethnicity from self-report, public records, and proprietary ethnic code tables. Geographic region was based on the US Census Bureau’s groupings of states (http://www2.census.gov/geo/docs/maps-data/maps/reg_div.txt). Index year was included as a covariate to examine the possibility of cohort effect, given increasing evidence during the study period of immunomodulatory treatment effectiveness in youth with SLE. The presence of SLE nephritis was identified with a previously validated algorithm using ICD-9 diagnosis and procedure codes (8,9). The presence of seizure/stroke disorder was identified by at least 1 ICD-9 code for seizures or cerebrovascular disease, a previously validated method used with pediatric administrative claims (10,11).
Statistical analysis.
Descriptive statistics were used to estimate the proportion of subjects with prescription fills within 3, 6, and 12 months from the index date for hydroxychloroquine, an immunosuppressant, either hydroxychloroquine or an immunosuppressant, and glucocorticoids. In the primary analysis, multivariable Cox proportional hazards regression was used to examine associations between demographic/disease characteristics and time to first glucocorticoid-sparing immunomodulatory prescription fill. For the secondary analysis, logistic regression was used to estimate the association between covariates and having a glucocorticoid-sparing immunomodulatory fill any time after the index date.
A sensitivity analysis was performed for subjects who had all 3 SLE diagnosis codes assigned within 12 months from the index date to address the possibility that some patients may have had a tentative diagnosis of SLE during the first year of care and therefore had not received immediate immunosuppression. Additional subanalyses were performed to examine demographic/disease characteristics for subcohorts with specific immunomodulatory medication use patterns, as well as demographic/disease characteristics and immunomodulatory prescription fills for subjects with and without lupus nephritis.
RESULTS
We identified 532 youth with an incident diagnosis of SLE. Cohort characteristics are provided in Table 1. Most were female (87%). Mean ± SD age was 18.5 ± 3.7 years. White youth comprised 62%, African American youth 17%, and Hispanic youth 14%. Nephritis was present in 25%, and 12% had central nervous system (CNS) disease. Childhood-onset disease comprised 199 (37%), and adult-onset comprised 333 (63%). Nephritis was more common in the childhood-onset group (35% versus 19%; P < 0.001), but other demographic characteristics were similar.
Table 1.
Demographic and disease characteristics by immunomodulatory medication use*
| Characteristic | Total cohort (n = 532) | No IM (n = 119) | IM (n = 413) | P † |
|---|---|---|---|---|
|
| ||||
| Female | 462 (87) | 107 (90) | 355 (86) | 0.26 |
| Age, mean ± SD years | 18.5 ± 3.7 | 19.2 ± 3.7 | 18.3 ± 3.7 | 0.02 |
| Age at SLE onset, years | ||||
| 10–17 | 199 (37) | 32 (27) | 167 (40) | 0.01 |
| 18–24 | 333 (63) | 87 (73) | 246 (60) | – |
| Race/ethnicity | ||||
| White | 307 (62) | 62 (58) | 245 (63) | 0.63 |
| African American | 86 (17) | 23 (21) | 63 (16) | – |
| Hispanic | 71 (14) | 15 (14) | 56 (14) | – |
| Asian | 34 (7) | 7 (7) | 27 (7) | – |
| Region | ||||
| Midwest | 143 (27) | 35 (29) | 108 (26) | 0.34 |
| Northeast | 57 (11) | 17 (14) | 40 (10) | – |
| South | 261 (49) | 51 (43) | 210 (51) | – |
| West | 71 (13) | 16 (13) | 55 (13) | – |
| Highest household education | ||||
| High school or less | 144 (27) | 35 (29) | 109 (26) | 0.42 |
| Less than bachelor’s degree | 271 (51) | 52 (44) | 219 (53) | – |
| Bachelor’s degree or more | 98 (18) | 23 (19) | 75 (18) | – |
| Unknown/missing | 19 (4) | 9 (8) | 10 (2) | – |
| Seizure/stroke | 62 (12) | 16 (13) | 46 (11) | 0.49 |
| Nephritis | 133 (25) | 23 (19) | 110 (27) | 0.11 |
| Oral glucocorticoid use‡ | 340 (64) | 32 (27) | 308 (75) | <0.01 |
| Number of fills, median (IQR) | 7 (4–15) | 8 (3–11) | 7 (4–16) | 0.43 |
| Total days supply, median (IQR) | 128 (60–213) | 105 (40–180) | 140 (60–240) | 0.12 |
Values are the number (%) unless indicated otherwise. Immunomodulatory (IM) medication fill was within 12 months after the index date; IMs include hydroxychloroquine or oral immunosuppressants (including mycophenolate [17% of total cohort], azathioprine [9%], methotrexate [8%], leflunomide [1%], cyclophosphamide [1%], and calcineurin inhibitors [1%]).
IQR = interquartile range; SLE = systemic lupus erythematosus.
By chi-square test, Student’s t-test, or Wilcoxon’s rank sum tests as appropriate.
At least 1 oral glucocorticoid prescription filled within 12 months after the index date.
In the 12 months following the index date, 413 youth (78% of 532) filled a prescription for an immunomodulatory medication, 366 (69%) filled hydroxychloroquine, and 182 (34%) filled an immunosuppressant. Glucocorticoid prescriptions were filled by 340 youth (64%) within 12 months of the index date, more commonly in subjects receiving immunomodulatory medications than in those who were not (Table 1). A greater proportion of the childhood-onset group had immunosuppressant fills (84% versus 74%; P = 0.007) and glucocorticoid fills (74% versus 58%; P < 0.001) at 12 months. Medication fill patterns at 3, 6, and 12 months after the index date are shown in Figure 1.
Figure 1.
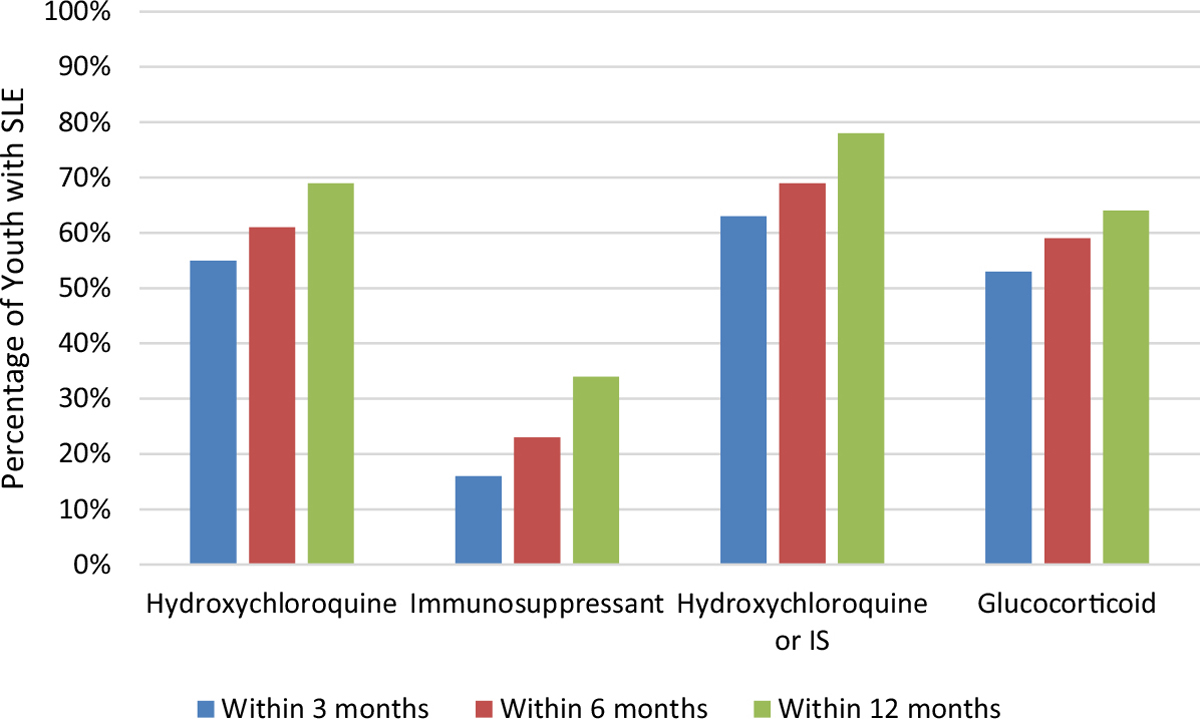
Proportion of youth with systemic lupus erythematosus (SLE) with an immunomodulatory medication prescription filled within the first year after diagnosis. The graph shows the percentage of youth with at least 1 prescription filled for an immunomodulatory medication (hydroxychloroquine, oral immunosuppressants [IS]) or oral glucocorticoids within the first year after SLE diagnosis. Of 532 youth with SLE, 295 (55%), 324 (61%), and 366 (69%) filled a prescription for hydroxychloroquine at 3, 6, and 12 months, respectively. Only 83 (16%), 124 (23%), and 182 (34%) youth filled a prescription for oral immunosuppressants at 3, 6, and 12 months, respectively, while 280 (53%), 313 (59%), and 340 (64%) youth filled a prescription for any oral glucocorticoid within the respective time points.
The median time to immunomodulatory prescription fill was generally short but varied. The median days to fill an immunomodulatory prescription was 12 (interquartile range [IQR] 1–25) within 3 months, 14 (IQR 2–33) within 6 months, and 21 (IQR 3–56) within 12 months of observation from the index date. No statistically significant associations between demographic/disease factors and time to immunomodulatory prescription fill were identified, although there was an increased rate of immunomodulatory fills each calendar year (hazard ratio 1.07 [95% confidence interval (95% CI) 1.03–1.10], P < 0.001). Similarly, the year of the index date was associated with an immunomodulatory fill within any time after the index date (odds ratio [OR] 1.13 [95% CI 1.06–1.21], P < 0.001). With inclusion of a binary covariate for age (instead of continuous age) in the multivariable regression models, the results for time to prescription fill did not change. However, immunomodulatory fill in the first year was less likely for those with adult-onset versus childhood-onset SLE (OR 0.56 [95% CI 0.34–0.92], P = 0.021), and there was an increased odds of immunomodulatory fills each calendar year (OR 1.10 [95% CI 1.06–1.20], P < 0.001) (Table 2).
Table 2.
Factors associated with the presence of or time to first glucocorticoid-sparing immunomodulatory prescription filled*
| Time to first IM† |
IM within 12 months‡ |
|||
|---|---|---|---|---|
| Factor | HR (95% CI) | P | OR (95% CI) | P |
|
| ||||
| Female | 1.0 (0.8–1.4) | 0.93 | 0.7 (0.4–1.5) | 0.40 |
| Childhood-onset SLE | 1.1 (0.9–1.4) | 0.26 | 1.8 (1.1–2.9) | 0.02 |
| Race/ethnicity | ||||
| White | – | – | – | – |
| African American | 0.8 (0.6–1.1) | 0.25 | 0.6 (0.3–1.1) | 0.09 |
| Hispanic | 1.0 (0.8–1.4) | 0.78 | 0.9 (0.4–1.7) | 0.66 |
| Asian | 1.0 (0.7–1.5) | 0.95 | 0.9 (0.4–2.2) | 0.80 |
| Region | ||||
| Midwest | – | – | – | – |
| Northeast | 0.8 (0.6–1.2) | 0.35 | 0.7 (0.3–1.5) | 0.33 |
| South | 1.1 (0.8–1.4) | 0.68 | 1.1 (0.6–1.9) | 0.71 |
| West | 0.9 (0.7–1.3) | 0.74 | 0.8 (0.4–1.7) | 0.53 |
| Household education | ||||
| High school or less | – | – | – | – |
| Less than bachelor’s degree | 1.0 (0.8–1.3) | 0.84 | 1.2 (0.7–2) | 0.49 |
| Bachelor’s degree or higher | 1.2 (0.9–1.6) | 0.31 | 1.0 (0.5–2) | 0.93 |
| Seizure/stroke | 0.8 (0.6–1.1) | 0.19 | 0.6 (0.3–1.3) | 0.22 |
| Nephritis | 1.1 (0.9–1.4) | 0.30 | 1.7 (0.9–3.0) | 0.09 |
| Calendar year | 1.1 (1.0–1.1) | <0.01 | 1.1 (1.1–1.2) | <0.01 |
95% CI = 95% confidence interval; HR = hazard ratio; IM = immunomodulatory; OR = odds ratio; SLE = systemic lupus erythematosus.
Multivariable Cox proportional hazards regression model estimating associations between subject characteristics and time to first glucocorticoid-sparing IM prescription fill.
Multivariable logistic regression model estimating associations between subject characteristics and the presence of ≥1 glucocorticoid-sparing IM prescription fill within 12 months after SLE diagnosis.
Restricting the analysis to the 462 youth who had a third SLE diagnosis code within 12 months of the index date did not significantly change the results; 309 (67%) had an immunomodulatory prescription fill within the first 3 months, 336 (73%) within the first 6 months, and 380 (82%) within the first 12 months after their index date. Additional subanalysis exploring specific immunomodulatory prescription fill patterns showed that those with an immunosuppressant fill but no hydroxychloroquine were more likely to be male and to have nephritis (see Supplementary Table 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24392/abstract). Subanalysis comparing subjects with and without nephritis revealed that ~60% filled hydroxychloroquine and/or an immunosuppressant medication by 12 months (see Supplementary Table 2, at http://onlinelibrary.wiley.com/doi/10.1002/acr.24392/abstract).
DISCUSSION
Youth with SLE are at high risk for organ damage in the first years following diagnosis. Early treatment with glucocorticoid-sparing immunomodulatory medication can mitigate effects of SLE while minimizing the adverse effects of glucocorticoids (1–4). This study investigated patterns of immunomodulatory medication use in youth with newly diagnosed SLE. We found that hydroxychloroquine use was common but not universal, and that prescription immunosuppressant use was low during the first year of care for youth with SLE.
Specifically, we found that only 55% of the cohort filled a prescription for hydroxychloroquine in the first 3 months after diagnosis, and only 69% obtained the medication by 1 year. Hydroxychloroquine has been shown to prevent accrual of organ damage and potentially decrease morbidity (12). Its use is indicated for almost all children with SLE; an international multispecialty task force urged consideration of antimalarial use irrespective of other treatment, and some task force members stated that treatment should be universal unless contraindicated (8). The proportion of youth filling a prescription for hydroxychloroquine, therefore, is grossly low.
In addition to suboptimal rates of hydroxychloroquine use, we found that prescription immunosuppressant use was notably low during the first year of care for youth with SLE. Only 16% of the study cohort filled a prescription for an immunosuppressant in the first 3 months after diagnosis, and only 34% did so within the first year. While we had limited clinical information with which to determine which patients in the cohort required immunosuppressive treatment, 12% of the cohort had seizure/stroke disorder, and 25% had nephritis within 12 months of the index date. Patients with renal and/or CNS disease typically present with these features within the first year following diagnosis and have high risk for rapid accrual of organ damage, indicating a need for early treatment with immunosuppressants (3,9). Although the proportion of subjects filling an immunosuppressant prescription in the first year mirrored those with renal and/or CNS disease in our cohort, we expect that a portion of those with other SLE manifestations, such as arthritis, cytopenias, and cutaneous disease, also require immunosuppressants. Furthermore, additional analysis confirmed that only 60% of subjects with nephritis filled a prescription for hydroxychloroquine and/or an immunosuppressant medication within 12 months. Therefore, our findings suggest that the rate of immunosuppressant use is lower than expected.
This study also found that youth with disease onset as young adults had fewer immunosuppressant fills compared to those with childhood-onset disease. This finding may reflect greater disease activity in the childhood-onset group, although we could not measure this result directly in the claims data, and the effect was independent of major organ disease (CNS or nephritis). Our results highlight potential vulnerability of young adults with new-onset lupus for suboptimal medication use, and further study of this group is warranted.
The low rate of immunomodulatory use could be due to suboptimal physician treatment practices and/or poor patient adherence to filling prescribed medication. Although little is known about the former, we interestingly found that an increasing year of index date was independently associated with both decreased time to fill and increased odds of fill within 12 months of diagnosis. Encouragingly, this finding may represent the anticipated cohort effect due to increasing uptake of evidence for immunomodulatory treatment effectiveness during the study period. Regarding medication adherence, however, prior studies have demonstrated poor rates in the SLE population. Most studies report that >50% of patients with SLE are nonadherent to treatment, presumably varying with cohort characteristics and method of measurement (13–15). There are 2 etiologies of medication nonadherence: some patients never get medication, and others, while in possession of medication, do not properly take it. The latter is a known issue based on high rates of medication nonadherence as documented by self-reports and electronic monitoring methods. The current study explores the former using pharmacy refill data and demonstrates that a notable proportion of patients never obtain immunomodulatory medication. This finding suggests that interventions to increase prescription fills in the year following diagnosis would be merited.
In adults with SLE, factors that are associated with increased medication nonadherence include depression, rural residence, being single, low educational level, the presence of other comorbidities, limited comprehension of physician instructions, having to take medication more than once daily, side effects experienced, dissatisfaction with treatment, and better physical health (15). However, in youth with SLE, further research is needed to identify factors associated with decreased prescription use.
Several study limitations should be mentioned. First, the cohort included only privately insured patients. Results do not necessarily apply to Medicaid-enrolled or uninsured patients. Second, the cohort does not reflect the whole population with regard to race/ethnicity because a disproportionately low percentage of African American and Latino American youth with SLE were in this cohort. The underrepresentation of racial/ethnic minorities may bias interpretation of these data, given associations with increased disease severity and mortality in minority populations with SLE (4). Third, the prescription fill data may overestimate medication use because youth may fill the prescription but not take the medication. Conversely, we were unable to accurately assess intravenous medication use. Therefore, members of this cohort may have received treatment not captured by prescription fill data. Fourth, the data permit limited assessment of SLE disease severity/activity and time course (besides the presence/absence of nephritis and CNS disease). Although hydroxychloroquine was indicated for the entire cohort, we could not determine for how many patients immunosuppressants were indicated during the observation period. However, our sensitivity analysis of the subcohort with a firm SLE diagnosis comprising 3 diagnosis codes within the first year found that prescription fill rates were comparable to those of the full cohort. Finally, we could not determine the time from SLE diagnosis to provider prescription of immunomodulatory medication, nor could we determine the prescriber specialty. Therefore, the relative contributions of provider prescribing practices versus patient adherence to the observed low rates of immunomodulatory medication fills remains unclear.
Despite these limitations, there are important implications of the study findings. The use of glucocorticoid-sparing immunomodulatory medication among youth with newly diagnosed SLE is lower than evidence-based practice would suggest is desirable. Further work is needed to better characterize physician prescribing practices and medication adherence in this population. This work will help identify factors contributing to suboptimal use and inform development of targeted interventions to improve health outcomes for youth with SLE.
Supplementary Material
SIGNIFICANCE & INNOVATIONS.
This study examined glucocorticoid-sparing immunomodulatory treatment patterns in the first year of care for youth with SLE, who are at high risk for early onset of major organ involvement and subsequent damage due to disease activity and glucocorticoid treatment.
The findings indicated that hydroxychloroquine use is prevalent, although not universal, and that prescription immunosuppressant use is low during the first year of care for youth with SLE.
Multivariable analysis showed that those with adult-onset (versus childhood-onset) disease were less likely to fill an immunomodulatory medication by 12 months. No other statistically significant and clinically relevant associations were found between demographic/disease characteristics and time to prescription fill in the first year or prescription fill at any time after the index date.
ACKNOWLEDGMENTS
The authors thank the Childhood Arthritis and Rheumatology Research Alliance (CARRA), and the ongoing Arthritis Foundation financial support of CARRA.
Supported by the Childhood Arthritis and Rheumatology Research Alliance (Large Grant award and Publication Grant award). Dr. Knight’s work was supported by the Rheumatology Research Foundation and the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Footnotes
No potential conflicts of interest relevant to this article were reported.
Study conception and design. Davis, Klein-Gitelman, Knight.
Acquisition of data. Chang, Faerber, Katcoff, Knight.
Analysis and interpretation of data. Davis, Chang, Shapiro, Klein-Gitelman, Faerber, Katcoff, Cidav, Mandell, Knight.
REFERENCES
- 1.Brunner HI, Gladman DD, Ibañez D, Urowitz MD, Silverman ED. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum 2008;58:556–62. [DOI] [PubMed] [Google Scholar]
- 2.Hersh AO, Trupin L, Yazdany J, Panopalis P, Julian L, Katz P, et al. Childhood-onset disease as a predictor of mortality in an adult cohort of patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2010;62:1152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiraki LT, Benseler SM, Tyrrell PN, Herbert D, Harvey E, Silverman ED. Clinical and laboratory characteristics and long-term outcome of pediatric systemic lupus erythematosus: a longitudinal study. J Pediatr 2008;152:550–6. [DOI] [PubMed] [Google Scholar]
- 4.Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum 2006;54:2550–7. [DOI] [PubMed] [Google Scholar]
- 5.Hanly JG, Thompson K, Skedgel C. Identification of patients with systemic lupus erythematosus in administrative healthcare databases. Lupus 2014;23:1377–82. [DOI] [PubMed] [Google Scholar]
- 6.Hiraki LT, Feldman CH, Liu J, Alarcón GS, Fischer MA, Winkelmayer WC, et al. Prevalence, incidence, and demographics of systemic lupus erythematosus and lupus nephritis from 2000 to 2004 among children in the US Medicaid beneficiary population. Arthritis Rheum 2012;64:2669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus 2010;19:741–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Vollenhoven RF, Mosca M, Bertsias G, Isenberg D, Kuhn A, Lerstrøm K, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis 2014;73:958–67. [DOI] [PubMed] [Google Scholar]
- 9.Bertsias GK, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JH, et al. Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis 2012;71:1771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jette N, Reid AY, Quan H, Hill MD, Wiebe S. How accurate is ICD coding for epilepsy? Epilepsia 2010;51:62–9. [DOI] [PubMed] [Google Scholar]
- 11.Golomb MR, Garg BP, Saha C, Williams LS. Accuracy and yield of ICD-9 codes for identifying children with ischemic stroke. Neurology 2006;67:2053–5. [DOI] [PubMed] [Google Scholar]
- 12.Fessler BJ, Alarcón GS, McGwin G Jr, Roseman J, Bastian HM, Friedman AW, et al. Systemic lupus erythematosus in three ethnic groups. XVI: association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum 2005;52:1473–80. [DOI] [PubMed] [Google Scholar]
- 13.Ting TV, Kudalkar D, Nelson S, Cortina S, Pendl J, Budhani S, et al. Usefulness of cellular text messaging for improving adherence among adolescents and young adults with systemic lupus erythematosus. J Rheumatol 2011;39:174–9. [DOI] [PubMed] [Google Scholar]
- 14.Davis AM, Graham TB, Zhu Y, McPheeters ML. Depression and medication non-adherence in childhood-onset systemic lupus erythematosus. Lupus 2018;27:1532–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehat P, Atiquzzaman M, Esdaile JM, Aviña-Zubieta A, De Vera MA. Medication nonadherence in systemic lupus erythematosus: a systematic review. Arthritis Care Res (Hoboken) 2017;69:1706–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


