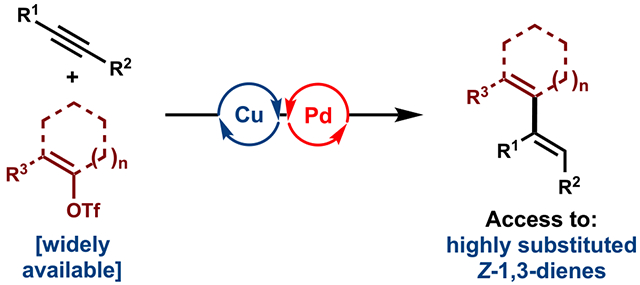
Abstract
Conjugated dienes are versatile building blocks and prevalent substructures in synthetic chemistry. Herein, we report a method for the stereoselective hydroalkenylation of alkynes, utilizing readily available enol triflates. We leveraged an in situ-generated and geometrically pure vinyl-Cu(I) species to form the Z,Z- or Z,E-1,3-dienes in excellent stereoselectivity and yield. This approach allowed for the synthesis of highly substituted Z-dienes, including pentasubstituted 1,3-dienes, which are difficult to prepare by existing approaches.
Graphical Abstract
Conjugated dienes are prevalent structural elements present in numerous biologically active small molecules1–3 and constitute a major feedstock for industrial polymer production.4,5 Because of their unique chemical reactivity, 1,3-dienes are versatile building blocks with the potential to form new C–C and C–heteroatom bonds at all four encompassing carbons.6 The utility of conjugated dienes has been demonstrated in a variety of critical synthetic processes, including cycloadditions,7,8 hydrofunctionalizations,9–13 and difunctionalizations.14–17 The stereochemical outcome of these methods is typically influenced by the olefin geometry of the 1,3-diene substrate.8,14,17 Accordingly, methods to access substituted 1,3-dienes in a stereoselective manner are paramount for their use in fine chemical synthesis.6,18 While various methods exist for the synthesis of E,E-dienes,18–20 a general, highly stereoselective process to produce Z-dienes is desirable.
Due to the utility of 1,3-dienes in organic synthesis, a variety of strategies to access these compounds have been developed.6,18 Olefination of carbonyl compounds with stoichiometric allyl nucleophiles has been widely employed in the synthesis of conjugated dienes;21–28 however, the products are generally obtained as inseparable E/Z mixtures (Figure 1A).28,29 Although considerable advances have been made toward stereoselective olefination of carbonyl substrates, most methods to access 1,3-dienes result in the E,E-isomer.20,26,28,30 To avoid the formation of isomeric product mixtures, transition-metal-catalyzed cross-coupling utilizing preformed organometallic reagents and vinyl (pseudo)halides has emerged as a practical route to stereoselectively synthesize dienes (Figure 1B).31–36 In these processes, the geometry of the diene product is dictated by the stereochemistry of the coupling partners. Complementary approaches to prepare 1,3-dienes, including C–H activation of olefin starting materials,37,38 rearrangements of allenes or alkynes,39 and ene-yne metathesis of acyclic precursors,40,41 have also been developed.42,43
Figure 1.

(A) Olefination employing stoichiometric allylation reagents. (B) Cross-coupling of vinyl-metal species with stereodefined coupling partners. (C) Proposed dual CuH- and Pd-catalyzed alkyne hydroalkenylation. (D) Potential undesired reactions.
Our group and others have demonstrated the potential of CuH-catalyzed hydrofunctionalization reactions to enable unsaturated substrates to serve as surrogates for preformed organometallic reagents.44,45 Hydrocupration of an olefinic precursor results in a catalytically generated Cu(I) species [I (Figure 1C)] that can engage in bond-forming reactions with a range of electrophiles, including carbonyls,46,47 heterocycles,48,49 and LPd(II) complexes.50–53 However, the equivalent transformations employing alkyne pronucleophiles have been underexplored.54–62 Recently, we developed a dual CuH- and Pd-catalyzed hydroalkenylation of olefins (Figure 1C), employing widely available enol sulfonates to synthesize highly substituted α-chiral olefins.53 We reasoned that an analogous approach to generate the otherwise elusive Z,E- and Z,Z-1,3-dienes could be realized by exploiting the syn-selective hydrocupration of alkynes and the rapid transmetalation of a vinyl-Cu(I) species (II) with an LPd(II)-alkenyl complex.63 We anticipated several specific challenges for the dual-catalytic alkyne hydroalkenylation (Figure 1D). It was evident that the 1,3-diene products are competent substrates for hydrofunctionalization reactions. Subsequent reduction,44,45 isomerization, or oligomerization reactions of the conjugated diene product were also conceivable. Hydrolysis or reduction of the enol triflate to generate the corresponding olefin is also possible. We reasoned that tuning the rates of the catalytic cycles (e.g., hydrocupration, oxidative addition, and transmetalation) would be crucial to suppress off-cycle reactivity and enabling construction of the C–C bond at the resulting diene 2 position.53
We focused on developing a set of dual-catalytic conditions for the stereoselective alkyne hydroalkenylation, using 1-phenyl-1-hexyne (1a) as a model substrate and 1-cyclohexenyl trifluoromethanesulfonate (2a) as the alkenyl coupling partner (Table 1).64 Utilizing our previously described reaction conditions for olefin hydroalkenylation,53 we observed the Z-diene (3a) in moderate yield (entry 1, 33% yield, as determined by 1H NMR). Contrary to our olefin hydroalkenylation process, which was ineffective at room temperature,53 we found that conducting the alkyne hydroalkenylation at room temperature resulted in moderate yield of 3a (entry 3). As hydrocupration of a vinyl arene and 1a readily occurs at room temperature, this dichotomy may arise from the more facile transmetalation of a vinyl-Cu(I) species (II) to a LPd(II) complex, relative to I.63 However, 45 °C was identified as the optimal temperature for the formation of the diene product (entries 1–4). Although similar results were seen with a vinyl bromide (2b), as compared with 2a, the use of the corresponding vinyl iodide (2c) or enol tosylate (2d) was less effective (entries 5–7) and resulted primarily in reduction of the alkenyl coupling partner. Increasing the reaction concentration resulted in an improved yield of 3a (entries 8 and 9). Examination of alternative solvents, ancillary ligands for Cu or Pd, and Cu salts did not improve the yield of 3a (see Tables SI1 and SI2 for details). When the reaction was performed in the absence of a Pd or Cu catalyst, a trace of product or no product was observed, respectively (see Table SI3).
Table 1.
Optimization of the Stereoselective Hydroalkenylation of Alkynesa

|
Reaction conditions: 0.2 mmol of alkyne (1a), alkenyl coupling partner (2) (0.3 mmol, 1.5 equiv). Yields were determined by 1H NMR spectroscopy of the crude reaction mixtures, using 1,3,5-trimethoxybenzene as an internal standard.
With our optimized protocol for the synthesis of 1,3-dienes, we sought to explore the range of alkynes that could be utilized in this transformation (Scheme 1). When 2a was employed with 1-phenyl-1-hexyne or diphenyl acetylene, the corresponding dienes (3a and 3i) were accessed in good yield with excellent Z selectivity (>20:1 Z:E). A variety of heteroarylcontaining Z-dienes could be prepared with excellent selectivities, including a thiophene (3b), a quinoline (3d), a pyrrole (3h), and an indole (3f). A thiazole containing diene (3g) was the only product for which the E-isomer was detected (2.3:1 Z:E). An ester (3c) was tolerated under the reaction conditions; however, a diethyl acetal was hydrolyzed to the corresponding aldehyde (3e) upon isolation. When unsymmetrical diaryl alkynes were subjected to the reaction conditions, regioisomeric mixtures of diene products were observed (3j and 3k). An electron-deficient 1-aryl alkyne resulted in the expected 1,3-diene (3k) in conjunction with isomer 3l. This isomerized product may arise from a subsequent hydrocupration, to form an allyl-Cu(I) species, followed by β-hydride elimination. A series of 1-silyl-substituted acetylenes, including -TMS and -TIPS, did not result in the diene adduct (3), although a -TES-substituted butadiene (3m) was formed as a minor product, favoring hydride addition β to silicon.65 This regiochemical reversal is likely due to stereoelectronic effects exerted by the nearby silicon atom, increasing cationic character at the β position.66 When a 1,2-dialkyl alkyne, 4-octyne, was employed as a substrate in the alkyne hydroalkenylation process, only reduction of 2a was observed (see Scheme SI1). This result can possibly be attributed to the more challenging hydrocupration of 1,2-dialkyl alkynes, relative to 1-aryl-2-alkyl alkynes.54
Scheme 1. Substrate Scope of Alkyne Coupling Partnersa.

aAll yields represent the average of at least two isolated yields of reactions conducted with 0.5 mmol of alkyne (1); the corresponding enol triflate was used unless otherwise noted. The yields in parentheses were determined by 1H NMR spectroscopy of the crude reaction mixtures using 1,1,2,2-tetrachloroethane as an internal standard. The position of the minor regioisomer is denoted by a 1. bThe corresponding propargylic diethyl acetal was utilized. cIsolated as a 2.3:1 Z:E mixture. dIsolated separately from 3k.
The scope with respect to the enol triflate coupling partner was evaluated with a selection of differentially substituted alkynes, as depicted in Scheme 2. A range of alkenyl groups, including benzo-fused (3q), heterocyclic (3p and 3r), and acyclic (3u and 3v) groups, could be appended to the 2 position of the resulting diene. Pentasubstituted 1,3-dienes, such as 3n and 3u, could be prepared with high yield and selectivity (>20:1 Z:E). A variety of functional groups were tolerated in this process, including nitriles (3o and 3q), carbamates (3o and 3x), a tertiary amine (3r), and a ketal (3w). While an alkyne with an unprotected alcohol was a suitable substrate (3s), the corresponding benzyl ether resulted in the 1,3-diene product in improved yield (3t), with 42% and 74% yields, respectively. Despite their increased steric hindrance, acyclic enol triflates enabled access to 3u and 3v in excellent yield and selectivity. Heterocycles such as quinoline (3o), pyridine (3u and 3x), pyrimidine (3v), and indole (3p) were effectively converted to the corresponding Z-dienes. Pharmaceutical derivatives, including a substituted loratadine (3x) and a steroid-derived triene (3y), are readily prepared via this method. A sterically congested α-spirocyclic vinyl bromide resulted in a 4:1 regioisomeric mixture of products (3w), which is in accord with our previous observations.53 Despite olefins and dienes being suitable substrates for hydrofunctionalization reactions, no subsequent dimerization or oligomerization of the products was observed.
Scheme 2. Substrate Scope of Stereoselective Hydroalkenylation of Alkynes with Various Enol Triflate Coupling Partnersa.

aAll yields represent the average of at least two isolated yields with 0.5 mmol of alkyne (1); the corresponding enol triflate was used unless otherwise noted. The position of the minor regioisomer is denoted by a 1. bThe corresponding vinyl bromide was used.
To further demonstrate the utility of this alkyne hydroalkenylation method, we conducted the process on a gram scale (eq 1). Using a commercially available alkyne (1b) and enol triflate (2a), diene 3i could be isolated in 86% yield and high selectivity (>20:1 Z:E).
 |
(1) |
In summary, we have developed a highly stereoselective process to prepare substituted Z-1,3-dienes, employing widely available alkynes and enol triflates. Instead of relying on conferring the olefin geometry of the starting material to the product, we leverage an in situ-generated and geometrically pure vinyl-Cu(I) species to access exclusively Z-conjugated dienes. The reaction conditions tolerated numerous important functional groups and enabled the synthesis of highly substituted 1,3-dienes, including pentasubstituted dienes, which are difficult to prepare by complementary strategies.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the Arnold and Mabel Beckman Foundation for a postdoctoral fellowship to A.W.S., the National Science Foundation Graduate Research Fellowship Program (1122374, to J.L.K.), the National Institutes of Health (R35-GM122483 and Diversity Supplement Fellowship R35-GM122483-03S to J.L.K.), Dalian Polytechnic University (DPU, to C.-J.H.), and MIT UROP Direct Funding to A.Z.N. The authors thank Millipore-Sigma for the generous donation of biarylphosphine ligands. The authors are grateful to Drs. Veronika Kottisch, Simon Rössler, and Christine Nguyen (Massachusetts Institute of Technology, Cambridge, MA) for advice on the preparation of this manuscript.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.1c03324.
Experimental details and characterization of the products and starting materials (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.orglett.1c03324
The authors declare no competing financial interest.
Contributor Information
Chuan-Jin Hou, Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States.
Alexander W. Schuppe, Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States.
James Levi Knippel, Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States.
Anton Z. Ni, Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States
Stephen L. Buchwald, Department of Chemistry, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, United States.
REFERENCES
- (1).Rychnovsky SD Oxo Polyene Macrolide Antibiotics. Chem. Rev 1995, 95, 2021–2040. [Google Scholar]
- (2).Zotchev SB Polyene Macrolide Antibiotics and Their Applications in Human Therapy. Curr. Med. Chem 2003, 10, 211–223. [DOI] [PubMed] [Google Scholar]
- (3).Thirsk C; Whiting A Polyene Natural Products. J. Chem. Soc., Perkin Trans.1 2002, 999–1023. [Google Scholar]
- (4).White WC Butadiene Production Process Overview. Chem.-Biol. Interact 2007, 166, 10–14. [DOI] [PubMed] [Google Scholar]
- (5).Thiele SK-H; Wilson DR Alternate Transition Metal Complex Based Diene Polymerization. J. Macromol. Sci., Polym. Rev 2003, 43, 581–628. [Google Scholar]
- (6).De Paolis M; Chataigner I; Maddaluno J Recent Advances in Stereoselective Synthesis of 1,3-Dienes. In Stereoselective Alkene Synthesis; Wang J, Ed.; Topics in Current Chemistry; Springer: Berlin, 2012; pp 87–146. [DOI] [PubMed] [Google Scholar]
- (7).Nicolaou KC; Snyder SA; Montagnon T; Vassilikogiannakis G The Diels–Alder Reaction in Total Synthesis. Angew. Chem., Int. Ed 2002, 41, 1668–1698. [DOI] [PubMed] [Google Scholar]
- (8).Corey EJ Catalytic Enantioselective Diels–Alder Reactions: Methods, Mechanistic Fundamentals, Pathways, and Applications. Angew. Chem., Int. Ed 2002, 41, 1650–1667. [DOI] [PubMed] [Google Scholar]
- (9).Perry GJP; Jia T; Procter DJ Copper-Catalyzed Functionalization of 1,3-Dienes: Hydrofunctionalization, Borofunctionalization, and Difunctionalization. ACS Catal. 2020, 10, 1485–1499. [Google Scholar]
- (10).Huang L; Arndt M; Gooßen K; Heydt H; Gooßen LJ Late Transition Metal-Catalyzed Hydroamination and Hydroamidation. Chem. Rev 2015, 115, 2596–2697. [DOI] [PubMed] [Google Scholar]
- (11).McNeill E; Ritter T 1,4-Functionalization of 1,3-Dienes With Low-Valent Iron Catalysts. Acc. Chem. Res 2015, 48, 2330–2343. [DOI] [PubMed] [Google Scholar]
- (12).Li MML; Cheng L; Xiao L-J; Xie J-H; Zhou Q-L Palladium-Catalyzed Asymmetric Hydrosulfonylation of 1,3-Dienes with Sulfonyl Hydrazides. Angew. Chem., Int. Ed 2021, 60, 2948–2051. [DOI] [PubMed] [Google Scholar]
- (13).Cheng L; Li M-M; Xiao L-J; Xie J-H; Zhou Q-L Nickel(0)-Catalyzed Hydroalkylation of 1,3-Dienes with Simple Ketones. J. Am. Chem. Soc 2018, 140, 11627–11630. [DOI] [PubMed] [Google Scholar]
- (14).Xiong Y; Sun Y; Zhang G Recent Advances on Catalytic Asymmetric Difunctionalization of 1,3-Dienes. Tetrahedron Lett. 2018, 59, 347–355. [Google Scholar]
- (15).Smith KB; Brown MK Regioselective Arylboration of Isoprene and Its Derivatives by Pd/Cu Cooperative Catalysis. J. Am. Chem. Soc 2017, 139, 7721–7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Smith KB; Huang Y; Brown KM Copper-Catalyzed Heteroarylboration of 1,3-Dienes with 3- Bromopyridines: A cine Substitution. Angew. Chem., Int. Ed 2018, 57, 6146–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wu X; Gong L-Z Palladium(0)-Catalyzed Difunctionalization of 1,3-Dienes: From Racemic to Enantioselective. Synthesis 2019, 51, 122–134. [Google Scholar]
- (18).Soengas RG; Rodríguez-Solla H Modern Synthetic Methods for the Stereoselective Construction of 1,3-Dienes. Molecules 2021, 26, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Wen Z-K; Xu Y-H; Loh T-P Palladium(II)-catalyzed cross-coupling of simple alkenes with acrylates: A direct approach to 1,3-dienes through C–H activation. Chem. Sci 2013, 4, 4520–4524. [Google Scholar]
- (20).Dong D-J; Li H-H; Tian S-K A highly tunable stereoselective olefination of semistabilizedtriphenylphosphonium ylides with N-sulfonyl imines. J. Am. Chem. Soc 2010, 132, 5018–5020. [DOI] [PubMed] [Google Scholar]
- (21).Wittig G; Geissler G Zur Reaktionsweise Des Pentaphenyl-Phosphors Und Einiger Derivate. Justus Liebigs Ann. Chem 1953, 580, 44–57. [Google Scholar]
- (22).Maryanoff BE; Reitz AB The Wittig Olefination Reaction and Modifications Involving Phosphoryl-Stabilized Carbanions. Stereochemistry, Mechanism, and Selected Synthetic Aspects. Chem. Rev 1989, 89, 863–927. [Google Scholar]
- (23).Julia M; Paris J-M Syntheses a l’aide de Sulfones v(+)-Methode de SyntheseGenerale de Doubles Liaisons. Tetrahedron Lett. 1973, 14, 4833–4836. [Google Scholar]
- (24).Blakemore PR The Modified Julia Olefination: Alkene Synthesis via the Condensation of MetallatedHeteroarylalkylsulfones with Carbonyl Compounds. J. Chem. Soc., Perkin Trans. 1 2002, 2563–2585. [Google Scholar]
- (25).Chatterjee B; Bera S; Mondal D Julia–Kocienski Olefination: A Key Reaction for the Synthesis of Macrolides. Tetrahedron: Asymmetry 2014, 25, 1–55. [Google Scholar]
- (26).Vedejs E; Fang HW An E-Selective 1,3-Diene Synthesis from Moderated Ylides and Aldehydes. J. Org. Chem 1984, 49, 210–212. [Google Scholar]
- (27).White JD; Jensen MS Synthesis of 1,3-Dienes of (E,Z) Configuration by a Three-Component Coupling Strategy. Tetrahedron 1995, 51, 5743–5756. [Google Scholar]
- (28).Billard F; Robiette R; Pospíšil J Julia–Kocienski Reaction-Based 1,3-Diene Synthesis: Aldehyde-Dependent (E, E/E, Z)-Selectivity. J. Org. Chem 2012, 77, 6358–6364. [DOI] [PubMed] [Google Scholar]
- (29).Hubert P; Seibel E; Beemelmanns C; Campagne J-M; Figueiredo R. M. de. Stereoselective Construction of (E,Z)-1,3-Dienes and Its Application in Natural Product Synthesis. Adv. Synth. Catal 2020, 362, 5532–5575. [Google Scholar]
- (30).McNulty J; McLeod D; Das P; Zepeda-Velázquez C Wittig Reactions of Trialkylphosphine-Derived Ylides: New Directions and Applications in Organic Synthesis. Phosphorus, Sulfur Silicon Relat. Elem 2015, 190, 619–632. [Google Scholar]
- (31).Wang G; Mohan S; Negishi E Highly selective synthesis of conjugated dienoic and trienoic esters via alkyne elementometalation-Pd-catalyzed cross-coupling. Proc. Natl. Acad. Sci. U. S. A 2011, 108, 11344–11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Kurosawa F; Nakano T; Soeta T; Endo K; Ukaji Y (Z)-Selective enol triflation of α-alkoxyacetoaldehydes: Application to synthesis of (Z)-allylic alcohols via cross-coupling reaction and [1,2]-Wittig rearrangement. J. Org. Chem 2015, 80, 5696–5703. [DOI] [PubMed] [Google Scholar]
- (33).McAdam CA; McLaughlin MG; Cook MJ An alkyne hydrosilylation–Hiyama coupling approach to highly functionalized 1,3-dienes. Org. Chem. Front 2015, 2, 510–514. [Google Scholar]
- (34).Hornillos V; Giannerini M; Vila C; Fañanás-Mastral M; Feringa BL Direct catalytic cross-coupling of alkenyllithium compounds. Chem. Sci 2015, 6, 1394–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Fiorito D; Folliet S; Liu Y; Mazet C A general nickel-catalyzed Kumada vinylation for the preparation of 2-substituted 1,3-dienes. ACS Catal. 2018, 8, 1392–1398. [Google Scholar]
- (36).Scaringi S; Mazet C Kinetically Controlled Stereoselective Access to Branched 1,3-Dienes by Ru-Catalyzed Remote Conjugative Isomerization. ACS Catal. 2021, 11, 7970–7977. [Google Scholar]
- (37).Wen Z-K; Xu Y-H; Loh T-P Palladium(II)-catalyzed cross-coupling of simple alkenes with acrylates: A direct approach to 1,3-dienes through C–H activation. Chem. Sci 2013, 4, 4520–4524. [Google Scholar]
- (38).Liu M; Yang P; Karunananda MK; Wang Y; Liu P; Engle KM C(alkenyl)-H activation via six-membered palladacycles: Catalytic 1,3-diene synthesis. J. Am. Chem. Soc 2018, 140, 5805–5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Horii S; Ishimaru I; Ukaji Y; Inomata K Stereoselective one-pot 1,4-elimination and the [1,2]-Wittig rearrangement of (E)-δ-(arylmethoxy or 3-silyl-2-propynyloxy)-substituted allylic sulfones. Chem. Lett 2011, 40, 521–523. [Google Scholar]
- (40).Bauer RA; Diblasi CM; Tan DS The tert-butylsulfinamide lynchpin in transition-metal-mediated multiscaffold library synthesis. Org. Lett 2010, 12, 2084–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Dolan MA; Dixon ADC; Chisholm JD; Clark DA Ruthenium dihydride complexes as enyne metathesis catalysts. Tetrahedron Lett. 2018, 59, 4471–4474. [Google Scholar]
- (42).Dang HT; Nguyen VD; Haug GC; Vuong NTH; Arman HD; Larionov OV Z -Selective Dienylation Enables Stereodivergent Construction of Dienes and Unravels a Ligand-Driven Mechanistic Dichotomy. ACS Catal. 2021, 11, 1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Olivares AM; Weix DJ Multimetallic Ni- and Pd-catalyzed Cross-Electrophile Coupling to Form Highly Substituted 1,3-Dienes. J. Am. Chem. Soc 2018, 140, 2446–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Pirnot MT; Wang Y-M; Buchwald SL Copper Hydride Catalyzed Hydroamination of Alkenes and Alkynes. Angew. Chem., Int. Ed 2016, 55, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Liu RY; Buchwald SL CuH-Catalyzed Olefin Functionalization: From Hydroamination to Carbonyl Addition. Acc. Chem. Res 2020, 53, 1229–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Li C; Shin KY; Liu R; Buchwald SL Engaging Aldehydes in CuH-Catalyzed Reductive Coupling Reactions: Stereoselective Allylation with Unactivated 1,3-Diene Pronucleophiles. Angew. Chem., Int. Ed 2019, 58, 17074–17080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Li C; Liu RY; Jesikiewicz LT; Yang Y; Liu P; Buchwald SL CuH-Catalyzed Enantioselective Ketone Allylation with 1,3-Dienes: Scope, Mechanism, and Applications. J. Am. Chem. Soc 2019, 141, 5062–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Gribble MW; Liu RY; Buchwald SL Evidence for Simultaneous Dearomatization of Two Aromatic Rings under Mild Conditions in Cu(I)-Catalyzed Direct Asymmetric Dearomatization of Pyridine. J. Am. Chem. Soc 2020, 142, 11252–11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Knippel JL; Ye Y; Buchwald SL Enantioselective C2-Allylation of Benzimidazoles Using 1,3-Diene Pronucleophiles. Org. Lett 2021, 23, 2153–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Friis SD; Pirnot MT; Buchwald SL Asymmetric Hydroarylation of Vinylarenes Using a Synergistic Combination of CuH and Pd Catalysis. J. Am. Chem. Soc 2016, 138, 8372–8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Schuppe AW; Borrajo-Calleja GM; Buchwald SL Enantioselective Olefin Hydrocyanation without Cyanide. J. Am. Chem. Soc 2019, 141, 18668–18672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Lu Z; Buchwald SL Enantioselective Preparation of Arenes with β-Stereogenic Centers: Confronting the 1,1-Disubstituted Olefin Problem Using CuH/Pd Cooperative Catalysis. Angew. Chem., Int. Ed 2020, 59, 16128–16132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Schuppe AW; Knippel JL; Borrajo-Calleja GM; Buchwald SL Enantioselective Hydroalkenylation of Olefins with Enol Sulfonates Enabled by Dual Copper Hydride and Palladium Catalysis. J. Am. Chem. Soc 2021, 143, 5330–5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Shi S-L; Buchwald SL Copper-catalysed selective hydroamination reactions of alkynes. Nat. Chem 2015, 7, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Rao SA; Periasamy M Hydrocupration of Alkynes: A Simple Synthesis of (E,E)-1,3-Dienes. J. Chem. Soc., Chem. Commun 1987, 495–496. [Google Scholar]
- (56).Uehling MR; Suess AM; Lalic G Copper-Catalyzed Hydroalkylation of Terminal Alkynes. J. Am. Chem. Soc 2015, 137, 1424. [DOI] [PubMed] [Google Scholar]
- (57).Suess AM; Uehling MR; Kaminsky W; Lalic G Mechanism of Copper-Catalyzed Hydroalkylation of Alkynes: An Unexpected Role of Dinuclear Copper Complexes. J. Am. Chem. Soc 2015, 137, 7747. [DOI] [PubMed] [Google Scholar]
- (58).Kortman GD; Hull KL Copper-Catalyzed Hydroarylation of Internal Alkynes: Highly Regio- and Diastereoselective Synthesis of 1,1-Diaryl, Trisubstituted Olefins. ACS Catal. 2017, 7, 6220–6224. [Google Scholar]
- (59).Cheng L-J; Mankad NP Cu-Catalyzed Hydrocarbonylative C-C Coupling of Terminal Alkynes with Alkyl Iodides. J. Am. Chem. Soc 2017, 139, 10200–10203. [DOI] [PubMed] [Google Scholar]
- (60).Hazra A; Chen J; Lalic G Stereospecific Synthesis of E-Alkenes through Anti-Markovnikov Hydroalkylation of Terminal Alkynes. J. Am. Chem. Soc 2019, 141, 12464–12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Gao D-W; Gao Y; Shao H; Qiao T-Z; Wang X; Sanchez BB; Chen JS; Liu P; Engle KM Cascade CuH-catalysed conversion of alkynes into enantioenriched 1,1-disubstituted products. Nat. Catal 2020, 3, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Hazra A; Kephart JA; Velian A; Lalic G Hydroalkylation of Alkynes: Functionalization of the Alkenyl Copper Intermediate through Single Electron Transfer Chemistry. J. Am. Chem. Soc 2021, 143, 7903–7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Garcïa-Melchor M; Braga AAC; Lledós A; Ujaque G; Maseras F Computational Perspective on Pd-Catalyzed C–C Cross-coupling Reaction Mechanisms. Acc. Chem. Res 2013, 46, 2626–2634. [DOI] [PubMed] [Google Scholar]
- (64).The use of a chiral ligand is not necessary; however, rac-L1 is not commercially available, therefore (S)-L1 was employed. See the Supporting Information for additional details.
- (65).Niljianskul N; Zhu S; Buchwald SL Enantioselective Synthesis of α-Aminosilanes by Copper-Catalyzed Hydroamination of Vinylsilanes. Angew. Chem., Int. Ed 2015, 54, 1638–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).White JM; Clark CI Stereoelectronic Effects of the Group 4 Metal Substituents in Organic Chemistry. In Topics in Stereochemistry, Vol. 22; John Wiley & Sons, 2009; pp 137–200. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


