Abstract
Background
The emphasis on better pain control with less narcotic use represents an ongoing challenge for outpatient plastic surgery procedures. Intravenous (IV) bolus opioids during surgery can lead to short-term relief, but often repeat dosing is required in the postanesthesia care unit (PACU), prolonging recovery time. The sufentanil sublingual tablet (SST) has recently shown efficacy in reducing overall opioid use and postsurgical recovery time for outpatient general surgery procedures.
Objectives
To examine the effect of SST on PACU opioid use, adverse events, and recovery time compared with traditional IV opioid drug regimens in patients undergoing aesthetic surgical procedures.
Methods
A retrospective chart review was performed on SST patients (n = 61) receiving a single 30 mcg SST 30 minutes before surgery (for short procedures) or 45 minutes before surgical extubation (longer procedures). A control group (n = 32) underwent similar surgical procedures utilizing standard IV opioid treatment without SST.
Results
Control and study groups were of similar age and sex. Procedure duration (approximately 3 hours) and intraoperative opioid administration were similar in both groups, with 92% of patients receiving SST before extubation due to the length of the case. Almost all control patients (90.6%) required rescue opioids during recovery in the PACU compared with a few SST patients (16.4%; P < 0.001), averaging 5-fold higher dosing in the control group. Recovery duration did not differ between groups as factors other than pain management and adverse events affected discharge.
Conclusions
SST substantially reduced opioid administration in the PACU for patients undergoing outpatient plastic surgery procedures.
Level of Evidence: 3

As more plastic and reconstructive surgery cases are performed on an outpatient basis, there has been a focused effort to limit the overall opioid use during and after surgery while enhancing the effectiveness of pain management strategies.1,2 Multiple cosmetic procedures, such as abdominoplasty, liposuction, and breast-lift (“mommy makeover”), are now commonly combined into a single surgery, resulting in not only longer but also more painful and invasive surgeries. Maintaining adequate postoperative analgesia to allow timely discharge of the patient becomes more challenging as surgery duration increases.
Studies suggest that inadequate postoperative pain management is common among adults.3 In a national study of more than 300 adults undergoing surgery, 86% reported postoperative pain and 75% described the pain as being moderate to severe.4 Uncontrolled postanesthesia care unit (PACU) pain is one of the main reasons for delayed discharge from the same-day surgery.5,6 Delayed discharge can limit the number of patients that can be accommodated daily, add expense for nursing care overtime, and leave the patient and their family members dissatisfied. Furthermore, postoperative pain increases morbidities and risk of chronic postsurgical pain and, subsequently, risk of opioid addiction.7-9
Typically, intravenous (IV) fentanyl or less commonly IV hydromorphone or IV morphine is administered intraoperatively and postoperatively for pain management in outpatient procedures. Most notably, IV fentanyl has a rapid onset of effect but a very short duration of action, so it often must be administered repeatedly.10 The high peak plasma concentrations following bolus administration of fentanyl can cause a variety of cardiovascular and respiratory side effects.11 Furthermore, with repeated dosing often required in the PACU, nausea and vomiting are common adverse effects that may delay recovery and discharge.12
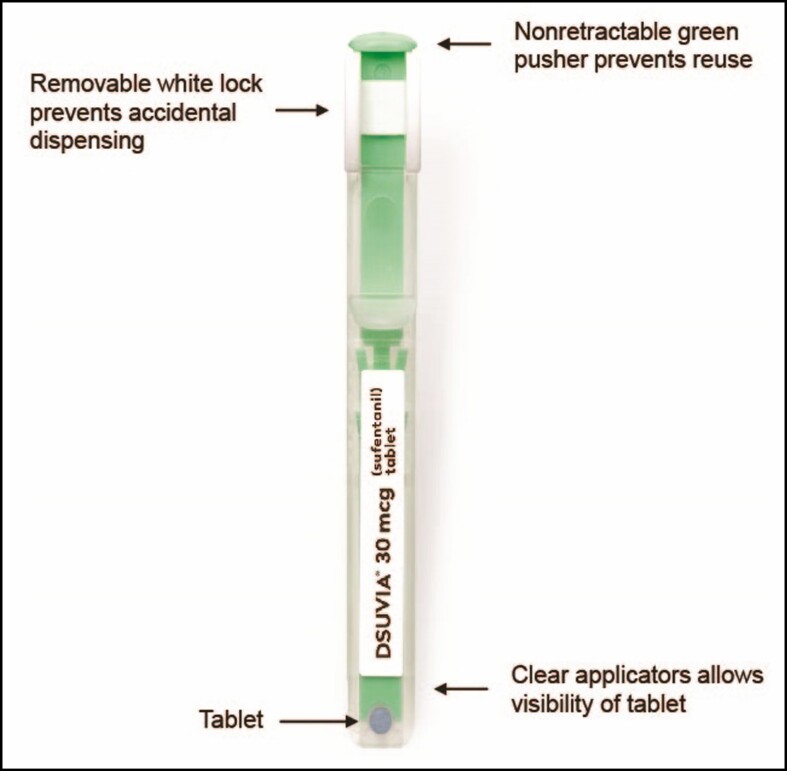
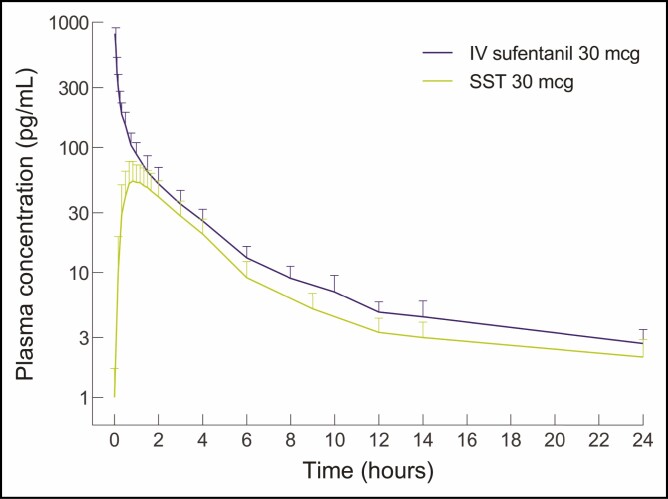
Sufentanil sublingual tablet (SST) 30 mcg (DSUVIA, AcelRx Pharmaceuticals, Inc., Hayward, CA) is an alternative novel sublingual opioid analgesic housed in a prefilled applicator (Figure 1), approximately equivalent to 5 morphine milligram equivalents (MME).13 Once administered under the tongue, the tablet typically dissolves within 5 minutes.14 Sufentanil is highly lipophilic, which allows for rapid transmucosal absorption into the sublingual tissues.15 The transit of the drug from the sublingual tissue depot to the plasma occurs over time, avoiding the high peak levels obtained with bolus IV opioids (Figure 2).16 The pharmacokinetic profile is consistent with the clinical profile, with an onset of analgesia by 15 minutes, a peak plasma concentration by 60 minutes, and a duration of action of approximately 3 hours.16,17
Figure 1.
Sufentanil sublingual tablet (SST; DSUVIA, AcelRx Pharmaceuticals, Inc., Hayward, CA) 30 mcg in a disposable single-dose applicator for sublingual administration. Reproduced with permission from AcelRx Pharmaceuticals, Inc.
Figure 2.
Plasma concentration profile of sufentanil administered as sufentanil sublingual tablet 30 mcg (SST 30 mcg; lower curve; DSUVIA, AcelRx Pharmaceuticals, Inc., Hayward, CA) or intravenous (IV) sufentanil 30 mcg (upper curve). Reproduced with permission from Fisher et al. 2018.16
Two recent studies have shown SST to be associated with reduced overall opioid use and postsurgical recovery time.18,19 A single dose of SST administered approximately 30 minutes before incision in an outpatient general surgery setting reduced the overall opioid dose (during surgery and recovery) by more than 50% and reduced recovery time by 34% compared with controls undergoing similar short-duration (<1 hour) surgeries.18 In a second larger study, when dosed either preoperatively or intraoperatively among inpatients and outpatients matched across a wide variety of surgeries, patients receiving SST required 50% less opioid dosing in the PACU and a 14-minute shorter recovery duration than controls.19 Other studies have shown that SST, unlike traditional IV opioids, has minimal effects on cognitive and respiratory function, which can also influence recovery times in ambulatory settings.20,21
SST is approved for the management of acute pain in adults in medically supervised settings.22 It has not yet been studied among plastic surgery patients undergoing general anesthesia for long-duration procedures. In this study, SST was evaluated in an outpatient plastic surgery setting for its ability to minimize PACU opioid dosing in order to possibly reduce PACU adverse events and shorten PACU recovery times compared with matched historical controls.
METHODS
Following IRB approval (WIRB-Copernicus Group; WCG IRB #20210729), a retrospective chart review was performed on patients who underwent general anesthesia for plastic surgery procedures at our ambulatory surgery center between January 2020 and March 2021, with control patients treated within the first 6 months and SST-treated patients mainly falling into the remaining 9-month period. Written consent was provided, by which the patients agreed to the use and analysis of their data.
The standard anesthetic regimen for all patients (controls and SST-treated) consisted of administration of midazolam 2 mg IV preoperatively and induction of anesthesia using IV propofol and IV fentanyl. Sevoflurane and nitrous oxide were utilized to maintain anesthesia for the duration of the procedure. Additional IV opioids were used as needed intraoperatively and postoperatively. All patients had local field block with bupivacaine or lidocaine. Patients undergoing breast procedures had pectoral I and II blocks.23 Prophylactic antiemetics were provided in both groups, with additional antiemetics administered in the PACU for nausea and/or vomiting. Additional key elements of Enhanced Recovery After Surgery (ERAS) recommendations were implemented, including the preoperative elements of patient and family education, patient optimization before admission, minimal fasting, antibiotic prophylaxis, and venous thromboembolism prophylaxis.24 Following the procedure, patients were prescribed oxycodone/acetaminophen orally as needed for 5 days.
Sufentanil Sublingual Tablet Treatment Group Procedures
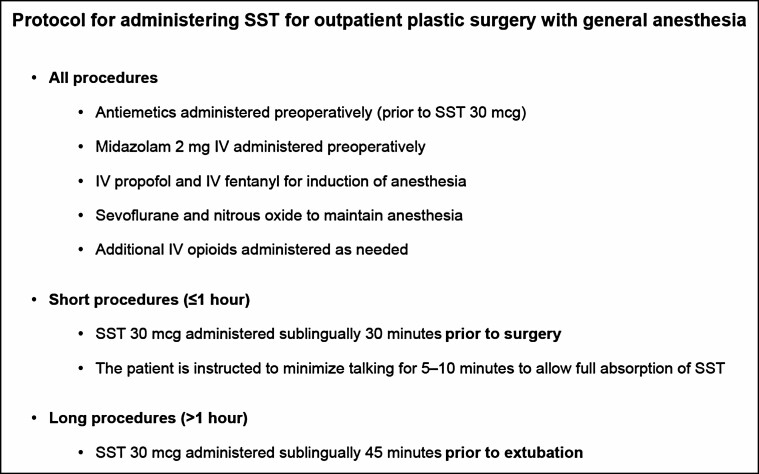
SST administration was designed to allow peak plasma concentrations to be achieved as the patient arrived in the PACU to maximize PACU analgesia and minimize PACU opioid dosing to facilitate timely discharge. SST-treated patients were administered a single SST 30 mcg approximately 30 minutes before incision for shorter-duration procedures to allow the 3-hour analgesic duration of a single dose to cover the intraoperative and postoperative period. For longer-duration procedures (>1 hour), which were the majority of the cases, standard IV opioid dosing was utilized for analgesia intraoperatively and a single dose of SST 30 mcg was administered approximately 45 minutes before the end of anesthesia. The study protocol for the SST patients is shown in Figure 3.
Figure 3.
Study protocol for administering sufentanil sublingual tablet (SST; DSUVIA, AcelRx Pharmaceuticals, Inc., Hayward, CA) 30 mcg for outpatient plastic surgery with general anesthesia. IV, intravenous.
Assessments
Age, sex, procedure duration, use of intraoperative opioids, time spent in the PACU before discharge, and additional opioids or antiemetics in the PACU were examined. Adverse events and medical treatment for these events were also recorded. Primary outcomes of interest were the percent of patients requiring PACU opioids and the average IV MME administered in the PACU.
Statistics
Descriptive statistics were calculated and reviewed for all patients. The univariate analysis compared groups on demographics, surgical details, and perioperative drug administration. Intraoperative and postoperative opioid doses were converted to MME to allow for a consistent unit of opioid utilization. MME conversion calculations were based on the Practical Pain Management online opioid calculator (https://opioidcalculator.practicalpainmanagement.com).25 The statistical level of significance was set at 5% (P < 0.05) for all univariate analyses for continuous data and the chi-square test for categorical variables. Analyses were conducted using SPSS version 28.0 (IBM Corp., Armonk, NY).
RESULTS
Patient Demographics
The chart reviews of aesthetic surgery patients undergoing general anesthesia yielded 32 outpatient cases receiving typical IV opioid analgesics perioperatively (control group) and 61 outpatient cases receiving SST. Groups did not differ in demographic variables (Table 1), with the vast majority of patients being female with an average age in the mid-40s. No patients in either cohort were taking chronic outpatient opioids before the procedure. The most common surgical procedures in both groups were bilateral breast augmentations (eg, capsulectomy with mastopexy) or reductions, often combined with an additional procedure (eg, liposuction and/or abdominoplasty). There was a trend for procedure duration to be slightly longer for controls (3 hours and 12 minutes) than for the SST group (2 hours and 40 minutes; P = 0.10). As a result, the duration of surgery was used as a covariate for subsequent analyses comparing opioid MME administration between groups.
Table 1.
Patient Characteristics
| Variable | Control (n = 32) |
SST (n = 61) |
P-value |
|---|---|---|---|
| Females, n (%) | 31 (96.9%) | 56 (91.8%) | 0.43 |
| Age (years), mean (SD) | 44.1 (9.6) | 46.1 (13.4) | 0.45 |
| Duration of surgery (h:min), mean (SD) | 3:12 (1:32) | 2:40 (1:23) | 0.10 |
SD, standard deviation; SST, sufentanil sublingual tablet.
Sufentanil Sublingual Tablet Dosing
The majority of patients in the SST group (56/61) had surgical durations > 1 hour and, therefore, received a dose of SST sublingually 45 minutes before extubation, while the 5 remaining patients underwent short-duration procedures and received a dose of SST before the start of the procedure.
Opioid MME Dosing
Not surprisingly, the dosing of IV opioids intraoperatively did not differ significantly between groups as presented in Table 2, because the majority of patients were administered SST toward the end of the procedure when most intraoperative IV analgesics had already been administered. This calculation of IV MME administered during surgery does not include the SST opioid equivalent of 5 MME, which brings the total in the SST-treated group to the same administered dose of opioids before the patient enters the recovery room. In the PACU, 29 of the 32 (90.6%) patients in the control group required opioids during the recovery period vs 10 of the 61 (16.4%) SST-treated patients (Pearson chi-square = 47.49; P < 0.001). This resulted in the groups differing in the average dose of opioids administered in the PACU, with controls receiving more MME (3.6 ± 2.65) than SST patients (0.64 ± 2.31; P < 0.001).
Table 2.
Intravenous Morphine Milligram Equivalent Utilization
| Variable mean (SD) |
Control (n = 32) |
Study (n = 61) |
P-value |
|---|---|---|---|
| IV opioid MME during surgery | 23.54 (9.76) | 18.66 (9.17) | 0.07 |
| IV opioid MME during recovery | 3.60 (2.65) | 0.64 (2.31) | <0.001 |
IV, intravenous; MME, morphine milligram equivalent; SD, standard deviation.
Antiemetic Dosing and Adverse Events
Patients received prophylactic antiemetics, with ondansetron being the most commonly administered drug, followed by metoclopramide, famotidine, and promethazine. During recovery, 3 patients in the control group required additional antiemetics in the PACU for nausea (9.4%), whereas only 1 SST patient (1.6%) had nausea and required an antiemetic in the PACU. Aside from the cases of nausea in the PACU, there were no additional adverse events reported during the recovery period, and no patient in either treatment group required naloxone for respiratory depression.
Postanesthesia Care Unit Time
The average duration of time spent in the PACU did not differ between the 2 groups, even though the SST-treated patients required less PACU opioids and did not have any adverse events delaying discharge. The control group stayed an average of 59 ± 15 minutes before discharge compared with 57 ± 20 minutes for SST-treated patients. It was observed by the clinical staff that discharge time was more governed by nurse routine, availability of patient transport, and additional factors other than patient readiness for discharge.
DISCUSSION
With the recent opioid-sparing approach to perioperative pain control across all surgical specialties, the challenge has been providing effective pain control while minimizing opioid dosing. The idea is not to practice opioid-free surgery but rather to carefully select the appropriate opioid and dosing regimen to optimize postoperative pain control and decrease the need for recovery room administration of opioids to allow for more rapid discharge.
The current evaluation of SST for pain management in outpatient aesthetic surgery patients undergoing general anesthesia aimed to address the challenge of reducing opioid dosing and associated negative sequelae in the PACU across a wide range of cosmetic surgeries, including those of extended duration (4 or more hours of procedural time). Previously published studies examining SST for acute pain management have highlighted its safety and efficacy with procedures of relatively shorter duration, typically averaging under an hour.18,19,26
For outpatient plastic surgery procedures, SST may be the “optimal” opioid to use because a single sublingual dose, which is the equivalent of 5 MME,14 provides 3 to 4 hours of analgesia while not exposing patients to the high peak plasma concentrations that are inherent with typical IV bolus dosing of opioids.16,27 SST has been found to have minimal effect on cognitive functioning. In a study of SST administered to patients with moderate-to-severe pain in the emergency department, cognition as measured by the 6-item screener showed no decrease from baseline in 97% (73/75) of patients and a single point drop in 3% (2/75) of patients 1 hour after SST dosing, which is the time of peak plasma concentration.20 The almost complete lack of effect of SST on cognition is why it is able to be used for plastic surgery procedures conducted in the minimal sedation setting.28 Although only a single dose is often needed for surgical procedures,19 multiple doses of SST can be administered with a minimum of 1 hour between doses.13
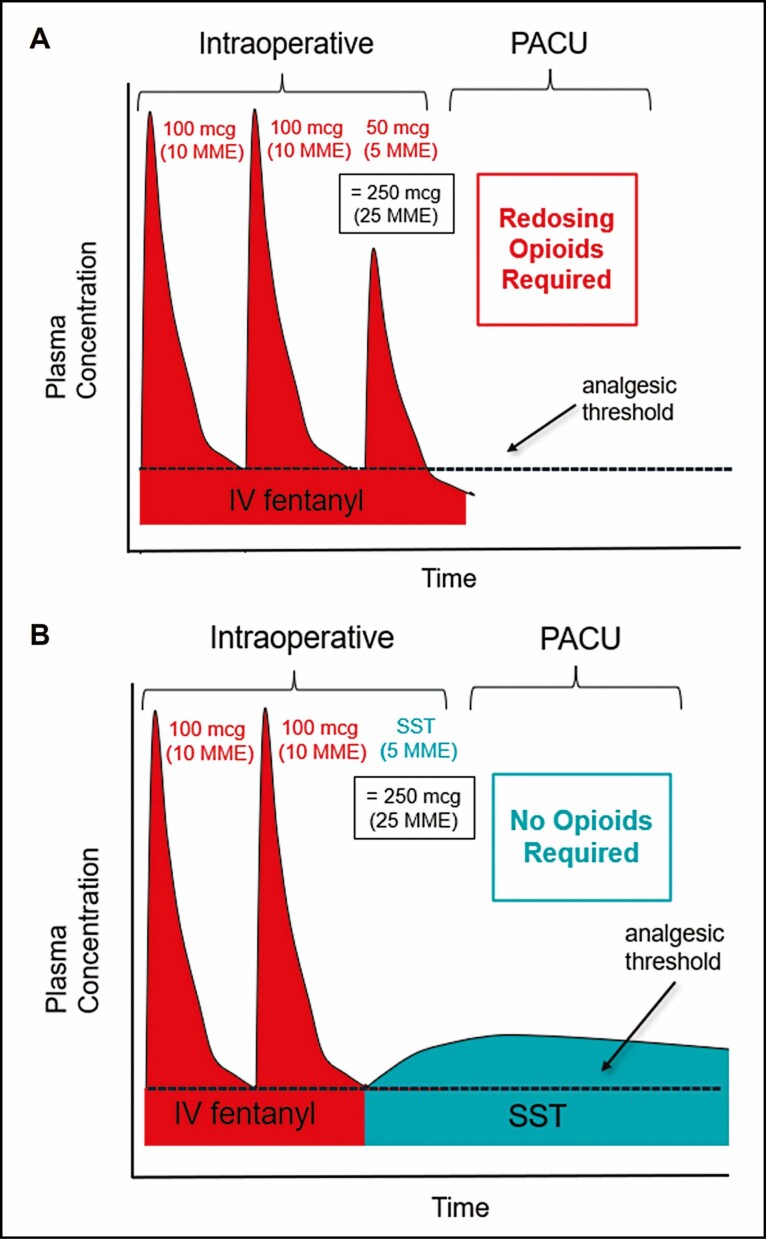
Administering a single SST approximately 45 minutes before extubation for cases over an hour in duration (and 30 minutes before intubation for shorter-duration cases) allowed this relatively low MME dose to provide significant analgesia throughout the immediate postoperative period. Only 16.4% of the SST group required additional opioids in the PACU compared with nearly all (90.6%) of the controls who underwent similar lengthy procedures with both groups averaging surgical durations of approximately 3 hours. This may not be surprising given that the typical IV fentanyl boluses administered intraoperatively suffer from a very rapid decay in plasma concentrations such that patients have pain returning within 30 minutes of dosing, due to the initial distribution half-life of only 1.7 minutes for IV fentanyl.10,29,30 The sublingual absorption of sufentanil in the SST occurs over time such that the duration of analgesia is greatly extended compared with the equivalent dose of IV fentanyl.31 Figure 4 illustrates how intraoperative dosing of SST can provide analgesia into the PACU period without increasing the intraoperative MME.
Figure 4.
Illustration representing the pharmacokinetic profiles of IV fentanyl and sufentanil sublingual tablet (SST; DSUVIA, AcelRx Pharmaceuticals, Inc., Hayward, CA). (A) Intravenous (IV) fentanyl has a rapid decay in plasma concentrations such that repeated doses are often needed during the intraoperative period and the analgesic effect is not maintained in the postanesthesia care unit (PACU) period. (B) Replacing 5 morphine milligram equivalents (MME) of IV fentanyl with SST can provide analgesia in the PACU period due to a slow decay in plasma concentrations.
While the dosing of PACU opioids was dramatically reduced, which may underlie the 3-fold lower incidence of nausea in the SST-treated group, interestingly, SST had no effect on time to discharge. Both control and SST-treated patients were in the PACU for approximately 1 hour before being discharged. As this was a retrospective study in our surgical center, “readiness for discharge” was not measured but likely would have shown a difference. The standard recovery protocols used in this specific clinical setting include a monitoring period, discussion of postdischarge instructions, and arranging patient discharge. In this outpatient unit, the patient is discharged from the PACU with no step-down or phase 2 unit, and the patient family members were not instructed to arrive earlier than an hour following the end of the procedure. Importantly, we have recently been revamping our PACU routine to allow for more rapid discharge of patients dosed with SST, as the large majority of these patients are comfortable and do not require IV opioids in recovery, even following extensive procedures.
Our clinical experience from using our SST protocol (Figure 3) for perioperative pain management has been that patients have an easier recovery with a smoother extubation and transfer to the recovery room compared with our years of experience with standard IV opioid administration intraoperatively. Good communication between the different team members is essential, as opioid requirements in the PACU greatly diminished following SST dosing and standard approaches to PACU pain management and patient flow had to be reconsidered. The cost of SST is under $60 per dose, so while it is more expensive than IV opioids, the increased patient satisfaction that we have observed makes the relative cost compared with the overall procedure negligible.
The rapid onset (within 15 minutes)17 and ease of administration of SST with the prefilled applicator (Figure 1) may be a time- and money-saving feature in the fast-paced outpatient aesthetic setting. Not all procedures need to be performed with general anesthesia or even conscious or deep sedation, which all require the setup of an IV line, which can take up nursing staff time. For those procedures with a duration well within the efficacy duration of a single SST dose (3-4 hours),27 effective pain management without IV propofol, midazolam, or fentanyl may be economically beneficial. Our clinical practice utilizes SST, along with local anesthetic infiltration, without an IV for awake aesthetic procedures, such as liposuction and facelifts.28 Although emergency equipment is available, including naloxone to be given intramuscularly if necessary, it has not been needed to date.
Limitations of the study include that it was a chart review and not a prospectively designed, randomized study. The variety of procedures conducted reflect real-world clinical practice; however, study and control groups were not matched for procedure type or procedure duration. Indeed, the mean duration of surgery was slightly longer for control patients than for SST-treated patients, although the difference was not statistically significant. The use of surgery duration as a statistical covariate in all analyses addressed the issue, but matching patients on surgical duration in future studies would provide a more rigorous control for potential group differences. This study also did not employ a metric of pain or readiness for discharge in the PACU, which could have been used to assess the potential of SST to facilitate a timely discharge. Additionally, with only 5 SST patients receiving a preoperative dose for short procedures, a comparison of the efficacy of SST between preoperative and intraoperative dosing was not possible in this study.
CONCLUSIONS
SST significantly reduced both the percentage of patients requiring PACU opioids and the overall average dose administered in the PACU compared with control patients receiving standard IV opioids. In addition, SST was associated with a lower rate of nausea requiring antiemetics in the PACU. As outpatient aesthetic surgeries increase in complexity and duration, SST may be a powerful pain management adjunct when dosed before extubation to allow for patient comfort in the PACU with minimal need for additional opioids to be administered.
Acknowledgments
The author would like to acknowledge the editorial support provided by Ann E. Todd, PhD.
Disclosures
Dr Seify is a paid consultant to AcelRx Pharmaceuticals, Inc. (Hayward, CA) and Syntr Health Technologies (Irvine, CA); has received research support from AcelRx; and is a member of the AcelRx Speaker Bureau.
Funding
The conduct of the study and manuscript preparation were funded by an investigator-initiated research grant from AcelRx Pharmaceuticals, Inc (Hayward, CA).
REFERENCES
- 1. Barker JC, DiBartola K, Wee C, et al. Preoperative multimodal analgesia decreases postanesthesia care unit narcotic use and pain scores in outpatient breast surgery. Plast Reconstr Surg. 2018;142(4):443e-450e. doi: 10.1097/PRS.0000000000004804 [DOI] [PubMed] [Google Scholar]
- 2. Faulkner HR, Coopey SB, Sisodia R, Kelly BN, Maurer LR, Ellis D. Does an ERAS protocol reduce postoperative opiate prescribing in plastic surgery? JPRAS Open. 2022;31:22-28. doi: 10.1016/j.jpra.2021.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017;10:2287-2298. doi: 10.2147/JPR.S144066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149-160. doi: 10.1185/03007995.2013.860019 [DOI] [PubMed] [Google Scholar]
- 5. Seago JA, Weitz S, Walczak S. Factors influencing stay in the postanesthesia care unit: a prospective analysis. J Clin Anesth. 1998;10(7):579-587. doi: 10.1016/s0952-8180(98)00084-1 [DOI] [PubMed] [Google Scholar]
- 6. Ganter MT, Blumenthal S, Dubendorfer S, et al. The length of stay in the post-anaesthesia care unit correlates with pain intensity, nausea and vomiting on arrival. Perioper Med (Lond). 2014;3(1):10. doi: 10.1186/s13741-014-0010-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248-273. doi: 10.1097/ALN.0b013e31823c1030 [DOI] [PubMed] [Google Scholar]
- 8. Carr DB, Goudas LC. Acute pain. Lancet. 1999;353(9169): 2051-2058. doi: 10.1016/S0140-6736(99)03313-9 [DOI] [PubMed] [Google Scholar]
- 9. Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425-430. doi: 10.1001/archinternmed.2011.1827 [DOI] [PubMed] [Google Scholar]
- 10. Fentanyl Citrate Injection. Prescribing Information. Hospira, Inc; 2016. [Google Scholar]
- 11. Harris M, Chung F. Complications of general anesthesia. Clin Plast Surg. 2013;40(4):503-513. doi: 10.1016/j.cps.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 12. Manahan MA, Johnson DJ, Gutowski KA, et al. Postoperative nausea and vomiting with plastic surgery: a practical advisory to etiology, impact, and treatment. Plast Reconstr Surg. 2018;141(1):214-222. doi: 10.1097/PRS.0000000000003924 [DOI] [PubMed] [Google Scholar]
- 13. Dsuvia. Prescribing Information. AcelRx Pharmaceuticals, Inc.; 2021. [Google Scholar]
- 14. Miner JR, Melson TI, Leiman D, et al. Pooled phase III safety analysis of sufentanil sublingual tablets for short-term treatment of moderate-to-severe acute pain. Pain Manag. 2019;9(3):259-271. doi: 10.2217/pmt-2018-0090 [DOI] [PubMed] [Google Scholar]
- 15. Bernards CM. Clinical implications of physiochemical properties of opioids. In: Stein C, ed. Opioids in Pain Control: Basic and Clinical Aspects. Cambridge University Press; 1998:166-187. [Google Scholar]
- 16. Fisher DM, Chang P, Wada DR, Dahan A, Palmer PP. Pharmacokinetic properties of a sufentanil sublingual tablet intended to treat acute pain. Anesthesiology. 2018;128(5):943-952. doi: 10.1097/ALN.0000000000002145 [DOI] [PubMed] [Google Scholar]
- 17. Minkowitz HS, Leiman D, Melson T, Singla N, DiDonato KP, Palmer PP. Sufentanil sublingual tablet 30 mcg for the management of pain following abdominal surgery: a randomized, placebo-controlled, phase-3 study. Pain Pract. 2017;17(7):848-858. doi: 10.1111/papr.12531 [DOI] [PubMed] [Google Scholar]
- 18. Tvetenstrand CD, Wolff ME. Reduced opioid use and reduced time in the postanesthesia care unit following preoperative administration of sublingual sufentanil in an ambulatory surgery setting. J Clin Anesth Pain Manag. 2020;4(2):123-128. doi: 10.36959/377/341 [DOI] [Google Scholar]
- 19. Cassavaugh KM, Hogan SM, Sensak JC, Cady MD. A medication use evaluation of sufentanil sublingual tablet 20 mcg for perioperative management of surgical pain. J Univ Surg. 2020;8(5):6): 1-5. doi: 10.36648/2254-6758.8.5.136 [DOI] [Google Scholar]
- 20. Miner JR, Rafique Z, Minkowitz HS, DiDonato KP, Palmer PP. Sufentanil sublingual tablet 30 mcg for moderate-to-severe acute pain in the ED. Am J Emerg Med. 2018;36(6):954-961. doi: 10.1016/j.ajem.2017.10.058 [DOI] [PubMed] [Google Scholar]
- 21. Leiman D, Jove M, Spahn GR, Palmer P. Patient and healthcare professional satisfaction ratings and safety profile of sufentanil sublingual tablets for treatment of acute pain: a pooled demographic analysis. J Pain Res. 2021;14:805-813. doi: 10.2147/JPR.S291359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. AcelRx Pharmaceuticals, Inc. Risk evaluation and mitigation strategy (REMS). Accessed September 29, 2021. http://dsuviarems.com/
- 23. Battista C, Krishnan S. Pectoralis nerve block. In: StatPearls [Internet]. StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 24. Temple-Oberle C, Shea-Budgell MA, Tan M, et al. Consensus review of optimal perioperative care in breast reconstruction: Enhanced Recovery after Surgery (ERAS) Society Recommendations. Plast Reconstr Surg. 2017;139(5):1056e-1071e. doi: 10.1097/PRS.0000000000003242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Practical Pain Management. Opioid calculator. Accessed August 30, 2021. https://opioidcalculator.practicalpainmanagement.com/
- 26. Thangaraju P, Varthya SB, Venkatesan S, Tamilselvan T, Singh S. Efficacy and safety of sufentanil sublingual tablet system in postoperative pain management: a systematic review and meta-analysis. BMJ Support Palliat Care. 2021:bmjspcare-2020-002693. doi: 10.1136/bmjspcare-2020-002693 [DOI] [PubMed] [Google Scholar]
- 27. Hutchins JL, Leiman D, Minkowitz HS, Jove M, DiDonato KP, Palmer PP. An open-label study of sufentanil sublingual tablet 30 mcg in patients with postoperative pain. Pain Med. 2018;19(10):2058-2068. doi: 10.1093/pm/pnx248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seify H. Awake plastic surgery procedures: the use of a sufentanil sublingual tablet to improve patient experience. Aesthet Surg J Open Forum. 2022;4:1-8. doi: 10.1093/asjof/ojab056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kandasamy J, Carlo WA. Pharmacologic therapies IV: other medications. In: Goldsmith JP, Karotkin E, Suresh G, Keszler M, eds. Assisted Ventilation of the Neonate, 6th ed. Elsevier Inc; 2017:366-379. [Google Scholar]
- 30. Simmons B, Kuo A. Analgesics, tranquilizers, and sedatives. In: Brown DL, ed. Cardiac Intensive Care, 3rd ed. Elsevier Inc; 2019:421-431. [Google Scholar]
- 31. Fudin J, Bettinger JJ, Dasta JF. A commentary on opioid stewardship: fentanyl, sufentanil, and perioperative pain. Pract Pain Manag. 2020;20(6). [Google Scholar]